ABSTRACT
Treatment of anthrax is challenging, especially during the advanced stages of the disease. Recently, the Centers for Disease Control and Prevention (CDC) updated its recommendations for postexposure prophylaxis and treatment of exposed populations (before and after symptom onset). These recommendations distinguished, for the first time, between systemic disease with and without meningitis, a common and serious complication of anthrax. The CDC considers all systemic cases meningeal unless positively proven otherwise. The treatment of patients suffering from systemic anthrax with suspected or confirmed meningitis includes the combination of three antibiotics, i.e., a fluoroquinolone (levofloxacin or ciprofloxacin), a β-lactam (meropenem or imipenem), and a protein synthesis inhibitor (linezolid or clindamycin). In addition, treatment with an antitoxin (anti-protective antigen antibodies) and dexamethasone should be applied. Since the efficacy of most of these treatments has not been demonstrated, especially in animal meningitis models, we developed an anthrax meningitis model in rabbits and tested several of these recommendations. We demonstrated that, in this model, ciprofloxacin, linezolid, and meropenem were ineffective as single treatments, while clindamycin was highly effective. Furthermore, combined treatments of ciprofloxacin and linezolid or ciprofloxacin and dexamethasone failed in treating rabbits with meningitis. We demonstrated that dexamethasone actually hindered blood-brain barrier penetration by antibiotics, reducing the effectiveness of antibiotic treatment of anthrax meningitis in this rabbit model.
KEYWORDS: Bacillus anthracis, anthrax, antibiotic, ciprofloxacin, clindamycin, dexamethasone, linezolid, meningitis, meropenem, rabbits
INTRODUCTION
Bacillus anthracis, a spore-forming Gram-positive bacterium, is the etiological cause of anthrax in humans and animals (1, 2). Naturally infecting mainly grazing animals, B. anthracis causes disease in humans by two major infectious routes (3), i.e., the contact of spores with compromised skin (cutaneous) and the consumption of infected meat (1). Skin infection is the most common form of anthrax, with typical skin lesions in the form of black eschars that are usually painless (1, 4). These lesions represent local inflammation that may or may not constrain the bacteria to the infection site. Left untreated, cutaneous infection leads to bacteremia and metastatic infection in about 30% of the cases, resulting in death. A variation of cutaneous infection is the soft tissue form, an artificial infection that results from the injection of spore-containing heroin (5). This injection/inoculation results in diffuse inflammation and edema, which, if left untreated, rapidly progresses to lethal systemic infection (6). Digestion of infected meat can manifest in two forms, namely, oropharyngeal or gastrointestinal infection, both of which are lethal without prompt treatment (7). In the oropharyngeal form, lesions and edema result in suffocation (8). Gastrointestinal infection begins with severe gastroenteritis, followed by systemic bacterial spread, which is usually fatal without treatment (9). A third route of infection is inhalation of an aerosol of spores. This form of infection was known as an occupational disease associated with animal skin and wool processing, but modern biosafety practices have all but eradicated it (10). Therefore, inhalational anthrax is considered today less a natural occurrence and more an artificial malicious form of biothreat (11). An example of this threat is the 2001 letter attacks, which resulted in mortality rates of about 50% despite intensive antibiotic and supportive treatment (12). Inhalational anthrax develops through spore deposition in the lower respiratory tract, followed by spore uptake through phagocytic sampling and migration to a nearby lymph node. During this process, the spores germinate and overcome phagocytic killing due to the poly-γ-d-glutamic capsule and immunosuppressive toxins, i.e., the lethal toxin (LT) and the edema toxin (ET) (13–15). The phagocytes' migration facilitates systemic spread of the bacteria (Trojan horse model). Early symptoms of inhalational anthrax resemble those of common viral or bacterial lung infections, a similarity that usually results in preliminary misdiagnosis and ineffective antibiotic treatment (1). Having escaped the immune system, the bacteria spread to the bloodstream. Once in the blood, the bacteria multiply and spread into the organs, reaching high concentrations of up to 108 to 109 CFU per gram of tissue or milliliter of blood. B. anthracis has a predilection for crossing the blood-brain barrier (BBB) and infecting the central nervous system (CNS) (16). In more than 50% of human cases and in experimental nonhuman primate (NHP) models, CNS infection is typically associated with meningeal hemorrhage (“cardinal's cap”) (16–18). This hemorrhagic gross pathology common in NHPs is relatively rare in mice, guinea pigs, and rabbits. However, brain histopathological analysis reveals inflammations and hemorrhage in those animals as well (18, 19). Effective antibiotic treatment of CNS infections depends on two major parameters, i.e., BBB penetration and antibacterial activity. In 2014, the Centers for Disease Control and Prevention (CDC) acknowledged that effective anthrax treatment must take into consideration the treatment of anthrax meningitis (20). The CDC guidelines define three types of situations, i.e., postexposure prophylaxis (PEP), systemic disease for which meningitis was excluded, and systemic disease with assumed meningitis. While the treatments for PEP and systemic disease for which meningitis was excluded were not modified (ciprofloxacin or doxycycline for PEP and the combination of ciprofloxacin and linezolid or clindamycin for systemic disease), the recommendations for treating anthrax with the possibility of meningitis were updated (21). Those guidelines recommended triple-antibiotic treatment with ciprofloxacin, linezolid, and meropenem. To this triple-antibiotic treatment, the CDC experts recommended addition of antitoxin (protective antigen [PA]-neutralizing antibodies) and dexamethasone (20, 21) treatments. With the lack of evidence regarding the efficacy of these treatments for human anthrax patients, these recommendations were based on treatment of non-B. anthracis CNS infections. Therefore, validation of these recommendations in relevant animal models is required to determine the best treatment for patients with systemic disease in the case of anthrax meningitis.
Data regarding the efficacy of PEP for anthrax were previously reported for humans and animal models. In 2001, PEP with ciprofloxacin or doxycycline prevented the development of anthrax in individuals with suspected or confirmed exposure (22). In addition, this treatment was effective in exposed mice (23), Guinea pigs (24), rabbits (25, 26), and NHPs (27). The efficacy of treating systemic anthrax decreased with disease progression in humans (28) and animal models (24, 26). Diagnosing meningitis in animal models is complicated and relies mainly on cerebrospinal fluid (CSF) testing for bacteria, immune cells, or elevated protein content. In these experiments, it is impossible to determine whether a specific animal had meningitis at treatment initiation, making it impossible to determine the efficacy of specific treatments for CNS infections. Previously, we demonstrated in the guinea pig and rabbit models that intracranial (IC) injection of B. anthracis resulted in meningitis and death (29, 30). The pathophysiology was toxin independent, and injection of a toxin-deficient mutant resulted in meningitis and death similar to that caused by the wild-type strain. Since 100% of the animals have meningitis in this model, we used this rabbit model to test the efficacy of different antibiotic and nonantibiotic treatments that are recommended by the CDC for anthrax meningitis. We have since improved the model by infecting the rabbits by injecting the bacteria directly into the CSF via the cisterna magna (i.e., intra-cisterna magna [ICM] injection), thereby reducing the invasiveness of the infection protocol, as well as allowing drug administration via the same route. In this report, we describe the testing of ciprofloxacin, meropenem, linezolid, and clindamycin (which were found to be efficacious in treating systemic anthrax [23, 24, 26]) as single or combined treatments, as well as the effect of combining dexamethasone with the ciprofloxacin treatment in our animal model of induced anthrax meningitis.
