Abstract
The effects of obesity on bone metabolism are complex, and may be mediated by consumption of a high fat diet and/or by obesity-induced metabolic dysregulation. To test the hypothesis that both high fat (HF) diet and diet-induced metabolic disease independently decrease skeletal acquisition, we compared effects of HF diet on bone mass and microarchitecture in two mouse strains: diet-induced obesity (DIO)-susceptible C57BL/6J (B6) and DIO-resistant FVB/NJ (FVB). At 3 wks of age we weaned 120 female FVB and B6 mice onto normal (N, 10% Kcal/fat) or HF diet (45% Kcal/fat) and euthanized them at 6, 12 and 20 weeks of age (N = 10/grp). Outcomes included body mass; percent fat and whole-body bone mineral density (WBBMD, g/cm2) via DXA; cortical and trabecular bone architecture at the midshaft and distal femur via μCT; and marrow adiposity via histomorphometry. In FVB HF, body mass, percent body fat, WBBMD and marrow adiposity did not differ vs. N, but trabecular bone mass was lower at 6 wks of age only (p < 0.05), cortical bone geometric properties were lower at 12 wks only, and bone strength was lower at 20 wks of age only in HF vs. N (p < 0.05). In contrast, B6 HF had higher body mass, percent body fat, and leptin vs. N. B6 HF also had higher WBBMD (p < 0.05) at 9 and 12 wks of age but lower distal femur trabecular bone mass at 12 wks of age, and lower body mass-adjusted cortical bone properties at 20 wks of age compared to N (p < 0.05). Marrow adiposity was also markedly higher in B6 HF vs. N. Overall, HF diet negatively affected bone mass in both strains, but was more deleterious to trabecular bone microarchitecture and marrow adiposity in B6 than in FVB mice. These data suggest that in addition to fat consumption itself, the metabolic response to high fat diet independently alters skeletal acquisition in obesity.
Keywords: High fat diet, Leptin, Bone, Mouse, Bone density, Bone acquisition
Highlights
-
•
High fat diet causes metabolic dysfunction and bone loss in obesity-prone mice.
-
•
Obesity-resistant mice on high fat diet have few metabolic or skeletal changes.
-
•
Diet-induced obesity and metabolic disease decrease skeletal acquisition in mice.
1. Introduction
Obesity has complex effects on bone mass and skeletal fragility. High body mass increases bone mineral density (BMD, g/cm2) via greater mechanical loading, and soft tissue padding dampens impact forces during falls (Felson et al., 1993; Bouxsein et al., 2007), such that body mass index (BMI) is correlated positively with BMD (Morin et al., 2009; Looker et al., 2007; Reid et al., 1992; Albala et al., 1996) and negatively with fragility fractures of the hip (De Laet et al., 2005). However, recent studies have challenged the notion that high body mass is unequivocally beneficial to bone. First, the skeleton adapts most closely to lean body mass, such that individuals with low lean mass and high percent body fat may have lower BMD than expected for their total body mass (Greco et al., 2010; Aguirre et al., 2014; Wang et al., 2005; Nunez et al., 2007; Beck et al., 2009). Second, obese men and women are at elevated risk for humerus and ankle fractures, suggesting lower resistance to fracture (Nielson et al., 2011; Compston et al., 2011; Premaor et al., 2010; Prieto-Alhambra et al., 2012). Finally, obesity-induced inflammation increases adipocytokine levels and decreases BMD in humans (Aguirre et al., 2014).
Potential detrimental effects of fat mass on bone health are of particular concern in adolescents who are obese during the time of peak bone mass acquisition (Bailey, 1997; Bonjour et al., 1991). Obese children have higher fracture risk and lower bone mass than expected for their height and weight (Goulding et al., 2000; Eliakim et al., 2001; Whiting, 2002; Petit et al., 2005), suggesting a failure of skeletal adaptation to total body mass (Dimitri et al., 2012; Pollock et al., 2011; Pollock et al., 2007; Hoy et al., 2012), and obesity-induced chronic inflammation is associated with lower adolescent BMD (Russell et al., 2010; Lucas et al., 2012). Understanding the mechanisms by which high fat mass impairs skeletal acquisition is of critical importance because low young adult bone mass is a risk factor for skeletal fragility later in life (Seeman, 1994; Hui et al., 1990). By one estimate, a 10% increase in peak bone mass may decrease adult fracture risk by up to 50% (Bonjour et al., 2007). Conversely, failure to reach peak bone mass likely increases the incidence of later osteoporosis and fracture (Loud and Gordon, 2006).
Understanding the interrelationships between obesity and bone mass is challenging because several of the factors contributing to obesity, including diet, heredity, activity level, socioeconomic status, and metabolic disease, can also directly affect bone. The goal of this study is to delineate the impact of high fat intake vs. the impact of diet-induced obesity (DIO) due to a high calorie diet on skeletal acquisition. Although obesity results from overconsumption, and is often linked to high carbohydrate diets, Americans also tend to have a high fat intake (Millen et al., 2016), which is associated with lower BMD and higher fracture risk compared to unsaturated fats (Longo and Ward, 2016). Previous studies using rodent models to test the effects of DIO on bone have focused on high saturated fat (HF) diets, and report such diets increase (Lecka-Czernik et al., 2015; Ma et al., 2011), decrease (Fujita et al., 2012; Gautam et al., 2014; Shu et al., 2015; Cao et al., 2009; Bornstein et al., 2017), or do not change (Doucette et al., 2015; Ackert-Bicknell et al., 2008) bone mass ign wildtype mice, likely due to differences in experimental design. Fewer experimental studies have used diets high in polyunsaturated fats, although HF corn oil diets rich in omega 6 fatty acids decrease bone mass in mice (Halade et al., 2010) and rats (Yan et al., 2015), whereas omega 3 fatty acids are beneficial to bone mass in rodent models (Bonnet et al., 2014; Lukas et al., 2011).
Here we sought to test whether it is the type of calories (primarily saturated fat vs. primarily carbohydrate) or the number of calories (with chronic overconsumption leading to obesity) that causes bone loss. The overall hypothesis is that both high fat diet itself and the resulting metabolic dysfunction, including obesity, inflammation and impaired glucose tolerance, independently decrease skeletal acquisition. To test this hypothesis, we compared the effect of high saturated fat (HF) diet on bone mass and microarchitecture during rapid skeletal growth in two inbred mouse strains: FVB/J, which do not overeat on HF diet and thus are resistant to DIO, and C57BL/6J (B6), which overeat on HF diet and are DIO-susceptible (Hu et al., 2004; Lin et al., 2000). The prediction is that the combination of high saturated fat diet, obesity, and metabolic dysfunction in B6 mice will be more deleterious to skeletal acquisition compared to high saturated fat diet in DIO-resistant FVB mice.
2. Materials and methods
2.1. Dietary intervention
We obtained 3-wk-old female FVB/NJ (FVB) and C57BL/6J (B6) mice (The Jackson Laboratory, Bar Harbor, ME) and randomized them to normal (N) or high fat (HF) diet. N diet mice were fed a purified, phytoestrogen-free diet ad libitum (Research Diets 12450B, 10% kcal/fat (4% lard +6% soybean oil), 20% protein, 70% carbohydrate). HF mice were fed a high-fat purified, phytoestrogen-free diet ad libitum (Research Diets 12451, 45% kcal/fat (39% lard +6% soybean oil), 20% protein, 35% carbohydrate). The HF diet was 50% lower in carbohydrate vs. the N diet (7% vs. 31% corn starch, 17% vs. 35% sucrose, 10% vs. 3% maltodextrin). Fiber and micronutrient content of the experimental diets were identical. Mouse body mass and food intake were measured three times per week on a digital scale. Mice were sacrificed by CO2 inhalation at 6, 12 or 20 wks of age (N = 10/group for each strain, diet, and age).
2.2. Glucose tolerance
Glucose tolerance tests (GTT) were performed at 12 wks of age using a OneTouch Ultra blood glucose monitor (LifeScan, Inc., Milpitas, CA). Briefly, after an overnight fast, a baseline blood glucose measurement was made via tail nick, mice were injected IP with sterile glucose solution (1 g/kg), and blood glucose was measured at 15, 30, 60, and 120 min following injection (Heikkinen et al., 2007).
2.3. Bone mineral density and body composition
Longitudinal in vivo assessment of whole body (exclusive of the head) bone mineral density (WBBMD, g/cm2), bone mineral content per body mass (WBBMC/BM, g/g) and body composition (percent body fat) was performed at 3, 6, 9, 12, and 20 wks of age using peripheral dual-energy x-ray absorptiometry (pDXA, PIXImusII, GE Lunar Corp.), as previously described (Bouxsein et al., 2009; Bouxsein et al., 2005; Ferrari et al., 2005).
2.4. Specimen harvesting/preparation
Gonadal white adipose depots were excised and weighed. Femurs and lumbar vertebrae were harvested and cleaned of soft tissue. The right femur and L5 vertebral body were prepared for imaging and biomechanical testing by wrapping in saline-soaked gauze and freezing at −20 °C as previously described (Devlin et al., 2010). The left femur was prepared for histology in 70% ethanol at 4 °C until processing.
