CsMYB6 interacts with CsTRY and they work cooperatively to suppress fruit trichome initiation in cucumber.
Keywords: CsMYB6, CsTRY, cucumber, fruit spine, regulatory module, trichome initiation
Abstract
Fruit epidermal features such as the number and size of trichomes or spines are important fruit quality traits in cucumber production. Little is known about the molecular mechanisms underlying fruit spine formation in cucumber. Here, we report functional characterization of the cucumber CsMYB6 gene, which encodes a MIXTA-like MYB transcription factor that plays an important role in regulating fruit trichome development. Spatial-temporal expression analyses revealed high-level expression of CsMYB6 in the epidermis of cucumber ovaries during fruit spine initiation, which was similar to the expression of CsTRY, a homolog of the Arabidopsis TRY gene that also plays a key role in trichome development. Overexpression of CsMYB6 and CsTRY in cucumber and Arabidopsis revealed that CsMYB6 and CsTRY act as negative regulators of trichome initiation in both species, and that CsMYB6 acted upstream of CsTRY in this process. CsMYB6 was found to bind to the three MYB binding sites inside the promoter region of CsTRY, and protein–protein interaction assays suggested that CsTRY also directly interacted with CsMYB6 protein. The results also revealed conserved and divergent roles of CsMYB6 and its Arabidopsis homolog AtMYB106 in trichome development. Collectively, our results reveal a novel mechanism in which the CsMYB6-CsTRY complex negatively regulates fruit trichome formation in cucumber.
Introduction
Cucumber (Cucumis sativus L., 2n=2x=14) is one of the most economically important vegetable crops in the world, and it is the main vegetable grown in protected environments in China. The cucumber fruit, a pepo that develops from the ovary and receptacle, is covered with a thick cuticle, tubercules, and trichomes (or spines) (Roth, 1977; Wang et al., 2015). The trichomes are widely distributed on leaves, stems, flowers, tendrils, and ovaries (Chen et al., 2014). Two types of trichomes have been observed on cucumber fruits, both of which are multicellular (Chen et al., 2014; Li et al., 2015). Type I, or bloom trichomes, are small and glandular, and produce fine white powdery secretions that are composed primarily of silicon dioxide (SiO2). Type I trichomes give cucumber fruits a coarse outer appearance (Yamamoto et al., 1989; Samuels et al., 1993; Chen et al., 2014; Li et al., 2015). Type II, or spines, are much larger than bloom trichomes. Trichomes can serve as barriers to protect plants from stresses, excessive transpiration, ultraviolet light, herbivores, and parasites (Neal et al., 1989; Bodnaryk, 1996; Mauricio and Rausher, 1997; Eisner et al., 1998; Werker, 2000). Warty fruits have both spines and tubercules on their surface. The presence or absence, number, and size of spines and tubercles are important fruit quality traits that define different cucumber market classes. For example, fruits of North American pickles usually have large but sparse spines. The North China fresh market cucumber, with dense and small spines, is more popular in China fresh markets, while the fruits of the European greenhouse-type or the Mediterranean-type (mini) cucumbers are in general smooth and glossy, and lack visible spines and tubercules (Zhang et al., 2010; Yang et al., 2014; Li et al., 2015; Pan et al., 2015). As such, there is considerable interest in understanding the regulatory mechanisms of fruit spine organogenesis in cucumber in order to enhance breeding programs and the economic value of cucumber production.
The model plant Arabidopsis thaliana (Arabidopsis) does not bear warty fruits, and the trichomes on its rosette leaves are unicellular, highly branched, and non-glandular. The molecular mechanisms of Arabidopsis trichome formation have been extensively studied. More than 30 genes have been identified that contribute to different aspects of trichome development (Hülskamp et al., 1999; Hülskamp et al., 2004; Pattanaik et al., 2014). Among them, a trimeric complex that is composed of GLABRA1 (GL1; Oppenheimer et al., 1991), TRANSPARENT TESTA GLABRA1 (TTG1; Galway et al., 1994; Walker et al., 1999), and GLABRA 3/ENHANCER OF GLABRA3 (GL3/EGL3; Payne et al., 2000; Szymanski et al., 2000; Zhang et al., 2003) activates trichome initiation by promoting the expression of the genes GL2 and EGL2 (Larkin et al., 2003). In this complex, R3MYB transcription factors also play vital roles in controlling trichome formation and development. One of these, TRY, is important because it can compete with GL1 for interaction with GL3/EGL3 and can suppress the initiation of trichome formation (Schellmann et al., 2002; Esch et al., 2003; Zhang et al., 2003).
Compared with Arabidopsis, little is known about the molecular mechanisms of trichome formation in cucumber. So far, only two genes, Cucumber glabrous-1 (CsGL1) [syn. tiny branched hair (tbh), micro-trichome (Mict)] and CsGL3 [syn. trichome-less (Tril)] (Chen et al., 2014; Li et al., 2015; Pan et al., 2015; Zhao et al., 2015) have been identified. The csgl1 mutant has no visible trichomes on any above-ground organs, but instead has many extremely small trichomes with a stunted morphology that can be seen only under a scanning electron microscope. CsGL1 may be involved in cucumber trichome development but not in their initiation (Chen et al., 2014; Li et al., 2015; Pan et al., 2015; Zhao et al., 2015; Liu et al., 2016). The csgl3 mutant is completely glabrous on all aerial organs, and CsGL3 may be involved in cucumber trichome initiation (Pan et al., 2015; Wang et al., 2016). More recently, we showed that the CsTTG1 gene, which encodes a WD-repeat protein, plays an important role in regulating fruit bloom trichome and wart formation in cucumber (Chen et al., 2016). Nonetheless, the regulatory network underlying the formation of cucumber fruit trichomes remains poorly understood.
The MIXTA gene in snapdragon (Antirrhinum majus) (Noda et al. 1994) and its homologs in Arabidopsis, cotton (Gossypium hirsutum), and many other species have been reported to be both negative and positive regulators of trichome differentiation (Glover et al., 1998; Perez-Rodriguez et al., 2005; Baumann et al., 2007; Jaffé et al., 2007; Jakoby et al., 2008; Machado et al., 2009; Dubos et al., 2010; Walford et al., 2011; Du et al., 2012). MIXTA-like genes therefore seem to be involved in trichome development across major core eudicot lineages (Scoville et al., 2011). There are two MIXTA-like homologs in the cucumber genome. Phylogenetic analysis of the R2R3MYB family members in cucumber, Arabidopsis, snapdragon, and cotton showed that the MIXTA-like homologs CsMYB6 and CsMYB26 were in the same clade with the Arabidopsis MIXTA-like proteins AtMYB106 (NOK) and AtMYB16 (MIXTA), which have been shown to regulate epidermal cell differentiation, cuticular wax biosynthesis, and trichome morphogenesis (Jakoby et al., 2008; Matus et al., 2008; Wilkins et al., 2009; Li et al., 2012; Yoshimi et al., 2013). In previous transcriptome profiling studies, CsMYB6 was the only MIXTA-like homolog that was significantly down- regulated in both csgl1 and csgl3 mutants (Chen et al., 2014; Li et al., 2015; Zhao et al., 2015). Thus, we hypothesize that CsMYB6 may be involved in cucumber trichome formation. We developed CsMYB6-overexpressing cucumber transgenic lines. We found that CsMYB6 regulates trichome formation by directly interacting with CsTRY, which exhibited the same expression pattern as CsMYB6. We also examined the molecular link between CsMYB6 and CsGL1 during trichome development.
Materials and methods
Plant material and growth conditions
Two monoecious cucumber inbred lines, R3407 [wild-type (WT)] and its ‘glabrous’ and wart-free csgl1 mutant, were used in the present study. All plants were grown in a greenhouse under natural light in Beijing, China.
The Arabidopsis nok mutant (Col background) was obtained from The Arabidopsis Information Resource (http://www.arabidopsis.org/index.jsp; stock number SALK_025449) and Col was used as the WT control. Arabidopsis seeds were germinated on Murashige and Skoog (MS) medium containing 1% sucrose and 0.2% phytagar. Seeds were kept at 4 °C for 3 d and then were moved to 22 °C under a 16 h light/8 h dark regime. Seedlings were transferred to soil 7–10 d after germination.
Sequence alignments and phylogenetic analysis
The coding sequence (CDS) of CsMYB6 was amplified by PCR from female flower bud cDNA using gene-specific primers (Supplementary Table S1 at JXB online). The amino acid sequences of the related R2R3MYB proteins from A. majus, Arabidopsis, and cotton were obtained by BLAST searches of the National Center for Biotechnology Information nucleotide database (http://www.ncbi.nlm.nih.gov/nucleotide/) with the deduced amino acid sequence of CsMYB6. A multiple sequence alignment of CsMYB6 and the related R2R3MYB proteins was carried out as previously described (Zhang et al., 2014). The phylogenetic tree was created with MEGA5 software, using the neighbor-joining method (Saitou and Nei, 1987) with 1000 bootstrap replicates.
Spatial and temporal expression analysis by quantitative real-time RT–PCR
Total RNA was extracted from the roots, stems, male and female flower buds, and fruits of different stages using a Quick RNA isolation Kit (Huayueyang, China). cDNA was synthesized using a PrimeScript First Strand cDNA Synthesis Kit (TaKaRa). SYBR® Premix Ex Taq from TaKaRa was used for quantitative real-time reverse transcription–PCR (qRT–PCR) with an Applied Biosystems 7500 real-time PCR system (Applied Biosystems). The cucumber α-TUBULIN (TUA) gene was used as an internal control (Zhang et al., 2014) in all qRT–PCRs. Three biological replicates and three technical replicates were performed to verify the accuracy of the expression data. The gene-specific qRT–PCR primers are listed in Supplementary Table S1.
