Abstract
The testis is an organ that maintains an immune suppressive environment. We previously revealed that exposure of pre-pubertal rats to an acute dose of a well-described Sertoli cell toxicant, mono-(2-ethylhexyl) phthalate (MEHP), leads to an accumulation of CD11b+ immune cells in the testicular interstitial space that closely correlates with a robust incidence of germ cell (GC) apoptosis. Here we test the hypothesis that the infiltrating immune cells contribute to GC apoptosis. Postnatal day 28 Fischer rats that received an oral dose of 700 mg/kg MEHP showed a significant infiltration of both CD11bc+/CD68+/CD163- macrophages and neutrophils. The infiltration peaked at 12 hours, but had reduced by 48 hours. Testicular macrophages from MEHP-treated rats showed significantly upregulated expression of Tnfa and Il6, and the Arg1/Nos2 ratio was reduced compared to controls. However, small increases in anti-inflammatory genes Il10 and Tgfb1 were also observed. Depletion of circulating monocytes with clodronate liposomes prior to MEHP-treatment reduced the macrophage influx into the testis, but did not lower GC apoptosis. Additionally, depletion of neutrophils using an anti-polymorphonuclear cell antibody prevented both macrophage and neutrophil infiltration into the testis, but also did not affect GC apoptosis. Together, these results show that exposure to MEHP leads to a rapid and temporary influx of pro-inflammatory monocytes and neutrophils in the interstitium of the testis. However, with this acute dosing paradigm, these infiltrating leukocytes do not appear to contribute to MEHP-induced testicular GC apoptosis leaving the functional significance of these infiltrating cells in the pathogenesis of MEHP-induced testicular injury unresolved.
3. Introduction
The testis has a specialized immune suppressed environment in order to protect developing antigenic haploid germ cells (GCs) from immune recognition. This “immune privilege” state is created through both testicular structure, including the blood testis barrier (BTB) (Li et al., 2012; 2016), and by immunosuppressive signals produced by testicular cells. For example, Leydig cells produce testosterone, which has been shown to suppress the development of autoimmune orchitis in rats (Fijak et al., 2011), and Sertoli cells (SCs) produce immunosuppressive signals such as activins (Meehan et al., 2000; Hedger and Winnall, 2012), TGF-β (Avallet et al., 1994), SerpinG1/E1 (Doyle et al., 2012), and Jagged1 (Campese et al., 2014), and can survive as allografts as well as protect co-transplanted allogeneic and xenogeneic cells from rejection (reviewed in (Mital et al., 2010)). Additionally, corticosterone present in the interstitial fluid has been shown to contribute to the immunosuppressive environment by mediating testicular macrophage polarization (Wang et al., 2017).
Despite the immunosuppressive environment of the testis, immune cells do reside in the interstitial space of the testis. Of these cells, macrophages are the most predominant making up approximately 25% of interstitial cells (Niemi et al., 1986), with lower numbers of dendritic cells, mast cells, and T cells also present (Zhao et al., 2014). Testicular macrophages have been shown to play a key supportive role in the immune tolerant environment within the testis. These macrophages constitutively produce anti-inflammatory cytokines such as IL-10 (Winnall et al., 2011), and have a limited response to inflammatory stimuli (Bhushan et al., 2011; 2015). They respond to both classical (IFN-γ + LPS) and alternative (IL-4) activation by increasing expression of anti-inflammatory cytokines such as IL-10 and TGF-β 1 (Kern et al., 1995; Winnall et al., 2011; Bhushan et al., 2015). In the rat, these immune tolerant macrophages are generally recognized by their expression of the alternative activation marker CD163 (ED2) (Wang et al., 1994). However, some macrophages lacking CD163 expression are also present in the testis. These are generally considered to be pro-inflammatory and it is hypothesized that these are newly-recruited monocytes from the circulation (Fijak et al., 2016). Recent studies in mice have shown that there are two spatial locations of macrophages in the testis; interstitial macrophages which are found in the interstitial space, and peritubular macrophages, which are found lying on the surface of the seminiferous tubules. The latter were hypothesized to contribute to the spermatogonial niche (DeFalco et al., 2015).
In conditions of testicular inflammation, the immunosuppressive environment of the testis is disrupted by increased pro-inflammatory cytokine signaling and/or an increase in leukocytes in the interstitium. For instance, increases in pro-inflammatory type macrophages or neutrophils are seen in response to exposure to lipopolysaccharide (LPS), testicular torsion repair, GC homogenate, spondyloarthritis, and also in clinical analysis of human male infertility (Lysiak et al., 2001; Gerdprasert et al., 2002; Hussein et al., 2005; Rival et al., 2008; Taurog et al., 2012). In many of these models, these immunoreactive macrophages or neutrophils have been implicated in infertility or disruption of spermatogenesis that occurs simultaneously. For example, in models of experimental autoimmune orchitis or testicular inflammation, spermatogenesis is protected when either monocytes are depleted or the effector molecules from pro-inflammatory macrophages are blocked (Rival et al., 2008; Jarazo-Dietrich et al., 2015). Furthermore, neutropenic and E-selectin-deficient mice failed to stimulate GC apoptosis in response to testicular torsion (Lysiak et al., 2001). Accumulation of immunoreactive macrophages has also been shown to be responsible for tissue damage in other organs, such as lungs (Pendino et al., 1993; Redente et al., 2010) and liver (Laskin et al., 1995).
Recently, we reported an age- and species-dependent influx of CD11b+ immune cells into the testicular interstitium after pre-pubertal rodent exposure to mono-(2-ethylhexyl) phthalate (MEHP) (0.7g/kg) which coincided with high levels of GC apoptosis (Murphy et al., 2014) and increased IL-1α and IL-6 expression in the seminiferous tubules (Stermer et al., 2017). This influx of immune cells was specific for pre-pubertal aged rats, as adult rats (PND56) or pre-pubertal mice (PND21) did not demonstrate immune cell infiltration. Additionally, adult rats and pre-pubertal mice both presented with low levels of GC apoptosis, suggesting a possible functional connection between the infiltrating immune cells and GC apoptosis.
Here we test the hypothesis that MEHP exposure leads to an influx of pro-inflammatory immune cells that directly contribute to the extent of GC apoptosis. Unlike other models of testicular inflammation, our findings indicate that although MEHP exposure of pre-pubertal rats leads to a rapid but temporary influx of neutrophils and pro-inflammatory macrophages, depletion of monocytes or neutrophils from the circulation did not reduce the levels of GC apoptosis. These results suggest that these two pathological events (GC apoptosis and leukocyte infiltration) triggered by MEHP exposure are largely independent.
4. Materials and Methods
4.1. Animals and MEHP treatment
Male Fischer CDF344 rats were purchased from Charles River (Wilmington MA, USA). Animals were maintained in a controlled temperature (22°C± 0.5°C) and lighting (12L:12D) environment and allowed to acclimate for 1 week before experimental procedures. Standard laboratory chow (Purina Mills LabDiet #5LL2, St. Louis, MO, USA) and water were supplied ad libitum. All animal procedures were performed in accordance with the guidelines and approval of The University of Texas at Austin’s Institutional Animal Care and Use Committee, which abides by NIH Publications NO. 8023, revised 1978. Exact age PND 27 male Fischer F344 rats were treated with a single oral dose of MEHP (0.7g/kg in corn oil, per os (p.o.); 97.3% purity; Wako Chemicals, Richmond, VA, USA) or equivalent volume of vehicle (corn oil, 2ml/kg, p.o.). For macrophage depletion studies, an i.p. injection of 0.4ml clodronate liposomes (Liposoma B.V., Amsterdam, the Netherlands) or PBS liposomes (Liposoma B.V.) or phosphate buffered saline (PBS; Thermo Fisher Scientific, Waltham, MA, USA) was given at 48, 24 and 0 hours (hrs) prior to MEHP treatment. For neutrophil depletion studies, an i.v. tail vein injection of polyclonal rabbit anti-rat polymorphonuclear neutrophil (anti-PMN) antibody (0.2ml at 5.0 mg/ml CLAG51140; Cedarlane Labs, Burlington, NC, USA; or 0.2ml PBS for control) was given at 24 and 0 hr before MEHP treatment. At 3, 6, 12, 24, and 48 h after MEHP treatment, rats (at least 3 animals/time point/treatment) were anesthetized using a ketamine (1 mg/10g body weight; Animal Health International, Greeley, CO, USA) and xylazine (0.1mg/10g body weight; Animal Health International) cocktail and perfused with phosphate buffered saline and heparin (10 units/ml; Thermo Fisher Scientific) until complete exsanguination. The testes of the rats were removed, weighed, and testes were snap frozen in liquid nitrogen and stored at −80°C (for immunofluorescence staining and RNA extraction), fixed in Bouin’s fixative (Fisher Scientific; for MPO, H&E, and TUNEL staining), or used for interstitial cell collection as described below.
