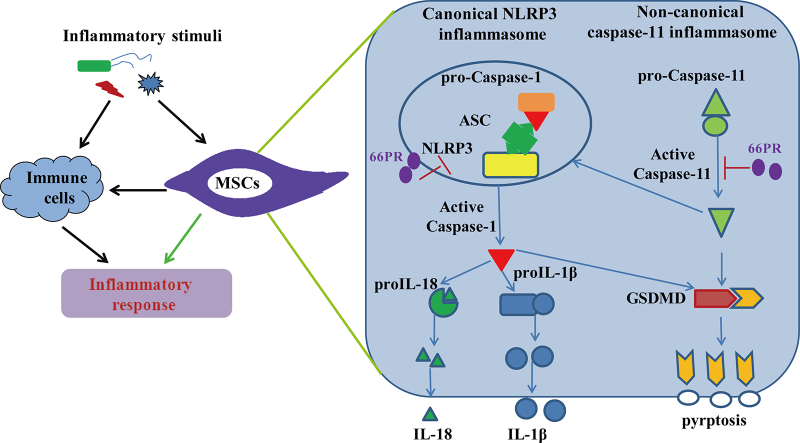
Abstract
Mesenchymal stromal cells (MSCs) based therapy is a promising approach to treat inflammatory disorders. However, therapeutic effect is not always achieved. Thus the mechanism involved in inflammation requires further elucidation. To explore the mechanisms by which MSCs respond to inflammatory stimuli, we investigated whether MSCs employed inflammasomes to participate in inflammation. Using in vitro and in vivo models, we found that canonical NLRP3 and non-canonical caspase-11 inflammasomes were activated in bone-associated MSCs (BA-MSCs) to promote the inflammatory response. The NLRP3 inflammasome was activated to mainly elicit IL-1β/18 release, whereas the caspase-11 inflammasome managed pyroptosis. Furthermore, we sought a small molecule component (66PR) to inhibit the activation of inflammasomes in BA-MSCs, which consequently improved their survival and therapeutic potential in inflammation bowel diseases. These current findings indicated that MSCs themselves could directly promote the inflammatory response by an inflammasome-dependent pathway. Our observations suggested that inhibition of the proinflammatory property may improve MSCs utilization in inflammatory disorders.
Keywords: Mesenchymal stromal cells, Inflammasome, NLRP3, Caspase-11, Pyroptosis
Abbreviations: MSCs, mesenchymal stromal cells; BA-MSCs, bone-associated MSCs; TLRs, toll-like receptors; NLRP3, nucleotide-binding domain-like receptor protein 3; ASC, apoptotic speck protein; LPS, lipopolysaccharide; IBD, inflammatory bowel disease; P.a, Pseudomonasaeruginosa; E.coli, Escherichia coli; MCL, micheliolide; DSS, dextran sulphate sodium; CFSE, 5-(and 6)-Carboxyfluorescein diacetate succinimidyl ester; GSDMD, Gasdermin-D; MCL, micheliolide
Graphical Abstract
Highlights
-
•
NLRP3 and caspase-11 inflammasomes were activated in bone associated MSCs after stimulation.
-
•
NLRP3 inflammasome mainly secreted IL-1β/18, whereas caspase-11 inflammasome managed pyroptosis in bone associated MSCs.
-
•
Inhibition of inflammasomes in bone associated MSCs benefits their utilization for inflammatory diseases therapy.
Abnormal inflammations cause currently high incidence of diseases worldwide, such as sepsis, allergic reactions, and even cancer. But the therapy of inflammatory diseases is far from satisfaction heretofore. MSCs are great interest to treat inflammatory disorders. However, many studies found their therapeutic effects were not always achieved. Further studies on the molecular mechanisms by which MSCs respond to the inflammatory microenvironment will undoubtedly promote applications in clinic. Here, we observed that MSCs promoted the inflammatory response by an inflammasome-dependent pathway. Regulation of this pathway improved MSCs to counter against inflammatory disorders.
1. Introduction
Inflammation is an essential response to various stimuli, including pathogen infection, self-tissue injury, and other endogenous danger signals. In spite of the protective effect on tissues, excessive and persistent inflammation is associated with septic shock or autoimmune pathologies, which underlie a large group of inflammatory diseases, such as sepsis, allergic reactions, myopathies, colitis, and even cancer [39]. Mesenchymal stromal cells (MSCs) are fibroblast-like multipotent cells characterized by their ability to differentiate into cells of mesodermal origin, such as adipocytes, chondroblast, and osteoblasts [3]. MSCs can be expanded from a variety of tissues including bone marrow, adipose tissue, umbilical cord blood, skin, tendon, muscle, dental pulp and endosteum [3,14,20]. In addition to their stem/progenitor properties, MSCs have also been shown to possess broad immunoregulatory abilities and are capable of influencing both adaptive and innate immune responses by suppressing T lymphocytes and dendritic cell maturation, reducing B cell activation and proliferation, inhibiting the proliferation and cytotoxicity of NK cells, and promoting the generation of regulatory T cells [3,18,42]. Therefore, MSCs have attracted great interest for clinical application in treating various inflammatory diseases. Some studies have already demonstrated the beneficial effects of MSCs in ameliorating various inflammatory disorders, including sepsis, inflammatory bowel disease and experimental allergy encephalomyelitis [7,8,27,29]. However, therapeutic effects were not always achieved [8,26,29]. Consequently, further studies on the molecular mechanisms by which MSCs respond to the inflammatory microenvironment may have a dramatic impact on the clinical application of these unique cells.
Most studies have reported that MSCs participate in inflammation mainly by direct cell-to-cell contact with immune cells or paracrine soluble cytokines [3,27]. Some investigations reported that Toll-like receptors (TLRs) on the cell membrane of MSCs are involved in their contribution to sense inflammation [9,33]. MSCs are able to transduct inflammatory signals into immune cells to fight inflammatory diseases. However, the intracellular pathway within MSCs responding to inflammatory stimuli is far from clarified.
Inflammasomes, a set of intracellular protein complexes, drive host and immune responses by releasing cytokines and by inducing pyroptosis [21,38]. Nucleotide-binding domain (NOD)-like receptor protein 3 (NLRP3) inflammasome is an essential inflammasome in immune responses and can be activated by diverse stimuli [22]. The NLRP3 inflammasome contains NLRP3, apoptotic speck protein (ASC) and pro-caspase-1 [38]. The NLRP3 inflammasome cleaves pro-caspase-1 into p20 and p10 subunits, inducing maturation and release of pro-inflammatory cytokines, such as IL-1β and IL-18 [22,30]. The NLRP3 inflammasome also induces pyroptotic cell death, which is characterized by the formation of membrane pores [34,51]. Pyroptosis is not only induced by the activation of caspase-1 but also by the activation of murine caspase-11 and human caspase-4/5-key players of non-canonical inflammasomes [35]. Non-canonical inflammasomes also release IL-1β with the help of the NLRP3 inflammasome and are crucial to innate immune defense [19,51]. The primary effectors of inflammasome-mediated inflammation are classically believed to be immune cells, such as monocytes, macrophages, dendritic cells, and neutrophils [13,41,52]. Recently, Wang et al. reported that the expression of NLRP3 and caspase-1 was increased in human MSCs derived from the human umbilical cord in vitro under lipopolysaccharide (LPS) treatment [47]. Moreover, it was reported that IL-1β was expressed in MSCs upon shear stress [10], and our previous study revealed that the transcription level of IL-1α in bone-associated MSCs was very high [14]. These results support the idea that MSCs may participate in inflammation through inflammasomes. Nevertheless, whether inflammasomes play a role in MSCs response to inflammatory stimuli is not fully elucidated yet.
In the present study, we identified an inflammasome-dependent pathway in bone associated MSCs (BA-MSCs), by which BA-MSCs directly participated in inflammation under inflammatory stimuli. Inhibition of the pro-inflammatory property induced by this pathway may improve MSCs utilization in various inflammatory disorders. These findings also supplement data regarding the activation of inflammasomes in non-immune cells, which is helpful to understand and control inflammation.
2. Materials and Methods
2.1. Mice
Adult (8–12 weeks) C57BL/6 mice and GFP C57BL/6 mice were purchased from Animal Resources Center of Fourth Military Medical University (Xi'an, China). The gene knockout C57BL/6 mice (NLRP3-/-, caspase-11-/- and caspase-1/11-/- mice) were what had been described [5,25,41]. Bacterial infection models were induced by vein injection with Pseudomonasaeruginosa (P.a, ATCC 35218) or Escherichia coli (E. coli, ATCC 27853). The model of inflammatory bowel disease (IBD) was induced with C57BL/6 mice through the oral ingestion of 3% dextran sulphate sodium (DSS) for 7 days. All the mice were bred in a special pathogen-free environment, and randomly assigned to the treatment and control groups.
All animal experimental protocols were reviewed and approved by the Animal Care Committee of Fourth Military Medical University of China.
2.2. Reagents
LPS (E. coli O111:B4) and nigericin (14K05-MM) were from Invitrogen. DSS (#118K7374V) were from Sigma-aldrich. Anti-mouse antibodies used for immunoblot analysis were: IL-1β (#12426, Cell Signaling Technologies), NLRP3 (#AG-20B-0014, Adipogen), Caspase-1 p20 (#AG-20B-0042, Adipogen), alpha Tubulin (#66031-1-1g, Proteintech), Caspase-11 p20 (#AG-20B-0061, Adipogen). Anti-mouse antibodies used for immunofluorescent staining analysis were: GSDMDC1 (#sc-393656, Santa Cruz Biotechnology), FITC Goat Anti-Mouse IgG Antibody (L146A, Gene Copoeia), AF647TM Goat Anti-Mouse IgG Antibody (L125A, Gene Copoeia). Anti-mouse antibodies used for flow analysis were: APC-CD45 (#30-F11, Biolegend), APC-Ter119 (#17-5921, eBioscience), PE-CD44 (#IM7, BD, USA), PE-CD51 (#RMV-7, eBioscience), APC-CD90 (#OX-7, BD), APC-CD105 (#MJ7/18, Biolegend), PE-CD146 (#P1H12, eBioscience), PE-CD166 (#105902, R&D), FITC-Sca-1 (#122505, Biolegend), APC-CD3 (#17A2, Biolegend), FITC-CD11c (#117306, Biolegend), APC-CD11b (#101212, Biolegend), APC-Gr1 (#17-5931, eBioscience), FITC-F4/80 (#123108, Biolegend), APC-CD19 (#17-5921, eBioscience), and FITC-CD14 (#Sa14-2, Biolegend).
