Abstract
Anti-cancer tyrosine kinase inhibitors (TKIs) are effective in many types of cancers including non-small cell lung cancer, while appearance of TKI-resistant tumors suggests a need for the development of their potentiation strategies. We have previously shown that a methoxyflavanone derivative from the Asian medicinal herb Perilla frutescens (Perilla-derived methoxyflavanone; PDMF) shows a prominent anti-tumor activity against A549 human lung adenocarcinoma. Here we show that PDMF and anti-cancer TKIs (nilotinib, bosutinib, dasatinib, and ponatinib) synergistically suppress proliferation of A549 cells. Flow cytometric analysis indicated that co-stimulation with nilotinib (4 μM) and PDMF induced G2/M cell cycle arrest in low PDMF doses (10–50 μM), whereas this combination triggered de novo G1 arrest in higher PDMF dosages (50–125 μM). We also found that co-administration with nilotinib and PDMF significantly suppressed in vivo tumorigenicity of A549 cells in athymic nude mice.
Electronic supplementary material
The online version of this article (doi:10.1007/s10616-017-0124-1) contains supplementary material, which is available to authorized users.
Keywords: A549 cells, Lung cancer, Methoxyflavanone, Perilla frutescens, Tyrosine kinase inhibitors
Introduction
Lung cancer is the leading cause of cancer-associated mortality worldwide (Siegel et al. 2013). In the clinical practice, lung carcinomas are divided into two main histological types: small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC), where the other accounts for 80% of total lung cancer cases (Meoni et al. 2013). The main characteristic of NSCLC is its high aggressive invasion and metastatic properties. Therefore, the majority of advanced NSCLC cases require receiving combination of chemotherapy, and/or radiation treatment (Molina et al. 2008). Tyrosine kinase inhibitors (TKIs) are now the golden stone of treatment in NSCLC (Gridelli et al. 2014). These drugs target biological functions that cancer cells are critically reliant on, ultimately causing tumor shrinkage (Krause and Van Etten 2005). However, a serious problem is that many of patients who especially receive the epidermal growth factor receptor (EGFR)-TKI therapy get resistance to the agents (Rosell et al. 2009), suggesting a need for the development of other chemotherapeutic drugs. Thus, there remains a need for strategies to safely increase the effectiveness of TKIs.
Recently, a considerable attention has been given to the identification of new therapeutic agents that show synergistic effect with TKIs as a promising direction to overcome the above-mentioned problems. In general, combination therapies not only potentiate the therapeutic efficacy of each agent alone, but also enable the use of reduced doses (Milano et al. 2009; Nautiyal et al. 2011). In this context, plant flavonoids, especially those from dietary sources, are commonly perceived as non-toxic, well-tolerated, easily available, and inexpensive compounds that can target multiple cellular pathways (Surh 2003; Sak 2012). It has been shown that flavonoids have anti-tumor activities through induction of apoptosis and cell cycle arrest of tumor cells (Szliszka et al. 2008; Kim et al. 2013). In particular, flavanones are a type of flavonoids that exhibit chemo-preventive and anticancer properties (Graf et al. 2005). Previous in vitro studies showed that naturally-occurring flavanones have anti-tumor activity against human cancer cells; e.g. naringenin in THP-1 and U937 leukemia cells (Park et al. 2008b; Jin et al. 2009), hesperidin in SNUC4 colon cancer and NALM6 leukemia cells (Park et al. 2008a; Ghorbani et al. 2012), and liquiritigenin in SMMC7721 hepatocarcinoma and HeLa cervical cancer cells (Zhang et al. 2009; Liu et al. 2011). Flavanones also received a considerable attention as these agents may potentiate the cytotoxic effects of chemotherapy and radiotherapy, protect normal cells from therapy-associated toxicity, increase a systemic bioavailability of cytostatic agents, and in some cases, even overcome chemoresistance (Garg et al. 2005; Kuno et al. 2012).
