Summary
Visceral leishmaniasis (VL) is a disease transmitted by phlebotomine sand flies, fatal if untreated, and with no available human vaccine. In rodents, cellular immunity to Leishmania parasite proteins as well as salivary proteins of the sand fly is associated with protection, making them worthy targets for further exploration as vaccines. This review discusses the notion that a combination vaccine including Leishmania and vector salivary antigens may improve vaccine efficacy by targeting the parasite at its most vulnerable stage just after transmission. Furthermore, we put forward the notion that better modeling of natural transmission is needed to test efficacy of vaccines. For example, the fact that individuals living in endemic areas are exposed to sand fly bites and will mount an immune response to salivary proteins should be considered in pre-clinical and clinical evaluation of leishmaniasis vaccines. Nevertheless, despite remaining obstacles there is good reason to be optimistic that safe and effective vaccines against leishmaniasis can be developed.
Keywords: Leishmania, vaccine, sand fly, saliva, adjuvant
1. Introduction
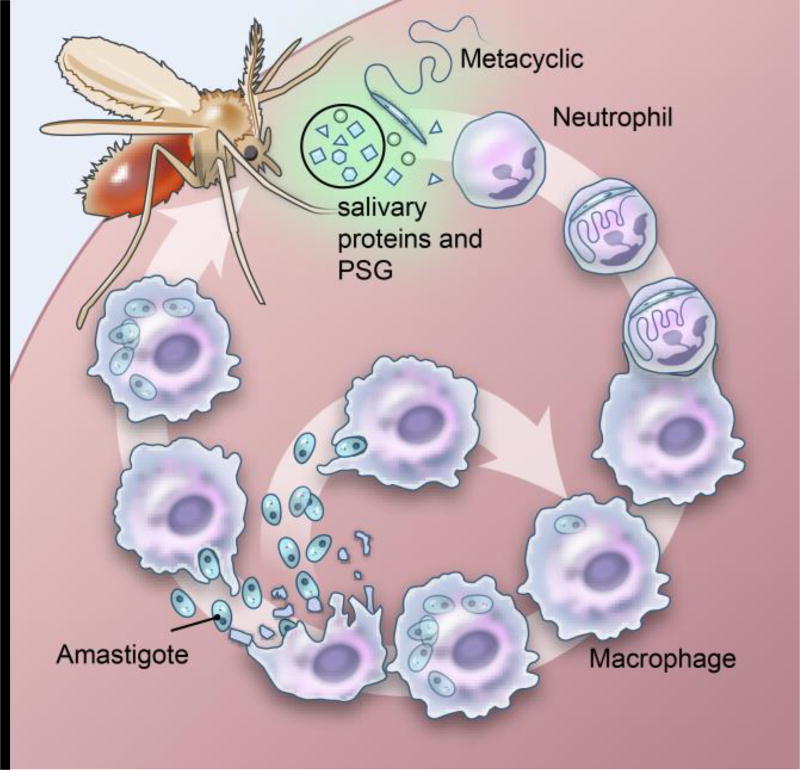
Leishmaniasis is a complex of parasitic diseases caused by several different species of Leishmania. The parasites are transmitted to and from animals or humans through the bite of infected phlebotomine sand flies leading to an intracellular infection in the mammalian host, predominantly within macrophages (Figure 1) (1, 2). Together with parasites and other parasite-derived molecules, such as the promastigote secretory gel (PSG) (3), an infected sand fly injects saliva, composed of biologically active components, mostly proteins, with anti-hemostatic and immunomodulatory properties that help the insect to acquire a blood meal and consequently benefits Leishmania parasite establishment in mammalian hosts (4).
Figure 1.
Leishmania species are inoculated into the skin together with vector-derived factors after an infected sand fly bite where they infect macrophages and form skin lesions (cutaneous forms) or migrate, typically to the spleen, liver, and bone marrow (visceral forms).
The main clinical manifestations of the disease include visceral leishmaniasis (VL), also known as kala azar, cutaneous leishmaniasis (CL), and mucosal leishmaniasis (ML). Post-kala-azar dermal leishmaniasis is considered a complication of VL in areas where Leishmania donovani is endemic. Fortunately for vaccine development the parasite species are highly related antigenically, raising the potential for developing cross-protective vaccines. In addition, some of the vector salivary gland components are also conserved among vector species.
VL, the most serious form of leishmaniasis, is primarily caused by parasites of the Leishmania donovani complex, including L. donovani and L. infantum (5, 6). VL is characterized by fever, wasting, anemia, hepato-splenomegaly, and a depressed immune response (7). VL has a very high fatality rate in the absence of treatment. After malaria, VL is the second most common parasitic cause of death and is prevalent in 47 countries with about 200 million people at risk and an estimated annual incidence of approximately 500,000 cases (8, 9). In South Asia and East Africa, VL is anthroponotic and caused by L. donovani (10). In the Mediterranean region, and in Central and South America, the disease is zoonotic and caused by L. infantum (= L. chagasi) where the main reservoir is dogs (11). Recently, cats and leporids have also been considered as possible alternative reservoirs of VL in certain foci in the Mediterranean region (12). Ninety percent of VL cases occur in five countries – India, Bangladesh, Nepal, Sudan, and Brazil – most often in remote regions without ready access to medical care. In the state of Bihar where more than 90% of all the cases in India are reported, high prevalence of asymptomatic persons and clinical VL cases has led to increased transmission of VL (8).
In this opinion piece, we present an additional consideration for vaccine development, i.e. the inclusion of vector components and the relevance of immunity to vector components in endemic populations.
2. Development of a Vaccine for Leishmaniasis
Efforts to develop a safe, effective, and practical vaccine for one or more leishmanial diseases have been driven by a largely unmet need. Measures such as vector control by insecticide spraying or impregnated bed nets have been insufficient, impractical, or difficult to sustain. Adding to the problem are issues with the drugs used to treat VL that are toxic (pentavalent antimonials, amphotericin B), or expensive (AmBisome) (8, 13–15). Similar issues exist for CL, particularly for the virulent L. braziliensis (CL, ML) and L. tropica (CL) (16).
Development of effective defined vaccines for the leishmaniases should be possible for the following reasons:
Unlike many other protozoa, Leishmania do not undergo significant antigenic variation.
Leishmania have a single host cell, the macrophage, although it interacts with neutrophils and dendritic cells. There are ample immunologic data on enhancing macrophage killing of pathogenic organisms.
A single morphologic form, the amastigote, is associated with pathology in the mammalian host.
Although there are many distinct species of Leishmania, most pathogenic species share many important antigens.
Safe and effective adjuvants that preferentially induce TH1 responses are now available.