GUS expression and staining of transgenic cucumber plants
The putative promoter regions of CsMYB6 and CsTRY, 2184 bp and 2000 bp fragments upstream of the start codon (ATG), respectively, were used to generate pCsMYB6-GUS and pCsTRY-GUS constructs, using gene-specific primers containing the PstI (5ʹ end) and BamHI (3ʹ end) sites, and the BamHI (5ʹ end) and NcoI (3ʹ end) sites, respectively. The constructs were transformed into cucumber as described previously (Wang et al., 2014). Histochemical staining for β-glucuronidase (GUS) activity was performed following Chen et al. (2016).
RNA in situ hybridization
Female cucumber flower buds, carpopodium samples, and petioles were fixed, embedded, sectioned, and hybridized with digoxigenin-labeled probes as previously described (Zhang et al., 2014). Digoxigenin-labeled sense and antisense RNA probes were obtained using the T7 and SP6 RNA polymerases (Roche). The primer pairs used are listed in Supplementary Table S1.
Subcellular localization of CsMYB6 and CsTRY in onion epidermal cells
The full-length coding regions of CsMYB6 and CsTRY were cloned without the stop codon and inserted into the pEZS-NL vector (Chen et al., 2016) between the XbaI and SmaI sites. An empty pEZS-NL vector was used as the positive control. Transient expression of the fusion proteins in onion epidermal cells was performed according to Varagona et al. (1992). An Olympus BX 51 fluorescence microscope was used to visualize the fluorescent signals.
Scanning electron microscopy
Cucumber ovary samples were fixed, washed, postfixed, dehydrated, coated (Chen et al., 2014), and observed using a Hitachi S-4700 scanning electron microscope with a 2 kV accelerating voltage.
Ectopic expression of CsMYB6 in Arabidopsis
To generate the CsMYB6-overexpressing lines, the full-length coding region of CsMYB6 was amplified using specific primers containing the XbaI (5ʹ end) and SmaI (3ʹ end) endonuclease sites, and inserted in the reverse orientation into the pCAMBIA1305.1 vector (Chen et al., 2016). The recombinant plasmids were transformed into Col (WT) and nok mutant plants using the floral dip method (Clough and Bent, 1998). The transgenic Arabidopsis plants were screened on MS medium with 25 mg l−1 hygromycin.
Cucumber transformation
The full-length CsTRY coding region was amplified and inserted in the reverse orientation into the XbaI and SmaI sites of the pCAMBIA1305.1 vector (Chen et al., 2016). The CsMYB6 and CsTRY overexpression constructs were used for cucumber transformation. The recombinant plasmids were transformed into the cucumber R3407 (WT) line and csgl1 mutant plants using a cotyledon transformation method as previously described (Wang et al., 2014). The primers are listed in Supplementary Table S1.
Dual-luciferase transient expression assay in tobacco leaves
The promoters of pCsTRY (1500 bp) were inserted into the vector pGreenII 0800-Luc (Hellens et al., 2005) and the CDS of CsMYB6 was inserted into pGreenII 62-SK (Hellens et al., 2005). Tobacco (Nicotiana benthamiana) leaves were used for co-expression studies as previously described (Yotsui et al., 2013). A no-effector construct was used as a negative control. The expression of firefly luciferase and renilla luciferase was examined using dual-luciferase assay reagents (Promega, USA). Data were collected as the ratio of firefly luciferase to renilla luciferase.
Yeast one-hybrid assay
A yeast one-hybrid (Y1H) assay was performed using the Matchmaker Gold Yeast One-Hybrid System (Clontech). The three MYB binding site (MBS) motifs in the CsTRY promoter were cloned into the pAbAi vector. The full-length CsTRY coding region was amplified and inserted in the pGADT7 vector. The recombinant plasmids were transformed into the Y1H Gold strain. DNA–protein interaction was assessed based on the growth of the co-transformants on synthetic dextrose plates lacking leucine, but containing aureobasidin A. The primers are listed in Supplementary Table S1.
Electrophoretic mobility shift assay
The full-length CsMYB6 protein sequence was fused in frame with a GST tag and expressed in Escherichia coli BL21 cells by the addition of isopropyl β-D-1-thiogalactopyranoside to a final concentration of 1 mM, before the cultures were incubated at 18 °C for 16 h. The recombinant protein was purified by using glutathione Sepharose 4B beads (GE Healthcare). An electrophoretic mobility shift assay (EMSA) was performed using the Light Shift Chemiluminescent EMSA kit (Thermo Fisher Scientific, Shanghai, China) according to the manufacturer’s instructions. The biotin-labeled DNA fragments were used as probes, while unlabeled fragments of the same sequence were used as the competitors.
CsMYB6 transcriptional activation analysis
The nuclear localization signal (NLS) and GAL4 AD sequences were amplified from the pGADT7 vector (Clontech) by PCR and ligated into the pGBKT7 vector (Clontech) fused to the GAL4 DNA BD. The fusion vector, pGBKT7-NLSAD, was used as a positive control and the pGBKT7 vector was used as a negative control. To determine the transcriptional activation portion of CsMYB6, the full-length or partial CDS [corresponding to deletions of amino acids 1–11 (ΔN1-11), 1–64 (ΔN1-64), or 1–128 (ΔN1-128) at the N-terminus; 12–234 (ΔC12-234) or 129–234 (ΔC129-234) at the C-terminus; and ΔN1-11, 129-234 (ΔN1-11, ΔC129-234)] of CsMYB6 were fused to the BD in the pGBKT7 vector. The recombinant constructs were separately transformed into yeast strain AH109. The transformed yeast cells were grown on synthetic defined (SD) plates lacking tryptophan and histidine (SD/-Trp-His) and lacking tryptophan, histidine, and adenine (SD/-Trp-His-Ade) with α-gal, and on control plates lacking only tryptophan (SD/-Trp). All experiments were performed according to the manufacturer’s user manual (Clontech).
Yeast two-hybrid assay
A yeast two-hybrid (Y2H) library derived from the RNA from –7 days post anthesis (DPA) to 4 DPA cucumber ovaries was constructed using a commercial service (Oebiotech; Shanghai, China). The library was screened using the truncated C-terminal CsMYB6 protein (N65-234) as the bait according to the manufacturer’s instructions (Clontech). For the yeast two-hybrid assay, we cloned the cDNA sequence of CsTRY (full-length) and fused it into the pGBKT7 and pGADT7 vectors, respectively. The combination of TTG1-AD and GL1-BD was used as a positive control (Chen et al., 2016). All recombinant constructs were separately transformed into the yeast strain AH109. The growth conditions of the yeast cells were as described above.
Bimolecular fluorescence complementation assay
To generate the bimolecular fluorescence complementation (BiFC) constructs, the full-length cDNA sequences of CsTRY and CsMYB6 were cloned and fused with the pSPYCE and pSPYNE vectors (Walter et al., 2004). Tobacco (N. benthamiana) leaves were used for co-expression studies as previously described (Schütze et al., 2009). The fluorescence signal was detected 2 to 4 d after infiltration, using an Olympus BX 51 fluorescence microscope to acquire fluorescent images. Yellow fluorescent protein (YFP) imaging was performed at an excitation wavelength of 488 nm.
Co-immunoprecipitation assay
Tobacco (N. benthamiana) leaves were infiltrated with Agrobacterium strain GV3101 harboring CsMYB6-6×His and CsTRY-HA. Three days later, tobacco leaves were collected and total proteins were extracted from them. CsMYB6-6×His and CsTRY-HA proteins were immunoprecipitated by anti-HA and anti-His agarose conjugate (Sigma) separately. Proteins bound to the beads were resolved by lysis buffer and dissolved with 2× loading buffer. After being boiled, the samples were resolved via sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred on to nitrocellulose membranes (Millipore). The membranes were incubated with anti-HA and anti-His at 4 °C.
Accession numbers
GenBank accession numbers of R2R3MYB protein sequences used in this study included Cucumber CsMYB26 (Csa009688); snapdragon AmMYBML1 (CAB43399), AmMYBML2 (AAV70655), AmMYBML3 (AAU13905), and AmMIXTA (CAA55725); Petunia PhMYB1 (CAA78386); Arabidopsis AtMYB0 (GL1) (NP_189430), AtMYB5 (NP_187963.1), AtMYB23 (NP_198849.1), AtMYB16 (NP_197035), AtMYB17 (NP_191684), AtMYB106 (NP_186763), and AtMYB66(Wer) (NP_196979); cotton GaMYB2 (AAU12248), GhMYB109 (CAD71140) GhMYB25 (AAK19616), and GhMYB25-like (EST AY464066); and tomato ShMYB1 (EST241733).
