Introduction
Pharmacological recanalization with recombinant tissue plasminogen activator (rt-PA) has been the mainstay for acute ischemic stroke (IS) treatment.1 Recent randomized controlled trials have additionally demonstrated the efficacy of mechanical thrombectomy (MT).2-4 While the restoration of blood flow is a major goal in acute treatment, if this occurs too late, worse damage can ensue, compared to no revascularization.5 This worsening results due to the generation of excess reactive oxygen species (ROS) which leads to direct cellular damage and indirect damage through the triggering of inflammation. Inflammation causes the generation of damaging immune mediators, effector molecules and more ROS.6 ROS can also lead to apoptosis/necrosis via DNA/RNA damage and lipid peroxidation. This cycle is known as reperfusion injury (R/I) (Figure). Experimental studies have shown that durations of more than 2-3h transient middle cerebral artery occlusion (tMCAO) lead to worsened injury compared to permanent MCAO.7 At the clinical level, delayed revascularization can sometimes lead to worsened outcomes.8 Hyperintense acute reperfusion marker (HARM) seen on MRI in some stroke patients has been associated with hemorrhagic transformation (HTf) and clinical worsening, suggesting the existence of R/I in humans.9 Hence, adjunctive treatments to recanalization to target R/I has the potential to improve current outcomes, while reducing complications of rt-PA.
Figure.
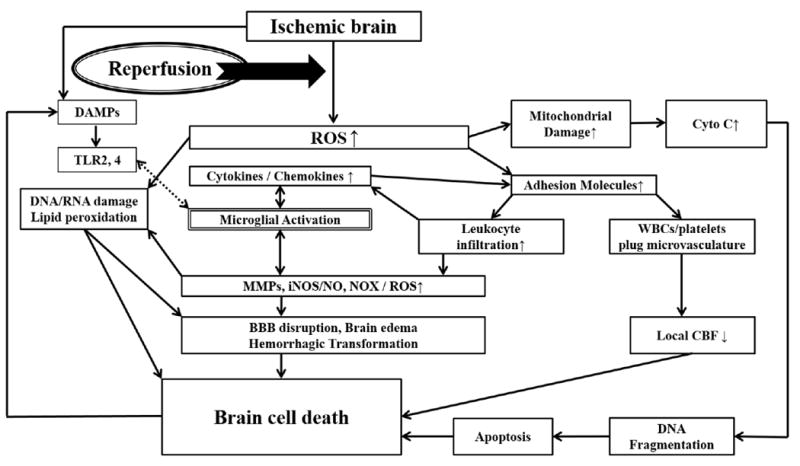
Reperfusion injury is thought to occur when a sudden influx of oxygenated blood introduces reactive oxygen species (ROS) into critically damaged ischemic brain. Ischemically damaged mitochondria become unable to efficiently neutralize ROS. Elevated ROS can directly damage DNA, RNA and cause lipid peroxidation. ROS lead to immune cell activation including brain resident microglia. Ischemic brain may also elaborate damage-associated molecular patterns (DAMPs) that act on Toll-like receptors (TLR) present on the surface of microglia. TLR activation triggers immune signalling with upregulation of cytokines and chemokines, which upregulate adhesion molecules involved in the recruitment and infiltration of circulating leukocytes. Once inside of the brain, leukocytes potentiate immune responses already established by microglia. Some leukocytes and platelets may remain in the intravascular space, and form plugs which compromises local blood flow. Activated immune cells elaborate various toxic mediators including matrix metalloproteinases (MMPs) which can disrupt the extracelluar matrix and blood brain barrier (BBB) leading to brain edema and haemorrhage. Other immune molecules include iNOS (inducible nitric oxide synthase) and NADPH oxidase-2 (NOX2) which generate nitric oxide (NO) and superoxide, respectively. Mitochondria also release pro-death factors such as cytochrome c (cyto c) which ultimately lead to DNA damage and apoptosis.
We will focus on the underlying mechanisms of R/I and laboratory studies which targeted these mechanisms in experimental ‘revascularization models’ such as tMCAO or thromboembolic stroke (Table 1).10-18 We will also review past and present clinical trials that attempt to study these targets, some in the setting of combined use with revascularization treatments (Table 2).19-30
Table 1.
Laboratory studies of antioxidant and anti-inflammatory combination therapies with rt-PA.
