Abstract
BACKGROUND
Previous research has shown that hypoventilation therapy reduces panic symptoms in part by increasing basal PCO2 levels. We tested an additional pathway by which hypoventilation therapy could exert its therapeutic effects: through repeated interoceptive exposure to sensations of dyspnea.
METHODS
Thirty-five patients with panic disorder and agoraphobia were trained to perform exercises to raise their end-tidal PCO2 levels using a portable capnometry device. Anxiety, dyspnea, end-tidal PCO2, and respiratory rate were assessed during each exercise across four weeks of training. Mixed model analysis examined whether within-exercise levels of dyspnea were predictive of reduction of panicogenic cognitions.
RESULTS
As expected, within-exercise anxiety and respiratory rate decreased over time. Unexpectedly, PCO2 dropped significantly from the beginning to the end of exercise, these drops becoming progressively smaller across weeks. Dyspnea increased and remained consistently above basal levels across weeks. As hypothesized, greater dyspnea was related to significantly lower panicogenic cognitions over time, even after controlling for anxiety and PCO2. Additional exploratory analyses showed that within-exercise increases in dyspnea were related to within-exercise increases in anxiety, but not related to within-exercise increases in PCO2.
CONCLUSIONS
In support of the interoceptive exposure model, we found that greater dyspnea during hypoventilation exercises resulted in lower panicogenic cognitions, even after the effect of PCO2 was taken into account. The findings offer an additional, important target in panic treatment.
Keywords: panic, interoception, dyspnea, therapy, respiration, exposure
Panic Disorder (PD) is unique among the anxiety disorders in that its reported symptoms are primarily of a physical nature (1). Attacks are discrete periods of intense uncontrollable fear, whose symptoms can be roughly divided as originating from one of three systems: the autonomic nervous system (e.g., pounding heart, sweating), the respiratory system (e.g., shortness of breath, chest tightness), and a cognitive system (e.g., depersonalization, fear of losing control, fear of dying). Among these symptoms, heart palpitations, dizziness, and dyspnea (shortness of breath) are rated as the most severe during the attacks themselves (2). The strikingly physical nature of the symptoms has led to decades of research on the possible biological causes of panic disorder. One prominent theory is the suffocation false alarm theory (3), which postulates a causal relationship between a faulty respiratory control system and panic. Specifically, hypersensitive medullary chemoreceptors result in a lower set-point for a suffocation alarm. Triggering that alarm, by means of even small rises in PCO2, leads to compensatory hyperventilation, a state in which rate and depth of breathing exceeds metabolic demands, which in turn leads to dyspnea (air hunger, shortness of breath, feelings of suffocation) and a cascade of succeeding panic symptoms. In this context, chronic hyperventilation is an adaptation to a lowered suffocation alarm threshold, keeping PCO2 low enough to avoid triggering the alarm. However, hyperventilation itself can also create symptoms typical for panic: air hunger, dizziness, tingling sensations, chest tightness, shortness of breath, and heart palpitations. Support for the notion of heightened chemosensitivity comes from animal research demonstrating chemosensitivity of cells in the amygdala (4), which, with increased PCO2, trigger intense fear responses. Support also comes from human research on breath holding (5,6), CO2 inhalation challenges (7,8), basal PCO2 levels (9,10), and PCO2 rises before out-of-the-blue panic attacks (11) in individuals with high suffocation fears or panic. However, interoceptive sensory (e.g., cardiac) or non-sensory (e.g., cognitive) pathways, other than chemosensory ones, can also elicit panic, as demonstrated in an isoproterenol paradigm in twins with bilateral amygdala lesions (12).
To test whether normalization of hypocapnia (defined as low arterial PCO2) would lead to reduction of panic symptom severity, we devised an intervention to systematically increase basal PCO2 into a normocapnic range (hypoventilation therapy). We speculated that by increasing basal PCO2, panic patients would have less risk of hyperventilation-induced panic as well as exhibit a desensitization of a hypersensitive suffocation alarm system. To achieve this goal, patients received a portable capnometer with breath-by-breath feedback and storage of their end-tidal CO2 (along with respiratory rate). Patients were instructed to follow audio-guided exercises twice daily for 4 weeks, with the goal of increasing PCO2 through slower, shallower breathing (i.e., less tidal volume). Findings from three randomized controlled studies (13,14,15) showed that this hypoventilation therapy resulted in sustained increases in PCO2 and significant reductions in panic symptom severity. Importantly, rises in PCO2 mediated reductions of panicogenic cognitions and improvements of perceived control (14,16). By contrast, changes in respiratory rate were unrelated to therapeutic improvements, and, notably, respiratory rate was not related to PCO2. Similar improvements were found in patients who had PCO2 increases during psychological treatments that neither targeted PCO2 nor offered PCO2 feedback (cognitive therapy (14)), or treatments that targeted hypocapnia in asthma, but not panic/anxiety symptoms (17). These findings argue against simple demand characteristics and/or expectations as being responsible for the observed improvements.
