Abstract
Purpose of Review
Immune-mediated necrotizing myopathy (IMNM) is a type of autoimmune myopathy characterized by relatively severe proximal weakness, myofiber necrosis with minimal inflammatory cell infiltrate on muscle biopsy, and infrequent extra-muscular involvement. Here, we will review the characteristics of patients with IMNM.
Recent Findings
Anti-signal recognition particle (SRP) and anti-hydroxy-3-methylglutaryl-CoA reductase (HMGCR) autoantibodies are closely associated with IMNM and define unique subtypes of patients. Importantly, the new European Neuromuscular Centre criteria recognize anti-SRP myopathy, anti-HMGCR myopathy, and autoantibody-negative IMNM as three distinct subtypes of IMNM. Anti-SRP myopathy patients have more severe muscle involvement, have more common extra-muscular features, and may respond best to immunosuppressive regimens that include rituximab. In contrast, anti-HMGCR myopathy is often associated with statin exposure and intravenous immunoglobulin treatment may be an effective treatment, even as monotherapy. Both anti-SRP and anti-HMGCR myopathy tend to be most severe in younger patients. Furthermore, children with these forms of IMNM may present with dystrophy-like features which are potentially reversible with immunosuppressant treatment. IMNM patients with either autoantibody may experience fatty replacement of muscle soon after disease onset, suggesting that intense and early immunosuppressant therapy may provide the best chance to avoid long-term disability.
Summary
IMNM is composed of anti-SRP myopathy, anti-HMGCR myopathy, and autoantibody-negative IMNM. Both anti-SRP and anti-HMGCR myopathy can cause severe weakness, especially in younger patients. Anti-SRP myopathy patients tend to have the most severe weakness and most prevalent extra-muscular features. Autoantibody-negative IMNM remains poorly described.
Keywords: Myositis, Autoantibodies, Signal recognition particle, HMGCR protein, human, Necrotizing myositis, Polymyositis
Introduction
The inflammatory myopathies are a heterogeneous family of diseases characterized by muscle weakness, elevated serum muscle enzyme levels, autoantibodies, and inflammatory muscle biopsies. Dermatomyositis (DM), the antisynthetase syndrome, and inclusion body myositis (IBM) have long been recognized as distinct subtypes of inflammatory myopathy; each has prominent lymphocytic infiltrates on muscle biopsy [1]. However, in the last 15 years, it has been acknowledged that muscle biopsies from some patients with myositis have significant myofiber necrosis and minimal, if any, lymphocytic infiltrates. These patients are now widely recognized to have immune-mediated necrotizing myopathy (IMNM), sometimes referred to as necrotizing autoimmune myopathy (NAM), a distinct type of myositis included in most recent classification schemes for the inflammatory myopathies [2, 3••, 4].
Autoantibodies have proven useful to define distinct subtypes of myositis patients that share immunogenetic markers, environmental exposures, clinical features, histological features, and prognosis. To date, two different autoantibodies have been described in association with IMNM, those recognizing the signal recognition particle (SRP) and those targeting hydroxy-3-methylglutaryl-CoA reductase (HMGCR). The SRP complex is composed of a 7S RNA and six protein subunits with molecular weights of 9, 14, 19, 54, 68, and 72 kDa. The function of the SRP complex is to recognize and target proteins to the endoplasmic reticulum. Autoantibodies recognizing the SRP were first identified in the 1980s [5, 6] by RNA immunoprecipitation using serum from myositis patients. Using this technique, it was found that all patients with anti-SRP immunoreactivity immunoprecipitated the 7S RNA associated with the SRP complex. Further studies demonstrated that autoantibodies from most, but not all, anti-SRP-positive patients recognize the 54-kDa protein of the SRP complex. Many or all of the rest of the protein subunits of the SRP complex could also be immunoprecipitated from most patients [6, 7].
Anti-HMGCR autoantibodies were discovered in 2010 by screening serum from “autoantibody-negative” necrotizing myositis patients for novel immunospecificities [8]. It was initially found that serum from some of these patients immunoprecipitated 100- and 200-kDa proteins from HeLa cell extracts. The 100-kDa protein was subsequently identified as the monomeric form of HMGCR, the rate-controlling enzyme of the cholesterol pathway. HMGCR dimers are thought to form the 200-kDa protein [9].
Previous myositis classification criteria relied exclusively on muscle biopsy features to categorize patients as having IMNM [2]. However, it is now appreciated that predominant necrosis on muscle biopsy may be found in a significant number of patients with other types of myositis. For example, necrosis is the predominant feature in 16% of those with DM [10], 15% of those with anti-Jo1-positive antisynthetase syndrome [10], and 21% of those with scleroderma-myositis [11], as well as some hereditary myopathy patients [12]. Conversely, 15–30% of patients with anti-SRP or anti-HMGCR myopathy have significant perivascular infiltrates in their muscle biopsies, but are otherwise clinically indistinguishable from other IMNM patients [8, 13••]. Given the current limitations of the muscle biopsy to classify patients accurately and the utility of autoantibodies to define relatively homogeneous subsets of patients, myositis-specific autoantibodies are increasingly relied upon as disease subtype-defining markers. Following this trend, the most recent European Neuromuscular Centre (ENMC) criteria for IMNM (2017) divide this syndrome into three subtypes: anti-SRP myopathy, anti-HMGCR myopathy, and antibody-negative IMNM [3••]. The presence of elevated creatine kinase (CK) levels and proximal weakness is sufficient to diagnose the disease subtype in those patients that are positive for anti-SRP or anti-HMGCR autoantibodies; for those that are autoantibody-negative, it is necessary to obtain a muscle biopsy to demonstrate the characteristic features of a necrotizing myopathy (Table 1) [3••]. Consistent with the latest ENMC criteria, we will recognize these three subtypes of IMNM as separate entities, focusing our attention on the two best-characterized subtypes, anti-SRP and anti-HMGCR myopathy.
Table 1.
2017 ENMC criteria for immune-mediated necrotizing myopathy. Drug/toxin-induced myopathy should be excluded
| Serologic criteria | Muscle biopsy features | Clinical criteria | |
|---|---|---|---|
| Anti-SRP myositis | Anti-SRP antibody | Not required | High creatine kinase |
| Anti-HMGCR myositis | Anti-HMGCR antibody | Proximal weakness | |
| Antibody-negative IMNM | No myositis-specific antibody |
|
Epidemiology
The autoimmune myopathies are rare diseases, with a prevalence of just 9–14 cases per 100,000 people [14, 15], and only ~ 10% of these have either anti-SRP or anti-HMGCR myopathy [9, 16••]. As will be discussed in more detail, both anti-SRP and anti-HMGCR myopathies can occur in children [17••, 18••, 19•, 20]. Among adult patients, those with anti-SRP myopathy tend to be younger [16••] than those with anti-HMGCR myopathy (~ 40 vs. ~ 55 years of age). Of note, the mean age of non-statin-exposed anti-HMGCR myopathy patients (~ 40 years old) [21••] is similar to the mean age of those with anti-SRP myopathy, suggesting the possibility that anti-HMGCR myositis may be composed of two epidemiologically different populations of patients, those triggered by medical statins and those that are not. At least two additional epidemiological observations support this hypothesis. First, in some Asian cohorts [22] where statin exposure in anti-HMGCR myopathy is infrequent, the age of disease onset is similar to that seen in anti-SRP myopathy [21••, 22, 23]. Second, although both anti-SRP and anti-HMGCR myopathy are more common in women, this gender bias is reduced in statin-exposed anti-HMGCR patients [13••, 16••, 21••].