Results
CsMYB6 is a homolog of Arabidopsis MIXTA transcription factor
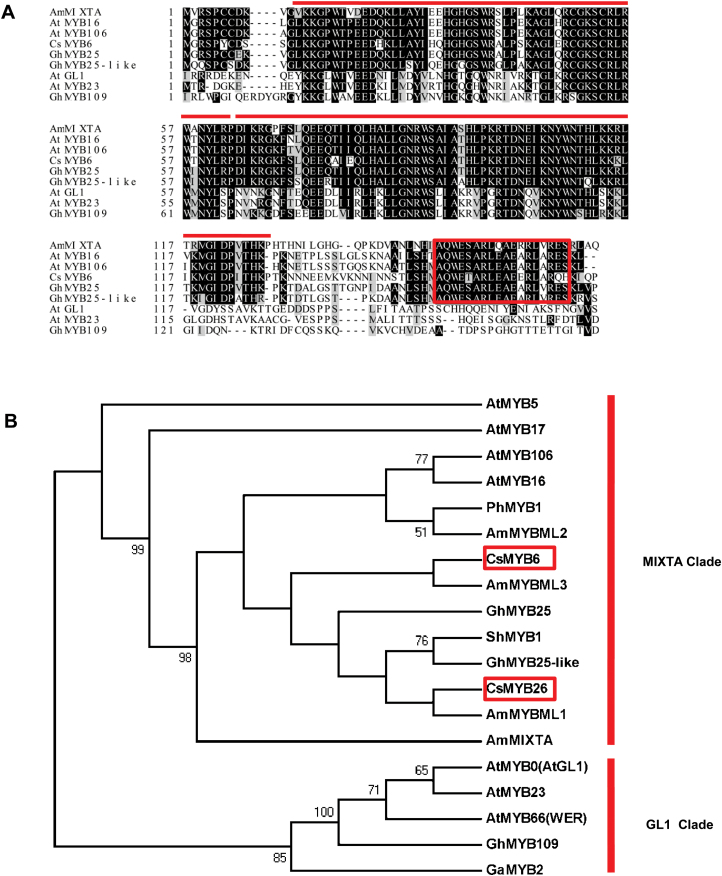
Alignment of CsMYB6 and its homologs from Arabidopsis (AtMYB106, AtMYB16, AtGL1, AtMYB23), snapdragon (AmMIXTA), and cotton (GhMYB109, GhMYB25, GhMYB25-like) revealed that the CsMYB6 protein shared the conserved R2R3MYB repeat region and amino acid sequence of R2R3MYB subgroup 9, and had the highest similarity with cotton GhMYB25, followed by AtMYB106 and AtMYB16. CsMYB6 was more distantly related to AtGL1, AtMYB23, and cotton GhMYB109 (Fig. 1A). A phylogenetic analysis of the cucumber MYB6 protein and the R2R3MYB proteins from Arabidopsis, snapdragon, petunia, tomato, and cotton, which have been shown to be involved in trichome development (Perez-Rodriguez et al., 2005; Jaffé et al., 2007; Jakoby et al., 2008; Pu et al., 2008; Li et al., 2012; Yoshimi et al., 2013), showed that it clustered within the MIXTA clade, which includes other known MIXTA-like transcription factors, such as AmMIXTA, AmMYBML1, GhMYB25, and GhMYB25-like, and was distinct from the MYBs, such as AtGL1, AtWER, and the cotton GL1-like MYB GhMYB109 (Fig. 1B). Expression analysis with qRT-PCR confirmed lower expression of CsMYB6 in various organs of the csgl1 mutant than in the WT line (Supplementary Fig. S1). These observations indicated that CsMYB6 may be involved in trichome development in cucumber.
Fig. 1.
Phylogenetic analyses of CsMYB6 and its homologs in various species. (A) Protein sequence alignment of MYB6 homologs from Arabidopsis (AtMYB106, AtMYB16, AtGL1, AtMYB23), snapdragon (AmMIXTA), cotton (GhMYB109, GhMYB25, GhMYB25-like), and cucumber (CsMYB6). The lines above the sequence indicate the conserved R2 and R3 MYB repeats. The box indicates the conserved MIXTA motif in subgroup 9 of R2R3MYB (AQWESARXXAEXRLXRES). (B) Phylogram of CsMYB6, CsMYB26, and related R2R3MYB proteins from Arabidopsis, snapdragon, Petunia, tomato, cotton, and cucumber. The number at each node is the probability supporting each node with 1000 bootstrapping values.
CsMYB6 is highly expressed in cucumber ovary epidermal cells
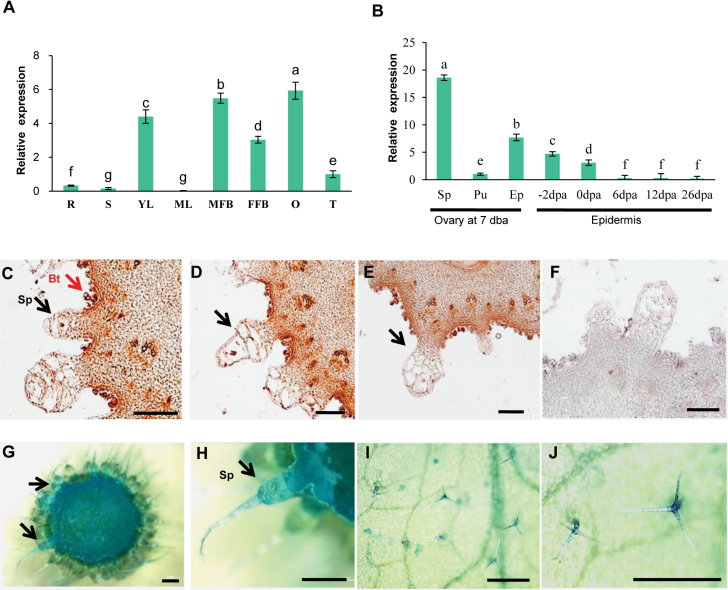
To better understand the biological function of CsMYB6, we evaluated its expression in various organs (roots, stems, young leaves, mature leaves, female flower buds, male flower buds, fruits, and tendrils) using qRT–PCR. The highest expression of CsMYB6 occurred in young leaves and both male and female flower buds (Fig. 2A). We analyzed CsMYB6 transcript levels in different parts of the cucumber ovary at 7 days before anthesis (DBA; the stage of fruit spine initiation and development); higher expression was detected in the epidermis (including peel and spines) than in the pulp (Fig. 2B). Transcript levels were also assessed during different cucumber fruit developmental stages. CsMYB6 was abundantly expressed in the peel at the stage of fruit spine initiation (7 DBA), but expression declined rapidly as the spines began to elongate (Fig. 2B). When the expression patterns of CsMYB6 in 7 DBA ovaries were analyzed by in situ hybridization, CsMYB6 transcripts were detected in spines, tubercules, bloom trichomes, the epidermis, and the pulp adjacent to the epidermis, which was consistent with the qRT–PCR results (Fig. 2C–E). In addition, these results were supported by CsMYB6 promoter GUS reporter gene analysis, in which pCsMYB6-GUS-expressing transgenic cucumber lines showed GUS activity mainly in the spines, tubercules, epidermis, and pulp, and pCsMYB6-GUS-expressing Arabidopsis lines showed GUS activity mainly in the trichomes (Fig. 2G–I). These observations suggested that CsMYB6 may be involved in epidermal cell differentiation and fruit trichome initiation in cucumber.
Fig. 2.
Spatial-temporal expression patterns of CsMYB6. (A) CsMYB6 expression in different organs based on the results of qRT–PCR. (B) CsMYB6 expression in different parts and developmental stages of ovaries. The cucumber gene α-TUBULIN (TUA) was used as an internal control. Significant differences were determined according to Duncan’s multiple range test (P<0.05). Error bars represent the SD based on three biological replicates. (C–E) mRNA in situ hybridization of CsMYB6 in cucumber ovaries at 7 days before anthesis (DBA). Strong signals are detected in spines, bloom trichomes, epidermis, and pulp adjacent to the epidermis. Arrows indicate the locations of expression of CsMYB6 in fruit trichomes. (F) Negative control using the sense probe at 7 DBA. (G–J) GUS expression patterns in pCsMYB6-GUS transgenic lines. GUS staining in trichomes from cucumber ovaries (G, H) and Arabidopsis leaves (I, J). Bt, bloom trichome; DPA, days post anthesis; Ep, epidermis including fine spines; FFB, female flower bud; MFB, male flower bud; O, ovary (at 7 DBA); Pu, pulp; R, Root; S, stem; Sp, spines; T, tendril; YL, young leaf. Scale bars=200 µm in C–F, 1 mm in G and H, and 500 µm in I and J.
Overexpression of CsMYB6 reduces cucumber trichome density
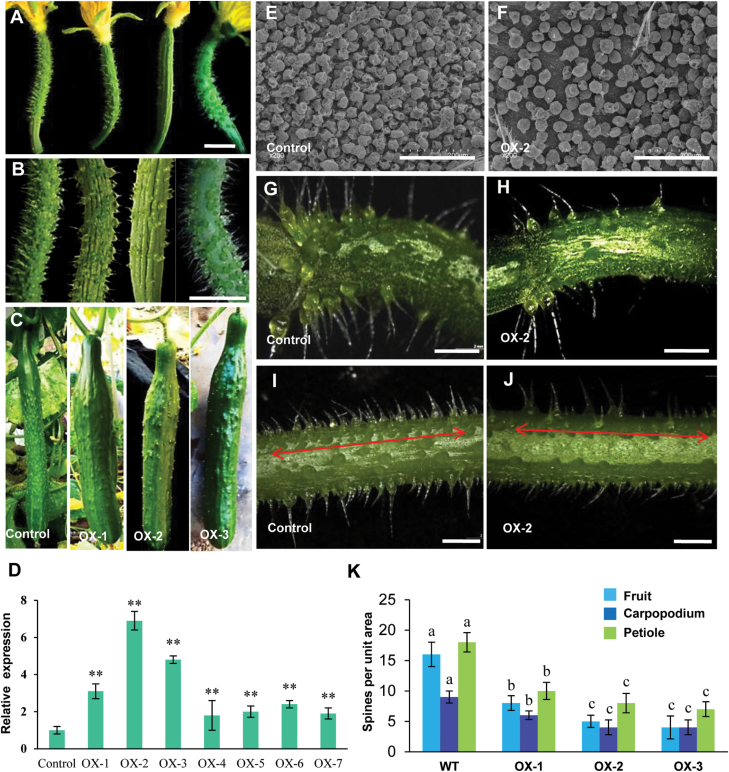
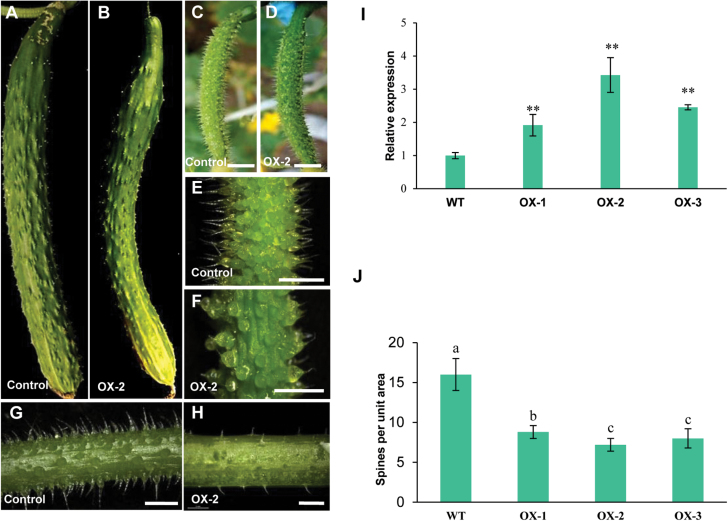
In order to verify the function of CsMYB6 in cucumber, the 35S:CsMYB6 overexpression vector was introduced into the cucumber inbred line R3407 by Agrobacterium-mediated cotyledon transformation. Antibiotic selection and genomic PCR were used to screen transgenic plants (Zhang et al., 2014; Cheng et al., 2015; Chen et al., 2016). Seven positive overexpression lines were obtained (Fig. 3D). Three representative overexpression lines (OX-1, OX-2, and OX-3), whose CsMYB6 expression levels were much higher than the levels in the WT line, were selected for detailed studies. We observed that the numbers of trichomes on the fruit surface, carpopodium, and petiole were substantially lower in all three lines compared with the WT (Fig. 3A–C, G–J), and the numbers of spines on fruits at 0 d post pollination were 50%, 75%, and 69% lower in line OX-1, OX-2, and OX-3, respectively, than in WT plants (Fig. 3I). Moreover, there were substantially fewer bloom trichomes on the surface of the fruit of the transgenic lines than on WT plants (Fig. 3E, F). There were no obvious differences between the overexpression lines and WT plants in terms of spine appearance or morphological characteristics (Supplementary Fig. S2).