Another mechanism through which hypoventilation therapy could exert its therapeutic effects is repeated exposure to sensations of dyspnea, which in turn could lead to desensitization of the central fear network (18). Dyspnea is a complex sensation (19) and various aspects of hypoventilation therapy could cause dyspnea. Dyspnea could be caused by either high or low PCO2 levels during the exercises, since both can cause shortness of breath and/or suffocation symptoms. In addition, the strong voluntary control over breathing required by the exercises recruits respiratory muscles, which are known to induce dyspnea sensations independent from respiratory gas exchange (20). In line with possible mechanisms in interoceptive exposure (21), prolonged and repeated exposure to dyspnea triggered by the breathing exercises could lead to reduction of panicogenic cognitions through desensitization of the central fear network. This additional mechanism would be supported if dyspnea increased during breathing exercises (Hypothesis 1) and if greater within-exercise exposure to dyspnea was related to subsequently fewer panicogenic cognitions, over and above within-exercise PCO2 changes (Hypothesis 2). This study is the first to assess the impact of within-exercise changes in dyspnea and PCO2 as alternative targets to improvement in panic pathology. Prior interoceptive exposure studies have only studied the effects of longer-term (i.e., pre-treatment to post-treatment) changes of PCO2 and dyspnea in interoceptive exposure (15,22).
Method and Materials
Sample
The sample comprised 35 patients (22 women, 13 men) with a principal DSM-IV diagnosis of panic disorder with (n=19) or without (n=16) agoraphobia (23). The present paper reports unpublished data from a randomized-controlled trial testing the efficacy of capnometry-assisted respiratory training (13). Inclusion criteria were: (i) age 18 to 60; (ii) if on psychotropic medications, on stable doses for 3+ months prior to the study with an agreement not to change dosage until after the 2-month follow-up; (iii) no evidence of any organic mental disorder, suicidality, schizophrenia, alcohol or drug dependence, cardiovascular disease, pulmonary disease, epilepsy, or pregnancy; and (iv) no additional psychological treatment until after the 2-month follow-up. Mean age was 41 (SD=8.6, range 23–54). The majority of the sample was married (n=20), employed (n=26), and well-educated (mean: 17 years, range: 12–25). Race/ethnicities included White (n = 30), Hispanic (n = 1), African American (n = 1), and Asian (n = 3). PD duration averaged 8 years (range 0.5–32). Agoraphobic avoidance was assessed by item 4 of the Panic Disorder Severity Scale (24) and was reported by 82.9% of participants, with 31.4% classified as mild, 31.4% moderate, 11.4% severe, and 8.6% extreme. Seventeen participants had at least one secondary current DSM-IV Axis I diagnosis: 13 had another anxiety disorder, and 4 had both an anxiety and mood disorder. Diagnosis was assessed using the structured clinical interview for DSM-IV patient edition (25). Interrater reliability was high for PD and other Axis I diagnoses (K = 1.00, K = .83). Eleven patients were on a stable dose of psychotropic medications (benzodiazepines (n=6), antidepressants (n=3), beta-blockers (n=1), and other anxiolytics (n=1)). Basal pre-treatment PCO2 was 32.3 (SD=4.57, range=20.6–39.0), with 68.6% falling into hypocapnic range (PCO2<35 mmHg (26)). Mean resting respiratory rate was 12.4 (SD: 4.37, range: 4.3–24.6).
The study was approved by the Institutional Review Boards at Stanford University and VA Palo Alto Health Care System. All subjects signed an informed consent form prior to enrollment.
Intervention
Capnometry-assisted respiratory training (CART) is based on the idea that sustained levels of hypocapnia contribute to symptom development and maintenance of PD (13). The four-week training included weekly 1-hr treatment sessions. The initial session included (a) educating patients about the exacerbation of panic symptoms through hypocapnia, (b) directing their attention to problematic respiratory patterns, (c) instructing them to perform different breathing maneuvers with capnometer feedback to understand how changes in breathing affect physiological symptoms and mood, and (d) teaching them how to modify PCO2 and respiratory rate and instructions in between-session practices. Between-session exercises using a portable capnometer were to be performed twice-daily for 17-min at home or elsewhere. The exercises consisted of three phases. Participants followed tape-recorded instructions that included time information and pacing tones: Baseline Phase of the exercises: a 2-min baseline during which patients sat quietly with their eyes closed; Paced Breathing Phase: a 10-min paced breathing period, during which patients breathed in synchrony with tones while occasionally checking their PCO2 level and respiratory rate on a feedback device. The paced breathing served to guide patients to gradually slow their breathing across the weeks of treatment. The tones were set to correspond to a respiratory rate of 13 breaths per minute in the first week, and rates of 11, 9, and 6 breaths per minute in successive weeks; Unpaced Phase: a 5-min breathing period without pacing tones, during which patients were to continue breathing at the targeted respiratory rate and PCO2 using the feedback device. The patient’s goal was to maintain a constant breathing rate, while gradually inhaling less air (shallower breathing) to reach and maintain PCO2 in a normocapnic range of 40 +/−3 mmHg.