Interestingly, unlike other types of myositis [24], neither anti-SRP nor anti-HMGCR myopathy shows a clear predominance for Black patients over Caucasian or Asian populations.
Clinical Features
The hallmark feature of patients with IMNM is the presence of proximal muscle weakness. Indeed, most IMNM patients experience only this clinical manifestation and finding significant skin or lung involvement should suggest the possibility of another type of inflammatory myopathy, even if the muscle biopsy shows prominent necrosis [13••, 16••, 21••].
Specifically, interstitial lung disease occurs in just 10–20% of patients with anti-SRP myopathy and in less than 5% of those with anti-HMGCR myopathy [13••, 16••, 21••]. Furthermore, skin involvement or other extra-muscular features occur in less than 10% of patients in both groups [13••, 16••, 21••]. The first reports of anti-SRP myopathy stressed the high prevalence of severe cardiac involvement in this IMNM group [25–27]. However, more recent descriptions of large cohorts have only shown this clinical manifestation in a relatively small number of anti-SRP myopathy patients [13••, 16••, 21••]. Cardiac involvement has only rarely been described in anti-HMGCR myopathy.
In children, anti-SRP and anti-HMGCR myopathy may present with slowly progressive proximal muscle weakness that can be difficult to differentiate clinically from a limb girdle muscle dystrophy. Thus, testing for these autoantibodies should be an essential part of the evaluation of children thought to have a muscular dystrophy but who have no family history or genetically confirmed diagnosis [17••, 18••, 19•, 20, 28, 29•].
Cancer Association
In patients with some types of inflammatory myopathy, especially in dermatomyositis patients with anti-TIF1g autoantibodies, there is an increased risk of cancer within 3 years of the myositis diagnosis; these patients are defined as having cancer-associated myositis. In those with IMNM, the risk of cancer depends on the disease subtype. Specifically, autoantibody-negative IMNM has been associated with a markedly increased risk of malignancy (95%SCR CI: 8.35–24.41) [30••]. In contrast, anti-HMGCR myopathy may have a relatively weak association with cancer (95%SCR CI: from 0.4–2.4 to 1.02–6.07) [30••], although this has not been demonstrated in all cohorts [21••, 31•, 32•, 33••]. Finally, anti-SRP myopathy is not associated with cancer [13••, 16••, 30••, 33••, 34•].
Diagnosis and Management
Autoantibodies
Autoantibody testing is necessary to classify patients with IMNM since the division of patients into subtypes (e.g., anti-HMGCR myopathy) relies on autoantibody status. Unfortunately, uniform methods to test for myositis autoantibodies have not been validated and widely adopted. Thus, there is significant variability between autoantibody testing techniques in laboratories around the world.
As is the case for many other autoantibodies, IMNM autoantibody testing methods can be divided in screening and confirmatory procedures. Among screening techniques, the most commonly used are the enzyme-linked immunosorbent assay (ELISA), the line blot assay, the dot blot assay, and the addressable laser bead immunoassay (ALBIA). In general, screening tests have a high sensitivity but may lack specificity compared with the confirmatory techniques. Thus, positive results using these methods may have to be considered provisional until confirmed by another technique. The most common confirmatory techniques include using patient serum to immunoprecipitate radioactively labeled proteins from cell extracts or purified proteins that have been produced by in vitro transcription and translation (IVTT). Alternatively, RNA immunoprecipitation methodologies can be used as confirmatory techniques in cases where the autoantibodies recognize a complex including RNA (e.g., the 7S RNA of the SRP).
Anti-SRP autoantibodies are usually screened by ELISA or line blot techniques. However, it should be noted that commercially available kits often only test for the 54-kDa SRP subunit. Since a small number of anti-SRP-positive patients do not have reactivity against this protein, a few false negatives may result [13••].
The presence of anti-SRP autoantibodies can be confirmed either by RNA-IP or by IP of radioactively labeled whole cell extract or IVTT protein products. Since the 7S RNA should be immunoprecipitated regardless of the protein subunit recognized by the autoantibodies, RNA-IP may be the most reliable confirmatory technique for anti-SRP autoantibodies [13••]. However, considering that RNA-IP is labor intensive, not easily automated, and requires expertise to perform and interpret, its use is limited to specialized labs or research settings.
Anti-HMGCR autoantibodies are usually screened by means of an ELISA assay. However, it should be noted that the anti-HMGCR ELISA may have a false positive rate of up to 0.7% [35, 36••]. Thus, we recommend against testing for these autoantibodies in the large number of patients with statin-associated muscle symptoms. Rather, in clinical practice, we suggest routine screening for anti-HMGCR autoantibodies only when the pre-test probability of anti-HMGCR myopathy is high. This would include patients with significant CK elevations and muscle weakness that does not improve following statin discontinuation. Additionally, screening for anti-HMGCR autoantibodies would be indicated in any patient with a necrotizing muscle biopsy. Recently, a new and distinct immunofluorescence pattern (HALIP) has been described in patients with anti-HMGCR autoantibodies. This pattern could be useful as a screening technique in settings where the specific immunologic tests are not readily available [37•].
In contrast, in the research setting, when screening large numbers of patients with a relatively low pre-test probability, the presence of anti-HMGCR autoantibodies should be confirmed by another method, such as immunoprecipitation of purified HMGCR protein.
Muscle Strength
Since proximal muscle weakness is the predominant clinical feature in patients with IMNM, documenting the degree of weakness is a critical aspect of managing these patients. The medical research council (MRC) scale is the most widely accepted system to measure the strength level in individual muscles [38, 39]. However, the MRC scale has a limited capacity for documenting the full spectrum of strength in those with moderate weakness (i.e., those with an MRC strength score of 4, who have “active movement against gravity and resistance”). In addition, it has a ceiling effect, such that some patients with diminished strength may still be scored as having normal power (an MRC score of 5). In our experience, including quantitative muscle strength testing, especially of the arm abductors and hip flexors, using a hand-held dynamometer has several advantages over using the MRC scale alone. For example, it may be possible to document moderate, but important, changes in muscle strength that are not fully captured using the MRC scale.