4.2. Interstitial Cell Collection and Flow Cytometry
Cells from the interstitial space of rat testis were collected as previously described (Murphy, 2014). Briefly, one testis was decapsulated and seminiferous tubules were gently teased apart using fine forceps in Dulbecco-modified Eagle medium (DMEM; Thermo Fisher Scientific) supplemented with 0.1% collagenase (Sigma-Aldrich, St. Louis, MO, USA). Single cell suspensions were obtained by filtering through a 70μm cell strainer. Cells were counted using a hemocytometer, and stained with CD11bc-APC (1:200 dilution; clone OX-42; BioLegend, San Diego, CA, USA), CD163-RPE (1:20 dilution; MCA342PE; Bio-Rad, Hercules, CA, USA) and rabbit anti-rat PMN (1:2000 dilution), followed by staining with Alexa Fluor 488 goat anti-rabbit IgG (H+L) antibody (Thermo Fisher Scientific, A11008, 1:500 dilution). Cells were then fixed and permeabilized (Biolegend kit) before staining for CD68-APC-Vio770 (Miltenyi Biotec, Aubern, CA, USA, clone REA237, 1:20 dilution). Cell staining was analyzed on the BD LRSFortessa flow cytometer with BD FACSDiva software (version 6.1.3) and interpreted with FlowJo software (version 10.1). 50,000 events were collected for each sample, and cell debris was excluded from the analysis following the gating strategy. (data not shown). The total number of stained cells was determined for each rodent by taking the percentage of stained cells and multiplying it by the number of interstitial cells counted.
4.3. Histology
Tissue sections (5μm) were stained with standard hematoxylin and eosin or were used in immunohistochemistry (IHC). Following heat-treatment in a sodium citrate-based antigen retrieval solution, Bouin’s fixed, paraffin-embedded tissue sections were incubated with 3% hydrogen peroxidase (Thermo Fisher Scientific) to block endogenous peroxidase activity and then incubated in blocking buffer (10% normal horse serum (Sigma-Aldrich) and 0.3% Triton X-100 (Sigma-Aldrich) in PBS). Sections were probed with polyclonal antibody (Ab) to myeloperoxidase (1:100 dilution; PA5-16672; Thermo Fisher Scientific) in 2% normal horse serum and 0.3% Triton X-100 in PBS. Immunhistochemical (IHC) staining was detected by standard procedure using VectaStain ABC kit (Vector Laboratories, Burlingame, CA, USA) and 3,3’-diaminobenzidine (Vector Laboratories) with hematoxylin counterstain and mounted using Cytoseal 60 (Thermo Fisher Scientific). Images were acquired using a Nikon Eclipse microscope, with a x20 objective and a Nikon DS-Fi1 camera and NIS Elements software (version 3.22). The number of positively-stained cells were counted for five randomly chosen images per animal and the mean was calculated.
For CD68 and CD163-staining of frozen sections, cross-sections (6μm) of frozen rat testes embedded in Optimal Cutting Temperature compound (OCT; Tissue-Tek, Electron Microscopy Sciences, Hatfield, PA, USA) were mounted on Superfrost Plus glass slides and air-dried. Frozen sections were washed in PBS, then fixed in acetone at −20°C for 2 min. Sections were incubated with 3% hydrogen peroxidase to block endogenous peroxidase activity and then incubated in blocking buffer (PBS supplemented with 10% horse serum). Sections were incubated with mouse anti-mononuclear phagocyte monoclonal Ab (1:200 dilution; clone IC7; BD Biosciences, San Jose, CA, USA), or mouse anti-rat CD68 (1:200 dilution, MCA341R, Bio-Rad), or mouse anti-CD163 monoclonal Ab (1:200 dilution; clone ED2; Thermo Fischer Scientific) for 1hr at room temperature. Sections were then incubated in Alexa Fluor 488 anti-mouse antibody (1:500 dilution; A11011; Thermo Fisher Scientific) for 1 hr and mounted with Vectashield Mounting Medium (Vector Laboratories). Fluorescent and differential interference contrast (DIC) images were acquired using a Nikon Eclipse microscope, with a 10x objective and captured with Nikon Cool-SNAP digital camera. Images were processed and analyzed using NIS Elements software. The number of positively-stained cells were counted for four randomly chosen images per animal and the mean was calculated.
4.4. TUNEL assay
The presence of apoptotic fragmentation of DNA in frozen or paraffin-embedded testis cross-sections was determined by terminal deoxynucleotidyl Transferase-Mediated Digoxigenin-dUTP Nick End Labeling (TUNEL) analysis using the ApopTag kit (EMD Millipore, Burlington, MA, USA). The apoptotic index (AI) was calculated as the percentage of essentially round seminiferous tubules containing more than three TUNEL-positive GCs in each cross section, in accordance with previous studies (Yao et al., 2007; 2009; Lin et al., 2010). For each rat, at least 100 seminiferous tubules were analyzed.
4.5. Ex vivo isolation and primary cell culture of testicular macrophages
Interstitial cells were collected from both testes of untreated or 12 hour MEHP-treated PND 28 Fisher rats in the manner described for flow cytometry above. For cell culture, interstitial cells were suspended in DMEM supplemented with 1% penicillin/ streptomycin (TM media), seeded at 1×106 cells/well in 6-well plates (at 2mL media per well), and incubated at 37°C, 5% CO2, for 1-2 hrs. For interstitial cells from untreated animals, cells from 2 or 3 animals were combined. After 1-2 hrs, media and non-adherent cells were aspirated, and cells were either washed with PBS and lysed for RNA extraction, or further cultured for in vitro MEHP treatment as described below. The purity of the macrophages is routinely tested and is >80% pure (data not shown).
4.6. In vitro MEHP treatment
Cell cultures were treated with 200 μM MEHP diluted in dimethyl sulfoxide (DMSO; final concentration 0.04%) or equivalent volume of DMSO only in TM media, for 3, 6, or 12 hrs. The viability of MEHP-treated cells was evaluated using the trypan blue dye exclusion method to determine the appropriate dosage. At each time point cells were washed with PBS, lysed and RNA was extracted as described below.
4.7. RNA extraction and real-time qPCR
Tissue RNA was isolated using lysis buffer from PureLink RNA mini kit (Thermo Fisher Scientific) per manufacturer’s protocol. RNA quantity and quality were assessed using a ThermoScientific Nanodrop 1000. Extracted RNA was DNase treated using Amplification Grade DNase 1 (Sigma-Aldrich) and cDNA was generated from 500ng of total RNA with Multiscribe reverse transcriptase and random hexameters (Thermo Fisher Scientific) into a 25μl reaction volume. Real-time qPCR was performed in triplicates in a Bio-Rad C1000 Thermal cycler system using iTaq Universal SYBR GreenSupermix (Bio-Rad) and gene-specific primers. Primers were ordered from Sigma-Aldrich and are listed in Table 1. Relative mRNA expression for genes of interest was assessed in comparison with control samples, following normalization to the reference genes α-tubulin (Tuba1), β2 microglobulin (B2m), and β-actin (Actb) using the ΔΔCT method as described in Applied Biosystems User Bulletin No. 2 (P/N 4303859) (Livak and Schmittgen, 2001). Appropriateness of reference genes was tested using NormFinder (version 20) (Andersen et al., 2004). The relative quantification data are presented in graphs showing fold increase expression ratio relative to control samples with error bars corresponding to the S.E.M. Statistical analysis was performed on biological replicates.
Table 1. Sequences of rat primers used in real-time qPCR.
