Abstract
Recent genetic tools have allowed researchers to visualize and manipulate memory traces (i.e. engrams) in small brain regions. However, the ultimate goal is to visualize memory traces across the entire brain in order to better understand how memories are stored in neural networks and how multiple memories may coexist. Intact tissue clearing and imaging is a new and rapidly growing area of focus that could accomplish this task. Here, we utilized the leading protocols for whole-brain clearing and applied them to the ArcCreERT2 mice, a murine line that allows for the indelible labeling of memory traces. We found that CLARITY and PACT greatly distorted the tissue, and iDISCO quenched enhanced yellow fluorescent protein (EYFP) fluorescence and hindered immunolabeling. Alternative clearing solutions, such as tert-Butanol, circumvented these harmful effects, but still did not permit whole-brain immunolabeling. CUBIC and CUBIC with Reagent 1A produced improved antibody penetration and preserved EYFP fluorescence, but also did not allow for whole-brain memory trace visualization. Modification of CUBIC with Reagent-1A resulted in EYFP fluorescence preservation and immunolabeling of the immediate early gene (IEG) Arc in deep brain areas; however, optimized memory trace labeling still required tissue slicing into mm-thick tissue sections. In summary, our data show that CUBIC with Reagent-1A* is the ideal method for reproducible clearing and immunolabeling for the visualization of memory traces in mm-thick tissue sections from ArcCreERT2 mice.
Keywords: Arc, engram, CUBIC, memory, hippocampus, c-Fos
Introduction
Visualization of molecular tissue structures is an essential tool in many biological and medical disciplines. Tissue visualization is made possible by immunolabeling of molecular structures. However, the application of these techniques has historically been limited to thin tissue sections due to the inability of photons and molecular labels to penetrate deep tissue. Moreover, tissue-slicing techniques often compromise the structural integrity of the tissue and the molecular circuitry within it. To avoid these problems and instead image intact tissue without slicing, the molecules that prevent photons and antibodies from deep tissue penetration—lipids—must be cleared from the tissue.
In order to accomplish this, several techniques have been developed in recent years: Scale (Hama et al., 2011), ScaleS (Hama et al., 2015), 3DISCO (Ertürk et al., 2012; 2014), ClearT2 (Kuwajima et al., 2013), SeeDB (Ke et al., 2013), CLARITY (Chung et al., 2013; Tomer et al., 2014), PACT (Yang et al., 2014), CUBIC (Susaki et al., 2014; 2015), iDISCO (Renier et al., 2014), and SWITCH (Murray et al., 2015). By optimizing for time, cost, preservation of structural integrity, and endogenous and/or exogenous fluorescence, these techniques render the intact tissue transparent while preserving fine-wiring and molecular structures. These methods grant unprecedented access to neural activity, neural projections, and gene expression across the entire brain (Belle et al., 2014; Kim et al., 2015; Wang et al., 2015; Renier et al., 2016).
The brain-wide insights offered by tissue clearing are particularly important for the study of memory traces or engrams. Through extensive investigation in recent years, memory traces have been found in several cross-brain regions, including the hippocampus (HPC) and various neocortical regions (Wiltgen et al., 2006; Wiltgen and Silva, 2007; Wiltgen et al., 2010; Bonnici et al., 2013; Tayler et al., 2013; Denny et al., 2014). However, much is still unknown about the mechanisms by which memories are formed and retrieved, or how memories are stored together across different brain regions. To better study memories, we previously developed an indelible system, the ArcCreERT2 transgenic mouse (Denny et al., 2014), that allows for the comparison between permanently labeled EYFP+ neurons activated during learning and recently labeled Arc+ or c-Fos+ cells activated during memory retrieval. By using this mouse line in combination with a clearing technique that allows imaging of the entire mouse brain, the cross-brain circuitry of memory traces can be studied. Therefore, such a clearing technique must allow for successful, thorough, and precise antibody penetration for EYFP and Arc/c-Fos.
In this study, we thoroughly tested and modified clearing methods using the aforementioned clearing protocols for immunolabeling of EYFP and Arc/c-Fos in mm-thick tissue slices from the ArcCreERT2 mice. iDISCO and CUBIC were superior to CLARITY and PACT for labeling Arc and c-Fos; however, without modification, none of these methods yielded satisfactory labeling. To overcome this, we: 1) improved the solutions used during iDISCO immunolabeling and clearing to decrease fluorescence quenching; 2) identified a set of antibodies termed nanobodies that achieved relatively uniform labeling; and 3) modified incubation periods, antibodies, and antibody concentrations. Our results show that a modified protocol based on CUBIC with Reagent-1A, which we term CUBIC with Reagent-1A*, achieves satisfactory co-labeling. Together, these results provide optimized clearing protocols that yield representative EYFP fluorescence and Arc/c-Fos immunolabeling for use in memory trace studies.
Materials and Methods
Mice
The ArcCreERT2 mice were generated and genotyped as previously described (Denny et al., 2014). ArcCreERT2(+) mice were bred with ROSA26-CAG-stopflox-ChR2(H134R)-EYFP (Ai32) (Madisen et al., 2012). ArcCreERT2 mice were also bred with a Rosa26-LSL-H2B-mCherry line (Peron et al., 2015). Mice were housed 4-5 per cage in a 12-h (06:00-18:00) light-dark colony room at 22°C. Food and water were provided ad libitum. The procedures described herein were conducted in accordance with National Institutes of Health regulations and approved by the Institutional Animal Care and Use Committees of the New York State Psychiatric Institute (NYSPI).
Contextual Fear Conditioning (CFC)
ArcCreERT2 mice were administered a CFC procedure as previously described (Denny et al., 2014). For context exposure, mice were re-exposed to the training context and euthanized 1 h later in order for optimal Arc or c-Fos protein expression.
4-hydroxytamoxifen (4-OHT)
Five hours before CFC, ArcCreERT2 mice were injected with 2 mg of 4-OHT as previously described (Cazzulino et al., 2016). ArcCreERT2 × H2B-mCherry mice were injected with vehicle (Veh) or 1 mg of 4-OHT.
Brain processing
Mice were transcardially perfused with 4% paraformaldehyde (PFA). Brains were further fixed in 4% PFA at 4°C overnight. Next, brains were cut into half-brain samples or into 4-mm-, 2-mm-, or 1-mm-thick coronal slices using a manual brain cutter. All prescreening experiments were performed on 35- or 100-μm thick coronal slices, which were obtained by immersing whole brains in 4% PFA overnight, then in 30% sucrose at 4°C for 3 d, followed by snap-freezing in OCT medium and slicing on a Leica CM3050 cryostat.
