Abstract
Calcification of the coronary artery is a complex pathophysiologic process that is intimately associated with atherosclerosis. Extensive investigation has demonstrated the value of identifying and quantifying coronary artery calcium (CAC) in atherosclerotic cardiovascular disease (CVD) prognostication. However, over the last several years, an increasing body of evidence has suggested that CAC has underappreciated aspects that modulate, and at times attenuate, future CVD risk. The most commonly used measure of CAC, the Agatston unit, effectively models both higher density and higher area of CAC as risk factors for future CVD events. Recent findings from the Multi‐Ethnic Study of Atherosclerosis (MESA) have challenged this assumption, demonstrating that higher density of CAC is protective for coronary heart disease and CVD events. Statins may be associated with an increase in CAC, an unexpected finding given their clear benefits in the prevention and treatment of CVD. Studies utilizing intracoronary ultrasound and coronary computed tomography angiography have demonstrated that calcified atherosclerotic plaque—as compared with noncalcified or sparsely calcified plaque—is associated with fewer CVD events. These studies lend support to the often‐asserted (but as yet unvalidated) view that calcification may play a role in plaque stabilization. Furthermore, vascular calcification, though a surrogate for atherosclerotic plaque burden, may also possess identifiable aspects that can refine CVD risk assessment.
Keywords: Calcium Density, Cardiovascular Disease, Coronary Artery Calcium, Coronary Computed Tomography Angiography
1. INTRODUCTION
Despite sophisticated means of detecting coronary artery calcium (CAC), the impact of CAC on the natural history of coronary artery disease (CAD) remains uncertain. Early pathologic studies showed CAC to correlate closely with the presence and extent of atherosclerotic plaque.1 More recent in vivo studies utilizing computed tomography (CT) found both high levels of CAC and progression of CAC to be associated with cardiovascular disease (CVD).2, 3 These findings support CAC as a robust, noninvasive surrogate measure of noncalcified atherosclerosis of the coronary arteries. Yet calcification of the coronary arteries and other vascular structures appears to be a regulated process of mineral deposition, akin to bone formation.4 This latter observation spurred the hypothesis that CAC, beyond simply denoting the underlying presence of noncalcified plaque, may also play a role in modulating the natural history of CAD.5
Utilizing noninvasive and invasive imaging modalities, several recent studies have identified select characteristics of CAC that appear to be associated with a lower risk of CVD. These characteristics include the density of CAC, the pattern of calcification within a plaque, and the proportion of atherosclerotic plaque that is calcified. In attenuating the risk of CVD, these characteristics refine the present view of the prognostic implications of CAC.
In this review, we will evaluate a growing body of evidence suggesting that certain CAC characteristics may mitigate the risk of the atherosclerosis that CAC denotes.
2. CAC QUANTIFICATION AND CAC DENSITY
As an assessment of atherosclerotic burden, the quantification of CAC as identified by multidetector and electron‐beam CT has proven to be a powerful predictor of future myocardial infarction (MI).2 CAC scores provide improvement in risk prediction when added to the Framingham Risk Score (FRS) and other risk scores and have been recommended in select clinical settings to improve risk stratification.6 Additionally, the progression of CAC scores over time is significantly associated with incident coronary heart disease (CHD) and mortality.3, 7 CAC is also a robust marker of CVD risk both in comparison and complementary to other CT‐derived risk scores. For instance, CAC scoring was equivalent to coronary stenosis measurement by coronary CT angiography (CCTA) in predicting mortality and MI in asymptomatic patients.8 Among patients with chest pain undergoing CCTA, assessing the amount of noncalcified plaque in addition to the extent and severity of coronary atherosclerosis (the latter quantified by coronary segment involvement scores and segment stenosis scores) resulted in significant improvement in the discrimination of acute coronary syndrome (ACS) events.9 The addition of CAC scoring to CCTA in patients suspected of having obstructive coronary atherosclerosis resulted in a significant improvement in total mortality risk prediction.10
A long‐established and now standard approach to scoring CAC identified on CT is the Agatston method (Figure 1). Using this method, CAC is defined as any area within the course of the coronary artery that is >1 mm2 in size and >130 Hounsfield units (HU) of CT attenuation. Each CAC area is then multiplied by a density factor determined from the maximal attenuation present within each area of calcium. Calcium areas with maximal HU of 130 to 199, 200 to 299, 300 to 399, and ≥400 are given density factors of 1, 2, 3, and 4, respectively. The products of the CAC area and density factor for each lesion at every CT slice level are then summed to produce the Agatston score.11
Figure 1.
