Abstract
Lactoferrin, an iron-binding glycoprotein found in mammalian mucosal secretions and granules of neutrophils, possesses several immune modulatory properties. Published reports indicate that lactoferrin enhances the efficacy of the tuberculosis vaccine, BCG (Bacillus Calmette Guerin), both by increasing macrophage and dendritic cell ability to stimulate receptive T cells and by modulating the inflammatory response. This report is the first to demonstrate the effects of a recombinant human lactoferrin (10 μg/ mL) on human PBMC derived CD14+ and CD16+ macrophages stimulated with a strong (LPS, 10 ng/mL) or weaker (BCG, MOI 1:1) stimulator of inflammation. After 3 days culture, LPS and human lactoferrin treated CD14+ cells significantly increased production of IL-10, IL-6, and MCP-1 compared to the LPS only group. In contrast, similarly treated CD16+ macrophages increased production of IL-12p40 and IL-10 and decreased TNF-α. Limited changes were observed in BCG stimulated CD14+ and CD16+ macrophages with and without lactoferrin. Analysis of surface expression of antigen presentation and co-stimulatory molecules demonstrated that CD14+ macrophages, when stimulated with BCG or LPS and cultured with lactoferrin, increased expression of CD86. CD16+ macrophages treated with lactoferrin showed a similar trend of increase in CD86 expression, but only when stimulated with BCG.
Keywords: Recombinant human lactoferrin, Tuberculosis, PBMC macrophages
1. Introduction
The challenge to discover and develop more efficacious vaccines and therapeutics to successfully treat tuberculosis (TB) disease is complicated by the roles that the host immune response plays in progression of lung pathology and eventual disease transmission [1]. Leukocytes are recruited to the site of Mycobacterium tuberculosis (MTB) infection and contribute to disease progression [2,3], yet the macrophage is believed to be a key player in establishing both innate and adaptive immunity. Thus, one potential method to control dissemination and spread of TB disease is to modulate macrophage responses to mycobacterial antigens.
Lactoferrin, a naturally occurring protein, produced by epithelial cells and neutrophils, is a first line defense protein involved in protection against microbial infections and subsequent development of systemic inflammation [4]. It possesses several immune modulating properties [5,6], including pleiotropic effects on macrophage activity and function [7–10]. Published reports demonstrated that addition of lactoferrin to macrophages stimulated with BCG (Bacillus Calmette Guerin), the current TB vaccine, enhanced the expression of presentation and co-stimulatory molecules, leading to enhanced stimulation of receptive CD4+ T cells. At the same time, lactoferrin limits the proinflammatory environment by enhancing the production of TGF-β1, allowing the potential to increase regulatory T cells to control the destructive inflammatory response during MTB infection and developing disease pathology [11,12]. These studies demonstrate that lactoferrin is capable of enhancing the effect of the BCG vaccine. Furthermore, in vivo experiments showed that the addition of lactoferrin to BCG does enhance vaccine efficacy by protecting against MTB induced lung disease progression by limiting the inflammation within the lung environment [13–17].
Much of lactoferrin’s ability to enhance BCG efficacy occurs through modulation of macrophage activity. Reported immune-related studies utilized the bovine form, which is not ideal for use in the human clinical setting. In addition, variability in the isolation and purification process of bovine lactoferrin from different manufactures has led to differences seen in immune modulatory properties between batches of bovine lactoferrin [18]. Recently, a recombinant human lactoferrin was produced in Chinese hamster ovary (CHO) cells, allowing mammalian glycosylation patterns which are known to be critical for proper immune function [19]. The CHO derived recombinant human lactoferrin exhibited similar activity as bovine lactoferrin in enhancing efficacy of the BCG vaccine, as measured by both increased splenic recall IFN-γ production (an indication of enhanced CD4+ T cell helper type 1 response critical for MTB control) and decreased TB disease pathology associated with overall decrease in lung inflammation [20].
Monocyte derived macrophages are tissue macrophages that are differentiated from circulating monocytes that have migrated into tissue. In humans, there are two distinct sets of monocytes, CD14+ and CD16+, distributed 90–95% and 5–10% respectively. While the classical monocytes (CD14+) are believed to be the primary responders during infection, the non-classical monocytes (CD16+) have been linked to several inflammatory disease states [21–24], including TB. Reports indicate that CD16+ expression is increased in monocytes isolated from TB patients compared to healthy controls [25]. The increase in CD16+ monocytes is correlated with severity of active TB disease [26]. CD16+ monocytes isolated from TB patients with poorer clinical outcomes are less responsive to stimulation with MTB antigens or LPS, producing significantly less TNF-α [27], a cytokine whose decrease has been linked to TB disease activation [28,29]. This increase in CD16+ monocytes could be a direct result of MTB infection altering the CD14+ monocytes. Culturing of CD14+ monocytes in supernatants collected from MTB infected macrophages pushed for enhanced expression of CD16 [30]. This elevation in CD16+ monocyte population and its immune dysfunction are believed to be involved in the disease process during chronic inflammation.
Monocytes are matured into macrophages once they are recruited into tissue where there are active sites of infection and/or inflammation. Monocytes normally do not encounter mycobacterial antigens in circulation, which during TB pulmonary disease are restricted to the lung tissue. During TB pulmonary disease, monocytes are recruited to the lung tissue where they are activated [31]. The sequence of events once the monocytes are in tissue, whether they mature into macrophage before encountering MTB antigens or the monocytes encounter MTB antigens prior to maturation is unknown.
The BCG vaccine is an attenuated strain of Mycobacterium bovis and possesses many of the host immune evasion properties of virulent MTB [32–35]. Once injected, BCG is hypothesized to establish a localized infection where monocytes and other leukocytes are recruited, and this transient infection is critical for establishment of the adaptive T cell response required to protect against subsequent MTB assault. This manuscript will explore the hypothesis that CD16+ monocyte derived macrophages play a role in response to the BCG vaccine, and that the adjuvant effect of lactoferrin observed in vivo is attributed to its ability to modulate CD16+ monocyte derived macrophage activity during BCG infection. In these studies we demonstrate utility of fully compatible recombinant human lactoferrin in the human macrophage system under stimulation by BCG or LPS.
2. Materials and method
2.1. Lactoferrin, BCG, LPS
CHO expressed recombinant human LF (endotoxin is < 0.2 EU/mg) was kindly provided by PharmaReview Corporation (Houston, TX) [20,36].
Mycobacterium bovis, Bacillus Calmette Guerin (BCG), Pasteur strain, (TMC 1011, ATCC, Manassas, VA) was grown in Dubos base (without addition of glycerol) with 10% supplement (5% BSA and 7.5% dextrose in saline) on an orbital shaker at 37 C for 2 weeks before use. BCG was diluted with 1x Dulbecco’s phosphate-buffered saline (PBS) (Cellgro, Herndon, VA) to 3 × 108 organisms/mL, the concentration was estimated using McFarland standards (Sigma, St. Louis, MO), and confirmed by plating dilutions onto 7H11 agar plates (Remel, Lenesa, KS). Plates were incubated at 37 °C with 5% CO2 for 3–4 weeks, and resulting colonies counted.
Lipopolysaccharide (LPS) from Escherichia coli O111:B4 was purchased from Sigma.