Antibodies and Nanobodies
Antibody and nanobody information is summarized in the Supplementary Information and in Supplement Table S1.
iDISCO-based screening of thirty-five μm-thick sections
Floating 35 μm-thick sections screened for nanobody compatibility with iDISCO underwent a 2.5 h MeOH dehydration sequence, followed by a bleaching step for 10 min. Then, samples screened for compatibility with various organic solvents underwent a 2.5 h dehydration sequence. Samples undergoing an organic solvent-free pretreatment (referred to here as iDISCO without MeOH) were incubated in a detergent mixture for 2.5 h. Control samples were incubated in 1× PBS for 2.5 h. Finally, all samples were washed in 0.2% PBST 3 times for 10 min each. For EYFP immunohistochemistry, samples were blocked for 2 h, washed in 1× PBS/0.2% Tween-20 with 10 μg/ml heparin (PTwH) 3 times for 10 min each, and then incubated in primary antibody dilutions in PTwH/3% normal donkey serum (NDS) for 48 h at 4°C. Samples were washed again in PTwH 3 times for 10 min each, placed in secondary antibody dilutions in PTwH/3% normal donkey serum (NDS) for 2 h, and then washed in PTwH 3 times for 10 min each before mounting and imaging. All steps except the primary antibody incubation were performed at room temperature (RT).
iDISCO
The iDISCO protocol was followed as previously described (Renier et al., 2014). Briefly, prior to whole antibody immunolabeling, 4-mm thick tissue slices were permeabilized by a MeOH pre-treatment step. Samples labeled with the anti-GFP nanobody were pretreated with an organic solvent-free protocol, consisting of series of incubations in detergents (iDISCO without MeOH) (Renier et al., 2014). Next, samples were immunolabeled with 2 ml of primary antibody or nanobody mixture for 4-5 days at 37°C, followed by 1 day of washing at RT. Samples were immunolabeled with 2 ml of secondary antibody mixture for 4 days at 37°C.
To test if iDISCO quenches endogenous EYFP fluorescence, 4-mm-thick sections were treated only with clearing steps of the iDISCO protocol. After 4% PFA fixation, samples were washed in 1× PBS 3 times for 1 h each, and dehydrated with 50% THF (in ddH2O), 80% THF, 100% THF, and 100% THF as described in the original iDISCO protocol (Renier et al., 2014). To test the effect of other organic dehydration solvents, THF was replaced with MeOH, tert-Butanol, or buffered tert-Butanol. After dehydration, samples were further cleared with DCM and DBE as previously described (Renier et al., 2014). To decrease tissue oxidation, all organic solvent steps were performed under vacuum at RT. tert-Butanol was buffered to pH 9.5 using triethylamine (3EtN) (1:118 dilution). We also pretreated samples with the iDISCO without MeOH procedure.
CUBIC with Reagent-1A / CUBIC with Reagent-1A*
CUBIC with Reagent-1A protocol was previously reported to optimize endogenous fluorescence preservation from reporter proteins after tissue clearing (Susaki and Ueda, 2016). One-mm thick tissue slices were treated with the same tissue treatment, immunolabeling (Cy3 secondary antibody for Arc immunolabeling), and clearing procedure as in the original CUBIC with Reagent-1A protocol, with its decreased amount of Triton (10% weight (wt)), Urea (10% wt), and Quadrol (5% wt). To achieve more uniform thick-tissue Arc immunolabeling, we made a series of modifications that we now termed CUBIC with Reagent-1A*. We utilized a lower dilution factor of the rabbit anti-Arc primary antibody (1:500) and a 5-day incubation period for the primary and secondary antibody immunolabeling steps. For deeper imaging and reduced autofluorescence, a secondary donkey anti-rabbit antibody conjugated to red-shifted Alexa Fluor 647 (1:500, Life Technologies, Carlsbad, CA) was instead used. Refer to Supplement Table S1 for full immunolabeling details.
Confocal Microscopy Imaging
All samples were imaged on a confocal scanning microscope (Leica TCS SP8, Leica Microsystems Inc.) with 2 simultaneous PMT detectors. Fluorescence from 1) endogenous EYFP, tissue autofluorescence, Cy2, or Atto-488 was excited at 488-nm and detected at 500-545 nm, 2) Cy3 was excited at 552- and detected at 565-610 nm, and 3) Alexa Fluor 647 and TO-PRO®-3 Iodide were excited at 634 nm and detected at 645-700 nm.
Thirty-five μm-thick sections were imaged with a dry Leica 20× objective (NA 0.70, working distance 0.5 mm), with a pixel size of 1.08 × 1.08 μm, a z step of 1.5 μm, and at 1 f/s. Thick tissue samples were imaged with a dry Leica 10× objective (NA 0.30, working distance 10 mm), with a pixel size of 2.16 × 2.16 μm, a z step of 10-100 μm, and at 1 f/s. Several fields of view were stitched together to form tiled images by using an automated stage as well as the tiling function and algorithm of the LAS × software.
Image Processing and Analysis
Maximum projections of z stacks were performed using ImageJ (NIH, https://imagej.nih.gov/ij/,RRID:SCR_003070). An unpaired t-test determined statistical differences in average image intensities between experimental groups. CellProfiler (http://cellprofiler.org) was used for automated cell counting.
Results
ArcCreERT2 mice allow for memory trace labeling in mm-thick tissue slices
The cross-brain circuitry of memory traces can be studied by utilizing our indelible labeling system, the ArcCreERT2 (Denny et al., 2014) × channelrhodopsin 2 (ChR2)-EYFP mice (Madisen et al., 2012) (Figure 1a) with a thick tissue clearing and immunolabeling technique. Our transgenic system offers robust and permanent EYFP labeling of neurons activated during learning (Figure 1b-c). This population can then be compared with cells expressing Arc or c-Fos protein that are activated during memory retrieval or expression (Figure 1d-g).
Figure 1. ArcCreERT2 × ChR2-EYFP mice allow for robust memory trace labeling.
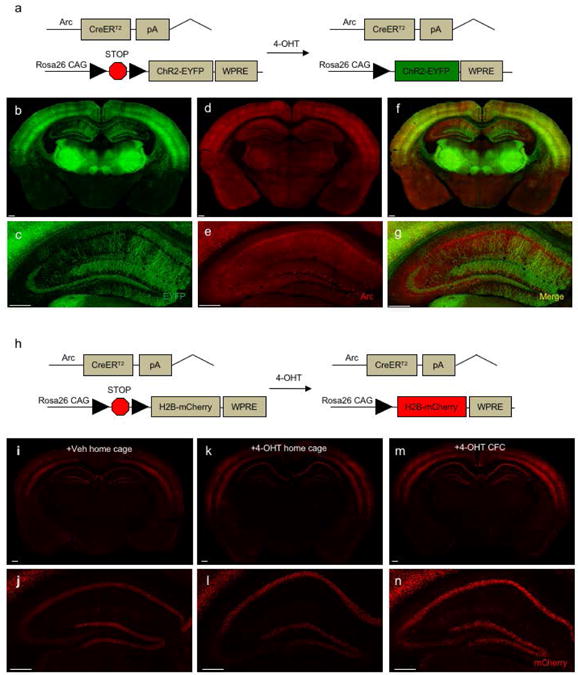
(a) Genetic design for ArcCreERT2 × ChR2-EYFP mice. (b-c) EYFP, (d-e) Arc, and (f-g) merged immunolabeling in a 35 μm section. Scale bar represents 0.25 mm. (h) Genetic design for ArcCreERT2 × H2B-mCherry mice. Endogenous expression of reporter mCherry in 100 μm sections from a mouse administered (i-j) Veh injections in the home cage; (k-l) a 4-OHT injection (1 mg) in the home cage; or (m-n) 4-OHT injection (1 mg) prior to CFC. Scale bar represents 0.25 mm.