Creatine Kinase
In general, patients with IMNM have the greatest muscle enzyme elevations among those with various forms of myositis. Specifically, the median peak CK is around 4700 IU/L in anti-HMGCR and anti-SRP myopathy, around 700 IU/L in DM (including clinically amyopathic forms), and around 1300 IU/L in the antisynthetase syndrome (data not published).
It is well known that in patients with some forms of myositis, serum CK levels may not accurately reflect disease activity. For example, some dermatomyositis patients can have significant disease activity, including muscle weakness, without serum CK elevations. Presumably, in these patients, myofiber atrophy, perivascular inflammation, and other insults result in muscle dysfunction without disruption of the muscle cell membrane and consequent release of CK. In contrast, IMNM patients have prominent myofiber necrosis and, to the best of our knowledge, disease activity is almost always associated with elevated CK levels.
Several additional points are worth mentioning regarding CK levels in patients with IMNM. First, muscle enzyme elevations may precede the development of weakness in IMNM. Consequently, some anti-HMGCR myopathy patients may masquerade as “benign hyperCKemia.” Also, CK elevations may be used to detect incipient flares in patients who are being weaned from therapy; thus, we recommend that CK levels be followed closely in this context. Second, after starting therapy, CK levels often decline first, with muscle regeneration and the recovery of strength following weeks or even months later. Therefore, we would usually recommend against escalating therapy in an IMNM patient whose CK has normalized. Third, in patients who have had longstanding or poorly treated disease, a significant amount of muscle tissue may have been permanently replaced by fat and connective tissue. These patients may not have CK elevations even if they have active disease in their remaining muscles. Since they will also likely have permanent weakness from chronic muscle damage, the only way to assess disease activity in such patients may be to obtain a muscle magnetic resonance imaging (MRI) study.
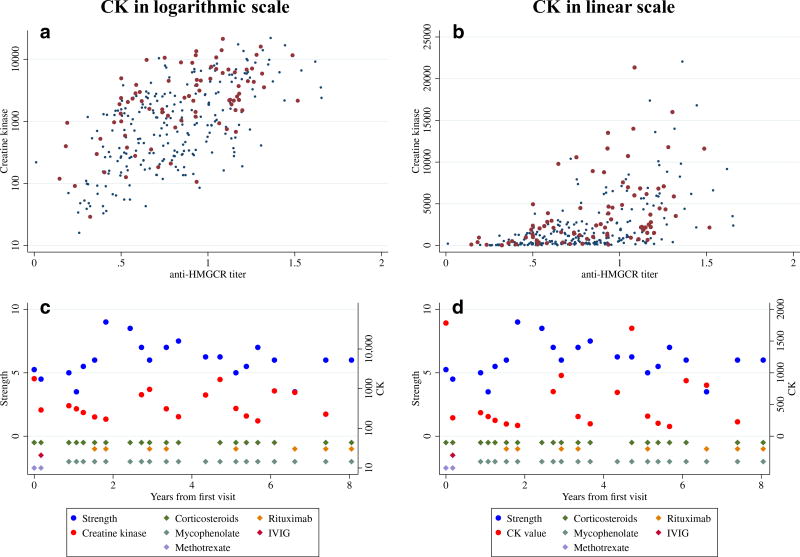
Although it applies to patients with any form of myopathy, another point regarding serum CK levels should be mentioned. Namely, muscle enzyme levels increase exponentially in muscle diseases. Thus, an increase of the CK level from 100 to 1000 IU/L represents the same fold change in disease activity as when CK levels increase from 1000 to 10,000 IU/L (Fig. 1). This is important to keep in mind when caring for patients in the clinic. Furthermore, this means that in the research setting, logarithmic transformation of the CK is required for statistical analyses so as not to (a) miss detecting clinically relevant associations or (b) bias the results due to extremely high individual CK levels.
Fig. 1.
Association of creatine kinase (CK) levels with anti-HMGCR titers (a and b) and examples of the evolution of strength and CK over time in a patient with immune-mediated necrotizing myopathy (c and d). Expressing the CK levels in a logarithmic scale (a and c) correlates better with the activity of the disease (autoantibody levels and strength) than when using it in a linear scale (b and d)
Muscle Magnetic Resonance Imaging
MRI can be used to help with the management of patients with IMNM as it can show the distribution and severity of both active disease and chronic muscle damage. Both T1-weighted and short tau inversion recovery (STIR) sequences should be obtained in patients with IMNM. Intramuscular hyperintensities on the STIR sequences reflect active muscle edema associated with inflammation or myofiber necrosis; this is expected to resolve with successful treatment of the underlying autoimmune muscle disease. In contrast, T1-weighted images can be used to assess fatty replacement, which, to the best of our knowledge, is an irreversible consequence of a chronic and/or severe myopathic process.
Muscle MRI has only limited value in diagnosing patients with IMNM because it discriminates poorly between patients with IMNM compared to other types of myositis (e.g., dermatomyositis) [40••]. However, muscle MRI may be a useful tool to monitor the evolution of muscle disease over time. In this regard, we have recently shown that in all types of myositis, including IMNM, fatty replacement begins very early after the onset of the disease. This suggests the possibility that early and intense induction therapy may help to decrease long-term disability in these patients [40••].
As muscle involvement may be patchy, muscle MRI can be used to increase the diagnostic accuracy of a muscle biopsy by allowing the surgeon to target an affected region; muscles with edema but minimal fatty replacement are preferred biopsy locations. A recent study showed that performing a biopsy in regions with edema increases the likelihood of obtaining a diagnostic biopsy whereas non-diagnostic biopsies may be more likely when a muscle without edema or with significant fatty replacement is selected [41].
In general, patients with active IMNM have MRI evidence of generalized muscle edema, muscle atrophy, and fatty replacement of muscle with minimal fascial edema. Compared with other types of myositis patients, IMNM patients have relatively less involvement of the anterior compartment, which is especially striking compared with IBM patients, where involvement of the anterior compartment is extremely common. Overall, the extent of muscle involvement as assessed by MRI is higher in IMNM patients than in those with DM or polymyositis (PM) and similar to IBM (Fig. 2) [40••, 42].
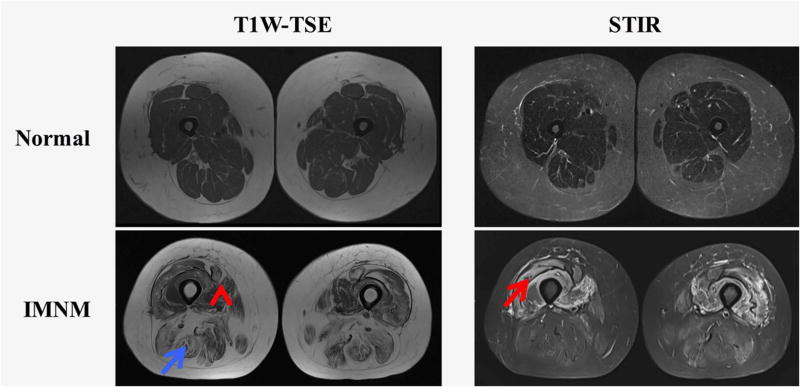
Fig. 2.
