Abstract
Rationale: Recent evidence suggests that obstructive sleep apnea (OSA) may be a risk factor for developing mild cognitive impairment and Alzheimer’s disease. However, how sleep apnea affects longitudinal risk for Alzheimer’s disease is less well understood.
Objectives: To test the hypothesis that there is an association between severity of OSA and longitudinal increase in amyloid burden in cognitively normal elderly.
Methods: Data were derived from a 2-year prospective longitudinal study that sampled community-dwelling healthy cognitively normal elderly. Subjects were healthy volunteers between the ages of 55 and 90, were nondepressed, and had a consensus clinical diagnosis of cognitively normal. Cerebrospinal fluid amyloid β was measured using ELISA. Subjects received Pittsburgh compound B positron emission tomography scans following standardized procedures. Monitoring of OSA was completed using a home sleep recording device.
Measurements and Main Results: We found that severity of OSA indices (AHIall [F1,88 = 4.26; P < 0.05] and AHI4% [F1,87 = 4.36; P < 0.05]) were associated with annual rate of change of cerebrospinal fluid amyloid β42 using linear regression after adjusting for age, sex, body mass index, and apolipoprotein E4 status. AHIall and AHI4% were not associated with increases in ADPiB-mask (Alzheimer’s disease vulnerable regions of interest Pittsburg compound B positron emission tomography mask) most likely because of the small sample size, although there was a trend for AHIall (F1,28 = 2.96, P = 0.09; and F1,28 = 2.32, not significant, respectively).
Conclusions: In a sample of cognitively normal elderly, OSA was associated with markers of increased amyloid burden over the 2-year follow-up. Sleep fragmentation and/or intermittent hypoxia from OSA are likely candidate mechanisms. If confirmed, clinical interventions for OSA may be useful in preventing amyloid build-up in cognitively normal elderly.
Keywords: obstructive sleep apnea, amyloid burden, Pittsburgh compound B positron emission tomography scan, cerebrospinal fluid amyloid β, cognitive impairment
At a Glance Commentary
Scientific Knowledge on the Subject
Recent literature in both mice and humans suggests that disturbed sleep leads to higher levels of brain soluble β amyloid peptides, which aggregate to form senile plaques, a hallmark of Alzheimer’s disease. This pathologic process might be present before cognitive decline, indicating that disturbed sleep can be both a consequence of and a risk factor for Alzheimer’s disease.
What This Study Adds to the Field
This longitudinal study shows that obstructive sleep apnea, very common in elderly, can be a risk factor for developing Alzheimer’s disease.
Obstructive sleep apnea (OSA) and Alzheimer’s disease (AD) are chronic disease conditions that are highly prevalent, cause significant morbidity and mortality to those afflicted (1, 2), and have an enormous socioeconomic impact. OSA is typified by recurrent partial or complete obstructions of the upper airway during sleep leading to intermittent hypoxia and/or sleep fragmentation. OSA is associated with hypertension, cardiovascular risk, cognitive decline (3), and multiple inflammatory and metabolic effects (4–6) (for a review, see Reference 7). OSA affects 30–80% of the elderly (8, 9) depending on how OSA is defined. The clinical relevance of these high rates in the elderly is unclear, because some studies demonstrate increased rates of mortality, whereas others suggest that sleepiness, cognitive impairment, hypertension, and mortality associated with OSA decline with age (10). However, in a recent study of older women where nocturnal polysomnography was collected at baseline and cognition was evaluated 5 years later, patients with OSA were more likely to develop mild cognitive impairment (MCI) or dementia at follow-up (3). In a similar study using the Alzheimer’s Disease Neuroimaging Initiative database, we found that reported patients with OSA had an earlier age of cognitive decline to MCI and to AD than non-OSA control subjects (11). Furthermore, in a meta-analysis of cross-sectional studies, patients with AD were five times more likely to present with OSA than cognitively unimpaired individuals of similar age (12). Although OSA could be a consequence of events in the progression of AD pathology, alternatively, OSA may precipitate AD pathogenesis. The latter would present an exciting opportunity to slow AD pathology with sleep interventions.
The link between severity of OSA and risk for AD could be mediated by an increase in amyloid deposition as a small number of cross-sectional studies suggest. Greater Aβ burden using amyloid positron emission tomography (PET) globally and regionally in the precuneus has been associated with OSA severity among patients with MCI (13). We also demonstrated a trend toward decreased cerebrospinal fluid (CSF) amyloid β42 (Aβ42) levels in cognitively normal apolipoprotein E4 (ApoE4)-positive carriers with OSA (14), and a recent cross-sectional study showed that patients with OSA had lower CSF Aβ42 levels when compared with control subjects (15), suggesting that OSA might contribute to amyloid deposition and accelerate cognitive decline in those at risk for AD. However, so far it has been challenging to verify causality for these associations because OSA and AD may share common risk factors (16, 17) and neurodegenerative consequences (17) (e.g., vascular damage, hippocampal atrophy).
Based on the existing literature, the aims of this study were to use the New York University (NYU) Center for Brain Health cohort of cognitively normal healthy elderly to investigate the cross-sectional and longitudinal associations between OSA severity and changes in CSF and PET biomarkers of AD.
Methods
NYU Cohort
The NYU cohort consists of community-dwelling healthy cognitively normal volunteers and was derived from three NIH/National Institute on Aging and one Alzheimer’s Association supported studies. All subjects received medical, neurologic, and psychiatric evaluations; clinical laboratory studies; home monitoring for OSA; structural magnetic resonance imaging scans; a lumbar puncture (LP); and/or a Pittsburgh compound B (PiB) PET scan. As such, sleep complaints were not part of the inclusion or exclusion criteria of these protocols nor were subjects referred to the studies from any sleep disorders clinic. All subjects were administered a standard neuropsychological test battery that has published norm values (18).
Subjects
Subjects were between the ages of 55 and 90, English speaking, with a minimum of 12 years of education, had Mini-Mental State Exam (19) scores between 25 and 30 (inclusive), a Clinical Dementia Rating (20) of 0, were nondepressed, and had a consensus clinical diagnosis of cognitively normal. Because of known CSF batch variations, only values that were either batch corrected or from the same assay date were included. Individuals using continuous positive airway pressure (CPAP) or with significant medical conditions that could affect brain structure or function and/or magnetic resonance imaging evidence of intracranial mass or infarcts were excluded. Written informed consent was obtained from all participants.
Sleep Evaluation
The sleep evaluation included a sleep interview, detailed snoring history, and self-administration of the Epworth Sleepiness Scale (21). Home monitoring of OSA was completed using either an ARES Unicorder (Watermark) (22) or an Embletta MPR (Natus Medical Inc.) (23) system during a two-night period. For most subjects, home sleep evaluations were completed before the baseline LP and amyloid PET scan. However, there were few subjects (n = 21) whose sleep evaluations were done after the baseline LP and amyloid PET scan. Out of these 21 subjects only five completed their follow-up LP and amyloid PET scan of whom were included in the longitudinal analyses. The variables used in this study were 1) the apnea/hypopnea index with 4% desaturation (AHI4%), defined as the sum of all apneas (>90% reduction in airflow for >10 s) and all hypopneas (>30% reduction in airflow for 10 s) associated with greater than 4% oxygen desaturation divided by the total time where both flow and oximetry signals were valid; 2) the AHIall, which was defined as the sum of all apneas and all hypopneas identified plus events with visible reduction in airflow amplitude and presence of inspiratory flattening ending in breaths with normalization of airflow as a surrogate for arousal (24), divided by the total time where there was a valid flow signal irrespective of SaO2; and, 3) mean SaO2 during the night. Although the systems used different techniques of oximetry measurement, we have previously shown that OSA indices between these two devices are highly correlated (22). Both systems and AHI indices have been compared with the recommended definitions of AHI (22). Reported total sleep time (TST) duration was assessed using one question: “During the past month, how many hours of sleep did you usually get each night”?