Recently, our group has identified a new class of methoxyflavanone derivative (8-hydroxy-5,7-dimethoxyflavanone) from the Asian dietary herb, Perilla frutescens (named Perilla-derived methoxyflavanone, PDMF). PDMF selectively suppresses Akt activation to inhibit type I hypersensitivity reactions (Kamei et al. 2017). We also have found that PDMF shows prominent tumor suppressive activity on A549 human adenocarcinoma through induction of p53-driven G2/M cell cycle arrest and apoptosis (Abd El-Hafeez et al. unpublished data). In the present study, we show a synergistic tumor-suppressive potency of PDMF and anti-cancer TKIs on A549 cells in vitro as well as in vivo.
Materials and methods
Cell cultures and reagents
The A549 human lung adenocarcinoma was obtained from the RIKEN CELL BANK (Tsukuba, Japan), and was cultured in Dulbecco’s-modified Eagle’s medium (DMEM; Life Technologies, Tokyo, Japan) containing 10% fetal bovine serum (FBS; Sigma-Aldrich, St. Louis, MO, USA), 100 U/ml penicillin, and 100 μg/ml streptomycin (Life Technologies) in a humidified atmosphere with 5% CO2 at 37 °C. Perilla-derived methoxyflavanone (PDMF; 8-hydroxy-5,7-dimethoxyflavanone) (Kamei et al. 2017) was chemically synthesized by Tokyo Chemical Industry (Tokyo, Japan). Nilotinib, bosutinib, dasatinib, and ponatinib were purchased from LC laboratories (Woburn, MA, USA). Doxorubicin was purchased from Sigma-Aldrich. All chemicals used in this study were of analytical or cell culture grade.
Cell proliferation assay
Cell proliferation was analyzed by BrdU incorporation using a Colorimetric Cell Proliferation ELISA, BrdU Kit (Roche Diagnostics, Indianapolis, IN, USA) according to the manufacturer’s instructions. A549 cells were seeded at 5 × 103 cells/well and cultured overnight in a 96-well plate. The cells were then treated with sub-optimal doses of PDMF alone (10, 50, 100 or 125 μM), or in combination with sub-optimal doses of Bcr-Abl TKIs (4 μM nilotinib, 1 μM bosutinib, 50 nM dasatinib or 0.5 μM ponatinib) for 24 h. The doses of PDMF and TKIs were determined by a preliminary dose–response study on A549 cells, in which reduction in cell proliferation is limited up to 20%. A549 cells were also treated with either 1 μM doxorubicin as a positive control or dimethyl sulfoxide (DMSO, vehicle) as a negative control. BrdU was added to a final concentration of 10 μM and cultured for the last 2 h.
Drug interaction analysis
To evaluate whether the anti-tumor effects of the combination of PDMF and TKIs were synergistic, the drug interactions were analyzed based on the combination index method of Chou and Talalay (1984) using a CompuSyn software (ComboSyn, Paramus, NJ, USA). The dose response curves for single agents and their combinations were generated, and the combination index (CI) values were calculated. The resulting CI values of 0.1–0.3 indicate strong synergism, those of 0.3–0.7 for synergism, 0.7–0.85 for moderate synergism, and 0.85–0.90 for slight synergism.
Cell cycle and apoptosis analyses
Cell cycle progression or induction of apoptosis of A549 cells was analyzed by a FACSCalibur flow cytometer (BD Biosciences, San Jose, CA, USA) using the BD Pharmingen™ FITC-BrdU Flow Kit (BD Biosciences) or the Annexin V/Propidium Iodide (PI) Staining Kit (BioLegend, San Diego, CA, USA), respectively. A549 cells were treated with 10, 50, 100 or 125 μM PDMF alone, or in combination with 4 μM nilotinib. A549 cells were also treated with 8 μM nilotinib alone or 1 μM doxorubicin for positive controls. Quantitative analysis of those flow cytometry data was performed with FlowJo software (FlowJo, Ashland, OR, USA).