2.3. BA-MSCs Isolation, Purification, Characterization and Culture
BA-MSCs cells were isolated as our previous reports [1,6,14], and were enriched according to mouse mesenchymal stromal cells enrichment kit for compact bone (#19771, Stem cell EasySep). The purity of sorted cells was confirmed with monoclonal antibodies against CD45/Ter119 by FACS analysis to be >90% CD45-/Ter119− cells. The characteristics of BA-MSCs were identified as previously described [1,6,14] by staining with monoclonal antibodies against CD44, CD51, CD90, CD105, CD146, CD166, Sca-1, CD11c, CD11b, F4/80, Gr1, CD3, CD19 and CD14 (Fig. S1). BA-MSCs were cultured in the MEM-alpha medium (Gibco) containing 15% FBS at 37 °C with 5% CO2. Cells were used at the 2th or 3th passages. For their trace after transplantation, BA-MSCs were stained with CFSE [5-(and 6)-Carboxyfluorescein diacetate succinimidyl ester], according to the procedure of CFSE cell division tracker kit (#B214561, Biolegend).
2.4. Enzyme-Linked Immunosorbent Assay
Paired (capture and detection) antibodies and standard recombinant mouse IL-1β (from R&D Systems), IL-18 (from AbcanSystems) and IL-6 (from Neobioscience) were used to determine IL-1β, IL-18 and IL-6 concentration in cell culture supernatants.
2.5. Immunoblot Analysis
Cells were lysed in RIPA buffer. Protein concentrations were quantified using BCA Protein Assay Kit (Pierce, #23225). Proteins in supernatant were precipitated by addition of 10% trichloroacetic acid (9:1, v/v) and centrifuged to obtain a pellet, and then washed three times in ethanol-acetone (1:1, v/v). Equal amounts of protein were loaded into SDS-PAGE and transferred onto PVDF membranes. The membranes were incubated, followed by appropriate secondary HRP–conjugated antibodies, with antibodies against NLRP3, caspase-1, caspase-11, IL-1β and α-Tubulin (as described above), and then washed and detected by using a chemiluminescence detection kit (#34077, Thermo Scientific). Band intensities were determined by Image J software.
2.6. Morphological Analysis
Scanning electron microscopy: Cells were seeded at 5 × 104 cells per well in 24 well glass slides, and rested overnight for proper attachment. Then, the cells were treated as above. BA-MSCs were fixed in 2.5% glutaraldehyde in 0.1 M cacodylate buffer. The samples were sent to the center of electron microscopy in Fourth Military Medical University of China. Samples were imaged through a scanning electron microscope (Olympus N300M, Shinjuku-ku, Tokyo, Japan).
Immunofluorescent staining and confocal microscopy: Bone sections from animals were prepared as standard protocol by CryoJane taping system (Leica). The cells in cell culture dishes (# 801002, NEST) or bone sections were washed twice with sterile PBS and fixed with 4% paraformaldehyde (PFA), and blocked in 5% BSA. The cells or bone sections were then incubated overnight with primary antibody, anti-GSDMDC1(Gasdermin-D, N-terminal). Secondary fluorescent antibodies were added for 1 h and DAPI was used for nuclear counterstaining. Samples were imaged through a confocal microscope (IBX81, Olympus).
2.7. Synthesis of 66PR
The synthesis of compound 66PR was conducted in a manner similar to the literature procedure [44]. Characterization data for the new compound is listed below. (11R)-13-(5-Fluorouracil)-11, 13-dihydro micheliolide. Yield 97.5%; mp = 0C·1H NMR (500 MHz, DMSO) δ 11.84 (d, J = 4.4 Hz, 1H), 8.02 (d, J = 6.8 Hz, 1H), 4.28 (s, 1H), 3.97 (dd, J = 14.3, 6.6 Hz, 1H), 3.86 (dt, J = 11.0, 8.0 Hz, 2H), 2.90 (dt, J = 12.3, 6.1 Hz, 1H), 2.54 (d, J = 10.4 Hz, 1H), 2.31–2.22 (m, 1H), 2.12–1.95 (m, 4H), 1.76 (d, J = 11.9 Hz, 1H), 1.62 (s, 3H), 1.61–1.55 (m, 2H), 1.30–1.25 (m, 1H), 1.15 (s, 3H)·13C NMR (126 MHz, DMSO) δ 176.20, 157.46, 149.73, 140.05, 138.23, 132.76, 130.53, 82.88, 79.38, 57.42, 49.52, 46.12, 44.27, 40.22, 34.63, 29.51, 26.53, 23.54, 22.42.
2.8. Treatment of IBD Mice
66PR was administered intraperitoneally in IBD mice at a dosage of 5 mg/kg body weight once daily. Control IBD animals received PBS once daily. BA-MSCs (wild type-CFSE; NLRP3-KO-CFSE, GFP, 66PR-GFP) were transplanted intraperitoneally into IBD mice at 2 × 106 cells/per mice. Control animals received PBS. The weight, feces and motility were recorded every day. According to these records, clinical scores were made refer to the reports [23,48]. After sacrifice, colons were collected. The length of colons was recorded and H&E sections of colons were made. The histological analysis and assessment of inflammation were performed in a blinded way by a pathologist, according to the standard of analysis as previously described [23,48]. Some colons were collected to obtain cells for FLOW analysis and colon sections were made to analysis by immunofluorescence confocal microscopy.
2.9. Statistical Analysis
All experiments have been repeated at least three times and in vivo experiments include the indicated number of mice. Statistical analyses were performed using SPSS16.0 soft. All data are presented as mean ± SD. Two group comparisons were performed with a two-tailed ANOVA with all data points showing a normal distribution with a similar variance between compared groups. A value of p < 0.05 was taken as statistically significant. *p < 0.05;**p < 0.01;***p < 0.001. nd: none detected; ns: no statistical significance.
3. Results
3.1. Nigericin and LPS Activated NLRP3 Inflammasome in BA-MSCs ex vivo
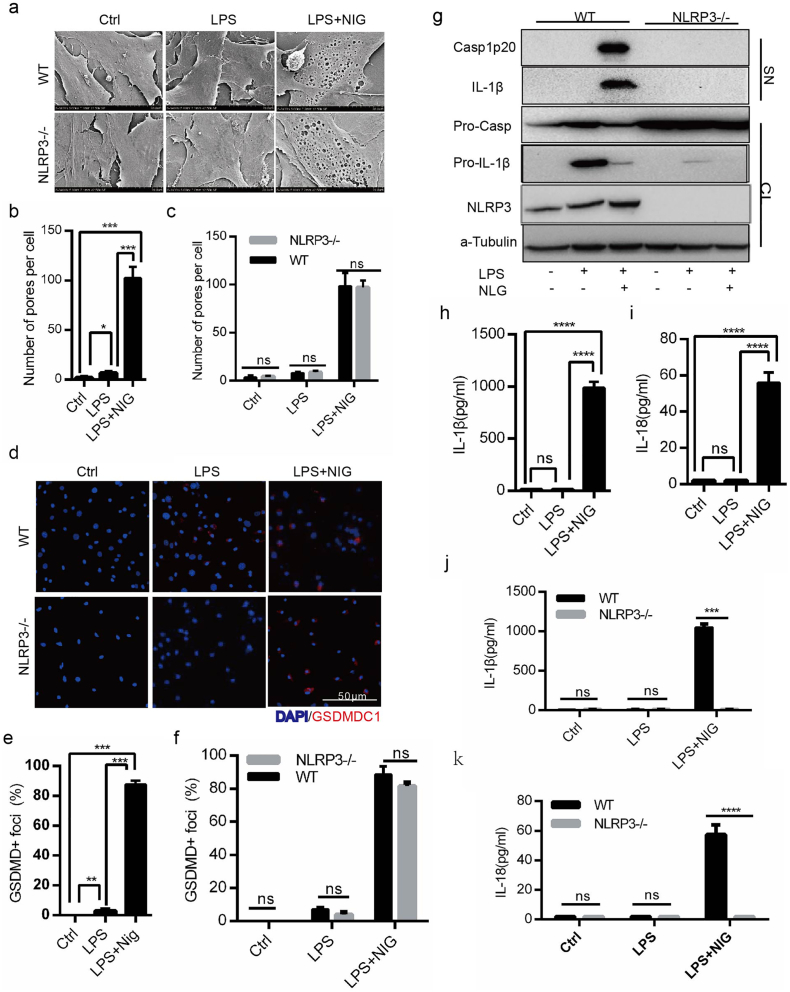
To identify the potential mechanism of MSCs participation in inflammation, we focused on the activation of NLRP3 inflammasome in BA-MSCs. Under nigericin and LPS stimulation, we observed that the BA-MSC number decreased macroscopically (Fig. S2a and b) and dead cells increased (Fig. S2c). Given that we used nigericin and LPS-the activator of NLRP3 inflammasome, we postulated that the dead BA-MSCs may a result of pyroptosis. As expected, we found that the inflammatory stimulation resulted in the formation of many membrane pores in BA-MSCs (Fig. 1a and b). We also found that the percentage of GSDMDC1 foci significantly increased in BA-MSCs (Fig. 1d and e), and the GSDMD gene transcript was up-regulated after stimulation (Fig. S2e). These data suggested that inflammatory stimulation induces pyroptosis of BA-MSCs in vitro.