Although counting cells with dendritic labeling is possible, we hypothesized utilizing a line with a nuclear tag rather than a dendritic label may better facilitate automated counting. Therefore, we also bred the ArcCreERT2 mice with H2B-mCherry mice (Peron et al., 2015), which allow for permanent nuclear mCherry labeling of neurons activated during learning (Figure 1h). To verify the specificity of mCherry expression, ArcCreERT2 mice were injected with Veh (Figure 1i-j) or 4-OHT (Figure 1k-l) in the home cage or 4-OHT before CFC (Figure 1m-n). mCherry+ cells were visible in the HPC of both Veh- and 4-OHT-injected home cage mice, and the mCherry expression was similar to that observed in 4-OHT-injected CFC mice, indicating that mCherry expression induced by 4-OHT injection is not specific to activated neurons during CFC. Therefore, because the ArcCreERT2 × H2B mCherry mice have significant labeling in the absence of drug and do not produce representative reporter expression, the ArcCreERT2 × ChR2-EYFP mice were determined to best meet experimental needs.
We next determined that automated counting of EYFP + and Arc/c-Fos+ cells is possible using the ArcCreERT2 × ChR2-EYFP mice (Supplement Figure S1), suggesting that it is an appropriate model for labeling and automated quantification of whole-brain memory traces.
CLARITY and PACT do not permit uniform Arc immunolabeling or preserve endogenous EYFP fluorescence
Intact-tissue immunolabeling for EYFP and Arc was first attempted on 4-mm mouse brain tissue sections using CLARITY (Tomer et al., 2014). CLARITY, a hydrogel embedding clearing method, clears the brain in 5 main steps: 1) fixation, 2) hydrogel embedding, 3) lipid clearing with SDS detergent and electrophoresis tissue clearing (ETC), 4) immunolabeling, and 5) refractive index (RI) matching with FocusClear (Supplement Figure S2a). As expected, brains cleared using CLARITY become relatively clear initially and then turn opaque when released from the clearing state prior to RI matching homogenization (Supplement Figure S2b). To improve transparency, hydrogel concentration was varied from 4% PFA/4% acrylamide/0.025% bis-acrylamide to 4% PFA/1% acrylamide/0.025% bis-acrylamide (Supplement Figure S2c). While optimal clearing was achieved with a 4% PFA/2% acrylamide/0.025% bis-acrylamide hydrogel concentration and ETC for 5 days at 37°C and 0.6-0.7 A (Supplement Figure S2d), no deep tissue Arc immunolabeling was observed with this protocol, as the antibody remained in the tissue periphery (Supplement Figure S2e).
In an additional attempt to improve transparency, a modified CLARITY procedure was performed on 4 mm-thick tissue slices as previously described (Epp et al., 2015). A 3% PFA/3% acrylamide /0.025% bis-acrylamide hydrogel concentration was utilized, and ETC was performed first for 5 days at 37°C and 0.78 A, and next for 1 day at 50°C and 0.78 A (Supplement Figure S3a). However, samples that were processed using this ETC procedure showed no improved transparency or integrity compared to CLARITY and showed quenched endogenous EYFP fluorescence compared with samples that were passively cleared (Supplement Figure S3b-c). We again varied the hydrogel concentrations in order to potentially preserve EYFP fluorescence, but there was still no fluorescence remaining post-clearing (Supplement Figure S2d-e). These data indicate that the modified CLARITY protocol does not preserve endogenous EYFP fluorescence in the ArcCreERT2 × ChR2-EYFP mice.
PACT (Yang et al., 2014), another hydrogel embedding clearing method, was also screened, but exhibited unsatisfactory preservation of tissue integrity (i.e. greatly distorted the tissue) and did not allow for Arc or GFP immunolabeling (Supplement Figure S4). It also quenched the endogenous EYFP fluorescence signal (data not shown). In summary, these data indicate that CLARITY and PACT are not ideal clearing methods for preserving endogenous EYFP fluorescence or for uniform immunolabeling in the ArcCreERT2 × ChR2-EYFP mice.
iDISCO results in satisfactory immunolabeling of Arc, but not EYFP
iDISCO, an organic solvent-based clearing method, renders the brain transparent through 4 main steps: 1) fixation, 2) dehydration with MeOH, 3) immunolabeling, and 4) lipid clearing with THF, DCM, and DBE (Supplement Figure S5a). iDISCO produced extremely clear tissue, with less tissue distortion relative to CLARITY (Supplement Figure S5b-d). Arc immunolabeling in iDISCO-cleared tissue was satisfactory (Figure 2a-c); deep-tissue labeling of distinct neurons in the dentate gyrus (DG) region was achieved, even at 1000 μm below cut surface (Figure 2c). However, this labeling was not uniform, producing a visible intensity gradient that results in the middle of the tissue being less labeled than the periphery at 1000 μm. Arc immunolabeling further decreased when tissue was co-labeled for EYFP with anti-GFP antibodies (Figure 2d-f). GFP immunolabeling was satisfactory only at the tissue periphery, but was poor in deep tissue (Figure 2g-h), producing an “edge effect.” Compared to PACT and CLARITY, iDISCO resulted in uniform immunolabeling of Arc+, but not EYFP+ cells.
Figure 2. iDISCO results in uniform intact-tissue Arc, but not GFP immunolabeling.

Arc antibody staining penetrates throughout the brain tissue. Representative micrographs of Arc immunolabeling in coronal sections at (a) 500 μm, (b) 750 μm, and (c) 1000 μm below the cut surface of a 4-mm thick tissue section. Representative micrographs of (d) GFP, (e) Arc, and (f) merged immunolabeling in iDISCO-processed brains at 200 μm below the cut surface. (g-h) Micrographs of GFP, Arc, and TO-PRO®-3 immunolabeling in the DG at (g) 100 μm and (h) 200 μm below the cut surface. GFP immunolabeling is only present on the edge of the tissue. Scale bar represents 0.25 mm.
Strategies for improving EYFP immunolabeling in iDISCO
Two aspects of the iDISCO pipeline were hypothesized to improve deep-tissue EYFP immunolabeling: 1) the amount or size of the antibody, and 2) the organic solvents used for tissue dehydration. First, we tested if the GFP “edge effect” could be resolved by optimizing the anti-GFP antibody concentration (Supplement Figure S6). While the GFP “edge effect” was more pronounced at higher antibody concentrations, changes in antibody concentration did not lead to any improvements in deep-tissue EYFP immunolabeling. This is in contrast to the Arc antibody, for which higher concentrations produced better labeling.
We then tested if the “edge effect” was a result of the GFP antibody being too large to penetrate deep tissue and instead accumulating on the brain's periphery. To test this, we utilized a smaller-sized alternative: a GFP nanobody (Fridy et al., 2014), which functions as the isolated binding region of the antibody (Figure 3a-b). Thirty-five μm-thick sections stained with GFP nanobody exhibited significantly greater fluorescence intensity than unlabeled sections, but significantly lower fluorescence intensity than whole-antibody labeled sections (ANOVA, F (2,21) = 157.916, p < 0.0001). (Figure 3c-f). Despite this intensity difference, the nanobody yielded robust staining in ArcCreERT2 × ChR2-EYFP sections.
Figure 3. GFP nanobody yields effective immunostaining but is impacted by MeOH dehydration.