2.2. PBMC derived macrophages
Buffy coats from healthy blood donors were purchased from Gulf Coast Regional Blood Center (Houston, TX). Peripheral blood monocytes (PBMC) were isolated using Histopaque 1077 (Sigma) according to manufacturer’s instructions. Collected cells were treated with ACK buffer (Cambrex Bio Sciences, East Rutherford, NJ) to lyse the remaining red blood cells. PBMCs were washed 3xs with 1x Dulbecco’s phosphate buffer saline (PBS, Cellgro, Manassas, VA).
CD16+ monocytes were isolated from donor PBMCs using magnetic beads (Miltenyi Biotec, San Diego, CA). The CD16+ monocyte isolation kit depletes granulocytes and NK cells prior to positive selection for CD16+ monocytes. The remaining CD16 cells were collected and isolated for CD14+ monocytes by positive selection using magnetic beads (Miltenyi Biotec). Both CD16+ and CD14+ monocytes were differentiated at 1 × 106 cells/mL in RPMI 1640 (Sigma) supplemented with 10% fetal bovine serum (FBS, Sigma) and 20 ng/mL human GM-CSF (Cell Sciences, Canton, MA) at 37 °C with 5% CO2 for 6 days, with one media change at day 3. At day 6, the adherent CD14+ and CD16+ monocyte derived macrophages were washed and rested in RPMI 1640 with 10% FBS overnight. The naïve macrophages were infected with BCG (MOI 1:1) or stimulated with LPS (5 ng/mL) with or without recombinant human lactoferrin (10 μg/mL). At day 3 post-BCG infection, supernatants were collected and analyzed by ELISA. The macrophages were released from the culture plate by incubating with 0.25% trypsin-EDTA (Sigma) at 37 °C for 5–10 min. Trypsin is neutralized using 1% bovine serum albumin (BSA, Gemini Bioproducts, West Sacramento, CA) in 1x PBS at a 2:1 v/v ratio. Macrophages were collected by flushing, not scraping, and immediately placed on ice. Collected macrophages were stained for surface molecule expression and analyzed by flow cytometry.
2.3. ELISA analysis
Supernatants were assayed for cytokine production using the human DuoSet ELISA kits (R&D Systems, Minneapolis, MN), according to manufacturer’s instructions. Human macrophage supernatants were assayed for production of proinflammatory mediators, TNF-α, IL-1β and IL-6, the TH1 mediators IL-12p40 and IL-10, and chemokines IL-8 and MCP-1. Limits of detection are between 1.95 and 31.25 pg/mL depending on the analytic assay.
2.4. Flow cytometry
Collected macrophages were blocked using CD16/32 antibody (BioLegend, San Diego, CA) on ice for 15 min. The macrophages were then stained with anti-human HLA-A,B,C-FITC, HLA-DR-AlexaFluor 700, CD80-PE/Cy7, CD86-PerCP/Cy5.5, CD40-PE, CD1b-APC, and CD1c-pacific blue (BioLegend) on ice for 45 min. Staining buffer and washing buffer was 1% BSA in 1x PBS. Stained cells were fixed in 2% paraformaldehyde in 1x PBS on ice for 15 min. Fixed cells were stored in staining buffer and read on Gallios Flow Cytometer (Beckman-Coulter, Brea, CA). For each sample, 30,000 to 50,000 events were assessed. Analysis was conducted with Kaluza flow cytometry analysis software (Beckman-Coulter).
2.5. Statistics
Buffy coats from four individuals were used to isolate PBMC derived CD14+ and CD16+ monocytes. For each buffy coat sample, 1 to 2 separate macrophage cultures were setup for each stimulation condition. ELISA and flow cytometry data were compared using paired Student’s T test. Significance is determined at p < 0.05.
3. Results
3.1. Recombinant human lactoferrin modulates inflammatory cytokine production from BCG stimulated CD14+ and CD16+ derived macrophages
Macrophages, as a critical responder of the innate immune system, produce several cytokines and chemokines to prepare and influence activity of leukocytes arriving to a localized microenvironment. Production of TNF-α and IL-6 directs formation and maintenance of the granuloma [37], a structure that prevents dissemination of MTB and BCG bacteria from the localized site. MCP-1 (monocyte chemotactic factor) attracts monocytes into the site, and IL-8 recruits neutrophils [38]. Production of high levels of IL-12 and low levels IL-10 influences the differentiation of CD4+ T cells into T cell helper type 1, which upon activation secrete IFN-γ which is critical towards protection against MTB infection [39]. Macrophage responses were therefore investigated in the presence or absence of recombinant human lactoferrin when stimulated with BCG, a known low stimulator of macrophage subset. Comparisons in response were made to LPS, a high macrophage activator of inflammatory responses.
There was a significant difference between the effect of recombinant human lactoferrin on CD14+ and CD16+ monocyte derived macrophages stimulated with BCG or LPS. The effect of recombinant human lactoferrin was observed mainly under LPS stimulation in CD14+ monocyte derived macrophages and under BCG stimulation in CD16+ macrophages. With the exception of IL-1β, generally, CD14+ derived macrophages produced higher levels of cytokines compared to CD16+ derived macrophages under LPS stimulation.
CD14+ monocyte derived macrophages in the presence of human lactoferrin increased production of IL-10, IL-6, IL-1β, and MCP-1 compared to LPS cultured macrophages without lactoferrin. CD16+ monocyte derived macrophages decreased production of IL-12p40, IL-10, TNF-α, and IL-6 when cultured with BCG and human lactoferrin compared to the no lactoferrin cultures (Fig. 1). The addition of recombinant human lactoferrin demonstrated a non-significant decrease in the production of IL-6 and IL-8 in CD16+ monocyte derived macrophages.
Fig. 1. Recombinant human lactoferrin modulation of cytokine and chemokine production from BCG or LPS stimulated CD14+ and CD16+ macrophages.