Both parasite and vector proteins may have protective capabilities
2.1 Brief History of Vaccination
There is evidence for natural immunity in human leishmaniasis. Sero-epidemiological studies indicate that the majority of infections with L. donovani or L. infantum, the causative agents of VL, remain asymptomatic with natural immunity to disease being the norm rather than the exception (7, 17, 18). Individuals cured from certain forms of CL are resistant to subsequent development of CL disease (though this is not true for all forms, particularly CL caused by L. braziliensis) (19). There are clearly parasite, as well as host factors that may influence the development of immunity.
The history of leishmaniasis vaccine development and a description of tried vaccine candidates have been reviewed (20). Much as in the case of small pox, early attempts at vaccination against CL involved the use of live parasite inoculation, leading to self-healing lesions and subsequent immunity. However, this approach was only used for CL, particularly L. major (21). Attempts to modernize the approach included using killed Leishmania preparations, with only partial and sporadic success in clinical studies (22–25). Experience, generally failures, with developing CL vaccine candidates has led scientists to question how to best mimic natural infection, and indeed how to do so for not only different forms of CL but for VL as well. One approach is obviously to continue efforts on attenuated live vaccines (26, 27), including genetic knockout strains (28), but manufacturing and regulatory issues will likely add additional barriers to the development of a commercially viable vaccine. Despite setbacks, past vaccine studies have been instructive. In this article, we present evolving concepts; which may lead to practical vaccine approaches that take aspects of natural infection into consideration.
2.2 New Approaches to Vaccine Development: Antigen Components
Immunity to the intracellular Leishmania parasites appears to be dominated by appropriate CD4+ T cell responses, particularly those with TH1 dominant profiles, characterized by the production of IFNγ, IL-2 and TNFα by polyfunctional CD4+ T cells (29–31). Negative effects on immunity to leishmaniasis have been associated with excess production of IL-10 and TGF-β (32–36). Understanding the positive and negative aspects of immunity are important considerations for vaccine development, including both antigen and adjuvant selection. Several parasite proteins have been evaluated as vaccine candidates in a variety of animal models, including mice, hamsters, dogs, and non-human primates. A summary of Leishmania antigens tested in experimental studies was recently reviewed (20).
Several Leishmania protein antigens induce potent antibody and T cell responses, yet it is clear that they are unable to induce protection in animal models, regardless of adjuvant formulation or delivery method used. Indeed, it appears that the majority of immunodominant parasite components, such as the repeat antigens represented by k39 (37), are not able to induce protection.
It has been appreciated that phlebotomine sand fly vectors of Leishmania can greatly affect the immune response to the parasite as well as the development of disease (3, 4, 38–40). This has rarely been considered in vaccine development. Two important paradigms establishing the influence of vector-derived factors on transmission and disease progression of leishmaniasis should be of interest to vaccinologists. The first concerns the heightened virulence of Leishmania transmission by vector bites resulting in enhanced disease pathology. Currently, this is mostly attributed to the immunomodulatory properties of saliva and the PSG (3, 4, 40). Other mechanical and behavioral factors such as the site of parasite deposition and altered feeding behavior of infected flies (41) may also contribute to the observed virulence of vector-transmitted leishmaniasis. Importantly, this virulence overrides protection provided by vaccines tested against needle-injected parasites (42) revealing a weakness in current preclinical evaluation of the efficacy of most vaccines. The second paradigm involves the use of such vector-derived factors to protect against Leishmania infections. Indeed, immunity generated against salivary proteins or PSG protected rodents against vector-transmitted leishmaniasis (3, 4, 40, 43) demonstrating their potential as vaccines against leishmaniasis.
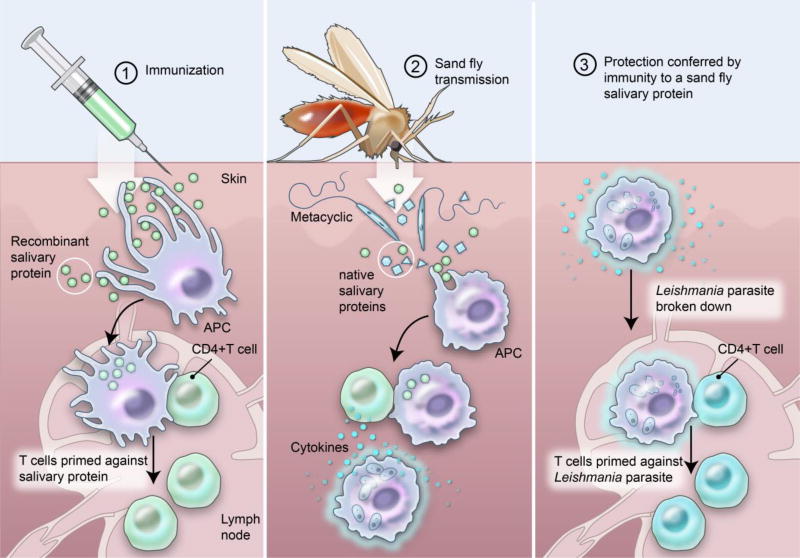
The concept of vector salivary proteins as vaccines against human leishmaniasis has been gaining traction in recent years. Evidence to date indicates that salivary molecules inducing a TH1-delayed type hypersensitivity response in immunized animals create an adverse immunological environment at the bite site that impacts the co-deposited parasites, controlling disease and promoting Leishmania-specific immunity (Figure 2). Importantly, there is no cross reaction between vector salivary proteins and parasite antigens. The immunological environment (TH1) developed at the bite site to the salivary protein promotes a protective TH1 response against the parasite (Figure 2). These sand fly salivary proteins have emerged as candidates for a vaccine or components of a vaccine against leishmaniasis. Table 2 lists the three most promising candidates to date: PdSP15, a 15 kDa salivary protein member of the family of small odorant binding proteins from P. duboscqi (4), the vector of L. major in Sub-Saharan Africa, as a candidate antigen against cutaneous leishmaniasis (39); LJM19, an 11 kDa salivary protein with unknown function, and LJL143, a 38 kDa salivary protein with anti-coagulant activity (44), both from saliva of the sand fly Lutzomyia longipalpis, the vector of L. infantum in Latin America, as candidates against visceral leishmaniasis (38).
Figure 2.
(1) Immunizing with sand fly salivary proteins induces a TH1 delayed type hypersensitivity response specific to the salivary protein (2) recalled to the bite site after the sand fly deposits parasites and salivary proteins while feeding where it activates nearby macrophages and (3) promotes Leishmania-specific immunity.
Table 2.