Fig. 3.
Phenotypes and gene expression analysis of 35S:CsMYB6 transgenic cucumber plants. Appearance of female flowers of different 35S:CsMYB6 lines (A) and fruits at 2 (B) or 12 (C) days post anthesis. (D) qRT–PCR analyses of CsMYB6 in wild-type (WT) plants and transgenic overexpression (OX) lines. The cucumber gene α-TUBULIN (TUA) was used as an internal control. Error bars represent the SD of three biological replicates. (E, F) Scanning electron microscopic images of the fruit surface of a WT plant (E) and a 35S:CsMYB6-overexpressing line (F) at anthesis. (G, H) Carpopodium of a WT plant (G) and the 35S:CsMYB6-overexpressing line OX-2 (H). (I, J) Petiole of a WT plant (I) and the 35S:CsMYB6-overexpressing line OX-2 (J). (K) Number of trichomes in WT and 35S:CsMYB6-overexpressing lines. Error bars represent the SE. Significant differences were determined according to Duncan’s multiple range test (P<0.05) or Student’s t-test (*P<0.05, **P<0.01). Scale bars=0.5 cm in A and B, 200 μm in E and F, and 2 mm in G–J.
CsMYB6 suppresses fruit trichome initiation in the csgl1 mutant
The spontaneous csgl1 (mict) glabrous cucumber mutant has fruit trichomes with a massive reduction in size and a stunted morphology (Zhao et al., 2015). However, further characterization of csgl1 revealed many obvious macro-size trichomes on the epidermis of the carpopodium and petiole (Fig. 4I, J). In a previous study, the expression of CsMYB6 in the csgl1 mutant was found to be reduced to 11% of that in the WT (Li et al., 2015). We characterized the expression of CsMYB6 in the csgl1 mutant using RNA in situ hybridization and a promoter::GUS assay, and found that CsMYB6 was still expressed in the peel, carpopodium, and petiole (Fig. 4A, B, D–F). To understand the function of CsMYB6 in fruit trichome formation in the csgl1 mutant, the CsMYB6 gene was overexpressed in the csgl1 mutant. We found that overexpression of CsMYB6 reduced trichome density on the fruit surface, carpopodium, and petiole compared with the csgl1 mutant; however, the abnormal morphologic phenotype of the trichomes was not rescued (Fig. 4I–N). These results suggested that CsMYB6 suppresses the initiation of fruit trichomes in the csgl1 mutant.
Fig. 4.
Expression pattern and functional analysis of CsMYB6 in the csgl1 mutant. (A, B) mRNA in situ hybridization of CsMYB6 in ovaries from the csgl1 mutant at 7 days before anthesis (DBA). A strong signal was detected in the epidermis (A) and trichome (B). (C) Negative control using the sense probe at 7 DBA. (D–F) GUS expression patterns in ovaries (D), petiole (E), and carpopodium (F) from the pCsMYB6-GUS transgenic csgl1 plants. (G, H) Negative control of GUS staining in petiole and carpopodium from the csgl1 mutant. (I–N) Phenotype of the csgl1 mutant (I–K) and the CsMYB6-overexpressing csgl1 mutant (L–N). Morphological observations of petiole (I, L) and carpopodium (J, M), and scanning electron microscopic images of the fruit surface (K, N). Overexpression of CsMYB6 resulted in a lower density of trichomes on the fruit surface, carpopodium, and petiole than in the csgl1 mutant. Scale bars=500 µm in A and C, 50 µm in B, 1 mm in D–H, 2 mm in I, J, L, and M, and 200 µm in K and N.
To further verify the function of CsMYB6 in regulating the initiation of spines, the CsMYB6-RNAi construct was introduced into the WT line and the csgl1 mutant. However, phenotypic analysis of CsMYB6-RNAi transgenic cucumber plants showed no difference in trichome morphology (Supplementary Fig. S3).
CsTRY exhibits a similar expression pattern to CsMYB6 and overexpression of CsTRY reduces trichome numbers on fruits and petioles
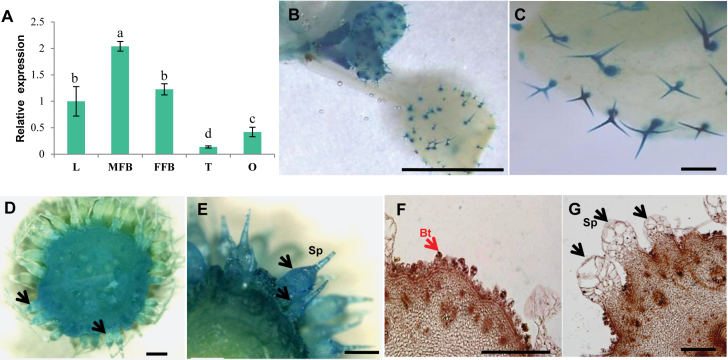
Our previous study found that CsTRY was the only cucumber homolog of Arabidopsis AtTRY, AtETC1, and AtCPC, and that, additionally, ectopic overexpression of CsTRY in WT Arabidopsis significantly reduced the number of leaf trichomes (Tan et al., 2012). We hypothesize that CsTRY may perform a similar function to CsMYB6 in cucumber trichome development. We examined the expression pattern of CsTRY in leaves, female and male flower buds, ovaries, and tendrils using qRT–PCR. We observed that CsTRY was mainly expressed in leaves, male and female flower buds, and ovaries, which was consistent with the expression pattern of CsMYB6 (Fig. 5A). Furthermore, in the pCsTRY::GUS assay, pCsTRY-GUS-expressing Arabidopsis lines showed GUS activity mainly in the trichomes (Fig. 5B, C), while GUS expression in pCsTRY-GUS-expressing transgenic cucumber lines occurred in the trichomes, epidermis, and pulp adjacent to the epidermis of the ovaries (Fig. 5D, E). We examined the expression pattern of CsTRY in 7 DBA ovaries by using RNA in situ hybridization; results were consistent with those of the pCsTRY::GUS assay, indicating that CsTRY transcripts were expressed in spines, tubercules, epidermis, and pulp adjacent to the epidermis (Fig. 5F, G). These data suggested that CsTRY may be involved in fruit trichome development in cucumber.
Fig. 5.
Expression analysis of CsTRY. (A) qRT–PCR analysis of CsTRY expression in different organs of cucumber. The cucumber gene α-TUBULIN (TUA) was used as the internal control. Significant differences were determined according to Duncan’s multiple range test (P<0.05). Error bars represent the SD of three biological replicates. (B–E) GUS expression patterns in pCsTRY-GUS transgenic lines. GUS staining in Arabidopsis leaf trichomes (B, C) and cucumber ovaries (D, E). (F, G) mRNA in situ hybridization of CsTRY in cucumber ovaries at 7 days before anthesis (DBA). FFB, female flower bud; L, leaf; MFB, male flower bud; O, ovary at 7 DBA; T, tendril. Scale bars=2 mm in B, 200 µm in C, F, and G, and 1 mm in D and E.
The CsTRY coding sequence (CsTRY-OE) was fused to the CaMV 35S promoter and introduced into the cucumber inbred line R3407. Five positive overexpression lines were obtained (Fig. 6I). We found that the numbers of trichomes on the fruits and petioles of all transgenic lines were lower than those in the WT line (Fig. 6A–H). Consistent with this observation, the numbers of fruit spines at 0 d post pollination in three overexpressing lines (OX-1, OX-2, and OX-3) were 38%, 44%, and 41% of those in the WT, respectively (Fig. 6J), suggesting that CsTRY may suppresses the formation of trichomes in cucumber fruits, similar to CsMYB6.
Fig. 6.
