Impairment of Lhca4, a subunit of LHCI, causes high chlorophyll content and LHCII accumulation in rice, suggesting a novel functional interaction between LHCI and LHCII.
Keywords: Chlorophyll, Lhca4, light-harvesting complex, long-term acclimation, rice, state transition, stay-green
Abstract
Chlorophyll is an essential molecule for acquiring light energy during photosynthesis. Mutations that result in chlorophyll retention during leaf senescence are called ‘stay-green’ mutants. One of the several types of stay-green mutants, Type E, accumulates high levels of chlorophyll in the pre-senescent leaves, resulting in delayed yellowing. We isolated delayed yellowing1-1 (dye1-1), a rice mutant whose yellowing is delayed in the field. dye1-1 accumulated more chlorophyll than the wild-type in the pre-senescent and senescent leaves, but did not retain leaf functionality in the ‘senescent green leaves’, suggesting that dye1-1 is a Type E stay-green mutant. Positional cloning revealed that DYE1 encodes Lhca4, a subunit of the light-harvesting complex I (LHCI). In dye1-1, amino acid substitution occurs at the location of a highly conserved amino acid residue involved in pigment binding; indeed, a severely impaired structure of the PSI-LHCI super-complex in dye1-1 was observed in a blue native PAGE analysis. Nevertheless, the biomass and carbon assimilation rate of dye1-1 were comparable to those in the wild-type. Interestingly, Lhcb1, a trimeric LHCII protein, was highly accumulated in dye1-1, in the chlorophyll–protein complexes. The high accumulation of LHCII in the LHCI mutant dye1 suggests a novel functional interaction between LHCI and LHCII.
Introduction
Chlorophyll synthesis and breakdown are strictly regulated in plants not only because it is an essential photosynthetic molecule, but also because in its free form it photo-oxidatively damages cells (Tanaka et al., 2011). Mutants that retain the greenness of leaves under senescence-inducing conditions are called stay-green mutants, and they are classified into two types: functional and non-functional. Functional stay-green mutants retain the green of leaves via delayed senescence, whereas the greenness of leaves is not necessarily correlated with leaf functionality in the non-functional mutants. The non-functional stay-green mutants can be further classified (Thomas and Howarth, 2000). The Type C mutants show impairment in chlorophyll degradation, with the majority of the mutations being in the genes that encode chlorophyll-degrading enzymes, such as the Chla-degrading enzyme SGR/NYE1, Chlb-degrading enzymes NYC1 and NOL, and pheophytinase PPH/NYC3 (Ren et al., 2007; Kusaba et al., 2007; Park, 2007; Morita et al., 2009; Sato et al., 2009; Schelbert et al., 2009; Shimoda et al., 2016). In addition, mutants of a chloroplast protein THF1/NYC4 and a small subunit of the PSII core complex, PsbM/CytG, show the stay-green phenotype via inhibition of the degradation of chlorophyll–protein complexes (Huang et al., 2013; Yamatani et al.1, 2013; Kohzuma et al., 2017). Although the genes associated with these mutations do not encode chlorophyll-degrading enzymes, the mutants are classified as Type C because chlorophyll degradation is impaired during senescence. Another class of non-functional stay-green mutants, Type E, accumulates high levels of chlorophyll in the pre-senescent leaves, resulting in longer retention of greenness than in those of the wild-type. In other words, Type E can be designated as a mutant with impairments in the proper regulation of chlorophyll accumulation.
Chlorophyll is contained in the photosystems in the thylakoid membranes. PSII uses light energy for the extraction of electrons from water, resulting in the production of oxygen. The photo-excited electrons are transferred to PSI through the cytochrome b6f complex and are used to produce NADPH. The proton gradient formed during the electron transfer process is used for the production of ATP. The light-harvesting complex I (LHCI) is an antenna complex for PSI, and consists of four subunits, Lhca1–Lhca4, in algae and land plants. Lhca1/Lhca4 and Lhca2/Lhca3, respectively, form dimers. The light-harvesting complex II (LHCII) is an antenna complex for PSII, encoded by Lhcb1–Lhcb6. Lhcb1, 2, and 3 are the major LHCII subunits and form trimers. Lhcb4, 5, and 6 are minor LHCII subunits that exist as monomers. Higher plants contain Chla and Chlb. Chla is a major chlorophyll found in all chlorophyll–protein complexes, whereas Chlb is found only in the light-harvesting complexes, LHCI and LHCII.
For optimal photosynthesis, the performances of PSI and PSII should be balanced (Minagawa, 2011). Photosynthetic organisms have several mechanisms to tune the performances of PSI and PSII. State transition is one such mechanism, wherein trimeric LHCII moves to PSI via phosphorylation by STN7 kinase, and functions as an antenna of PSI under ‘PSII-excess’ light conditions (Depège et al., 2003; Bellafiore et al., 2005). This condition is called State 2, while State 1 is induced by a ‘PSI-excess’ situation. State transition is a short-term acclimation under changing light conditions. In addition, plants perform long-term acclimation as well. For example, under lengthy PSII-excess conditions, Arabidopsis increases PSI by increasing the psaA and psaB mRNA levels (Pesaresi et al., 2009).
LHCII is typically considered an antenna of PSII, except under specific conditions, such as State 2. However, accumulating evidence suggests that LHCII acts as an antenna of PSI even under moderate light conditions (Wientjes, et al., 2013; Grieco et al., 2015). LHCII could be an efficient antenna for PSI, and trimeric LHCII interacts with PSI even in State 1, where LHCII phosphorylation is absent (Benson et al., 2015; Grieco et al., 2015).
In this study, we isolated a novel rice mutant that retains green of leaves in the field in autumn. This stay-green mutant, delayed yellowing1 (dye1), accumulates a higher amount of chlorophyll than the wild-type in the pre-senescent leaves, suggesting that it is a Type E mutant. To our knowledge, this is the first empirical report on Type E stay-green mutants. Positional cloning revealed that DYE1 encodes Lhca4, a subunit of LHCI. Recent reports using Lhca mutants in Arabidopsis have revealed various functions of LHCI in addition to its role as an antenna of PSI, such as its involvement in state transition (Benson et al., 2015; Bressan et al., 2016). Interestingly, Lhcb1 was highly accumulated among the chlorophyll–protein complexes in the pre-senescent dye1-1 leaves, suggesting that there is a novel functional interaction between LHCI and LHCII.
Materials and methods
Plant material
dye1-1 was isolated from a rice M2 population (Oryza sativa L. ‘Nipponbare’) irradiated with carbon ion beams (1.6 GeV). dye1-2 was isolated from the N-methyl-N-nitrosourea-mutagenized Nipponbare pool by using TILLING-based screening (Suzuki et al., 2008). The plants were cultivated in pots under field conditions.
Photosynthetic parameters
Foliar chlorophyll content was measured non-destructively using a SPAD-502 Plus instrument (KONICA MINOLTA; http://www.konicaminolta.jp, last accessed 24 December 2017). For pigment extraction, leaves were ground in a mortar in liquid nitrogen and extracted using 80% acetone. Chla and Chlb levels were determined as described by Porra et al. (1989). Maximum quantum yield (Fv/Fm) and the carbon fixation rate were measured using a LI-6400XT portable photosynthesis system (LI-COR; http://www.licor.com/, last accessed 24 December 2017) at 30 °C, with a CO2 concentration of 400 ppm, humidity of 70–80%, and photon flux density of 2000 μmol m–2 s–1. Oxidation of P700 was measured using a Dual-PAM-100 chlorophyll fluorescence and P700 photosynthesis analyser (Walz; http://www.walz.com/, last accessed 24 December 2017). The antenna function of LHCI was estimated as the time required to reach two-thirds of the maximum P700 oxidation with far-red light (intensity 2). State transition (Fr) was determined as described by Lunde et al. (2000) using a JUNIORPAM fluorometer (Walz). Fr is calculated using the following formula: Fr=[(Fi′–Fi)–(Fii′– Fii)]/(Fi′–Fi). Fi and Fii are the fluorescence in the presence of PSI light in States 1 and 2, respectively, whereas Fi′ and Fii′ are the fluorescence in the absence of PSI light in State 1 and 2, respectively.