The three most promising sand fly salivary vaccine candidates to date
| Vaccine Candidate | Origin | Protection | Animal Model |
Homology | Potential vaccine coverage |
|---|---|---|---|---|---|
|
| |||||
| PdSP15 | Ph. duboscqi | CL1 | NHP | P. Papatasi1 | MENA and Sub-Saharan Africa |
| P. sergenti1 | |||||
|
| |||||
| LJM19 | Lu. longipalpis | VL2,3, CL4 | Hamsters | Lu. intermedia5 | Latin America |
| Lu. ayacuchensis5 | |||||
|
| |||||
| LJL143 | Lu. longipalpis | VL (in vitro)6 | Dogs | Ubiquitous5 | Global |
da Silva et al., 2001;
Abdeladhim et al., 2015;
PdSP15 protected non-human primates against vector-transmitted parasites (39). Rhesus macaques immunized with PdSP15, a major component of the saliva of this insect, showed a significant reduction in lesion pathology, a decrease in parasite number, and the induction of Leishmania-specific immunity. Importantly, individuals from a CL endemic area in Mali recognized the PdSP15 salivary protein suggesting this molecule is also immunogenic in humans (39). Mice immunized with PpSP15, the homologue of PdSP15 in Ph. papatasi, the vector of L. major in the Middle East and North Africa, were also protected against CL (45). PdSP15 also shares homology with PsSP15 from Ph. sergenti, a principal vector of L. tropica (39) and as such is a good candidate antigen for a vaccine against Old World CL. LJM19 protected hamsters against the fatality of VL caused by L. infantum (38) and against CL caused by L. braziliensis infection when challenged together with Lu. Longiplapis saliva (46). LJL143 is immunogenic in dogs, was detrimental to the parasites in vitro (47). Importantly, these salivary proteins are mostly unique to sand flies and do not have human homologues (39, 48).
2.3 Vaccine Development: Inclusion and Selection of Adjuvant
Clinical grade adjuvants that induce preferential and durable TH1 responses in experimental animal models and in humans are now available. Recombinant proteins often induce only weak T cell responses without the inclusion of adjuvant. Distinctively, salivary antigens induce a robust T cell response in mice without adjuvant (49). Specific adjuvant molecules may directly activate innate immune receptors, for example, Toll-like receptors (TLRs) (50, 51). Other formulation systems may include both delivery and immunostimulatory components. Thus, adjuvants may be broadly classified into three groups of delivery systems: immunomodulatory molecules, particulate formulation and combinations of the former two classes (combination systems) (Table 3).
Table 3.
List of Adjuvants
| Adjuvant | Class | Mechanism of action | Type of immune response |
|---|---|---|---|
|
| |||
| Aluminum mineral salts | Particulate formulation antigen depot | NACHT, LRR and PYD domains-containing protein 3 (NALP3), immunereceptor tyrosine-based activation motif (ITAM), antigen delivery, Interleukin-1 secretion, Inflammasome, necrosis | Antibody, T helper 2 |
|
| |||
| Lipid A analogues (for example, Monophosphoryl Lipid A (MPL,) Glucopyranosyl Lipid A (GLA)) | Immunomodulatory molecule | Toll-like receptor 4 | Antibody, T helper 1 |
|
| |||
| Imidazoquinolines (for example Imiquimod, R848) | Immunomodulatory molecule | Toll-like receptor 7 | Antibody, T helper 1 |
| Toll-like receptor 8 | |||
|
| |||
| CpG oligodeoxynucleotides (CpG ODN) | Immunomodulatory molecule | Toll-like receptor 9 | Antibody, T helper 1, T helper 2, CD8+ T cells |
|
| |||
| Saponins (for example, QS21) | Immunomodulatory molecule | Unknown | |
|
| |||
| Virosomes | Particulate formulation | Antigen delivery | Antibody, T helper 1, T helper 2 |
|
| |||
| GLA-SE (GLA, stable emulsion) | Combination | Toll-like receptor 4 | Antibody, T helper 1 |
In leishmaniasis vaccine development, a synthetic toll-like receptor 4 (TLR-4) ligand, Glucopyranosyl Lipid A (GLA) is in clinical development (52). When formulated in a stable oil-in-water nano-emulsion (SE), the GLA-SE adjuvant system induced antigen-specific TH1 immune responses that are associated with vaccine or protective efficacy in several animal models of infection, including tuberculosis, malaria, and influenza (53–55).
A clinical study that evaluated LEISH-F3, a fusion of nucleoside hydrolase (NH) and sterol 24-c-methyltransferase (SMT), combined into a fusion construct, combined with GLA-SE was safe and immunogenic. T cell cytokines and TH1 biased Ab responses were seen in subjects immunized with protein/adjuvant, but not protein alone, demonstrating the need for adjuvant (56). This result, along with the results of several clinical studies with GLA-SE in nearly 1000 human subjects demonstrates the potential for a safe and efficacious adjuvant that can practically be applied to development of a human vaccine against leishmaniasis. This, along with the potential of using a sand fly protein as adjuvant, perhaps alone or in combination with GLA-SE, opens exciting and practical development opportunities.
2.4 New Approaches to Vaccine Development: Formulating Parasite and Vector Salivary Antigens
Considering that both parasites and salivary proteins are co-deposited at the bite site, and that immunity to a sand fly salivary protein promotes protective immunity against the parasite in rodents and non-human primates (39, 45), it is possible that a combination of antigens from different sources may be complementary. Recently, a study provided evidence that combining a salivary molecule and a recombinant Leishmania tarentolae parasite confers a stronger protection against CL compared to the efficacy of each antigen alone (57). For this combination approach, the best immunization strategy consisted of priming with a sand fly salivary antigen and boosting with the salivary antigen and L. tarentolae (57). It is likely that the salivary antigen rapidly recalled an immune response to itself during the boost resulting in a TH1-type environment against L. tarentolae parasites that favored the development of a protective immunity (57). Importantly, the immunity generated against a salivary protein and a Leishmania antigen is likely to be amplified when the immunized animals encounter an infective bite. Additionally, this immune response will be localized to the skin interface when the parasites are at their most vulnerable stage, in low numbers and transitioning from the insect to the vertebrate host.
