Metabolic changes in Arabidopsis induced by periods of elevated heat and drought stress under ambient and elevated CO2, are dynamic and specific to different classes of molecules.
Keywords: Amino acids, carbohydrates, climate change, extreme events, gene expression, lipids, membrane composition, primary metabolism
Abstract
As a consequence of global change processes, plants will increasingly be challenged by extreme climatic events, against a background of elevated atmospheric CO2. We analysed responses of Arabidopsis thaliana to periods of a combination of elevated heat and water deficit at ambient and elevated CO2 in order to gain mechanistic insights regarding changes in primary metabolism. Metabolic changes induced by extremes of climate are dynamic and specific to different classes of molecules. Concentrations of soluble sugars and amino acids increased transiently after short (4-d) exposure to heat and drought, and readjusted to control levels under prolonged (8-d) stress. In contrast, fatty acids showed persistent changes during the stress period. Elevated CO2 reduced the impact of stress on sugar and amino acid metabolism, but not on fatty acids. Integrating metabolite data with transcriptome results revealed that some of the metabolic changes were regulated at the transcriptional level. Multivariate analyses grouped metabolites on the basis of stress exposure time, indicating specificity in metabolic responses to short and prolonged stress. Taken together, the results indicate that dynamic metabolic reprograming plays an important role in plant acclimation to climatic extremes. The extent of such metabolic adjustments is less under high CO2, further pointing towards the role of high CO2 in stress mitigation.
Introduction
Extreme heat and drought often co-occur and dramatically reduce plant growth. They are predicted to become more frequent and severe as a consequence of global climate change (IPCC, 2012). Simultaneously, the predicted future atmosphere will contain higher CO2 levels, impacting plant growth and development. Heat, water deficit, and CO2 effects on plants are relatively well studied, although most often as separate factors (Ainsworth and Long, 2005; Dieleman et al., 2012; Feng et al., 2014; Pandey et al., 2015). Considerably less is known about combined heat extremes and drought stress effects under elevated CO2, a scenario very relevant to global climate change.
Plant responses to heat and drought depend on the intensity and frequency of these events, and on plant-specific factors such as developmental stage and adaptation potential. Inhibition of photosynthesis, changes in cell metabolism, and deterioration of membranes and proteins are frequently observed under severe stress. Metabolic changes may lead to imbalances in redox homeostasis and elevated levels of reactive oxygen species (ROS), causing oxidative stress (Mittler, 2002; Foyer and Noctor, 2005; Krasensky and Jonak, 2012; Munné-Bosch et al., 2013). Defences against short-term exposure to extreme heat or drought include regulation of stomatal opening and induction of protective molecules (e.g. osmolytes, heat shock proteins, and antioxidants) (Wang et al., 2003; Vinocur and Altman, 2005; Wahid et al., 2007; Ashraf, 2010).
Elevated atmospheric CO2 stimulates biomass production, particularly in plants with C3-carbon metabolism (fertilization effect) (Drake et al., 1997; Long et al., 2004; Ainsworth and Long, 2005; Pandey et al., 2015). In addition, high CO2 stimulates respiration and alters flowering time (Springer and Ward, 2007; Leakey et al., 2009). Elevated CO2 also reduces the impact of abiotic stresses on plants, such as ozone, heat, and drought (AbdElgawad et al., 2016). The stress-mitigating effect on drought responses is in part caused by stomatal factors such increased stomatal closure and reduced stomatal density, which improve plant water-use efficiency (Ghannoum et al., 2003). However, non-stomatal factors, including changes in photosynthetic enzymes, reduction of photorespiration (Aranjuelo et al., 2008; Mishra et al., 2013; Zinta et al., 2014; AbdElgawad et al., 2015), and increased levels of defence molecules (e.g. proline, antioxidants) are also important (Geissler et al., 2010; Pintó Marijuan et al., 2013; Li et al., 2015).
To gain a mechanistic understanding of heat and drought effects under a predicted future climate, it is necessary to not only focus on a selected set of defence parameters, but also to obtain a broader view of metabolic changes. Determining system-wide changes in gene expression level has become relatively affordable, and studies that address transcriptome-level effects of abiotic stress are becoming increasingly common (Rogers et al., 2006; Osuna et al., 2007; Usadel et al., 2008; Kanani et al., 2010; Sulpice et al., 2013). However, transcriptome changes do not result in linear alterations in protein activity and metabolite changes (Stitt and Gibon, 2014). Therefore, additional determination of changes in metabolite and enzyme activities provides a more conclusive view of the physiological reprogramming of the plant.
Analyses of metabolic changes have been performed for some abiotic stresses (Kaplan et al., 2004; Rizhsky et al., 2004; Sanchez et al., 2008; Usadel et al., 2008; Cramer et al., 2011). These studies have revealed that plants respond to stresses by transient, sustained, early- and late-metabolic adjustments. For example, raffinose and proline accumulate to high levels over the course of several days of salt, drought, or cold treatment, whereas carbohydrate metabolism changes rapidly in a complex, time-dependent manner (Krasensky and Jonak, 2012). Moreover, some metabolic changes are common among stresses, whereas others are more stress-specific. For example, proline accumulates upon drought, salt, and low-temperature treatments, but not upon high-temperature stress (Krasensky and Jonak, 2012). Such responses highlight the complexity of metabolic adjustments in natural environments. However, there is little or no information on the metabolic alterations induced by climatic extremes (e.g. heat and drought) under current and predicted future climate CO2 levels.
In previous work, we analysed the effects of climate extremes (periods of elevated heat combined with drought) at the level of growth, photosynthesis, and oxidative stress responses (ROS, antioxidants) under ambient and elevated CO2 (Zinta et al., 2014). Based on transcriptome and enzyme activity data, we concluded that the stress-mitigating CO2 effect is mediated by increased antioxidant capacity and reduced photorespiration. However, the transcriptome data also suggested significant changes in primary metabolism (Zinta et al., 2014). Given the importance of elevated CO2 on plant growth and metabolism, here we further quantified levels of sugars, amino acids, and fatty acids in Arabidopsis exposed to a combination of periods of elevated heat and drought stress at ambient and elevated CO2. Such knowledge is essential for understanding plant stress responses under complex climate change scenarios.
Materials and methods
Plant material and growth conditions
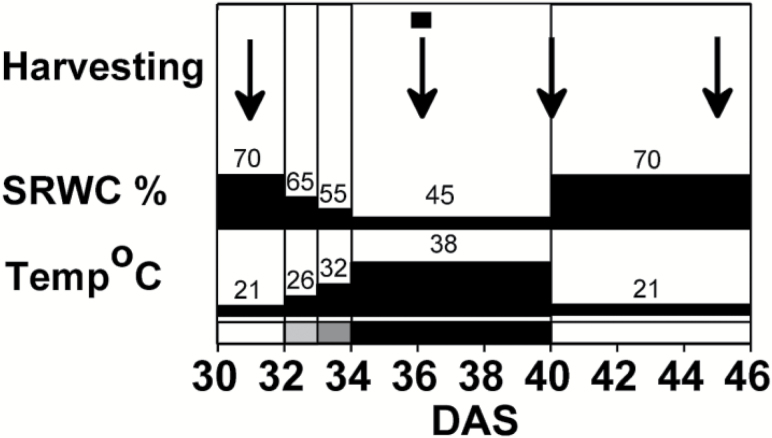
Arabidopsis thaliana L. (Columbia) seeds were sown and stratified in potting mix (Tref EGO substrates, Moerdijk, The Netherlands; 5 × 5-cm pots), and grown in walk-in climate chambers (Weiss Technik, Liedekerke, Belgium) at ambient (380 ppm, two chambers) or elevated CO2 (730 ppm, two chambers), supplied with 150 µmol PAR m–2 s–1, 16/8 h day/night photoperiod, 21/18 °C air temperature, and 60/70% humidity (Zinta et al., 2014). One-week-old seedlings were thinned to one plant per pot. To avoid pseudo-replication and in-chamber heterogeneity, the positions of pots within a chamber were rotated on a daily basis, and pots were switched between the two chambers within a treatment every 2 d from the start of the experiment. Soil relative water content (RWC) was adjusted by weighing the pots daily and watering them to 70% RWC, the optimal water requirement. At 32 d after sowing (DAS) plants were subjected to the experimental extreme climate conditions by imposing a combination of heat and drought treatments (see Fig. 1). The temperature was increased step-wise to 26/22 °C (day/night) on 32 DAS (light-grey bar at the base of Fig. 1); to 32/26 °C on 33 DAS (dark-grey bar); and to 38/30 °C at 34 DAS (black bar), and kept at this level until 40 DAS. Water was withheld from 32 DAS until the soil RWC reached 45%, which was maintained until 40 DAS. After the stress, plants were re-watered to 70% RWC and the temperature was reset to 21/18 °C. The four experimental treatments were: (i) ambient CO2 (labelled ‘C’); (ii) elevated CO2 (‘CO2’); (iii) heat and drought under ambient CO2 (‘HD’); and (iv) heat and drought under elevated CO2 (‘HD+CO2’). Whole-plant rosettes (without inflorescences) were harvested between 10:00–12:00 (the photoperiod started at 07:00), at 32 DAS (i.e. before stress exposure), at 4 d and 8 d of stress exposure (36 DAS and 40 DAS, respectively), and after recovery (45 DAS). For each treatment, five independent samples of 12 rosettes were collected, making a sample size of 60 individual plants. Harvested samples were immediately frozen in liquid nitrogen, and stored at –80 °C before analysis.