2.5. Trabecular and cortical bone morphology by μCT
Bone morphology and microarchitecture were assessed with high-resolution microcomputed tomography (μCT40, Scanco Medical, Brüttisellen, Switzerland), as previously described (Glatt et al., 2007). In brief, the distal femoral metaphysis and L5 vertebral body were scanned using a 12 μm3 isotropic voxel size, 70 kVP, 114 mA, and 200 ms integration time, and were subjected to Gaussian filtration and segmentation. At the distal femur, evaluation of transverse CT slices began at 360 μm proximal to the distal growth plate and continued 1800 μm proximally. Images were thresholded using an adaptive-iterative algorithm.(Ridler and Calvard, 1978; Meinel et al., 2005; Rajagopalan et al., 2005) The average adaptive-iterative threshold of all specimens from each strain was applied as a fixed threshold to segment bone from soft tissue (FVB: 296 mg hydroxyapatite (HA)/cm3, B6: 256 mg HA/cm3). At the femoral mid-diaphysis, transverse μCT slices spanning 600 μm were obtained to assess cortical bone parameters and segmented using a fixed threshold of 670 mg HA/cm3. In the fifth lumbar vertebra, transverse CT slices were evaluated throughout the body of each vertebra (2400–3000 μm), excluding the superior and inferior growth plates. As in the distal femur, the average adaptive-iterative threshold of all specimens from each strain was used as a fixed threshold to segment bone from soft tissue (FVB: 296 mg hydroxyapatite (HA)/cm3, B6: 318 mg HA/cm3). In the trabecular bone region we assessed bone volume fraction (BV/TV, %), trabecular thickness (Tb.Th, μm), trabecular separation (Tb.Sp, μm), trabecular number (Tb.N, 1/mm), connectivity density (ConnD 1/mm3), and structure model index (SMI). At the femoral midshaft we measured total cross-sectional area, cortical bone area, and medullary area (TA, BA and MA, mm2); bone area fraction (BA/TA, %), cortical thickness (μm), antero-posterior and medio-lateral diameters; and area moments of inertia. Procedures followed the 2010 recommendations of the American Society for Bone and Mineral Research (Bouxsein et al., 2010).
2.6. Bone strength testing
Following μCT scanning, the strength of the femoral midshaft was assessed by 3-point bending (by applying a flexion moment in the anterior-posterior plane) using previously described methods (35,40–42). Briefly, specimens were thawed to room temperature in a calcium-buffered saline bath, to ensure adequate hydration. A low force mechanical testing system (Bose Electroforce 3230, with 100 N load cell) was used to apply constant displacement at a rate of 0.03 mm/s. The distance between load supports was 8.0 mm. Force-displacement data were used to determine structural properties (ultimate moment (N*mm), bending stiffness (N*mm/mm) and post-yield displacement (mm)), and then data for each specimen were adjusted for the appropriate femoral midshaft area moment of inertia, as measured on the μCT scans, to derive estimated elastic modulus (GPa).
2.7. Serum hormones
Blood was collected at sacrifice by cardiac puncture and ELISA was used to measure serum leptin, as previously described (Devlin et al., 2010; Devlin et al., 2014).
2.8. Marrow adiposity
Marrow adiposity was quantified using static histomorphometry as previously described (Devlin et al., 2010). Terminology and units followed the recommendations of the Histomorphometry Nomenclature Committee of the American Society for Bone and Mineral Research (Dempster et al., 2013).
2.9. Statistical analyses
The effects of diet, strain and age on bone variables were evaluated using a full factorial General Linear Model (GLM) in SPSS (IBM, Armonk, NY). If the overall model indicated a significant effect of diet, diet*age, diet*strain, or diet*age*strain, we then stratified the GLM by age and strain to obtain p values for diet (HF vs. N) specific to a given strain at a given age. Only such significant pairwise differences are reported in the results (alpha = 0.05). To adjust for body mass differences, we regressed the variable of interest (i.e., bone cross-sectional properties) vs. body mass and compared residuals (Devlin et al., 2010).
3. Results
3.1. Body size and composition, whole-body BMD, and hormone levels
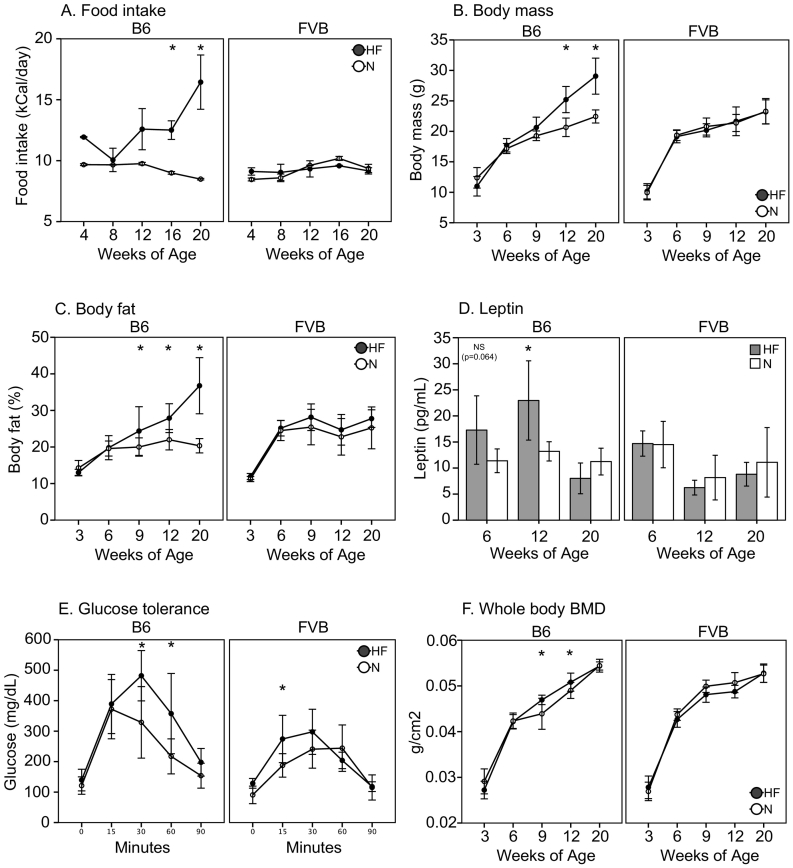
HF diet had strain-dependent effects on food intake, body mass, body composition, leptin levels, and bone density. In DIO-resistant FVB mice, caloric intake (kCal/day) did not differ between N and HF diets, and food consumption remained stable over time (Fig. 1A). There were no differences between FVB N and HF in body mass, body fat (%) by DXA, femur length, gonadal white adipose tissue mass, or serum leptin (Fig. 1B–D, Table 1). FVB HF mice had modestly impaired glucose tolerance at 0–15 min by GTT (p < 0.05, Fig. 1E). Longitudinal in vivo DXA showed no differences in WBBMD or in whole body bone mineral content per body mass (WBBMC/BM) in FVB HF vs. N (Fig. 1F).
Fig. 1.
A. Food intake (kCal/day), B. Body mass (g), C. Body fat (%) via Piximus, D. Leptin (ng/ml), E. Glucose tolerance at 12 wks of age (mg/dl), F. Whole body bone mineral density (WBBMD, g/cm2). Significant differences as shown (*p < 0.05).
Table 1.
Body size and body composition in female FVB/J and C57BL/6J mice fed a normal (N) or high fat (HF) diet from 3 wks of age to 6, 12, or 20 wks of age (mean ± SD).
| B6 |
FVB |
Pdiet | Pstrain⁎ diet | Pstrain⁎diet⁎age | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 6 wks |
12 wks |
20 wks |
6 wks |
12 wks |
20 wks |
||||||||||||||||||||||
| N |
HF |
N |
HF |
N |
HF |
N |
HF |
N |
HF |
N |
HF |
||||||||||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||||
| Femur length (mm) | 13.88 | 0.64 | 14.21 | 0.26 | 15.01 | 0.21 | 15.29 | 0.22 | 15.77 | 0.26 | 15.91 | 0.17 | 13.28 | 0.34 | 13.59 | 0.35 | 14.59 | 0.22 | 14.62 | 0.18 | 15.06 | 0.24 | 15.01 | 0.23 | 0.002 | NS | NS |
| WBBMC/BM (g/g * 100) | 1.58 | 0.19 | 1.62 | 0.12 | 1.86 | 0.12 | 1.64 | 0.16 | 2.10 | 0.07 | 1.61 | 0.16 | 1.55 | 0.12 | 1.52 | 0.11 | 1.88 | 0.19 | 1.84 | 0.22 | 1.94 | 0.12 | 1.78 | 0.12 | ≤0.002 | NS | NS |
| Gonadal WAT (g) | 0.28 | 0.08 | 0.27 | 0.10 | 0.34 | 0.09 | 0.79 | 0.29 | 0.27 | 0.04 | 1.30 | 0.45 | 0.47 | 0.16 | N/A | N/A | 0.58 | 0.17 | 0.66 | 0.20 | 0.77 | 0.30 | 0.96 | 0.35 | <0.001 | <0.001 | 0.028 |
Bolded values indicate significant pairwise differences within each strain at a given age (p < 0.05). Pairwise comparisons were performed for variables with overall significance in the GLM, as shown at right.
In contrast, DIO-prone B6 mice exhibited hyperphagia on HF diet from 16 to 20 wks of age, consuming 40–93% more calories vs. N (p < 0.05 for both, Fig. 1A). B6 mice on HF diet were heavier, had higher percent body fat by DXA and higher gonadal white adipose tissue mass, and had longer femurs vs. mice on N diet (p < 0.05 for all, Fig. 1B–C, Table 1). B6 HF mice also had 74% higher leptin levels vs. B6 N at 12 wks of age (p = 0.012, Fig. 1D). Glucose tolerance was substantially impaired in B6 HF vs. N from 30 to 90 min (p < 0.05, Fig. 2E). WBBMD was higher in B6 HF vs. N mice from 6 to 12 wks of age (p < 0.05, Fig. 1F), whereas WBBMC/BM was lower from 9 to 20 wks of age in B6 HF vs. N (p < 0.05, Table 1).