2.5 New Paradigms for Vaccine Design and Evaluation: Modeling Natural Transmission
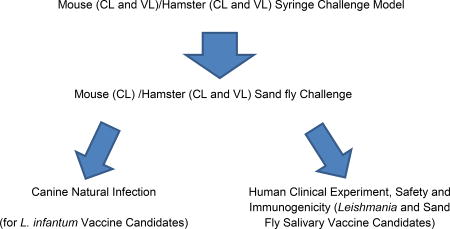
The use of challenge models that mimic natural transmission has been a neglected aspect of vaccine development for vector-borne diseases. Nevertheless, developing such models is vital to vaccine assessment. In addition to the pathogen itself, a vector inoculum is complex comprising a multitude of vector-derived factors of pathogen and vector origin. In leishmaniasis, the infected sand fly salivates into the bite site in the skin and egests molecules alongside the parasites that modulate the bite site and participate in enhancement of disease (3, 4, 40). Sand flies are telmophagic feeders that lacerate skin capillaries creating a shallow pool of blood and lymph mixed with tissue into which the parasites are deposited. The site of parasite deposition in the skin, the first barrier to infection and an immunologically rich compartment, most likely influences the nature of the early host immune response. This response is further modulated by vector-derived factors that are egested by the fly into the bite site together with the parasites. These include a plethora of salivary proteins to facilitate feeding (58). Studies, reviewed in (4, 59) have established the immunomodulatory properties of several of these proteins, all of which promote Leishmania establishment. The infected sand fly also egests a proteophosphoglycan-containing PSG that modulates the environment to the benefit of the parasites (3). Additional non-biological factors such as the modified persistent feeding behavior of infected flies (41) also contribute to the complexity and virulence of a vector-challenge. We are therefore proponents of incorporating a vector-challenge study at the final stages of preclinical testing of a vaccine. The need for this additional selection step has been validated by the failure of a vaccine considered efficacious after a needle-initiated infection when challenged by infected sand flies (42). We have developed vector-transmission models for both CL (39, 60) and VL (61). These models provide a more stringent evaluation of vaccines and a more accurate readout of their expected performance under field conditions. We are currently using these models to prioritize promising vaccines for further development.
Proposed Sequence of Testing Vaccine Candidates
2.6 Clinical Development: A New Strategy to Demonstrate Vaccine Efficacy in the Field
A vaccine cannot be useful if not used, not used if not approved, and not approved without demonstration of safety and efficacy in field studies. Following research efforts to demonstrate pre-clinical rationale, early clinical studies may involve, for example antigen and adjuvant dose finding to optimize safety and immunogenicity. Such studies may be critical, as recent studies have led to an improved understanding of how to increase potency and efficacy of adjuvanted proteins by lowering the dose, a concept described in leishmaniasis more than 20 years ago (62). We have recently demonstrated that a lower dose (2ug) of a recombinant adjuvanted tuberculosis vaccine candidate was more potent than a higher dose (10ug) when used to immunized Mycobacterium tuberculosis-exposed individuals (Coler et al., unpublished),
Classically, testing vaccine candidates is performed in naïve animals. However field testing of VL vaccine candidates will likely be conducted in adults in endemic areas, and these individuals have been exposed to vector salivary proteins through sand fly bites. Following natural challenge via a sand fly bite, the parasites will most likely encounter a site that is already primed to salivary proteins as well as by the injected vaccine. The same applies to a vaccine that includes a salivary molecule where the salivary antigen injected during vaccination will encounter a site naturally primed to sand fly salivary proteins. In exposed populations, therefore, the first injection of a salivary antigen may act as a boost of a preexisting immune response. Even if testing solely a Leishmania antigen, the immunological environment will be different (from naïve animals or naïve healthy individuals) due to the immune response to salivary proteins induced by a sand fly bite (in sand fly exposed individuals in endemic areas). The presence of immune responses to sand fly salivary proteins in humans living in endemic areas is well documented (4). Exposure to sand fly bites is known to generate specific antibodies to sand fly salivary proteins (63–65) but more importantly individuals experimentally or naturally exposed to sand fly bites develop a cellular immune response to these salivary proteins (66, 67). It was recently shown that a TH1 response at the bite site was developed in individuals from endemic areas in Mali after exposure to small number of uninfected sand flies (68) suggesting that the immunological site for any given vaccine to be tested has to be considered also in the context of the immune response in the skin to vector salivary proteins.
These scenarios are often neglected when designing or testing vaccines against leishmaniasis and may be also of consequence for other vector-borne diseases including malaria and dengue. Using animal models previously exposed to vector bites as part of pre-clinical testing may provide a more stringent approach to vaccine evaluation and also provides insights into the early immunological events following an infection event in vaccinated animals. In addition to previous exposure to vector salivary proteins, individuals living in endemic areas may harbor subclinical Leishmania infections. The prevention of disease in individuals with asymptomatic infections raises an interesting vaccine development issue, as rather than inducing de novo T cell responses, a vaccine candidate may act as a boost to an immune response primed by asymptomatic infection. This may affect the choice of adjuvant for example, as well as dose of both antigen and adjuvant components.
Using VL as an example, the desired effects of a vaccine would be to prevent clinical disease and to prevent or decrease infection to reduce the human reservoir. Demonstrating prevention of infection may be possible in areas with high rates of transmission, but will require surveillance with sensitive and specific tools to monitor asymptomatic infection and progression from negative to positive during one or more transmission seasons (69, 70). Although effective VL diagnostics for detecting disease have been widely available for years (71–76), only recently has the promise of sensitive and specific tools for monitoring early and low level infection been demonstrated (77).
Rather than attempting to demonstrate prevention of infection during initial stages of vaccine development, a more practical approach to evaluating VL vaccines will be to perform clinical studies in individuals with latent L. donovani infection to prevent progression to disease. In India and Bangladesh, it has been estimated that 5–7% of recently infected individuals may progress to acute VL within 12–18 months. This raises the possibility of demonstrating vaccine efficacy in relatively small clinical studies, using development of disease (and potential stabilization or decrease of parasite replication in asymptomatic individuals) as an endpoint(s).
Regardless of the approach used to evaluate vaccine efficacy, vaccine recipients are likely to have been exposed (repeatedly) to sand fly proteins or to both sand fly and parasite proteins, and these factors should be considered in the approach to prevention of infection and to prevention of disease progression, respectively, in pre-clinical modeling.
3. Expert commentary and five-year view
There is every reason to be optimistic that safe and effective vaccines against leishmaniasis can be developed. Many of the developmental hurdles can be overcome with a transition from first generation vaccines to defined vaccines that induce long-lasting protection. As we learn more about the appropriate interplay between host and parasite, host and vector, vector and parasite we should be able to design safe and effective vaccine candidates that can be delivered at low cost. Critical to this is, for the first time, availability of a safe and effective T cell adjuvant formulation not controlled by big pharmaceutical companies that can be produced in large volume at low cost. Sand fly components with both antigen and adjuvant activity have been identified and are now undergoing process development for clinical testing, which may be performed in conjunction with other promising clinical stage vaccine candidates, such as Leish F3. Perhaps of critical importance will be the design of clinical trials that will enable a more direct and affordable path toward demonstration of efficacy and ultimate approval. These trials will take into account the realization that we will be in most cases boosting, not priming, an immune response capable of preventing disease development in healthy individuals previously exposed to both parasite and vector. Identification of these individuals is now feasible with new diagnostic tools, enabling targeting of a vaccine to the most susceptible population.
Table 1.
