Abstract
Non-alcoholic fatty liver disease (NAFLD), the most common cause of liver disease, affects approximately 75 to 100 million Americans. Patients with concurrent NAFLD and type 2 diabetes mellitus have a higher risk of progressing to advanced fibrosis and non-alcoholic steatohepatitis compared to non-diabetics. Lifestyle modifications, including weight loss, remain the mainstay of treatment for NAFLD, as there are no medications currently indicated for this disease state. Anti-diabetic pharmacologic therapies aimed at improving insulin sensitivity and decreasing insulin production have been studied to determine their potential role in slowing the progression of NAFLD. In this review, we focus on the evidence surrounding anti-diabetic medications and their ability to improve disease progression in patients with NAFLD.
Keywords: Non-alcoholic fatty liver disease, Non-alcoholic steatohepatitis, Diabetes mellitus
Introduction
Non-alcoholic fatty liver disease (NAFLD), the most common cause of liver disease, affects approximately 75 to 100 million Americans.1,2 The hepatic injury resulting from NAFLD ranges from intrahepatic accumulation of fat (steatosis or non-alcoholic fatty liver) to necrotic inflammation (non-alcoholic steatohepatitis; NASH).3 Although NAFLD rarely progresses to advanced disease, approximately 20% of patients with NASH will develop progressive fibrosis, cirrhosis, and hepatocellular carcinoma (HCC).3,4 Due to advances in the treatment of hepatitis C virus and the rising rate of obesity, NASH is predicted to become the leading indication for liver transplantation in the United States within the next 5 to 15 years.5
The pathogenesis of NAFLD is a multifactorial and complex process. The intrahepatic regulation of free fatty acid uptake, synthesis, degradation, and secretion is altered, which leads to accumulation of triglycerides in the hepatocytes. These changes in morphology cause the liver to be susceptible to injury from inflammatory responses which aid in the progression of the disease. Additionally, there is a strong association between NAFLD and insulin resistance. Studies have demonstrated a decrease in whole body insulin sensitivity, as well as increased insulin resistance in hepatic and adipose tissues.6 This diminished response to insulin within adipocytes leads to increased free fatty acids flux to the liver, which may contribute to hepatic steatosis.6
There is a strong relationship between NAFLD and the components of metabolic syndrome, including type 2 diabetes mellitus (T2DM).7 Up to 70% of patients with T2DM may have concurrent NAFLD and studies have demonstrated that these patients are at higher risk of progression to NASH and advanced fibrosis.8–10 Moreover, NAFLD increases cardiovascular disease risk independent of traditional risk factors.11
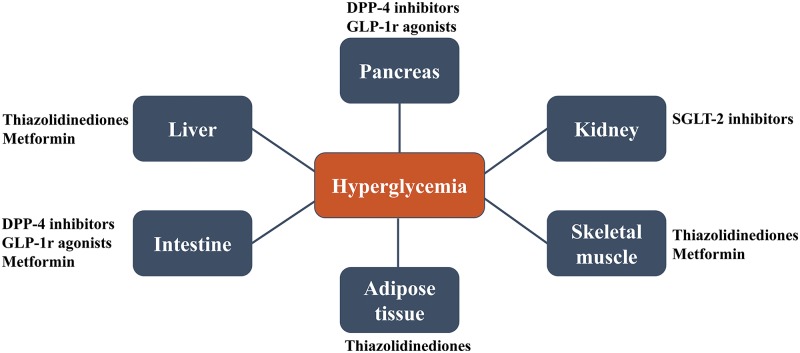
Recent recommendations for diabetic patients include increased screening and early interventions for NASH.12 Lifestyle changes, including weight loss, remain the mainstay of treatment for NAFLD, as there are no medications currently approved by the United States Food and Drug Administration (FDA) for this disease.13,14 Drug therapies would ideally decrease the disease activity score and delay the progression of fibrosis. Current treatment options target the mechanisms leading to NAFLD development and progression. Therefore, diabetes medications aimed at improving insulin sensitivity and decreasing insulin production have been extensively studied to evaluate their ability to slow the progression of NAFLD. Various studies have focused on the use of anti-diabetic therapies with different mechanisms of action (Fig. 1) for NAFLD, including metformin, thiazolidinediones (TZDs), glucagon-like peptide 1 receptor (GLP-1r) agonists, dipeptidyl peptidase 4 (DPP-4) inhibitors, and sodium/glucose cotransporter 2 (SGLT2) inhibitors. In this review, we focus on the evidence surrounding these agents and their ability to improve disease progression in patients with NAFLD.
Fig. 1. Site of action of anti-diabetic medications in NASH/NAFLD.
Metformin
Due to its ability to ameliorate hyperglycemia by improving peripheral sensitivity to insulin, reducing gastrointestinal glucose absorption and hepatic glucose production, metformin has been utilized as first-line therapy for the treatment of T2DM for several decades. As insulin resistance appears to have a strong role in the pathogenesis of NAFLD, therapeutic options to enhance insulin sensitivity in this patient population have been of interest to researchers. Several studies have assessed the potential role of metformin to alter the progression of NAFLD.
