Five genetic loci were found to be associated with the fuzzless seed trait in Gossypium barbadense, one of them containing MYB25-like_Dt, the best candidate for the N2 gene.
Keywords: Bulk segregant analysis, fuzzless, fuzz percentage, G. barbadense, lintless, mapping-by-sequencing
Abstract
Cotton fibres are single-celled trichomes arising from the epidermal cells of the seed coat and may be either long (lint) or very short (fuzz). The dominant fuzzless N1 of Gossypium hirsutum is a defective allele of the At-subgenome homoeolog of MYB25-like, but the genetic components underlying the recessive fuzzless trait from G. barbadense (Gb) are unknown. We have identified five genetic loci, including a major contributing locus containing MYB25-like_Dt, associated with Gb fuzzless seeds based on genotyping of fuzzy and fuzzless near isogenic lines (NILs) from an interspecies cross (G. barbadense × G. hirsutum). At 3 d post-anthesis when fuzz fibres are initiating, expression of MYB25-like_Dt was significantly lower in fuzzless NILs than in fuzzy seeded NILs, while higher MYB25-like_Dt expression was associated with more seed fuzz across different cotton genotypes. Phenotypic and genotypic analysis of MYB25-like homoeoalleles in cottons showing different fibre phenotypes and their crossing progeny indicated that both MYB25-like_At and MYB25-like_Dt are associated with lint development, and that fuzz development is mainly determined by the expression level of MYB25-like_Dt at ~3 d post-anthesis. Expression of Gb fuzzless seeds depends on genetic background and interactions amongst the multiple loci identified. MYB25-like_Dt is one of the best candidates for N2.
Introduction
Mature cotton seeds are covered with two types of fibres: lint (up to ~3.5 cm) and fuzz (<0.5 cm). Both are single-celled, tubular outgrowths that arise from the epidermal cells of the seed coat and are indistinguishable in appearance during the early stages of their growth (Lang, 1938; Stewart, 1975), suggesting that their growth may involve the same physiological and biochemical processes. Lint fibre initials in Gossypium hirsutum (Gh) and G. barbadense (Gb) start developing on the day of anthesis, i.e. 0 d post-anthesis (dpa), with approximately a quarter to a third of the epidermal cells becoming fibre initials and finally lint fibres (Lang, 1938; Stewart, 1975). Fuzz fibres start developing after the lint at ~4 dpa, do not elongate to the same extent as the lint, and are variable in length and abundance among different genotypes (Lang, 1938; Joshi et al., 1967; Lee et al., 2006), with some cottons having no fuzz fibres, but still producing normal lint fibres.
Fuzzless cotton seeds are referred to as ‘naked seeds’ and have advantages during ginning because they generally require much less force to remove the lint from the seed than fuzzy seeded cottons, and hence less power consumption at the gin and less breakage of the lint fibres (Boykin, 2007; Bechere et al., 2009, 2012). A number of fuzzless loci have been reported in cotton, including the dominant N1 and the recessive n2 (Percy & Kohel, 1999). Homozygous N1N1 mutants are completely fuzzless, and lack any tuft (seen in the recessive mutants) at the micropylar tip of the seed and also have a significantly reduced lint percentage (Ware, 1940; Turley & Kloth, 2002; Turley et al., 2007) probably because of delayed lint initiation and their shorter lint (Lee et al., 2006; Turley et al., 2007; Zhang et al., 2007; Romano et al., 2011). In earlier genetic studies, the recessive n2 fuzzless mutant was believed to have a genotype of n2n2. However, Turley & Kloth (2002) demonstrated that a third unlinked recessive locus, n3, is required for expression of the fuzzless trait in accessions carrying n2. This second locus may have confounded some earlier genetic studies of the n2 mutant, which shows variable fuzz development that is influenced by genetic background and environmental conditions (Turley & Kloth, 2002; Rong et al., 2005). Compared with the wild-type TM-1, in which fuzz initiation was observed at 4 dpa, few or no epidermal protrusions were observed in the N1 and n2n3 mutants at the same time point (Zhang et al., 2007). Cotton plants homozygous for all three fuzzless loci (N1N1n2n2n3n3) are fibreless (i.e. lack both lint and fuzz; Turley & Kloth, 2002), so clearly the genes at these loci have central roles in the development of both types of fibres. A new ethyl methanesulfonate-induced mutant, n4t, was recently reported that appears to be different from the other naked seed loci (Bechere et al., 2009, 2012). The n4t mutation has been reported to have a less negative effect on lint percentage and hence lint development (Bechere et al., 2012). In addition, no fuzzy seeded but lintless phenotype has ever been observed in cotton, suggesting that fuzz genes are epistatic to lint genes (Du et al., 2001) and/or that development of fuzz and lint are temporally and spatially regulated by the same regulatory genes. The genetic control of fuzz development is clearly very complex and there are many different genes other than these major naked seed genes that modify the amount of fuzz on the seed (Turley & Kloth, 2002) even on the same plant (Kearney & Harrison, 1928), and few of these have been characterized. Cotton breeders have long aimed to develop cotton cultivars with fuzzless seeds and a high lint percentage to capitalize on their ginning advantages, but to achieve that goal, it is essential to identify the genes underlying the regulation of fuzz and lint development and to understand their biological roles and genetic interactions.
Cotton is an allotetraploid with At and Dt subgenomes. The N1 and the n2 genes have been localized to chromosomes 12 (A12) and 26 (D12) (a pair of homoeologous chromosomes), respectively (Endrizzi & Ramsay, 1980; Samora et al. 1994). The n3 locus has not yet been mapped, but is reported to be unlinked to n2 (Turley & Kloth, 2002). Commercial Gb cultivars produce some of the highest quality lint fibres of any cotton species, but all appear to contain the n2 gene making them almost universally fuzzless, although there is considerable environmental effect on the amount of fuzz (Kearney & Harrison, 1928). It is not known if all Gb cultivars also carry the n3 locus, but this is highly likely. A recent study using a map-based cloning strategy has identified the dominant N1 gene to be a defective allele of the At-subgenome homoeolog of MYB25-like (i.e. MYB25-like_At) that generates small interfering RNAs from its 3′ end due to production of an overlapping antisense transcript that post-transcriptionally silences both homoeologs of this gene (Wan et al., 2016). MYB25-like is a master regulator involved in fibre initiation and development as silencing MYB25-like results in cotton seeds lacking both fuzz and lint fibres (Walford et al., 2011). However, the identities of the n2 and n3 genes are as yet unknown.
High-throughput genotyping and next-generation sequencing technologies are revolutionizing the speed and ability to fine-map and clone genes underlying agronomically important traits, which are usually controlled by multiple genes with complex interactions (Schneeberger, 2014; Zhu et al., 2014a). In cotton, a large number of single nucleotide polymorphism (SNP) markers have been reported based on whole genome re-sequencing of diverse cotton cultivars and transcriptome sequencing (Byers et al., 2012; Zhu et al., 2014b; Hulse-Kemp et al., 2015a; Wang et al., 2015; Fang et al., 2017). A cotton SNP array containing ~63 000 SNPs, mainly from US and Australian cotton cultivars, was recently developed (Hulse-Kemp et al., 2015b) and has been widely used by the cotton community for a diversity of studies (Ellis et al., 2016; Li et al., 2016; Wang et al., 2016; Gapare et al., 2017; Hinze et al., 2017; Huang et al., 2017a), including identification of the gene responsible for okra leaf shape (Zhu et al., 2016). One of the applications of next-generation sequencing in identification of genes underlying specific phenotypes is mapping-by-sequencing (MBS) where pools of segregating progeny are bulked according to their mutant phenotype and their genome sequenced to identify genomic regions inherited predominantly from the parent displaying the phenotype (Zhu et al., 2017). This approach has been applied in cotton to map genes related to the development and properties of fibres (Thyssen et al., 2014, 2015, 2016, 2017; Islam et al., 2016) and branching architectures (Chen et al., 2015). In model plant species, such as Arabidopsis, it is possible to narrow down the resolution of MBS to the single gene level as demonstrated recently by the cloning of an imprinted gene involved in endosperm cellularization (Huang et al., 2017b), but this is still difficult in allopolyploid species such as cotton, and therefore final identification of the causative genes usually requires other additional approaches once a genomic region containing the gene is identified.
In this study, we aimed to uncover the genetic basis of the fuzzless trait from G. barbadense and to explore the possibility of breeding G. hirsutum cottons with fuzzless seeds and without the lint percentage penalty normally associated with this trait that has so far prevented its adoption in commercial breeding programmes. To this end, we developed and used near isogenic lines (NILs) for identification of genetic loci tightly linked to the fuzzless trait using the Cotton SNP63K array and the MBS approach. We found that the fuzzless seed trait in Pima S-7, a Gb cultivar, is controlled by multiple recessive loci, including a locus containing MYB25-like_Dt. Our results indicate that lint development is associated with the expression levels of both MYB25-like_At and MYB25-like_Dt at ~0 dpa, while fuzz development is mainly determined by the expression level of MYB25-like_Dt at ~3 dpa.
Methods and materials
Plant materials, development of nearly isogenic lines, and segregating populations
One G. barbadense (Pima S-7, fuzzless) and four G. hirsutum accessions (Sicala 40, Sicala V-2, T586, and Xu142fl) were used in genetic and gene expression analyses. Sicala 40 and Sicala V-2 are normal fuzz commercial cultivars producing copious amounts of lint, T586 is a lint bearing cotton genetic standard line containing the dominant fuzzless gene N1 (Endrizzi & Taylor, 1968), while Xu142fl is a fibreless mutant known to carry n2 amongst other fibre mutations (Zhang & Pan, 1991; Du et al., 2001). In addition, 134 Gb accessions with variable fuzz phenotypes were selected from the National Mid-term Genetic Bank of Cotton at the Institute of Cotton Research (ICR) of the Chinese Academy of Agricultural Sciences, Anyang, China, and used for association analysis.