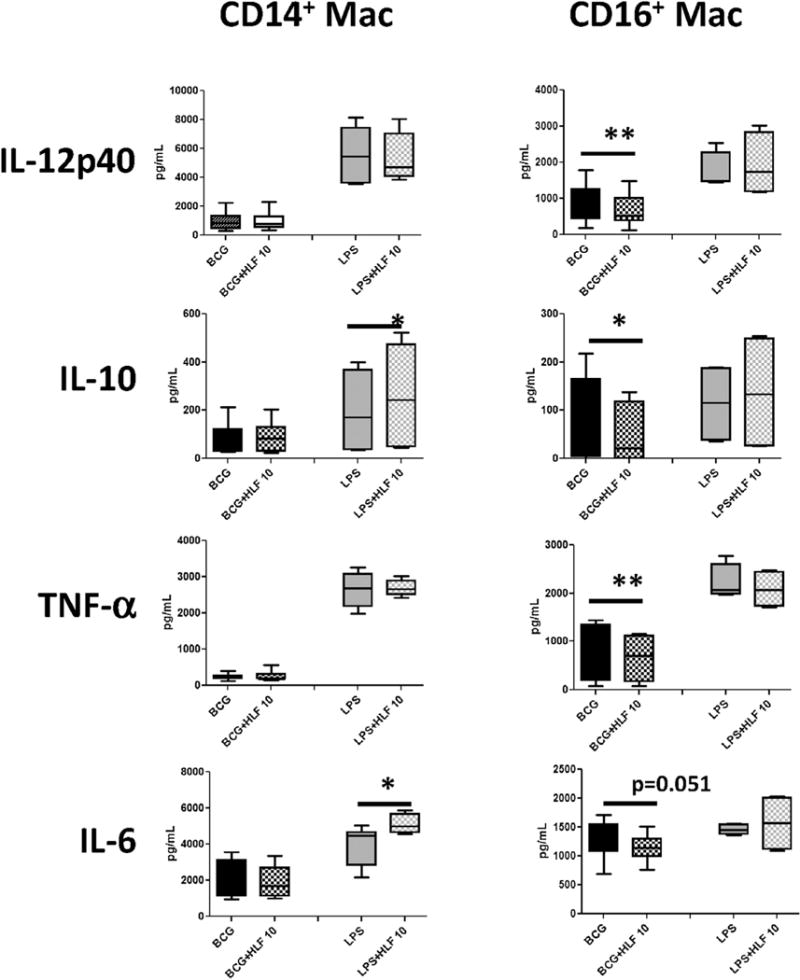
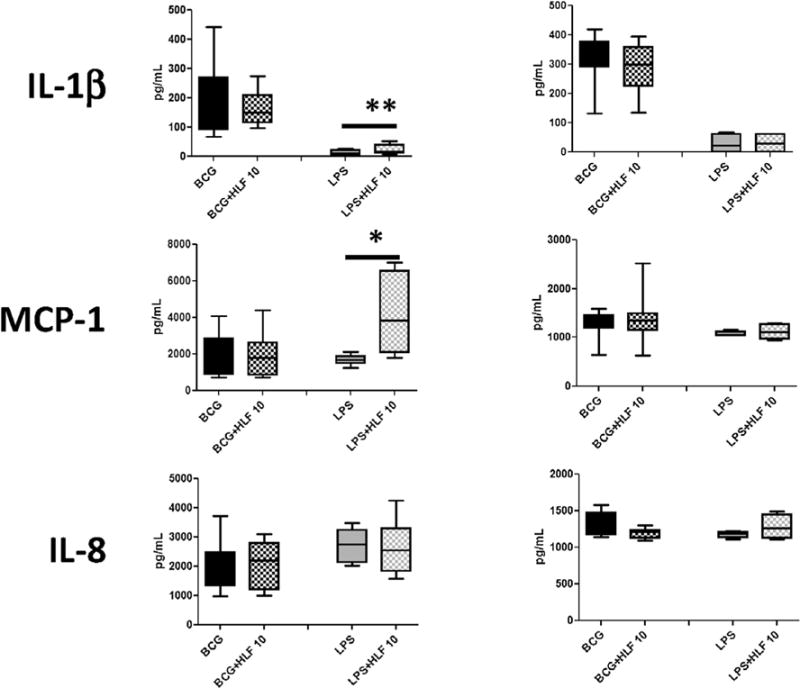
CD14+ and CD16+ monocytes derived macrophages were stimulated with BCG (MOI 1:1) or LPS (5 ng/mL) with or without human lactoferrin (10 μg/mL) for 72 h. Supernatants were collected and analyzed by ELISA for indicated molecules. Data represented as box and whiskers: mean, the box is the 1st and 3rd quartiles, and the error bar is the maximum and minimum. * = p < 0.05 ** = p < 0.01.
Cytokine production from non-stimulated CD14+ and CD16+ derived macrophages produced little to no detectable levels of IL-12p40, IL-10, TNF-α, IL-6, and IL-1β with or without the presence of human lactoferrin. Naïve CD14+ and CD16+ derived macrophages do produce measureable levels of MCP-1 and IL-8 (Supplemental Tables 1 and 2). In the presence of human lactoferrin, naïve CD16+ macrophages demonstrated a significant increase in MCP-1 (Supplemental Table 2).
3.2. Human lactoferrin increased co-stimulatory molecule CD86 in BCG and LPS stimulated macrophages
Macrophages surface expression of presentation and co-stimulatory molecules are responsible for the antigen presentation and activation of receptive T cells, initiation and the expanding memory immunity. Human leukocyte antigen (HLA)-A, B, C presents antigens to CD8+ T cells, whereas HLA-DR presents antigens to CD4+ T cells. Co-stimulatory molecules CD80 and CD86 are the secondary signal required for T cell activation at the time of antigen presentation. CD40 is important for proper macrophage activation during antigen presentation and T cell stimulation [40]. CD1b and CD1c are presentation molecules present lipid antigens to CD1 restricted T cells [41].
The gating strategies are demonstrated in Supplemental Figs. 1–3. Using forward (FS) and side scatter (SS), the intact macrophage population was gated (gate A), with elimination of dead cells and cell fragments that are low for both FS and SS (Supplemental Fig. 1). Macrophages express high levels of class II presentation molecules. Naive CD14+ macrophages express high levels of HLA-DR for nearly 100% of the population (Supplemental Fig. 2). A representative sample of CD14+ monocyte derived macrophages stained with the CD0, CD86, CD40, CD1b, and CD1c are demonstrated in Supplemental Fig. 3. Data not shown for CD16+ monocyte derived macrophages as the same gating strategy was used.
Generally, LPS stimulation increased surface expression of co-stimulatory and CD1 presentation molecules compared to macrophage stimulated with BCG. Both CD14+ and CD16+ monocyte derived macrophages are >99% positive for expression of HLA-A,B,C and HLA-DR (Figs. 2 and 3). CD14+ monocyte derived macrophages stimulated with BCG and LPS in the presence of human lactoferrin demonstrated an increase in CD86 percent positive cells and the CD86 geometric mean (the mean fluorescent intensity) compared to those cultured in the absence of lactoferrin. Interestingly, expression of CD1c showed a trend of increase in LPS/lactoferrin cultured CD14+ monocyte derived macrophages compared to the no lactoferrin macrophages (Fig. 2).
Fig. 2. Surface expression of CD86 altered by recombinant human lactoferrin in both BCG and LPS stimulated CD14+ macrophages.
