Abstract
Aims
The secreted form of the α-Klotho gene (S-Klotho), which is considered a powerful biomarker of longevity, makes it an attractive target as an anti-ageing therapy against functional decline, sarcopenic obesity, metabolic and cardiovascular diseases, osteoporosis, and neurodegenerative disorders. The S-Klotho plasma levels could be related to physical exercise inasmuch physical exercise is involved in physiological pathways that regulate the S-Klotho plasma levels. FIT-AGEING will determine the effect of different training modalities on the S-Klotho plasma levels (primary outcome) in sedentary healthy adults. FIT-AGEING will also investigate the physiological consequences of activating the klotho gene (secondary outcomes).
Methods
FIT-AGEING will recruit 80 sedentary, healthy adults (50% women) aged 45–65 years old. Eligible participants will be randomly assigned to a non-exercise group, i.e. the control group, (n = 20), a physical activity recommendation from World Health Organization group (n = 20), a high intensity interval training group (n = 20), and a whole-body electromyostimulation group (n = 20). The laboratory measurements will be taken at the baseline and 12 weeks later including the S-Klotho plasma levels, physical fitness (cardiorespiratory fitness, muscular strength), body composition, basal metabolic rate, heart rate variability, maximal fat oxidation, health blood biomarkers, free-living physical activity, sleep habits, reaction time, cognitive variables, and health-related questionnaires. We will also obtain dietary habits data and cardiovascular disease risk factors.
1. Introduction
The global elderly population will dramatically increase by 2050 [1]. Despite the current rise in life expectancy, the increases in disease-free years are higher, concentrating the prevalence of chronic diseases in old age [2]. Age-related diseases, such as cardiovascular diseases, osteoporosis, cancer and neurodegenerative disorders, represent the major cause of morbidity and mortality in developed countries, with an exponential growth of health costs [3,4]. However, significant inter-individual variability exists in the health impact of ageing [5]. Therefore, it is necessary to find strategies which can attenuate the ageing process and improve the quality of life [6].
The α-Klotho gene encodes a type I single-pass transmembrane protein of 1014 amino acid long, and it expresses itself in three functionally different family members: the intra-cellular form which binds sodium-potassium adenosine triphosphatase, the cell-membrane form which forms a complex with fibroblast growth factor 23 and fibroblast growth factor receptor 1 and the secreted form (S-Klotho), identified in blood, plasma, urine, and cerebrospinal fluid [7]. The S-Klotho is considered a powerful biomarker of longevity [8] and has several anti-ageing functions such as regulating the metabolism of calcium and phosphate metabolism [avoiding cell's precipitation of calcium phosphate and reducing apoptosis mechanism [[8], [9], [10], [11]]], reducing the inflammatory process [12] and the oxidative stress [13], and protecting the oxidative stress cells, obtaining an anti-ageing effect derived from these actions. Recent findings have discovered a pool of novel α-Klotho gene activators such as Na+-dependent Pi cotransporter type IIa (NaPi-IIa) and Na+-dependent Pi cotransporter type IIb (NaPi-IIb) [[9], [10], [11]], insulin, insulin-like growth factor-1 (IGF-1), transforming growth factor beta (TGF-β), Wnt signaling pathway, and interferon gamma (IFNγ) [14,15], and fibroblast growth factor 21 (FGF21) [16].
Physical fitness, which includes cardiorespiratory fitness [17,18] and muscular strength [19], is an excellent predictor of both cardiovascular and all-cause mortality [20]. In addition, it is an important predictor of living independently at older ages in both men and women [21]. Moreover, it is well known that exercise improves physical fitness in healthy [22] and unhealthy people [23], and it may also be effective in preventing, delaying, or reversing the ageing effects on tissue health and functioning [24].