In 2004, Nair et al.15 conducted an open label study in patients with histologically-confirmed NAFLD, in which patients were given metformin (20 mg/kg, maximum of 2 g) for 48 weeks. During the treatment period, laboratory parameters including alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase, serum bilirubin, international normalized ratio, gamma glutamyl transferase, and blood glucose levels were assessed at 12-week intervals. Fifteen patients (age 51±12 years; six females) completed 1 year of treatment, and 10 underwent a post-treatment biopsy. The authors noted improvements in ALT and AST, and in insulin sensitivity. However, no additional improvement in insulin sensitivity was seen after 3 months and aminotransferases gradually increased to pre-treatment levels. Among patients with post-treatment biopsy, three (33%), showed improvement in steatosis and only one (10%) showed improvement in fibrosis.15 A study by Haukeland et al.16 yielded similar results in a randomized placebo-controlled trial of 48 patients with biopsy-proven NAFLD. Patients were treated with either metformin (n = 24) or placebo (n = 24) for 6 months. There were no significant differences between groups for liver steatosis, which was assessed either histologically or by computed tomography (CT). The metformin group experienced reductions in serum lipid and glucose levels, therefore the authors suggested that metformin may still benefit patients with NAFLD.16
Additionally, the use of metformin in combination with lifestyle modifications versus lifestyle changes alone has been studied by various authors. Ugyn et al.17 assessed the ability of metformin to attenuate the necrotizing inflammatory process of NAFLD by comparing sonographic and histological parameters in 36 patients who utilized dietary modifications versus those who received metformin 850 mg twice daily in addition to dietary modifications for 6 months. Although there was some improvement in necroinflammatory activity in the metformin group, it was not statistically significant and, more importantly, there was no difference in fibrosis between the two groups.17 Similarly, a small randomized placebo-controlled trial including 19 patients compared metformin plus dietary changes and exercise to diet and exercise alone in non-diabetic patients with insulin resistance and NAFLD.18 There was no significant difference in histopathology between groups on follow-up liver biopsy at 12 months. Therefore, due to the lack of evidence demonstrating significant histological improvement, metformin is not currently recommended for the treatment of liver disease in patients with NAFLD.13,14
Thiazolidinediones (TZDs)
TZDs activate peroxisome proliferator-activated receptor γ, which improves insulin sensitivity in the liver, muscle and adipose tissue.19 TZDs also increase adiponectin levels, which counter the effects of tumor necrosis factor-α and promote oxidation of fatty acids. This oxidation coupled with decreased lipogenesis decrease gluconeogenesis.19
The TZDs, including rosiglitazone and pioglitazone, have been well studied for the treatment of NASH. Ratziu et al.20 conducted the FLIRT trial, which compared 12 months of rosiglitazone (titrated to 8 mg/day) to placebo in 63 NASH patients. Normalization of ALT by the end of treatment was higher in the rosiglitazone group versus placebo (38% vs. 7%, respectively; p = 0.005), but these levels returned to baseline 4 months after the end of treatment. More patients treated with rosiglitazone had greater than 30% reduction in steatosis compared to placebo (47% vs. 16%, respectively; p = 0.014), but there were no significant improvements in other histological parameters.20 A 2-year extension study of the FLIRT trial was conducted to determine if prolonged rosiglitazone treatment improved histological response. This study, however, found no additional improvement in steatosis after 12 months of therapy and no effects on inflammation and liver injury.21
A randomized placebo-controlled trial comparing 6 months of a hypocaloric diet plus pioglitazone (titrated to 45 mg/day) to a hypocaloric diet plus placebo was conducted in 55 patients with biopsy-proven NASH and either pre-diabetes or T2DM. When compared to the placebo arm, patients treated with diet plus pioglitazone had decreased hepatic fat content, improved insulin sensitivity, and normalization of liver aminotransferase levels. While the pioglitazone group experienced improvements in inflammation, ballooning necrosis, and steatosis compared to placebo, there was no significant difference in reduction of fibrosis between the groups.22 The PIVENS trial was a large double-blind, placebo-controlled trial that compared the effects of pioglitazone to vitamin E in NASH patients without diabetes.23 The 247 patients included were randomized to one of the three following groups for 96 weeks of treatment: pioglitazone 30 mg/day; vitamin E 800 IU/day; or placebo. The primary outcome was a composite endpoint which included improvement in the NAFLD activity score (NAS), improvement in hepatocellular ballooning, and no increase in fibrosis. More patients receiving pioglitazone compared to placebo achieved the primary composite endpoint (34% vs. 19%; p = 0.004), but the difference between these two groups did not reach the pre-specified 0.025 level of significance. Although pioglitazone did not significantly reduce fibrosis, resolution of NASH was found in 47% of patients treated with pioglitazone compared to 21% with placebo (p = 0.001). Similarly, a recent meta-analysis reviewing 8 randomized controlled trials found that pioglitazone is associated with resolution of NASH (odds ratio; OR: 3.22, 95% confidence interval; CI: 2.17–4.79; p < 0.001). This study also found that pioglitazone improves fibrosis of any stage (OR: 1.66, 95% CI: 1.12–2.47; p = 0.01), including advanced fibrosis, with similar results in patients without diabetes.24
Based on the histological improvements seen with pioglitazone therapy, the American Association for the Study of Liver Diseases (AASLD) and European Association for the Study of Liver (EASL) guidelines recommend that this medication can be considered for use in patients with biopsy-proven NASH.13,14 However, use of pioglitazone is limited by the lack of sufficient data evaluating the long-term efficacy and safety in this patient population. Long-term effects are still debatable, largely due to different intervention strategies, reporting methods, and histological scoring.25 The side effect profile of pioglitazone remains a significant concern and may limit its use. TZDs are known to cause weight gain, sodium and water retention, and osteoporosis and bone fractures, especially in post-menopausal women. Additionally, these medications have been associated with an increased risk of cardiovascular events.26
Glucagon-like peptide 1 (GLP-1) receptor (GLP-1r) agonists
GLP-1 is a naturally occurring gastrointestinal hormone secreted by the enteroendocrine L cells of the distal small intestine and proximal colon that binds to GLP-1r expressed in various organs, including the liver.27,28 Its primary function is to regulate blood glucose in systemic and splanchnic vessels via stimulation of glucose-dependent insulin secretion and inhibition of glucagon secretion.29,30 In addition to insulin regulation, GLP-1r agonists approximately double the gastric emptying time and enhance early satiety, thus leading to weight loss in a majority of treated patients.31,32 GLP-1r agonists have also been found to reverse hepatic steatosis in mice by suppressing key regulatory genes in hepatocytes that are associated with NASH.33 Due to its degradation by dipeptidyl peptidase-4, GLP-1 has a short half-life. Synthetic GLP-1r agonists were first tested in 2001 and approved for treatment of T2DM in the United States in April 2005.34,35 Current FDA approved GLP-1r agonists include exenatide, liraglutide, exenatide extended-release, albiglutide, and lixisenatide.