Gene symbols, Genbank IDs, forward (F) and reverse (R) oligonucleotide sequences and amplicon size in base pairs (bp) are shown.
| Gene | Genbank accession | Primer sequence (5’ ➞ 3’) | Amplicon size (bp) |
|---|---|---|---|
| Actb | NM_031144.3 | F: GCAGGAGTACGATGAGTCCG R: ACGCAGCTCAGTAACAGTCC |
74 |
| Arg1 | NM_017134.3 | F: ACAAGACAGGGCTACTTTCAGG R: ACAAGACAAGGTCAACGCCA |
116 |
| B2m | NM_012512 | F: GCCTGTGTGCGGTTTTCATC R: CCCTTTCCTCTGGACCCTTG |
95 |
| Ccl5 | NM_031116.3 | F: ATATGGCTCGGACACCACTC R: GCGGTTCCTTCGAGTGACAA |
136 |
| Cxcl1 | NM_030845.1 | F: CCACACTCAAGAATGGTCGC R: ACTTGGGGACACCCTTTAGC |
93 |
| Cxcl6 | NM_022214.1 | F: CATTGCCCCAAGGTGGAAGT R: GCAAGTGCATTCCGCTTTGTT |
143 |
| Cxcl10 | NM_139089.1 | F: TGCAAGTCTATCCTGTCCGC R: CTCTGCTGTCCATCGGTCTC |
190 |
| Il6 | NM_012589.2 | F: TTCTCTCCGCAAGAGACTTCC R: TCTCCTCTCCGGACTTGTGAA |
94 |
| Il10 | NM_012854.2 | F: TTGAACCACCCGGCATCTAC R: CCAAGGAGTTGCTCCCGTTA |
91 |
| Nos2 | NM_012611.3 | F: GGGGACTGGACTTTTAGAGACG R: TCCGTGGGGCTTGTAGTTGA |
80 |
| Tgfb1 | NM_021578.2 | F: CAATTCCTGGCGTTACCTTGG R: CCCTGTATTCCGTCTCCTTGG |
120 |
| Tnfa | NM_012675.3 | F: ATGGGCTCCCTCTCATCAGT R: GCTTGGTGGTTTGCTACGAC |
106 |
| Tuba1a | NM_022298.1 | F: TCACAAGGTGCTGCTTTCAC R: GCTCGGGTCTCTGACAAATCA |
142 |
4.8. Protein Extraction and CXCL1 ELISA
Whole testis tissue was homogenized in 500μl RIPA buffer supplemented with protease inhibitors (Pierce, 88666) and incubated on ice for 30 min. Tissue extracts were cleared by sequential centrifugation at 18,000g for 20 min at 4°C. Protein concentration was determined using the Pierce BCA Protein Assay Kit (Thermo Fisher Scientific). CXCL1 concentrations of 2.5μg protein were measured using the Rat CXCL1/CINC-1 ELISA kit (R&D Systems, Minneapolis, MN, USA, RCN100) as per the manufacturer’s protocol.
4.9. Statistical Analysis
Results from experiments are presented as individual data points and means ± S.E.M. Each replicate represents a biological replicate and the number of replicates is indicated in each figure. Data analysis was performed using Prism software (version 6; GraphPad, La Jolla, CA, USA). The statistical tests and appropriate post hoc tests used on the data which were assumed to be normally distributed are indicated in the figure legends. P-values of less than 0.05 were considered statistically significant.
5. Results
5.1. MEHP induces an influx of both macrophages and neutrophils to the testicular interstitium
Pre-pubertal rats (PND27) were exposed to 0.7g/kg MEHP (p.o.) and testis interstitial cells were analyzed at 12, 24, and 48 hrs for expression of CD11b, CD68 (pan-macrophage marker) and CD163 using flow cytometry. MEHP exposure led to a significant increase in inflammatory (CD11bc+/CD68+/CD163-) macrophages, as well as other leukocytes (CD11bc+/CD68- cells) (Figure 1, A-C). The influx peaked at 12 hrs, remained significantly elevated by 24 hours, and returned to near control levels after 48 hrs. Flow cytometry analysis of the CD11bc+/CD68+/CD163+ macrophages did not indicate an increase in their number, but a significant increase in the expression of CD163 by these macrophages was measured by flow cytometry at both 12 and 24 hrs (Fig 1, B & D). Staining of testis cross-sections for CD68 and CD163 showed similar increases in CD68-expressing cells, although a small, yet significant increase of both CD68+ and CD163+ macrophages numbers were also observed 48 hrs after MEHP exposure (Figure 1, E-F).
Figure 1. MEHP exposure induces an influx of CD11bc+/CD68+/CD163- macrophages and CD11bc+/CD68-negative immune cells into the testicular interstitium.
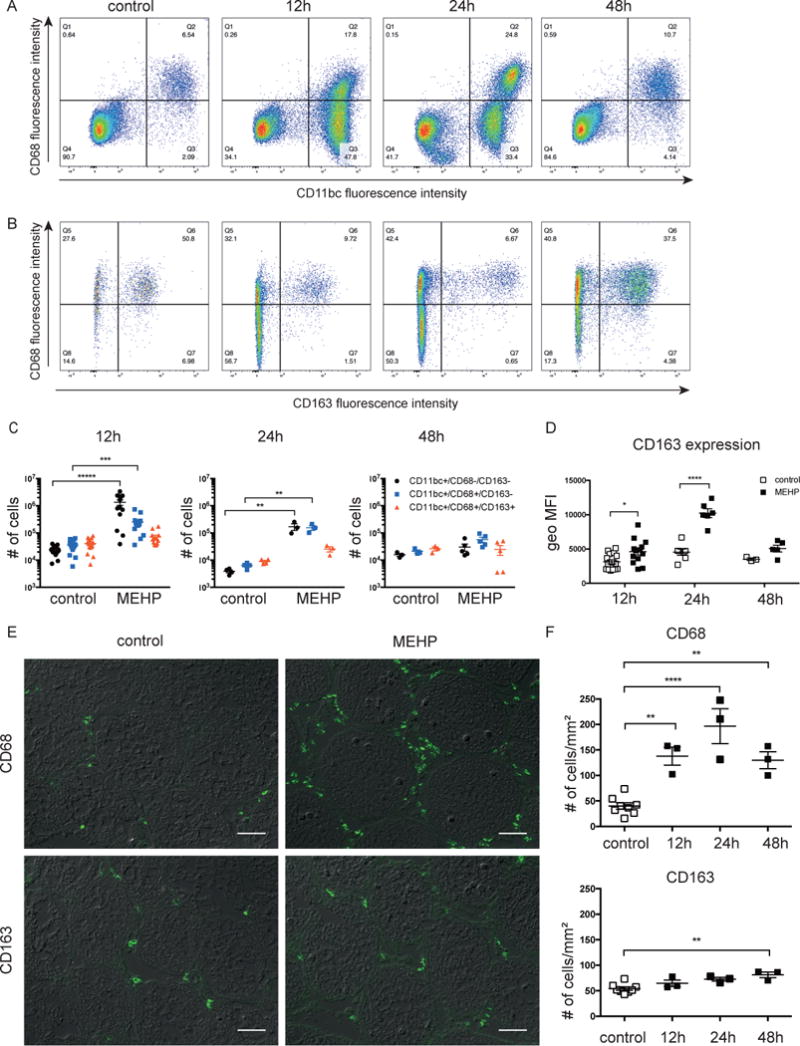
Expression of CD11bc, CD68 and CD163 by interstitial cells was measured 12, 24, and 48 hrs after MEHP treatment (0.7 g/kg, p.o.) or corn oil control. Flow plots indicating staining for (A) CD68 and CD11bc and (B) CD68 and CD163 of CD11bc-positive cells after MEHP treatment or control. Gates indicate the percentage of cells that are double-positive, single-positive, or unstained. Flow plots are representative of n ≥ 3 for each treatment. (C) Bar chart indicating the total number of CD11bc-expressing cells that are negative, single-positive, or double-positive for CD68 and CD163 expression. (D) Bar chart indicating the geometric mean fluorescence intensity (geo MFI) of CD163 cell surface expression of CD11bc+/CD68+/CD163+ cells. (C-D) Statistical analysis was performed by comparing each group of cells with those of control rats for each time point using the unpaired t-test and corrected for multiple comparisons using the Holm-Sidak method. (E) Immunofluorescent detection of cells labelled with anti-CD68 or anti-CD163 antibody in testicular cross sections 12hrs after exposure to MEHP. Images show immunofluorescence overlaid on differential interference contrast (DIC) images and are representative of n ≥ 3 for each treatment. Scale bars = 50μm. (F) Quantification of CD68-positive and CD163-positive cells from immunofluorescent images. Individual data points are shown and means ± S.E.M for n≥3. Statistical analysis was performed using one-way ANOVA and Tukey’s multiple comparisons test. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001.
To identify the CD11bc+/CD68- leukocytes that infiltrate into the testis after MEHP exposure, Hematoxylin and Eosin (H&E) staining was performed on testis cross-sections of control and MEHP-treated (0.7 g/kg, p.o.) rats at 12, 24 and 48 hrs after exposure. A large number of polymorphonuclear cells, highly characteristic of neutrophils, was observed to have accumulated in the interstitium of the MEHP-treated animals (Fig 2A). To confirm that these were indeed neutrophils, additional tissue sections were stained with myeloperoxidase (MPO), which is abundantly expressed in neutrophil granules (Kinkade et al., 1983) (Fig 2B). Quantification of MPO-positive cells showed that neutrophil expression was significantly increased at both 12 and 24hrs after MEHP treatment (Fig 2, B-C).
Figure 2. MEHP exposure also induces neutrophil infiltration into the testis.