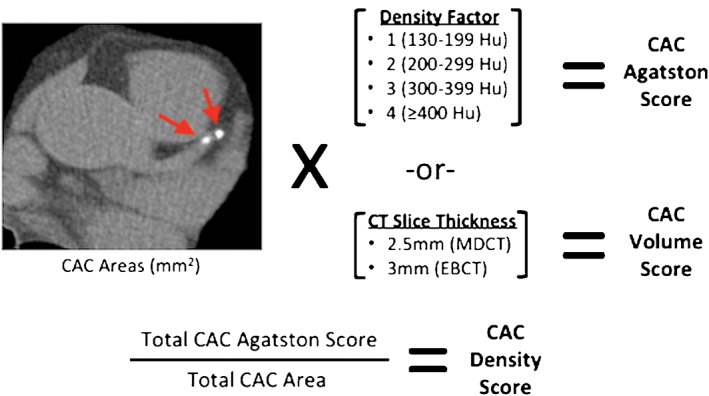
Agatston, volume, and density CAC scores in MESA. CAC is identified on axial CT images as areas of attenuation >130 HU and ≥1 mm2 in size. Each CAC area is multiplied by its density factor to produce an Agatston score for each CAC area. The density factor corresponds to the maximal HU attenuation within each CAC area, ranging from 1 to 4. The Agatston scores of all CAC areas throughout the coronary tree are then summed to produce the total CAC Agatston score. The CAC density score is the average density factor of all CAC areas. It is equal to the total CAC Agatston score divided by total CAC area. The CAC volume score is equal to the sum of all CAC areas multiplied by the slice thickness of the CT scanner used (2.5 mm for MDCT and 3 mm for EBCT). Abbreviations: CAC, coronary artery calcium; CT, computed tomography; EBCT, electron beam computed tomography; HU, Hounsfield units; MDCT, multidetector computed tomography; MESA, Multi‐Ethnic Study of Atherosclerosis
Thus, Agatston scores are derived from area and density, 2 features of coronary calcium that are, in fact, both separate and distinct. By weighting CAC area for higher density, the Agatston method models higher density of CAC as a CVD hazard. Despite over 2 decades of research into the application of CAC scoring, this model had not been challenged until recently.
In an effort to elucidate the independent role of CAC density in CVD risk prediction, participants in the Multi‐Ethnic Study of Atherosclerosis (MESA), a multicenter prospective cohort study designed to investigate the natural history of subclinical CVD, were evaluated.12 By dividing the CAC Agatston score by the CAC area score, the investigators obtained a CAC density score, which reflected the average of lesion density factors throughout the coronary tree, ranging from 1 to 4 (Figure 1). The investigators compared the predictive value of the CAC density score with other parameters found on standard CAC scans—CAC area, volume, and Agatston scores—and reported several novel findings.
The investigators observed that whereas CAC area, volume, and Agatston scores were all highly correlated (r = 0.99 for each correlation), the density score was only moderately correlated with these parameters (r = 0.62 with Agatston, r = 0.56 with volume, and r = 0.54 with area). After adjustment for major CVD risk factors, CAC area, volume, and Agatston scores all showed significant associations with CHD and CVD outcomes. However, CAC density had a significant inverse association with CHD and CVD outcomes (Figure 2). Thus, because higher CAC density appears to be inversely associated with CHD and CVD, the standard Agatston method of weighting CAC scores for increased density may be inappropriate for CVD risk prediction. In fact, in this study superior event discrimination was demonstrated by considering the CAC volume score (positively related) and the CAC density score (inversely related) separately. The addition of CAC volume and density to a base model of the Framingham Risk Score (FRS), race/ethnicity, and statin use resulted in incremental improvements in the area under the ROC curves when compared with CAC Agatston score. For CHD, the addition of CAC Agatston to the base model improved the c‐statistic from 0.668 to 0.696 (P = 0.02 vs base), whereas the sequential additions of CAC volume and density to the base model resulted in c‐statistics of 0.700 (P = 0.01 vs base) and 0.711 (P = 0.006 vs base), respectively.12 Although increases in the AUC were modest after inclusion of CAC volume and density, greater improvement may occur with development of CAC density scores that are not derived from the Agatston score, which caps density scores for any attenuation >400 HU at a maximum of 4.
Figure 2.
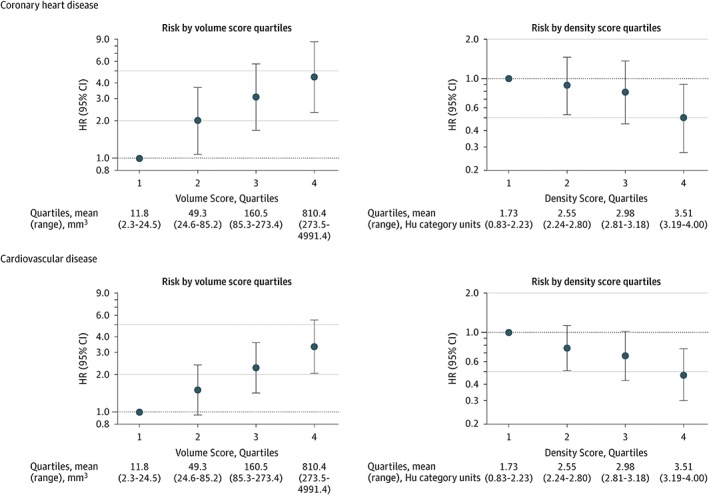
The associations of CAC volume and density with CHD and CVD outcomes in MESA. Risk of hard CHD events and hard CVD events increases among higher quartiles of CAC volume and decreases among higher quartiles of CAC density. HRs for volume score quartiles were adjusted for density score, race/ethnicity, statin use, and general FRS. HRs for density score quartiles were adjusted for natural logarithm volume score, race/ethnicity, statin use, and general FRS. Error bars indicated 95% CIs. Reproduced from Criqui et al.12 Abbreviations: CAC, coronary artery calcium; CHD, coronary heart disease; CI, confidence interval; CVD, cardiovascular disease; FRS, Framingham Risk Score; HR, hazard ratio; HU, Hounsfield units; MESA, Multi‐Ethnic Study of Atherosclerosis
