ABSTRACT
The bacterium Burkholderia thailandensis possesses three N-acyl-l-homoserine lactone (AHL) quorum sensing (QS) systems designated BtaI1/BtaR1 (QS-1), BtaI2/BtaR2 (QS-2), and BtaI3/BtaR3 (QS-3). These QS systems are associated with the biosynthesis of N-octanoyl-homoserine lactone (C8-HSL), N-3-hydroxy-decanoyl-homoserine lactone (3OHC10-HSL), and N-3-hydroxy-octanoyl-homoserine lactone (3OHC8-HSL), which are produced by the LuxI-type synthases BtaI1, BtaI2, and BtaI3 and modulated by the LuxR-type transcriptional regulators BtaR1, BtaR2, and BtaR3. The btaR1-btaI1 and btaR2-btaI2 gene clusters each carry an additional gene encoding a homologue of the QS repressor RsaM originally identified in the phytopathogen Pseudomonas fuscovaginae and thus here named rsaM1 and rsaM2, respectively. We have characterized the functions of these two conserved rsaM homologues and demonstrated their involvement in the regulation of AHL biosynthesis in B. thailandensis strain E264. We quantified the production of C8-HSL, 3OHC10-HSL, and 3OHC8-HSL by liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS) in the wild-type strain and in the rsaM1 and rsaM2 mutants, and we monitored btaI1, btaI2, and btaI3 expression using chromosomal mini-CTX-lux transcriptional reporters. The transcription of btaR1, btaR2, and btaR3 was also measured by quantitative reverse transcription-PCR (qRT-PCR). We observed that RsaM1 mainly represses the QS-1 system, whereas RsaM2 principally represses the QS-2 system. We also found that both rsaM1 and rsaM2 are QS controlled and negatively autoregulated. We conclude that RsaM1 and RsaM2 are an integral part of the QS circuitry of B. thailandensis and play a major role in the hierarchical and homeostatic organization of the QS-1, QS-2, and QS-3 systems.
IMPORTANCE Quorum sensing (QS) is commonly involved in the coordination of gene transcription associated with the establishment of host-pathogen interactions and acclimatization to the environment. We present the functional characterization of two rsaM homologues in the regulation of the multiple QS systems coexisting in the nonpathogenic bacterium Burkholderia thailandensis, which is widely used as a model system for the study of the human pathogen Burkholderia pseudomallei. We found that inactivation of these rsaM homologues, which are clustered with the other QS genes, profoundly affects the QS circuitry of B. thailandensis. We conclude that they constitute essential regulatory components of the QS modulatory network and provide additional layers of regulation to modulate the transcription of QS-controlled genes, particularly those linked to environmental adaptation.
KEYWORDS: Burkholderia pseudomallei, acyl-homoserine lactone, gene regulation, repressor
INTRODUCTION
Quorum sensing (QS) is a widespread cell-cell communication system that coordinates the expression of specific genes in a bacterial population density-dependent manner (1). QS is mediated by diffusible signaling molecules called autoinducers, which are synthesized and secreted in response to fluctuations in cell density. They accumulate in the environment as bacterial growth progresses until a threshold concentration is reached that allows bacteria to synchronize their activities and to function as multicellular communities. Gram-negative bacteria commonly possess homologues of the LuxI/LuxR system initially characterized in the bioluminescent marine bacterium Vibrio fischeri (2). The signaling molecules N-acyl-l-homoserine lactones (AHLs) are produced by the LuxI-type synthases. These AHLs activate the LuxR-type transcriptional regulators that modulate the expression of QS target genes, which usually contain a lux box sequence in their promoter region. These genes frequently include a luxI homologue encoding the AHL synthase, resulting in a typical self-inducing loop of AHLs (3).
The Burkholderia genus encompasses heterogeneous species colonizing diverse ecological niches, such as soil, water, plants, and animals, including humans (4, 5). The Burkholderia cepacia complex (Bcc), for instance, comprises notable opportunistic human pathogens deleterious to both cystic fibrosis (CF) patients and immunocompromised individuals (6). Bcc members carry luxI and luxR homologues, namely, cepI and cepR, respectively, coding for the AHL-based QS system CepI/CepR (7). CepI is a LuxI-type synthase responsible for N-octanoyl-homoserine lactone (C8-HSL) biosynthesis, which generally is the predominant AHL found in members of the Burkholderia genus (7). The LuxR-type transcriptional regulator CepR modulates the expression of QS target genes in conjunction with C8-HSL, including the cepI gene itself, creating the typical QS autoregulation loop (7). The genetic organization of cepI and cepR is conserved among Burkholderia spp. (8). Interestingly, they are generally separated by a gene encoding an RsaM-like protein originally identified in the plant pathogen Pseudomonas fuscovaginae (9, 10), which was shown to be a major negative regulator of both AHL biosynthesis and expression of AHL synthase-coding genes (9). RsaM actually acts as a global regulator mediating the transcription of numerous genes through and out of the QS regulon in P. fuscovaginae (10). The function of RsaM-like proteins could therefore be important for balancing and fine-tuning QS-dependent regulation in members of the Burkholderia genus (11). These proteins do not present any sequence similarity with biochemically or structurally characterized proteins, such as DNA-binding motifs, and constitute single-domain proteins with unique topology presenting a novel fold (12). Their precise underlying regulatory mechanism thus remains unknown.
The nonpathogenic soil saprophyte Burkholderia thailandensis and the closely related human pathogen Burkholderia pseudomallei (13) both encode two conserved RsaM-like proteins of uncharacterized function (8). The genome of B. thailandensis contains three LuxI/LuxR-type QS systems designated BtaI1/BtaR1 (QS-1), BtaI2/BtaR2 (QS-2), and BtaI3/BtaR3 (QS-3). These QS systems are also found in B. pseudomallei and were reported to be involved in the regulation of several virulence genes and to be essential to its pathogenicity (14, 15). We recently thoroughly dissected the QS circuitry of B. thailandensis and found that the QS-1, QS-2, and QS-3 systems are hierarchically and homeostatically organized, and they are integrated into an intricate modulatory network, including transcriptional and posttranscriptional interactions (16). The QS-1 system is responsible for C8-HSL production (17). The BtaR1 transcriptional regulator activates the expression of the btaI1 gene encoding the BtaI1 synthase (16, 18). The QS-2 system is responsible for the biosynthesis of both N-3-hydroxy-decanoyl-homoserine lactone (3OHC10-HSL) and N-3-hydroxy-octanoyl-homoserine lactone (3OHC8-HSL) (19). The btaI2 gene, which codes for the BtaI2 synthase, is positively and directly controlled by the BtaR2 transcriptional regulator in association with 3OHC10-HSL and 3OHC8-HSL (16, 19). The QS-3 system is composed of the BtaR3 transcriptional regulator and the BtaI3 synthase responsible for 3OHC8-HSL production (17). The btaI3 gene is activated by BtaR3 (16). While both the QS-1 and QS-2 gene clusters include an rsaM homologue (8), here named rsaM1 and rsaM2, respectively, no homologue of rsaM is present in the vicinity of btaR3 or btaI3 (8).
The central aim of this study was to further elucidate the QS modulatory network of B. thailandensis E264 by characterizing the roles of RsaM1 and RsaM2 in the regulation of its components. We established that they negatively affect the biosynthesis of AHLs and that they are central to the homeostasis of the QS circuitry of B. thailandensis E264. This study provides new insights on the intricate interplay existing between the various elements of B. thailandensis QS systems and is essential in unraveling the regulatory mechanism underlying QS-dependent gene expression in this bacterium.
RESULTS
The QS-1 and QS-2 gene clusters of B. thailandensis each carry an rsaM homologue.
The B. thailandensis E264 QS-1 system btaI1 (BTH_II1512) and btaR1 (BTH_II1510) genes, encoding the BtaI1 synthase and the BtaR1 transcriptional regulator, respectively, are separated by the BTH_II1511 gene that codes for a hypothetical protein conserved in members of the Burkholderia genus (8, 11, 12, 20–22). This hypothetical protein of 147 amino acids is similar to RsaM-like proteins and displays 35.8% identity with the QS repressor RsaM of the phytopathogen P. fuscovaginae UPB0736 (http://www.uniprot.org/uniprot/Q2T542) (see Fig. S1A in the supplemental material). Interestingly, another rsaM homologue, encoding a hypothetical protein of uncharacterized function, is present on the genome of B. thailandensis E264 between the QS-2 system btaI2 (BTH_II1227) and btaR2 (BTH_II1231) genes that code for the BtaI2 synthase and the BtaR2 transcriptional regulator, respectively. This hypothetical protein of 135 amino acids encoded by the BTH_II1228 gene is 32.4% identical to P. fuscovaginae UPB0736 RsaM (http://www.uniprot.org/uniprot/Q2T5X5) (Fig. S1A). Therefore, the putative proteins encoded by the BTH_II1511 and BTH_II1228 genes were designated RsaM1 and RsaM2, respectively.
Since the rsaM1 and rsaM2 genes are directly adjacent to btaI1 and btaI2 on the genome of B. thailandensis E264, respectively, and are transcribed in the same direction (Fig. S1B), we asked whether they could be cotranscribed. rsaM2 is indeed predicted to be arranged in a operon with btaI2 (http://www.burkholderia.com/). According to our transcriptomic analyses obtained by RNA-sequencing (RNA-seq) (S. Le Guillouzer, M. C. Groleau, F. Mauffrey, R. Villemur, and E. Déziel, unpublished data), neither rsaM1 nor rsaM2 is cotranscribed with the btaI1 or btaI2 gene, respectively (Fig. S1B), as confirmed by reverse transcription-PCR (RT-PCR) experiments (Fig. S2).
The functions of the rsaM1 and rsaM2 genes are unknown. While rsaM2 is located within a cluster responsible for bactobolin biosynthesis (19, 23, 24), its involvement was actually not demonstrated. To determine whether rsaM1 and rsaM2 are functionally similar to the RsaM-encoding gene of P. fuscovaginae UPB0736, which was described as an important repressor of AHL production (9), we investigated the impact of these genes on the biosynthesis of the following predominant AHLs produced by B. thailandensis E264: 3OHC10-HSL and, to lesser extents, C8-HSL and 3OHC8-HSL (16–19). Liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS) was used to measure the total concentrations of these AHLs at various time intervals of the bacterial growth in the B. thailandensis E264 wild-type strain and in rsaM1 and rsaM2 null mutants. These mutants both overproduced AHLs compared to the wild-type strain (Fig. 1). Interestingly, the impact of RsaM1 on total AHL concentrations was more pronounced than the effect of RsaM2 (Fig. 1). Of note, the rsaM1 mutant displayed a delayed growth phenotype (Fig. 1), and more cell aggregation was observed in this background (Fig. S3). Altogether, these observations indicate that the RsaM1 and RsaM2 proteins of B. thailandensis E264 constitute negative regulators of QS, as previously reported for P. fuscovaginae UPB0736 RsaM (9).