Fig. 1.
The activation of NLRP3 inflammsome in BA-MSCs was induced by nigericin combined with LPS.
Wild type or NLRP3 gene knockout BA-MSCs were primed for 4 h with LPS (500 ng/ml) or not, and then stimulated with or without nigericin (5 μM) for 1.5 h, before the following observations. (a) Scanning electron microscopic observation of NLRP3−/-BA-MSCs and wild type BA-MSCs (n = 8). (b) Analysis of pore number counts of wild type BA-MSCs according to (a). (c) Comparison of pore number counts between wild type BA-MSCs and NLRP3−/-BA-MSCs according to (a). (d) Confocal laser scanning microscopic analysis of nuclei (DAPI, blue) and GSDMDC1 (red) foci of NLRP3-/-BA-MSCs and wild type BA-MSCs. (e) Quantity of wild type BA-MSCs containing GSDMDC1+ foci from (d). (f) Quantity of both BA-MSCs type containing GSDMDC1+ foci from (d). (g) Western blot analysis of NLRP3, caspase-1and IL-1β proteins in total lysates (CL) and supernatants (SN) of NLRP3-/- BA-MSCs and wild type BA-MSCs using the indicated antibodies. n = 3, pro-IL-1β: IL-1β precursor; IL-1β: mature IL-1β; pro-Casp1: caspase-1 precursor; Casp1p20: caspase-1p20 subset. (h-i) ELISA analysis of IL-1β/18 levels in the supernatants of wild type BA-MSCs, n = 5. (j-k) ELISA analysis of IL-1β/18 levels in the supernatants of NLRP3-/-BA-MSCs and wild type BA-MSCs, n = 5. At least 300 cells were counted for morphological analyses, mean ± SD, t-test, *P < 0.05,**P < 0.01,***P < 0.001, ****P < 0.0001, ns: not significant. Ctrl: control; LPS: BA-MSCs were stimulated by LPS; LPS + NIG: BA-MSCs were stimulated by LPS and nigericin.
To explore the activation of NLRP3 inflammasome, we further focused on the transcriptional level of NLRP3 inflammasome complex genes. We found that NLRP3, pro-IL-1β, and caspase-1 gene transcripts were up-regulated after stimulation (Fig. S2f–h). Consistently, the protein levels of NLRP3, pro-IL-1β, and pro-caspase-1 also increased (Fig. 1g, S2i), indicating that the expression of the components of NLRP3 inflammasome increased after nigericin challenge. Once the components of NLRP3 inflammasome were assembled, pro-caspase-1was cleaved into caspase-1 p20, which could process pro-IL-1β and pro-IL-18 into mature IL-1β and IL-18[30]. Following the stimulation of BA-MSCs with nigericin, we observed that caspase-1 p20 in the supernatant significantly increased (Fig. 1g), which indicated that NLRP3 inflammasome was successfully assembled. At the same time, mature IL-1β was released (Fig. 1g and h), as well as IL-18 (Fig. 1i), suggesting that NLRP3 inflammasome was activated in BA-MSCs in this system.
To further confirm the function of NLRP3 inflammasome in BA-MSCs, we employed NLRP3 gene knockout mice to analyze IL-1β/18 release and pyroptosis. Under the stimulation of nigericin primed by LPS, the pro-caspase-1 protein was not cleaved into the caspase-1 p20 subunit in the NLRP3 gene knockout BA-MSCs (Fig. 1g). This result demonstrated that the cleavage of pro-caspase-1 in BA-MSCs was dependent on the NLRP3 inflammasome. Meanwhile, we found that the levels of IL-1β and IL-18 released from NLRP3−/-BA-MSCs were also significantly lower than those released from wild type BA-MSCs (Fig. 1j and k), showing that NLRP3 gene knockout inhibited IL-1β and IL-18 release. These results suggested that the release of mature IL-1β and IL-18 relies on the activation of the NLRP3 inflammasome. However, the cell death observed in NLRP3 knockout BA-MSCs and wild type BA-MSCs was not significantly different (Fig. S2a and d). Moreover, we found that the membrane pore numbers were not significantly different between NLRP3 knockout and wild type BA-MSCs (Fig. 1a and c). In addition, the GSDMDC1 foci observed in NLRP3 gene knockout BA-MSCs were similar to those in the controls (Fig. 1d and f). These data demonstrated that NLRP3 gene deficiency did not decrease pyroptosis in BA-MSCs.
Taken together, LPS and nigericin induced the activation of NLRP3 inflammasome in BA-MSCs, and IL-1β/18 release was dependent on NLRP3, while pyroptosisis was independent of NLRP3.
3.2. Caspase-11 Inflammasome was also Activated in BA-MSCs by Nigericin and LPS
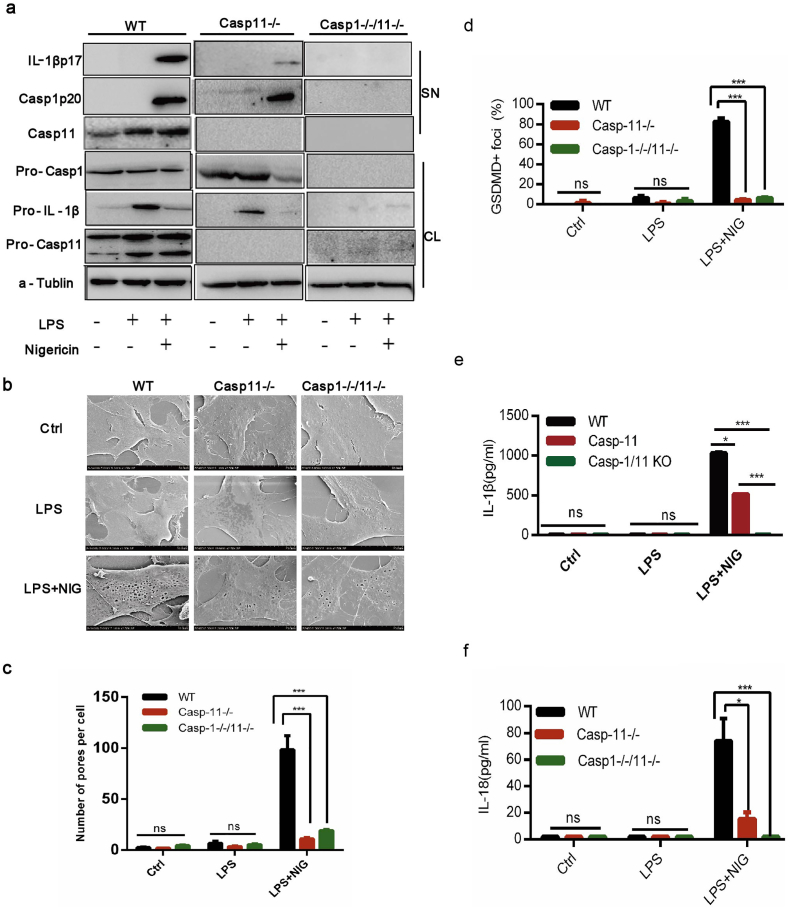
Given that the pro-caspase-1 protein was not cleaved into activated caspase-1 p20 in NLRP3-/-BA-MSCs (Fig. 1g), we investigated whether pyroptosis could be induced by another inflammasome independent on caspase-1. Non-canonical inflammasome can also trigger pyroptosis, which is dependent on caspase-11 in mice rather than caspase-1 [51]. We observed that the caspase-11 gene transcript level was up-regulated by LPS and nigericin (Fig. S3a). Moreover, the active caspase-11 protein was significant increased by LPS and nigericin (Fig. 2a and Fig. S3b). In NLRP3 gene knockout BA-MSCs, we observed that the caspase-11 gene transcript level showed no significant change compared with that in wild type cells (Fig. S3c). Additionally, the amount of capase-11 protein in NLRP3 knockout BA-MSCs was nearly equal to that in control BA-MSCs after LPS and nigericin stimulation (Fig. S3d and e). These results indicated that the non-canonical capase-11 inflammasome could be activated in BA-MSCs.
Fig. 2.
The caspase-11 inflammsome in BA-MSCs was activated by nigericin combined with LPS.
BA-MSCs from caspase-11 gene knockout mice, caspase-1/11 double gene knockout mice and wild type mice were primed for 4 h with LPS (500 ng/ml) and then stimulated with or without nigericin (5 μM) for 1.5 h, before the following observations. (a) Western blot analysis of caspase-11, caspase-1 and IL-1β proteins in total lysates (CL) and supernatants (SN) of the three groups of BA-MSCs. n = 3. (b) Electron microscopic scanning on the three groups of BA-MSCs. n = 8. (c) Analysis of pore number counts of three groups of BA-MSCs according to (b). (d) Quantity of BA-MSCs containing GSDMDC1+ foci from confocal laser scanning microscopic analysis. n = 3. (e-f) ELISA analyses of IL-1β/18 release from the three groups of BA-MSCs. At least 300 cells were counted for morphological analyses, mean ± SD, t-test, *P < 0.05,**P < 0.01,***P < 0.001, ns: not significant.
We further detected the function of caspase-11 inflammasome in BA-MSCs, using caspase-11 gene knockout mice (Fig. 2a and Fig. S3f). As expected, the number of membrane pores was significantly decreased in caspase-11 gene knockout BA-MSCs, compared with those in wild type BA-MSCs (Fig. 2b and c), Moreover, we consistently observed that fewer GSDMDC1 foci appeared in caspase-11 gene deficient BA-MSCs compared with those in normal BA-MSCs (Fig. 2d). Taken together, these observations indicated that capase-11 inflammasome in BA-MSCs played a key role in pyroptosis. In addition, we found that the pro-caspase-1 protein expression and cleavage were normal in caspase-11-/-BA-MSCs and similar to those in wild type BA-MSCs (Fig. S3g and h), indicating that the NLRP3 inflammasome was activated in caspase-11-/-BA-MSCs. However, the release of IL-1β and IL-18 was reduced in caspase-11 gene knockout BA-MSCs (Fig. 2e–f and Fig. S3i). These data suggested that the caspase-11 gene defect hindered the release of IL-1β and IL-18.