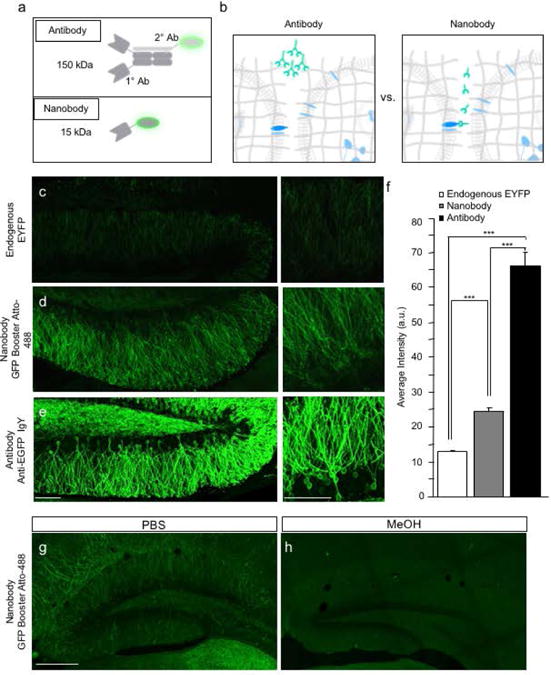
(a) Schematic. (b) Proposed diagram of antibodies and nanobodies diffusing through brain tissue. (c) Endogenous EYFP, (d) GFP-booster Atto-488 nanobody, and (e) GFP antibody staining in 35 μm-thick tissues. Scale bar represents 0.25 mm. (f) GFP antibody staining produces the most robust signal when compared with the endogenous signal and nanobody staining (unpaired t-test, p < 0.0001, p < 0.0001) (n = 8 sections per group). Error bars represent + SEM. (g-h) MeOH treatment impacts nanobody staining. Scale bar represents 0.25 mm.
Since the binding properties of some antibodies are affected by tissue dehydration using organic solvents such as MeOH, we tested whether the nanobody was compatible with the iDISCO MeOH dehydration step prior to immunolabeling. When the thin tissue sections were dehydrated in MeOH, GFP nanobody staining was lost, indicating that MeOH negatively impacts nanobody labeling (Figure 3g-h). In summary, these experiments suggest that if the GFP IgY antibody is indeed too large to penetrate deep tissue, the smaller GFP nanobody may be a viable alternative, but will require using a dehydration solvent different than MeOH.
Improving dehydration solvents for compatibility with the GFP nanobody
We identified three solutions as alternatives to MeOH in the dehydration step of the iDISCO protocol (Figure 4a). First, we selected a detergent-based solution offered by Renier et al., (2014) for use with MeOH-incompatible antibodies (referred to here as iDISCO without MeOH). We also selected tert-Butanol and THF, as they appear to be less harsh on organic tissue (Schwarz et al., 2015). Next, we assessed the intensity of GFP nanobody and GFP whole antibody staining in 35-μm-thick tissue sections that were first dehydrated in one of the above solutions (1× PBS, MeOH, THF, iDISCO without MeOH solutions, or tert-Butanol) and then treated with the subsequent iDISCO pre-screening protocol steps (Figure 4b-p). Compared with 1× PBS-treated slices (Figure 4b-d) and MeOH-dehydrated slices (Figure 4e-g), THF-dehydrated tissue slices exhibited exceptionally poor GFP nanobody staining (Figure 4h-j). In contrast, tissue slices treated with iDISCO without MeOH solutions (Figure 4k-m) and those dehydrated with tert-Butanol (Figure 4n-p) exhibited significantly greater GFP nanobody staining compared to MeOH-dehydrated slices (Figure 4e-4g) and similar intensity staining compared to 1× PBS-treated slices (Figure 4b-d).
Figure 4. Organic solvents used in iDISCO differentially affect GFP staining.
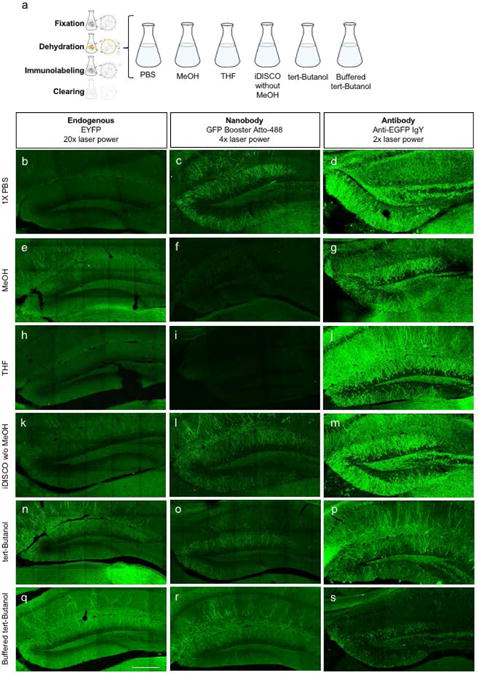
(a) Experimental schematic. iDISCO immunolabeling of the DG in 35-μm thick sections from ArcCreERT2 × ChR2-EYFP mice. Micrographs of coronal sections for endogenous EYFP label, GFP Booster nanobody immunostaining, and GFP antibody immunostaining when dehydrated in: (b-d) 1× PBS, (e-g) MeOH, (h-j) THF, (k-m) iDISCO without MeOH solutions, (n-p) tert-Butanol, or (q-s) buffered tert-Butanol. Laser power was increased as noted for optimal comparison. The scale bar represents 0.25 mm.
Buffering organic solvents has also been shown to improve fluorescent labeling (Schwarz et al., 2015). Therefore, we assessed the intensity of GFP nanobody and GFP whole antibody labeling in 35-μm-thick tissue sections that were dehydrated with buffered (to pH 9.5) tert-Butanol (Figure 4q-s), showing that buffered tert-Butanol did improve GFP nanobody labeling compared to un-buffered tert-Butanol (Figure 4n-p). Based on these results, the iDISCO without MeOH solutions and buffered tert-Butanol were identified as nanobody-compatible alternatives to the MeOH dehydration step in the iDISCO protocol.
We tested the validity of these results on thick tissues by treating 4-mm thick tissue sections with this modified iDISCO protocol; MeOH in the pre-immunolabeling dehydration step was replaced with either iDISCO without MeOH solutions or buffered tert-Butanol (Figure 5), labeling was performed with GFP nanobody, and the top 100 μm of tissue was imaged both immediately after immunolabeling (pre-clearing) and after clearing (post-clearing) (Figure 5a-d). GFP nanobody labeling was observed only in the tissue treated with the iDISCO without MeOH solutions (Figure 5d), suggesting that that the longer dehydration with buffered tert-Butanol negatively affects GFP nanobody binding. Moreover, there was significant autofluorescence seen following the buffered tert-Butanol procedure compared to the iDISCO without MeOH procedure (Figure 5c-d). Therefore, based on these results, the iDISCO without MeOH solutions, rather than the buffered tert-Butanol, was identified as the best nanobody-compatible alternative to MeOH dehydration.
Figure 5. GFP nanobodies readily diffuse through thick tissue sections, however, are negatively impacted by iDISCO.