Overexpression of CsTRY results in fewer trichomes on cucumber fruit. The fruit spine numbers of the 35S:CsTRY-overexpressing line OX-2 (B, D, F) were smaller than those of the wild type (WT) (A, C, E); this was also the case for the petiole of the WT (G) and the OX-2 line (H). (I) qRT–PCR analyses of CsTRY in WT plants and three transgenic 35S:CsTRY-overexpressing lines. The cucumber gene α-TUBULIN (TUA) was used as the internal control. Error bars represent the SD of three biological replicates. (J) The number of trichomes in WT and 35S:CsTRY-overexpressing lines. Significant differences were determined according to Duncan’s multiple range test (P<0.05) or Student’s t-test (**P<0.01). Error bars represent the SE. Scale bars=0.5 cm in C–F and 2 mm in G and H.
CsTRY is directly regulated by CsMYB6
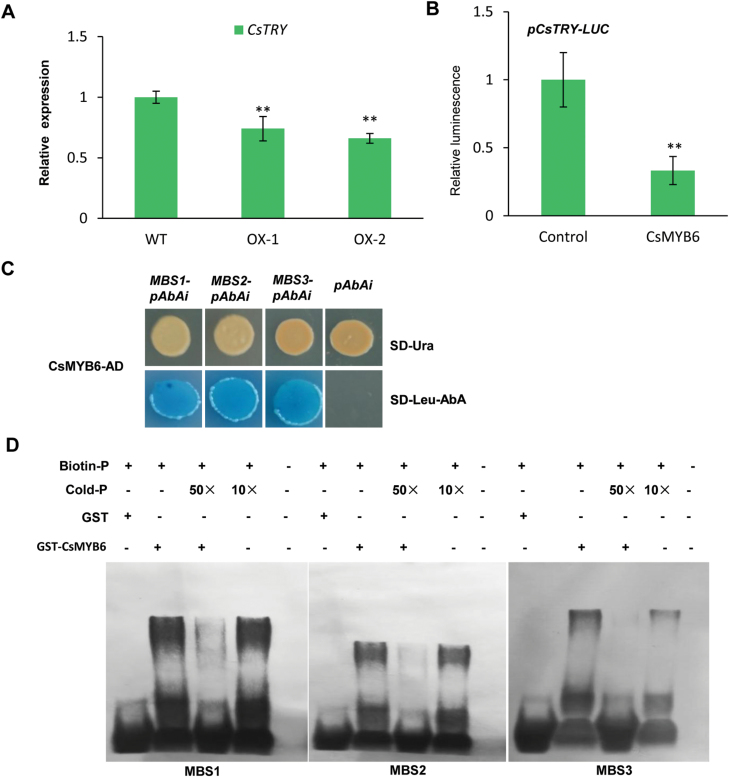
To further reveal the links between CsTRY and CsMYB6 in cucumber trichome development, we examined the expression of CsTRY in CsMYB6-overexpressing cucumber lines, and found significantly lower expression of CsTRY in the peel of the transgenic CsMYB6-overexpressing plants (Fig. 7A). The dual-luciferase assay also showed that CsMYB6 attenuated the promoter activity of CsTRY in N. benthamiana leaves (Fig. 7B).This observation and the fact that three MYB binding sites are present in the CsTRY promoter region suggested the possibility that CsMYB6 may directly bind to the CsTRY promoter. To test this hypothesis, we performed Y1H assays and found that CsMYB6 bound to all the three MBS elements in the CsTRY promoter (Fig. 7C); this finding was further confirmed with EMSA (Fig. 7D).
Fig. 7.
(A) Gene expression changes of CsTRY in CsMYB6-overexpressing cucumber plants. Asterisks indicate statistically significant differences according to Student’s t-test (**P<0.01) WT, wild type. (B) Transient transcriptional activity assay showing the repression of the CsTRY promoter by CsMYB6. Asterisks indicate statistically significant differences according to Student’s t-test (**P<0.01). (C) Transactivation activity of CsMYB6 to the three MBS motifs in the promoter of CsTRY in yeast. (D) An electrophoretic mobility shift assay was used to analyze the interaction of GST-CsMYB6 and the three MBS motifs in the promoter of CsTRY. Purified GST-CsMYB6 protein samples (3 µg) were incubated with 25 pM of the biotin-labeled WT probe. Non-labeled probes at 50-fold concentrations were added for the competition test.
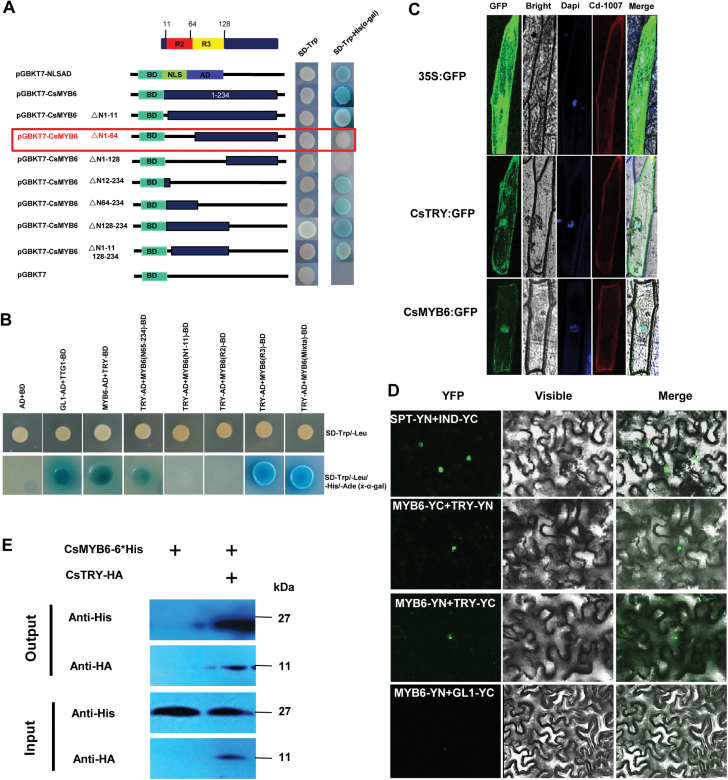
To better understand the regulatory mechanism of CsMYB6, Y2H assays were used to screen interaction partners of CsMYB6 in the cucumber genome. Because of the strong transcriptional self-activation of CsMYB6, a deletion analysis was performed; we found that the activation domain (AD) was localized in residues 1–64 at the N-terminus (Fig. 8A). Consequently, a Y2H assay was performed using the truncated C-terminal protein containing amino acids 65–234 of CsMYB6 as the bait protein to screen a cucumber cDNA library fused to the yeast GAL4 AD. Eighteen putative interacting proteins were identified, which included, unexpectedly, CsTRY (Supplementary Table S2). Additional Y2H assays indicated that CsTRY interacted directly with the R3 and MIXTA domains of CsMYB6 (Fig. 8B). Subcellular localization of CsMYB6 and CsTRY in onion epidermal cells revealed that both proteins were localized mainly to the nucleus and plasma membrane (Fig. 8C). In further BiFC and co-immunoprecipitation assays, the interaction between CsMYB6 and CsTRY was proven in planta (Fig. 8D, E). Therefore, all these data supported the existence of direct interactions between CsMYB6 and CsTRY.
Fig. 8.
CsMYB6 interacts with CsTRY. (A) Deletion analysis for screening the transcriptional activation regions of CsMYB6. Different parts of CsMYB6 were fused with the GAL4 DNA-binding domain and transformed into yeast strain AH109 containing the His3 and LacZ reporter genes. Three repeats were performed and each showed similar patterns. AD, GAL4 activation domain; BD, GAL4 DNA-binding domain; NLS, nuclear localization signal. (B) Yeast two-hybrid assay showing that the R3 and MIXTA domains of CsMYB6 interacted with CsTRY by growth on SD/-Leu/-Trp/-His/X-α-gal plates. The empty vector was used as a negative control, and the interaction between TTG1-BD and GL1-AD was used as a positive control. (C) Subcellular localization of CsTRY and CsMYB6 protein in onion epidermal cells. (D) BiFC analysis of the physical interaction between CsMYB6 (fused with the N-terminal or C-terminal fragment of YFP) and CsTRY (fused with the C-terminal or N-terminal fragment of YFP). INDEHISCENT (IND)-YFPC and SPATULA (SPT)-YFPN were used as positive controls. Different combinations of the fused constructs were co-expressed in leaves of N. benthamiana, and the cells were then visualized using confocal microscopy. (E) Co-immunoprecipitation of transiently co-expressed CsMYB6-6×His and HA-CsTRY in N. benthamiana leaves. Protein extracts before (input) and after (output) immunoprecipitation with anti-His antibody-conjugated beads were detected by western blot with anti-HA antibody.
Overexpression of CsMYB6 in Arabidopsis results in fewer trichomes
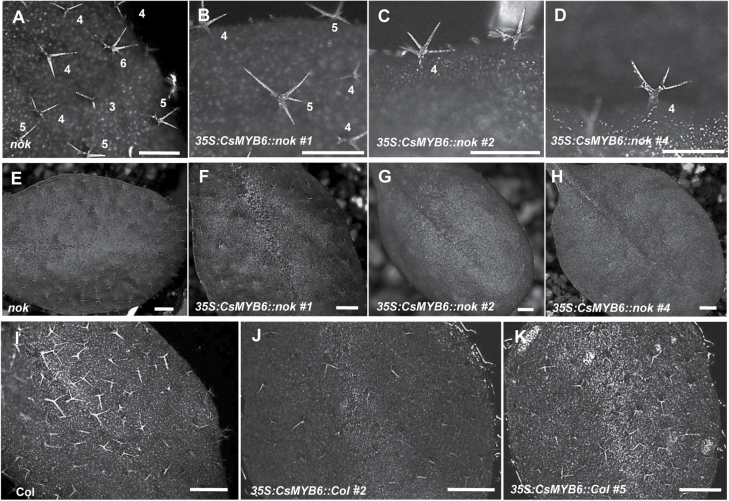
To study the extent to which CsMYB6 function is conserved among core eudicots, we tested the ability of CsMYB6 to recover the mutant phenotype of nok/myb106 (Fig. 9A; Folkers et al., 1997). The 35S promoter was used instead of the AtMYB106 promoter because AtMYB106 shows a different expression pattern from CsMYB6 (Jakoby et al., 2008). Six independent transgenic plants were obtained, all of which showed partial rescued of the over-branched trichome phenotype of the nok mutant (Fig. 9B–D, Supplementary Table S3). All the overexpression lines had a 10–20% higher proportion of trichomes with four branches than the nok mutant, while the number of trichomes with five branches in the overexpression lines decreased by 10–15% (Supplementary Table S3). Interestingly, the total number of trichomes on these transgenic plants was reduced compared with the nok mutant (Fig. 9E–H). For example, plants from transgenic line 4 had 41% fewer trichomes than the nok plants (Supplementary Table S3).