To further clarify the function of these two inflammasomes in BA-MSCs, we employed caspase-1/11 double knockout mice to assess the secretion of cytokines and pyroptosis (Fig. 2a, Fig. S3f–g). There was a significant difference in the number of membrane pore induced by pyroptosis between the wild type and caspase-1/11 knockout BA-MSCs (Fig. 2b–c). Additionally, fewer GSDMDC1 foci were observed in caspase-1/11 knockout BA-MSCs than in wild type BA-MSCs (Fig. 2d). These data indicated that very little pyroptosis occurred in caspase-1/11 defected BA-MSCs under nigericin and LPS stimulation. The levels of IL-1β and IL-18 were very low in the supernatant of caspase-1/11 knockout BA-MSCs (Fig. 2a, e-f, and S3i), illustrating that the production of IL-1β/18 was blocked. These data indicated that caspase-1/11 genes control the secretion of IL-1β/18 and pyroptosis in BA-MSCs. Moreover, when comparing pyroptosis and the secretion of cytokines between caspase-11 and caspase-1/11 knockout BA-MSCs, we found no significant difference related pyroptosis between them (Fig. 2b–d). But the secretion of IL-1β/18 was blocked because of caspase-1 gene knockout (Fig. 2a, e–f). This result suggested that the release of IL-1β/18 was dependent on caspase-1 in BA-MSCs.
Altogether, these findings indicated that stimulation with LPS and nigericin could also activate capase-11 inflammasome in BA-MSCs, which induced pyroptosis and promoted the secretion of IL-1β/18. Moreover, the activation of inflammasomes in BA-MSCs was dependent on caspase-1/11 genes and caspase-1 gene control the release of IL-1β/18.
3.3. NLRP3 and Caspase-11 Inflammasomes of BA-MSCs were Activated in vivo
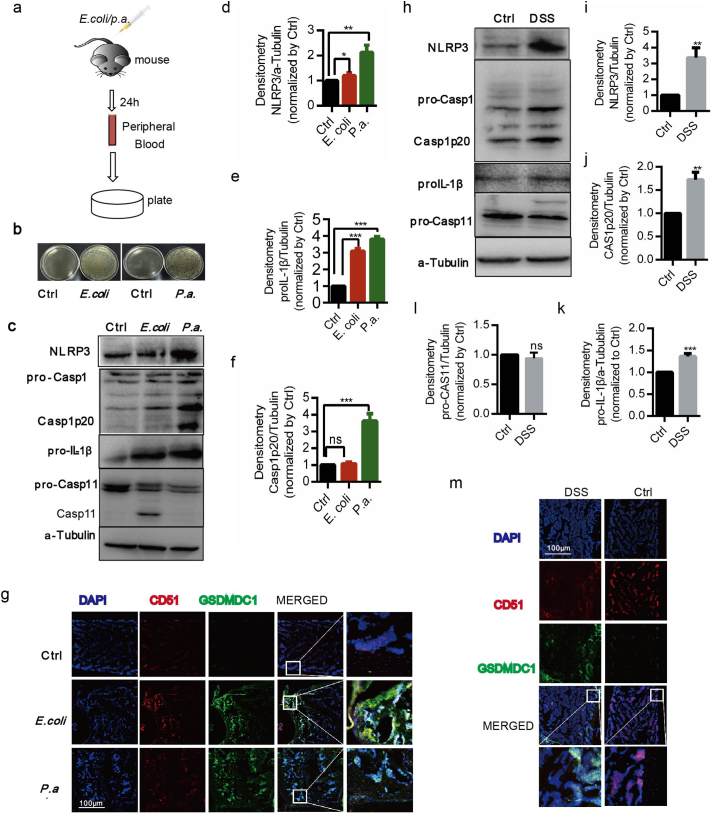
In view of the activation of NLRP3 and caspase-11 inflammasomes in BA-MSCs in vitro, we further assessed whether they are activated in vivo. We first challenged mice with P.a and E.coli (Fig. 3a) and tested the bacteria in peripheral blood to identify the infected model. We found higher bacterial burden in the mice injected with bacteria than that in control mice (Fig. 3b), indicating that the infected inflammation models were successfully established. Compared with those from healthy subjects, BA-MSCs from the infected mice had increased expression of NLRP3 and pro-IL-1β (Fig. 3c–e). Additionally, activated caspase-1 p20 was increased in BA-MSCs infected with P.a compared with that in control cells (Fig. 3c, f), suggesting that the NLRP3 inflammasome is activated by P.a. We also noted that the active caspase-11 was significantly increased in response to E.coli (Fig. 3c), indicating that the non-canonical caspase-11 inflammasome was activated by E.coli infection. Meanwhile, the bone sections from mice infected with E. coli or P.a had significantly more GSDMDC1 merged with anti-CD51(a surface marker of BA-MSCs) [24,36]foci than the control bone sections did (Fig. 3g), showing that E.coli or P.a infection induced BA-MSCs pyroptosis. These data demonstrated that the canonical NLRP3 inflammasome and capase-11 inflammasome of BA-MSCs can be activated by bacterial infection-related inflammation in vivo.
Fig. 3.
P.a and E.coli activated inflammsomes in BA-MSCs of mice model.
(a) Mice were infected with P.a and E.coli via caudal vein, and then the bacterial burden in peripheral blood was analyzed. n = 4. (b) Plates cultured with E.coli and P.a. from peripheral blood. n = 3. (c) Western blot analysis of NLRP3, caspase-1, caspase-11, and IL-1β proteins in BA-MSCs from mice after bacterial challenge. n = 3. (d-f) Densitometry analyses of NLRP3, pro-IL-1β and caspase-1 p20, based on (c). mean ± SD, t-test, *P < 0.05,**P < 0.01,***P < 0.001. (g) Confocal laser scanning microscopic analysis of GSDMDC1 (green), CD51 (red) and nuclei (DAPI, blue) of bone sections from mice infected with E.coli and P.a. n = 4. (h) Western blot analysis of NLRP3, caspase-1, caspase-11, IL-1β proteins in BA-MSCs from colitis and control mice. n = 3. (i-l) Densitometry analysis of NLRP3, caspase-1, caspase-11 and IL-1β proteins based on (h). mean ± SD, t-test, *P < 0.05,**P < 0.01,***P < 0.001. (m) Confocal laser scanning microscopic analysis of GSDMD (green), CD51 (red) and nuclei (DAPI, blue) in bone sections from colitis and control mice. Each experiment represents at least four mice per group.
To determine whether the activation of inflammasomes in BA-MSCs is universal, we investigated inflammasomes in tissue damaged settings. We used DSS to establish a murine IBD model (Fig. S4a), which exhibited significant clinical symptoms including diarrhea, bloody feces, body weight loss, and inflamed section of colon (Fig.S4b–f). Compared with controls, BA-MSCs from DSS-treated mice showed up-regulated protein levels of NLRP3, pro-IL-1β, and the caspase-1 p20 subunit (Fig. 3h–k), suggesting that NLRP3 inflammasome was activated in these animals. However, the pro-caspase-11 protein was not cleaved, and no difference was observed between the IBD and control groups (Fig. 3h,l); thus, non-canonical caspase-11 inflammasome was not activated in this situation. Then, we explored the pyroptosis of BA-MSCs, and found more GSDMDC1 merged with CD51 foci in the bone sections, compared with those in controls (Fig. 3m). These results indicated that pyroptosis was induced in the BA-MSCs from mice with colitis. Altogether, these data demonstrated that canonical NLRP3 inflammasome was activated to participate in the inflammatory response in BA-MSCs from IBD mice, whereas non-canonical caspase-11 inflammasome might not be involved.
3.4. NLRP3 Gene Deficiency Inhibited BA-MSCs' Pyroptosis in IBD
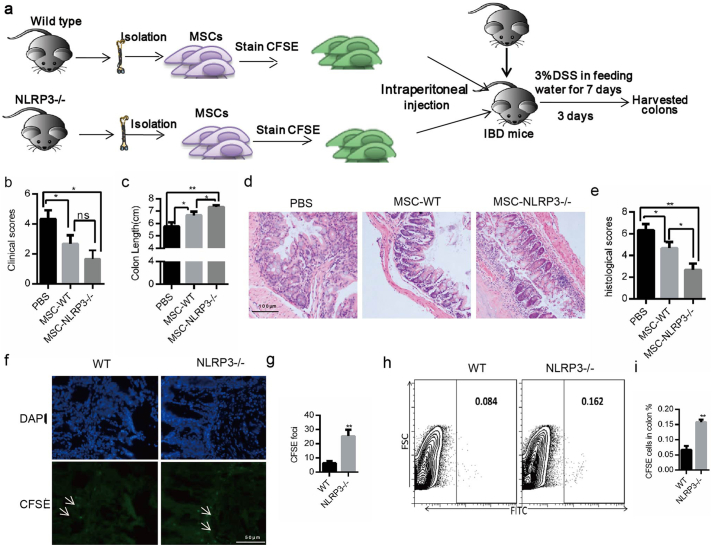
We then utilized the IBD mouse model, in which only the NLRP3 inflammasome was activated (Fig. 3), to further examine whether pyroptosis was inhibited in NLRP3 gene deficient BA-MSCs (Fig. 4a). Interestingly, we found that symptoms of colitis in mice were relieved after transplant with NLRP3-/-BA-MSCs, based on clinical scores, and colon length extension measurement (Fig. 4b–c), implying that NLRP3-/- BA-MSCs better suppress colitis. Similarly, based on the histologic analysis of colon sections, inflammation was better ameliorated after transplant with NLRP3-/- BA-MSCs than with wild type BA-MSCs (Fig. 4d–e). These results indicated that the inhibition of NLRP3 inflammasome in BA-MSCs was deeply involved in self-tissue-damage inflammation.