Armstrong and colleagues36 performed a meta-analysis of six, 26-week, phase III, randomized controlled trials encompassing the LEAD program to evaluate the effect of liraglutide on liver parameters compared to active placebo in patients with T2DM. Of the 4442 patients analyzed, 50.8% had baseline ALT abnormality. Liraglutide 1.8 mg/day led to significant reductions in ALT compared to placebo (p = 0.003). Although differences in hepatic steatosis on CT imaging were not statistically significant, a sub-study found that liraglutide 1.8 mg/day trended towards improving hepatic steatosis when compared to placebo. However, both effects were lost after adjusting for weight reduction and hemoglobin A1C (HbA1C), which suggests that liraglutide’s hepatic effects correlate to changes in weight and glycemic control.36 To further evaluate the safety and efficacy of liraglutide treatment in patients with NASH, the authors of the LEAN study conducted a double-blinded, randomized, placebo-controlled phase 2 trial at four medical centers in the United Kingdom. Overweight NASH patients with and without diabetes were treated with 48 weeks of liraglutide 1.8 mg/day or placebo. The primary endpoint was resolution of steatohepatitis without worsening of fibrosis from baseline. Nine of 23 (39%) patients in the liraglutide group achieved the primary outcome compared to 2 of 22 (9%) in the placebo group (p = 0.019). In addition, 9% of patients in the liraglutide group had progression of fibrosis compared to 36% of patients in the placebo group (p = 0.04).37
The effects of exenatide on hepatic biomarkers were also studied in 217 patients with T2DM. Patients from three placebo-controlled trials were enrolled into one open-label extension trial. Patients received exenatide 5 to 10 mcg twice daily in addition to metformin and/or sulfonylureas for at least 3 years. In patients with elevated ALT at baseline, ALT and AST levels improved by week 156, with 41% of patients achieving normalization of ALT after 3 years of treatment. Of these patients with elevated ALT at baseline, the 25% with the greatest weight reduction also experienced the largest decrease in ALT and AST. The remaining 75% had similar ALT reductions independent of weight loss or change in HbA1C.38 Garcia et al.39 conducted an observational, pilot study that compared treatment with exenatide, liraglutide, or other anti-diabetic agents for 6 months on laboratory and ultrasonographic markers of NAFLD in T2DM patients. All 58 patients included in the study were on metformin and 57.8% had NAFLD at baseline. There was ultrasonographic improvement in 80% of the patients receiving exenatide compared to 33% with liraglutide, 33.3% with gliclazide, 37.5% with pioglitazone, and 45.5% with sitagliptin (p = 0.28).39 Shao et al.40 further evaluated 12-weeks exenatide for the treatment of NAFLD in 60 obese patients with elevated liver enzymes and T2DM. Subjects were randomized to exenatide (titrated to 10 mcg twice daily) plus insulin glargine or the intensive insulin therapy group (insulin aspart plus insulin glargine). Exenatide treatment led to a significant decrease in weight and hepatic biomarkers compared to the intensive insulin group (p < 0.001). Similar to previous findings, the decrease in ALT and AST correlated with the degree of weight loss. Exenatide also resulted in a higher reversal rate of fatty liver (93.3%) in comparison to the intensive insulin group (66.7%) (p < 0.01).40
The remaining GLP-1r agonists have only recently been approved and therefore limited data is available regarding their effects on NAFLD. A retrospective study evaluated the efficacy and safety of dulaglutide 0.75 mg weekly for 12 weeks in 15 NAFLD patients with T2DM refractory to dietary interventions. There was a significant decrease from baseline in transaminases and liver stiffness, in addition to body weight and HbA1C.41 However, additional placebo-controlled or head to head trials are required to investigate these newer agents.
Dipeptidyl peptidase-4 (DPP-4) inhibitors
DPP-4 is an enzyme that breaks down bioactive peptides, including glucose-dependent insulinotropic polypeptide (GIP) and GLP-1, and renders them inactive; therefore, its inhibition increases insulin secretion and suppresses glucagon release from the pancreas. DPP-4 inhibitors were developed after the identification of the therapeutic effects of GLP-1, in order to delay its quick inactivation in plasma and thereby increase the incretin effect. Four DPP-4 inhibitors (sitagliptin, saxagliptin, linagliptin, and alogliptin) are currently available in the United States and many other countries. Vildagliptin is available in several countries but not in the United States. In patients with NAFLD, the hepatic expression of DPP-4 is increased significantly, when compared to normal subjects.42 Serum DPP-4 activity and hepatic DPP-4 expression are correlated with NAFLD grading.43
Most of the studies evaluating DPP-4 inhibitors in NAFLD have utilized sitagliptin due to its widespread use and because it was the earliest DPP-4 inhibitor available on the market. Earlier trials evaluated the effect of DPP-4 inhibitors on liver enzymes in patients with T2DM and NAFLD. Initially, Iwasaki et al.44 found that 4 months of treatment with sitagliptin 50 mg/day in 30 NAFLD patients was associated with significant decreases in AST, ALT and γ-GTP levels, in addition to improvement in the parameters of diabetes. In an open-label, single arm observational pilot study of 15 patients conducted by Yilmaz et al.45, treatment with sitagliptin for 1 year was associated with significant reduction in NASH scores and a trend towards improved hepatic steatosis. Significant reductions in AST and ALT levels and body mass index were also observed.45 Two retrospective reviews of patients with T2DM and liver dysfunction also showed that treatment with DPP-4 inhibitors was associated with improvements in liver enzymes. Although both studies included different types of chronic liver injury, the majority of these patients had NAFLD.46 In contrast, a study conducted by Fukuhara and colleagues47 in 44 patients with biopsy-proven NAFLD followed for 12 months demonstrated that liver transaminases did not change significantly during treatment with sitagliptin despite a reduction in HbA1C levels. Similarly, no significant changes in liver enzymes were observed with sitagliptin treatment during 48 weeks of follow-up in a case-control study conducted by Arase and colleagues.48
More recently, two randomized, double-blinded, placebo-controlled studies evaluated the effect of sitagliptin on histologic and non-histologic parameters of NASH. Cui and colleagues49 included 50 NAFLD patients with pre-diabetes or early stages of diabetes randomized to sitagliptin 100 mg/day versus placebo and followed for 24 weeks. There was not a statistically significant difference between the two groups in liver fat reduction, as measured by magnetic resonance imaging (MRI)-based biomarker of proton density-fat fraction (MRI-PDFF) in several liver segments (mean difference between the two groups: −1.3%; p = 0.4). End-of-treatment MRI-PDFF was also not different between the two groups, when compared to baseline [sitagliptin (18.1% to 16.9%; p = 0.27); placebo (16.6% to 14.0%; p = 0.07)]. Other biomarkers, such as changes in ALT, AST, low-density lipoprotein, insulin resistance as measured by homeostasis model, and MRE-derived liver stiffness, were not different between groups.49 In another trial conducted by Joy et al.,50 12 patients with biopsy-proven NASH were randomized to sitagliptin 100 mg/day (n = 6) versus placebo (n = 6) and followed for 24 weeks. At the end of the trial period, there was no difference between the groups in reduction of liver fibrosis score, as measured on liver biopsy. Also, secondary histologic outcomes of NAS or the individual components of NAS (hepatocyte ballooning, lobular inflammation, and steatosis) did not differ between the two groups. However, sitagliptin use was associated with improved HbA1C and a trend towards improved triglyceride and adiponectin levels. No significant changes in liver enzymes or other biomarkers were found.50
Other DPP-4 inhibitors studied include alogliptin, which showed a decrease in NASH, ferritin, insulin, type 4 collagen 7S (NAFIC) score in a single arm, multi-center, non-randomized study of NAFLD patients with T2DM followed for 12 months.51 In another double-blind, placebo-controlled trial of 44 patients with T2DM on a stable metformin regimen, the use of vildagliptin was associated with a 27% decrease in mean fasting liver triglyceride levels and improvements in ALT regardless of any changes in body weight. No changes in peripheral insulin sensitivity were observed.52
In summary, current evidence suggests that DPP-4 inhibitors do not improve histologic features of NAFLD/NASH. However, DPP-4 inhibitors appear to be well-tolerated in this patient population, improve glycemic control as measured through HbA1C, and may improve liver enzymes as demonstrated in some of the studies. Further randomized, placebo-controlled trials of larger sample size over longer follow-up periods are needed to assess the role of DPP-4 inhibitors in patients with T2DM and NAFLD.