Animal experiments
All animal experiments were carried out using protocols approved by the Committee on Animal Experimentation of Hiroshima University, Japan. Four-week-old female BALB/c nude mice were purchased from Charles River Laboratories Japan (Kanagawa, Japan), and kept under specific pathogen-free conditions.
For construction of A549 cell xenograft model, BALB/c nude mice (n = 42) were subcutaneously (s.c.) injected with 5 × 106 A549 cells into their dorsal skins. The mice were then randomly divided into seven groups (six mice each), and s.c. administered with 40 µl of 0.5% DMSO in PBS (control), 10 µM PDMF (6.4 ng/g murine body weight), 125 µM PDMF (79.7 ng/g body weight), 4 µM nilotinib (4.5 ng/g body weight), 4 μM nilotinib with 10 µM PDMF, 4 µM nilotinib with 125 µM PDMF, or 20 µM nilotinib (22.5 ng/g body weight). Those agents were started to be injected every three days from the next day of the A549 cell transplantation. On day 18, tumor volumes were started to be monitored every three days. Tumor volume was calculated as (wide2 × length)/2 of formulated tumor (Tomayko and Reynolds 1989). On day 36, mice were sacrificed and their final tumor weights were quantified.
Statistical analysis
Data are represented as mean ± SD. Student’s t test was performed to determine the statistical significance compared to corresponding controls. Statistical significance was defined as P < 0.05 or P < 0.005. The data shown in the figures are representative data for three independent experimental results.
Results and discussion
PDMF and anti-cancer TKIs synergistically suppress cell proliferation of A549 human lung cancer cells
A549 human lung adenocarcinoma cells are used as a model of EGFR-TKI-resistant NSCLC cells (Ito et al. 2014; Cao et al. 2016). For those EGFR-TKI-resistant cells, the Bcr-Abl type of TKIs may offer alternative anti-cancer agent, since the EGFR-TKI-resistant A549 cells indeed show a good response to a Bcr-Abl TKI (Zhang et al. 2003), probably because of its cross-reaction to a wide array of oncogenic TKs. For those reasons, here we decided to analyze the combination anti-tumor effect of Bcr-Abl TKIs (nilotinib, bosutinib, dasatinib and ponatinib) and PDMF on A549 cells.
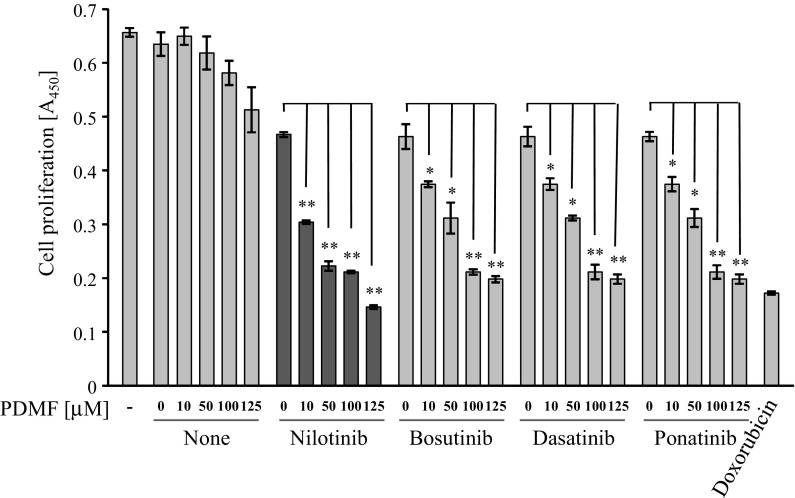
To test whether combination of PDMF and TKIs presents a synergistic anti-cancer effect, A549 cells were stimulated with serial doses of PDMF (10, 50, 100, and 125 μM) and sub-optimal doses of TKIs (nilotinib, bosutinib, dasatinib and ponatinib). BrdU incorporation assay revealed that those combination regimens resulted in a greater loss of the cell proliferation compared to the individual agents, suggesting a synergistic growth inhibitory effect of PDMF and TKIs on A549 cells (Fig. 1). To further confirm the synergism, interactions between PDMF and TKIs were analyzed with the median-effect principle of Chou and Talalay (1984). The resulting combination index (CI) theorem of the Chou–Talalay analysis offers quantitative definition for additive effect (CI = 1), synergism (CI < 1), and antagonism (CI > 1) in drug combinations. The CI values in the experimental points indicated that all the combination regimens of PDMF and TKIs showed strong synergism (with CI of 0.1–0.3) or synergism (with CI of 0.3–0.7) (Supplementary Table 1), clearly indicating that PDMF and those TKIs synergistically suppress proliferation of A549 cells. Among four TKIs tested, the overall maximal synergism was noted when PDMF was combined with nilotinib (shown underlined in Supplementary Table 1). We therefore selected nilotinib for further analysis.