Quantitative RT-PCR
Total RNA was extracted from the leaves of the wild-type and dye1-1 plants using a total RNA extraction kit (RBC Bioscience; http://www.rbcbioscience.com/, last accessed 24 December 2017). First-strand cDNA was synthesized from 500 ng total RNA using ReverTra ACE qPCR RT Master Mix with gRNA Remover (TOYOBO; http://www.toyobo.co.jp/, last accessed 24 December 2017). The transcript level was determined by quantitative RT-PCR using a KAPA SYBR FAST qPCR kit (KAPA Biosystems; http://www.kapabiosystems.com/, last accessed 24 December 2017) and a Rotor-Gene Q real-time PCR cycler (Qiagen; http://www.qiagen.com/, last accessed 24 December 2017). The primers used for amplification are listed in Supplementary Table S1 at JXB online.
Protein analysis
Total protein was extracted from a 100-mg (FW) sample of leaves from both the wild-type and dye1-1, using 400 µl of 2×SDS buffer [0.125 M Tris, pH 6.8, 4% SDS, 4% mercaptoethanol, 1% bromophenol blue (BPB), 20% glycerol]. The extracted proteins were diluted to one-fifth concentration using 1×SDS buffer (62.5 mM Tris, pH 6.8, 2% SDS, 2% mercaptoethanol, 0.5% BPB, 10% glycerol) and subjected to SDS-PAGE with or without boiling. The antibodies against Lhca1–Lhca4, and Lhcb1 for western blot analysis were purchased from Agrisera (http://www.agrisera.com/en/info/home.html, last accessed 24 December 2017), while the anti-PsaF antibody was provided by Y. Takahashi (Graduate School of Natural Science and Technology, Okayama University, Japan). The antibody against D1 was described previously by Kato et al. (2012). Detection of each protein was performed using an ECL Prime western blotting detection system (GE Healthcare; http://www3.gehealthcare.com/, last accessed 24 December 2017) and an ODYSSEY Fc imaging system (LI-COR). Quantification of band intensity in the western blot analysis was performed using Image Studio Ver 5.2 (LI-COR). An SDS-PAGE gel was stained by Coomassie Brilliant Blue R-250 to detect the Rubisco large subunit. Blue native PAGE analysis was performed using thylakoids solubilized in 1% β-dodecyl-maltoside, as described by Yamatani et al. (2013).
Positional cloning
Twenty-two stay-green F2 segregants from a cross between dye1-1 and the japonica rice Gimbozu EG4 were used for coarse-mapping. For fine-mapping, about 3000 F2 plants from a cross between dye1-1 and a line from the chromosome segment substitution line (CSSL) between Koshihikari and Kasalath (SL224) were used (Ebitani et al., 2005). The DNA markers used in positional cloning are described in Supplementary Table S2.
Whole-genome sequencing
Whole-genome sequencing of dye1-1 was performed with HiSeq2000 (Illumina; https://www.illumina.com/, last accessed 24 December 2017). Three candidate mutations against the Nipponbare genome sequence with quality over 50 were detected within the 43.1-kb DYE1 candidate region. Among the three candidate mutations, the G-to-A substitution in the second exon of Lhca4 was the only ‘homozygous’ mutation.
Transformation experiments
For complementation analysis, the 6-kb genomic fragment that contains the entire Os08g0435900 gene was amplified by PCR using Prime STAR GXL polymerase (TaKaRa; http://www.takara-bio.com/, last accessed 24 December 2017) and the primers DYE1 F1 (5′-TAGGCGCGCCAAGCTTA TGCAGTATGCTGTGACGGT-3′) and DYE1 R1 (5′-TTAATTAAGAATT CGAGCTCCACGCGAGG CCGCGAGAGGG-3′). Amplified DNA was cloned into the HindIII-SacI site of pZH2B, a binary vector derived from pPZP202 (Hajdukiewicz et al., 1994), using the In-fusion HD cloning kit (TaKaRa). dye1-1 calli were transformed with this construct by Agrobacterium-mediated transformation, as described by Fukuoka et al. (2000).
Accession numbers
The following rice genes were used in the analysis: NYC3 (Os06g0354700), SGR (Os09g0532000), a senescence-inducible NAC transcription factor gene (Os03g0327800), Lhcb1a (Os01g0600900), Lhcb1b (Os09g0346500), Actin2 (Os03g0654600), HemA1 (Os10g0502400), CAO (Os10g0567400), and Lhca4 (Os08g0435900). The following genes of other species were used: AtLhca4 (AT3G47470), GmLhca4 (Glyma.04G167900), and SlLhca4 (Solyc06g069730).
Results
Isolation of a rice stay-green mutant, dye1
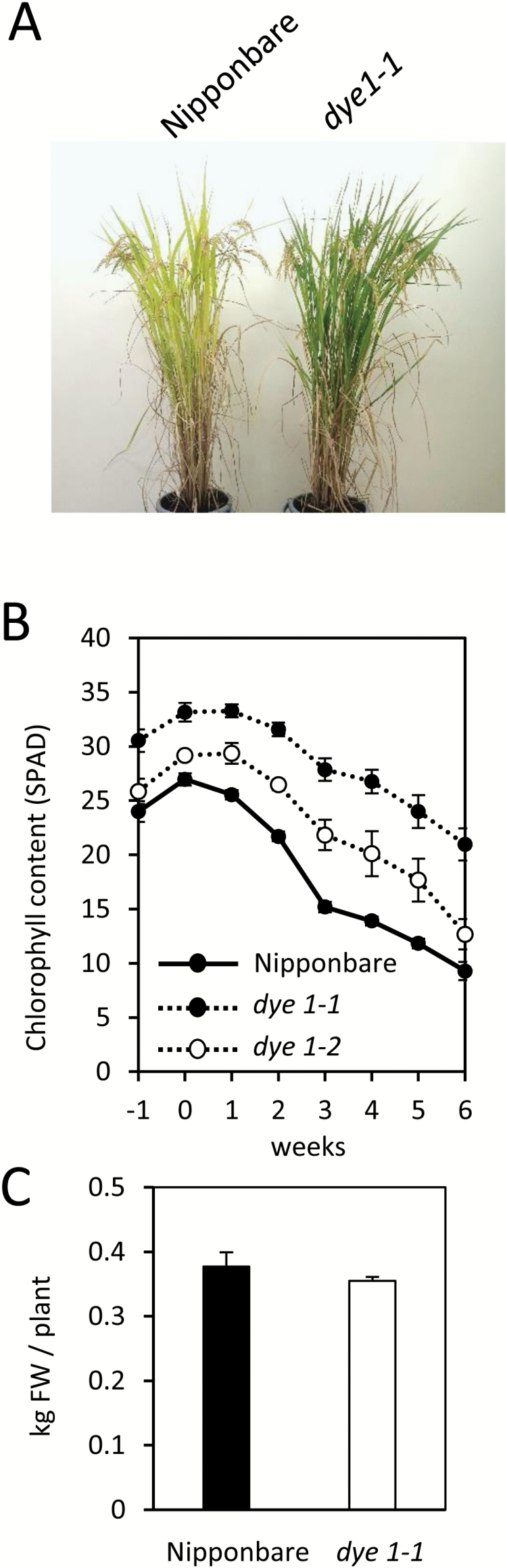
In a screening of rice mutants in the field, we isolated one that showed delayed yellowing during natural senescence. This recessive mutant, named delayed yellowing1-1 (dye1-1), was greener than the wild-type cultivar, Nipponbare, 5 weeks after heading, when most leaves are senescent (Fig. 1A). Measurement of the chlorophyll content of flag leaves during the ripening period showed that dye1-1 had higher chlorophyll contents not only 6 weeks after heading (SPAD units: 9.28 ± 0.83 in Nipponbare, 20.96 ± 1.48 in dye1-1) but also 1 week before heading, i.e. before leaf senescence set in (23.98 ± 0.96 in Nipponbare, 30.54 ± 1.04 in dye1-1) (Fig. 1B). The shoots of Nipponbare and dye1-1 plants were harvested 1 week before heading and there was no significant difference between their weights (Fig. 1C).
