Abstract
Background
Nicotinic acetylcholine receptors (nAChRs) belong to the Cys-loop ligand-gated ion-channel (LGIC) superfamily, which also includes the GABA, glycine, and serotonin receptors. Many nAChR subunits have been identified and shown to be involved in signal transduction on binding to them of either the neurotransmitter acetylcholine or exogenous ligands such as nicotine. The nAChRs are pentameric assemblies of homologous subunits surrounding a central pore that gates cation flux, and they are expressed at neuromuscular junctions throughout the nervous system.
Methods and Results
Because different nAChR subunits assemble into a variety of pharmacologically distinct receptor subtypes, and different nAChRs are implicated in various physiological functions and pathophysiological conditions, nAChRs represent potential molecular targets for drug addiction and medical therapeutic research. This review intends to provide insights into recent advances in nAChR signaling, considering the subtypes and subunits of nAChRs and their roles in nicotinic cholinergic systems, including structure, diversity, functional allosteric modulation, targeted knockout mutations, and rare variations of specific subunits, and the potency and functional effects of mutations by focusing on their effects on nicotine addiction (NA) and smoking cessation (SC). Furthermore, we review the possible mechanisms of action of nAChRs in NA and SC based on our current knowledge.
Conclusion
Understanding these cellular and molecular mechanisms will lead to better translational and therapeutic operations and outcomes for the prevention and treatment of NA and other drug addictions, as well as chronic diseases, such as Alzheimer’s and Parkinson’s. Finally, we put forward some suggestions and recommendations for therapy and treatment of NA and other chronic diseases.
Keywords: nAChRs, nicotinic acetylcholine receptor, nicotinic cholinergic system, smoking dependence, smoking-related diseases, signaling, SNPs
1. Introduction
Smoking or other tobacco use is the leading cause of preventable death in both developed and developing countries, being responsible for about six million deaths worldwide each year [1-7]. Tobacco-related diseases are predicted to become the largest health problem worldwide by 2020, resulting in about 8.4 million deaths each year [5, 8]. Despite the well-known harmful consequences associated with tobacco use, only a few smokers attempt to quit smoking each year, [5] and only 3%–5% are successful without nicotine replacement therapies, and no more than one-third are successful even with these therapies [9, 10].
Tobacco is a commonly abused legal substance, in which nicotine is the principle addictive component [11-15]. Although almost all of the toxicity of smoking is attributable to other substances [16-18]. There are mainly the pharmacologic effects of nicotine that lead to NA [11-15, 19]. Nicotine is a tertiary amine alkaloid which binds to diverse subtypes of the nicotinic acetylcholine receptors (nAChRs) that have unique expression patterns in the central nervous system (CNS). The nAChRs are pentameric proteins composed of identical or homologous subunits that belong to the Cys-loop family of ligand-gated ion channels (LGICs), which also include serotonin (5-hydroxytryptamine; 5-HT), γ-aminobutyric acid (GABA), dopamine (DA), and glycine [20-22]. Neuronal nAChRs are expressed during brain development and contribute to neurogenesis, neurite outgrowth, and synaptic maturation [23-26]. These receptors mediate the effects of the conventional neurotransmitter/agonist acetylcholine [14, 27, 28] and are crucial to the normal functioning of the brain, being amenable to various endogenous and exogenous modulating factors. Along with the progress of recent research, emerging evidence suggests that neuronal nAChRs in the mesocorticolimbic–dopamine system mediate both the rewarding effects of nicotine and the development of NA [1].
The nAChRs are excitatory receptors for the endogenous neurotransmitter acetylcholine and are widely expressed in the pre- and post-synaptic sites of the CNS [29, 30] whereas nicotine is membrane-permeable in its uncharged form. It can influence intracellular processes indirectly through nAChRs and directly by entering the cytoplasm. These receptors are distributed in both the peripheral nervous system (PNS) and the CNS areas involved in neuronal transmission and modulation of acetylcholine and the rapid physiological responses to that compound [25, 31-39]. Nicotine diffuses readily into brain tissue and binds to nAChRs. These receptors also can respond to low acetylcholine concentrations and are the target of regionally released acetylcholine and systemically applied pharmacological agents such as nicotine [1, 40]. Activation of brain nAChRs causes enhanced release of various neurotransmitters, including acetylcholine, dopamine, 5-HT, glutamate, and GABA. Recent studies have shown that nicotine-induced addiction, reward, and withdrawal involve a wide range of nAChR subtypes that are expressed in the diverse neural systems, including both neuronal and non-neuronal tissues [1, 41]. Genetic and clinical studies have identified nAChRs and brain membrane-bound ion channels in the mesolimbic–dopamine system as being involved in NA and SC, and their dysfunctions have been implicated in many neurological diseases. Therefore, nAChRs are considered potential molecular targets for curtailing the abuse of nicotine or tobacco, alcohol, and other substances. Furthermore, nAChRs have been implicated in various neuromuscular, neurological, and psychiatric disorders, such as Alzheimer’s and Parkinson’s diseases, lung cancer, and schizophrenia [42-54]. Various types of neuronal nAChRs have been identified as critical targets for drug discovery for the treatment of those psychiatric disorders. In this review, we intend to provide insights into recent advances in nicotinic receptor signaling, examining the subtypes and subunits of nAChRs and their roles in nicotinic cholinergic systems, including structures, diversity, functional allosteric modulation, targeted knockout (KO) mutations, and rare variants of specific nAChR subunits, as well as the potency and functional effects of mutations, by focusing on their effects on the dependence and cessation of nicotine use. We further review the possible mechanisms of action of nAChRs in NA and SC based on current knowledge. Understanding these mechanisms will lead to a better translational and therapeutic operation and possible methods for the prevention and treatment of NA and related diseases. Finally, we put forward some suggestions and recommendations showing promise for the treatment of NA and other NA-related chronic diseases.
2. The structure and diversity of NAChRS
The nAChRs are integral membrane receptors encoded by 17 subunit genes with a single molecular mass of approximately 290 kDa [55-57]. They are of fundamental importance in human disorders such as Alzheimer’s disease, Parkinson’s disease, schizophrenia, and NA [1, 41, 51, 58]. The homologous nAChR subunits are symmetrically arranged around a central ionic channel and are expressed in muscle, nerve, and sensory cells [59-63]. They are hetero-pentameric or homo-pentameric with a five-fold axis of pseudo-symmetry. Both the muscle and the neuronal nAChR subunits are composed of an N-terminal extracellular domain (ECD) that contributes to agonist binding, as well as four hydrophobic ion-pored transmembrane domains (TMDs or M1–M4), [20, 54, 64-69] a short extracellular C-terminal ECD, and a large intracellular cytoplasmic loop of various lengths and sequence identities between M3 and M4 [70, 71]. The channel’s ion pore is lined by M2 from five co-assembled subunits [72-74] whereas the ECDs carry the acetylcholine binding sites at the boundary between α and β subunits, and those TMDs delineate an axial cation-specific channel [20, 40]. Each nAChR subunit contains a β-sheet-rich N-terminal extracellular portion of approximately 200 residues, a 4-α-helical transmembrane segment of about 150 residues, and a variable C-terminal intracellular segment that forms contacts with the cytoskeleton [40, 72]. The receptor spans 150Å or so in its longest axis, with 60–70Å of this being attributable to the ECD and 40Å to the TMD [40, 72]. The ion conduction and channel gating function of the nAChR are localized to the TMD, whereas the five subunits form a donut structure around a central pore that conducts ions when it is in the open state [40, 72]. Thus, nAChRs possess a structure that converts the chemical signal of neurotransmitters into an electrical signal by opening the ion channel, such as in the presence of increased extracellular acetylcholine. The acute effect of acetylcholine consists of the fast opening of a cationic channel of nAChRs, lasting from microseconds to milliseconds, that is permeable to Na+, K+ and sometimes Ca2+ ions.
A total of 17 nAChR subunit genes, coding for subunits α1–α10, β1–β4, γ, δ, and ε, have been identified in various vertebrate species (Table 1). In mammals, nAChRs are found in both the CNS and the PNS, with nine α-subunits and three β-subunits being expressed in the brain. Up to now, 16 human nAChR subunits (α1–α7, α9, α10, β1–β4, γ, δ, and ϵ) have been identified [75, 76]. As noted above, nAChR receptors are broadly categorized as either neuronal (n = 12) or muscle (n = 5) subtypes on the basis of their specific subunit composition and stoichiometry (see Table 1). The α1, β1, γ, δ, and ϵ subtypes are expressed in muscle, whereas α2–α7, α9, α10, and β1–β4 are expressed widely and are commonly referred to as “neuronal” subunits [75, 76]. Muscle type nAChRs, found in the PNS, are composed of (α1)2βγδ or (α1)2βεδ, in which the γ subunit originally is embryonic, being replaced by the ε subunit in adult tissue [40]. The muscular and neuronal nAChRs containing α7 or α8 subunits are sensitive to the antagonist α-bungarotoxin (BTx), whereas the heteropentameric neuronal nAChRs are insensitive [77]. Two neuronal type subunits, α5 and β3, are found to be functional homologs of β1 subunits (muscle type) that are able to co-assemble with other subunits as structural subunits. Curiously, α8 has been only found in chickens.
Table 1.
A list of nAChR subunits and representative nAChR subtypes.
| Heteromeric nAChR subtypes | Muscle type receptors | |
| Subunit composition of ligand binding pentamers | Structural subunit | |
| α1, β, γ, δ (embryonic type); α1, β, ε, δ (adult type) | β (also called β1) | |
| Neuronal type receptors | ||
| Subunit composition of ligand binding pentamers | Structural subunit | |
| α2, α3, α4, α5, α6, β2, β3, β4 | α5, β3 | |
| Homomeric nAChR subtypes | Neuronal type receptors | |
| α7, α8, α9, α10 | ||
| Representative nAChR subtypes in CNS | α2β2, α3β2, α3β4, α3β3β4, α4β2, α4α5β2, α6β2β3, α6α4β2β3, α7. | |
Notes: CNS is the abbreviation of central nervous system. The subunit α8 is currently only found in chick. Those homomeric nAChR subunits, i.e., α10 and α9, may form heteromeric receptors, whereas other three subunits, i.e. α5 and β3 and β1, are able to co-assemble with other subunits as structural subunits.
There are diverse subtypes of nAChRs, and neuronal nAChRs alone comprise several subtypes, with each having distinctive pharmacological and biophysical properties [78]. Generally, neuronal nAChR subtypes can be divided into the αBgtx-sensitive (i.e., α7, α8, α7/α8, α9, and α10) and the heteromeric αBgtx-insensitive subtypes, including the combinations of α2–α6 with β2–β4 [79]. To date, nearly 30 neuronal nAChR subtypes have been identified in brain tissues. These nAChRs are generated from α (α2–α10) and β (β2–β4) subunits, [39, 40] and the three most abundant brain nAChR subtypes are composed of α7, α4β2, and α3β4 subunits [80]. There are homopentamers of neuronal nAChRs composed only of α7 subunits and heteropentamers composed of α4 and β2 subunits in the form of (α4)3(β2)2 (high affinity for agonist) or (α4)2(β2)3 (low affinity for agonist) [39]. Rarer combinations of α3β4, α3β2, and αβ2β3 subunits have been found in specific brain tissues [81]. Among these subtypes, each in the heteromeric neuronal nAChRs has a different affinity for agonists and antagonists [82, 83]. In contrast, the interfaces of homomeric nAChRs composed of α7, α8, or α9 subunits are identical and express five equivalent agonist-binding sites. Studies indicate significant expression of α3, α5, and β4 subunits in the brain [78, 84]. Neuronal nAChRs also are expressed in numerous other cell types and tissues, including endothelial cells, gastrointestinal tissues, glia, immune cells, keratinocytes, urinary bladder tissues, reproductive organs, and respiratory tissues [85-96]. In addition to the acetylcholine binding sites [20, 40] other binding sites on nAChRs, such as the cholesterol-binding sites in M1–M4 segments [74, 97] the α-neurotoxin-binding sites in the central loop C of the α subunit, [98] and binding sites for methyllycaconitine and synthetic antagonists [99] have been identified. As a result of their roles in the propagation of action potentials, cognitive function, and diverse central nervous system pathologies, they are hypothetical targets for many drugs and toxins [84, 100].
3. The NAChR signaling in NA and withdrawal during smoking cessation
It is well known that binding of nicotine to nAChRs creates the molecular basis for the reward effects of nicotine and thus the development of NA. Most of the neuronal nAChRs are heteromeric, being composed of different isoforms of alpha (α2–α9) and beta (β1–β4) subunits. The receptors differ in their pharmacological responses according to the particular combination of α and β receptor subunits [31, 101, 102]. Genetic variations in the genes encoding the heteromeric nAChRs contribute to differences in the risk of a smoker’s developing NA. The most compelling human genetic associations with NA are located in chromosomal region 15q25, which encompasses the α5-α3-β4 nAChR subunit gene cluster (CHRNA5-CHRNA3-CHRNB4) [103, 104] and on chromosome 8, in the region encompassing the β3–α6 nAChR subunit gene cluster (CHRNB3-CHRNA6) [105]. nAChR subunits also play critical roles in the reinforcing and withdrawal effects of nicotine, whereas chronic nicotine exposure induces neuroadaptations in receptor expression, resulting in either upregulation or downregulation of the nAChRs in different brain regions [106-108]. Some nAChR subtypes or subunits also mediate the annoying stresses and affective somatic effects in animals treated with nicotine [109-111]. The development of enhanced therapeutics and treatment of NA will result from the identification of specific nAChR subtypes or subunit compositions that are necessary for the expression of NA and SC.
4. Insight from allosteric modulation of nicotinic receptor signaling with NAChR subunits
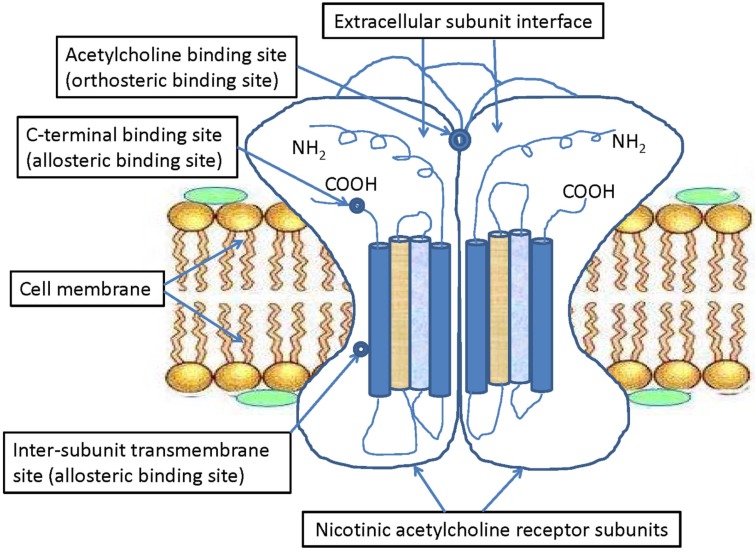
nAChRs are typical allosteric membrane proteins and historically pharmacological targets for diverse natural drug ligands or chemical compounds, including agonists and antagonists isolated from plants and vertebrate animals. These receptors can mediate signal transduction between particular sites through their conformational transition and equilibrium [28, 112] which makes them the most interesting model for studying the molecular mechanisms of drug–receptor or ligand–receptor interactions [20, 39, 40, 112, 113]. Early studies on functional nAChRs of Torpedo pioneered the discovery and subsequent development of a new class of pharmacological agents referred to as “allosteric ligands” that bind to sites distinct from the classic acetylcholine binding site (i.e., orthosteric sites; see Fig. 1). Most of the common drug molecules and their metabolites interact with the inner ion channel surfaces of nAChRs through a non-competitive inhibition mechanism, whereas the distinct binding of allosteric ligands to outer or inner surfaces of nAChR ECDs plays critical roles in coupling agonists. Previous studies have identified many nAChR-selective ligands, including both agonists and antagonists that link to the same extracellular binding sites of acetylcholine at the interface between nAChR subunits [72, 77, 114-120]. The nAChRs also can be activated by allosteric ligands binding to other transmembrane sites [121, 122]. Some studies suggest that allosteric ligands bind to different regions and recognition sites other than those where acetylcholine binds and affect the function of nAChRs [26, 64, 69, 77, 114, 115, 119, 123-126]. For example, five amino acids, located within the α-helical transmembrane domains TM1 (S222, A225), TM2 (M253), and TM4 (F455, C459), were identified as the binding sites of allosteric ligands of α7 nAChRs [127]. When these amino acids were changed by site-directed mutagenesis, the potentiation of α7 nAChRs was significantly reduced after treatment with two allosteric ligands (PNU-120596 and LY-2087101) [127]. Another study revealed that the N-terminal ECD of α7 nAChR plays a critical role in the modulation of nAChRs activity by allosteric ligand NS-1738, whereas α7-5HT(3) chimeras harboring an ECD M2-M3 segment show spontaneous activity in response to NS-1738 [128]. Furthermore, amino acid mutations in the receptor transmembrane cavity are regarded as the binding site for allosteric ligands of α7 nAChRs [123, 129, 130]. These data illustrate the existence of distinct allosteric recognition and binding sites and the critical role of the nAChR extracellular transmembrane domain in receptor gating function. Other than direct agonists and antagonists that exert their actions at the conventional agonist binding sites at the interface of two subunits [72, 77, 130] many newly identified allosteric modulating drugs act elsewhere on functional nAChRs [69, 114-116, 123, 124, 131].
Fig. (1).
Simplified schematic illustration of the orthosteric and allosteric binding sites of nAChR subunits. A simplified schematic representation showing both the orthosteric and allosteric binding sites of nAChR subunits, including acetylcholine binding sites (orthosteric binding sites) located between the extracellular subunit interfaces, and the c-terminal binding sites and inter-subunit transmembrane sites (allosteric binding sites).
The concepts of an allosteric modulator (i.e., ligand) and allosteric site describe a particular allosteric ligand and nAChR ligand-binding site distinct from the conventional agonist (acetylcholine) and its binding sites [64]. Although allosteric modulators usually have no intrinsic activity and merely modulate the effects of agonists or ligands, the response and activity of nAChRs (i.e., activation and desensitization) to non-modulatory orthosteric agonists are largely transformed by allosteric modulators into the binding of modulating molecules to specific allosteric sites on nAChRs, whereas the allosteric sites were recently reported to be distinct from the extracellular orthosteric sites for acetylcholine (Fig. 1), with some modulatory binding sites being located in the transmembrane domain [64, 69, 132]. Furthermore, some allosteric modulators display a mixed activity profile, as they both enhance the potency of endogenous orthosteric agonists and directly activate the receptor by binding to specific orthosteric or non-orthosteric sites [69, 121, 133]. Other allosteric ligands do not exhibit any detectable intrinsic efficacy for receptor activation. These allosteric modulators produce conformational changes in the receptor that positively increase or negatively decrease the activation potency of orthosteric agonists by binding to and acting on allosteric sites [64, 69, 121]. As to the effects on nAChRs, these allosteric modulators generally are classified as positive allosteric modulators (PAMs), negative allosteric modulators (NAMs), silent allosteric modulators (SAMs), or other novel compounds (neutral allosteric ligands) activating or desensitizing nAChRs via allosteric sites [64, 69, 134]. As for neuronal nAChRs, the endogenous neurotransmitter acetylcholine acts as a conventional agonist and binds the receptor’s extracellular domain at the interface between two nAChR subunits [77, 135]. In contrast, some allosteric modulators potentiate/activate the nAChR conformation and agonist-activation (PAMs) or inhibit/desensitize the nAChR conformation and non-competitive agonist-activation (NAMs). Yet other non-competitive allosteric modulating antagonists (SAMs) are capable of blocking channel pores and inhibiting agonist-evoked responses through distinct binding sites other than those used by PAMs and NAMs [134]. Among these modulators, PAMs often potentiate the effect of agonist binding and thus are called “co-agonists” or “non-competitive agonists” [64, 69, 132]. In the case of α7 nAChRs, these compounds usually are further subdivided functionally into type I PAMs, with minimal to no effects on the receptor’s desensitization, and type II PAMs, with some but reduced effects on the receptor’s desensitization [64, 69, 125, 132, 134, 136]. Furthermore, structurally similar allosteric modulators exhibit diverse pharmacological effects on α7 nAChRs [116, 125, 134]. For instance, nondesensitizing activation (allosteric agonists), which enhance the agonist-induced activation without altering desensitization kinetics (type I PAMs), stabilize the receptor’s open state with slow desensitization (type II PAMs), noncompetitive antagonism (NAMs), and blocking allosteric modulation without effects on orthosteric agonists (SAMs) [134]. There also are a few allosteric modulators that exhibit features of both these subclasses; i.e., they possess PAM effects on some nAChR subtypes and NAM effects on other subtypes. In addition to synthetic compounds, several endogenous compounds, including neurosteroids and some fatty acids, have NAM effects [90, 137, 138]. In contrast, NAMs can stabilize non-conducting conformations of the receptor, decrease agonist affinity, or increase the receptor’s desensitization rate [139]. Both PAMs and NAMs exert effects at a variety of locations, including non-canonical intersubunit interfaces in the ECDs [130, 140] the coupling region between ECDs and TMDs [141] and within the TMD helices [127, 142]. It has been suggested that large molecules act as PAMs by binding at an inter-subunit transmembrane site and preventing the transition from the open to the closed states [64, 71]. These various binding sites differ in chemistry across different nAChR subunits, leading to variable sensitivities to modulation by allosteric modulators, whereas the intrasubunit TMD sites are the most prominent and conserved ones [64, 69, 143]. Furthermore, PAMs can potentiate lower agonist concentration selectively by facilitating the agonist binding and can change the efficacy of agonists by reducing or increasing the energy barrier between the closed and open states of nAChR subunits [125, 134, 144]. Moreover, the combined usage of therapeutic orthosteric agonists and PAMs might be reasonable because of the ubiquitous expression of nAChRs [26, 121, 144]. In animal models, behavioral studies with α7 nAChR PAMs, such as PNU-120596, have revealed their ability to influence cognitive function directly, and those studies with α4β2 nAChR PAMs focused mainly on reducing pain and improving cognitive function [26]. In addition, the functional expression and degree of activity of nAChRs decline only in a subunit- or tissue-specific manner but do not vanish in the age-, disease- or trauma-related pathological states [121]. Therefore, PAMs are proposed as promising functional allosteric ligands to act as therapeutic orthosteric agonists of nAChRs.