LP, CSF Collection, and Assays
The procedures for the NYU LP are published (25, 26). CSF amyloid β (Aβ42), total-tau (T-tau), and tau phosphorylated at threonine 181 (P-tau) concentrations were measured using sandwich ELISA (INNOTEST). All assays were conducted at Sahlgrenska University Hospital. Batch wise rescaling of CSF Aβ42 was performed using linear regression with a reference batch. Before rescaling Aβ42, the coefficient of variation was 20%, and was reduced to 10% after rescaling. P-tau and T-tau were not rescaled because the coefficient of variation between batches was already relatively low (9%). CSF assays were done blind to clinical or sleep data.
PiB Scans
All subjects received PiB PET scans following standardized published procedures (27). Parametric standardized uptake value ratio (SUVR) images were generated by normalizing PiB uptake by cerebellar gray matter uptake (28). PiB SUVR images were processed using automated regions of interest (ROIs) (27). These ROIs were used to sample AD-vulnerable brain regions from the PiB SUVR images, including hippocampus, inferior parietal lobule, lateral temporal lobe, medial frontal gyrus, posterior cingulate cortex/precuneus, prefrontal cortex, occipital cortex, and thalamus. The cortical PiB meta-ROI retention mask (Alzheimer’s disease vulnerable regions of interest Pittsburgh compound B positron emission tomography mask; ADPiB-mask) was created by combining amyloid-vulnerable inferior parietal lobule, lateral temporal lobe, medial frontal gyrus, posterior cingulate cortex/precuneus, and prefrontal cortex regions (29).
Statistical Analyses
Statistical analyses were performed using SPSS version 23 (SPSS, Inc.). Baseline measures between OSA groups (normal, mild, and moderate–severe) were examined based on AHI4% cutoff values (<5, 5–14.9, and ≥15, respectively) using ANOVA with post hoc Tukey tests for continuous variables and chi-square test for categorical variables. Regression-based z-scores corrected for age, sex, race, and education, derived from our normative sample (18), were used for OSA group comparisons of cognitive variables (Logic 2, animal fluency, vegetable fluency, Boston Naming Test, Digit Symbol Substitution Test, Trails Making Test-A, and Trails Making Test-B), and for correlation analyses between annual rate of change of CSF Aβ42 and annual change in cognitive z-scores. For comparison between OSA severity groups, univariate analysis was used after adjusting for age, sex, body mass index (BMI), ApoE4, and time interval between procedures.
To test whether normal elderly subjects with OSA showed evidence of positive PET/CSF AD biomarkers, first we calculated the correlation coefficients between AD biomarkers and OSA indices at cross-section. Direct and partial correlations were computed, the latter adjusting for relevant cofactors, such as age, sex, BMI, and ApoE4 status. A similar approach was used for longitudinal analyses using Δ in amyloid biomarkers. We decided to control for these factors a priori given the well-documented association between decreased levels of CSF Aβ42, old age, and presence of the ApoE4 allele. Male sex and obesity were similarly included because they are the most important risk factors for OSA, whereas female sex is also a well-known risk factor for AD.
To calculate the annual rate of change of CSF Aβ42 or ADPiB-mask for each subject, we used the change in outcome from baseline to follow-up divided by the elapsed time from baseline to follow-up. We then applied a hierarchical linear regression, with annual rate of change of CSF Aβ42 or ADPiB-mask as dependent variables and OSA indices as independent, adjusting first for age, sex, BMI, and ApoE4 status. To control for the type of sleep recording device, we included it as a covariate in the model. Because of the skewness and heavy tails in the distributions of ADPIB-mask, nonparametric correlations were performed for comparisons between ADPiB-mask and OSA indices. Logarithm transformations were applied to continuous measures of Aβ42, P-tau, T-tau, Δ ADPiB-mask, and AHI indices because of their right-skewed distributions. All statistical analyses were tested for violations of the model assumptions and any conflicts and resolutions are reported. Statistical significance was set at P less than 0.05 using two-sided tests.
Results
Baseline Demographics and Sleep Characteristics
Baseline demographic and raw values of sleep characteristics are summarized in Table 1. Among the 208 participants, 97 were free of OSA (AHI4% <5) and considered healthy control subjects, 76 had mild OSA (AHI4% 5–14.9), and 35 had moderate to severe OSA (AHI4% ≥15). Within the moderate to severe group only, 14 subjects had an AHI4% greater than 30 and six subjects had an AHI4% greater than 45. Patients with OSA were more commonly male and older (χ2[2,n = 208] = 4.26, P = 0.11, and F2,205 = 2.36, P = 0.09, respectively), and had significantly higher BMI than healthy control subjects (F2,206 = 9.67; P < 0.01). However it was not an obese group (mean BMI, 26.68 ± 5.35; only 14 subjects of the 208 with a BMI >35). Moreover, using repeated measures ANOVA, BMI within subjects did not change significantly at follow-up (F1,105 = 0.68, not significant [NS]). We did not find significant differences across healthy control subjects and OSA groups in years of education, hypertension, diabetes, cardiovascular, thyroid disease, or ApoE4 status. Excessive daytime sleepiness was remarkably low in the entire sample (median Epworth Sleepiness Scale of 5; interquartile range, 3.8), with only 19 subjects with an Epworth Sleepiness Scale greater than 10. On univariate analysis there were no significant differences between OSA groups regarding TST. Overall TST was 7.03 ± 1.12 hours.
Table 1.
Baseline Demographic and Sleep Characteristics of the Subjects
| Characteristics | All | Normal | Mild OSA | Moderate–Severe OSA |
|---|---|---|---|---|
| Participants, n (%) | 208 (100) | 97 (46.63) | 76 (36.53) | 35 (16.82) |
| Female sex, n (%) | 129 (62) | 67 (69.1) | 44 (57.9) | 18 (51.4) |
| BMI, kg/m2, median (IQR) | 25.79 (22.7–29.87) | 24.61 (22.32–28.17)* | 26.89 (23.32–29.9) | 29.76 (23.49–33.51)* |
| Age, yr, mean ± SD | 68.46 ± 7.38 | 67.56 ± 7.32 | 68.60 ± 7.19 | 70.68 ± 7.69 |
| Education, yr, median (IQR) | 17 (16–18) | 16.5 (16–18) | 17 (16–18) | 16 (14–19) |
| Hypertension, n (%) | 86 (41.3) | 34 (35.1) | 32 (42.1) | 20 (57.1) |
| Diabetes, n (%) | 12 (5.8) | 4 (4.1) | 4 (5.3) | 4 (11.4) |
| Cardiovascular disease, n (%) | 9 (4.3) | 1 (1) | 7 (9.2) | 1 (2.9) |
| Thyroid disease, n (%) | 34 (16.3) | 16 (16.5) | 11 (14.5) | 7 (20) |
| ApoE4 positive, n (%) | 71 (34.1) | 34 (35.1) | 25 (32.9) | 12 (34.3) |
| AHI4%, median (IQR) | 5 (1.55–11.40) | 1.45 (0.725–3.00)* | 7.75 (5.81–10.52)* | 25.00 (19.3–37.00)* |
| AHIall, median (IQR) | 17 (10.85–24.00) | 10.40 (6.75–13.65)* | 20.05 (17.05– 24.00)* | 39.00 (31–57)* |
| Mean SaO2, median (IQR) | 94.19 (93.15–95.6) | 94.57 (93.78–95.6)* | 94.9 (92.77–95.71)*† | 93.47 (92.1–94.5)*† |
| ESS, median (IQR) | 5 (3–8) | 4 (3–7) | 6 (3.5–8.5) | 6 (4–9) |
| TST, h, median (IQR) | 7 (6.5–8) | 7.48 (6.75–8) | 7.00 (6.5–8) | 7.50 (6.5–8) |
Definition of abbreviations: AHI4% = sum of all apneas and hypopneas associated with greater that 4% oxygen desaturation; AHIall = sum of all apneas, hypopneas, or arousals; ApoE4 = apolipoprotein E4; BMI = body mass index; ESS = Epworth Sleepiness Scale; IQR = interquartile range; OSA = obstructive sleep apnea; TST = total sleep time.
Statistically significant difference between the groups.