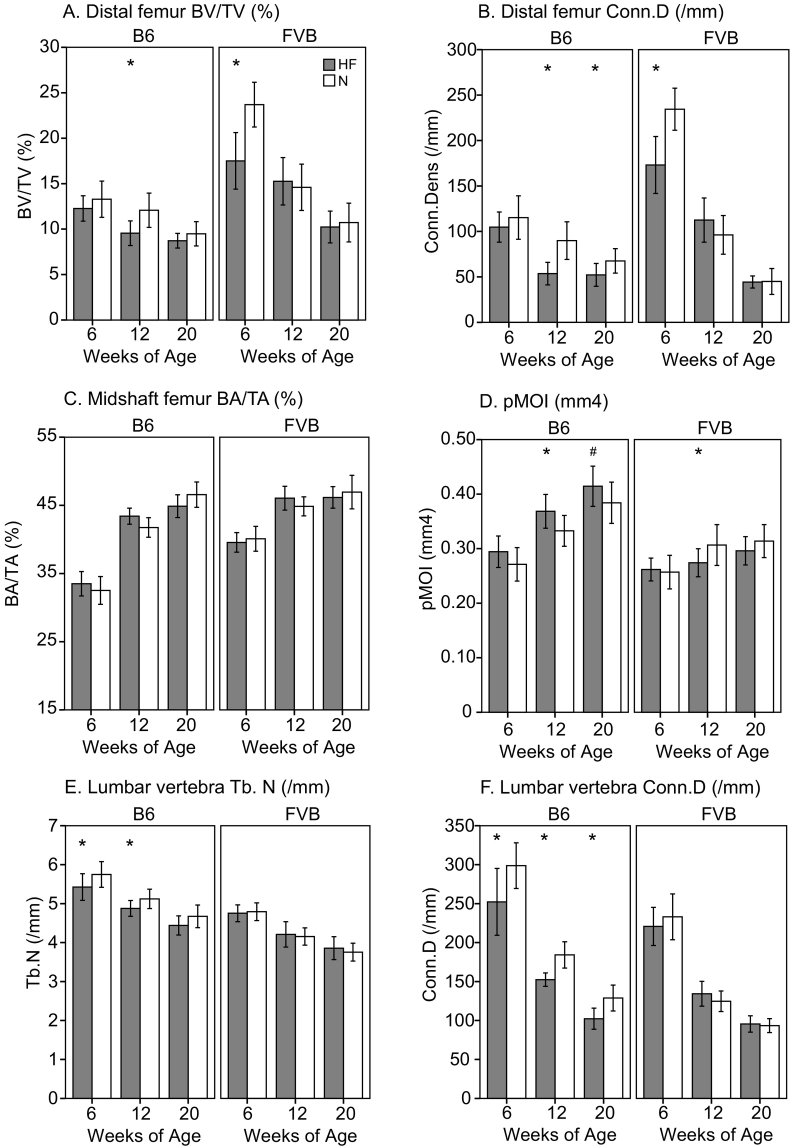
Fig. 2.
A. Distal femur BV/TV (%), B. Distal femur connectivity density (/mm), C. Midshaft femur BA/TA (%), D. Midshaft femur pMOI (mm4), E. Lumbar vertebra Tb.N (/mm), F. Lumbar vertebra connectivity density (/mm). Significant differences (p < 0.05) as shown for unadjusted data (*); following body mass adjustment, pMOI was lower in B6 HF vs. N mice (#).
3.2. Bone morphology: Trabecular bone at distal femur and cortical bone at midshaft femur
In DIO-resistant FVB, HF diet was associated with early deficits in trabecular bone at the distal femur that attenuated at later ages. FVB HF had worse trabecular architecture than N mice at 6 weeks of age (p < 0.05 for all, Figs. 2A–B and Table 2), but not at 12 and 20 wks of age. In contrast, in B6 mice, HF diet was associated with consistent decrements in trabecular bone at 12 and 20 wks of age, with significantly lower Tb.BV/TV (−21%), Tb.N (−14%), and Conn.D (−40%) and higher Tb.Sp (17%) and SMI (+10%) at 12 weeks of age, and lower Tb.N (−6%) and Conn.D (−23%) and higher Tb.Sp (+7%) at 20 weeks of age (p ≤ 0.04 for all, Fig. 2A–B and Table 2).
Table 2.
Trabecular & cortical bone properties in female FVB/NJ and C57BL/6J mice fed a normal (N) or high fat (HF) diet from 3 wks of age to 6, 12, or 20 wks of age (mean ± SD).
| B6 |
FVB |
Pdiet | Pstrain⁎diet | Pstrain⁎diet⁎age | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 6 wks |
12 wks |
20 wks |
6 wks |
12 wks |
20 wks |
||||||||||||||||||||||
| N |
HF |
N |
HF |
N |
HF |
N |
HF |
N |
HF |
N |
HF |
||||||||||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||||
| Distal femur (trabecular) | |||||||||||||||||||||||||||
| Tb.N (/mm) | 4.46 | 0.23 | 4.39 | 0.23 | 4.15 | 0.26 | 3.59 | 0.17 | 3.47 | 0.16 | 3.28 | 0.21 | 5.65 | 0.29 | 5.09 | 0.31 | 4.01 | 0.21 | 4.17 | 0.37 | 3.20 | 0.37 | 3.13 | 0.23 | <0.001 | NS | <0.001 |
| Tb.Th (mm) | 0.050 | 0.002 | 0.050 | 0.001 | 0.054 | 0.002 | 0.056 | 0.002 | 0.055 | 0.003 | 0.058 | 0.004 | 0.056 | 0.002 | 0.052 | 0.002 | 0.056 | 0.002 | 0.055 | 0.001 | 0.059 | 0.002 | 0.058 | 0.002 | NS | <0.001 | NS |
| Tb.Sp (mm) | 0.223 | 0.014 | 0.226 | 0.014 | 0.238 | 0.016 | 0.277 | 0.013 | 0.283 | 0.015 | 0.302 | 0.021 | 0.175 | 0.012 | 0.195 | 0.015 | 0.249 | 0.015 | 0.241 | 0.025 | 0.318 | 0.044 | 0.324 | 0.029 | 0.001 | 0.07 | 0.005 |
| SMI | 2.63 | 0.21 | 2.77 | 0.14 | 2.85 | 0.18 | 3.12 | 0.18 | 2.86 | 0.22 | 2.99 | 0.19 | 1.72 | 0.19 | 2.31 | 0.29 | 2.32 | 0.28 | 2.31 | 0.23 | 2.60 | 0.23 | 2.52 | 0.11 | <0.001 | NS | <0.001 |
| Midshaft femur (cortical) | |||||||||||||||||||||||||||
| BA (mm2) | 0.56 | 0.05 | 0.59 | 0.03 | 0.72 | 0.03 | 0.78 | 0.04 | 0.83 | 0.04 | 0.84# | 0.04 | 0.64 | 0.05 | 0.64 | 0.03 | 0.75 | 0.05 | 0.72 | 0.03 | 0.78 | 0.06 | 0.75 | 0.04 | NS | 0.001 | NS |
| TA (mm2) | 1.73 | 0.09 | 1.78 | 0.10 | 1.73 | 0.09 | 1.79 | 0.07 | 1.78 | 0.08 | 1.87 | 0.08 | 1.60 | 0.08 | 1.62 | 0.07 | 1.66 | 0.08 | 1.57 | 0.09 | 1.66 | 0.07 | 1.62 | 0.07 | NS | 0.001 | NS |
| Cort.Th (mm) | 0.129 | 0.010 | 0.133 | 0.008 | 0.168 | 0.006 | 0.179 | 0.005 | 0.193 | 0.007 | 0.189# | 0.007 | 0.157 | 0.009 | 0.157 | 0.006 | 0.183 | 0.009 | 0.185 | 0.007 | 0.195 | 0.013 | 0.188 | 0.007 | NS | 0.053 | NS |
| Imax (mm4) | 0.176 | 0.020 | 0.195 | 0.019 | 0.222 | 0.019 | 0.249 | 0.025 | 0.259 | 0.033 | 0.281# | 0.030 | 0.162 | 0.020 | 0.165 | 0.012 | 0.195 | 0.027 | 0.170 | 0.015 | 0.193 | 0.018 | 0.187 | 0.017 | NS | <0.001 | NS |
| Imin (mm4) | 0.095 | 0.012 | 0.100 | 0.013 | 0.110 | 0.011 | 0.120 | 0.007 | 0.125 | 0.009 | 0.133# | 0.009 | 0.095 | 0.011 | 0.097 | 0.009 | 0.112 | 0.011 | 0.104 | 0.011 | 0.120 | 0.014 | 0.109 | 0.010 | NS | 0.001 | NS |
| Fifth lumbar vertebra (trabecular) | |||||||||||||||||||||||||||
| BV/TV (%) | 26.3 | 2.1 | 24.8 | 4.4 | 29.2 | 2.4 | 29.9 | 1.7 | 33.7 | 2.7 | 31.4 | 2.9 | 23.6 | 3.0 | 25.7 | 3.3 | 27.3 | 1.8 | 26.5 | 2.7 | 26.5 | 3.7 | 25.0 | 4.8 | NS | NS | NS |
| Tb.Th (mm) | 0.048 | 0.002 | 0.050 | 0.003 | 0.056 | 0.002 | 0.062 | 0.002 | 0.066 | 0.003 | 0.067 | 0.004 | 0.052 | 0.003 | 0.053 | 0.004 | 0.063 | 0.004 | 0.060 | 0.002 | 0.066 | 0.004 | 0.062 | 0.005 | NS | 0.003 | 0.062 |
| Tb.Sp (mm) | 0.167 | 0.011 | 0.179 | 0.014 | 0.187 | 0.010 | 0.199 | 0.010 | 0.206 | 0.015 | 0.218 | 0.016 | 0.211 | 0.011 | 0.212 | 0.010 | 0.245 | 0.015 | 0.241 | 0.020 | 0.269 | 0.016 | 0.260 | 0.020 | NS | 0.003 | NS |
| SMI | 0.78 | 0.20 | 0.96 | 0.43 | 0.44 | 0.22 | 0.40 | 0.17 | −0.10 | 0.22 | 0.11 | 0.26 | 1.14 | 0.38 | 0.63 | 0.37 | 0.41 | 0.18 | 0.57 | 0.27 | 0.49 | 0.32 | 0.64 | 0.42 | NS | 0.10 | 0.004 |
Bolded values indicate significant pairwise differences within each strain at a given age (p < 0.05). Pairwise comparisons were performed for variables with overall significance in the GLM, as shown at right. Cortical bone variables that were significantly lower in HF vs. N mice (p < 0.05) after body mass adjustment are indicated by #.