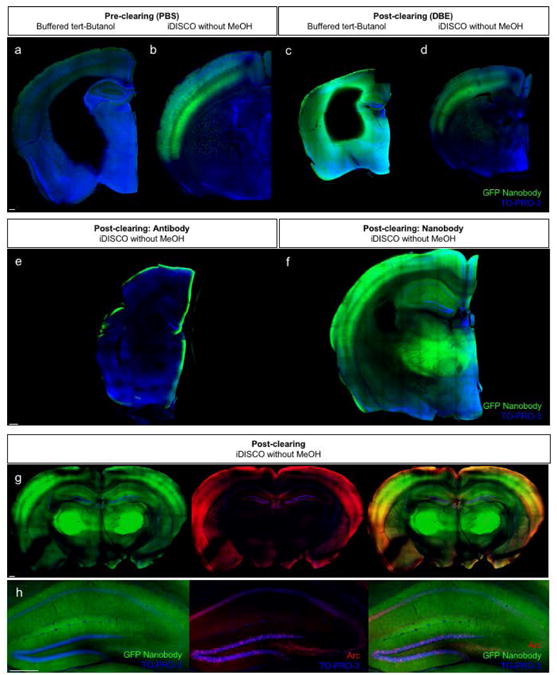
Pre-clearing confocal images of 4-mm thick ArcCreERT2 × ChR2-EYFP tissue sections immunolabeled with GFP-booster Atto-488 nanobodies and treated with (a) buffered tert-Butanol or (b) iDISCO without MeOH. Post-clearing confocal images of 4-mm thick ArcCreERT2 × ChR2-EYFP tissue sections immunolabeled with GFP-booster Atto-488 nanobodies and treated with (c) buffered tert-Butanol or (d) iDISCO without MeOH. (e-f) Post-clearing confocal images of 4-mm thick ArcCreERT2 × ChR2-EYFP tissue sections immunolabeled with (e) Anti-GFP antibody or (f) Nanobody. (g-h) Confocal images of a 2-mm thick ArcCreERT2 × ChR2-EYFP tissue section immunolabeled with GFP-booster Atto-488 nanobody and anti-Arc antibodies. Images were taken after clearing at 0.5 mm below the cut surface. Scale bar represents 0.25 mm.
When the iDISCO without MeOH detergents and GFP nanobody immunolabeling were tested on 4-mm thick tissue sections, the sections exhibited uniform labeling throughout the tissue (Figure 5e-f), resolving the “edge effect” seen with the GFP whole antibody. However, when performed with Arc co-staining (Figure 5g-h), sections again exhibited GFP nanobody immunolabeling prior to final clearing, but the labeling was diffuse and indistinct post-clearing, most likely due to the nanobody's already lower fluorescence intensity relative to the GFP whole antibody. These findings suggest that, while it can decrease the “edge effect,” nanobody labeling does not result in robust labeling in ArcCreERT2 × ChR2-EYFP brain tissue.
iDISCO solvents increase autofluorescence and quench endogenous EYFP fluorescence
Because satisfactory EYFP and Arc/c-Fos co-immunolabeling could not be achieved with any of the aforementioned protocols, we asked whether we could achieve satisfactory signal from endogenous EYFP fluorescence, which has not been previously reported using the iDISCO technique (Renier et al., 2014). To test this, 4-mm-thick tissue sections were treated with the fixation and clearing steps of the iDISCO protocol and imaged before (in 1× PBS) and after clearing (in DBE) (Supplement Figure S7). Endogenous EYFP fluorescence in ArcCreERT2(+) × ChR2-EYFP heterozygous (+/f) mice was observed pre-clearing, but was lost post-clearing and disrupted by an excess amount of autofluorescence.
We hypothesized that limiting the amount of oxygen exposure may decrease this autofluorescence. To test this, we performed the iDISCO protocol on un-stained (ArcCreERT2(-) × ChR2-EYFP (+/f)) thick tissue sections in air and under vacuum conditions (Supplement Figure S7) and imaged them both pre-clearing and post-clearing. Increased autofluorescence appeared in the in-air sample post-clearing, but decreased in the sample cleared under vacuum when oxygen exposure was limited.
Next, we hypothesized that the endogenous EYFP fluorescence is primarily quenched during the THF dehydration step of iDISCO clearing. We tested if other dehydration solvents—such as MeOH, tert-Butanol, and buffered tert-Butanol—prevent endogenous EYFP quenching at this clearing sub-step (Supplement Figure S8). Images of 4-mm-thick tissue sections post-dehydration reveal that MeOH drastically quenched endogenous EYFP fluorescence when compared to THF. Dehydration with tert-Butanol and buffered tert-Butanol also appear to quench endogenous EYFP fluorescence, but to a lesser extent than THF. However, regardless of the dehydration solvent used, post-clearing images (in DBE) reveal that endogenous EYFP signal is minimal in cleared tissue at depths greater than 500 μm. This is likely due to a combination of endogenous EYFP signal quenching caused by organic solvents and of additional light absorption by the brown color of iDISCO-cleared tissue. These results confirm that iDISCO-based clearing, even with modified solvents, is not compatible with deep-tissue imaging of endogenous EYFP signal in the ArcCreERT2 × ChR2-EYFP mice.
CUBIC preserves endogenous EYFP fluorescence, but yields unsatisfactory Arc immunolabeling
To identify a clearing protocol that preserves endogenous EYFP fluorescence in ArcCreERT2 × ChR2-EYFP tissue, we treated 4-mm-thick tissue sections with CUBIC (Susaki et al., 2014; 2015). CUBIC, a hyperhydration clearing technique, renders the brain transparent through 4 main steps: 1) fixation, 2) hyperhydration with an aqueous clearing solution, 3) immunolabeling, and 4) RI matching with a urea-containing solution. Our results show that while CUBIC (Supplement Figure S9a-c) preserved endogenous EYFP fluorescence better than iDISCO (Supplement Figure S9d-e) and permitted deep-tissue imaging (∼1000 μm below cut surface) of endogenous EYFP signal, CUBIC is not compatible with GFP nanobody immunolabeling (Supplement Figure S9f-g) and does not allow uniform Arc immunolabeling in tissue deeper than 500 μm below the cut surface (Supplement Figure S9h).
CUBIC with Reagent-1A* best preserves endogenous EYFP fluorescence and yields uniform Arc immunolabeling
We hypothesized that the limited Arc immunolabeling may be the result of incompatibility with the hyperhydrating aqueous clearing solution. To test this, CUBIC with Reagent-1A was performed (Susaki and Ueda, 2016) (Supplement Figure S10). CUBIC with Reagent-1A preserved endogenous EYFP fluorescence up to 400 μm below the surface and allowed for Arc immunolabeling, but not uniformly throughout the tissue.
To further improve Arc immunolabeling, we modified CUBIC with Reagent-1A, terming it CUBIC with Reagent-1A*. A new far-red secondary antibody was utilized for Arc, and the incubation time was increased. CUBIC with Reagent-1A* resulted in preservation of endogenous EYFP fluorescence (Figure 6a), allowing for visualization of cell bodies and dendrites in both the HPC (Figure 6b) and cortex (Figure 6c) up to 600 μm below the surface (Figure 6d-e) and uniform Arc immunolabeling in HPC and throughout the tissue section (Figure 6f-i). CUBIC with Reagent-1A* also permitted c-Fos immunolabeling in a 1-mm thick tissue section (Supplement Figure S11). In comparison, organic solvent clearing, such as iDISCO and the recently developed uDISCO (Pan et al., 2016) (Supplement Figure S12), did not preserve endogenous EYFP fluorescence in our mouse line. These data suggest that the CUBIC with Reagent-1A* is the best clearing technique to utilize with the ArcCreERT2 × ChR2-EYFP mouse model for preserving endogenous EYFP signal and for allowing Arc immunolabeling throughout the tissue.