Agatston scores and volume scores are the most commonly reported measures of CAC on CT, but they appear to be unsatisfactory in reflecting the true impact of coronary calcification on future CVD risk. Alternative CAC measures have demonstrated improvements in precision over traditional scoring methods. The volumetric calcium score utilizes the method of isotropic interpolation to sample cardiac CT at several intermediate cross‐sections between the original scan sections, allowing for more precise CAC volume reconstruction.13 Using this method, the precision of CAC volume scoring was improved and, in a landmark study, CAC volume was shown to decrease in patients treated with statin medications.14 The mass score has been advocated that utilizes CT phantoms of known calcium hydroxyapatite concentration to estimate the absolute mineral mass of a calcium lesion, expressed in milligrams.15 The mass score is felt to be a better reflection of the true physical properties of each calcified lesion and has enabled improved accuracy and reproducibility of CAC quantification compared with Agatston scores.16 However, the widespread use of the mass score has been limited by a relative paucity of literature correlating the mass score with the risk of clinical outcomes, though age and sex distributions of CAC mass scores among a large population of patients undergoing EBCT have been reported.17
The above findings from the MESA suggest that the volume of CAC and density of CAC have divergent prognostic implications. What is not clear from these findings is precisely why denser CAC is associated with a reduced risk of future events in individuals with CAC. At the level of the atherosclerotic lesion, higher CAC density may simply reflect a lower atheroma lipid content, or the absence of a high‐risk necrotic core. On the other hand, higher CAC density may also reflect a prior subclinical coronary plaque rupture followed by vessel‐wall healing accompanied by calcification, which at high densities may reflect quiescence of the inflammatory process in that lesion.18 In this vein, some studies have suggested a role for CAC as a marker of coronary plaque stabilization.
3. CAC AND THE VULNERABLE PLAQUE HYPOTHESIS
One area of recent intense focus has been the identification of coronary plaque compositions that might predict subsequent MI. So‐called vulnerable or high‐risk plaques are focal areas of atherosclerosis prone to disruption and subsequent thrombosis. The most commonly suspected vulnerable plaque is the thin‐cap fibroatheroma (TCFA), and by utilizing increasingly sophisticated imaging technologies, investigators have assessed several characteristics of these lesions such as plaque area, luminal stenosis, and plaque composition and morphology.
The value of this approach in predicting CVD events has thus far been limited. In a review of the topic, Arbab‐Zadeh et al.19 found that there is no conclusive evidence that the identification of high‐risk coronary plaques provides improved CVD risk prediction in comparison with traditional approaches. Nevertheless, in investigating TCFA, studies utilizing intravascular ultrasound (IVUS) to identify vulnerable plaques have suggested that the presence of calcium does not necessarily mark a greater risk of future coronary events.
In the Providing Regional Observations to Study Predictors of Events in the Coronary Tree (PROSPECT) study, coronary arteries were evaluated by IVUS at the time of percutaneous coronary intervention (PCI) in the setting of the ACS, and patients were followed prospectively for major adverse cardiovascular events (MACE), a composite of death from cardiac causes, cardiac arrest, MI, or hospitalization due to unstable or progressive angina.20 The principal findings of the study were the significant independent associations of plaque burden (defined as the cross‐sectional area of the plaque and media divided by the cross‐sectional area enclosed by the external elastic membrane), TCFA, and minimal luminal area, with future MACE (hazard ratios [HR] of 5.03, 3.35, and 3.21, respectively).20
In an analysis of PROSPECT, Xu et al.21 reported the association of calcified nodules found on IVUS with future MACE. Calcified nodules were identified as distinct calcifications with irregular, protruding, and convex luminal surfaces. The investigators reported that the presence of ≥1 calcified nodule per patient was an independent predictor of freedom from nonculprit events, with no death, cardiac arrest, or MI occurring in this group. The investigators theorized that the reason for this association was that in this cohort, calcified nodules were rarely a component of TCFAs, and that calcified nodules were not associated with TCFA elsewhere in the coronary arteries.
On the other hand, limited or “spotty” calcification tends to be associated with unstable plaques. Ehara et al22 reported that in an IVUS study of culprit lesions prior to PCI, the patterns of calcification tended to be spotty in lesions associated with acute MI and unstable angina (UA). The investigators defined spotty calcification as calcium deposits involving an arc of <90°, or one‐fourth of the coronary circumference. In contrast, in patients with stable angina, calcium deposits were significantly longer, and a significantly smaller proportion of calcium deposits was spotty. Using intracoronary optical coherence tomography (OCT), Mizukoshi et al23 demonstrated similar findings in patients with stable and unstable coronary lesions undergoing coronary angiography. Patients with UA and acute MI had more frequent spotty calcification that was closer to the plaque‐lumen interface, whereas patients with stable angina had larger calcium deposits that were deeper within the plaque.