HF diet decreased femoral cortical bone acquisition in both strains. In particular, while there were no differences at 6 wks of age, by 12 wks of age FVB HF mice had lower cortical cross-sectional properties, including TA (−5%), Imax (−13%), and pMOI (−11%), and at 20 wks of age FVB HF had lower Imin (−9%) (p < 0.05 for all, Fig. 2C–D and Table 2). These differences were no longer statistically significant after body mass adjustment. In B6, HF diet appeared to improve cortical bone geometry, but this effect disappeared after adjustment for body mass. Specifically, prior to body mass adjustment, at 6 wks of age B6 HF mice had higher Imax (+11%); at 12 wks of age B6 HF had higher BA (+8%), Imax (+12%), Imin (+9%), and pMOI (+11%); and at 20 wks of age B6 HF had higher TA (+5%) and Imin (+6%) (p < 0.05 for all, Fig. 2C–D and Table 2). After body mass adjustment, there were no significant effects of HF diet on cortical bone geometry until 20 weeks of age, at which time B6 HF had significantly lower BA, Cort.Th, Imax, Imin, and pMOI vs. N (p < 0.05 for all, indicated by # in Fig. 2D and Table 2).
3.3. Bone morphology: Trabecular bone at lumbar vertebrae
As with the femur, the fifth lumbar vertebra exhibited strain-dependent effects of HF diet. In DIO-resistant FVB, vertebral trabecular bone properties were similar in HF vs. N mice at 6, 12, and 20 wks of age, although SMI was lower in FVB HF vs. N at 6 wks of age (−45%, p = 0.009, Fig. 2E–F and Table 2). However, in DIO-prone B6, HF mice had 5–6% lower Tb.N at 6 and 12 wks of age and 16–21% lower Conn.D at all ages, with 10% higher Tb.Th at 12 wks and 2-fold higher (i.e. worse) SMI at 20 wks of age vs. N mice (p < 0.05 for all, Fig. 2E–F and Table 2).
3.4. Distal femur bone marrow adiposity
At 12 wks of age, B6 HF mice had a twofold higher adipocyte volume per total volume (Ad.V/TV, %) vs. B6 N (p = 0.006), but there was no effect of HF diet on marrow adiposity in FVB mice (Table 3).
Table 3.
Distal femur bone marrow adiposity in female FVB/NJ and C57BL/6J mice fed a normal (N) or high fat (HF) diet from 3 wks of age to 12 wks of age (mean ± SD).
| B6 |
FVB |
Pdiet |
Pstrain⁎diet |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N |
HF |
N |
HF |
|||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |||
| Ad.V/TV (%) | 2.14 | 1.52 | 4.52 | 2.35 | 0.11 | 0.08 | 0.03 | 0.02 | 0.057 | 0.044 |
Bolded values indicate significant pairwise differences within each strain at a given age (p < 0.05). Pairwise comparisons were performed for variables with overall significance in the GLM, as shown at right.
3.5. Bone strength
Mechanical testing showed that femora from B6 HF mice had higher stiffness (N) at 12 weeks of age (p < 0.05, Table 4). In contrast, femora from FVB HF had higher stiffness at 6 wks of age, but lower stiffness and maximum force vs. N at 20 wks of age (p < 0.05 for all, Table 4). There were no effects of HF diet on the apparent elastic modulus or postyield displacement in femora from either strain.
Table 4.
Midshaft femoral cortical bone mechanical properties in female FVB/J and C57BL/6J mice fed a normal (N) or high fat (HF) diet from 3 wks of age to 6, 12, or 20 wks of age (mean ± SD).
| B6 |
FVB |
Pdiet | Pstrain⁎diet | Pstrain⁎diet⁎age | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 6 wks |
12 wks |
20 wks |
6 wks |
12 wks |
20 wks |
||||||||||||||||||||||
| N |
HF |
N |
HF |
N |
HF |
N |
HF |
N |
HF |
N |
HF |
||||||||||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||||
| Stiffness (N/mm) | 42.4 | 9.0 | 41.2 | 9.1 | 61.2 | 9.4 | 71.6 | 4.9 | 77.1 | 10.5 | 82.7 | 9.8 | 52.3 | 7.0 | 61.2 | 8.7 | 76.6 | 11.1 | 78.6 | 7.9 | 97.1 | 8.9 | 85.5 | 12.1 | NS | 0.073 | 0.004 |
| Maximum Force (N) | 10.6 | 1.3 | 10.4 | 1.5 | 14.5 | 1.1 | 15.4 | 1.1 | 17.6 | 1.1 | 17.6 | 1.9 | 12.0 | 1.1 | 12.9 | 1.5 | 17.4 | 0.9 | 17.1 | 1.3 | 19.9 | 1.8 | 17.9 | 1.7 | NS | NS | 0.040 |
| Estimated Young's modulus (GPa) | 3.82 | 2.34 | 3.54 | 2.00 | 5.83 | 1.53 | 6.43 | 1.17 | 6.49 | 1.08 | 6.38 | 1.26 | 4.77 | 2.43 | 5.25 | 2.67 | 7.72 | 1.99 | 8.55 | 1.26 | 9.13 | 0.97 | 9.00 | 1.64 | NS | NS | NS |
| Postyield Displacement (mm) | 1.41 | 0.32 | 1.19 | 0.66 | 1.24 | 0.57 | 1.31 | 0.54 | 0.69 | 0.32 | 0.80 | 0.16 | 0.54 | 0.18 | 0.56 | 0.32 | 0.39 | 0.11 | 0.42 | 0.15 | 0.30 | 0.07 | 0.33 | 0.12 | NS | NS | NS |
Bolded values indicate significant pairwise differences within each strain at a given age (p < 0.05). Pairwise comparisons were performed for variables with overall significance in the GLM, as shown at right.
4. Discussion
In this study, we tested the effects of high fat (HF) diet on metabolism and skeletal acquisition in young, rapidly growing female mice from two strains: FVB, which resists diet-induced obesity, and B6, which readily develops diet-induced obesity (Hu et al., 2004). We hypothesized that the combination of high saturated fat diet, obesity, and metabolic dysfunction in B6 mice would be more deleterious to skeletal acquisition compared to high saturated fat diet alone in DIO-resistant FVB mice.
In keeping with previous studies, we found that high fat diet induced a metabolic phenotype including obesity and glucose intolerance, higher leptin, and higher marrow adiposity in B6 mice, while FVB were largely unaffected (Lecka-Czernik et al., 2015; Ma et al., 2011; Fujita et al., 2012; Gautam et al., 2014; Shu et al., 2015; Cao et al., 2009; Bornstein et al., 2017; Doucette et al., 2015; Ackert-Bicknell et al., 2008; Hu et al., 2004; Lin et al., 2000). We also observed strain-specific effects of HF diet on the skeleton: B6 on high fat diet had higher bone mineral density from 6 to 12 wks of age, but lower bone mineral content for their body mass from 9 to 20 wks of age, indicating bone mineral deposition did not keep pace with their weight gain. In contrast, FVB mice on a high fat diet had no changes in bone mineral density or content. B6 mice on a high fat diet had worse trabecular architecture at 12 and 20 wks of age, whereas FVB mice on high fat diet had worse trabecular bone architecture at 6 wks of age but not thereafter, perhaps because bone loss was slower in HF vs. N mice after 6 wks of age. B6 HF mice had lower body mass-adjusted cortical bone properties vs. normal diet controls at 12 and 20 wks of age, but FVB HF mice did not, although at 20 wks of age FVB HF had lower femoral bone strength vs. N. These data provide partial support for our hypothesis that most of the effects of high fat diet on the skeleton are mediated via diet-induced obesity and metabolic dysfunction, particularly in trabecular bone. However, these effects were complex and appeared to attenuate with age, such that by 20 wks of age, there were few significant skeletal differences between HF and N mice of either strain.
4.1. Comparison to prior studies of high fat diet and bone mass
Previous studies of the influence of high fat diet on bone in mice found a range of effects from positive to negative. Two studies of male C57BL/6J mice from 12 to 23 or 7 to 28 wks of age found HF diet was associated with higher femoral trabecular thickness and trabecular bone mineral content, as well as higher cortical bone area, total area, cortical thickness, and polar moment of inertia (unadjusted for body mass), despite lower bone formation and high marrow adiposity (Lecka-Czernik et al., 2015; Ma et al., 2011). In contrast, other studies reported HF diet from 3 wks or 8 wks to 14 to 17 wks of age had little effect on femoral trabecular or cortical bone in wildtype mice, although marrow adiposity increased (Doucette et al., 2015; Ackert-Bicknell et al., 2008; Styner et al., 2014), and osteocalcin decreased while TRACP5b increased (Yan et al., 2015). Finally, several studies of HF diet in male and female B6 beginning at 4 to 7 wks and ending at 14 to 31 wks of age found deleterious effects in the distal femur and proximal tibia including lower trabecular BV/TV, increased bone turnover, and higher RANKL, TNF, and PPAR-gamma along with higher marrow adiposity and lower fracture resistance (Fujita et al., 2012; Gautam et al., 2014; Shu et al., 2015; Cao et al., 2009; Scheller et al., 2016; Patsch et al., 2011). It is challenging to integrate these disparate findings, although it should be noted that the studies reporting positive effects of HF diet on bone mass tended to be initiated in mice that were older (i.e. 7 to 12 wks of age) than those studies reporting neutral or negative effects (i.e. initiated at 3 to 8 wks of age). This difference is critical because skeletal acquisition in B6 mice peaks between 3 and 6 wks of age (Glatt et al., 2007), such that bone may be particularly susceptible to metabolic insult during this interval. Our data in female B6 from 3 to 20 wks of age concur with the finding that HF is deleterious to bone mass during rapid skeletal acquisition. In terms of marrow adiposity, the association of HF diet with higher BMAT is remarkably consistent across studies, regardless of whether bone mass is higher, lower, or unchanged (Lecka-Czernik et al., 2015; Fujita et al., 2012; Gautam et al., 2014; Shu et al., 2015; Doucette et al., 2015; Ackert-Bicknell et al., 2008; Styner et al., 2014; Scheller et al., 2016). Thus our finding of higher BMAT in B6 HF at 12 wks of age is likely representative of marrow adiposity at other ages as well. To the best of our knowledge, this is the first study to test the effects of HF diet on bone mass in FVB mice, so our data cannot be compared to prior work in this strain. The mechanisms underlying the age-related effects of HF diet on distal femur trabecular architecture in this strain are unclear and should be tested in future studies. One possibility is that genetic differences between strains mediate the effects of HF diet on the skeleton. For example, several recent studies have demonstrated significant strain-related differences in the skeletal response to saturated fats and unsaturated omega 3 vs. omega 6 fats involving nutrition-by-gene interactions with peroxisome proliferator-activated receptor-gamma (PPARγ) (Ackert-Bicknell et al., 2008; Bonnet et al., 2014).
