Abstract
Introduction
Photobiomodulation was assessed as a novel treatment of Alzheimer’s disease (AD) by the use of a new device RGn500 combining photonic and magnetic emissions in a mouse model of AD.
Methods
Following the injection of amyloid β 25-35 peptide in male Swiss mice, RGn500 was applied once a day for 7 days either on the top of the head or the center of abdomen or both.
Results
RGn500 daily application for 10 min produced a neuroprotective effect on the neurotoxic effects of amyloid β 25-35 peptide injection when this type of photobiomodulation was applied both on the head and on the abdomen. Protection was demonstrated by memory restoration and on the normalization of key markers of AD (amyloid β 1-42, pTau), oxidative stress (lipid peroxidation), apoptosis (Bax/Bcl2) and neuroinflammation.
Discussion
RGn500 displays therapeutic efficacy similar to other pharmacological approaches evaluated in this model of AD.
Keywords: Alzheimer’s disease, Neurodegenerescence, Memory, Neuroinflammation, Amyloid β, Phosphorylated tau, Photobiomodulation, Electromagnetic, LLLT, Magnetic, Photonic, Oxidative stress
1. Introduction
Alzheimer's disease (AD), the main cause of dementia, is a major public health issue [1] in light of the burden it places on patients, families, and caregivers as well as its socioeconomic impact. Despite the considerable research advances made and promising data obtained recently with aducanumab [2], no real effective treatment has been identified to date. Therefore, combination of innovative treatments, engaging not only one target but several ones, is currently mandatory to hope to treat this complex neurodegenerative disorder. In this article, we describe a treatment based on a noninvasive electromagnetic technology, called photobiomodulation (PBM). The use of light emitting diodes or lasers in PBM has been shown to induce a photochemical reaction within the cell [3], causing an increase in mitochondrial function and adenosine triphosphate synthesis. Such parameters suggest a neuroprotective effect, especially relevant to AD pathogenesis where mitochondrial proteostasis is particularly affected [4]. Several studies report astonishing properties of transcranial PBM, such as inflammation downregulation, repair processes, and tissue healing stimulation [5], for treatments in neurology and neuropsychiatry [6]. Neuroprotective efficacy of PBM is now well established and adds a unique value to promising therapies because it has been proven safe and effective in humans [7]. PBM treatment ameliorates neuropathology and disease progression in a mouse transgenic model of AD [8], [9]. Recently, pilot clinical trials on patients with dementia and AD indicate significant cognitive improvement [10], [11], [12], suggesting that transcranial PBM is a potential candidate for treatment of AD. In this article, we describe an innovative device, RGn500, combining different technologies producing photonic and magnetic field emissions. This neuro-magneto-photonic treatment combines PBM with a static magnetic field in addition. Low static magnetic field has been studied in animal models, and transcranial magnetic stimulation with a high magnetic field has been investigated as a noninvasive therapeutic tool to treat neurological and psychiatric diseases [13]. The implication of various altered processes in AD, such as mitochondrial dysfunction [14] or inflammatory processes [15], support transcranial stimulation. Alternative hypotheses point the importance of brain-gut axis in neurodegenerative diseases [16] and suggest the interest of an abdominal stimulation.
To test the hypothesis of multiple possible strategies of treatment of the pathology, RGn500 was tested, with applications both on the head and abdomen, on the neurotoxic effects produced by the intracerebroventricular (i.c.v.) injection of oligomeric amyloid β peptide 25–35 (Aβ25–35) in mice. That procedure has been described as reproducing some of the features of AD pathology [17] and may give some indication on the interest of this new treatment in humans. The details of the materials and methods used in this study are described in Appendix 1.
2. Results
2.1. Behavior
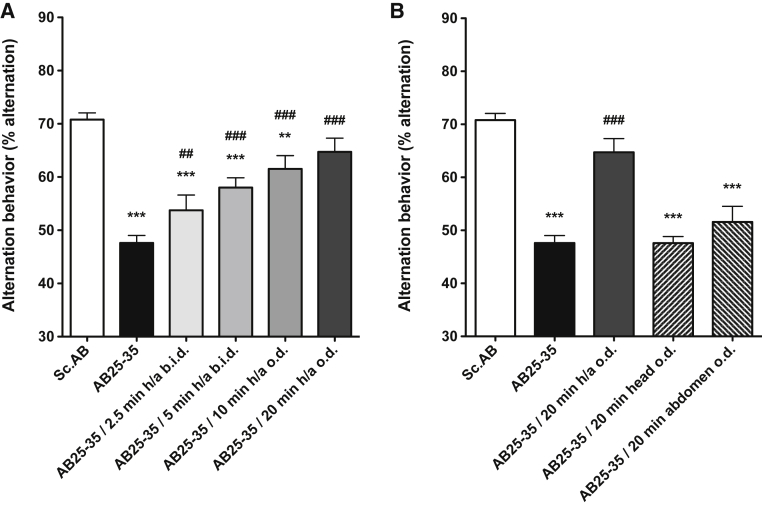
The Y-maze evaluates short-term memory, by measurement of spontaneous alternation. As compared to scramble Aβ (Sc Aβ)-injected mice, which showed normal percentage of alternation (70.8% ± 1.3), Aβ25–35 peptide very significantly induced spatial working memory deficits by decreasing the percentage of alternation (47.6% ± 1.4) (Fig. 1A). RGn500 very significantly alleviated deficits and fully restored the cognitive performance in a time of exposure-dependent manner when the animals were treated from 2.5 to 20 minutes both on the head (10 Hz) and abdomen (1000 Hz) by restoring a normal percentage of alternation [(64.7% ± 2.6), F (5, 86) = 24.26, P < .001], Dunnett's test after one-way analysis of variance (ANOVA) (Fig. 1A).
Fig. 1.
Effect of RGn500 treatment on Aβ25–35 (AB25–35)–induced deficits in the spontaneous alternation test. Different times of exposure, the frequency (once a day [o.d.] or twice a day [b.i.d.]) (A), and the mode of delivery with exposure of head alone (at 10 Hz), of abdomen alone (at 1000 Hz), or of both body parts (h/a) (B) were examined. Data are presented as mean ± SEM. ∗∗P < .01, ***P < .001 versus Sc Aβ (Sc.AB) group; ##P < .01, ###P < .001 versus Aβ25–35 group. n = 12–21 per group. Abbreviations: Aβ25–35, amyloid β peptide 25–35; SEM, standard error of mean.