An F2 population derived from Pima S-7 × Sicala 40 (PS; 169 plants) was used in segregation and fuzz percentage analysis. Pima S-7 and two genetically related Gh cultivars, Sicala V-2 and Sicala 40, were used in the development of a set of NILs each showing fuzzless or fuzzy seeded phenotypes that were selected from a breeder’s population aimed at generating a fuzzless seed in an elite commercial Gh background. After four backcrosses, homozygous fuzzless and normal fuzz NILs were identified and confirmed in BC4F4 and BC4F5 generations (see Supplementary Fig. S1 at JXB online). Selected BC4F4 and BC4F5 plants showing normal fuzz (eight NILs) or reduced fuzz (20 fuzzless or intermediate fuzz NILs) were genotyped using the Cotton SNP63K array. BC4F6 progeny showing uniform fuzzless or segregating fuzz phenotypes were used in bulk segregant sequencing (Supplementary Fig. S1A). A BC5F2 population (designated FLNS) derived from a cross between one of the BC4F5 fuzzless NILs, FLN1-10, and Sicala 40 was used in fuzz segregation and lint percentage analyses. Gene expression analysis was performed using Pima S-7, Sicala 40, T586, Xu142fl, and different BC4F5 NILs that had normal fuzz or were fuzzless. The contributing effects of MYB25-like homoeoalleles on fuzz and lint development were evaluated using two more F2 populations: PX (92 plants) from Pima S-7 × Xu142fl and FLNX (87 plants) from FLN1-10 × Xu142fl. The reason for use of Xu142fl was that both of its MYB25-like homoeologs are very lowly expressed and therefore potentially dysfunctional. All cotton plants, except the 134 Gb accessions, which were planted in the field of ICR’s Experiment Station in Xinjiang, China (2015), were grown in a glasshouse (Canberra, Australia) at 28 ± 2 °C with natural lighting.
Phenotyping
Fuzzy, intermediate, and fuzzless seed phenotypes were scored based on visual inspection. The fuzz phenotype of the PS F2 population and the 134 Gb accessions was measured quantitatively using fuzz percentage, which was calculated using at least 100 seeds and the formula of [(weight of ginned seeds−weight of delinted seeds)/weight of ginned seeds]×100, where the seeds were equilibrated to a constant moisture content before and after sulfuric acid delinting to burn off the fuzz fibres.
DNA sample preparation and SNP genotyping
DNA extraction, SNP genotyping using the Cotton SNP63K array or KASP (Kompetitive Allele Specific PCR) were performed as previously described (Zhu et al., 2016). For calculation of SNP frequencies based on the SNP array, Pima S-7, Sicala 40 and heterozygous alleles were designated 1, 0 and 0.5, respectively. For MYB25-like_Dt, a KASP marker was designed using a SNP located within its coding region (see Supplementary Fig. S2A). The MYB25-like_At alleles of Pima S-7 and Xu142fl were distinguished using species-specific SNPs (Gb vs Gh) and a non-synonymous SNP within the coding region of the Xu142fl MYB25-like_At (Supplementary Fig. S2B), respectively. KASP oligos are shown in Supplementary Table S1.
Bulk segregant analysis and mapping-by-sequencing
DNA was extracted from 24 randomly selected BC4F6 progeny derived from NILs segregating for the fuzz phenotype and bulked to form the Recessive Fuzzless Bulk1 (RFB1) DNA pool. DNA was extracted from 15 BC4F6 progeny derived from fuzzless NILs and bulked to form the RFB2 DNA pool. Two barcoded DNA sequencing libraries were created using RFB1 and RFB2, and sequenced in a single lane using the Illumina HiSeq2500 platform at the Australian Genome Research Facility (Melbourne, Australia). Approximately 50 Gb of 100-bp paired-end reads were generated.
After quality check using FastQC, the clean reads of the two bulks were separately mapped to the Sicala 40 genome sequence, which was generated by mapping re-sequenced Sicala 40 reads to the TM-1 (G. hirsutum) reference genome (Zhang et al., 2015) using CLC Genomics Workbench (version 7.5.1) with the following parameter settings: mismatch cost, 2; insertion and deletion cost, 3; length fraction, 0.5; similarity fraction, 0.95; and non-specifically matched reads ignored. Variants (including SNPs and indels) between RFB1 or RFB2 and Sicala 40 were identified using the ‘basic variant detection’ module implemented in CLC Genomics Workbench with the following settings: ploidy, 2; default read quality filters (i.e. neighbourhood radius, 5; minimum neighbourhood quality, 15; minimum central quality, 20); minimum read coverage, 10; minimum variant read count, 3; minimum variant frequency, 35%. SNPs were filtered from the results. Average SNP, i.e. Pima S-7 allele, frequency of a 1-Mbp window (500 kb overlapping between the two adjacent windows) was calculated for RFB1 and RFB2 using a sliding-window-based approach and plotted for each chromosome using the physical co-ordinates of the TM-1 genome (Zhang et al., 2015). Candidate regions associated with the recessive fuzzless trait should be homozygous for Pima S-7 in the RFB2 pool and hence have a Pima S-7 allele frequency of 1 or close to 1, while in the segregating RFB1 pool the Pima S-7 allele frequency should be ~0.5 because this pool contains plants that are homozygous Pima S-7, heterozygous Pima S-7 and homozygous Sicala 40.
Gene expression analysis using quantitative real-time PCR
Two types of tissues, whole ovules and ovule outer integuments (a 3–4-cell layer including the epidermis that produces the lint and fuzz fibres) were used in gene expression analysis. Whole ovules of different developmental stages were collected from normal fuzz (NFN) or fuzzless (FLN) NILs and used for dissection of outer integuments according to a previously reported protocol (Bedon et al., 2014). For the four cotton accessions (Sicala 40, Pima S-7, T586, and Xu142fl), whole ovules of different developmental stages were directly used for RNA extraction. Total RNA extraction and quantitative real-time PCR (qRT-PCR) procedures were performed as previously described (Zhu et al., 2009, 2013) except that the reference gene used was the cotton ubiquitin gene (GenBank accession no. EU604080), and the reactions were run on the ViiA7 Real-Time PCR System (Life Technologies) using the FasterStart Universal SYBR Green Master Mix (ROX) (Roche). Gene expression levels were determined based on three biological replicates each with three technical replicates. Primers specifically amplifying MYB25-like_At could not be designed, so the expression level of MYB25-like_At was thus determined by subtraction of the expression level of MYB25-like_Dt from the total expression level of MYB25-like. Primers used in qRT-PCR are shown in Supplementary Table S1. All primer pairs had a similar PCR efficiency (89.9–99.2%) determined by LinRegPCR (http://www.hartfaalcentrum.nl/index.php?main=files&sub=LinRegPCR).
Results
The fuzzless trait in G. barbadense is controlled by multiple recessive genetic loci
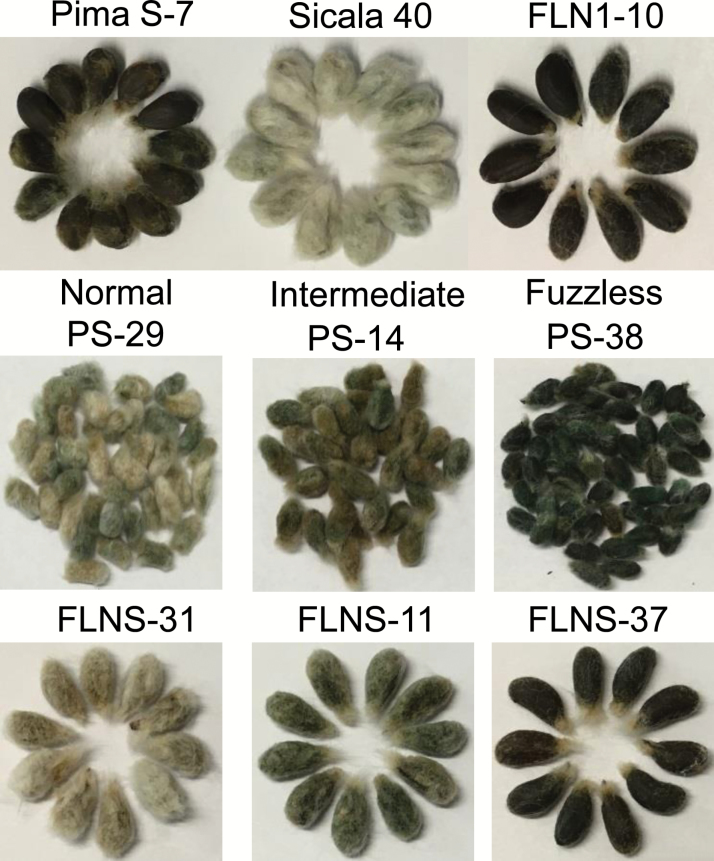
F3 seeds of 169 F2 plants derived from Pima S-7 (Gb) × Sicala 40 (Gh; hereafter named PS) showed a continuous gradation of the fuzz phenotype, from Pima S-7-like (fuzzless) through to Sicala 40-like (normal fuzz) (Fig. 1). Of the 169 F2:F3 families, seeds of only three families (e.g. PS-38; Fig. 1) looked similar to the naked seeds of Pima S-7, fitting a model with three recessive genes controlling fuzz formation (χ263:1,1=0.0497, P=0.8236). However, for the FLNS F2 population created using Sicala 40 and a fuzzless NIL (FLN1-10; Fig. 1; Supplementary Fig. S1), five out of the 60 F2:F3 families showed a Pima S-7-like naked seed phenotype (e.g. FLNS-37; Fig. 1), fitting a model with two recessive genes (χ215:1,1=0.4444, P=0.5050). We noticed that the three fuzzless F2:F3 families observed in the PS population were not completely fuzzless and had a little more fuzz than Pima S-7, and we also observed transgressive segregants (with more fuzz than Sicala 40) in the PS but not in the FLNS F2 populations. These results indicate that (i) fuzz development is complex with at least three recessive genes being involved in the regulation of the fuzzless trait in Pima S-7, and (ii) Pima S-7 must contain additional epistatic modifier(s) affecting the function and/or the interaction of the three loci responsible for its fuzzless phenotype that have not been identified here due to the small population sizes used.