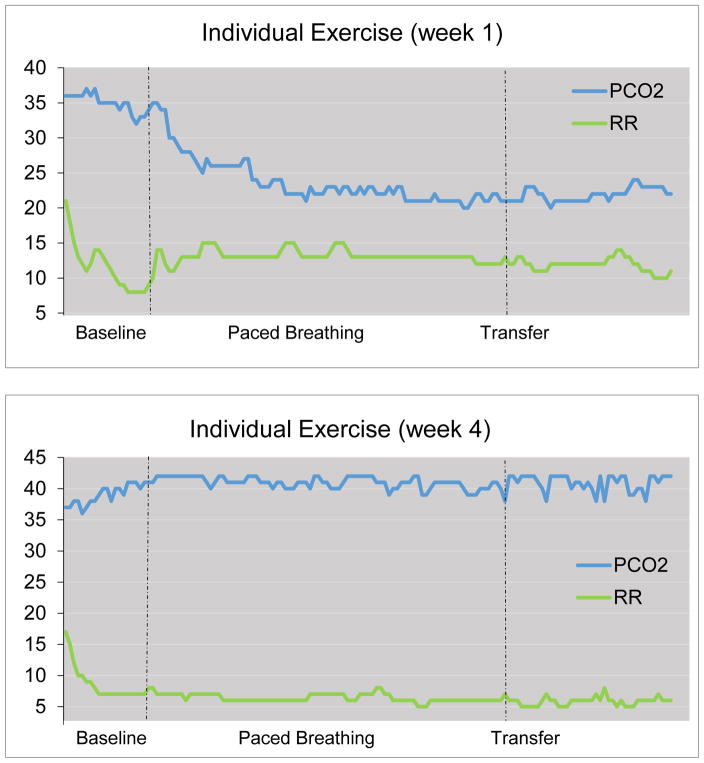
Patients completed home-training exercises using a handheld, battery-operated sidestream capnometry device (Capnocount mini, Weinmann, Germany), which analyzes exhaled air drawn through a nasal cannula into the infrared chamber of the device. The device provides a breath-by-breath digital display of PCO2 and respiratory rate, which are stored along with the time, date, and duration of the entire exercise. Electronic storage facilitated the verification of treatment compliance and progress. At each weekly session, therapists presented and discussed the printout of each exercise with the patients (see Figure 1 for illustration of an individual exercise at the beginning [week 1] and at end [week 4] of training). Patients completed an average of 44.8 (86.2%) of the 52 homework exercises assigned over the course of the four-week treatment. Baseline PCO2 changed from hypocapnic levels at the first treatment exercise (32.3mmHg) to normocapnic levels at the last exercise (38.2mmHg). Mean baseline respiratory rate was 14.3 breaths/min during the first and 8.3 breaths/min during the last exercise.
Figure 1.
Within exercise changes in end-tidal PCO2 (PCO2 in mmHg) and respiratory rate (RR in breaths/min) (representative individual exercise, at week 1 and at week 4).
Respiratory and Symptom Measures
PCO2 and respiratory rate were collected continuously during exercises (twice daily for four weeks). For the purpose of the analysis, means of the last minute of the baseline phase, the last 3 minutes of the paced breathing phase, and the last 3 minutes of the unpaced phase were extracted and averaged within each week for each participant.
Dyspnea (and anxiety) were rated separately before and after the exercises (twice daily for four weeks) on a scale from 0=none to 10=extreme (“Please rate the maximum severity of the symptoms you experienced”). These before and after ratings were averaged separately within each week for each participant.
Panicogenic cognitions were assessed once per week, at the beginning of each weekly treatment session using the 16-item Anxiety Sensitivity Index (ASI) (27). ASI assesses fear of anxiety-related physical sensations resulting from the belief that these sensations may have potentially harmful somatic, psychological, or social consequences (e.g., “When I notice that my heart is beating rapidly, I worry that I might have a heart attack”). Responses are on a 5-point rating scale (range: 0=very little, to 4=very much). At baseline, ASI was significantly correlated with the Panic Disorder Severity Scale (24), r(33)=.49, p<.001.
Statistical Analyses
To test Hypothesis 1, we examined the within-exercise change in dyspnea (and, for descriptive information, the other study variables) from the beginning (baseline phase) to the end of the practice exercises (unpaced phase), across the four weeks. The change score was analyzed using repeated measures ANOVAs with time (4 weeks) as the independent variable. For all repeated measures ANOVAs, P-levels were corrected for nonsphericity using the Greenhouse-Geisser procedure when necessary.
We used mixed models to examine whether higher average levels of dyspnea during each week of exercises led to lower panicogenic cognitions at the end of the week (across the four weeks) (Hypothesis 2). In this analysis, we controlled for average level of anxiety during the exercises, since anxiety may act as a suppressor of the relation between dyspnea and panicogenic cognitions (higher within-exercise dyspnea may be related to higher levels of within-exercise anxiety, and higher levels of anxiety might lead to higher levels of next-session panicogenic cognitions). To enhance causal inference in this analysis, we used a cross-lag format, controlling for prior session panicogenic cognitions, and controlling for other variables that might account for the relation (baseline panicogenic cognitions, week, gender, age, and age of PD onset). Finally, we included weekly within-exercise change in PCO2 as an additional control variable, to determine if dyspnea affected panicogenic cognitions over and above PCO2 effects. We used each weekly measure of dyspnea, anxiety, and PCO2 as time-varying predictors (TVPs) of panicogenic cognitions at the next treatment session. However, since TVPs can confound the effects of between-subjects differences in overall level of the TVP with within-subjects changes in the TVP over time, we disaggregated the between- and within-subjects components of the TVPs into the average level of the TVP (e.g., average level of dyspnea) and the deviations each week from the average of the TVP (e.g., the difference between a person’s dyspnea in a particular week and the average of their dyspnea over the 4 weeks (28 pp. 327–392, 29 pp. 69–75, 30)). Where the relations between an outcome and both the mean and the deviations of the TVP were not different, the TVP was re-aggregated into a single predictor (28). For more details about this analysis, see prior studies (14,31) and the Online supplement.