Fig. 1.
PDMF and anti-cancer TKIs synergistically suppress proliferation of A549 human lung adenocarcinoma. Inhibitory effects of sub-optimal doses of PDMF (10–125 μM), sub-optimal doses of Bcr-Abl TKIs (4 μM nilotinib, 1 μM bosutinib, 0.05 μM dasatinib, or 0.5 μM ponatinib), and their combinations on the proliferation of A549 cells were determined by BrdU incorporation assay. The sub-optimal doses of PDMF and TKIs were determined by a preliminary dose–response study on A549 cells, in which a reduction in cell proliferation is limited up to 20%. Doxorubicin (1 μM, DNA damaging agent) and dimethyl sulfoxide (DMSO, vehicle) are set as a positive and negative vehicle controls, respectively. Representative data of three independent experiments are shown as mean ± SD of three replicates. *P < 0.05 and **P < 0.005 indicate significant differences assessed by Student’s t test
Co-stimulation with PDMF and anti-cancer TKI (nilotinib) synergistically induces cell cycle arrest at G1 and G2/M phases
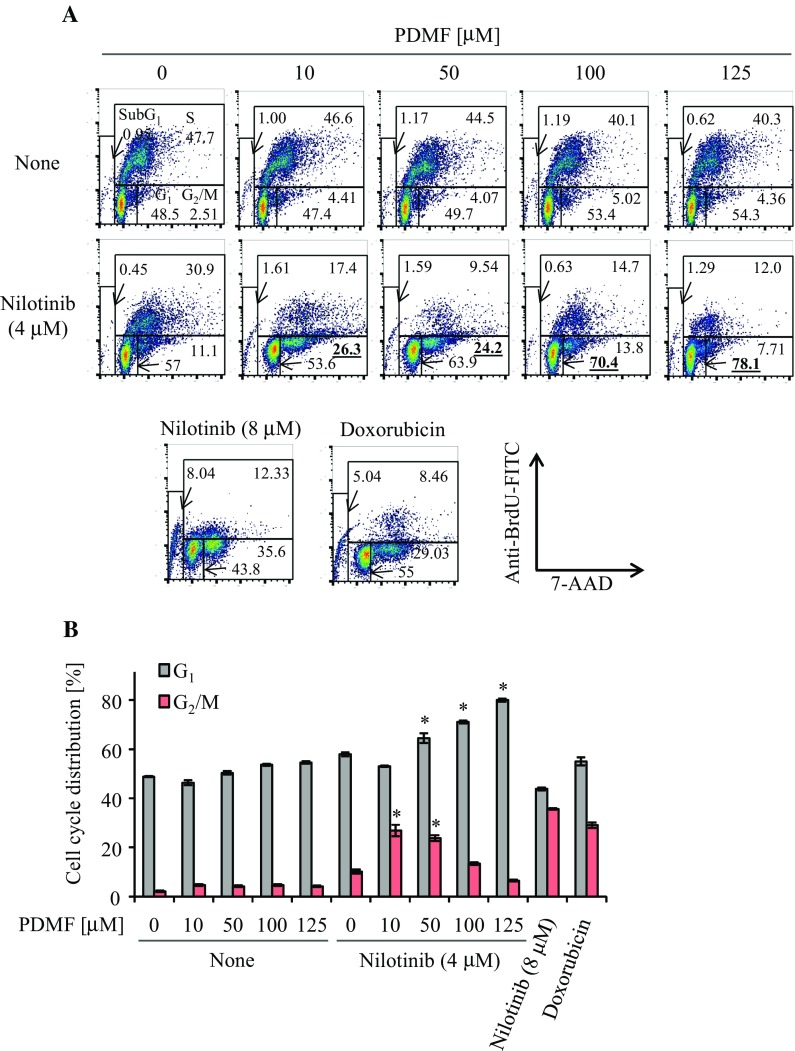
We next analyzed impact of the PDMF/nilotinib co-stimulation on cell cycle progression of A549 cells. Flow cytometric analysis indicated that low doses of PDMF (10–50 µM) and nilotinib (4 µM) synergistically induced G2/M cell cycle arrest in A549 cells (Fig. 2a, b). Intriguingly, we found that co-stimulation with higher doses of PDMF (50–125 µM) synergized with nilotinib to induce de novo G1 cell cycle arrest (Fig. 2a, b), suggesting that the synergistic anti-cancer effect of PDMF and nilotinib is executed by a two-stage cell cycle arrest depending on the PDMF dosages. We also found that the PDMF co-stimulation had no effect on apoptosis induction in A549 cells (Supplementary Fig. 1), suggesting that the synergistic tumor suppressive effect is mainly fulfilled through augmentation of G1 and G2/M cell cycle arrest.