| Therapy (Author / Year) | Animal Model | Therapeutic Target | Treatment | Outcomes |
|---|---|---|---|---|
| Minocycline (Fan et al. 2013)10 | Thromboembolic stroke, rat | p38 MAPK | rt-PA alone (1.5h post emboliztion) vs rt-PA + minocycline 1h, post embolization) | Minocycline ↓ infarct volume, hemorrhage, & edema |
| BB-94 (Lapchak et al. 2000)11 | Large clot embolic Stroke, rabbit | MMPs | rt-PA (1h post embolization) vs. rt-PA + BB-94 5 min after embolization. | BB-94 ↓rt-PA-induced hemorrhage |
| EGCG (You et al. 2016)12 | Thromboembolic stroke, rat | MMPs, ROS | EGCG every 4h after embolization treated + rt-PA | EGCG ↓rt-PA extended therapeutic window, ↓ infarct volume, edema, & BBB disruption |
| Progranulin (Kanazawa et al. 2015)13 | Thromboembolic stroke, rat | inflammation | rt-PA (4h post MCAO), vs. PA +progranulin immediately before rt-PA rt- | Progranulin ↓infarct size, cerebral edema, rt-PA induced hemorrhagic transformation; improved motor outcome |
| G-CSF (dela Peña et al. 2015)14 | tMCAO x 1 h, rat | IL-1β, iNOS, apoptosis | IV rt-PA vs. rt-PA + G-CSF immediately before reperfusion | rt-PA+G-CSF ↓neurological deficit & hemorhhagic transformation vs rt-PA alone |
| NXY-059G (Lapchak et al. 2002)15 | Large clot embolic Stroke rabbit | ROS | NXY-059G 5min after embolization vs. rt-PA 1h after embolization + NXY-059G | NXY-059G + rt-PA ↓ rt-PA-induced hemorrhagic transformation and ↑behavioral function. |
| Uric Acid (Romanos et al. 2007)16 | Thromboembolic stroke, rat | ROS | rt-PA + uric acid 20 mins after occlusion | Uric acid+rt-PA ↓infarct volume, ↑neurological function |
| Edaravone (Yagi et al. 2009)17 | tMCAO x 3h, rat | ROS and MMP-9 | rt-PA alone vs rt-PA + edaravone immediately after reperfusion | Edaravone ↓ rt-PA-induced hemorrhagic transformation |
| Hypothermia (Tang et al. 2013)18 | tMCAO x 1 or 3 h, rat | multiple mechanisms | rt-PA 1h or 3h after ischemia vs. rt-PA plus cooling (33 °C) prior to or concurrent with rt-PA | hypothermia ↓infarct size, neurological deficits, brain hemorrhage, BBB disruption |
Abbreviations: rt-PA=recombinant tissue plasminogen activator; MMP=matrix metalloproteinase; tMCAO=transient middle cerebral artery occlusion; EGCG=epigallocatechin gallate; ROS=reactive oxygen species; BBB=blood brain barrier; G-CSF=granulocyte-colony stimulating factor; iNOS=inducible nitric oxide synthase;
Table 2.
Clinical studies of antioxidant and anti-inflammatory combination therapies with acute revascularization.
| Therapy/Trial | Study Design | Patients Number / inclusion criteria | Intervention Time | Treatments | Outcomes |
|---|---|---|---|---|---|
| Edaravone |
|
|
< 4.5hours |
|
Edaravone+rt-PA safe; timing of edaravone did not affect recanalization; edaravone+rt- PA may be superior to MT |
| Uric acid URICO-ICTUS22 | Randomized, double-blind, placebo-controlled, phase 2b/3 | n=411 (NIHSS>6, ≤25, premorbid mRS ≤2 | < 4.5hours | 1000 mg UA during rt-PA infusion | UA safe, did not affect outcomes from rt-PA treatment |
| Hypothermia |
|
|
|
|
|
| Verapamil SAVER-I26 | Phase I | n=11 (MT with a TICI 2A or better) | < 8hours | 10mg verapamil over 20 min into the previously occluded vessel | Combination is safe & feasible |
| Minocycline Kohler et al. 201327 | Multicenter, prospective, randomized, open-label, blinded, pilot study | n=95 | < 24hours | rt-PA vs. rt-PA +minocycline (100mg daily X 3d) | Minocycline safe but not efficacious |
| EGCG Wang et al. 201728 | Randomized, double-blind, placebo-controlled | n=371 (clearly defined time of onset, measurable NIHSS deficit, no HTf) | < 4.5hours | rt-PA vs. rt-PA + EGCG (500mg daily X 7d) | EGCG improved NIHSS; extended the time window for rt-PA |
| Fingolimod Zhu et al. 201529 | Randomized, open-label, evaluator-blind, multicenter pilot study | n=47 (NIHSS ≥5) | 4.5-72hours | rt-PA vs. rt-PA +fingolimod (0.5mg every 12h X 5d) | Fingolimod +rt-PA well tolerated; improved outcomes |
| Simvastatin STARS0730 | multicentre, phase IV, prospective, randomized, double-blind, placebo-controlled | n=104 (NIHSS 4-22, premorbid mRS 0-1; 55 patients received rt-PA therapy) | <12hours | rt-PA vs. rt-PA +simvastatin (40mg once daily X 90d) | Simvastatin+rt-PA safe & ↓HTf. |
Abbreviations: MCA, middle cerebral artery; rt-PA=recombinant tissue plasminogen activator; MT=mechanical thrombectomy; UA=uric acid; NIHSS=National Institutes of Health Stroke Scale; ASPECTS=Alberta Stroke Program Early CT Score; HTf=hemorrhagic transformation; TICI=Thrombolysis in Cerebral Infarction; EGCG=epigallocatechin gallate.