Fig. 9.
Ectopic expression of CsMYB6 in the nok mutant and wild-type Arabidopsis. (A–K) Phenotypic comparison of leaf trichomes from nok (A, E), 35S::CsMYB6/nok lines (B–D, F–H), Col (I), and 35S:CsMYB6/Col lines (J, K) Arabidopsis plants. Overexpression of CsMYB6 in nok and Col both resulted in fewer trichomes. Scale bars=1 mm in A–D and 2 mm in E–K.
To further examine the function of CsMYB6, we overexpressed CsMYB6 in WT Arabidopsis (Col). In all six independent transgenic lines obtained, the number of trichomes was lower than in WT plants (Fig. 9I–K). Moreover, in 35S:CsMYB6 lines, trichomes exhibiting two branches emerged, which were similar to the trichomes of AtMYB106/NOK-overexpressing Arabidopsis plants (Supplementary Fig. S4). These results suggested that CsMYB6 also has a suppressive role in the regulation of trichome development in Arabidopsis.
Discussion
Trichomes are specialized epidermal cells that are located in the aerial parts of plants. Several MYB transcription factors have been identified that are involved in the regulation of epidermal cell patterning in Arabidopsis and cotton, species in which trichomes are unicellular structures (Loguerico et al., 1999; Suo et al., 2003; Wilkins and Arpat, 2005; Lee et al., 2006; Lee et al., 2007; Wu et al., 2006). Little is known about the function of MYB transcription factors in the development of cucumber multicellular trichomes. In this study, we provide evidence to show that the R2R3MYB transcription factor gene CsMYB6 plays a key role in cucumber fruit trichome formation. We found that, unlike the function of its homologs in Arabidopsis and cotton, both of which have single-celled trichomes, CsMYB6 negatively regulates fruit trichome formation through an interaction with CsTRY, which is independent of CsGL1.
CsMYB6 is a MIXTA-like gene of the R2R3MYB family
Since the first cloning of the MIXTA R2R3MYB transcription factor gene in snapdragon (Noda et al., 1994), MIXTA homologs have been identified in many plant species, such as Thalictrum thalictroides, Erythranthe lewisii, tomato (Solanum lycopersicum), cotton, and Arabidopsis. MIXTA homologs have been shown to regulate epidermal cell morphology, including the differentiation of trichome cells (e.g. Glover et al., 1998; Perez-Rodriguez et al., 2005; Jaffé et al., 2007; Baumann et al., 2007; Yoshimi et al., 2013). Here, we studied CsMYB6, a MIXTA homolog in cucumber (Fig. 1). Protein sequence alignment among MIXTA homologs of different species revealed that the CsMYB6 protein shared the conserved R2R3MYB repeat region and amino acid sequence of R2R3MYB subgroup 9 (Fig. 1A). Overexpression of CsMYB6 reduced trichome density in both cucumber and Arabidopsis. These data provided convincing evidence for a role of CsMYB6, like other MIXTA-like MYBS, in regulating epidermal cell differentiation and fruit trichome formation (Figs 3, 9). Phylogenetic analysis revealed that CsMYB6 was in the MIXTA clade, which also included Arabidopsis AtMYB106 and cotton GhMYB25-like (Fig. 1B). However, AtMYB106-overexpressing Arabidopsis plants showed trichomes that were less branched, suggesting that AtMYB106 suppresses the formation of trichome branching, rather than trichome formation itself (Folkers et al., 1997; Jakoby et al., 2008). Unlike AtMYB106, GhMYB25-like is a key regulator in cotton fiber initiation (Walford et al., 2011). In the present study, overexpression of CsMYB6 affected the number of trichomes rather than their morphology, a function more like that of the cotton GhMYB25-like gene. These observations suggested that the role of CsMYB6 in trichome development is more similar to that of GhMYB25-like than AtMYB106.
CsMYB6 is involved in the regulation of fruit trichome initiation in cucumber
In this study, expression analysis revealed spatial-temporal dynamics of CsMYB6 expression in cucumber. We found that CsMYB6 transcript was more abundant in ovaries at the stage of fruit spine initiation and development, and declined rapidly as the spines began to elongate (Fig. 2B). Furthermore, CsMYB6 was expressed at a much higher level in the epidermis than in the pulp; both in situ hybridization and pCsMYB6::GUS assays clearly showed that CsMYB6 was expressed in the fruit epidermis and trichomes (Fig. 2C–J). These data are consistent with the role of CsMYB6 in regulating epidermal cell differentiation and fruit trichome formation. Since the overexpression of CsMYB6 in both WT and csgl1 mutant cucumber lines caused a significant decrease in trichome numbers in the fruit, petiole, and carpopodium (Figs 3, 4), CsMYB6 may suppress fruit trichome initiation. This was further supported by ectopic expression of CsMYB6 in Arabidopsis WT (Col) and nok (AtMYB106) mutant lines, which resulted in a reduced number of trichomes (Fig. 9). However, altered trichome morphology was observed in transgenic Arabidopsis plants overexpressing CsMYB6; this observation may have two possible explanations. Unlike the simple unicellular, highly branched trichomes of Arabidopsis, cucumber trichomes are multicellular and unbranched. Therefore, CsMYB6 does not seem to be involved in trichome branching. In addition, the regulatory mechanisms for trichome formation in cucumber may differ from those in Arabidopsis, and CsMYB6 and AtMYB106 may play distinct roles in this process.
Phenotypic analysis of CsMYB6-RNAi-WT and CsMYB6-RNAi-csgl1 transgenic cucumber plants showed no difference in the number and morphology of trichomes compared with their respective controls (Supplementary Fig. S3). The expression of CsMYB26 was increased in CsMYB6-RNAi lines, and the phylogenetic analysis of CsMYB6 and CsMYB26 showed that they both clustered within the MIXTA clade (Fig. 1), indicating that CsMYB26 might have some functional redundancy with CsMYB6.
CsMYB6 acts upstream of CsTRY to regulate fruit trichome formation in cucumber
In Arabidopsis, AtTRY suppresses the formation of trichomes through interaction with GL3/EGL3 in competition with GL1 (Schellmann et al., 2002; Esch et al., 2003; Zhang et al., 2003). The SQUAMOSA PROMOTER BINDING PROTEIN LIKE 9 transcription factors suppress trichome formation by directly binding to the TRY gene promoter and activating its expression (Nan et al., 2010). The WRKY protein TTG2 binds to the promoter of TRY and regulates TRY expression through the enhancement of activator complex-triggered activation (Pesch et al., 2014). TRY appears to play an essential role in the gene regulatory network underlying trichome patterning. Previous studies suggested that CsTRY is the only putative cucumber homolog of the Arabidopsis genes AtTRY, AtETC1, and AtCPC, and ectopic overexpression of CsTRY in Arabidopsis WT plants significantly reduced the number of leaf trichomes, indicating that CsTRY may have the same function as AtTRY (Tan et al., 2012). Here, we generated CsTRY-overexpressing transgenic cucumber lines, which exhibited significantly reduced trichome initiation on the fruit (Fig. 6J). Thus, it is reasonable to conclude that, similar to its Arabidopsis homolog AtTRY, CsTRY suppresses fruit trichome initiation in cucumber. CsTRY was predominantly expressed in the trichomes, epidermis, and pulp adjacent to the ovary epidermis, showing overlapping expression with CsMYB6. In 35S:CsMYB6 plants, the CsTRY transcript level was lower than in WT plants (Fig. 7A). The dual-luciferase assay also showed that CsMYB6 can attenuate the promoter activity of CsTRY in N. benthamiana leaves (Fig. 7B). In further Y1H and EMSA assays, CsMYB6 was able to bind to the promoter of CsTRY (Fig. 7C, D). From these data, it seems that there is a novel mechanism in which a CsMYB6-CsTRY complex negatively regulates fruit trichome formation in cucumber. CsMYB6 acted upstream of CsTRY in this process. Interestingly, we identified that overexpression of CsMYB6 or CsTRY results in fewer trichomes. However, overexpression of CsMYB6 inhibited, rather than promoted, the expression of CsTRY. Thus, the relationship between CsMYB6 and CsTRY seems not to be simple, and the details of the relationship remain to be identified. In Arabidopsis, AtGL3, AtTTG1, and AtGL1 were found to compose a trimeric complex and to activate trichome initiation (Oppenheimer et al., 1991; Walker et al., 1999; Zhang et al., 2003). AtGL3 and AtTTG1 can enhance the activation of the AtTRY promoter, while AtGL1 can repress the activation of the AtTRY promoter by AtGL3 and AtTTG1. AtTTG1 inhibited the activation of the promoter of AtCPC by AtGL3 and AtGL1 (Pesch et al., 2015). Consequently, CsMYB6 acts as a negative regulator of cucumber spine initiation, and attenuates the promoter activity of CsTRY, while some other proteins that interact with CsMYB6 may regulate the inhibition of the CsTRY promoter. Protein–protein interaction assays suggested that CsTRY also interacts directly with CsMYB6 protein, indicating that CsTRY may participate in the regulation of the inhibition of its promoter by CsMYB6. Recently, our research demonstrated that CsTTG1 regulated fruit spine formation in cucumber (Chen et al., 2016), which suggested that CsTTG1 may also participate in the modulation of the activity of the CsMYB6-CsTRY complex. In conclusion, our results uncover a new role for the CsMYB6-CsTRY complex in regulating trichome initiation. However, the molecular mechanisms underlying how and why CsMYB6 represses CsTRY transcriptional activity require further study.