Examples of Parasites Causing Human Leishmaniases
| Parasite | Diseases | Distribution |
|---|---|---|
| L. donovani | visceral leishmaniasis, post-kalaazar dermal leishmaniasis Human | India, Nepal, Bangladesh, Sudan, Ethiopia |
| L infantum | visceral leishmaniasis Human, Canine | Brazil, Mediterranean Region |
| L. major | cutaneous leishmaniasis Human | Middle East |
| L. tropica | cutaneous leishmaniasis Human | Middle East |
| L. braziliensis | cutaneous leishmaniasis, mucocutaneous leishmaniasis Human | Brazil |
Key Issues.
Leishmania delivered into the skin of humans by sand bites are accompanied by immunomodulatory vector-derived factors forming a complex infectious inoculum that enhances the virulence of transmitted parasites. Importantly, the immune response to vector-derived factors has a negative impact on parasite establishment.
Certain Leishmania and sand fly salivary antigens induce a cellular immune response that protects animals against leishmaniasis. Combining antigens form these two sources may produce a more effective vaccine as recently demonstrated in rodent models.
Leishmania vaccines are tested in naïve animals neglecting the fact that individuals living in endemic areas are constantly exposed to sand fly bites. Potentially, these individuals may react differently to a Leishmania vaccine as they concurrently mount an immune response to vector saliva.
New models for testing Leishmania vaccines are needed that account for virulence of natural transmission as well as pre-existing confounders including an underlying immunity to vector saliva in target populations.
Acknowledgments
The authors want to thank Ryan Kissinger, Research and technologies branch, NIAID, NIH, for the artwork.
Support for this work was provided by the Intramural Research Program at the National Institute of Allergy and Infectious Diseases, NIH (JG Valenzuela and S Kamhawi). This research was supported in part with funding from the Bill & Melinda Gates Foundation, under grants 631, 39129 and 49932 and by Grant # R01AI025038 from NIAID of the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Bill & Melinda Gates Foundation or the National Institutes of Health. SG Reed is a co-inventor on a patent of leishmaniasis vaccine development.
Abbreviations used
- IFNγ
interferon gamma
- IL
interleukin
- TLR
Toll-like receptor
- VL
visceral leishmaniasis
- TH1
T helper 1
- PSG
Promastigote secretory gel
Footnotes
Financial and competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References
Reference annotations
* Of interest
** Of considerable interest
- 1.Maroli M, Feliciangeli MD, Bichaud L, Charrel RN, Gradoni L. Phlebotomine sandflies and the spreading of leishmaniases and other diseases of public health concern. Med Vet Entomol. 2013;27(2):123–47. doi: 10.1111/j.1365-2915.2012.01034.x. [DOI] [PubMed] [Google Scholar]
- 2.Kevric I, Cappel MA, Keeling JH. New World and Old World Leishmania Infections: A Practical Review. Dermatol Clin. 2015;33(3):579–93. doi: 10.1016/j.det.2015.03.018. [DOI] [PubMed] [Google Scholar]
- 3.Rogers ME. The role of leishmania proteophosphoglycans in sand fly transmission and infection of the Mammalian host. Frontiers in microbiology. 2012;3:223. doi: 10.3389/fmicb.2012.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4*.Abdeladhim M, Kamhawi S, Valenzuela JG. What's behind a sand fly bite? The profound effect of sand fly saliva on host hemostasis, inflammation and immunity. Infection, genetics and evolution : journal of molecular epidemiology and evolutionary genetics in infectious diseases. 2014;28:691–703. doi: 10.1016/j.meegid.2014.07.028. A detailed review of the different biological activities reported for sand fly salivary proteins and their potential as components of a leishmaniasis vaccine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Magill AJ, editor. Hunter's Tropical Medicine and Emerging Infectious Diseases. Eighth. Philadelphia: W.B. Saunders Company; 2000. [Google Scholar]
- 6.Kedzierski L, Sakthianandeswaren A, Curtis JM, Andrews PC, Junk PC, Kedzierska K. Leishmaniasis: current treatment and prospects for new drugs and vaccines. Curr Med Chem. 2009;16(5):599–614. doi: 10.2174/092986709787458489. [DOI] [PubMed] [Google Scholar]
- 7.Kumar R, Nylen S. Immunobiology of visceral leishmaniasis. Front Immunol. 2012;3:251. doi: 10.3389/fimmu.2012.00251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alvar J, Velez ID, Bern C, Herrero M, Desjeux P, Cano J, et al. Leishmaniasis worldwide and global estimates of its incidence. PLoS One. 2012;7(5):e35671. doi: 10.1371/journal.pone.0035671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization, Expert Committee. Control of leishmaniasis. World health Organization Technical Report Series. 1990:793. [PubMed] [Google Scholar]
- 10.Ready PD. Epidemiology of visceral leishmaniasis. Clinical epidemiology. 2014;6:147–54. doi: 10.2147/CLEP.S44267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roatt BM, Aguiar-Soares RD, Coura-Vital W, Ker HG, Moreira N, Vitoriano-Souza J, et al. Immunotherapy and Immunochemotherapy in Visceral Leishmaniasis: Promising Treatments for this Neglected Disease. Front Immunol. 2014;5:272. doi: 10.3389/fimmu.2014.00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dominguez-Bernal G, Jimenez M, Molina R, Ordonez-Gutierrez L, Martinez-Rodrigo A, Mas A, et al. Characterisation of the ex vivo virulence of Leishmania infantum isolates from Phlebotomus perniciosus from an outbreak of human leishmaniosis in Madrid, Spain. Parasit Vectors. 2014;7:499. doi: 10.1186/s13071-014-0499-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhattacharya SK, Sur D, Sinha PK, Karbwang J. Elimination of leishmaniasis (kala-azar) from the Indian subcontinent is technically feasible & operationally achievable. The Indian journal of medical research. 2006;123(3):195–6. [PubMed] [Google Scholar]
- 14.Joshi AB, Banjara MR, Pokhrel S, Jimba M, Singhasivanon P, Ashford RW. Elimination of visceral leishmaniasis in Nepal: pipe-dreams and possibilities. Kathmandu Univ Med J (KUMJ) 2006;4(4):488–96. [PubMed] [Google Scholar]