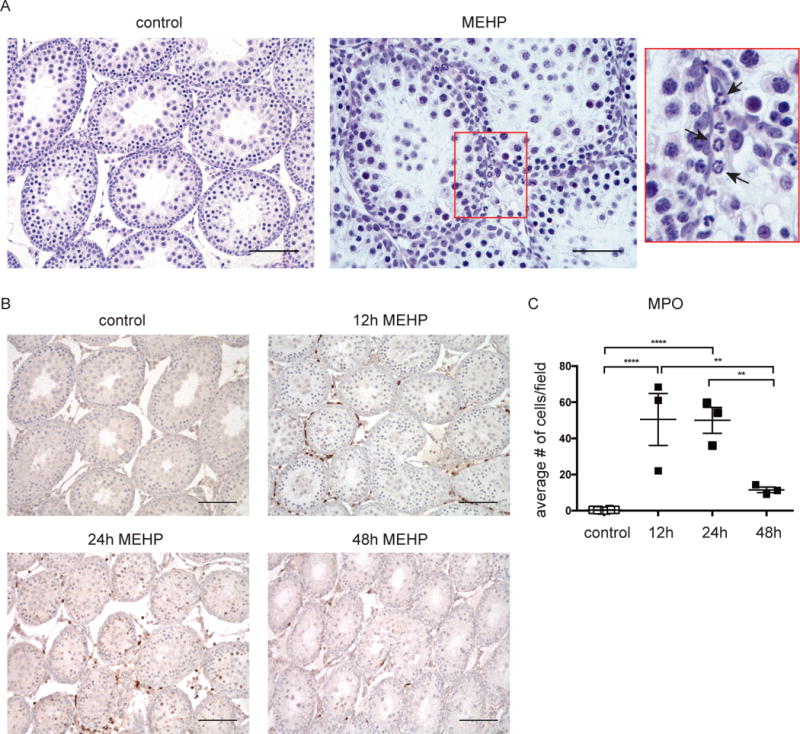
(a) Representative images of (A) hematoxylin and eosin staining at 12h and (B) myeloperoxidase staining (brown) of Fisher rats 12, 24, and 48 hours after receiving an oral dose of 0.7g/kg MEHP or equivalent volume corn oil. (A) Cells showing multilobular nuclear staining typical of neutrophils are indicated by an arrow in the highlighted box. (C) Summarized data of myeloperoxidase staining. Data are means ± S.E.M for n≥3. Statistical analysis was performed by comparing MPO cell counts of MEHP-exposed animals for each time point with those of controls using one-way ANOVA and Tukey’s multiple comparisons test. **P<0.01, ****P<0.0001. Scale bars = 100μm (or 50μm for Figure 2A - MEHP).
5.2. Neutrophil chemoattractant factors Cxcl1 and Cxcl10 are expressed in the testis of MEHP-treated rats
To investigate how neutrophils might be recruited to the testicular interstitium, gene expression of several neutrophil chemoattractant factors (Ccl5, Cxcl1, Cxcl6, and Cxcl10), known to be expressed in the testes, and some of which have previously been shown expressed in response to phthalates (Lahousse et al., 2006; Johnson et al., 2007), was measured for whole testis 3, 6, and 12 hrs after MEHP or control treatment (Fig 3A). The expression of Cxcl1 and Cxcl6 was significantly upregulated at 3 hours in MEHP-treated rats, and Cxcl10 expression was significantly elevated at all measured time points, but peaked at 6 hrs. Expression of Ccl5 was unchanged. Because the most significant upregulation was observed for Cxcl1 (134-fold increase at 3h; notice difference in y-axis for the different chemoattractants), protein levels of CXCL1 were measured by ELISA. Protein levels of CXCL1 in all control testis tissues were below the detection limit of the assay, but measurable levels of CXCL1 were detected in testes of MEHP-treated rats at all time points, peaking at 6 hrs, corroborating the gene expression results (Figure 3B).
Figure 3. MEHP exposure induces expression of Cxcl1 and Cxcl10 in the testis.
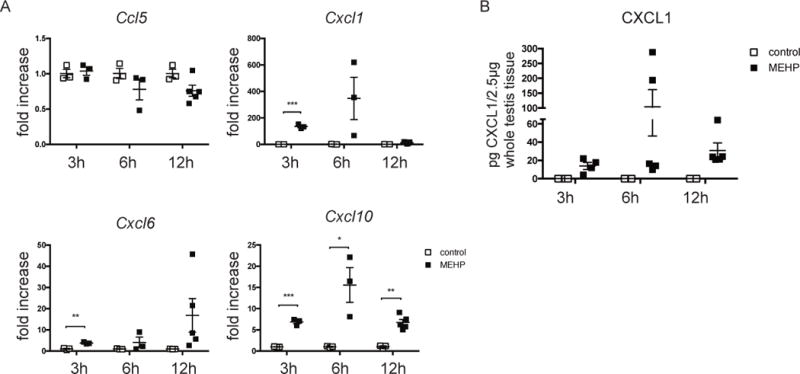
(A) mRNA expression of Ccl5, Cxcl6, Cxcl1, and Cxcl10 was assessed for whole testis of PND28 Fisher rats that were exposed to MEHP or control for 3, 6, and 12 hours. Expression by rats treated with MEHP is presented as fold increase compared to expression by rats from untreated animals. Data are means ± S.E.M. for n≥3. Statistical analysis was performed by comparing each group of cells with those of control rats for each time point using the unpaired t-test and corrected for multiple comparisons using the Holm-Sidak method. *P<0.05, **P<0.01, ***P<0.001. (B) Protein expression of CXCL1 in whole testis tissue of rats exposed to MEHP or control for 3, 6, and 18 hrs as measured by ELISA. CXCL1 levels in all control-treated rats was below the detection limit of the assay and are shown as 0. Data are means ± S.E.M. for n≥3.
5.3. Interstitial macrophages of MEHP-treated rats show increased pro-inflammatory gene expression as well as moderate increases in the expression of anti-inflammatory genes
To further investigate the phenotype of the infiltrating macrophages, gene expression of pro- and anti-inflammatory genes was analyzed for macrophages isolated from the testicular interstitium of MEHP and control-treated rats. The pro-inflammatory genes Tnfa and Il6 were significantly upregulated by the macrophages of MEHP-treated animal testis and the Arg1/Nos2 ratio was reduced (Figure 4A), indicating an increase in pro-inflammatory macrophages. However, significant, but smaller increases in the anti-inflammatory genes Il10 and Tgfb1 were also measured (Figure 4B).
Figure 4. Infiltrating mononuclear immune cells are pro-inflammatory.
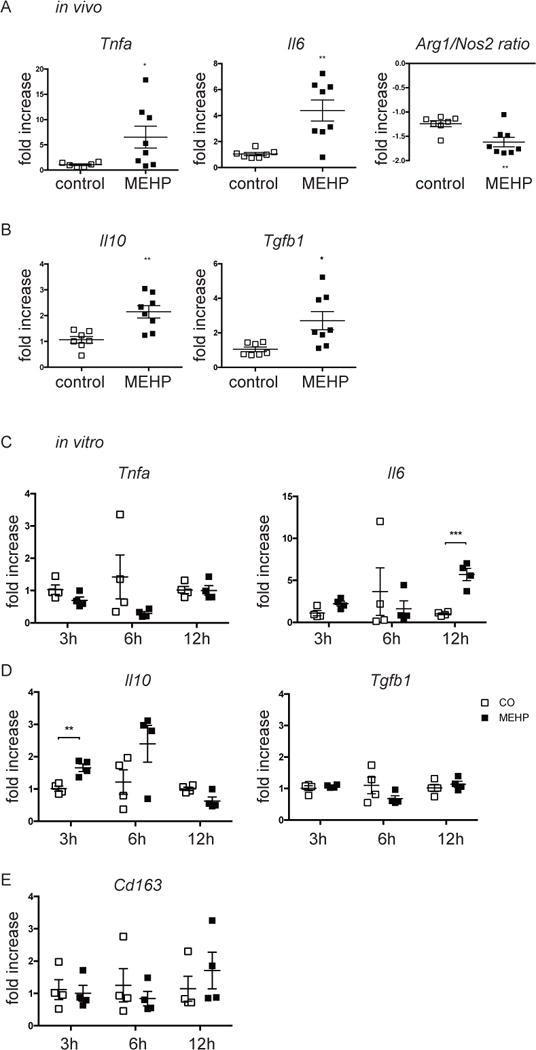
mRNA expression by (A-B) macrophages from animals 12 hours after exposure to MEHP or control or (C-E) testicular macrophages exposed to 200μM MEHP in vitro for 3, 6, or 12 hrs of (A,C) pro-inflammatory, (B,D) anti-inflammatory genes, or (E) Cd163, is presented as fold increase compared to gene expression of control macrophages. Data are means ± S.E.M. for n≥4. Statistical analysis was performed by the (A-B) unpaired two-tailed t-test and (C-E) the unpaired t-test and corrected for multiple comparisons using the Holm-Sidak method. *P<0.05, **P<0.01, ***P<0.001.