Fig. 1.
The ‘stay-green’ phenotype of dye1 during natural leaf senescence. (A) Natural senescence of dye1-1, which has greener leaves than wild-type Nipponbare 5 weeks after heading. (B) Changes in the chlorophyll content over time in the flag leaves during natural senescence. The x-axis indicates weeks before or after heading. Nipponbare, dye1-1, and dye1-2 are as indicated in the key. Data are means ±SE (n=5). (C) Biomass of dye1-1 and wild-type Nipponbare at 1 week before heading (means and SE; n=5).
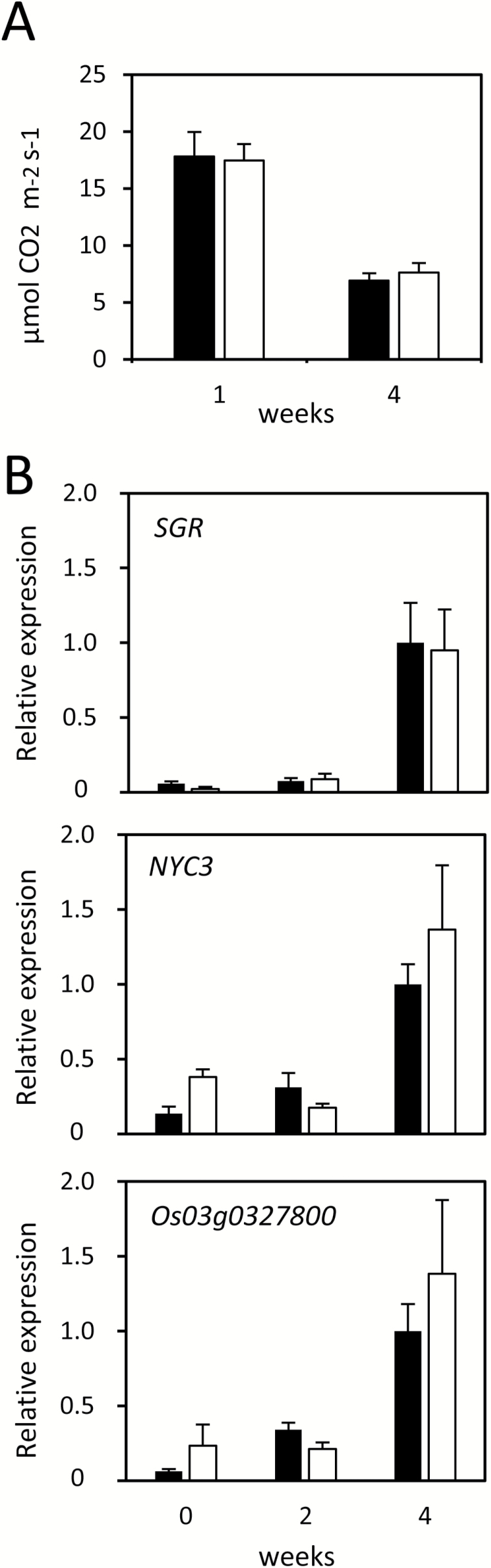
Examination of the leaf functionality of dye1-1 during leaf senescence showed that, in terms of carbon assimilation rate, there was no significant difference between Nipponbare and dye1-1, either for pre-senescent leaves (1 week after heading: Nipponbare, 17.81 ± 2.15 μmol CO2 m–2 s–1; dye1-1, 17.47 ± 1.43 μmol CO2 m–2 s–1) or for senescent leaves (4 weeks after heading: Nipponbare, 6.94 ± 0.62 μmol CO2 m–2 s–1; dye1-1, 7.65 ± 0.82 μmol CO2 m–2 s–1) (Fig. 2A). In addition, we examined the expression of senescence-inducible genes during leaf senescence (Fig. 2B). The expression of the chlorophyll-degrading pathway enzyme-coding genes SGR and NYC3, as well as Os03g0327800, a senescence-inducible NAC transcription factor gene, were low at heading but were significantly induced 4 weeks after heading in both Nipponbare and dye1-1, with no significant differences between them. These observations show that dye1-1 does not have higher photosynthetic capacity and delayed leaf senescence, despite having greener leaves during senescence.
Fig. 2.
Physiological characterization of dye1-1 during senescence. (A) Carbon assimilation rates of dye1-1 (open bars) and wild-type Nipponbare (filled bars) flag leaves 1 week (pre-senescent) and 4 weeks (senescent) after heading. (B) Expression of senescence-associated genes during natural senescence in flag leaves of dye1-1 (open bars) and Nipponbare (filled bars). Data are means and SE (n=4).