Figure 6. CUBIC with Reagent-1A* best preserves endogenous EYFP fluorescence and permits Arc immunolabeling in ArcCreERT2 × ChR2-EYFP brain tissue.
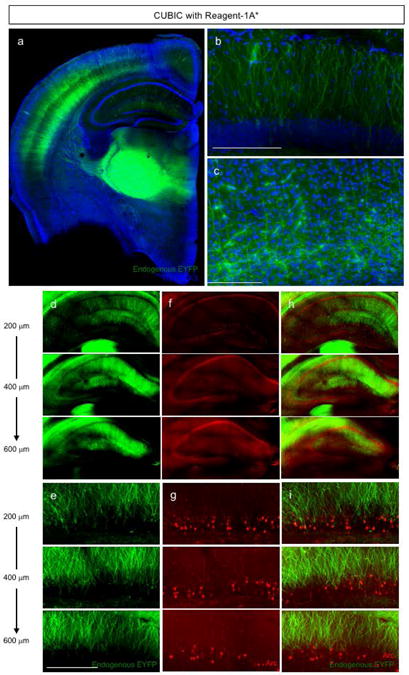
Confocal micrographs of a 2-mm thick tissue section showing endogenous EYFP in (a) half of a brain, (b) the DG, and (c) cortex region following CUBIC with Reagent-1A* treatment. CUBIC with Reagent-1A* (d-e) preserves endogenous EYFP fluorescence, (f-g) permits Arc immunolabeling up to 600 μm below the surface, and (h-i) allows for co-labeling of endogenous EYFP and Arc immunolabeling in the HPC and DG, respectively. Scale bars represent 0.25 mm.
Discussion
Here, we have piloted numerous whole-brain clearing techniques—CLARITY, PACT, iDISCO, uDISCO, and CUBIC—to effectively label and image memory traces in ArcCreERT2 × ChR2-EYFP mouse brains by preserving both endogenous EYFP fluorescence or immunolabeling for EYFP and co-labeling with Arc or c-Fos. The ChR2-EYFP reporter line can be used to study memory across long time periods via the indelible EYFP labeling that is representative of experience-dependent Arc expression. The versatility and specificity of this line is not offered by all reporter lines, such as the mCherry reporter line tested here; however, we recognize that, because of its characteristic dense and dendritic labeling, the ChR2-EYFP line brings many challenges, which we worked toward resolving here.
iDISCO and CUBIC were superior to CLARITY and PACT in clearing and labeling ArcCreERT2 × ChR2-EYFP mouse brains. Yet, neither of the original methods—iDISCO nor CUBIC—offered satisfactory Arc and EYFP co-labeling. Published data have shown that these methods efficiently clear whole brains with relatively sparsely-expressed antigens and reporters (Renier et al., 2014; Renier et al., 2016; Liebmann et al., 2016). However, thick-tissue immunolabeling of densely-expressed antigens, such as in ArcCreERT2 × ChR2-EYFP mice brains, is challenging, an issue that has been recognized in recent literature (Murray et al., 2015). We believe the original clearing protocols are tremendously useful, but our results show that they are not optimized for studying whole-brain memory traces in densely labeled lines, such as in the ArcCreERT2 × ChR2-EYFP mice.
In iDISCO-treated mm-thick tissue slices, Arc antibodies stained uniformly, but GFP staining did not fully penetrate the tissue, resulting in an “edge effect”. The observed “edge effect” resulted from the dense cortical EYFP label characteristic of the ArcCreERT2 × ChR2-EYFP line, making thick-tissue antibody penetration and uniform labeling more difficult than in other mouse lines typically used for iDISCO studies, which have less dense cortical labeling (Renier et al., 2014; Liebmann et al., 2016). To resolve this problem and allow GFP staining, several modifications were piloted, including modifications of 1) antibodies and 2) organic solvents used for dehydrating, fixing, and clearing. As an antibody alternative, a GFP nanobody was piloted (Fridy et al., 2014), which stained uniformly but with significantly less intensity than a GFP IgY antibody. The observed intensity discrepancy between the nanobody and whole antibody is not surprising, as the nanobody contains only one antigen-binding region while the whole antibody contains two, and the nanobody is not enhanced with a secondary antibody while the whole antibody is. Still, this discrepancy is important because the less intense fluorescence of the GFP nanobody may cause the GFP nanobody to fail at labeling and report dimmer signals on less fluorescent neurons. Regardless, considering that GFP nanobody did stain in sufficient quantities, results suggest that GFP nanobody may be used as a viable alternative to the whole antibody following future studies to enhance nanobody fluorescence.
Some of the organic solvents used in the original iDISCO protocol (Renier et al., 2014), especially MeOH and THF, cause not only fluorescence quenching, but also changes in antigenicity, especially for monoclonal antibodies and nanobodies. tert-Butanol, a higher-level organic solvent, negated the harmful effects of the MeOH on nanobody staining in thin tissue sections, and functioned even better when buffered to a higher pH. Our data in thin sections are in agreement with recent studies assessing the stability of endogenous fluorescent proteins when treated with various organic solvents (Schwarz et al., 2015; Pan et al., 2016). Future buffering of iDISCO solutions holds promise in promoting immunolabeling and decreasing autofluorescence.
In thick tissue sections, GFP nanobody staining was not observed after tert-Butanol dehydration. This suggests that either 1) the longer tert-Butanol incubation period needed for thicker samples negatively affects the tissue and disrupts nanobody binding, or 2) tert-Butanol does not adequately dehydrate the tissue, rendering the nanobody unable to penetrate thick tissue. The latter possibility is supported by the finding that MeOH and THF cleared lipids better than tert-Butanol. Nevertheless, our results in thin and thick sections are in accord with past studies showing that, although MeOH is comparatively better at lipid clearing than its alternatives, MeOH is also more disruptive to proteins (Herskovits et al., 1970; Schwarz et al., 2015). These studies also found that tert-Butanol preserves protein fluorescence significantly better than MeOH and other alternatives, which aligns with our thin section results. Likewise, studies showing that THF and MeOH clear lipids and water more aggressively than tert-Butanol may explain the poor nanobody penetration in thick sections treated with tert-Butanol (Herskovits et al., 1970; Ertürk et al., 2012; 2014; Schwarz et al., 2015). Nevertheless, tert-Butanol is still an organic solvent, so the possibility that it, too, like MeOH and THF, may not be gentle enough to preserve nanobody labeling remains. Unlike tert-Butanol, the iDISCO without MeOH solutions preserved nanobody fluorescence in thick sections. However, the nanobody labeling was diffuse, and determining co-labeling when the tissue was co-stained with Arc was infeasible.