Fig. 1.
Schematic representation of the exposure regime of Arabidopsis thaliana (Col-0) to extremes of climate (elevated heat combined with drought, HD). At 32 d after sowing (DAS) the temperature was increased gradually and water was withheld. The grey-scale on the bottom axis indicates the step-wise temperature increase: light grey, 26/22 ºC (day/night) on 32 DAS; dark grey, 32/26 ºC on 33 DAS; black, 38/30 ºC on 34 DAS. After decreasing to 45%, soil RWC was kept constant. Recovery started at 40 DAS. Sampling time-points for metabolite analyses are indicated by the arrows, and the microarray analysis is indicated by the square (at 36 DAS).
Metabolite and enzyme determinations
To examine changes in primary metabolism at the biochemical level, cellular concentrations of sugars, amino acids, and fatty acids were determined. Soluble sugar concentrations were determined using high-performance anion-exchange chromatography with pulsed amperometric detection (HPAEC-PAD; Dionex, Sunnyvale, CA, USA) (Vergauwen et al., 2000). Briefly, 100 mg (FW) plant material was ground in liquid nitrogen using a MagNA Lyser (Roche, Vilvoorde, Belgium). One ml of 50 mM TAE extraction buffer (0.02% sodium azide, 10 mM mannitol, 0.1% polyclar, 10 mM NaHSO3, 1 mM mercapto-ethanol, 1 mM phenylmethanesulfonylfluoride, pH 7.5) was added and the mixture was further homogenized with the MagNA Lyser. The extract was centrifuged and heated, and glucose, fructose, sucrose, and raffinose were determined after separation on a mixed bed Dowex ion exchange column (Acros Organics, Morris Plains, NJ, USA) (Vergauwen et al., 2000; AbdElgawad et al., 2014). Total soluble sugar was calculated as the sum of the measured individual soluble sugars. Starch content in the pellet remaining after soluble sugar extraction was determined enzymatically (Galtier et al., 1995).
The α-amylase activity was determined in extracts of 100 mg (FW) frozen leaf material homogenized using a MagNA Lyser in 1 ml of 50 mM cold phosphate buffer (pH 5.2). After centrifugation (14 000 g, 4 °C, 5 min), the supernatant was used for assaying α-amylase, and an aliquot of the extract was heated (70 °C for 5 min with 3 mmol l–1 CaCl2) to inactivate β-amylase (Tárrago and Nicolás, 1976). Using a 0.2 % (w/v) boiled starch solution and 0.05 % (w/v) I2/KI in 0.05 % (v/v) HCl (Marambe et al., 1992), the α -amylase activity was assayed as the decrease in the absorbance at 620 nm. For β-amylase activity, 100 mg (FW) leaf material was homogenized in 50 mM phosphate buffer (pH 7.0, 1% PVP, 1 mM benzamidine, 20 mM cysteine) (McCleary and Codd, 1989). After centrifugation, 50 µl of the supernatant was incubated at 37 °C with 100 µl reaction mixture (36 mM sodium phosphate buffer pH 7.0, 2U alpha-glucosidase, 0.25 µmol p-nitrophenyl-maltopentaoside). The reaction was stopped after 1 h with 1% Tris. The p-nitrophenol released from the substrate was measured at 410 nm in a microplate reader (Synergy Mx, Biotek Instruments Inc., Vermont, VT, USA).
Invertase enzyme activities were determined by homogenizing (MagNA Lyser) frozen leaf tissue in ice-cold TAE extraction buffer (pH 7.5) as described by AbdElgawad et al. (2014). After centrifugation (14 000 g, 4 °C, 15 min), the supernatant and pellet extracts were processed separately. Pellets were re-dissolved in ice-cold 50 mM Na-acetate buffer (pH 5.0) and an aliquot was subsequently used to determine cell wall invertase activity. The supernatant samples were split into two parts and precipitated with 80% saturated (NH4)2SO4 (incubation on ice for 30 min). After centrifugation, the pellet obtained from one part was re-dissolved in 80% (NH4)2SO4-saturated in TAE buffer (pH 8.5), and the pellet from the second part was re-dissolved in Na-acetate buffer (pH 5.0); aliquots were used to determine neutral and soluble acid invertase activities, respectively. Aliquots obtained for neutral, soluble, and cell wall invertase extracts were incubated (at 30 °C) with 100 μl reaction mixture containing 100 mM sucrose in TAE buffer pH 8.5 (neutral invertase) or Na-acetate buffer pH 5.0 (cell wall and soluble acid invertases), and 0.02% (w/v) Na-azide. Reactions were stopped by keeping an aliquot for 5 min in a water bath at 90 °C. The formation of fructose as a product of sucrose degradation was determined in the reaction mixture using HPAEC-PAD (Dionex, Sunnyvale, CA, USA). Protein concentrations were determined by the method of Sedmak and Grossberg (1977).
Amino acids were determined after extraction (100 mg FW, MagNA Lyser) in 1 ml 80% (v/v) ethanol, spiked with norvaline as an internal control (Sinha et al., 2013). Quantitative determination was performed using a Waters Acquity UPLC-tqd chromatography system (Milford, Massachusetts, USA), equipped with an ethylene-bridged hybrid (BEH) amide 2.1 × 50 column. Total amino acid content was calculated as the sum of all individual amino acids.
For lipid profiling (Torras-Claveria et al., 2014), plant samples (300 mg FW), were extracted in 10 ml methanol at room temperature until discoloration of the tissues using a MagNA Lyser. Codeine and nonadecanoic acids were added as internal standards. GC/MS analysis was carried out on a Hewlett-Packard 6890, MSD 5975 mass spectrometer (Hewlett Packard, Palo Alto, CA, USA), with a HP-5 MS column (30 m × 0.25 mm × 0.25 mm). Lipids were identified using the NIST 05 database and plant-specific databases (e.g. Golm Metabolome Database, http://gmd.mpimp-golm.mpg.de/). Total lipid content (saturated fatty acids, unsaturated fatty acids) was calculated as the sum of the individual lipids (e.g. saturated fatty acids, SFA = ∑12:0 + 13:0 + 14:0 + 15:0 + 18:0+....+26:0). The double-bond index was calculated as DBI = ∑ mol % of unsaturated fatty acids × number of double-bonds of each unsaturated fatty acid (Pamplona et al., 1998).
Concentrations of metabolites are commonly expressed on a tissue fresh weight (FW), or dry weight (DW) basis, and both approaches can be justified. Our statistical analyses of the data expressed on FW or DW basis resulted in identical conclusions (data not shown); however, we prefer to present the results on a FW basis to more accurately reflect the actual cytoplasmic concentration changes impacting cell metabolism. Note that a progressive decrease in biomass during stress exposure could potentially result in ‘artificially’ elevated values, unrelated to primary metabolism changes; however, our previous data (Zinta et al., 2014) indicated that there were no significant changes in biomass over the stress exposure times that we used. Moreover, many metabolite levels only transiently increased, or even decreased, over time, which is hard to explain on the basis of a progressively decreasing biomass. We are therefore confident that the metabolite changes reported here represent changes in metabolism.
Transcriptome analysis
Transcriptome analysis was carried out on tissue samples from plants at 36 DAS using Agilent Arabidopsis (V4) 4 × 44 K arrays (Zinta et al., 2014). Microarray data have been deposited at NCBI’s Gene Expression Omnibus (GEO, accession GSE57035, http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE57035). Genes with known functions that were significantly up- or down-regulated were organized into pathways using MapMan (Thimm et al., 2004).
Statistical analyses
The effects of high CO2, periods of combined elevated heat and drought, exposure time, and their interactions, were assessed by three-way ANOVA using SPSS 16.0 (SPSS Science, Woking, UK). Significant differences between means of treatments were identified using Duncan’s test (P<0.05). To classify metabolites into groups according to their stress response, hierarchical clustering was performed and visualized as heat maps generated with MultiExperiment Viewer (MeV) (TM4 software, Dana-Farber Cancer Institute, Boston, USA), using the Euclidian distance metric. Principal component analysis (PCA) was performed (OriginLab 9 software, OriginLab, Northampton, MA, USA) and projection on the two components with the highest explanatory values was used to make plots.