Examples of T1-weighted (T1W) turbo spin echo (TSE) and short tau inversion recovery (STIR) sequences showing edema (red arrow), atrophy (red arrow head), and fatty replacement (blue arrow) in a patient with immune-mediated necrotizing myositis (IMNM)
Compared to anti-HMGCR myopathy patients, the muscle MRI of anti-SRP myopathy patients demonstrates a more severe form of myopathy as reflected by higher rates of atrophy and fatty replacement. Specifically, anti-SRP patients show more extensive edema, atrophy, and fatty replacement in the lateral rotator group; more atrophy and fatty replacement in the anterior compartment; and more atrophy in the medial compartment [40••]. To date, the muscle MRI characteristics of autoantibody-negative IMNM have not been described.
Muscle Biopsy
Muscle biopsies in patients with each subtype of IMNM include examples of muscle cell necrosis and muscle cell regeneration; these features are often widespread, but may be infrequent in patients with less severe disease [1, 2, 8, 13••, 34•, 43•]. As in other forms of myositis, anti-HMGCR and anti-SRP myopathy cases usually have multifocal upregulation of class I major histocompatibility complex (~ 50%) and the deposition of membrane attack complex (MAC) on the sarcolemma of non-necrotic muscle fibers (~ 20–50%) [1, 2, 8, 13••, 32•, 33••, 34•, 43•]. The presence of perifascicular atrophy or non-necrotic fibers surrounded and invaded by lymphocytes (primary inflammation) is uncommon, if ever present [1, 2, 8, 13••, 32•, 33••, 34•, 43•]. In anti-HMGCR myopathy, it has been shown that the inflammatory infiltrate is mainly composed of macrophages, but that scant CD4+ or CD8+ (both ~ 50%) lymphocytes as well as CD123+ plasmacytoid dendritic cells (~ 70%) can also be present (Fig. 3) [43•].
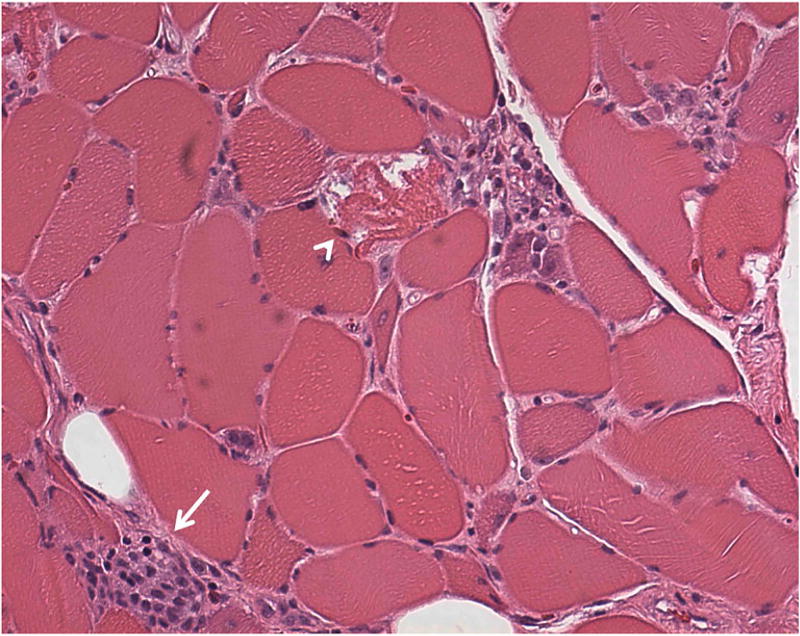
Fig. 3.
Representative muscle biopsy of a patient with immune-mediated necrotizing myopathy, showing degenerating fibers (arrow head), myophagocytosis (arrow), and many atrophic fibers
Few studies have directly compared the pathologic features of the different IMNM subtypes. However, one analysis suggested that sarcolemmal MAC deposition may be more common in anti-HMGCR (65%) than in anti-SRP patients (22%) [33••]. The detailed pathologic features of autoantibody-negative IMNM patients have not been described.
As stated previously, the recent ENMC criteria do not strictly require a muscle biopsy to diagnose anti-HMGCR or anti-SRP myopathy. However, as serological studies may take weeks or longer to become available, a muscle biopsy may still be useful to help establish an autoimmune etiology (e.g., by showing MHC-I upregulation) and to exclude other diagnoses. Furthermore, a muscle biopsy is required to establish the diagnosis of antibody-negative IMNM.
Other Diagnostic Tests
The electromyogram (EMG) may be useful early in the diagnostic workup to confirm the presence of a myopathic pattern and to rule out other causes of muscle weakness, like neuropathy or myasthenia gravis.
If pulmonary involvement is suspected, pulmonary function tests and high-resolution computed tomography (HRCT) of the chest may be useful to detect and monitor this. HRCT should be performed at diagnosis if there is any suspicion of lung involvement and repeated if there are changes in the clinical course of the patient from a pulmonary standpoint. Even though interstitial lung disease is uncommon in IMNM, severely affected patients may have a restrictive pattern on PFTs due to weakness of the respiratory muscles. In this scenario, besides the classical pulmonary function test parameters and the DLCO determination, the maximum inspiratory and expiratory pressures can be helpful to monitor the progression of disease.
No evidence-based guidelines indicate what type of cancer screening should be performed in newly diagnosed myositis patients, including those with IMNM. Nonetheless, given that cancer may be associated with IMNM, we recommend chest and abdomen computed tomography as well as age- and gender-appropriate cancer screening (i.e., colonoscopy in those over 50 years old, and mammogram, PAP smear, and a gynecologic examination in women). Of note, one study has suggested that a single PET scan may be just as sensitive to detect cancer in other myositis patients compared to the combination of all of the other tests [44]. Future studies will be required to determine if this is the case for IMNM patients as well.