RESULTS
Establishment of CNS infection model.
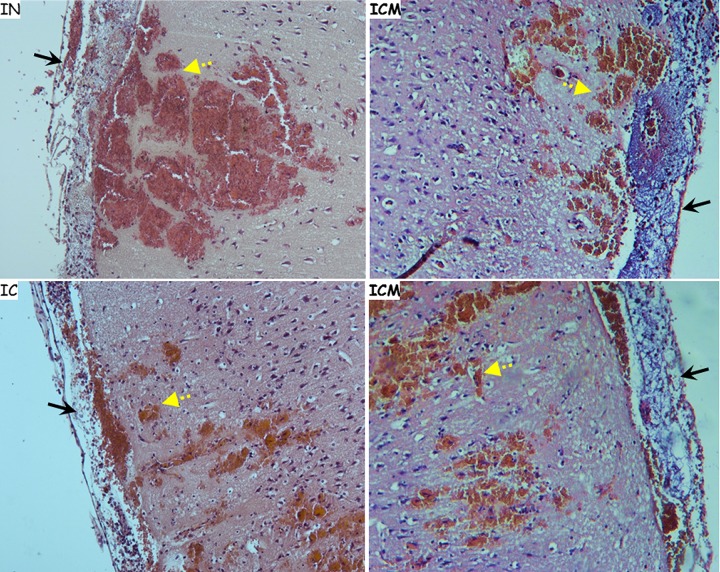
In order to establish a relevant animal model for CNS infections, we compared the pathology of brains from animals that succumbed to three different infection forms, namely, intranasal (IN) spore instillation and IC and ICM injection of B. anthracis encapsulated bacteria, focusing the analysis on meningeal pathology. While IN instillation and ICM injection are minimally invasive techniques, IC injection is an invasive method that physically damages the BBB and possibly the brain cortex at the point of injection. Indeed, pathological analysis of animals injected IC revealed a small lesion at the point of injection. However, overall cranial hemorrhaging was not significantly greater in animals infected IC, compared to that observed in animals infected IN or ICM. Histological findings for all three infection models (Fig. 1) included massive bacterial growth within the meninges, the definitive marker of meningitis. Cortical hemorrhaging was also ubiquitous and was estimated to be of comparable severity. Interestingly, both direct CNS infection routes led to bacteremia and systemic spread of the infection in a time-dependent manner. This probably resulted from damage to the BBB due to inflammation, increasing permeability and resulting in bacterial leaking into the circulation (30).
FIG 1.
Histology of brains from rabbits that had been infected by IN instillation, IC injection, or direct injection into the CSF in the cisterna magna (ICM injection), showing hemorrhagic areas (yellow dashed arrows) and inflamed meninges (black arrows). H&E staining of the meninges and cortical region of the brain from rabbits that succumbed to B. anthracis infection via the specified route of infection is shown. Magnification, ×10.
Establishment of treatment model.
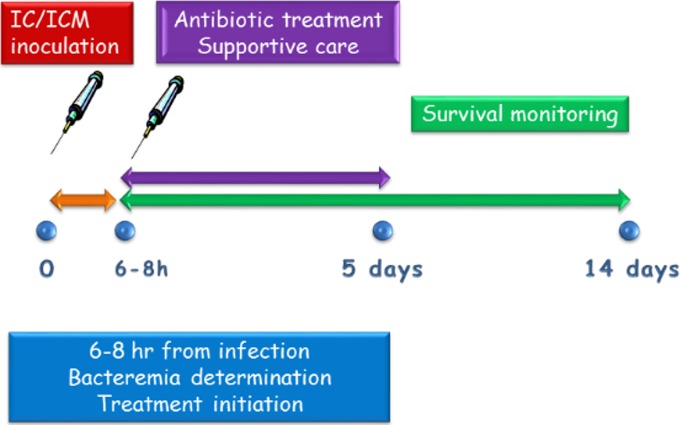
The treatment model was set up based on our previously reported IN model (24, 26) (Fig. 2). Rabbits were inoculated IC or ICM; 6 to 8 h later, the animals were bled to determine bacteremia levels and were immediately treated intravenously (i.v.) with antibiotics, at the same doses that were used previously for treatment of airway-infected rabbits (26). Following the initial i.v. dose, treatment continued with administration of the antibiotics subcutaneously (s.c.) twice a day for a total of 5 days, a treatment that we found to be sufficient to clear the CNS infection. In cases in which the physical condition of the animal deteriorated, the animal received supportive care in the form of concentrated food (oral) and fluids (s.c.). The animals were monitored for 14 days, at which time the rabbits were considered fully recovered.
FIG 2.
Experimental design.
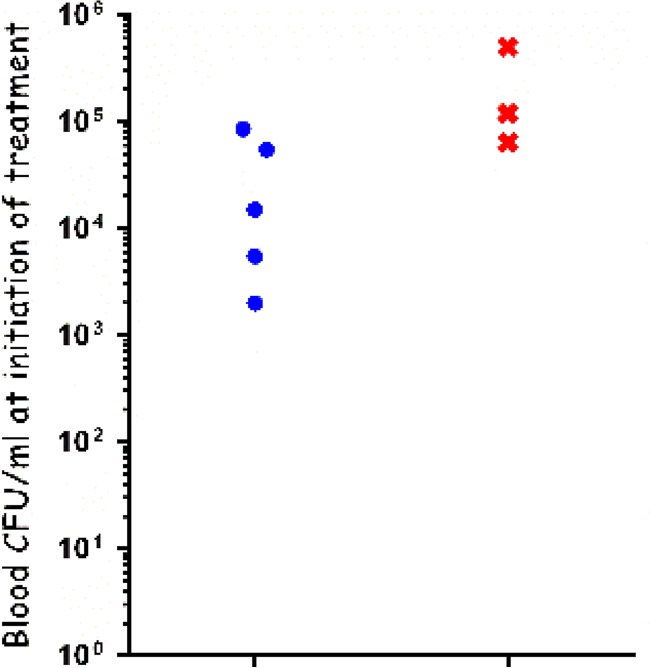
Since the toxins have been shown to play a minor role in CNS infections (29, 30), and in order to avoid unexpected neurotoxicity effects, we decided to test the efficacy of ciprofloxacin treatment (16 mg/kg) of CNS infections following IC inoculation of the VollumΔTox mutant. As shown in Fig. 3, all of the animals were bacteremic at treatment initiation, with bacterial concentrations of 2 × 103 to 5 × 105 CFU/ml. The ciprofloxacin treatment was effective in approximately 60% of the cases, preventing death for 5 of 8 infected rabbits. The treatment seemed effective up to a bacterial concentration of ∼105 CFU/ml, similar to the results obtained when rabbits and guinea pigs infected IN were treated with ciprofloxacin (24). The animals that succumbed to the infection died within 24 h after inoculation (Fig. 4A), similar to the untreated controls. These results validate our model as suitable for testing the efficacy of specific treatments against CNS anthrax infections.