If the treatment was localized only on the head or abdomen, RGn500 failed to yield beneficial effects (Fig. 1B).
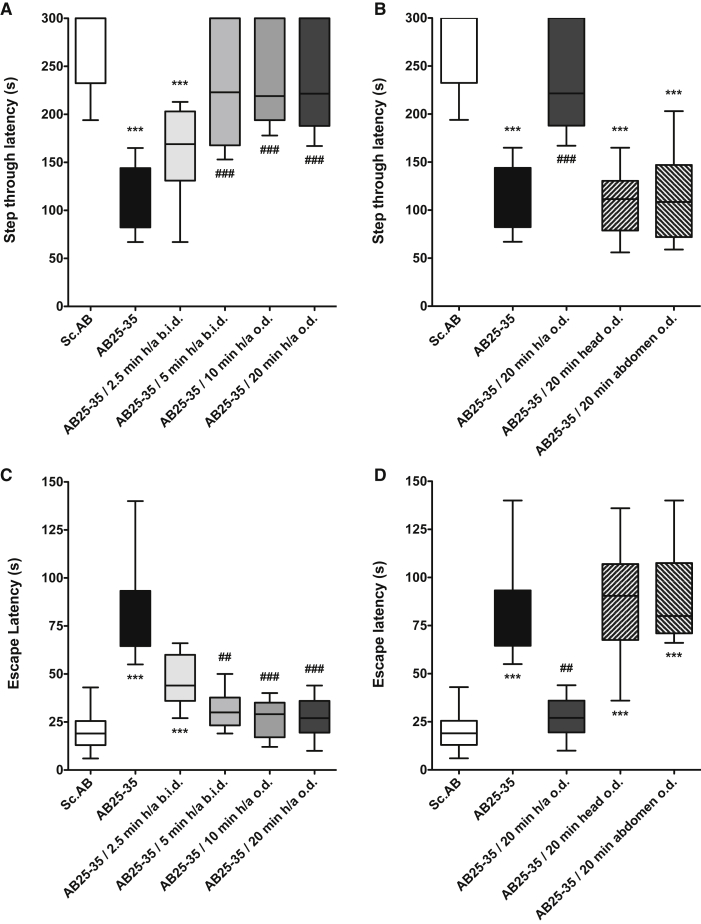
Eight days after the i.c.v. injection, Aβ25–35 peptide induced very significant contextual long-term memory deficits in terms of step-through latency (123.5 s [median], 82.3, 144.0 [25%, 75% percentile]) (Fig. 2A) and escape latency (Fig. 2C). RGn500 alleviated deficits in a time-dependent manner and fully and very significantly restored the cognitive performance when the animals were treated 20 minutes simultaneously on the head (10 Hz) and abdomen (1000 Hz) (h/a) in terms of step-through latency (221.5 s [median], 188.0, 300.0 [25%, 75% percentile], H = 58.60, P < .001) (Fig. 2A) and escape latency (27.0 s [median], 19.5, 36.0 [25%, 75% percentile], H = 57.38, P < .001) (Fig. 2C), Dunnett's test after two-way nonparametric ANOVA (Fig. 2C).
Fig. 2.
Effect of RGn500 treatment on Aβ25–35 (AB25–35)–induced deficits in the step-through latency (A and C) and the escape latency (B and D) in the passive avoidance task. Different times of exposure, the frequency (once a day [o.d.] or twice a day [b.i.d.]), and the mode of delivery with exposure of head alone (at 10 Hz), of abdomen alone (at 1000 Hz), or of both body parts (h/a) were examined. Data are presented as mean ± SEM. ***P < .001 versus Sc Aβ (Sc.AB) group; ##P < .01, ###P < .001 versus Aβ25–35 group. n = 12–21 per group. Abbreviations: Aβ25–35, amyloid β peptide 25–35; SEM, standard error of mean.
When the treatment was localized only on the head or abdomen alone, RGn500 failed to yield beneficial effects in terms of step-through latency (Fig. 2D).
2.2. Oxidative stress
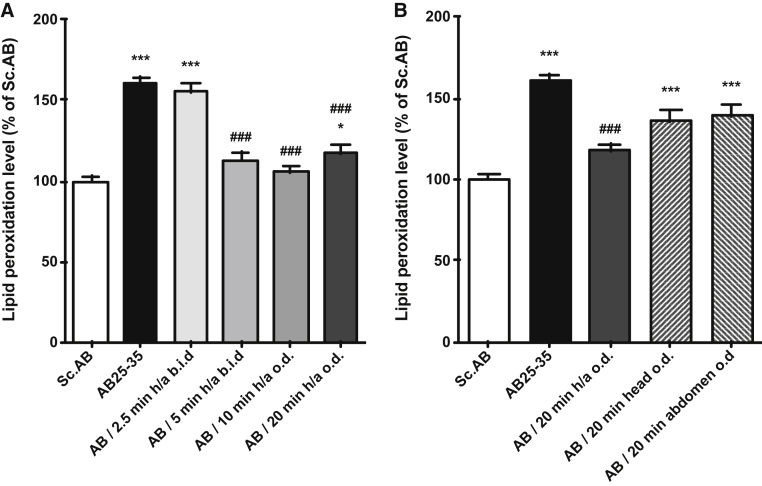
Compared to Sc Aβ-injected mice, Aβ25–35 peptide induced a very significant elevation of lipid peroxidation (160.0% ± 4.3) (Fig. 3A). RGn500 very significantly reduced oxidative stress in a time of exposure-dependent manner when the animals were treated simultaneously on the head (10 Hz) and abdomen (1000 Hz) (117.4% ± 4.4), F (5, 29) = 14.75, P < .001, Dunnett's test after one-way ANOVA (Fig. 3A).
Fig. 3.
Effect of RGn500 treatment on Aβ25–35 (AB25–35)–induced increase of lipid peroxidation. Different times of exposure, the frequency (once a day [o.d.] or twice a day [b.i.d.]) (A), and the mode of delivery with exposure of head alone (at 10 Hz), of abdomen alone (at 1000 Hz), or of both body parts (h/a) (B) were examined. Data are presented as mean ± SEM. *P < .05, ***P < .001 versus Sc Aβ (Sc.AB) group; ###P < .001 versus Aβ25–35 group. n = 6 per group. Abbreviations: Aβ25–35, amyloid β peptide 25–35; SEM, standard error of mean.