Prognosis
Muscle prognosis in IMNM is worse than in most other types of myositis, and the percentage of IMNM patients who have persistent weakness despite intense immunosuppressant treatment is significant [13••, 16••, 21••]. Specifically, around half of anti-SRP and anti-HMGCR myopathy patients continue to have significant weakness after 2 years of treatment. In both groups, younger age seems to be the factor most closely associated with a worse prognosis. In this regard, some reports of IMNM in children have suggested that juvenile forms of this disease may be considerably resistant to treatment [17••, 18••, 19•, 20]. However, a recent case report and small case series have shown that children with anti-HMGCR and anti-SRP myopathy, respectively, can have a dramatic improvement with immunosuppressive therapy [28, 29•]. Also, one report demonstrated that Black patients have a trend towards more severe forms of the disease compared to White patients, although this needs to be confirmed in further studies [13••, 16••, 21••]
Treatment
Importantly, there are no clinical trials to guide therapeutic decisions in IMNM and most of the following recommendations are derived from case series, observational studies, and the personal experience of clinicians. Nonetheless, most experts agree that treatment of IMNM should be initiated early and may need to be intense to avoid long-term disability. As in other forms of myositis, treatment intensity in IMNM should be individualized on a case by case basis with the most intensive therapy reserved for those with the most severe and/or rapidly progressive disease. Of note, an ENCM working group recently recommended that corticosteroids plus methotrexate may be a good initial treatment (Table 2) [3]. Alternatives to methotrexate would include azathioprine, mycophenolate, tacrolimus, cyclosporine, or cyclophosphamide, but the evidence to support the use of one of these over another is limited.
Table 2.
2017 ENMC treatment recommendations for immune-mediated necrotizing myopathy
| Induction |
|
| Maintenance |
|
The ENMC treatment guidelines, based on case series and observational cohort studies, suggest that rituximab should be used in anti-SRP patients who fail to respond to steroids and another agent [3, 16••, 45]. In anti-HMGCR myopathy, intravenous immunoglobulin (IVIG) was recommended for refractory disease and, based on a small case series [46••], may even be considered as monotherapy for patients who have contraindications to the use of steroids. In our experience, a combination of treatments (e.g., high-dose corticosteroids, methotrexate, rituximab, and IVIG) may be required to achieve a clinical response in the most severely affected individuals, especially in young patients with anti-SRP myopathy.
Etiopathogenesis and Physiopathology
Although the causes of IMNM are still unknown, strong immunogenetic predisposing factors have been identified in anti-HMGCR myopathy. In adults, the class II MHC allele DRB1*11:01 is strongly associated with anti-HMGCR myopathy [32•, 47, 48•], with an increased odds ratio (OR) of 24.5 in Whites, 56.5 in Blacks, and 3.7 in Asian populations [32•, 47, 48•]. In contrast, children with anti-HMGCR have an increased prevalence of the DRB1*07:01 allele [17••, 28]. Of note, in the Japanese population, it has been shown that the risk of developing anti-SRP myopathy is associated with the HLA-DRB1*08:03 allele (OR 2.5, 95% CI 1.4–4.3) [48•].
In those with anti-HMGCR myopathy, the preponderance of evidence has established that statin exposure is a risk factor for developing the disease. Thus, in the original description of the anti-HMGCR autoantibodies, the prevalence of statin exposure at the onset of the disease was 89% in anti-HMGCR patients over age 50, compared to < 37% in DM, PM, or IBM groups [8]. Also, compared to anti-SRP patients, statin exposure was highly associated with anti-HMGCR autoantibodies both in American (OR 32.9, p < 0.001) [21••] and in Asian cohorts (4 vs. 18%, p = 0.02) [33••].
Nonetheless, the prevalence of statin exposure in anti-HMGCR myopathy patients differs depending on the origin of the cohort. For example, the percentage of HMGCR patients with statin exposure ranged from 38 to 63% in US cohorts [8, 34•], was 44% in a European cohort [23], and was only 14–38% in Asian cohorts [22, 49]. Interestingly, statins are present in some foods and dietary supplements including certain types of fungus (like the oyster mushroom) [50], red yeast rice [51], or pu-erh tea [52]. These alternative sources of statins are common in Asian cooking, suggesting the possibility that the weaker association between anti-HMGCR and medical statin exposure in Asian cohorts may be explained by the higher exposure to these other alimentary sources of statins.
Statins could contribute to breaking immune tolerance to HMGCR by several mechanisms. For example, statins are known to increase the expression of HMGCR [53] and this could lead to aberrant processing of the protein with the subsequent production of neoantigens. Alternatively, statin binding might change the conformation of the HMGCR protein, making it more immunogenic or more effectively presented by MHC molecules. Further studies will be required to test these possibilities.
As discussed earlier, some IMNM patients have an increased risk of cancer. This raises the possibility that some IMNM subtypes, especially antibody-negative IMNM, could be triggered by tumors. However, the association with cancer is probably not as strong as in those with the anti-TIF1g-positive dermatomyositis [30••, 54, 55].
Viral infections represent another possible trigger of IMNM. In support of this, it has been observed that there is a seasonal pattern to the development of anti-SRP myopathy, with a peak in the month of November [56]. Furthermore, since both the 54-kDa subunits of SRP and the HMGCR protein share regions of homology with proteins of the varicella zoster virus and the human papillomavirus type 58, respectively (data not published), it is feasible that exposure to these viruses could generate an immune response against the human protein through molecular mimicry.
Although the mechanisms underlying myofiber necrosis have not been elucidated, given that IMNM patients have MAC on the surface of non-necrotic muscle fibers [8, 23, 43•], it could be that myofiber toxicity occurs as a result of antibody-dependent complement-mediated cell death. In this regard, it is noteworthy that recent in vitro data suggest that anti-HMGCR and anti-SRP autoantibodies may have a direct toxic effect on muscle cells. However, rather than causing necrosis in vitro, these autoantibodies were proposed to induce muscle fiber atrophy, increase levels of reactive oxygen species and proinflammatory cytokines like the tumor necrosis factor and IL-6, and impair myoblast fusion by decreasing the production of IL-4 and IL-13. Furthermore, the toxic effects anti-SRP and/or anti-HMGCR autoantibodies have not yet been demonstrated in animal models [57••].
Conclusions
IMNM, a type of autoimmune myopathy, is now recognized to include at least three distinct serologically defined subtypes: anti-SRP myopathy, anti-HMGCR myopathy, and antibody-negative IMNM. While each of these has myofiber necrosis as the predominant histological feature on muscle biopsy, the different IMNM subtypes have different environmental risk factors, genetic risk factors, cancer risks, extra-muscular manifestations, and prognoses. This raises the possibility that the different IMNM subtypes represent fundamentally different diseases. As such, they may have distinct pathological mechanisms and may respond differently to various therapeutic modalities. This would have important consequences for clinical trials to define optimal treatment strategies for IMNM patients, some of whom have severe and sometimes refractory muscle disease.
Footnotes
Compliance with Ethical Standards
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
Conflict of Interest The authors declare that they have no conflict of interest.