FIG 1.
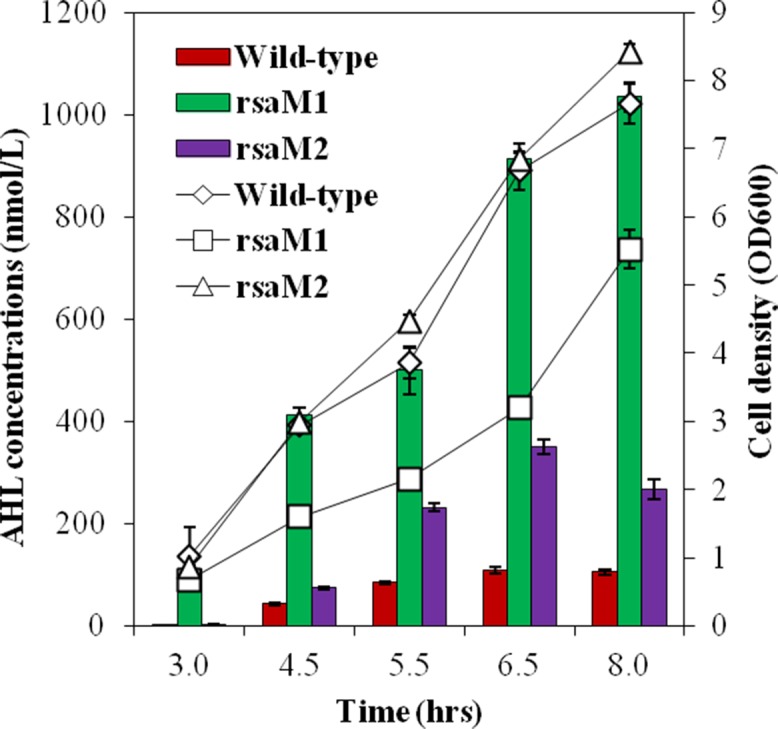
AHLs are overproduced by rsaM1 and rsaM2 mutants. Total concentrations of AHLs (3OHC10-HSL, C8-HSL, and 3OHC8-HSL) (bars) were monitored by LC-MS/MS at various times during growth (lines) in cultures of the B. thailandensis E264 wild-type strain and isogenic rsaM1 and rsaM2 mutants. The error bars represent the standard deviations of the averages for three replicates.
RsaM1 mainly represses the QS-1 system, and RsaM2 principally represses the QS-2 system.
Since we confirmed the involvement of the BtaI1, BtaI2, and BtaI3 synthases in C8-HSL, 3OHC10-HSL, and 3OHC8-HSL biosynthesis, respectively (Fig. 2), we determined the effects of RsaM1 and RsaM2 on the QS-1, QS-2, and QS-3 systems by measuring the respective production of C8-HSL, 3OHC10-HSL, and 3OHC8-HSL in the wild-type strain and in the rsaM1 and rsaM2 mutants of B. thailandensis E264 throughout the bacterial growth phases. To gain additional insights, we also monitored the expression of the AHL synthase-coding genes btaI1, btaI2, and btaI3 in the same backgrounds using the chromosomal btaI1-lux, btaI2-lux, and btaI3-lux transcriptional reporters, respectively.
FIG 2.
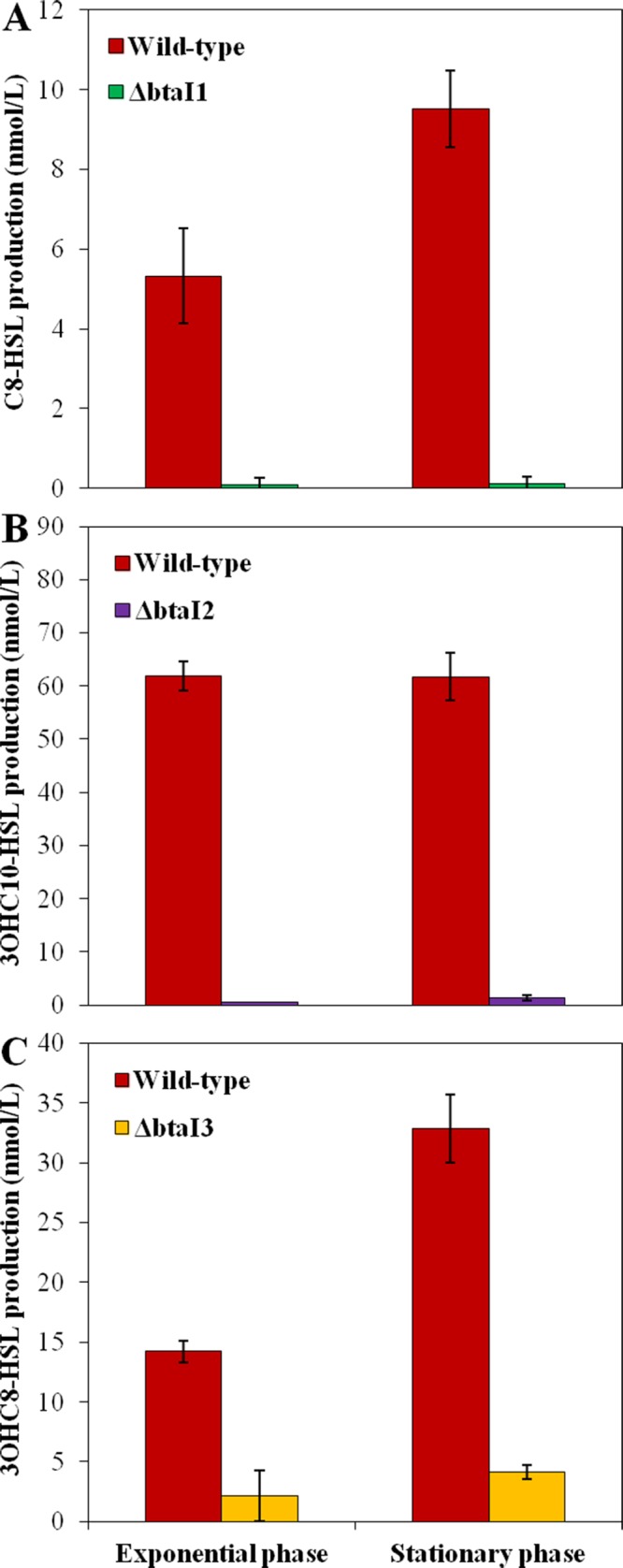
AHL biosynthesis in the wild-type strain of B. thailandensis E264 and the ΔbtaI1, ΔbtaI2, and ΔbtaI3 mutants. The production of C8-HSL (A), 3OHC10-HSL (B), and 3OHC8-HSL (C) was quantified using LC-MS/MS during the exponential and stationary phases in cultures of the wild-type strain of B. thailandensis E264 and the ΔbtaI1 (A), ΔbtaI2 (B), and ΔbtaI3 (C) mutants, respectively. The error bars represent the standard deviations of the averages for three replicates.
We observed a dramatic overproduction of C8-HSL in the rsaM1 mutant compared to the wild-type strain during the early exponential (optical density at 600 nm [OD600], ≈3.0) and late-exponential (OD600, ≈5.0) phases, indicating that RsaM1 represses the biosynthesis of C8-HSL (Fig. 3A). The transcription of the btaI1 gene was accordingly enhanced in the absence of RsaM1, suggesting that RsaM1 intervenes in the modulation of C8-HSL production by regulating the transcription of btaI1 (Fig. 3C). Interestingly, the impact of RsaM1 on C8-HSL biosynthesis (approximately 200-fold) was larger than its effect on btaI1 transcription (approximately 2-fold) (Fig. 3). We also detected a small, but reproducible, augmentation of C8-HSL concentrations from the stationary phase (OD600, ≈6.0) in the rsaM2 mutant compared to the wild-type strain, highlighting that the production of C8-HSL is negatively modulated by RsaM2 as well (Fig. 3B). However, no discernible difference in the transcription of btaI1 was detected in the absence of RsaM2 (Fig. 3C). Thus, the negative impact of RsaM2 on C8-HSL production might not result from regulation of btaI1 transcription.
FIG 3.