So far, the hypothetical mechanism of conformational equilibrium and functional switch of nAChRs into high activable affinity receptors is accepted as modulatable by both endogenous and exogenous compounds [68, 69, 131, 145] which makes nAChRs prime targets for pharmaceuticals for the treatment of neurological diseases [64, 84, 144-148]. In particular, α7 nAChRs selectively bind the II PAM PNU-120596 type, which slows desensitization in a cavity formed by the α-helices of M1, M2, and M4 [127]. PNU-120596 is a highly selective potentiator or PAM of α7 nAChRs [144, 149, 150] and it significantly reduces cerebral infarct volume and neurological deficits in a middle cerebral artery occlusion model of ischemic stroke in rats [150, 151]. Recently, a significant therapeutic potential of PNU-120596 after traumatic brain injury in rats has been reported [144, 150] and more representative nAChR ligands with multifunctional activities and effects can be found in a recent review [152]. These trials will provide helpful guidance for the practical design of α7 nAChR antagonists and shed new light on the antagonistic mechanism. Additionally, the core question in therapeutic drug development is how drug ligands will affect the endogenous signaling that is mediated by natural activators, including both agonists and antagonists. Long-term exposure to nicotine leads to an activity increase, amount upregulation, or both of nicotine binding sites in the brain of smokers [153, 154] and rodents subjected to repeated nicotine administration [155, 156]. It has been suggested that receptor desensitization is a major contributor to the upregulation of nAChR subunits that is observed in chronic users of nicotine-containing products [154, 157]. Because of the relatively conserved receptor binding sites of nAChRs for conventional agonists such as acetylcholine, [143] the subtype selectivity of allosteric modulators may be achieved easily by designing drug ligands that bind to various allosteric sites in diverse nAChR subunits [116, 123, 124, 158-165].
5. Insight from knockout of NAChR subunits in transgenic mice
Although NA is a complex condition influenced by multiple factors, including both genetic and environmental elements, several specific subtypes and subunits of nAChRs mediate the bothersome somatic stresses and affective symptoms associated with NA and withdrawal in SC of nicotine-treated animals [109, 166, 167]. Furthermore, a crucial goal of therapeutic drug discovery for NA is the identification or design of receptor ligands that bind to highly selected nAChR subtypes. Studies on NA have revealed that many nAChR subtypes and subunits are involved in the processes of self-administration, reward behavior, and withdrawal effects of nicotine [167-170]. These effects include genetic deletions of known nAChR subunits in the brain, targeted knockout (KO) mice, and knockin (KI) mutations yielding specific transgenic gain-of-function receptor phenotypes of nAChR subunits [171]. The subtypes and subunits of nAChRs can mediate the unpleasant somatic stresses and affective effects associated with NA and SC. Among the nAChR subunits, β2, α4, and α7 account for many of the brain nicotine-binding sites. In general, genetic KO of β2, β3, β4, α2, α4, α5, α6, or α7 subunits generally attenuate or weaken somatic signs of nicotine withdrawal in nicotine-dependent mice compared with their wild-type counterparts [109, 172-174]. Moreover, recent reports of transgenic mice with specific KO of subunits of nAChRs have provided important information about both the function of neuronal nAChRs and the mediation of addiction-related behavior [4, 5, 175]. These KO animal models with mutant nAChR subunits are helpful to identify and evaluate the nAChR subtype selectivity associated with many symptoms of nicotine addiction, reward, and withdrawal (Table 2).
Table 2.
Main findings in nAChR mutant KO mice.
| Deleted Gene | Critical Phenotype in nAChR Null Mutant Mice | Refs. |
|---|---|---|
| CHRNA2 | Increased self-administration; no somatic signs and hyperalgesia with conditioned CPA; context-dependent nicotine abstinence | [174, 210, 211] |
| CHRNA4 | More hypersensitive to nicotine than the wild-type; increased basal anxiety (EPM); blunted basal level and nicotine-stimulated DA release. | [184, 197-200] |
| CHRNA5 | Increased nicotine intake and self-administration; enhanced anxiety during nicotine withdrawal; reduced somatic symptoms; no signs of hyperalgesia or anhedonia. | [109, 174, 176-179] |
| CHRNA6 | Blocked nicotine withdrawal-induced CPA and anxiety; nicotine self-administration blocked; blunted nicotine-stimulated DA release; reduced anxiety and aversion in pharmacological blockade. | [186, 189-193] |
| CHRNA7 | Unaffected anxiety-like behavior; unaffected nicotine self-administration; increased nicotine-stimulated DA release; blunted nicotine self-administration; delayed onset of nicotine withdrawal-induced anhedonia-like behavior; significantly reduced chronic oral nicotine intake and nicotine withdrawal-induced somatic symptoms. | [109, 182, 213-216] |
| CHRNB2 | No somatic signs and abstinence-induced hyperalgesia; no nicotine-stimulated DAergic neuron firing; no anxiety or anxiety-related behavior (EPM); blocked nicotine-evoked DA release; blocked nicotine self-administration and conditioned reinforcement. | [109, 110, 201-206] |
| CHRNB3 | Decreased anxiety (EPM); altered hypothalamic pituitary–adrenal axis responses; altered locomotor activity, prepulse inhibition, and other behaviors. | [195, 196] |
| CHRNB4 | Decreased anxiety (EPM); dose-dependent tolerance development; reduced somatic symptoms and hyperalgesia in nicotine withdrawal; delayed onset of nicotine withdrawal-induced behaviors; decreased somatic signs of nicotine withdrawal-induced symptoms; highly resistant to nicotine-induced seizures. | [109, 172, 181-186, 213, 216, 253] |
5.1. The α3*, β4*, and α5* nAChRs
The β4, α3, and α5 nAChR subunits (* indicates the presence of additional subunits) are densely expressed in the medial habenula (MHb) and the interpeduncular nucleus (IPN) [176]. They are encoded by the CHRNA5-CHRNA3-CHRNB4 cluster, which was revealed in association with the behavior of smoking addiction and NA. The α5* nAChRs were found to be diffusely expressed in brain tissues (e.g., VTA, MHb, and IPN) and implicated in the effects of nicotine addiction [177]. Importantly, MHb and IPN were suggested as the locations of action of α5* nAChRs in mediating physical behaviors and symptoms of NA and SC [174]. Nevertheless, α5 subunit KO mice display no obvious physical behaviors linked to mecamylamine-induced nicotine addiction [174] or somatic symptoms related to nicotine withdrawal-elicited hyperalgesia [109]. Other observations suggested that α5* nAChRs do not influence anhedonia [109, 178]. Similarly, α5 null mutant mice display no uncomfortable symptoms such as nicotine withdrawal-induced conditioned place aversion (CPA) and increased anxiety during NA and SC [109]. Study of CHRNA5 KO mice indicated a null mutation in CHRNA5 markedly increased nicotine intake but no disassociation between reinforcing and aversive properties of such intake [179]. Notably, α5 subunit null mutation in MHb did not alter the rewarding effects of nicotine but did abolish the inhibitory effects of higher nicotine doses, suggesting a critical role for the α5 subunit in the aversion to nicotine intake [179]. It was suggested that nicotine activates the habenulo–interpeduncular pathway through α5* nAChRs, triggering an inhibitory motivational signal that limits nicotine intake [179]. However, there is no further evidence of KO mice exhibiting physical effects or somatic symptoms associated with α5* nAChRs for NA and SC.
Because of developmental abnormalities, α3 subunit-null mutant mice exhibit impaired growth and a higher perinatal mortality rate, so there are few data on α3 subunit KO mice [180] whereas clinical studies suggest that nAChRs containing the α3 and β4 subunits influence both the nicotine reward and the somatic symptoms of NA and SC [109, 181].
Early study observed a significant role for β4* nAChRs in nicotine withdrawal-induced symptoms [181] and there are fewer or less-intense signs of nicotine withdrawal in β4 subunit-null KO mice [181]. The KO mice lacking either β4 or α7 subunits showed a delayed onset of nicotine withdrawal-induced anhedonia-like behavior or state [182]. Furthermore, neuronal α4, α5, and α7 nAChR subunits are all involved in nicotine-induced seizures in KO mice [183] whereas α3β4* nAChRs mediate nicotine reward and physical nicotine withdrawal signs independently of the α5 nAChR subunit [181]. In addition, β4-null mutant mice and β4/α5-deleted KO mice (lacking both α5 and β4 subunits) are highly resistant to nicotine-induced seizures [183, 184]. Therefore, animal aversion to nicotine probably is regulated by the balanced activity of β4 and α5 nicotinic subunits in the medial habenula [184]. Although β4 KO mice showed few somatic signs of nicotine withdrawal or hyperalgesia after nicotine withdrawal [109, 172, 182] other studies reported that different β4* nAChR subtypes are involved in NA and SC, and α3β4* nAChRs are postulated to be the main contributors to tolerance [178, 181]. Moreover, β4* nAChR KO mice display a dose-dependent tolerance during chronic nicotine treatment [185]. In particular, β4-null mutant (β4–) mice develop more significant tolerance than either β4 wild-type (β4++) or β4 heterozygote (β4+−) mice [185]. These data indicate that β4* nAChRs are the critical response mediators and are resistant to both acute and chronic nicotine administration [185].
5.2. α6* and β3* nAChRs
Current research suggests that β3* and α6* nAChRs are involved in the unpleasant phenotypes of NA and SC [186]. The transgenic mouse model overexpressing α6β4* and α3β4* nAChRs displayed altered nicotine consumption and addiction and nicotine conditioned place aversion (CPA) [181, 184, 187]. A significant role was also recorded for α6* nAChRs with nicotine induced CPA in NA and SC [188]. Moreover, the α6 nAChR subunit plays crucial roles in the syndromes of NA and SC, as DA release is regulated in part by α6* nAChRs after nicotine administration [189-192] whereas α6 KO mice demonstated an important role of the α6 nAChR subunit in nicotine-affected DA neurons [193]. Inhibition of [3H]epibatidine binding to striatal membranes revealed the absence of α-CtxMII (a toxin inhibiting nicotine-induced DA release)-sensitive and cytosine-resistant [3H]epibatidine binding sites in α6-/- mice [193]. By modulating DA release in the nucleus accumbens (NAc) and modulating GABA release onto the DAergic neurons in the ventral tegmental area (VTA), α6* nAChRs may play important roles in nicotine reward and addiction [193] as well as in the selective preventive treatment of Parkinson’s disease in the nigrostriatal DAergic system (NDS) [194]. In addition, comparison of previously reported tolerance development in β2-null mutant mice (less tolerance) with that of β4-null mutant mice (more tolerance) supports a differential role for the α6β2* nAChR subtypes in regulating tolerance after chronic nicotine treatment [188]. The intracerebral infusion of α6β2* nAChR-selective antagonists blocks CPA and nicotine-elicited anxiety in the elevated plus maze (EPM), whereas there is no effect on the somatic symptoms in SC [105, 188]. In contrast, conditionally blocked α6* nAChRs weaken the nicotine-induced anxiety and aversion of KO mice in SC [105, 188].
Similar to the situation in α5-null mutant mice [173] β3-subunit KO mice exhibit lower baseline anxiety-related behavior, and β3-null mutant mice show altered hypothalamic pituitary–adrenal axis responses [195]. Changes also were observed in locomotor activity, prepulse inhibition, and some behavior that is controlled partly by nigrostriatal and mesolimbic dopaminergic activity [196].
5.3. α4* and β2* nAChRs
Previous studies of KO mice suggested that α4/β2* nAChRs are critical for nicotine-related reward behavior [197, 198] and the α4 nAChR subunit usually assembles with the β2 subunit in major heteromeric nAChRs. In the α4-subunit-KO animal line, the behavior of mutant mice in the elevated plus-maze assay is consistent with greater basal anxiety relative to the phenotype of wild-type mice [197]. In response to nicotine, wild-type mice exhibit early reductions in a number of behavioral topographies in any habitat, whereas increased behavioral topographies in unhabituated mutant mice are reduced by nicotine [197]. Similarly, α4-subunit-KI mice have α4* receptors that are hypersensitive to nicotine [198-200]. Actually, selective activation of α4* nAChRs with low doses of agonist recapitulates the nicotine effects proved to be important in NA, including reinforced responses to nicotine administration, tolerance, and sensitization to SC induced by chronic nicotine administration [198-200]. These data indicate that the activation of α4* nAChRs is sufficient for nicotine-elicited reward, tolerance, and sensitization.
The KO mice lacking the β2 subunit do not exhibit any nicotine-associated responses in SC, with absence of nicotine-elicited DA release and the raised firing rate of DA neurons in the dorsal striatum (DS) and ventral striatum (VS) [110, 201]. The β2-KO mice display more somatic signs of nicotine withdrawal and the abstinence-induced hyperalgesia than do wild-type mice [172] whereas the mutant mice do not display anxiety-related behavior that normally is associated with NA and SC in animals having chronic nicotine exposure [109]. Furthermore, the upregulation of nAChRs in vivo requires the presence of β2 subunit to modulate the animal’s adaptation to nicotine exposure, and β2+/+ mice develop dose-dependent tolerance with upregulation of [3H]epibatidine-binding sites [202]. Moreover, null-mutant analysis of β2-KO mice revealed that deletion of this subunit, but not the α7 subunit, reduced sensitivity to nicotine-induced locomotor depression and hypothermia [203]. Also, the β2 nAChR subunit mediates to some extent the dose-dependent effects of nicotine on locomotor activity and body temperature after drug injection [203]. In other reports, the systemic administration of DhβE (a α4β2* nAChR antagonist) in the VTA increased the intracranial self-stimulation (ICSS) thresholds of nicotine-dependent animals [204-206] whereas α6β2* nAChRs are expressed in both the VTA and the VS [204-206]. Additionally, KO mouse studies targeting the β6 subunit indicated that β6β2* nAChRs might play a critical role in nicotine-induced behavior [207]. These β2* nAChRs therefore might be targets for drugs to treat the affective and somatic effects of NA and SC. In practice, varenicline is a non-nicotine pharmaceutical that can act as a partial agonist at α6β2* nAChRs, and it is currently approved for the treatment of NA and SC [208, 209].
5.4. α2* and α7* nAChRs
α2-subunit KO mice self-administer higher doses of nicotine than wild-type control animals [210] and α2-null mutant mice show many somatic symptoms of NA and SC in a new habitat but few somatic signs in a familiar habitat [210]. Furthermore, α2-subunit deletions or null mutations abolish the somatic deficits and symptoms of NA and SC in KO mice [174, 178]. Variants in CHRNA2 have proved to be strongly associated with the results of the Fagerström Test for Nicotine Dependence (FTND) in a genome-wide association analysis (GWAS) of European-Americans and African-Americans [211].
In another report, the hyperalgesia symptoms emerging during mecamylamine-elicited nicotine withdrawal were reduced significantly in α7-subunit-KO mice [212]. Compared with wild-type mice, α7-null mutant mice displayed no elevated ICSS thresholds during 3–6 h after nicotine exposure [109, 182]. It appeared that KO mice lacking α7 subunits might have a delay in the somatic symptoms induced by NA and SC [109, 182]. Moreover, genetic deletion of the adenosine A2A receptor prevents nicotine-induced upregulation of α7 in the brain [213] whereas α7-null mice exhibit significantly decreased chronic oral nicotine intake [214] and somatic signs of nicotine-induced symptoms of nicotine abstinence [215] but displayed unaffected nicotine self-administration and anxiety-like behaviors after nicotine withdrawal [215, 216]. However, no definite role of the β7 nAChR subunit in nicotine addiction and reward was detectable or in conditioning observed in KO mice [175] despite the wide distribution of β7 nAChR subunits in the brain, especially in the mesocorticolimbic system.
Because of many research issues, there are few preclinical or clinical data available on the null mutation effects of β3-, α3-, and α5-KO on NA- and SC-related behaviors in mice. Nevertheless, these studies suggest that many subunits may be useful in understanding the mechanisms of NA and SC effects in nAChR-targeted KO mice. Those unpleasant physical signs and symptoms associated with NA and SC may be mediated by nAChRs containing the β2, β4, β6, α4, α6, or α7 subunits. Studies of transgenic KO mice have revealed some genetic regulatory roles of nAChR subunits in NA and SC involving several neuronal subtypes of nAChRs and the relevant neurobiological mechanisms. These studies also have demonstrated that mutations in the nAChR subunits of KO mice increase the risk of NA and SC and decrease the functional response of nAChRs.
6. Insight from specific rare variations of NAChR subunits
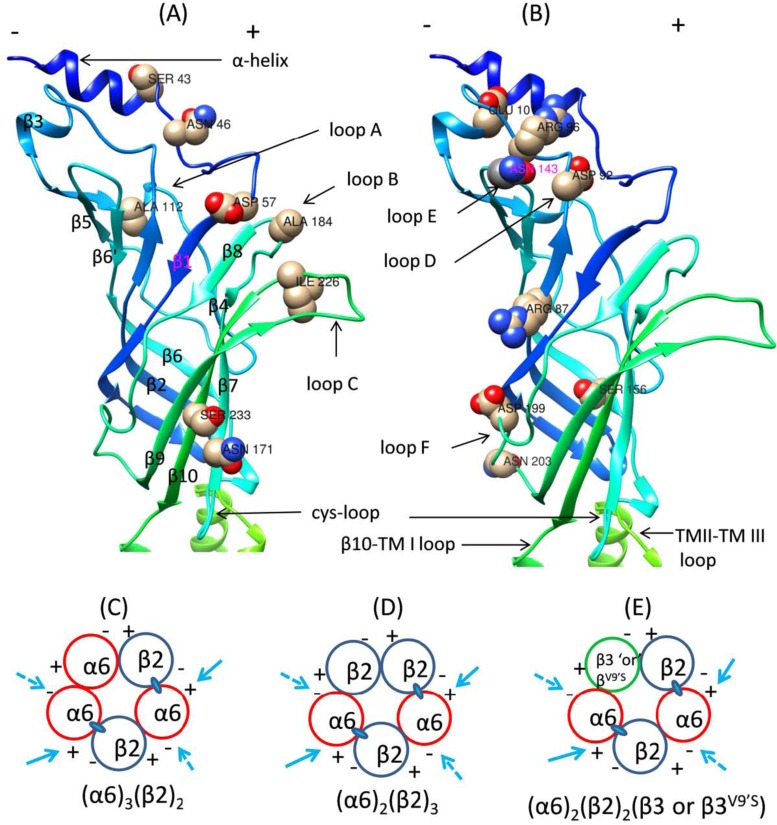
Human genetic association studies with robust tests have identified significant rare variants contributing to nicotine addiction and their location, structure, and risk information in several nAChR subunit genes [103, 105]. At present, the roles of those rare variants in nicotine-induced risks have not been well characterized. However, using the genetic data, together with advanced biotechnology methods, we can evaluate the functional effective subunits (e.g., the human α6 nAChR subunit shown in Fig. 2) and critical amino acid sites to explore possible mechanisms. Meanwhile, there have been some molecular studies related to nicotine addiction probing these rare variants of nAChR subunit genes with site-directed mutagenesis and transient transfection and other methods in artificial gene expression systems (Table 3). This part of the review focuses on the genetic effects of specific rare variants on the functional expression and sensitivity to nicotine-induced receptor upregulation and desensitization of nAChR subunits and subtypes.
Fig. (2).
Ribbon diagrams of amino acid variation localizations to secondary structures and interfaces of human α6 nAChR subunit. (A) and (B) illustrate the amino acid residues undergoing variation in a 3D model of the secondary structures of the hα6 nAChR subunit. (C-E) illustrate the interfaces contributed by α6 and other subunits to formation of α6* nAChRs. Adapted from Fig. 2 of Dash and Li (2014) [244].
Table 3.
Representative rare variants in nAChR subunits.
| Subunit | Critical Substitution of nAChR Amino Acid Residue | Refs. |
|---|---|---|
| Alpha 2 | T22I (rs2472553), D478N (rs141072985), D478E (rs563447740) | [222, 223] |
| Alpha 4 | R336C, P451L, R487Q, S438D, S469A, Y576A, S589A, S516D, T536A, S247F, S252L, 776ins3, T529A | [199, 225-229] |
| Alpha 5 | N143D, M145V, D398N (rs16969968) | [154, 233, 234, 239] |
| Alpha 6 | S43P, N46K, D57N, R87C, D92E, R96H, E101K, A112V, S156R, N171K, A184D, D199Y, N203T, I226T, S233C. | [244-246] |
| Alpha 7 | α7345-348A, D44A | [259-261] |
| Beta 2 | V286L, V337G, V287L, V287M. | [199, 229-231] |
| Beta 3 | V273S | [248, 255, 258] |
| Beta 4 | R349C | [262] |
6.1. α2 nAChR Subunit
To date, several low-frequency or rare variants; e.g., rs2472553, rs2043063, and rs104894063, have been identified in the α2 nAChR subunit gene and proved to be associated with drug dependence (including nicotine) and physiological disorders [217-220]. In particular, rs2472553 was found to be strongly associated with NA. It encodes a functional variant in the N-terminal ECD signal peptide, in which a thymidine missense mutation (C→T) leads to a threonine-to-isoleucine substitution at residue 22 (T22I or Thr22Ile). This changes the nicotine sensitivity of α2β4* nAChRs [220-222]. The critical mutant effect of T22I was indicated to favor the formation of low-sensitivity α2* nAChRs in two-electrode voltage clamp analysis [222]. Meanwhile, two rare variants (rs141072985: D478N and rs563447740: D478E) were found in the putative cytoplasmic amphipathic α-helices of the α2 nAChR subunit [223]. These variants were identified as a result of mutations in the 1st (G→A: rs141072985) and 3rd (C→A: rs56344740) nucleotide of its 478th triplet codon (GAC) [223, 224]. These two variants affect primarily the agonist-induced peak current responses of the α2β2* and α2β4* nAChRs [223]. In particular, an agonist (acetylcholine/nicotine) induced peak Imax current responses of α2β2* nAChRs, and those of α2β4* nAChRs were increased 1.3–4.7 fold, whereas mutant α2 subunit with the D478N variant increased the Imax of IS about 2 fold or HS 1.4–2.1 fold in α2β2* nAChRs [223].