We then performed additional exploratory analyses to further examine the relations among the study variables. Mixed models were used to study the associations between the within-exercise changes in the study variables (see Online supplement for more details). For each of the 4 weeks, mean within-exercise change in each parameter (from the baseline phase to the unpaced phase) was calculated. But, instead of using raw change scores, which are subject to regression to the mean and have high variability (32), we used residualized change scores (the residual from the regression in which levels during the baseline phase predicted levels during the unpaced phase, see Online supplement).
Finally, we used mixed models to explore if these within-exercise changes in PCO2, respiratory rate, dyspnea, and anxiety during weekly practice exercises were predictive of panicogenic cognitions assessed at the next weekly therapy session. Again, for better causal inference, we also controlled for prior session panicogenic cognitions, baseline panicogenic cognitions, and week, gender, age, and age of PD onset.
RESULTS
Treatment efficacy
As reported previously (13), treatment led to sustained increases in PCO2 levels (Cohen’s d=0.59) and decreases in respiratory rate (d=1.23), and was successful in substantially reducing panicogenic cognitions (d=0.97), disability (33) (d=1.22), and panic symptom severity (24) (d=2.21). Mean ASI was 30.7 at pre-treatment and 16.0 at post-treatment.
Within-exercise change in dyspnea across weeks (Hypothesis 1)
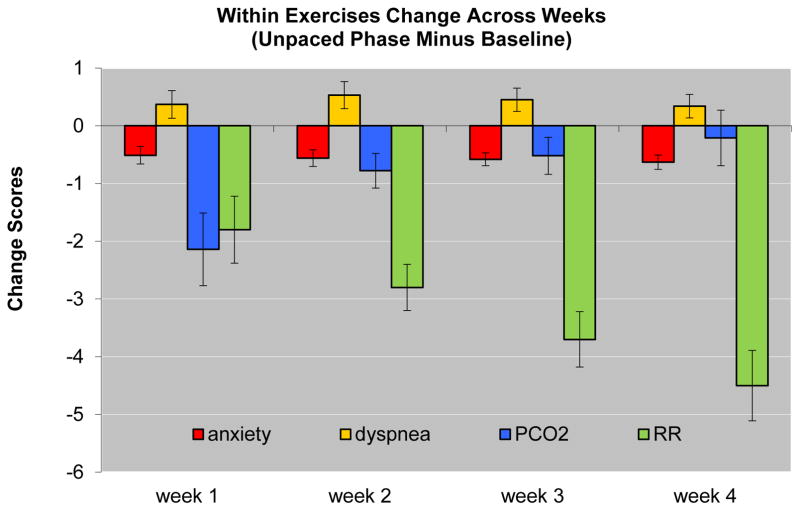
Consistent with Hypothesis 1, dyspnea increased from the beginning to the end of the individual exercises (F(1,34)=6.31, p<.01) and this increase did not differ across weeks (p>.10) (Figure 2). Exploratory analyses of the other study variables showed that anxiety, PCO2, and respiratory rate all decreased from the beginning to the end of each exercise (F(1,34)=15.83, p<.001; F(1,34)=19.20, p<.001; F(1,34)=65.93, p<.001, respectively), but their change over time differed: While within-exercise decreases in anxiety did not change over the 4 weeks (p>.10), within-exercise decreases in PCO2 got smaller over time (F(1,34)=8.87, p<.01) and within-exercise decreases in respiratory rate got larger over time (F(1,34)=7.12, p<.01) (Figure 2).
Figure 2.
Mean within exercise changes (and standard errors) across treatment weeks: end of exercise minus baseline phase at the start of exercise, for anxiety (0–10 scale), shortness of breath (0–10 scale), end-tidal PCO2 (PCO2 in mmHg), and respiratory rate (RR in breaths/min).
Association between level of dyspnea and next-session panicogenic cognitions (Hypothesis 2)
More intense interoceptive exposure (operationalized as higher average levels of dyspnea) during weekly exercises predicted lower levels of panicogenic cognitions at the next session, when anxiety and PCO2 were controlled (b=−.07, t(87)=−2.64, p<.010, d=.57). This analysis also showed that higher average levels of anxiety during the exercises were related to higher panicogenic cognitions at the next session (b=.08, t(78)=2.52, p=.014, d=.57), while the within-exercise changes in PCO2 were not significantly related to next-session panicogenic cognitions (p=.108). The relation between dyspnea and panicogenic cognitions was not significant when anxiety levels were not controlled (b=−.03, t(91)=−1.08, p=.283). This latter finding is consistent with our expectation that the effect of dyspnea on anxiety during exercises would have an indirect effect on later panicogenic cognitions and hence would act as a suppressor of the direct effect of dyspnea (interoceptive exposure) on subsequent panicogenic cognitions.