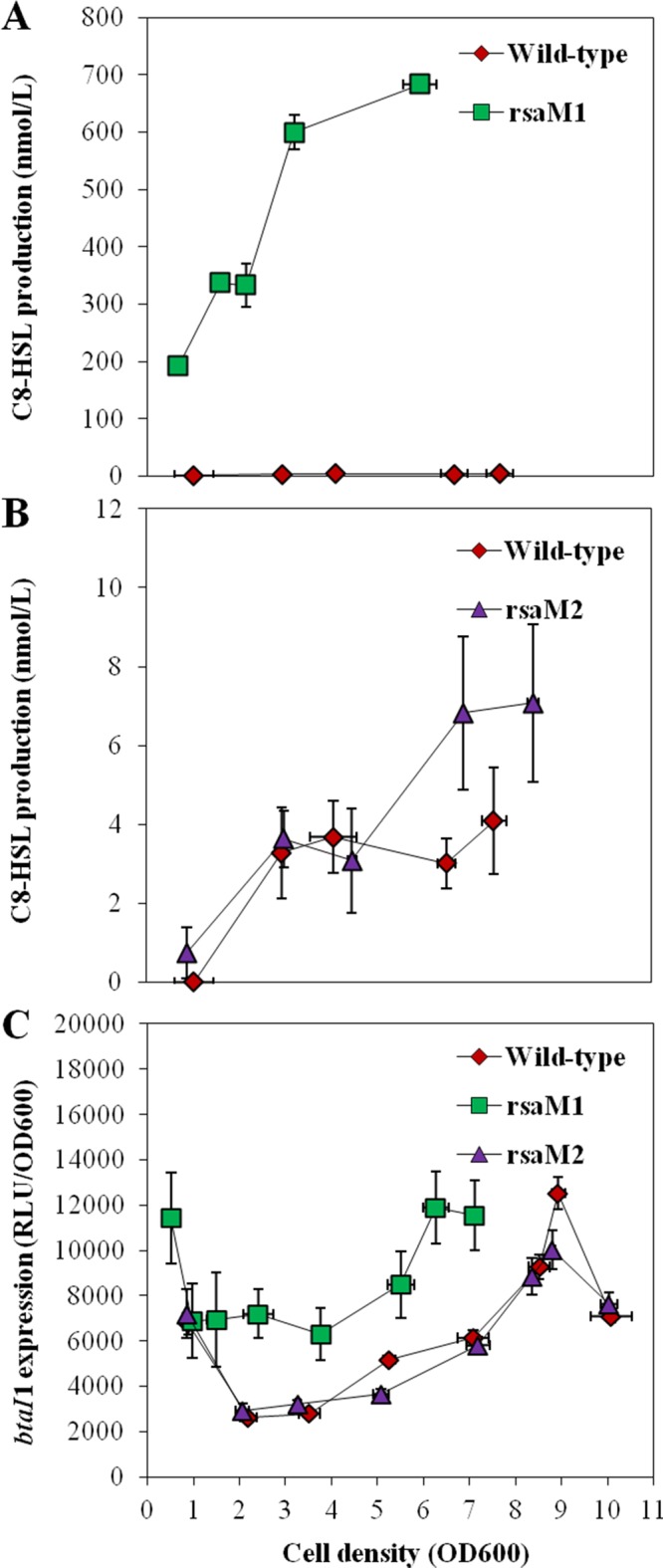
C8-HSL biosynthesis and expression from the btaI1 promoter in the wild-type and the rsaM1 and rsaM2 mutant strains of B. thailandensis E264. (A and B) The production of C8-HSL was quantified using LC-MS/MS at various times during growth in cultures of the wild-type strain and of the rsaM1 (A) and rsaM2 (B) mutant strains of B. thailandensis E264. The error bars represent the standard deviations of the averages for three replicates. (C) The luminescence of the chromosomal btaI1-lux transcriptional fusion was monitored in cultures of the B. thailandensis E264 wild-type strain and the rsaM1 and rsaM2 mutants. The luminescence is expressed in relative light units per optical density of the culture at 600 nm (RLU/OD600).
While 3OHC10-HSL production, as well as the transcription of btaI2, was unaffected in the absence of RsaM1 (Fig. 4), the concentrations of 3OHC10-HSL were strongly increased in the rsaM2 mutant compared with the wild-type strain throughout both the late-exponential and stationary phases (Fig. 4A), and btaI2 transcription was similarly upregulated (Fig. 4B). These data suggest that RsaM2 represses 3OHC10-HSL biosynthesis by modulating the transcription of btaI2.
FIG 4.
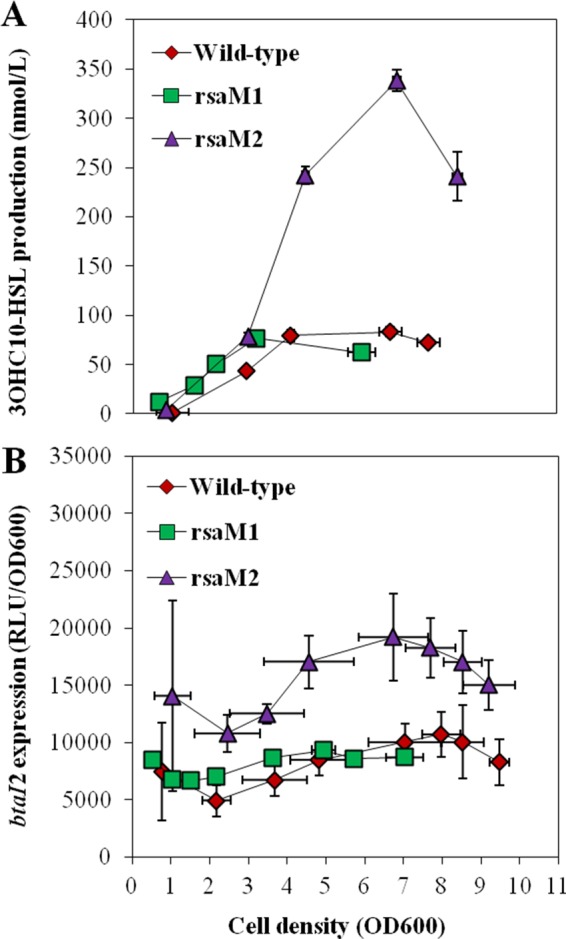
3OHC10-HSL biosynthesis and expression from the btaI2 promoter in the wild-type and the rsaM1 and rsaM2 mutant strains of B. thailandensis E264. (A) The production of 3OHC10-HSL was quantified using LC-MS/MS at various times during growth in cultures of the wild-type and the rsaM1 and rsaM2 mutant strains of B. thailandensis E264. The error bars represent the standard deviations of the averages for three replicates. (B) The luminescence of the chromosomal btaI2-lux transcriptional fusion was monitored in cultures of the B. thailandensis E264 wild-type strain and the rsaM1 and rsaM2 mutants. The luminescence is expressed in relative light units per optical density of the culture at 600 nm (RLU/OD600).
The levels of 3OHC8-HSL were also higher from the logarithmic growth in the rsaM1 mutant than in the wild-type strain (Fig. 5A). Unexpectedly, the transcription of the btaI3 gene was not increased, suggesting that the negative impact of RsaM1 on 3OHC8-HSL production does not involve the regulation of btaI3 transcription (Fig. 5C). Additionally, 3OHC8-HSL concentrations were augmented during the stationary phase in the rsaM2 mutant in comparison with the wild-type strain, showing that the production of 3OHC8-HSL is repressed by RsaM2 as well (Fig. 5B). Nevertheless, no visible change in the transcription of btaI3 was noticed in the absence of RsaM2, revealing that the RsaM2-dependent control on 3OHC8-HSL biosynthesis might not be linked to modulation of btaI3 transcription (Fig. 5C).
FIG 5.
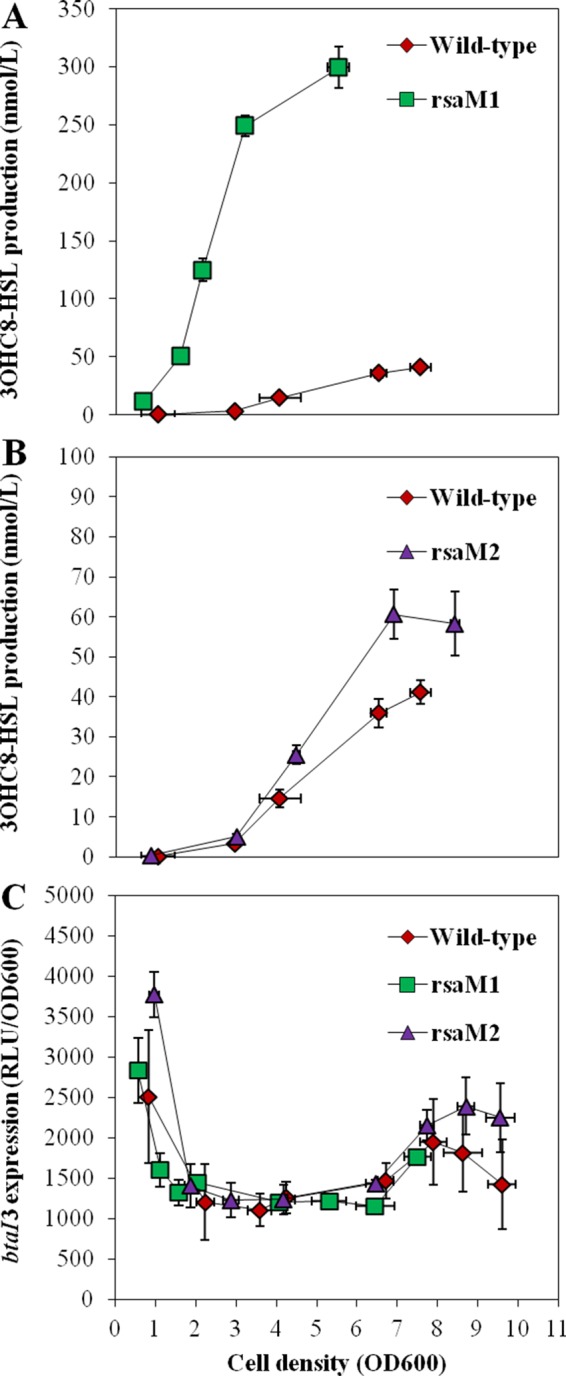
3OHC8-HSL biosynthesis and expression from the btaI3 promoter in the wild-type and the rsaM1 and rsaM2 mutant strains of B. thailandensis E264. (A and B) The production of 3OHC8-HSL was quantified using LC-MS/MS at various times during growth in cultures of the wild-type strain and of the rsaM1 (A) and rsaM2 (B) mutant strains of B. thailandensis E264. The error bars represent the standard deviations of the averages for three replicates. (C) The luminescence of the chromosomal btaI3-lux transcriptional fusion was monitored in cultures of the B. thailandensis E264 wild-type strain and the rsaM1 and rsaM2 mutants. The luminescence is expressed in relative light units per optical density of the culture at 600 nm (RLU/OD600).
While the concentrations of both C8-HSL and 3OHC8-HSL were enhanced in the rsaM1 mutant background, the impact on C8-HSL biosynthesis was more pronounced than the effect on 3OHC8-HSL production (Fig. S4A and B). Additionally, the amounts of C8-HSL, 3OHC10-HSL, and 3OHC8-HSL were all increased in the rsaM2 mutant background; however, 3OHC10-HSL levels were the most affected (Fig. S4A and C). Collectively, these findings indicate that RsaM1 mainly represses the QS-1 system, whereas RsaM2 principally represses the QS-2 system.
RsaM1 negatively regulates btaR1 gene transcription, but transcription of the btaR2 gene is not modulated by RsaM2.