Anti-inflammatory approaches to reperfusion injury
Post-stroke inflammation has largely been thought to exacerbate ischemic injury. Several immune molecules to contribute to this worsening, including inflammatory cytokines, chemokines, and immune cell-produced reactive species. Immune cell activation is thought to first occur in microglia following release of molecules elaborated by ischemic brain cells. Leukocyte activation and infiltration into the brain soon follows. Several laboratory studies showed that preventing leukocyte infiltration led to better outcomes in stroke models, although its efficacy has not yet been shown clinically.31
Inflammation begins after stroke as ischemic brain cells elaborate molecules collectively known as damage-associated molecular patterns (DAMPs). These include high mobility group box-1 (HMGB-1), peroxiredoxin, purines, nucleotides such as ATP and UDP, and nucleic acid fragments.6, 31 DAMPs bind innate immune receptors such as Toll-like (TLRs) and purinergic receptors on microglia and leukocytes leading to their activation followed by activation of inflammatory transcription factors NF-κB and MAPK. Deficiency or pharmacological inhibition of these factors have largely been shown to protect against experimental stroke.32, 33 These factors give rise to cytokines, chemokines, adhesion molecules, matrix metalloproteinases-9 (MMP-9), inducible nitric oxide synthase (iNOS) and NADPH oxidase (NOX), leading to exacerbation of ischemic injury. Proinflammatory cytokine IL-1β has moved the farthest forward in terms of translation. In a phase II clinical trial, human recombinant IL-1 receptor antagonist (IL-1Ra), IL-1 β’s endogenous inhibitor, reduced infarct volume and improved neurological outcome at 3 months.34 T-cell releasing pro-inflammatory cytokines (IFN-γ, IL-17, and IL-23) have recently emerged as therapeutic targets. IL-17 promotes TNF-α, IL-1β, and MMP-9 expression, whereas IL-23 induces the expression of IL-17.35 Inhibiting these cytokines improves neurological outcome in experimental R/I. TNF-α-inducible protein 8-like 2 (TIPE2), expressed in microglia/macrophages following tMCAO, contributes to anti-inflammatory effects, and TIPE2-deficient mice subjected to tMCAO have exacerbated neurological and inflammatory outcomes.36 IL-10, IL-4 and TGF-β1 are anti-inflammatory cytokines, and all seem associated with improved neurological outcomes in stroke models.6, 37 MMP-9, which is expressed by immune cells, contributes to inflammation by disrupting the blood brain barrier (BBB). Furthermore, endogenous tissue plasminogen activator (t-PA) activates plasmin, which activates MMP-9. Thus, administration of rt-PA may accelerate hemorrhage and edema should it enter the brain, making MMP-9 a relevant target.38
Several anti-inflammatory treatments have been studied in stroke models, especially in a combination therapy with rt-PA. Minocycline, with its pleiotropic effects against cell death, improved neurological outcome and decreased rt-PA related HTf.10 One mechanism of minocycline’s protective effect may be its ability to suppress microglial activation by inhibiting p38-MAPK. Minocycline also improved BBB integrity via MMP inhibition.39 BB-94, a MMP-9 inhibitor, reduced rt-PA-induced HTf in a rabbit stroke model.11 However, MMPs are involved in neurovascular remodeling, and longterm inhibition may impede repair.40 Epigallocatechin gallate (EGCG), found in green tea, has gained interested for its antioxidant and neuroprotective properties. EGCG downregulated MMP-2 and MMP-9 while upregulating plasminogen activator inhibitor-1 (PAI-1) in stroke models.12 EGCG combined with rt-PA extended the therapeutic time window of rt-PA and reduced brain edema and BBB disruption.12 Progranulin (PGRN), a growth factor found in the brain, is thought to contribute anti-inflammatory and vasoprotective properties. It is particularly increased in microglia and endothelial cells after ischemia.13 rt-PA plus PGRN in a stroke model was shown to improve neurological outcomes and reduce brain hemorrhage and edema. Granulocyte-colony stimulating factor (G-CSF) may provide neuroprotection through anti-inflammatory effects.41 In a model of tMCAO, rt-PA plus G-CSF reduced HTf and improved of neurological function compared to rt-PA alone.14
Anti-oxidative/nitrosative approaches to reperfusion injury
Reperfusion after IS induces oxidative stress through the mitochondrial respiratory chain (MRC) and NOX. Ischemic mitochondria, overwhelmed by ROS introduced by oxygenated blood, become unable to efficiently neutralize these species. Overexpression of endogenous antioxidant enzymes superoxide dismutase (SOD), glutathione peroxidase and catalase have been shown to improve outcome from experimental stroke. Transgenic mice overexpressing SOD had significantly reduced infarct size in experimental tMCAO, while SOD-deficient mice had worsened outcomes.42 Overexpressing other endogenous antioxidants such as glutathione peroxidase and catalase were similarly neuroprotective.43 At the clinical level, ebselen, a glutathione peroxidase mimic, improved neurological outcomes in IS patients treated within 6h of symptom onset.44
Superoxide generated by immune cells occurs via NOX, leading to more oxidative stress.45 NOX inhibition has been shown to improve outcome in experimental stroke45, but NOX may also play an important role in hyperglycemia-induced stroke exacerbation. Glucose can be metabolized via the hexose monophosphate shunt to produce NADPH, thereby providing substrate to generate NOX. Apocynin, a NOX inhibitor, improved outcome from experimental hyperglycemic tMCAO46, and reduced hyperglycemia-induced worsening of BBB disruption and HTf due to rt-PA use.47
Other related strategies have been investigated over the years. Free radical scavengers such as tirilazad and 4-benzene-1,3-disulphonate N-oxide (NXY-059) plus rt-PA showed efficacy in experimental stroke15, but were negative when studied clinically.48, 49 Postsynaptic density-95 (PSD-95) protein is associated with the N-methyl-D-aspartate receptor (NMDAR). It recruits neuronal NOS (nNOS), responsible for generating neurotoxic NO.50 NA-1 inhibits PSD-95 and improves outcome in experimental R/I. Insulin-like growth factor (IGF-1), a pleiotropic peptide involved in pro-survival signaling, may also inhibit oxidative and nitrosative stress.51 Uric acid (UA), through its antioxidant properties, improved outcomes in a thromboembolic stroke model.16 ROS scavenger edaravone also improved neurological outcome in experimental models in combination with rt-PA17, and is used clinically in Japan for acute IS.19
Anti-apoptotic approach to reperfusion injury
ROS-mediated damage leads to apoptosis, and may be especially relevant to R/I, as apoptosis requires the presence of cellular energy stores to drive cell death.52 Mitochondrial initiation of apoptosis is referred to as the intrinsic pathway. This occurs when mitochondria release cytochrome c to the cytosol, and forms a complex with apoptotic protease-activating factor 1 (APAF-1) and pro-caspase-9 to form the apoptosome.53 The apoptosome activates caspase-9 and activates effector caspase-3 which promotes DNA cleavage.52 SOD-overexpressing mice showed reduced apoptosis and cytochrome c translocation in R/I53, as did blocking both second mitochondria-derived activator of caspase/direct IAP-binding protein of low pI (Smac/DIABLO) and Omi stress-regulated endoprotease/high temperature requirement protein A2 (Omi/HtrA2).54
Bcl-2 family molecules involved in apoptosis include BAX, BAD, and BID which trigger cytochrome c release, whereas Bcl-2 and Bcl-XL prevent it.52 Changing the balance of these molecules to favor anti-apoptotic isoforms has been shown to improve outcome in experimental stroke. The extrinsic apoptotic pathway is activated when death receptors are bound by their ligands. The best studied of these receptor-ligand pairs is Fas/FasL. FasL ligates Fas and leads to caspase-8 activation, followed by eventual caspase-3 activation and DNA cleavage.52 Mice with Fas mutations seem protected from R/I.55
Finally, estrogen is known to factor in IS. Female animals are known to have better outcomes after experimental stroke compared to male, and 17- β estradiol (E2)’s protective effect seems related to Bcl-2 upregulation, and suppression of apoptosis.56 Thus, neuroprotective effects for sex-specific steroids may be linked to the modulation of apoptosis.