6.2. α4 nAChR Subunit
nAChRs containing α4 and β2 subunits are the most abundant subtypes in the mammalian brain, and cumulative experimental evidence shows that these nAChRs can mediate nicotine-elicited rewarding and reinforcing effects [76, 201, 225, 226]. Three rare missense mutations (i.e., R336C, P451L, and R487Q) of the α4 subunit render the expression and function of high-affinity α4β2 nAChR desensitized or upregulated after chronic nicotine exposure, resulting in altered functional receptors most sensitive to the drug [226]. Significant mutant effects of α4 subunit variants also were observed on the subcellular expression and functional sensitivity of α4β2 nAChRs to nicotine-induced receptor upregulation [225]. Similar mutations of 11 residues (S364, T417, S438, S469, S471, S490, S504, S516, T536, Y576, and S589) in the α4 subunit M3-M4 cytoplasmic loop decreased the receptor activation for major mutations of α4β2 nAChRs with acetylcholine as agonist, whereas four mutations (S438D, S469A, Y576A, and S589A) caused statistically significant hypersensitivity to the agonist nicotine [227]. Furthermore, the disruption of specific interactions at putative consensus sites for protein kinase C (PKC) rendered α4β2 nAChRs almost completely insensitive to nicotine [227] and these PKC consensus sites of the α4 nAChR subunit are potential targets for SC-related drugs acting on α4β2 nAChRs. Interestingly, the upregulated α4* nAChRs in VTA GABAergic neurons increases murine sensitivity to nicotine, and these nAChRs might be targets for SC therapeutics [200]. Additionally, KI mice with a α4 subunit mutation (T529A in CHRNA4 A529) exhibited altered sensitivity of their nAChRs to nicotine [228]. Similar α4 subunit mutations also were reported in the channel-lining M2 domain (S247F, S252L, and 776INS3) [199] and in the intracellular cytoplasmic loop C2 (R336H) [229]. nAChRs formed by mutant α3 and α4 and wild-type β4 nAChR subunits displayed altered affinity for nicotine and reduced nicotine-activated current and desensitization [226].
6.3. β2 nAChR Subunit
The rare variants of the β2 subunit gene (CHRNB2) are found in smokers. In situ hybridization indicates that transgenic rats having β2* nAChRs with a V286L mutation express the protein to a greater degree in the cortex, hippocampus, and cerebellum of V286L-TG (V286L-transgenic) than did wild-type (non-transgenic) rats [230]. In comparison with wild-type littermates, V286L-TG rats display nicotine-induced abnormal motor activity, including seizures, whereas the response time for seizures after nicotine administration is shorter in V286L-TG rats than in the wild type [230]. Recently, it was discovered that a β2 mutant subunit (β2V287L) suppresses low-sensitivity expression of α4β2* nAChRs in KI mice and that α4β2* nAChRs might regulate nicotine addiction and brain reward and other behaviors [199, 231]. The β2V287L mutation reduces the EC50 values of high and low sensitivity of α4β2 nAChRs to acetylcholine and suppresses low-sensitivity α4β2 expression by cognate agonists [231]. Similar β2 subunit mutant effects were reported for two mutations (V287L and V287M) in the channel-lining M2 domain [199] and two mutations in the intracellular cytoplasmic loop C2 (V337G) [229].
6.4. α5 nAChR Subunit
When human (h) and mouse (m) chimeric α5* nAChRs were jointly expressed in Xenopus oocytes, the mutant chimeric hα5/hβ3V9′S nAChR allowed the agonist-activated function of hα6hβ4* or chimeric nAChR (mα6/hβ4* nAChR) to be expressed [232, 233]. These chimeric hα6/hα3 subunits were more sensitive to agonists and exhibited much higher functional activity when coexpressed with mutant hα5V9′S or chimeric hα5/hβ3V9′S subunits [233]. Mutations in the N-terminal domain of the hα6 subunit (N143D and M145V) could enable hα5/hβ3V9′S subunits to have gain-of-function effects on hα6hβ2* nAChRs [233, 234]. Furthermore, the α5 subunit might play a crucial modulatory role in the expression of functional nAChRs, regulating their activation and desensitization kinetics [235, 236]. Inserting the α5 subunit into α4β2, α3β2, or α3β4 combined nAChRs increases the receptor’s desensitization in response to nicotine [237, 238]. Interestingly, the substitution D398N (rs16969968) of α5* nAChRs also influences the receptor’s maximum response to agonist nicotine [239]. Moreover, mutations in the stretch region of the α5 nAChR subunit membrane can influence the function of nAChRs [236]. An in vitro study found that (α4β2)2α5 receptors containing a mutant α5 subunit with the high-risk allele of rs16969968 display less response to nicotine agonist compared with those containing the low genetic-risk mutant allele [239]. Further functional studies demonstrated that nAChRs harboring amino acids encoded by a minor risk allele (N398) of rs16969968 show reduced or unaltered responses to nicotinic agonists [239-242]. Human carriers of the minor allele N398 (Asp398Asn) of rs16969968 also display decreased intrinsic resting connectivity strength in the dorsal anterior cingulate–ventral striatum/extended amygdala circuit [154] whereas an adjacent mutation (S435R) in the β4 subunit abolishes the specific activity of α3β4* nAChRs [184]. Additional studies showed similar effects of mutations causing amino acid changes on the function of other nAChRs containing the α5 subunit, such as (α4β2)2α5 [240] and (α3β4)2α5 [243].
6.5. α6 nAChR Subunit
The specific residues of N-terminal ECDs of the human α6 nAChR subunit were investigated with fifteen rare variants in a site-directed mutagenesis analysis (Fig. 2) [244]. One variant in the N-terminal α-helix (Asp57), two variants in the complementary face/inner β fold (Arg87 or Asp92 residues), and two variants in the principal face/outer β fold (Ser156 or Asn171 residues) are crucial for the functional expression of human α6* nAChRs [244]. Variations at residues Ser43 or Asn46 (N-terminal α-helix) reduce the functional activity of human α6hβ2* nAChRs, whereas those at residues Arg96 (β2–β3 loop), Asp199 (loop F), or Ser233 (β10-strand) increase the function activity of human α6β2* nAChRs [244]. Interestingly, mammalian α6* nAChRs exist naturally in combination with β2 and β4 or other subunits [245, 246]. Besides well-known formations of α6β2* and α6β4* nAChRs [232, 247-249] the combinations of α4, β3, or α5 nAChR subunits could yield more complex α6* nAChRs [232, 248, 250, 251].
6.6. β3 nAChR Subunit
The β3 or α5 nAChR subunits were once classified as “accessory subunits” in dopaminergic regions. However, integration of the β3 nAChR subunit in the accessory positions was suggested to be a critical final step in the assembly and stability of mature α6β3* nAChRs [196, 252]. The integration of the β3 nAChR subunit into the mature process of nAChRs also is crucial for the formation of α6* nAChRs, α5* nAChRs, and other functional nAChRs [177, 240, 244, 253-258]. Particularly, h and m chimeric α6* nAChRs containing a mutant β3 subunit (V273S) (or β3V9′S) yield highly functional nAChRs in Xenopus oocytes [258] whereas hybrid mα6mβ4hβ3- (about 5-8 fold) or wild-type mα6mβ4mβ3-nAChRs (about 2 fold) yield higher functional activity than mα6mβ4-nAChRs [258]. Although agonist potency is markedly increased, the coexpression of α6 and β4 subunits with the mutant β3 subunit (V273S) blocks the spontaneous channel opening with an atropine sensitivity [248]. Moreover, coexpression with wild-type β3 subunits abolishes the typical function observed for all h or m α6β4* nAChRs [248]. Additionally, negative effects are seen when h mutant β3V9'S subunits are incorporated into functional α2β2*, α2β4*, α3β2*, α3β4*, α4β2*, or α4β4* nAChRs, [255] although a positive role for the β3 subunit in functional α6* nAChRs has been reported in KO animals [196]. Incorporation of wild-type β3 subunits generally (except for α3* nAChRs) reduces the magnitude of agonist-induced currents by 20%–50%, an effect mediated by β2* or β4* nAChRs, and modestly affects the agonist potency (less than 2 fold) and other agonist-specific effects [255]. Co-expression with mutant β3V9'S subunits generally (except for α4β2* nAChRs) increases agonist potencies [255].
6.7. α7 nAChR Subunit
There are a few reports concerning mutations of α7 nAChRs [259-261]. A single mutation (D44A) in the ECD of the receptor can reduce the permeability of α7 nAChR to calcium [259-261] whereas a mutation (α7345-348A) of the G protein-binding cluster in the M3-M4 loop of the receptor significantly attenuates the α7 nAChR-induced Gαq calcium signaling response [259, 260]. In Xenopus oocytes, the expressions of 14 SNPs of α7 nAChRs revealed many interesting functional activities, including six nonfunctional mutants, four mutants reducing current expression, one mutant altering the efficacy of acetylcholine and nicotine in opposite directions, and another mutant slightly reduces agonist sensitivity [150]. Interestingly, the function of most of these nonfunctional mutants can be rescued by the α7 nAChR-positive allosteric modulator PNU-120596 and agonist-PAM 4BP-TQS [150]. Therefore, these mutant changes of α7 nAChR properties could have an impact on α7 nAChR-mediated cholinergic synaptic transmission and anti-inflammatory effects in human SNP carriers, whereas rescuing the nonfunctional mutants could provide a novel way to treat related disorders [150].
6.8. Other nAChR Subunits
When coexpressed with wild-type α3 or α4 subunits, the β4R349C (R349C) subunit, a mutant nAChR subunit found in some patients, independently reduces the potency of acetylcholine/nicotine and decreases the density of whole-cell current evoked by the transmitter concentrations [262]. In contrast, none of the functional activities examined showed differences between α4β4* and α4R487Qβ4* nAChRs [262]. In particular, there was no function change when β2 subunits were expressed alone or in the presence of wild-type or mutant β3 subunits [248, 263]. It was revealed that β3 point mutation resulted in a lower current decay rate and increased acetylcholine agonist potency of α6* nAChRs [263]. These studies illuminated the structural bases and genetic variants for the effects of β3 subunits on α6* nAChR function and also characterized those unique nAChR subunit interfaces involving the complementary rather than the primary face of α6 subunits.
In brief, these studies present a novel insight into the assembly, structure, and function of functional nAChR subtypes, which could be exploited for developing new ligands to affect drug dependence (including NA). Almost all β2 or β4 subunits can form functional nAChRs with the α2, α3, or α4 subunits in vitro, whereas other subunits are more likely to be the additional third subunit in heteromeric nAChRs with combinations of α and β subunits [264-266]. However, α4*, α5*, α6*, α7*, and β3* nAChRs, but not α2* nAChRs, may be most physiologically relevant. Furthermore, the rare-variant hypothesis suggests that a significant proportion of human inherited susceptibility to common chronic diseases is attributable mostly to the effects of low-frequency dominant variants of particular genes [267, 268]. Subsequently, each gene confers a moderate but readily detectable increase in the relative risk of chronic human diseases, and thus these rare variants will be relatively population specific because of the founder effects resulting from genetic drift. Additionally, the above data indicate that rare genetic variants in the nAChR subunits may increase the risk of NA and can decrease the functional responses of nAChRs.
Conclusions and remarks
To date, nAChRs and other homologous ion channels are recognized as important mediators of cellular functions and crucial drug targets for treatment of a variety of diseases. Indeed, a diversity of neuronal nAChR subtypes and subunits may provide novel targets for assisting smokers to quit permanently. In particular, the modulating mechanisms such as allosteric modulation are involved mainly in the upregulation or desensitization of the quantity or activity of nAChR subtypes or subunits. Unlike the effect of other drugs of abuse, chronic use of nicotine usually leads to increased expression or upregulation of nAChRs, a hallmark of nicotine addiction, or is a major contributor to the upregulation observed in chronic users of nicotine-containing products [68, 154, 157]. Furthermore, long-term exposure to nicotine usually leads to an activity increase, or amount upregulation, of nicotine-binding sites in the brains of smokers [153, 154] and rodents [155, 156] subjected to repeated nicotine administration. Although some nAChRs render the receptor complexes more prone to be upregulated, desensitization is a prominent mechanism that contributes to this upregulation [269]. Because desensitized receptor conformations have a higher affinity for an agonist, nAChRs will increasingly adopt desensitized conformations in response to chronic nicotine exposure [270]. Consequently, those receptors undergo transitions between different conformations in response to ligand binding and dissociation [68] whereas agonists tend to stabilize particular conformations. Notably, several neuronal nAChR subtypes offer promising targets for the treatment of disorders such as NA, Alzheimer’s and Parkinson’s diseases, schizophrenia, depression, and other cognitive deficits [42-54]. Eventually, the development of enhanced therapeutics and treatment promoting SC will result from the identification of particular nAChR subtypes or subunit compositions that are necessary for the expression of NA and SC symptoms.
One of the goals of drug discovery is the identification and selection of receptor ligands with high-affinity nAChR subtypes. Fortunately, several neuronal subtypes of nAChRs (e.g., α6β2*, α6β4*, α4β2*, α3β4*, and α7) offer promising targets for the treatment of nicotine-elicited disorders. In particular, α7 and α4β2 subtype-selective nAChR agonists and partial agonists have been developed as potential candidates for the treatment of schizophrenia and some cognitive disorders. Therefore, the selectivity of nAChR subtypes might be achieved more readily by designing ligands that bind to allosteric sites rather than to the conventional orthosteric binding sites. For instance, the low intrinsic activity of PAMs of α7 nAChRs may be useful in reducing toxicity and off-target effects in comparison with agonists. Those characteristics may be advantageous because they can enable retention of the spatial and temporal pattern of signaling of endogenous acetylcholine-evoked responses [124, 271, 272]. Furthermore, exploration and analysis of the nAChR-targeted KO mice containing mutant subtypes and subunits will be crucial to understanding the unpleasant effects and specific behaviors and the underlying mechanisms of NA and SC. Moreover, there are many rare variations of nAChR subunits displaying normal activation and remarkably changed sensitivity to nicotine. These functional variants may be used as critical genetic markers to design effective therapeutic drugs or pharmacochemical ligands. A critical step in translating these genetic findings to clinical practice is identifying the genetic factors affecting NA and withdrawal and specific receptor interaction sites of nAChRs in order to enhance current SC treatments. Although upregulation and desensitization of nAChRs are two critical aspects in understanding the physiological observation of chronic nicotine exposure and the pharmacological interaction of both orthosteric and allosteric ligands in drug design, the behavioral consequences of NA and how it relates to the syndromes of SC are largely unknown.
Cigarette smoking is highly addictive, and recent research has identified a number of robust rare genetic variants that affect nicotine addiction. Some rare nAChR variants have proved to be significantly associated with nicotine-induced risks, and there are already studies on the effects of specific rare variants on the functional expression and sensitivity to nicotine-induced receptor upregulation or desensitization of nAChR subunits and subtypes. These mutations or rare variants can be used as markers in preclinical and clinical studies. They also are great references for the design of effective agonists, competitive antagonists, or allosteric modulators for the treatment of the syndromes of NA and SC. The genetic association between these mutations and nicotine addiction differs, with many potential moderators and influencing factors for genetic risks, such as impact on nicotine addiction, ethanol-nicotine co-use and co-abuse, therapeutic medication development for SC, and relevant environmental risk factors. Clearly, additional studies are needed to provide a better understanding of the mechanisms that are involved in NA and SC, ethanol-nicotine couse and coabuse, and other environmental risk factors. Importantly, emerging evidence indicates that SNPs within genes encoding nAChR subunits are associated with phenotypes of NA and SC, although further systematic studies and much work are needed to understand how these SNPs modulate the effects of nicotine on nAChRs in animal models of NA and SC.
Additionally, the challenges and limitations associated with SC and the development of medications for treating NA might be unlike those for other CNS disorders, and the chances for success have been relatively low. According to previous studies on SC and NA, it is found that lack of knowledge about the harmful effects of tobacco smoking and some misconceptions on smoking hazards might contribute to the low SC rate. Another important fact is that pre-clinical and clinical studies on SC and relevant effective measures remain inadequate in the field.
Consent for Publication
Not applicable.
Acknowledgements
This study was supported in part by the China Precision Medicine Initiative (2016YFC0906300), Research Center for Air Pollution and Health of Zhejiang University, the State Key Laboratory for Diagnosis and Treatment of Infectious Diseases of the First Affiliated Hospital of Zhejiang University, and National Institutes of Health grant DA012844. We thank Dr. David L. Bronson for excellent editing of this manuscript.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
References
- 1.De Biasi M., Dani J.A. Reward, addiction, withdrawal to nicotine. Annu. Rev. Neurosci. 2011;34:105–130. doi: 10.1146/annurev-neuro-061010-113734. [http://dx.doi.org/10. 1146/annurev-neuro-061010-113734]. [PMID: 21438686]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crooks P.A., Bardo M.T., Dwoskin L.P. Nicotinic receptor antagonists as treatments for nicotine abuse. Adv. Pharmacol. 2014;69:513–551. doi: 10.1016/B978-0-12-420118-7.00013-5. [http://dx.doi.org/10.1016/B978-0-12-420118-7.00013-5]. [PMID: 24484986]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. The World Health Organization W. WHO report on the global tobacco epidemic, 2015: Raising taxes on tobacco. 2015.