When the treatment was only localized on the head or abdomen, RGn500 application did not produce any significant beneficial effect (Fig. 3B).
2.3. Inflammation
Inflammatory processes were measured in the hippocampus 9 days after Aβ25–35 peptide i.c.v. injection in mice, in terms of glial fibrillary protein, tumor necrosis factor α, interleukin 1β (IL-1β), interleukin 6 level measurement by enzyme linked immunosorbent assay and astrocyte and microglia activation evaluated by immunohistochemical analyses of brain slices in the CA1 region of the hippocampus.
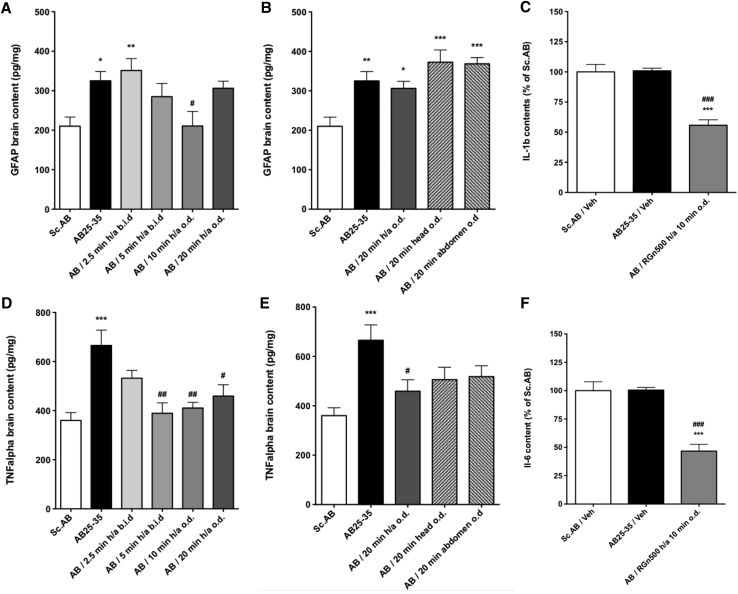
Aβ25–35 peptide injection induced a significant increase of glial fibrillary protein levels (154.8% ± 11.3) (Fig. 4A). The RGn500 application on both the head (10 Hz) and abdomen (1000 Hz) (h/a) triggered a reduction of the inflammation with a total reversal for 10 minutes, whereas a 20-minute treatment was inactive [(145.8% ± 8.6), F (5, 40) = 4.283, P < .01, Dunnett's test after one-way ANOVA; Fig. 4A]. When the treatment was localized only on the head or abdomen, RGn500 failed to produce any beneficial effect (respectively, 177.4% ± 14.8, and 175.4% ± 7.6, F (4, 33) = 6.692, P < .001, Dunnett's test after one-way ANOVA) (Fig. 4B).
Fig. 4.
Effect of RGn500 treatment on Aβ25–35 (AB25-35)–induced increase of inflammatory processes, GFAP (A, B), IL-1β (IL-1b) (C), TNFα (TNF alpha) (D, E), and IL6 (IL-6) (F). Different times of exposure, the frequency (once a day [o.d.] or twice a day [b.i.d.]), and the mode of delivery with exposure of head alone (at 10 Hz), of abdomen alone (at 10 Hz), or of both body parts (h/a) were examined. Data are presented as mean ± SEM. *P < .05, **P < .01, ***P < .001 versus Sc Aβ (Sc.AB) group; #P < .05, ##P < .01, ###P < .001 versus Aβ25–35 group. n = 6 per group. Abbreviations: Aβ25–35, amyloid β peptide 25–35; GFAP, glial fibrillary protein; IL-1β, interleukin 1β; IL-6, interleukin 6; SEM, standard error of mean; TNFα, tumor necrosis factor.
Aβ25–35 peptide induced a very significant increase of tumor necrosis factor α levels (184.8% ± 17.3) (Fig. 4D). RGn500 application reduced this increase after 5, 10, and 20 minutes of simultaneous application on the head (10 Hz) and abdomen (1000 Hz) (127.5% ± 12.9), F (5, 51) = 6.761, P < .001, Dunnett's test after one-way ANOVA, (Fig. 4D).
When the treatment was only localized on the head or the abdomen, RGn500 failed to yield any beneficial effect (respectively, 140.5% ± 13.9, and 143.9% ± 12.2, F (4, 43) = 6.073, P < .001, Dunnett's test after one-way ANOVA) (Fig. 4E). Similar results were found after an application of RGn500 on both the head (10 Hz) and abdomen (10 Hz) during 10 minutes (results not shown). The following experiments were conducted under these conditions.
IL-1β and IL6 hippocampal levels were not modified by Aβ25–35 peptide injection as compared to Sc Aβ injection (Fig. 4C and 4F). However, RGn500 very significantly reduced the level of IL-1β and interleukin 6 when animals were treated simultaneously during 10 minutes on the head (10 Hz) and abdomen (10 Hz) (for IL-1β 55.7% ± 4.5, F (2, 17) = 31.22, P < .001, for interleukin 6 46.7% ± 6.0, F (2, 17) = 27.97, P < .001, Dunnett's test after one-way ANOVA).
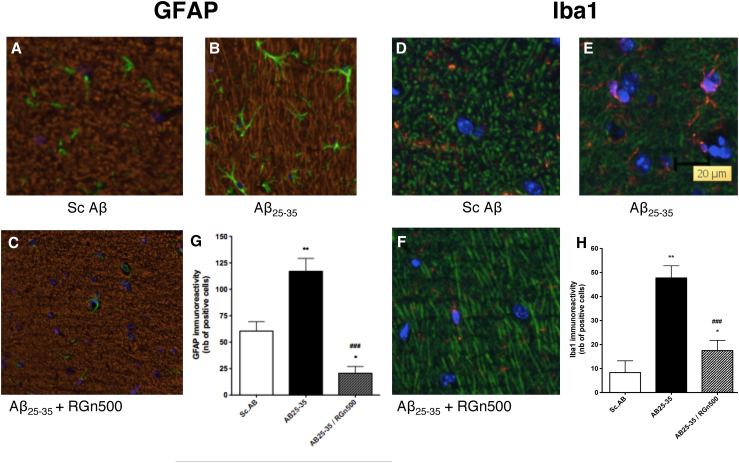
The injection of Aβ25–35 peptide oligomers produced the appearance of typical activated astrocytes (Fig. 5B) characterized by a change in shape consisting particularly in an important ramification compared to resting astrocytes in the Sc Aβ group (Fig. 5A). The significant increase in the number of activated astrocytes was normalized after daily treatment with RGn500 applied during 10 minutes on both the head and abdomen (Fig. 5G).