Clinical studies of combination therapy with revascularization
While several drugs have been studied in combination with rt-PA in experimental stroke models to prevent R/I, clinical studies have also been carried out in combination with rt-PA and/or MT. Although not specifically designed to target reperfusion injury, these studies may provide a framework to design future trials.
Combination treatment with rt-PA/MT plus edaravone is already being used clinically in Japan. Although lacking control groups, two observational studies were suggestive. PROTECT4.5 evaluated edaravone plus rt-PA, and suggested that this combination might increase the chances for better outcomes and reduce HTf.19 YAMATO indicated that favorable outcomes after rt-PA were not related to the timing of edaravone infusion.20 Finally, a subanalysis of a stroke registry (RESCUE Japan Registry) indicated that edaravone was more effective in patients treated with rt-PA than MT.21
The URICO-ICTUS trial, which assessed the efficacy of UA plus rt-PA/MT in acute IS, confirmed safety in patients treated within 4.5h of symptom onset. This study also reported that UA was associated with reduced infarct growth and improved outcome in IS patients with early recanalization and hyperglycemia.16 Efficacy was also demonstrated when UA was given in addition to MT.22
Therapeutic hypothermia (TH), which is thought to target multiple R/I mechanisms, is already indicated to improve neurological outcomes following cardiac arrest and neonatal hypoxia-ischemia.57 In combination with rt-PA in experimental tMCAO, TH led to reduced BBB disruption and HTf.18 The ReCCLAIM (Reperfusion and Cooling in Cerebral Acute Ischemia)23 and ICTuS224 trials examined this combination in stroke patients, and both showed that this approach was safe and feasible, although RECCLAIM-II, which additionally examined MT, was stopped early for lack of funding.25 Pilot studies of MT plus selective brain cooling via intra-arterial chilled saline infusion are ongoing, and appear feasible and safe.58
The ACTION-I trial studied safety and efficacy of natalizumab in acute IS. Natalizumab, an antibody to α4 integrin is used in multiple sclerosis. Natalizumab is thought to reduce lymphocyte invasion and adhesion molecule upregulation. Although nataliizumab failed to show efficacy in experimental stroke59, ACTION-I included patients receiving rt-PA.60 Infarct volume was not significantly different with the addition of natalizumab, but neurological outcomes were improved.
Other anti-inflammatory and anti-oxidant drugs, all of which are used clinically for other indications, have also been studied clinically in combination with rt-PA. The phase I trial, Superselective Administration of VErapamil During Recanalization in Acute Ischemic Stroke (SAVER-I) studied the therapeutic potential of verapamil with rt-PA and MT and found that this combination was safe.26 Similarly, minocycline with rt-PA appears safe, although efficacy is unclear.27 EGCG plus rt-PA seemed to extend the temporal therapeutic window of rt-PA and improve outcome.28 Oral fingolimod in a small study indicated that it could be given safely with improved neurological recovery at 90d61, while a pilot study of fingolimod plus rt-PA demonstrated safety and trends towards favorable clinical outcomes and reduced HTf.29 Recently, the Stroke Treatment with Acute Reperfusion and Simvastatin (STARS07) trial, which was a phase IV trial to demonstrate the efficacy and safety of simvastatin treatment in acute stroke, showed that simvastatin plus rt-PA were safe and reduced HTf.30
There are currently several ongoing trials specifically evaluating the safety and efficacy of revascularization plus neuroprotection. Compared to earlier trials, these studies directly examined whether adjunctive treatments to rt-PA and/or MT improve outcome, and may inadvertently study R/I. Activated protein C (APC), which is thought to suppress inflammation and prevent BBB disruption, is being studied in combination with rt-PA/MT in The “Safety Evaluation of 3K3A-APC in Ischemic Stroke (RHAPSODY)” trial (NCT02222714).62 Another clinical trial of atorvastatin combined with MT, The Safety and Efficacy Study of High Dose Atorvastatin After Thrombolytic Treatment in Acute Ischemic Stroke (SEATIS)” trial (NCT02452502) is also ongoing.63 Combined therapy with NA-1 and MT in the “Safety and Efficacy of NA-1 in Subjects Undergoing Endovascular Thrombectomy for Stroke (ESCAPE-NA1)” trial (NCT02930018) plans to assess safety and efficacy. Although it did not limit enrollment to IS patients receiving rt-PA/MT, ACTION II (NCT02730455) evaluates the safety and efficacy of intravenous natalizmab. Finally, “Fingolimod with Alteplase bridging with Mechanical Thrombectomy in Acute Ischemic Stroke (FAMTAIS, NCT02956200)”, a phase II trial of bridging therapy (fingolimod plus rt-PA/MT), was recently started to assess safety and efficacy in large vessel occlusion.64
Conclusions
The broad field of R/I in acute stroke may identify potential treatment targets and lead to clinical translation. The phenomenon of R/I is well established in the laboratory. While it is less clear clinically, recent advances in acute revascularization may make it possible to establish whether this occurs in humans. Regardless, pharmacological thrombolysis carries an increased risk of brain hemorrhage, and adjunctive therapies against the same targets that contribute to reperfusion injury in the laboratory could reduce HTf, lengthen the time window for intervention and further improve outcomes. We propose that such approaches may also increase the numbers of stroke patients eligible for treatment.