4.2. Mechanisms for deleterious effects of obesity on bone mass
Several potential mechanisms link obesity and metabolic dysregulation to bone metabolism, including chronic inflammation, hyperleptinemia, and hyperglycemia. Cytokine production in adipose tissue induces chronic inflammation that has been implicated in atherosclerosis and insulin resistance (Shoelson et al., 2007; Wellen and Hotamisligil, 2003), as well as bone loss (Ouchi et al., 2011) and lower bone mineral density in adolescents (Russell et al., 2010; Lucas et al., 2012). Inflammatory markers including tumor necrosis factor-alpha (TNF-α), interleukin-1beta (IL-1β), and interleukin-6 (IL-6) can stimulate osteoclast activity via increased proliferation of preosteoclastic cells and upregulated RANKL expression (Khosla, 2001). A recent comparison of B6 and FVB metabolism following 8 wks of HF diet also found that although B6 mice had smaller adipocytes, they exhibited higher levels of proinflammatory cytokines and macrophages, along with higher tissue hypoxia and expression of angiogenic genes compared to FVB (Kim et al., 2013). The authors concluded that B6 mice have a greater capacity to expand their adipose depots in response to high fat diet compared to FVB, contributing to their DIO but also potentially contributing to inflammation-induced metabolic dysregulation and bone loss (Kim et al., 2013). It is also possible that these metabolic differences simply reflect the fact that B6 overeat on HF diet whereas FVB do not. Recently, Bornstein et al. tested the effect of metformin in C57BL/6J mice after HF diet, and found that DIO was not deleterious to trabecular bone architecture but led to decreased midshaft cortical bone area fraction and higher marrow adiposity in the femur and tibia (Bornstein et al., 2017). Metformin treatment did not affect body mass or percent body fat, but partially reversed the skeletal phenotype, normalized blood glucose, increased osteoblast number, and caused alterations in serum metabolites and in the gut microbiome (Nunez et al., 2007). These data support the hypothesis that metabolic dysfunction significantly contributes to the skeletal phenotype in DIO.
The adipokine leptin also modulates both cortical and trabecular bone mass, both directly via osteoblast leptin receptors and indirectly via neural pathways (Upadhyay et al., 2015). Direct effects of leptin in peripheral tissues are generally osteogenic, including stimulation of periosteal bone apposition and promoting differentiation of mesenchymal stem cells (MSCs) to osteoblasts rather than adipocytes (Cornish et al., 2002; Thomas et al., 1999; Hamrick and Ferrari, 2008). In addition, reports indicate that leptin can reduce bone loss following ovariectomy or hindlimb unloading (Hamrick and Ferrari, 2008; Martin et al., 2005; Gimble et al., 2006). Thus we might expect that high circulating leptin due to DIO would maintain or increase bone mass in B6 mice (Cornish et al., 2002; Thomas et al., 1999; Ducy et al., 2000; Takeda et al., 2002). However, in this study DIO-induced hyperleptinemia in B6 mice at 12 wks of age was associated with lower cortical and trabecular bone mass. At 20 wks of age, B6 HF were not hyperleptinemic, despite high body mass and percent body fat, and their trabecular and cortical bone mass did not differ from B6 N. The decrease in leptin in B6 HF from 12 to 20 wks of age was unexpected given the high adiposity of these mice. One potential mechanism involves a high fat diet-induced increase in peroxisome proliferator-activated receptor gamma,
In terms of the skeletal phenotype, these findings are consistent with prior studies demonstrating the osteogenic effects of leptin in humans (Welt et al., 2004; Sienkiewicz et al., 2011) and in animal models (Goldstone et al., 2002; Iwaniec et al., 2007; Philbrick et al., 2017; Turner et al., 2013) are most pronounced in hypoleptinemic individuals. Hyperleptinemia may induce leptin resistance (Hamrick, 2007) and upregulate deleterious inflammatory pathways in bone (Yang et al., 2014), an intriguing possibility that deserves further study.
Finally, HF diet also induced impaired glucose tolerance, particularly in B6 mice. Although few studies in animal models have isolated the effects of impaired glucose tolerance on bone mass, in humans there is a link between impaired glucose tolerance and lower bone mineral content in adolescents (Pollock et al., 2010; Afghani et al., 2005). More broadly, hyperglycemia is known to impair osteoblast function in vitro, and has been implicated in diabetes-induced osteoporosis and skeletal fragility (McCabe et al., 2011; Botolin and McCabe, 2006; Hamann et al., 2012; Dhaliwal and Rosen, 2016). These data suggest that treating the metabolic consequences of obesity, such as inflammation and impaired glucose tolerance, might help maximize peak bone mass and reduce future osteoporosis risk in adolescents.
4.3. Limitations and future directions
The strengths of our approach include a focus on young, rapidly growing animals, the use of high fat diet to physiologically induce metabolic dysregulation, and the use of inbred mouse strains with contrasting responses to diet induced obesity. However, a limitation of this approach is the possibility that genetic differences between FVB and B6, for example in quantitative trait loci for feeding behavior (Smith Richards et al., 2002; Kumar and Smith Richards, 2008) or for metabolic response to macronutrient intake (Ackert-Bicknell et al., 2008), mediate strain-specific responses to HF diet. It is particularly interesting that in FVB mice, HF diet caused worse trabecular bone architecture at 6 wks of age but not thereafter. Further study of resistance vs. susceptibility to overeating across inbred mouse strains may be relevant for understanding why some humans do not gain weight even in an obesogenic environment (Mercer, 2001; Blundell et al., 2005). Future studies should further investigate the unexpected apparent decrease in leptin in B6 HF females from 12 wks to 20 wks of age, and should include leptin sensitivity tests to assess the impact of leptin resistance on feeding behavior and body composition (Krawczewski Carhuatanta et al., 2011). An additional limitation is that we currently include only female mice, and there may be sex differences in the response to high fat diet. In addition, our physiological approach to diet-induced obesity induced multiple metabolic changes including hyperleptinemia, high fat mass, and impaired glucose tolerance, thus making it challenging to determine the contributions of each factor to the changes in skeletal acquisition we observed. Finally, there is evidence that the gut microbiome varies across inbred mouse strains (Hildebrand et al., 2013) and can increase or decrease bone strength (Guss et al., 2017; Yan et al., 2016; Sjogren et al., 2012). Future studies will include males and will test the effects of treatments including diet and exercise and anti-inflammatory agents on both metabolic dysregulation and skeletal acquisition in high fat diet-induced obesity within a given mouse strain. Future work should also continue to test the role of the gut microbiome in mediating the effects of HF diet on the skeleton (Bornstein et al., 2017), particularly the strain-related differences observed in this study.
5. Conclusions
In this study, we sought to test whether the effects of high fat diet on skeletal acquisition differ in the presence or absence of excessive caloric intake leading to obesity and metabolic disease. We did this by comparing the effects of high fat diet on metabolism and skeletal acquisition in FVB mice, which resist diet-induced obesity, and B6 mice, which are susceptible to diet-induced obesity. The results indicated that in FVB mice, high fat diet impairs skeletal acquisition at a young age, but has little effect on bone mass, architecture, or marrow adiposity in older animals. In B6 mice, high fat diet leads to overconsumption of food, increased body mass and fat mass, decreased trabecular number and connectivity density in the distal femur at 12 and 20 wks of age, and increased marrow adiposity at 12 wks of age. These results suggest that both high fat diet and diet-induced metabolic disease cause subtle alterations in bone mass and microarchitecture, although these effects are complex and vary in an age- and strain-dependent manner. Trabecular bone appears to be more sensitive to dietary and metabolic perturbations than cortical bone, perhaps due to its faster turnover. Protection of bone mass is an additional rationale for treating metabolic dysregulation, particularly during peak bone mass acquisition in adolescents.
Acknowledgements
Funding for this project was provided by RC1AR058389, S10RR017868, T32DK007028, and F32HD060419. We thank two anonymous reviewers for constructive feedback that improved the manuscript.
Footnotes
This work was supported by the National Institutes of HealthRC1AR058389, S10RR017868, T32DK007028, and F32HD060419.