These and other observations suggest that higher calcium deposition in coronary artery plaques could result in a stabilizing effect on plaques. However, these study findings relating to calcium should be interpreted in context of the limitations of IVUS. Because of the inability of ultrasound to penetrate calcium, the area and thickness of calcium plaques cannot be accurately assessed.24 OCT does not have this same limitation, and as utilization of this modality continues, further insights into CAC may be gained.
4. EFFECTS OF STATINS ON CALCIFICATION
One of the more unexpected findings in studies of CAC has been the possible association of statins on CAC progression. Although several observational studies have shown statin therapy to result in slowing of CAC progression identified by coronary CT,14, 25, 26 many studies, including randomized trials, have shown that high‐intensity statin therapy does not attenuate (and may actually increase) the progression of CAC.27, 28, 29, 30, 31 The benefits of statins in the prevention of CVD events are clearly established, yet these agents do not appear to conclusively reduce CT‐identified CAC progression. Arguelles et al32 evaluated the association of CT‐identified CAC Agatston score progression with modification of traditional CVD risk factors in MESA. Contrary to expectation, greater reductions in low‐density lipoprotein cholesterol (LDL‐C) and blood pressure at the hands of lipid‐lowering and antihypertensive medications resulted in greater CAC progression as measured by the Agatston score. One possible explanation for this finding may be that although CAC volume progression is lessened with statins, density of plaque calcium increases, which could lead to higher Agatston scores, as they are weighted higher for calcium density.
In a meta‐analysis of 8 randomized clinical trials assessing the effects of statin and nonstatin therapies on coronary disease burden assessed via serial coronary IVUS, Puri et al33 reported on changes in coronary plaque volume and the calcium index, a metric of CAC used in their study that incorporates the presence of calcium along the length of the coronary artery and the arc of the vessel wall with calcium involvement on cross‐section. In addition to a reduction in total plaque volume, patients receiving high‐intensity and low‐intensity statins had a significantly greater increase in their calcium index compared with patients not on statin therapy. In a meta‐analysis of clinical studies of the effects of statins on coronary plaque composition via IVUS assessment, Banach et al similarly reported that statin use was associated with both a reduction in overall plaque volume as well as an increase in dense calcium.34
5. CALCIFIED, PARTIALLY CALCIFIED, AND NONCALCIFIED CORONARY PLAQUE
Though CAC can be detected through several noninvasive imaging modalities such as chest radiography, fluoroscopy, echocardiography, and cardiac magnetic resonance imaging, CT has become the standard for noninvasive detection in clinical practice. The addition of intravenous contrast agents to coronary CT allows for the assessment of noncalcified plaque in addition to calcified plaque. CCTA, though less sensitive than IVUS and OCT for the identification of histological characteristics of coronary atherosclerosis, is nonetheless a valuable tool for the rapid and noninvasive identification of coronary lesions. In a study of patients with ACS and stable angina evaluated by CCTA prior to PCI, Motoyama et al35 reported characteristics of high‐risk coronary lesions similar to those identified with IVUS. In their study, spotty calcification of atherosclerotic coronary plaques was associated with ACS; however, in contrast, large calcification was associated with stable angina.
Similarly, Ferencik et al36 reported that in patients presenting with chest pain and found to have significant coronary stenosis identified on CCTA, spotty coronary calcium was one of 4 morphologic features of coronary plaque associated with ACS. In a study comparing patients with stable angina with those with acute MI undergoing CCTA, Leber et al37 reported that patients with stable angina had a significantly higher number and area of heavily calcified plaque. Furthermore, patients with acute MI had a significantly increased number and area of noncalcified plaques.
Noncalcified coronary plaques identified by CCTA portend a poor prognosis. In a study of 1102 patients with nonobstructive CAD identified on CCTA, Ahmadi et al38 reported that the rate of all‐cause mortality at 10 years varied significantly based on the presence, absence, and degree of calcification in coronary plaques assessed throughout the coronary tree. For the purposes of their analysis, patients with multiple types of plaque morphologies were excluded, and the remaining patients were categorized as having calcified plaque, noncalcified plaque, or mixed plaque (Figure 3). Patients with calcified plaque had the lowest risk of all‐cause mortality, followed by mixed plaque, with noncalcified plaque being associated with the greatest risk (Figure 4). Notably, patients with only noncalcified plaque had a nearly 3‐fold greater risk of death compared to the group with the most extensive coronary calcification (CAC Agatston scores >400).
Figure 3.
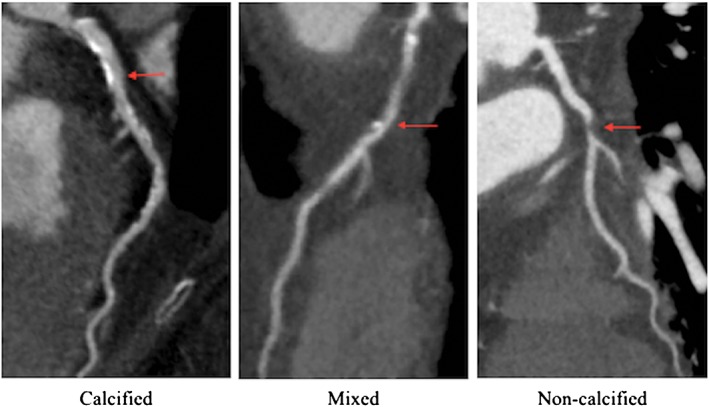
Calcified, mixed, and noncalcified plaque on CCTA. Images courtesy of Andrew Kahn, MD, PhD, University of California San Diego. Abbreviations: CCTA, coronary computed tomography angiography
Figure 4.