To investigate whether MEHP can directly affect the phenotype of interstitial macrophages, macrophages from the interstitium of untreated animals were isolated and stimulated with MEHP in vitro for 3, 6, and 12 hrs. Gene expression of Il6 was increased after 12h stimulation with MEHP (Figure 4C). Additionally, Il10 was significantly increased at 3hrs (Figure 4D). Tnfa and Tfgb1 expression were unaffected by stimulation with MEHP (Figure 4C-D). As cell surface expression of CD163 was shown to be increased by CD11bc+/CD68+/CD163+ testicular macrophages (Figure 1D) Cd163 gene expression was also assessed in response to MEHP, but no differences were measured (Figure 4E).
5.4. Macrophage or neutrophil infiltration following MEHP exposure does not exacerbate the incidence of GC apoptosis
To assess the functional significance of the immune cell infiltrate following MEHP, macrophages or neutrophils were depleted from the circulation and leukocyte infiltration and GC apoptosis levels in the testis were evaluated following MEHP exposure by flow cytometry and IHC. A 78% depletion of macrophages from the blood was accomplished by three i.p. injections of clodronate liposomes prior to gavage with MEHP (Figure 5A). I.p. injections of clodronate liposomes did not eliminate macrophages from the untreated testis (not shown), but it did appear to reduce macrophage recruitment to the testis 24 hrs after MEHP treatment (Figure 5B), although this was not significant. The infiltration of CD11bc+/CD68-/CD163- cells was not affected. Assessment of apoptosis by TUNEL staining showed that the apoptotic index was not lowered when macrophage infiltration was lowered (Figure 5, C-D), suggesting that the infiltrating macrophages do not contribute to GC apoptosis. Expression of CD163 on the outer membrane of CD11bc+/CD68+/CD163+ testicular macrophages following MEHP treatment remained high even when macrophage infiltration into the testis was inhibited (Figure 5E).
Figure 5. Preventing macrophage infiltration into the testis does not reduce germ cell apoptosis.
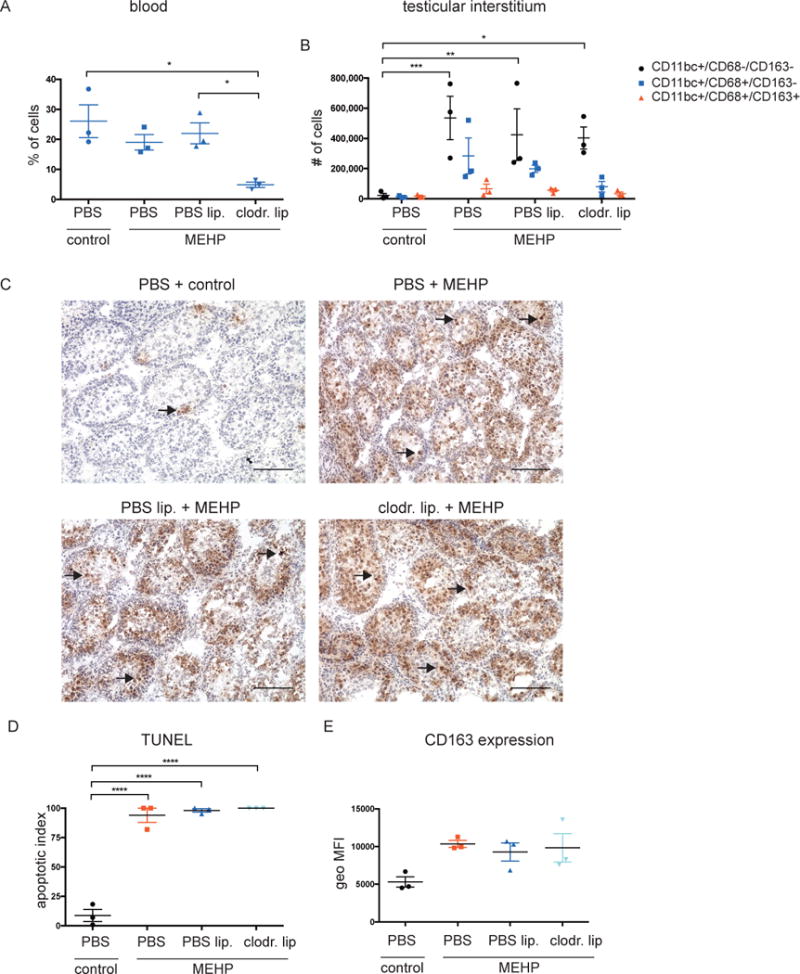
Before MEHP-exposure of PND28 rats, monocytes were depleted from the circulation using clodronate liposomes as described in materials and methods. (A) Quantification of flow cytometric counts of CD68+ macrophages in the blood. (B) Bar chart indicating the total number of CD11bc-expressing cells that are negative, single-positive, or double-positive for CD68 and CD163 expression. (C) Representative TUNEL images and d) quantification of untreated rats, and rats treated with MEHP that had been pretreated with clodronate liposomes to deplete monocytes from circulation, or control PBS liposomes. Scale bars = 100μm. (D) Bar chart indicating the geometric mean fluorescence intensity (geo MFI) of CD163 cell surface expression of CD11bc+/CD68+/CD163+ cells. Data are means ± S.E.M. for n=3. Statistical analysis was performed using (A,D) one-way ANOVA and (B) two-way ANOVA and Tukey’s multiple comparisons test. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001.
Neutrophil depletion from the blood was accomplished by two i.v. injections with an anti-polymorphonuclear neutrophil antibody (anti-PMN), resulting in a significant reduction of neutrophils in the blood compared to animals injected with PBS (Figure 6A). Numbers of CD68+ monocytes in the blood were not affected (Figure 6A). Anti-PMN Ab treatment alone did not affect neutrophil or macrophage numbers in the control testis, but prevented both neutrophil and macrophage recruitment to the testis after MEHP treatment (Figure 6, B-D). Assessment of apoptosis by TUNEL staining showed that no differences in GC apoptosis were observed compared to control animals (Figure 6, E-F).
Figure 6. Neutrophil depletion does not reduce germ cell apoptosis.
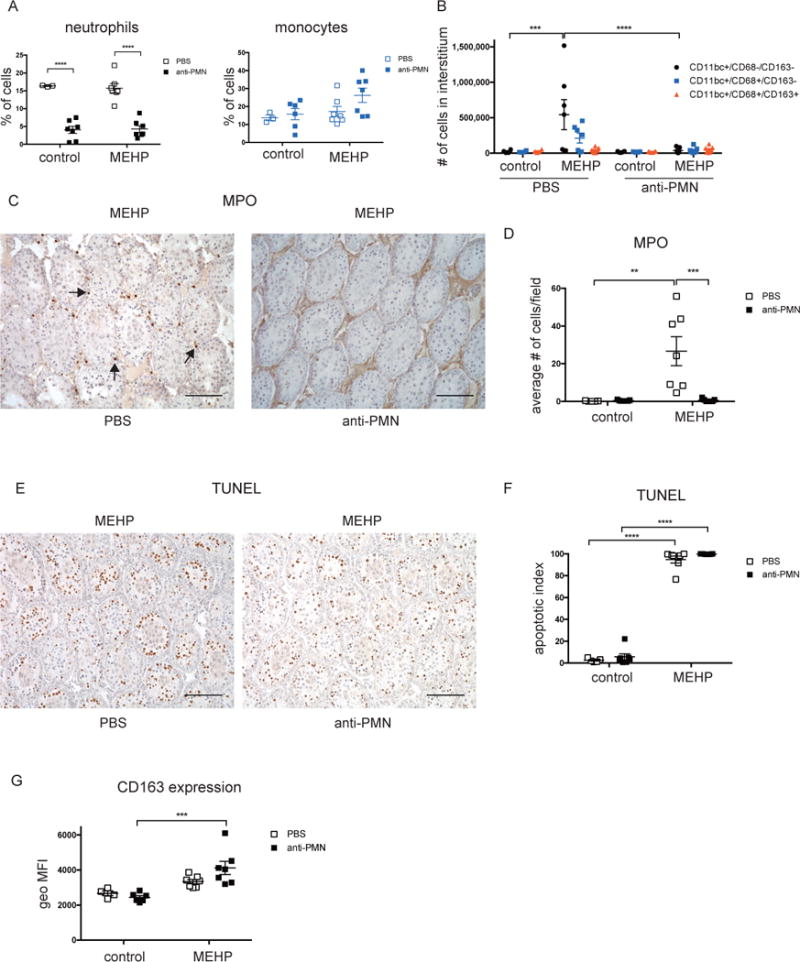
Before MEHP-treatment of PND28 rats, monocytes and/or neutrophils were depleted from the circulation by two i.v. injections of an anti-PMN Ab as described in materials and methods. (A) Quantification of flow cytometric counts of neutrophils and CD68+ macrophages in the blood. (B) Bar chart indicating the total number of CD11bc-expressing cells that are negative, single-positive, or double-positive for CD68 and CD163 expression. (C) Representative images and (D) summarized data of myeloperoxidase staining. Scale bars = 100μm. (E) Representative TUNEL images and (F) quantification of TUNEL-positive tubules of rats treated with MEHP with or without neutrophil depletion from the circulation. (G) Bar chart indicating the geometric mean fluorescence intensity (geo MFI) of CD163 cell surface expression of CD11bc+/CD68+/CD163+ cells. Data are means ± S.E.M. for n= 4-7. Statistical analysis was performed using two-way ANOVA and Tukey’s multiple comparisons test. **P<0.01, ***P<0.001, ****P<0.0001.