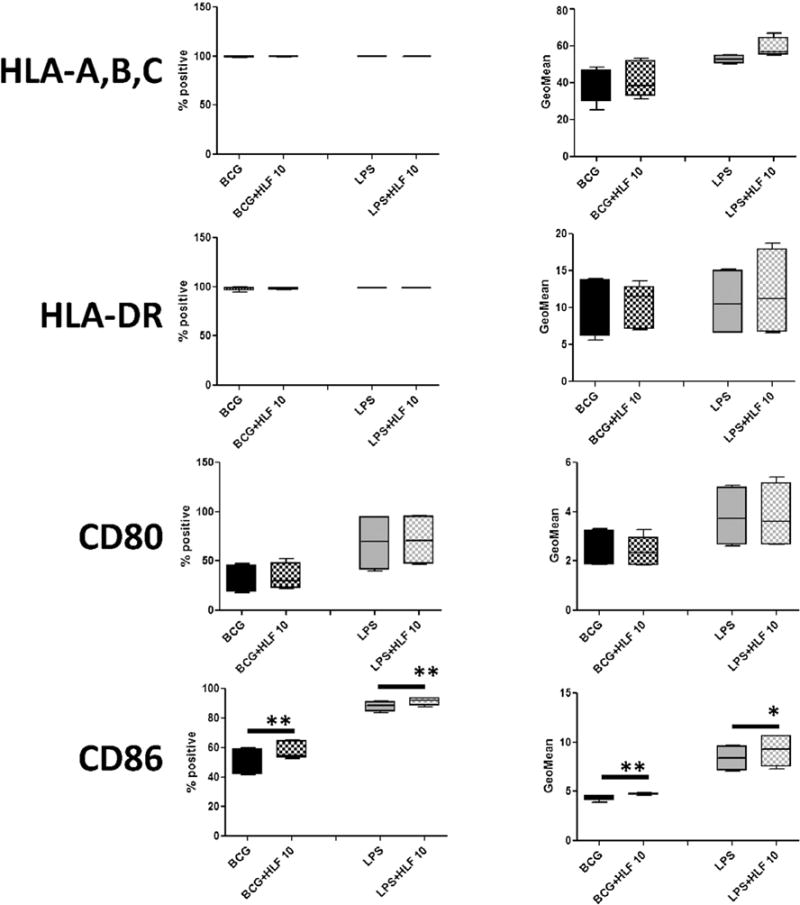
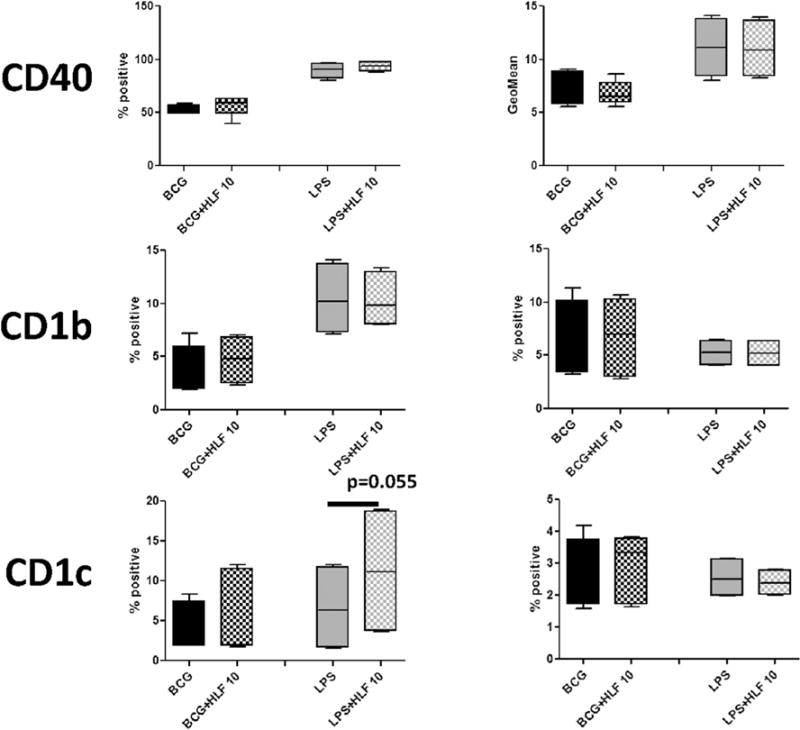
CD14+ monocytes derived macrophages were stimulated with BCG (MOI 1:1) or LPS (5 ng/mL) with or without human lactoferrin (10 μg/mL) for 72 h. Cells were isolated and analyzed by flow cytometry for multiple surface molecules involved with antigen presentation. Data represented as box and whiskers: mean, the box is the 1st and 3rd quartiles, and the error bars is the maximum and minimum. * = p < 0.05 ** = p < 0.01.
Fig. 3. Influence of recombinant human lactoferrin to alter surface molecules involved in antigenic presentation post BCG stimulation of CD16+ macrophages.
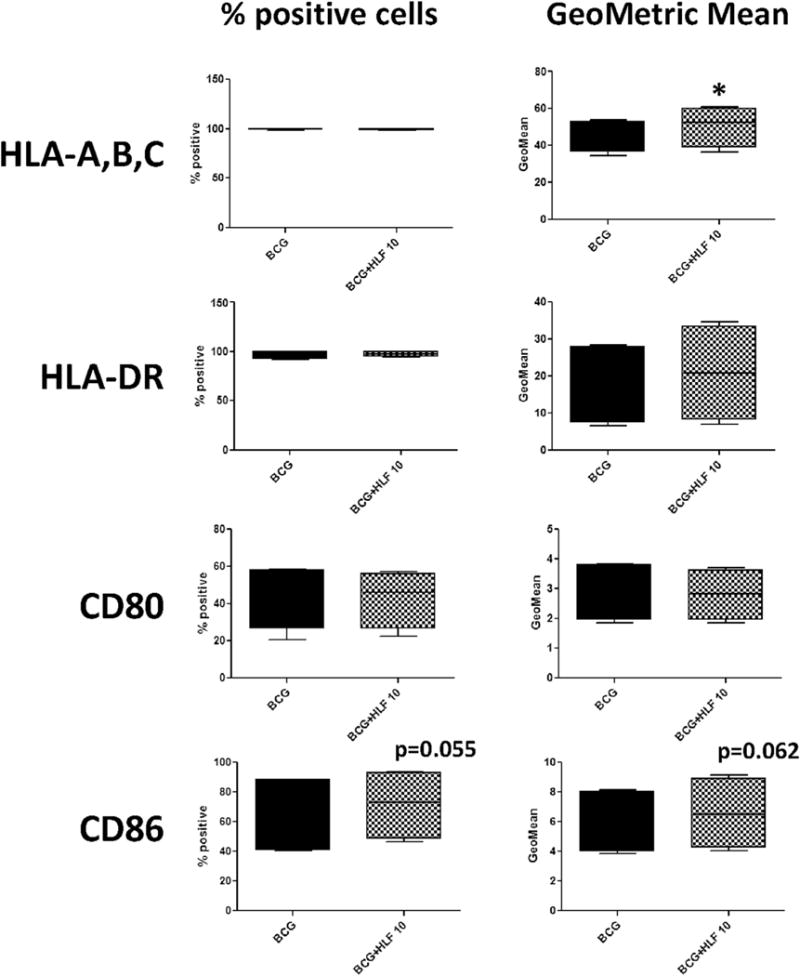
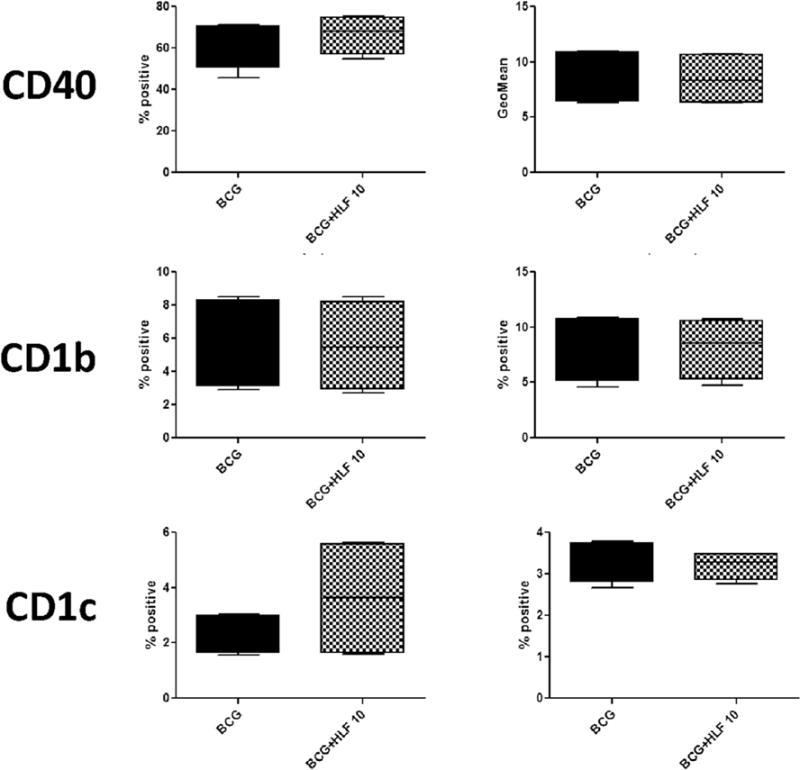
CD14+ monocytes derived macrophages were stimulated with BCG (MOI 1:1) with or without human lactoferrin (10 μg/mL) for 72 h. Cells were isolated and analyzed by flow cytometry for indicated surface antigens. Data represented as box and whiskers: mean, the box is the 1st and 3rd quartiles, and the error bar is the maximum and minimum. * = p < 0.05.