- 15.Kumar V, Kesari S, Dinesh DS, Tiwari AK, Kumar AJ, Kumar R, et al. A report on the indoor residual spraying (IRS) in the control of Phlebotomus argentipes, the vector of visceral leishmaniasis in Bihar (India): an initiative towards total elimination targeting 2015 (Series-1) J Vector Borne Dis. 2009;46(3):225–9. [PubMed] [Google Scholar]
- 16.Mears ER, Modabber F, Don R, Johnson GE. A Review: The Current In Vivo Models for the Discovery and Utility of New Anti-leishmanial Drugs Targeting Cutaneous Leishmaniasis. PLoS Negl Trop Dis. 2015;9(9):e0003889. doi: 10.1371/journal.pntd.0003889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Joshi S, Rawat K, Yadav NK, Kumar V, Siddiqi MI, Dube A. Visceral Leishmaniasis: Advancements in Vaccine Development via Classical and Molecular Approaches. Front Immunol. 2014;5:380. doi: 10.3389/fimmu.2014.00380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khadem F, Uzonna JE. Immunity to visceral leishmaniasis: implications for immunotherapy. Future microbiology. 2014;9(7):901–15. doi: 10.2217/fmb.14.43. [DOI] [PubMed] [Google Scholar]
- 19.Kedzierski L, Evans KJ. Immune responses during cutaneous and visceral leishmaniasis. Parasitology. 2014:1–19. doi: 10.1017/S003118201400095X. [DOI] [PubMed] [Google Scholar]
- 20.Alvar J, Croft SL, Kaye P, Khamesipour A, Sundar S, Reed SG. Case study for a vaccine against leishmaniasis. Vaccine. 2013;31(Suppl 2):B244–9. doi: 10.1016/j.vaccine.2012.11.080. [DOI] [PubMed] [Google Scholar]
- 21.Khamesipour A, Dowlati Y, Asilian A, Hashemi-Fesharki R, Javadi A, Noazin S, et al. Leishmanization: use of an old method for evaluation of candidate vaccines against leishmaniasis. Vaccine. 2005;23(28):3642–8. doi: 10.1016/j.vaccine.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 22.Alimohammadian MH, Khamesipour A, Darabi H, Firooz A, Malekzadeh S, Bahonar A, et al. The role of BCG in human immune responses induced by multiple injections of autoclaved Leishmania major as a candidate vaccine against leishmaniasis. Vaccine. 2002;21(3–4):174–80. doi: 10.1016/s0264-410x(02)00458-9. [DOI] [PubMed] [Google Scholar]
- 23.Khamesipour A, Rafati S, Davoudi N, Maboudi F, Modabber F. Leishmaniasis vaccine candidates for development: a global overview. Indian J MedRes. 2006;123(3):423–38. [PubMed] [Google Scholar]
- 24.Momeni AZ, Jalayer T, Emamjomeh M, Khamesipour A, Zicker F, Ghassemi RL, et al. A randomised, double-blind, controlled trial of a killed L. major vaccine plus BCG against zoonotic cutaneous leishmaniasis in Iran. Vaccine. 1999;17(5):466–72. doi: 10.1016/s0264-410x(98)00220-5. [DOI] [PubMed] [Google Scholar]
- 25.Sharifi I, FeKri AR, Aflatonian MR, Khamesipour A, Nadim A, Mousavi MR, et al. Randomised vaccine trial of single dose of killed Leishmania major plus BCG against anthroponotic cutaneous leishmaniasis in Bam, Iran. Lancet. 1998;351(9115):1540–3. doi: 10.1016/S0140-6736(98)09552-X. [DOI] [PubMed] [Google Scholar]
- 26.Selvapandiyan A, Dey R, Gannavaram S, Lakhal-Naouar I, Duncan R, Salotra P, et al. Immunity to visceral leishmaniasis using genetically defined live-attenuated parasites. J Trop Med. 2012;2012:631460. doi: 10.1155/2012/631460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Selvapandiyan A, Duncan R, Debrabant A, Lee N, Sreenivas G, Salotra P, et al. Genetically modified live attenuated parasites as vaccines for leishmaniasis. The Indian journal of medical research. 2006;123(3):455–66. [PubMed] [Google Scholar]
- 28.Selvapandiyan A, Dey R, Gannavaram S, Solanki S, Salotra P, Nakhasi HL. Generation of growth arrested Leishmania amastigotes: a tool to develop live attenuated vaccine candidates against visceral leishmaniasis. Vaccine. 2014;32(31):3895–901. doi: 10.1016/j.vaccine.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 29.Carvalho EM, Badaro R, Reed SG, Jones TC, Johnson WD., Jr Absence of gamma interferon and interleukin 2 production during active visceral leishmaniasis. The Journal of clinical investigation. 1985;76(6):2066–9. doi: 10.1172/JCI112209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carvalho EM, Johnson WD, Barreto E, Marsden PD, Costa JL, Reed S, et al. Cell mediated immunity in American cutaneous and mucosal leishmaniasis. J Immunol. 1985;135(6):4144–8. [PubMed] [Google Scholar]
- 31.Darrah PA, Patel DT, De Luca PM, Lindsay RW, Davey DF, Flynn BJ, et al. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nature medicine. 2007;13(7):843–50. doi: 10.1038/nm1592. [DOI] [PubMed] [Google Scholar]
- 32.Olivier M, Gregory DJ, Forget G. Subversion mechanisms by which Leishmania parasites can escape the host immune response: a signaling point of view. Clin Microbiol Rev. 2005;18(2):293–305. doi: 10.1128/CMR.18.2.293-305.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saha S, Mondal S, Ravindran R, Bhowmick S, Modak D, Mallick S, et al. IL-10- and TGF-beta-mediated susceptibility in kala-azar and post-kala-azar dermal leishmaniasis: the significance of amphotericin B in the control of Leishmania donovani infection in India. J Immunol. 2007;179(8):5592–603. doi: 10.4049/jimmunol.179.8.5592. [DOI] [PubMed] [Google Scholar]
- 34.Barral A, Barral-Netto M, Yong EC, Brownell CE, Twardzik DR, Reed SG. Transforming growth factor beta as a virulence mechanism for Leishmania braziliensis. ProcNatlAcadSciUSA. 1993;90(8):3442–6. doi: 10.1073/pnas.90.8.3442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barral-Netto M, Barral A, Brownell CE, Skeiky YA, Ellingsworth LR, Twardzik DR, et al. Transforming growth factor-beta in leishmanial infection: a parasite escape mechanism. Science. 1992;257(5069):545–8. doi: 10.1126/science.1636092. [DOI] [PubMed] [Google Scholar]
- 36.Ghalib HW, Piuvezam MR, Skeiky YA, Siddig M, Hashim FA, el-Hassan AM, et al. Interleukin 10 production correlates with pathology in human Leishmania donovani infections. The Journal of clinical investigation. 1993;92(1):324–9. doi: 10.1172/JCI116570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Duthie MS, Raman VS, Piazza FM, Reed SG. The development and clinical evaluation of second-generation leishmaniasis vaccines. Vaccine. 2012;30(2):134–41. doi: 10.1016/j.vaccine.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gomes R, Teixeira C, Teixeira MJ, Oliveira F, Menezes MJ, Silva C, et al. Immunity to a salivary protein of a sand fly vector protects against the fatal outcome of visceral leishmaniasis in a hamster model. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(22):7845–50. doi: 10.1073/pnas.0712153105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39**.Oliveira F, Rowton E, Aslan H, Gomes R, Castrovinci PA, Alvarenga PH, et al. A sand fly salivary protein vaccine shows efficacy against vector-transmitted cutaneous leishmaniasis in nonhuman primates. Sci Transl Med. 2015;7(290):290ra90. doi: 10.1126/scitranslmed.aaa3043. The first report of a salivary-based vaccine that protects non-human primates against Leishmania transmitted by sand fly bites. [DOI] [PubMed] [Google Scholar]