6. Discussion
Here we tested the functional significance of the influx of leukocytes into the testis following the disruption of SC function by an acute exposure to MEHP. Our previous findings indicated that immune cell infiltration and levels of GC apoptosis in response to MEHP exposure are correlated with age and is species-specific (Murphy et al., 2014). Furthermore, others have indicated that inflammation of the testis disrupts spermatogenesis (Lysiak et al., 2001; Rival et al., 2008; Jarazo-Dietrich et al., 2015). Taken together, these findings led us to hypothesize that the influx of immune cells after MEHP may exacerbate the extent of MEHP-induced GC apoptosis.
Our previous results indicated an increase in CD11b+ cells and macrophages in the testis in response to MEHP, but not T lymphocytes (Murphy et al., 2014). Here we further analyzed the CD11b infiltrate and show for the first time that MEHP exposure leads to a rapid and temporary increase of neutrophils and CD11bc+/CD68+/CD163- macrophages, in line with previous findings that phthalates can also induce neutrophil infiltration (Creasy et al., 1983; Lahousse et al., 2006), although additional infiltration of dendritic cells was not ruled out. Gene expression of several neutrophil chemoattractant factors highlighted CXCL1, and to a lesser extent CXCL10 and CXLC6, as candidates for recruiting neutrophils to the testis. CXCL1 protein was indeed found to be upregulated in the testes of MEHP-treated rats. These findings are in agreement with other reports that phthalates (DBP and DEHP) can upregulate Cxcl1 and Cxcl10 gene expression in the testis in vivo (Lahousse et al., 2006; Johnson et al., 2007), and in an in vitro testicular co-culture model (Harris et al., 2016). How expression of CXCL1 is induced remains to be investigated, but TNFα and Il-1β are likely candidates, as they have been reported to induce CXCL1 expression in peritubular myoid cells (PTMCs) and SCs, respectively (Aubry et al., 2000), and we have previously shown both cytokines to be increased upon MEHP exposure (Stermer et al., 2017).
Recent findings suggest that differentiated peritoneal macrophages can mobilize and infiltrate other injured tissues quickly after an injury or infection (Wang and Kubes, 2016). However, we did not find increased expression of Gata6, highly expressed by peritoneal macrophages (Miller et al., 2012) in the testis (data not shown), suggesting that instead the macrophages are recruited from the circulation. We have previously hypothesized that monocytes are recruited from the blood in response to release of CCL2, a strong chemotactic factor for monocytes, which we found upregulated in the seminiferous tubules and by PTMCs in response to MEHP (Murphy et al., 2014; Stermer et al., 2017). Proliferation of testicular macrophages (Schlatt et al., 1999), may account for the small yet significant increase in CD163-expressing macrophages at 48 hrs measured by immunofluorescence. However, alternatively the increase in CD163-positive macrophages could be due to phenotypic switching of some of the newly arrived CD68+ macrophages (Winnall and Hedger, 2013). The increase in CD163-expressing macrophages at 48hrs was not observed by flow cytometry, which is likely because of the lower sensitivity of this assay, due to the variability in interstitial cell recovery and testis size between rats.
The significant increases in pro-inflammatory gene expression of the macrophage population in the MEHP-treated testes compared to control, together with their lack of CD163 expression, a resident or alternative activation marker, suggest that the accumulating macrophages are pro-inflammatory. Interestingly, a small but significant increase in the anti-inflammatory genes Tgfb1 and Il10 were measured as well. One explanation for this observation is that the interstitium of the testis of MEHP-treated rats includes both the infiltrating cells as well as the ‘resident’ macrophages that are normally present in the testis. We predict that MEHP leads to the recruitment of pro-inflammatory macrophages, but that the phenotype of these macrophages is not further affected by MEHP, which would explain the increase in Tnfa expression observed in vivo, but not in vitro. In contrast, the resident testicular macrophages may respond to MEHP exposure directly by increasing gene expression of proteins that are involved in anti-inflammatory, tissue repair and/or regeneration processes. Indeed, we show that testicular macrophages produce both Il10 and Il6 in response to MEHP in vitro, and that cell surface expression of CD163 by CD11bc+/CD68+/CD163+ macrophages was increased in response to MEHP in vivo. This is in line with findings that testicular macrophages produce IL-10 in response to inflammatory stimuli (Bhushan et al., 2015). Additionally, IL-10, IL-6, and MEHP have all been shown to induce CD163-expression by macrophages (Buechler et al., 2000; Bølling et al., 2012).
Given the correlation between the infiltration of inflammatory cells into the testicular interstitium and GC apoptosis we observed in response to MEHP previously (Murphy et al., 2014), and the potential of these immune cells to cause testicular damage; e.g. through the release of TNF-α and ROS (Lysiak et al., 2001; Rival et al., 2008; Theas et al., 2008; Jarazo-Dietrich et al., 2015), it was surprising to find that preventing the influx of immune cells into the testis did not lower the GC apoptotic index, suggesting that the inflammatory cells do not contribute to GC apoptosis. It has previously been shown that phthalate-induced SC injury leads to Fas/FasL paracrine signaling that results in GC apoptosis, which is further triggered by the release of soluble TNFα from GCs, which activates the TNFRSF1A (TNFR1) signaling pathway in SCs that further stimulates FASL expression by SCs (Giammona et al., 2002; Yao et al., 2007). Although in mice this resulted in only approximately 11% of the tubules showing increased GC apoptosis (Murphy et al., 2014), it is possible that in the rat, the acute dose of MEHP used is so detrimental to SC function that it alone is responsible for the increased GC apoptosis in all tubules. Any potential additional contribution to the damage from harmful infiltrating immune cells is then unmeasurable. Interestingly, removal of neutrophils from the blood prevented not only neutrophil, but also macrophage recruitment to the testis, suggesting that the influx of neutrophils is necessary to trigger macrophage infiltration.
An alternate hypothesis for the role of infiltrating leukocytes in response to MEHP-induced SC injury is that rather than contributing to GC apoptosis, the macrophages and neutrophils actually facilitate the protection of the testis. MEHP-induced SC damage has also been shown to alter the expression and localization of junctional proteins that make up the BTB (Yao et al., 2010). Fragments from apoptotic GCs may breach the damaged BTB and could induce an autoimmune response. In the uninjured testis, SCs are generally considered to be the phagocytes of apoptotic GCs (Miething, 1992; Shiratsuchi et al., 1997; Nakanishi and Shiratsuchi, 2004) but with the SCs injured and their ability to maintain an anti-inflammatory environment compromised, neutrophils and macrophages may contribute in the clearance of escaping apoptotic fragments, thereby preventing autoimmune responses. Additionally, as macrophages in particular have been shown to play key roles in repair in many tissues (Mantovani et al., 2012; Novak and Koh, 2013; Chazaud, 2014; Wynn and Vannella, 2016), they may contribute to the repair of the testis following MEHP-induced injury.
In conclusion, although inflammatory neutrophils and macrophages are recruited to the interstitium of the testis following MEHP exposure, they do not exacerbate the incidence of MEHP-induced GC apoptosis in the testis. With the growing reports and understanding of the key role of leukocytes in both repair and resolution of injury in many tissues, it is possible that these cells are supporting other functions in the testis. Therefore, future studies are targeted to evaluate the non-inflammatory role of the infiltrating immune cells and provide insights into the functional significance of testicular leukocyte infiltration in the clinical analysis of human male infertility.
Acknowledgments
8. Funding
This work was funded, in part, by a grant from the National Institutes of Health/ National Institute of Environmental Health Sciences (NIH/NIEHS; 1R01 ES016591) and the Center for Molecular Carcinogenesis and Toxicology.
Footnotes
7. Declaration of interest
The authors declare no conflict of interest.