Exploratory Analyses
Associations among within-exercise changes in dyspnea, anxiety, PCO2, and respiratory rate
As noted above, PCO2 unexpectedly decreased during the exercises (even though basal PCO2 increased over weeks). These analyses found that greater within-exercise decreases in PCO2 were related to greater within-exercise increases in anxiety (b=−.04, t(87)=−2.25, p=.027, d=.48), but were unrelated to within-exercise dyspnea changes (p=.997). Greater within-exercise increases in dyspnea were related to greater within-exercise increases in anxiety (b=.17, t(104)=3.48, p<.001, d=.68). Greater within-exercise decreases in respiratory rate were not related to changes in either dyspnea or anxiety, but were related to smaller within-exercise decreases in PCO2 (b=−.40, t(87)=−4.52, p<.001, d=.97).
Within-exercise changes in PCO2, respiratory rate, dyspnea, and anxiety predicting next session’s panicogenic cognitions
Consistent with previous studies on basal PCO2 (14,15), participants with greater within-exercise mean levels of PCO2 decreases had higher (worse) panicogenic cognitions (b=−.04, t(88)=−2.02, p=.047, d=.43). Also, participants with greater within-exercise increases in mean levels of anxiety had higher (worse) panicogenic cognitions (b=.20, t(84)=2.88, p=.005, d=.63). On the other hand, changes in respiration rate were not related to next session’s panicogenic cognitions (ps>.698).
DISCUSSION
We tested whether an intervention reducing hypocapnia in panic sufferers might act as an interoceptive exposure therapy. Over the course of four weeks, patients completed twice-daily 17-minute exercises, during which they breathed shallowly and slowly with the goal to increase PCO2 levels. We have shown that such training can result in clinically significant and long-lasting reductions in panic pathology (13,14,15), and that the benefit appears to be driven, in part, by increases in basal PCO2, over and above perceived control (expectancy) (14) and reappraisal (14,16). In this study, we investigated whether an additional therapeutic target of panic symptom reduction, other than the therapeutic effect of reducing hypocapnia, could be in play. In particular, we hypothesized that the repeated and intense exposure (an average of 11.2 hours over the 4-weeks of training) to physical alterations triggered by the exercises would act to desensitize participants to physical sensations of dyspnea and hence reduce panicogenic cognitions.
In line with an interoceptive therapy model, our findings showed that patients were significantly more dyspneic after the exercises than before. We further confirmed that more intensive interoceptive exposure, operationalized as higher average levels of dyspnea during weekly exercises, predicted lower panic symptoms at the next session, as long as the relation between dyspnea and anxiety was controlled. Thus, to the extent that exercises were associated with more intense dyspnea, but not more anxiety, patients improved more. Our findings point to a potentially important qualification of the usefulness of interoceptive exposure: only patients that manage to keep their anxiety low during exercises are awarded the benefit of reduced panicogenic cognitions. These findings contradict the common idea that exposure training requires substantial anxiety mobilization to be effective (34). Indeed, recent reviews of the literature show that elevated anxiety during exposure is not critical for therapy success, and may even hinder it (35). Furthermore, our findings confirm that a lack of reduction in anxiety during exercises, or greater average anxiety levels during exercises, is associated with poorer therapeutic outcome, support for which has come from other exteroceptive (in-vivo) exposure trials. For instance, in a previous study we found that greater increases in anxiety (and panic symptoms) during repeated in-vivo exposure to agoraphobic situations predicted less improvement in panic disorder severity (36). And Smits et al. (37) found that patients who reported high fear at the end of a social anxiety exposure session, and had received D-cycloserine prior to session, showed significantly lower clinical improvement at the next session. Similar results were found for socially anxious patients receiving yohimbine-enhanced exposure therapy (38).
We had speculated that dyspnea could be caused by 1) attempting to control breathing by adopting a slow and shallow breathing pattern, which likely involves recruitment of respiratory muscles or 2) by causing hypocapnia or hypercapnia through the breathing pattern adopted. Given that dyspnea could be caused by 1), which is unrelated to blood gases, or by 2) with actual high or low PCO2, it is not surprising that PCO2 changes were not clearly related to dyspnea changes. Notably, in a study on isoproterenol challenge in twins with bilateral amygdala lesions (12), only the twin who reported dyspnea panicked. The twin who did not report dyspnea sensations did not. Thus, respiratory muscle activation can also be a source of dyspnea independent of PCO2 levels, and individuals with elevated anxiety sensitivity are particularly sensitive to manipulations of tension in these muscles (20).
Taken together, our findings support the conclusion that repeated hypoventilation exercises act as a form of interoceptive exposure therapy. Although greater dyspnea was related to greater concurrent anxiety during the exercises, it induced lower panicogenic cognition scores at the subsequent weekly therapy session (as long as anxiety was controlled) over and above what was related to changes in PCO2. Indeed, that dyspnea during breathing therapy could serve as an interoceptive exposure may partially explain why one previous study found improved panic symptoms both with an intervention that increased PCO2 and one that decreased PCO2 (15). Notwithstanding, dyspnea induction was not the goal, but a “side-effect,” of the exercises in the present study. It remains unknown exactly what triggered within-exercise dyspnea sensation in CART since both PCO2 and respiratory rate were unrelated to it. Future studies should examine mechanisms of change in interventions involving direct manipulation of dyspnea by experimental means, such as inspiratory resistive loads (39).