Fig. 5.
Effect of RGn500 treatment on Aβ25–35–induced activation of astrocytes (A–C, and G) and microglia (D–F, and H). Exposure was once a day of both the head and abdomen for 10 minutes at 10 Hz on both body parts. **P < .01 versus Sc.Aβ (Sc.AB) group; ***P < .001 versus Sc AB group; ###P < .001 versus Aβ25–35 (AB25–35) group. Abbreviation: Aβ25–35, amyloid β peptide 25–35.
Iba-1 immunolabeling showed a significantly modified microglial activity. Strongly labeled fluorescent cells exhibited typical amoeboid morphology and few ramifications indicative of activated microglia as compared to surveilling type (Fig. 5E).
The daily treatment with RGn500 applied during 10 minutes on both the head (10 Hz) and abdomen (10 Hz) inhibited this microglial activation (Fig. 5F) in a highly significant manner (P < .001, Fig. 5H).
2.4. Mitochondrial markers of apoptosis
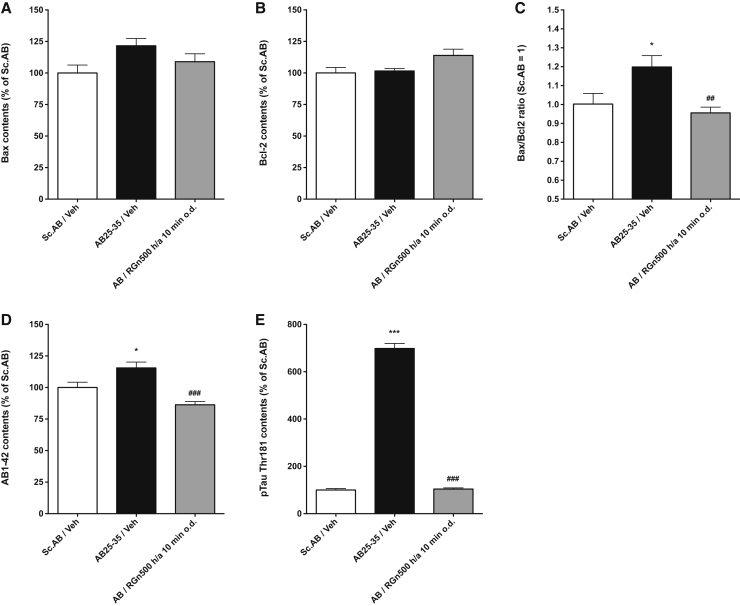
The level of pro-apoptotic Bax and the anti-apoptotic Bcl2 markers were measured in the hippocampus 10 days after Aβ25–35 peptide oligomer i.c.v. injection in mice, by enzyme linked immunosorbent assay. An increase of the ratio Bax/Bcl2 translates an induction of apoptosis due to the heterodimerization of Bax and Bcl2.
Aβ25–35 peptide oligomers injection produced an increase of Bax levels (121.6% ± 5.8) that was not significant. RGn500 reduced the increase of Bax when the animals were treated 10 minutes on the head (10 Hz) and on the abdomen (10 Hz) [(108.9% ± 6.3), F (2, 17) = 3.110, P > .05, Dunnett's test after one-way ANOVA; Fig. 6A].
Fig. 6.
Effect of RGn500 on Aβ25–35 (AB25–35)–induced increase of Bax and Bcl2 (A–C), Aβ1-42 (AB1–42) (D) and pTau-Thr181 (E). Exposure was once a day of both the head and abdomen for 10 minutes at 10 Hz on both body parts. Data are mean ± SEM. *P < .05, ***P < .001 versus Sc Aβ (Sc.AB) group; ###P < .001 versus Aβ25–35 (AB25–35) group. n = 6 per group. Abbreviations: Aβ25–35, amyloid β peptide 25–35; SEM, standard error of mean.
No effect on Bcl2 levels was seen after Aβ25–35 peptide oligomers injection and RGn500 application (Fig. 6B). The Bax/Bcl2 ratio was significantly increased (1.199 ± 0.060) (Fig. 6C). RGn500 significantly reduced the increase of the ratio when animals were treated daily for 10 minutes on the head (10 Hz) and the abdomen (10 Hz) [(0.956 ± 0.030), F (2, 17) = 6.437, P < .01, Dunnett's test after one-way ANOVA; Fig. 6C].
2.5. Amyloid processing and hyperphosphorylation of tau protein
Aβ25–35 peptide induced a very significant augmentation of Aβ1–42 (115.5% ± 4.6) measured in the hippocampus 10 days after Aβ25–35 peptide i.c.v. injection (Fig. 6D). RGn500 inhibited this increase when the animals were treated for 10 minutes on the head (10 Hz) and the abdomen (10 Hz) (86.3% ± 2.6), F (2, 17) = 13.92, P < .001, Dunnett's test after one-way ANOVA, (Fig. 6D).
The levels of pTau-Thr181 were very highly increased (698.3% ± 21.4) (Fig. 6E). RGn500 reduced this rise when animals were treated for 10 minutes both on the head (10 Hz) and on the abdomen (10 Hz) (104.1% ± 5.1), F (2, 17) = 686.8, P < .001, Dunnett's test after one-way ANOVA (Fig. 6E).
3. Discussion
Our results clearly indicate that RGn500 application produces a neuroprotective effect in the Aβ25–35 mouse model when the light beam, with described parameters, was applied both to the head and abdomen but not when only to the head or abdomen. Although this rodent model is far away from reproducing the complexity of the physiopathological characteristics of dementia in humans, its features may be of interest for better understanding some of the underlying mechanisms. The toxicity induced by the Aβ25–35 peptide in oligomeric form has been repeatedly shown to result 7 days after in neuroinflammation and reactive gliosis [18], [19], pro-apoptotic caspases activity enhancement [18], oxidative stress [20], endogenously produced amyloid protein deposition [18], tau protein hyperphosphorylation [18], and increase of kinases [21], [22], a reduction in the number of neurons measured in hippocampal pyramidal cell layers [17], [23], loss of cholinergic neurons [24], and memory deficits [17], [18], [23], [25], [26], [27].