- 40.Gomes R, Oliveira F. The immune response to sand fly salivary proteins and its influence on leishmania immunity. Front Immunol. 2012;3:110. doi: 10.3389/fimmu.2012.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rogers ME, Bates PA. Leishmania manipulation of sand fly feeding behavior results in enhanced transmission. PLoS pathogens. 2007;3(6):e91. doi: 10.1371/journal.ppat.0030091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peters NC, Kimblin N, Secundino N, Kamhawi S, Lawyer P, Sacks DL. Vector transmission of leishmania abrogates vaccine-induced protective immunity. PLoS pathogens. 2009;5(6):e1000484. doi: 10.1371/journal.ppat.1000484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rogers ME, Sizova OV, Ferguson MA, Nikolaev AV, Bates PA. Synthetic glycovaccine protects against the bite of leishmania-infected sand flies. The Journal of infectious diseases. 2006;194(4):512–8. doi: 10.1086/505584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Collin N, Assumpcao TC, Mizurini DM, Gilmore DC, Dutra-Oliveira A, Kotsyfakis M, et al. Lufaxin, a novel factor Xa inhibitor from the salivary gland of the sand fly Lutzomyia longipalpis blocks protease-activated receptor 2 activation and inhibits inflammation and thrombosis in vivo. Arteriosclerosis, thrombosis, and vascular biology. 2012;32(9):2185–98. doi: 10.1161/ATVBAHA.112.253906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oliveira F, Lawyer PG, Kamhawi S, Valenzuela JG. Immunity to distinct sand fly salivary proteins primes the anti-Leishmania immune response towards protection or exacerbation of disease. PLoS neglected tropical diseases. 2008;2(4):e226. doi: 10.1371/journal.pntd.0000226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tavares NM, Silva RA, Costa DJ, Pitombo MA, Fukutani KF, Miranda JC, et al. Lutzomyia longipalpis saliva or salivary protein LJM19 protects against Leishmania braziliensis and the saliva of its vector, Lutzomyia intermedia. PLoS neglected tropical diseases. 2011;5(5):e1169. doi: 10.1371/journal.pntd.0001169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Collin N, Gomes R, Teixeira C, Cheng L, Laughinghouse A, Ward JM, et al. Sand fly salivary proteins induce strong cellular immunity in a natural reservoir of visceral leishmaniasis with adverse consequences for Leishmania. PLoS pathogens. 2009;5(5):e1000441. doi: 10.1371/journal.ppat.1000441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Valenzuela JG, Garfield M, Rowton ED, Pham VM. Identification of the most abundant secreted proteins from the salivary glands of the sand fly Lutzomyia longipalpis, vector of Leishmania chagasi. The Journal of experimental biology. 2004;207(Pt 21):3717–29. doi: 10.1242/jeb.01185. [DOI] [PubMed] [Google Scholar]
- 49.Gomes R, Oliveira F, Teixeira C, Meneses C, Gilmore DC, Elnaiem DE, et al. Immunity to sand fly salivary protein LJM11 modulates host response to vector-transmitted leishmania conferring ulcer-free protection. The Journal of investigative dermatology. 2012;132(12):2735–43. doi: 10.1038/jid.2012.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Badiee A, Heravi Shargh V, Khamesipour A, Jaafari MR. Micro/nanoparticle adjuvants for antileishmanial vaccines: present and future trends. Vaccine. 2013;31(5):735–49. doi: 10.1016/j.vaccine.2012.11.068. [DOI] [PubMed] [Google Scholar]
- 51.Gurung P, Kanneganti TD. Innate immunity against Leishmania infections. Cellular microbiology. 2015;17(9):1286–94. doi: 10.1111/cmi.12484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Coler RN, Bertholet S, Moutaftsi M, Guderian JA, Windish HP, Baldwin SL, et al. Development and characterization of synthetic glucopyranosyl lipid adjuvant system as a vaccine adjuvant. PLoS One. 2011;6(1):e16333. doi: 10.1371/journal.pone.0016333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bertholet S, Ireton GC, Ordway DJ, Windish HP, Pine SO, Kahn M, et al. A defined tuberculosis vaccine candidate boosts BCG and protects against multidrug-resistant Mycobacterium tuberculosis. Sci Transl Med. 2010;2(53):53ra74. doi: 10.1126/scitranslmed.3001094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wiley SR, Raman VS, Desbien A, Bailor HR, Bhardwaj R, Shakri AR, et al. Targeting TLRs expands the antibody repertoire in response to a malaria vaccine. Sci Transl Med. 2011;3(93):93ra69. doi: 10.1126/scitranslmed.3002135. [DOI] [PubMed] [Google Scholar]
- 55.Coler RN, Baldwin SL, Shaverdian N, Bertholet S, Reed SJ, Raman VS, et al. A synthetic adjuvant to enhance and expand immune responses to influenza vaccines. PLoS One. 2010;5(10):e13677. doi: 10.1371/journal.pone.0013677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56*.Coler RN, Duthie MS, Hofmeyer KA, Guderian J, Jayashankar L, Vergara J, et al. From Mouse to Man: Safety, immunogenicity and efficacy of a candidate leishmaniasis vaccine LEISH-F3 + GLA-SE. Clin Transl Immunology. 2015;4(e35) doi: 10.1038/cti.2015.6. This shows development of a vaccine candidate from benchtop through regulatory approval to clinical testing. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57*.Zahedifard F, Gholami E, Taheri T, Taslimi Y, Doustdari F, Seyed N, et al. Enhanced Protective Efficacy of Nonpathogenic Recombinant Leishmania tarentolae Expressing Cysteine Proteinases Combined with a Sand Fly Salivary Antigen. PLoS neglected tropical diseases. 2014;8(3):e2751. doi: 10.1371/journal.pntd.0002751. The first report to demonstrate that combining salivary and Leishmania antigens produces a better vaccine against cutaneous leishmaniasis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ribeiro JM, Francischetti IM. Role of arthropod saliva in blood feeding: sialome and post-sialome perspectives. Annual review of entomology. 2003;48:73–88. doi: 10.1146/annurev.ento.48.060402.102812. [DOI] [PubMed] [Google Scholar]