Positional cloning of DYE1
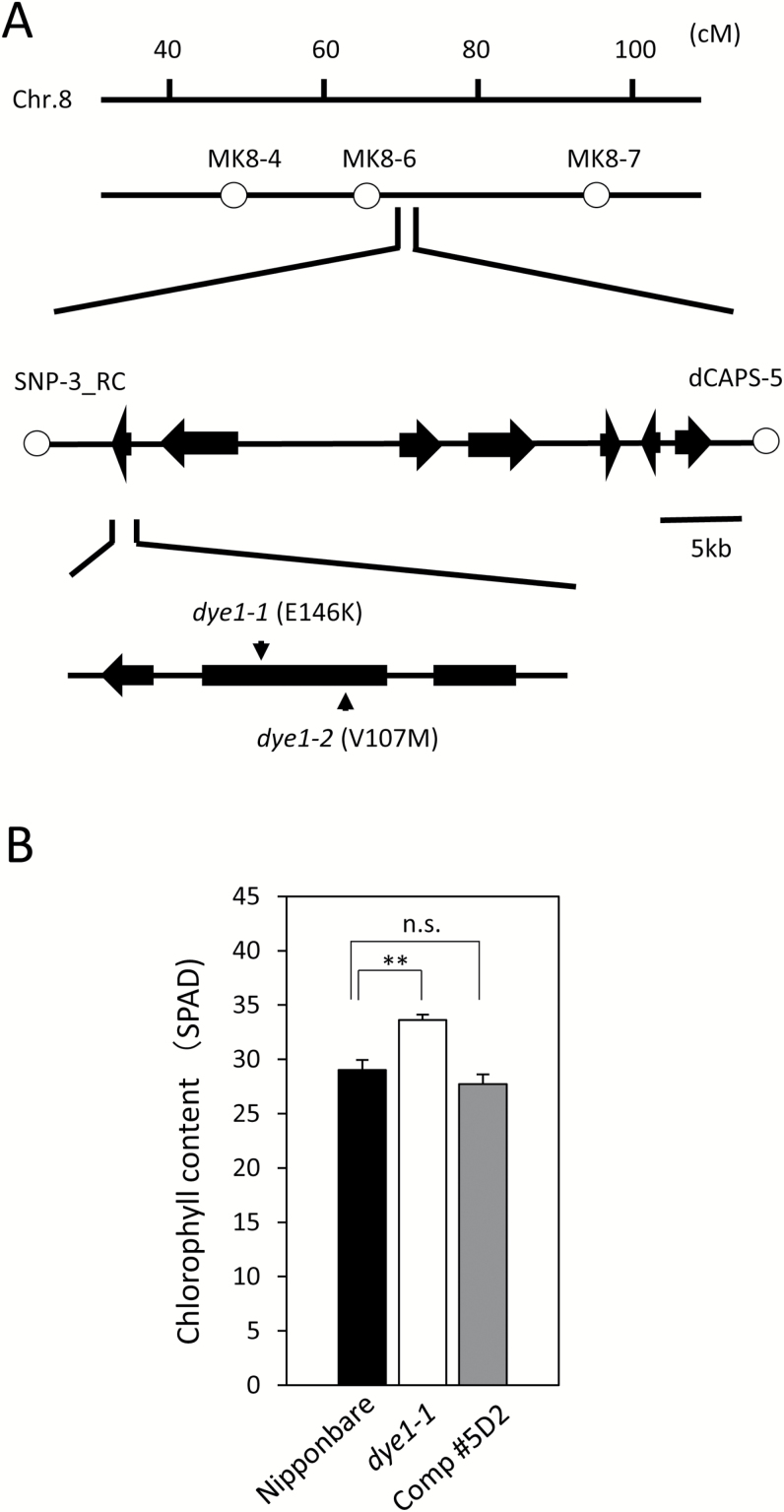
To isolate the DYE1 gene, we obtained an F2 population between dye1-1 and Gimbozu EG4, a japonica strain that harbors over a 1000 copies of mPing, a mobile MITE in rice (Nakazaki et al., 2003; Naito et al., 2006). Transposed mPings can be used as sequence characterized amplified region (SCAR) makers for gene mapping (Monden et al., 2009). Coarse-mapping of 22 plants showing the stay-green phenotype selected from this segregating population detected a linkage between DYE1 and the mPing-SCAR marker MK8-6 (77.6 cM) on Chromosome 8 (Fig. 3A). For fine-mapping, we generated an F2 population from a cross between dye1-1 and a CSSL that had its Chromosome 8 replaced with that of the indica cultivar Kasalath. Genotyping around 3000 F2 plants and their progeny revealed several recombinants near the DYE1 candidate region (see Supplementary Fig. S1). Analysis of the genotype and phenotype of the recombinants revealed that DYE1 is located between the derived cleaved-amplified polymorphic sequence (dCAPS) markers SNP-3 _RC and dCAPS5 (Fig. 3A). This 43.1-kb candidate region contains eight functional genes. Whole-genome sequencing of dye1-1 using Illumina HiSeq2000 revealed a G-to-A substitution in the second exon of Lhca4 (Os08g0435900), which was the only mutation in the candidate region. This single-base change could cause amino acid substitution from Glu to Lys at position 146 from the first Met in Lhca4, which is a subunit of LHCI. This amino acid residue is highly conserved among the Lhca and Lhcb subunits, and is involved in pigment binding (Melkozernov and Blankenship, 2003). Taken together with the fact that rice has only one copy of the Lhca4 gene in its genome, it suggests that this amino acid substitution causes a severe impairment of Lhca4 function (see Supplementary Fig. S2) (Melkozernov and Blankenship, 2003; Klimmek et al., 2006). Western blot analysis revealed that the content of the Lhca4 apoprotein was severely reduced in dye1-1, suggesting that E146K substitution drastically reduces the stability of the Lhca4 protein (Supplementary Fig. S3).
Fig. 3.
Positional cloning of DYE1. (A) Fine-mapping of DYE1. Analysis of the F2 population and its progeny revealed that DYE1 is located between the markers SNP-3_RC and dCAPS-5 on Chromosome 8. The open circles indicate makers tightly linked with DYE1. dye1-1 and dye1-2 have single-nucleotide substitutions causing amino acid substitution in Lhca4. (B) Complementation of dye1-1 by a Lhca4 genomic clone. The chlorophyll contents of the flag leaves at heading are shown for wild-type Nipponbare, dye1-1, and the complementation transgenic line. Data are means and SE (n=4). **P<0.01; n.s., not significant (Student’s t-test).
dye1-2, another allele of dye1, isolated by TILLING-based screening (Suzuki et al., 2008) of the Nipponbare mutant population, was found to have a single-base change, causing substitution from Val to Met at position 107 from the first Met (Fig. 3A). dye1-2 showed higher chlorophyll content than Nipponbare 1 week before heading, and the stay-green phenotype during natural senescence, albeit to a weaker degree than dye1-1 (Fig. 1B).
A complementation experiment was designed to confirm that DYE1 encodes Lhca4, and was performed via the Agrobacterium-mediated transformation method, wherein dye1-1 was transformed with a 6-kb genomic fragment carrying the entire coding region of the wild-type Lhca4 gene. This showed that Lhca4 content was significantly reduced in dye1-1, but the complementation lines accumulated normal amounts of Lhca4, as expected (see Supplementary Fig. S3). In these complementation lines, the chlorophyll contents of the flag leaves 1 week before heading were similar to those in Nipponbare (Fig. 3B). These results confirmed that DYE1 encodes Lhca4.