FIG 3.
Efficacy of ciprofloxacin treatment of rabbits infected IC with the VollumΔTox strain. The bacterial levels at treatment initiation are indicated. Animals that survived are indicated by blue circles, and animals that succumbed to infection are indicated by red Xs.
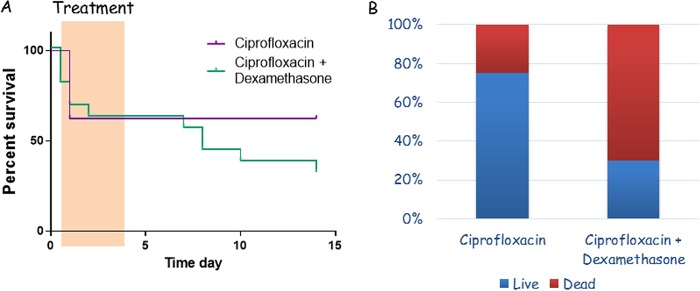
FIG 4.
Effect of adding dexamethasone to ciprofloxacin treatment of IC VollumΔTox infections in rabbits. (A) Survival curves for the two treatments. The highlighted region is the treatment period. (B) Percentages of successful (blue) or failed (red) treatments, of the total number of rabbits infected IC.
Efficacy of treatment combining ciprofloxacin and dexamethasone.
Using this model, we tested the incorporation of dexamethasone into the ciprofloxacin treatment, as recommended by the CDC (20, 21). Dexamethasone was administered s.c., at a dose of 1 mg/kg (as recommended for treating rabbits), 30 min prior to the first ciprofloxacin administration and simultaneously with the antibiotic administration for the rest of the treatment course. The effects of dexamethasone had two phases, early and late. While all of the deaths with ciprofloxacin as the sole treatment occurred within 12 h after treatment initiation, the deaths with ciprofloxacin and dexamethasone treatment started within 30 min and continued up to 48 h after treatment initiation (Fig. 4A). With the combined treatment, unlike with the antibiotic alone, a second wave of deaths started at day 9, 3 days after the end of treatment. Those deaths continued up to day 14, the last day of the experiment (Fig. 4A). Overall, the rate of survival with ciprofloxacin alone was 62.5% (5 of 8). The rate of survival with the combination of ciprofloxacin and dexamethasone was 33.3% (5 of 15 rabbits) (Fig. 4B).
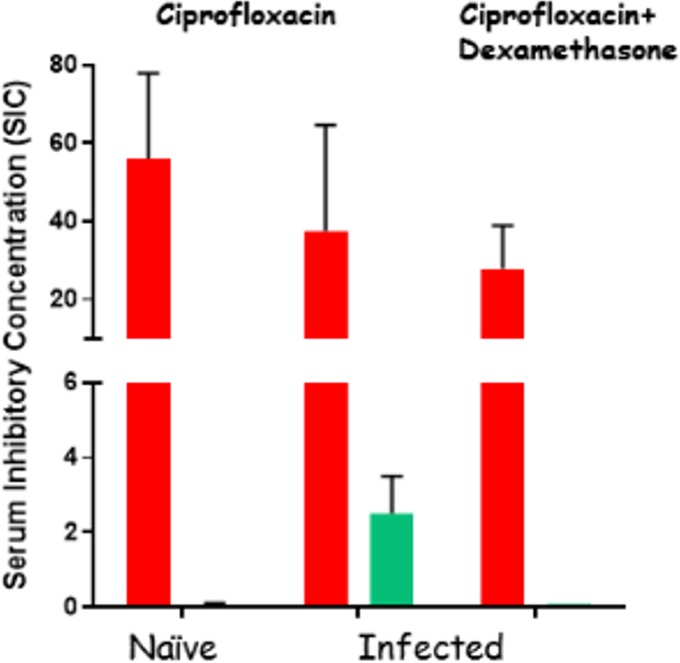
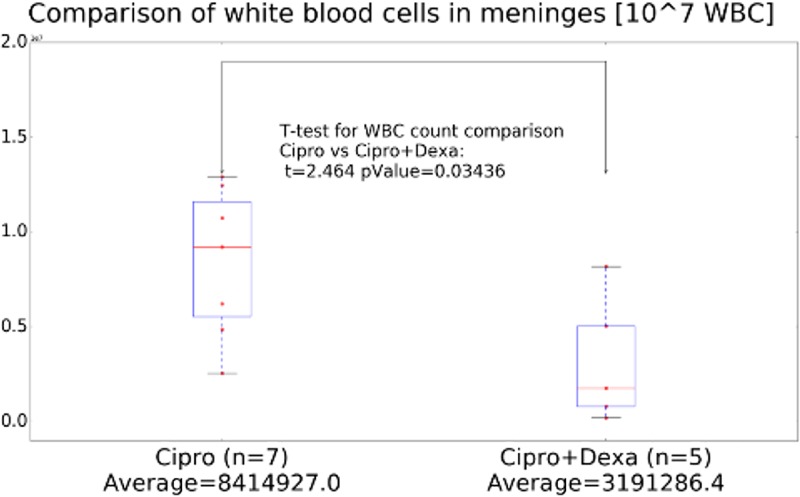
The second wave of deaths may possibly be explained as the effect of dexamethasone on the permeability of the BBB to antibiotic drugs, reducing inflammation and restoring proper barrier functions and thus interfering with the eradication of bacteria. To test this possibility, we examined the CNS concentrations of ciprofloxacin, as the sole treatment or in combination with dexamethasone, in naive and infected rabbits. The experimental protocol included five subsequent treatments, twice a day over 3 days. Thirty minutes after the last treatment, serum and CSF were sampled and their bacterial growth inhibitory capacities were tested as a measure of antibiotic concentration. In the infected groups, the data represent only animals that survived the full treatment course; in all cases, only clear CSF samples (without blood contamination) were tested. The ciprofloxacin contents of CSF from naive animals were below the test's detection limit (Fig. 5). CNS infection increased antibiotic penetration, and significant levels of antibiotic could be detected in the CSF (Fig. 5). In the infected rabbits that were treated with the combination of ciprofloxacin and dexamethasone, however, the antibiotic activity in the CSF was dramatically reduced to below the limit of detection. We tested the possibility that these differences might be due to the immunosuppressive effect of dexamethasone. We performed a quantitative histological analysis of white blood cell (WBC) infiltration in the brains of rabbits that had succumbed to IC infection with the VollumΔTox strain, during treatment with ciprofloxacin as monotherapy or in combination with dexamethasone. The results presented in Fig. 6 show significantly lower levels of WBC infiltration (P = 0.03) following dexamethasone treatment for 3 days. These findings offer an explanation for the lower efficacy of combined treatment with ciprofloxacin plus dexamethasone.