- 4.Changeux J.P. Nicotine addiction and nicotinic receptors: lessons from genetically modified mice. Nat. Rev. Neurosci. 2010;11(6):389–401. doi: 10.1038/nrn2849. [http://dx.doi.org/10.1038/nrn2849]. [PMID: 20485364]. [DOI] [PubMed] [Google Scholar]
- 5.Fowler C.D., Arends M.A., Kenny P.J. Subtypes of nicotinic acetylcholine receptors in nicotine reward, dependence, and withdrawal: evidence from genetically modified mice. Behav. Pharmacol. 2008;19(5-6):461–484. doi: 10.1097/FBP.0b013e32830c360e. [http://dx.doi.org/10.1097/FBP.0b013 e32830c360e]. [PMID: 18690103]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bierut L J, Johnson E O, Saccone N L. A glimpse into the future - Personalized medicine for smoking cessation. Neuropharmacology, . 2014;(76 Pt B):592–599. doi: 10.1016/j.neuropharm.2013.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brunzell D.H., Stafford A.M., Dixon C.I. Nicotinic receptor contributions to smoking: insights from human studies and animal models. Curr. Addict. Rep. 2015;2(1):33–46. doi: 10.1007/s40429-015-0042-2. [http://dx.doi.org/ 10.1007/s40429-015-0042-2]. [PMID: 26301171]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murray C.J., Lopez A.D. Alternative projections of mortality and disability by cause 1990-2020: Global burden of disease study. Lancet. 1997;349(9064):1498–1504. doi: 10.1016/S0140-6736(96)07492-2. [http://dx.doi.org/10.1016/ S0140-6736(96)07492-2]. [PMID: 9167458]. [DOI] [PubMed] [Google Scholar]
- 9.Stead L.F., Perera R., Bullen C., Mant D., Lancaster T. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst. Rev. 2008;(1):CD000146. doi: 10.1002/14651858.CD000146.pub3. [PMID: 18253970]. [DOI] [PubMed] [Google Scholar]
- 10.Stead L.F., Perera R., Bullen C., Mant D., Hartmann-Boyce J., Cahill K., Lancaster T. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst. Rev. 2012;11:CD000146. doi: 10.1002/14651858.CD000146.pub4. [PMID: 23152200]. [DOI] [PubMed] [Google Scholar]
- 11.Garrett B.E., Rose C.A., Henningfield J.E. Tobacco addiction and pharmacological interventions. Expert Opin. Pharmacother. 2001;2(10):1545–1555. doi: 10.1517/14656566.2.10.1545. [http://dx.doi.org/10.1517/14656566.2.10. 1545]. [PMID: 11825298]. [DOI] [PubMed] [Google Scholar]
- 12.Cornuz J. Smoking cessation interventions in clinical practice. Eur. J. Vasc. Endovasc. Surg. 2007;34(4):397–404. doi: 10.1016/j.ejvs.2007.06.009. [http://dx.doi. org/10.1016/j.ejvs.2007.06.009]. [PMID: 17681834]. [DOI] [PubMed] [Google Scholar]
- 13.Benowitz N.L. Pharmacology of nicotine: addiction, smoking-induced disease, and therapeutics. Annu. Rev. Pharmacol. Toxicol. 2009;49:57–71. doi: 10.1146/annurev.pharmtox.48.113006.094742. [http://dx.doi.org/10.1146/annurev.pharmtox.48. 113006.094742]. [PMID: 18834313]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Balfour D.J. The role of mesoaccumbens dopamine in nicotine dependence. Curr. Top. Behav. Neurosci. 2015;24:55–98. doi: 10.1007/978-3-319-13482-6_3. [http:// dx.doi.org/10.1007/978-3-319-13482-6_3]. [PMID: 25638334]. [DOI] [PubMed] [Google Scholar]
- 15.Rose J.E. Multiple brain pathways and receptors underlying tobacco addiction. Biochem. Pharmacol. 2007;74(8):1263–1270. doi: 10.1016/j.bcp.2007.07.039. [http://dx.doi.org/10.1016/j.bcp.2007.07.039]. [PMID: 17826746]. [DOI] [PubMed] [Google Scholar]
- 16.Scherer G. Smoking behaviour and compensation: a review of the literature. Psychopharmacology (Berl.) 1999;145(1):1–20. doi: 10.1007/s002130051027. [http:// dx.doi.org/10.1007/s002130051027]. [PMID: 10445368]. [DOI] [PubMed] [Google Scholar]
- 17.Kotlyar M., Hatsukami D.K. Managing nicotine addiction. J. Dent. Educ. 2002;66(9):1061–1073. [PMID: 12374267]. [PubMed] [Google Scholar]
- 18.Foulds J., Burke M., Steinberg M., Williams J.M., Ziedonis D.M. Advances in pharmacotherapy for tobacco dependence. Expert Opin. Emerg. Drugs. 2004;9(1):39–53. doi: 10.1517/eoed.9.1.39.32951. [http://dx.doi.org/ 10.1517/14728214.9.1.39]. [PMID: 15155135]. [DOI] [PubMed] [Google Scholar]
- 19.Balfour D.J. The neurobiology of tobacco dependence: a preclinical perspective on the role of the dopamine projections to the nucleus accumbens. Nicotine Tob. Res. 2004;6(6):899–912. doi: 10.1080/14622200412331324965. [corrected]. [corrected]. [http://dx.doi.org/10.1080/14622200412331324965]. [PMID: 15801566]. [DOI] [PubMed] [Google Scholar]
- 20.Corringer P.J., Le Novère N., Changeux J.P. Nicotinic receptors at the amino acid level. Annu. Rev. Pharmacol. Toxicol. 2000;40:431–458. doi: 10.1146/annurev.pharmtox.40.1.431. [http://dx.doi.org/10.1146/annurev.pharmtox.40.1.431]. [PMID: 10836143]. [DOI] [PubMed] [Google Scholar]
- 21.Karlin A. Emerging structure of the nicotinic acetylcholine receptors. Nat. Rev. Neurosci. 2002;3(2):102–114. doi: 10.1038/nrn731. [http://dx.doi.org/ 10.1038/nrn731]. [PMID: 11836518]. [DOI] [PubMed] [Google Scholar]
- 22.Chen L. In pursuit of the high-resolution structure of nicotinic acetylcholine receptors. J. Physiol. 2010;588(Pt 4):557–564. doi: 10.1113/jphysiol.2009.184085. [http://dx.doi.org/10.1113/jphysiol.2009.184085]. [PMID: 19995851]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leonard S., Adams C., Breese C.R., Adler L.E., Bickford P., Byerley W., Coon H., Griffith J.M., Miller C., Myles-Worsley M., Nagamoto H.T., Rollins Y., Stevens K.E., Waldo M., Freedman R. Nicotinic receptor function in schizophrenia. Schizophr. Bull. 1996;22(3):431–445. doi: 10.1093/schbul/22.3.431. [http://dx.doi.org/10.1093/ schbul/22.3.431]. [PMID: 8873294]. [DOI] [PubMed] [Google Scholar]
- 24.Adler L.E., Olincy A., Waldo M., Harris J.G., Griffith J., Stevens K., Flach K., Nagamoto H., Bickford P., Leonard S., Freedman R. Schizophrenia, sensory gating, and nicotinic receptors. Schizophr. Bull. 1998;24(2):189–202. doi: 10.1093/oxfordjournals.schbul.a033320. [http://dx.doi.org/ 10.1093/oxfordjournals.schbul.a033320]. [PMID: 9613620]. [DOI] [PubMed] [Google Scholar]
- 25.Dineley K.T., Pandya A.A., Yakel J.L. Nicotinic ACh receptors as therapeutic targets in CNS disorders. Trends Pharmacol. Sci. 2015;36(2):96–108. doi: 10.1016/j.tips.2014.12.002. [http://dx.doi.org/10.1016/j.tips.2014.12.002]. [PMID: 25639674]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pandya A.A., Yakel J.L. Effects of neuronal nicotinic acetylcholine receptor allosteric modulators in animal behavior studies. Biochem. Pharmacol. 2013;86(8):1054–1062. doi: 10.1016/j.bcp.2013.05.018. [http://dx.doi.org/10. 1016/j.bcp.2013.05.018]. [PMID: 23732296]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Changeux J., Edelstein S.J. Allosteric mechanisms in normal and pathological nicotinic acetylcholine receptors. Curr. Opin. Neurobiol. 2001;11(3):369–377. doi: 10.1016/s0959-4388(00)00221-x. [http://dx.doi.org/10.1016/S0959-4388 (00)00221-X]. [PMID: 11399437]. [DOI] [PubMed] [Google Scholar]
- 28.Changeux J.P., Edelstein S.J. Allosteric mechanisms of signal transduction. Science. 2005;308(5727):1424–1428. doi: 10.1126/science.1108595. [http://dx.doi. org/10.1126/science.1108595]. [PMID: 15933191]. [DOI] [PubMed] [Google Scholar]
- 29.Gotti C., Clementi F., Fornari A., Gaimarri A., Guiducci S., Manfredi I., Moretti M., Pedrazzi P., Pucci L., Zoli M. Structural and functional diversity of native brain neuronal nicotinic receptors. Biochem. Pharmacol. 2009;78(7):703–711. doi: 10.1016/j.bcp.2009.05.024. [http://dx.doi. org/10.1016/j.bcp.2009.05.024]. [PMID: 19481063]. [DOI] [PubMed] [Google Scholar]
- 30.Wu J., Lukas R.J. Naturally-expressed nicotinic acetylcholine receptor subtypes. Biochem. Pharmacol. 2011;82(8):800–807. doi: 10.1016/j.bcp.2011.07.067. [http://dx.doi.org/10.1016/j.bcp.2011.07.067]. [PMID: 21787755]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Galzi J.L., Revah F., Bessis A., Changeux J.P. Functional architecture of the nicotinic acetylcholine receptor: from electric organ to brain. Annu. Rev. Pharmacol. Toxicol. 1991;31:37–72. doi: 10.1146/annurev.pa.31.040191.000345. [http:// dx.doi.org/10.1146/annurev.pa.31.040191.000345]. [PMID: 2064379]. [DOI] [PubMed] [Google Scholar]
- 32.Sargent P.B. The diversity of neuronal nicotinic acetylcholine receptors. Annu. Rev. Neurosci. 1993;16:403–443. doi: 10.1146/annurev.ne.16.030193.002155. [http://dx. doi.org/10.1146/annurev.ne.16.030193.002155]. [PMID: 7681637]. [DOI] [PubMed] [Google Scholar]
- 33.Decker M.W., Brioni J.D., Bannon A.W., Arneric S.P. Diversity of neuronal nicotinic acetylcholine receptors: lessons from behavior and implications for CNS therapeutics. Life Sci. 1995;56(8):545–570. doi: 10.1016/0024-3205(94)00488-e. [http://dx.doi.org/10.1016/0024-3205(94)00488-E]. [PMID: 7869835]. [DOI] [PubMed] [Google Scholar]
- 34.McGehee D.S. Molecular diversity of neuronal nicotinic acetylcholine receptors. Ann. N. Y. Acad. Sci. 1999;868:565–577. doi: 10.1111/j.1749-6632.1999.tb11330.x. [http://dx.doi.org/10.1111/j.1749-6632.1999.tb11330.x]. [PMID: 10414338]. [DOI] [PubMed] [Google Scholar]
- 35.Stokes C., Treinin M., Papke R.L. Looking below the surface of nicotinic acetylcholine receptors. Trends Pharmacol. Sci. 2015;36(8):514–523. doi: 10.1016/j.tips.2015.05.002. [http://dx.doi.org/10.1016/j.tips.2015.05.002]. [PMID: 26067101]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Corringer P.J., Bertrand S., Bohler S., Edelstein S.J., Changeux J.P., Bertrand D. Critical elements determining diversity in agonist binding and desensitization of neuronal nicotinic acetylcholine receptors. J. Neurosci. 1998;18(2):648–657. doi: 10.1523/JNEUROSCI.18-02-00648.1998. [PMID: 9425007]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leslie F.M., Mojica C.Y., Reynaga D.D. Nicotinic receptors in addiction pathways. Mol. Pharmacol. 2013;83(4):753–758. doi: 10.1124/mol.112.083659. [http://dx.doi.org/10.1124/mol.112.083659]. [PMID: 23247824]. [DOI] [PubMed] [Google Scholar]
- 38.McGehee D.S., Role L.W. Physiological diversity of nicotinic acetylcholine receptors expressed by vertebrate neurons. Annu. Rev. Physiol. 1995;57:521–546. doi: 10.1146/annurev.ph.57.030195.002513. [http://dx.doi.org/10.1146/ annurev.ph.57.030195.002513]. [PMID: 7778876]. [DOI] [PubMed] [Google Scholar]
- 39.Dani J.A., Bertrand D. Nicotinic acetylcholine receptors and nicotinic cholinergic mechanisms of the central nervous system. Annu. Rev. Pharmacol. Toxicol. 2007;47:699–729. doi: 10.1146/annurev.pharmtox.47.120505.105214. [http://dx.doi. org/10.1146/annurev.pharmtox.47.120505.105214]. [PMID: 17009926]. [DOI] [PubMed] [Google Scholar]
- 40.Taly A., Corringer P.J., Guedin D., Lestage P., Changeux J.P. Nicotinic receptors: allosteric transitions and therapeutic targets in the nervous system. Nat. Rev. Drug Discov. 2009;8(9):733–750. doi: 10.1038/nrd2927. [http://dx.doi.org/10.1038/nrd2927]. [PMID: 19721446]. [DOI] [PubMed] [Google Scholar]
- 41.Brunzell D.H., McIntosh J.M., Papke R.L. Diverse strategies targeting α7 homomeric and α6β2* heteromeric nicotinic acetylcholine receptors for smoking cessation. Ann. N. Y. Acad. Sci. 2014;1327(1):27–45. doi: 10.1111/nyas.12421. [PMID: 24730978]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Spindel E.R. Cholinergic targets in lung cancer. Curr. Pharm. Des. 2016;22(14):2152–2159. doi: 10.2174/1381612822666160127114237. [http://dx.doi.org/10.2174/ 1381612822666160127114237]. [PMID: 26818857]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dang N., Meng X., Song H. Nicotinic acetylcholine receptors and cancer. Biomed. Rep. 2016;4(5):515–518. doi: 10.3892/br.2016.625. [http://dx.doi.org/ 10.3892/br.2016.625]. [PMID: 27123240]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Egea J., Buendia I., Parada E., Navarro E., León R., Lopez M.G. Anti-inflammatory role of microglial alpha7 nAChRs and its role in neuroprotection. Biochem. Pharmacol. 2015;97(4):463–472. doi: 10.1016/j.bcp.2015.07.032. [http://dx.doi.org/10.1016/j.bcp.2015.07.032]. [PMID: 26232730]. [DOI] [PubMed] [Google Scholar]
- 45.Improgo M.R., Tapper A.R., Gardner P.D. Nicotinic acetylcholine receptor-mediated mechanisms in lung cancer. Biochem. Pharmacol. 2011;82(8):1015–1021. doi: 10.1016/j.bcp.2011.05.020. [http://dx.doi.org/10.1016/j. bcp.2011.05.020]. [PMID: 21640716]. [DOI] [PubMed] [Google Scholar]
- 46.Liu Y., Zeng X., Hui Y., Zhu C., Wu J., Taylor D.H., Ji J., Fan W., Huang Z., Hu J. Activation of α7 nicotinic acetylcholine receptors protects astrocytes against oxidative stress-induced apoptosis: implications for Parkinson’s disease. Neuropharmacology. 2015;91:87–96. doi: 10.1016/j.neuropharm.2014.11.028. [http://dx.doi.org/10.1016/j.neuropharm.2014. 11.028]. [PMID: 25486621]. [DOI] [PubMed] [Google Scholar]
- 47.Lombardo S, Maskos U. Role of the nicotinic acetylcholine receptor in Alzheimer's disease pathology and treatment. 2015. [DOI] [PubMed]
- 48.Meyer P.M., Tiepolt S., Barthel H., Hesse S., Sabri O. Radioligand imaging of α4β2* nicotinic acetylcholine receptors in Alzheimer’s disease and Parkinson’s disease. Q. J. Nucl. Med. Mol. Imaging. 2014;58(4):376–386. [PMID: 25387119]. [PubMed] [Google Scholar]
- 49.Mucchietto V., Crespi A., Fasoli F., Clementi F., Gotti C. Neuronal acetylcholine nicotinic receptors as new targets for lung cancer treatment. Curr. Pharm. Des. 2016;22(14):2160–2169. doi: 10.2174/1381612822666160203144114. [http://dx.doi.org/10.2174/1381612822666160203144114]. [PMID: 26845123]. [DOI] [PubMed] [Google Scholar]
- 50.Quik M., Zhang D., McGregor M., Bordia T. Alpha7 nicotinic receptors as therapeutic targets for Parkinson’s disease. Biochem. Pharmacol. 2015;97(4):399–407. doi: 10.1016/j.bcp.2015.06.014. [http://dx.doi.org/10.1016/j.bcp. 2015.06.014]. [PMID: 26093062]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Srinivasan R., Henderson B.J., Lester H.A., Richards C.I. Pharmacological chaperoning of nAChRs: a therapeutic target for Parkinson’s disease. Pharmacol. Res. 2014;83:20–29. doi: 10.1016/j.phrs.2014.02.005. [http://dx.doi. org/10.1016/j.phrs.2014.02.005]. [PMID: 24593907]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Srinivasan R., Henley B.M., Henderson B.J., Indersmitten T., Cohen B.N., Kim C.H., McKinney S., Deshpande P., Xiao C., Lester H.A. Smoking-Relevant Nicotine Concentration Attenuates the Unfolded Protein Response in Dopaminergic Neurons. J. Neurosci. 2016;36(1):65–79. doi: 10.1523/JNEUROSCI.2126-15.2016. [http://dx.doi.org/10.1523/JNEUROSCI. 2126-15.2016]. [PMID: 26740650]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wallace T.L., Bertrand D. Alpha7 neuronal nicotinic receptors as a drug target in schizophrenia. Expert Opin. Ther. Targets. 2013;17(2):139–155. doi: 10.1517/14728222.2013.736498. [http://dx.doi.org/10.1517/14728222.2013.736498]. [PMID: 23231385]. [DOI] [PubMed] [Google Scholar]
- 54.Colquhoun L.M., Patrick J.W. Pharmacology of neuronal nicotinic acetylcholine receptor subtypes. Adv. Pharmacol. 1997;39:191–220. doi: 10.1016/s1054-3589(08)60072-1. [http://dx.doi.org/10.1016/S1054-3589(08)60072-1]. [PMID: 9160116]. [DOI] [PubMed] [Google Scholar]
- 55.Sine S.M., Engel A.G. Recent advances in Cys-loop receptor structure and function. Nature. 2006;440(7083):448–455. doi: 10.1038/nature04708. [http://dx.doi.org/10.1038/nature04708]. [PMID: 16554804]. [DOI] [PubMed] [Google Scholar]
- 56.Lester H.A., Dibas M.I., Dahan D.S., Leite J.F., Dougherty D.A. Cys-loop receptors: new twists and turns. Trends Neurosci. 2004;27(6):329–336. doi: 10.1016/j.tins.2004.04.002. [http://dx.doi.org/10.1016/j.tins.2004.04. 002]. [PMID: 15165737]. [DOI] [PubMed] [Google Scholar]
- 57.Corringer P.J., Poitevin F., Prevost M.S., Sauguet L., Delarue M., Changeux J.P. Structure and pharmacology of pentameric receptor channels: from bacteria to brain. Structure. 2012;20(6):941–956. doi: 10.1016/j.str.2012.05.003. [http://dx.doi.org/10.1016/j.str.2012.05.003]. [PMID: 22681900]. [DOI] [PubMed] [Google Scholar]
- 58.Steinlein O.K., Bertrand D. Neuronal nicotinic acetylcholine receptors: from the genetic analysis to neurological diseases. Biochem. Pharmacol. 2008;76(10):1175–1183. doi: 10.1016/j.bcp.2008.07.012. [http://dx.doi.org/ 10.1016/j.bcp.2008.07.012]. [PMID: 18691557]. [DOI] [PubMed] [Google Scholar]
- 59.Matsunaga K., Klein T.W., Friedman H., Yamamoto Y. Involvement of nicotinic acetylcholine receptors in suppression of antimicrobial activity and cytokine responses of alveolar macrophages to Legionella pneumophila infection by nicotine. J. Immunol. 2001;167(11):6518–6524. doi: 10.4049/jimmunol.167.11.6518. [http://dx.doi.org/10.4049/ jimmunol.167.11.6518]. [PMID: 11714820]. [DOI] [PubMed] [Google Scholar]
- 60.Heeschen C., Jang J.J., Weis M., Pathak A., Kaji S., Hu R.S., Tsao P.S., Johnson F.L., Cooke J.P. Nicotine stimulates angiogenesis and promotes tumor growth and atherosclerosis. Nat. Med. 2001;7(7):833–839. doi: 10.1038/89961. [http://dx.doi.org/10.1038/89961]. [PMID: 11433349]. [DOI] [PubMed] [Google Scholar]
- 61.Lee J., Cooke J.P. Nicotine and pathological angiogenesis. Life Sci. 2012;91(21-22):1058–1064. doi: 10.1016/j.lfs.2012.06.032. [http://dx.doi.org/10.1016/j.lfs. 2012.06.032]. [PMID: 22796717]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang H., Yu M., Ochani M., Amella C.A., Tanovic M., Susarla S., Li J.H., Wang H., Yang H., Ulloa L., Al-Abed Y., Czura C.J., Tracey K.J. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature. 2003;421(6921):384–388. doi: 10.1038/nature01339. [http://dx.doi.org/10.1038/nature01339]. [PMID: 12508119]. [DOI] [PubMed] [Google Scholar]
- 63.Gotti C., Clementi F. Neuronal nicotinic receptors: from structure to pathology. Prog. Neurobiol. 2004;74(6):363–396. doi: 10.1016/j.pneurobio.2004.09.006. [http:// dx.doi.org/10.1016/j.pneurobio.2004.09.006]. [PMID: 15649582]. [DOI] [PubMed] [Google Scholar]
- 64.Chatzidaki A., Millar N.S. Allosteric modulation of nicotinic acetylcholine receptors. Biochem. Pharmacol. 2015;97(4):408–417. doi: 10.1016/j.bcp.2015.07.028. [http://dx.doi.org/10.1016/j.bcp.2015.07.028]. [PMID: 26231943]. [DOI] [PubMed] [Google Scholar]
- 65.Hénault C M, Sun J, Therien J P D, Dacosta C J B, Carswell C L, Labriola J M, Juranka P F, Baenziger J E. The role of the M4 lipid-sensor in the folding, trafficking, and allosteric modulation of nicotinic acetylcholine receptors. 2015. [DOI] [PubMed]
- 66.Le Novère N., Changeux J.P. Molecular evolution of the nicotinic acetylcholine receptor: an example of multigene family in excitable cells. J. Mol. Evol. 1995;40(2):155–172. doi: 10.1007/BF00167110. [http://dx.doi.org/ 10.1007/BF00167110]. [PMID: 7699721]. [DOI] [PubMed] [Google Scholar]
- 67.Picciotto M.R., Caldarone B.J., Brunzell D.H., Zachariou V., Stevens T.R., King S.L. Neuronal nicotinic acetylcholine receptor subunit knockout mice: physiological and behavioral phenotypes and possible clinical implications. Pharmacol. Ther. 2001;92(2-3):89–108. doi: 10.1016/s0163-7258(01)00161-9. [http://dx.doi.org/10.1016/S0163-7258(01)00161-9]. [PMID: 11916531]. [DOI] [PubMed] [Google Scholar]