CsTTG1 regulated further differentiation of spines via interaction with CsGL1, while CsTTG1 seemed to be independent of Mict/CsGL1 in regulating fruit trichome initiation (Chen et al., 2016). Overexpressing CsMYB6 in the csgl1 mutant caused a lower density of fruit trichomes; however, the morphology of the trichomes was not rescued, indicating that CsMYB6 is involved only in the initiation of fruit trichomes (Fig. 4I–N). However, the expression of CsMYB6 is strongly decreased in the csgl1 mutant. The interaction between CsGL1 and CsTTG1 influences the expression of CsTTG1, which may affect the expression of CsMYB6. Future studies to obtain knockout transgenic lines of CsMYB6 and CsTTG1 by using CRISPR/Cas9 technology would help research into the molecular mechanism of trichome formation in cucumber.
Supplementary data
Supplementary data are available at JXB online.
Fig. S1. Relative transcript abundance of CsMYB6 in different tissues of wild-type and csgl1 mutant.
Fig. S2. Morphological characterization of CsMYB6-overexpressing cucumber plants.
Fig. S3. Phenotypic analysis of CsMYB6-RNAi cucumber plants.
Fig. S4. Ectopic expression of CsMYB6 in wild-type Arabidopsis caused the emergence of trichomes with two branches.
Table S1. Primers used in this study.
Table S2. Proteins interacting with CsMYB6.
Table S3. Effect of CsMYB6 on trichome and its branch numbers on leaves.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (grant no. 31672159), the National Key Research and Development Program of China (grant no. 2016YFD0101705), and Beijing Agricultural Innovation Consortium (grant no. BAIC01-2016).
References
- Baumann K, Perez-Rodriguez M, Bradley D, Venail J, Bailey P, Jin H, Koes R, Roberts K, Martin C. 2007. Control of cell and petal morphogenesis by R2R3 MYB transcription factors. Development 134, 1691–1701. [DOI] [PubMed] [Google Scholar]
- Bodnaryk RP. 1996. Physical and chemical defenses of pods and seeds of white mustard (Sinapis alba L.) against tarnished plant bugs, Lygus lineolaris (Palisor de Baeuvois) (Heteroptera: Miridae). Canadian Journal of Plant Science 76, 33–36. [Google Scholar]
- Cheng J, Wang Z, Yao F, Gao L, Ma S, Sui X, Zhang Z. 2015. Down-regulating CsHT1, a cucumber pollen-specific hexose transporter, inhibits pollen germination, tube growth, and seed development. Plant Physiology 168, 635–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Liu M, Jiang L, Liu X, Zhao J, Yan S, Yang S, Ren H, Liu R, Zhang X. 2014. Transcriptome profiling reveals roles of meristem regulators and polarity genes during fruit trichome development in cucumber (Cucumis sativus L.). Journal of Experimental Botany 65, 4943–4958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Yin S, Liu X, et al. 2016. The WD-repeat protein CsTTG1 regulates fruit wart formation through interaction with the homeodomain-leucine zipper I protein Mict. Plant Physiology 171, 1156–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough SJ, Bent AF. 1998. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. The Plant Journal 16, 735–743. [DOI] [PubMed] [Google Scholar]
- Du H, Yang SS, Liang Z, Feng BR, Liu L, Huang YB, Tang YX. 2012. Genome-wide analysis of the MYB transcription factor superfamily in soybean. BMC Plant Biology 12, 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubos C, Stracke R, Grotewold E, Weisshaar B, Martin C, Lepiniec L. 2010. MYB transcription factors in Arabidopsis. Trends in Plant Science 15, 573–581. [DOI] [PubMed] [Google Scholar]
- Eisner T, Eisner M, Hoebeke ER. 1998. When defence backfires: detrimental effect of a plant’s protective trichomes on an insect beneficial to the plant. Proceedings of the National Academy of Sciences, USA 95, 4410–4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esch JJ, Chen M, Sanders M, Hillestad M, Ndkium S, Idelkope B, Neizer J, Marks MD. 2003. A contradictory GLABRA3 allele helps define gene interactions controlling trichome development in Arabidopsis. Development 130, 5885–5894. [DOI] [PubMed] [Google Scholar]
- Folkers U, Berger J, Hülskamp M. 1997. Cell morphogenesis of trichomes in Arabidopsis: differential control of primary and secondary branching by branch initiation regulators and cell growth. Development 124, 3779–3786. [DOI] [PubMed] [Google Scholar]
- Galway ME, Masucci JD, Lloyd AM, Walbot V, Davis RW, Schiefelbein JW. 1994. The TTG gene is required to specify epidermal cell fate and cell patterning in the Arabidopsis root. Developmental Biology 166, 740–754. [DOI] [PubMed] [Google Scholar]
- Glover BJ, Perez-Rodriguez M, Martin C. 1998. Development of several epidermal cell types can be specified by the same MYB-related plant transcription factor. Development 125, 3497–3508. [DOI] [PubMed] [Google Scholar]
- Hellens RP, Allan AC, Friel EN, Bolitho K, Grafton K, Templeton MD, Karunairetnam S, Gleave AP, Laing WA. 2005. Transient expression vectors for functional genomics, quantification of promoter activity and RNA silencing in plants. Plant Methods 1, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hülskamp M. 2004. Plant trichomes: a model for cell differentiation. Nature Reviews Molecular Cell Biology 5, 471–480. [DOI] [PubMed] [Google Scholar]
- Hülskamp M, Misŕa S, Jürgens G. 1994. Genetic dissection of trichome cell development in Arabidopsis. Cell 76, 555–566. [DOI] [PubMed] [Google Scholar]
- Hülskamp M, Schnittger A. 1998. Spatial regulation of trichome formation in Arabidopsis thaliana. Seminars in Cell & Developmental Biology 9, 213–220. [DOI] [PubMed] [Google Scholar]
- Jaffé FW, Tattersall A, Glover BJ. 2007. A truncated MYB transcription factor from Antirrhinum majus regulates epidermal cell outgrowth. Journal of Experimental Botany 58, 1515–1524. [DOI] [PubMed] [Google Scholar]
- Jakoby MJ, Falkenhan D, Mader MT, Brininstool G, Wischnitzki E, Platz N, Hudson A, Hülskamp M, Larkin J, Schnittger A. 2008. Transcriptional profiling of mature Arabidopsis trichomes reveals that NOECK encodes the MIXTA-like transcriptional regulator MYB106. Plant Physiology 148, 1583–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin JC, Brown ML, Schiefelbein J. 2003. How do cells know what they want to be when they grow up? Lessons from epidermal patterning in Arabidopsis. Annual Review of Plant Biology 54, 403–430. [DOI] [PubMed] [Google Scholar]
- Lee JJ, Hassan OS, Gao W, Wei NE, Kohel RJ, Chen XY, Payton P, Sze SH, Stelly DM, Chen ZJ. 2006. Developmental and gene expression analyses of a cotton naked seed mutant. Planta 223, 418–432. [DOI] [PubMed] [Google Scholar]
- Lee JJ, Woodward AW, Chen ZJ. 2007. Gene expression changes and early events in cotton fibre development. Annals of Botany 100, 1391–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Zhang C, Li J, Wang L, Ren Z. 2012. Genome-wide identification and characterization of R2R3MYB family in Cucumis sativus. PLoS One 7, e47576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Cao C, Zhang C, Zheng S, Wang Z, Wang L, Ren Z. 2015. The identification of Cucumis sativus Glabrous 1 (CsGL1) required for the formation of trichomes uncovers a novel function for the homeodomain-leucine zipper I gene. Journal of Experimental Botany 66, 2515–2526. [DOI] [PubMed] [Google Scholar]
- Liu XW, Ezra B, Cai YL, Ren HZ. 2016. Trichome-related mutants provide a new perspective on multicellular trichome initiation and development in cucumber (Cucumis sativus L). Frontiers in Plant Science 7, 1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loguerico LL, Zhang JQ, Wilkins TA. 1999. Differential regulation of six novel MYB-domain genes defines two distinct expression patterns in allotetraploid cotton (Gossypium hirsutum L.). Molecular & General Genetics 261, 660–671. [DOI] [PubMed] [Google Scholar]
- Machado A, Wu Y, Yang Y, Llewellyn DJ, Dennis ES. 2009. The MYB transcription factor GhMYB25 regulates early fibre and trichome development. The Plant Journal 59, 52–62. [DOI] [PubMed] [Google Scholar]
- Matus JT, Aquea F, Arce-Johnson P. 2008. Analysis of the grape MYB R2R3 subfamily reveals expanded wine quality-related clades and conserved gene structure organization across Vitis and Arabidopsis genomes. BMC Plant Biology 8, 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauricio R, Rausher MD. 1997. Experimental manipulation of putative selective agents provides evidence for the role of natural enemies in the evolution of plant defense. Evolution 51, 1435–1444. [DOI] [PubMed] [Google Scholar]
- Nan Y, Wen JC, Shu CW, Chun MS, Ling JW, Xiao YC. 2010. Temporal control of trichome distribution by microRNA156-targeted SPL genes in Arabidopsis thaliana. The Plant Cell 22, 2322–2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal JJ, Steffens JC, Tingey WM. 1989. Glandular trichomes of Solanum berhaultii and resistance to the Colorado potato beetle. Entomologia Experimentalis et Applicata 51, 133–140. [Google Scholar]