Fig. 4.
The impact of NLRP3-/-BA-MSCs on IBD mice.
(a) The experimental scheme. IBD mice were transplanted with NLRP3-/-BA-MSCs or wild type BA-MSCs, n = 5, 2 × 106 cells per mouse. After transplantation for 3 days, these mice were sacrificed. (b) The clinical scores of the three groups of colitis mice. (c) The colon length of the three groups of colitis mice. (d) The H&E staining colon sections from the three groups of colitis mice. (e) These histological scores of three groups of colitis mice. (f) The location of NLRP3-/-BA-MSCs or wild type BA-MSCs stained with CFSE in colon. (g) CFSE+ foci in colon sections from colitis animals treated with two types of BA-MSCs. Eight sections were observed per group. (h) Flow plots of the CFSE staining in colons from colitis mice treated with different BA-MSCs. (i) Quantity of CFSE+ cells according to (H). PBS: mice injected with PBS; MSC-WT: mice injected with wild type BA-MSCs; MSCs-NLRP3-/-: mice injected with NLRP3-/-BA-MSCs. Each experiment represents four or five mice per group. Mean ± SD, t-test, *P < 0.05,**P < 0.01,***P < 0.001.
To fully interpret this scenario, we tracked the survival of transplanted BA-MSCs (stained with CFSE) in IBD mice. More CFSE signals were found in the colon sections of mice injected with NLRP3-/- BA-MSCs than with control cells (Fig. 4f–g). Moreover, the FACS analysis showed more NLRP3-/- BA-MSCs than normal BA-MSCs in the colon (Fig. 4h–i), indicating that the survival of NLRP3-/- BA-MSCs was higher than that of control cells. Taken together, these results were consistent with the idea that only the NLRP3 inflammasome is activated in BA-MSCs when responding to self-tissue-damage inflammation, which implied that NLRP3 gene deficiency enhances BA-MSC survival by inhibiting pyroptosis in animals with colitis.
3.5. A Synthesized Product Inhibited the Activation of Inflammasomes in BA-MSCs
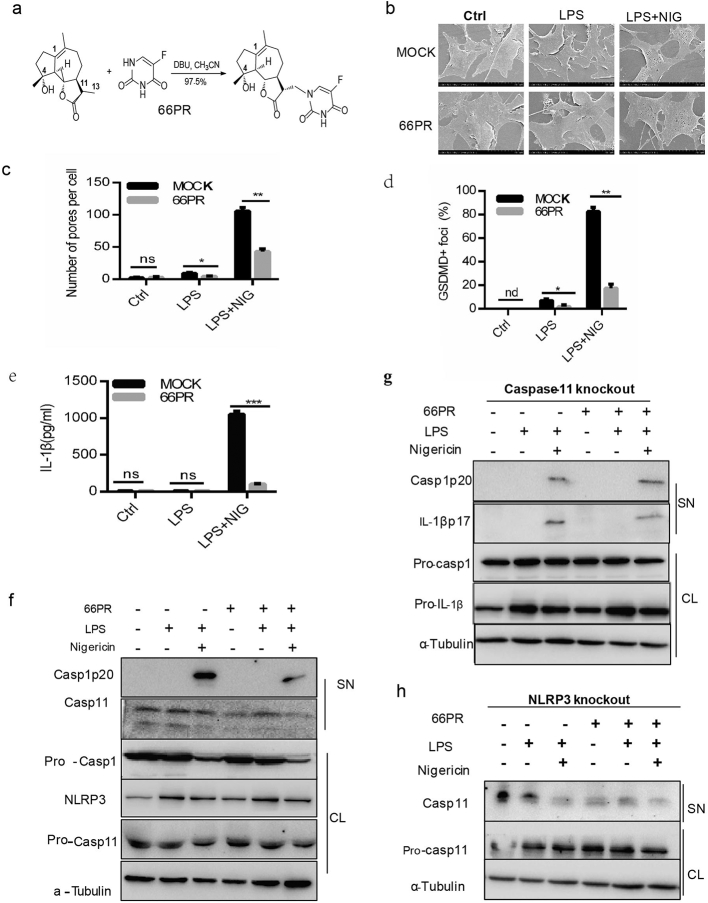
Given that the activation of NLRP3 and caspase-11 inflammasomes in BA-MSCs was observed in inflammation, an inhibitor of inflammasomes in BA-MSCs is highly desired for therapy. We thus tried to synthesize a compound (designated 66PR) based on micheliolide (MCL), which possessed anti-inflammatory activity and could inhibit IL-1β processing in our previous research [37]. The synthesis scheme and structure of 66PR are described in Fig. 5a and S5a. First, we investigated the effect of 66PR on pyroptosis. As expected, we found the ratio of dead BA-MSCs to be significantly decreased in the 66PR-treated groups (Fig. S5b). Meanwhile, we observed that the number of membrane pores on BA-MSCs (Fig. 5b–c) and the percentage of GSDMDC1 foci in BA-MSCs were significantly reduced owing to 66PR administration (Fig. 5d), indicating that 66PR could inhibit pyroptosis. Moreover, the IL-1β concentration in the supernatant of BA-MSCs was lower in the 66PR-treated group than in the mock group(Fig. 5e), indicating that 66PR inhibited the release of IL-1β. In addition, 66PR had no effect on production of IL-6 (Fig. S5c), which is one of the inflammasome-independent cytokines, suggesting that 66PR may not impair their role in host defense. Altogether, these results suggested that 66PR has an inhibitory effect on pyroptosis and the secretion of IL-1β from BA-MSCs in vitro.
Fig. 5.
The change of inflammsomes in BA-MSCs after priming with 66PR in vitro.
A small-molecular compound, 66PR, was synthesized, characterized, and used to treat BA-MSCs. Then the cells were primed for 4 h with LPS and 66PR (20 μM) and stimulated with or without nigericin for 1.5 h before the following observations. (a) Synthesis of 66PR. (b) Scanning electron microscopic observation of BA-MSCs treated with 66PR. (c)Analysis of pore number counts of BA-MSCs after 66PR and mock treatment according to (b). (d) Quantity of GSDMDC1+ foci from BA-MSCs after 66PR and mock treatment based on confocal laser scanning microscopic analysis. (e) ELISA analyses of IL-1β in BA-MSCs supernatant after 66PR and mock treatment. (f) Western blot analysis of NLRP3, caspase-1, and caspase-11 in wild type BA-MSCs after 66PR and mock treatment. (g) Western blot analysis of caspase-1 and IL-1β in caspase-11-/-BA-MSCs after 66PR treatment; (h) Western blot analysis of caspase-11 in NLRP3-/-BA-MSCs after 66PR treatment. CL: cell lysates, SN: supernatants. At least 300 cells were counted for morphological analyses, mean ± SD, two-way ANOVA, *P < 0.05,**P < 0.01,***P < 0.001, ns: not significant, nd: no detected.
To determine whether inhibition by 66PR was relevant to inflammasomes, we detected the expression of NLRP3, caspase-1, and caspase-11. Western blot analysis showed that NLRP3 and activated caspase-1 expression was significantly decreased after treatment with 66PR, whereas pro-caspase-1 expression showed no difference between 66PR-primed BA-MSCs and mock cells (Fig. 5f, Fig. S5f–g). The transcript level of the NLRP3 gene was also decreased by 66PR (Fig. S5d), which led to the inhibition of NLRP3 expression. The cleavage of caspase-11 protein obviously decreased by 66PR (Fig. 5f and Fig. S5h), suggesting that 66PR inhibits the activation of non-canonical caspase-11 inflammasome. Furthermore, the expression and transcript level of pro-caspase-11 was also obviously decreased by 66PR treatment (Fig. S5e). Taken together, 66PR could inhibit the NLRP3 and capase-11 inflammasomes in BA-MSCs.
According to the cleavage of caspase-1 and the secretion of IL-1β in caspase-11 knockout BA-MSCs (Fig. 2a,e), the activation of NLRP3 inflammasome remained in these cells. Thus, to primary investigate the specificity of 66PR inhibition on NLRP3 inflammasome, we used caspase-11 gene knockout murine BA-MSCs. After treatment by 66PR, pro-caspase-1 expression did not significant change, but the caspase-1 p20 decreased in the supernatant, similar to pro-IL-1β and IL-1β p17 profile in BA-MSCs administrated with 66PR (Fig. 5g and Fig. S6a–b). These results stated that 66PR inhibition targeted, at least partially, on the NLRP3 inflammasome.
Then we resorted to determinate the specificity of 66PR inhibition on caspase-11 with NLRP3 knockout murine BA-MSCs, because in this BA-MSCs the pyroptosis remained (Fig. 1a,c,d,f). We found the cleavage caspase-11 was significantly reduced by 66PR treatment (Fig. 5h and Fig. S7a) in NLRP3 gene knockout BA-MSCs, and GSDMDC1 foci was also reduced (Fig. 5h, and Fig. S7b–c), suggesting that 66PR, if not all, suppresses caspase-11 inflammasome.