- 68.Wilson G., Karlin A. Acetylcholine receptor channel structure in the resting, open, and desensitized states probed with the substituted-cysteine-accessibility method. Proc. Natl. Acad. Sci. USA. 2001;98(3):1241–1248. doi: 10.1073/pnas.031567798. [http://dx.doi.org/10.1073/pnas.98.3.1241]. [PMID: 11158624]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Uteshev V.V. Allosteric modulation of nicotinic acetylcholine receptors: The concept and therapeutic trends. Curr. Pharm. Des. 2016;22(14):1986–1997. doi: 10.2174/1381612822666160201115341. [http://dx.doi.org/10.2174/1381612822666160 201115341]. [PMID: 26831463]. [DOI] [PubMed] [Google Scholar]
- 70.Karlin A., Akabas M.H. Toward a structural basis for the function of nicotinic acetylcholine receptors and their cousins. Neuron. 1995;15(6):1231–1244. doi: 10.1016/0896-6273(95)90004-7. [http://dx.doi.org/10.1016/0896-6273(95) 90004-7]. [PMID: 8845149]. [DOI] [PubMed] [Google Scholar]
- 71.Cecchini M, Changeux J P. The nicotinic acetylcholine receptor and its prokaryotic homologues: Structure, conformational transitions alloster-ic modulation. Neuropharmacology, 2015. pp. 137–149. [DOI] [PubMed]
- 72.Unwin N. Refined structure of the nicotinic acetylcholine receptor at 4A resolution. J. Mol. Biol. 2005;346(4):967–989. doi: 10.1016/j.jmb.2004.12.031. [http://dx.doi.org/10.1016/j.jmb.2004.12.031]. [PMID: 15701510]. [DOI] [PubMed] [Google Scholar]
- 73.Mordvintsev D.Y., Polyak Y.L., Levtsova O.V., Tourleigh Y.V., Kasheverov I.E., Shaitan K.V., Utkin Y.N., Tsetlin V.I. A model for short alpha-neurotoxin bound to nicotinic acetylcholine receptor from Torpedo californica: comparison with long-chain alpha-neurotoxins and alpha-conotoxins. Comput. Biol. Chem. 2005;29(6):398–411. doi: 10.1016/j.compbiolchem.2005.08.007. [http://dx.doi.org/10.1016/j.compbiolchem.2005. 08.007]. [PMID: 16290328]. [DOI] [PubMed] [Google Scholar]
- 74.Brannigan G., Hénin J., Law R., Eckenhoff R., Klein M.L. Embedded cholesterol in the nicotinic acetylcholine receptor. Proc. Natl. Acad. Sci. USA. 2008;105(38):14418–14423. doi: 10.1073/pnas.0803029105. [http://dx.doi. org/10.1073/pnas.0803029105]. [PMID: 18768796]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Le Novère N., Corringer P.J., Changeux J.P. The diversity of subunit composition in nAChRs: evolutionary origins, physiologic and pharmacologic consequences. J. Neurobiol. 2002;53(4):447–456. doi: 10.1002/neu.10153. [http://dx.doi.org/10.1002/neu.10153]. [PMID: 12436412]. [DOI] [PubMed] [Google Scholar]
- 76.Millar N.S., Gotti C. Diversity of vertebrate nicotinic acetylcholine receptors. Neuropharmacology. 2009;56(1):237–246. doi: 10.1016/j.neuropharm.2008.07.041. [http:// dx.doi.org/10.1016/j.neuropharm.2008.07.041]. [PMID: 18723036]. [DOI] [PubMed] [Google Scholar]
- 77.Arias H.R. Localization of agonist and competitive antagonist binding sites on nicotinic acetylcholine receptors. Neurochem. Int. 2000;36(7):595–645. doi: 10.1016/s0197-0186(99)00154-0. [http://dx.doi.org/10.1016/S0197-0186(99) 00154-0]. [PMID: 10771117]. [DOI] [PubMed] [Google Scholar]
- 78.Wessler I.K., Kirkpatrick C.J. The Non-neuronal cholinergic system: an emerging drug target in the airways. Pulm. Pharmacol. Ther. 2001;14(6):423–434. doi: 10.1006/pupt.2001.0313. [http://dx.doi.org/10.1006/pupt.2001. 0313]. [PMID: 11782122]. [DOI] [PubMed] [Google Scholar]
- 79.Gotti C., Moretti M., Gaimarri A., Zanardi A., Clementi F., Zoli M. Heterogeneity and complexity of native brain nicotinic receptors. Biochem. Pharmacol. 2007;74(8):1102–1111. doi: 10.1016/j.bcp.2007.05.023. [http://dx. doi.org/10.1016/j.bcp.2007.05.023]. [PMID: 17597586]. [DOI] [PubMed] [Google Scholar]
- 80.Lindstrom J.M. Nicotinic acetylcholine receptors of muscles and nerves: comparison of their structures, functional roles, and vulnerability to pathology. Ann. N. Y. Acad. Sci. 2003;998:41–52. doi: 10.1196/annals.1254.007. [http://dx.doi.org/10.1196/annals.1254.007]. [PMID: 14592862]. [DOI] [PubMed] [Google Scholar]
- 81.Gotti C., Moretti M., Zanardi A., Gaimarri A., Champtiaux N., Changeux J.P., Whiteaker P., Marks M.J., Clementi F., Zoli M. Heterogeneity and selective targeting of neuronal nicotinic acetylcholine receptor (nAChR) subtypes expressed on retinal afferents of the superior colliculus and lateral geniculate nucleus: identification of a new native nAChR subtype alpha3beta2(alpha5 or beta3) enriched in retinocollicular afferents. Mol. Pharmacol. 2005;68(4):1162–1171. doi: 10.1124/mol.105.015925. [http://dx.doi.org/10.1124/mol.105.015925]. [PMID: 16049166]. [DOI] [PubMed] [Google Scholar]
- 82.Sine S.M. Identification of equivalent residues in the gamma, delta, and epsilon subunits of the nicotinic receptor that contribute to alpha-bungarotoxin binding. J. Biol. Chem. 1997;272(38):23521–23527. doi: 10.1074/jbc.272.38.23521. [http://dx.doi.org/10.1074/jbc.272.38.23521]. [PMID: 9295287]. [DOI] [PubMed] [Google Scholar]
- 83.Sine S.M., Kreienkamp H.J., Bren N., Maeda R., Taylor P. Molecular dissection of subunit interfaces in the acetylcholine receptor: identification of determinants of alpha-conotoxin M1 selectivity. Neuron. 1995;15(1):205–211. doi: 10.1016/0896-6273(95)90077-2. [http://dx.doi.org/10.1016/ 0896-6273(95)90077-2]. [PMID: 7619523]. [DOI] [PubMed] [Google Scholar]
- 84.Lloyd G.K., Williams M. Neuronal nicotinic acetylcholine receptors as novel drug targets. J. Pharmacol. Exp. Ther. 2000;292(2):461–467. [PMID: 10640281]. [PubMed] [Google Scholar]
- 85.Nguyen V.T., Hall L.L., Gallacher G., Ndoye A., Jolkovsky D.L., Webber R.J., Buchli R., Grando S.A. Choline acetyltransferase, acetylcholinesterase, and nicotinic acetylcholine receptors of human gingival and esophageal epithelia. J. Dent. Res. 2000;79(4):939–949. doi: 10.1177/00220345000790040901. [http://dx.doi.org/10.1177/00220345000790040901]. [PMID: 10831096]. [DOI] [PubMed] [Google Scholar]
- 86.Kawashima K., Fujii T. Basic and clinical aspects of non-neuronal acetylcholine: overview of non-neuronal cholinergic systems and their biological significance. J. Pharmacol. Sci. 2008;106(2):167–173. doi: 10.1254/jphs.fm0070073. [http://dx.doi.org/10.1254/jphs.FM0070073]. [PMID: 18285657]. [DOI] [PubMed] [Google Scholar]
- 87.Battaglioli E., Gotti C., Terzano S., Flora A., Clementi F., Fornasari D. Expression and transcriptional regulation of the human alpha3 neuronal nicotinic receptor subunit in T lymphocyte cell lines. J. Neurochem. 1998;71(3):1261–1270. doi: 10.1046/j.1471-4159.1998.71031261.x. [http://dx.doi.org/10. 1046/j.1471-4159.1998.71031261.x]. [PMID: 9721752]. [DOI] [PubMed] [Google Scholar]
- 88.Macklin K.D., Maus A.D., Pereira E.F., Albuquerque E.X., Conti-Fine B.M. Human vascular endothelial cells express functional nicotinic acetylcholine receptors. J. Pharmacol. Exp. Ther. 1998;287(1):435–439. [PMID: 9765366]. [PubMed] [Google Scholar]
- 89.Wang Y., Pereira E.F., Maus A.D., Ostlie N.S., Navaneetham D., Lei S., Albuquerque E.X., Conti-Fine B.M. Human bronchial epithelial and endothelial cells express alpha7 nicotinic acetylcholine receptors. Mol. Pharmacol. 2001;60(6):1201–1209. doi: 10.1124/mol.60.6.1201. [http:// dx.doi.org/10.1124/mol.60.6.1201]. [PMID: 11723227]. [DOI] [PubMed] [Google Scholar]
- 90.Arias H.R., Richards V.E., Ng D., Ghafoori M.E., Le V., Mousa S.A. Role of non-neuronal nicotinic acetylcholine receptors in angiogenesis. Int. J. Biochem. Cell Biol. 2009;41(7):1441–1451. doi: 10.1016/j.biocel.2009.01.013. [http://dx.doi.org/10.1016/j.biocel.2009.01.013]. [PMID: 19401144]. [DOI] [PubMed] [Google Scholar]
- 91.Kawashima K., Fujii T. The lymphocytic cholinergic system and its contribution to the regulation of immune activity. Life Sci. 2003;74(6):675–696. doi: 10.1016/j.lfs.2003.09.037. [http://dx.doi.org/10.1016/j.lfs.2003.09.037]. [PMID: 14654162]. [DOI] [PubMed] [Google Scholar]
- 92.Maus A.D., Pereira E.F., Karachunski P.I., Horton R.M., Navaneetham D., Macklin K., Cortes W.S., Albuquerque E.X., Conti-Fine B.M. Human and rodent bronchial epithelial cells express functional nicotinic acetylcholine receptors. Mol. Pharmacol. 1998;54(5):779–788. doi: 10.1124/mol.54.5.779. [http://dx.doi.org/10.1124/mol.54.5.779]. [PMID: 9804613]. [DOI] [PubMed] [Google Scholar]
- 93.Arredondo J., Nguyen V.T., Chernyavsky A.I., Jolkovsky D.L., Pinkerton K.E., Grando S.A. A receptor-mediated mechanism of nicotine toxicity in oral keratinocytes. Lab. Invest. 2001;81(12):1653–1668. doi: 10.1038/labinvest.3780379. [http://dx.doi.org/10.1038/labinvest.3780379]. [PMID: 11742036]. [DOI] [PubMed] [Google Scholar]
- 94.Gahring L.C., Rogers S.W. Nicotinic acetylcholine receptor expression in the hippocampus of 27 mouse strains reveals novel inhibitory circuitry. Hippocampus. 2008;18(8):737–749. doi: 10.1002/hipo.20430. [http://dx. doi.org/10.1002/hipo.20430]. [PMID: 18446824]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Arredondo J., Chernyavsky A.I., Marubio L.M., Beaudet A.L., Jolkovsky D.L., Pinkerton K.E., Grando S.A. Receptor-mediated tobacco toxicity: regulation of gene expression through alpha3beta2 nicotinic receptor in oral epithelial cells. Am. J. Pathol. 2005;166(2):597–613. doi: 10.1016/s0002-9440(10)62281-x. [http://dx.doi.org/10.1016/S0002-9440(10) 62281-X]. [PMID: 15681842]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kawashima K., Fujii T. Expression of non-neuronal acetylcholine in lymphocytes and its contribution to the regulation of immune function. Front. Biosci. 2004;9:2063–2085. doi: 10.2741/1390. [http://dx.doi.org/ 10.2741/1390]. [PMID: 15353271]. [DOI] [PubMed] [Google Scholar]
- 97.Hamouda A.K., Chiara D.C., Sauls D., Cohen J.B., Blanton M.P. Cholesterol interacts with transmembrane alpha-helices M1, M3, and M4 of the Torpedo nicotinic acetylcholine receptor: photolabeling studies using [3H]Azicholesterol. Biochemistry. 2006;45(3):976–986. doi: 10.1021/bi051978h. [http://dx.doi.org/10.1021/bi051978h]. [PMID: 16411773]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kasheverov I.E., Kryukova E.V., Kudryavtsev D.S., Ivanov I.A., Egorova N.V., Zhmak M.N., Spirova E.N., Shelukhina I.V., Odinokov A.V., Alfimov M.V., Tsetlin V.I. Analysis of binding centers in nicotinic receptors with the aid of synthetic peptides. Dokl. Biochem. Biophys. 2016;470(1):338–341. doi: 10.1134/S1607672916050070. [http://dx. doi.org/10.1134/S1607672916050070]. [PMID: 27817023]. [DOI] [PubMed] [Google Scholar]
- 99.Peng W., Ding F. Biomolecular recognition of antagonists by α7 nicotinic acetylcholine receptor: Antagonistic mechanism and structure-activity relationships studies. Eur. J. Pharm. Sci. 2015;76:119–132. doi: 10.1016/j.ejps.2015.05.005. [http://dx.doi.org/10.1016/j.ejps.2015.05.005]. [PMID: 25963024]. [DOI] [PubMed] [Google Scholar]
- 100.Marubio L.M., del Mar Arroyo-Jimenez M., Cordero-Erausquin M., Léna C., Le Novère N., de Kerchove d’Exaerde A., Huchet M., Damaj M.I., Changeux J.P. Reduced antinociception in mice lacking neuronal nicotinic receptor subunits. Nature. 1999;398(6730):805–810. doi: 10.1038/19756. [http://dx.doi.org/10.1038/19756]. [PMID: 10235262]. [DOI] [PubMed] [Google Scholar]
- 101.Changeux J.P. Allosteric receptors: from electric organ to cognition. Annu. Rev. Pharmacol. Toxicol. 2010;50:1–38. doi: 10.1146/annurev.pharmtox.010909.105741. [http://dx.doi. org/10.1146/annurev.pharmtox.010909.105741]. [PMID: 20055696]. [DOI] [PubMed] [Google Scholar]
- 102.Zoli M, Pistillo F, Gotti C. Diversity of native nicotinic receptor subtypes in mammalian brain. 2015. [DOI] [PubMed]
- 103.Saccone S.F., Hinrichs A.L., Saccone N.L., Chase G.A., Konvicka K., Madden P.A., Breslau N., Johnson E.O., Hatsukami D., Pomerleau O., Swan G.E., Goate A.M., Rutter J., Bertelsen S., Fox L., Fugman D., Martin N.G., Montgomery G.W., Wang J.C., Ballinger D.G., Rice J.P., Bierut L.J. Cholinergic nicotinic receptor genes implicated in a nicotine dependence association study targeting 348 candidate genes with 3713 SNPs. Hum. Mol. Genet. 2007;16(1):36–49. doi: 10.1093/hmg/ddl438. [http://dx.doi.org/10.1093/hmg/ddl438]. [PMID: 17135278]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Thorgeirsson T.E., Geller F., Sulem P., Rafnar T., Wiste A., Magnusson K.P., Manolescu A., Thorleifsson G., Stefansson H., Ingason A., Stacey S.N., Bergthorsson J.T., Thorlacius S., Gudmundsson J., Jonsson T., Jakobsdottir M., Saemundsdottir J., Olafsdottir O., Gudmundsson L.J., Bjornsdottir G., Kristjansson K., Skuladottir H., Isaksson H.J., Gudbjartsson T., Jones G.T., Mueller T., Gottsäter A., Flex A., Aben K.K., de Vegt F., Mulders P.F., Isla D., Vidal M.J., Asin L., Saez B., Murillo L., Blondal T., Kolbeinsson H., Stefansson J.G., Hansdottir I., Runarsdottir V., Pola R., Lindblad B., van Rij A.M., Dieplinger B., Haltmayer M., Mayordomo J.I., Kiemeney L.A., Matthiasson S.E., Oskarsson H., Tyrfingsson T., Gudbjartsson D.F., Gulcher J.R., Jonsson S., Thorsteinsdottir U., Kong A., Stefansson K. A variant associated with nicotine dependence, lung cancer and peripheral arterial disease. Nature. 2008;452(7187):638–642. doi: 10.1038/nature06846. [http://dx.doi.org/10.1038/nature06846]. [PMID: 18385739]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wen L., Yang Z., Cui W., Li M.D. Crucial roles of the CHRNB3-CHRNA6 gene cluster on chromosome 8 in nicotine dependence: update and subjects for future research. Transl. Psychiatry. 2016;6(6):e843. doi: 10.1038/tp.2016.103. [http://dx.doi.org/10.1038/tp.2016.103]. [PMID: 27327258]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Balfour D.J. The neuronal pathways mediating the behavioral and addictive properties of nicotine. Handb. Exp. Pharmacol. 2009;(192):209–233. doi: 10.1007/978-3-540-69248-5_8. [http://dx.doi.org/10.1007/978-3-540-69248-5_8]. [PMID: 19184651]. [DOI] [PubMed] [Google Scholar]
- 107.Maldonado R., Berrendero F. Endogenous cannabinoid and opioid systems and their role in nicotine addiction. Curr. Drug Targets. 2010;11(4):440–449. doi: 10.2174/138945010790980358. [http://dx.doi.org/10.2174/138945010790980358]. [PMID: 20017727]. [DOI] [PubMed] [Google Scholar]
- 108.Colombo S.F., Mazzo F., Pistillo F., Gotti C. Biogenesis, trafficking and up-regulation of nicotinic ACh receptors. Biochem. Pharmacol. 2013;86(8):1063–1073. doi: 10.1016/j.bcp.2013.06.023. [http://dx.doi.org/10.1016/ j.bcp.2013.06.023]. [PMID: 23830821]. [DOI] [PubMed] [Google Scholar]
- 109.Jackson K.J., Martin B.R., Changeux J.P., Damaj M.I. Differential role of nicotinic acetylcholine receptor subunits in physical and affective nicotine withdrawal signs. J. Pharmacol. Exp. Ther. 2008;325(1):302–312. doi: 10.1124/jpet.107.132977. [http://dx.doi.org/10.1124/jpet.107.132977]. [PMID: 18184829]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Picciotto M.R., Zoli M., Rimondini R., Léna C., Marubio L.M., Pich E.M., Fuxe K., Changeux J.P. Acetylcholine receptors containing the beta2 subunit are involved in the reinforcing properties of nicotine. Nature. 1998;391(6663):173–177. doi: 10.1038/34413. [http://dx.doi.org/ 10.1038/34413]. [PMID: 9428762]. [DOI] [PubMed] [Google Scholar]
- 111.Picciotto M.R., Kenny P.J. Molecular mechanisms underlying behaviors related to nicotine addiction. Cold Spring Harb. Perspect. Med. 2013;3(1):a012112. doi: 10.1101/cshperspect.a012112. [http://dx.doi.org/10.1101/ cshperspect.a012112]. [PMID: 23143843]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Li M. Nicotinic acetylcholine receptor technologies. Neuromethods. 2016 [http://dx.doi.org/10.1007/978-1-4939-3768-4]. [Google Scholar]
- 113.Changeux J.P., Corringer P.J., Maskos U. The nicotinic acetylcholine receptor: From molecular biology to cognition. Neuropharmacology. Pt B. 2015;96:135–136. doi: 10.1016/j.neuropharm.2015.03.024. [http://dx.doi.org/10.3389/fnmol. 2015.00071]. [DOI] [PubMed] [Google Scholar]
- 114.Mohamed T.S., Jayakar S.S., Hamouda A.K. Orthosteric and allosteric ligands of nicotinic acetylcholine receptors for smoking cessation. Front. Mol. Neurosci. 2015;8:71. doi: 10.3389/fnmol.2015.00071. [http://dx.doi.org/ 10.3389/fnmol.2015.00071]. [PMID: 26635524]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gill J.K., Chatzidaki A., Ursu D., Sher E., Millar N.S. Contrasting properties of α7-selective orthosteric and allosteric agonists examined on native nicotinic acetylcholine receptors. PLoS One. 2013;8(1):e55047. doi: 10.1371/journal.pone.0055047. [http://dx.doi.org/10.1371/journal.pone.0055047]. [PMID: 23383051]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gill J.K., Dhankher P., Sheppard T.D., Sher E., Millar N.S. A series of α7 nicotinic acetylcholine receptor allosteric modulators with close chemical similarity but diverse pharmacological properties. Mol. Pharmacol. 2012;81(5):710–718. doi: 10.1124/mol.111.076026. [http://dx.doi.org/10. 1124/mol.111.076026]. [PMID: 22328718]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Short C.A., Cao A.T., Wingfield M.A., Doers M.E., Jobe E.M., Wang N., Levandoski M.M. Subunit interfaces contribute differently to activation and allosteric modulation of neuronal nicotinic acetylcholine receptors. Neuropharmacology. 2015;91:157–168. doi: 10.1016/j.neuropharm.2014.11.027. [http://dx.doi.org/10.1016/j.neuropharm.2014.11.027]. [PMID: 25486620]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Christensen D.Z., Mikkelsen J.D., Hansen H.H., Thomsen M.S. Repeated administration of alpha7 nicotinic acetylcholine receptor (nAChR) agonists, but not positive allosteric modulators, increases alpha7 nAChR levels in the brain. J. Neurochem. 2010;114(4):1205–1216. doi: 10.1111/j.1471-4159.2010.06845.x. [PMID: 20533993]. [DOI] [PubMed] [Google Scholar]
- 119.Arias H.R., Xing H., Macdougall K., Blanton M.P., Soti F., Kem W.R. Interaction of benzylidene-anabaseine analogues with agonist and allosteric sites on muscle nicotinic acetylcholine receptors. Br. J. Pharmacol. 2009;157(2):320–330. doi: 10.1111/j.1476-5381.2009.00156.x. [http://dx.doi.org/ 10.1111/j.1476-5381.2009.00156.x]. [PMID: 19338581]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Auerbach A. Agonist activation of a nicotinic acetylcholine receptor. 2015. [DOI] [PMC free article] [PubMed]