Sodium/glucose cotransporter-2 (SGLT2) inhibitors
The SGLT2 transporters are expressed in proximal renal tubules and are responsible for a majority of glucose reabsorption from the tubular lumen. The gliflozins represent a newer class of oral anti-diabetic drugs that inhibit SGLT2, thereby promoting urinary glucose excretion. These medications have been shown to reduce body weight and blood pressure with a low risk of hypoglycemia. The reduction of glucose without impacting insulin secretion allows these medications to be used alone or in combination with other agents that may cause hypoglycemia, such as insulin and sulfonylureas.
The benefits of SGLT2 inhibitors in the prevention or reversal of NAFLD have been demonstrated in animal and human studies. Ipragliflozin is an SGLT2 inhibitor currently available in Japan, but not in the United States. This agent improved hepatic steatosis and prevented hepatic triglyceride accumulation and fibrosis in mice models. The human studies in patients with T2DM demonstrated that treatment with SGLT2 inhibitors led to a decrease in serum ALT levels.53–55 Studies evaluating the efficacy of SGLT2 inhibitors in NAFLD patients are limited. Seko et al.56 conducted a retrospective study comparing 24-weeks of treatment with ipragliflozin 50 mg or canagliflozin 300 mg to sitagliptin in T2DM patients with biopsy-proven NAFLD. Both groups had significant improvements in serum AST and ALT levels from baseline. The SGLT2 inhibitor group also experienced significant weight loss compared to the DPP-4 inhibitor group.56 Ohki et al.57 conducted a retrospective review of Japanese NAFLD patients with T2DM who had abnormal ALT levels despite treatment with GLP-1r agonists or DPP-4 inhibitors. When ipragliflozin was added as second-line treatment, there was a significant decrease in ALT (62 to 38 IU/L; p < 0.01), with 58.3% of patients achieving normalization of ALT levels. The addition of an SGLT2 inhibitor also improved the fibrosis-4 index, from 1.75 to 1.39 (p = 0.04).57 A 21-patient investigation by Takase et al.58 showed that steatosis, measured by the fatty liver index, improved after 16 weeks of ipragliflozin therapy. Patients also had reductions in fat mass, visceral adipose tissue, and subcutaneous adipose tissue, but there was no correlation in these changes with fatty liver index.58
Data evaluating the long-term safety and tolerability of this drug class are lacking, due to their recent introduction to the market, with dapagliflozin receiving the earliest FDA approval in 2013. In 2015, the FDA released a warning cautioning prescribers to screen for urinary tract infections in patients on SGLT2 inhibitors. The warning was based on 19 reported cases of urosepsis or pyelonephritis requiring hospitalization, with some patients also requiring hemodialysis. In a pooled safety analysis of 12 randomized, placebo-controlled trials, Johnson and colleagues59 found an increased risk of urinary tract infections with dapagliflozin compared to placebo. A similar increase was observed among patients treated with empagliflozin and canagliflozin.60–62 Additional trials need to be conducted evaluating the histological effects of SGLT2 inhibitors to determine their role in the treatment of NAFLD. Similar to the other agents being investigated, long-term safety and efficacy data should also be considered in this patient population.
Farnesoid X receptor (FXR) agonist
FXR is a bile acid nuclear receptor that plays a role in lipoprotein and glucose metabolism, hepatic regeneration, and regulating hepatic inflammation.63 Mouse models deficient in FXR have increasingly developed NASH and HCC.64,65 Studies in humans have also found decreased FXR expression in patients with NAFLD.66 Obeticholic acid (OCA) is an FXR receptor agonist that is a semi-synthetic variant of the natural bile acid chenodeoxycholic acid.67 Although this agent is not approved for the treatment of T2DM, it has been studied in patients with T2DM and NAFLD.
A randomized, double-blind, placebo-controlled, phase 2 trial was conducted in NAFLD patients with T2DM over 6 weeks to evaluate OCA effects on insulin resistance and hepatic steatosis. When given a low-dose insulin infusion, insulin sensitivity increased by 28% in patients treated with OCA 25 mg and 20.1% in those treated with OCA 50 mg compared to a 5.5% decrease in the placebo group. The OCA 25 mg group also experienced a significant decrease in markers of liver fibrosis.68 Based on these results, a phase 2, randomized, double-blind, placebo-controlled investigation called the FLINT trial explored the effect of 72-week OCA therapy in 283 biopsy-proven NAFLD patients. The primary outcome was improvement in liver histology, defined as a decrease in NAS by at least 2 points without worsening of fibrosis. Remarkably, 45.4% of patients in the OCA group experienced histological improvement compared to 21.1% in the placebo group (p = 0.0002). The mean change in NAS was greater in the OCA arm compared to placebo (−1.7 vs. −0.7; p < 0.0001). Despite these improvements, there was no significant difference in the resolution of definite NASH (22% in the OCA group vs. 13% in the placebo group; p = 0.08). OCA also significantly decreased ALT and AST levels during treatment when compared to placebo, but there was no significant difference in levels at 24 weeks after treatment discontinuation.69 Another remarkable result from this trial which was overlooked was the different histological responses to OCA between diabetic and non-diabetic patients. Liver histology for diabetic patients improved in 53% of the OCA group and 19% for placebo (OR: 4.6, 95% CI: 2.0–10.6; p = 0.0003), while for non-diabetic patients liver histology improved in 37% of patients in the OCA group compared to 23% with placebo (OR: 2.0, 95% CI: 0.8–4.7; p = 0.12).70 These results suggest that OCA may only provide beneficial effects on NAFLD in patients with T2DM.
Conclusions
Due to the obesity epidemic in the United States, end-stage liver disease due to NASH is predicted to become the leading indication for liver transplantation over the next decade. Compared to non-diabetics, NAFLD patients with concomitant T2DM are at increased risk of progression to advanced fibrosis and NASH. While lifestyle modifications remain the mainstay of treatment for NAFLD and T2DM, patients often have difficulty achieving and sustaining adequate weight loss. Due to various shared pathogenic mechanisms leading to the development of NAFLD and T2DM, anti-diabetic agents are potential treatment options for the management of both disease states. Based on the current literature, TZD and GLP-1r agonists are the only anti-hyperglycemics with histological improvement of NASH (Table 1). However, the addition of SGLT2 inhibitors may improve liver enzymes and promote weight loss. Continued research and additional randomized controlled trials are warranted in this evolving landscape to develop newer agents to prevent disease progression and/or result in resolution of NASH. Additionally, these studies should focus on evaluating the long-term efficacy and safety of these therapeutic agents in NASH patients.