Statistically significant difference between mild OSA and moderate–severe OSA groups for mean SaO2.
Psychometric Assessment
Cross-sectional and longitudinal cognitive characteristics of all subjects are shown in Table 2. We did not find any statistically significant differences between OSA indices and cognition across healthy and OSA groups at baseline or longitudinally. To assess the relationship between longitudinal changes in CSF Aβ42 and cognitive performance, we performed Pearson correlation analyses comparing annual rate of change of CSF Aβ42 and annual change in cognitive z-scores. No statistically significant correlations were found: Logic 2 (r = −0.12, NS), animal fluency (r = 0.15, NS), vegetable fluency (r = 0.09, NS), Boston Naming Test (r = 0.006, NS), Digit Symbol Substitution Test (r = 0.16, NS), Trails Making Test-A (r = 0.001, NS), and Trails Making Test-B (r = −0.08, NS).
Table 2.
Cognitive Characteristics of NYU Cohort at Baseline and Follow-up Evaluations
| All (n = 108) | Normal (n = 50) | Mild OSA (n = 43) | Moderate–Severe OSA (n = 15) | |
|---|---|---|---|---|
| MMSE baseline | 29.31 ± 0.99 | 29.40 ± 0.93 | 29.18 ± 0.98 | 29.33 ± 1.30 |
| MMSE follow-up | 29.36 ± 0.85 | 29.51 ± 0.718 | 29.29 ± 0.867 | 29.00 ± 1.206 |
| CDR baseline | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| CDR follow-up | 0.010 ± 0.071 | 0 ± 0 | 0 ± 0 | 0.083 ± 0.19 |
| Animal fluency (z-scores) | 0.207 ± 0.99 | 0.24 ± 1.14 | 0.05 ± 0.81 | 0.50 ± 0.95 |
| Animal fluency (Δ z-scores) | −0.23 ± 0.87 | −0.30 ± 0.98 | −0.20 ± 0.85 | −0.11 ± 0.54 |
| Vegetable fluency (z-scores) | −0.042 ± 1.1 | −0.023 ± 0.98 | −0.14 ± 1.28 | 0.15 ± 0.96 |
| Vegetable fluency (Δ z-scores) | −0.14 ± 0.99 | −0.39 ± 0.87 | 0.087 ± 1.08 | −0.02 ± 0.98 |
| Boston Naming Test (z-scores) | −0.20 ± 1.03 | −0.10 ± 1.06 | −0.38 ± 0.98 | −0.017 ± 1.07 |
| Boston Naming Test (Δ z-scores) | 0.11 ± 0.71 | 0.24 ± 0.69 | 0.12 ± 0.71 | −0.28 ± 0.69 |
| Logic 1 (z-scores) | 0.19 ± 0.96 | 0.11 ± 1.0 | 0.24 ± 0.90 | 0.29 ± 1.05 |
| Logic 1 (Δ z-scores) | −0.007 ± 0.86 | −0.03 ± 0.87 | −0.07 ± 0.82 | 0.23 ± 0.96 |
| Logic 2 (z-scores) | 0.10 ± 1.0 | 0.11 ± 1.07 | 0.008 ± 0.97 | 0.33 ± 0.88 |
| Logic 2 (Δ z-scores) | −0.012 ± 0.75 | 0.042 ± 0.8 | −0.06 ± 0.75 | −0.04 ± 0.67 |
| Trails Making Test-A time (z-scores) | 0.062 ± 1.06 | −0.14 ± 0.88 | −0.33 ± 1.04 | 0.12 ± 0.89 |
| Trails Making Test-A time (Δ z-scores) | 0.048 ± 0.88 | 0.025 ± 0.14 | 0.127 ± 0.7 | −0.093 ± 1.03 |
| Trails Making Test-B time (z-scores) | −0.17 ± 0.96 | −0.14 ± 0.89 | −0.33 ± 1.04 | 0.12 ± 0.9 |
| Trails Making Test-B time (Δ z-scores) | −0.034 ± 0.72 | −0.007 ± 0.65 | −0.002 ± 0.63 | −0.19 ± 0.64 |
| DSST (z-scores) | 0.2 ± 0.95 | 0.2 ± 0.83 | 0.14 ± 1.03 | 0.36 ± 1.11 |
| DSST (Δ z-scores) | 0.07 ± 0.44 | 0.1 ± 0.44 | −0.003 ± 0.45 | 0.18 ± 0.37 |
Definition of abbreviations: CDR = Clinical Dementia Rating; DSST = Digit Symbol Substitution Test; MMSE = Mini-Mental State Exam; NYU = New York University; OSA = obstructive sleep apnea.
Data are shown as mean ± SD.
Lower z-scores represent worse cognitive function.
CSF and PET Assessment
From the 208 participants, 179 subjects performed an LP at baseline. A second LP was obtained at follow-up in 104 subjects 2.42 ± 0.88 years later. A total of 86 subjects performed PiB scans at baseline. A second PiB scan evaluation was obtained at follow-up in 34 subjects 2.50 ± 0.39 years later. A total of 57 participants performed both the LP and the PET scans at baseline. Twenty-five participants performed the LP and PET scans at both baseline and follow-up. We refer to participants with both baseline and follow-up biomarker data available as “completers,” whereas subjects with only baseline biomarkers data are referred to as “noncompleters.” There were no differences between completers and noncompleters in terms of age (t = −0.27, NS), sex (χ2 = 0.002, NS), BMI (t = 0.40, NS), Mini-Mental State Exam (t = 0.00, NS), years of education (t = 0.17, NS), ApoE4 status (χ2 = 0.93, NS), TST (t = 1.18, NS), AHIall (t = 0.82, NS), or AHI4% (t = 0.88, NS). Summary statistics of baseline, and annual changes of AD biomarkers are shown in Table 3. No significant associations were observed between annual changes in CSF Aβ42 and age (F1,93 = 2.23; P = 0.13; β = −1.68; 95% confidence interval [CI], −0.39 to 0.55; P = 0.13), sex (F1,93 = 0.64; P = 0.42; β = 13.64; 95% CI, −20.17 to 47.47; P = 0.42), BMI (F1,93 = 0.16; P = 0.69; β = −0.61; 95% CI, −3.67 to 2.44; P = 0.69) or ApoE4 (F1,93 = 0.42; P = 0.51; β = −11.35; 95% CI, −46.03 to 23.32; P = 0.51). At cross-section and longitudinally, we did not find any significant differences among the three OSA severity groups for CSF P-tau or T-tau. Similarly, no cross-sectional or longitudinal effects were found for CSF Aβ42 across OSA severity groups using univariate analysis. No significant correlation between CSF Aβ42 and AHI indices were observed at cross-section.
Table 3.
Alzheimer’s Disease Biomarker Characteristics
| All (n = 208) | Normal (n = 97) | Mild OSA (n = 76) | Moderate–Severe OSA (n = 35) | |
|---|---|---|---|---|
| CSF Aβ42 baseline (n = 179), mean ± SD | 681.31 ± 236.43 | 681.88 ± 243.18 | 690.61 ± 233.99 | 657.48 ± 224.79 |
| CSF Aβ42 annual change (n = 104), median (IQR) | 29.40 (−9.53 to 71.06) | 40.59 (4.23 to 80.80) | 26.97 (−29.99 to 66.71) | −4.088 (−18.97 to 27.92) |
| CSF P-tau baseline (n = 179), median (IQR) | 41 (31 to 52) | 42.50 (31.5 to 52.05) | 43.55 (30 to 55) | 40.97 (31.71 to 49) |
| CSF P-tau annual change (n = 104), mean ± SD | 1.42 ± 3.93 | 1.35 ± 3.18 | 0.73 ± 4.27 | 3.43 ± 4.90 |
| CSF T-tau baseline (n = 179), median (IQR) | 257.96 (202 to 360.91) | 268.04 (217.65 to 362) | 244.85 (198 to 382) | 248.14 (174 to 343) |
| CSF T-tau annual change (n = 104), mean ± SD | 8.24 ± 21.42 | 7.52 ± 18.86 | 5.85 ± 21.83 | 17.04 ± 27.53 |
| ADPiB PET mask baseline (n = 86), median (IQR) | 1.05 (1.02 to 1.11) | 1.047 (1.02 to 1.09) | 1.061 (1.00 to 1.11) | 1.06 (1.01 to 1.14) |
| ADPiB PET mask annual change (n = 34), median (IQR) | 0.0005 (−0.009 to 0.014) | −0.0020 (−0.0095 to 0.0078) | −0.0022 (−0.0126 to 0.0224) | 0.014 (0.006 to 0.028) |
Definition of abbreviations: Aβ42 = amyloid β42; ADPiB PET mask = Alzheimer’s disease vulnerable regions of interest Pittsburgh compound B PET mask; CSF = cerebrospinal fluid; IQR = interquartile range; OSA = obstructive sleep apnea; PET = positron emission tomography; P-tau = tau phosphorylated at threonine 181; T-tau = total tau.