Results
Carbohydrate metabolism
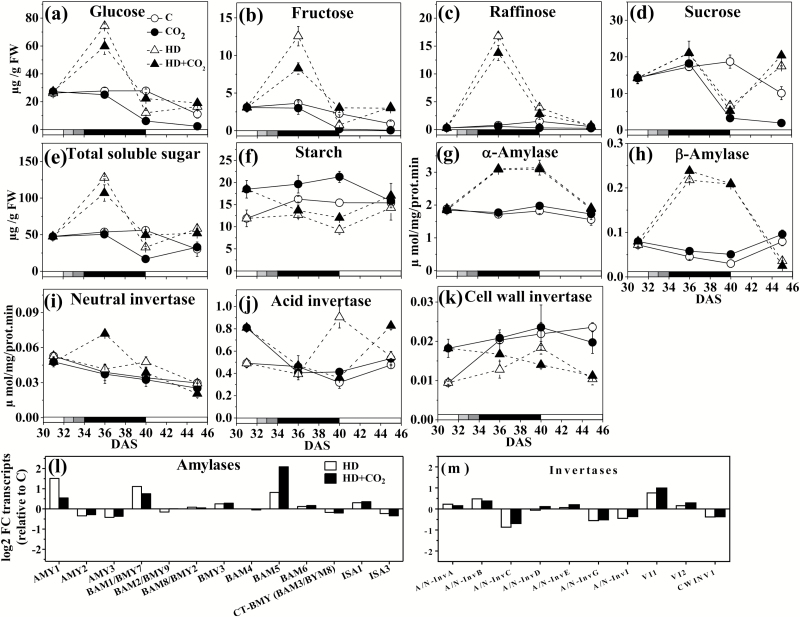
Saccharides are important in primary carbon metabolism, and their levels and those of the enzymes controlling them were determined. Glucose, fructose, and raffinose did not change significantly between 31 and 45 DAS under non-stress conditions (Fig. 2a–c; for results of ANOVA see Supplementary Table S1 at JXB online). Notably, for most sugars, the heat and drought treatment caused a strong, significant (2- to 5-fold) transient increase in concentration. For glucose, fructose, and raffinose this increase occurred early after the onset of exposure to stress (36 DAS). In case, the increase was less under elevated CO2. The similarity in the responses of glucose, fructose, raffinose, and the total soluble sugar content (Fig. 2e) was also reflected in the cluster analysis, in which these molecules were grouped closely together (in cluster 2, see below).
Fig. 2.
Sugar concentrations in Arabidopsis thaliana (Col-0) exposed to a combination of elevated heat and drought at ambient and elevated atmospheric CO2. (a) Glucose, (b) fructose, (c) raffinose, (d) sucrose, (e) total soluble sugars, (f) starch, (g) α-amylase activity, (h) β-amylase activity, (i) neutral invertase activity, (j) acid invertase activity, and (k) cell wall invertases activity. (l, m) Microarray-derived transcript levels at 36 d after sowing (DAS) of (l) amylases (AMY, α-amylase; BAM, β-amylase; ISA, iso-amylase) and (m) invertases (A/N-Inv, acid/neutral; VI, vacuolar; CWINV, cell wall). Data are means (±SE) (n=5). Treatments: C, ambient CO2 (control); CO2, elevated CO2; HD, combined heat and drought at ambient CO2; HD+CO2, combined heat and drought at elevated CO2. The shading on the bottom axes corresponds to the treatments as shown in Fig. 1.
Sucrose and starch showed different profiles. The sucrose concentration showed a strong decline after 36 DAS under elevated CO2 and stress, but relatively little change in ambient non-stressed conditions (Fig. 2d). Cellular sucrose levels are partially determined by the activities of invertase (EC 3.2.1.26). The activities of neutral and acid invertases decreased over time (P<0.05) in the absence of stress at both CO2 levels (Fig. 2i, j); however, they increased under stress at specific time points in ambient or elevated CO2. Cell wall (CW) invertase activity decreased under stress conditions (Fig. 2k). At the transcript level, decreased CW invertase activity corresponds to reduced expression levels of CW invertase 1 (CWINV1, at3g13790). For the acid and neutral invertases, increases and decreases were observed in the expression of isoform genes (Fig. 2m).
Starch content was significantly higher (P<0.05) under elevated CO2 (31–40 DAS) in non-stressed conditions (Fig. 2f). It decreased in response to heat and drought exposure, and recovered within 5 d after removing stress. The activities of α-amylase (EC 3.2.1.1) and β-amylase (EC 3.2.1.2), which are involved in determining starch levels, changed little (31–45 DAS) in ambient or elevated CO2 (Fig. 2g, h); however, their activities were strongly increased in the stressed plants both under ambient and elevated CO2. The transcripts of the amylase isoforms AMY1 (at4g25000), BAM1/BMY7 (at3g23920), BAM5 (at4g15210), and ISA1 (at2g39930) increased, whereas other amylases were somewhat less expressed under stress [e.g. AMY2 (at1g76130), AMY3 (at1g69830), ISA3 (at4g09020), Fig. 2l]. Elevated CO2 generally reduced the impact of stress on expression, except for BAM5 (at4g15210) where expression was further increased.
Additional results regarding stress-induced changes in carbohydrate metabolism, i.e. for sucrose–starch metabolism, glycolysis, and raffinose synthesis, were obtained from our transcriptome data at 36 DAS (ontology as defined in MapMan: see Supplementary Fig. S1, ‘Minor CHO’;) (Zinta et al., 2014). Sucrose–starch metabolism heat maps showed up-regulation of amylase gene expression under stress, and a simultaneous down-regulation of starch and sucrose synthesis enzymes (sucrose-phosphate synthase, EC 2.4.1.14, at4g10120; ADP glucose pyrophosphorylase, EC 2.7.7.27, at5g19220), and sugar transporters (sucrose-proton symporter 1/plastidic GLC translocator, at5g16150; glucose-6-phosphate translocator, at5g46110). Elevated CO2 dampened this effect (see Supplementary Fig. S1a). Glycolysis-related genes were generally down-regulated under stress (Fig. S1b). Transcripts of raffinose synthesis genes were either up-regulated (galactinol synthase 1 and 2, at1g56600 and at2g47180; myoinositol monophosphatase-like 1, at1g31190) or down-regulated (raffinose synthases, at5g20250; galactinol synthase 3, at1g09350; Fig. S1c).
Amino acid metabolism
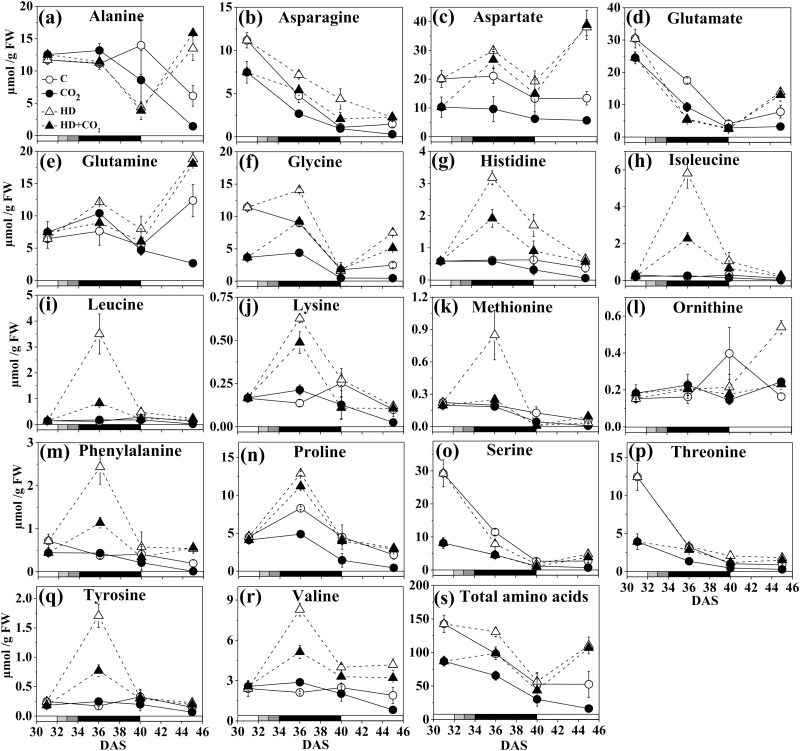
Distinct profiles of inductions and decreases were observed in the concentrations of 18 amino acids over time (Fig. 3a–r). These profiles were also identified as separate clusters in a hierarchical analysis (see below). A first group included amino acids that decreased in non-stressed and stressed plants (31–40 DAS) at both ambient and elevated CO2, without recovery (Asn, Glu, Ser, Thr; cluster 1 in the hierarchical analysis). In contrast, a second group showed little or no change in the absence of stress whereas a strong transient increase under heat and drought at 36 DAS was observed, which returned to pre-stress levels at 40 DAS (Gly, His, Ile, Leu, Lys, Met, Phe, Pro, Tyr, Val; cluster 2). This effect was generally dampened under elevated CO2. The total amino acid concentration decreased with progressing development, both in the absence of stress and under stress (Fig. 3s).