Fig. 1.
Fuzz phenotypes of the parental lines (Pima S-7, Sicala 40, and the fuzzless NIL, FLN1-10) used in crosses and representative normal fuzz, intermediate, and fuzzless seed phenotypes of the segregating PS and FLNS F2 population. PS represents Pima S-7 × Sicala 40. FLN1-10 is a BC4F5 fuzzless NIL described in Fig. S1A.
Identification of genetic loci, including the MYB25-like_Dt containing locus, associated with low seed fuzz
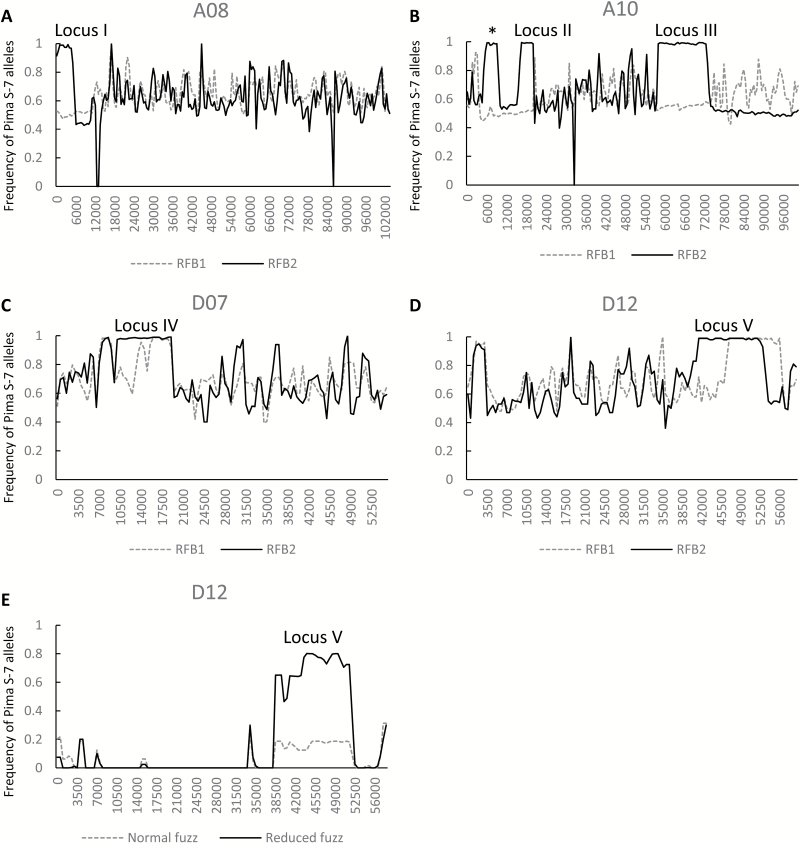
We used NILs with different fuzz phenotypes to identify genetic loci controlling fuzz development (see Supplementary Fig. S1). Eight normal fuzz and 20 reduced fuzz (fuzzless or intermediate) BC4F4 or BC4F5 NILs were used in the SNP array analysis. Of the ~63 000 SNPs, 5426 were polymorphic between Pima S-7 and Sicala 40, and these were distributed across all 26 chromosomes, although chromosomes A02, A04, and D04 had less than 20 SNPs each (Supplementary Table S2). The Pima S-7 allele frequency of the polymorphic SNPs was plotted for each chromosome based on their genomic coordinates relative to the TM-1 genome. For the pool of the 20 reduced fuzz NILs, each candidate locus associated with low fuzz would have a Pima S-7 allele frequency close to 1 because the fuzzless NILs should be homozygous for the Pima S-7 allele and the NILs close to being fuzzless could be still heterozygous for the Pima S-7 allele. For the pool of the eight normal fuzz NILs, each candidate locus would have a Pima S-7 allele frequency <0.5 because these NILs could contain heterozygous or null Pima S-7 alleles. Three regions on chromosomes A12, D05, and D12 met these criteria (Supplementary Table S3; Fig. 2E; Supplementary Fig. S3). The A12 locus contained only three SNP markers and all of them were mapped to the D12 interval based on previous interspecific mapping results (Hulse-Kemp et al., 2015b) and so can be excluded as their positions were incorrectly assigned based on blasting. We further created and sequenced two bulked DNA libraries, RFB1 (progeny of normal fuzz NILs) and RFB2 (progeny of fuzzless NILs). Genome-wide SNP identification was performed for RFB1 and RFB2 using Sicala 40 as a reference. Candidate loci associated with low fuzz seeds would be expected to have a Pima S-7 allele frequency of ~0.5 and 1 in RFB1 and RFB2, respectively. Nine regions on seven different chromosomes met these criteria (Supplementary Table S3; Fig. 2A–D; Supplementary Fig. S4). The D12 locus was identified in both MBS and SNP array analyses, and so represents the best candidate for a major locus contributing to fuzzless seeds.
Fig. 2.
Identification of genetic loci associated with fuzz development. (A–D) Frequency distribution of the Pima S-7 alleles on chromosomes A08, A10, D07, and D12. The results were based on sequencing of bulked segregants showing fuzzless (RFB2) or segregating fuzz phenotypes (RFB1). The graphs were generated using a sliding-window-based approach (1 Mbp window length and 500 kb overlap between the two adjacent windows) with the x-axis representing chromosomal coordinates (kb). The regions indicated by I, II, III, IV and V were the five loci associated with fuzz development, whereas the region indicated by an asterisk was not associated with fuzz development based on fuzz percentage analysis of the PS F2 population and the results from other segregating NILs genotyped by the Cotton SNP63K array. (E) The Pima S-7 allele frequency of the SNP markers polymorphic between Pima S-7 and Sicala 40 on D12 (267 markers). The result was based on the genotyping of eight normal fuzz and 20 reduced fuzz (including fuzzless) BC4F4 or BC4F5 NILs using the Cotton SNP63K array. The graph was generated using the same approach as those in (A–D).
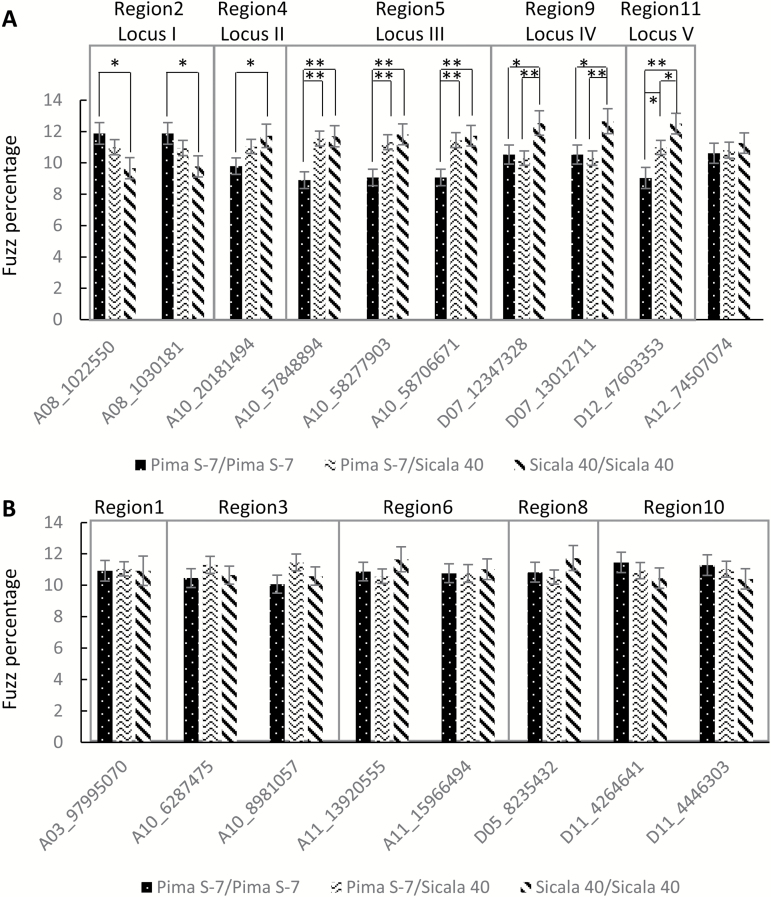
Because the fuzzless NILs used were self-pollinated for a few generations, some of the ten candidate regions identified could thus be false positives due to fixation of Pima S-7 alleles not related to the fuzzless phenotype. To refine these candidate loci we designed KASP marker assays (between 1 and 3 assays for each region) across all the ten candidates and genotyped the PS F2 population with those markers and also measured fuzz percentage for each F2 individual. For each SNP marker, F2 plants were grouped based on their genotypes (i.e. homozygous Pima S-7 or Sicala 40, and heterozygous) and the average fuzz percentage was compared for each genotype. Of the ten candidate regions, five (regions 2, 4, 5, 9, and 11) were found to be positively or negatively correlated with the amount of seed fuzz. They were designated loci I–V (Supplementary Table S3; Fig. 3). For loci II–V, plants with a genotype of homozygous Pima S-7 regions had a significantly lower fuzz percentage than those with a genotype of homozygous Sicala 40, but for locus I (region 2), plants with a genotype of homozygous Pima S-7 had a significantly higher fuzz percentage than those with a genotype of homozygous Sicala 40 (Fig. 3A), suggesting Pima S-7 contained an enhancer rather than an inhibitor of fuzz development within that locus. For locus V (region 11), the fuzz percentage of heterozygous plants was intermediate and significantly different from either homozygous Pima S-7 or Sicala 40 (Fig. 3A), suggesting a major and additive effect of locus V on fuzz percentage, whereas the other loci were closer to one or other of the homozygous parental genotypes. This was supported by the fuzz phenotypes of the segregants within the FLNS population, as its F2:F3 families with a homozygous Pima S-7 region at locus V always had less fuzz than those with a homozygous Sicala 40 region (see Supplementary Fig. S5), and this trend was not observed for the other loci.