CD16+ monocyte derived macrophages showed a non-significant increase of CD86 percent positive cells and CD86 geometric mean in BCG stimulated cells in the presence of human lactoferrin. In addition, human lactoferrin increased the geometric mean of HLA-A,B,C in CD16+ macrophages (Fig. 3). No effect on any surface markers examined was affected by human lactoferrin the cell stimulated with LPS (Table 1).
Table 1. Lactoferrin effect on surface marker expression post LPS treatment of CD16+ macrophages.
CD16+ monocyte derived macrophages were cultured with and without recombinant human lactoferrin (10 μg/mL) in the presence of LPS (5 ng/mL) for 72 h. Cells were collected and analyzed by flow cytometry for surface marker expression. Data reported as mean ± standard deviation.
| CD16 | % Positive cells
|
Geometric MFI
|
||
|---|---|---|---|---|
| LPS | LPS + HLF 10 | LPS | LPS + HLF 10 | |
| HLA-A,B,C | 99.46 ± 0.51 | 99.52 ± 0.46 | 49.7 ± 0.83 | 51.83 ± 2.35 |
| HLA-DR | 97.73 ± 0.79 | 97.24 ± 0.21 | 10.56 ± 1.68 | 10.49 ± 1.94 |
| CD80 | 68.88 ± 25.85 | 63.93 ± 34.75 | 3.48 ± 0.84 | 3.33 ± 1.17 |
| CD86 | 92.55 ± 2.49 | 91.13 ± 6.03 | 10.98 ± 1.91 | 10.48 ± 2.79 |
| CD40 | 88.86 ± 7.44 | 88.15 ± 11.92 | 11.24 ± 2.43 | 10.34 ± 3.49 |
| CD1b | 11.07 ± 2.79 | 9.09 ± 6.59 | 5.41 ± 0.95 | 5.52 ± 1.34 |
| CD1c | 1.18 ± 0.49 | 2.23 ± 0.13 | 2.77 ± 0.14 | 2.76 ± 0.24 |
In unstimulated macrophages, the addition of lactoferrin did not affect any changes in surface expression of any molecules examined, with the exception of CD86. In naïve CD14+ and CD16+ macrophages, the addition of human lactoferrin increased CD86 percent positive cells and CD86 geometric mean (Supplemental Tables 3 and 4).
4. Discussion
This is the first report of recombinant human lactoferrin to affect BCG and LPS stimulation of CD14+ and CD16+ monocyte derived macrophages, leading to modulation of cytokine production post stimulation. Molecules involved in both antigen presentation and co-stimulatory activity were examined. There was a distinct difference between the effect of human lactoferrin to modulate production of pro-inflammatory mediators between the CD14+ and CD16+ monocyte derived macrophage populations. The presence of human lactoferrin significantly increased cytokine and chemokine production from CD14+ macrophages when a robust stimulant (LPS) was used. In contrast, effect of human lactoferrin was observed primarily to decrease BCG stimulated cytokine and chemokine production in the CD16+ macrophages. There was no difference in the effect of lactoferrin on surface marker expression during BCG or LPS stimulation in either CD14+ or CD16+ monocyte derived macrophages. This preliminary data strongly suggests that human lactoferrin can differentially affect macrophage subsets depending on the relative “strength” of the immunogen.
One consistent effect of human lactoferrin on both BCG and LPS stimulated CD14+ and CD16+ macrophages was the increase in surface expression of CD86. The co-stimulatory molecules, CD86 and CD80, expressed by antigen presenting cells, act as secondary signals necessary for activation of specific T lymphocytes. On T cells, surface molecules CD28 and CTLA-4 are the ligands for CD80 and CD86; interaction with CD28 will activate the T cell during antigen presentation, while binding of CTLA-4 leads to T cell anergy. The regulation of co-stimulatory signals is complex and yet to be fully elucidated. Naïve T cells express CD28, which is downregulated during activation concurrently with upregulation of CTLA-4, as a regulatory step to prevent chronic T cell activation [42]. Recent analysis also suggests that CTLA-4 ligation with CD80 or CD86 can remove expression of CD80 and CD86 from the surface of antigen presenting cells, preventing further interaction with the activating signal, CD28 [43]. Expression of CD86 is expressed at low levels, which is upregulated during activation along with CD80. This, along with biochemical studies suggesting that CD86 prefers CD28, and CD80 prefers CTLA-4 [44–47], indicate that CD86 upregulation can lead to increased T cell activation and increased protection against pathogenic infection. A decrease in expression of CD86 has been correlated with increased severity of infection and sepsis [48–50]. Thus, increased expression of CD86 via addition of recombinant human lactoferrin suggests increased capability of macrophages to activate antigen specific T cells. This subsequently can enhance the host memory response to BCG stimulation to protect against MTB challenge. This activity of lactoferrin has been well documented with bovine, recombinant human and recombinant mouse lactoferrins on mouse bone marrow derived macrophages and dendritic cells. Co-culture experiments demonstrated that CD86 high expressing cells do increase T cell activation [11,12,51,52]. Additionally, this increase in CD86 has been correlated with in vivo data that demonstrated mice immunized with BCG and lactoferrin (bovine and human) enhanced memory recall responses that led to enhanced pathological protection against MTB challenge [13–17,20,53]. Thus, this in vitro human macrophage model is a good model to predict the effect of lactoferrin in vivo.
The non-conventional monocyte (CD16+) population, due to its correlation with inflammatory diseases, is often referred to as the inflammatory monocyte, suggesting that these monocytes demonstrate a more mature phenotype and are more readily activated. Maturation of CD16+ monocytes with GM-CSF has been reported to be directed towards dendritic cell phenotype, rather than the macrophage, which follows the maturation pattern of CD14+ monocytes under GM-CSF [54,55]. However, naïve CD16+ macrophages did not demonstrate any increase in expression of class I (HLA-A,B,C) or class II (HLA-DR) presentation molecules or co-stimulatory molecules CD80, CD86, or CD40 (Supplemental Tables 3 and 4) compared to CD14+ macrophages. The maturation state of CD16+ monocytes differs depending on the culturing condition. In vitro differentiation of CD16+ monocytes without any recombinant protein growth factors, led to a cell phenotype with low surface expression of class II molecules and a pronounced apoptotic state [56].
Of interest, CD16+ monocytes are associated with endotoxin tolerance; LPS induced cytokine production may shift to a MyD88 independent mechanism [57] and a TRIF dependent TLR-4 pathway [58]. This limited data suggest that the CD16+ macrophage may demonstrate changes in LPS induced TLR-4 signaling. Lactoferrin’s mechanism to regulate LPS induced inflammation is thought to occur through the LPS-TLR-4 signaling cascade [59–65]. One potential hypothesis for why recombinant human lactoferrin did not affect CD16+ macrophage cytokine/chemokine production maybe due to the innate changes in the LPS-TLR-4 signaling cascade, compared to CD14+ macrophage response.