Of interest is that several studies have reported that exercise induces the activation of various factors that also increase the expression of the α-Klotho gene [25,26]. Several studies have shown that a single bout of exercise produces an S-Klotho plasma levels increase both in animal models [27] and in humans [28,29]. Although Matsubara el al. (2014) showed that aerobic exercise training increased the S-Klotho plasma levels in postmenopausal women [30], little is known about which are the chronic effects of different training modalities on the S-Klotho plasma levels in sedentary healthy adults.
The overall objective and primary outcome of the FIT-AGEING randomized controlled trial (RCT) is to quantify the effects of different training modalities on the S-Klotho plasma levels in sedentary healthy adults. The training modalities will be (i) no exercise, (ii) physical activity recommendation from the World Health Organization, (iii) High Intensity Interval Training, and (iv) Whole-Body Electromyostimulation training. The secondary outcomes of this study will focus on the quantification of the effects of the above-mentioned training modalities on physical fitness components, body composition and anthropometric measurements, energy expenditure and nutrients oxidation, heart rate variability (HRV), health blood biomarkers, free-living physical activity, cognitive variables, health-related quality of life, dietary habits, and cardiovascular disease risk factors.
2. Methods
The present study is a RCT (ClinicalTrials.gov ID: NCT03334357) approved by The Human Research Ethics Committee of the “Junta de Andalucia” [0838-N-2017]. All participants will have to provide an informed consent. The participants will be randomly allocated to a control group, a physical activity recommendation group (PAR), high intensity interval training group (HIIT), and whole-body electromyostimulation group (WB-EMS). All of the baseline and follow-up examinations will be performed at the same setting [Instituto Mixto Universitario Deporte y Salud (IMUDS) at the University of Granada]. The study will follow the revised ethical guidelines of the Declaration of Helsinki.
2.1. Participants and selection criteria
The participants will be adults from the province of Granada (Spain). Granada has ≈885,000 population, of which ≈190,000 are adults aged 45–65. The eligible participants should be 45–65 years old, and they must have a body mass index (BMI) between 18.5 and 35 kg/m2. The inclusion and exclusion criteria are listed in Table 1. We decided to conduct the intervention on overweight and obese adults because overweight and obesity accelerate the ageing of adipose tissue, increase the formation of reactive oxygen species in fat cells, shorten telomeres, and produce the inhibition of the p53 tumor suppressor [31], which are factors that could be related to the S-Klotho plasma levels. Considering that aerobic exercise is able to increase the S-Klotho plasma levels in normal-weight young and senior adults according to literature [32], we will also include participants with a BMI between 18.5 and 24.9 kg/m2. Including people with different weight status and body composition allows to study S-Klotho plasma levels differences across different categories (i.e. normal-weight, overweight, and obese based on BMI, and on body fat measures).
Table 1.
Selection criteria.
| Inclusion criteria | Exclusion criteria |
|---|---|
| Age: 45–65 years old | History of cardiovascular disease |
| Body mass index: 18.5–35 kg/m2 | Diabetes |
| Not engaged in regular physical activity >20min on >3 day/week | Pregnancy or planning to get pregnant during the study period |
| Not participating in a weight-loss program | Beta blockers or benzodiazepines use |
| Stable weight over the last 5 months (body weight changes>5 kg) | Taking medication for thyroid |
| The participants must be capable and willing to provide consent, understand the exclusion criteria, and accept the randomized group assignment | Other significant conditions that are life-threatening or that can interfere with or be aggravated by exercise |
| Normal electrocardiogram | Unwillingness to either complete the study requirements or to be randomized into the control or training group |
All participants will have a health history and a medical examination done prior to the intervention program to minimize risks by ruling out contraindications to the testing and training protocols. If any participant suffers any injury or medical problem, a medical evaluation will be performed and, if necessary, they will be excluded from the study.