Photosynthetic properties of dye1
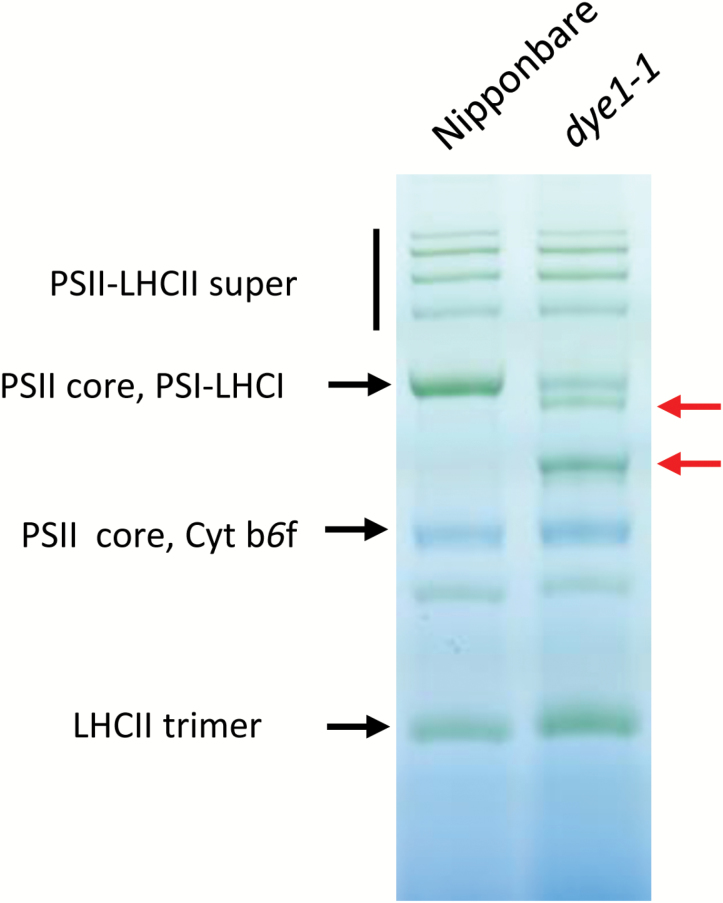
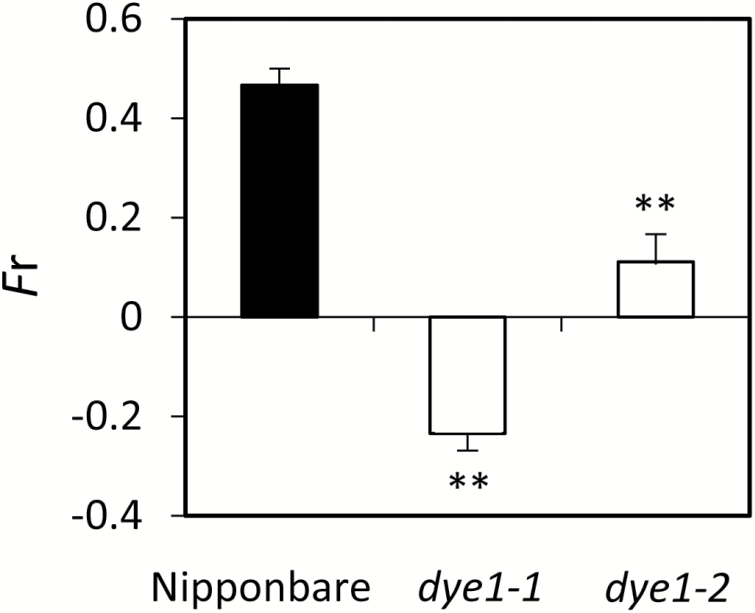
We examined the photosynthetic properties of dye1-1 using flag leaves at heading, since DYE1 encodes a subunit of LHCI. Both Chla and Chlb contents were higher in dye1-1 than in Nipponbare (1.85 ± 0.09 and 0.57 ± 0.02 nmol mg–1 FW for Chla and Chlb, respectively, in Nipponbare; 2.66 ± 0.08 and 0.79 ± 0.04 nmol mg–1 FW for Chla and Chlb, respectively, in dye1-1), but the Chla/b ratio was similar (see Supplementary Table S3). Fv/Fm was slightly higher in dye1-1 than in Nipponbare (Supplementary Table S3). To examine the structure of PSI–LHCI super-complexes, blue native PAGE analysis was performed using mature leaves (Fig. 4). In dye1-1, two bands emerged (indicated by red arrows in Fig. 4), which were not observed in Nipponbare. The upper band is thought to correspond with the PSI–LHCI super-complex lacking Lhca4, and the lower band corresponds with the PSI core complex lacking all Lhca subunits. The PSI core complex band was prominent in dye1-1, suggesting a severely defective organization of the PSI–LHCI super-complex. Consistent with this observation, the kinetics of P700 oxidation induced by far-red light, which reflects the antenna function of LHCI (Gobets and van Grondelle, 2001; Bonente et al., 2012), was found to be much slower in dye1-1 (Supplementary Table S3). Interestingly, the Fr value, an indicator of state transition, was very low in dye1-1 and moderately low in dye1-2, which was consistent with the severity of their phenotype (Fig. 5) (Lunde et al., 2000). A similar observation has been reported in Lhca4 and other Lhca subunit mutants in Arabidopsis (Benson et al., 2015). It is likely that the reduced state transition in dye1-1 was due to the reduced function of LHCI, and was not specific to Lhca4.
Fig. 4.
Blue native PAGE analysis of thylakoid proteins obtained from wild-type Nipponbare and dye1-1 pre-senescent leaves. The thylakoid was solubilized in 1% (w/v) n-Dodecyl-β-D-maltoside and subjected to blue native PAGE analysis. Red arrows indicate the newly emerged bands in dye1-1. Similar results were obtained across independent experiments.
Fig. 5.
State transition in wild-type Nipponbare, dye1-1, and dye1-2. Fr values, which reflect state transition, were measured using mature leaves. Data are means and SE (n=3). **P<0.01 (Student’s t-test).
Analysis of chlorophyll–protein complexes in dye1
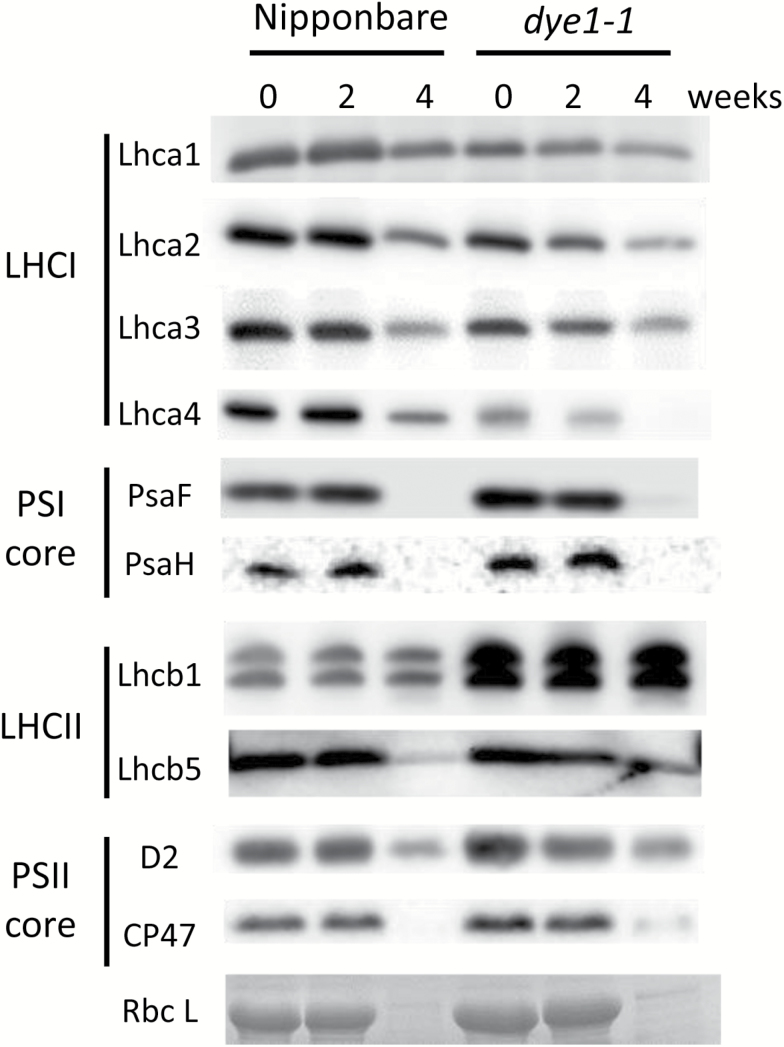
The results of western blot analyses, performed for a number of photosynthetic proteins (Fig. 6), showed that the Lhca4 content was severely reduced in pre-senescent (at heading) and senescent (4 weeks after heading) leaves of dye1-1. E146K substitution is thought to influence the stability of the Lhca4 protein, as mentioned above. Other Lhca subunits, Lhca1–Lhca3, were also reduced in content in the pre-senescent and senescent leaves of dye1-1. It is very likely that the impairment of Lhca4 destabilizes other Lhca subunits because of defects in the proper formation of the PSI–LHCI super-complex. Slight increases in the subunits of the PSII core, D2 (1.4-fold) and CP47 (1.3-fold), were observed in the dye1-1 flag leaves at heading, suggesting that the amount of PSII core complex increased slightly. In addition, a slight increase in the PSI core subunits PsaF (1.5-fold) and PsaH (1.5-fold) were observed in the dye1-1 flag leaves at heading, suggesting a slight increase in the PSI core complex. Interestingly, a more prominent increase was observed in a trimeric LHCII subunit in dye1-1. The content of Lhcb1 in the flag leaves at heading in dye1-1 was 2.6-fold that of the equivalent leaves in Nipponbare. In contrast, there was no significant increase in the content of Lhcb5, a monomeric LHCII subunit, in dye1-1. Levels of several chlorophyll–protein complexes were elevated in dye1-1, suggesting that the higher chlorophyll content in the pre-senescent dye1-1 leaves was due to an increase in such complexes, particularly trimeric LHCII.