References
Recently published papers of particular interest have been highlighted as:
• Of importance
•• Of major importance
- 1.Dalakas MC. Inflammatory muscle diseases. N Engl J Med. 2015;373(4):393–4. doi: 10.1056/NEJMc1506827. [DOI] [PubMed] [Google Scholar]
- 2.Hoogendijk JE, Amato AA, Lecky BR, Choy EH, Lundberg IE, Rose MR, et al. 119th ENMC international workshop: trial design in adult idiopathic inflammatory myopathies, with the exception of inclusion body myositis, 10–12 October 2003, Naarden, The Netherlands. Neuromuscul Disord. 2004;14(5):337–45. doi: 10.1016/j.nmd.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 3••.Allenbach Y, Mammen AL, Stenzel W, Benveniste O. Immune-mediated necrotizing myopathies working G. 224th ENMC International Workshop:: Clinico-sero-pathological classification of immune-mediated necrotizing myopathies Zandvoort, The Netherlands, 14–16 October 2016. Neuromuscul Disord. 2017 doi: 10.1016/j.nmd.2017.09.016. Most recent classification criteria in IMNM. It includes consensus treatment recommendations for the different IMNM subsets. [DOI] [PubMed] [Google Scholar]
- 4.Senecal JL, Raynauld JP, Troyanov Y. Editorial: a new classification of adult autoimmune myositis. Arthritis Rheumatol. 2017;69(5):878–84. doi: 10.1002/art.40063. [DOI] [PubMed] [Google Scholar]
- 5.Nakao Y, Mukai R, Kabashima T, Ohshima Y, Hamaguchi H, Kashiwagi H, et al. A novel antibody which precipitates 7.5S RNA is isolated from a patient with autoimmune disease. Biochem Biophys Res Commun. 1982;109(4):1332–8. doi: 10.1016/0006-291x(82)91923-4. [DOI] [PubMed] [Google Scholar]
- 6.Okada N, Mimori T, Mukai R, Kashiwagi H, Hardin JA. Characterization of human autoantibodies that selectively precipitate the 7SL RNA component of the signal recognition particle. J Immunol. 1987;138(10):3219–23. [PubMed] [Google Scholar]
- 7.Reeves WH, Nigam SK, Blobel G. Human autoantibodies reactive with the signal-recognition particle. Proc Natl Acad Sci U S A. 1986;83(24):9507–11. doi: 10.1073/pnas.83.24.9507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Christopher-Stine L, Casciola-Rosen LA, Hong G, Chung T, Corse AM, Mammen AL. A novel autoantibody recognizing 200-kd and 100-kd proteins is associated with an immune-mediated necrotizing myopathy. Arthritis Rheum. 2010;62(9):2757–66. doi: 10.1002/art.27572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mammen AL, Chung T, Christopher-Stine L, Rosen P, Rosen A, Doering KR, et al. Autoantibodies against 3-hydroxy-3-methylglutaryl-coenzyme A reductase in patients with statin-associated autoimmune myopathy. Arthritis Rheum. 2011;63(3):713–21. doi: 10.1002/art.30156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pinal-Fernandez I, Casciola-Rosen LA, Christopher-Stine L, Corse AM, Mammen AL. The prevalence of individual histopathologic features varies according to autoantibody status in muscle biopsies from patients with dermatomyositis. J Rheumatol. 2015;42(8):1448–54. [PMC free article] [PubMed] [Google Scholar]
- 11.Paik JJ, Wigley FM, Lloyd TE, Corse AM, Casciola-Rosen L, Shah AA, et al. Spectrum of muscle histopathologic findings in forty-two scleroderma patients with weakness. Arthritis Care Res (Hoboken) 2015;67(10):1416–25. doi: 10.1002/acr.22620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schneider I, Stoltenburg G, Deschauer M, Winterholler M, Hanisch F. Limb girdle muscular dystrophy type 2L presenting as necrotizing myopathy. Acta Myol. 2014;33(1):19–21. [PMC free article] [PubMed] [Google Scholar]
- 13••.Suzuki S, Nishikawa A, Kuwana M, et al. Inflammatory myopathy with anti-signal recognition particle antibodies: case series of 100 patients. Orphanet J Rare Dis. 2015;10:61. doi: 10.1186/s13023-015-0277-y. Large study on the clinical features of anti-SRP patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dobloug C, Garen T, Bitter H, Stjärne J, Stenseth G, Grøvle L, et al. Prevalence and clinical characteristics of adult polymyositis and dermatomyositis; data from a large and unselected Norwegian cohort. Ann Rheum Dis. 2015;74(8):1551–6. doi: 10.1136/annrheumdis-2013-205127. [DOI] [PubMed] [Google Scholar]
- 15.Svensson J, Arkema EV, Lundberg IE, Holmqvist M. Incidence and prevalence of idiopathic inflammatory myopathies in Sweden: a nationwide population-based study. Rheumatology (Oxford) 2017;56(5):802–10. doi: 10.1093/rheumatology/kew503. [DOI] [PubMed] [Google Scholar]
- 16••.Pinal-Fernandez I, Parks C, Werner JL, et al. Longitudinal course of disease in a large cohort of myositis patients with autoantibodies recognizing the signal recognition particle. Arthritis care res (Hoboken) 2017;69(2):263–70. doi: 10.1002/acr.22920. Longitudinal cohort study of anti-SRP patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17••.Kishi T, Rider LG, Pak K, et al. Association of anti-3-hydroxy-3-methylglutaryl-coenzyme A reductase autoantibodies with DRB1*07:01 and severe myositis in juvenile myositis patients. Arthritis Care Res (Hoboken) 2017;69(7):1088–94. doi: 10.1002/acr.23113. Report of juvenile anti-HMGCR cases from the USA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18••.Tansley SL, Betteridge ZE, Simou S, et al. Anti-HMGCR autoantibodies in juvenile idiopathic inflammatory myopathies identify a rare but clinically important subset of patients. J Rheumatol. 2017;44(4):488–92. doi: 10.3899/jrheum.160871. Report of juvenile anti-HMGCR cases from the UK. [DOI] [PubMed] [Google Scholar]