FIG 5.
Serum and CSF inhibitory concentrations of ciprofloxacin in naive rabbits and rabbits infected IC with VollumΔTox. The infected rabbits were inoculated IC and treated for 3 days. Serum (red) and CSF (green) samples were collected 30 min after the last treatment with ciprofloxacin or ciprofloxacin and dexamethasone. The inhibitory concentrations were determined by a growth inhibition test and are presented as the maximal dilution of serum (or CSF) to inhibit growth.
FIG 6.
Quantitative histology detecting CNS infiltration of immune cells in brains from animals treated with ciprofloxacin (Cipro) or ciprofloxacin and dexamethasone (Dexa).
Efficacy of antibiotic treatment following CNS infection with the wild-type Vollum strain.
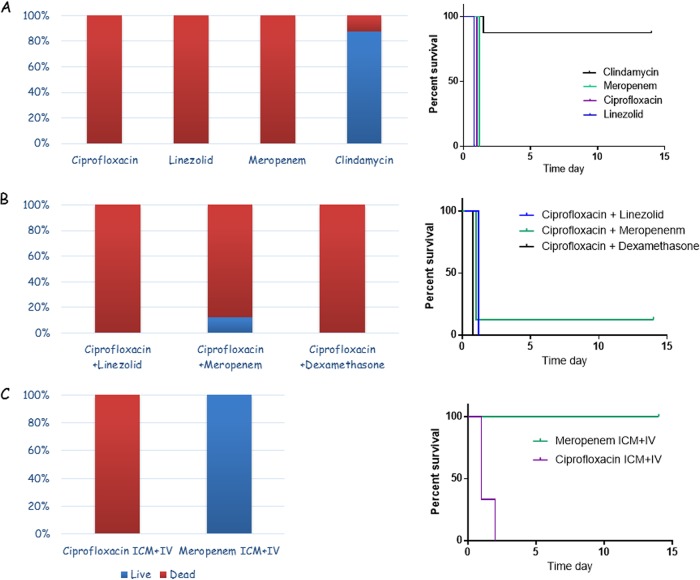
Since the meningitis model should represent the natural inhalation disease, we tested our model using the wild-type Vollum strain. The experimental design was not changed, since the time to death for the wild-type strain in this system was the same as that for the toxin-null mutant. Rabbits were inoculated IC or ICM with encapsulated vegetative Vollum bacteria, and the rabbits were treated i.v. with ciprofloxacin, linezolid, meropenem, or clindamycin 6 to 8 h postinfection. Subsequently, to reduce animal stress, the treatments were administered s.c. twice a day for a total of 5 days. While ciprofloxacin treatment was effective in about 60% of the VollumΔTox infections, this treatment failed completely with the wild-type strain and all of the animals died within 24 h after infection (Fig. 7A). Similar to the ciprofloxacin treatment, treatments with linezolid and meropenem were ineffective and all of the animals died within 24 h after infection (Fig. 7A), similar to untreated animals. In contrast to these failures, the efficacy of clindamycin was high, protecting 87.5% of infected animals (7 of 8 animals) (Fig. 7A).
FIG 7.
(A) Efficacy of ciprofloxacin, linezolid, meropenem, and clindamycin as a single treatment in rabbits infected IC/ICM with the fully virulent, wild-type Vollum strain. (B) Efficacy of combined treatment with ciprofloxacin and linezolid, meropenem, or dexamethasone in rabbits infected IC/ICM with the fully virulent, wild-type Vollum strain. (C) Additive effect of administering the antibiotics directly to the CSF (ICM injection) with systemic treatment with ciprofloxacin or meropenem in rabbits infected ICM with the fully virulent, wild-type Vollum strain. (Left) Percentages of surviving (blue) and deceased (red) animals, out of the infected animals. (Right) Survival curves for the treated animals.
Due to the failure of three of the first-line antibiotics for treatment of CNS infections as the sole antibiotic treatment, we tested the efficacy of combining two antibiotics. The combinations of ciprofloxacin and linezolid or ciprofloxacin and meropenem were tested in CNS-infected rabbits. In addition, the effect of dexamethasone was retested in combination with ciprofloxacin, this time for treatment of wild-type CNS infections. We could not find any synergistic effect with any of the combinations we tested (Fig. 7B). Using ciprofloxacin and linezolid, a combination that is recommended by the CDC (21), no animal survived the infection (n = 8). Treatment with ciprofloxacin and meropenem had a 12.5% survival rate (1 of 8). Ciprofloxacin and dexamethasone together also completely failed to save the infected animals (n = 4). All deaths occurred within 24 h after infection, the same as for untreated animals.
Efficacy of intrathecal antibiotic treatment.
Failure to treat CNS infections could result from insufficient CSF antibiotic concentrations, due to poor BBB penetration, or low specific activity of the antibiotic given. To test the possibility of poor BBB penetration, we established a treatment protocol in which we injected the antibiotic directly into the CSF via the cisterna magna (ICM or intrathecal injection), similar to the method used to inoculate the CNS with bacteria. This type of CSF injection is limited by volume and antibiotic concentration. Injections of doses in the range of those used for i.v. treatment resulted in severe adverse effects, including seizures and CSF hemorrhage, and in most cases were lethal. Eventually, a 1:10 to 1:20 dilution of the systemic dose was used in these experiments. We tested the effect of adding a single ICM administration to treatment with ciprofloxacin or meropenem on the efficacy of this treatment in our CNS infection model. Rabbits were infected and, 6 h postinfection, they were anesthetized and treated i.v. and ICM with antibiotics. This initial treatment was followed by s.c. treatment as described previously. While this ICM administration had only minor effects with ciprofloxacin treatment (slight extension of the time to death), this treatment protocol resulted in full protection of the meropenem-treated animals (Fig. 7C).
Effects of preimmunization with PA (simulating antitoxin treatment) on the outcome of antibiotic treatment.