- 121.Uteshev V.V. The therapeutic promise of positive allosteric modulation of nicotinic receptors. Eur. J. Pharmacol. 2014;727:181–185. doi: 10.1016/j.ejphar.2014.01.072. [http://dx.doi.org/10.1016/j.ejphar.2014.01.072]. [PMID: 24530419]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Spurny R., Debaveye S., Farinha A., Veys K., Vos A.M., Gossas T., Atack J., Bertrand S., Bertrand D., Danielson U.H., Tresadern G., Ulens C. Molecular blueprint of allosteric binding sites in a homologue of the agonist-binding domain of the α7 nicotinic acetylcholine receptor. Proc. Natl. Acad. Sci. USA. 2015;112(19):E2543–E2552. doi: 10.1073/pnas.1418289112. [http://dx.doi.org/10.1073/pnas.1418289112]. [PMID: 25918415]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gill J.K., Savolainen M., Young G.T., Zwart R., Sher E., Millar N.S. Agonist activation of alpha7 nicotinic acetylcholine receptors via an allosteric transmembrane site. Proc. Natl. Acad. Sci. USA. 2011;108(14):5867–5872. doi: 10.1073/pnas.1017975108. [http://dx.doi.org/10.1073/pnas. 1017975108]. [PMID: 21436053]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bortz D.M., Upton B.A., Mikkelsen J.D., Bruno J.P. Positive allosteric modulators of the α7 nicotinic acetylcholine receptor potentiate glutamate release in the prefrontal cortex of freely-moving rats. Neuropharmacology. 2016;111:78–91. doi: 10.1016/j.neuropharm.2016.08.033. [http://dx.doi.org/ 10.1016/j.neuropharm.2016.08.033]. [PMID: 27569994]. [DOI] [PubMed] [Google Scholar]
- 125.Thomsen M.S., Mikkelsen J.D. Type I and II positive allosteric modulators differentially modulate agonist-induced up-regulation of α7 nicotinic acetylcholine receptors. J. Neurochem. 2012;123(1):73–83. doi: 10.1111/j.1471-4159.2012.07876.x. [http://dx.doi.org/10.1111/j.1471-4159.2012.07876.x]. [PMID: 22804734]. [DOI] [PubMed] [Google Scholar]
- 126.Pandya A., Yakel J.L. Allosteric modulators of the α4β2 subtype of neuronal nicotinic acetylcholine receptors. Biochem. Pharmacol. 2011;82(8):952–958. doi: 10.1016/j.bcp.2011.04.020. [http://dx.doi.org/10.1016/j.bcp.2011. 04.020]. [PMID: 21596025]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Young G.T., Zwart R., Walker A.S., Sher E., Millar N.S. Potentiation of alpha7 nicotinic acetylcholine receptors via an allosteric transmembrane site. Proc. Natl. Acad. Sci. USA. 2008;105(38):14686–14691. doi: 10.1073/pnas.0804372105. [http://dx.doi.org/10.1073/pnas.0804372105]. [PMID: 18791069]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bertrand D., Bertrand S., Cassar S., Gubbins E., Li J., Gopalakrishnan M. Positive allosteric modulation of the alpha7 nicotinic acetylcholine receptor: ligand interactions with distinct binding sites and evidence for a prominent role of the M2-M3 segment. Mol. Pharmacol. 2008;74(5):1407–1416. doi: 10.1124/mol.107.042820. [http://dx.doi.org/ 10.1124/mol.107.042820]. [PMID: 18678621]. [DOI] [PubMed] [Google Scholar]
- 129.Chatzidaki A., D’Oyley J.M., Gill-Thind J.K., Sheppard T.D., Millar N.S. The influence of allosteric modulators and transmembrane mutations on desensitisation and activation of α7 nicotinic acetylcholine receptors. Neuropharmacology. 2015;97:75–85. doi: 10.1016/j.neuropharm.2015.05.006. [http://dx.doi.org/10.1016/j.neuropharm.2015.05.006]. [PMID: 25998276]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Seo S., Henry J.T., Lewis A.H., Wang N., Levandoski M.M. The positive allosteric modulator morantel binds at noncanonical subunit interfaces of neuronal nicotinic acetylcholine receptors. J. Neurosci. 2009;29(27):8734–8742. doi: 10.1523/JNEUROSCI.1859-09.2009. [http://dx.doi.org/10.1523/ JNEUROSCI.1859-09.2009]. [PMID: 19587280]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Papke R.L. Merging old and new perspectives on nicotinic acetylcholine receptors. Biochem. Pharmacol. 2014;89(1):1–11. doi: 10.1016/j.bcp.2014.01.029. [http://dx.doi.org/10.1016/j.bcp.2014.01.029]. [PMID: 24486571]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bertrand D., Gopalakrishnan M. Allosteric modulation of nicotinic acetylcholine receptors. Biochem. Pharmacol. 2007;74(8):1155–1163. doi: 10.1016/j.bcp.2007.07.011. [http://dx.doi.org/10.1016/j.bcp.2007.07.011]. [PMID: 17707779]. [DOI] [PubMed] [Google Scholar]
- 133.Schwartz T.W., Holst B. Ago-allosteric modulation and other types of allostery in dimeric 7TM receptors. J. Recept. Signal Transduct. Res. 2006;26(1-2):107–128. doi: 10.1080/10799890600567570. [http://dx.doi.org/10. 1080/10799890600567570]. [PMID: 16595341]. [DOI] [PubMed] [Google Scholar]
- 134.Gill-Thind J.K., Dhankher P., D’Oyley J.M., Sheppard T.D., Millar N.S. Structurally similar allosteric modulators of α7 nicotinic acetylcholine receptors exhibit five distinct pharmacological effects. J. Biol. Chem. 2015;290(6):3552–3562. doi: 10.1074/jbc.M114.619221. [http://dx.doi.org/ 10.1074/jbc.M114.619221]. [PMID: 25516597]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sine S.M. The nicotinic receptor ligand binding domain. J. Neurobiol. 2002;53(4):431–446. doi: 10.1002/neu.10139. [http://dx.doi.org/10.1002/neu.10139]. [PMID: 12436411]. [DOI] [PubMed] [Google Scholar]
- 136.Grønlien J.H., Håkerud M., Ween H., Thorin-Hagene K., Briggs C.A., Gopalakrishnan M., Malysz J. Distinct profiles of alpha7 nAChR positive allosteric modulation revealed by structurally diverse chemotypes. Mol. Pharmacol. 2007;72(3):715–724. doi: 10.1124/mol.107.035410. [http://dx.doi.org/10.1124/mol.107.035410]. [PMID: 17565004]. [DOI] [PubMed] [Google Scholar]
- 137.Arias H.R., Bhumireddy P., Bouzat C. Molecular mechanisms and binding site locations for noncompetitive antagonists of nicotinic acetylcholine receptors. Int. J. Biochem. Cell Biol. 2006;38(8):1254–1276. doi: 10.1016/j.biocel.2006.01.006. [http://dx.doi.org/10.1016/j.biocel.2006.01.006]. [PMID: 16520081]. [DOI] [PubMed] [Google Scholar]
- 138.Arias H.R., Bhumireddy P., Spitzmaul G., Trudell J.R., Bouzat C. Molecular mechanisms and binding site location for the noncompetitive antagonist crystal violet on nicotinic acetylcholine receptors. Biochemistry. 2006;45(7):2014–2026. doi: 10.1021/bi051752e. [http://dx.doi.org/ 10.1021/bi051752e]. [PMID: 16475790]. [DOI] [PubMed] [Google Scholar]
- 139.Pavlovicz R.E., Henderson B.J., Bonnell A.B., Boyd R.T., McKay D.B., Li C. Identification of a negative allosteric site on human α4β2 and α3β4 neuronal nicotinic acetylcholine receptors. PLoS One. 2011;6(9):e24949. doi: 10.1371/journal.pone.0024949. [http://dx.doi.org/10.1371/journal. pone.0024949]. [PMID: 21949802]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hansen S.B., Taylor P. Galanthamine and non-competitive inhibitor binding to ACh-binding protein: evidence for a binding site on non-alpha-subunit interfaces of heteromeric neuronal nicotinic receptors. J. Mol. Biol. 2007;369(4):895–901. doi: 10.1016/j.jmb.2007.03.067. [http://dx.doi.org/ 10.1016/j.jmb.2007.03.067]. [PMID: 17481657]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hosie A.M., Wilkins M.E., da Silva H.M., Smart T.G. Endogenous neurosteroids regulate GABAA receptors through two discrete transmembrane sites. Nature. 2006;444(7118):486–489. doi: 10.1038/nature05324. [http://dx.doi.org/10.1038/nature05324]. [PMID: 17108970]. [DOI] [PubMed] [Google Scholar]
- 142.Collins T., Millar N.S. Nicotinic acetylcholine receptor transmembrane mutations convert ivermectin from a positive to a negative allosteric modulator. Mol. Pharmacol. 2010;78(2):198–204. doi: 10.1124/mol.110.064295. [http://dx.doi.org/10.1124/mol.110.064295]. [PMID: 20463059]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Barrantes F.J. Phylogenetic conservation of protein-lipid motifs in pentameric ligand-gated ion channels. Biochim. Biophys. Acta. 2015;1848(9):1796–1805. doi: 10.1016/j.bbamem.2015.03.028. [http://dx.doi.org/10.1016/j.bbamem. 2015.03.028]. [PMID: 25839355]. [DOI] [PubMed] [Google Scholar]
- 144.Gatson J.W., Simpkins J.W., Uteshev V.V. High therapeutic potential of positive allosteric modulation of α7 nAChRs in a rat model of traumatic brain injury: proof-of-concept. Brain Res. Bull. 2015;112:35–41. doi: 10.1016/j.brainresbull.2015.01.008. [http://dx.doi.org/10.1016/j.brainresbull.2015. 01.008]. [PMID: 25647232]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Iturriaga-Vásquez P., Alzate-Morales J., Bermudez I., Varas R., Reyes-Parada M. Multiple binding sites in the nicotinic acetylcholine receptors: An opportunity for polypharmacolgy. Pharmacol. Res. 2015;101:9–17. doi: 10.1016/j.phrs.2015.08.018. [http://dx.doi.org/10.1016/j.phrs.2015.08. 018]. [PMID: 26318763]. [DOI] [PubMed] [Google Scholar]
- 146.Suto M.J., Zacharias N. Neuronal nicotinic acetylcholine receptors as drug targets. Expert Opin. Ther. Targets. 2004;8(2):61–64. doi: 10.1517/14728222.8.2.61. [http://dx.doi.org/10.1517/14728222.8.2.61]. [PMID: 15102549]. [DOI] [PubMed] [Google Scholar]
- 147.Holladay M.W., Dart M.J., Lynch J.K. Neuronal nicotinic acetylcholine receptors as targets for drug discovery. J. Med. Chem. 1997;40(26):4169–4194. doi: 10.1021/jm970377o. [http://dx.doi.org/10.1021/jm970377o]. [PMID: 9435889]. [DOI] [PubMed] [Google Scholar]
- 148.D’hoedt D., Bertrand D. Nicotinic acetylcholine receptors: an overview on drug discovery. Expert Opin. Ther. Targets. 2009;13(4):395–411. doi: 10.1517/14728220902841045. [http://dx.doi.org/10.1517/14728220902841045]. [PMID: 19335063]. [DOI] [PubMed] [Google Scholar]
- 149.Hurst R.S., Hajós M., Raggenbass M., Wall T.M., Higdon N.R., Lawson J.A., Rutherford-Root K.L., Berkenpas M.B., Hoffmann W.E., Piotrowski D.W., Groppi V.E., Allaman G., Ogier R., Bertrand S., Bertrand D., Arneric S.P. A novel positive allosteric modulator of the alpha7 neuronal nicotinic acetylcholine receptor: in vitro and in vivo characterization. J. Neurosci. 2005;25(17):4396–4405. doi: 10.1523/JNEUROSCI.5269-04.2005. [http://dx.doi.org/10.1523/JNEUROSCI.5269-04.2005]. [PMID: 15858066]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zhang Q., Du Y., Zhang J., Xu X., Xue F., Guo C., Huang Y., Lukas R.J., Chang Y. Functional impact of 14 single nucleotide polymorphisms causing missense mutations of human α7 nicotinic receptor. PLoS One. 2015;10(9):e137588. doi: 10.1371/journal.pone.0137588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Sun F., Jin K., Uteshev V.V. A type-II positive allosteric modulator of α7 nAChRs reduces brain injury and improves neurological function after focal cerebral ischemia in rats. PLoS One. 2013;8(8):e73581. doi: 10.1371/journal.pone.0073581. [http://dx.doi.org/10.1371/journal.pone.0073581]. [PMID: 23951360]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Terry A.V., Jr, Callahan P.M., Hernandez C.M. Nicotinic ligands as multifunctional agents for the treatment of neuropsychiatric disorders. Biochem. Pharmacol. 2015;97(4):388–398. doi: 10.1016/j.bcp.2015.07.027. [http://dx. doi.org/10.1016/j.bcp.2015.07.027]. [PMID: 26231940]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Benwell M.E., Balfour D.J., Anderson J.M. Evidence that tobacco smoking increases the density of (-)-[3H]nicotine binding sites in human brain. J. Neurochem. 1988;50(4):1243–1247. doi: 10.1111/j.1471-4159.1988.tb10600.x. [http://dx.doi.org/10.1111/j.1471-4159.1988.tb10600.x]. [PMID: 3346676]. [DOI] [PubMed] [Google Scholar]
- 154.Hong L.E., Hodgkinson C.A., Yang Y., Sampath H., Ross T.J., Buchholz B., Salmeron B.J., Srivastava V., Thaker G.K., Goldman D., Stein E.A. A genetically modulated, intrinsic cingulate circuit supports human nicotine addiction. Proc. Natl. Acad. Sci. USA. 2010;107(30):13509–13514. doi: 10.1073/pnas.1004745107. [http://dx.doi.org/10.1073/pnas. 1004745107]. [PMID: 20643934]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Staley J.K., Krishnan-Sarin S., Cosgrove K.P., Krantzler E., Frohlich E., Perry E., Dubin J.A., Estok K., Brenner E., Baldwin R.M., Tamagnan G.D., Seibyl J.P., Jatlow P., Picciotto M.R., London E.D., O’Malley S., van Dyck C.H. Human tobacco smokers in early abstinence have higher levels of beta2* nicotinic acetylcholine receptors than nonsmokers. J. Neurosci. 2006;26(34):8707–8714. doi: 10.1523/JNEUROSCI.0546-06.2006. [http://dx.doi.org/10.1523/JNEUROSCI.0546-06.2006]. [PMID: 16928859]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Marks M.J., Burch J.B., Collins A.C. Effects of chronic nicotine infusion on tolerance development and nicotinic receptors. J. Pharmacol. Exp. Ther. 1983;226(3):817–825. [PMID: 6887012]. [PubMed] [Google Scholar]
- 157.Benowitz N.L. Neurobiology of nicotine addiction: implications for smoking cessation treatment. Am. J. Med. 2008;121(4) Suppl. 1:S3–S10. doi: 10.1016/j.amjmed.2008.01.015. [http://dx.doi.org/10.1016/j.amjmed.2008.01.015]. [PMID: 18342164]. [DOI] [PubMed] [Google Scholar]
- 158.Philpot R.M. Potential use of nicotinic receptor agonists for the treatment of chemotherapy-induced cognitive deficits. Neurochem. Res. 2015;40(10):2018–2031. doi: 10.1007/s11064-015-1528-y. [http://dx.doi.org/10.1007/s11064-015-1528-y]. [PMID: 25652578]. [DOI] [PubMed] [Google Scholar]
- 159.Faghih R., Gfesser G.A., Gopalakrishnan M. Advances in the discovery of novel positive allosteric modulators of the alpha7 nicotinic acetylcholine receptor. Recent Patents CNS Drug Discov. 2007;2(2):99–106. doi: 10.2174/157488907780832751. [http://dx.doi.org/10.2174/157488907780832751]. [PMID: 18221220]. [DOI] [PubMed] [Google Scholar]
- 160.Faghih R., Gopalakrishnan M., Briggs C.A. Allosteric modulators of the α7 nicotinic acetylcholine receptor. J. Med. Chem. 2008;51(4):701–712. doi: 10.1021/jm070256g. [http://dx.doi.org/10.1021/jm070256g]. [PMID: 18198823]. [DOI] [PubMed] [Google Scholar]
- 161.Guerrini G., Ciciani G. Benzodiazepine receptor ligands: a patent review (2006-2012). Expert Opin. Ther. Pat. 2013;23(7):843–866. doi: 10.1517/13543776.2013.782005. [http://dx.doi.org/10.1517/13543776.2013.782005]. [PMID: 23521498]. [DOI] [PubMed] [Google Scholar]
- 162.Lightfoot A.P., Kew J.N., Skidmore J. Alpha7 nicotinic acetylcholine receptor agonists and positive allosteric modulators. Prog. Med. Chem. 2008;46:131–171. doi: 10.1016/S0079-6468(07)00003-3. [http://dx.doi.org/10.1016/S0079-6468(07)00003-3]. [PMID: 18381125]. [DOI] [PubMed] [Google Scholar]
- 163.Mazurov A.A., Speake J.D., Yohannes D. Discovery and development of α7 nicotinic acetylcholine receptor modulators. J. Med. Chem. 2011;54(23):7943–7961. doi: 10.1021/jm2007672. [http://dx.doi.org/10.1021/ jm2007672]. [PMID: 21919481]. [DOI] [PubMed] [Google Scholar]
- 164.Rex E.B., Shukla N., Gu S., Bredt D., DiSepio D. A genome-wide arrayed cDNA screen to identify functional modulators of α7 nicotinic acetylcholine receptors. SLAS Discov. 2017;22(2):155–165. doi: 10.1177/1087057116676086. [http://dx.doi.org/10.1177/1087057116676086]. [PMID: 27789755]. [DOI] [PubMed] [Google Scholar]
- 165.Gündisch D., Eibl C. Nicotinic acetylcholine receptor ligands, a patent review (2006-2011). Expert Opin. Ther. Pat. 2011;21(12):1867–1896. doi: 10.1517/13543776.2011.637919. [http://dx.doi.org/10.1517/13543776.2011.637919]. [PMID: 22098319]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Chen L.S., Horton A., Bierut L. Pathways to precision medicine in smoking cessation treatments. 2016. [DOI] [PMC free article] [PubMed]
- 167.Renda A., Penty N., Komal P., Nashmi R. Vulnerability to nicotine self-administration in adolescent mice correlates with age-specific expression of α4* nicotinic receptors. Neuropharmacology. 2016;108:49–59. doi: 10.1016/j.neuropharm.2016.04.019. [http://dx.doi.org/10.1016/j.neuropharm. 2016.04.019]. [PMID: 27102349]. [DOI] [PubMed] [Google Scholar]
- 168.Jackson A., Bagdas D., Muldoon P.P., Lichtman A.H., Carroll F.I., Greenwald M., Miles M.F., Damaj M.I. In vivo interactions between α7 nicotinic acetylcholine receptor and nuclear peroxisome proliferator-activated receptor-α: Implication for nicotine dependence. Neuropharmacology. 2017;118:38–45. doi: 10.1016/j.neuropharm.2017.03.005. [http://dx.doi. org/10.1016/j.neuropharm.2017.03.005]. [PMID: 28279662]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Hendrickson L.M., Guildford M.J., Tapper A.R. Neuronal nicotinic acetylcholine receptors: common molecular substrates of nicotine and alcohol dependence. Front. Psychiatry. 2013;4:29. doi: 10.3389/fpsyt.2013.00029. [http://dx.doi.org/10.3389/fpsyt.2013.00029]. [PMID: 23641218]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Korpi E.R., den Hollander B., Farooq U., Vashchinkina E., Rajkumar R., Nutt D.J., Hyytiä P., Dawe G.S., Koulu M. Mechanisms of action and persistent neuroplasticity by drugs of abuse. Pharmacol. Rev. 2015;67(4):872–1004. doi: 10.1124/pr.115.010967. [http://dx.doi.org/ 10.1124/pr.115.010967]. [PMID: 26403687]. [DOI] [PubMed] [Google Scholar]
- 171.Drenan R.M., Lester H.A. Insights into the neurobiology of the nicotinic cholinergic system and nicotine addiction from mice expressing nicotinic receptors harboring gain-of-function mutations. Pharmacol. Rev. 2012;64(4):869–879. doi: 10.1124/pr.111.004671. [http://dx.doi.org/10.1124/ pr.111.004671]. [PMID: 22885704]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Salas R., Pieri F., De Biasi M. Decreased signs of nicotine withdrawal in mice null for the beta4 nicotinic acetylcholine receptor subunit. J. Neurosci. 2004;24(45):10035–10039. doi: 10.1523/JNEUROSCI.1939-04.2004. [http://dx.doi. org/10.1523/JNEUROSCI.1939-04.2004]. [PMID: 15537871]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Gangitano D., Salas R., Teng Y., Perez E., De Biasi M. Progesterone modulation of alpha5 nAChR subunits influences anxiety-related behavior during estrus cycle. Genes Brain Behav. 2009;8(4):398–406. doi: 10.1111/j.1601-183X.2009.00476.x. [http://dx.doi.org/10.1111/j.1601-183X.2009.00476. x]. [PMID: 19220484]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Salas R., Sturm R., Boulter J., De Biasi M. Nicotinic receptors in the habenulo-interpeduncular system are necessary for nicotine withdrawal in mice. J. Neurosci. 2009;29(10):3014–3018. doi: 10.1523/JNEUROSCI.4934-08.2009. [http:// dx.doi.org/10.1523/JNEUROSCI.4934-08.2009]. [PMID: 19279237]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Mineur Y.S., Picciotto M.R. Genetics of nicotinic acetylcholine receptors: Relevance to nicotine addiction. Biochem. Pharmacol. 2008;75(1):323–333. doi: 10.1016/j.bcp.2007.06.010. [http://dx.doi.org/10.1016/j.bcp.2007.06.010]. [PMID: 17632086]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Grady S.R., Moretti M., Zoli M., Marks M.J., Zanardi A., Pucci L., Clementi F., Gotti C. Rodent habenulo-interpeduncular pathway expresses a large variety of uncommon nAChR subtypes, but only the alpha3beta4* and alpha3beta3beta4* subtypes mediate acetylcholine release. J. Neurosci. 2009;29(7):2272–2282. doi: 10.1523/JNEUROSCI.5121-08.2009. [http:// dx.doi.org/10.1523/JNEUROSCI.5121-08.2009]. [PMID: 19228980]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Salas R., Orr-Urtreger A., Broide R.S., Beaudet A., Paylor R., De Biasi M. The nicotinic acetylcholine receptor subunit alpha 5 mediates short-term effects of nicotine in vivo. Mol. Pharmacol. 2003;63(5):1059–1066. doi: 10.1124/mol.63.5.1059. [http://dx.doi.org/10.1124/mol.63.5.1059]. [PMID: 12695534]. [DOI] [PubMed] [Google Scholar]