References
- Ackert-Bicknell C.L., Demissie S., Marin de Evsikova C., Hsu Y.H., DeMambro V.E., Karasik D., Cupples L.A., Ordovas J.M., Tucker K.L., Cho K., Canalis E., Paigen B., Churchill G.A., Forejt J., Beamer W.G., Ferrari S., Bouxsein M.L., Kiel D.P., Rosen C.J. PPARG by dietary fat interaction influences bone mass in mice and humans. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2008;23(9):1398–1408. doi: 10.1359/JBMR.080419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afghani A., Cruz M.L., Goran M.I. Impaired glucose tolerance and bone mineral content in overweight Latino children with a family history of type 2 diabetes. Diabetes Care. 2005;28(2):372–378. doi: 10.2337/diacare.28.2.372. [DOI] [PubMed] [Google Scholar]
- Aguirre L., Napoli N., Waters D., Qualls C., Villareal D.T., Armamento-Villareal R. Increasing adiposity is associated with higher adipokine levels and lower bone mineral density in obese older adults. J. Clin. Endocrinol. Metab. 2014;99(9):3290–3297. doi: 10.1210/jc.2013-3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albala C., Yanez M., Devoto E., Sostin C., Zeballos L., Santos J.L. Obesity as a protective factor for postmenopausal osteoporosis. International Journal of Obesity and Related Metabolic Disorders: Journal of the International Association for the Study of Obesity. 1996;20(11):1027–1032. [PubMed] [Google Scholar]
- Bailey D.A. The Saskatchewan Pediatric Bone Mineral Accrual Study: bone mineral acquisition during the growing years. Int. J. Sports Med. 1997;18(Suppl. 3):S191–4. doi: 10.1055/s-2007-972713. [DOI] [PubMed] [Google Scholar]
- Beck T.J., Petit M.A., Wu G., LeBoff M.S., Cauley J.A., Chen Z. Does obesity really make the femur stronger? BMD, geometry, and fracture incidence in the women's health initiative-observational study. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2009;24(8):1369–1379. doi: 10.1359/JBMR.090307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blundell J.E., Stubbs R.J., Golding C., Croden F., Alam R., Whybrow S., Le Noury J., Lawton C.L. Resistance and susceptibility to weight gain: individual variability in response to a high-fat diet. Physiol. Behav. 2005;86(5):614–622. doi: 10.1016/j.physbeh.2005.08.052. [DOI] [PubMed] [Google Scholar]
- Bonjour J.P., Theintz G., Buchs B., Slosman D., Rizzoli R. Critical years and stages of puberty for spinal and femoral bone mass accumulation during adolescence. J. Clin. Endocrinol. Metab. 1991;73(3):555–563. doi: 10.1210/jcem-73-3-555. [DOI] [PubMed] [Google Scholar]
- Bonjour J.P., Chevalley T., Rizzoli R., Ferrari S. Gene-environment interactions in the skeletal response to nutrition and exercise during growth. Medicine and Sport Science. 2007;51:64–80. doi: 10.1159/000103005. [DOI] [PubMed] [Google Scholar]
- Bonnet N., Somm E., Rosen C.J. Diet and gene interactions influence the skeletal response to polyunsaturated fatty acids. Bone. 2014;68:100–107. doi: 10.1016/j.bone.2014.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein S., Moschetta M., Kawano Y., Sacco A., Huynh D., Brooks D., Manier S., Fairfield H., Falank C., Roccaro A.M., Nagano K., Baron R., Bouxein M., Vary C., Ghobrial I.M., Rosen C.J., Reagan M.R. Metformin affects cortical bone mass and marrow adiposity in diet-induced obesity in male mice. Endocrinology. 2017;158(10):3369–3385. doi: 10.1210/en.2017-00299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botolin S., McCabe L.R. Chronic hyperglycemia modulates osteoblast gene expression through osmotic and non-osmotic pathways. J. Cell. Biochem. 2006;99(2):411–424. doi: 10.1002/jcb.20842. [DOI] [PubMed] [Google Scholar]
- Bouxsein M.L., Pierroz D.D., Glatt V., Goddard D.S., Cavat F., Rizzoli R., Ferrari S.L. beta-Arrestin2 regulates the differential response of cortical and trabecular bone to intermittent PTH in female mice. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2005;20(4):635–643. doi: 10.1359/JBMR.041204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouxsein M.L., Szulc P., Munoz F., Thrall E., Sornay-Rendu E., Delmas P.D. Contribution of trochanteric soft tissues to fall force estimates, the factor of risk, and prediction of hip fracture risk. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2007;22(6):825–831. doi: 10.1359/jbmr.070309. [DOI] [PubMed] [Google Scholar]
- Bouxsein M.L., Devlin M.J., Glatt V., Dhillon H., Pierroz D.D., Ferrari S.L. Mice lacking beta-adrenergic receptors have increased bone mass, but are not protected from deleterious skeletal effects of ovariectomy. Endocrinology. 2009;15(1):144–152. doi: 10.1210/en.2008-0843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouxsein M.L., Boyd S.K., Christiansen B.A., Guldberg R.E., Jepsen K.J., Muller R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2010;25(7):1468–1486. doi: 10.1002/jbmr.141. [DOI] [PubMed] [Google Scholar]
- Cao J.J., Gregoire B.R., Gao H. High-fat diet decreases cancellous bone mass but has no effect on cortical bone mass in the tibia in mice. Bone. 2009;44(6):1097–1104. doi: 10.1016/j.bone.2009.02.017. [DOI] [PubMed] [Google Scholar]
- Compston J.E., Watts N.B., Chapurlat R., Cooper C., Boonen S., Greenspan S., Pfeilschifter J., Silverman S., Diez-Perez A., Lindsay R., Saag K.G., Netelenbos J.C., Gehlbach S., Hooven F.H., Flahive J., Adachi J.D., Rossini M., Lacroix A.Z., Roux C., Sambrook P.N., Siris E.S., Glow I. Obesity is not protective against fracture in postmenopausal women: GLOW. Am. J. Med. 2011;124(11):1043–1050. doi: 10.1016/j.amjmed.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish J., Callon K.E., Bava U., Lin C., Naot D., Hill B.L., Grey A.B., Broom N., Myers D.E., Nicholson G.C., Reid I.R. Leptin directly regulates bone cell function in vitro and reduces bone fragility in vivo. J. Endocrinol. 2002;175(2):405–415. doi: 10.1677/joe.0.1750405. [DOI] [PubMed] [Google Scholar]
- De Laet C., Kanis J.A., Oden A., Johanson H., Johnell O., Delmas P., Eisman J.A., Kroger H., Fujiwara S., Garnero P., McCloskey E.V., Mellstrom D., Melton L.J., 3rd, Meunier P.J., Pols H.A., Reeve J., Silman A., Tenenhouse A. Body mass index as a predictor of fracture risk: a meta-analysis. Osteoporosis International: A Journal Established As Result of Cooperation Between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2005;16(11):1330–1338. doi: 10.1007/s00198-005-1863-y. [DOI] [PubMed] [Google Scholar]
- Dempster D.W., Compston J.E., Drezner M.K., Glorieux F.H., Kanis J.A., Malluche H., Meunier P.J., Ott S.M., Recker R.R., Parfitt A.M. Standardized nomenclature, symbols, and units for bone histomorphometry: a 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2013;28(1):2–17. doi: 10.1002/jbmr.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin M.J., Cloutier A.M., Thomas N.A., Panus D.A., Lotinun S., Pinz I., Baron R., Rosen C.J., Bouxsein M.L. Caloric restriction leads to high marrow adiposity and low bone mass in growing mice. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2010;25(9):2078–2088. doi: 10.1002/jbmr.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin M.J., Van Vliet M., Motyl K., Karim L., Brooks D.J., Louis L., Conlon C., Rosen C.J., Bouxsein M.L. Early-onset type 2 diabetes impairs skeletal acquisition in the male TALLYHO/JngJ mouse. Endocrinology. 2014;155(10):3806–3816. doi: 10.1210/en.2014-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhaliwal R., Rosen C.J. Type 2 diabetes and aging: a not so sweet scenario for bone. Horm. Metab. Res. 2016;48(11):771–778. doi: 10.1055/s-0042-117719. [DOI] [PubMed] [Google Scholar]
- Dimitri P., Bishop N., Walsh J.S., Eastell R. Obesity is a risk factor for fracture in children but is protective against fracture in adults: a paradox. Bone. 2012;50(2):457–466. doi: 10.1016/j.bone.2011.05.011. [DOI] [PubMed] [Google Scholar]
- Doucette C.R., Horowitz M.C., Berry R., MacDougald O.A., Anunciado-Koza R., Koza R.A., Rosen C.J. A high fat diet increases bone marrow adipose tissue (MAT) but does not alter trabecular or cortical bone mass in C57BL/6J mice. J. Cell. Physiol. 2015;230(9):2032–2037. doi: 10.1002/jcp.24954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducy P., Amling M., Takeda S., Priemel M., Schilling A.F., Beil F.T., Shen J., Vinson C., Rueger J.M., Karsenty G. Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell. 2000;100(2):197–207. doi: 10.1016/s0092-8674(00)81558-5. [DOI] [PubMed] [Google Scholar]
- Eliakim A., Nemet D., Wolach B. Quantitative ultrasound measurements of bone strength in obese children and adolescents. J. Pediatr. Endocrinol. Metab. 2001;14(2):159–164. doi: 10.1515/jpem.2001.14.2.159. [DOI] [PubMed] [Google Scholar]
- Felson D.T., Zhang Y., Hannan M.T., Anderson J.J. Effects of weight and body mass index on bone mineral density in men and women: the Framingham study. Journal of Bone and Mineral Research: The Official Journal of the American Society fsssor Bone and Mineral Research. 1993;8(5):567–573. doi: 10.1002/jbmr.5650080507. [DOI] [PubMed] [Google Scholar]
- Ferrari S.L., Pierroz D.D., Glatt V., Goddard D.S., Bianchi E.N., Lin F.T., Manen D., Bouxsein M.L. Bone response to intermittent parathyroid hormone is altered in mice null for {beta}-Arrestin2. Endocrinology. 2005;146(4):1854–1862. doi: 10.1210/en.2004-1282. [DOI] [PubMed] [Google Scholar]
- Fujita Y., Watanabe K., Maki K. Serum leptin levels negatively correlate with trabecular bone mineral density in high-fat diet-induced obesity mice. J. Musculoskelet. Neuronal Interact. 2012;12(2):84–94. [PubMed] [Google Scholar]
- Gautam J., Choudhary D., Khedgikar V., Kushwaha P., Singh R.S., Singh D., Tiwari S., Trivedi R. Micro-architectural changes in cancellous bone differ in female and male C57BL/6 mice with high-fat diet-induced low bone mineral density. Br. J. Nutr. 2014;111(10):1811–1821. doi: 10.1017/S0007114514000051. [DOI] [PubMed] [Google Scholar]