Table 1. Comparison of anti-diabetic medications in NASH/NAFLD patients.
| Drug class | Body weight | Body fat | AST/ALT | Liver histology | Cost | Pros | Cons |
| Biguanides, metformin | Decreased | Decreased | ? | ? | Low | 1st line therapy for DM, CV benefit | GI side effects Lactic acidosis |
| Thiazolidinediones | Increased | Increased | Decreased | Decreased | Low | Evidence exists for NASH | Weight gain Edema/ heart failure |
| GLP-1r agonists | Decreased | Decreased | Decreased | Decreased/Unchanged | High | Evidence exists for NASH | Injectable |
| DPP-4 inhibitors | Unchanged | Unchanged | Decreased/Unchanged | Unchanged | High | Well tolerated | Lacks evidence for NASH/NAFLD |
| SGLT2 inhibitors | Decreased | Decreased | Decreased | ? | High | Reduced CV outcome | Genitourinary infections |
Abbreviations: CV, cardiovascular; DM, diabetes mellitus; DPP-4, dipeptidyl peptidase-4 inhibitors; GI, gastrointestinal; GLP-1r agonists, glucagon-like peptide 1 receptor agonists; NAFLD, non-alcoholic fatty liver disease; NASH, non-alcoholic steatohepatitis; SGLT2, sodium/glucose cotransporter-2 inhibitors.
Abbreviations
- AASLD
American Association for the Study of Liver Diseases
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- CI
confidence interval
- CT
computed tomography
- DPP-4
dipeptidyl peptidase 4
- EASL
European Association for the Study of Liver
- FDA
Food and Drug Administration
- FXR
farnesoid X receptor
- GLP-1r
glucagon-like peptide 1 receptor
- HbA1C
hemoglobin A1C
- HCC
hepatocellular carcinoma
- MRI
magnetic resonance imaging
- NAFIC
NASH, ferritin, insulin, type 4 collagen 7S
- NAFLD
non-alcoholic fatty liver disease
- NAS
NAFLD activity score
- NASH
non-alcoholic steatohepatitis
- OCA
obeticholic acid
- OR
odds ratio
- SGLT2
sodium/glucose cotransporter 2
- T2DM
type 2 diabetes mellitus
- TZD
thiazolidinedione.
References
- 1.Watanabe S, Hashimoto E, Ikejima K, Uto H, Ono M, Sumida Y, et al. Evidence-based clinical practice guidelines for nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. Hepatol Res. 2015;45:363–377. doi: 10.1111/hepr.12511. doi: 10.1111/hepr.12511. [DOI] [PubMed] [Google Scholar]
- 2.Rinella ME. Nonalcoholic fatty liver disease: a systematic review. JAMA. 2015;313:2263–2273. doi: 10.1001/jama.2015.5370. doi: 10.1001/jama.2015.5370. [DOI] [PubMed] [Google Scholar]
- 3.Yu J, Marsh S, Hu J, Feng W, Wu C. The pathogenesis of nonalcoholic fatty liver disease: interplay between diet, gut microbiota, and genetic background. Gastroenterol Res Pract. 2016;2016:2862173. doi: 10.1155/2016/2862173. doi: 10.1155/2016/2862173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sawangjit R, Chongmelaxme B, Phisalprapa P, Saokaew S, Thakkinstian A, Kowdley KV, et al. Comparative efficacy of interventions on nonalcoholic fatty liver disease (NAFLD): A PRISMA-compliant systematic review and network meta-analysis. Medicine (Baltimore) 2016;95:e4529. doi: 10.1097/MD.0000000000004529. doi: 10.1097/MD.0000000000004529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Charlton MR, Burns JM, Pedersen RA, Watt KD, Heimbach JK, Dierkhising RA. Frequency and outcomes of liver transplantation for nonalcoholic steatohepatitis in the United States. Gastroenterology. 2011;141:1249–1253. doi: 10.1053/j.gastro.2011.06.061. doi: 10.1053/j.gastro.2011.06.061. [DOI] [PubMed] [Google Scholar]
- 6.Utzschneider KM, Kahn SE. Review: The role of insulin resistance in nonalcoholic fatty liver disease. J Clin Endocrinol Metab. 2006;91:4753–4761. doi: 10.1210/jc.2006-0587. doi: 10.1210/jc.2006-0587. [DOI] [PubMed] [Google Scholar]
- 7.Almeda-Valdés P, Cuevas-Ramos D, Aguilar-Salinas CA. Metabolic syndrome and non-alcoholic fatty liver disease. Ann Hepatol. 2009;8:S18–S24. [PubMed] [Google Scholar]
- 8.Hazlehurst JM, Woods C, Marjot T, Cobbold JF, Tomlinson JW. Non-alcoholic fatty liver disease and diabetes. Metabolism. 2016;65:1096–1108. doi: 10.1016/j.metabol.2016.01.001. doi: 10.1016/j.metabol.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koehler EM, Plompen EP, Schouten JN, Hansen BE, Darwish Murad S, Taimr P, et al. Presence of diabetes mellitus and steatosis is associated with liver stiffness in a general population: The Rotterdam study. Hepatology. 2016;63:138–147. doi: 10.1002/hep.27981. doi: 10.1002/hep.27981. [DOI] [PubMed] [Google Scholar]
- 10.Fracanzani AL, Valenti L, Bugianesi E, Andreoletti M, Colli A, Vanni E, et al. Risk of severe liver disease in nonalcoholic fatty liver disease with normal aminotransferase levels: a role for insulin resistance and diabetes. Hepatology. 2008;48:792–798. doi: 10.1002/hep.22429. doi: 10.1002/hep.22429. [DOI] [PubMed] [Google Scholar]
- 11.Targher G, Day CP, Bonora E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N Engl J Med. 2010;363:1341–1350. doi: 10.1056/NEJMra0912063. doi: 10.1056/NEJMra0912063. [DOI] [PubMed] [Google Scholar]
- 12.Bril F, Cusi K. Management of nonalcoholic fatty liver disease in patients with type 2 diabetes: a call to action. Diabetes Care. 2017;40:419–430. doi: 10.2337/dc16-1787. doi: 10.2337/dc16-1787. [DOI] [PubMed] [Google Scholar]
- 13.Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55:2005–2023. doi: 10.1002/hep.25762. doi: 10.1002/hep.25762. [DOI] [PubMed] [Google Scholar]
- 14.EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol. 2016;64:1388–1402. doi: 10.1016/j.jhep.2015.11.004. doi: 10.1016/j.jhep.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 15.Nair S, Diehl AM, Wiseman M, Farr GH, Jr, Perrillo RP. Metformin in the treatment of non-alcoholic steatohepatitis: a pilot open label trial. Aliment Pharmacol Ther. 2004;20:23–28. doi: 10.1111/j.1365-2036.2004.02025.x. doi: 10.1111/j.1365-2036.2004.02025.x. [DOI] [PubMed] [Google Scholar]