Fig. 3.
Amino acid concentrations in Arabidopsis thaliana (Col-0) exposed to a combination of elevated heat and drought at ambient and elevated CO2. (a) Alanine, (b) asparagine, (c) aspartate, (d) glutamate, (e) glutamine, (f) glycine, (g) histidine, (h) isoleucine, (i) leucine, (j) lysine, (k) methionine, (l) ornithine, (m) phenylalanine, (n) proline, (o) serine, (p) theronine, (q) tyrosine, (r) valine, and (s) total amino acids. DAS, days after sowing. Data are means (±SE) (n=5). Treatments: C, ambient CO2, i.e. control; CO2, control plus elevated CO2; HD, combined heat and drought at ambient CO2; HD + CO2, combined heat and drought at elevated CO2. The shading on the bottom axes corresponds to the treatments as shown in Fig. 1.
The transcriptome data at 36 DAS (Supplementary Fig. S2) showed that transcripts of pyrroline-5-carboxylate synthase (EC 1.5.1.12, at2g39800) and pyrroline-5-carboxylate reductase (EC 1.5.1.2, at5g14800) increased considerably (Supplementary Fig. S2b). Expression of proline dehydrogenase decreased. These expression changes were consistent with increases in Pro. On the other hand, a putative threonine synthase transcript (EC 4.2.3.1, at1g72810), a pyridoxal-5′-phosphate-dependent enzyme Supplementary Fig. S2c), was up-regulated, but threonine levels tended to decrease. Transcripts for Met-synthesis enzymes, i.e. homocysteine methyltransferases (EC 2.1.1.10, at3g22740), SAM-dependent methyltransferase (at3g60910), and methionine adenosyltransferase (EC 2.5.1.6, at2g36880), were both up and down-regulated (Supplementary Fig. S2c). With respect to N-metabolism, transcripts of nitrate transporter (at1g12110), ammonium transporter (at2g38290), nitrate reductase 1 (EC 1.6.6.1, at1g77760), nitrite reductase (EC1.7.7.1, at2g15620), glutamine synthetase (EC 6.3.1.2, at5g16570), glutamate synthase (EC 1.4.1.13, at1g23310), and glutamate dehydrogenase (EC 1.4.1.2, at5g07440) were significantly down-regulated, whereas nitrate reductase 2 (at1g37130) was up-regulated (Supplementary Fig. S2g).
Fatty acid metabolism
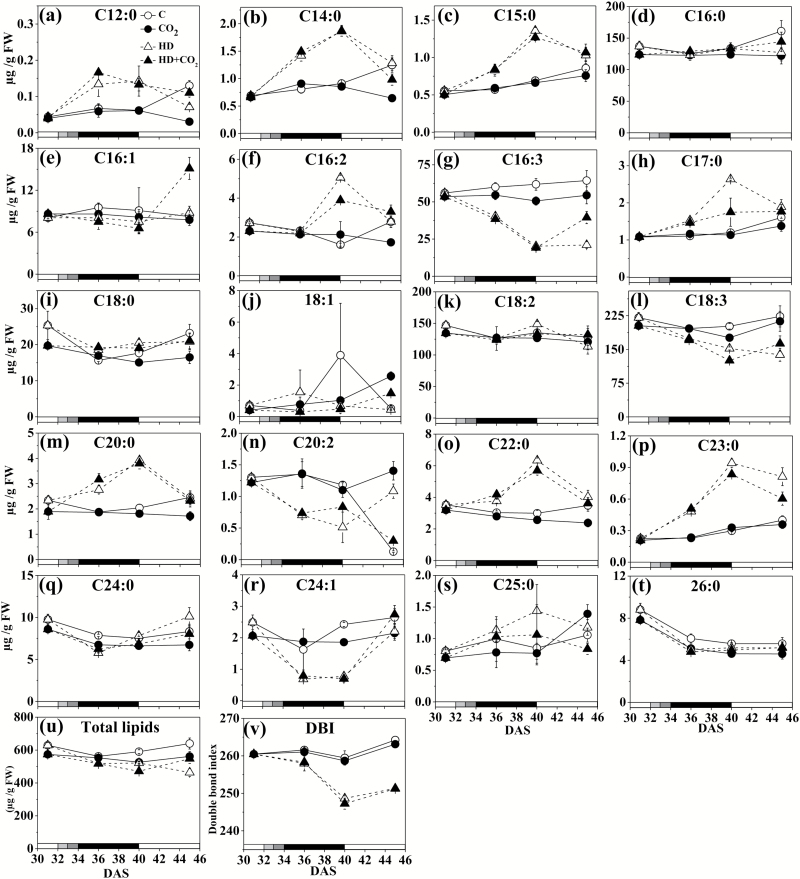
Like amino acids, saturated (SFA) and unsaturated fatty acids (UFA) showed particular temporal patterns (Fig. 4a–v), and hierarchical analysis classified them into two broad groups (see below). In one group, concentrations of mostly SFAs (C12:0, C14:0, C15:0, C16:2, C17:0, C20:0, C22:0, C23:0, C25:0) increased during exposure to stress, and recovered upon re-watering (cluster 3 in the hierarchical analysis). This cluster also contain C16:0, C18:0, and C18:2, but their increases under stress was less pronounced. A second group mostly contained mono- and poly-UFAs (C16:3, C18:1, C18:3, C20:2, C24:1; cluster 4) whose concentrations decreased or remained unchanged during stress. The degree of saturation, expressed as the double-bond index (DBI), decreased significantly (P<0.05) during the heat and drought stress (Fig. 4v). The total lipid concentration varied little in control plants at ambient and elevated CO2, and decreased somewhat under stress (Fig. 4u).
Fig. 4.
Fatty acid concentrations in Arabidopsis thaliana (Col-0) exposed to a combination of elevated heat and drought at ambient and elevated CO2. (a) Dodecanoic (C12:0), (b) tetradecanoic (C14:0), (c) pentadecanoic (C15:0), (d) hexadecanoic (C16:0), (e) hexadecenoic (C16:1), (f) hexadecadienoic (C16:2), (g) hexadecatrienoic (C16:3), (h) heptadecanoic (C17:0), (i) octadecanoic (C18:0), (j) octadecenoic (18:1), (k) octadecadienoic (C18:2), (l) octadecatrienoic (C18:3), (m) eicosanoic (C20:0), (n) eicosadienoic (C20:2), (o) docosanoic (C22:0), (p) tricosanoic (C23:0), (q) tetracosanoic (C24:0), (r) tetracosenoic (C24:1), (s) pentacosanoic (C25:0), (t) hexacosanoic (26:0), (u) total lipids, and (v) double-bond index (DBI). Data are means (±SE) (n=5). Treatments: C, ambient CO2, i.e. control; CO2, control plus elevated CO2; HD, combined heat and drought at ambient CO2; HD + CO2, combined heat and drought at elevated CO2. The shading on the bottom axes corresponds to the treatments as shown in Fig. 1.
Gene expression related to fatty acid chain length and saturation (MapMan bins, see Supplementary Fig S3 a, b) showed decreases in transcripts of fatty acid desaturases involved in Δ9, Δ12, and Δ15 desaturation [i.e. delta 9 desaturase 2 (at2g31360), fatty acid desaturase family protein (at1g06360), fatty acid desaturases 2, 3, 5, 7 and 8 (at3g12120, at2g29980, at3g15850, at3g11170, and at5g05580), and delta 8 sphingolipid desaturase (at3g61580)]. Decreased desaturase activity was consistent with a lower DBI (Fig. 4v). A considerable number of transcripts related to fatty acid chain length decreased in the stress treatment (e.g. acetyl-CoA carboxylase, at5g16390, EC: 6.4.1.21) (Supplementary Fig. S3a). These changes were less pronounced under elevated CO2. Analysis of the distribution of chain lengths showed that the proportion of fatty acids with short chains (C12, C14, C15) increased more under stress (36 and 40 DAS), whereas C16 and C18 fatty acids increased less under stress conditions (Supplementary Fig. S4).