In order to determine whether the impact of RsaM1 and RsaM2 on AHL biosynthesis also implicates the BtaR transcriptional regulators, we monitored the levels of transcription of btaR1, btaR2, and btaR3 by quantitative reverse transcription-PCR (qRT-PCR) in the wild-type strain and in the rsaM1 and rsaM2 mutants of B. thailandensis E264 during the exponential phase. We observed an increase in btaR1 transcription in the absence of RsaM1 (Fig. 6A), but no significant variation was noticed in the rsaM2 mutant compared to the wild-type strain (Fig. 6A), correlating with the transcription profiles of btaI1 in these backgrounds (Fig. S5). Thus, the transcription of both btaR1 and btaI1 is negatively regulated by RsaM1, suggesting that the negative impact of RsaM1 on the production of C8-HSL involves the regulation of btaR1 and btaI1 transcription, whereas RsaM2 does not apparently impact the QS-1 system genes transcription to repress C8-HSL biosynthesis. Furthermore, no significant difference was detected in btaR2 transcription in both the rsaM1 and rsaM2 mutant strains compared to the wild-type strain, showing that neither RsaM1 nor RsaM2 modulates the transcription of btaR2 (Fig. 6B). Consequently, while RsaM1 seems to have no effect on the QS-2 system, the RsaM2-dependent control on 3OHC10-HSL biosynthesis is not likely linked to the regulation of btaR2 transcription and therefore appears to solely result from modulation of btaI2 transcription. Moreover, neither RsaM1 nor RsaM2 had a significant impact on the transcription of btaR3 (Fig. 6C). These observations indicate that the production of 3OHC8-HSL is not controlled by RsaM1 and RsaM2 through modulation of the transcription of the QS-3 system genes.
FIG 6.
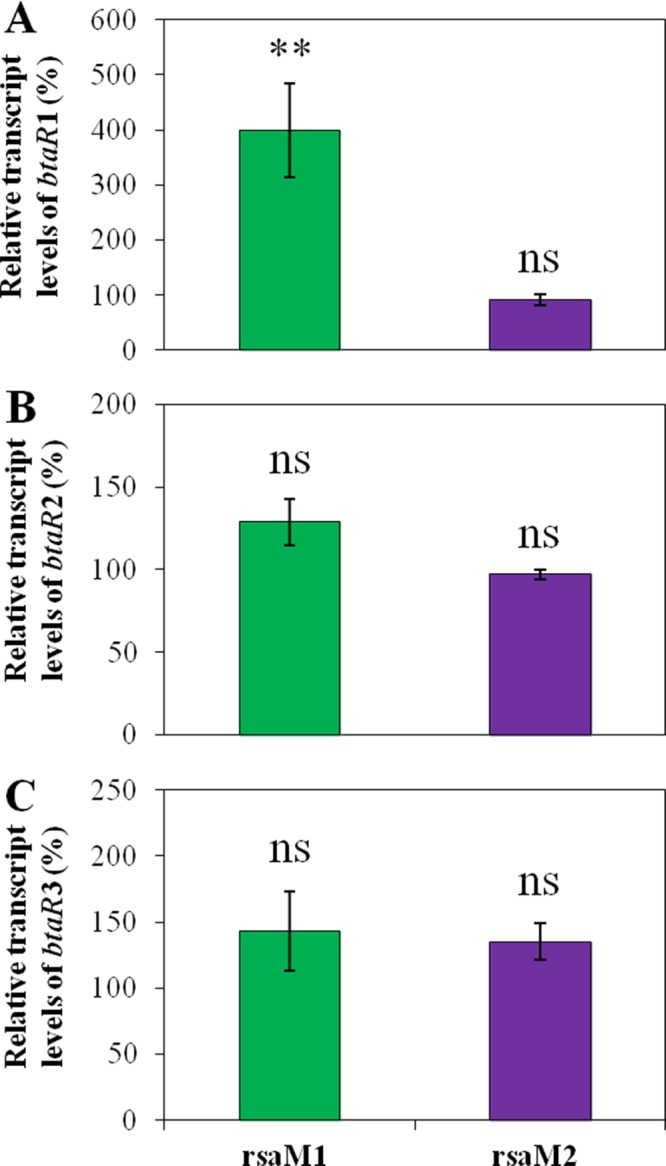
RsaM1 negatively regulates the transcription of btaR1, but btaR2 transcription is not modulated by RsaM2. The relative transcript levels of btaR1 (A), btaR2 (B), and btaR3 (C) were assessed by qRT-PCR in cultures of the wild-type and the rsaM1 and rsaM2 mutant strains of B. thailandensis E264. The results are presented as relative quantification of transcription of the gene compared to the wild-type strain, which was set at 100%. The values are means ± standard deviations (error bars) for three replicates. Values that are significantly different are indicated by asterisks as follows: **, P < 0.01. Values that are not significantly different (ns) are also indicated.
The rsaM1 and rsaM2 genes are QS controlled.
The transcription of the rsaM2 gene, but not rsaM1 gene transcription, was reported to be activated by QS (18). Our transcriptomic analyses indicate that QS indeed stimulates rsaM2 transcription, as well as the transcription of rsaM1 (Le Guillouzeret al., unpublished).
In order to ascertain that the transcription of rsaM1 is positively controlled by QS, we monitored rsaM1 transcription by qRT-PCR in the B. thailandensis E264 wild-type strain and in the AHL-defective ΔbtaI1 ΔbtaI2 ΔbtaI3 mutant supplemented with exogenous AHLs or not supplemented with AHLs during the exponential phase. We observed that the transcription of rsaM1 was reduced in the absence of AHLs, confirming that QS activates rsaM1 transcription (Fig. 7A). Furthermore, the transcription of rsaM1 was significantly enhanced in cultures of the ΔbtaI1 ΔbtaI2 ΔbtaI3 triple-mutant strain supplemented with C8-HSL, 3OHC10-HSL, or 3OHC8-HSL (Fig. 7A). To gain insights into the QS-dependent regulation of rsaM1, we also measured the transcription of rsaM1 in the ΔbtaR1, ΔbtaR2, and ΔbtaR3 mutants versus the B. thailandensis E264 wild-type strain during the exponential phase. While no obvious change in rsaM1 transcription was visible in the absence of neither BtaR2 nor BtaR3, the transcription of rsaM1 was decreased in the ΔbtaR1 mutant compared to the wild-type strain (Fig. 7B). Taken together, these data indicate that the transcription of rsaM1 is positively regulated by the QS-1 system, whereas the QS-2 and QS-3 systems are likely not involved in the modulation of rsaM1 transcription.
FIG 7.
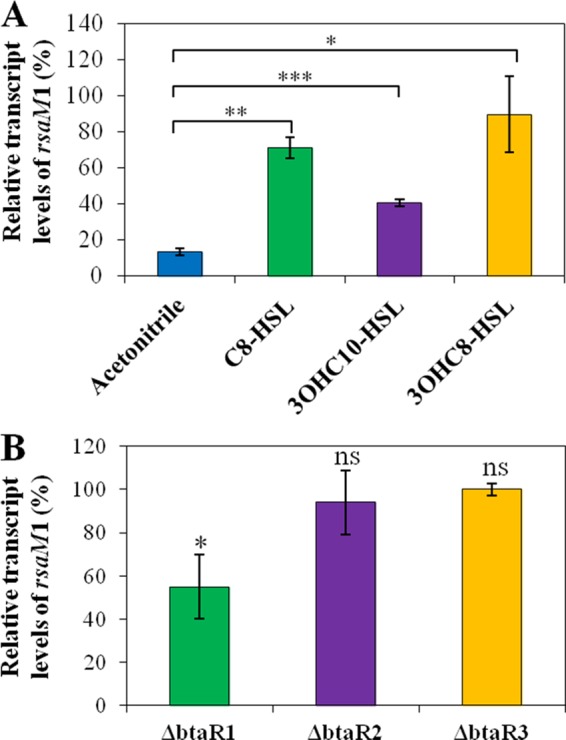
QS positively regulates rsaM1 transcription. (A) The relative transcript levels of rsaM1 from the B. thailandensis E264 wild-type strain and its ΔbtaI1 ΔbtaI2 ΔbtaI3 mutant strain were estimated by qRT-PCR. Cultures were supplemented with 10 μM C8-HSL, 3OHC10-HSL, or 3OHC8-HSL. Acetonitrile only was added to the controls. The results are presented as relative quantification of transcription of the gene compared to the wild-type strain, which was set at 100%. The error bars represent the standard deviations of the averages for three replicates. (B) The relative transcript levels of rsaM1 were assessed by qRT-PCR in cultures of the wild-type and the ΔbtaR1, ΔbtaR2, and ΔbtaR3 mutant strains of B. thailandensis E264. ***, P < 0.0001; **, P < 0.01; *, P < 0.05; ns, nonsignificant.
The transcription of rsaM2 was lowered in the absence of AHLs, confirming that the rsaM2 gene is activated by QS as well (Fig. 8A). Moreover, rsaM2 transcription was significantly enhanced in cultures of the ΔbtaI1 ΔbtaI2 ΔbtaI3 triple-mutant strain supplemented with 3OHC10-HSL or 3OHC8-HSL (Fig. 8A). Interestingly, we observed that the transcription of rsaM2 was also downregulated in the ΔbtaR2 mutant compared to the wild-type strain, meaning that the rsaM2 gene is positively controlled by BtaR2, whereas no discernible difference in rsaM2 transcription was detected in the absence of BtaR1 or BtaR3 (Fig. 8B). Altogether, our results indicate that the transcription of rsaM2 is positively modulated by the QS-2 system, whereas the QS-1 and QS-3 systems apparently do not intervene in the regulation of rsaM2 transcription.
FIG 8.
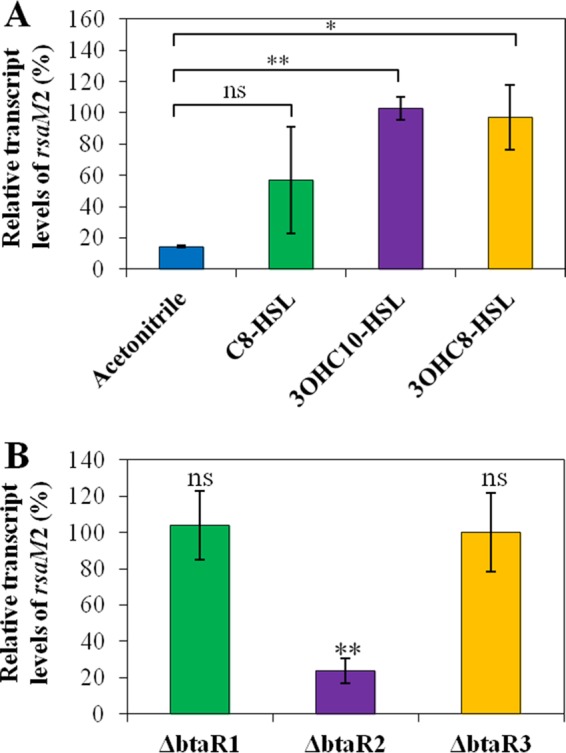
The transcription of rsaM2 is activated by QS. (A) The relative transcript levels of rsaM2 from the B. thailandensis E264 wild-type strain and its ΔbtaI1 ΔbtaI2 ΔbtaI3 mutant strain were monitored by qRT-PCR. Cultures were supplemented with 10 μM C8-HSL, 3OHC10-HSL, or 3OHC8-HSL. Acetonitrile only was added to the controls. The results are presented as relative quantification of transcription of the gene compared to the wild-type strain, which was set at 100%. The error bars represent the standard deviations of the averages for three replicates. (B) The relative transcript levels of rsaM2 were quantified by qRT-PCR in cultures of the wild-type and the ΔbtaR1, ΔbtaR2, and ΔbtaR3 mutant strains of B. thailandensis E264. **, P < 0.01; *, P < 0.05; ns, nonsignificant.