- 16.Haukeland JW, Konopski Z, Eggesbø HB, von Volkmann HL, Raschpichler G, Bjøro K, et al. Metformin in patients with non-alcoholic fatty liver disease: a randomized, controlled trial. Scand J Gastroenterol. 2009;44:853–860. doi: 10.1080/00365520902845268. doi: 10.1080/00365520902845268. [DOI] [PubMed] [Google Scholar]
- 17.Uygun A, Kadayifci A, Isik AT, Ozgurtas T, Deveci S, Tuzun A, et al. Metformin in the treatment of patients with non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2004;19:537–544. doi: 10.1111/j.1365-2036.2004.01888.x. [DOI] [PubMed] [Google Scholar]
- 18.Shields WW, Thompson KE, Grice GA, Harrison SA, Coyle WJ. The effect of metformin and standard therapy versus standard therapy alone in nondiabetic patients with insulin resistance and nonalcoholic steatohepatitis (NASH): a pilot trial. Therap Adv Gastroenterol. 2009;2:157–163. doi: 10.1177/1756283X09105462. doi: 10.1177/1756283X09105462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Wagner LB, Rinella ME. The role of insulin-sensitizing agents in the treatment of nonalcoholic steatohepatitis. Therap Adv Gastroenterol. 2011;4:249–263. doi: 10.1177/1756283X11403809. doi: 10.1177/1756283X11403809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ratziu V, Giral P, Jacqueminet S, Charlotte F, Hartemann-Heurtier A, Serfaty L, et al. Rosiglitazone for nonalcoholic steatohepatitis: one-year results of the randomized placebo-controlled Fatty Liver Improvement with Rosiglitazone Therapy (FLIRT) Trial. Gastroenterology. 2008;135:100–110. doi: 10.1053/j.gastro.2008.03.078. doi: 10.1053/j.gastro.2008.03.078. [DOI] [PubMed] [Google Scholar]
- 21.Ratziu V, Charlotte F, Bernhardt C, Giral P, Halbron M, Lenaour G, et al. Long-term efficacy of rosiglitazone in nonalcoholic steatohepatitis: results of the fatty liver improvement by rosiglitazone therapy (FLIRT 2) extension trial. Hepatology. 2010;51:445–453. doi: 10.1002/hep.23270. doi: 10.1002/hep.23270. [DOI] [PubMed] [Google Scholar]
- 22.Belfort R, Harrison SA, Brown K, Darland C, Finch J, Hardies J, et al. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med. 2006;355:2297–2307. doi: 10.1056/NEJMoa060326. doi: 10.1056/NEJMoa060326. [DOI] [PubMed] [Google Scholar]
- 23.Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675–1685. doi: 10.1056/NEJMoa0907929. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Musso G, Cassader M, Paschetta E, Gambino R. Thiazolidinediones and advanced liver fibrosis in nonalcoholic steatohepatitis: a meta-analysis. JAMA Intern Med. 2017;177:633–640. doi: 10.1001/jamainternmed.2016.9607. doi: 10.1001/jamainternmed.2016.9607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mahady SE, Webster AC, Walker S, Sanyal A, George J. The role of thiazolidinediones in non-alcoholic steatohepatitis - a systematic review and meta analysis. J Hepatol. 2011;55:1383–1390. doi: 10.1016/j.jhep.2011.03.016. doi: 10.1016/j.jhep.2011.03.016. [DOI] [PubMed] [Google Scholar]
- 26.Murphy CE, Rodgers PT. Effects of thiazolidinediones on bone loss and fracture. Ann Pharmacother. 2007;41:2014–2018. doi: 10.1345/aph.1K286. doi: 10.1345/aph.1K286. [DOI] [PubMed] [Google Scholar]
- 27.Körner M, Stöckli M, Waser B, Reubi JC. GLP-1 receptor expression in human tumors and human normal tissues: potential for in vivo targeting. J Nucl Med. 2007;48:736–743. doi: 10.2967/jnumed.106.038679. doi: 10.2967/jnumed.106.038679. [DOI] [PubMed] [Google Scholar]
- 28.Gupta NA, Mells J, Dunham RM, Grakoui A, Handy J, Saxena NK, et al. Glucagon-like peptide-1 receptor is present on human hepatocytes and has a direct role in decreasing hepatic steatosis in vitro by modulating elements of the insulin signaling pathway. Hepatology. 2010;51:1584–1592. doi: 10.1002/hep.23569. doi: 10.1002/hep.23569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Naftalin RJ. A computer model simulating human glucose absorption and metabolism in health and metabolic disease states. F1000Res. 2016;5:647. doi: 10.12688/f1000research.8299.1. doi: 10.12688/f1000research.8299.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Drucker DJ. The biology of incretin hormones. Cell Metab. 2006;3:153–165. doi: 10.1016/j.cmet.2006.01.004. doi: 10.1016/j.cmet.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 31.Turton MD, O’Shea D, Gunn I, Beak SA, Edwards CM, Meeran K, et al. A role for glucagon-like peptide-1 in the central regulation of feeding. Nature. 1996;379:69–72. doi: 10.1038/379069a0. doi: 10.1038/379069a0. [DOI] [PubMed] [Google Scholar]
- 32.Wettergren A, Schjoldager B, Mortensen PE, Myhre J, Christiansen J, Holst JJ. Truncated GLP-1 (proglucagon 78-107-amide) inhibits gastric and pancreatic functions in man. Dig Dis Sci. 1993;38:665–673. doi: 10.1007/BF01316798. [DOI] [PubMed] [Google Scholar]
- 33.Ding X, Saxena NK, Lin S, Gupta NA, Anania FA. Exendin-4, a glucagon-like protein-1 (GLP-1) receptor agonist, reverses hepatic steatosis in ob/ob mice. Hepatology. 2006;43:173–181. doi: 10.1002/hep.21006. doi: 10.1002/hep.21006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Edwards CM, Stanley SA, Davis R, Brynes AE, Frost GS, Seal LJ, et al. Exendin-4 reduces fasting and postprandial glucose and decreases energy intake in healthy volunteers. Am J Physiol Endocrinol Metab. 2001;281:E155–E161. doi: 10.1152/ajpendo.2001.281.1.E155. doi: 10.1152/ajpendo.2001.281.1.E155. [DOI] [PubMed] [Google Scholar]