- Gimble J.M., Zvonic S., Floyd Z.E., Kassem M., Nuttall M.E. Playing with bone and fat. J. Cell. Biochem. 2006;98(2):251–266. doi: 10.1002/jcb.20777. [DOI] [PubMed] [Google Scholar]
- Glatt V., Canalis E., Stadmeyer L., Bouxsein M.L. Age-related changes in trabecular architecture differ in female and male C57BL/6J mice. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2007;22(8):1197–1207. doi: 10.1359/jbmr.070507. [DOI] [PubMed] [Google Scholar]
- Goldstone A.P., Howard J.K., Lord G.M., Ghatei M.A., Gardiner J.V., Wang Z.L., Wang R.M., Girgis S.I., Bailey C.J., Bloom S.R. Leptin prevents the fall in plasma osteocalcin during starvation in male mice. Biochem. Biophys. Res. Commun. 2002;295(2):475–481. doi: 10.1016/s0006-291x(02)00697-6. [DOI] [PubMed] [Google Scholar]
- Goulding A., Taylor R.W., Jones I.E., McAuley K.A., Manning P.J., Williams S.M. Overweight and obese children have low bone mass and area for their weight. International Journal of Obesity and Related Metabolic Disorders: Journal of the International Association for the Study of Obesity. 2000;24(5):627–632. doi: 10.1038/sj.ijo.0801207. [DOI] [PubMed] [Google Scholar]
- Greco E.A., Fornari R., Rossi F., Santiemma V., Prossomariti G., Annoscia C., Aversa A., Brama M., Marini M., Donini L.M., Spera G., Lenzi A., Lubrano C., Migliaccio S. Is obesity protective for osteoporosis? Evaluation of bone mineral density in individuals with high body mass index. Int. J. Clin. Pract. 2010;64(6):817–820. doi: 10.1111/j.1742-1241.2009.02301.x. [DOI] [PubMed] [Google Scholar]
- Guss J.D., Horsfield M.W., Fontenele F.F., Sandoval T.N., Luna M., Apoorva F., Lima S.F., Bicalho R.C., Singh A., Ley R.E., van der Meulen M.C., Goldring S.R., Hernandez C.J. Alterations to the gut microbiome impair bone strength and tissue material properties. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2017;32(6):1343–1353. doi: 10.1002/jbmr.3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halade G.V., Rahman M.M., Williams P.J., Fernandes G. High fat diet-induced animal model of age-associated obesity and osteoporosis. J. Nutr. Biochem. 2010;21(12):1162–1169. doi: 10.1016/j.jnutbio.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann C., Kirschner S., Gunther K.P., Hofbauer L.C. Bone, sweet bone—osteoporotic fractures in diabetes mellitus. Nat. Rev. Endocrinol. 2012;8(5):297–305. doi: 10.1038/nrendo.2011.233. [DOI] [PubMed] [Google Scholar]
- Hamrick M.W. Leptin and bone: a consensus emerging? IBMS BoneKEy. 2007;4(3):99–107. [Google Scholar]
- Hamrick M.W., Ferrari S.L. Leptin and the sympathetic connection of fat to bone. Osteoporosis International: A Journal Established as Result of Cooperation Between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2008;19(7):905–912. doi: 10.1007/s00198-007-0487-9. [DOI] [PubMed] [Google Scholar]
- Heikkinen S., Argmann C.A., Champy M.F., Auwerx J. Evaluation of glucose homeostasis. Curr Protoc Mol Biol. 2007;77(1) doi: 10.1002/0471142727.mb29b03s77. Chapter 29. (Unit 29B 3) [DOI] [PubMed] [Google Scholar]
- Hildebrand F., Nguyen T.L., Brinkman B., Yunta R.G., Cauwe B., Vandenabeele P., Liston A., Raes J. Inflammation-associated enterotypes, host genotype, cage and inter-individual effects drive gut microbiota variation in common laboratory mice. Genome Biol. 2013;14(1):R4. doi: 10.1186/gb-2013-14-1-r4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoy C.L., Macdonald H.M., McKay H.A. How does bone quality differ between healthy-weight and overweight adolescents and young adults? Clin. Orthop. Relat. Res. 2012;471(4):1214–1225. doi: 10.1007/s11999-012-2576-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu C.C., Qing K., Chen Y. Diet-induced changes in stearoyl-CoA desaturase 1 expression in obesity-prone and -resistant mice. Obes. Res. 2004;12(8):1264–1270. doi: 10.1038/oby.2004.160. [DOI] [PubMed] [Google Scholar]
- Hui S.L., Slemenda C.W., Johnston C.C., Jr. The contribution of bone loss to postmenopausal osteoporosis. Osteoporosis International: A Journal Established as Result of Cooperation Between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 1990;1(1):30–34. doi: 10.1007/BF01880413. [DOI] [PubMed] [Google Scholar]
- Iwaniec U.T., Boghossian S., Lapke P.D., Turner R.T., Kalra S.P. Central leptin gene therapy corrects skeletal abnormalities in leptin-deficient ob/ob mice. Peptides. 2007;28(5):1012–1019. doi: 10.1016/j.peptides.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khosla S. Minireview: the OPG/RANKL/RANK system. Endocrinology. 2001;142(12):5050–5055. doi: 10.1210/endo.142.12.8536. [DOI] [PubMed] [Google Scholar]
- Kim D.H., Gutierrez-Aguilar R., Kim H.J., Woods S.C., Seeley R.J. Increased adipose tissue hypoxia and capacity for angiogenesis and inflammation in young diet-sensitive C57 mice compared with diet-resistant FVB mice. Int. J. Obes. 2013;37(6):853–860. doi: 10.1038/ijo.2012.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krawczewski Carhuatanta K.A., Demuro G., Tschop M.H., Pfluger P.T., Benoit S.C., Obici S. Voluntary exercise improves high-fat diet-induced leptin resistance independent of adiposity. Endocrinology. 2011;152(7):2655–2664. doi: 10.1210/en.2010-1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar K.G., Smith Richards B.K. Transcriptional profiling of chromosome 17 quantitative trait Loci for carbohydrate and total calorie intake in a mouse congenic strain reveals candidate genes and pathways. J Nutrigenet Nutrigenomics. 2008;1(4):155–171. doi: 10.1159/000113657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecka-Czernik B., Stechschulte L.A., Czernik P.J., Dowling A.R. High bone mass in adult mice with diet-induced obesity results from a combination of initial increase in bone mass followed by attenuation in bone formation; implications for high bone mass and decreased bone quality in obesity. Mol. Cell. Endocrinol. 2015;410:35–41. doi: 10.1016/j.mce.2015.01.001. [DOI] [PubMed] [Google Scholar]
- Lin S., Thomas T.C., Storlien L.H., Huang X.F. Development of high fat diet-induced obesity and leptin resistance in C57Bl/6J mice. International Journal of Obesity and Related Metabolic Disorders: Journal of the International Association for the Study of Obesity. 2000;24(5):639–646. doi: 10.1038/sj.ijo.0801209. [DOI] [PubMed] [Google Scholar]
- Longo A.B., Ward W.E. PUFAs, bone mineral density, and fragility fracture: findings from human studies. Adv. Nutr. 2016;7(2):299–312. doi: 10.3945/an.115.009472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Looker A.C., Flegal K.M., Melton L.J., 3rd Impact of increased overweight on the projected prevalence of osteoporosis in older women. Osteoporosis International: A Journal Established as Result of Cooperation Between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2007;18(3):307–313. doi: 10.1007/s00198-006-0241-8. [DOI] [PubMed] [Google Scholar]
- Loud K.J., Gordon C.M. Adolescent bone health. Arch. Pediatr. Adolesc. Med. 2006;160(10):1026–1032. doi: 10.1001/archpedi.160.10.1026. [DOI] [PubMed] [Google Scholar]
- Lucas R., Ramos E., Oliveira A., Monjardino T., Barros H. Low-grade systemic inflammation and suboptimal bone mineral density throughout adolescence: a prospective study in girls. Clin. Endocrinol. 2012;77(5):665–671. doi: 10.1111/j.1365-2265.2012.04430.x. [DOI] [PubMed] [Google Scholar]
- Lukas R., Gigliotti J.C., Smith B.J., Altman S., Tou J.C. Consumption of different sources of omega-3 polyunsaturated fatty acids by growing female rats affects long bone mass and microarchitecture. Bone. 2011;49(3):455–462. doi: 10.1016/j.bone.2011.05.029. [DOI] [PubMed] [Google Scholar]
- Ma H., Turpeinen T., Silvennoinen M., Torvinen S., Rinnankoski-Tuikka R., Kainulainen H., Timonen J., Kujala U.M., Rahkila P., Suominen H. Effects of diet-induced obesity and voluntary wheel running on the microstructure of the murine distal femur. Nutrition & Metabolism. 2011;8(1):1. doi: 10.1186/1743-7075-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A., de Vittoris R., David V., Moraes R., Begeot M., Lafage-Proust M.H., Alexandre C., Vico L., Thomas T. Leptin modulates both resorption and formation while preventing disuse-induced bone loss in tail-suspended female rats. Endocrinology. 2005;146(8):3652–3659. doi: 10.1210/en.2004-1509. [DOI] [PubMed] [Google Scholar]
- McCabe L., Zhang J., Raehtz S. Understanding the skeletal pathology of type 1 and 2 diabetes mellitus. Crit. Rev. Eukaryot. Gene Expr. 2011;21(2):187–206. doi: 10.1615/critreveukargeneexpr.v21.i2.70. [DOI] [PubMed] [Google Scholar]
- Meinel L., Fajardo R., Hofmann S., Langer R., Chen J., Snyder B., Vunjak-Novakovic G., Kaplan D. Silk implants for the healing of critical size bone defects. Bone. 2005;37(5):688–698. doi: 10.1016/j.bone.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Mercer J.G. Dietary and genetic influences on susceptibility or resistance to weight gain on a high fat diet. Nutr Metab Cardiovasc Dis. 2001;11(4 Suppl):114–117. [PubMed] [Google Scholar]
- Millen B.E., Abrams S., Adams-Campbell L., Anderson C.A., Brenna J.T., Campbell W.W., Clinton S., Hu F., Nelson M., Neuhouser M.L., Perez-Escamilla R., Siega-Riz A.M., Story M., Lichtenstein A.H. The 2015 dietary guidelines advisory committee scientific report: development and major conclusions. Adv. Nutr. 2016;7(3):438–444. doi: 10.3945/an.116.012120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin S., Leslie W.D., Manitoba Bone Density Program High bone mineral density is associated with high body mass index. Osteoporosis International: A Journal Established as Result of Cooperation Between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2009;20(7):1267–1271. doi: 10.1007/s00198-008-0797-6. [DOI] [PubMed] [Google Scholar]