Fig. 6.
Western blot analysis of photosynthetic proteins in wild-type Nipponbare and dye1-1 pre-senescent and senescent leaves. Proteins were extracted from the same weight of flag leaves with the same volume of extraction buffer. Similar results were obtained across independent experiments. The Rubisco large subunit was visualized using Coomassie brilliant blue G-250 staining.
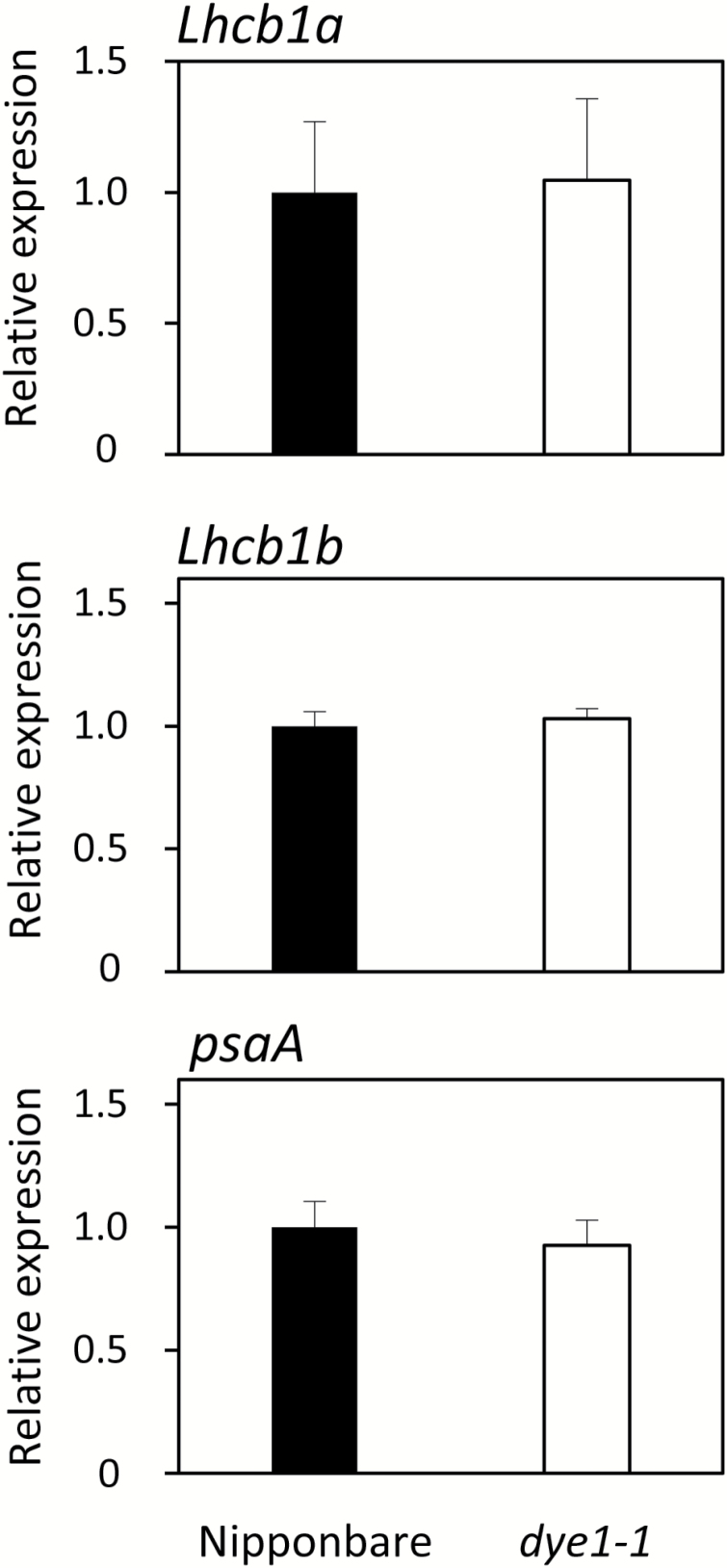
In rice, there are two copies of the Lhcb1 gene, Lhcb1a and Lhcb1b. qPCR analysis revealed no significant up-regulation of Lhcb1a and Lhcb1b expression in dye1-1, suggesting that the increase in Lhcb1 protein in dye1-1 was not regulated at the mRNA level (Fig.7). Similarly, there was no increase in the mRNA of the PSI core subunit PsaA in dye1-1. As it is known that LHCII accumulation is regulated by chlorophyll content, particularly Chlb, the expression of the genes involved in chlorophyll synthesis was investigated. The mRNA level of HemA1, which encodes the rate-limiting enzyme of tetrapyrrole synthesis, Glu-tRNA reductase, was not significantly different between Nipponbare and dye1-1 (Tanaka et al., 2011) (see Supplementary Fig. S4). Similarly, the mRNA level of CAO, which encodes the Chlb-synthesizing enzyme chlorophyllide a oxygenase, was comparable between Nipponbare and dye1-1 (Supplementary Fig. S4).
Fig. 7.
Expression of Lhcb1 and psaA in wild-type Nipponbare and dye1-1. Quantitative RT-PCR analysis of Lhcb1a, Lhcb1b, and psaA was performed using flag leaves at heading. Data are means and SE (n=4).
Discussion
dye1 was isolated as a delayed-yellowing mutant of rice in a field experiment. The pre-senescent mature leaves of dye1 contained a higher level of chlorophyll compared with those of the wild-type, suggesting that this elevated chlorophyll content causes the ‘stay-green’ phenotype of the senescent leaves. However, dye1 showed comparable levels of CO2 assimilation rate and expression of senescence-inducible genes in the senescent leaves, suggesting that it is a non-functional stay-green mutant. Taken together, the results suggest that dye1 is a Type E stay-green mutant (Thomas and Howarth, 2000). To our knowledge, this is the first empirical report on such a mutant.