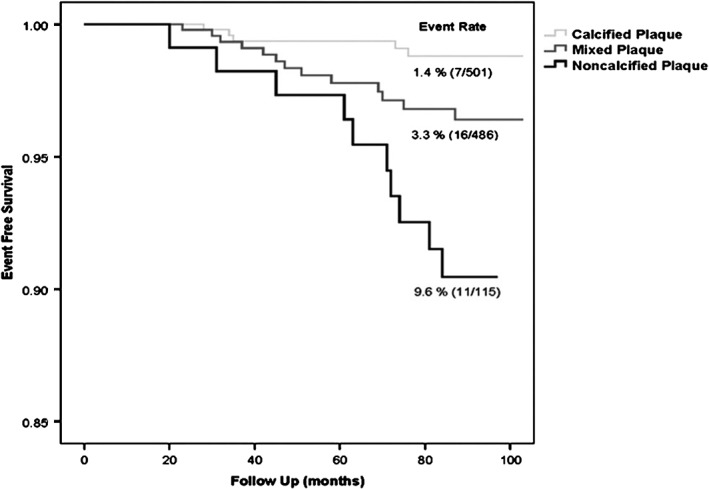
Kaplan–Meier estimates of survival for patients with calcified, mixed, and noncalcified plaque identified on CCTA. Reproduced from Ahmadi et al.38 Abbreviations: CCTA, coronary computed tomography angiography
In a similar study, Hou et al39 reported the outcomes of 4425 patients with suspected CAD who underwent CCTA. Patients were categorized as having calcified, noncalcified, or mixed plaques depending on the appearance of the most stenotic plaque visualized on CCTA. The cohort was followed for approximately 3 years and evaluated for MACE. The investigators found that patients with calcified plaque had a significantly lower risk of MACE at 3 years than did patients with noncalcified and mixed plaque (Figure 5). Patients with calcified plaque had only a modestly higher risk of MACE when compared with patients with CAC scores of 0. Patients with noncalcified and mixed plaque had a similar risk of MACE as patients with CAC scores >400.
Figure 5.
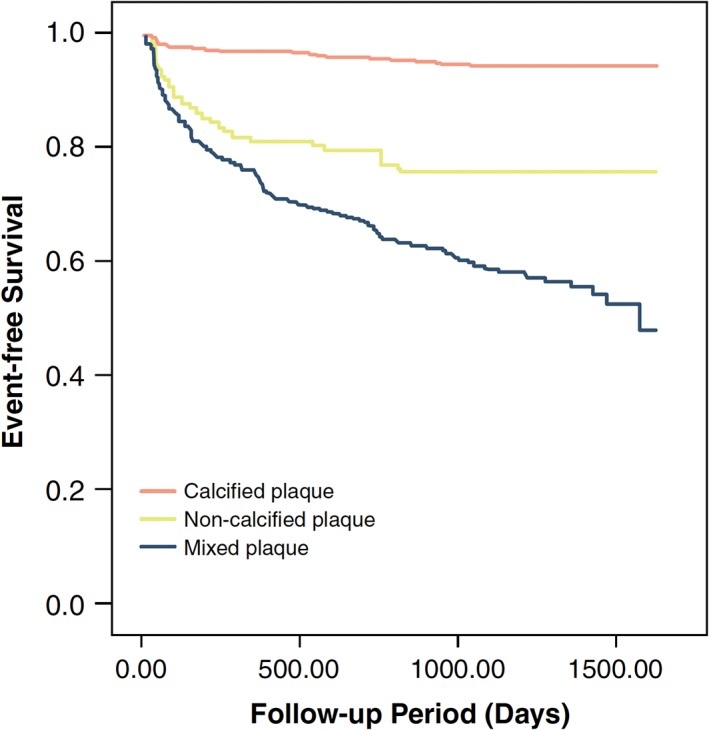
Kaplan–Meier estimates of survival from MACE for patients with calcified, mixed, and noncalcified plaque identified on CCTA. Reproduced from Hou et al.39 Abbreviations: CCTA, coronary computed tomography angiography; MACE, major adverse cardiac events
Several studies utilizing noncontrast cardiac CT have established the presence and extent of CAC as strongly predictive of CVD events. However, relatively fewer studies have utilized CCTA to evaluate the extent of both calcified and noncalcified coronary artery plaque. The presence of CAC indicates underlying atherosclerosis and, unsurprisingly, an increased risk of CVD in comparison with an absence of CAC. However, in evaluating the morphology and extent of both calcified and noncalcified coronary artery plaque, the above CCTA studies suggest that CAC may be protective for any given plaque area, and the extent of noncalcified plaque may pose the highest risk. Autopsy studies have demonstrated that plaque volume can far exceed CAC volume, and diffuse coronary plaque can be present without detectable CAC.1, 40, 41 As studies of statin therapies have demonstrated, plaque volume can change independently of CAC, with plaque regression being associated with CAC progression.33, 42, 43, 44
6. FUTURE DIRECTIONS
Although CVD remains the leading cause of death worldwide, the age‐standardized rate of CVD mortality has been on the decline in recent decades45 due to the identification and modification of CVD risk factors, as well as improvements in the arsenal of tools available to diagnose and treat CVD.46 Despite increasingly accurate risk prediction and contemporary therapies, CVD events still occur with significant frequency. As such, the pursuit of novel methods of CVD risk prediction and prevention continues.