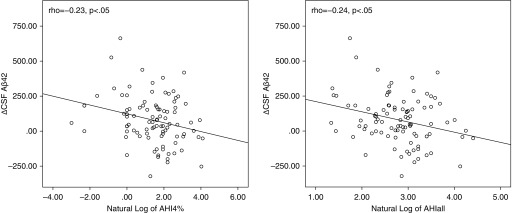
However, significant correlations were observed between longitudinal change in CSF Aβ42 levels and AHIall/AHI4% (r = −0.24, P < 0.05; and r = −0.23, P < 0.05, respectively) and after controlling for age, sex, BMI, and ApoE4 (r = −0.27, P < 0.05; and r = −0.24, P < 0.05, respectively). Significant associations were also observed between annual rate of change of CSF Aβ42 and AHI indices at baseline using hierarchical linear regression model (shown in Table 4), including annual rate of change of CSF Aβ42 as dependent and AHI indices (lnAHI4% and lnAHIall) as independent variables, before (F1,92 = 5.41, P < 0.05; and F1,93 = 4.72, P < 0.05, respectively) and after accounting for age, sex, BMI, and ApoE4 (F1,88 = 4.26, P < 0.05; and F1,87 = 4.36, P < 0.05, respectively). The effect of the type of sleep recording device and TST were not significant, thus we excluded them from the final model. Figure 1 shows the relationship between Δ in CSF Aβ42 and the AHI indices at baseline. Sensitivity analyses were performed excluding five subjects whose baseline sleep evaluation was done after their first CSF measurements. Association between lnAHI4%, lnAHIall, and annual Δ CSF Aβ42 remained unchanged.
Table 4.
Final Model Showing Relationship of Annual ΔCSF Aβ42 and Annual ΔPiB with AHIall and AHI4%
| Dependent variable | Models | R2 | ΔR2 | Independent Variables | β | 95% CI | P Value |
|---|---|---|---|---|---|---|---|
| Annual ΔCSF Aβ42 | Model | −0.008 | 0.035 | Age | −1.36 | −3.67 to 0.95 | 0.24 |
| Sex | 6.63 | −27.72 to 40.99 | 0.70 | ||||
| BMI | 0.88 | −2.36 to 4.12 | 0.59 | ||||
| ApoE4 | −15.54 | −50.69 to 8.81 | 0.36 | ||||
| Model + AHI4%* | 0.028 | 0.046 | AHI4% | −13.35 | −26.06 to −0.64 | 0.04 | |
| Model | −0.008 | 0.035 | Age | −1.57 | −3.86 to 0.70 | 0.17 | |
| Sex | 4.07 | −30.36 to 38.51 | 0.81 | ||||
| BMI | 1.0 | −2.27 to 4.27 | 0.54 | ||||
| ApoE4 | −17.89 | −52.58 to 16.79 | 0.30 | ||||
| Model + AHIall | 0.027 | 0.044 | AHIall | −29.08 | −57.08 to −1.08 | 0.04 | |
| Annual ΔPiB | Model | −0.068 | 0.062 | Age | 0.001 | −0.001 to 0.004 | 0.28 |
| Sex | 0.001 | −0.036 to 0.038 | 0.96 | ||||
| BMI | −0.001 | −0.004 to 0.002 | 0.37 | ||||
| ApoE4 | 0.01 | −0.026 to 0.046 | 0.36 | ||||
| Model + AHI4% | 0.134 | 0.072 | AHI4% | 0.013 | −0.004 to 0.03 | 0.13 | |
| Model | −0.068 | 0.062 | Age | 0.001 | −0.001 to 0.004 | 0.25 | |
| Sex | 0.001 | −0.036 to 0.038 | 0.96 | ||||
| BMI | −0.001 | −0.004 to 0.002 | 0.37 | ||||
| ApoE4 | 0.01 | −0.026 to 0.046 | 0.56 | ||||
| Model + AHIall | 0.151 | 0.09 | AHIall | 0.026 | −005 to 0.057 | 0.09 |
Definition of abbreviations: Aβ42 = amyloid β42; AHI = apnea–hypopnea index; AHI4% = the sum of all apneas and hypopneas associated with greater that 4% oxygen desaturation; AHIall = the sum of all apneas, hypopneas, or arousals; ApoE4 = apolipoprotein E4; BMI = body mass index; CI = confidence interval; CSF = cerebrospinal fluid; PiB = Pittsburgh compound B.
AHI4% is missing in 1 subject owing to errors in home sleep pulse oximetry measurements.
Figure 1.
Relationship between longitudinal change in cerebrospinal fluid amyloid β42 (Aβ42) and the natural log of apnea–hypopnea indices at baseline. AHI = apnea–hypopnea index; AHI4% = sum of all apneas and hypopneas associated with greater that 4% oxygen desaturation; AHIall = sum of all apneas, hypopneas, or arousals; CSF = cerebrospinal fluid.
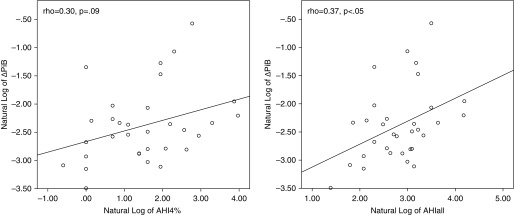
Similarly, on univariate analysis no difference in ADPiB-mask was observed between OSA severity groups, and no significant correlation between ADPiB-mask and AHI indices were observed at cross-section. However, correlations were observed between longitudinal change in ADPiB-mask and AHIall or AHI4% (r = 0.374, P < 0.05; and r = 0.302, P = 0.09, respectively) after controlling for age, sex, BMI, and ApoE4. Using the same hierarchical linear regression model as for CSF Aβ42, no statistically significant associations were observed between annual rate of change of ADPiB-mask and AHIs, including annual rate of change of ADPiB-mask as dependent and AHI indices at baseline as independent variables after accounting for age, sex, BMI, and ApoE4. lnAHIall and lnAHI4% were not associated with increases in ADPiB-mask most likely because of the small sample size because there was a trend for lnAHIall (F1,28 = 2.96, P = 0.09; and F1,28 = 2.32, NS, respectively). Figure 2 shows the relationship between Δ in ADPiB-mask and the AHIall index at baseline; both variables were corrected for normal distribution by log transformation.
Figure 2.
Relationship between longitudinal change in ADPiB-mask and the apnea–hypopnea indices at baseline. ADPiB-mask = Alzheimer’s disease vulnerable regions of interest Pittsburg compound B positron emission tomography mask; AHI = apnea–hypopnea index; AHI4% = sum of all apneas and hypopneas associated with greater that 4% oxygen desaturation; AHIall = sum of all apneas, hypopneas, or arousals; PiB = Pittsburgh compound B.