- Nielson C.M., Marshall L.M., Adams A.L., LeBlanc E.S., Cawthon P.M., Ensrud K., Stefanick M.L., Barrett-Connor E., Orwoll E.S., Osteoporotic Fractures in Men Study Research Group BMI and fracture risk in older men: the osteoporotic fractures in men study (MrOS) J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2011;26(3):496–502. doi: 10.1002/jbmr.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez N.P., Carpenter C.L., Perkins S.N., Berrigan D., Jaque S.V., Ingles S.A., Bernstein L., Forman M.R., Barrett J.C., Hursting S.D. Extreme obesity reduces bone mineral density: complementary evidence from mice and women. Obesity (Silver Spring) 2007;15(8):1980–1987. doi: 10.1038/oby.2007.236. [DOI] [PubMed] [Google Scholar]
- Ouchi N., Parker J.L., Lugus J.J., Walsh K. Adipokines in inflammation and metabolic disease. Nat. Rev. Immunol. 2011;11(2):85–97. doi: 10.1038/nri2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patsch J.M., Kiefer F.W., Varga P., Pail P., Rauner M., Stupphann D., Resch H., Moser D., Zysset P.K., Stulnig T.M., Pietschmann P. Increased bone resorption and impaired bone microarchitecture in short-term and extended high-fat diet-induced obesity. Metab. Clin. Exp. 2011;60(2):243–249. doi: 10.1016/j.metabol.2009.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit M.A., Beck T.J., Shults J., Zemel B.S., Foster B.J., Leonard M.B. Proximal femur bone geometry is appropriately adapted to lean mass in overweight children and adolescents. Bone. 2005;36(3):568–576. doi: 10.1016/j.bone.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Philbrick K.A., Wong C.P., Branscum A.J., Turner R.T., Iwaniec U.T. Leptin stimulates bone formation in ob/ob mice at doses having minimal impact on energy metabolism. J. Endocrinol. 2017;232(3):461–474. doi: 10.1530/JOE-16-0484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock N.K., Laing E.M., Baile C.A., Hamrick M.W., Hall D.B., Lewis R.D. Is adiposity advantageous for bone strength? A peripheral quantitative computed tomography study in late adolescent females. Am. J. Clin. Nutr. 2007;86(5):1530–1538. doi: 10.1093/ajcn/86.5.1530. [DOI] [PubMed] [Google Scholar]
- Pollock N.K., Bernard P.J., Wenger K., Misra S., Gower B.A., Allison J.D., Zhu H., Davis C.L. Lower bone mass in prepubertal overweight children with prediabetes. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2010;25(12):2760–2769. doi: 10.1002/jbmr.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock N.K., Bernard P.J., Gutin B., Davis C.L., Zhu H., Dong Y. Adolescent obesity, bone mass, and cardiometabolic risk factors. J. Pediatr. 2011;158(5):727–734. doi: 10.1016/j.jpeds.2010.11.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premaor M.O., Pilbrow L., Tonkin C., Parker R.A., Compston J. Obesity and fractures in postmenopausal women. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2010;25(2):292–297. doi: 10.1359/jbmr.091004. [DOI] [PubMed] [Google Scholar]
- Prieto-Alhambra D., Premaor M.O., Fina Aviles F., Hermosilla E., Martinez-Laguna D., Carbonell-Abella C., Nogues X., Compston J.E., Diez-Perez A. The association between fracture and obesity is site-dependent: a population-based study in postmenopausal women. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2012;27(2):294–300. doi: 10.1002/jbmr.1466. [DOI] [PubMed] [Google Scholar]
- Rajagopalan S., Lu L., Yaszemski M.J., Robb R.A. Optimal segmentation of microcomputed tomographic images of porous tissue-engineering scaffolds. J. Biomed. Mater. Res. A. 2005;75(4):877–887. doi: 10.1002/jbm.a.30498. [DOI] [PubMed] [Google Scholar]
- Reid I.R., Plank L.D., Evans M.C. Fat mass is an important determinant of whole body bone density in premenopausal women but not in men. J. Clin. Endocrinol. Metab. 1992;75(3):779–782. doi: 10.1210/jcem.75.3.1517366. [DOI] [PubMed] [Google Scholar]
- Ridler T., Calvard S. Picture thresholding using an iterative selection method. IEEE Trans on Systems, Man and Cybernetics. 1978;SMC-8(8):630–632. [Google Scholar]
- Russell M., Mendes N., Miller K.K., Rosen C.J., Lee H., Klibanski A., Misra M. Visceral fat is a negative predictor of bone density measures in obese adolescent girls. J. Clin. Endocrinol. Metab. 2010;95(3):1247–1255. doi: 10.1210/jc.2009-1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheller E.L., Khoury B., Moller K.L., Wee N.K., Khandaker S., Kozloff K.M., Abrishami S.H., Zamarron B.F., Singer K. Changes in skeletal integrity and marrow adiposity during high-fat diet and after weight loss. Front Endocrinol (Lausanne) 2016;7:102. doi: 10.3389/fendo.2016.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman E. Reduced bone density in women with fractures: contribution of low peak bone density and rapid bone loss. Osteoporosis International: A Journal Established as Result of Cooperation Between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 1994;4(Suppl. 1):15–25. doi: 10.1007/BF01623430. [DOI] [PubMed] [Google Scholar]
- Shoelson S.E., Herrero L., Naaz A. Obesity, inflammation, and insulin resistance. Gastroenterology. 2007;132(6):2169–2180. doi: 10.1053/j.gastro.2007.03.059. [DOI] [PubMed] [Google Scholar]
- Shu L., Beier E., Sheu T., Zhang H., Zuscik M.J., Puzas E.J., Boyce B.F., Mooney R.A., Xing L. High-fat diet causes bone loss in young mice by promoting osteoclastogenesis through alteration of the bone marrow environment. Calcif. Tissue Int. 2015;96(4):313–323. doi: 10.1007/s00223-015-9954-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sienkiewicz E., Magkos F., Aronis K.N., Brinkoetter M., Chamberland J.P., Chou S., Arampatzi K.M., Gao C., Koniaris A., Mantzoros C.S. Long-term metreleptin treatment increases bone mineral density and content at the lumbar spine of lean hypoleptinemic women. Metab. Clin. Exp. 2011;60(9):1211–1221. doi: 10.1016/j.metabol.2011.05.016. [DOI] [PubMed] [Google Scholar]
- Sjogren K., Engdahl C., Henning P., Lerner U.H., Tremaroli V., Lagerquist M.K., Backhed F., Ohlsson C. The gut microbiota regulates bone mass in mice. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2012;27(6):1357–1367. doi: 10.1002/jbmr.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith Richards B.K., Belton B.N., Poole A.C., Mancuso J.J., Churchill G.A., Li R., Volaufova J., Zuberi A., York B. QTL analysis of self-selected macronutrient diet intake: fat, carbohydrate, and total kilocalories. Physiol. Genomics. 2002;11(3):205–217. doi: 10.1152/physiolgenomics.00037.2002. [DOI] [PubMed] [Google Scholar]
- Styner M., Thompson W.R., Galior K., Uzer G., Wu X., Kadari S., Case N., Xie Z., Sen B., Romaine A., Pagnotti G.M., Rubin C.T., Styner M.A., Horowitz M.C., Rubin J. Bone marrow fat accumulation accelerated by high fat diet is suppressed by exercise. Bone. 2014;64:39–46. doi: 10.1016/j.bone.2014.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda S., Elefteriou F., Levasseur R., Liu X., Zhao L., Parker K.L., Armstrong D., Ducy P., Karsenty G. Leptin regulates bone formation via the sympathetic nervous system. Cell. 2002;111(3):305–317. doi: 10.1016/s0092-8674(02)01049-8. [DOI] [PubMed] [Google Scholar]
- Thomas T., Gori F., Khosla S., Jensen M.D., Burguera B., Riggs B.L. Leptin acts on human marrow stromal cells to enhance differentiation to osteoblasts and to inhibit differentiation to adipocytes. Endocrinology. 1999;140(4):1630–1638. doi: 10.1210/endo.140.4.6637. [DOI] [PubMed] [Google Scholar]
- Turner R.T., Kalra S.P., Wong C.P., Philbrick K.A., Lindenmaier L.B., Boghossian S., Iwaniec U.T. Peripheral leptin regulates bone formation. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2013;28(1):22–34. doi: 10.1002/jbmr.1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhyay J., Farr O.M., Mantzoros C.S. The role of leptin in regulating bone metabolism. Metab. Clin. Exp. 2015;64(1):105–113. doi: 10.1016/j.metabol.2014.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M.C., Bachrach L.K., Van Loan M., Hudes M., Flegal K.M., Crawford P.B. The relative contributions of lean tissue mass and fat mass to bone density in young women. Bone. 2005;37(4):474–481. doi: 10.1016/j.bone.2005.04.038. [DOI] [PubMed] [Google Scholar]
- Wellen K.E., Hotamisligil G.S. Obesity-induced inflammatory changes in adipose tissue. J. Clin. Invest. 2003;112(12):1785–1788. doi: 10.1172/JCI20514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welt C.K., Chan J.L., Bullen J., Murphy R., Smith P., DePaoli A.M., Karalis A., Mantzoros C.S. Recombinant human leptin in women with hypothalamic amenorrhea. N. Engl. J. Med. 2004;351(10):987–997. doi: 10.1056/NEJMoa040388. [DOI] [PubMed] [Google Scholar]
- Whiting S.J. Obesity is not protective for bones in childhood and adolescence. Nutr. Rev. 2002;60(1):27–30. doi: 10.1301/002966402760240327. [DOI] [PubMed] [Google Scholar]
- Yan L., Graef G.L., Nielsen F.H., Johnson L.K., Cao J. Soy protein is beneficial but high-fat diet and voluntary running are detrimental to bone structure in mice. Nutr. Res. 2015;35(6):523–531. doi: 10.1016/j.nutres.2015.04.012. [DOI] [PubMed] [Google Scholar]
- Yan J., Herzog J.W., Tsang K., Brennan C.A., Bower M.A., Garrett W.S., Sartor B.R., Aliprantis A.O., Charles J.F. Gut microbiota induce IGF-1 and promote bone formation and growth. Proc. Natl. Acad. Sci. U. S. A. 2016;113(47):E7554–E7563. doi: 10.1073/pnas.1607235113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W.H., Tsai C.H., Fong Y.C., Huang Y.L., Wang S.J., Chang Y.S., Tang C.H. Leptin induces oncostatin M production in osteoblasts by downregulating miR-93 through the Akt signaling pathway. Int. J. Mol. Sci. 2014;15(9):15778–15790. doi: 10.3390/ijms150915778. [DOI] [PMC free article] [PubMed] [Google Scholar]