Fig. 2.
PDMF synergizes with nilotinib to induce G1 and G2/M cell cycle arrest on A549 cells. a Representative data are shown for the cell cycle distribution analyzed by flow cytometry. A549 cells were treated with a serial concentration of PDMF (10–125 μM) in the presence or absence of nilotinib (4 µM). 8 µM nilotinib (TKI only at higher dose) and 1 µM doxorubicin are set as positive controls. b Quantification of G1 and G2/M cell cycle arrest of A549 cells upon co-stimulation with PDMF and nilotinib. The values are the mean ± SD of three different experiments. *P < 0.05 and **P < 0.005 indicate significant differences compared with nilotinib only (4 µM)
Mechanisms underlying the PDMF/nilotinib-driven G1 (high PDMF doses) and G2/M (low PDMF doses) cell cycle arrest are currently unknown. Our preliminary data indicate that co-stimulation with a high dose PDMF (125 μM) synergized with nilotinib to induce cyclin-dependent kinase (CDK) inhibitor p21, whereas a low dose PDMF (10 μM) and nilotinib synergistically decreased protein levels of CDK1 and cyclin B1, that form a hallmark cyclin/CDK complex in the G2/M cell cycle checkpoint (data not shown). Thus, one possible explanation is that the synergistic two-stage cell cycle arrest could be fulfilled through direct up- and down-modulation of those G1 and G2/M cell cycle checkpoint molecules. Another critical issue is that a possible anti-cancer effect after more than 24 h remains to be investigated in the present study. Further rigorous analysis is needed to elucidate the precise mechanisms by which the co-stimulation with nilotinib and PDMF fulfills anti-tumor potency.