Collectively, these observations highlight that the transcription of rsaM1 is activated by the QS-1 system, which is negatively regulated by RsaM1, whereas rsaM2 transcription is stimulated by the QS-2 system, which is negatively regulated by RsaM2, showing that these repressors are deeply integrated into the QS modulatory network of B. thailandensis E264.
rsaM1 and rsaM2 are negatively autoregulated.
To further explore the RsaM1 and RsaM2 molecular mechanisms of action, the levels of transcription of rsaM1 and rsaM2 were assessed by qRT-PCR in the B. thailandensis E264 wild-type strain and in the rsaM1 and rsaM2 mutants during the exponential phase. The transcription of rsaM1 was strongly increased in the absence of RsaM1 (Fig. 9A), and the same was observed for rsaM2 transcription in the rsaM2 mutant compared to the wild-type strain (Fig. 9B). However, the absence of RsaM2 had no impact on rsaM1 transcription (Fig. 9A), and the transcription of rsaM2 was unchanged in the rsaM1 mutant in comparison with the wild-type strain (Fig. 9B). Altogether, our results indicate that RsaM1 and RsaM2 repress their own transcription but do not influence each other.
FIG 9.
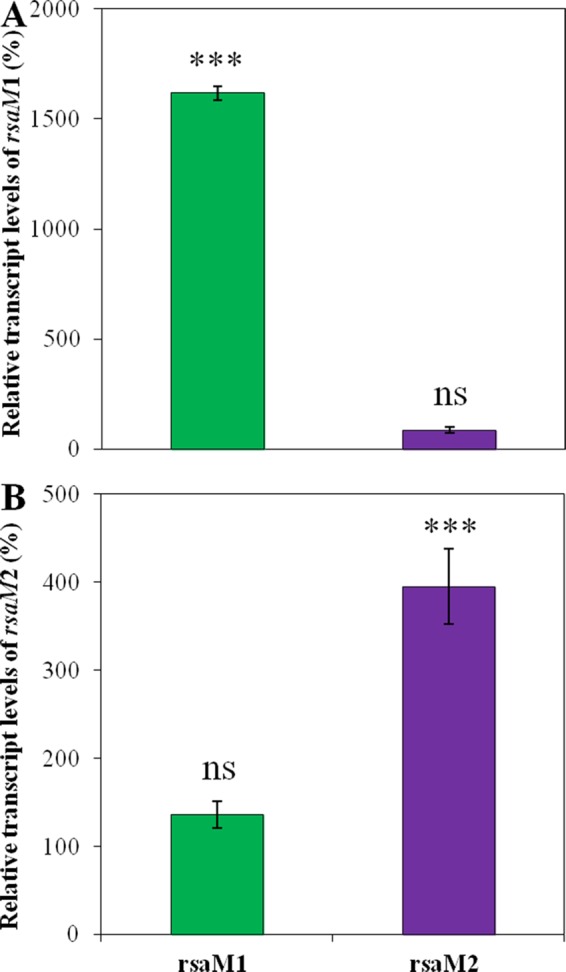
The rsaM1 and rsaM2 genes are negatively autoregulated. The relative transcript levels of rsaM1 (A) and rsaM2 (B) from the wild-type B. thailandensis E264 strain and its rsaM1 and rsaM2 mutant strains were estimated by qRT-PCR. The results are presented as relative quantification of transcription of the gene compared to the wild-type strain, which was set at 100%. The error bars represent the standard deviations of the averages for three replicates. ***, P < 0.001; ns, nonsignificant.
DISCUSSION
While the function of RsaM-like proteins was previously investigated in a few Burkholderia species (11, 12, 21), their involvement in the complex organization of the multiple QS circuitries found in the closely related species of the Burkholderia pseudomallei-B. thailandensis-B. mallei group had not been addressed. Here, we initiated a study of the two rsaM homologues present on the genome of B. thailandensis E264.
The rsaM1 gene, which is divergently transcribed from btaR1 and oriented in the same direction as btaI1, encodes an RsaM-like protein initially characterized in the plant pathogen P. fuscovaginae (8, 11, 12, 20–22) (see Fig. S1A and B in the supplemental material). The RsaM protein of P. fuscovaginae UPB0736 was reported to negatively control the AHL-based QS systems PfsI/PfsR and PfvI/PfvR (9, 22). It is hypothesized to directly repress the transcription of the LuxI-type synthase PfsI- and PfvI-encoding genes. However, it could also act indirectly, for instance, by inhibiting the functionality of the LuxR-type transcriptional regulators PfsR and PfvR, which are required for activation of pfsI and pfvI gene transcription, respectively. In the Bcc member Burkholderia cenocepacia, an RsaM-like protein homologue, namely, BcRsaM, was described as an important repressor of the production of C8-HSL affiliated with the CepI/CepR QS system and proposed to regulate the activity and/or stability of the LuxI-type synthase CepI and the LuxR-type transcriptional regulator CepR, as well as the orphan LuxR-type transcriptional regulator CepR2 (11, 12). The transcription of the cepI, cepR, and cepR2 genes of B. cenocepacia H111 was seen to be lowered in an rsaM mutant in comparison with the wild-type strain (11). However, in B. thailandensis E264, we found that the transcription of both btaI1 and btaR1 was increased in the absence of RsaM1 (Fig. 3C and 6A), correlating with the accumulation of C8-HSL in this background (Fig. 3A). Consequently, RsaM1 could repress the transcription of btaI1 and btaR1, suggesting that its mode of action in B. thailandensis E264 differs from that of BcRsaM. However, we noticed that the impact of RsaM1 on C8-HSL biosynthesis was dramatically greater than its effect on btaI1 transcription, which hints that RsaM1 could also act at posttranscriptional levels, as proposed for BcRsaM. Thus, RsaM1 could directly repress the transcription of the btaI1 and btaR1 genes or indirectly do so, for instance, by modulating the activity and/or stability of BtaI1 or by controlling the functionality of BtaR1. This demonstrates that while BtaR1 is considered the principal regulator of the QS-1 system, RsaM1 plays a major role in modulating the production of C8-HSL (Fig. 10). Strikingly, the absence of RsaM1 was associated with a growth defect in tryptic soy broth (TSB) medium (Fig. 1) and leads to an aggregative growth phenotype in modified M9 medium (see Fig. S3 in the supplemental material). This could be linked to the prominent levels of C8-HSL produced in the rsaM1 mutant compared to the wild-type strain, and thus overactivation of phenotypes controlled by the QS-1 system, such as autoaggregation, biofilm development, and oxalate production (17, 18, 25–27).
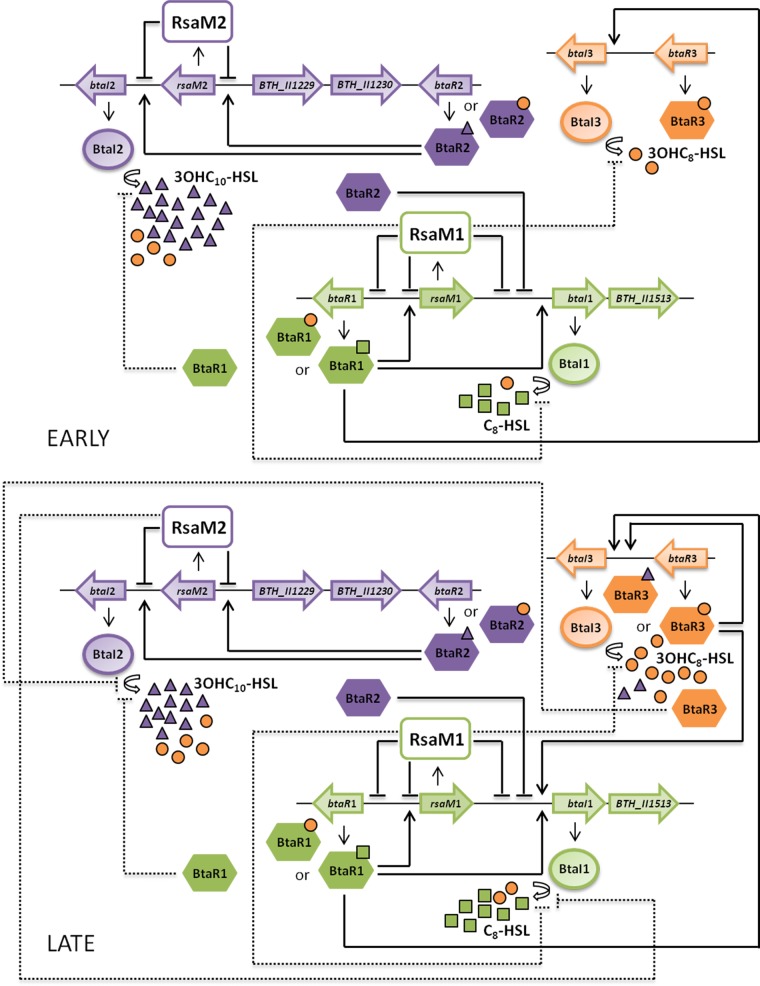
FIG 10.