The findings have important therapeutic implications. Traditional respiratory training has always operated under the assumption that simply teaching patients to breathe slower (or worse, deeper) would alleviate hypocapnia and arousal (40). Our findings suggest that just the opposite may take place: breathing slower and regularly leads to compensatory deeper breathing, which increases the risk for more hyperventilation. This result was observed previously. For example, Conrad and colleagues (41) demonstrated that brief breathing instructions (slowly, shallowly, or both) for managing stress led to more, not less, respiratory and autonomic reactivity, including decreases in PCO2. Similarly, in a randomized-controlled trial for asthma, patients assigned to breathe progressively more slowly were successful in reducing their respiratory rate, but achieved only minor increases in PCO2 levels (17). Last, we found that successfully lowering respiratory rate during the exercises was unrelated to reduction in panicogenic cognitions. These results are in line with prior studies that found that improvements in basal respiratory rate were unrelated to therapeutic outcome (14,16) and did not contribute to hyperventilatory states during phobic exposures (42). However, we did find that greater reduction in respiration rate was related to less PCO2 decrease. Thus, no assumptions should be made about the effects of respiratory training in the absence of PCO2 monitoring. A recent example (43) illustrates this dilemma: slower breathing following Cdh9/Dbx1 neural ablation in mice resulted in more sedative (i.e., grooming, sitting), and less exploratory behavior (interpreted as “calm behaviors” by the authors). Since tidal volume remained unchanged, it is likely that the slower breaths led to PCO2 changes, but without PCO2 measurement, that is uncertain. Possibly the “calm behaviors” were actually freezing behaviors due to hypercapnic levels. In any case, to understand the complexities of the impact of therapeutic instructions, detailed assessment of physiological and psychological changes within the treatment session is unavoidable.
Supplementary Material
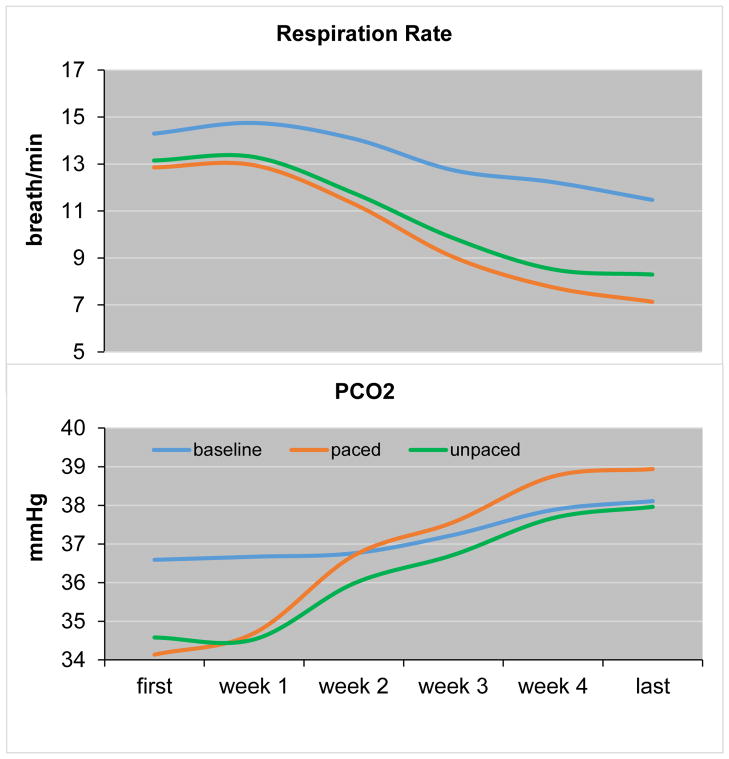
Figure 3.
Mean changes in end-tidal PCO2 (PCO2 in mmHg) and respiratory rate (RR in breaths/min) across treatment weeks (including first exercise of week 1 and last exercise of week 4 for illustration).
Acknowledgments
This research was partly supported by the National Institutes of Mental Health and the Department of Veterans Affairs.