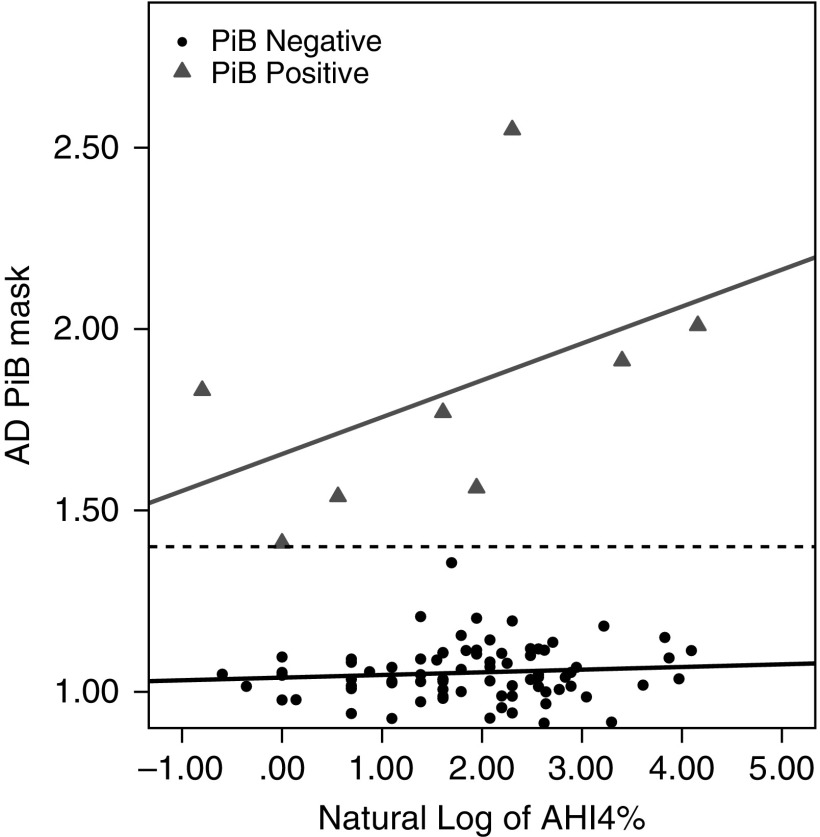
Furthermore, we analyzed the association between longitudinal change in CSF Aβ42 and ADPiB-mask. Using a Pearson correlation, a significant negative correlation between longitudinal change in CSF Aβ42 and ADPiB-mask was observed (r = −0.44; P < 0.05). Using an ADPiB-mask SUVR greater than or equal to 1.4 to define presence of brain amyloid deposition (PiB-positive) (30–32), a secondary analysis performed only in the initial cross-sectional cases, revealed a significant difference between the slopes of PiB-positive and PiB-negative cases (Figure 3). This was confirmed by the presence of an interaction between PiB status and lnAHI4% (F1,29 = 5.54; P < 0.05) and a positive trend between AHI4% and PiB uptake in PiB-positive subjects (r = 0.67; P = 0.07). Similar findings were observed for AHIall (data not shown). Figure 3 shows the relationships between the AHI4% and PiB SUVR uptake when comparing PiB-positive with PiB-negative groups.
Figure 3.
At cross-section, severity of obstructive sleep apnea is associated with greater brain amyloid β deposition in PiB-positive participants, whereas no such association is found in participants with PiB-negative scans. AD PiB mask = Alzheimer’s disease vulnerable regions of interest Pittsburgh compound B positron emission tomography mask; AHI4% = sum of all apneas and hypopneas associated with greater that 4% oxygen desaturation; PiB = Pittsburgh compound B.
Discussion
The primary objective of this study was to determine if severity of OSA in cognitively normal elderly is associated with CSF and PET AD biomarkers at cross-section and their longitudinal change across an approximate 2-year period. Our initial finding revealed that OSA was common and affected 53% of our cognitively normal community-dwelling cohort. Second, we demonstrated that baseline OSA severity was associated with 2-year longitudinal decreases in CSF Aβ42 and a trend toward increases in cortical PiB-PET uptake. Such changes are potentially consistent with increased brain amyloid burden, which were also observed in our cohort (i.e., a negative correlation between longitudinal change in CSF Aβ42 and ADPiB-mask), suggesting that OSA may play a role in amyloid deposition in late-life. Moreover, the magnitude of these changes was higher than the one predicted by the presence of the ApoE4 allele alone (Table 4), which to date is considered the most important risk factor for sporadic AD. AHIall, which includes hypopneas associated with oxygen desaturation or arousals, was a better predictor of longitudinal increases in amyloid burden than AHI4%, which includes only hypopneas associated with 4% oxygen desaturation. This raises the possibility that sleep fragmentation is a more critical pathophysiologic mechanism by which OSA contributes to AD risk. However, AHIall and AHI4% were highly correlated in our cohort (r = 0.91; P < 0.01) and this study was unable to differentiate the individual effects of sleep fragmentation versus intermittent hypoxia.
Although OSA severity was associated with increases in brain amyloid burden, it was not predictive of cognitive deterioration based on neuropsychological performance, which is in agreement with prior studies (33, 34). This is not completely surprising given that the relationship between amyloid burden and cognition is probably nonlinear and dependent on additional factors, such as tau pathology and microvascular changes. Low sensitivity of the neuropsychological tests used may have been another factor. Sensitivity could be increased in the future by using cognitive tasks that are known to be sleep dependent.
Current evidence suggests that cognitive decline in AD is associated with decreases in CSF Aβ42 and increases in amyloid PET uptake (35). However, little is known about the temporal course of CSF Aβ42 in the preclinical or early stages of the disease, with some recent animal and human studies showing Aβ42 elevations before Aβ42 reductions (36, 37), suggesting an intermediate stage of increased soluble Aβ levels before amyloid deposition. Interestingly, we and others have shown that reduced slow wave activity at cross-section and one night of slow wave sleep (SWS) disruption, are associated with increases in CSF Aβ levels, potentially as a consequence of increases in neuronal firing and/or decreases in amyloid clearance (38–41). It remains to be determined how universal a period of elevated CSF Aβ42 in humans is observed before a decline, but the previously mentioned studies suggest that sleep disruption might be associated with elevations of CSF Aβ42, which in chronic sleep disorders, such as OSA, could foster its aggregation and manifest as longitudinal decreases in CSF Aβ42 over time, such as the one observed in our study. This hypothesis would also explain the absence of significant associations at cross-section.
Whether OSA-related sleep fragmentation increases AD-risk through disruption of SWS or other sleep stages is unknown. The ends of apneas are associated with arousals or awakenings that prevent sleep (42) and these are more commonly observed in non-REM1–2 and REM sleep. Apneic episodes are less common in SWS, which has been associated with a higher respiratory arousal threshold (43, 44) and more stable breathing (45). However, the temporal course of slow wave activity has been shown to be slower in mild OSA (46), whereas severe patients with OSA show up to a 40% rebound in SWS duration during OSA treatment with CPAP (47), which suggest that changes in SWS quality may also be involved. However, a recent prospective study reported the association between decreased percentage of REM sleep and increased risk of dementia, implicating also REM sleep as a possible mediator for AD risk (48). In addition, actigraphy-assessed arousals and circadian rhythm disruption have also been shown to increase the risk of MCI/dementia in the elderly (49), indicating that the relationship between OSA-related sleep fragmentation and amyloid deposition might not be stage-specific.
Another possible mechanism by which OSA might increase amyloid deposition is through impairment in the CSF–interstitial fluid (ISF) exchange promoted by the glymphatic system (40) resulting in decreased clearance of ISF Aβ42. This mechanism was suggested in a recent study of 31 control subjects and 10 severe OSA middle-age subjects where neuronally derived proteins were decreased in the OSA group when compared with control subjects (40). The authors propose that elevations in the intrathoracic and intracranial pressure as well as a sudden pressure reversal at the end of the apnea would impede the glymphatic flow of metabolites from ISF into CSF (40). Another potential pathway of impairment of CSF–ISF exchange could be cerebral edema secondary to intermittent hypoxia, as proposed recently in a study in which severity of OSA correlated with increased volume and thickness of the left lateral prefrontal cortex and increased thickness of the right frontal pole, the right lateral parietal lobules, and the left posterior cingulate cortex (50). Similar findings were observed as brain volume reductions after 6 months of treatment with CPAP, which also suggests the existence of brain edema in OSA (51).