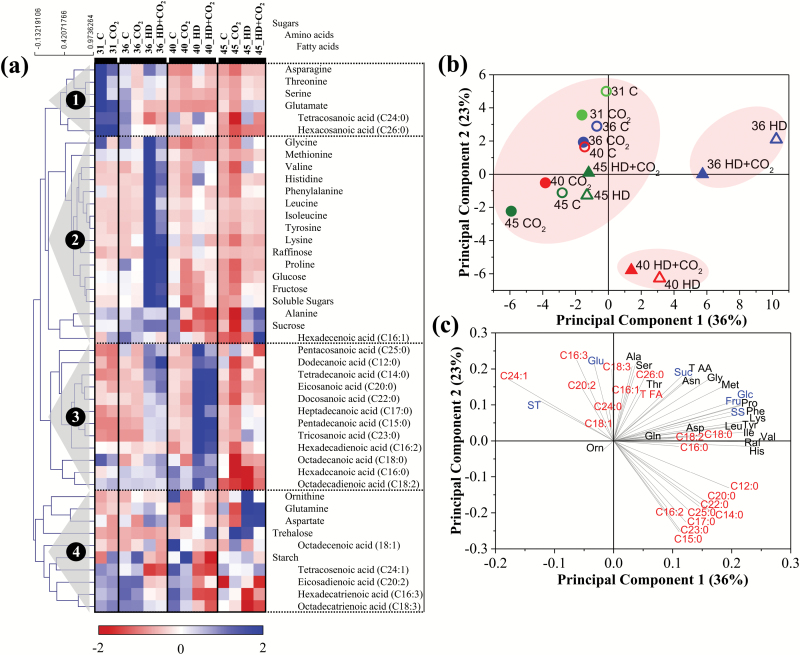
Hierarchical clustering and principal component analysis
Hierarchical clustering analysis of all the metabolite data resulted in the separation of four groups (Fig. 5a). As described above, these groups coincided well with particular patterns of time-dependent changes in metabolite concentrations. The cluster analysis also clearly illustrated the separation in response patterns between amino acids and fatty acids. To independently test the deductions of the hierarchical cluster analysis, metabolite data were subjected to principal component analysis (PCA) (Fig. 5b). The PCA plot based on the tissue sampling time-points showed a clear separation of the stress treatments from the non-stressed and recovered plants along the first two principal components (PC1 and PC2), which together explained 59% of the variability (Fig. 5b). PC1 primarily separated the treatments, whereas PC2 appeared to relate primarily to the age of the plants. PC1 (36% of the variance) was heavily determined by SFAs (C12:0, C14:0, C15:0, C17:0, C18:0, C20:0, C22:0, C23:0, C25:0), whereas PC2 (23% of the variance) showed high loading for sugars, amino acids, and poly-UFAs (Fig. 5c).
Fig. 5.
Hierarchical clustering and principal component analysis (PCA) of primary metabolite concentrations in Arabidopsis thaliana (Col-0) in response to a combination of elevated heat and drought under ambient and elevated CO2. (a) Heat map and cluster tree representation of the normalized metabolite levels, and PCA plots separating (b) the sampling time-points (c) and the measured metabolites. Treatments: C, ambient CO2, i.e. control; CO2, control plus elevated CO2; HD, combined heat and drought at ambient CO2; HD + CO2, combined heat and drought at elevated CO2.
Discussion
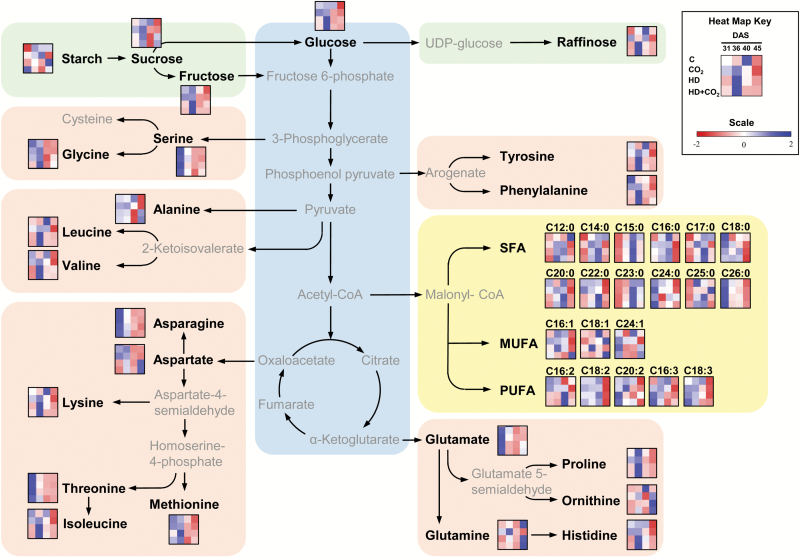
Understanding plant responses to predicted future climate stress conditions requires in-depth analysis. While many studies have focused on oxidative stress and related defence responses, much less attention has been given to effects on primary metabolism. In this study we quantified sugars, amino acids, and lipids in Arabidopsis plants exposed to climate extremes (a period of elevated heat combined with drought) at ambient and elevated CO2. In addition, as metabolic responses are dynamic, we sampled rosette leaves after short (4-d) and long (8-d) term stress exposure, and recovery. An overview of all metabolite changes, organised by class of molecule and biosynthetic origin is presented in Fig. 6. The results provide insights into metabolite-type and time-specific responses, and lead to new conclusions that complement our previous work (Zinta et al., 2014).
Fig. 6.
Overview of all metabolite concentrations, organised by class of molecule and biosynthetic origin. The 4 × 4 heat maps represent normalized metabolite levels at different time-points [horizontal: 31, 36, 40, 45 d after sowing (DAS)] and treatments (vertical: C, ambient CO2, i.e. control; CO2, control plus elevated CO2; HD, combined heat and drought at ambient CO2; HD + CO2, combined heat and drought at elevated CO2).
Short-term stress responses are dominated by changes in sugars and amino acids
Elevated CO2 alone is often observed to increase starch concentrations of plant tissues (Teng et al., 2006; Ainsworth and Rogers, 2007; Ekman et al., 2007; Ainsworth, 2008). We also observed that plants grown under high CO2 had increased starch content. Plants exposed to heat and drought stress showed lower starch levels, which was consistent with increased amylase activities, and up-regulation of α- and β-amylase transcripts. In addition, elevated ADP-glucose pyrophosphorylase (AGPase), which catalyses the first committed reaction of starch synthesis, could also have contributed to lower starch levels. Increases in amylase transcripts under heat stress have also been observed previously (Rizhsky et al., 2004).
Short-term exposure to stress resulted in strong, transient increases in soluble sugars and various amino acids. Transient increases in sugars and amino acids have been observed in Arabidopsis exposed to high irradiance and sulphur depletion (Wulff-Zottele et al., 2010), and to different light intensities (Jänkänpää et al., 2012). This raises the question of the mechanisms that underlie metabolite changes during the stress period. It seems plausible that transient increases in the levels of sugars and amino acids are the result of decreased plant growth rate and reduced photosynthesis causing a reduction in the demand for primary metabolites for biomass. Increased levels of soluble sugars have also been observed in other species under exposure to stress (Krasensky and Jonak 2012; Hossain et al., 2017), suggesting that this is a more common response in plants. It is notably that our previous analysis showed a decrease in photosynthetic activity in stressed plants (Zinta et al., 2014), indicating that the elevated sugars were not the result of extra C-fixation, but instead came from starch breakdown.
For some amino acids, the increased level was supported by altered transcript levels of key biosynthesis genes. For example, transcripts of pyrroline-5-carboxylate synthase and pyrroline-5-carboxylate reductase increased under stress conditions. Both enzymes function as positive regulators of Pro biosynthesis (AbdElgawad et al., 2015). In addition, transcripts for proline dehydrogenase decreased, consistent with the increase in Pro, and the changes in transcript levels for all these genes were suppressed under elevated CO2. On the other hand, however, the expression of methionine-synthesis enzymes was both up- and down-regulated, and therefore did not clearly explain the increased methionine levels. The expression of putative threonine synthase was up-regulated, while Thr levels tended to decrease. These observations illustrate the limitations in using gene transcription analysis to explain metabolite-level changes, and extensive enzyme activity measurements are necessary to understand the discrepancies. It should be noted that the plant material harvested for transcript and metabolite analyses was not homogeneous, as it contained old and young leaves, and mature and dividing tissues, and responses may vary at different developmental stages (Beemster et al., 2005; Avramova et al., 2015, 2017). Nevertheless, the full rosette of Arabidopsis mostly contains mature leaves and hence the data most likely reflect metabolic changes in fully differentiated cells. It was notable that transiently elevated amino acids occurred in each of the main amino acid biosynthesis branches (Fig. 6) (Buchanan et al., 2015); however, not all amino acids in each branch followed this transient induction pattern.