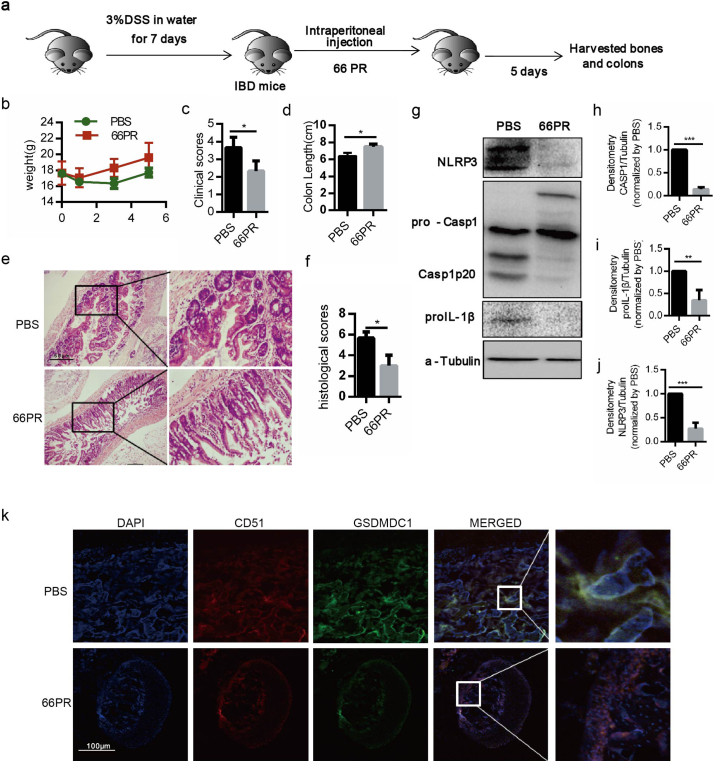
To further validate the 66PR inhibition of NLRP3 inflammasome in vivo, we injected 66PR into IBD mice (Fig. 6a), in which only NLRP3 inflammasome in BA-MSCs was activated (Fig. 3). As expected, 66PR obviously relieved symptoms of colitis in the animals (Fig. 6b–f). Meanwhile, 66PR also inhibited caspase-1 protein cleavage in the BA-MSCs of mice with colitis (Fig. 6g–j). Consistently, we found significantly fewer GSDMDC1 signals merged with CD51 foci in the bone sections from mice treated with 66PR than that in control bone sections (Fig. 6k), suggesting that 66PR also inhibits BA-MSCs pyroptosis in vivo. These data further validated that 66PR could alleviate self-tissue-damage inflammation.
Fig. 6.
The change of NLRP3 inflammsome in BA-MSCs after administration with 66PR in vivo.
(a) The experimental animal scheme. (b) The body weight analysis of colitis mice injected with 66PR and PBS. (c) The clinical scores of colitis mice injected with 66PR and PBS. (d) The colon length of colitis mice injected with 66PR and PBS. (e) The H&E stained colon sections from colitis mice injected with 66PR and PBS. (f) The histological scores of colitis and control mice. (g) Western blot analysis of NLRP3, caspase-1, IL-1β proteins in BA-MSCs from colitis mice injected with 66PR and PBS. (h-j) Densitometry analyses of NLRP3, caspase-1 and IL-1β according to (g). (k) Confocal laser scanning microscopic analysis of GSDMDC1 (green), CD51 (red) and nuclei (DAPI, blue) of bone sections from IBD mice injected with 66PR and PBS. Each experiment represents at least four mice per group. Eight sections were observed per group. Mean ± SD, t-test, *P < 0.05,**P < 0.01,***P < 0.001, ns: not significant, nd: not detected.
3.6. 66PR Improved BA-MSCs Survival by Restraining Pyroptosis in IBD
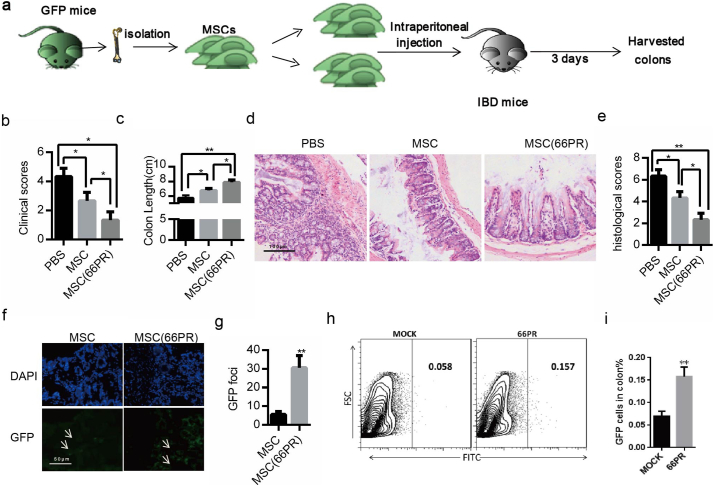
Considering MSCs' potential clinical application in inflammation control, we further investigated whether 66PR could improve BA-MSCs' inhibition on self-tissue-damage inflammation. Using an IBD animal model (Fig. 7a), we found that the clinical score, body weight, and colon length of mice had been greatly improved after transplantation with 66PR-treated BA-MSCs compared with normal BA-MSCs (Fig. 7b–c). Moreover, the histological analysis showed that 66PR-treated BA-MSCs significantly decreased the inflammatory features of colitis in colon sections (Fig. 7d–e). Consistently, the viability of mice transplanted with 66PR-treated BA-MSCs was greater than that of mice transplanted with normal BA-MSCs (Videos S1–S3). Given that both normal and 66PR-treated BA-MSCs were from GFP mice (Fig. 7a), we screened the GFP+BA-MSCs, analyzed the GFP fluorescence intensity, and found more GFP+foci in colon sections from mice transplanted with 66PR-treated BA-MSCs than those from mice transplanted with control BA-MSCs (Fig. 7f–g). Likewise, FACS analysis showed more green signals from single-cell suspensions of colon from the 66PR-treated BA-MSCs group than those in the control group (Fig. 7h–i), implying that 66PR can improve the survival of BA-MSCs by inhibiting pyroptosis. Altogether, these results demonstrated that 66PR could improve BA-MSCs survival by restraining pyroptosis in a self-tissue-damage inflammation environment.
Fig. 7.
The therapeutic effect of BA-MSCs treated with 66PR on IBD mice.
(a) The experimental animal scheme. (b) The clinical scores of the three groups of colitis mice. (c) The colon length of colitis mice transplanted with 66PR- or mock-treated BA-MSCs. (d) H&E stained colon section from colitis mice transplanted with 66PR- or mock-treated BA-MSCs. (e) The histological scores of colitis mice transplanted with 66PR- or mock-treated BA-MSCs. (f) The location of nuclei (DAPI, blue) staining and 66PR- or mock-treated CFSE+BA-MSCs. Eight sections were observed per group. (g) CFSE+ foci analysis of 66PR- or mock-treated BA-MSCs. (h) Flow plots of the CFSE staining in colons from colitis mice injected with 66PR- or mock-treated BA-MSCs. (i) Quantity of CFSE+ cells according to (h). PBS: injection with PBS; MSC: transplantation with BA-MSCs; MSCs(66PR): transplantation with MSCs treated with 66PR. Each experiment represents at least four mice per group. Mean ± SD, t-test, *P < 0.05,**P < 0.01,***P < 0.001.
4. Discussion
In the current study, we uncovered an inflammasome-dependent pathway of MSCs directly participating in inflammation in response to inflammatory stimuli. Under in vitro stimulation and in an inflammation animal model, we found that canonical NLRP3 inflammation and non-canonical caspase-11 inflammasome were activated in BA-MSCs. The NLRP3 inflammasome in BA-MSCs dominated IL-1β and IL-18 release, whereas caspase-11 inflammasome managed pyroptosis. These findings indicated that BA-MSCs themselves could directly promote the inflammatory response without the help of immune cells. Inhibition of the activation of inflammasomes in BA-MSCs by a small-molecule compound improved their survival in the inflammatory environment, which might optimize BA-MSCs therapy against inflammation diseases.
We previously reported that BA-MSCs' progenitors supported the maintenance, self-renewal, and differentiation of HSCs [14]. In this study, our findings uncovered that BA-MSCs secreted IL-1β/18 and induced pyroptosis by activation of NLRP3 inflammasome and caspase-11 inflammasome. IL-1β and IL-18, members of the pro-inflammatory IL-1 cytokine superfamily, are released in the earliest stage of immune response and act as a trigger for the subsequent cascade of pro-inflammatory cytokines [2]. Some studies reported that IL-1β stimulated the release of IL-6 and IL-17a, whereas IL-18 promoted the production of IFN-γ, IL-2, and IL-12 [4,31]. Thus, BA-MSCs could also develop inflammation via IL-1β and IL-18. Pyroptosis is characterized by pore formation in the plasma membrane, cell welling, and rupture of the membrane, causing massive leakage of cytosolic contents [40]. Accumulating evidence suggests a critical role of pyroptosis in the inflammatory response [17]. Thus, our data showed that BA-MSCs could directly promote inflammation via IL-1β/18 and pyroptosis. This finding indicated a key role for BA-MSCs in the initiation and development of inflammation.
The activation of the canonical inflammasomes mainly occurs in macrophage/dendritic cells [38,51], whereas non-canonical inflammasome activation occurs in both macrophage and non-macrophage cells [17,41]. Here, our observations showed that canonical NLRP3 inflammasome and non-canonical caspase-11 inflammasome were activated in BA-MSCs. our finding demonstrated that inflammasomes employed to respond to inflammatory stimulation are not restricted to immune cells. This finding was consistent with other reports. For example, the activation of non-canonical caspase-4 and caspase-11 inflammasomes occurred in intestinal epithelial cells [19]. The NLRP1 inflammasome is expressed in hematopoietic progenitor cells and its activation triggers their pyroptotic death [28]. Furthermore, the NLRP3 inflammasome has been activated in skin epithelial stem cells, hepatocytes and human dental pulp fibroblasts[16,25,32,53]. Thus, inflammasome signal transduction maybe a general phenomenon in mammalian cells. As a consequence, inflammasomes could be potential targets on non-immune cells to fight against relevant diseases, rather than only classical immune cells. Although inflammasomes exist in different cells, the types of inflammasomes in different cells were not identical, which suggesting the function of inflammasomes may be dependent on the types of cells. Although it was similar that the activation of NLRP3 and caspase-11 inflammasomes happened in macrophages and MSCs, the roles of NLRP3 and caspase-11 inflammasomes between macrophages and MSCs may be different.