References
- Andersen CL, Jensen JL, Ørntoft TF. Normalization of real-time quantitative reverse transcription-PCR data: a model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Research. 2004;64:5245–5250. doi: 10.1158/0008-5472.CAN-04-0496. [DOI] [PubMed] [Google Scholar]
- Aubry F, Habasque C, Satie AP, Jégou B, Samson M. Expression and regulation of the CXC-chemokines, GRO/KC and IP-10/mob-1 in rat seminiferous tubules. European Cytokine Network. 2000;11:690–698. [PubMed] [Google Scholar]
- Avallet O, Vigier M, Leduque P, Dubois PM, Saez JM. Expression and regulation of transforming growth factor-beta 1 messenger ribonucleic acid and protein in cultured porcine Leydig and Sertoli cells. Endocrinology. 1994;134:2079–2087. doi: 10.1210/endo.134.5.8156908. [DOI] [PubMed] [Google Scholar]
- Bhushan S, Tchatalbachev S, Lu Y, Frohlich S, Fijak M, Vijayan V, Chakraborty T, Meinhardt A. Differential Activation of Inflammatory Pathways in Testicular Macrophages Provides a Rationale for Their Subdued Inflammatory Capacity. The Journal of Immunology. 2015;194:5455–5464. doi: 10.4049/jimmunol.1401132. [DOI] [PubMed] [Google Scholar]
- Bhushan S, Hossain H, Lu Y, Geisler A, Tchatalbachev S, Mikulski Z, Schuler G, Klug J, Pilatz A, Wagenlehner F, et al. Uropathogenic E. coli induce different immune response in testicular and peritoneal macrophages: implications for testicular immune privilege. PLoS ONE. 2011;6:e28452. doi: 10.1371/journal.pone.0028452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buechler C, Ritter M, Orsó E, Langmann T, Klucken J, Schmitz G. Regulation of scavenger receptor CD163 expression in human monocytes and macrophages by pro- and antiinflammatory stimuli. Journal of Leukocyte Biology. 2000;67:97–103. [PubMed] [Google Scholar]
- Bølling AK, Ovrevik J, Samuelsen JT, Holme JA, Rakkestad KE, Mathisen GH, Paulsen RE, Korsnes MS, Becher R. Mono-2-ethylhexylphthalate (MEHP) induces TNF-α release and macrophage differentiation through different signalling pathways in RAW264.7 cells. Toxicology Letters. 2012;209:43–50. doi: 10.1016/j.toxlet.2011.11.016. [DOI] [PubMed] [Google Scholar]
- Campese AF, Grazioli P, de Cesaris P, Riccioli A, Bellavia D, Pelullo M, Padula F, Noce C, Verkhovskaia S, Filippini A, et al. Mouse Sertoli cells sustain de novo generation of regulatory T cells by triggering the notch pathway through soluble JAGGED1. Biology of Reproduction. 2014;90:53. doi: 10.1095/biolreprod.113.113803. [DOI] [PubMed] [Google Scholar]
- Chazaud B. Macrophages: supportive cells for tissue repair and regeneration. Immunobiology. 2014;219:172–178. doi: 10.1016/j.imbio.2013.09.001. [DOI] [PubMed] [Google Scholar]
- Creasy DM, Foster JR, Foster PMD. The morphological development of di‐n‐pentyl phthalate induced testicular atrophy in the rat. The Journal of Pathology. 1983;139:309–321. doi: 10.1002/path.1711390307. [DOI] [PubMed] [Google Scholar]
- DeFalco T, Potter SJ, Williams AV, Waller B, Kan MJ, Capel B. Macrophages Contribute to the Spermatogonial Niche in the Adult Testis. Cell Reports. 2015;12:1107–1119. doi: 10.1016/j.celrep.2015.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle TJ, Kaur G, Putrevu SM, Dyson EL, Dyson M, McCunniff WT, Pasham MR, Kim KH, Dufour JM. Immunoprotective properties of primary Sertoli cells in mice: potential functional pathways that confer immune privilege. Biology of Reproduction. 2012;86:1–14. doi: 10.1095/biolreprod.110.089425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fijak M, Bhushan S, Meinhardt A. The Immune Privilege of the Testis. In: Krause WKH, Naz RK, editors. Immune Infertility. 2nd. Cham: Springer International Publishing: 2016. pp. 97–107. [Google Scholar]
- Fijak M, Schneider E, Klug J, Bhushan S, Hackstein H, Schuler G, Wygrecka M, Gromoll J, Meinhardt A. Testosterone replacement effectively inhibits the development of experimental autoimmune orchitis in rats: evidence for a direct role of testosterone on regulatory T cell expansion. The Journal of Immunology. 2011;186:5162–5172. doi: 10.4049/jimmunol.1001958. [DOI] [PubMed] [Google Scholar]
- Gerdprasert O, O’Bryan MK, Muir JA, Caldwell AM, Schlatt S, de Kretser DM, Hedger MP. The response of testicular leukocytes to lipopolysaccharide-induced inflammation: further evidence for heterogeneity of the testicular macrophage population. Cell and Tissue Research. 2002;308:277–285. doi: 10.1007/s00441-002-0547-6. [DOI] [PubMed] [Google Scholar]
- Giammona CJ, Sawhney P, Chandrasekaran Y, Richburg JH. Death receptor response in rodent testis after mono-(2-ethylhexyl) phthalate exposure. Toxicology and Applied Pharmacology. 2002;185:119–127. doi: 10.1006/taap.2002.9536. [DOI] [PubMed] [Google Scholar]
- Harris S, Shubin SP, Wegner S, Van Ness K, Green F, Hong SW, Faustman EM. The presence of macrophages and inflammatory responses in an in vitro testicular co-culture model of male reproductive development enhance relevance to in vivo conditions. Toxicology in Vitro. 2016;36:210–215. doi: 10.1016/j.tiv.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedger MP, Winnall WR. Regulation of activin and inhibin in the adult testis and the evidence for functional roles in spermatogenesis and immunoregulation. Molecular and Cellular Endocrinology. 2012;359:30–42. doi: 10.1016/j.mce.2011.09.031. [DOI] [PubMed] [Google Scholar]
- Hussein MR, Abou-Deif ES, Bedaiwy MA, Said TM, Mustafa MG, Nada E, Ezat A, Agarwal A. Phenotypic characterization of the immune and mast cell infiltrates in the human testis shows normal and abnormal spermatogenesis. Fertility and Sterility. 2005;83:1447–1453. doi: 10.1016/j.fertnstert.2004.11.062. [DOI] [PubMed] [Google Scholar]
- Jarazo-Dietrich S, Fass MI, Jacobo PV, Sobarzo CMA, Lustig L, Theas MS. Inhibition of NOS-NO System Prevents Autoimmune Orchitis Development in Rats: Relevance of NO Released by Testicular Macrophages in Germ Cell Apoptosis and Testosterone Secretion. PLoS ONE. 2015;10:e0128709. doi: 10.1371/journal.pone.0128709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KJ, Hensley JB, Kelso MD, Wallace DG, Gaido KW. Mapping gene expression changes in the fetal rat testis following acute dibutyl phthalate exposure defines a complex temporal cascade of responding cell types. Biology of Reproduction. 2007;77:978–989. doi: 10.1095/biolreprod.107.062950. [DOI] [PubMed] [Google Scholar]
- Kern S, Robertson SA, Mau VJ, Maddocks S. Cytokine secretion by macrophages in the rat testis. Biology of Reproduction. 1995;53:1407–1416. doi: 10.1095/biolreprod53.6.1407. [DOI] [PubMed] [Google Scholar]
- Kinkade JM, Pember SO, Barnes KC, Shapira R, Spitznagel JK, Martin LE. Differential distribution of distinct forms of myeloperoxidase in different azurophilic granule subpopulations from human neutrophils. Biochemical and Biophysical Research Communications. 1983;114:296–303. doi: 10.1016/0006-291x(83)91627-3. [DOI] [PubMed] [Google Scholar]
- Lahousse SA, Wallace DG, Liu D, Gaido KW, Johnson KJ. Testicular gene expression profiling following prepubertal rat mono-(2-ethylhexyl) phthalate exposure suggests a common initial genetic response at fetal and prepubertal ages. Toxicological Sciences. 2006;93:369–381. doi: 10.1093/toxsci/kfl049. [DOI] [PubMed] [Google Scholar]
- Laskin DL, Gardner CR, Price VF, Jollow DJ. Modulation of macrophage functioning abrogates the acute hepatotoxicity of acetaminophen. Hepatology. 1995;21:1045–1050. [PubMed] [Google Scholar]