CAC identified by CT scanning is the most robust marker of subclinical CVD in predicting future CVD events,47 yet uncertainties remain in what CAC represents in the natural history of atherosclerotic CVD, and how facets of CAC impact overall prognosis. An abundance of CAC throughout the coronary tree reflects a high atherosclerotic burden and, as multiple studies have demonstrated, a poor prognosis. What the presence of CAC represents in the pathophysiology of coronary artery atherosclerosis remains unclear. Is calcification the inexorable result of long‐standing and uncontrolled proliferation of atherosclerosis? The presence of CAC denotes high atherosclerotic plaque burden, but does its significance extend beyond this, to a stabilizing effect that converts vulnerable plaques to less vulnerable plaques? CAC has been associated with traditional risk factors for atherosclerosis, yet the effect of calcium in the plaque may limit plaque vulnerability and reduce the chance of subsequent rupture. Efforts toward plaque stabilization may become a novel avenue in CVD prevention, and certain features of CAC may play a role.
For the clinician assessing CVD risk in an asymptomatic patient, the 2013 ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults and the 2013 ACC/AHA Guideline on the Assessment of Cardiovascular Risk support a CAC Agatston score ≥ 300 or ≥75th percentile for age, sex, and ethnicity as a cutoff for revising a patient's risk assessment upward.6, 48 Given the association of calcium density with the risk of CVD, Agatston score cutoffs may not be using the most accurate calcium metric in determining risk. Furthermore, when available, knowledge of the distribution of calcium within a plaque and the proportion of plaque that is calcified can potentially refine our assessment of the impact of CAC on CVD outcomes. Recent reviews of CAC scoring have posited that optimization of CT scanner parameters relevant to CAC scoring—such as slice thickness, tube current, and the attenuation threshold for calcium detection— as well as an overhaul of the traditional CAC score itself into one that incorporates additional metrics such as the regional distribution of CAC, the number of CAC lesions, and density, have the potential to improve the value of CAC scoring.49, 50 Efforts are needed to explore a comprehensive approach incorporating these, and possibly undiscovered, characteristics of CAC that may improve CVD risk stratification and prevention.
Conflicts of interest
The authors declare no potential conflicts of interest.
Thomas IC, Forbang NI, Criqui MH. The evolving view of coronary artery calcium and cardiovascular disease risk. Clin Cardiol. 2018;41:144–150. 10.1002/clc.22842
REFERENCES
- 1. Rumberger JA, Simons DB, Fitzpatrick LA, et al. Coronary artery calcium area by electron‐beam computed tomography and coronary atherosclerotic plaque area: a histopathologic correlative study. Circulation. 1995;92:2157–2162. [DOI] [PubMed] [Google Scholar]
- 2. Detrano R, Guerci AD, Carr JJ, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358:1336–1345. [DOI] [PubMed] [Google Scholar]
- 3. Budoff MJ, Young R, Lopez VA, et al. Progression of coronary calcium and incident coronary heart disease events: the Multi‐Ethnic Study of Atherosclerosis. J Am Coll Cardiol. 2013;61:1231–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wexler L, Brundage B, Crouse J, et al. Coronary artery calcification: pathophysiology, epidemiology, imaging methods, and clinical implications: a statement for health professionals from the American Heart Association Writing Group. Circulation. 1996;94:1175–1192. [DOI] [PubMed] [Google Scholar]
- 5. Demer LL. A skeleton in the atherosclerosis closet. Circulation. 1995;92:2029–2032. [DOI] [PubMed] [Google Scholar]
- 6. Goff DC Jr, Lloyd‐Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines [published correction appears in Circulation. 2014;129(25 suppl 2):S74–S75]. Circulation. 2014;129(25 suppl 2):S49–S73. [DOI] [PubMed] [Google Scholar]
- 7. Budoff MJ, Hokanson JE, Nasir K, et al. Progression of coronary artery calcium predicts all‐cause mortality. JACC Cardiovasc Imaging. 2010;3:1229–1236. [DOI] [PubMed] [Google Scholar]
- 8. Cho I, Chang HJ, Sung JM, et al; CONFIRM Investigators . Coronary computed tomographic angiography and risk of all‐cause mortality and nonfatal myocardial infarction in subjects without chest pain syndrome from the CONFIRM Registry (coronary CT angiography evaluation for clinical outcomes: an international multicenter registry). Circulation. 2012;126:304–313. [DOI] [PubMed] [Google Scholar]
- 9. Tesche C, Caruso D, De Cecco CN, et al. Coronary computed tomography angiography–derived plaque quantification in patients with acute coronary syndrome. Am J Cardiol. 2017;119:712–718. [DOI] [PubMed] [Google Scholar]