Proposed involvement of RsaM1 and RsaM2 in the QS circuitry of B. thailandensis E264. The QS-1 system is composed of the BtaI1 synthase, which is principally responsible for C8-HSL biosynthesis (17) and is hypothesized to produce 3OHC8-HSL, as well as the BtaR1 transcriptional regulator that stimulates the transcription of btaI1 in association with C8-HSL (16, 18). The BtaI2 synthase, which synthesizes both 3OHC10-HSL and 3OHC8-HSL (19), as well as the BtaR2 transcriptional regulator that activates btaI2 transcription in conjunction with these AHL signaling molecules (16, 18, 19), constitute the QS-2 system. Furthermore, the QS-1 and QS-2 systems contain rsaM homologues designated rsaM1 and rsaM2, respectively. The RsaM1 protein mainly represses the production of C8-HSL. It could act directly by repressing the transcription of btaI1 and btaR1 or indirectly, for instance, by modulating the activity and/or stability of BtaI1 or by controlling the functionality of BtaR1. The RsaM2 protein principally represses the production of 3OHC10-HSL, as well as btaI2 transcription but not the transcription of btaR2. The rsaM1 and rsaM2 genes are negatively autoregulated and activated by the QS-1 and QS-2 systems, respectively, showing an important homeostatic modulation of AHL biosynthesis. Moreover, an interdependence between the QS-1 and QS-2 systems was observed. The production of C8-HSL, as well as btaI1 transcription, but not the transcription of btaR1, is indeed negatively controlled by BtaR2 (16). Since RsaM2 seems to have no impact on the transcription of btaI1 and btaR1, the negative modulation of C8-HSL biosynthesis by RsaM2 might involve other regulatory elements, underscoring an additional modulatory layer connecting the QS-1 and QS-2 systems. While neither the transcription of btaI2 nor btaR2 transcription are under BtaR1 control, BtaR1 appears to repress the production of 3OHC10-HSL (16, 18). Similarly, 3OHC10-HSL biosynthesis was shown to be negatively controlled by the QS-3 system (16, 18), which is composed of the BtaI3 synthase and the BtaR3 transcriptional regulator. BtaI3 is mainly responsible for 3OHC8-HSL biosynthesis (17) and is hypothesized to produce 3OHC10-HSL (16), whereas the transcription of btaI3 is stimulated by BtaR3 in association with 3OHC8-HSL and 3OHC10-HSL (16). An interdependence between the QS-1 and QS-3 systems was observed as well, since btaI3 transcription is likely activated by BtaR1/C8-HSL from the exponential phase, and BtaR3, in conjunction with 3OHC8-HSL and 3OHC10-HSL, was suggested to positively modulate the transcription of btaI1 from the stationary phase (16). Additionally, RsaM1 could repress the production of 3OHC8-HSL by targeting the QS-1 and/or QS-3 systems, thus further connecting these QS circuitries.
We recently reported that the transcription of btaI1 is activated by BtaR1/C8-HSL, meaning that the QS-1 system is positively autoregulated (16) (Fig. 10). We indeed confirmed that btaI1 transcription is downregulated in the ΔbtaR1 mutant compared to in the wild-type strain (16, 18). However, we observed an accumulation of C8-HSL in the absence of BtaR1 (16). We thus hypothesized that additional regulatory elements are involved in the modulation of C8-HSL production (16). The finding that BtaR1 and C8-HSL activate the transcription of rsaM1 might explain why more C8-HSL is detected in the absence of BtaR1 (Fig. 7). In fact, it is possible that the mutation in btaR1, which appears to affect rsaM1 transcription, results indirectly in C8-HSL overproduction. Moreover, it reveals that the QS-1 system is also negatively autoregulated through RsaM1, presumably counteracting with the positive-feedback loop mediated by BtaR1/C8-HSL for the biosynthesis of C8-HSL. This could be necessary to modulate the QS response depending on specific environmental conditions, as previously suggested for other negative regulators of QS. For instance, the QteE and RsaL repressors in the human opportunistic pathogen Pseudomonas aeruginosa modulate the timing and extent of the QS response and likely increase P. aeruginosa phenotypic plasticity and population fitness, ultimately facilitating the colonization of challenging environments, including higher organisms (22, 28–33). The RsaL protein is also found ubiquitously in the group of nonpathogenic plant-associated nitrogen-fixing Burkholderia spp., such as Burkholderia kururiensis, and its role is hypothesized to be a switch to turn on/off the AHL signaling system under various environmental conditions (22, 34). We found a putative lux box sequence in the promoter region of rsaM1 that might be specifically recognized by BtaR1/C8-HSL to stimulate rsaM1 transcription (Fig. S1C). Consistently, the CepR transcriptional regulator of B. cenocepacia K56-2 was shown to positively and directly control the transcription of the rsaM gene in association with C8-HSL (35, 36). Nevertheless, rsaM1 displayed different transcriptional profiles in the ΔbtaR1 mutant and in the ΔbtaI1 ΔbtaI2 ΔbtaI3 mutant backgrounds (Fig. 7), indicating that the QS-dependent regulation of rsaM1 transcription might be more complex and will need further investigation.
We suppose that RsaM1 does not control the QS-2 system, since neither the biosynthesis of 3OHC10-HSL (Fig. 4A), which we confirmed constitutes the main AHL produced by BtaI2 (19) (Fig. 2B), nor the transcription of btaI2 and btaR2 is affected in the rsaM1 mutant compared to the wild-type strain (Fig. 4B and 6B). Therefore, we must deduce that the effect of RsaM1 on 3OHC8-HSL production (Fig. 5A), which is also synthesized by BtaI2 (19), rather involves modulation of the QS-1 and/or QS-3 systems. We indeed confirmed that BtaI3 principally synthesizes 3OHC8-HSL (17) (Fig. 2C). However, RsaM1 seems to have no impact on btaI3 and btaR3 transcription (Fig. 5C and 6C). An explanation could be that RsaM1 indirectly modulates the QS-3 system through the control of other regulatory elements that would affect the production of 3OHC8-HSL, thus further connecting the QS-1 and QS-3 systems in B. thailandensis E264 that were shown to be transcriptionally linked (16, 18) (Fig. 10). Interestingly, the QS repressor RsaM of P. fuscovaginae UPB0736 was reported to control several genes encoding transcriptional factors and could consequently intervene directly in the modulation of gene expression, as well as indirectly via auxiliary regulators (10, 22). In order to further understand the molecular mechanism of action of RsaM1, we propose to define the RsaM1 regulon, for instance, by performing RNA-seq analyses and/or chromatin immunoprecipitation sequencing (ChIP-Seq) analyses. Still, it is also conceivable that RsaM1 affects 3OHC8-HSL biosynthesis by directly regulating the QS-1 system. In fact, 3OHC8-HSL could be produced via BtaI1 in the wild-type strain in concentrations under our detection limit, and then those levels become detectable in the QS-1 system-boosted rsaM1 mutant. We previously reported that besides C8-HSL, the homologue of this AHL synthase can produce trace amounts of 3OHC8-HSL in the Bcc member Burkholderia ambifaria (37). Additionally, the B. pseudomallei KHW BpsI and Burkholderia mallei ATCC 23344 BmaI1 synthases, which are homologous to BtaI1 (38–40), were both shown to produce 3OHC8-HSL in addition to C8-HSL, albeit at lower concentrations (41, 42), and the B. pseudomallei KHW BpsR and B. mallei ATCC 23344 BmaR1 transcriptional regulators, which are homologous to BtaR1 (38–40), were reported to specifically respond to both C8-HSL and 3OHC8-HSL (41, 42). Accordingly, the BtaR1-controlled genes identified in transcriptomic analyses were generally affected by both C8-HSL and 3OHC8-HSL (18). This then explains why these AHLs exhibit similar production profiles (16). Additional experiments, however, will be necessary to confirm the possible production of 3OHC8-HSL by BtaI1.
The rsaM2 gene, which is found directly adjacent to btaI2 and is transcribed in the same direction, encodes an additional RsaM-like protein (Fig. S1A and B). The transcription of btaI2 and 3OHC10-HSL production are activated by BtaR2, which constitutes the main regulator of the QS-2 system (16, 18, 19). Additionally, we demonstrated that the QS-2 system is negatively modulated by RsaM2 (Fig. 4), whereas the transcription of rsaM2 is stimulated by the QS-2 system (Fig. 8). Consequently, while btaI2 transcription is directly activated by BtaR2, it seems that BtaR2 also represses the transcription of btaI2 indirectly through RsaM2 control (Fig. 10). We assume that the negative regulation exerted by RsaM2 restrains the QS-2 system response by limiting the self-inducing loop that leads to the accumulation of 3OHC10-HSL, showing again an important homeostatic modulation of AHL production in B. thailandensis E264. The negative impact of RsaM2 on the production of 3OHC8-HSL, as for the RsaM2-dependent regulation of 3OHC10-HSL biosynthesis, might result from modulation of btaI2 transcription (Fig. 10). Remarkably, we noticed that the production of 3OHC10-HSL is repressed by RsaM2 from the exponential phase (Fig. 4A), whereas 3OHC8-HSL biosynthesis is repressed by RsaM2 from the stationary phase (Fig. 5B). This is consistent with our proposal that 3OHC8-HSL is produced by BtaI2 at the expense of 3OHC10-HSL in the stationary phase (16). Since the transcription of neither btaI3 nor btaR3 seems to be under RsaM2 control, we conclude that RsaM2 does not influence 3OHC8-HSL biosynthesis by modulating the QS-3 system gene transcription.
It is not clear how C8-HSL biosynthesis is repressed by RsaM2 when no matching overexpression of btaI1 is observed in the absence of RsaM2 (Fig. 3B and C), as we confirmed the loss of C8-HSL production in the ΔbtaI1 mutant in comparison with the wild-type strain, indicating that this AHL is exclusively synthesized by BtaI1 (17) (Fig. 2A). We recently reported that the QS-1 and QS-2 systems are transcriptionally linked (16) and indeed determined that C8-HSL biosynthesis and the transcription of btaI1, but not btaR1, are repressed by BtaR2 (Fig. S6). Therefore, while the QS-2 system appears to directly repress the production of C8-HSL by modulating btaI1 transcription, it is possible that C8-HSL biosynthesis is also negatively and indirectly controlled by the QS-2 system, underscoring an additional modulatory layer connecting the QS-1 and QS-2 systems in B. thailandensis E264 (Fig. 10). In fact, the negative impact of RsaM2 on the production of C8-HSL could involve additional transcriptional and/or posttranscriptional regulators. More experiments will thus be necessary to determine the precise underlying molecular mechanism of action of RsaM2.
We demonstrated that RsaM1 and RsaM2 repress their own transcription (Fig. 9). Negative autoregulation of these repressors could be necessary to maintain AHLs at appropriate levels depending on particular environmental conditions, likely contributing further to the hierarchical and homeostatic expression of the QS-1, QS-2, and QS-3 systems (Fig. 10).