Acknowledgments
Funding Sources: This work was supported by grants from the Veteran’s Merit Award I01 BX000589 and NIH National Institute of Neurological Disorders and Stroke R03 NS101246 (to MY), SENSHIN Medical Research Foundation (to AM), and the Basic Science Research Program of the National Research Foundation of Korea (NRF) funded by the Ministry of Science, Information and Communications Technology (ICT) & Future Planning NRF-2015R1C1A1A01054641(to JY). Grants to MY were administered by the Northern California Institute for Research and Education, and supported by resources from the Veterans Affairs Medical Center, San Francisco, California.
Footnotes
Conflict of Interest
The authors state that they have no conflict of interest.
Author Contributions
This manuscript was written by AM and JY. MY developed the outline, made revisions and approved the final version.
Disclosures
None.
References
- 1.Wardlaw JM, Murray V, Berge E, del Zoppo GJ. Thrombolysis for acute ischaemic stroke. Cochrane Database Syst Rev. 2014;7 doi: 10.1002/14651858.CD000213.pub3. CD000213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goyal M, Menon BK, van Zwam WH, Dippel DW, Mitchell PJ, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387:1723–1731. doi: 10.1016/S0140-6736(16)00163-X. [DOI] [PubMed] [Google Scholar]
- 3.Nogueira RG, Jadhav AP, Haussen DC, Bonafe A, Budzik RF, Bhuva P, et al. Thrombectomy 6 to 24 Hours after Stroke with a Mismatch between Deficit and Infarct. N Engl J Med. 2018;378:11–21. doi: 10.1056/NEJMoa1706442. [DOI] [PubMed] [Google Scholar]
- 4.Albers GW, Marks MP, Kemp S, Christensen S, Tsai JP, Ortega-Gutierrez S, et al. Thrombectomy for Stroke at 6 to 16 Hours with Selection by Perfusion Imaging. N Engl J Med. 2018;378:708–718. doi: 10.1056/NEJMoa1713973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aronowski J, Strong R, Grotta JC. Reperfusion injury: demonstration of brain damage produced by reperfusion after transient focal ischemia in rats. J Cereb Blood Flow Metab. 1997;17:1048–1056. doi: 10.1097/00004647-199710000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Amantea D, Micieli G, Tassorelli C, Cuartero MI, Ballesteros I, Certo M, et al. Rational modulation of the innate immune system for neuroprotection in ischemic stroke. Front Neurosci. 2015;9:147. doi: 10.3389/fnins.2015.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang GY, Betz AL. Reperfusion-induced injury to the blood-brain barrier after middle cerebral artery occlusion in rats. Stroke. 1994;25:1658–1664. doi: 10.1161/01.str.25.8.1658. [DOI] [PubMed] [Google Scholar]
- 8.Lees KR, Bluhmki E, von Kummer R, Brott TG, Toni D, Grotta JC, et al. Time to treatment with intravenous alteplase and outcome in stroke: an updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials. Lancet. 2010;375:1695–1703. doi: 10.1016/S0140-6736(10)60491-6. [DOI] [PubMed] [Google Scholar]
- 9.Warach S, Latour LL. Evidence of reperfusion injury, exacerbated by thrombolytic therapy, in human focal brain ischemia using a novel imaging marker of early blood-brain barrier disruption. Stroke. 2004;35:2659–2661. doi: 10.1161/01.STR.0000144051.32131.09. [DOI] [PubMed] [Google Scholar]
- 10.Fan X, Lo EH, Wang X. Effects of minocycline plus tissue plasminogen activator combination therapy after focal embolic stroke in type 1 diabetic rats. Stroke. 2013;44:745–752. doi: 10.1161/STROKEAHA.111.000309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lapchak PA, Chapman DF, Zivin JA. Metalloproteinase inhibition reduces thrombolytic (tissue plasminogen activator)-induced hemorrhage after thromboembolic stroke. Stroke. 2000;31:3034–3040. doi: 10.1161/01.str.31.12.3034. [DOI] [PubMed] [Google Scholar]