3.1. Efficacy of PBM treatment
The efficacy of PBM treatment can be compared to what has been found in the same model by daily treatment with various pharmacological substances (Appendix 2). The fact that PBM treatment produces a clear normalization of all parameters that are strongly modified in the model, including memory performances associated to oxidative stress, neuroinflammation, or apoptosis markers and not only specific markers related to the amyloid or tau processes, suggests that this treatment is mobilizing a large number of mechanisms. Only a very specific and limited mechanism would be expected from a classical pharmacological intervention. From that observation, and given the complexity of the aimed pathology, it can be expected that the application of PBM may produce better outcomes than a precise and limited pharmacological treatment.
3.2. Dual mechanism of action
Our results suggest two different mechanisms by which near infrared (NIR) light can induce neuroprotection: a direct stimulation of the damaged cells and an indirect stimulation of as yet unidentified circulating mediators that transduce a protective effect to the brain.
There is strong evidence that photons that have wavelengths in red or NIR spectrum are absorbed in central nervous system structures by specific cellular chromophores localized in the mitochondria. The crucial chromophore identified in the mitochondria is cytochrome c oxydase also known as complex IV. Photonic exposure causes an increase in electron transport and mitochondrial products, such as adenosine triphosphate, β-nicotinamide adenine dinucleotide, reduced, nitric oxide, proteins, and ribonucleic acid [28], leading to the self-repair of the damaged cells, accelerating wound healing and tissue regeneration, increasing circulation, and reducing inflammation and pain [7].
Several studies have shown a real interest for transcranial PBM treatment in neurology and neuropsychiatry with promising results on animals and humans in stroke [29], traumatic brain injury [30], depression [31], anxiety [32], and cognitive enhancement [33], [34]. No peer reviewed study has been published to date in patients with Parkinson's disease or AD except a recent placebo-controlled clinical trial in dementia patients [11]. However, in agreement with our findings, a conclusive study on an amyloid β protein precursor transgenic mouse of AD suggests that transcranial low level laser therapy is a potential candidate for treatment of AD [9].
Indirect stimulation was first evidenced in patients with metastatic cancer: it was reported that localized radiation therapy delivered to the patients can occasionally result in the regression of tumors distant from the irradiation site. Termed “abscopal effect”, this phenomenon is rare but widely reported and is believed to be mediated by a systemic cytokine and/or immune response [35]. Bone marrow–derived stem cells, possibly mesenchymal stem cells, have been identified as possible prime candidates in a model of myocardial infarction in rats [36]. Furthermore, intravenous transplantation of exogenous mesenchymal stem cells was shown to protect mouse dopaminergic neurons against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine toxicity [37] and rat dopaminergic neurons against degeneration due to the proteasome inhibitor MG-132 [38].
Alternative hypotheses to the actions of remote NIR include the modulation of immune cells and effects on inflammatory mediators, such as the upregulation of anti-inflammatory cytokines (IL-4, IL-10) [39].
3.3. Brain-gut axis and AD
The contribution of gut microbiome also needs to be considered and leads to novel hypotheses about new possible therapeutic approaches for AD treatment. First, a direct neural communication occurs between the gut and the brain via the vagal nerve, as bacteria can stimulate afferent neurons of the enteric nervous system [16]. Vagal signals from the gut could induce an anti-inflammatory reaction in a nicotinic acetylcholine receptor α7 subunit–dependent manner [40]. Furthermore, many metabolites have been reported to be produced by gut bacteria that play an important role in the central nervous function not only such as gamma-amino butyric acid [41], serotonin [42], norepinephrine [43], acetylcholine [44], but also indole-3-propionic acid [45], controlling inflammation through interaction with G-protein-coupled receptor 43 expressed on neutrophils and eosinophils [46].
Recent data have demonstrated the link between gut microbiota (GMB) and Parkinson's disease as well as AD. Alterations in the human microbiome have been shown to represent a risk factor for Parkinson's disease [47]. In a mouse model of Parkinson's disease, GMB have been shown to be able to regulate motor deficits and neuroinflammation [48]. Regarding AD, a recent article indicates that an increase in the abundance of a pro-inflammatory GMB taxon, Escherichia/Shigella, and a reduction in the abundance of an anti-inflammatory taxon Eubacterium rectale are possibly associated with a peripheral inflammatory state in patients with cognitive impairment and brain amyloidosis [49]. This finding leads to the hypothesis that the GMB composition may drive peripheral inflammation, contributing to brain amyloidosis and, possibly, neurodegeneration and cognitive symptoms in AD. Conversely, a recent study demonstrated that a 12-week probiotic supplement positively affected cognitive functions and some metabolic statuses in AD patients [50]. It might be possible that the stimulation of the abdomen could change the microbiota with the release of unknown factors producing a neuroprotective action on the brain.
3.4. Future directions
Future studies will be needed to explore various lines of research to test the hypothesis of a dual mechanism underlying the systemic response to NIR treatment and describe its nature. Novel data are being generated in partnership with Institut Fresnel (CNRS, Aix-Marseille Université) aiming at elucidating the interaction of photonic and magnetic exposure with neurons. Furthermore, we plan to devote a particular interest to circulating mediators of inflammation and more particularly to anti-inflammatory cytokines like IL-1, IL-4, IL-10, IL-11, or IL-13. As complementary studies, a metabolomic analysis of caecal content, using proteomics and mass spectrometry, will help to identify metabolic changes produced by NIR and particularly bacterial byproducts, such as short-chain fatty acids and tryptophan, exerting a number of neuromodulatory effects. For example, short-chain fatty acids have been shown to be produced by a number of GMB, such as Bifidobacterium, Propionibacterium, Eubacterium, Lactobacillus, Clostridium, Roseburia, or Prevotella, promoting the secretion of neurotransmitters and hormones and reducing inflammation [51]. Better understanding of the mechanism underlying the effects of RGn500 treatment will be possible by the 16SrRNA high-throughput gene sequencing of GMB allowing the precise analysis of present bacterial communities [52] and possibly understanding the changes produced by the treatment. These studies will allow us to build a solid hypothesis supporting the testing of RGn500 in phase 2B trials.