To test the effect of the adaptive anti-PA immune response on the efficacy of the antibiotic treatment, we tested the ability of ciprofloxacin or meropenem to treat CNS infections in PA-immunized rabbits. The rabbits were immunized with a PA-based vaccine two times, 4 weeks apart, and the experiment was performed 2 weeks after the last injection. Normally, this vaccination regimen fully protects against lethal spore challenges with the fully virulent, wild-type Vollum strain given IN or s.c. (31). The immunized rabbits were CNS infected and treated as described previously, 6 h postinfection, by i.v. administration of ciprofloxacin or meropenem. As a control, immunized rabbits were CNS infected at the same time and left untreated, to test the vaccine's efficacy against this infection modality. As shown in Fig. 8, PA vaccination alone did not protect rabbits from CNS infections. However, this immunization improved the efficacy of the antibiotic treatment. For ciprofloxacin treatment, it appeared that the immunization delayed the time of death, with 25% of the rabbits dying after termination of antibiotic treatment. Although the hypothesis is untested, this late death possibly could be prevented by prolonging antibiotic treatment (Fig. 8B). However, these rabbits exhibited significant behavioral symptoms of CNS damage, including disorientation, tilted head position, and uncontrolled eye movements. These rabbits received s.c. saline injections and concentrated food orally. In contrast, meropenem treatment of immune rabbits fully protected the animals from lethal CNS infection (Fig. 8).
FIG 8.
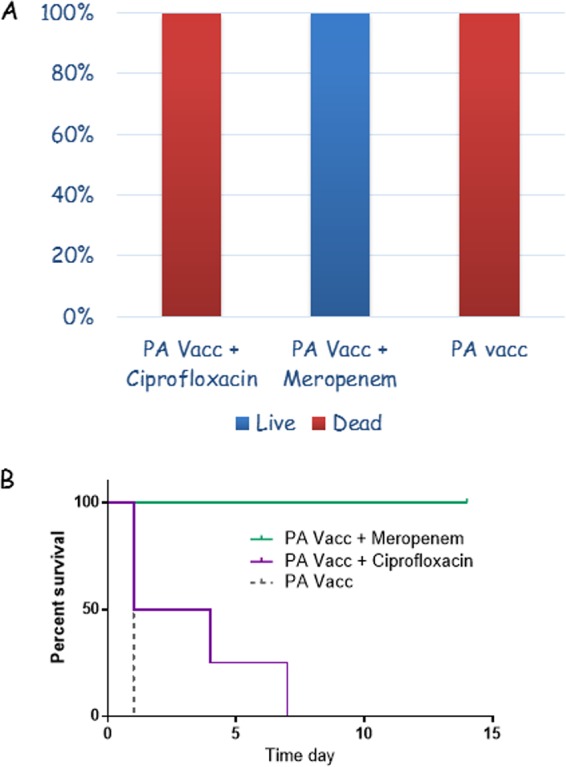
Additive effect of protective anti-PA immunity with systemic treatment with ciprofloxacin or meropenem in rabbits infected ICM with the fully virulent, wild-type Vollum strain. (A) Percentages of surviving (blue) or deceased (red) animals, of the infected animals. (B) Survival curves for the treated animals.
DISCUSSION
The normal course of a systemic anthrax infection includes the spread of bacteria from the point of infection to the blood and from there to the body's organs, with the CNS probably being one of the last organs to be infected, due to the need to overcome the BBB (1, 29). By the time this occurs following IN or s.c. infection, the disease has progressed so far that effective treatment is difficult and death could arise from a multitude of organ failures (24, 26). Therefore, in order to address the issue of anthrax CNS infections, there was a need for the development of an animal model that would allow the establishment of anthrax CNS infections in a controlled repeatable manner. In inhalation anthrax animal models, the most accurate marker for progression of the disease is bacteremia (32). We previously demonstrated that, in rabbits, significant bacterial accumulation in the brain starts at bacterial levels of ∼104 CFU/ml (29). Still, there is no assurance that all animals with bacterial levels above 104 CFU/ml in fact suffer from meningitis. Therefore, we developed an experimental setup based on direct CNS inoculation, which produces CNS infections with meningeal pathologies, followed by antibiotic treatment initiated at certain time points. This allowed us to test different treatments for their efficacy in treating anthrax CNS infections. As expected, the time to death with such CNS infections is short, compared to that in the inhalation model (26, 32), taking into consideration that this model represents the terminal phase of the disease, which is short even in humans (28). Nevertheless, antibiotic treatment can be effective even if it is initiated up to 10 h postinoculation, mere hours before death (death occurs 12 to 18 h after infection in this model). We used brain pathological analyses to determine that this model resembles the natural disease, by comparing inhalational infections with CNS inoculation and finding similar CNS pathologies at the time of death. Having initially established the direct IC injection method, followed by the minimally invasive ICM inoculation technique, we compared the treatment efficacies of the two infection models and did not see any significant differences. Due to the less traumatic nature of ICM injection, we preferred this inoculation method, despite the greater skill required for its execution.
The CDC recommendations for the treatment of anthrax meningitis (20, 21) include the combination of a fluoroquinolone (levofloxacin or ciprofloxacin) with a protein synthesis inhibitor (linezolid [as the first choice] or clindamycin) and a β-lactam (meropenem [as the first choice] or imipenem). In addition, the treatment should include dexamethasone and antitoxin antibodies (either polyclonal or monoclonal). We tested four of these antibiotic substances, i.e., ciprofloxacin, linezolid, meropenem, and clindamycin, in our CNS infection model as monotherapies or as combined treatments of ciprofloxacin plus linezolid or ciprofloxacin plus meropenem. Ciprofloxacin administration failed in treating anthrax meningitis. Ciprofloxacin treatment increases the toxin concentration in the serum of sick animals (26); therefore, we tested whether this was the cause of failure by adding a protein synthesis inhibitor or an antitoxin to the treatment. Neither preimmunization of the rabbits nor the addition of linezolid improved the efficacy of the ciprofloxacin treatment. The low efficacy of the ciprofloxacin treatment of anthrax meningitis was probably not due to slow BBB penetration, since ICM administration (CSF) did not improve the treatment. These results, in addition to the failure of linezolid as a single treatment, are disturbing, since the combination of ciprofloxacin and linezolid is the first treatment choice recommended by the CDC for anthrax meningitis (20). The failure of linezolid as monotherapy could be due to a relatively high MIC (33) for B. anthracis (1 to 2 μg/ml), which in some cases is defined as borderline sensitivity (34). Although ciprofloxacin and linezolid treatment failed in the CNS infection model, this treatment was efficacious in treating rabbits following IN infection (24, 26). This result implies that this combination will probably work in treating systemic disease but has no advantage for anthrax meningitis.