- 178.McLaughlin I., Dani J.A., De Biasi M. Nicotine withdrawal. Curr. Top. Behav. Neurosci. 2015;24:99–123. doi: 10.1007/978-3-319-13482-6_4. [http://dx.doi.org/ 10.1007/978-3-319-13482-6_4]. [PMID: 25638335]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Fowler C.D., Lu Q., Johnson P.M., Marks M.J., Kenny P.J. Habenular α5 nicotinic receptor subunit signalling controls nicotine intake. Nature. 2011;471(7340):597–601. doi: 10.1038/nature09797. [http://dx.doi.org/10. 1038/nature09797]. [PMID: 21278726]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Xu W., Gelber S., Orr-Urtreger A., Armstrong D., Lewis R.A., Ou C.N., Patrick J., Role L., De Biasi M., Beaudet A.L. Megacystis, mydriasis, and ion channel defect in mice lacking the alpha3 neuronal nicotinic acetylcholine receptor. Proc. Natl. Acad. Sci. USA. 1999;96(10):5746–5751. doi: 10.1073/pnas.96.10.5746. [http://dx.doi.org/10.1073/pnas.96. 10.5746]. [PMID: 10318955]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Jackson K.J., Sanjakdar S.S., Muldoon P.P., McIntosh J.M., Damaj M.I. The α3β4* nicotinic acetylcholine receptor subtype mediates nicotine reward and physical nicotine withdrawal signs independently of the α5 subunit in the mouse. Neuropharmacology. 2013;70:228–235. doi: 10.1016/j.neuropharm.2013.01.017. [http://dx.doi.org/10.1016/j.neuropharm. 2013.01.017]. [PMID: 23416040]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Stoker A.K., Olivier B., Markou A. Role of α7- and β4-containing nicotinic acetylcholine receptors in the affective and somatic aspects of nicotine withdrawal: studies in knockout mice. Behav. Genet. 2012;42(3):423–436. doi: 10.1007/s10519-011-9511-0. [http://dx.doi.org/10.1007/ s10519-011-9511-0]. [PMID: 22009521]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Kedmi M., Beaudet A.L., Orr-Urtreger A. Mice lacking neuronal nicotinic acetylcholine receptor beta4-subunit and mice lacking both alpha5- and beta4-subunits are highly resistant to nicotine-induced seizures. Physiol. Genomics. 2004;17(2):221–229. doi: 10.1152/physiolgenomics.00202.2003. [http:// dx.doi.org/10.1152/physiolgenomics.00202.2003]. [PMID: 14996991]. [DOI] [PubMed] [Google Scholar]
- 184.Frahm S., Slimak M.A., Ferrarese L., Santos-Torres J., Antolin-Fontes B., Auer S., Filkin S., Pons S., Fontaine J.F., Tsetlin V., Maskos U., Ibañez-Tallon I. Aversion to nicotine is regulated by the balanced activity of β4 and α5 nicotinic receptor subunits in the medial habenula. Neuron. 2011;70(3):522–535. doi: 10.1016/j.neuron.2011.04.013. [http://dx. doi.org/10.1016/j.neuron.2011.04.013]. [PMID: 21555077]. [DOI] [PubMed] [Google Scholar]
- 185.Meyers E.E., Loetz E.C., Marks M.J. Differential expression of the beta4 neuronal nicotinic receptor subunit affects tolerance development and nicotinic binding sites following chronic nicotine treatment. Pharmacol. Biochem. Behav. 2015;130:1–8. doi: 10.1016/j.pbb.2014.12.013. [http:// dx.doi.org/10.1016/j.pbb.2014.12.013]. [PMID: 25560939]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Thorgeirsson T.E., Gudbjartsson D.F., Surakka I., Vink J.M., Amin N., Geller F., Sulem P., Rafnar T., Esko T., Walter S., Gieger C., Rawal R., Mangino M., Prokopenko I., Mägi R., Keskitalo K., Gudjonsdottir I.H., Gretarsdottir S., Stefansson H., Thompson J.R., Aulchenko Y.S., Nelis M., Aben K.K., den Heijer M., Dirksen A., Ashraf H., Soranzo N., Valdes A.M., Steves C., Uitterlinden A.G., Hofman A., Tönjes A., Kovacs P., Hottenga J.J., Willemsen G., Vogelzangs N., Döring A., Dahmen N., Nitz B., Pergadia M.L., Saez B., De Diego V., Lezcano V., Garcia-Prats M.D., Ripatti S., Perola M., Kettunen J., Hartikainen A.L., Pouta A., Laitinen J., Isohanni M., Huei-Yi S., Allen M., Krestyaninova M., Hall A.S., Jones G.T., van Rij A.M., Mueller T., Dieplinger B., Haltmayer M., Jonsson S., Matthiasson S.E., Oskarsson H., Tyrfingsson T., Kiemeney L.A., Mayordomo J.I., Lindholt J.S., Pedersen J.H., Franklin W.A., Wolf H., Montgomery G.W., Heath A.C., Martin N.G., Madden P.A., Giegling I., Rujescu D., Järvelin M.R., Salomaa V., Stumvoll M., Spector T.D., Wichmann H.E., Metspalu A., Samani N.J., Penninx B.W., Oostra B.A., Boomsma D.I., Tiemeier H., van Duijn C.M., Kaprio J., Gulcher J.R., McCarthy M.I., Peltonen L., Thorsteinsdottir U., Stefansson K. Sequence variants at CHRNB3-CHRNA6 and CYP2A6 affect smoking behavior. Nat. Genet. 2010;42(5):448–453. doi: 10.1038/ng.573. [http://dx.doi.org/10.1038/ng.573]. [PMID: 20418888]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Sanjakdar S.S., Maldoon P.P., Marks M.J., Brunzell D.H., Maskos U., McIntosh J.M., Bowers M.S., Damaj M.I. Differential roles of α6β2* and α4β2* neuronal nicotinic receptors in nicotine- and cocaine-conditioned reward in mice. Neuropsychopharmacology. 2015;40(2):350–360. doi: 10.1038/npp.2014.177. [http://dx.doi.org/10.1038/ npp.2014.177]. [PMID: 25035086]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Jackson K.J., McIntosh J.M., Brunzell D.H., Sanjakdar S.S., Damaj M.I. The role of alpha6-containing nicotinic acetylcholine receptors in nicotine reward and withdrawal. J. Pharmacol. Exp. Ther. 2009;331(2):547–554. doi: 10.1124/jpet.109.155457. [http://dx.doi.org/10.1124/jpet.109. 155457]. [PMID: 19644040]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Exley R., Clements M.A., Hartung H., McIntosh J.M., Cragg S.J. Alpha6-containing nicotinic acetylcholine receptors dominate the nicotine control of dopamine neurotransmission in nucleus accumbens. Neuropsychopharmacology. 2008;33(9):2158–2166. doi: 10.1038/sj.npp.1301617. [http://dx.doi.org/10.1038/sj.npp.1301617]. [PMID: 18033235]. [DOI] [PubMed] [Google Scholar]
- 190.Grady S.R., Salminen O., Laverty D.C., Whiteaker P., McIntosh J.M., Collins A.C., Marks M.J. The subtypes of nicotinic acetylcholine receptors on dopaminergic terminals of mouse striatum. Biochem. Pharmacol. 2007;74(8):1235–1246. doi: 10.1016/j.bcp.2007.07.032. [http://dx. doi.org/10.1016/j.bcp.2007.07.032]. [PMID: 17825262]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Zhao-Shea R., Liu L., Soll L.G., Improgo M.R., Meyers E.E., McIntosh J.M., Grady S.R., Marks M.J., Gardner P.D., Tapper A.R. Nicotine-mediated activation of dopaminergic neurons in distinct regions of the ventral tegmental area. Neuropsychopharmacology. 2011;36(5):1021–1032. doi: 10.1038/npp.2010.240. [http://dx.doi.org/10.1038/npp. 2010.240]. [PMID: 21289604]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Zhao-Shea R., Liu L., Pang X., Gardner P.D., Tapper A.R. Activation of GABAergic neurons in the interpeduncular nucleus triggers physical nicotine withdrawal symptoms. Curr. Biol. 2013;23(23):2327–2335. doi: 10.1016/j.cub.2013.09.041. [http://dx.doi.org/10.1016/j.cub.2013.09.041]. [PMID: 24239118]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Champtiaux N., Han Z.Y., Bessis A., Rossi F.M., Zoli M., Marubio L., McIntosh J.M., Changeux J.P. Distribution and pharmacology of alpha 6-containing nicotinic acetylcholine receptors analyzed with mutant mice. J. Neurosci. 2002;22(4):1208–1217. doi: 10.1523/JNEUROSCI.22-04-01208.2002. [PMID: 11850448]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Yang K.C., Jin G.Z., Wu J. Mysterious alpha6-containing nAChRs: function, pharmacology, and pathophysiology. Acta Pharmacol. Sin. 2009;30(6):740–751. doi: 10.1038/aps.2009.63. [http://dx.doi.org/10.1038/ aps.2009.63]. [PMID: 19498417]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Booker T.K., Butt C.M., Wehner J.M., Heinemann S.F., Collins A.C. Decreased anxiety-like behavior in beta3 nicotinic receptor subunit knockout mice. Pharmacol. Biochem. Behav. 2007;87(1):146–157. doi: 10.1016/j.pbb.2007.04.011. [http://dx.doi.org/10.1016/j.pbb.2007.04.011]. [PMID: 17509676]. [DOI] [PubMed] [Google Scholar]
- 196.Cui C., Booker T.K., Allen R.S., Grady S.R., Whiteaker P., Marks M.J., Salminen O., Tritto T., Butt C.M., Allen W.R., Stitzel J.A., McIntosh J.M., Boulter J., Collins A.C., Heinemann S.F. The beta3 nicotinic receptor subunit: a component of alpha-conotoxin MII-binding nicotinic acetylcholine receptors that modulate dopamine release and related behaviors. J. Neurosci. 2003;23(35):11045–11053. doi: 10.1523/JNEUROSCI.23-35-11045.2003. [PMID: 14657161]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Ross S.A., Wong J.Y., Clifford J.J., Kinsella A., Massalas J.S., Horne M.K., Scheffer I.E., Kola I., Waddington J.L., Berkovic S.F., Drago J. Phenotypic characterization of an alpha 4 neuronal nicotinic acetylcholine receptor subunit knock-out mouse. J. Neurosci. 2000;20(17):6431–6441. doi: 10.1523/JNEUROSCI.20-17-06431.2000. [PMID: 10964949]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Tapper A.R., McKinney S.L., Nashmi R., Schwarz J., Deshpande P., Labarca C., Whiteaker P., Marks M.J., Collins A.C., Lester H.A. Nicotine activation of alpha4* receptors: sufficient for reward, tolerance, and sensitization. Science. 2004;306(5698):1029–1032. doi: 10.1126/science.1099420. [http://dx.doi.org/10.1126/science.1099420]. [PMID: 15528443]. [DOI] [PubMed] [Google Scholar]
- 199.Son C.D., Moss F.J., Cohen B.N., Lester H.A. Nicotine normalizes intracellular subunit stoichiometry of nicotinic receptors carrying mutations linked to autosomal dominant nocturnal frontal lobe epilepsy. Mol. Pharmacol. 2009;75(5):1137–1148. doi: 10.1124/mol.108.054494. [http://dx. doi.org/10.1124/mol.108.054494]. [PMID: 19237585]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Ngolab J., Liu L., Zhao-Shea R., Gao G., Gardner P.D., Tapper A.R. Functional upregulation of α4* nicotinic acetylcholine receptors in VTA GABAergic neurons increases sensitivity to nicotine reward. J. Neurosci. 2015;35(22):8570–8578. doi: 10.1523/JNEUROSCI.4453-14.2015. [http://dx.doi.org/ 10.1523/JNEUROSCI.4453-14.2015]. [PMID: 26041923]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Picciotto M.R., Zoli M., Léna C., Bessis A., Lallemand Y., Le Novère N., Vincent P., Pich E.M., Brûlet P., Changeux J.P. Abnormal avoidance learning in mice lacking functional high-affinity nicotine receptor in the brain. Nature. 1995;374(6517):65–67. doi: 10.1038/374065a0. [http://dx.doi.org/10.1038/374065a0]. [PMID: 7870173]. [DOI] [PubMed] [Google Scholar]
- 202.McCallum S.E., Collins A.C., Paylor R., Marks M.J. Deletion of the beta 2 nicotinic acetylcholine receptor subunit alters development of tolerance to nicotine and eliminates receptor upregulation. Psychopharmacology (Berl.) 2006;184(3-4):314–327. doi: 10.1007/s00213-005-0076-6. [http://dx. doi.org/10.1007/s00213-005-0076-6]. [PMID: 16001112]. [DOI] [PubMed] [Google Scholar]
- 203.Tritto T., McCallum S.E., Waddle S.A., Hutton S.R., Paylor R., Collins A.C., Marks M.J. Null mutant analysis of responses to nicotine: deletion of beta2 nicotinic acetylcholine receptor subunit but not alpha7 subunit reduces sensitivity to nicotine-induced locomotor depression and hypothermia. Nicotine Tob. Res. 2004;6(1):145–158. doi: 10.1080/14622200310001656966. [http://dx.doi.org/10.1080/14622200310001656966]. [PMID: 14982698]. [DOI] [PubMed] [Google Scholar]
- 204.Epping-Jordan M.P., Watkins S.S., Koob G.F., Markou A. Dramatic decreases in brain reward function during nicotine withdrawal. Nature. 1998;393(6680):76–79. doi: 10.1038/30001. [http://dx.doi.org/10.1038/ 30001]. [PMID: 9590692]. [DOI] [PubMed] [Google Scholar]
- 205.Bruijnzeel A.W., Markou A. Adaptations in cholinergic transmission in the ventral tegmental area associated with the affective signs of nicotine withdrawal in rats. Neuropharmacology. 2004;47(4):572–579. doi: 10.1016/j.neuropharm.2004.05.005. [http://dx.doi.org/10.1016/j.neuropharm.2004.05. 005]. [PMID: 15380374]. [DOI] [PubMed] [Google Scholar]
- 206.Leri F., Vaccarino F.J. Tribute to: Self-administered nicotine activates the mesolimbic dopamine system through the ventral tegmental area. Brain Res. 2016;1645:61–64. doi: 10.1016/j.brainres.2015.12.064. [William Corrigall, Kathleen Coen and Laurel Adamson, Brain Res., 1994, 653, 278-284]. [William Corrigall, Kathleen Coen and Laurel Adamson, Brain Res., 1994, 653, 278-284]. [http://dx.doi.org/10.1016/j. brainres.2015.12.064]. [PMID: 26867702]. [DOI] [PubMed] [Google Scholar]
- 207.Champtiaux N., Gotti C., Cordero-Erausquin M., David D.J., Przybylski C., Léna C., Clementi F., Moretti M., Rossi F.M., Le Novère N., McIntosh J.M., Gardier A.M., Changeux J.P. Subunit composition of functional nicotinic receptors in dopaminergic neurons investigated with knock-out mice. J. Neurosci. 2003;23(21):7820–7829. doi: 10.1523/JNEUROSCI.23-21-07820.2003. [PMID: 12944511]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Grady S.R., Drenan R.M., Breining S.R., Yohannes D., Wageman C.R., Fedorov N.B., McKinney S., Whiteaker P., Bencherif M., Lester H.A., Marks M.J. Structural differences determine the relative selectivity of nicotinic compounds for native alpha 4 beta 2*-, alpha 6 beta 2*-, alpha 3 beta 4*- and alpha 7-nicotine acetylcholine receptors. Neuropharmacology. 2010;58(7):1054–1066. doi: 10.1016/j.neuropharm.2010.01.013. [http://dx.doi.org/10.1016/j.neuropharm.2010.01.013]. [PMID: 20114055]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Bordia T., Hrachova M., Chin M., McIntosh J.M., Quik M. Varenicline is a potent partial agonist at α6β2* nicotinic acetylcholine receptors in rat and monkey striatum. J. Pharmacol. Exp. Ther. 2012;342(2):327–334. doi: 10.1124/jpet.112.194852. [http://dx.doi.org/10.1124/jpet.112. 194852]. [PMID: 22550286]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Lotfipour S., Byun J.S., Leach P., Fowler C.D., Murphy N.P., Kenny P.J., Gould T.J., Boulter J. Targeted deletion of the mouse α2 nicotinic acetylcholine receptor subunit gene (Chrna2) potentiates nicotine-modulated behaviors. J. Neurosci. 2013;33(18):7728–7741. doi: 10.1523/JNEUROSCI.4731-12.2013. [http://dx.doi.org/10.1523/JNEUROSCI.4731-12.2013]. [PMID: 23637165]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Wang S. D van der Vaart, A.; Xu, Q.; Seneviratne, C.; Pomerleau, O.F.; Pomerleau, C.S.; Payne, T.J.; Ma, J.Z.; Li, M.D. Significant associations of CHRNA2 and CHRNA6 with nicotine dependence in European American and African American populations. Hum. Genet. 2014;133(5):575–586. doi: 10.1007/s00439-013-1398-9. [http://dx.doi.org/10.1007/s00439-013-1398-9]. [PMID: 24253422]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Grabus S.D., Martin B.R., Batman A.M., Tyndale R.F., Sellers E., Damaj M.I. Nicotine physical dependence and tolerance in the mouse following chronic oral administration. Psychopharmacology (Berl.) 2005;178(2-3):183–192. doi: 10.1007/s00213-004-2007-3. [http://dx.doi.org/10.1007/s00213-004-2007-3]. [PMID: 15365686]. [DOI] [PubMed] [Google Scholar]
- 213.Metaxas A., Al-Hasani R., Farshim P., Tubby K., Berwick A., Ledent C., Hourani S., Kitchen I., Bailey A. Genetic deletion of the adenosine A(2A) receptor prevents nicotine-induced upregulation of α7, but not α4β2* nicotinic acetylcholine receptor binding in the brain. Neuropharmacology. 2013;71:228–236. doi: 10.1016/j.neuropharm.2013.03.023. [http://dx. doi.org/10.1016/j.neuropharm.2013.03.023]. [PMID: 23583933]. [DOI] [PubMed] [Google Scholar]
- 214.Levin E.D., Petro A., Rezvani A.H., Pollard N., Christopher N.C., Strauss M., Avery J., Nicholson J., Rose J.E. Nicotinic alpha7- or beta2-containing receptor knockout: effects on radial-arm maze learning and long-term nicotine consumption in mice. Behav. Brain Res. 2009;196(2):207–213. doi: 10.1016/j.bbr.2008.08.048. [http://dx.doi.org/10.1016/j.bbr. 2008.08.048]. [PMID: 18831991]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Salas R., Main A., Gangitano D., De Biasi M. Decreased withdrawal symptoms but normal tolerance to nicotine in mice null for the alpha7 nicotinic acetylcholine receptor subunit. Neuropharmacology. 2007;53(7):863–869. doi: 10.1016/j.neuropharm.2007.08.017. [http://dx.doi.org/10.1016/j. neuropharm.2007.08.017]. [PMID: 17920082]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Pons S., Fattore L., Cossu G., Tolu S., Porcu E., McIntosh J.M., Changeux J.P., Maskos U., Fratta W. Crucial role of alpha4 and alpha6 nicotinic acetylcholine receptor subunits from ventral tegmental area in systemic nicotine self-administration. J. Neurosci. 2008;28(47):12318–12327. doi: 10.1523/JNEUROSCI.3918-08.2008. [http://dx.doi.org/10.1523/ JNEUROSCI.3918-08.2008]. [PMID: 19020025]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Kim J. Association of CHRNA2 polymorphisms with overweight/obesity and clinical characteristics in a Korean population. Clin. Chem. Lab. Med. 2008;46(8):1085–1089. doi: 10.1515/CCLM.2008.230. [http://dx.doi.org/ 10.1515/CCLM.2008.230]. [PMID: 18588430]. [DOI] [PubMed] [Google Scholar]
- 218.Corley R.P., Zeiger J.S., Crowley T., Ehringer M.A., Hewitt J.K., Hopfer C.J., Lessem J., McQueen M.B., Rhee S.H., Smolen A., Stallings M.C., Young S.E., Krauter K. Association of candidate genes with antisocial drug dependence in adolescents. Drug Alcohol Depend. 2008;96(1-2):90–98. doi: 10.1016/j.drugalcdep.2008.02.004. [http://dx.doi.org/10. 1016/j.drugalcdep.2008.02.004]. [PMID: 18384978]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Himes B.E., Lasky-Su J., Wu A.C., Wilk J.B., Hunninghake G.M., Klanderman B., Murphy A.J., Lazarus R., Soto-Quiros M.E., Avila L., Celedón J.C., Lange C., O’Connor G.T., Raby B.A., Silverman E.K., Weiss S.T. Asthma-susceptibility variants identified using probands in case-control and family-based analyses. BMC Med. Genet. 2010;11:122. doi: 10.1186/1471-2350-11-122. [http://dx.doi.org/10.1186/ 1471-2350-11-122]. [PMID: 20698975]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Philibert R.A., Todorov A., Andersen A., Hollenbeck N., Gunter T., Heath A., Madden P. Examination of the nicotine dependence (NICSNP) consortium findings in the Iowa adoption studies population. Nicotine Tob. Res. 2009;11(3):286–292. doi: 10.1093/ntr/ntn034. [http://dx.doi. org/10.1093/ntr/ntn034]. [PMID: 19307444]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Wessel J., McDonald S.M., Hinds D.A., Stokowski R.P., Javitz H.S., Kennemer M., Krasnow R., Dirks W., Hardin J., Pitts S.J., Michel M., Jack L., Ballinger D.G., McClure J.B., Swan G.E., Bergen A.W. Resequencing of nicotinic acetylcholine receptor genes and association of common and rare variants with the Fagerström test for nicotine dependence. Neuropsychopharmacology. 2010;35(12):2392–2402. doi: 10.1038/npp.2010.120. [http://dx.doi.org/10.1038/npp.2010.120]. [PMID: 20736995]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Dash B., Lukas R.J., Li M.D. A signal peptide missense mutation associated with nicotine dependence alters α2*-nicotinic acetylcholine receptor function. Neuropharmacology. 2014;79:715–725. doi: 10.1016/j.neuropharm.2014.01.021. [http://dx.doi.org/10.1016/j.neuropharm.2014.01.021]. [PMID: 24467848]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Dash B., Li M.D. Two rare variations, D478N and D478E, that occur at the same amino acid residue in nicotinic acetylcholine receptor (nAChR) α2 subunit influence nAChR function. Neuropharmacology. 2014;85:471–481. doi: 10.1016/j.neuropharm.2014.05.014. [http://dx.doi.org/10.1016/ j.neuropharm.2014.05.014]. [PMID: 24950454]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Li M.D., Xu Q., Lou X.Y., Payne T.J., Niu T., Ma J.Z. Association and interaction analysis of variants in CHRNA5/ CHRNA3/CHRNB4 gene cluster with nicotine dependence in African and European Americans. Am. J. Med. Genet. B. Neuropsychiatr. Genet. 2010;153B(3):745–756. doi: 10.1002/ajmg.b.31043. [PMID: 19859904]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.McClure-Begley T.D., Papke R.L., Stone K.L., Stokes C., Levy A.D., Gelernter J., Xie P., Lindstrom J., Picciotto M.R. Rare human nicotinic acetylcholine receptor α4 subunit (CHRNA4) variants affect expression and function of high-affinity nicotinic acetylcholine receptors. J. Pharmacol. Exp. Ther. 2014;348(3):410–420. doi: 10.1124/jpet.113.209767. [http://dx.doi.org/10.1124/jpet.113.209767]. [PMID: 24385388]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Sabatelli M., Eusebi F., Al-Chalabi A., Conte A., Madia F., Luigetti M., Mancuso I., Limatola C., Trettel F., Sobrero F., Di Angelantonio S., Grassi F., Di Castro A., Moriconi C., Fucile S., Lattante S., Marangi G., Murdolo M., Orteschi D., Del Grande A., Tonali P., Neri G., Zollino M. Rare missense variants of neuronal nicotinic acetylcholine receptor altering receptor function are associated with sporadic amyotrophic lateral sclerosis. Hum. Mol. Genet. 2009;18(20):3997–4006. doi: 10.1093/hmg/ddp339. [http://dx.doi.org/10. 1093/hmg/ddp339]. [PMID: 19628475]. [DOI] [PubMed] [Google Scholar]