- Li N, Tang EI, Cheng CY. Regulation of blood-testis barrier by actin binding proteins and protein kinases. Reproduction. 2016;151:R29–R41. doi: 10.1530/REP-15-0463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Wang T, Han D. Structural, cellular and molecular aspects of immune privilege in the testis. Frontiers in Immunology. 2012;3:152. doi: 10.3389/fimmu.2012.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y-C, Yao P-L, Richburg JH. FasL gene-deficient mice display a limited disruption in spermatogenesis and inhibition of mono-(2-ethylhexyl) phthalate-induced germ cell apoptosis. Toxicological Sciences. 2010;114:335–345. doi: 10.1093/toxsci/kfq015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods (San Diego, Calif) 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lysiak JJ, Turner SD, Nguyen QA, Singbartl K, Ley K, Turner TT. Essential role of neutrophils in germ cell-specific apoptosis following ischemia/reperfusion injury of the mouse testis. Biology of Reproduction. 2001;65:718–725. doi: 10.1095/biolreprod65.3.718. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Biswas SK, Galdiero MR, Sica A, Locati M. Macrophage plasticity and polarization in tissue repair and remodelling. The Journal of Pathology. 2012;229:176–185. doi: 10.1002/path.4133. [DOI] [PubMed] [Google Scholar]
- Meehan T, Schlatt S, O’Bryan MK, de Kretser DM, Loveland KL. Regulation of germ cell and Sertoli cell development by activin, follistatin, and FSH. Developmental Biology. 2000;220:225–237. doi: 10.1006/dbio.2000.9625. [DOI] [PubMed] [Google Scholar]
- Miething A. Germ-cell death during prespermatogenesis in the testis of the golden hamster. Cell and Tissue Research. 1992;267:583–590. doi: 10.1007/BF00319381. [DOI] [PubMed] [Google Scholar]
- Miller J, Greter M, Ivanov S, Helft J, Chow A, Elpek KG, Gordonov S, Mazloom AR, Ma’ayan A, Chua W-J, et al. Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nature Immunology. 2012;13:1118–1128. doi: 10.1038/ni.2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mital P, Kaur G, Dufour JM. Immunoprotective sertoli cells: making allogeneic and xenogeneic transplantation feasible. Reproduction. 2010;139:495–504. doi: 10.1530/REP-09-0384. [DOI] [PubMed] [Google Scholar]
- Murphy CJ, Stermer AR, Richburg JH. Age- and species-dependent infiltration of macrophages into the testis of rats and mice exposed to mono-(2-Ethylhexyl) phthalate (MEHP) Biology of Reproduction. 2014;91:18. doi: 10.1095/biolreprod.113.115527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi Y, Shiratsuchi A. Phagocytic removal of apoptotic spermatogenic cells by Sertoli cells: mechanisms and consequences. Biological & Pharmaceutical Bulletin. 2004;27:13–16. doi: 10.1248/bpb.27.13. [DOI] [PubMed] [Google Scholar]
- Niemi M, Sharpe RM, Brown WR. Macrophages in the interstitial tissue of the rat testis. Cell and Tissue Research. 1986;243:337–344. doi: 10.1007/BF00251049. [DOI] [PubMed] [Google Scholar]
- Novak ML, Koh TJ. Phenotypic transitions of macrophages orchestrate tissue repair. The American Journal of Pathology. 2013;183:1352–1363. doi: 10.1016/j.ajpath.2013.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendino KJ, Laskin JD, Shuler RL, Punjabi CJ, Laskin DL. Enhanced production of nitric oxide by rat alveolar macrophages after inhalation of a pulmonary irritant is associated with increased expression of nitric oxide synthase. Journal of Immunology. 1993;151:7196–7205. [PubMed] [Google Scholar]
- Redente EF, Higgins DM, Dwyer-Nield LD, Orme IM, Gonzalez-Juarrero M, Malkinson AM. Differential polarization of alveolar macrophages and bone marrow-derived monocytes following chemically and pathogen-induced chronic lung inflammation. Journal of Leukocyte Biology. 2010;88:159–168. doi: 10.1189/jlb.0609378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rival C, Theas MS, Suescun MO, Jacobo P, Guazzone V, van Rooijen N, Lustig L. Functional and phenotypic characteristics of testicular macrophages in experimental autoimmune orchitis. The Journal of Pathology. 2008;215:108–117. doi: 10.1002/path.2328. [DOI] [PubMed] [Google Scholar]
- Schlatt S, de Kretser DM, Hedger MP. Mitosis of resident macrophages in the adult rat testis. Journal of Reproduction and Fertility. 1999;116:223–228. doi: 10.1530/jrf.0.1160223. [DOI] [PubMed] [Google Scholar]
- Shiratsuchi A, Umeda M, Ohba Y, Nakanishi Y. Recognition of phosphatidylserine on the surface of apoptotic spermatogenic cells and subsequent phagocytosis by Sertoli cells of the rat. The Journal of Biological Chemistry. 1997;272:2354–2358. doi: 10.1074/jbc.272.4.2354. [DOI] [PubMed] [Google Scholar]
- Stermer AR, Murphy CJ, Ghaffari R, Di Bona KR, Voss JJ, Richburg JH. Mono-(2-ethylhexyl) phthalate-induced Sertoli cell injury stimulates the production of pro-inflammatory cytokines in Fischer 344 rats. Reproductive Toxicology. 2017;69:150–158. doi: 10.1016/j.reprotox.2017.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taurog JD, Rival C, van Duivenvoorde LM, Satumtira N, Dorris ML, Sun M, Shelton JM, Richardson JA, Hamra FK, Hammer RE, et al. Autoimmune epididymoorchitis is essential to the pathogenesis of male-specific spondylarthritis in HLA-B27-transgenic rats. Arthritis and Rheumatism. 2012;64:2518–2528. doi: 10.1002/art.34480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theas MS, Rival C, Jarazo-Dietrich S, Jacobo P, Guazzone VA, Lustig L. Tumour necrosis factor-alpha released by testicular macrophages induces apoptosis of germ cells in autoimmune orchitis. Human Reproduction. 2008;23:1865–1872. doi: 10.1093/humrep/den240. [DOI] [PubMed] [Google Scholar]
- Wang J, Wreford NG, Lan HY, Atkins R, Hedger MP. Leukocyte populations of the adult rat testis following removal of the Leydig cells by treatment with ethane dimethane sulfonate and subcutaneous testosterone implants. Biology of Reproduction. 1994;51:551–561. doi: 10.1095/biolreprod51.3.551. [DOI] [PubMed] [Google Scholar]
- Wang J, Kubes P. A Reservoir of Mature Cavity Macrophages that Can Rapidly Invade Visceral Organs to Affect Tissue Repair. Cell. 2016;165:668–678. doi: 10.1016/j.cell.2016.03.009. [DOI] [PubMed] [Google Scholar]
- Wang M, Fijak M, Hossain H, Markmann M, Nüsing RM, Lochnit G, Hartmann MF, Wudy SA, Zhang L, Gu H, et al. Characterization of the Micro-Environment of the Testis that Shapes the Phenotype and Function of Testicular Macrophages. The Journal of Immunology. 2017;198:4327–4340. doi: 10.4049/jimmunol.1700162. [DOI] [PubMed] [Google Scholar]
- Winnall WR, Hedger MP. Phenotypic and functional heterogeneity of the testicular macrophage population: a new regulatory model. Journal of Reproductive Immunology. 2013;97:147–158. doi: 10.1016/j.jri.2013.01.001. [DOI] [PubMed] [Google Scholar]
- Winnall WR, Muir JA, Hedger MP. Rat resident testicular macrophages have an alternatively activated phenotype and constitutively produce interleukin-10 in vitro. Journal of Leukocyte Biology. 2011;90:133–143. doi: 10.1189/jlb.1010557. [DOI] [PubMed] [Google Scholar]
- Wynn TA, Vannella KM. Macrophages in Tissue Repair, Regeneration, and Fibrosis. Immunity. 2016;44:450–462. doi: 10.1016/j.immuni.2016.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao PL, Lin YC, Richburg JH. Mono-(2-Ethylhexyl) Phthalate-Induced Disruption of Junctional Complexes in the Seminiferous Epithelium of the Rodent Testis Is Mediated by MMP2. Biology of Reproduction. 2010;82:516–527. doi: 10.1095/biolreprod.109.080374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao P-L, Lin Y-C, Richburg JH. TNF alpha-mediated disruption of spermatogenesis in response to Sertoli cell injury in rodents is partially regulated by MMP2. Biology of Reproduction. 2009;80:581–589. doi: 10.1095/biolreprod.108.073122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao P-L, Lin Y-C, Sawhney P, Richburg JH. Transcriptional regulation of FasL expression and participation of sTNF-alpha in response to sertoli cell injury. The Journal of Biological Chemistry. 2007;282:5420–5431. doi: 10.1074/jbc.M609068200. [DOI] [PubMed] [Google Scholar]
- Zhao S, Zhu W, Xue S, Han D. Testicular defense systems: immune privilege and innate immunity. Cellular and Molecular Immunology. 2014;11:428–437. doi: 10.1038/cmi.2014.38. [DOI] [PMC free article] [PubMed] [Google Scholar]