- 12.You YP. Epigallocatechin Gallate Extends the Therapeutic Window of Recombinant Tissue Plasminogen Activator Treatment in Ischemic Rats. J Stroke Cerebrovasc Dis. 2016;25:990–997. doi: 10.1016/j.jstrokecerebrovasdis.2016.01.014. [DOI] [PubMed] [Google Scholar]
- 13.Kanazawa M, Kawamura K, Takahashi T, Miura M, Tanaka Y, Koyama M, et al. Multiple therapeutic effects of progranulin on experimental acute ischaemic stroke. Brain. 2015;138:1932–1948. doi: 10.1093/brain/awv079. [DOI] [PubMed] [Google Scholar]
- 14.dela Peña IC, Yoo A, Tajiri N, Acosta SA, Ji X, Kaneko Y, et al. Granulocyte colony-stimulating factor attenuates delayed tPA-induced hemorrhagic transformation in ischemic stroke rats by enhancing angiogenesis and vasculogenesis. J Cereb Blood Flow Metab. 2015;35:338–346. doi: 10.1038/jcbfm.2014.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lapchak PA, Araujo DM, Song D, Wei J, Purdy R, et al. Effects of the spin trap agent disodium- [tert-butylimino]methyl]benzene-1,3-disulfonate N-oxide (generic NXY-059) on intracerebral hemorrhage in a rabbit Large clot embolic stroke model: combination studies with tissue plasminogen activator. Stroke. 2002;33:1665–1670. doi: 10.1161/01.str.0000017145.22806.aa. [DOI] [PubMed] [Google Scholar]
- 16.Romanos E, Planas AM, Amaro S, Chamorro A. Uric acid reduces brain damage and improves the benefits of rt-PA in a rat model of thromboembolic stroke. J Cereb Blood Flow Metab. 2007;27:14–20. doi: 10.1038/sj.jcbfm.9600312. [DOI] [PubMed] [Google Scholar]
- 17.Yagi K, Kitazato KT, Uno M, Tada Y, Kinouchi T, Shimada K, et al. Edaravone, a free radical scavenger, inhibits MMP-9-related brain hemorrhage in rats treated with tissue plasminogen activator. Stroke. 2009;40:626–631. doi: 10.1161/STROKEAHA.108.520262. [DOI] [PubMed] [Google Scholar]
- 18.Tang XN, Liu L, Koike MA, Yenari MA. Mild hypothermia reduces tissue plasminogen activator-related hemorrhage and blood brain barrier disruption after experimental stroke. Ther Hypothermia Temp Manag. 2013;3:74–83. doi: 10.1089/ther.2013.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamaguchi T, Awano H, Matsuda H, Tanahashi N PROTECT4.5 Investigators. Edaravone with and without .6 Mg/Kg Alteplase within 4.5 Hours after Ischemic Stroke: A Prospective Cohort Study (PROTECT4.5) J Stroke Cerebrovasc Dis. 2017;26:756–765. doi: 10.1016/j.jstrokecerebrovasdis.2016.10.011. [DOI] [PubMed] [Google Scholar]
- 20.Aoki J, Kimura K, Morita N, Harada M, Metoki N, Tateishi Y, et al. YAMATO Study (Tissue-Type Plasminogen Activator and Edaravone Combination Therapy) Stroke. 2017;48:712–719. doi: 10.1161/STROKEAHA.116.015042. [DOI] [PubMed] [Google Scholar]
- 21.Miyaji Y, Yoshimura S, Sakai N, Yamagami H, Egashira Y, Shirakawa M, et al. Effect of edaravone on favorable outcome in patients with acute cerebral large vessel occlusion: subanalysis of RESCUE-Japan Registry. Neurol Med Chir (Tokyo) 2015;55:241–247. doi: 10.2176/nmc.ra.2014-0219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Amaro S, Llull L, Renú A, Laredo C, Perez B, Vila E, et al. Uric acid improves glucose-driven oxidative stress in human ischemic stroke. Ann Neurol. 2015;77:775–783. doi: 10.1002/ana.24378. [DOI] [PubMed] [Google Scholar]
- 23.Horn CM, Sun CH, Nogueira RG, Patel VN, Krishnan A, Glenn BA, et al. Endovascular Reperfusion and Cooling in Cerebral Acute Ischemia (ReCCLAIM I) J Neurointerv Surg. 2014;6:91–95. doi: 10.1136/neurintsurg-2013-010656. [DOI] [PubMed] [Google Scholar]
- 24.Lyden P, Hemmen T, Grotta J, Rapp K, Ernstrom K, Rzesiewicz T, et al. Results of the ICTuS 2 Trial (Intravascular Cooling in the Treatment of Stroke 2) Stroke. 2016;47:2888–2895. doi: 10.1161/STROKEAHA.116.014200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.REperfusion with Cooling in CerebraL Acute IscheMia II “RECCLAIM-II”. [April 17 2018];The internet stroke center. http://www.strokecenter.org/trials/clinicalstudies/reperfusion-with-cooling-in-cerebral-acute-ischemia-ii--2.