- 35.Drucker DJ, Sherman SI, Gorelick FS, Bergenstal RM, Sherwin RS, Buse JB. Incretin-based therapies for the treatment of type 2 diabetes: evaluation of the risks and benefits. Diabetes Care. 2010;33:428–433. doi: 10.2337/dc09-1499. doi: 10.2337/dc09-1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Armstrong MJ, Houlihan DD, Rowe IA, Clausen WH, Elbrønd B, Gough SC, et al. Safety and efficacy of liraglutide in patients with type 2 diabetes and elevated liver enzymes: individual patient data meta-analysis of the LEAD program. Aliment Pharmacol Ther. 2013;37:234–242. doi: 10.1111/apt.12149. doi: 10.1111/apt.12149. [DOI] [PubMed] [Google Scholar]
- 37.Armstrong MJ, Gaunt P, Aithal GP, Barton D, Hull D, Parker R, et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet. 2016;387:679–690. doi: 10.1016/S0140-6736(15)00803-X. doi: 10.1016/S0140-6736(15)00803-X. [DOI] [PubMed] [Google Scholar]
- 38.Klonoff DC, Buse JB, Nielsen LL, Guan X, Bowlus CL, Holcombe JH, et al. Exenatide effects on diabetes, obesity, cardiovascular risk factors and hepatic biomarkers in patients with type 2 diabetes treated for at least 3 years. Curr Med Res Opin. 2008;24:275–286. doi: 10.1185/030079908x253870. doi: 10.1185/030079908X253870. [DOI] [PubMed] [Google Scholar]
- 39.García Díaz E, Guagnozzi D, Gutiérrez V, Mendoza C, Maza C, Larrañaga Y, et al. Effect of incretin therapies compared to pioglitazone and gliclazide in non-alcoholic fatty liver disease in diabetic patients not controlled on metformin alone: An observational, pilot study. Endocrinol Nutr. 2016;63:194–201. doi: 10.1016/j.endonu.2016.01.006. doi: 10.1016/j.endonu.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 40.Shao N, Kuang HY, Hao M, Gao XY, Lin WJ, Zou W. Benefits of exenatide on obesity and non-alcoholic fatty liver disease with elevated liver enzymes in patients with type 2 diabetes. Diabetes Metab Res Rev. 2014;30:521–529. doi: 10.1002/dmrr.2561. doi: 10.1002/dmrr.2561. [DOI] [PubMed] [Google Scholar]
- 41.Seko Y, Sumida Y, Tanaka S, Mori K, Taketani H, Ishiba H, et al. Effect of 12-week dulaglutide therapy in Japanese patients with biopsy-proven non-alcoholic fatty liver disease and type 2 diabetes mellitus. Hepatol Res. 2017;47:1206–1211. doi: 10.1111/hepr.12837. doi: 10.1111/hepr.12837. [DOI] [PubMed] [Google Scholar]
- 42.Miyazaki M, Kato M, Tanaka K, Tanaka M, Kohjima M, Nakamura K, et al. Increased hepatic expression of dipeptidyl peptidase-4 in non-alcoholic fatty liver disease and its association with insulin resistance and glucose metabolism. Mol Med Rep. 2012;5:729–733. doi: 10.3892/mmr.2011.707. doi: 10.3892/mmr.2011.707. [DOI] [PubMed] [Google Scholar]
- 43.Balaban YH, Korkusuz P, Simsek H, Gokcan H, Gedikoglu G, Pinar A, et al. Dipeptidyl peptidase IV (DDP IV) in NASH patients. Ann Hepatol. 2007;6:242–250. [PubMed] [Google Scholar]
- 44.Iwasaki T, Yoneda M, Inamori M, Shirakawa J, Higurashi T, Maeda S, et al. Sitagliptin as a novel treatment agent for non-alcoholic Fatty liver disease patients with type 2 diabetes mellitus. Hepatogastroenterology. 2011;58:2103–2105. doi: 10.5754/hge11263. doi: 10.5754/hge11263. [DOI] [PubMed] [Google Scholar]
- 45.Yilmaz Y, Yonal O, Deyneli O, Celikel CA, Kalayci C, Duman DG. Effects of sitagliptin in diabetic patients with nonalcoholic steatohepatitis. Acta Gastroenterol Belg. 2012;75:240–244. [PubMed] [Google Scholar]
- 46.Asakawa M, Mitsui H, Akihisa M, Sekine T, Niitsu Y, Kobayashi A, et al. Efficacy and safety of sitagliptin for the treatment of diabetes mellitus complicated by chronic liver injury. Springerplus. 2015;4:346. doi: 10.1186/s40064-015-1135-z. doi: 10.1186/s40064-015-1135-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fukuhara T, Hyogo H, Ochi H, Fujino H, Kan H, Naeshiro N, et al. Efficacy and safety of sitagliptin for the treatment of nonalcoholic fatty liver disease with type 2 diabetes mellitus. Hepatogastroenterology. 2014;61:323–328. [PubMed] [Google Scholar]
- 48.Arase Y, Kawamura Y, Seko Y, Kobayashi M, Suzuki F, Suzuki Y, et al. Efficacy and safety in sitagliptin therapy for diabetes complicated by non-alcoholic fatty liver disease. Hepatol Res. 2013;43:1163–1168. doi: 10.1111/hepr.12077. doi: 10.1111/hepr.12077. [DOI] [PubMed] [Google Scholar]
- 49.Cui J, Philo L, Nguyen P, Hofflich H, Hernandez C, Bettencourt R, et al. Sitagliptin vs. placebo for non-alcoholic fatty liver disease: A randomized controlled trial. J Hepatol. 2016;65:369–376. doi: 10.1016/j.jhep.2016.04.021. doi: 10.1016/j.jhep.2016.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Joy TR, McKenzie CA, Tirona RG, Summers K, Seney S, Chakrabarti S, et al. Sitagliptin in patients with non-alcoholic steatohepatitis: A randomized, placebo-controlled trial. World J Gastroenterol. 2017;23:141–150. doi: 10.3748/wjg.v23.i1.141. doi: 10.3748/wjg.v23.i1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mashitani T, Noguchi R, Okura Y, Namisaki T, Mitoro A, Ishii H, et al. Efficacy of alogliptin in preventing non-alcoholic fatty liver disease progression in patients with type 2 diabetes. Biomed Rep. 2016;4:183–187. doi: 10.3892/br.2016.569. doi: 10.3892/br.2016.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Macauley M, Hollingsworth KG, Smith FE, Thelwall PE, Al-Mrabeh A, Schweizer A, et al. Effect of vildagliptin on hepatic steatosis. J Clin Endocrinol Metab. 2015;100:1578–1585. doi: 10.1210/jc.2014-3794. doi: 10.1210/jc.2014-3794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Komiya C, Tsuchiya K, Shiba K, Miyachi Y, Furuke S, Shimazu N, et al. Ipragliflozin improves hepatic steatosis in obese mice and liver dysfunction in type 2 diabetic patients irrespective of body weight reduction. PLoS One. 2016;11:e0151511. doi: 10.1371/journal.pone.0151511. doi: 10.1371/journal.pone.0151511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bailey CJ, Gross JL, Pieters A, Bastien A, List JF. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with metformin: a randomised, double-blind, placebo-controlled trial. Lancet. 2010;375:2223–2233. doi: 10.1016/S0140-6736(10)60407-2. doi: 10.1016/S0140-6736(10)60407-2. [DOI] [PubMed] [Google Scholar]