Fig. 3.
Association analysis between SNP markers and fuzz percentages of the PS F2:F3 seeds. F2 plants were genotyped using the markers shown below the x-axis. For each marker, F2 plants were grouped based on their genotypes, i.e. homozygous Pima S-7 (Pima S-7/Pima S-7) or Sicala 40 (Sicala 40/Sicala 40), or heterozygous (Pima S-7/Sicala 40) individuals. Error bars represent standard error. (A) Regions or genetic loci associated with fuzz percentage. Asterisks denote significant difference determined by Student’s t-test: *P<0.05, **P<0.01. A12_74507074 was a randomly selected SNP marker and used as a negative control. (B) Regions not associated with fuzz development.
By checking the co-segregation of markers on the individual plants used with the Cotton SNP63K array analysis and the overlap between the SNP array and MBS analyses, we were able to deduce the intervals containing the candidate gene(s) for the five individual loci that contained between 20 and >300 annotated genes. For example, the size of the overlapping interval of locus V is ~1.4 Mbp containing 84 annotated genes (see Supplementary Tables S3 and S4). Fine mapping and/or other strategies will be required to pinpoint the actual causal gene(s) within these loci. However, one of the locus V genes is MYB25-like_Dt (Gh_D12G1628), a homoeolog of MYB25-like_At that has been shown to be critical for fuzz development (Wan et al., 2016). MYB25-like_Dt is thus the best candidate at this locus.
We also found that the average fuzz percentage of the 134 Gb accessions used in this study was low at 2.68% (with a range of 0.29–9.20%), and generally considerably less than the 10–12% found with normal fuzz Gh cultivars. Although the majority of these Gb accessions were fuzzless or close to fuzzless, a few were normal fuzzy seeded (e.g. M210353 in Supplementary Fig. S6). Despite their variable fuzz phenotypes, all of these Gb accessions had a homozygous Pima S-7 genotype in the five loci we have identified, suggesting that there could be additional modifiers of those five fuzzless loci in the rare fuzzy seeded Gb accessions, but this will have to await a more detailed genetic and molecular analysis to ensure that these accessions have not been misclassified or are partial hybrids with Gh.
Expression of MYB25-like_Dt is down-regulated in outer integuments of fuzzless NILs during fuzz initiation
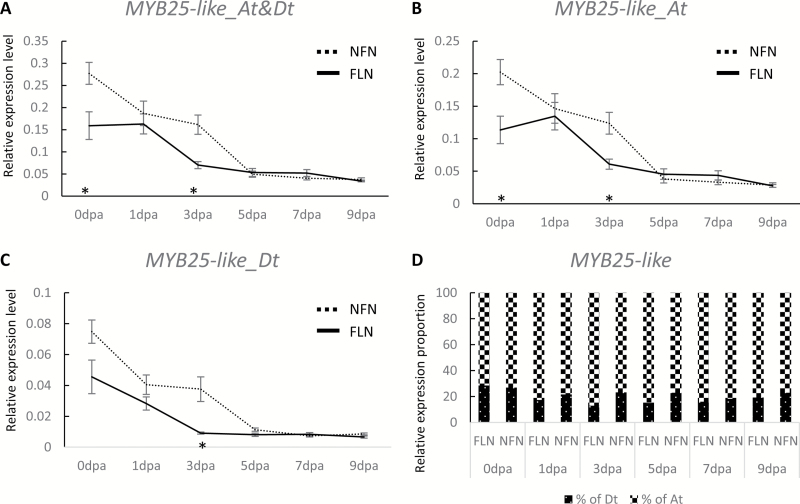
In 0–9 dpa ovule outer integuments of NILs with normal fuzz (NFN) or fuzzless (FLN) seeds, MYB25-like_At and MYB25-like_Dt had a similar expression profile to each other, but were differentially expressed in both seed phenotypes, with approximately 70–80% of the total expression of MYB25-like contributed by its At homoeolog (Fig. 4). In NFN, the expression level of MYB25-like_Dt dropped steadily from 0 to 5 dpa, and then remained relatively low thereafter. The fuzzless FLN had a lower expression level of MYB25-like_Dt from 0 dpa and declined more rapidly than in the normal fuzz NFN NILs (Fig. 4C), such that there was little expression at 3 dpa, just prior to when fuzz fibres would normally initiate. Although the significantly reduced fuzz percentage observed in F2 plants homozygous for the Pima S-7 allele of MYB25-like_Dt (i.e. MYB25-like_DtPimaS7/PimaS7; Fig. 3A) could be contributed by other gene(s) within the same linkage block as MYB25-like_Dt, this expression profile of MYB25-like_Dt, together with the previously reported role of its At homoeolog in fuzz development (Wan et al., 2016), provides additional support for MYB25-like_Dt being one of the best candidates for a gene involved in the reduced fuzz of Pima S-7.
Fig. 4.
Expression profile of MYB25-like in 0–9 dpa outer integuments of the normal fuzz NIL (NFN, homozygous Sicala 40 allele for MYB25-like_Dt) and fuzzless NIL (FLN, homozygous Pima S-7 allele for MYB25-like_Dt). (A–C) Expression level changes of MYB25-like, MYB25-like_At and MYB25-like_Dt. *Significant difference at P<0.05 determined by one-way ANOVA. (D) Relative expression proportion of the At and Dt homoeologs of MYB25-like in 0–9 dpa outer integuments of NFN and FLN.
The protein coding sequences of MYB25-like_Dt between the reference sequence of TM-1 (fuzzy seeded) and Pima S-7 differ at three nucleotides with two of them giving rise to changed amino acids in Pima S-7 (see Supplementary Fig. S2A). In both cases, however, the changed bases are all found in either the Dt-subgenome or the At-subgenome homoeologs from other cultivars including TM-1, Sicala 40, and Xu142 (Supplementary Figs S2A and S7) that have fuzzy seeds, so these SNPs are unlikely to lead to a loss of function of MYB25-like_Dt in Pima S-7. The core promoters (−1 to −250 bp) of MYB25-like_Dt and MYB25-like_At in Pima S-7, Sicala 40, and TM-1 were very similar, but the remaining upstream sequences of their promoters were quite different. Whether any of these differences is the cause of their differential expression remains to be investigated by fusing different lengths of promoter to reporter genes. The promoter of MYB25-like_Dt in Pima S-7, compared with that in Sicala 40 and TM-1, had seven differences, including three SNPs, two small indels (1 bp deletion or insertion), and two deletions (>20 bp) (Supplementary Fig. S8).
Expression levels of MYB25-like_Dt but not of MYB25-like_At predominantly determine the fuzz phenotypes
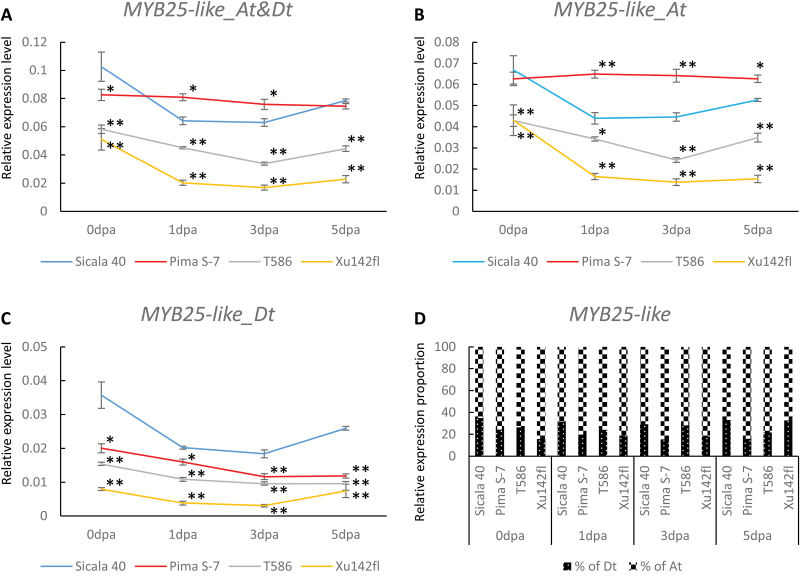
We compared the expression levels of the two MYB25-like homoeologs in 0–5 dpa whole ovules of the linted-fuzzless T586 (N1) and fibreless Xu142fl lines with those of Pima S-7 and Sicala 40 (Fig. 5). At 0 dpa when lint fibres have started to initiate, the total expression level of MYB25-like was highest in Sicala 40 and lowest in Xu142fl with a ranking of Sicala 40>Pima S-7>T586>Xu142fl (Fig. 5A), which is consistent with their rankings in final lint percentage (Sicala 40: 41.58%; Pima S-7: 34.51%; T586: 11.37%; Xu142fl: 0), suggesting that the expression level of MYB25-like at ~0 dpa is correlated with lint fibre initiation and development. In 1–5 dpa ovules, Pima S-7 had a more lowly expressed MYB25-like_Dt than Sicala 40, but a significantly more highly expressed MYB25-like_At (Fig. 5B, C) despite being fuzzless, supporting the association between the expression of MYB25-like_Dt, but not MYB25-like_At, and the poor fuzz development in Pima S-7. From 0 to 5 dpa, the expression levels of both MYB25-like homoeologs were consistently lower in Xu142fl and T586 than in Sicala 40 and Pima S-7. Around the time of fuzz initiation at 3 dpa, Xu142fl had the lowest expression of both homoeologs in any of the genotypes, but particularly the Dt homoeolog. In addition, there is a non-conservative substitution at position 314 within the conserved MYB DNA binding domain of MYB25-like_At in Xu142fl (see Supplementary Fig. S2B) that is likely to negatively impact on the activity of this transcription factor, supporting the assertion previously reported (Walford et al., 2011) that this fibreless mutant line has two dysfunctional MYB25-like homoeologs.