- 26.Fraser JF, Maniskas M, Trout A, Lukins D, Parker L, Stafford WL, et al. Intra-arterial verapamil post-thrombectomy is feasible, safe, and neuroprotective in stroke. J Cereb Blood Flow Metab. 2017;37:3531–3543. doi: 10.1177/0271678X17705259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kohler E, Prentice DA, Bates TR, Hankey GJ, Claxton A, van Heerden J, et al. Intravenous minocycline in acute stroke: a randomized, controlled pilot study and meta-analysis. Stroke. 2013;44:2493–2499. doi: 10.1161/STROKEAHA.113.000780. [DOI] [PubMed] [Google Scholar]
- 28.Wang XH, You YP. Epigallocatechin Gallate Extends Therapeutic Window of Recombinant Tissue Plasminogen Activator Treatment for Brain Ischemic Stroke: A Randomized Double-Blind and Placebo-Controlled Trial. Clin Neuropharmacol. 2017;40:24–28. doi: 10.1097/WNF.0000000000000197. [DOI] [PubMed] [Google Scholar]
- 29.Zhu Z, Fu Y, Tian D, Sun N, Han W, Chang G, et al. Combination of the Immune Modulator Fingolimod With Alteplase in Acute Ischemic Stroke: A Pilot Trial. Circulation. 2015;132:1104–1112. doi: 10.1161/CIRCULATIONAHA.115.016371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Montaner J, Bustamante A, García-Matas S, Martínez-Zabaleta M, Jiménez C, de la Torre J, et al. Combination of Thrombolysis and Statins in Acute Stroke Is Safe: Results of the STARS Randomized Trial (Stroke Treatment With Acute Reperfusion and Simvastatin) Stroke. 2016;47:2870–2873. doi: 10.1161/STROKEAHA.116.014600. [DOI] [PubMed] [Google Scholar]
- 31.Danton GH, Dietrich WD. Inflammatory mechanisms after ischemia and stroke. J Neuropathol Exp Neurol. 2003;62:127–136. doi: 10.1093/jnen/62.2.127. [DOI] [PubMed] [Google Scholar]
- 32.Harari OA, Liao JK. NF-κB and innate immunity in ischemic stroke. Ann N Y Acad Sci. 2010;1207:32–40. doi: 10.1111/j.1749-6632.2010.05735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun J, Nan G. The Mitogen-Activated Protein Kinase (MAPK) Signaling Pathway as a Discovery Target in Stroke. J Mol Neurosci. 2016;59:90–98. doi: 10.1007/s12031-016-0717-8. [DOI] [PubMed] [Google Scholar]
- 34.Emsley HC, Smith CJ, Georgiou RF, Vail A, Hopkins SJ, Rothwell NJ, et al. Acute Stroke Investigators. A randomised phase II study of interleukin-1 receptor antagonist in acute stroke patients. J Neurol Neurosurg Psychiatry. 2005;76:1366–1372. doi: 10.1136/jnnp.2004.054882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yago T, Nanke Y, Kawamoto M, Furuya T, Kobashigawa T, Kamatani N, et al. IL-23 induces human osteoclastogenesis via IL-17 in vitro, and anti-IL-23 antibody attenuates collagen-induced arthritis in rats. Arthritis Res Ther. 2007;9:R96. doi: 10.1186/ar2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang Y, Wei X, Liu L, Liu S, Wang Z, Zhang B, et al. TIPE2, a novel regulator of immunity, protects against experimental stroke. J Biol Chem. 2012;287:32546–32555. doi: 10.1074/jbc.M112.348755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao X, Wang H, Sun G, Zhang J, Edwards NJ, Aronowski J. Neuronal Interleukin-4 as a Modulator of Microglial Pathways and Ischemic Brain Damage. J Neurosci. 2015;35:11281–11291. doi: 10.1523/JNEUROSCI.1685-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chaturvedi M, Kaczmarek L. Mmp-9 inhibition: a therapeutic strategy in ischemic stroke. Mol Neurobiol. 2014;49:563–573. doi: 10.1007/s12035-013-8538-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Machado LS, Kozak A, Ergul A, Hess DC, Borlongan CV, Fagan SC. Delayed minocycline inhibits ischemia-activated matrix metalloproteinases 2 and 9 after experimental stroke. BMC Neurosci. 2006;7:56. doi: 10.1186/1471-2202-7-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang Y, Rosenberg GA. Matrix metalloproteinases as therapeutic targets for stroke. Brain Res. 2015;1623:30–38. doi: 10.1016/j.brainres.2015.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abe K, Yamashita T, Takizawa S, Kuroda S, Kinouchi H, Kawahara N. Stem cell therapy for cerebral ischemia: from basic science to clinical applications. J Cereb Blood Flow Metab. 2012;32:1317–1331. doi: 10.1038/jcbfm.2011.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nizuma K, Endo H, Chan PH. Oxidative stress and mitochondrial dysfunction as determinants of ischemic neuronal death and survival. J Neurochem. 2009;109:133–138. doi: 10.1111/j.1471-4159.2009.05897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Armogida M, Nistico R, Mercuri NB. Therapeutic potential of targeting hydrogen peroxide metabolism in the treatment of brain ischaemia. Br J Pharmacol. 2012;166:1211–1124. doi: 10.1111/j.1476-5381.2012.01912.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ogawa A, Yoshimoto T, Kikuchi H, Sano K, Saito I, Yamaguchi T, et al. Ebselen in acute middle cerebral artery occlusion: a placebo-controlled, double-blind clinical trial. Cerebrovasc Dis. 1999;9:112–118. doi: 10.1159/000015908. [DOI] [PubMed] [Google Scholar]