4. Conclusions
Results obtained in the hereby study suggest that PBM may represent a novel nonpharmacological therapeutic approach for the treatment of AD. The specific combination of PBM/static magnetic field of the so-called emerging therapy we propose, including, furthermore, two application sites showing synergistic effects, points out very promising results, considering especially the extremely severe phenotype of Aβ25–35 mouse model of AD. Yet, a complex human disease such as AD cannot be fully recapitulated in this context. Replicating this alleviated AD phenotype in other preclinical models will be our next step to support clinical investigations. It is however worthy of note to acknowledge that multiple studies have demonstrated that transcranial PBM is safe and well tolerated [7], [53], [54], [55] and clinical investigations have been already reported [10], [12]. Finally, the hypothesis that a dual mechanism of action is involved opens new perspectives for the treatment of AD by widening the therapeutic approach limited up to now by a too reductionistic logic.
Research in context.
-
1.
Systematic review: New therapies are urgently needed to treat affected patients and to prevent, defer, and slow the decline or improve the symptoms of Alzheimer's disease. As no really effective treatment is available to date and as Alzheimer's disease drug development pipeline is unsatisfactory, novel therapeutic approaches differing from traditional pharmacological approaches are worth being considered.
-
2.
Interpretation: Photobiomodulation treatment using the RGn500 device application has shown its efficacy to fully reverse the memory deficits and the biochemical changes occurring in a murine model of Alzheimer's disease. As best efficacy is obtained when both the head and abdomen are exposed, we hypothesize that several mechanisms are involved, comprising direct activation of cellular chromophores at the neuronal level and indirect effects resulting from abdominal exposure.
-
3.
Future directions: We plan to test the hypothesis of dual mechanism involved by devoting a particular interest to circulating anti-inflammatory cytokines, metabolomic analysis of caecal content, and the changes of gut bacterial communities in mice exposed to RGn500.
Acknowledgments
The authors thank Dr. Patrick Lemoine for his constant support from the beginning of the project and for his valuable advice.
Footnotes
The authors have declared that no conflict of interest exists.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.trci.2017.12.003.
Supplementary data
References
- 1.Ando T., Xuan W., Xu T., Dai T., Sharma S.K., Kharkwal G.B. Comparison of therapeutic effects between pulsed and continuous wave 810-nm wavelength laser irradiation for traumatic brain injury in mice. PLoS One. 2011;6:e26212. doi: 10.1371/journal.pone.0026212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Budd Haeberlein S., O'Gorman J., Chiao P., Bussiere T., von Rosenstiel P., Tian Y. Clinical development of Aducanumab, an anti-abeta human monoclonal antibody being investigated for the treatment of early Alzheimer's disease. J Prev Alzheimers Dis. 2017;4:255–263. doi: 10.14283/jpad.2017.39. [DOI] [PubMed] [Google Scholar]
- 3.Sutherland J.C. Biological effects of polychromatic light. Photochem Photobiol. 2002;76:164–170. doi: 10.1562/0031-8655(2002)076<0164:beopl>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 4.Sorrentino V., Romani M., Mouchiroud L., Beck J.S., Zhang H., D'Amico D. Enhancing mitochondrial proteostasis reduces amyloid-beta proteotoxicity. Nature. 2017;552:187–193. doi: 10.1038/nature25143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang Y.Y., Sharma S.K., Carroll J., Hamblin M.R. Biphasic dose response in low level light therapy - an update. Dose Response. 2011;9:602–618. doi: 10.2203/dose-response.11-009.Hamblin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rojas J.C., Gonzalez-Lima F. Neurological and psychological applications of transcranial lasers and LEDs. Biochem Pharmacol. 2013;86:447–457. doi: 10.1016/j.bcp.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 7.Chung H., Dai T., Sharma S.K., Huang Y.Y., Carroll J.D., Hamblin M.R. The nuts and bolts of low-level laser (light) therapy. Ann Biomed Eng. 2012;40:516–533. doi: 10.1007/s10439-011-0454-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farfara D., Tuby H., Trudler D., Doron-Mandel E., Maltz L., Vassar R.J. Low-level laser therapy ameliorates disease progression in a mouse model of Alzheimer's disease. J Mol Neurosci. 2015;55:430–436. doi: 10.1007/s12031-014-0354-z. [DOI] [PubMed] [Google Scholar]
- 9.De Taboada L., Yu J., El-Amouri S., Gattoni-Celli S., Richieri S., McCarthy T. Transcranial laser therapy attenuates amyloid-beta peptide neuropathology in amyloid-beta protein precursor transgenic mice. J Alzheimers Dis. 2011;23:521–535. doi: 10.3233/JAD-2010-100894. [DOI] [PubMed] [Google Scholar]
- 10.Saltmarche A.E., Naeser M.A., Ho K.F., Hamblin M.R., Lim L. Significant improvement in cognition in mild to moderately severe dementia cases treated with transcranial plus intranasal photobiomodulation: case series report. Photomed Laser Surg. 2017;35:432–441. doi: 10.1089/pho.2016.4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berman M.H., Halper J.P., Nichols T.W., Jarrett H., Lundy A., Huang J.H. Photobiomodulation with near infrared light helmet in a pilot, placebo controlled clinical trial in dementia patients testing memory and cognition. J Neurol Neurosci. 2017;8 doi: 10.21767/2171-6625.1000176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee J., Choi B.H., Oh E., Sohn E.H., Lee A.Y. Treatment of Alzheimer's disease with repetitive transcranial magnetic stimulation combined with cognitive training: a prospective, randomized, double-Blind, placebo-controlled study. J Clin Neurol. 2016;12:57–64. doi: 10.3988/jcn.2016.12.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ziemann U. Thirty years of transcranial magnetic stimulation: where do we stand? Exp Brain Res. 2017;235:973–984. doi: 10.1007/s00221-016-4865-4. [DOI] [PubMed] [Google Scholar]