Conclusion.
We recently reported the complex organization of the QS-1, QS-2, and QS-3 systems in B. thailandensis E264 and we observed that these QS systems are integrated into an intricate modulatory network, including the required involvement of additional regulators (16). The study described here uncovers the central role of RsaM1 and RsaM2 in the modulation of AHL signaling in this bacterium (Fig. 10). We demonstrated that RsaM1 mainly represses the QS-1 system, whereas RsaM2 principally represses the QS-2 system. Additionally, RsaM1 and RsaM2 were shown to be an integral part of the QS circuitry in B. thailandensis, contributing to the temporal activation of its multiple QS systems by modulating the production of AHLs. The precise underlying molecular mechanism of action of these proteins is, however, currently unknown and has to be further investigated in the future given their importance in the regulation of QS-controlled genes in the Burkholderia genus and other proteobacteria (8–12, 20–22).
MATERIALS AND METHODS
Bacterial strains and culture conditions.
The bacterial strains used in this study are listed in Table S1 in the supplemental material. Unless stated otherwise, all bacteria were cultured at 37°C in tryptic soy broth (TSB; BD Difco, Mississauga, Ontario, Canada), with shaking (240 rpm) in a TC-7 roller drum (New Brunswick, Canada), or on petri dishes containing TSB solidified with 1.5% agar. When required, antibiotics were used at the following concentrations: 200 μg/ml tetracycline (Tc) and 100 μg/ml trimethoprim (Tp) for B. thailandensis E264, and 15 μg/ml Tc for Escherichia coli DH5α. All measurements of optical density at 600 nm (OD600) were acquired with a Thermo Fisher Scientific NanoDrop ND-1000 spectrophotometer.
Construction of plasmids.
All plasmids used in this study are described in Table S2. Amplification of btaR2 was performed from genomic DNA from B. thailandensis E264 using the appropriate primers (Table S3). The amplified product was digested with the FastDigest restriction enzymes BamHI and HindIII (Thermo Fisher Scientific) and inserted by T4 DNA ligase (Bio Basic, Inc., Markham, Ontario, Canada) within the corresponding restriction sites in the pME6000 plasmid (43), generating the constitutive expression vector pMCG21. All primers were purchased from Alpha DNA (Montreal, Quebec, Canada).
Construction of recombinant strains.
The pME6000 and pM6000-btaR2 constitutive expression vectors were introduced in B. thailandensis E264 strains by electroporation. Briefly, bacterial cultures were grown to an OD600 of 1.0, pelleted by centrifugation, and washed several times with 1 ml of sterile water. The pellets were concentrated 100-fold in 100 μl of sterile water and electroporated using a 1-mm-gap disposable electroporation cuvette at 1.8 kV with an Eppendorf Electroporator 2510 (Eppendorf Scientific, Inc., Westbury, NY). Cells were grown for 1 h in 1 ml lysogeny broth (LB) (Alpha Biosciences, Inc., Baltimore, MD) at 37°C then plated on Tc-selective medium.
Construction of reporter strains.
Chromosomal integration of the mini-CTX-btaI1-lux, mini-CTX-btaI2-lux, and mini-CTX-btaI3-lux transcriptional reporters at the attB locus in B. thailandensis E264 strains was performed through conjugation with the auxotrophic E. coli χ7213. Overnight bacterial cultures of B. thailandensis E264 strains were diluted in 1.5 ml TSB to an initial OD600 of 0.1 and incubated as described above. Overnight bacterial cultures of E. coli χ7213 carrying the corresponding chromosomal reporters were diluted in 1.5 ml TSB supplemented with 62.5 μg/ml diaminopimelic acid (DAP) to an initial OD600 of 0.1 and statically grown at 37°C. When the cultures reached an OD600 of 0.5, they were pelleted by centrifugation. The pellets were resuspended together in 100 μl TSB and then spotted onto TSB agar plates containing DAP and incubated overnight at 37°C. The bacterial strains were suspended in 1 ml TSB and then plated on Tc-selective medium. Successful chromosomal insertion of the btaI1-lux, btaI2-lux, and btaI3-lux plasmids was confirmed by PCR using appropriate primers.
LC-MS/MS quantification of AHLs.
The concentration of AHLs was determined from samples of B. thailandensis E264 cultures obtained at different time points during bacterial growth, using liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS). The samples were prepared and analyzed as described previously (37). 5,6,7,8-Tetradeutero-4-hydroxy-2-heptylquinoline (HHQ-d4) was used as an internal standard. All experiments were performed in triplicate and conducted at least twice independently.
Measurement of the activities of btaI1-lux, btaI2-lux, and btaI3-lux reporters.
The levels of transcription from the promoter regions of btaI1, btaI2, or btaI3 were quantified by measuring the luminescence of B. thailandensis E264 cultures carrying the corresponding chromosomal reporters, as described previously (16). Overnight bacterial cultures were diluted in TSB to an initial OD600 of 0.1 and incubated as indicated above. The luminescence was regularly determined from culture samples using a multimode microplate reader (Cytation 3; BioTek Instruments, Inc., Winooski, VT) and expressed in relative light units per optical density of the culture at 600 nm (RLU/OD600). All experiments were performed with three biological replicates and repeated at least twice.
Quantitative reverse transcription-PCR experiments.
Total RNA of B. thailandensis E264 cultures at an OD600 of 4.0 was extracted with the PureZOL RNA isolation reagent (Bio-Rad Laboratories, Mississauga, Ontario, Canada) and treated twice with the Turbo DNA-free kit (Ambion Life Technologies, Inc., Burlington, Ontario, Canada), according to the manufacturer's instructions. Extractions were done on three different bacterial cultures. Quality and purity controls were confirmed by agarose gel electrophoresis and UV spectrophotometric analysis, respectively. cDNA synthesis was performed using the iScript reverse transcription supermix (Bio-Rad Laboratories), and amplification was accomplished on a Corbett Life Science Rotor-Gene 6000 thermal cycler using the SsoAdvanced universal SYBR green supermix (Bio-Rad Laboratories), according to the manufacturer's protocol. The reference gene was ndh (44). The ndh gene displayed stable transcription under the different genetic contexts tested. All primers used for cDNA amplification are presented in Table S4. Differences in gene transcription between B. thailandensis E264 strains were calculated using the 2−ΔΔCT formula (45). A threshold of 0.5 was chosen as significant. For experiments with AHL additions, cultures were supplemented with 10 μM C8-HSL, 3OHC10-HSL, and 3OHC8-HSL (Sigma-Aldrich Co., Oakville, Ontario, Canada) or not supplemented with AHLs from stocks prepared in high-performance liquid chromatography (HPLC)-grade acetonitrile. Acetonitrile only was added to the controls. All experiments were performed in triplicate and conducted at least twice independently.
Data analysis.
Unless stated otherwise, data are reported as means ± standard deviations (SD). Statistical analyses were performed with the R software version 3.3.3 (http://www.R-project.org/) using one-way analysis of variance (ANOVA) or a t test. Probability values of less than 0.05 were considered significant.
Supplementary Material
ACKNOWLEDGMENTS
We thank Everett Peter Greenberg (Department of Microbiology, University of Washington School of Medicine, Seattle, WA) for providing the B. thailandensis E264 strains. We especially thank Sylvain Milot and François D'Heygere for their technical help.
This study was supported by Canadian Institutes of Health Research (CIHR) operating grants MOP-97888 and MOP-142466 to Eric Déziel. Eric Déziel holds the Canada Research Chair in Sociomicrobiology.
The funders had no role in the study design, data collection and interpretation, or the decision to submit the work for publication.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/JB.00727-17.