The effect of human lactoferrin is distinctly different between CD14+ and CD16+ macrophages in the production of inflammatory mediators. CD14+ monocyte derived macrophages demonstrate an effect of lactoferrin to increase LPS induced IL-10, IL-6, IL-1β, and MCP-1. In contrast, in CD16+ monocyte derived macrophages, presence of lactoferrin decreased BCG promotion of IL-12p40, IL-10, TNF-α, and IL-6. LPS is a stronger stimulant of pro-inflammatory responses compared to BCG, as evident by increased cytokine production. This difference in the effect of recombinant human lactoferrin between the CD14+ and CD16+ macrophages may be due to the inherent mature/inflammatory activation status of the cells. Current published studies suggest that CD16+ monocytes possess an enhanced inflammatory status [66–68], and are thus potentially easier to activate upon antigenic stimulation. Thus, in CD14+ macrophages, BCG stimulation may be below the activation threshold for lactoferrin to be effective.
The studies reported here indicate that recombinant human lactoferrin is effective to modulate the inflammatory responses of CD14+ and CD16+ macrophages during low and high stimulating conditions. The ability of recombinant human lactoferrin to decrease inflammatory cytokine production by CD16+ macrophages during BCG stimulation suggest that a potential mechanism for human lactoferrin mechanism to improve BCG vaccine efficacy may indeed be through decreased activity of the pathological CD16+ macrophage population.
Supplementary Material
Appendix A. Supplementary data
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.tube.2016.09.011.
Footnotes
This work was supported by NIH Grant 1R41-AI117990-01.
References
- 1.Korb VC, Chuturgoon AA, Moodley D. Mycobacterium tuberculosis: manipulator of protective immunity. Int J Mol Sci. 2016;17 doi: 10.3390/ijms17030131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jasenosky LD, Scriba TJ, Hanekom WA, Goldfeld AE. T cells and adaptive immunity to Mycobacterium tuberculosis in humans. Immunol Rev. 2015;264:74–87. doi: 10.1111/imr.12274. [DOI] [PubMed] [Google Scholar]
- 3.Sia JK, Georgieva M, Rengarajan J. Innate immune defenses in human tuberculosis: an overview of the interactions between Mycobacterium tuberculosis and innate immune cells. J Immunol Res. 2015;2015:747543. doi: 10.1155/2015/747543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kruzel ML, Actor JK, Boldogh I, Zimecki M. Lactoferrin in health and disease. Postepy Hig i Med doswiadczalnej. 2007;61:261–7. [PubMed] [Google Scholar]
- 5.Actor JK, Hwang SA, Kruzel ML. Lactoferrin as a natural immune modulator. Curr Pharm Des. 2009;15:1956–73. doi: 10.2174/138161209788453202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siqueiros-Cendon T, Arevalo-Gallegos S, Iglesias-Figueroa BF, Garcia-Montoya IA, Salazar-Martinez J, Rascon-Cruz Q. Immunomodulatory effects of lactoferrin. Acta Pharmacol Sin. 2014;35:557–66. doi: 10.1038/aps.2013.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cutone A, Frioni A, Berlutti F, Valenti P, Musci G, Bonaccorsi di Patti MC. Lactoferrin prevents LPS-induced decrease of the iron exporter ferroportin in human monocytes/macrophages. Biometals. 2014;27:807–13. doi: 10.1007/s10534-014-9742-7. [DOI] [PubMed] [Google Scholar]
- 8.Puddu P, Valenti P, Gessani S. Immunomodulatory effects of lactoferrin on antigen presenting cells. Biochimie. 2009;91:11–8. doi: 10.1016/j.biochi.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 9.Wakabayashi H, Takakura N, Teraguchi S, Tamura Y. Lactoferrin feeding augments peritoneal macrophage activities in mice intraperitoneally injected with inactivated Candida albicans. Microbiol Immunol. 2003;47:37–43. doi: 10.1111/j.1348-0421.2003.tb02783.x. [DOI] [PubMed] [Google Scholar]
- 10.Hwang SA, Wilk KM, Bangale YA, Kruzel ML, Actor JK. Lactoferrin modulation of IL-12 and IL-10 response from activated murine leukocytes. Med Microbiol Immunol. 2007;196:171–80. doi: 10.1007/s00430-007-0041-6. http://dx.doi.org/10.1007/s00430-007-0041-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hwang SA, Actor JK. Lactoferrin modulation of BCG-infected dendritic cell functions. Int Immunol. 2009;21:1185–97. doi: 10.1093/intimm/dxp084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hwang SA, Kruzel ML, Actor JK. Influence of bovine lactoferrin on expression of presentation molecules on BCG-infected bone marrow derived macrophages. Biochimie. 2009;91:76–85. doi: 10.1016/j.biochi.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hwang SA, Arora R, Kruzel ML, Actor JK. Lactoferrin enhances efficacy of the BCG vaccine: comparison between two inbred mice strains (C57BL/6 and BALB/c) Tuberc (Edinb) 2009;89(Suppl 1):S49–54. doi: 10.1016/S1472-9792(09)70012-5. [DOI] [PubMed] [Google Scholar]
- 14.Hwang SA, Kruzel ML, Actor JK. Lactoferrin augments BCG vaccine efficacy to generate T helper response and subsequent protection against challenge with virulent Mycobacterium tuberculosis. Int Immunopharmacol. 2005;5:591–9. doi: 10.1016/j.intimp.2004.11.006. http://dx.doi.org/10.1016/j.intimp.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 15.Hwang SA, Welsh KJ, Boyd S, Kruzel ML, Actor JK. Comparing efficacy of BCG/lactoferrin primary vaccination versus booster regimen. Tuberc (Edinb) 2011;91(Suppl 1):S90–5. doi: 10.1016/j.tube.2011.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hwang SA, Welsh KJ, Kruzel ML, Actor JK. Lactoferrin augmentation of the BCG vaccine leads to increased pulmonary integrity. Tuberc Res Treat. 2011;2011:835410. doi: 10.1155/2011/835410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hwang SA, Wilk KM, Budnicka M, Olsen M, Bangale YA, Hunter RL, et al. Lactoferrin enhanced efficacy of the BCG vaccine to generate host protective responses against challenge with virulent Mycobacterium tuberculosis. Vaccine. 2007;25:6730–43. doi: 10.1016/j.vaccine.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang R, Lonnerdal B. Transcriptomic profiling of intestinal epithelial cells in response to human, bovine and commercial bovine lactoferrins. Biometals. 2014;27:831–41. doi: 10.1007/s10534-014-9746-3. [DOI] [PubMed] [Google Scholar]