Given that the activation of inflammasomes leads to IL-1β/18 release and pyroptosis, triggering strong inflammation, the inflammasomes in BA-MSCs are a potential disadvantage for BA-MSCs applied in the treatment of inflammatory diseases. Moreover, our finding showed that inhibition of inflammasome activation in BA-MSCs increased their survival rate and improved their therapeutic effect on IBD. Therefore, inflammasomes in BA-MSCs need to be subtly controlled to improve their potential clinical application in inflammation control. Currently, many researchers are focusing on identification of a small-molecule inflammasome inhibitor. Dopamine[50], MCC950[43,45], and CY-9 [15] were reported to inhibit the NLRP3 inflammasome based on caspase-1 cleavage and mature IL-1β release, whereas their inhibition of pyroptosis was not detected. Accumulating evidence suggests that pyroptosis, as well as IL-1β release, plays a critical role in the inflammatory response[17]. Thus, the ideal inhibitors of inflammasomes should be designed to inhibit pyroptosis and IL-1β.
Natural products not only play a significant role in the design, synthesis, and discovery of new drugs but also act as the most promising source of bioactive substances and innovative drugs [12]. MCL, a sesquiterpenelactone isolated from Micheliacompressa (Magnoliaceae), has an anti-inflammatory effect on intestinal inflammation, colitis-associated cancer, rheumatoid arthritis, and diabetes nephropathy [46,49,54]. On the other hand, N-substituted pyrimidine nucleobases have attracted much interest because of their potential use as antineoplastic, antiviral, and anticancer agents[11]. Meanwhile, the incorporation of fluorine into molecules is a powerful strategy in medicinal chemistry to modulate their hydrophobic character and, hence, decrease their metabolism[11]. On this basis, we designed and synthesized one adduct of MCL (66PR) with more potency and better drug-like properties than those of MCL. Fortunately, 66PR could inhibit IL-1β release and pyroptosis in vitro and in vivo. When BA-MSCs were used to treat IBD, 66PR pretreatment of the BA-MSCs improved their survival at local sites and their therapeutic effect on IBD. 66PR is an exciting compound for two important reasons. Firstly, we believe that pretreatment of MSCs with 66PR will increase their pro-inflammatory response and broaden their potential for treatment of various inflammatory disorders. Secondly, the inhibitory effect of 66PR on inflammasomes may also be applied to other cells containing inflammasomes, to control inflammatory diseases.
Although our results suggested that the inhibitory effect of 66PR on inflammasomes may lie in reducing the expression of NLRP3 and caspase-11 in BA-MSCs, the detailed mechanism of 66PR inhibition of inflammasomes needs to be further explored. The activation pathway, of the canonical NLRP3 inflammasome in BA-MSCs, involving two signals, was similar to that in some innate immune cells, such as macrophages or neutrophils. However, the activation pathway of the non-canonical caspase-11 inflammasome was different from that reported in other studies. In our study, the pro-caspase-11 protein in BA-MSCs was activated by LPS, but pyroptosis needed further stimulation with nigericin to occur. Shi et al. reported that only intracellular LPS activated caspase-11-dependent non-canonical inflammasome, inducing pyroptosis [41]. The discrepancies among in different studies suggest that there might be multiple pathways for the activation of non-canonical caspase-11 inflammasome. We are planning to explore the underlying mechanism of the activation of non-canonical caspase-11 inflammasome.
Collectively, the present investigation determined that BA-MSCs directly participated in the inflammatory reaction via inflammasome signaling and supplemented the knowledge on inflammasome activation in non-immune cells. The findings serve to remind us that the maintenance of a proper inflammatory balance should not only involve targeting of immune cells but also non-immune cells. The collected data extends our understanding of the subtle functions of BA-MSCs other than hematopoiesis or tissue repair, which is important for BA-MSCs use in clinical settings. Pretreatment of BA-MSCs with our small-molecule inhibitor might improve their potential therapeutic effect on inflammatory disorders.
The following are the supplementary data related to this article.
The viability of IBD mice.
The viability of IBD mice transplanted with BA-MSCs.
The viability of IBD mice transplanted with 66PR-treated BA-MSCs.
Supplementary material
Acknowledgments
Acknowledgments
We are grateful to Dr. Qin Liu (East China University of Science and Technology) for kindly revising and constructive suggestions. We thank the Institute of Neurosciences, and Department of Clinical Diagonistics Laboratory in Xijing Hospital of Fourth Military Medical University of China for technical assistance.
Funding Sources
This work was supported by Key Science and Technology Programs of Shaanxi Province of China NO·S2018-YF-YBSF-0556 (X.H.) and 2015SF195(X.Q.), and National Natural Science Foundation of China NO.81202421(X.Q.). The information contained in the manuscript is the sole responsibility of the authors, no funding source was involved in the collection, analysis, or interpretation of the data nor the manuscript preparation.
Author Contributions
Y. C., X. Q and Q.A. conducted most of the experiments. Y. C, Z.L, L.W. and X.H. wrote the manuscript. J. Y., F. F., D.Y., N. A. S. C. and J. X. helped to perform experiments. X.H. and W. Y. designed and supervised this study.
Declaration of Interests
The authors have declared that no conflict of interest exists.
Contributor Information
Xingbin Hu, Email: hxbyqh@fmmu.edu.cn.
Wen Yin, Email: yinwen@fmmu.edu.cn.
References
- 1.An N., Chen Y.Z., Yin D.D., Zhang H.J., Liu Z., Feng F. Melanoma-induced anemia could be rescued by Sca-1(+) mesenchymal stromal cells in mice. Stem Cells Dev. 2017;26:495–502. doi: 10.1089/scd.2016.0139. [DOI] [PubMed] [Google Scholar]
- 2.Arend W.P., Palmer G., Gabay C. IL-1, IL-18, and IL-33 families of cytokines. Immunol. Rev. 2008;223:20–38. doi: 10.1111/j.1600-065X.2008.00624.x. [DOI] [PubMed] [Google Scholar]
- 3.Bernardo M.E., Fibbe W.E. Mesenchymal stromal cells: sensors and switchers of inflammation. Cell Stem Cell. 2013;13:392–402. doi: 10.1016/j.stem.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 4.Cahill C.M., Rogers J.T. Interleukin (IL) 1 beta induction of IL-6 is mediated by a novel phosphatidylinositol 3-kinase-dependent AKT/I kappa B kinase alpha pathway targeting activator protein-1. J. Biol. Chem. 2008;283:25900–25912. doi: 10.1074/jbc.M707692200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen H., Yang D.H., Han F.J., Tan J.C., Zhang L.Z., Xiao J.F. The bacterial T6SS effector EvpP prevents NLRP3 inflammasome activation by inhibiting the Ca2+−dependent MAPK-Jnk pathway. Cell Host Microbe. 2017;21:47–58. doi: 10.1016/j.chom.2016.12.004. [DOI] [PubMed] [Google Scholar]
- 6.Chen Y.Z., Yang J.L., Zhang H.J., Fan H., An N., Xin J.J. Sca-1(+)mesenchymal stromal cells inhibit splenic marginal zone B lymphocytes commitment through caspase-3. Cell Biol. Int. 2016;40:549–559. doi: 10.1002/cbin.10591. [DOI] [PubMed] [Google Scholar]
- 7.Chung E., Son Y. Crosstalk between mesenchymal stem cells and macrophages in tissue repair. Tissue Eng. Regen. Med. 2014;11:431–438. [Google Scholar]
- 8.Dang S.P., Xu H.B., Xu C.F., Cai W., Li Q., Cheng Y.J. Autophagy regulates the therapeutic potential of mesenchymal stem cells in experimental autoimmune encephalomyelitis. Autophagy. 2014;10:1301–1315. doi: 10.4161/auto.28771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DelaRosa O., Dalemans W., Lombardo E. Toll-like receptors as modulators of mesenchymal stem cells. Front. Immunol. 2012;3 doi: 10.3389/fimmu.2012.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glossop J.R., Cartmell S.H. Effect of fluid flow-induced shear stress on human mesenchymal stem cells: differential gene expression of IL1B and MAP3K8 in MAPK signaling. Gene Expr. Patterns. 2009;9:381–388. doi: 10.1016/j.gep.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 11.Hagmann W.K. The many roles for fluorine in medicinal chemistry. J. Med. Chem. 2008;51:4359–4369. doi: 10.1021/jm800219f. [DOI] [PubMed] [Google Scholar]