β-Lactams are considered effective for treating nonanthrax CNS infections (35). Therefore, the low efficacy of meropenem in treating anthrax CNS infections was unexpected. No synergism was achieved by adding meropenem to the ciprofloxacin treatment (the fact that this treatment protected 1 animal of the 8 treated animals was not statistically significant). The failure of meropenem in this case is probably a result of low BBB penetration, since injection of the antibiotic into the CSF (ICM) in addition to the systemic treatment resulted in full protection of the infected animals.
The humoral immune response has no protective effect regarding CNS infection, since antibodies do not cross an intact BBB to neutralize the toxins. We demonstrated that the BBB becomes permeable to antibiotics in response to inflammation. During this inflammatory process, innate immune cells cross from the bloodstream to the CNS, compromising the barrier and enabling the crossing of substances that normally do not penetrate. The augmenting effects of PA immunization on the outcomes of meropenem (protection) and ciprofloxacin (mainly effects on the time to death) treatments could represent enhanced penetration of immune cells and antibodies, resulting in improved antibiotic penetration. The failure of ciprofloxacin to protect the immunized animals while delaying the time of death could indicate that PA-neutralizing antibodies enter as well, creating an infection similar to that of the ΔTox mutant. In addition, it is possible that the systemic toxin neutralization contributed to the host tolerance but did not confer protection.
Dexamethasone, as an anti-inflammatory drug, acts to suppress the immune response. This suppression can explain the interference of dexamethasone with the treatment by reducing antibiotic penetration to the CSF. Any effect on the innate immune response that might interfere with the recruitment of immune cells to the infection site in the CNS would affect the BBB entry of these cells. Dexamethasone was previously shown to interfere with antibiotic penetration to the CSF in an animal model (36). According to our findings, the recommendation of adding dexamethasone to the antibiotic treatment should be reconsidered.
Clindamycin was found to be the only antibiotic that conferred nearly full protection to the CNS-infected rabbits (7/8 infected animals survived). Clindamycin in combination with ciprofloxacin was extensively used to treat anthrax patients in 2001 and afterwards (28). It is unclear why clindamycin is effective while linezolid is not and what the logic was for choosing linezolid as a first priority to treat anthrax meningitis (20). However, we find clindamycin to be significantly superior to linezolid in treating this phase of the disease.
To conclude, we are presenting a robust rabbit CNS infection system that enables testing of specific treatments for this stage of the disease. Using this model, we demonstrated that clindamycin is highly effective as monotherapy, while ciprofloxacin, linezolid, and meropenem are ineffective. In the case of meropenem, we demonstrated that the low efficacy is probably due to poor BBB penetration; if the BBB is compromised by direct CSF injection or by elevated immune responses, then the efficacy of the treatment increases dramatically. The addition of dexamethasone interferes with the antibiotic treatment, probably by decreasing the amounts of antibiotics that cross through the BBB, thus affecting the CSF antibiotic concentration. We propose that any recommended treatment of anthrax meningitis must be validated in this or a similar model to test for preclinical efficacy.
MATERIALS AND METHODS
Bacterial strains, media, and growth conditions.
The B. anthracis strains used in this study were Vollum (ATCC 14578) and VollumΔTox (Vollum Δpag Δlef Δcya) (37). B. anthracis strains were cultivated at 37°C in terrific broth (38), with vigorous shaking (250 rpm). For the induction of toxins and capsule production, Dulbecco's modified Eagle's medium (DMEM) with 10% normal rabbit serum (NRS) was used.
Infection of rabbits.
New Zealand White rabbits (2.5 to 3.5 kg) were obtained from Charles River (Canada). The animals received food and water ad libitum. Prior to infection, spores were germinated by incubation in terrific broth for 0.5 h and then were incubated in DMEM-10% NRS for 2 h, to induce capsule formation. The capsule was visualized by negative staining with India ink. The encapsulated vegetative bacteria were used to infect rabbits intracranially (IC). For IC administration, rabbits were anesthetized. A 3- to 4-cm longitudinal incision was made along the sagittal suture of the skull, centered around the bregma (the coronal suture's transection of the sagittal suture). The underlying connective tissue was removed to expose the skull. A small hole (1 mm in diameter) was drilled 5 mm caudal and 5 mm lateral to the bregma, using a handheld power drill (Dremel 300; Dremel, Mount Prospect, IL, USA). Encapsulated vegetative bacteria (30 μl) were injected through the drilled hole, at a depth of 4 mm from the dural surface, and the scalp was closed using surgical clips. For ICM administration, the animals were anesthetized and the fur at the back of the head and neck was shaved. Using a 23-gauge blood collection set, 350 μl of encapsulated vegetative bacteria was injected into the cisterna magna (ICM injection). In all cases, the remaining sample was plated for determination of total viable counts (CFU per milliliter). The animals were observed daily for 14 days or for the indicated period. Upon death, blood samples were plated and DNA was extracted, followed by PCR analysis in order to determine the identity of the strain responsible for the animals' death.
Antibiotic treatment.
Groups of 8 New Zealand White rabbits (2.5 to 3 kg) were inoculated IC or ICM with 1 × 105 Vollum or VollumΔTox encapsulated bacteria. At 6 to 8 h postinoculation, blood samples were drawn from the rabbits' ear veins to determine the level of bacteremia (32). Animals were immediately treated i.v. with antibiotics, in the same doses that were used previously for treatment of airway-infected rabbits (26). Antibiotic treatment continued twice daily s.c. for a period of 5 days. The animals were then monitored for survival for 9 additional days. Antibiotic doses were as follows: ciprofloxacin, 16 mg/kg; linezolid, 50 mg/ml; meropenem, 40 mg/kg; clindamycin, 60 mg/kg (26).
Serum and CSF inhibitory concentrations of antibiotics.
The ciprofloxacin levels in the serum and CSF of treated rabbits were determined by a growth inhibition test. Blood and CSF samples were drawn from rabbits after five antibiotic administrations, and the concentration of the antibiotic in the serum was defined by determining the highest dilution that inhibited the growth of VollumΔpXO1ΔpXO2. The inhibitory concentration is presented as the reciprocal value of the maximal inhibitory dilution.
Tissue processing for histopathological analysis.
Brains chosen for histological analysis were harvested postmortem from rabbits that succumbed to the infection or were euthanized at designated time points (controls and nonlethal infections) (29, 30). The brains were immediately placed in 50-ml tubes containing ∼30 ml of 3.7% formaldehyde in phosphate-buffered saline (PBS), for fixation. After fixation, brains were cross-sectioned into 3.5-mm-thick slabs, each of which was placed in a separate histological cassette and paraffinized overnight in a Leica APS200 system (Leica Biosystems, Wetzlar, Germany). The tissue slices were then embedded in paraffin blocks, and slides were prepared by mounting 5-μm-thick sections prepared using a rotary microtome (Leica Biosystems, Wetzlar, Germany).