- Noda K, Glover BJ, Linstead P, Martin C. 1994. Flower colour intensity depends on specialized cell shape controlled by a Myb-related transcription factor. Nature 369, 661–664. [DOI] [PubMed] [Google Scholar]
- Oppenheimer DG, Herman PL, Sivakumaran S, Esch J, Marks MD. 1991. A myb gene required for leaf trichome differentiation in Arabidopsis is expressed in stipules. Cell 67, 483–493. [DOI] [PubMed] [Google Scholar]
- Pan Y, Bo K, Cheng Z, Weng Y. 2015. The loss-of-function GLABROUS 3 mutation in cucumber is due to LTR-retrotransposon insertion in a class IV HD-ZIP transcription factor gene CsGL3 that is epistatic over CsGL1. BMC Plant Biology 15, 302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattanaik S, Patra B, Singh SK, Yuan L. 2014. An overview of the gene regulatory network controlling trichome development in the model plant, Arabidopsis. Frontiers in Plant Science 5, 259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne CT, Zhang F, Lloyd AM. 2000. GL3 encodes a bHLH protein that regulates trichome development in Arabidopsis through interaction with GL1 and TTG1. Genetics 156, 1349–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Rodriguez M, Jaffe FW, Butelli E, Glover BJ, Martin C. 2005. Development of three different cell types is associated with the activity of a specific MYB transcription factor in the ventral petal of Antirrhinum majus flowers. Development 132, 359–370. [DOI] [PubMed] [Google Scholar]
- Pesch M, Dartan B, Birkenbihl R, et al. 2014. Arabidopsis TTG2 regulates TRY expression through enhancement of activator complex-triggered activation. The Plant Cell 26, 4067–4083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesch M, Schultheiß I, Klopffleisch K, Uhrig JF, Koegl M, Clemen CS, Simon R, Weidtkamp-Peters S, Hülskamp M. 2015. TRANSPARENT TESTA GLABRA1 and GLABRA1 compete for binding to GLABRA3 in Arabidopsis. Plant Physiology 168, 584–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pu L, Li Q, Fan X, Yang W, Xue Y. 2008. The R2R3 MYB transcription factor GhMYB109 is required for cotton fiber development. Genetics 180, 811–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth I. 1977. Fruits of Cucurbitaceae. Encyclopedia of Plant Anatomy 10, 471–477. [Google Scholar]
- Saitou N, Nei M. 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Molecular Biology and Evolution 4, 406–425. [DOI] [PubMed] [Google Scholar]
- Samuels ALM, Glass AD, Ehret DL, Menzies JG. 1993. The effects of silicon supplementation on cucumber fruit: changes in surface characteristics. Annals of Botany 72, 433–440. [Google Scholar]
- Schellmann S, Schnittger A, Kirik V, Wada T, Okada K, Beermann A, Thumfahrt J, Jürgens G, Hülskamp M. 2002. TRIPTYCHON and CAPRICE mediate lateral inhibition during trichome and root hair patterning in Arabidopsis. The EMBO Journal 21, 5036–5046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schütze K, Harter K, Chaban C. 2009. Bimolecular fluorescence complementation (BiFC) to study protein-protein interactions in living plant cells. Methods in Molecular Biology 479, 189–202. [DOI] [PubMed] [Google Scholar]
- Scoville AG, Barnett LL, Bodbyl-Roels S, Kelly JK, Hileman LC. 2011. Differential regulation of a MYB transcription factor is correlated with transgenerational epigenetic inheritance of trichome density in Mimulus guttatus. New Phytologist 191, 251–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suo J, Liang X, Pu L, Zhang Y, Xue Y. 2003. Identification of GhMYB109 encoding a R2R3 MYB transcription factor that expressed specifically in fiber initials and elongating fibers of cotton (Gossypium hirsutum L.). Biochimica et Biophysica Acta 1630, 25–34. [DOI] [PubMed] [Google Scholar]
- Szymanski DB, Lloyd AM, Marks MD. 2000. Progress in the molecular genetic analysis of trichome initiation and morphogenesis in Arabidopsis. Trends in Plant Science 5, 214–219. [DOI] [PubMed] [Google Scholar]
- Tan Z, Guo F, Yang FQ, Liu LY, Zhang XL, Ren HZ. 2012. Overexpression of cucumber CsTRY greatly represses trichome formation in Arabidopsis. Acta Horticulturae Sinica 39, 91–100. [Google Scholar]
- Varagona MJ, Schmidt RJ, Raikhel NV. 1992. Nuclear localization signal(s) required for nuclear targeting of the maize regulatory protein Opaque-2. The Plant Cell 4, 1213–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walford SA, Wu Y, Llewellyn DJ, Dennis ES. 2011. GhMYB25-like: a key factor in early cotton fibre development. The Plant Journal 65, 785–797. [DOI] [PubMed] [Google Scholar]
- Walker AR, Davison PA, Bolognesi-Winfield AC, James CM, Srinivasan N, Blundell TL, Esch JJ, Marks MD, Gray JC. 1999. The TRANSPARENT TESTA GLABRA1 locus, which regulates trichome differentiation and anthocyanin biosynthesis in Arabidopsis, encodes a WD40 repeat protein. The Plant Cell 11, 1337–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter M, Chaban C, Schütze K, et al. 2004. Visualization of protein interactions in living plant cells using bimolecular fluorescence complementation. The Plant Journal 40, 428–438. [DOI] [PubMed] [Google Scholar]
- Wang H, Sui X, Guo J, Wang Z, Cheng J, Ma S, Li X, Zhang Z. 2014. Antisense suppression of cucumber (Cucumis sativus L.) sucrose synthase 3 (CsSUS3) reduces hypoxic stress tolerance. Plant, Cell & Environment 37, 795–810. [DOI] [PubMed] [Google Scholar]
- Wang W, Zhang Y, Xu C, Ren J, Liu X, Black K, Gai X, Wang Q, Ren H. 2015. Cucumber ECERIFERUM1 (CsCER1), which influences the cuticle properties and drought tolerance of cucumber, plays a key role in VLC alkanes biosynthesis. Plant Molecular Biology 87, 219–233. [DOI] [PubMed] [Google Scholar]
- Wang YL, Nie JT, Chen HM, Guo CL, Pan J, He HL, Pan JS, Cai R. 2016. Identification and mapping of Tril, a homeodomain-leucine zipper gene involved in multicellular trichome initiation in Cucumis sativus. Theoretical and Applied Genetics 129, 305–316. [DOI] [PubMed] [Google Scholar]
- Werker E. 2000. Trichome diversity and development. Advances in Botanical Research 31, 1–35. [Google Scholar]
- Wilkins O, Nahal H, Foong J, Provart NJ, Campbell MM. 2009. Expansion and diversification of the Populus R2R3-MYB family of transcription factors. Plant Physiology 149, 981–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins TA, Arpat AB. 2005. The cotton fibre transcriptome. Physiologia Plantarum 124, 295–300. [Google Scholar]
- Wu Y, Machado A, White RG, Llewellyn DJ, Dennis ES. 2006. Identification of early genes expressed during cotton fiber initiation using cDNA microarrays. Plant Cell Physiology 47, 107–127. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Hayashi M, Kanamaru T, Watanabe T, Mametsuka S, Tanaka Y. 1989. Studies on bloom on the surface of cucumber fruits, relation between the degree of bloom occurrence and contents of mineral elements. Bulletin of the Fukuoka Agricultural Research Center B 9, 1–6. [Google Scholar]
- Yang X, Zhang W, He H, et al. 2014. Tuberculate fruit gene Tu encodes a C2H2 zinc finger protein that is required for the warty fruit phenotype in cucumber (Cucumis sativus L.). The Plant Journal 78, 1034–1046. [DOI] [PubMed] [Google Scholar]
- Yoshimi O, Masahito S, Tomotsugu K, Norihiro O, Nobutaka M, Masaru OT. 2013. MIXTA-like transcription factors and WAX INDUCER1/SHINE1 coordinately regulate cuticle development in Arabidopsis and Torenia fournieri. Plant Cell 25, 1609–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yotsui I, Saruhashi M, Kawato T, et al. 2013. ABSCISIC ACID INSENSITIVE3 regulates abscisic acid-responsive gene expression with the nuclear factor Y complex through the ACTT-core element in Physcomitrella patens. New Phytologist 199, 101–109. [DOI] [PubMed] [Google Scholar]
- Zhang F, Gonzalez A, Zhao M, Payne CT, Lloyd A. 2003. A network of redundant bHLH proteins functions in all TTG1-dependent pathways of Arabidopsis. Development 130, 4859–4869. [DOI] [PubMed] [Google Scholar]
- Zhang W, He H, Guan Y, Du H, Yuan L, Li Z, Yao D, Pan J, Cai R. 2010. Identification and mapping of molecular markers linked to the tuberculate fruit gene in the cucumber (Cucumis sativus L.). Theoretical and Applied Genetics 120, 645–654. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Zhang X, Liu B, Wang W, Liu X, Chen C, Liu X, Yang S, Ren H. 2014. A GAMYB homologue CsGAMYB1 regulates sex expression of cucumber via an ethylene-independent pathway. Journal of Experimental Botany 65, 3201–3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao JL, Pan JS, Guan Y, Zhang WW, Bie BB, Wang YL, He HL, Lian HL, Cai R. 2015. Micro-trichome as a class I homeodomain-leucine zipper gene regulates multicellular trichome development in Cucumis sativus. Journal of Integrative Plant Biology 57, 925–935. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.