Finally, the effects of OSA directly increasing ISF Aβ42 burden as suggested by some intermittent hypoxia animals models (52, 53), or indirectly through other intermediate mechanisms, such as oxidative stress, sympathetic activation, inflammation, hypercoagulability, endothelial dysfunction, or metabolic dysregulation, cannot be discarded, although it is feasible that these and other consequences of OSA may decline with age (10, 54) and might not be as relevant in the elderly as in middle age.
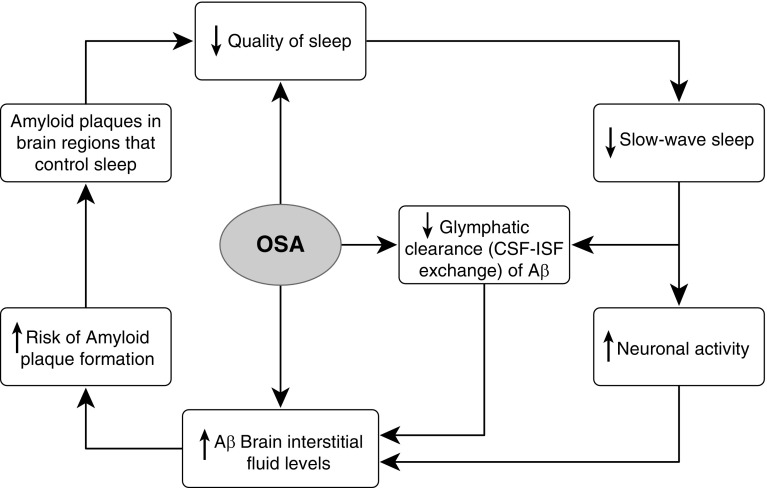
Among participants with initial PiB-positive scans at cross-section, Figure 3 suggest that a higher severity of OSA is associated with greater brain Aβ deposition, whereas no such association is found in participants with PiB-negative scans, implying that presence or absence of amyloid burden might act as a moderator in these relationships. This would be in agreement with previous studies showing increased amyloid deposition associated with higher AHI indices in patients with MCI but not in cognitively normal control subjects at cross-section (13). We did not observe this effect in the CSF sample when we compared amyloid-positive with amyloid-negative cases based on the NYU Center for Brain Health CSF bank Aβ42 cutoffs (i.e., CSF Aβ42 ng/ml <500), so this finding should be interpreted with caution. It may be that the effects of OSA/hypoxia on Aβ aggregation are most pronounced after significant Aβ accumulation has already occurred, leading to an acceleration of further Aβ deposition in a feed–forward cycle (13) (Figure 4) with OSA-related arousals worsening sleep quality and increasing amyloid deposition. In addition, 33 of 34 of the subjects that had PiB PET follow-up scans were PiB-negative at baseline, indicating that the observed longitudinal increases in PiB uptake were not dependent on amyloid status.
Figure 4.
Obstructive sleep apnea–related arousals may worsen sleep quality and increase amyloid deposition in a feed-forward cycle. Aβ = amyloid β; CSF = cerebrospinal fluid; ISF = interstitial fluid; OSA = obstructive sleep apnea.
Our observations are consistent with our hypothesis that there is an association between severity of OSA-related sleep fragmentation and longitudinal increase in amyloid burden in cognitively normal elderly. This implies that existing therapies for OSA, such as CPAP, could delay the progression to MCI or dementia in elderly with OSA, as was suggested by our previous epidemiologic studies using the Alzheimer’s Disease Neuroimaging Initiative database (11) and a recent cross-sectional study in which patients with OSA showed lower CSF Aβ42 concentrations, and higher T-tau/Aβ42 ratio when compared with patients with OSA-CPAP (15).
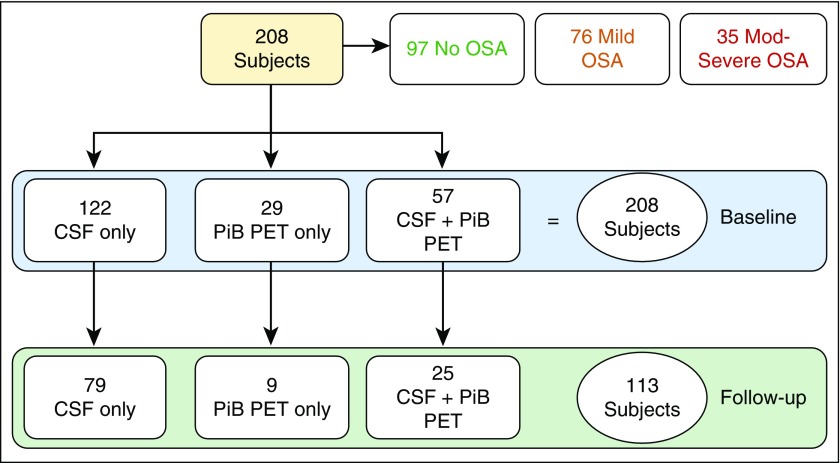
The high prevalence of mild and moderate-to-severe OSA in cognitively normal elderly in asymptomatic adults undergoing screening for OSA as part of a protocol on memory and normal aging adds to the importance of these findings. Strengths of our study include that our community-residing subjects were not recruited for the study based on sleep complaints, and thus should have been free of selection biases potentially affecting sleep clinic–based cohorts, which typically include younger, more frequently male, obese, and symptomatic (e.g., excessive daytime sleepiness, treatment-resistant hypertension). We also used state-of-the-art methods for home-monitoring of OSA, and longitudinal standardized CSF and PET biomarkers. Potential weaknesses of the study were the relative short duration and the lack of longitudinal sleep data, which did not allow us to test whether preclinical-AD brain lesions increase the risk for OSA, or the lack of a longer clinical assessment to test whether amyloid deposition is followed by cognitive decline to MCI or AD. Another limitation of the study was that not all subjects had a longitudinal follow-up, although both completers and noncompleters (Figure 5) were not different in terms of sociodemographics, BMI, Mini-Mental State Exam, AHIall, or AHI4%.
Figure 5.
Breakdown of completers and noncompleters. CSF = cerebrospinal fluid; Mod = moderate; OSA = obstructive sleep apnea; PET = positron emission tomography; PiB = Pittsburgh compound B.
In summary, to our knowledge this study is the first to document that OSA is associated with longitudinal changes in amyloid burden in a sample of cognitively normal elderly. The implication of these findings is that we have identified a contribution of OSA in increasing the Aβ burden before significant cognitive decline occurs. Our data support testing whether clinical interventions aimed at OSA, such as treatment with CPAP or dental appliances, could be implemented during the early phase in which tissue damage precedes clinical symptoms and neuronal dysfunction, to mitigate the progression of cognitive impairment.
Acknowledgments
Acknowledgment
The authors thank the study subjects for their patience, and for their participation in and contribution to the research. The authors acknowledge contributions to patient recruitment and data collection by Ms. Kimberly Clay, Mr. Michael Yablon, Ms. Christine Grosso, and Ms. Gabriella Petrongolo. They also thank Dr. Pauline McHugh for her assessment of research subjects.
Footnotes
This work was supported by grants from NIH/National Institute on Aging/NHLBI (R01HL118624, R01HL111724, R21AG049348, R01AG035137, R01AG022374, R01AG13616, R01AG12101, and P30AG008051), Foundation for Research in Sleep Disorders, the American Sleep Medicine Foundation Junior Faculty Award, and the Friedman Brain Institute. I.R. is supported by the Wellcome Trust (103952/Z/14/Z). H.Z. is supported by the Swedish Research Council (2013-2546) and the European Research Council (681712). N.G. is supported by a salary award from the Fonds pour la recherche du Québec–Santé.