It is pertinent to try to understand the physiological importance of the transient accumulation of these particular sugars and amino acids. Their elevated levels may possibly be related to stress defence. For example, raffinose can act as a chaperone as well as an osmoprotectant (Panikulangara et al., 2004; Egert et al., 2013), and some monosaccharides may provide protection against specific reactive oxygen species (Valluru and Van den Ende, 2008; Keunen et al., 2013). As for the amino acids, 10 out of the 18 quantified molecules were in cluster 2, and included hydrophobic, polar, and positively charged examples. Changes in amino acids may be related to changes in N-metabolism (Rogers et al., 2006; Gargallo‐Garriga et al., 2015). Analysing transcriptome data revealed that stress resulted in the down-regulation of key genes related to nitrate uptake, translocation, and assimilation, which suggested that N-metabolism was adversely affected by stress exposure, as observed previously (Goel and Singh, 2015). On the other hand, Pro is well known as an osmolyte and possibly also has antioxidant capacity (Szabados and Savouré, 2010), and Gly and Met are involved in the synthesis of the osmolyte glycine-betaine (Kanani et al., 2010). In addition, other branched-chain amino acids (e.g. Leu, Ile, Val) are known to be osmoprotectants and accumulate under stress (Obata and Fernie, 2012). As these functions of amino acids have been demonstrated in very different species, it appears that this protective role is fairly common in plants. In summary, it appears that the rapid responses to stress exposure are related to the provision of energy and the synthesis of defense molecules.
Long-term stress responses are dominated by changes in lipids
Two main metabolite clusters contained saturated (cluster 3) and unsaturated (cluster 4) fatty acids (Fig. 5a). SFAs mostly increased under combined heat and drought stress, with the highest level at 40 DAS, whereas UFAs mostly decreased. Elevated CO2 had little impact on these responses. The shift in saturation was also reflected in the DBI, and was consistent with decreased transcript levels of desaturase at 36 DAS. The strong increases in SFAs and decreases in UFAs after prolonged stress may possibly be related to adaptation of membranes to control temperature-induced increases in fluidity. Consistent with this observation, it has been previously shown that short exposure of Arabidopsis to high temperature did not cause alterations in membrane lipids, whereas prolonged stress significantly modified them (Falcone et al., 2004). In addition, increase in the levels of SFAs may provide protection against heat stress (Horváth et al., 1998; Nishiyama et al., 1999; Grover et al., 2000; Larkindale and Huang, 2004; Burgos et al., 2011). Decreases in poly-UFAs may constitute a strategy to reduce high temperature-induced oxidative membrane damage (Falcone et al., 2004; Liu and Bingru, 2004). Moreover, stress induced an increase in the proportion of the shorter acids in the total fatty acid fraction. This was consistent with the reduced expression of genes responsible for elongating chain length. Taken together, the prolonged impact of high temperature and water deficit stress was particularly apparent in the lipids, and occurred at the level of saturation and chain elongation. Presumably, such changes in lipids counter the impact of stress.
Elevated CO2 lessens the impact of climate extremes
Elevated CO2 affects plant metabolism at many levels (Takatani et al., 2014; Noguchi et al., 2015; Abadie et al., 2016), and generally has a dampening effect on the impact of abiotic stress responses (Zinta et al., 2014; Roy et al., 2016). This effect is caused by stomatal (e.g. transpiration) and non-stomatal (e.g. antioxidants, osmolytes, photorespiration) factors (Ghannoum et al., 2003; AbdElgawad et al., 2016). Elevated CO2 suppresses photorespiration, resulting in less ROS (mostly H2O2) and lower oxidative pressure. Increases in antioxidants have also been observed in stress- and CO2-treated plants, and contribute to the reduced impact of stress (Erice et al., 2007; Geissler et al., 2010; Farfan-Vignolo and Asard, 2012; Pintó-Marijuan et al., 2013; Zinta et al., 2014). Together, these factors are the basis for the reduced response to stress that were observed for the sugars and amino acids. However, it should be noted that elevated CO2 had little or no effect on the response of fatty acids, irrespective of stage of exposure. This points to a rather specific effect of high CO2, which is conceivable given that changes in H2O2 are likely to result in changes in (redox) signalling. We are not aware of previously published reports that demonstrate an effect of CO2 that is specific to a class of molecule. It would be of considerable interest to investigate this specific metabolic CO2 effect in other species, for example in crop plants, and species with a C4-fixation pathway.
In summary, we identified temporal changes in the primary metabolism of Arabidopsis during exposure to extreme climate conditions under both ambient and elevated CO2. Responses to heat and water deficit varied across different stress exposure times, and the dynamics were specific to particular classes of molecules. Sugars and the majority of amino acids tended to increase transiently after shorter exposure times, whereas fatty acid levels increased more gradually. The transient increases in the sugars and amino acids may be related to the arrest of growth under stress, causing a reduction in demand for primary metabolites. Fatty acids also showed decreasing levels of saturation, possibly to control membrane fluidity. Elevated CO2 reduced the impact of stress and, interestingly this effect was also specific to different classes of molecules.
Supplementary data
Supplementary data are available at JXB online.
Fig. S1. Expression changes of genes related to sugar metabolism in Arabidopsis exposed to a combination of elevated heat and drought at ambient and elevated CO2.
Fig. S2. Expression changes of genes related to amino acid metabolism in Arabidopsis exposed to a combination of elevated heat and drought at ambient and elevated CO2.
Fig. S3. Expression changes of genes related to lipid metabolism in Arabidopsis exposed to a combination of elevated heat and drought at ambient and elevated CO2.
Fig. S4. Chain length distribution of fatty acids in Arabidopsis exposed to a combination of elevated heat and drought at ambient and elevated CO2.
Table S1. Results of three-way ANOVA for the changes in metabolites in relation to high CO2, stress, and time.
Acknowledgements
The authors acknowledge the Flemish Fund for Scientific Research (FWO) for support (THERMOTOL project, G.0.248.08.N.10). GZ acknowledges support from the Methusalem Funding to the Centre of Excellence ‘PLECO’, University of Antwerp. We thank Dr Karine Vandermeiren (CODA-CERVA, Belgium) for providing the high-CO2 facility, Prof. Dries Knapen and Dr Lucia Vergauwen (University of Antwerp, Belgium), for their assistance with the microarray data analyses, and Dr Strahil Berkov (IBER, Bulgaria) for analyses of fatty acids.
Author contributions
GZ, IN, IAJ, GB, and HA planned and designed the research; GZ HAb, and DP performed the experiments; GZ, HAb, JW, and HA analysed the data; WVE, IAJ, GB, and HA contributed to the reagents/chemicals. GZ provided a draft version of the manuscript, and HA revised and finalized the manuscript.