- 12.Harvey A.L. Natural products in drug discovery. Drug Discov. Today. 2008;13:894–901. doi: 10.1016/j.drudis.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 13.He Y., Zeng M.Y., Yang D.H., Metro B., Nunez G. NEK7 is an essential mediator of NLRP3 activation downstream of potassium efflux. Nature. 2016;530:354. doi: 10.1038/nature16959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu X.B., Garcia M., Weng L.H., Jung X.M., Murakami J.L., Kumar B. Identification of a common mesenchymal stromal progenitor for the adult haematopoietic niche. Nat. Commun. 2016;7 doi: 10.1038/ncomms13095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang H., He H., Chen Y., Huang W., Cheng J., Ye J. Identification of a selective and direct NLRP3 inhibitor to treat inflammatory disorders. J. Exp. Med. 2017;214(11):3219–3238. doi: 10.1084/jem.20171419. jem.20171419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang W.K., Lv H.P., Wang H.J., Wang D.Y., Sun S.K., Jia Q. Activation of the NLRP3/caspase-1 inflammasome in human dental pulp tissue and human dental pulp fibroblasts. Cell Tissue Res. 2015;361:541–555. doi: 10.1007/s00441-015-2118-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jorgensen I., Miao E.A. Pyroptotic cell death defends against intracellular pathogens. Immunol. Rev. 2015;265:130–142. doi: 10.1111/imr.12287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keating A. Mesenchymal stromal cells: new directions. Cell Stem Cell. 2012;10:709–716. doi: 10.1016/j.stem.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 19.Knodler L.A., Crowley S.M., Sham H.P., Yang H.J., Wrande M., Ma C.X. Noncanonical inflammasome activation of caspase-4/caspase-11 mediates epithelial defenses against enteric bacterial pathogens. Cell Host Microbe. 2014;16:249–256. doi: 10.1016/j.chom.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumar B., Garcia M., Weng L., Jung X., Murakami J.L., Hu X. Acute myeloid leukemia transforms the bone marrow niche into a leukemia-permissive microenvironment through exosome secretion. Leukemia. 2017;32(3):575–587. doi: 10.1038/leu.2017.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lamkanfi M., Dixit V.M. Inflammasomes and their roles in health and disease. Annu. Rev. Cell Dev. Bi. 2012;28:137–161. doi: 10.1146/annurev-cellbio-101011-155745. [DOI] [PubMed] [Google Scholar]
- 22.Lamkanfi M., Dixit V.M. Mechanisms and functions of inflammasomes. Cell. 2014;157:1013–1022. doi: 10.1016/j.cell.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 23.Lee S.Y., Lee S.H., Yang E.J., Kim E.K., Kim J.K., Shin D.Y. Metformin ameliorates inflammatory bowel disease by suppression of the STAT3 signaling pathway and regulation of the between Th17/treg balance. PLoS One. 2015;10 doi: 10.1371/journal.pone.0135858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee T.C., Lee T.H., Huang Y.H., Chang N.K., Lin Y.J., Chien P.W.C. Comparison of surface markers between human and rabbit mesenchymal stem cells. PLoS One. 2014;9 doi: 10.1371/journal.pone.0111390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu Y.H., Xu S.C., Chen H.Y., He M.D., Deng Y.C., Cao Z.W. CdSe/ZnS quantum dots induce hepatocyte pyroptosis and liver inflammation via NLRP3 inflammasome activation. Biomaterials. 2016;90:27–39. doi: 10.1016/j.biomaterials.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 26.Ma S., Xie N., Li W., Yuan B., Shi Y., Wang Y. Immunobiology of mesenchymal stem cells. Cell Death Differ. 2014;21:216–225. doi: 10.1038/cdd.2013.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mao F., Kang J.-j., Cai X., Ding N.-f., Wu Y.-b., Yan Y.-m. Crosstalk between mesenchymal stem cells and macrophages in inflammatory bowel disease and associated colorectal cancer. Współczesna Onkol. 2017;2:91–97. doi: 10.5114/wo.2017.68616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Masters S.L., Gerlic M., Metcalf D., Preston S., Pellegrini M., O'Donnell J.A. NLRP1 inflammasome activation induces pyroptosis of hematopoietic progenitor cells. Immunity. 2012;37:1009–1023. doi: 10.1016/j.immuni.2012.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matthay M.A., Pati S., Lee J.W. Concise review: mesenchymal stem (stromal) cells: biology and preclinical evidence for therapeutic potential for organ dysfunction following trauma or sepsis. Stem Cells. 2017;35:316–324. doi: 10.1002/stem.2551. [DOI] [PubMed] [Google Scholar]
- 30.Menu P., Vince J.E. The NLRP3 inflammasome in health and disease: the good, the bad and the ugly. Clin. Exp. Immunol. 2011;166:1–15. doi: 10.1111/j.1365-2249.2011.04440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mills K.H.G., Dungan L.S., Jones S.A., Harris J. The role of inflammasome-derived IL-1 in driving IL-17 responses. J. Leukoc. Biol. 2013;93:489–497. doi: 10.1189/jlb.1012543. [DOI] [PubMed] [Google Scholar]
- 32.Naik S., Larsen S.B., Gomez N.C., Alaverdyan K., Sendoel A., Yuan S.P. Inflammatory memory sensitizes skin epithelial stem cells to tissue damage. Nature. 2017;550:475. doi: 10.1038/nature24271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nemeth K., Mayer B., Mezey E. Modulation of bone marrow stromal cell functions in infectious diseases by toll-like receptor ligands. J. Mol. Med. 2010;88:5–10. doi: 10.1007/s00109-009-0523-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ng T.M., Kortmann J., Monack D.M. Policing the cytosol-bacterial-sensing inflammasome receptors and pathways. Curr. Opin. Immunol. 2013;25:34–39. doi: 10.1016/j.coi.2012.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ng T.M., Monack D.M. Revisiting caspase-11 function in host defense. Cell Host Microbe. 2013;14:9–14. doi: 10.1016/j.chom.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pinho S., Lacombe J., Hanoun M., Mizoguchi T., Bruns I., Kunisaki Y. PDGFR alpha and CD51 mark human nestin(+) sphere-forming mesenchymal stem cells capable of hematopoietic progenitor cell expansion. J. Exp. Med. 2013;210:1351–1367. doi: 10.1084/jem.20122252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qin X.Y., Jiang X.R., Jiang X., Wang Y.L., Miao Z.L., He W.G. Micheliolide inhibits LPS-induced inflammatory response and protects mice from. LPS Challenge Sci. Rep.-UK. 2016;6 doi: 10.1038/srep23240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schroder K., Tschopp J. The inflammasomes. Cell. 2010;140:821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 39.Shalapour S., Karin M. Immunity, inflammation, and cancer: an eternal fight between good and evil. J. Clin. Invest. 2015;125:3347–3355. doi: 10.1172/JCI80007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shi J.J., Zhao Y., Wang K., Shi X.Y., Wang Y., Huang H.W. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526:660–665. doi: 10.1038/nature15514. [DOI] [PubMed] [Google Scholar]
- 41.Shi J.J., Zhao Y., Wang Y.P., Gao W.Q., Ding J.J., Li P. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature. 2014;514:187. doi: 10.1038/nature13683. [DOI] [PubMed] [Google Scholar]
- 42.Singer N.G., Caplan A.I. Mesenchymal stem cells: mechanisms of inflammation. Annu. Rev. Pathol. Mech. 2011;6:457–478. doi: 10.1146/annurev-pathol-011110-130230. [DOI] [PubMed] [Google Scholar]
- 43.Siterman M., Lengier S., Zadik L., Ofir N., Nachmias N., Rotem M. Mcc950 a novel inhibitor of Nlrp3 inflammasome reduces migration and invasion of lung adenocarcinoma in-vitro. Am. J. Resp. Crit. Care. 2017;195 [Google Scholar]
- 44.Stéphane Guillarmea S.L. Anne-Marie aubertinb cécile olicardc nathalie bourgougnonc françois hueta. Tetrahedron. 2003;59:8. [Google Scholar]
- 45.van Hout G.P.J., Bosch L., Ellenbroek G.H.J.M., de Haan J.J., van Solinge W.W., Cooper M.A. The selective NLRP3-inflammasome inhibitor MCC950 reduces infarct size and preserves cardiac function in a pig model of myocardial infarction. Eur. Heart J. 2017;38:828. doi: 10.1093/eurheartj/ehw247. [DOI] [PubMed] [Google Scholar]
- 46.Viennois E., Xiao B., Ayyadurai S., Wang L.X., Wang P.G., Zhang Q. Micheliolide, a new sesquiterpene lactone that inhibits intestinal inflammation and colitis-associated cancer. Lab. Investig. 2014;94:950–965. doi: 10.1038/labinvest.2014.89. [DOI] [PubMed] [Google Scholar]
- 47.Wang L.H., Chen K., Wan X.X., Wang F., Guo Z., Mo Z.H. NLRP3 inflammasome activation in mesenchymal stem cells inhibits osteogenic differentiation and enhances adipogenic differentiation. Biochem. Bioph. Res. Co. 2017;484:871–877. doi: 10.1016/j.bbrc.2017.02.007. [DOI] [PubMed] [Google Scholar]
- 48.Wirtz S., Neufert C., Weigmann B., Neurath M.F. Chemically induced mouse models of intestinal inflammation. Nat. Protoc. 2007;2:541–546. doi: 10.1038/nprot.2007.41. [DOI] [PubMed] [Google Scholar]
- 49.Xu H., Wang J., Wang C.J., Chang G.Q., Lin Y.N., Zhang H.J. Therapeutic effects of micheliolide on a murine model of rheumatoid arthritis. Mol. Med. Rep. 2015;11:489–493. doi: 10.3892/mmr.2014.2767. [DOI] [PubMed] [Google Scholar]
- 50.Yan Y.Q., Jiang W., Liu L., Wang X.Q., Ding C., Tian Z.G. Dopamine controls systemic inflammation through inhibition of NLRP3 inflammasome. Cell. 2015;160:62–73. doi: 10.1016/j.cell.2014.11.047. [DOI] [PubMed] [Google Scholar]
- 51.Yang J.L., Zhao Y., Shao F. Non-canonical activation of inflammatory caspases by cytosolic LPS in innate immunity. Curr. Opin. Immunol. 2015;32:78–83. doi: 10.1016/j.coi.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 52.Zanoni I., Tan Y.H., Di Gioia M., Broggi A., Ruan J.B., Shi J.J. An endogenous caspase-11 ligand elicits interleukin-1 release from living dendritic cells. Science. 2016;352:1232–1236. doi: 10.1126/science.aaf3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang A.S., Wang P.N., Ma X.Y., Yin X., Li J.G., Wang H.J. Mechanisms that lead to the regulation of NLRP3 inflammasome expression and activation in human dental pulp fibroblasts. Mol. Immunol. 2015;66:253–262. doi: 10.1016/j.molimm.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 54.Zhao Y, Chen S.J., Wang J.C., Niu H.X., Jia Q.Q., Chen X.W. Sesquiterpene lactones inhibit advanced oxidation protein product-induced MCP-1 expression in podocytes via an IKK/NF-kappa B-dependent mechanism. Oxidative Med. Cell. Longev. 2015 doi: 10.1155/2015/934058. 934058. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The viability of IBD mice.
The viability of IBD mice transplanted with BA-MSCs.
The viability of IBD mice transplanted with 66PR-treated BA-MSCs.
Supplementary material