Positional cloning revealed that DYE1 encodes Lhca4, a subunit of the PSI antenna complex LHCI. While dye1 is the only Lhca mutant reported in rice so far, two Lhca mutants have been reported in Arabidopsis, including a quadruple-mutant of Lhca1–Lhca4 (ΔLhca) (Ganeteg et al., 2004; Benson et al., 2015; Bressan et al., 2016). These mutants are reported to show reduced state transition, and slightly higher Fv/Fm values and Chla/b ratios (Benson et al., 2015; Bressan et al., 2016), which are common to dye1-1. In addition to these characteristics, dye1-1 showed higher chlorophyll content, which has not been described in the Lhca mutants in Arabidopsis. The fact that dye1-1, a mutant of a chlorophyll–protein complex, has a higher chlorophyll content than the wild-type is thought to be due to a higher accumulation of other chlorophyll–protein complexes, particularly the trimeric LHCII.
dye1-1 has an E146K amino acid substitution in Lhca4. This residue corresponds to E154 in Arabidopsis Lhca4, which is a pigment-binding site conserved not only among the Lhca subunits, but also among the Lhcb subunits of different species (Melkozernov and Blankenship, 2003). Furthermore, western blot analysis revealed that the Lhca4 apoprotein content was severely reduced in dye1-1. Taken together, these results suggest a severe impairment of Lhca4 function in dye1-1. Interestingly, the phenotype of the Lhca4 mutant is the severest among the single Lhca-subunit mutants in Arabidopsis (Benson et al., 2015). This is partly because the impairment of Lhca4 drastically influences the stability of other LHCI subunits and the organization of the PSI–LHCI super-complex. Indeed, the kinetics of P700 oxidation by far-red light was much slower in dye1-1. Nevertheless, the biomass and CO2 assimilation efficiency in dye1-1 were comparable with those of the Nipponbare pre-senescent leaves.
Plants adapt to an imbalance in PSI/PSII through short- and long-term acclimations. In Arabidopsis, long-time exposure of PSII-activating light dramatically increases the transcription of psaA/pasB and the accumulation of PSI core proteins (Pesaresi et al., 2009). Because the LHCI antenna was reduced in dye1, the activity of PSI is thought to be continuously low. However, only a slight increase in the PSI core and no increase in psaA transcript accumulation were observed in dye1-1, suggesting that the mechanism of long term-acclimation in rice is different from that in Arabidopsis. In contrast, higher LHCII accumulation was observed in dye1-1. It is possible that the increase in trimeric LHCII accumulation is a kind of compensation for a reduced PSI antenna. LHCII has been classically thought to be the antenna of PSII, but increasing evidence suggests that it could be a major antenna of PSI, even under normal conditions. Indeed, Bressan et al. (2016) suggested that LHCII can compensate for the deficiency of LHCI, although not perfectly.
The mRNA levels of Lhcb1a and Lhcb1b did not increase in dye1-1, suggesting that the increase in Lhcb protein was regulated not at the mRNA level, but at the protein level. For instance, it is suggested that the gun-related retrograde signaling, which regulates Lhcb transcription, is not involved in this phenomenon (Kleine and Leister, 2016). Lhcb protein accumulation is known to be regulated by chlorophyll content, particularly by Chlb (Bellemare et al., 1982; Horn and Paulsen, 2004; Kusaba et al., 2007); however, expression of the enzyme genes HemA1 and CAO involved in chlorophyll synthesis did not differ significantly between Nippponbare and dye1-1, even though these enzymes are also under post-translational control (Nakagawara et al., 2007; Tanaka et al., 2011). Thus, the detailed mechanism behind the increase in LHCII content in dye1-1 remains unclear. An increase in the LHCII content in dye1-1 might be a compensation for the low activity of PSI, considering that the biomass and carbon assimilation rate of dye1-1 were comparable to those of the wild-type, despite very low antenna function of LHCI. Consistent with this idea, Lhca4-antisense plants in Arabidopsis show considerable growth defects under field and controlled conditions (Ganeteg et al., 2004). However, the possible involvement of reduced state transition cannot be excluded. In any case, our observation that the impairment of Lhca4 caused increased LHCII accumulation suggests that LHCI and LHCII functionally interact in a previously unknown manner.
Supplementary data
Supplementary data are available at JXB online.
Fig. S1. Genotype and phonotype of recombinant individuals in the DYE1 candidate region.
Fig. S2. Alignment of Lhca4 proteins from different species.
Fig. S3. Western blot analysis of Lhca4 in the complementation line.
Fig. S4. Expression of HemA1 and CAO in dye1-1.
Table 1. Primers used in the quantitative RT-PCR
Table 2. Information on dCAPS markers used in the positional cloning of DYE1.
Table 3. Analysis of the photosynthetic properties of the pre-senescent flag leaves in dye1-1.
Supplementary Material
Acknowledgments
We thank Yumi Nagashima for technical assistance, Ken Naito for experimental information, Yuichiro Takahashi for providing the anti-PsaF antibody, Masaharu Kuroda for providing the pZH2B binary vector, Zennosuke Katsuba for help with culturing rice in the field, and Yutaka Sato for helpful discussions. The CSSL lines were provided by the Rice Genome Resource Center. This work was supported by the Core Research for Evolutional Science and Technology program of the Japan Science and Technology Agency (to MK), JSPS KAKENHI Grant Numbers JP26292006 (to MK), JP15J03936 (to HY), Joint Research Program implemented at the Institute of Plant Science and Resources, Okayama University (to MK), and the Cross-ministerial Strategic Innovation Promotion Program (to TA).
Glossary
Abbreviations:
- CSSL
Chromosome segment substitution line
- dCAPS
derived cleaved-amplified polymorphic sequence
- dye1
delayed yellowing1
- LHC
light-harvesting complex
- MITE
miniature inverted–repeat transposable element
- SCAR
sequence characterized amplified region.