References
- Abadie C, Mainguet S, Davanture M, Hodges M, Zivy M, Tcherkez G. 2016. Concerted changes in the phosphoproteome and metabolome under different CO2/O2 gaseous conditions in Arabidopsis rosettes. Plant & Cell Physiology 57, 1544–1556. [DOI] [PubMed] [Google Scholar]
- AbdElgawad H, De Vos D, Zinta G, Domagalska MA, Beemster GT, Asard H. 2015. Grassland species differentially regulate proline concentrations under future climate conditions: an integrated biochemical and modelling approach. New Phytologist 208, 354–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AbdElgawad H, Peshev D, Zinta G, Van den Ende W, Janssens IA, Asard H. 2014. Climate extreme effects on the chemical composition of temperate grassland species under ambient and elevated CO2: a comparison of fructan and non-fructan accumulators. PLoS ONE 9, e92044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AbdElgawad H, Zinta G, Beemster GT, Janssens IA, Asard H. 2016. Future climate CO2 levels mitigate stress impact on plants: increased defense or decreased challenge?Frontiers in Plant Science 7, 556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ainsworth EA. 2008. Rice production in a changing climate: a meta‐analysis of responses to elevated carbon dioxide and elevated ozone concentration. Global Change Biology 14, 1642–1650. [Google Scholar]
- Ainsworth EA, Long SP. 2005. What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytologist 165, 351–371. [DOI] [PubMed] [Google Scholar]
- Ainsworth EA, Rogers A. 2007. The response of photosynthesis and stomatal conductance to rising [CO2]: mechanisms and environmental interactions. Plant, Cell & Environment 30, 258–270. [DOI] [PubMed] [Google Scholar]
- Aranjuelo I, Erice G, Nogués S, Morales F, Irigoyen JJ, Sánchez-Díaz M. 2008. The mechanism(s) involved in the photoprotection of PSII at elevated CO2 in nodulated alfalfa plants. Environmental and Experimental Botany 64, 295–306. [Google Scholar]
- Ashraf M. 2010. Inducing drought tolerance in plants: recent advances. Biotechnology Advances 28, 169–183. [DOI] [PubMed] [Google Scholar]
- Avramova V, AbdElgawad H, Vasileva I, Petrova AS, Holek A, Mariën J, Asard H, Beemster GT. 2017. High antioxidant activity facilitates maintenance of cell division in leaves of drought tolerant maize hybrids. Frontiers in Plant Science 8, 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avramova V, AbdElgawad H, Zhang Z et al. . 2015. Drought induces distinct growth response, protection, and recovery mechanisms in the maize leaf growth zone. Plant Physiology 169, 1382–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beemster GT, De Veylder L, Vercruysse S et al. . 2005. Genome-wide analysis of gene expression profiles associated with cell cycle transitions in growing organs of Arabidopsis. Plant Physiology 138, 734–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan BB, Gruissem W, Jones RL. eds 2015. Biochemistry and molecular biology of plants. Wiley Blackwell. [Google Scholar]
- Burgos A, Szymanski J, Seiwert B, Degenkolbe T, Hannah MA, Giavalisco P, Willmitzer L. 2011. Analysis of short-term changes in the Arabidopsis thaliana glycerolipidome in response to temperature and light. The Plant Journal 66, 656–668. [DOI] [PubMed] [Google Scholar]
- Cramer GR, Urano K, Delrot S, Pezzotti M, Shinozaki K. 2011. Effects of abiotic stress on plants: a systems biology perspective. BMC Plant Biology 11, 163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieleman WI, Vicca S, Dijkstra FA et al. . 2012. Simple additive effects are rare: a quantitative review of plant biomass and soil process responses to combined manipulations of CO2 and temperature. Global Change Biology 18, 2681–2693. [DOI] [PubMed] [Google Scholar]
- Drake BG, Gonzalez-Meler MA, Long SP. 1997. More efficient plants: a consequence of rising atmospheric CO2?Annual Review of Plant Physiology and Plant Molecular Biology 48, 609–639. [DOI] [PubMed] [Google Scholar]
- Egert A, Keller F, Peters S. 2013. Abiotic stress-induced accumulation of raffinose in Arabidopsis leaves is mediated by a single raffinose synthase (RS5, At5g40390). BMC Plant Biology 13, 218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekman A, Bülow L, Stymne S. 2007. Elevated atmospheric CO2 concentration and diurnal cycle induce changes in lipid composition in Arabidopsis thaliana. New Phytologist 174, 591–599. [DOI] [PubMed] [Google Scholar]
- Erice G, Aranjuelo I, Irigoyen JJ, Sánchez‐Díaz M. 2007. Effect of elevated CO2, temperature and limited water supply on antioxidant status during regrowth of nodulated alfalfa. Physiologia Plantarum 130, 33–45. [Google Scholar]
- Falcone DL, Ogas JP, Somerville CR. 2004. Regulation of membrane fatty acid composition by temperature in mutants of Arabidopsis with alterations in membrane lipid composition. BMC Plant Biology 4, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farfan-Vignolo ER, Asard H. 2012. Effect of elevated CO₂ and temperature on the oxidative stress response to drought in Lolium perenne L. and Medicago sativa L. Plant Physiology and Biochemistry 59, 55–62. [DOI] [PubMed] [Google Scholar]
- Feng G-Q, Li Y, Cheng Z-M. 2014. Plant molecular and genomic responses to stresses in projected future CO2 environment. Critical Reviews in Plant Sciences 33, 238–249. [Google Scholar]
- Foyer CH, Noctor G. 2005. Redox homeostasis and antioxidant signaling: a metabolic interface between stress perception and physiological responses. The Plant Cell 17, 1866–1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galtier N, Foyer CH, Murchie E, Aired R, Quick P, Voelker TA, Thépenier C, Lascève G, Betsche T. 1995. Effects of light and atmospheric carbon dioxide enrichment on photosynthesis and carbon partitioning in the leaves of tomato (Lycopersicon esculentum L.) plants over-expressing sucrose phosphate synthase. Journal of Experimental Botany 46, 1335–1344. [Google Scholar]
- Gargallo‐Garriga A, Sardans J, Pérez‐Trujillo M et al. . 2015. Warming differentially influences the effects of drought on stoichiometry and metabolomics in shoots and roots. New Phytologist 207, 591–603. [DOI] [PubMed] [Google Scholar]
- Geissler N, Hussin S, Koyro HW. 2010. Elevated atmospheric CO2 concentration enhances salinity tolerance in Aster tripolium L. Planta 231, 583–594. [DOI] [PubMed] [Google Scholar]
- Ghannoum O, Conroy JP, Driscoll SP, Paul MJ, Foyer CH, Lawlor DW. 2003. Nonstomatal limitations are responsible for drought‐induced photosynthetic inhibition in four C4 grasses. New Phytologist 159, 599–608. [DOI] [PubMed] [Google Scholar]
- Goel P, Singh AK. 2015. Abiotic stresses downregulate key genes involved in nitrogen uptake and assimilation in Brassica juncea L. PLoS ONE 10, e0143645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grover A, Agarwal M, Katiyar-Agarwal S, Sahi C, Agarwal S. 2000. Production of high temperature tolerant transgenic plants through manipulation of membrane lipids. Current Science 79, 557–559. [Google Scholar]
- Horváth I, Glatz A, Varvasovszki V et al. . 1998. Membrane physical state controls the signaling mechanism of the heat shock response in Synechocystis PCC 6803: identification of hsp17 as a “fluidity gene”. Proceedings of the National Academy of Sciences, USA 95, 3513–3518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain MS, Persicke M, ElSayed AI, Kalinowski J, Dietz KJ. 2017. Metabolite profiling at the cellular and subcellular level reveals metabolites associated with salinity tolerance in sugar beet. Journal of Experimental Botany 68, 5961–5976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IPCC 2012. Summary for policy makers. In: Field CB, Barros V, Stocker TF. et al. , eds. Managing the risks of extreme events and disasters to advance climate change adaptation. Cambridge, UK; New York, NY: Cambridge University Press, 1–19. [Google Scholar]
- Jänkänpää HJ, Mishra Y, Schröder WP, Jansson S. 2012. Metabolic profiling reveals metabolic shifts in Arabidopsis plants grown under different light conditions. Plant, Cell & Environment 35, 1824–1836. [DOI] [PubMed] [Google Scholar]
- Kanani H, Dutta B, Klapa MI. 2010. Individual vs. combinatorial effect of elevated CO2 conditions and salinity stress on Arabidopsis thaliana liquid cultures: comparing the early molecular response using time-series transcriptomic and metabolomic analyses. BMC Systems Biology 4, 177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan F, Kopka J, Haskell DW, Zhao W, Schiller KC, Gatzke N, Sung DY, Guy CL. 2004. Exploring the temperature-stress metabolome of Arabidopsis. Plant Physiology 136, 4159–4168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keunen E, Peshev D, Vangronsveld J, Van Den Ende W, Cuypers A. 2013. Plant sugars are crucial players in the oxidative challenge during abiotic stress: extending the traditional concept. Plant, Cell & Environment 36, 1242–1255. [DOI] [PubMed] [Google Scholar]
- Krasensky J, Jonak C. 2012. Drought, salt, and temperature stress-induced metabolic rearrangements and regulatory networks. Journal of Experimental Botany 63, 1593–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkindale J, Huang BR. 2004. Changes of lipid composition and saturation level in leaves and roots for heat-stressed and heat-acclimated creeping bentgrass (Agrostis stolonifera). Environmental and Experimental Botany 51, 57–67. [Google Scholar]