Footnotes
DISCLOSURES
All authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Meuret AE, Kroll J, Ritz T. Panic disorder comorbidity with medical conditions and treatment implications. Annu Rev Clin Psychol. 2017;13:209–240. doi: 10.1146/annurev-clinpsy-021815-093044. [DOI] [PubMed] [Google Scholar]
- 2.Meuret AE, White KS, Ritz T, Roth WT, Hofmann SG, Brown T. Panic attack symptom dimensions and their relationship to illness characteristics in panic disorder. J Psychiatr Res. 2006;40:520–27. doi: 10.1016/j.jpsychires.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 3.Klein DF. False suffocation alarms, spontaneous panics, and related conditions. An integrative hypothesis. Arch Gen Psychiatry. 1993;50:306–317. doi: 10.1001/archpsyc.1993.01820160076009. [DOI] [PubMed] [Google Scholar]
- 4.Ziemann AE, Allen JE, Dahdaleh NS, Drebot II, Coryell MW, Wunsch AM, et al. The amygdala is a chemosensor that detects carbon dioxide and acidosis to elicit fear behavior. Cell. 2009;139:1012–1021. doi: 10.1016/j.cell.2009.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roth WT, Wilhelm FH, Trabert W. Voluntary breath holding in panic and generalized anxiety disorders. Psychosom Med. 1998;60:671–679. doi: 10.1097/00006842-199811000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Benke C, Hamm AO, Pané-Farré CA. When dyspnea gets worse: Suffocation fear and the dynamics of defensive respiratory responses to increasing interoceptive threat. Psychophysiology. 2017;54:1266–1283. doi: 10.1111/psyp.12881. [DOI] [PubMed] [Google Scholar]
- 7.Blechert J, Wilhelm FH, Meuret AE, Wilhelm EM, Roth WT. Respiratory, autonomic, and experiential responses to repeated inhalations of 20% CO2 enriched air in panic disorder, social phobia, and healthy controls. Biol Psychol. 2010;84:104–111. doi: 10.1016/j.biopsycho.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Savage JE, McMichael O, Gorlin EI, Beadel JR, Teachman B, Vladimirov VI, Hettema JM, Roberson-Nay R. Validation of candidate anxiety disorder genes using a carbon dioxide challenge task. Biol Psychol. 2015;109:61–6. doi: 10.1016/j.biopsycho.2015.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilhelm FH, Trabert W, Roth WT. Physiologic instability in panic disorder and generalized anxiety disorder. Biol Psychiatry. 2001;49:596–605. doi: 10.1016/s0006-3223(00)01000-3. [DOI] [PubMed] [Google Scholar]
- 10.Meuret AE, Ritz T. Hyperventilation in panic disorder and asthma: Empirical evidence and clinical strategies. Int J Psychophysiol. 2010;78:68–79. doi: 10.1016/j.ijpsycho.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meuret AE, Rosenfield D, Wilhelm FH, Zhou E, Conrad A, Ritz T, Roth WT. Do unexpected panic attacks occur spontaneously? Biol Psychiatry. 2011;70:985–991. doi: 10.1016/j.biopsych.2011.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khalsa SS, Feinstein JS, Li W, Feusner JD, Adolphs R, Hurlemann R. Panic Anxiety in Humans with Bilateral Amygdala Lesions: Pharmacological Induction via Cardiorespiratory Interoceptive Pathways. J Neurosci. 2016;36:3559–66. doi: 10.1523/JNEUROSCI.4109-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meuret AE, Wilhelm FH, Ritz T, Roth WT. Feedback of end-tidal pCO2 as a therapeutic approach for panic disorder. J Psychiatr Res. 2008;42:560–568. doi: 10.1016/j.jpsychires.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meuret AE, Rosenfield D, Seidel A, Bhaskara L, Hofmann SG. Respiratory and cognitive mediators of treatment change in panic disorder: evidence for intervention specificity. J Consult Clin Psychol. 2010;78:691–704. doi: 10.1037/a0019552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim S, Wollburg E, Roth WT. Opposing breathing therapies for panic disorder: a randomized controlled trial of lowering vs raising end-tidal P(CO2) J Clin Psychiatry. 2012;73:931–939. doi: 10.4088/JCP.11m07068. [DOI] [PubMed] [Google Scholar]
- 16.Meuret AE, Rosenfield D, Hofmann SG, Suvak MK, Roth WT. Changes in respiration mediate changes in fear of bodily sensations in panic disorder. J Psychiatr Res. 2009;43:634–641. doi: 10.1016/j.jpsychires.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ritz T, Rosenfield D, Steele AM, Millard M, Meuret AE. Controlling asthma by training of capnometry-assisted hypoventilation (CATCH) versus slow breathing: a randomized controlled trial. Chest. 2014;146:1237–1247. doi: 10.1378/chest.14-0665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gorman JM, Kent JM, Sullivan GM, Coplan JD. Neuroanatomical hypothesis of panic disorder, revised. Am J Psychiatry. 2000;157:493–505. doi: 10.1176/appi.ajp.157.4.493. [DOI] [PubMed] [Google Scholar]
- 19.Davenport PW, Vovk A. Cortical and subcortical central neural pathways in respiratory sensations. Respir Physiol Neurobiol. 2009;167:72–86. doi: 10.1016/j.resp.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 20.Ritz T, Meuret AE, Bhaskara L, Petersen S. Respiratory muscle tension as symptom generator in individuals with high anxiety sensitivity. Psychosom Med. 2013;75:187–195. doi: 10.1097/PSY.0b013e31827d1072. [DOI] [PubMed] [Google Scholar]