- 19•.Binns EL, Moraitis E, Maillard S, et al. Effective induction therapy for anti-SRP associated myositis in childhood: a small case series and review of the literature. Pediatr Rheumatol Online J. 2017;15(1):77. doi: 10.1186/s12969-017-0205-x. Report of juvenile anti-SRP patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suzuki S, Ohta M, Shimizu Y, Hayashi YK, Nishino I. Anti-signal recognition particle myopathy in the first decade of life. Pediatr Neurol. 2011;45(2):114–6. doi: 10.1016/j.pediatrneurol.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 21••.Tiniakou E, Pinal-Fernandez I, Lloyd TE, et al. More severe disease and slower recovery in younger patients with anti-3-hydroxy-3-methylglutaryl-coenzyme A reductase-associated autoimmune myopathy. Rheumatology (Oxford) 2017;56(5):787–94. doi: 10.1093/rheumatology/kew470. Comprehensive longitudinal cohort study of patients with anti-HMGCR myositis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ge Y, Lu X, Peng Q, Shu X, Wang G. Clinical characteristics of anti-3-hydroxy-3-methylglutaryl coenzyme A reductase antibodies in Chinese patients with idiopathic inflammatory myopathies. PLoS One. 2015;10(10):e0141616. doi: 10.1371/journal.pone.0141616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Allenbach Y, Drouot L, Rigolet A, Charuel JL, Jouen F, Romero NB, et al. Anti-HMGCR autoantibodies in European patients with autoimmune necrotizing myopathies: inconstant exposure to statin. Medicine (Baltimore) 2014;93(3):150–7. doi: 10.1097/MD.0000000000000028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pinal-Fernandez I, Casal-Dominguez M, Huapaya JA, Albayda J, Paik JJ, Johnson C, et al. A longitudinal cohort study of the anti-synthetase syndrome: increased severity of interstitial lung disease in black patients and patients with anti-PL7 and anti-PL12 autoantibodies. Rheumatology (Oxford) 2017;56(6):999–1007. doi: 10.1093/rheumatology/kex021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Targoff IN, Johnson AE, Miller FW. Antibody to signal recognition particle in polymyositis. Arthritis Rheum. 1990;33(9):1361–70. doi: 10.1002/art.1780330908. [DOI] [PubMed] [Google Scholar]
- 26.Kao AH, Lacomis D, Lucas M, Fertig N, Oddis CV. Anti-signal recognition particle autoantibody in patients with and patients without idiopathic inflammatory myopathy. Arthritis Rheum. 2004;50(1):209–15. doi: 10.1002/art.11484. [DOI] [PubMed] [Google Scholar]
- 27.Hengstman GJ, Brouwer R, Egberts WT, et al. Clinical and serological characteristics of 125 Dutch myositis patients. Myositis specific autoantibodies aid in the differential diagnosis of the idiopathic inflammatory myopathies. J Neurol. 2002;249(1):69–75. doi: 10.1007/pl00007850. [DOI] [PubMed] [Google Scholar]
- 28.Mohassel P, Foley AR, Donkervoort S, Fequiere PR, Pak K, Bönnemann CG, et al. Anti-3-hydroxy-3-methylglutaryl-coenzyme A reductase necrotizing myopathy masquerading as a muscular dystrophy in a child. Muscle Nerve. 2017;56(6):1177–81. doi: 10.1002/mus.25567. [DOI] [PubMed] [Google Scholar]
- 29•.Zhao Y, Liu X, Zhang W, Yuan Y. Childhood autoimmune necrotizing myopathy with anti-signal recognition particle antibodies. Muscle Nerve. 2017;56(6):1181–7. doi: 10.1002/mus.25575. Report of juvenile cases of anti-SRP myositis. [DOI] [PubMed] [Google Scholar]
- 30••.Allenbach Y, Keraen J, Bouvier AM, et al. High risk of cancer in autoimmune necrotizing myopathies: usefulness of myositis specific antibody. Brain. 2016;139(Pt 8):2131–5. doi: 10.1093/brain/aww054. Study of cancer risk in the different IMNM subsets. [DOI] [PubMed] [Google Scholar]
- 31•.Kadoya M, Hida A, Hashimoto Maeda M, et al. Cancer association as a risk factor for anti-HMGCR antibody-positive myopathy. Neurol Neuroimmunol Neuroinflamm. 2016;3(6):e290. doi: 10.1212/NXI.0000000000000290. Cancer association in Japanese anti-HMGCR myositis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32•.Limaye V, Bundell C, Hollingsworth P, et al. Clinical and genetic associations of autoantibodies to 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase in patients with immune-mediated myositis and necrotizing myopathy. Muscle Nerve. 2015;52(2):196–203. doi: 10.1002/mus.24541. Report of the clinical features of anti-HMGCR patients from Australia. [DOI] [PubMed] [Google Scholar]
- 33••.Watanabe Y, Uruha A, Suzuki S, et al. Clinical features and prognosis in anti-SRP and anti-HMGCR necrotising myopathy. J Neurol Neurosurg Psychiatry. 2016;87(10):1038–44. doi: 10.1136/jnnp-2016-313166. Comparison of anti-HMGCR and anti-SRP myositis in Japanese patients. [DOI] [PubMed] [Google Scholar]
- 34•.Alshehri A, Choksi R, Bucelli R, Pestronk A. Myopathy with anti-HMGCR antibodies: perimysium and myofiber pathology. Neurol Neuroimmunol Neuroinflamm. 2015;2(4):e124. doi: 10.1212/NXI.0000000000000124. Clinical and pathologic features of anti-HMGCR patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mammen AL, Pak K, Williams EK, Brisson D, Coresh J, Selvin E, et al. Rarity of anti-3-hydroxy-3-methylglutaryl-coenzyme A reductase antibodies in statin users, including those with self-limited musculoskeletal side effects. Arthritis Care Res (Hoboken) 2012;64(2):269–72. doi: 10.1002/acr.20662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36••.Mammen AL. Statin-associated autoimmune myopathy. N Engl J Med. 2016;374(7):664–9. doi: 10.1056/NEJMra1515161. In-depth review about anti-HMGCR myositis. [DOI] [PubMed] [Google Scholar]
- 37•.Alvarado-Cardenas M, Marin-Sanchez A, Martinez MA, et al. Statin-associated autoimmunemyopathy: a distinct new IFL pattern can increase the rate of HMGCR antibody detection by clinical laboratories. Autoimmun Rev. 2016;15(12):1161–6. doi: 10.1016/j.autrev.2016.09.005. New IFL pattern in anti-HMGCR myositis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rider LG, Werth VP, Huber AM, et al. Measures of adult and juvenile dermatomyositis, polymyositis, and inclusion body myositis: Physician and Patient/Parent Global Activity, Manual Muscle Testing (MMT), Health Assessment Questionnaire (HAQ)/Childhood Health Assessment Questionnaire (C-HAQ), Childhood Myositis Assessment Scale (CMAS), Myositis Disease Activity Assessment Tool (MDAAT), Disease Activity Score (DAS), Short Form 36 (SF-36), Child Health Questionnaire (CHQ), physician global damage, Myositis Damage Index (MDI), Quantitative Muscle Testing (QMT), Myositis Functional Index-2 (FI-2), Myositis Activities Profile (MAP), Inclusion Body Myositis Functional Rating Scale (IBMFRS), Cutaneous Dermatomyositis Disease Area and Severity Index (CDASI), Cutaneous Assessment Tool (CAT), Dermatomyositis Skin Severity Index (DSSI), Skindex, and Dermatology Life Quality Index (DLQI) Arthritis Care Res (Hoboken) 2011;63(Suppl 11):S118–57. doi: 10.1002/acr.20532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Compston A. Aids to the investigation of peripheral nerve injuries. Medical Research Council: nerve injuries research committee. His Majesty’s stationery office: 1942; pp.48 (iii) and 74 figures and 7 diagrams; with aids to the examination of the peripheral nervous system. By Michael O'Brien for the Guarantors of Brain. Saunders Elsevier: 2010; pp. [8] 64 and 94 figures. Brain. 2010;133(10):2838–44. doi: 10.1093/brain/awq270. [DOI] [PubMed] [Google Scholar]