Co-administration with PDMF and nilotinib suppresses tumorigenicity of A549 cells in athymic nude mice
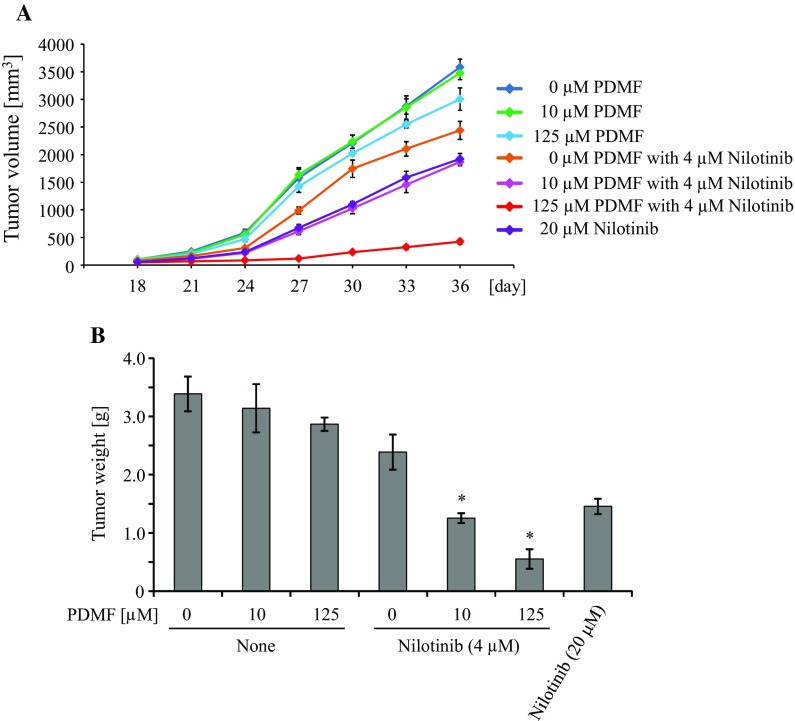
To further test the synergistic anti-tumor effect of PDMF and nilotinib in vivo, we co-administered PDMF and nilotinib to an A549 cell-xenograft model on athymic nude mice. We found that co-treatment with PDMF and nilotinib significantly impaired tumorigenicity of A549 cells as compared to nilotinib alone or PDMF alone (Fig. 3a); especially, co-administration with 125 µM PDMF markedly suppress in vivo tumor development. The PDMF co-treatment also significantly decreased final A549 tumor weight in a dose-dependent manner (Fig. 3b). These results suggest that co-administration with PDMF and nilotinib to cancer-adjacent sites prevent in vivo tumorigenesis of A549 cells. To further gain insight into the application of PDMF, oral and/or systemic administration study of PDMF and nilotinib should be conducted in the next studies. Another unsolved issue is safety and specificity of the PDMF/nilotinib combination. We preliminary observe that PDMF by itself is less cytotoxic to WI-38 human normal lung fibroblast cells than to A549 cells (Abd El-Hafeez et al., unpublished data), but cancer selectivity as well as safety issue of the combination regimen should be overcome in future studies.
Fig. 3.
Co-administration of PDMF and nilotinib impairs in vivo tumorigenicity of A549 cells in athymic nude mice. Six week-old BALB/c nude mice were subcutaneously (s.c.) injected with A549 cells (5 × 106 cells/head). Mice were then randomly assigned to seven groups (six mice per group) that were s.c. administered with 40 µl of 0.5% DMSO in PBS (control), 10 µM PDMF (6.4 ng/g murine body weight), 125 µM PDMF (79.7 ng/g body weight), 4 µM nilotinib (4.5 ng/g body weight), 4 μM nilotinib with 10 µM PDMF, 4 µM nilotinib with 125 µM PDMF, or 20 µM nilotinib (22.5 ng/g body weight). 18 days after xenotransplantation, tumor volumes were monitored each three days (a). On day 36, mice were euthanized and final tumor weights were quantified (b). *P < 0.05 indicates significant difference compared with nilotinib only (4 µM)
In conclusion, here we show that a P. frutescens-derived flavanone derivative (PDMF) synergizes with anti-cancer TKI to augment tumor suppressive potency on A549 human adenocarcinoma in vitro as well as in vivo. Mechanistically, the synergistic anti-tumor effect is fulfilled by the induction of a two-stage cell cycle arrest at G1 and G2/M phases. Further investigation is required to develop the combinational cancer preventive strategies utilizing PDMF and anti-cancer TKIs.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This work was financially supported by the Mishima Food Co., Ltd (to S. Kawamoto). N. Hirakawa and K. Baba are employees of the Mishima Food Co., Ltd. A. A. Abd El-Hafeez was supported by the Ministry of Education, Culture, Sports, Science, and Technology, MEXT, Japan.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1007/s10616-017-0124-1) contains supplementary material, which is available to authorized users.