A recent whole-brain clearing and imaging technique, uDISCO, champions the use of tert-Butanol as a dehydration solvent for preserving endogenous fluorescence from reporter proteins (Pan et al., 2016). We piloted this technique as well to determine if endogenous EYFP fluorescence is sufficiently preserved to allow detection of EYFP+ cells and if this clearing technique can be combined with an immunolabeling step to allow immunolabeling and detection of c-Fos+ cells. However, in the ArcCreERT2 × ChR2 mice, both endogenous EYFP fluorescence preservation and immunolabeling of c-Fos+ cells were unsatisfactory compared to CUBIC-based clearing, further strengthening our conclusion that protocols based on organic solvent clearing are not compatible with our mouse line.
The original CUBIC protocol preserves endogenous EYFP fluorescence in the HPC and cortex of the ArcCreERT2 line better than iDISCO. However, this protocol does not permit satisfactory Arc immunolabeling. The CUBIC with Reagent-1A protocol utilizes a modified hyperhydrating aqueous clearing solution, which preserves both endogenous EYFP and permits better Arc immunolabeling across the mm-thick tissue section, but labeling was not uniform. Difficulty with the CUBIC with Reagent-1A protocol has also been reported by other groups (Kubota et al., 2017), who have likewise improved delipidation efficiency in whole organs samples with a CUBIC-based reagent cocktail optimized for imaging and analysis of cancer cells in these samples.
To further improve Arc immunolabeling, we modified the CUBIC with Reagent-1A protocol, utilizing an Alexa Fluor 647 instead of a Cy3 based secondary antibody and longer incubation periods; we term this modified protocol CUBIC with Reagent-1A*. This protocol improves the depth and uniformity of endogenous EYFP and Arc immunolabeling and also permits c-Fos immunolabeling, thus providing a satisfactory clearing technique that is compatible with the ArcCreERT2 line. We hypothesized that the Alexa secondary antibody has better tissue diffusion than the Cy3 secondary antibody, improving the Arc immunolabeling with depth. Alexa Fluor 647 also has longer fluorescence excitation and emission compared to Cy3, allowing for deeper light propagation in thick tissue.
The higher dilution factor of the primary antibody used in the original CUBIC with Reagent-1A protocol may have exaggerated the negative effects of the ArcCreERT2 line's dense cortical labeling, causing a less uniform label. Thus, the lower primary antibody concentration in CUBIC with Reagent-1A* may account for the more uniform labeling of Arc. Finally, increasing the incubation periods for the primary and secondary antibody immunolabeling steps from 3 days to 5 days allowed the Arc antibody more time to completely and uniformly penetrate the tissue. Compared to other proposed methods, CUBIC with Reagent-1A* offers greater depth access (∼2 mm below surface) and more reliable Arc and c-Fos immunolabeling with endogenous EYFP labeling.
In summary, we have modified the CUBIC with Reagent-1A protocol to develop a clearing method optimized for endogenous EYFP fluorescence and co-staining with Arc or c-Fos in mm-thick tissue sections from the ArcCreERT2 mouse model. For experimental needs that require EYFP immunolabeling, we have also improved the iDISCO protocol with the use of a GFP nanobody for satisfactory GFP and Arc or c-Fos co-staining in the ArcCreERT2 line. Together, our modified clearing methods offer compatibility with a variety of specific experimental needs and ultimately enable the study of memory traces in intact mm-thick brain tissue. Combining the unique brain-wide insights offered by CUBIC and iDISCO with the uniquely long timescale permitted by the ArcCreERT2 indelible system establishes a novel and functional approach to studying memory via memory trace maps and cross-brain circuits. Such studies will be critical to characterizing many neuropathologies, injuries, and disorders, including the disturbances associated witdh aging and Alzheimer's disease, anterograde and retrograde amnesia, and post-traumatic stress disorder.
Supplementary Material
Acknowledgments
We greatly thank Drs. Nicolas Renier, Zhuaho Wu, and Marc Tessier-Lavigne at Rockefeller University for assistance with iDISCO. We thank members of the Denny laboratory for insightful comments on this project and manuscript, Sean C. Lim for assistance with genotyping and maintenance of mice, and Josephine C. McGowan for technical assistance early in the project.
Funding Information: I.P.P. was supported by a NYSTEM C-029157. S.C.S. was supported by the Columbia University I.I. Rabi Scholars Program award, a Columbia University Summer Undergraduate Research Fellowship award, and a Columbia University Work Exemption Program award. M.L. was supported by Medical Scientist Training Program 4T32GM007367. R.H. was supported by a NYSTEM C-029157. C.A.D. was supported by a NIH DP5 OD017908, a NARSAD Young Investigator Grant from the Brain and Behavior Research Foundation (P&S Fund Investigator), a NYSTEM C-029157, a Research Initiatives for Science and Engineering (RISE) grant, and a Robert N. Butler Columbia Aging Center of Columbia University Faculty Research Fellowship.
Footnotes
Supporting Information: Additional Supporting Information may be found online in the supporting information tab for this article.
Competing Financial Interests: There are no competing financial interests or conflicts of interest.
References
- Belle M, Godefroy D, Dominici C, Heitz-Marchaland C, Zelina P, Hellal F, et al. Chédotal1 A. A Simple Method for 3D Analysis of Immunolabeled Axonal Tracts in a Transparent Nervous System. Cell Reports. 2014;9:1191–1201. doi: 10.1016/j.celrep.2014.10.037. [DOI] [PubMed] [Google Scholar]
- Bonnici HM, Chadwick MJ, Maguire EA. Representations of recent and remote autobiographical memories in hippocampal subfields. Hippocampus. 2013;23:849–854. doi: 10.1002/hipo.22155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazzulino AS, Martinez R, Tomm NK, Denny CA. Improved specificity of hippocampal memory trace labeling. Hippocampus. 2016;26:752–762. doi: 10.1002/hipo.22556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung K, Wallace J, Kim SY, Kalyanasundaram S, Andalman AS, Davidson TJ, et al. Deisseroth K. Structural and molecular interrogation of intact biological systems. Nature. 2013;497:332–337. doi: 10.1038/nature12107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denny CA, Kheirbek MA, Alba EL, Tanaka KF, Brachman RA, Laughman KB, et al. Hen R. Hippocampal memory traces are differentially modulated by experience, time, and adult neurogenesis. Neuron. 2014;83:189–201. doi: 10.1016/j.neuron.2014.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epp JR, Niibori Y, Hsiang L, Mercaldo V, Deisseroth K, Josselyn SA, Frankland PW. Optimization of CLARITY for Clearing Whole-Brain and Other Intact Organs. eNeuro. 2015;2:1–15. doi: 10.1523/ENEURO.0022-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ertürk A, Becker K, Jährling N, Mauch CP, Hojer CD, Egen JG, et al. Dodt HU. Three-dimensional imaging of solvent-cleared organs using 3DISCO. Nature Protocols. 2012;7:1983–1995. doi: 10.1038/nprot.2012.119. [DOI] [PubMed] [Google Scholar]