- 14.Swerdlow R.H., Burns J.M., Khan S.M. The Alzheimer's disease mitochondrial cascade hypothesis. J Alzheimers Dis. 2010;20 Suppl 2:S265–S279. doi: 10.3233/JAD-2010-100339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guerriero F., Sgarlata C., Francis M., Maurizi N., Faragli A., Perna S. Neuroinflammation, immune system and Alzheimer disease: searching for the missing link. Aging Clin Exp Res. 2017;29:821–831. doi: 10.1007/s40520-016-0637-z. [DOI] [PubMed] [Google Scholar]
- 16.Forsythe P., Bienenstock J., Kunze W.A. Vagal pathways for microbiome-brain-gut axis communication. Adv Exp Med Biol. 2014;817:115–133. doi: 10.1007/978-1-4939-0897-4_5. [DOI] [PubMed] [Google Scholar]
- 17.Maurice T., Lockhart B.P., Privat A. Amnesia induced in mice by centrally administered beta-amyloid peptides involves cholinergic dysfunction. Brain Res. 1996;706:181–193. doi: 10.1016/0006-8993(95)01032-7. [DOI] [PubMed] [Google Scholar]
- 18.Klementiev B., Novikova T., Novitskaya V., Walmod P.S., Dmytriyeva O., Pakkenberg B. A neural cell adhesion molecule-derived peptide reduces neuropathological signs and cognitive impairment induced by Abeta25-35. Neuroscience. 2007;145:209–224. doi: 10.1016/j.neuroscience.2006.11.060. [DOI] [PubMed] [Google Scholar]
- 19.Chavant F., Deguil J., Pain S., Ingrand I., Milin S., Fauconneau B. Imipramine, in part through tumor necrosis factor alpha inhibition, prevents cognitive decline and beta-amyloid accumulation in a mouse model of Alzheimer's disease. J Pharmacol Exp Ther. 2010;332:505–514. doi: 10.1124/jpet.109.162164. [DOI] [PubMed] [Google Scholar]
- 20.Stepanichev M.Y., Zdobnova I.M., Zarubenko I.I., Moiseeva Y.V., Lazareva N.A., Onufriev M.V. Amyloid-beta(25-35)-induced memory impairments correlate with cell loss in rat hippocampus. Physiol Behav. 2004;80:647–655. doi: 10.1016/j.physbeh.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 21.Lahmy V., Long R., Morin D., Villard V., Maurice T. Mitochondrial protection by the mixed muscarinic/σ1 ligand ANAVEX2-73, a tetrahydrofuran derivative, in Aβ25–35 peptide-injected mice, a nontransgenic Alzheimer's disease model. Front Cell Neurosci. 2014;8:463. doi: 10.3389/fncel.2014.00463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Noh M.Y., Koh S.H., Kim S.M., Maurice T., Ku S.K., Kim S.H. Neuroprotective effects of donepezil against Abeta42-induced neuronal toxicity are mediated through not only enhancing PP2A activity but also regulating GSK-3beta and nAChRs activity. J Neurochem. 2013;127:562–574. doi: 10.1111/jnc.12319. [DOI] [PubMed] [Google Scholar]
- 23.Stepanichev M.Y., Zdobnova I.M., Zarubenko I.I., Lazareva N.A., Gulyaeva N.V. Studies of the effects of central administration of beta-amyloid peptide (25-35): pathomorphological changes in the Hippocampus and impairment of spatial memory. Neurosci Behav Physiol. 2006;36:101–106. doi: 10.1007/s11055-005-0167-1. [DOI] [PubMed] [Google Scholar]
- 24.Stepanichev M., Lazareva N., Tukhbatova G., Salozhin S., Gulyaeva N. Transient disturbances in contextual fear memory induced by Abeta(25-35) in rats are accompanied by cholinergic dysfunction. Behav Brain Res. 2014;259:152–157. doi: 10.1016/j.bbr.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 25.Villard V., Espallergues J., Keller E., Vamvakides A., Maurice T. Anti-amnesic and neuroprotective potentials of the mixed muscarinic receptor/sigma 1 (sigma1) ligand ANAVEX2-73, a novel aminotetrahydrofuran derivative. J Psychopharmacol. 2011;25:1101–1117. doi: 10.1177/0269881110379286. [DOI] [PubMed] [Google Scholar]
- 26.Zussy C., Brureau A., Delair B., Marchal S., Keller E., Ixart G. Time-course and regional analyses of the physiopathological changes induced after cerebral injection of an amyloid beta fragment in rats. Am J Pathol. 2011;179:315–334. doi: 10.1016/j.ajpath.2011.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meunier J., Borjini N., Gillis C., Villard V., Maurice T. Brain toxicity and inflammation induced in vivo in mice by the amyloid-beta forty-two inducer aftin-4, a roscovitine derivative. J Alzheimers Dis. 2015;44:507–524. doi: 10.3233/JAD-140711. [DOI] [PubMed] [Google Scholar]
- 28.de Freitas L.F., Hamblin M.R. Proposed mechanisms of photobiomodulation or low-level light therapy. IEEE J Sel Top Quantum Electron. 2016;22 doi: 10.1109/JSTQE.2016.2561201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lampl Y. Laser treatment for stroke. Expert Rev Neurother. 2007;7:961–965. doi: 10.1586/14737175.7.8.961. [DOI] [PubMed] [Google Scholar]
- 30.Thunshelle C., Hamblin M.R. Transcranial low-level laser (light) therapy for brain injury. Photomed Laser Surg. 2016;34:587–598. doi: 10.1089/pho.2015.4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cassano P., Petrie S.R., Hamblin M.R., Henderson T.A., Iosifescu D.V. Review of transcranial photobiomodulation for major depressive disorder: targeting brain metabolism, inflammation, oxidative stress, and Neurogenesis. Neurophotonics. 2016;3:031404. doi: 10.1117/1.NPh.3.3.031404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schiffer F., Johnston A.L., Ravichandran C., Polcari A., Teicher M.H., Webb R.H. Psychological benefits 2 and 4 weeks after a single treatment with near infrared light to the forehead: a pilot study of 10 patients with major depression and anxiety. Behav Brain Funct. 2009;5:46. doi: 10.1186/1744-9081-5-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gonzalez-Lima F., Barrett D.W. Augmentation of cognitive brain functions with transcranial lasers. Front Syst Neurosci. 2014;8:36. doi: 10.3389/fnsys.2014.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blanco N.J., Maddox W.T., Gonzalez-Lima F. Improving executive function using transcranial infrared laser stimulation. J Neuropsychol. 2017;11:14–25. doi: 10.1111/jnp.12074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bramhall R.J., Mahady K., Peach A.H. Spontaneous regression of metastatic melanoma - clinical evidence of the abscopal effect. Eur J Surg Oncol. 2014;40:34–41. doi: 10.1016/j.ejso.2013.09.026. [DOI] [PubMed] [Google Scholar]