Author Contributions: Conception and design, R.S.O., R.A.S., A.W.V., and O.M.B. Analysis and interpretation, R.S.O., R.A.S., A.W.V., O.M.B., E.P., A.P., K.K., M.W., M.D.M., C.L., S.T., P.L.Y., M.B., R.S., L.M., Y.L., T.B., L.G., E.F., J.S.B., K.B., H.Z., S.E.L., S.G.B., S.R., I.R., N.G., G.J.-L., D.M.R., M.J.d.L., I.A., and A.A. Drafting the manuscript for important intellectual content, R.S.O., R.A.S., A.W.V., O.M.B., T.B., A.A., L.G., E.F., J.S.B., K.B., H.Z., S.E.L., S.G.B., S.R., I.R., N.G., G.J.-L., D.M.R., M.J.d.L., and I.A.
Originally Published in Press as DOI: 10.1164/rccm.201704-0704OC on November 10, 2017
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Hebert LE, Beckett LA, Scherr PA, Evans DA. Annual incidence of Alzheimer disease in the United States projected to the years 2000 through 2050. Alzheimer Dis Assoc Disord. 2001;15:169–173. doi: 10.1097/00002093-200110000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Heinzer R, Marti-Soler H, Haba-Rubio J. Prevalence of sleep apnoea syndrome in the middle to old age general population. Lancet Respir Med. 2016;4:e5–e6. doi: 10.1016/S2213-2600(16)00006-0. [DOI] [PubMed] [Google Scholar]
- 3.Yaffe K, Laffan AM, Harrison SL, Redline S, Spira AP, Ensrud KE, et al. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA. 2011;306:613–619. doi: 10.1001/jama.2011.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ayas NT, Drager LF, Morrell MJ, Polotsky VY. Update in sleep-disordered breathing 2016. Am J Respir Crit Care Med. 2017;195:1561–1566. doi: 10.1164/rccm.201701-0048UP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gozal D, Kheirandish-Gozal L. Cardiovascular morbidity in obstructive sleep apnea: oxidative stress, inflammation, and much more. Am J Respir Crit Care Med. 2008;177:369–375. doi: 10.1164/rccm.200608-1190PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shahar E, Whitney CW, Redline S, Lee ET, Newman AB, Nieto FJ, et al. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;163:19–25. doi: 10.1164/ajrccm.163.1.2001008. [DOI] [PubMed] [Google Scholar]
- 7.Sánchez-de-la-Torre M, Campos-Rodriguez F, Barbé F. Obstructive sleep apnoea and cardiovascular disease. Lancet Respir Med. 2013;1:61–72. doi: 10.1016/S2213-2600(12)70051-6. [DOI] [PubMed] [Google Scholar]
- 8.Ancoli-Israel S, DuHamel ER, Stepnowsky C, Engler R, Cohen-Zion M, Marler M. The relationship between congestive heart failure, sleep apnea, and mortality in older men. Chest. 2003;124:1400–1405. doi: 10.1378/chest.124.4.1400. [DOI] [PubMed] [Google Scholar]
- 9.Mehra R, Stone KL, Varosy PD, Hoffman AR, Marcus GM, Blackwell T, et al. Nocturnal arrhythmias across a spectrum of obstructive and central sleep-disordered breathing in older men: outcomes of sleep disorders in older men (MrOS sleep) study. Arch Intern Med. 2009;169:1147–1155. doi: 10.1001/archinternmed.2009.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fung MM, Peters K, Redline S, Ziegler MG, Ancoli-Israel S, Barrett-Connor E, et al. Osteoporotic Fractures in Men Research Group. Decreased slow wave sleep increases risk of developing hypertension in elderly men. Hypertension. 2011;58:596–603. doi: 10.1161/HYPERTENSIONAHA.111.174409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Osorio RS, Gumb T, Pirraglia E, Varga AW, Lu SE, Lim J, et al. Alzheimer’s Disease Neuroimaging Initiative. Sleep-disordered breathing advances cognitive decline in the elderly. Neurology. 2015;84:1964–1971. doi: 10.1212/WNL.0000000000001566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Emamian F, Khazaie H, Tahmasian M, Leschziner GD, Morrell MJ, Hsiung GY, et al. The Association between obstructive sleep apnea and Alzheimer’s disease: a meta-analysis perspective. Front Aging Neurosci. 2016;8:78. doi: 10.3389/fnagi.2016.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spira AP, Yager C, Brandt J, Smith GS, Zhou Y, Mathur A, et al. Objectively measured sleep and β-amyloid burden in older adults: a pilot study. SAGE Open Med. 2014;2:2050312114546520. doi: 10.1177/2050312114546520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Osorio RS, Ayappa I, Mantua J, Gumb T, Varga A, Mooney AM, et al. Interaction between sleep-disordered breathing and apolipoprotein E genotype on cerebrospinal fluid biomarkers for Alzheimer’s disease in cognitively normal elderly individuals. Neurobiol Aging. 2014;35:1318–1324. doi: 10.1016/j.neurobiolaging.2013.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liguori C, Mercuri NB, Izzi F, Romigi A, Cordella A, Sancesario G, et al. Obstructive sleep apnea is associated with early but possibly modifiable Alzheimer’s disease biomarkers changes. Sleep. 2017;40:zsx011. doi: 10.1093/sleep/zsx011. [DOI] [PubMed] [Google Scholar]
- 16.Helzner EP, Luchsinger JA, Scarmeas N, Cosentino S, Brickman AM, Glymour MM, et al. Contribution of vascular risk factors to the progression in Alzheimer disease. Arch Neurol. 2009;66:343–348. doi: 10.1001/archneur.66.3.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de la Torre JC. Pathophysiology of neuronal energy crisis in Alzheimer’s disease. Neurodegener Dis. 2008;5:126–132. doi: 10.1159/000113681. [DOI] [PubMed] [Google Scholar]
- 18.De Santi S, Pirraglia E, Barr W, Babb J, Williams S, Rogers K, et al. Robust and conventional neuropsychological norms: diagnosis and prediction of age-related cognitive decline. Neuropsychology. 2008;22:469–484. doi: 10.1037/0894-4105.22.4.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Folstein MF, Robins LN, Helzer JE. The Mini-Mental State Examination. Arch Gen Psychiatry. 1983;40:812. doi: 10.1001/archpsyc.1983.01790060110016. [DOI] [PubMed] [Google Scholar]
- 20.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 21.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 22.Ayappa I, Norman RG, Seelall V, Rapoport DM. Validation of a self-applied unattended monitor for sleep disordered breathing. J Clin Sleep Med. 2008;4:26–37. [PMC free article] [PubMed] [Google Scholar]
- 23.Tiihonen P, Hukkanen T, Tuomilehto H, Mervaala E, Töyräs J. Evaluation of a novel ambulatory device for screening of sleep apnea. Telemed J E Health. 2009;15:283–289. doi: 10.1089/tmj.2008.0118. [DOI] [PubMed] [Google Scholar]
- 24.Ayappa I, Norman RG, Suryadevara M, Rapoport DM. Comparison of limited monitoring using a nasal-cannula flow signal to full polysomnography in sleep-disordered breathing. Sleep. 2004;27:1171–1179. doi: 10.1093/sleep/27.6.1171. [DOI] [PubMed] [Google Scholar]
- 25.Spiegel J, Pirraglia E, Osorio RS, Glodzik L, Li Y, Tsui W, et al. Greater specificity for cerebrospinal fluid P-tau231 over P-tau181 in the differentiation of healthy controls from Alzheimer’s disease. J Alzheimers Dis. 2016;49:93–100. doi: 10.3233/JAD-150167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vanderstichele H, Bibl M, Engelborghs S, Le Bastard N, Lewczuk P, Molinuevo JL, et al. Standardization of preanalytical aspects of cerebrospinal fluid biomarker testing for Alzheimer’s disease diagnosis: a consensus paper from the Alzheimer’s Biomarkers Standardization Initiative. Alzheimers Dement. 2012;8:65–73. doi: 10.1016/j.jalz.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 27.Mosconi L, Rinne JO, Tsui WH, Murray J, Li Y, Glodzik L, et al. Amyloid and metabolic positron emission tomography imaging of cognitively normal adults with Alzheimer’s parents. Neurobiol Aging. 2013;34:22–34. doi: 10.1016/j.neurobiolaging.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Price JC, Klunk WE, Lopresti BJ, Lu X, Hoge JA, Ziolko SK, et al. Kinetic modeling of amyloid binding in humans using PET imaging and Pittsburgh Compound-B. J Cereb Blood Flow Metab. 2005;25:1528–1547. doi: 10.1038/sj.jcbfm.9600146. [DOI] [PubMed] [Google Scholar]