Amer Ali Abd El-Hafeez and Takashi Fujimura have contributed equally to this work.
References
- Cao H, Yu S, Chen D, Jing C, Wang Z, Ma R, Liu S, Ni J, Feng J, Wu J. Liver X receptor agonist T0901317 reverses resistance of A549 human lung cancer cells to EGFR-TKI treatment. FEBS Open Bio. 2016;7:35–43. doi: 10.1002/2211-5463.12147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Reg. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Garg AK, Buchholz TA, Aggarwal BB. Chemosensitization and radiosensitization of tumors by plant polyphenols. Antioxid Redox Signal. 2005;7:1630–1647. doi: 10.1089/ars.2005.7.1630. [DOI] [PubMed] [Google Scholar]
- Ghorbani A, Nazari M, Jeddi-Tehrani M, Zand H. The citrus flavonoid hesperidin induces p53 and inhibits NF-κB activation in order to trigger apoptosis in NALM-6 cells: involvement of PPARγ-dependent mechanism. Eur J Nutr. 2012;51:39–46. doi: 10.1007/s00394-011-0187-2. [DOI] [PubMed] [Google Scholar]
- Graf BA, Milbury PE, Blumberg JB. Flavonols, flavones, flavanones, and human health: epidemiological evidence. J Med Food. 2005;8:281–290. doi: 10.1089/jmf.2005.8.281. [DOI] [PubMed] [Google Scholar]
- Gridelli C, De Marinis F, Cappuzzo F, Di Maio M, Hirsch FR, Mok T, Morgillo F, Rosell R, Spigel DR, Yang JC, Ciardiello F. Treatment of advanced non–small-cell lung cancer with epidermal growth factor receptor (EGFR) mutation or ALK gene rearrangement: results of an international expert panel meeting of the Italian Association of Thoracic Oncology. Clin Lung Cancer. 2014;15:173–181. doi: 10.1016/j.cllc.2013.12.002. [DOI] [PubMed] [Google Scholar]
- Ito K, Semba T, Uenaka T, Wakabayashi T, Asada M, Funahashi Y. Enhanced anti-angiogenic effect of E7820 in combination with erlotinib in epidermal growth factor receptor-tyrosine kinase inhibitor-resistant non-small-cell lung cancer xenograft models. Cancer Sci. 2014;105:1023–1031. doi: 10.1111/cas.12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin CY, Park C, Lee JH, Chung KT, Kwon TK, Kim GY, Choi BT, Choi YH. Naringenin-induced apoptosis is attenuated by Bcl-2 but restored by the small molecule Bcl-2 inhibitor, HA 14-1, in human leukemia U937 cells. Toxicol In Vitro. 2009;23:259–265. doi: 10.1016/j.tiv.2008.12.005. [DOI] [PubMed] [Google Scholar]
- Kamei R, Fujimura T, Matsuda M, Kakihara K, Hirakawa N, Baba K, Ono K, Arakawa K, Kawamoto S. A flavanone derivative from the Asian medicinal herb (Perilla frutescens) potently suppresses IgE-mediated immediate hypersensitivity reactions. Biochem Biophys Res Commun. 2017;483:674–679. doi: 10.1016/j.bbrc.2016.12.083. [DOI] [PubMed] [Google Scholar]
- Kim YA, Kim H, Seo Y. Antiproliferative effect of flavonoids from the halophyte Vitex rotundifolia on human cancer cells. Nat Prod Commun. 2013;8:1405–1408. [PubMed] [Google Scholar]
- Krause DS, Van Etten RA. Tyrosine kinases as targets for cancer therapy. N Engl J Med. 2005;353:172–187. doi: 10.1056/NEJMra044389. [DOI] [PubMed] [Google Scholar]
- Kuno T, Tsukamoto T, Hara A, Tanaka T. Cancer chemoprevention through the induction of apoptosis by natural compounds. J Biophys Chem. 2012;3:19431. [Google Scholar]