- 36.Tuby H., Maltz L., Oron U. Induction of autologous mesenchymal stem cells in the bone marrow by low-level laser therapy has profound beneficial effects on the infarcted rat heart. Lasers Surg Med. 2011;43:401–409. doi: 10.1002/lsm.21063. [DOI] [PubMed] [Google Scholar]
- 37.Chao Y.X., He B.P., Tay S.S. Mesenchymal stem cell transplantation attenuates blood brain barrier damage and neuroinflammation and protects dopaminergic neurons against MPTP toxicity in the substantia nigra in a model of Parkinson's disease. J Neuroimmunol. 2009;216:39–50. doi: 10.1016/j.jneuroim.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 38.Park H.J., Lee P.H., Bang O.Y., Lee G., Ahn Y.H. Mesenchymal stem cells therapy exerts neuroprotection in a progressive animal model of Parkinson's disease. J Neurochem. 2008;107:141–151. doi: 10.1111/j.1471-4159.2008.05589.x. [DOI] [PubMed] [Google Scholar]
- 39.Muili K.A., Gopalakrishnan S., Meyer S.L., Eells J.T., Lyons J.A. Amelioration of experimental autoimmune encephalomyelitis in C57BL/6 mice by photobiomodulation induced by 670 nm light. PLoS One. 2012;7:e30655. doi: 10.1371/journal.pone.0030655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Borre Y.E., Moloney R.D., Clarke G., Dinan T.G., Cryan J.F. The impact of microbiota on brain and behavior: mechanisms & therapeutic potential. Adv Exp Med Biol. 2014;817:373–403. doi: 10.1007/978-1-4939-0897-4_17. [DOI] [PubMed] [Google Scholar]
- 41.Barrett E., Ross R.P., O'Toole P.W., Fitzgerald G.F., Stanton C. gamma-Aminobutyric acid production by culturable bacteria from the human intestine. J Appl Microbiol. 2012;113:411–417. doi: 10.1111/j.1365-2672.2012.05344.x. [DOI] [PubMed] [Google Scholar]
- 42.Shishov V.A., Kirovskaia T.A., Kudrin V.S., Oleskin A.V. [Amine neuromediators, their precursors, and oxidation products in the culture of Escherichia coli K-12] Prikl Biokhim Mikrobiol. 2009;45:550–554. [PubMed] [Google Scholar]
- 43.Tsavkelova E.A., Botvinko I.V., Kudrin V.S., Oleskin A.V. Detection of neurotransmitter amines in microorganisms with the use of high-performance liquid chromatography. Dokl Biochem. 2000;372:115–117. [PubMed] [Google Scholar]
- 44.Kawashima K., Yoshikawa K., Fujii Y.X., Moriwaki Y., Misawa H. Expression and function of genes encoding cholinergic components in murine immune cells. Life Sci. 2007;80:2314–2319. doi: 10.1016/j.lfs.2007.02.036. [DOI] [PubMed] [Google Scholar]
- 45.Bendheim P.E., Poeggeler B., Neria E., Ziv V., Pappolla M.A., Chain D.G. Development of indole-3-propionic acid (OXIGON) for Alzheimer's disease. J Mol Neurosci. 2002;19:213–217. doi: 10.1007/s12031-002-0036-0. [DOI] [PubMed] [Google Scholar]
- 46.Maslowski K.M., Vieira A.T., Ng A., Kranich J., Sierro F., Yu D. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature. 2009;461:1282–1286. doi: 10.1038/nature08530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mulak A., Bonaz B. Brain-gut-microbiota axis in Parkinson's disease. World J Gastroenterol. 2015;21:10609–10620. doi: 10.3748/wjg.v21.i37.10609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sampson T.R., Debelius J.W., Thron T., Janssen S., Shastri G.G., Ilhan Z.E. Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson's disease. Cell. 2016;167:1469–1480.e12. doi: 10.1016/j.cell.2016.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cattaneo A., Cattane N., Galluzzi S., Provasi S., Lopizzo N., Festari C. Association of brain amyloidosis with pro-inflammatory gut bacterial taxa and peripheral inflammation markers in cognitively impaired elderly. Neurobiol Aging. 2017;49:60–68. doi: 10.1016/j.neurobiolaging.2016.08.019. [DOI] [PubMed] [Google Scholar]
- 50.Akbari E., Asemi Z., Daneshvar Kakhaki R., Bahmani F., Kouchaki E., Tamtaji O.R. Effect of probiotic supplementation on cognitive function and metabolic status in Alzheimer's disease: a randomized, double-blind and controlled trial. Front Aging Neurosci. 2016;8:256. doi: 10.3389/fnagi.2016.00256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Russell W.R., Hoyles L., Flint H.J., Dumas M.E. Colonic bacterial metabolites and human health. Curr Opin Microbiol. 2013;16:246–254. doi: 10.1016/j.mib.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 52.Lelouvier B., Servant F., Delobel P., Courtney M., Elbaz M., Amar J. Identification by highly sensitive 16S metagenomic sequencing of an unusual case of polymicrobial bacteremia. J Infect. 2017;75:278–280. doi: 10.1016/j.jinf.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 53.Hashmi J.T., Huang Y.Y., Osmani B.Z., Sharma S.K., Naeser M.A., Hamblin M.R. Role of low-level laser therapy in neurorehabilitation. PM R. 2010;2:S292–305. doi: 10.1016/j.pmrj.2010.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Avci P., Gupta A., Sadasivam M., Vecchio D., Pam Z., Pam N. Low-level laser (light) therapy (LLLT) in skin: stimulating, healing, restoring. Semin Cutan Med Surg. 2013;32:41–52. [PMC free article] [PubMed] [Google Scholar]
- 55.Oliviero A., Carrasco-Lopez M.C., Campolo M., Perez-Borrego Y.A., Soto-Leon V., Gonzalez-Rosa J.J. Safety study of transcranial Static Magnetic Field Stimulation (tSMS) of the human cortex. Brain Stimul. 2015;8:481–485. doi: 10.1016/j.brs.2014.12.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.