- Leakey AD, Xu F, Gillespie KM, McGrath JM, Ainsworth EA, Ort DR. 2009. Genomic basis for stimulated respiration by plants growing under elevated carbon dioxide. Proceedings of the National Academy of Sciences, USA 106, 3597–3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Ahammed GJ, Zhang YQ et al. . 2015. Carbon dioxide enrichment alleviates heat stress by improving cellular redox homeostasis through an ABA-independent process in tomato plants. Plant Biology 17, 81–89. [DOI] [PubMed] [Google Scholar]
- Liu X, Bingru H. 2004. Changes in fatty acid composition and saturation in leaves and roots of creeping bentgrass exposed to high soil temperature. Journal of American Society of Horticulture Science 129, 795–801. [Google Scholar]
- Long SP, Ainsworth EA, Rogers A, Ort DR. 2004. Rising atmospheric carbon dioxide: plants FACE the future. Annual Review of Plant Biology 55, 591–628. [DOI] [PubMed] [Google Scholar]
- Marambe B, Ando T, Kouno K. 1992. Alpha-amylase and protease activities and water relations in germinating sorghum (Sorghum bicolor Moench) seeds as affected by animal-waste composts. Soil Science and Plant Nutrition 38, 123–131. [Google Scholar]
- McCleary BV, Codd R. 1989. Measurement of beta-amylase in cereal flours and commercial enzyme preparations. Journal of Cereal Science 9, 17–33. [Google Scholar]
- Mishra AK, Rai R, Agrawal SB. 2013. Individual and interactive effects of elevated carbon dioxide and ozone on tropical wheat (Triticum aestivum L.) cultivars with special emphasis on ROS generation and activation of antioxidant defence system. Indian Journal of Biochemistry & Biophysics 50, 139–149. [PubMed] [Google Scholar]
- Mittler R. 2002. Oxidative stress, antioxidants and stress tolerance. Trends in Plant Science 7, 405–410. [DOI] [PubMed] [Google Scholar]
- Munné-Bosch S, Queval G, Foyer CH. 2013. The impact of global change factors on redox signaling underpinning stress tolerance. Plant Physiology 161, 5–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama Y, Los DA, Murata N. 1999. PsbU, a protein associated with photosystem II, is required for the acquisition of cellular thermotolerance in Synechococcus species PCC 7002. Plant Physiology 120, 301–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi K, Watanabe CK, Terashima I. 2015. Effects of elevated atmospheric CO2 on primary metabolite levels in Arabidopsis thaliana Col-0 leaves: an examination of metabolome data. Plant & Cell Physiology 56, 2069–2078. [DOI] [PubMed] [Google Scholar]
- Obata T, Fernie AR. 2012. The use of metabolomics to dissect plant responses to abiotic stresses. Cellular and Molecular Life Sciences 69, 3225–3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osuna D, Usadel B, Morcuende R et al. . 2007. Temporal responses of transcripts, enzyme activities and metabolites after adding sucrose to carbon-deprived Arabidopsis seedlings. The Plant Journal 49, 463–491. [DOI] [PubMed] [Google Scholar]
- Pamplona R, Portero-Otín M, Riba D, Ruiz C, Prat J, Bellmunt MJ, Barja G. 1998. Mitochondrial membrane peroxidizability index is inversely related to maximum life span in mammals. Journal of Lipid Research 39, 1989–1994. [PubMed] [Google Scholar]
- Pandey R, Zinta G, AbdElgawad H, Ahmad A, Jain V, Janssens IA. 2015. Physiological and molecular alterations in plants exposed to high [CO2] under phosphorus stress. Biotechnology Advances 33, 303–316. [DOI] [PubMed] [Google Scholar]
- Panikulangara TJ, Eggers-Schumacher G, Wunderlich M, Stransky H, Schöffl F. 2004. Galactinol synthase1. A novel heat shock factor target gene responsible for heat-induced synthesis of raffinose family oligosaccharides in Arabidopsis. Plant Physiology 136, 3148–3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pintó-Marijuan M, Joffre R, Casals I et al. . 2013. Antioxidant and photoprotective responses to elevated CO2 and heat stress during holm oak regeneration by resprouting, evaluated with NIRS (near-infrared reflectance spectroscopy). Plant Biology 15, 5–17. [DOI] [PubMed] [Google Scholar]
- Rizhsky L, Liang H, Shuman J, Shulaev V, Davletova S, Mittler R. 2004. When defense pathways collide. The response of Arabidopsis to a combination of drought and heat stress. Plant Physiology 134, 1683–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers A, Gibon Y, Stitt M, Morgan PB, Bernacchi CJ, Ort DR, Long SP. 2006. Increased C availability at elevated carbon dioxide concentration improves N assimilation in a legume. Plant, Cell & Environment 29, 1651–1658. [DOI] [PubMed] [Google Scholar]
- Roy J, Picon-Cochard C, Augusti A et al. . 2016. Elevated CO2 maintains grassland net carbon uptake under a future heat and drought extreme. Proceedings of the National Academy of Sciences, USA 113, 6224–6229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez DH, Siahpoosh MR, Roessner U, Udvardi M, Kopka J. 2008. Plant metabolomics reveals conserved and divergent metabolic responses to salinity. Physiologia Plantarum 132, 209–219. [DOI] [PubMed] [Google Scholar]
- Sedmak JJ, Grossberg SE. 1977. A rapid, sensitive, and versatile assay for protein using Coomassie brilliant blue G250. Analytical Biochemistry 79, 544–552. [DOI] [PubMed] [Google Scholar]
- Sinha AK, Giblen T, AbdElgawad H, De Rop M, Asard H, Blust R, De Boeck G. 2013. Regulation of amino acid metabolism as a defensive strategy in the brain of three freshwater teleosts in response to high environmental ammonia exposure. Aquatic Toxicology 130-131, 86–96. [DOI] [PubMed] [Google Scholar]
- Springer CJ, Ward JK. 2007. Flowering time and elevated atmospheric CO2. New Phytologist 176, 243–255. [DOI] [PubMed] [Google Scholar]
- Stitt M, Gibon Y. 2014. Why measure enzyme activities in the era of systems biology?Trends in Plant Science 19, 256–265. [DOI] [PubMed] [Google Scholar]
- Sulpice R, Nikoloski Z, Tschoep H et al. . 2013. Impact of the carbon and nitrogen supply on relationships and connectivity between metabolism and biomass in a broad panel of Arabidopsis accessions. Plant Physiology 162, 347–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabados L, Savouré A. 2010. Proline: a multifunctional amino acid. Trends in Plant Science 15, 89–97. [DOI] [PubMed] [Google Scholar]
- Takatani N, Ito T, Kiba T, Mori M, Miyamoto T, Maeda S, Omata T. 2014. Effects of high CO2 on growth and metabolism of Arabidopsis seedlings during growth with a constantly limited supply of nitrogen. Plant & Cell Physiology 55, 281–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tárrago JF, Nicolás G. 1976. Starch degradation in the cotyledons of germinating lentils. Plant Physiology 58, 618–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng N, Wang J, Chen T, Wu X, Wang Y, Lin J. 2006. Elevated CO2 induces physiological, biochemical and structural changes in leaves of Arabidopsis thaliana. New Phytologist 172, 92–103. [DOI] [PubMed] [Google Scholar]
- Thimm O, Bläsing O, Gibon Y, Nagel A, Meyer S, Krüger P, Selbig J, Müller LA, Rhee SY, Stitt M. 2004. MAPMAN: a user-driven tool to display genomics data sets onto diagrams of metabolic pathways and other biological processes. The Plant Journal 37, 914–939. [DOI] [PubMed] [Google Scholar]
- Torras-Claveria L, Berkov S, Codina C, Viladomat F, Bastida J. 2014. Metabolomic analysis of bioactive Amaryllidaceae alkaloids of ornamental varieties of Narcissus by GC–MS combined with k-means cluster analysis. Industrial Crops and Products 56, 211–222. [Google Scholar]
- Usadel B, Bläsing OE, Gibon Y et al. . 2008. Multilevel genomic analysis of the response of transcripts, enzyme activities and metabolites in Arabidopsis rosettes to a progressive decrease of temperature in the non-freezing range. Plant, Cell & Environment 31, 518–547. [DOI] [PubMed] [Google Scholar]
- Valluru R, Van den Ende W. 2008. Plant fructans in stress environments: emerging concepts and future prospects. Journal of Experimental Botany 59, 2905–2916. [DOI] [PubMed] [Google Scholar]
- Vergauwen R, Van den Ende W, Van Laere A. 2000. The role of fructan in flowering of Campanula rapunculoides. Journal of Experimental Botany 51, 1261–1266. [PubMed] [Google Scholar]
- Vinocur B, Altman A. 2005. Recent advances in engineering plant tolerance to abiotic stress: achievements and limitations. Current Opinion in Biotechnology 16, 123–132. [DOI] [PubMed] [Google Scholar]
- Wahid A, Gelani S, Ashraf M, Foolad MR. 2007. Heat tolerance in plants: an overview. Environmental and Experimental Botany 61, 199–223. [Google Scholar]
- Wang W, Vinocur B, Altman A. 2003. Plant responses to drought, salinity and extreme temperatures: towards genetic engineering for stress tolerance. Planta 218, 1–14. [DOI] [PubMed] [Google Scholar]
- Wulff-Zottele C, Gatzke N, Kopka J, Orellana A, Hoefgen R, Fisahn J, Hesse H. 2010. Photosynthesis and metabolism interact during acclimation of Arabidopsis thaliana to high irradiance and sulphur depletion. Plant, Cell & Environment 33, 1974–1988. [DOI] [PubMed] [Google Scholar]
- Zinta G, AbdElgawad H, Domagalska MA, Vergauwen L, Knapen D, Nijs I, Janssens IA, Beemster GT, Asard H. 2014. Physiological, biochemical, and genome-wide transcriptional analysis reveals that elevated CO2 mitigates the impact of combined heat wave and drought stress in Arabidopsis thaliana at multiple organizational levels. Global Change Biology 20, 3670–3685. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.