REFERENCES
- 1.Fuqua WC, Winans SC, Greenberg EP. 1994. Quorum sensing in bacteria: the LuxR-LuxI family of cell density-responsive transcriptional regulators. J Bacteriol 176:269–275. doi: 10.1128/jb.176.2.269-275.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nealson KH, Platt T, Hastings JW. 1970. Cellular control of the synthesis and activity of the bacterial luminescent system. J Bacteriol 104:313–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fuqua WC, Greenberg EP. 2002. Listening in on bacteria: acyl-homoserine lactone signalling. Nat Rev Mol Cell Biol 3:685–695. doi: 10.1038/nrm907. [DOI] [PubMed] [Google Scholar]
- 4.Coenye T, Vandamme P. 2003. Diversity and significance of Burkholderia species occupying diverse ecological niches. Environ Microbiol 5:719–729. doi: 10.1046/j.1462-2920.2003.00471.x. [DOI] [PubMed] [Google Scholar]
- 5.Vial L, Chapalain A, Groleau MC, Déziel E. 2011. The various lifestyles of the Burkholderia cepacia complex species: a tribute to adaptation. Environ Microbiol 13:1–12. doi: 10.1111/j.1462-2920.2010.02343.x. [DOI] [PubMed] [Google Scholar]
- 6.Mahenthiralingam E, Urban TA, Goldberg JB. 2005. The multifarious, multireplicon Burkholderia cepacia complex. Nat Rev Microbiol 3:144–156. doi: 10.1038/nrmicro1085. [DOI] [PubMed] [Google Scholar]
- 7.Eberl L. 2006. Quorum sensing in the genus Burkholderia. Int J Med Microbiol 296:103–110. doi: 10.1016/j.ijmm.2006.01.035. [DOI] [PubMed] [Google Scholar]
- 8.Choudhary KS, Hudaiberdiev S, Gelencser Z, Goncalves Coutinho B, Venturi V, Pongor S. 2013. The organization of the quorum sensing luxI/R family genes in Burkholderia. Int J Mol Sci 14:13727–13747. doi: 10.3390/ijms140713727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mattiuzzo M, Bertani I, Ferluga S, Cabrio L, Bigirimana J, Guarnaccia C, Pongor S, Maraite H, Venturi V. 2011. The plant pathogen Pseudomonas fuscovaginae contains two conserved quorum sensing systems involved in virulence and negatively regulated by RsaL and the novel regulator RsaM. Environ Microbiol 13:145–162. doi: 10.1111/j.1462-2920.2010.02316.x. [DOI] [PubMed] [Google Scholar]
- 10.Uzelac G, Patel HK, Devescovi G, Licastro D, Venturi V. 2017. Quorum sensing and RsaM regulons of the rice pathogen Pseudomonas fuscovaginae. Microbiology 163:765–777. doi: 10.1099/mic.0.000454. [DOI] [PubMed] [Google Scholar]
- 11.Inhülsen S. 2011. Investigations on the quorum sensing circuitry in Burkholderia cenocepacia H111. Ph.D dissertation, University of Zurich, Zurich, Switzerland. [Google Scholar]
- 12.Michalska K, Chhor G, Clancy S, Jedrzejczak R, Babnigg G, Winans SC, Joachimiak A. 2014. RsaM: a transcriptional regulator of Burkholderia spp. with novel fold. FEBS J 281:4293–4306. doi: 10.1111/febs.12868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brett PJ, DeShazer D, Woods DE. 1998. Burkholderia thailandensis sp. nov., a Burkholderia pseudomallei-like species. Int J Syst Bacteriol 48:317–320. doi: 10.1099/00207713-48-1-317. [DOI] [PubMed] [Google Scholar]
- 14.Valade E, Thibault FM, Gauthier YP, Palencia M, Popoff MY, Vidal DR. 2004. The PmlI-PmlR quorum-sensing system in Burkholderia pseudomallei plays a key role in virulence and modulates production of the MprA protease. J Bacteriol 186:2288–2294. doi: 10.1128/JB.186.8.2288-2294.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Song Y, Xie C, Ong YM, Gan YH, Chua KL. 2005. The BpsIR quorum-sensing system of Burkholderia pseudomallei. J Bacteriol 187:785–790. doi: 10.1128/JB.187.2.785-790.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Le Guillouzer S, Groleau MC, Déziel E. 2017. The complex quorum sensing circuitry of Burkholderia thailandensis is both hierarchically and homeostatically organized. mBio 8:e01861-. doi: 10.1128/mBio.01861-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chandler JR, Duerkop BA, Hinz A, West TE, Herman JP, Churchill ME, Skerrett SJ, Greenberg EP. 2009. Mutational analysis of Burkholderia thailandensis quorum sensing and self-aggregation. J Bacteriol 191:5901–5909. doi: 10.1128/JB.00591-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Majerczyk CD, Brittnacher M, Jacobs M, Armour CD, Radey M, Schneider E, Phattarasokul S, Bunt R, Greenberg EP. 2014. Global analysis of the Burkholderia thailandensis quorum sensing-controlled regulon. J Bacteriol 196:1412–1424. doi: 10.1128/JB.01405-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duerkop BA, Varga J, Chandler JR, Peterson SB, Herman JP, Churchill ME, Parsek MR, Nierman WC, Greenberg EP. 2009. Quorum-sensing control of antibiotic synthesis in Burkholderia thailandensis. J Bacteriol 191:3909–3918. doi: 10.1128/JB.00200-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gelencsér Z, Choudhary KS, Coutinho BG, Hudaiberdiev S, Galbats B, Venturi V, Pongor S. 2012. Classifying the topology of AHL-driven quorum sensing circuits in proteobacterial genomes. Sensors (Basel) 12:5432–5444. doi: 10.3390/s120505432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen R, Barphagha IK, Karki HS, Ham JH. 2012. Dissection of quorum-sensing genes in Burkholderia glumae reveals non-canonical regulation and the new regulatory gene tofM for toxoflavin production. PLoS One 7:e52150. doi: 10.1371/journal.pone.0052150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Venturi V, Rampioni G, Pongor S, Leoni L. 2011. The virtue of temperance: built-in negative regulators of quorum sensing in Pseudomonas. Mol Microbiol 82:1060–1070. doi: 10.1111/j.1365-2958.2011.07890.x. [DOI] [PubMed] [Google Scholar]
- 23.Seyedsayamdost MR, Chandler JR, Blodgett JA, Lima PS, Duerkop BA, Oinuma K, Greenberg EP, Clardy J. 2010. Quorum-sensing-regulated bactobolin production by Burkholderia thailandensis E264. Org Lett 12:716–719. doi: 10.1021/ol902751x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carr G, Seyedsayamdost MR, Chandler JR, Greenberg EP, Clardy J. 2011. Sources of diversity in bactobolin biosynthesis by Burkholderia thailandensis E264. Org Lett 13:3048–3051. doi: 10.1021/ol200922s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goo E, An JH, Kang Y, Hwang I. 2015. Control of bacterial metabolism by quorum sensing. Trends Microbiol 23:567–576. doi: 10.1016/j.tim.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 26.Goo E, Majerczyk CD, An JH, Chandler JR, Seo YS, Ham H, Lim JY, Kim H, Lee B, Jang MS, Greenberg EP, Hwang I. 2012. Bacterial quorum sensing, cooperativity, and anticipation of stationary-phase stress. Proc Natl Acad Sci U S A 109:19775–19780. doi: 10.1073/pnas.1218092109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tseng BS, Majerczyk CD, Passos da Silva D, Chandler JR, Greenberg EP, Parsek MR. 2016. Quorum sensing influences Burkholderia thailandensis biofilm development and matrix production. J Bacteriol 198:2643–2650. doi: 10.1128/JB.00047-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rampioni G, Bertani I, Zennaro E, Polticelli F, Venturi V, Leoni L. 2006. The quorum-sensing negative regulator RsaL of Pseudomonas aeruginosa binds to the lasI promoter. J Bacteriol 188:815–819. doi: 10.1128/JB.188.2.815-819.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rampioni G, Schuster M, Greenberg EP, Bertani I, Grasso M, Venturi V, Zennaro E, Leoni L. 2007. RsaL provides quorum sensing homeostasis and functions as a global regulator of gene expression in Pseudomonas aeruginosa. Mol Microbiol 66:1557–1565. doi: 10.1111/j.1365-2958.2007.06029.x. [DOI] [PubMed] [Google Scholar]
- 30.Rampioni G, Polticelli F, Bertani I, Righetti K, Venturi V, Zennaro E, Leoni L. 2007. The Pseudomonas quorum-sensing regulator RsaL belongs to the tetrahelical superclass of H-T-H proteins. J Bacteriol 189:1922–1930. doi: 10.1128/JB.01552-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bondi R, Messina M, De Fino I, Bragonzi A, Rampioni G, Leoni L. 2014. Affecting Pseudomonas aeruginosa phenotypic plasticity by quorum sensing dysregulation hampers pathogenicity in murine chronic lung infection. PLoS One 9:e112105. doi: 10.1371/journal.pone.0112105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gupta R, Schuster M. 2013. Negative regulation of bacterial quorum sensing tunes public goods cooperation. ISME J 7:2159–2168. doi: 10.1038/ismej.2013.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Siehnel R, Traxler B, An DD, Parsek MR, Schaefer AL, Singh PK. 2010. A unique regulator controls the activation threshold of quorum-regulated genes in Pseudomonas aeruginosa. Proc Natl Acad Sci U S A 107:7916–7921. doi: 10.1073/pnas.0908511107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suárez-Moreno ZR, Caballero-Mellado J, Venturi V. 2008. The new group of non-pathogenic plant-associated nitrogen-fixing Burkholderia spp. shares a conserved quorum-sensing system, which is tightly regulated by the RsaL repressor. Microbiology 154:2048–2059. doi: 10.1099/mic.0.2008/017780-0. [DOI] [PubMed] [Google Scholar]
- 35.Wei Y, Ryan GT, Flores-Mireles AL, Costa ED, Schneider DJ, Winans SC. 2011. Saturation mutagenesis of a CepR binding site as a means to identify new quorum-regulated promoters in Burkholderia cenocepacia. Mol Microbiol 79:616–632. doi: 10.1111/j.1365-2958.2010.07469.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O'Grady EP, Viteri DF, Malott RJ, Sokol PA. 2009. Reciprocal regulation by the CepIR and CciIR quorum sensing systems in Burkholderia cenocepacia. BMC Genomics 10:441. doi: 10.1186/1471-2164-10-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chapalain A, Groleau MC, Le Guillouzer S, Miomandre A, Vial L, Milot S, Déziel E. 2017. Interplay between 4-hydroxy-3-methyl-2-alkylquinoline and N-acyl-homoserine lactone signaling in a Burkholderia cepacia complex clinical strain. Front Microbiol 8:1021. doi: 10.3389/fmicb.2017.01021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ulrich RL, Deshazer D, Brueggemann EE, Hines HB, Oyston PC, Jeddeloh JA. 2004. Role of quorum sensing in the pathogenicity of Burkholderia pseudomallei. J Med Microbiol 53:1053–1064. doi: 10.1099/jmm.0.45661-0. [DOI] [PubMed] [Google Scholar]
- 39.Ulrich RL, Deshazer D, Hines HB, Jeddeloh JA. 2004. Quorum sensing: a transcriptional regulatory system involved in the pathogenicity of Burkholderia mallei. Infect Immun 72:6589–6596. doi: 10.1128/IAI.72.11.6589-6596.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ulrich RL, Hines HB, Parthasarathy N, Jeddeloh JA. 2004. Mutational analysis and biochemical characterization of the Burkholderia thailandensis DW503 quorum-sensing network. J Bacteriol 186:4350–4360. doi: 10.1128/JB.186.13.4350-4360.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gamage AM, Shui G, Wenk MR, Chua KL. 2011. N-Octanoylhomoserine lactone signalling mediated by the BpsI-BpsR quorum sensing system plays a major role in biofilm formation of Burkholderia pseudomallei. Microbiology 157:1176–1186. doi: 10.1099/mic.0.046540-0. [DOI] [PubMed] [Google Scholar]
- 42.Duerkop BA, Ulrich RL, Greenberg EP. 2007. Octanoyl-homoserine lactone is the cognate signal for Burkholderia mallei BmaR1-BmaI1 quorum sensing. J Bacteriol 189:5034–5040. doi: 10.1128/JB.00317-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maurhofer M, Reimmann C, Schmidli-Sacherer P, Heeb S, Haas D, Defago G. 1998. Salicylic acid biosynthetic genes expressed in Pseudomonas fluorescens strain P3 improve the induction of systemic resistance in tobacco against tobacco necrosis virus. Phytopathology 88:678–684. doi: 10.1094/PHYTO.1998.88.7.678. [DOI] [PubMed] [Google Scholar]
- 44.Subsin B, Chambers CE, Visser MB, Sokol PA. 2007. Identification of genes regulated by the cepIR quorum-sensing system in Burkholderia cenocepacia by high-throughput screening of a random promoter library. J Bacteriol 189:968–979. doi: 10.1128/JB.01201-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.