- 10. Ostrom MP, Gopal A, Ahmadi N, et al. Mortality incidence and the severity of coronary atherosclerosis assessed by computed tomography angiography. J Am Coll Cardiol. 2008;52:1335–1343. [DOI] [PubMed] [Google Scholar]
- 11. Agatston AS, Janowitz WR, Hildner FJ, et al. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. [DOI] [PubMed] [Google Scholar]
- 12. Criqui MH, Denenberg JO, Ix JH, et al. Calcium density of coronary artery plaque and risk of incident cardiovascular events [published correction appears in JAMA. 2015;313:1374]. JAMA. 2014;311:271–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Callister TQ, Cooil B, Raya SP, et al. Coronary artery disease: improved reproducibility of calcium scoring with an electron‐beam CT volumetric method. Radiology. 1998;208:807–814. [DOI] [PubMed] [Google Scholar]
- 14. Callister TQ, Raggi P, Cooil B, et al. Effect of HMG‐CoA reductase inhibitors on coronary artery disease as assessed by electron‐beam computed tomography. N Engl J Med. 1998;339:1972–1978. [DOI] [PubMed] [Google Scholar]
- 15. Hong C, Becker CR, Schoepf UJ, et al. Coronary artery calcium: absolute quantification in nonenhanced and contrast‐enhanced multi–detector row CT studies. Radiology. 2002;223:474–480. [DOI] [PubMed] [Google Scholar]
- 16. Ulzheimer S, Kalender WA. Assessment of calcium scoring performance in cardiac computed tomography. Eur Radiol. 2003;13:484–497. [DOI] [PubMed] [Google Scholar]
- 17. Rumberger JA, Kaufman L. A rosetta stone for coronary calcium risk stratification: Agatston, volume, and mass scores in 11 490 individuals. AJR Am J Roentgenol. 2003;181:743–748. [DOI] [PubMed] [Google Scholar]
- 18. Arbab‐Zadeh A, Nakano M, Virmani R, et al. Acute coronary events. Circulation. 2012;125:1147–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Arbab‐Zadeh A, Fuster V. The myth of the “vulnerable plaque”: transitioning from a focus on individual lesions to atherosclerotic disease burden for coronary artery disease risk assessment. J Am Coll Cardiol. 2015;65:846–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Stone GW, Maehara A, Lansky AJ, et al; PROSPECT Investigators . A prospective natural‐history study of coronary atherosclerosis [published correction appears in N Engl J Med 2011;365:2040]. N Engl J Med. 2011;364:226–235. [DOI] [PubMed] [Google Scholar]
- 21. Xu Y, Mintz GS, Tam A, et al. Prevalence, distribution, predictors, and outcomes of patients with calcified nodules in native coronary arteries: a 3‐vessel intravascular ultrasound analysis from Providing Regional Observations to Study Predictors of Events in the Coronary Tree (PROSPECT). Circulation. 2012;126:537–545. [DOI] [PubMed] [Google Scholar]
- 22. Ehara S, Kobayashi Y, Yoshiyama M, et al. Spotty calcification typifies the culprit plaque in patients with acute myocardial infarction: an intravascular ultrasound study. Circulation. 2004;110:3424–3429. [DOI] [PubMed] [Google Scholar]
- 23. Mizukoshi M, Kubo T, Takarada S, et al. Coronary superficial and spotty calcium deposits in culprit coronary lesions of acute coronary syndrome as determined by optical coherence tomography. Am J Cardiol. 2013;112:34–40. [DOI] [PubMed] [Google Scholar]
- 24. Mintz GS. Intravascular imaging of coronary calcification and its clinical implications. JACC Cardiovasc Imaging. 2015;8:461–471. [DOI] [PubMed] [Google Scholar]
- 25. Achenbach S, Ropers D, Pohle K, et al. Influence of lipid‐lowering therapy on the progression of coronary artery calcification: a prospective evaluation. Circulation. 2002;106:1077–1082. [DOI] [PubMed] [Google Scholar]
- 26. Budoff MJ, Yu D, Nasir K, et al. Diabetes and progression of coronary calcium under the influence of statin therapy. Am Heart J. 2005;149:695–700. [DOI] [PubMed] [Google Scholar]
- 27. Houslay ES, Cowell SJ, Prescott RJ, et al; Scottish Aortic Stenosis and Lipid‐Lowering Therapy, Impact on Regression Trial Investigators . Progressive coronary calcification despite intensive lipid‐lowering treatment: a randomised controlled trial. Heart. 2006;92:1207–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schmermund A, Achenbach S, Budde T, et al. Effect of intensive versus standard lipid‐lowering treatment with atorvastatin on the progression of calcified coronary atherosclerosis over 12 months: a multicenter, randomized, double‐blind trial. Circulation. 2006;113:427–437. [DOI] [PubMed] [Google Scholar]
- 29. Saremi A, Bahn G, Reaven PD; Investigators VADT. Progression of vascular calcification is increased with statin use in the Veterans Affairs Diabetes Trial (VADT). Diabetes Care. 2012;35:2390–2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Arad Y, Spadaro LA, Roth M, et al. Treatment of asymptomatic adults with elevated coronary calcium scores with atorvastatin, vitamin C, and vitamin E: the St. Francis Heart Study randomized clinical trial [published correction appears in J Am Coll Cardiol. 2011;58:1832]. J Am Coll Cardiol. 2005;46:166–172. [DOI] [PubMed] [Google Scholar]