- 29.Jack CR, Jr, Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, et al. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 2010;9:119–128. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mielke MM, Wiste HJ, Weigand SD, Knopman DS, Lowe VJ, Roberts RO, et al. Indicators of amyloid burden in a population-based study of cognitively normal elderly. Neurology. 2012;79:1570–1577. doi: 10.1212/WNL.0b013e31826e2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nordberg A, Carter SF, Rinne J, Drzezga A, Brooks DJ, Vandenberghe R, et al. A European multicentre PET study of fibrillar amyloid in Alzheimer’s disease. Eur J Nucl Med Mol Imaging. 2013;40:104–114. doi: 10.1007/s00259-012-2237-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Villeneuve S, Rabinovici GD, Cohn-Sheehy BI, Madison C, Ayakta N, Ghosh PM, et al. Existing Pittsburgh Compound-B positron emission tomography thresholds are too high: statistical and pathological evaluation. Brain. 2015;138:2020–2033. doi: 10.1093/brain/awv112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martin MS, Sforza E, Roche F, Barthélémy JC, Thomas-Anterion C PROOF study group. Sleep breathing disorders and cognitive function in the elderly: an 8-year follow-up study. the proof-synapse cohort. Sleep. 2015;38:179–187. doi: 10.5665/sleep.4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sforza E, Roche F, Thomas-Anterion C, Kerleroux J, Beauchet O, Celle S, et al. Cognitive function and sleep related breathing disorders in a healthy elderly population: the SYNAPSE study. Sleep. 2010;33:515–521. doi: 10.1093/sleep/33.4.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Palmqvist S, Zetterberg H, Mattsson N, Johansson P, Minthon L, Blennow K, et al. Alzheimer’s Disease Neuroimaging Initiative; Swedish BioFINDER Study Group. Detailed comparison of amyloid PET and CSF biomarkers for identifying early Alzheimer disease. Neurology. 2015;85:1240–1249. doi: 10.1212/WNL.0000000000001991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maia LF, Kaeser SA, Reichwald J, Lambert M, Obermüller U, Schelle J, et al. Increased CSF Aβ during the very early phase of cerebral Aβ deposition in mouse models. EMBO Mol Med. 2015;7:895–903. doi: 10.15252/emmm.201505026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shoji M, Kanai M, Matsubara E, Tomidokoro Y, Shizuka M, Ikeda Y, et al. The levels of cerebrospinal fluid Abeta40 and Abeta42(43) are regulated age-dependently. Neurobiol Aging. 2001;22:209–215. doi: 10.1016/s0197-4580(00)00229-3. [DOI] [PubMed] [Google Scholar]
- 38.Varga AW, Wohlleber ME, Giménez S, Romero S, Alonso JF, Ducca EL, et al. Reduced slow-wave sleep is associated with high cerebrospinal fluid Aβ42 levels in cognitively normal elderly. Sleep. 2016;39:2041–2048. doi: 10.5665/sleep.6240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ju YS, Ooms SJ, Sutphen C, Macauley SL, Zangrilli MA, Jerome G, et al. Slow wave sleep disruption increases cerebrospinal fluid amyloid-β levels. Brain. 2017;140:2104–2111. doi: 10.1093/brain/awx148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ju YE, Finn MB, Sutphen CL, Herries EM, Jerome GM, Ladenson JH, et al. Obstructive sleep apnea decreases central nervous system-derived proteins in the cerebrospinal fluid. Ann Neurol. 2016;80:154–159. doi: 10.1002/ana.24672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ooms S, Overeem S, Besse K, Rikkert MO, Verbeek M, Claassen JA. Effect of 1 night of total sleep deprivation on cerebrospinal fluid β-amyloid 42 in healthy middle-aged men: a randomized clinical trial. JAMA Neurol. 2014;71:971–977. doi: 10.1001/jamaneurol.2014.1173. [DOI] [PubMed] [Google Scholar]
- 42.Schwartz AR, Schneider H, Smith PL, McGinley BM, Patil SP, Kirkness JP. Physiologic phenotypes of sleep apnea pathogenesis. Am J Respir Crit Care Med. 2011;184:1105–1106. doi: 10.1164/rccm.201108-1573ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ratnavadivel R, Stadler D, Windler S, Bradley J, Paul D, McEvoy RD, et al. Upper airway function and arousability to ventilatory challenge in slow wave versus stage 2 sleep in obstructive sleep apnoea. Thorax. 2010;65:107–112. doi: 10.1136/thx.2008.112953. [DOI] [PubMed] [Google Scholar]
- 44.Saboisky J, Eckert D, Malhotra A. Stable breathing through deeper sleeping. Thorax. 2010;65:95–96. doi: 10.1136/thx.2009.127860. [DOI] [PubMed] [Google Scholar]
- 45.Wellman A, Jordan AS, Malhotra A, Fogel RB, Katz ES, Schory K, et al. Ventilatory control and airway anatomy in obstructive sleep apnea. Am J Respir Crit Care Med. 2004;170:1225–1232. doi: 10.1164/rccm.200404-510OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ondze B, Espa F, Dauvilliers Y, Billiard M, Besset A. Sleep architecture, slow wave activity and sleep spindles in mild sleep disordered breathing. Clin Neurophysiol. 2003;114:867–874. doi: 10.1016/s1388-2457(02)00389-9. [DOI] [PubMed] [Google Scholar]
- 47.Brillante R, Cossa G, Liu PY, Laks L. Rapid eye movement and slow-wave sleep rebound after one night of continuous positive airway pressure for obstructive sleep apnoea. Respirology. 2012;17:547–553. doi: 10.1111/j.1440-1843.2012.02147.x. [DOI] [PubMed] [Google Scholar]
- 48.Pase MP, Himali JJ, Grima NA, Beiser AS, Satizabal CL, Aparicio HJ, et al. Sleep architecture and the risk of incident dementia in the community. Neurology. 2017;89:1244–1250. doi: 10.1212/WNL.0000000000004373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lim AS, Kowgier M, Yu L, Buchman AS, Bennett DA. Sleep fragmentation and the risk of incident Alzheimer’s disease and cognitive decline in older persons. Sleep. 2013;36:1027–1032. doi: 10.5665/sleep.2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baril AA, Gagnon K, Brayet P, Montplaisir J, De Beaumont L, Carrier J, et al. Gray matter hypertrophy and thickening with obstructive sleep apnea in middle-aged and older adults. Am J Respir Crit Care Med. 2017;195:1509–1518. doi: 10.1164/rccm.201606-1271OC. [DOI] [PubMed] [Google Scholar]
- 51.O’Donoghue FJ, Briellmann RS, Rochford PD, Abbott DF, Pell GS, Chan CH, et al. Cerebral structural changes in severe obstructive sleep apnea. Am J Respir Crit Care Med. 2005;171:1185–1190. doi: 10.1164/rccm.200406-738OC. [DOI] [PubMed] [Google Scholar]
- 52.Shiota S, Takekawa H, Matsumoto SE, Takeda K, Nurwidya F, Yoshioka Y, et al. Chronic intermittent hypoxia/reoxygenation facilitate amyloid-β generation in mice. J Alzheimers Dis. 2013;37:325–333. doi: 10.3233/JAD-130419. [DOI] [PubMed] [Google Scholar]
- 53.Tabuchi M, Lone SR, Liu S, Liu Q, Zhang J, Spira AP, et al. Sleep interacts with aβ to modulate intrinsic neuronal excitability. Curr Biol. 2015;25:702–712. doi: 10.1016/j.cub.2015.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lavie P, Lavie L, Herer P. All-cause mortality in males with sleep apnoea syndrome: declining mortality rates with age. Eur Respir J. 2005;25:514–520. doi: 10.1183/09031936.05.00051504. [DOI] [PubMed] [Google Scholar]