Fig. 5.
Expression profile of MYB25-like in 0–5 dpa whole ovules of four cotton accessions with different fuzz and lint phenotypes. (A) Total expression level of MYB25-like (both homoeologs). (B) Expression level of MYB25-like_At. (C) Expression level of MYB25-like_Dt. Asterisks denote a significant difference compared with the expression level in Sicala 40 determined by one-way ANOVA: *P<0.05, **P<0.01. (D) Relative expression proportion of the At and Dt homoeologs of MYB25-like in 0–5 dpa whole ovules of the same four cotton accessions.
To investigate the effect of different MYB25-like homoeoalleles from Gh and Gb on lint and fuzz development, we generated two other segregating F2 populations, PX from Pima S-7 x Xu142fl and FLNX from FLN1-10 (MYB25-like_DtPimaS7/PimaS7 locus in an essentially Sicala 40 genetic background) × Xu142fl and examined lint and fuzz phenotypes of the F2 plants with different allele combinations determined by the presence of SNPs specific to each of the MYB25-like homoeologs and their parental origins (Table 1; Supplementary Fig. S9). The difference in these two populations was in the maternal allele of MYB25-like_At, which was from Pima S-7 in PX and from Sicala 40 in FLNX, both of which should be highly expressed and as demonstrated below, fully functional (Fig. 5B). For each F2 population, individual plants were classified into 9 types (1–9 and 10–18 for PX and FLNX, respectively) based on the homozygosity or heterozygosity of parental origin for the At and Dt homoeolog of MYB25-like. A number of observations were clear from this analysis:
Table 1.
Segregation of the fuzzless trait by MYB25-like homoeoallele combination in two F2 populations
| Type | MYB25-like_At a | MYB25-like_Dt a | No. of plants (%) | Lintd | Significancee | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Normal | Intermediate | Fuzzless | Tufted | Not tufted | (%) | P<0.05 | P<0.01 | |||
| The PX F 2 population (Pima S-7 × Xu142fl) | ||||||||||
| 1 | Xu142fl(M)/Xu142fl(M) | Xu142fl(M)/Xu142fl(M) | 0 (0) | 0 (0) | 5 (5.43)b | 0 (0) | 5 (5.43) | 0.77 | a | A |
| 2 | Xu142fl(M)/Xu142fl(M) | Xu142fl(M)/Pima S-7(M) | 0 (0) | 0 (0) | 11 (11.96)c | 0 (0) | 11 (11.96) | 17.17 | b | B |
| 3 | Xu142fl(M)/Xu142fl(M) | Pima S-7(M)/Pima S-7(M) | 0 (0) | 0 (0) | 3 (3.26) | 0 (0) | 3 (3.26) | 22.44 | bc | BC |
| 4 | Xu142fl(M)/Pima S-7(N) | Xu142fl(M)/Xu142fl(M) | 0 (0) | 0 (0) | 15 (16.30) | 10 (10.87) | 5 (5.43) | 24.21 | bc | CD |
| 5 | Xu142fl(M)/Pima S-7(N) | Xu142fl(M)/Pima S-7(M) | 0 (0) | 1 (1.09) | 24 (26.09) | 23 (25.00) | 2 (2.17) | 27.26 | d | CD |
| 6 | Xu142fl(M)/Pima S-7(N) | Pima S-7(M)/Pima S-7(M) | 1 (1.09) | 0 (0) | 6 (6.52) | 5 (5.43) | 2 (2.17) | 28.94 | de | D |
| 7 | Pima S-7(N)/Pima S-7(N) | Xu142fl(M)/Xu142fl(M) | 1 (1.09) | 1 (1.09) | 5 (5.43) | 7 (7.61) | 0 (0) | 29.61 | de | D |
| 8 | Pima S-7(N)/Pima S-7(N) | Xu142fl(M)/Pima S-7(M) | 3 (3.26) | 7 (7.61) | 2 (2.17) | 11 (11.96) | 1 (1.09) | 29.99 | de | D |
| 9 | Pima S-7(N)/Pima S-7(N) | Pima S-7(M)/Pima S-7(M) | 3 (3.26) | 2 (2.17) | 2 (2.17) | 7 (7.61) | 0 (0) | 31.03 | e | D |
| The FLNX F 2 population (FLN1-10 × Xu142fl) | ||||||||||
| 10 | Xu142fl(M)/Xu142fl(M) | Xu142fl(M)/Xu142fl(M) | 0 (0) | 0 (0) | 5 (5.49) | 0 (0) | 5 (5.49) | 0 | a | A |
| 11 | Xu142fl(M)/Xu142fl(M) | Xu142fl(M)/Pima S-7(M) | 0 (0) | 0 (0) | 14 (15.38) | 1 (1.10) | 13 (14.29) | 31.12 | b | B |
| 12 | Xu142fl(M)/Xu142fl(M) | Pima S-7(M)/Pima S-7(M) | 0 (0) | 0 (0) | 4 (4.40) | 2 (2.20) | 2 (2.20) | 36.50 | cd | CD |
| 13 | Xu142fl(M)/Sicala 40(N) | Xu142fl(M)/Xu142fl(M) | 0 (0) | 0 (0) | 11 (12.09) | 0 (0) | 11 (12.09) | 31.26 | b | B |
| 14 | Xu142fl(M)/Sicala 40(N) | Xu142fl(M)/Pima S-7(M) | 0 (0) | 0 (0) | 25 (27.47) | 13 (14.29) | 12 (13.19) | 36.72 | cd | CD |
| 15 | Xu142fl(M)/Sicala 40(N) | Pima S-7(M)/Pima S-7(M) | 0 (0) | 1 (1.10) | 4 (4.40) | 5 (5.49) | 0 (0) | 39.30 | d | D |
| 16 | Sicala 40(N)/Sicala 40(N) | Xu142fl(M)/Xu142fl(M) | 0 (0) | 0 (0) | 8 (8.79) | 5 (5.49) | 3 (3.30) | 34.74 | c | BC |
| 17 | Sicala 40(N)/Sicala 40(N) | Xu142fl(M)/Pima S-7(M) | 0 (0) | 1 (1.10) | 6 (6.59) | 7 (7.69) | 0 (0) | 36.58 | cd | CD |
| 18 | Sicala 40(N)/Sicala 40(N) | Pima S-7(M)/Pima S-7(M) | 0 (0) | 7 (7.69) | 5 (5.49) | 11 (12.09) | 1 (1.10) | 37.22 | cd | CD |
a M and N represent mutated and normal alleles, respectively; see Supplementary Table S1 for markers used in distinguishing different alleles.
b Four were completely lintless and one was close to lintless.
c Four were close to lintless.
d Weight of lint as a percentage of total seed cotton weight.
e Values with the same letter were not significantly different determined by Student’s t-test.
(i) Fuzzless-lintless seeds (all lacking a micropylar tuft) (types 1 and 10, Table 1) were only produced when the two homozygous defective homoeologs, MYB25-like_AtXu142fl/Xu142fl and MYB25-like_DtXu142fl/Xu142fl, were combined together, as in the original Xu142fl fibreless parent. Replacing a single copy of MYB25-like_DtXu142fl with the Pima S-7 Dt allele (types 2 and 11) or the more highly expressed At allele from Pima S-7 (type 4) was sufficient to allow lint, but not fuzz, to develop on the seeds. This suggests that MYB25-like_DtPimaS7 and MYB25-like_AtPimaS7 both produce fully functional MYB proteins able to activate lint fibre initiation. However, although MYB25-like_AtPimaS7 is highly expressed from 0 through to 5 dpa (Fig. 5B), a single copy (type 4) was unable to rescue fuzz fibre production (Table 1), although two copies (type 7) allowed the development of some intermediate and normal fuzzy seeds in some plants, suggesting that fuzz and lint development are both dependent on gene dosage and hence total expression of MYB25-like homoeoalleles at specific times during development. Interestingly, plants in the PX population with homozygous Pima S-7 alleles for both homoeologs (type 9) were not all fuzzless like the Pima S-7 parent, attesting to the influence of some of the other identified fuzzless loci. All plants in the two populations have a lowly expressed MYB25-like_Dt allele, i.e. MYB25-like_DtXu142fl or MYB25-like_DtPimaS7, and regardless of whether their MYB25-like_At allele was functional or mutant, produced mostly fuzzless or reduced fuzz seeds, suggesting it is the expression level of the Dt homoeolog that is critical for fuzz development, but either homoeolog can support lint fibre development provided that it expresses a functional protein during lint initiation. As Pima S-7 and Xu142fl are both believed to carry alleles of n2 causing their fuzzless seeds, this provides support for MYB25-like_Dt being a good candidate for N2.