- 55.Lavalle-González FJ, Januszewicz A, Davidson J, Tong C, Qiu R, Canovatchel W, et al. Efficacy and safety of canagliflozin compared with placebo and sitagliptin in patients with type 2 diabetes on background metformin monotherapy: a randomised trial. Diabetologia. 2013;56:2582–2592. doi: 10.1007/s00125-013-3039-1. doi: 10.1007/s00125-013-3039-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Seko Y, Sumida Y, Tanaka S, Mori K, Taketani H, Ishiba H, et al. Effect of sodium glucose cotransporter 2 inhibitor on liver function tests in Japanese patients with non-alcoholic fatty liver disease and type 2 diabetes mellitus. Hepatol Res. 2017;47:1072–1078. doi: 10.1111/hepr.12834. doi: 10.1111/hepr.12834. [DOI] [PubMed] [Google Scholar]
- 57.Ohki T, Isogawa A, Toda N, Tagawa K. Effectiveness of ipragliflozin, a sodium-glucose co-transporter 2 inhibitor, as a second-line treatment for non-alcoholic fatty liver disease patients with type 2 diabetes mellitus who do not respond to incretin-based therapies including glucagon-like peptide-1 analogs and dipeptidyl peptidase-4 inhibitors. Clin Drug Investig. 2016;36:313–319. doi: 10.1007/s40261-016-0383-1. doi: 10.1007/s40261-016-0383-1. [DOI] [PubMed] [Google Scholar]
- 58.Takase T, Nakamura A, Miyoshi H, Yamamoto C, Atsumi T. Amelioration of fatty liver index in patients with type 2 diabetes on ipragliflozin: an association with glucose-lowering effects. Endocr J. 2017;64:363–367. doi: 10.1507/endocrj.EJ16-0295. doi: 10.1507/endocrj.EJ16-0295. [DOI] [PubMed] [Google Scholar]
- 59.Johnsson KM, Ptaszynska A, Schmitz B, Sugg J, Parikh SJ, List JF. Urinary tract infections in patients with diabetes treated with dapagliflozin. J Diabetes Complications. 2013;27:473–478. doi: 10.1016/j.jdiacomp.2013.05.004. doi: 10.1016/j.jdiacomp.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 60.Rosenstock J, Seman LJ, Jelaska A, Hantel S, Pinnetti S, Hach T, et al. Efficacy and safety of empagliflozin, a sodium glucose cotransporter 2 (SGLT2) inhibitor, as add-on to metformin in type 2 diabetes with mild hyperglycaemia. Diabetes Obes Metab. 2013;15:1154–1160. doi: 10.1111/dom.12185. doi: 10.1111/dom.12185. [DOI] [PubMed] [Google Scholar]
- 61.Rosenstock J, Aggarwal N, Polidori D, Zhao Y, Arbit D, Usiskin K, et al. Dose-ranging effects of canagliflozin, a sodium-glucose cotransporter 2 inhibitor, as add-on to metformin in subjects with type 2 diabetes. Diabetes Care. 2012;35:1232–1238. doi: 10.2337/dc11-1926. doi: 10.2337/dc11-1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schernthaner G, Gross JL, Rosenstock J, Guarisco M, Fu M, Yee J, et al. Canagliflozin compared with sitagliptin for patients with type 2 diabetes who do not have adequate glycemic control with metformin plus sulfonylurea: a 52-week randomized trial. Diabetes Care. 2013;36:2508–2515. doi: 10.2337/dc12-2491. doi: 10.2337/dc12-2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee FY, Lee H, Hubbert ML, Edwards PA, Zhang Y. FXR, a multipurpose nuclear receptor. Trends Biochem Sci. 2006;31:572–580. doi: 10.1016/j.tibs.2006.08.002. doi: 10.1016/j.tibs.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 64.Kong B, Luyendyk JP, Tawfik O, Guo GL. Farnesoid X receptor deficiency induces nonalcoholic steatohepatitis in low-density lipoprotein receptor-knockout mice fed a high-fat diet. J Pharmacol Exp Ther. 2009;328:116–122. doi: 10.1124/jpet.108.144600. doi: 10.1124/jpet.108.144600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kim I, Morimura K, Shah Y, Yang Q, Ward JM, Gonzalez FJ. Spontaneous hepatocarcinogenesis in farnesoid X receptor-null mice. Carcinogenesis. 2007;28:940–946. doi: 10.1093/carcin/bgl249. doi: 10.1093/carcin/bgl249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang ZX, Shen W, Sun H. Effects of nuclear receptor FXR on the regulation of liver lipid metabolism in patients with non-alcoholic fatty liver disease. Hepatol Int. 2010;4:741–748. doi: 10.1007/s12072-010-9202-6. doi: 10.1007/s12072-010-9202-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hameed B, Terrault N. Emerging therapies for nonalcoholic fatty liver disease. Clin Liver Dis. 2016;20:365–385. doi: 10.1016/j.cld.2015.10.015. doi: 10.1016/j.cld.2015.10.015. [DOI] [PubMed] [Google Scholar]
- 68.Mudaliar S, Henry RR, Sanyal AJ, Morrow L, Marschall HU, Kipnes M, et al. Efficacy and safety of the farnesoid X receptor agonist obeticholic acid in patients with type 2 diabetes and nonalcoholic fatty liver disease. Gastroenterology. 2013;145:574–582.e1. doi: 10.1053/j.gastro.2013.05.042. doi: 10.1053/j.gastro.2013.05.042. [DOI] [PubMed] [Google Scholar]
- 69.Neuschwander-Tetri BA, Loomba R, Sanyal AJ, Lavine JE, Van Natta ML, Abdelmalek MF, et al. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet. 2015;385:956–965. doi: 10.1016/S0140-6736(14)61933-4. doi: 10.1016/S0140-6736(14)61933-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Musso G, Cassader M, Gambino R. Trials of obeticholic acid for non-alcoholic steatohepatitis. Lancet. 2015;386:27. doi: 10.1016/S0140-6736(15)61198-9. doi: 10.1016/S0140-6736(15)61198-9. [DOI] [PubMed] [Google Scholar]