- 31. Raggi P, Davidson M, Callister TQ, et al. Aggressive versus moderate lipid‐lowering therapy in hypercholesterolemic postmenopausal women: Beyond Endorsed Lipid Lowering with EBT Scanning (BELLES). Circulation. 2005;112:563–571. [DOI] [PubMed] [Google Scholar]
- 32. Arguelles W, Llabre MM, Penedo FJ, et al. Relationship of change in traditional cardiometabolic risk factors to change in coronary artery calcification among individuals with detectable subclinical atherosclerosis: the Multi‐Ethnic Study of Atherosclerosis. Int J Cardiol. 2014;174:51–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Puri R, Nicholls SJ, Shao M, et al. Impact of statins on serial coronary calcification during atheroma progression and regression. J Am Coll Cardiol. 2015;65:1273–1282. [DOI] [PubMed] [Google Scholar]
- 34. Banach M, Serban C, Sahebkar A, et al; Lipid and Blood Pressure Meta‐analysis Collaboration (LBPMC) Group . Impact of statin therapy on coronary plaque composition: a systematic review and meta‐analysis of virtual histology intravascular ultrasound studies. BMC Med. 2015;13:229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Motoyama S, Kondo T, Sarai M, et al. Multislice computed tomographic characteristics of coronary lesions in acute coronary syndromes. J Am Coll Cardiol. 2007;50:319–326. [DOI] [PubMed] [Google Scholar]
- 36. Ferencik M, Schlett CL, Ghoshhajra BB, et al. A computed tomography‐based coronary lesion score to predict acute coronary syndrome among patients with acute chest pain and significant coronary stenosis on coronary computed tomographic angiogram. Am J Cardiol. 2012;110:183–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Leber AW, Knez A, White CW, et al. Composition of coronary atherosclerotic plaques in patients with acute myocardial infarction and stable angina pectoris determined by contrast‐enhanced multislice computed tomography. Am J Cardiol. 2003;91:714–718. [DOI] [PubMed] [Google Scholar]
- 38. Ahmadi N, Nabavi V, Hajsadeghi F, et al. Mortality incidence of patients with non‐obstructive coronary artery disease diagnosed by computed tomography angiography. Am J Cardiol. 2011;107:10–16. [DOI] [PubMed] [Google Scholar]
- 39. Hou ZH, Lu B, Gao Y, et al. Prognostic value of coronary CT angiography and calcium score for major adverse cardiac events in outpatients. JACC Cardiovasc Imaging. 2012;5:990–999. [DOI] [PubMed] [Google Scholar]
- 40. Narula J, Nakano M, Virmani R, et al. Histopathologic characteristics of atherosclerotic coronary disease and implications of the findings for the invasive and noninvasive detection of vulnerable plaques. J Am Coll Cardiol. 2013;61:1041–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Simons DB, Schwartz RS, Edwards WD, et al. Noninvasive definition of anatomic coronary artery disease by ultrafast computed tomographic scanning: a quantitative pathologic comparison study. J Am Coll Cardiol. 1992;20:1118–1126. [DOI] [PubMed] [Google Scholar]
- 42. Puri R, Libby P, Nissen SE, et al. Long‐term effects of maximally intensive statin therapy on changes in coronary atheroma composition: insights from SATURN. Eur Heart J Cardiovasc Imaging. 2014;15:380–388. [DOI] [PubMed] [Google Scholar]
- 43. Pugliese F, Mollet NR, Runza G, et al. Diagnostic accuracy of non‐invasive 64‐slice CT coronary angiography in patients with stable angina pectoris. Eur Radiol. 2006;16:575–582. [DOI] [PubMed] [Google Scholar]
- 44. Kroft LJ, de Roos A, Geleijns J. Artifacts in ECG‐synchronized MDCT coronary angiography. AJR Am J Roentgenol. 2007;189:581–591. [DOI] [PubMed] [Google Scholar]
- 45. Moran AE, Forouzanfar MH, Roth GA, et al. Temporal trends in ischemic heart disease mortality in 21 world regions, 1980 to 2010: the Global Burden of Disease 2010 Study. Circulation. 2014;129:1483–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ford ES, Ajani UA, Croft JB, et al. Explaining the decrease in U.S. deaths from coronary disease, 1980–2000. N Engl J Med. 2007;356:2388–2398. [DOI] [PubMed] [Google Scholar]
- 47. Yeboah J, McClelland RL, Polonsky TS, et al. Comparison of novel risk markers for improvement in cardiovascular risk assessment in intermediate‐risk individuals. JAMA. 2012;308:788–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Stone NJ, Robinson J, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines [published correction appears in Circulation. 2014;129(25 suppl 2):S46–S48]. Circulation. 2014;129(25 suppl 2):S1–S45. [DOI] [PubMed] [Google Scholar]
- 49. Alluri K, Joshi PH, Henry TS, et al. Scoring of coronary artery calcium scans: history, assumptions, current limitations, and future directions. Atherosclerosis. 2015;239:109–117. [DOI] [PubMed] [Google Scholar]
- 50. Blaha MJ, Mortensen MB, Kianoush S, et al. Coronary artery calcium scoring: is it time for a change in methodology? JACC Cardiovasc Imaging. 2017;10:923–937. [DOI] [PubMed] [Google Scholar]