- Ertürk A, Lafkas D, Chalouni C. Imaging Cleared Intact Biological Systems at a Cellular Level by 3DISCO. Journal of Visualized Experiments. 2014:1–13. doi: 10.3791/51382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridy PC, Li Y, Keegan S, Thompson MK, Nudelman I, Scheid JF, et al. Rout MP. A robust pipeline for rapid production of versatile nanobody repertoires. Nature Methods. 2014;11:1253–1260. doi: 10.1038/nmeth.3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hama H, Kurokawa H, Kawano H, Ando R, Shimogori T, Noda H, et al. Miyawaki A. Scale: a chemical approach for fluorescence imaging and reconstruction of transparent mouse brain. Nature Neuroscience. 2011;16:1481–1488. doi: 10.1038/nn.2928. [DOI] [PubMed] [Google Scholar]
- Hama H, Hioki H, Namiki K, Hoshida T, Kurokawa H, Ishidate F, et al. Miyawaki A. ScaleS: an optical clearing palette for biological imaging. Nature Neuroscience. 2015;18:1518–1529. doi: 10.1038/nn.4107. [DOI] [PubMed] [Google Scholar]
- Herskovits TT, Gadegbeku B, Jaillet H. On the Structural Stability Denaturation of Proteins. Journal of Biological Chemistry. 1970;245:2588–2598. [PubMed] [Google Scholar]
- Ke MT, Fujimoto S, Imai T. SeeDB: a simple and morphology-preserving optical clearing agent for neuronal circuit reconstruction. Nature Neuroscience. 2013;16:1154–1161. doi: 10.1038/nn.3447. [DOI] [PubMed] [Google Scholar]
- Kim Y, Venkataraju KU, Pradhan K, Mende C, Taranda J, Turaga SC, … OstenP. Mapping Social Behavior-Induced Brain Activation at Cellular Resolution in the Mouse. Cell Reports. 2015;10:292–305. doi: 10.1016/j.celrep.2014.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota SI, Takahashi K, Nishida J, Morishita Y, Ehata S, Tainaka K, et al. Ueda HR. Whole-Body Profiling of Cancer Metastasis with Single-Cell Resolution. Cell Reports. 2017;20:236–250. doi: 10.1016/j.celrep.2017.06.010. [DOI] [PubMed] [Google Scholar]
- Kuwajima T, Sitko AA, Bhansali P, Jurgens C, Guido W, Mason C. ClearT: a detergent- and solvent-free clearing method for neuronal and non-neuronal tissue. Development. 2013;140:1364–1368. doi: 10.1242/dev.091844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebmann T, Renier N, Bettayeb K, Greengard P, Tessier-Lavigne M, Flajolet M. Three-Dimensional Study of Alzheimer's Disease Hallmarks Using the iDISCO Clearing Method. Cell Reports. 2016;16:1138–1152. doi: 10.1016/j.celrep.2016.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madisen L, Mao T, Koch H, Zhuo JM, Berenyi A, Fujisawa S, et al. Zeng H. A toolbox of Cre-dependent optogenetic transgenic mice for light-induced activation and silencing. Nature Neuroscience. 2012;15:793–802. doi: 10.1038/nn.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray E, Cho JH, Goodwin D, Ku T, Swaney J, Kim SY, et al. Chung K. Simple, Scalable Proteomic Imaging for High-Dimensional Profiling of Intact Systems. Cell. 2015;163:1500–1514. doi: 10.1016/j.cell.2015.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan C, Cai R, Quacquarelli FP, Ghasemigharagoz A, Lourbopoulos A, Matryba P, et al. Ertürk A. Shrinkage-mediated imaging of entire organs and organisms using uDISCO. Nature Methods. 2016;13:859–867. doi: 10.1038/nmeth.3964. [DOI] [PubMed] [Google Scholar]
- Peron PP, Freeman J, Iyer V, Guo C, Svoboda K. A Cellular Resolution Map of Barrel Cortex Activity during Tactile Behavior. Neuron. 2015;86:736–799. doi: 10.1016/j.neuron.2015.03.027. [DOI] [PubMed] [Google Scholar]
- Renier N, Wu Z, Simon DJ, Yang J, Ariel P, Tessier-Lavigne M. iDISCO: A Simple, Rapid Method to Immunolabel Large Tissue Samples for Volume Imaging. Cell. 2014;159:896–910. doi: 10.1016/j.cell.2014.10.010. [DOI] [PubMed] [Google Scholar]
- Renier N, Adams EL, Kirst C, Wu Z, Azevedo R, Kohl J, et al. Tessier-Lavigne M. Mapping of Brain Activity by Automated Volume Analysis of Immediate Early Genes. Cell. 2016;165:1789–1802. doi: 10.1016/j.cell.2016.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz MK, Scherbarth A, Sprengel R, Engelhardt J, Theer P, Giese G. Fluorescent-Protein Stabilization and High-Resolution Imaging of Cleared, Intact Mouse Brains. PLoS One. 2015;10:e0124650. doi: 10.1371/journal.pone.0124650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susaki EA, Tainaka K, Perrin D, Kishino F, Tawara T, Watanabe TM, et al. Ueda HR. Whole-brain imaging with single-cell resolution using chemical cocktails and computational analysis. Cell. 2014;157:726–739. doi: 10.1016/j.cell.2014.03.042. [DOI] [PubMed] [Google Scholar]
- Susaki EA, Tainaka K, Perrin D, Yukinaga H, Kuno A, Ueda HR. Advanced CUBIC protocols for whole-brain and whole-body clearing and imaging. Nature Protocols. 2015;10:1709–1727. doi: 10.1038/nprot.2015.085. [DOI] [PubMed] [Google Scholar]
- Susaki EA, Ueda HR. Whole-body and Whole-Organ Clearing and Imaging Techniques with Single-Cell Resolution: Toward Organism-Level Systems Biology in Mammals. Cell Chemical Biology. 2016;23:137–157. doi: 10.1016/j.chembiol.2015.11.009. [DOI] [PubMed] [Google Scholar]
- Tayler KK, Tanaka KZ, Reijmers LG, Wiltgen BJ. Reactivation of neural ensembles during the retrieval of recent and remote memory. Current Biology. 2013;23:99–106. doi: 10.1016/j.cub.2012.11.019. [DOI] [PubMed] [Google Scholar]
- Tomer R, Ye L, Hsueh B, Deisseroth K. Advanced CLARITY for rapid and high-resolution imaging of intact tissues. Nature Protocols. 2014;9:1682–1697. doi: 10.1038/nprot.2014.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, He X, Zhao Z, Feng Q, Lin R, Sun Y, et al. Zhan C. Whole-brain mapping of the direct inputs and axonal projections of POMC and AgRP neurons. Frontiers in Neuroanatomy. 2015;9:1–17. doi: 10.3389/fnana.2015.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiltgen BJ, Sanders MJ, Anagnostaras SG, Sage JR, Fanselow MS. Context fear learning in the absence of the hippocampus. Journal of Neuroscience. 2006;26:5484–5491. doi: 10.1523/JNEUROSCI.2685-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiltgen BJ, Silva AJ. Memory for context becomes less specific with time. Learning and Memory. 2007;14:313–317. doi: 10.1101/lm.430907. [DOI] [PubMed] [Google Scholar]
- Wiltgen BJ, Zhou M, Cai Y, Balaji J, Karlsson MG, Parivash SN, et al. Silva AJ. The hippocampus plays a selective role in the retrieval of detailed contextual memories. Current Biology. 2010;20:1336–1344. doi: 10.1016/j.cub.2010.06.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B, Treweek JB, Kulkarni RP, Deverman BE, Chen CK, Lubeck E, et al. Gradinaru V. Single-cell phenotyping within transparent intact tissue through whole-body clearing. Cell. 2014;158:945–958. doi: 10.1016/j.cell.2014.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