- 21.Boettcher H, Brake CA, Barlow DH. Origins and outlook of interoceptive exposure. J Behav Ther Exp Psychiatry. 2016;53:41–51. doi: 10.1016/j.jbtep.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 22.Deacon B, Kemp JJ, Dixon LJ, Sy JT, Farrell NR, Zhang AR. Maximizing the efficacy of interoceptive exposure by optimizing inhibitory learning: a randomized controlled trial. Behav Res Ther. 2013;51:588–596. doi: 10.1016/j.brat.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 23.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Press; 1994. [Google Scholar]
- 24.Shear MK, Brown TA, Barlow DH, Money R, Sholomskas, Woods, et al. Multicenter collaborative panic disorder severity scale. Am J Psychiatry. 1997;154:1571–1575. doi: 10.1176/ajp.154.11.1571. [DOI] [PubMed] [Google Scholar]
- 25.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM–IV, Patient Edition (SCID–I/P Version 2.0) New York, NY: Biometrics Research Department, New York State Psychiatric Institute; 1995. [Google Scholar]
- 26.Oakes DF, Jones S. Oakes’ Respiratory Care Pocket Guide. 9 Health Educator Publications; 2017. [Google Scholar]
- 27.Reiss S, Peterson RA, Gursky DM, McNally RJ. Anxiety sensitivity, anxiety frequency and the predictions of fearfulness. Behav Res Ther. 1986;24:1–8. doi: 10.1016/0005-7967(86)90143-9. [DOI] [PubMed] [Google Scholar]
- 28.Hoffman L. Longitudinal Analysis: Modeling Within-Person Fluctuation and Change. New York, NY: Routledge; 2015. [Google Scholar]
- 29.Hedeker D, Gibbons RD. Longitudinal Data Analysis. Hoboken, N.J: John Wiley & Sons; 2006. [Google Scholar]
- 30.Wang L, Maxwell SE. On disaggregating between-person and within-person effects in longitudinal data using multilevel models. Psychol Methods. 2015;20:63–83. doi: 10.1037/met0000030. [DOI] [PubMed] [Google Scholar]
- 31.Meuret AE, Rosenfield D, Bhaskara L, Auchus R, Liberzon I, Ritz T, et al. Timing matters: Endogenous cortisol mediates benefits from early-day psychotherapy. Psychoneuroendocrinology. 2016;74:197–202. doi: 10.1016/j.psyneuen.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 32.Tabachnick BG, Fidell LS. Using Multivariate Statistics. 6. Allyn & Bacon; Boston, MA: 2013. [Google Scholar]
- 33.Sheehan DV, Harnett-Sheehan K, Raj BA. The measurement of disability. Int Clin Psychopharmacol. 1996;11:89–95. doi: 10.1097/00004850-199606003-00015. [DOI] [PubMed] [Google Scholar]
- 34.Foa EB, Kozak MJ. Emotional processing of fear: Exposure to corrective information. Psych Bull. 1986;99:20–35. [PubMed] [Google Scholar]
- 35.Craske MG, Kircanski K, Zelikowsky M, Mystkowski J, Chowdhury N, Baker A. Optimizing inhibitory learning during exposure therapy. Behav Res Ther. 2008;46:25–27. doi: 10.1016/j.brat.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 36.Meuret AE, Seidel A, Rosenfield B, Hofmann SG, Rosenfield D. Does fear reactivity during exposure predict panic symptom reduction? J Consult Clin Psychol. 2012;80:773–785. doi: 10.1037/a0028032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smits JA, Rosenfield D, Otto MW, Marques L, Davis ML, Meuret AE, et al. D-cycloserine enhancement of exposure therapy for social anxiety disorder depends on the success of exposure sessions. J Psychiatr Res. 2013;47:1455–1461. doi: 10.1016/j.jpsychires.2013.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smits JA, Rosenfield D, Davis ML, Julian K, Handelsman PR, Otto MW, et al. Yohimbine enhancement of exposure therapy for social anxiety disorder: a randomized controlled trial. Biol Psychiatry. 2013;75:840–846. doi: 10.1016/j.biopsych.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 39.Benke C, Hamm AO, Pané-Farré CA. When dyspnea gets worse: Suffocation fear and the dynamics of defensive respiratory responses to increasing interoceptive threat. Psychophysiology. 2017;54:1266–1283. doi: 10.1111/psyp.12881. [DOI] [PubMed] [Google Scholar]
- 40.Meuret A, Wilhelm FH, Ritz T, Roth WT. Breathing training in panic disorder treatment - Useful intervention or impediment? Behav Modification. 2003;27:731–754. doi: 10.1177/0145445503256324. [DOI] [PubMed] [Google Scholar]
- 41.Conrad A, Müller A, Doberenz S, Kim S, Meuret AE, Wollburg E, et al. Psychophysiological effects of breathing instructions for stress management. Applied Psychophysiology and Biofeedback. 2007;32:89–98. doi: 10.1007/s10484-007-9034-x. [DOI] [PubMed] [Google Scholar]
- 42.Ritz T, Wilhelm FH, Meuret AE, Gerlach A, Roth WT. Do blood phobia patients hyperventilate during exposure by breathing faster, deeper, or both? Dep Anxiety. 2009;26:60–7. doi: 10.1002/da.20466. [DOI] [PubMed] [Google Scholar]
- 43.Yackle K, Schwarz LA, Kam K, Sorokin JM, Huguenard JR, Feldman JL, Luo L, Krasnow MA. Breathing control center neurons that promote arousal in mice. Science. 2017;355:1411–1415. doi: 10.1126/science.aai7984. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.