This study was carried out in strict accordance with the recommendations of the Guide for the Care and Use of Laboratory Animals of the National Research Council. The protocols were approved by the Committee on the Ethics of Animal Experiments of the Israel Institute for Biological Research. We used female rabbits in this experiment since there are not significant differences in B. anthracis pathogenicity between male and female rabbits (39).
Stereology.
WBC counting was performed on a Stereologer workstation (Stereology Resource Center, Inc.), based on a Nikon E400 microscope. Sampling from the 3.5-mm-thick slabs described above was performed using a systematic-uniform-random (SUR) sampling scheme, with sampling fractions of f1 = 1/2 for slabs, which were processed to blocks, and then f2 = 1/200 for microtome sections (section thickness, 25 μm). Counting was performed using an optical dissector (40) with 400-μm x-y spacing, on sections stained with hematoxylin and eosin (H&E).
Histopathological staining.
Prepared sections were subjected to either H&E staining (using a protocol modified for brain staining, with 15 min of hematoxylin treatment, instead of the usual duration) or immunofluorescence staining for bacteria (30).
Image acquisition.
H&E-stained slide images were acquired using a Zeiss Axiokop microscope (Zeiss, Oberkochen, Germany) equipped with a Nikon DS-Ri1 camera controlled by a DS-U3 digital sight and the Nis-Elements-Br software suite (Nikon, Tokyo, Japan).
Statistical analysis.
The significance of differences in survival rates between treated groups and untreated controls and differences in bacteremia and time to death was determined by Fisher's exact test (two-tailed), using Prism 6 software (GraphPad).
REFERENCES
- 1.Dixon TC, Meselson M, Guillemin J, Hanna PC. 1999. Anthrax. N Engl J Med 341:815–826. doi: 10.1056/NEJM199909093411107. [DOI] [PubMed] [Google Scholar]
- 2.Hanna P. 1998. Anthrax pathogenesis and host response. Curr Top Microbiol Immunol 225:13–35. [DOI] [PubMed] [Google Scholar]
- 3.Swartz MN. 2001. Recognition and management of anthrax: an update. N Engl J Med 345:1621–1626. doi: 10.1056/NEJMra012892. [DOI] [PubMed] [Google Scholar]
- 4.Doganay M, Metan G, Alp E. 2010. A review of cutaneous anthrax and its outcome. J Infect Public Health 3:98–105. doi: 10.1016/j.jiph.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 5.Hanczaruk M, Reischl U, Holzmann T, Frangoulidis D, Wagner DM, Keim PS, Antwerpen MH, Meyer H, Grass G. 2014. Injectional anthrax in heroin users, Europe, 2000–2012. Emerg Infect Dis 20:322–323. doi: 10.3201/eid2002.120921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berger T, Kassirer M, Aran AA. 2014. Injectional anthrax: new presentation of an old disease. Euro Surveill 19:20877. doi: 10.2807/1560-7917.ES2014.19.32.20877. [DOI] [PubMed] [Google Scholar]
- 7.Sirisanthana T, Brown AE. 2002. Anthrax of the gastrointestinal tract. Emerg Infect Dis 8:649–651. doi: 10.3201/eid0807.020062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sirisanthana T, Navachareon N, Tharavichitkul P, Sirisanthana V, Brown AE. 1984. Outbreak of oral-oropharyngeal anthrax: an unusual manifestation of human infection with Bacillus anthracis. Am J Trop Med Hyg 33:144–150. doi: 10.4269/ajtmh.1984.33.144. [DOI] [PubMed] [Google Scholar]
- 9.Owen JL, Yang T, Mohamadzadeh M. 2015. New insights into gastrointestinal anthrax infection. Trends Mol Med 21:154–163. doi: 10.1016/j.molmed.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brachman PC. 1980. Inhalation anthrax. Ann N Y Acad Sci 353:83–93. doi: 10.1111/j.1749-6632.1980.tb18910.x. [DOI] [PubMed] [Google Scholar]
- 11.Riedel S. 2017. Anthrax: a continuing concern in the era of bioterrorism. Baylor Univ Med Cent Proc 18:234–243. doi: 10.1080/08998280.2005.11928074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jernigan DB, Raghunathan PL, Bell BP, Brechner R, Bresnitz EA, Butler JC, Cetron M, Cohen M, Doyle T, Fischer M, Greene C, Griffith KS, Guarner J, Hadler JL, Hayslett JA, Meyer R, Petersen LR, Phillips M, Pinner R, Popovic T, Quinn CP, Reefhuis J, Reissman D, Rosenstein N, Schuchat A, Shieh WJ, Siegal L, Swerdlow DL, Tenover FC, Traeger M, Ward JW, Weisfuse I, Wiersma S, Yeskey K, Zaki S, Ashford DA, Perkins BA, Ostroff S, Hughes J, Fleming D, Koplan JP, Gerberding JL. 2002. Investigation of bioterrorism-related anthrax, United States, 2001: epidemiologic findings. Emerg Infect Dis 8:1019–1028. doi: 10.3201/eid0810.020353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu S, Moayeri M, Leppla SH. 2014. Anthrax lethal and edema toxins in anthrax pathogenesis. Trends Microbiol 22:317–325. doi: 10.1016/j.tim.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guidi-Rontani C. 2002. The alveolar macrophage: the Trojan horse of Bacillus anthracis. Trends Microbiol 10:405–409. doi: 10.1016/S0966-842X(02)02422-8. [DOI] [PubMed] [Google Scholar]
- 15.Weiner ZP, Glomski IJ. 2012. Updating perspectives on the initiation of Bacillus anthracis growth and dissemination through its host. Infect Immun 80:1626–1633. doi: 10.1128/IAI.06061-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abramova FA, Grinberg LM, Yampolskaya OV, Walker DH. 1993. Pathology of inhalational anthrax in 42 cases from the Sverdlovsk outbreak of 1979. Proc Natl Acad Sci U S A 90:2291–2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grinberg LM, Abramova FA, Yampolskaya OV, Walker DH, Smith JH. 2001. Quantitative pathology of inhalational anthrax. I. Quantitative microscopic findings. Mod Pathol 14:482–495. doi: 10.1038/modpathol.3880337. [DOI] [PubMed] [Google Scholar]
- 18.Twenhafel NA. 2010. Pathology of inhalational anthrax animal models. Vet Pathol 47:819–830. doi: 10.1177/0300985810378112. [DOI] [PubMed] [Google Scholar]