- 40••.Pinal-Fernandez I, Casal-Dominguez M, Carrino JA, et al. Thigh muscle MRI in immune-mediated necrotising myopathy: extensive oedema, early muscle damage and role of anti-SRP autoantibodies as a marker of severity. Ann Rheum Dis. 2017;76(4):681–7. doi: 10.1136/annrheumdis-2016-210198. Comprehensive MRI study in patients with immune-mediated necrotizing myositis compared to other myositis subtypes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Van De Vlekkert J, Maas M, Hoogendijk JE, De Visser M, Van Schaik IN. Combining MRI and muscle biopsy improves diagnostic accuracy in subacute-onset idiopathic inflammatory myopathy. Muscle Nerve. 2015;51(2):253–8. doi: 10.1002/mus.24307. [DOI] [PubMed] [Google Scholar]
- 42.Zheng Y, Liu L, Wang L, Xiao J, Wang Z, Lv H, et al. Magnetic resonance imaging changes of thigh muscles in myopathy with antibodies to signal recognition particle. Rheumatology (Oxford) 2015;54(6):1017–24. doi: 10.1093/rheumatology/keu422. [DOI] [PubMed] [Google Scholar]
- 43•.Chung T, Christopher-Stine L, Paik JJ, Corse A, Mammen AL. The composition of cellular infiltrates in anti-HMG-CoA reductase-associated myopathy. Muscle Nerve. 2015;52(2):189–95. doi: 10.1002/mus.24642. Pathologic features of anti-HMGCR patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Selva-O'Callaghan A, Grau JM, Gamez-Cenzano C, et al. Conventional cancer screening versus PET/CT in dermatomyositis/polymyositis. Am J Med. 2010;123(6):558–62. doi: 10.1016/j.amjmed.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 45.Valiyil R, Casciola-Rosen L, Hong G, Mammen A, Christopher-Stine L. Rituximab therapy for myopathy associated with anti-signal recognition particle antibodies: a case series. Arthritis Care Res (Hoboken) 2010;62(9):1328–34. doi: 10.1002/acr.20219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46••.Mammen AL, Tiniakou E. Intravenous immune globulin for statin-triggered autoimmune myopathy. N Engl J Med. 2015;373(17):1680–2. doi: 10.1056/NEJMc1506163. Case series suggesting that IVIG in monotherapy is effective in anti-HMGCR myopathy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mammen AL, Gaudet D, Brisson D, Christopher-Stine L, Lloyd TE, Leffell MS, et al. Increased frequency of DRB1*11:01 in anti-hydroxymethylglutaryl-coenzyme A reductase-associated autoimmune myopathy. Arthritis Care Res (Hoboken) 2012;64(8):1233–7. doi: 10.1002/acr.21671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48•.Ohnuki Y, Suzuki S, Shiina T, et al. HLA-DRB1 alleles in immune-mediated necrotizing myopathy. Neurology. 2016;87(18):1954–5. doi: 10.1212/WNL.0000000000003160. HLA in anti-SRP and anti-HMGCR myositis. [DOI] [PubMed] [Google Scholar]
- 49.Watanabe Y, Suzuki S, Nishimura H, Murata KY, Kurashige T, Ikawa M, et al. Statins and myotoxic effects associated with anti-3-hydroxy-3-methylglutaryl-coenzyme Areductase autoantibodies: an observational study in Japan. Medicine (Baltimore) 2015;94(4):e416. doi: 10.1097/MD.0000000000000416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lo YC, Lin SY, Ulziijargal E, Chen SY, Chien RC, Tzou YJ, et al. Comparative study of contents of several bioactive components in fruiting bodies and mycelia of culinary-medicinal mushrooms. Int J Med Mushrooms. 2012;14(4):357–63. doi: 10.1615/intjmedmushr.v14.i4.30. [DOI] [PubMed] [Google Scholar]
- 51.Klimek M, Wang S, Ogunkanmi A. Safety and efficacy of red yeast rice (Monascus purpureus) as an alternative therapy for hyperlipidemia. P T. 2009;34(6):313–27. [PMC free article] [PubMed] [Google Scholar]
- 52.Jeng KC, Chen CS, Fang YP, Hou RC, Chen YS. Effect of microbial fermentation on content of statin, GABA, and polyphenols in pu-erh tea. J Agric Food Chem. 2007;55(21):8787–92. doi: 10.1021/jf071629p. [DOI] [PubMed] [Google Scholar]
- 53.Morikawa S, Murakami T, Yamazaki H, Izumi A, Saito Y, Hamakubo T, et al. Analysis of the global RNA expression profiles of skeletal muscle cells treated with statins. J Atheroscler Thromb. 2005;12(3):121–31. doi: 10.5551/jat.12.121. [DOI] [PubMed] [Google Scholar]
- 54.Pinal-Fernandez I, Ferrer-Fabregas B, Trallero-Araguas E, et al. Tumour TIF1 mutations and loss of heterozygosity related to cancer-associated myositis. Rheumatology (Oxford) 2017 doi: 10.1093/rheumatology/kex413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Trallero-Araguas E, Rodrigo-Pendas JA, Selva-O'Callaghan A, et al. Usefulness of anti-p155 autoantibody for diagnosing cancer-associated dermatomyositis: a systematic review and meta-analysis. Arthritis Rheum. 2012;64(2):523–32. doi: 10.1002/art.33379. [DOI] [PubMed] [Google Scholar]
- 56.Leff RL, Burgess SH, Miller FW, Love LA, Targoff IN, Dalakas MC, et al. Distinct seasonal patterns in the onset of adult idiopathic inflammatory myopathy in patients with anti-Jo-1 and anti-signal recognition particle autoantibodies. Arthritis Rheum. 1991;34(11):1391–6. doi: 10.1002/art.1780341108. [DOI] [PubMed] [Google Scholar]
- 57••.Arouche-Delaperche L, Allenbach Y, Amelin D, et al. Pathogenic role of anti-signal recognition protein and anti-3-hydroxy-3-methylglutaryl-CoA reductase antibodies in necrotizing myopathies: Myofiber atrophy and impairment of muscle regeneration in necrotizing autoimmune myopathies. Ann Neurol. 2017;81(4):538–48. doi: 10.1002/ana.24902. Manuscript suggesting that in vitro, anti-SRP, and anti-HMGCCR autoantibodies are pathogenic. [DOI] [PubMed] [Google Scholar]