- 19.Kruzel ML, Actor JK, Zimecki M, Wise J, Ploszaj P, Mirza S, et al. Novel recombinant human lactoferrin: differential activation of oxidative stress related gene expression. J Biotechnol. 2013;168:666–75. doi: 10.1016/j.jbiotec.2013.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hwang SA, Kruzel ML, Actor JK. CHO expressed recombinant human lactoferrin as an adjuvant for BCG. Int J Immunopathol Pharmacol. 2015;28:452–68. doi: 10.1177/0394632015599832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baeten D, Boots AM, Steenbakkers PG, Elewaut D, Bos E, Verheijden GF, et al. Human cartilage gp-39+, CD16+ monocytes in peripheral blood and synovium: correlation with joint destruction in rheumatoid arthritis. Arthritis Rheum. 2000;43:1233–43. doi: 10.1002/1529-0131(200006)43:6<1233::AID-ANR6>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 22.Heine GH, Ulrich C, Seibert E, Seiler S, Marell J, Reichart B, et al. CD14(++) CD16+ monocytes but not total monocyte numbers predict cardiovascular events in dialysis patients. Kidney Int. 2008;73:622–9. doi: 10.1038/sj.ki.5002744. [DOI] [PubMed] [Google Scholar]
- 23.Moniuszko M, Bodzenta-Lukaszyk A, Kowal K, Lenczewska D, Dabrowska M. Enhanced frequencies of CD14++CD16+, but not CD14+CD16+, blood monocytes in peripheral severe asthmatic patients. Clin Immunol. 2009;130:338–46. doi: 10.1016/j.clim.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 24.Saleh MN, Goldman SJ, LoBuglio AF, Beall AC, Sabio H, McCord MC, et al. CD16+ monocytes in patients with cancer: spontaneous elevation and pharmacologic induction by recombinant human macrophage colony-stimulating factor. Blood. 1995;85:2910–7. [PubMed] [Google Scholar]
- 25.Vanham G, Edmonds K, Qing L, Hom D, Toossi Z, Jones B, et al. Generalized immune activation in pulmonary tuberculosis: co-activation with HIV infection. Clin Exp Immunol. 1996;103:30–4. doi: 10.1046/j.1365-2249.1996.907600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Balboa L, Romero MM, Basile JI, Sabio y Garcia CA, Schierloh P, Yokobori N, et al. Paradoxical role of CD16+CCR2+CCR5+ monocytes in tuberculosis: efficient APC in pleural effusion but also mark disease severity in blood. J Leukoc Biol. 2011;90:69–75. doi: 10.1189/jlb.1010577. [DOI] [PubMed] [Google Scholar]
- 27.Waitt CJ, Banda P, Glennie S, Kampmann B, Squire SB, Pirmohamed M, et al. Monocyte unresponsiveness and impaired IL1beta, TNFalpha and IL7 production are associated with a poor outcome in Malawian adults with pulmonary tuberculosis. BMC Infect Dis. 2015;15:513. doi: 10.1186/s12879-015-1274-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kisacik B, Pamuk ON, Onat AM, Erer SB, Hatemi G, Ozguler Y, et al. Characteristics predicting tuberculosis risk under tumor necrosis factor-alpha inhibitors: report from a large multicenter cohort with high background prevalence. J Rheumatol. 2016;43:524–9. doi: 10.3899/jrheum.150177. [DOI] [PubMed] [Google Scholar]
- 29.Lorenzetti R, Zullo A, Ridola L, Diamanti AP, Lagana B, Gatta L, et al. Higher risk of tuberculosis reactivation when anti-TNF is combined with immunosup-pressive agents: a systematic review of randomized controlled trials. Ann Med. 2014;46:547–54. doi: 10.3109/07853890.2014.941919. [DOI] [PubMed] [Google Scholar]
- 30.Lastrucci C, Benard A, Balboa L, Pingris K, Souriant S, Poincloux R, et al. Tuberculosis is associated with expansion of a motile, permissive and immunomodulatory CD16(+) monocyte population via the IL-10/STAT3 axis. Cell Res. 2015;25:1333–51. doi: 10.1038/cr.2015.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fenton MJ, Vermeulen MW. Immunopathology of tuberculosis: roles of macrophages and monocytes. Infect Immun. 1996;64:683–90. doi: 10.1128/iai.64.3.683-690.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee BY, Jethwaney D, Schilling B, Clemens DL, Gibson BW, Horwitz MA. The Mycobacterium bovis bacille Calmette-Guerin phagosome proteome. Mol Cell Proteom. 2010;9:32–53. doi: 10.1074/mcp.M900396-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sturgill-Koszycki S, Schlesinger PH, Chakraborty P, Haddix PL, Collins HL, Fok AK, et al. Lack of acidification in Mycobacterium phagosomes produced by exclusion of the vesicular proton-ATPase. Science. 1994;263:678–81. doi: 10.1126/science.8303277. [DOI] [PubMed] [Google Scholar]
- 34.Sun J, Deghmane AE, Soualhine H, Hong T, Bucci C, Solodkin A, et al. Mycobacterium bovis BCG disrupts the interaction of Rab7 with RILP contributing to inhibition of phagosome maturation. J Leukoc Biol. 2007;82:1437–45. doi: 10.1189/jlb.0507289. [DOI] [PubMed] [Google Scholar]
- 35.Via LE, Deretic D, Ulmer RJ, Hibler NS, Huber LA, Deretic V. Arrest of mycobacterial phagosome maturation is caused by a block in vesicle fusion between stages controlled by rab5 and rab7. J Biol Chem. 1997;272:13326–31. doi: 10.1074/jbc.272.20.13326. [DOI] [PubMed] [Google Scholar]
- 36.Actor JK. Lactoferrin: a modulator for immunity against tuberculosis related granulomatous pathology. Mediat Inflamm. 2015;2015:409596. doi: 10.1155/2015/409596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Perez RL, Roman J, Roser S, Little C, Olsen M, Indrigo J, et al. Cytokine message and protein expression during lung granuloma formation and resolution induced by the mycobacterial cord factor trehalose-6,6′-dimycolate. J interferon & cytokine Res official J Int Soc Interferon Cytokine Res. 2000;20:795–804. doi: 10.1089/10799900050151067. http://dx.doi.org/10.1089/10799900050151067. [DOI] [PubMed] [Google Scholar]
- 38.Rollins BJ. Chemokines. Blood. 1997;90:909–28. [PubMed] [Google Scholar]
- 39.Flynn JL, Chan J. Immunology of tuberculosis. Annu Rev Immunol. 2001;19:93–129. doi: 10.1146/annurev.immunol.19.1.93. [DOI] [PubMed] [Google Scholar]
- 40.Behar SM, Carpenter SM, Booty MG, Barber DL, Jayaraman P. Orchestration of pulmonary T cell immunity during Mycobacterium tuberculosis infection: immunity interruptus. Semin Immunol. 2014;26:559–77. doi: 10.1016/j.smim.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.De Libero G, Mori L. The t-cell response to lipid antigens of Mycobacterium tuberculosis. Front Immunol. 2014;5:219. doi: 10.3389/fimmu.2014.00219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen L, Flies DB. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol. 2013;13:227–42. doi: 10.1038/nri3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Qureshi OS, Zheng Y, Nakamura K, Attridge K, Manzotti C, Schmidt EM, et al. Trans-endocytosis of CD80 and CD86: a molecular basis for the cell-extrinsic function of CTLA-4. Science. 2011;332:600–3. doi: 10.1126/science.1202947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bhatia S, Edidin M, Almo SC, Nathenson SG. B7-1 and B7-2: similar costimulatory ligands with different biochemical, oligomeric and signaling properties. Immunol Lett. 2006;104:70–5. doi: 10.1016/j.imlet.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 45.Collins M, Ling V, Carreno BM. The B7 family of immune-regulatory ligands. Genome Biol. 2005;6:223. doi: 10.1186/gb-2005-6-6-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lang TJ, Nguyen P, Peach R, Gause WC, Via CS. In vivo CD86 blockade inhibits CD4+ T cell activation, whereas CD80 blockade potentiates CD8+ T cell activation and CTL effector function. J Immunol. 2002;168:3786–92. doi: 10.4049/jimmunol.168.8.3786. [DOI] [PubMed] [Google Scholar]