(ii) The Gb allele MYB25-like_AtPimaS7 was less effective than that from Gh, MYB25-like_AtSicala40, in suppressing fuzz development in the presence of mutated Dt homoeologs, producing fewer fuzzless and more intermediate and fuzzy seeded F2 segregants (comparing types 7–9 with types 16–18), perhaps because of its higher expression during fuzz initiation (Fig. 5B), or because of other modifier genes brought in with the different genetic backgrounds.
(iii) Lint percentage (and hence lint fibre development) was largely determined by the functionality and expression (gene dosage) of the At homoeolog of MYB25-like, but could also be influenced by the Dt homoeolog. The negative effects of the defective MYB25-like_Dt on lint percentage was less severe in the predominantly Gh background of the FLNX plants than in the Gb background of the PX plants that had lower lint percentages with all allele combinations other than in those with fibreless seeds (Table 1).
Breeding fuzzless cottons without a penalty on lint fibre production is possible
Fuzzless seeds have historically been associated with an undesirable lint percentage (Ware, et al. 1944), most likely due to both fuzz and lint fibres being regulated by differential expression of the same genes. The N1 gene, i.e. the dysfunctional MYB25-like_At, has a strong negative effect on lint development (Turley et al., 2007), and therefore our ability to breed cottons with both N1 and a high lint percentage is quite poor. Most commercial Gb cultivars have a relatively high lint percentage (~34%), so their fuzzless seed trait should have smaller deleterious effects on lint development. To know whether it is possible to combine the fuzzless seed trait of Gb with the high lint percentage of Gh, we created a BC5F2 population (FLNS from FLN1-10 × Sicala 40) and measured lint percentages of individual plants. These plants segregated for all five fuzzless associated loci, and contained homozygous MYB25-like_At alleles from Sicala 40, i.e. MYB25-like_AtSicala40/Sicala40, which is expressed at a similar level to MYB25-like_AtPimaS7/PimaS7 at 0 dpa. Overall, the average lint percentage of the BC5F2 plants with a genotype of MYB25-like_DtSicala40/Sicala40 (normal fuzz) and MYB25-like_DtPimaS7/PimaS7 (fuzzless) were similar (41.96% and 41.71%, respectively), and as high as that of the elite Gh cultivar Sicala 40 (41.58%). These results suggest that Sicala 40 contains alleles that are able to compensate for any negative effects of MYB25-like_DtPimaS7 on lint percentage. Individually, the Pima S-7 allele of loci V and II had a positive and negative effect on lint percentage, respectively, whereas the other three loci had no significant effect on lint percentage (see Supplementary Fig. S10).
Discussion
Identification and characterization of genes contributing to initiation of lint and fuzz fibres is essential for understanding the biological processes underlying fibre development and for improvement of lint yield through traditional breeding or transgenic approaches. Several fibreless or fuzzless mutants, such as Xu142fl, SL1-7-1, N1, and n2, have been reported and characterized (Zhang & Pan, 1991; Percy & Kohel, 1999; Turley & Kloth, 2008). They are valuable genetic resources for uncovering genes related to lint and fuzz fibre development. Transcriptome analyses using some of these fibre mutants have identified a large number of genes with a potential role in fibre development (Wu et al., 2006; Padmalatha et al., 2012; Wan et al., 2014); however, only a few of those genes (GhMYB25-like, GhVIN1, and GhJAZ2) have been shown to be essential for fibre initiation and development (Walford et al., 2011; Wang et al., 2014; Hu et al., 2016). Using a forward genetics approach, Wan et al. (2016) identified the dominant fuzzless N1 gene to be a dysfunctional allele of MYB25-like_At that generates siRNAs and showed that suppression of MYB25-like_At by virus-induced gene silencing phenocopied the fuzzless phenotype. In this study, we identified five loci associated with the fuzzless seed trait from Pima S-7 with the MYB25-like_Dt-containing locus having a more prominent effect on fuzz development than the other loci (Fig. 3A; Supplementary Fig. S5).
We propose that MYB25-like_Dt is a candidate for the N2 gene based on a number of pieces of evidence. First, N1 and n2 have previously been assigned to the two homoeologous chromosomes A12 and D12, respectively (Endrizzi & Ramsay, 1980). N1 has been shown to be localized to an interval containing MYB25-like_At (Wan et al., 2016), and here we show that there is a major locus associated with the fuzzless seed phenotype of Gb carrying n2 that maps to an interval containing its homoeolog, MYB25-like_Dt. While there are several other loci that associated with fuzzless seeds in different genetic populations segregating for n2, only the locus containing MYB25-like_Dt is on D12, known to contain n2. Second, in a highly introgressed population (FLNS) that is predominantly in a Sicala 40 background, those lines carrying the allele of MYB25-like_Dt from Pima S-7 all have much reduced fuzz relative to lines carrying the corresponding wild-type allele from Sicala 40, regardless of the combinations of loci for the other four fuzzless associated regions from Gb (see Supplementary Fig. S5). Third, expression of MYB25-like_Dt when fuzz fibres begin to initiate at 3 dpa is consistently much lower in NILs and other lines producing fuzzless seeds compared with those with fuzzy seeds, whereas the expression of MYB25-like_At in Pima S-7 is even higher than in the fuzzy seeded cultivar Sicala 40 and so is not indispensable for fuzz development. MYB25-like_Dt from Pima S-7 does not appear to contain any obvious deleterious mutations within its coding region, and a single copy of this gene is able to restore lint development in lines containing otherwise defective homoeoalleles of MYB25-like from Xu142fl. The Pima S-7 MYB25-like_Dt locus must thus be sufficiently expressed at 0 dpa and produce a functional protein to be able to activate transcription of its downstream gene(s), at least within the lint fibre pathway. There are a number of upstream sequence differences between the MYB25-like_Dt alleles from Pima S-7 and Sicala 40 (Supplementary Fig. S8) that may be responsible for the low expression of the Pima S-7 allele during fuzz initiation in lines carrying n2, but further experiments will be required to verify that they are the causal mutations that convert N2 into n2.
Taking advantage of those dysfunctional MYB25-like homoeoalleles in Xu142fl, we used it to infer both the functionality and the dosage effects of MYB25-like_DtPimaS7 in fuzz development by comparing fuzz phenotype of F2 segregants with MYB25-like_DtXu142fl/Xu142fl or MYB25-like_DtPimaS7/PimaS7 in common MYB25-like_At backgrounds (Table 1). This investigation allowed us to not only demonstrate a key role for MYB25-like_Dt in both fuzz and lint development, but also to uncover subtle differences in expression and dosage effects between MYB25-like_DtPimaS7 and MYB25-like_DtXu142fl alleles in fuzz and lint fibre development. In addition, the loss of fuzz production by MYB25-like_DtPimaS7 could be alleviated by the relatively highly expressed MYB25-like_AtPimaS7 allele that was more effective than the less expressed MYB25-like_AtSicala40 allele (Table 1; Fig. 5B).
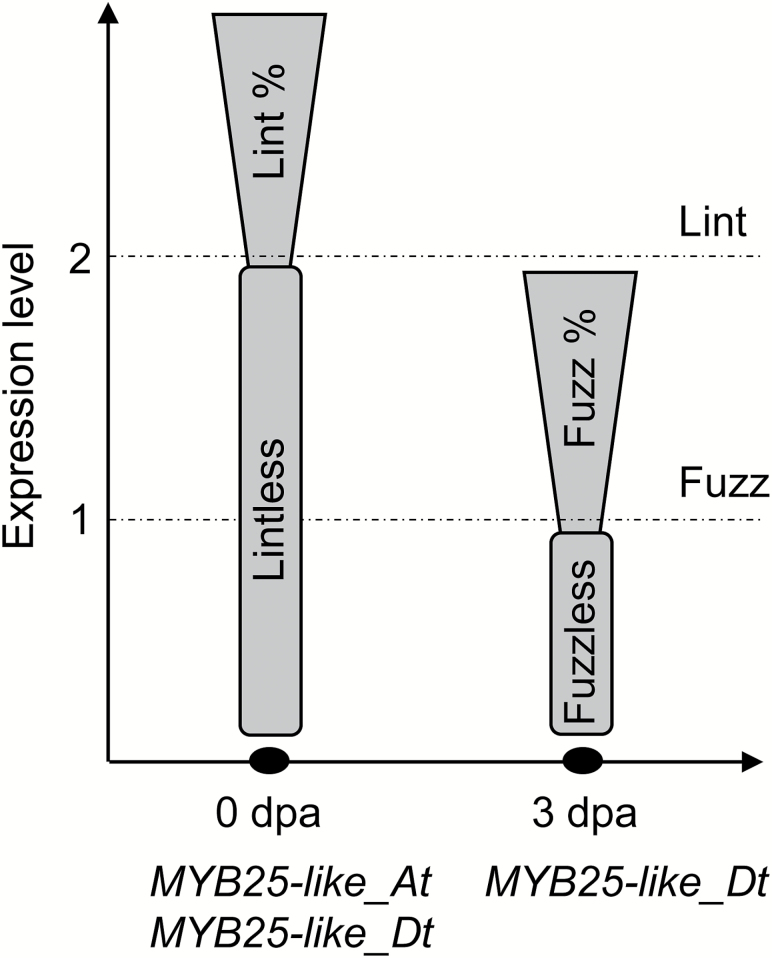
Based on our results, we proposed a working model for the role of MYB25-like homoeologs in lint and fuzz development (Fig. 6). According to this model, transferring a homozygous allele with low expression at ~3 dpa, such as MYB25-like_DtPimaS7/PimaS7, into a Gh background by backcrossing such that there is a high level of total MYB25-like at ~0 dpa to enhance lint fibre development should allow the breeding of elite commercial cotton lines with fuzzless seeds and without any penalty on lint yield, provided that other yield components (such as seeds per boll and bolls per plant) are not affected. We believe we are well on the way to achieving that result.