- 19.Savransky V, Sanford DC, Syar E, Austin JL, Tordoff KP, Anderson MS, Stark GV, Barnewall RE, Briscoe CM, Lemiale-Bierinx L, Park S, Ionin B, Skiadopoulos MH. 2013. Pathology and pathophysiology of inhalational anthrax in a guinea pig model. Infect Immun 81:1152–1163. doi: 10.1128/IAI.01289-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hendricks KA, Wright ME, Shadomy SV, Bradley JS, Morrow MG, Pavia AT, Rubinstein E, Holty JE, Messonnier NE, Smith TL, Pesik N, Treadwell TA, Bower WA. 2014. Centers for Disease Control and Prevention expert panel meetings on prevention and treatment of anthrax in adults. Emerg Infect Dis doi: 10.3201/eid2002.130687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bower WA, Hendricks K, Pillai S, Guarnizo J, Meaney-Delman D. 2015. Clinical framework and medical countermeasure use during an anthrax mass-casualty incident. MMWR Recomm Rep 64(RR04):1–28. doi: 10.15585/mmwr.rr6404a1. [DOI] [PubMed] [Google Scholar]
- 22.Bresnitz EA. 2005. Lessons learned from the CDC's post-exposure prophylaxis program following the anthrax attacks of 2001. Pharmacoepidemiol Drug Saf 14:389–391. doi: 10.1002/pds.1086. [DOI] [PubMed] [Google Scholar]
- 23.Heine HS, Bassett J, Miller L, Hartings JM, Ivins BE, Pitt ML, Fritz D, Norris SL, Byrne WR. 2007. Determination of antibiotic efficacy against Bacillus anthracis in a mouse aerosol challenge model. Antimicrob Agents Chemother 51:1373–1379. doi: 10.1128/AAC.01050-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weiss S, Kobiler D, Levy H, Pass A, Ophir Y, Rothschild N, Tal A, Schlomovitz J, Altboum Z. 2011. Antibiotics cure anthrax in animal models. Antimicrob Agents Chemother 55:1533–1542. doi: 10.1128/AAC.01689-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Altboum Z, Gozes Y, Barnea A, Pass A, White M, Kobiler D. 2002. Postexposure prophylaxis against anthrax: evaluation of various treatment regimens in intranasally infected guinea pigs. Infect Immun 70:6231–6241. doi: 10.1128/IAI.70.11.6231-6241.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weiss S, Altboum Z, Glinert I, Schlomovitz J, Sittner A, Bar-David E, Kobiler D, Levy H. 2015. Efficacy of single and combined antibiotic treatments of anthrax in rabbits. Antimicrob Agents Chemother 59:7497–7503. doi: 10.1128/AAC.01376-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Friedlander AM, Welkos SL, Pitt MLM, Ezzell JW, Worsham PL, Rose KJ, Ivins BE, Lowe JR, Howe GB, Mikesell P, Lawrence WB. 1993. Postexposure prophylaxis against experimental inhalation anthrax. J Infect Dis 167:1239–1242. doi: 10.1093/infdis/167.5.1239. [DOI] [PubMed] [Google Scholar]
- 28.Jernigan JA, Stephens DS, Ashford DA, Omenaca C, Topiel MS, Galbraith M, Tapper M, Fisk TL, Zaki S, Popovic T, Meyer RF, Quinn CP, Harper SA, Fridkin SK, Sejvar JJ, Shepard CW, McConnell M, Guarner J, Shieh WJ, Malecki JM, Gerberding JL, Hughes JM, Perkins BA. 2001. Bioterrorism-related inhalational anthrax: the first 10 cases reported in the United States. Emerg Infect Dis 7:933–944. doi: 10.3201/eid0706.010604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levy H, Glinert I, Weiss S, Bar-David E, Sittner A, Schlomovitz J, Altboum Z, Kobiler D. 2014. The central nervous system as target of Bacillus anthracis toxin independent virulence in rabbits and guinea pigs. PLoS One 9:e112319. doi: 10.1371/journal.pone.0112319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sittner A, Bar-David E, Glinert I, Ben-Shmuel A, Weiss S, Schlomovitz J, Kobiler D, Levy H. 2017. Pathology of wild-type and toxin-independent Bacillus anthracis meningitis in rabbits. PLoS One 12:e0186613. doi: 10.1371/journal.pone.0186613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weiss S, Kobiler D, Levy H, Marcus H, Pass A, Rothschild N, Altboum Z. 2006. Immunological correlates for protection against intranasal challenge of Bacillus anthracis spores conferred by a protective antigen-based vaccine in rabbits. Infect Immun 74:394–398. doi: 10.1128/IAI.74.1.394-398.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kobiler D, Weiss S, Levy H, Fisher M, Mechaly A, Pass A, Altboum Z. 2006. Protective antigen as a correlative marker for anthrax in animal models. Infect Immun 74:5871–5876. doi: 10.1128/IAI.00792-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Louie A, VanScoy BD, Heine HS, Liu W, Abshire T, Holman K, Kulawy R, Brown DL, Drusano GL. 2012. Differential effects of linezolid and ciprofloxacin on toxin production by Bacillus anthracis in an in vitro pharmacodynamic system. Antimicrob Agents Chemother 56:513–517. doi: 10.1128/AAC.05724-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clinical and Laboratory Standards Institute. 2017. Performance standards for antimicrobial susceptibility testing. M100, 27th ed. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 35.Quagliarello VJ, Scheld WM. 1997. Treatment of bacterial meningitis. N Engl J Med 336:708–716. doi: 10.1056/NEJM199703063361007. [DOI] [PubMed] [Google Scholar]
- 36.Paris MM, Hickey SM, Uscher MI, Shelton S, Olsen KD, McCracken GH Jr. 1994. Effect of dexamethasone on therapy of experimental penicillin- and cephalosporin-resistant pneumococcal meningitis. Antimicrob Agents Chemother 38:1320–1324. doi: 10.1128/AAC.38.6.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Levy H, Weiss S, Altboum Z, Schlomovitz J, Glinert I, Sittner A, Shafferman A, Kobiler D. 2012. Differential contribution of Bacillus anthracis toxins to pathogenicity in two animal models. Infect Immun 80:2623–2631. doi: 10.1128/IAI.00244-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sambrook J, Fritsch EF, Maniatis T. 1989. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- 39.Fellows PF, Linscott MK, Ivins BE, Pitt ML, Rossi CA, Gibbs PH, Friedlander AM. 2001. Efficacy of a human anthrax vaccine in guinea pigs, rabbits, and rhesus macaques against challenge by Bacillus anthracis isolates of diverse geographical origin. Vaccine 19:3241–3247. doi: 10.1016/S0264-410X(01)00021-4. [DOI] [PubMed] [Google Scholar]
- 40.Santos M, Dias-Pereira P, Correia-Gomes C, Marcos R, de Matos A, Rocha E, Lopes C. 2017. Use of the optical disector in canine mammary simple and complex carcinomas. APMIS 125:833–839. doi: 10.1111/apm.12717. [DOI] [PubMed] [Google Scholar]