References
- Bellafiore S, Barneche F, Peltier G, Rochaix JD. 2005. State transitions and light adaptation require chloroplast thylakoid protein kinase STN7. Nature 433, 892–895. [DOI] [PubMed] [Google Scholar]
- Bellemare G, Bartlett SG, Chua NH. 1982. Biosynthesis of chlorophyll a/b-binding polypeptides in wild type and the chlorina f2 mutant of barley. The Journal of Biological Chemistry 257, 7762–7767. [PubMed] [Google Scholar]
- Benson SL, Maheswaran P, Ware MA, Hunter CN, Horton P, Jansson S, Ruban AV, Johnson MP. 2015. An intact light harvesting complex I antenna system is required for complete state transitions in Arabidopsis. Nature Plants 1, 15176. [DOI] [PubMed] [Google Scholar]
- Bonente G, Pippa S, Castellano S, Bassi R, Ballottari M. 2012. Acclimation of Chlamydomonas reinhardtii to different growth irradiances. The Journal of Biological Chemistry 287, 5833–5847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bressan M, Dall’Osto L, Bargigia I, Alcocer MJ, Viola D, Cerullo G, D’Andrea C, Bassi R, Ballottari M. 2016. LHCII can substitute for LHCI as an antenna for photosystem I but with reduced light-harvesting capacity. Nature Plants 2, 16131. [DOI] [PubMed] [Google Scholar]
- Depège N, Bellafiore S, Rochaix JD. 2003. Role of chloroplast protein kinase Stt7 in LHCII phosphorylation and state transition in Chlamydomonas. Science 299, 1572–1575. [DOI] [PubMed] [Google Scholar]
- Ebitani T, Takeuchi Y, Nonoue Y, Yamamoto T, Takeuchi K, Yano M. 2005. Construction and evaluation of chromosome segment substitution lines carrying overlapping chromosome segments of indica rice cultivar ‘Kasalath’ in a genetic background of japonica elite cultivar ‘Koshihikari’. Breeding Science 55, 65–73. [Google Scholar]
- Fukuoka H, Ogawa T, Mitsuhara I. 2000. Agrobacterium-mediated transformation of monocot and dicot plants using the NCR promoter derived from soybean chlorotic mottle virus. Plant Cell Reports 19, 815–820. [DOI] [PubMed] [Google Scholar]
- Ganeteg U, Külheim C, Andersson J, Jansson S. 2004. Is each light-harvesting complex protein important for plant fitness?Plant Physiology 134, 502–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobets B, van Grondelle R. 2001. Energy transfer and trapping in photosystem I. Biochimica et Biophysica Acta 1507, 80–99. [DOI] [PubMed] [Google Scholar]
- Grieco M, Suorsa M, Jajoo A, Tikkanen M, Aro EM. 2015. Light-harvesting II antenna trimers connect energetically the entire photosynthetic machinery — including both photosystems II and I. Biochimica et Biophysica Acta 1847, 607–619. [DOI] [PubMed] [Google Scholar]
- Hajdukiewicz P, Svab Z, Maliga P. 1994. The small, versatile pPZP family of Agrobacterium binary vectors for plant transformation. Plant Molecular Biology 25, 989–994. [DOI] [PubMed] [Google Scholar]
- Horn R, Paulsen H. 2004. Early steps in the assembly of light-harvesting chlorophyll a/b complex: time-resolved fluorescence measurements. The Journal of Biological Chemistry 279, 44400–44406. [DOI] [PubMed] [Google Scholar]
- Huang W, Chen Q, Zhu Y, Hu F, Zhang L, Ma Z, He Z, Huang J. 2013. Arabidopsis thylakoid formation 1 is a critical regulator for dynamics of PSII-LHCII complexes in leaf senescence and excess light. Molecular Plant 6, 1673–1691. [DOI] [PubMed] [Google Scholar]
- Kato Y, Sun X, Zhang L, Sakamoto W. 2012. Cooperative D1 degradation in the photosystem II repair mediated by chloroplastic proteases in Arabidopsis. Plant Physiology 159, 1428–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleine T, Leister D. 2016. Retrograde signaling: organelles go networking. Biochimica et Biophysica Acta 1857, 1313–1325. [DOI] [PubMed] [Google Scholar]
- Klimmek F, Sjödin A, Noutsos C, Leister D, Jansson S. 2006. Abundantly and rarely expressed Lhc protein genes exhibit distinct regulation patterns in plants. Plant Physiology 140, 793–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohzuma K, Sato Y, Ito H et al. . 2017. The non-Mendelian green cotyledon gene in soybean encodes a small subunit of photosystem II. Plant Physiology 173, 2138–2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusaba M, Ito H, Morita R et al. . 2007. Rice NON-YELLOW COLORING1 is involved in light-harvesting complex II and grana degradation during leaf senescence. The Plant Cell 19, 1362–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunde C, Jensen PE, Haldrup A, Knoetzel J, Scheller HV. 2000. The PSI-H subunit of photosystem I is essential for state transitions in plant photosynthesis. Nature 408, 613–615. [DOI] [PubMed] [Google Scholar]
- Melkozernov AN, Blankenship RE. 2003. Structural modeling of the Lhca4 subunit of LHCI-730 peripheral antenna in photosystem I based on similarity with LHCII. The Journal of Biological Chemistry 278, 44542–44551. [DOI] [PubMed] [Google Scholar]
- Minagawa J. 2011. State transitions—the molecular remodeling of photosynthetic supercomplexes that controls energy flow in the chloroplast. Biochimica et Biophysica Acta 1807, 897–905. [DOI] [PubMed] [Google Scholar]
- Monden Y, Naito K, Okumoto Y et al. . 2009. High potential of a transposon mPing as a marker system in japonica × japonica cross in rice. DNA Research 16, 131–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita R, Sato Y, Masuda Y, Nishimura M, Kusaba M. 2009. Defect in NON-YELLOW COLORING 3, an α/β hydrolase-fold family protein, causes a stay-green phenotype during leaf senescence in rice. The Plant Journal 59, 940–952. [DOI] [PubMed] [Google Scholar]
- Naito K, Cho E, Yang G, Campbell MA, Yano K, Okumoto Y, Tanisaka T, Wessler SR. 2006. Dramatic amplification of a rice transposable element during recent domestication. Proceedings of the National Academy of Sciences, USA 103, 17620–17625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawara E, Sakuraba Y, Yamasato A, Tanaka R, Tanaka A. 2007. Clp protease controls chlorophyll b synthesis by regulating the level of chlorophyllide a oxygenase. The Plant Journal 49, 800–809. [DOI] [PubMed] [Google Scholar]
- Nakazaki T, Okumoto Y, Horibata A, Yamahira S, Teraishi M, Nishida H, Inoue H, Tanisaka T. 2003. Mobilization of a transposon in the rice genome. Nature 421, 170–172. [DOI] [PubMed] [Google Scholar]
- Park SY, Yu JW, Park JS et al. . 2007. The senescence-induced staygreen protein regulates chlorophyll degradation. The Plant Cell 19, 1649–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesaresi P, Hertle A, Pribil M et al. . 2009. Arabidopsis STN7 kinase provides a link between short- and long-term photosynthetic acclimation. The Plant Cell 21, 2402–2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porra RJ, Thompson WA, Kriedemann PE. 1989. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochimmica et Biophysica Acta 975, 384–394. [Google Scholar]
- Ren G, An K, Liao Y, Zhou X, Cao Y, Zhao H, Ge X, Kuai B. 2007. Identification of a novel chloroplast protein AtNYE1 regulating chlorophyll degradation during leaf senescence in Arabidopsis. Plant Physiology 144, 1429–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y, Morita R, Katsuma S, Nishimura M, Tanaka A, Kusaba M. 2009. Two short-chain dehydrogenase/reductases, NON-YELLOW COLORING 1 and NYC1-LIKE, are required for chlorophyll b and light-harvesting complex II degradation during senescence in rice. The Plant Journal 57, 120–131. [DOI] [PubMed] [Google Scholar]
- Schelbert S, Aubry S, Burla B, Agne B, Kessler F, Krupinska K, Hörtensteiner S. 2009. Pheophytin pheophorbide hydrolase (pheophytinase) is involved in chlorophyll breakdown during leaf senescence in Arabidopsis. The Plant Cell 21, 767–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimoda Y, Ito H, Tanaka A. 2016. Arabidopsis STAY-GREEN, Mendel’s green cotyledon gene, encodes magnesium-dechelatase. The Plant Cell 28, 2147–2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T, Eiguchi M, Kumamaru T, Satoh H, Matsusaka H, Moriguchi K, Nagato Y, Kurata N. 2008. MNU-induced mutant pools and high performance TILLING enable finding of any gene mutation in rice. Molecular Genetics and Genomics 279, 213–223. [DOI] [PubMed] [Google Scholar]
- Tanaka R, Kobayashi K, Masuda T. 2011. Tetrapyrrole metabolism in Arabidopsis thaliana. The Arabidopsis Book 9, e0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas H, Howarth CJ. 2000. Five ways to stay green. Journal of Experimental Botany 51, 329–337. [DOI] [PubMed] [Google Scholar]
- Wientjes E, van Amerongen H, Croce R. 2013. LHCII is an antenna of both photosystems after long-term acclimation. Biochimica et Biophysica Acta 1827, 420–426. [DOI] [PubMed] [Google Scholar]
- Yamatani H, Sato Y, Masuda Y et al. . 2013. NYC4, the rice ortholog of Arabidopsis THF1, is involved in the degradation of chlorophyll–protein complexes during leaf senescence. The Plant Journal 74, 652–662. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.