Fig. 6.
Working model for the role of MYB25-like homoeologs in lint and fuzz development. Initiation and development of lint are determined by the total expression level of both homoeologs of MYB25-like at ~0 dpa, while initiation and development of fuzz are mainly governed by the expression level of MYB25-like_Dt at ~3 dpa, although MYB25-like_At may have the ability to compensate for lack of MYB25-like_Dt at ~3 dpa. If the expression level of MYB25-like is below the threshold level 2 at ~0 dpa, no lint fibre will initiate; otherwise lint fibres develop and their amount is positively correlated with the total expression level of MYB25-like. If the expression level of MYB25-like_Dt is lower than the threshold level 1 at ~3 dpa, cotton seeds will be fuzzless; otherwise fuzz fibres develop and their amount is positively correlated with the expression level of MYB25-like_Dt. When the total expression levels of MYB25-like at ~0 dpa and of MYB25-like_Dt at ~3 dpa are lower than the threshold level 2 and 1, respectively, cotton seeds will be fibreless (e.g. Xu142fl). When the total expression level of MYB25-like at ~0 dpa is higher than the threshold level 2 but the expression level of MYB25-like_Dt at ~3 dpa is below the threshold level 1, cotton seeds will be fuzzless (e.g. T586). No mutant with lintless and fuzzy seed has ever been reported; that is probably because cottons with an expression level of MYB25-like_Dt at ~3 dpa high enough for fuzz development usually have a high enough total expression level of MYB25-like_Dt and MYB25-like_At at ~0 dpa for lint development.
Interactions between MYB25-like_At, MYB25-like_Dt, and other loci indicate that the genetic model regulating the fuzzless seed trait from Pima S-7 is genetic background dependent. For instance, the female parents of the PS and FLNS F2 populations (Pima S-7 and FLN1-10, respectively) had the same genotype at loci I–V (i.e. homozygous Pima S-7), but their F2 populations showed a three- and two-gene-model for the fuzzless trait, respectively. In addition, the PS but not the FLNS F2 population showed transgressive segregation, and a few Gb accessions (with homozygous Pima S-7 alleles at all five loci) produce considerable amounts of fuzz (see Supplementary Fig. S6). These results suggest the presence of as yet unknown modifier(s) affecting expression of the fuzzless genes or the interaction amongst the fuzzless genes in the Gb background, consistent with the previous finding that the genetics of the Pima S-7 fuzzless phenotype is complex (Rong et al., 2005).
In conclusion, the Gb fuzzless seed trait is regulated by multiple recessive genetic loci. The role of MYB25-like_Dt in regulating fuzz initiation and development is yet to be confirmed by specific silencing of its expression using gene editing, but the expression profile of MYB25-like_Dt and genetic analyses of cottons with variable lint and fuzz phenotypes provided quite convincing evidence for MYB25-like_Dt being the major fuzz gene in addition to its role in regulating lint development together with MYB25-like_At.
Supplementary data
Supplementary data are available at JXB online.
Fig. S1. Schematic of the development of the near isogeneic lines (NILs) used in SNP genotyping and mapping-by-sequencing.
Fig. S2. Alignment of the coding sequences of MYB25-like.
Fig. S3. The Cotton SNP63K array based frequency of the Pima S-7 allele of the polymorphic SNPs between Pima S-7 and Sicala 40 in the NILs with normal or reduced fuzz.
Fig. S4. Distribution of the Pima S-7 allele frequency across the 26 cotton chromosomes in the NILs showing fuzzless (RFB2) and segregating fuzz phenotype (RFB1) determined by MBS.
Fig. S5. Representative fuzz phenotype of the FLNS F2:F3 families.
Fig. S6. Seed fuzz phenotype of representative G. barbadense accessions.
Fig. S7. Alignment of the amino acid sequences of MYB25-like homoeoalleles from Xu142, Xu142fl, TM-1, and Pima S-7.
Fig. S8. Alignments of the promoter sequences of MYB25-like from mutant and wild-type lines.
Fig. S9. Distribution of fuzz phenotypes (F3 seeds) in the F2 populations PX and FLNX by MYB25-like homoeoalleles.
Fig. S10. Effects of fuzz associated loci on lint percentage of the FLNS F2:F3 families.
Table S1. Primers used in the study.
Table S2. Chromosomal distributions of the 5426 polymorphic SNPs between Pima S-7 and Sicala 40 based on the Cotton SNP63K array.
Table S3. Candidate regions identified based on mapping-by-sequencing (MBS) and SNP array association analysis.
Table S4. List of annotated genes in the five chromosomal regions associated with fuzz development.
Acknowledgements
This study was funded by Cotton Breeding Australia, a joint venture between Cotton Seed Distributors Ltd and CSIRO.
References
- Bechere E, Auld DL, Hequet E. 2009. Development of ‘naked-tufted’ seed coat mutants for potential use in cotton production. Euphytica 167, 333–339. [Google Scholar]
- Bechere E, Turley RB, Auld DL, Zeng L. 2012. A new fuzzless seed locus in an Upland cotton (Gossypium hirsutum L.) mutant. American Journal of Plant Science 3, 799–804. [Google Scholar]
- Bedon F, Ziolkowski L, Walford SA, Dennis ES, Llewellyn DJ. 2014. Members of the MYBMIXTA-like transcription factors may orchestrate the initiation of fiber development in cotton seeds. Frontiers in Plant Science 5, 179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boykin JC. 2007. Cultivar differences in gin stand energy utilization. Transactions of the ASABE 50, 733–743. [Google Scholar]
- Byers RL, Harker DB, Yourstone SM, Maughan PJ, Udall JA. 2012. Development and mapping of SNP assays in allotetraploid cotton. Theoretical and Applied Genetics 124, 1201–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Yao J, Chu L, Yuan Z, Li Y, Zhang Y. 2015. Genetic mapping of the nulliplex-branch gene (gb_nb1) in cotton using next-generation sequencing. Theoretical and Applied Genetics 128, 539–547. [DOI] [PubMed] [Google Scholar]
- Du XM, Pan JJ, Wang RH, Zhang TZ, Shi YZ. 2001. Genetic analysis of presence and absence of lint and fuzz in cotton. Plant Breeding 120, 519–522. [Google Scholar]
- Ellis MH, Stiller WN, Phongkham T, Tate WA, Gillespie VJ, Gapare WJ, Zhu Q-H, Llewellyn DJ, Wilson IW. 2016. Molecular mapping of bunchy top disease resistance in Gossypium hirsutum L. Euphytica 210, 135–142. [Google Scholar]
- Endrizzi JE, Ramsay G. 1980. Identification of ten chromosome deficiencies in cotton. Journal of Heredity 71, 45–48. [Google Scholar]
- Endrizzi JE, Taylor T. 1968. Cytogenetic studies of N Lc1yg2R2 marker genes and chromosome deficiencies in cotton. Genetics Research 12, 295–304. [Google Scholar]
- Fang L, Wang Q, Hu Y et al. 2017. Genomic analyses in cotton identify signatures of selection and loci associated with fiber quality and yield traits. Nature Genetics 49, 1089–1098. [DOI] [PubMed] [Google Scholar]
- Gapare W, Conaty W, Zhu Q-H, Liu S, Stiller W, Llewellyn D, Wilson I. 2017. Genome-wide association study of yield components and fibre quality traits in a cotton germplasm diversity panel. Euphytica 213, 66. [Google Scholar]
- Hinze LL, Hulse-Kemp AM, Wilson IW et al. 2017. Diversity analysis of cotton (Gossypium hirsutum L.) germplasm using the CottonSNP63K Array. BMC Plant Biology 17, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu HY, He X, Tu LL, Zhu LF, Zhu ST, Ge ZH, Zhang XL. 2016. GhJAZ2 negatively regulates cotton fiber initiation by interacting with the R2R3-MYB transcription factor GhMYB25-like. The Plant Journal 88, 921–935. [DOI] [PubMed] [Google Scholar]
- Huang C, Nie X, Shen C, You C, Li W, Zhao W, Zhang X, Lin Z. 2017a Population structure and genetic basis of the agronomic traits of upland cotton in China revealed by a genome-wide association study using high-density SNPs. Plant Biotechnology Journal 15, 1374–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang F, Zhu Q-H, Zhu A, Wu X, Xie L, Wu X, Helliwell C, Chaudhury A, Finnegan EJ, Luo M. 2017b Mutants in the imprinted PICKLE RELATED 2 gene suppress seed abortion of fertilization independent seed class mutants and paternal excess interploidy crosses in Arabidopsis. The Plant Journal 90, 383–395. [DOI] [PubMed] [Google Scholar]