- 47.Pentcheva-Hoang T, Egen JG, Wojnoonski K, Allison JP. B7-1 and B7-2 selectively recruit CTLA-4 and CD28 to the immunological synapse. Immunity. 2004;21:401–13. doi: 10.1016/j.immuni.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 48.Nolan A, Kobayashi H, Naveed B, Kelly A, Hoshino Y, Hoshino S, et al. Differential role for CD80 and CD86 in the regulation of the innate immune response in murine polymicrobial sepsis. PLoS One. 2009;4:e6600. doi: 10.1371/journal.pone.0006600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nolan A, Weiden M, Kelly A, Hoshino Y, Hoshino S, Mehta N, et al. CD40 and CD80/86 act synergistically to regulate inflammation and mortality in poly-microbial sepsis. Am J Respir Crit Care Med. 2008;177:301–8. doi: 10.1164/rccm.200703-515OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wolk K, Hoflich C, Zuckermann-Becker H, Docke WD, Volk HD, Sabat R. Reduced monocyte CD86 expression in postinflammatory immunodeficiency. Crit Care Med. 2007;35:458–67. doi: 10.1097/01.CCM.0000254724.54515.2F. [DOI] [PubMed] [Google Scholar]
- 51.Hwang SA, Kruzel ML, Actor JK. Effects of CHO-expressed recombinant lactoferrins on mouse dendritic cell presentation and function. Innate Immun. 2015;21:553–61. doi: 10.1177/1753425914564609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.O’Shea KM, Hwang SA, Actor JK. Immune activity of BCG infected mouse macrophages treated with a novel recombinant mouse lactoferrin. Ann Clin Lab Sci. 2015;45:487–94. [PubMed] [Google Scholar]
- 53.Hwang SA, Wilk K, Kruzel ML, Actor JK. A novel recombinant human lactoferrin augments the BCG vaccine and protects alveolar integrity upon infection with Mycobacterium tuberculosis in mice. Vaccine. 2009;27:3026–34. doi: 10.1016/j.vaccine.2009.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Arcila ML, Sanchez MD, Ortiz B, Barrera LF, Garcia LF, Rojas M. Activation of apoptosis, but not necrosis, during Mycobacterium tuberculosis infection correlated with decreased bacterial growth: role of TNF-alpha, IL-10, caspases and phospholipase A2. Cell Immunol. 2007;249:80–93. doi: 10.1016/j.cellimm.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 55.Grage-Griebenow E, Zawatzky R, Kahlert H, Brade L, Flad H, Ernst M. Identification of a novel dendritic cell-like subset of CD64(+)/CD16(+) blood monocytes. Eur J Immunol. 2001;31:48–56. doi: 10.1002/1521-4141(200101)31:1<48::aid-immu48>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 56.Castano D, Garcia LF, Rojas M. Increased frequency and cell death of CD16+ monocytes with Mycobacterium tuberculosis infection. Tuberc (Edinb) 2011;91:348–60. doi: 10.1016/j.tube.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 57.Dominguez-Nieto A, Zentella A, Moreno J, Ventura JL, Pedraza S, Velazquez JR. Human endotoxin tolerance is associated with enrichment of the CD14+ CD16+ monocyte subset. Immunobiology. 2015;220:147–53. doi: 10.1016/j.imbio.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 58.Shalova IN, Kajiji T, Lim JY, Gomez-Pina V, Fernandez-Ruiz I, Arnalich F, et al. CD16 regulates TRIF-dependent TLR4 response in human monocytes and their subsets. J Immunol. 2012;188:3584–93. doi: 10.4049/jimmunol.1100244. [DOI] [PubMed] [Google Scholar]
- 59.Ando K, Hasegawa K, Shindo K, Furusawa T, Fujino T, Kikugawa K, et al. Human lactoferrin activates NF-kappaB through the Toll-like receptor 4 pathway while it interferes with the lipopolysaccharide-stimulated TLR4 signaling. Febs J. 2010;277:2051–66. doi: 10.1111/j.1742-4658.2010.07620.x. [DOI] [PubMed] [Google Scholar]
- 60.Appelmelk BJ, An YQ, Geerts M, Thijs BG, de Boer HA, MacLaren DM, et al. Lactoferrin is a lipid A-binding protein. Infect Immun. 1994;62:2628–32. doi: 10.1128/iai.62.6.2628-2632.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Baveye S, Elass E, Fernig DG, Blanquart C, Mazurier J, Legrand D. Human lactoferrin interacts with soluble CD14 and inhibits expression of endothelial adhesion molecules, E-selectin and ICAM-1, induced by the CD14-lipopolysaccharide complex. Infect Immun. 2000;68:6519–25. doi: 10.1128/iai.68.12.6519-6525.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cohen MS, Mao J, Rasmussen GT, Serody JS, Britigan BE. Interaction of lactoferrin and lipopolysaccharide (LPS): effects on the antioxidant property of lactoferrin and the ability of LPS to prime human neutrophils for enhanced superoxide formation. J Infect Dis. 1992;166:1375–8. doi: 10.1093/infdis/166.6.1375. [DOI] [PubMed] [Google Scholar]
- 63.Elass-Rochard E, Legrand D, Salmon V, Roseanu A, Trif M, Tobias PS, et al. Lactoferrin inhibits the endotoxin interaction with CD14 by competition with the lipopolysaccharide-binding protein. Infect Immun. 1998;66:486–91. doi: 10.1128/iai.66.2.486-491.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Miyazawa K, Mantel C, Lu L, Morrison DC, Broxmeyer HE. Lactoferrin-lipo-polysaccharide interactions. Effect on lactoferrin binding to monocyte/ macrophage-differentiated HL-60 cells. J Immunol. 1991;146:723–9. [PubMed] [Google Scholar]
- 65.Spadaro M, Montone M, Arigoni M, Cantarella D, Forni G, Pericle F, et al. Recombinant human lactoferrin induces human and mouse dendritic cell maturation via Toll-like receptors 2 and 4. Faseb J. 2014;28:416–29. doi: 10.1096/fj.13-229591. [DOI] [PubMed] [Google Scholar]
- 66.Aguilar-Ruiz SR, Torres-Aguilar H, Gonzalez-Dominguez E, Narvaez J, Gonzalez-Perez G, Vargas-Ayala G, et al. Human CD16+ and CD16− monocyte subsets display unique effector properties in inflammatory conditions in vivo. J Leukoc Biol. 2011;90:1119–31. doi: 10.1189/jlb.0111022. [DOI] [PubMed] [Google Scholar]
- 67.Belge KU, Dayyani F, Horelt A, Siedlar M, Frankenberger M, Frankenberger B, et al. The proinflammatory CD14+CD16+DR++ monocytes are a major source of TNF. J Immunol. 2002;168:3536–42. doi: 10.4049/jimmunol.168.7.3536. [DOI] [PubMed] [Google Scholar]
- 68.Szaflarska A, Baj-Krzyworzeka M, Siedlar M, Weglarczyk K, Ruggiero I, Hajto B, et al. Antitumor response of CD14+/CD16+ monocyte subpopulation. Exp Hematol. 2004;32:748–55. doi: 10.1016/j.exphem.2004.05.027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