- 45.Kahles T, Brandes RP. NADPH oxidases as therapeutic targets in ischemic stroke. Cell Mol Life Sci. 2012;69:2345–2363. doi: 10.1007/s00018-012-1011-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Suh SW, Shin BS, Ma H, Van Hoecke M, Brennan AM, Yenari MA, et al. Glucose and NADPH oxidase drive neuronal superoxide formation in stroke. Ann Neurol. 2008;64:654–663. doi: 10.1002/ana.21511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Won SJ, Tang XN, Suh SW, Yenari MA, Swanson RA. Hyperglycemia promotes tissue plasminogen activator-induced hemorrhage by Increasing superoxide production. Ann Neurol. 2011;70:583–590. doi: 10.1002/ana.22538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chamorro A, Dirnagl U, Urra X, Planas AM. Neuroprotection in acute stroke: targeting excitotoxicity, oxidative and nitrosative stress, and inflammation. Lancet Neurol. 2016;15:869–881. doi: 10.1016/S1474-4422(16)00114-9. [DOI] [PubMed] [Google Scholar]
- 49.Green AR, Ashwood T. Free radical trapping as a therapeutic approach to neuroprotection in stroke: experimental and clinical studies with NXY-059 and free radical scavengers. Curr Drug Targets CNS Neurol Disord. 2005;4:109–118. doi: 10.2174/1568007053544156. [DOI] [PubMed] [Google Scholar]
- 50.Instrum R, Sun HS. Restoring neuroprotection through a new preclinical paradigm: translational success for NA-1 in stroke therapy. Acta Pharmacol Sin. 2013;34:3–5. doi: 10.1038/aps.2012.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kooijman R, Sarre S, Michotte Y, De Keyser J. Insulin-like growth factor I: a potential neuroprotective compound for the treatment of acute ischemic stroke? Stroke. 2009;40:e83–88. doi: 10.1161/STROKEAHA.108.528356. [DOI] [PubMed] [Google Scholar]
- 52.Broughton BRS, Reutens DC, Sobey CG. Apoptotic Mechanisms after Cerebral ischemia. Stroke. 2009;40:e331–339. doi: 10.1161/STROKEAHA.108.531632. [DOI] [PubMed] [Google Scholar]
- 53.Fujimura M, Morita-Fujimura Y, Noshita N, Sugawara T, Kawase M, et al. The cytosolic antioxidant copper/zinc-superoxide dismutase prevents the early release of mitochondrial cytochrome c in ischemic brain after transient focal cerebral ischemia in mice. J Neurosci. 2000;20:2817–2824. doi: 10.1523/JNEUROSCI.20-08-02817.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Althaus J, Siegelin MD, Dehghani F, Cilenti L, Zervos AS, Rami A. The serine protease Omi/HtrA2 is involved in XIAP cleavage and in neuronal cell death following focal cerebral ischemia/reperfusion. Neurochem Int. 2007;50:172–180. doi: 10.1016/j.neuint.2006.07.018. [DOI] [PubMed] [Google Scholar]
- 55.Rosenbaum DM, Gupta G, D’Amore J, Singh M, Weidenheim K, Zhang H, et al. Fas (CD95/APO-1) plays a role in the pathophysiology of focal cerebral ischemia. J Neurosci Res. 2000;61:686–692. doi: 10.1002/1097-4547(20000915)61:6<686::AID-JNR12>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 56.Cardona-Gomez GP, Mendez P, DonCarlos LL, Azcoitia I, Garcia-Sequra LM. Interactions of estrogens and insulin-like growth factor-I in the brain: implications for neuroprotection. Brain Res Brain Res Rev. 2001;37:320–334. doi: 10.1016/s0165-0173(01)00137-0. [DOI] [PubMed] [Google Scholar]
- 57.Yenari MA, Han HS. Neuroprotective mechanisms of hypothermia in brain ischaemia. Nat Rev Neurosci. 2012;13:267–278. doi: 10.1038/nrn3174. [DOI] [PubMed] [Google Scholar]
- 58.Chen J, Liu L, Zhang H, Geng X, Jiao L, Li G, et al. Endovascular Hypothermia in Acute Ischemic Stroke: Pilot Study of Selective Intra-Arterial Cold Saline Infusion. Stroke. 2016;47:1933–1935. doi: 10.1161/STROKEAHA.116.012727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Langhauser F, Kraft P, Göb E, Leinweber J, Schuhmann MK, Lorenz K, et al. Blocking of α4 integrin does not protect from acute ischemic stroke in mice. Stroke. 2014;45:1799–1806. doi: 10.1161/STROKEAHA.114.005000. [DOI] [PubMed] [Google Scholar]
- 60.Elkins J, Veltkamp R, Montaner J, Johnston SC, Singhal AB, Becker K, et al. Safety and efficacy of natalizumab in patients with acute ischaemic stroke (ACTION): a randomised, placebo-controlled, double-blind phase 2 trial. Lancet Neurol. 2017;16:217–226. doi: 10.1016/S1474-4422(16)30357-X. [DOI] [PubMed] [Google Scholar]
- 61.Fu Y, Zhang N, Ren L, Yan Y, Sun N, Li YJ, et al. Impact of an immune modulator fingolimod on acute ischemic stroke. Proc Natl Acad Sci U S A. 2014;111:18315–18320. doi: 10.1073/pnas.1416166111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Amar AP, Griffin JH, Zlokovic BV. Combined neurothrombectomy or thrombolysis with adjunctive delivery of 3K3A-activated protein C in acute ischemic stroke. Front Cell Neurosci. 2015;9:344. doi: 10.3389/fncel.2015.00344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cimino M, Gelosa P, Gianella A, Nobili E, Tremoli E, Sironi L. Statins: multiple mechanisms of action in the ischemic brain. Neuroscientist. 2007;13:208–213. doi: 10.1177/1073858406297121. [DOI] [PubMed] [Google Scholar]
- 64.Zhang S, Zhou Y, Zhang R, Zhang M, Campbell B, Lin L, et al. Rationale and design of combination of an immune modulator Fingolimod with Alteplase bridging with Mechanical Thrombectomy in Acute Ischemic Stroke (FAMTAIS) trial. Int J Stroke. 2017;12:906–909. doi: 10.1177/1747493017710340. [DOI] [PubMed] [Google Scholar]