- Liu C, Wang Y, Xie S, Zhou Y, Ren X, Li X, Cai Y. Liquiritigenin induces mitochondria-mediated apoptosis via cytochrome c release and caspases activation in HeLa Cells. Phytother Res. 2011;25:277–283. doi: 10.1002/ptr.3259. [DOI] [PubMed] [Google Scholar]
- Meoni G, Cecere FL, Lucherini E, Di Costanzo F. Medical treatment of advanced non-small cell lung cancer in elderly patients: a review of the role of chemotherapy and targeted agents. J Geriatr Oncol. 2013;4:282–290. doi: 10.1016/j.jgo.2013.04.005. [DOI] [PubMed] [Google Scholar]
- Milano V, Piao Y, LaFortune T, de Groot J. Dasatinib-induced autophagy is enhanced in combination with temozolomide in glioma. Mol Cancer Ther. 2009;8:394–406. doi: 10.1158/1535-7163.MCT-08-0669. [DOI] [PubMed] [Google Scholar]
- Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc. 2008;5:584–594. doi: 10.1016/S0025-6196(11)60735-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nautiyal J, Kanwar SS, Yu Y, Majumdar AP. Combination of dasatinib and curcumin eliminates chemo-resistant colon cancer cells. J Mol Signal. 2011;6:7. doi: 10.1186/1750-2187-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HJ, Kim MJ, Ha E, Chung JH. Apoptotic effect of hesperidin through caspase3 activation in human colon cancer cells, SNU-C4. Phytomedicine. 2008;15:147–151. doi: 10.1016/j.phymed.2007.07.061. [DOI] [PubMed] [Google Scholar]
- Park JH, Jin CY, Lee BK, Kim GY, Choi YH, Jeong YK. Naringenin induces apoptosis through downregulation of Akt and caspase-3 activation in human leukemia THP-1 cells. Food Chem Toxicol. 2008;46:3684–3690. doi: 10.1016/j.fct.2008.09.056. [DOI] [PubMed] [Google Scholar]
- Rosell R, Moran T, Queralt C, Porta R, Cardenal F, Camps C, Majem M, Lopez-Vivanco G, Isla D, Provencio M, Insa A, Massuti B, Gonzalez-Larriba JL, Paz-Ares L, Bover I, Garcia-Campelo R, Moreno MA, Catot S, Rolfo C, Reguart N, Palmero R, Sánchez JM, Bastus R, Mayo C, Bertran-Alamillo J, Molina MA, Sanchez JJ, Taron M, Spanish Lung Cancer Group Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med. 2009;361:958–967. doi: 10.1056/NEJMoa0904554. [DOI] [PubMed] [Google Scholar]
- Sak K. Chemotherapy and dietary phytochemical agents. Chemother Res Pract. 2012;2012:282570. doi: 10.1155/2012/282570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- Surh YJ. Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer. 2003;3:768–780. doi: 10.1038/nrc1189. [DOI] [PubMed] [Google Scholar]
- Szliszka E, Czuba ZP, Jernas K, Król W. Dietary flavonoids sensitize HeLa cells to tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) Int J Mol Sci. 2008;9:56–64. doi: 10.3390/ijms9010056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomayko MM, Reynolds CP. Determination of subcutaneous tumor size in athymic (nude) mice. Cancer Chemother Pharmacol. 1989;24:148–154. doi: 10.1007/BF00300234. [DOI] [PubMed] [Google Scholar]
- Zhang P, Gao WY, Turner S, Ducatman BS. Gleevec (STI-571) inhibits lung cancer cell growth (A549) and potentiates the cisplatin effect in vitro. Mol Cancer. 2003;2:1. doi: 10.1186/1476-4598-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SP, Zhou YJ, Liu Y, Cai YQ. Effect of liquiritigenin, a flavanone existed from Radix glycyrrhizae on pro-apoptotic in SMMC-7721 cells. Food Chem Toxicol. 2009;47:693–701. doi: 10.1016/j.fct.2008.12.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.