- 227.Biaggi-Labiosa N.M., Avilés-Pagán E., Caballero-Rivera D., Báez-Pagán C.A., Lasalde-Dominicci J.A. Engineering α4β2 nAChRs with reduced or increased nicotine sensitivity via selective disruption of consensus sites in the M3-M4 cytoplasmic loop of the α4 subunit. Neuropharmacology. 2015;99:273–284. doi: 10.1016/j.neuropharm.2015.04.022. [http://dx.doi. org/10.1016/j.neuropharm.2015.04.022]. [PMID: 25957813]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Wilking J.A., Hesterberg K.G., Crouch E.L., Homanics G.E., Stitzel J.A. Chrna4 A529 knock-in mice exhibit altered nicotine sensitivity. Pharmacogenet. Genomics. 2010;20(2):121–130. doi: 10.1097/FPC.0b013e3283369347. [http:// dx.doi.org/10.1097/FPC.0b013e3283369347]. [PMID: 20061993]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Weltzin M.M., Lindstrom J.M., Lukas R.J., Whiteaker P. Distinctive effects of nicotinic receptor intracellular-loop mutations associated with nocturnal frontal lobe epilepsy. Neuropharmacology. 2016;102:158–173. doi: 10.1016/j.neuropharm.2015.11.004. [http://dx.doi.org/10.1016/j.neuropharm. 2015.11.004]. [PMID: 26561946]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Shiba Y., Mori F., Yamada J., Migita K., Nikaido Y., Wakabayashi K., Kaneko S., Okada M., Hirose S., Ueno S. Spontaneous epileptic seizures in transgenic rats harboring a human ADNFLE missense mutation in the β2-subunit of the nicotinic acetylcholine receptor. Neurosci. Res. 2015;100:46–54. doi: 10.1016/j.neures.2015.06.003. [http://dx.doi. org/10.1016/j.neures.2015.06.003]. [PMID: 26091610]. [DOI] [PubMed] [Google Scholar]
- 231.Nichols W.A., Henderson B.J., Marotta C.B., Yu C.Y., Richards C., Dougherty D.A., Lester H.A., Cohen B.N. Mutation linked to autosomal dominant nocturnal frontal lobe epilepsy reduces low-sensitivity α4β2, and increases α5α4β2, nicotinic receptor surface expression. PLoS One. 2016;11(6):e158032. doi: 10.1371/journal.pone.0158032. [http:// dx.doi.org/10.1371/journal.pone.0158032]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Kuryatov A., Olale F., Cooper J., Choi C., Lindstrom J. Human alpha6 AChR subtypes: subunit composition, assembly, and pharmacological responses. Neuropharmacology. 2000;39(13):2570–2590. doi: 10.1016/s0028-3908(00)00144-1. [http://dx.doi.org/10.1016/S0028-3908(00)00144-1]. [PMID: 11044728]. [DOI] [PubMed] [Google Scholar]
- 233.Dash B., Chang Y., Lukas R.J. Reporter mutation studies show that nicotinic acetylcholine receptor (nAChR) α5 Subunits and/or variants modulate function of α6*-nAChR. J. Biol. Chem. 2011;286(44):37905–37918. doi: 10.1074/jbc.M111.264044. [http://dx.doi.org/10.1074/jbc.M111.264044]. [PMID: 21873428]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Dash B., Lukas R.J. Modulation of gain-of-function α6*-nicotinic acetylcholine receptor by β3 subunits. J. Biol. Chem. 2012;287(17):14259–14269. doi: 10.1074/jbc.M111.322610. [http://dx.doi.org/10.1074/jbc.M111.322610]. [PMID: 22315221]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Girod R., Crabtree G., Ernstrom G., Ramirez-Latorre J., McGehee D., Turner J., Role L. Heteromeric complexes of alpha 5 and/or alpha 7 subunits. Effects of calcium and potential role in nicotine-induced presynaptic facilitation. Ann. N. Y. Acad. Sci. 1999;868:578–590. doi: 10.1111/j.1749-6632.1999.tb11331.x. [http://dx.doi.org/10.1111/j.1749-6632.1999. tb11331.x]. [PMID: 10414339]. [DOI] [PubMed] [Google Scholar]
- 236.Nelson M.E., Lindstrom J. Single channel properties of human alpha3 AChRs: impact of beta2, beta4 and alpha5 subunits. J. Physiol. 1999;516(Pt 3):657–678. doi: 10.1111/j.1469-7793.1999.0657u.x. [http://dx.doi.org/10.1111/ j.1469-7793.1999.0657u.x]. [PMID: 10200416]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Wang F., Gerzanich V., Wells G.B., Anand R., Peng X., Keyser K., Lindstrom J. Assembly of human neuronal nicotinic receptor alpha5 subunits with alpha3, beta2, and beta4 subunits. J. Biol. Chem. 1996;271(30):17656–17665. doi: 10.1074/jbc.271.30.17656. [http://dx.doi.org/10.1074/ jbc.271.30.17656]. [PMID: 8663494]. [DOI] [PubMed] [Google Scholar]
- 238.Ramirez-Latorre J., Yu C.R., Qu X., Perin F., Karlin A., Role L. Functional contributions of alpha5 subunit to neuronal acetylcholine receptor channels. Nature. 1996;380(6572):347–351. doi: 10.1038/380347a0. [http://dx.doi.org/10.1038/380347a0]. [PMID: 8598930]. [DOI] [PubMed] [Google Scholar]
- 239.Bierut L.J., Stitzel J.A., Wang J.C., Hinrichs A.L., Grucza R.A., Xuei X., Saccone N.L., Saccone S.F., Bertelsen S., Fox L., Horton W.J., Breslau N., Budde J., Cloninger C.R., Dick D.M., Foroud T., Hatsukami D., Hesselbrock V., Johnson E.O., Kramer J., Kuperman S., Madden P.A., Mayo K., Nurnberger J., Jr, Pomerleau O., Porjesz B., Reyes O., Schuckit M., Swan G., Tischfield J.A., Edenberg H.J., Rice J.P., Goate A.M. Variants in nicotinic receptors and risk for nicotine dependence. Am. J. Psychiatry. 2008;165(9):1163–1171. doi: 10.1176/appi.ajp.2008.07111711. [http://dx.doi.org/10.1176/ appi.ajp.2008.07111711]. [PMID: 18519524]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Kuryatov A., Berrettini W., Lindstrom J. Acetylcholine receptor (AChR) α5 subunit variant associated with risk for nicotine dependence and lung cancer reduces (α4β2)2α5 AChR function. Mol. Pharmacol. 2011;79(1):119–125. doi: 10.1124/mol.110.066357. [http://dx.doi.org/10.1124/mol. 110.066357]. [PMID: 20881005]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.George A.A., Lucero L.M., Damaj M.I., Lukas R.J., Chen X., Whiteaker P. Function of human α3β4α5 nicotinic acetylcholine receptors is reduced by the α5(D398N) variant. J. Biol. Chem. 2012;287(30):25151–25162. doi: 10.1074/jbc.M112.379339. [http://dx.doi.org/10.1074/jbc.M112. 379339]. [PMID: 22665477]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Li P., McCollum M., Bracamontes J., Steinbach J.H., Akk G. Functional characterization of the α5(Asn398) variant associated with risk for nicotine dependence in the α3β4α5 nicotinic receptor. Mol. Pharmacol. 2011;80(5):818–827. doi: 10.1124/mol.111.073841. [http://dx.doi.org/10.1124/ mol.111.073841]. [PMID: 21856741]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Tammimäki A., Herder P., Li P., Esch C., Laughlin J.R., Akk G., Stitzel J.A. Impact of human D398N single nucleotide polymorphism on intracellular calcium response mediated by α3β4α5 nicotinic acetylcholine receptors. Neuropharmacology. 2012;63(6):1002–1011. doi: 10.1016/j.neuropharm.2012.07.022. [http://dx.doi.org/10.1016/j.neuropharm.2012. 07.022]. [PMID: 22820273]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Dash B., Li M.D. Analysis of rare variations reveals roles of amino acid residues in the N-terminal extracellular domain of nicotinic acetylcholine receptor (nAChR) alpha6 subunit in the functional expression of human alpha6*-nAChRs. Mol. Brain. 2014;7:35. doi: 10.1186/1756-6606-7-35. [http://dx.doi.org/10.1186/1756-6606-7-35]. [PMID: 24886653]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Lukas R.J., Changeux J.P., Le Novère N., Albuquerque E.X., Balfour D.J., Berg D.K., Bertrand D., Chiappinelli V.A., Clarke P.B., Collins A.C., Dani J.A., Grady S.R., Kellar K.J., Lindstrom J.M., Marks M.J., Quik M., Taylor P.W., Wonnacott S. International Union of Pharmacology. XX. Current status of the nomenclature for nicotinic acetylcholine receptors and their subunits. Pharmacol. Rev. 1999;51(2):397–401. [PMID: 10353988]. [PubMed] [Google Scholar]
- 246.Azam L., Maskos U., Changeux J.P., Dowell C.D., Christensen S., De Biasi M., McIntosh J.M. α-Conotoxin BuIA[T5A;P6O]: a novel ligand that discriminates between α6ß4 and α6ß2 nicotinic acetylcholine receptors and blocks nicotine-stimulated norepinephrine release. FASEB J. 2010;24(12):5113–5123. doi: 10.1096/fj.10-166272. [http://dx. doi.org/10.1096/fj.10-166272]. [PMID: 20739611]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Hone A.J., Meyer E.L., McIntyre M., McIntosh J.M. Nicotinic acetylcholine receptors in dorsal root ganglion neurons include the α6β4* subtype. FASEB J. 2012;26(2):917–926. doi: 10.1096/fj.11-195883. [http://dx.doi.org/ 10.1096/fj.11-195883]. [PMID: 22024738]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Dash B., Bhakta M., Chang Y., Lukas R.J. Identification of N-terminal extracellular domain determinants in nicotinic acetylcholine receptor (nAChR) α6 subunits that influence effects of wild-type or mutant β3 subunits on function of α6β2*- or α6β4*-nAChR. J. Biol. Chem. 2011;286(44):37976–37989. doi: 10.1074/jbc.M111.263673. [http://dx. doi.org/10.1074/jbc.M111.263673]. [PMID: 21832048]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Pérez-Alvarez A., Hernández-Vivanco A., McIntosh J.M., Albillos A. Native α6β4* nicotinic receptors control exocytosis in human chromaffin cells of the adrenal gland. FASEB J. 2012;26(1):346–354. doi: 10.1096/fj.11-190223. [http://dx.doi.org/10.1096/fj.11-190223]. [PMID: 21917987]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Drenan R.M., Nashmi R., Imoukhuede P., Just H., McKinney S., Lester H.A. Subcellular trafficking, pentameric assembly, and subunit stoichiometry of neuronal nicotinic acetylcholine receptors containing fluorescently labeled alpha6 and beta3 subunits. Mol. Pharmacol. 2008;73(1):27–41. doi: 10.1124/mol.107.039180. [http://dx.doi.org/10.1124/mol. 107.039180]. [PMID: 17932221]. [DOI] [PubMed] [Google Scholar]
- 251.Cox B.C., Marritt A.M., Perry D.C., Kellar K.J. Transport of multiple nicotinic acetylcholine receptors in the rat optic nerve: high densities of receptors containing alpha6 and beta3 subunits. J. Neurochem. 2008;105(5):1924–1938. doi: 10.1111/j.1471-4159.2008.05282.x. [http://dx.doi.org/10.1111/j. 1471-4159.2008.05282.x]. [PMID: 18266937]. [DOI] [PubMed] [Google Scholar]
- 252.Tumkosit P., Kuryatov A., Luo J., Lindstrom J. Beta3 subunits promote expression and nicotine-induced up-regulation of human nicotinic alpha6* nicotinic acetylcholine receptors expressed in transfected cell lines. Mol. Pharmacol. 2006;70(4):1358–1368. doi: 10.1124/mol.106.027326. [http://dx.doi.org/10.1124/mol.106.027326]. [PMID: 16835356]. [DOI] [PubMed] [Google Scholar]
- 253.Broadbent S., Groot-Kormelink P.J., Krashia P.A., Harkness P.C., Millar N.S., Beato M., Sivilotti L.G. Incorporation of the beta3 subunit has a dominant-negative effect on the function of recombinant central-type neuronal nicotinic receptors. Mol. Pharmacol. 2006;70(4):1350–1357. doi: 10.1124/mol.106.026682. [http://dx.doi.org/10.1124/mol.106. 026682]. [PMID: 16822928]. [DOI] [PubMed] [Google Scholar]
- 254.Brown R.W., Collins A.C., Lindstrom J.M., Whiteaker P. Nicotinic alpha5 subunit deletion locally reduces high-affinity agonist activation without altering nicotinic receptor numbers. J. Neurochem. 2007;103(1):204–215. doi: 10.1111/j.1471-4159.2007.04700.x. [PMID: 17573823]. [DOI] [PubMed] [Google Scholar]
- 255.Dash B., Bhakta M., Chang Y., Lukas R.J. Modulation of recombinant, α2*, α3* or α4*-nicotinic acetylcholine receptor (nAChR) function by nAChR β3 subunits. J. Neurochem. 2012;121(3):349–361. doi: 10.1111/j.1471-4159.2012.07685.x. [http://dx.doi.org/10.1111/j.1471-4159.2012. 07685.x]. [PMID: 22309577]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Jackson K.J., Marks M.J., Vann R.E., Chen X., Gamage T.F., Warner J.A., Damaj M.I. Role of alpha5 nicotinic acetylcholine receptors in pharmacological and behavioral effects of nicotine in mice. J. Pharmacol. Exp. Ther. 2010;334(1):137–146. doi: 10.1124/jpet.110.165738. [http://dx. doi.org/10.1124/jpet.110.165738]. [PMID: 20400469]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Kuryatov A., Onksen J., Lindstrom J. Roles of accessory subunits in alpha4beta2(*) nicotinic receptors. Mol. Pharmacol. 2008;74(1):132–143. doi: 10.1124/mol.108.046789. [http://dx.doi.org/10.1124/mol.108.046789]. [PMID: 18381563]. [DOI] [PubMed] [Google Scholar]
- 258.Dash B., Li M.D., Lukas R.J. Roles for N-terminal extracellular domains of nicotinic acetylcholine receptor (nAChR) β3 subunits in enhanced functional expression of mouse α6β2β3- and α6β4β3-nAChRs. J. Biol. Chem. 2014;289(41):28338–28351. doi: 10.1074/jbc.M114.566018. [http://dx. doi.org/10.1074/jbc.M114.566018]. [PMID: 25028511]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.King J.R., Nordman J.C., Bridges S.P., Lin M.K., Kabbani N. Identification and characterization of a G protein-binding cluster in α7 nicotinic acetylcholine receptors. J. Biol. Chem. 2015;290(33):20060–20070. doi: 10.1074/jbc.M115.647040. [http://dx.doi.org/10.1074/jbc.M115.647040]. [PMID: 26088141]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Colón-Sáez J.O., Yakel J.L. A mutation in the extracellular domain of the α7 nAChR reduces calcium permeability. Pflugers Arch. 2014;466(8):1571–1579. doi: 10.1007/s00424-013-1385-y. [http://dx.doi.org/10.1007/s00424-013-1385-y]. [PMID: 24177919]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.King J.R., Kabbani N. Alpha 7 nicotinic receptor coupling to heterotrimeric G proteins modulates RhoA activation, cytoskeletal motility, and structural growth. J. Neurochem. 2016;138(4):532–545. doi: 10.1111/jnc.13660. [http://dx.doi.org/10.1111/jnc.13660]. [PMID: 27167578]. [DOI] [PubMed] [Google Scholar]
- 262.Moriconi C., Di Angelantonio S., Piccioni A., Trettel F., Sabatelli M., Grassi F. Mutant human β4 subunit identified in amyotrophic lateral sclerosis patients impairs nicotinic receptor function. Pflugers Arch. 2011;461(2):225–233. doi: 10.1007/s00424-010-0905-2. [http://dx.doi.org/ 10.1007/s00424-010-0905-2]. [PMID: 21107856]. [DOI] [PubMed] [Google Scholar]
- 263.Rasmussen A.H., Strøbæk D., Dyhring T., Jensen M.L., Peters D., Grunnet M., Timmermann D.B., Ahring P.K. Biophysical and pharmacological characterization of α6-containing nicotinic acetylcholine receptors expressed in HEK293 cells. Brain Res. 2014;1542:1–11. doi: 10.1016/j.brainres.2013.10.024. [http://dx.doi.org/10.1016/j.brainres.2013.10.024]. [PMID: 24157862]. [DOI] [PubMed] [Google Scholar]
- 264.Chavez-Noriega L.E., Crona J.H., Washburn M.S., Urrutia A., Elliott K.J., Johnson E.C. Pharmacological characterization of recombinant human neuronal nicotinic acetylcholine receptors h alpha 2 beta 2, h alpha 2 beta 4, h alpha 3 beta 2, h alpha 3 beta 4, h alpha 4 beta 2, h alpha 4 beta 4 and h alpha 7 expressed in Xenopus oocytes. J. Pharmacol. Exp. Ther. 1997;280(1):346–356. [PMID: 8996215]. [PubMed] [Google Scholar]
- 265.Luetje C.W., Patrick J. Both alpha- and beta-subunits contribute to the agonist sensitivity of neuronal nicotinic acetylcholine receptors. J. Neurosci. 1991;11(3):837–845. doi: 10.1523/JNEUROSCI.11-03-00837.1991. [PMID: 1705971]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Luetje C.W. Getting past the asterisk: the subunit composition of presynaptic nicotinic receptors that modulate striatal dopamine release. Mol. Pharmacol. 2004;65(6):1333–1335. doi: 10.1124/mol.65.6.1333. [http://dx.doi.org/ 10.1124/mol.65.6.1333]. [PMID: 15155826]. [DOI] [PubMed] [Google Scholar]
- 267.Mackay T.F., Stone E.A., Ayroles J.F. The genetics of quantitative traits: challenges and prospects. Nat. Rev. Genet. 2009;10(8):565–577. doi: 10.1038/nrg2612. [http://dx.doi.org/10.1038/nrg2612]. [PMID: 19584810]. [DOI] [PubMed] [Google Scholar]
- 268.Mackay T.F. The genetic architecture of quantitative traits. Annu. Rev. Genet. 2001;35:303–339. doi: 10.1146/annurev.genet.35.102401.090633. [http://dx.doi.org/10.1146/annurev. genet.35.102401.090633]. [PMID: 11700286]. [DOI] [PubMed] [Google Scholar]
- 269.Fenster C.P., Whitworth T.L., Sheffield E.B., Quick M.W., Lester R.A. Upregulation of surface alpha4beta2 nicotinic receptors is initiated by receptor desensitization after chronic exposure to nicotine. J. Neurosci. 1999;19(12):4804–4814. doi: 10.1523/JNEUROSCI.19-12-04804.1999. [PMID: 10366615]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Picciotto M.R., Addy N.A., Mineur Y.S., Brunzell D.H. It is not “either/or”: activation and desensitization of nicotinic acetylcholine receptors both contribute to behaviors related to nicotine addiction and mood. Prog. Neurobiol. 2008;84(4):329–342. doi: 10.1016/j.pneurobio.2007.12.005. [http://dx.doi. org/10.1016/j.pneurobio.2007.12.005]. [PMID: 18242816]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Williams D.K., Wang J., Papke R.L. Positive allosteric modulators as an approach to nicotinic acetylcholine receptor-targeted therapeutics: advantages and limitations. Biochem. Pharmacol. 2011;82(8):915–930. doi: 10.1016/j.bcp.2011.05.001. [http://dx.doi.org/10.1016/j.bcp.2011.05.001]. [PMID: 21575610]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Mantione E., Micheloni S., Alcaino C., New K., Mazzaferro S., Bermudez I. Allosteric modulators of α4β2 nicotinic acetylcholine receptors: a new direction for antidepressant drug discovery. Future Med. Chem. 2012;4(17):2217–2230. doi: 10.4155/fmc.12.172. [http://dx.doi.org/10. 4155/fmc.12.172]. [PMID: 23190109]. [DOI] [PubMed] [Google Scholar]