- Hulse-Kemp AM, Ashrafi H, Stoffel K, Zheng X, Saski CA, Scheffler BE, Fang DD, Chen ZJ, Van Deynze A, Stelly DM. 2015a BAC-end sequence-based SNP mining in allotetraploid cotton (Gossypium) utilizing resequencing data, phylogenetic inferences, and perspectives for genetic mapping. G3 5, 1095–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulse-Kemp AM, Lemm J, Plieske J et al. 2015. b Development of a 63K SNP array for cotton and high-density mapping of intraspecific and interspecific populations of Gossypium spp. G3 5, 1187–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam MS, Zeng L, Thyssen GN, Delhom CD, Kim HJ, Li P, Fang DD. 2016. Mapping by sequencing in cotton (Gossypium hirsutum) line MD52ne identified candidate genes for fiber strength and its related quality attributes. Theoretical and Applied Genetics 129, 1071–1086. [DOI] [PubMed] [Google Scholar]
- Joshi PC, Wadhwani AM, Johri BM. 1967. Morphological and embryological studies of Gossypium L. Indian Journal of Agriculture Research 33, 37–93. [Google Scholar]
- Kearney TH, Harrison GJ. 1928. Variation in seed fuzziness on individual plants of Pima cotton. Journal of Agricultural Research 37, 465–472. [Google Scholar]
- Lang AG. 1938. The origin of lint and fuzz hairs of cotton. Journal of Agricultural Research 56, 507–521. [Google Scholar]
- Lee JJ, Hassan OS, Gao W, Wei NE, Kohel RJ, Chen XY, Payton P, Sze SH, Stelly DM, Chen ZJ. 2006. Developmental and gene expression analyses of a cotton naked seed mutant. Planta 223, 418–432. [DOI] [PubMed] [Google Scholar]
- Li C, Dong Y, Zhao T et al. 2016. Genome-wide SNP linkage mapping and QTL analysis for fiber quality and yield traits in the upland cotton recombinant inbred lines population. Frontiers in Plant Science 7, 1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmalatha KV, Patil DP, Kumar K et al. 2012. Functional genomics of fuzzless-lintless mutant of Gossypium hirsutum L. cv. MCU5 reveal key genes and pathways involved in cotton fibre initiation and elongation. BMC Genomics 13, 624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Percy RG, Kohel RJ. 1999. Qualitative genetics. In: Smith CW, Cothren JT, eds. Cotton: origin, history, technology, and production. New York: John Wiley & Sons, 319–360. [Google Scholar]
- Romano GB, Taliercio EW, Turley RB, Scheffler JA. 2011. Fiber initiation in 18 cultivars and experimental lines of three Gossypium species. The Journal of Cotton Science 15, 61–72. [Google Scholar]
- Rong JK, Pierce GJ, Waghmare VN et al. 2005. Genetic mapping and comparative analysis of seven mutants related to seed fiber development in cotton. Theoretical and Applied Genetics 111, 1137–1146. [DOI] [PubMed] [Google Scholar]
- Samora PJ, Stelly DJ, Kohel RJ. 1994. Localization and mapping of the Le1 and Gl2 loci of cotton (Gossypium hirsutum L.). Journal of Heredity 85, 152–157. [Google Scholar]
- Schneeberger K. 2014. Using next-generation sequencing to isolate mutant genes from forward genetic screens. Nature Reviews. Genetics 15, 662–676. [DOI] [PubMed] [Google Scholar]
- Stewart J. 1975. Fiber initiation on the cotton ovule (Gossypium hirsutum). American Journal of Botany 62, 723–730. [Google Scholar]
- Thyssen GN, Fang DD, Turley RB, Florane CB, Li P, Mattison CP, Naoumkina M. 2017. A Gly65Val substitution in an actin, GhACT_LI1, disrupts cell polarity and F-actin organization resulting in dwarf, lintless cotton plants. The Plant Journal 90, 111–121. [DOI] [PubMed] [Google Scholar]
- Thyssen GN, Fang DD, Turley RB, Florane C, Li P, Naoumkina M. 2014. Next generation genetic mapping of the Ligon-lintless-2 (Li₂) locus in upland cotton (Gossypium hirsutum L.). Theoretical and Applied Genetics 127, 2183–2192. [DOI] [PubMed] [Google Scholar]
- Thyssen GN, Fang DD, Turley RB, Florane C, Li P, Naoumkina M. 2015. Mapping-by-sequencing of Ligon-lintless-1 (Li1) reveals a cluster of neighboring genes with correlated expression in developing fibers of Upland cotton (Gossypium hirsutum L.). Theoretical and Applied Genetics 128, 1703–1712. [DOI] [PubMed] [Google Scholar]
- Thyssen GN, Fang DD, Zeng L, Song X, Delhom CD, Condon TL, Li P, Kim HJ. 2016. The immature fiber mutant phenotype of cotton (Gossypium hirsutum) is linked to a 22-bp frame-shift deletion in a mitochondria targeted pentatricopeptide repeat gene. G3 6, 1627–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turley RB, Kloth RH. 2002. Identification of a third fuzzless seed locus in upland cotton (Gossypium hirsutum L.). The Journal of Heredity 93, 359–364. [DOI] [PubMed] [Google Scholar]
- Turley RB, Kloth RH. 2008. The inheritance model for the fiberless trait in upland cotton (Gossypium hirsutum L.) line SL1-7-1: variation on a theme. Euphytica 164, 123–132. [Google Scholar]
- Turley RB, Vaughn KC, Scheffler JA. 2007. Lint development and properties of fifteen fuzzless seed lines of Upland cotton (Gossypium hirsutum L.). Euphytica 156, 57–65. [Google Scholar]
- Walford SA, Wu YR, Llewellyn DJ, Dennis ES. 2011. GhMYB25-like: a key factor in early cotton fibre development. The Plant Journal 65, 785–797. [DOI] [PubMed] [Google Scholar]
- Wan Q, Guan X, Yang N et al. 2016. Small interfering RNAs from bidirectional transcripts of GhMML3_A12 regulate cotton fiber development. New Phytologist 210, 1298–1310. [DOI] [PubMed] [Google Scholar]
- Wan Q, Zhang H, Ye W, Wu H, Zhang T. 2014. Genome-wide transcriptome profiling revealed cotton fuzz fiber development having a similar molecular model as Arabidopsis trichome. PLoS ONE 9, e97313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Chen J, Zhang W et al. 2015. Sequence-based ultra-dense genetic and physical maps reveal structural variations of allopolyploid cotton genomes. Genome Biology 16, 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Cook A, Patrick JW, Chen XY, Ruan YL. 2014. Silencing the vacuolar invertase gene GhVIN1 blocks cotton fiber initiation from the ovule epidermis, probably by suppressing a cohort of regulatory genes via sugar signaling. The Plant Journal 78, 686–696. [DOI] [PubMed] [Google Scholar]
- Wang X, Lu X, Wang J, Wang D, Yin Z, Fan W, Wang S, Ye W. 2016. Mining and analysis of SNP in response to salinity stress in upland cotton (Gossypium hirsutum L.). PLoS ONE 11, e0158142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware JO. 1940. Relation of fuzz pattern to lint in an upland cotton cross. Journal of Heredity 31, 489–496. [Google Scholar]
- Ware JO, Jenkins WH, Harrell DC. 1944. Seed characters and lint production. Journal of Heredity 35, 153–160. [Google Scholar]
- Wu Y, Machado AC, White RG, Llewellyn DJ, Dennis ES. 2006. Expression profiling identifies genes expressed early during lint fibre initiation in cotton. Plant & Cell Physiology 47, 107–127. [DOI] [PubMed] [Google Scholar]
- Zhang DY, Zhang TZ, Sang ZQ, Guo WZ. 2007. Comparative development of lint and fuzz using different cotton fiber-specific developmental mutants in Gossypium hirsutum. Journal of Integrative Plant Biology 49, 1038–1046. [Google Scholar]
- Zhang T, Hu Y, Jiang W et al. 2015. Sequencing of allotetraploid cotton (Gossypium hirsutum L. acc. TM-1) provides a resource for fiber improvement. Nature Biotechnology 33, 531–537. [DOI] [PubMed] [Google Scholar]
- Zhang TZ, Pan JJ. 1991. Genetic analysis of a fuzzless-lintless mutant in Gossypium hirsutum L. Jiangsu Journal of Agricultural Sciences 7, 13–16. [Google Scholar]
- Zhu Q-H, Fan L, Liu Y, Xu H, Llewellyn D, Wilson I. 2013. miR482 regulation of NBS-LRR defense genes during fungal pathogen infection in cotton. PLoS ONE 8, e84390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Q-H, Llewellyn D, Wilson I. 2014a Use of next-generation sequencing in genomic studies of polyploidcrops: cotton as an example. The Journal of Zhejiang University 40, 355–369. [Google Scholar]
- Zhu Q-H, Spriggs A, Taylor JM, Llewellyn D, Wilson I. 2014b Transcriptome and complexity-reduced, DNA-based identification of intraspecies single-nucleotide polymorphisms in the polyploid Gossypium hirsutum L. G3 4, 1893–1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Q-H, Upadhyaya NM, Gubler F, Helliwell CA. 2009. Over-expression of miR172 causes loss of spikelet determinacy and floral organ abnormalities in rice (Oryza sativa). BMC Plant Biology 9, 149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Q-H, Wilson I, Llewellyn D. 2017. Mapping-by-sequencing enabled fast forward genetics in crops with complex genomes. CAB Reviews 12, 16. [Google Scholar]
- Zhu Q-H, Zhang J, Liu D, Stiller W, Liu D, Zhang Z, Llewellyn D, Wilson I. 2016. Integrated mapping and characterization of the gene underlying the okra leaf trait in Gossypium hirsutum L. Journal of Experimental Botany 67, 763–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.