- 59.Prates DB, Araujo-Santos T, Brodskyn C, Barral-Netto M, Barral A, Borges VM. New Insights on the Inflammatory Role of Lutzomyia longipalpis Saliva in Leishmaniasis. Journal of parasitology research. 2012;2012:643029. doi: 10.1155/2012/643029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kamhawi S, Belkaid Y, Modi G, Rowton E, Sacks D. Protection against cutaneous leishmaniasis resulting from bites of uninfected sand flies. Science. 2000;290(5495):1351–4. doi: 10.1126/science.290.5495.1351. [DOI] [PubMed] [Google Scholar]
- 61.Aslan H, Dey R, Meneses C, Castrovinci P, Jeronimo SM, Oliva G, et al. A new model of progressive visceral leishmaniasis in hamsters by natural transmission via bites of vector sand flies. The Journal of infectious diseases. 2013;207(8):1328–38. doi: 10.1093/infdis/jis932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bretscher PA, Wei G, Menon JN, Bielefeldt-Ohmann H. Establishment of stable, cell-mediated immunity that makes "susceptible" mice resistant to Leishmania major. Science. 1992;257(5069):539–42. doi: 10.1126/science.1636090. [DOI] [PubMed] [Google Scholar]
- 63.Teixeira C, Gomes R, Collin N, Reynoso D, Jochim R, Oliveira F, et al. Discovery of markers of exposure specific to bites of Lutzomyia longipalpis, the vector of Leishmania infantum chagasi in Latin America. PLoS Negl Trop Dis. 2010;4(3):e638. doi: 10.1371/journal.pntd.0000638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gomes RB, Brodskyn C, de Oliveira CI, Costa J, Miranda JC, Caldas A, et al. Seroconversion against Lutzomyia longipalpis saliva concurrent with the development of anti-Leishmania chagasi delayed-type hypersensitivity. The Journal of infectious diseases. 2002;186(10):1530–4. doi: 10.1086/344733. [DOI] [PubMed] [Google Scholar]
- 65.Barral A, Honda E, Caldas A, Costa J, Vinhas V, Rowton ED, et al. Human immune response to sand fly salivary gland antigens: a useful epidemiological marker? The American journal of tropical medicine and hygiene. 2000;62(6):740–5. doi: 10.4269/ajtmh.2000.62.740. [DOI] [PubMed] [Google Scholar]
- 66.Belkaid Y, Valenzuela JG, Kamhawi S, Rowton E, Sacks DL, Ribeiro JM. Delayed-type hypersensitivity to Phlebotomus papatasi sand fly bite: An adaptive response induced by the fly? Proceedings of the National Academy of Sciences of the United States of America. 2000;97(12):6704–9. doi: 10.1073/pnas.97.12.6704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vinhas V, Andrade BB, Paes F, Bomura A, Clarencio J, Miranda JC, et al. Human anti-saliva immune response following experimental exposure to the visceral leishmaniasis vector, Lutzomyia longipalpis. European journal of immunology. 2007;37(11):3111–21. doi: 10.1002/eji.200737431. [DOI] [PubMed] [Google Scholar]
- 68*.Oliveira F, Traore B, Gomes R, Faye O, Gilmore DC, Keita S, et al. Delayed-type hypersensitivity to sand fly saliva in humans from a leishmaniasis-endemic area of Mali is Th1-mediated and persists to midlife. The Journal of investigative dermatology. 2013;133(2):452–9. doi: 10.1038/jid.2012.315. The first report showing a long-lasting cellular immune response to sand fly salivary proteins in subjects living in endemic areas. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sudarshan M, Singh T, Singh AK, Chourasia A, Singh B, Wilson ME, et al. Quantitative PCR in epidemiology for early detection of visceral leishmaniasis cases in India. PLoS Negl Trop Dis. 2014;8(12):e3366. doi: 10.1371/journal.pntd.0003366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sudarshan M, Sundar S. Parasite load estimation by qPCR differentiates between asymptomatic and symptomatic infection in Indian visceral leishmaniasis. Diagn Microbiol Infect Dis. 2014;80(1):40–2. doi: 10.1016/j.diagmicrobio.2014.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Badaro R, Benson D, Eulalio MC, Freire M, Cunha S, Netto EM, et al. rK39: a cloned antigen of Leishmania chagasi that predicts active visceral leishmaniasis. J InfectDis. 1996;173(3):758–61. doi: 10.1093/infdis/173.3.758. [DOI] [PubMed] [Google Scholar]
- 72.Houghton RL, Petrescu M, Benson DR, Skeiky YA, Scalone A, Badaro R, et al. A cloned antigen (recombinant K39) of Leishmania chagasi diagnostic for visceral leishmaniasis in human immunodeficiency virus type 1 patients and a prognostic indicator for monitoring patients undergoing drug therapy. Journal of Infectious Diseases. 1998;177(5):1339–44. doi: 10.1086/515289. [DOI] [PubMed] [Google Scholar]
- 73.Qu JQ, Guan LR, Shulidan I, Zuo XP, Chai JJ, Chen SB, et al. Rapid screening with a recombinant antigen (rK39) for diagnosis of visceral leishmaniasis using dipstick. Zhongguo JiSheng ChongXueYu JiSheng ChongBingZa Zhi. 2000;18(3):155–8. [PubMed] [Google Scholar]
- 74.Braz RF, Nascimento ET, Martins DR, Wilson ME, Pearson RD, Reed SG, et al. The sensitivity and specificity of Leishmania chagasi recombinant K39 antigen in the diagnosis of American visceral leishmaniasis and in differentiating active from subclinical infection. The American journal of tropical medicine and hygiene. 2002;67(4):344–8. doi: 10.4269/ajtmh.2002.67.344. [DOI] [PubMed] [Google Scholar]
- 75.Zijlstra EE, Daifalla NS, Kager PA, Khalil EA, El-Hassan AM, Reed SG, et al. rK39 enzyme-linked immunosorbent assay for diagnosis of Leishmania donovani infection. Clinical and diagnostic laboratory immunology. 1998;5(5):717–20. doi: 10.1128/cdli.5.5.717-720.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pattabhi S, Whittle J, Mohamath R, El-Safi S, Moulton GG, Guderian JA, et al. Design, development and evaluation of rK28-based point-of-care tests for improving rapid diagnosis of visceral leishmaniasis. PLoS Negl Trop Dis. 2010;4(9) doi: 10.1371/journal.pntd.0000822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vallur AC, Duthie MS, Reinhart C, Tutterrow Y, Hamano S, Bhaskar KR, et al. Biomarkers for intracellular pathogens: establishing tools as vaccine and therapeutic endpoints for visceral leishmaniasis. Clin Microbiol Infect. 2014;20(6):O374–83. doi: 10.1111/1469-0691.12421. [DOI] [PMC free article] [PubMed] [Google Scholar]