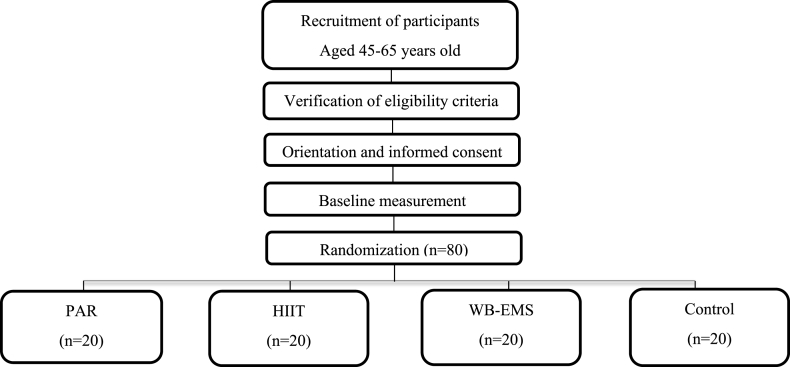
The study will be announced on social networks, local media, and posters at different points of Granada. We will also organize information meetings at the School of Medicine of the University of Granada. The people interested will be able to contact the research team by e-mail and phone. Later, they will visit our facilities to receive a thorough explanation about the study's aims, the test to be performed, the inclusion and exclusion criteria, and the types of intervention. What participants can expect from the study will be clarified, and any questions will be answered. The potentially interested participants meeting the inclusion criteria will be invited to a second orientation session; in this case, the participants will receive detailed written information about the study, and the informed consent. The participants will be cited for their baseline measurement. Fig. 1 shows the participant flow from the recruitment to the randomization stages.
Fig. 1.
Flow diagram of the study participants.
2.2. Participants and selection criteria
After completing the baseline measurements, the selected participants will be randomly assigned to either the control or the exercise training groups. The randomization will be computer-generated. We will use simple randomization [33], hence disparities in group size and in the number of men and women are possible. The assessment staff will be blinded to the participant randomization assignment. The participants will be explicitly informed of their assigned group, as well as of the study hypotheses. They will also be frequently reminded not to disclose their randomization assignments to the assessment staff in the follow-up measurements. For practical and feasibility reasons, the study will be conducted in two waves (maximum 45 participants).
2.3. Sample size
The determination of the sample size and power of the study are made based on the data of a pilot S-Klotho plasma levels sample [34]. We have considered S-Klotho plasma levels differences between pre and post-treatment in order to assess the sample size requirements for the one-way ANOVA [35]. As a result, we expect to detect an effect size of 100 pg/ml considering a type I error of 0.05 with a statistical power of 0.85 if we consider a minimum of 14 participants per group. Assuming a maximum loss at follow-up of 25%, we decided to recruit 20 participants (≈50% women) for each study group: control, PAR, HIIT, and WB-EMS. A total of 80 participants (≈40 women and ≈40 men) will be enrolled in FIT-AGEING. Based on previous RCT and on our own experience [36,37], we think that the sample size is realistic and accessible.
2.4. Statistical analysis
All outcome variables will be verified for normality, and the results will be expressed as mean and SD. For between group comparisons at the baseline (control group vs PAR vs HIIT vs WB-EMS), we will analyze continuous variables with the one-way analysis of variance or the non-parametric method of Kruskal–Wallis and Chi-square tests, as appropriate. We will use General Lineal Models to assess the training effects [time (pre-post 3-month intervention) × group interactions] on the primary and secondary study outcomes. We will adjust multiple comparisons for mass significance [38]. We will also examine the differences in the baseline measurements between the participants who remain in the study and the drop-outs. We will analyze the data according to the intention-to-treat principle [39], and we will handle missing data resulting from drop-outs or non-compliance using multiple imputation methods. To fully appreciate the potential influence of missing responses, we will perform sensitivity analyses to examine whether the imputation method affects the outcomes. In addition, we will perform multiple regression analyses to evaluate potential independent predictors of the α-Klotho protein concentration.
2.5. Participant retention and adherence
The participants will be allowed to withdraw at any time; however, to reduce participants drop-out and to maintain adherence to the training program, several strategies will be implemented. In anticipation of private commitments, vacations, etc., the intervention program will be carried out from September to December. All sessions will be accompanied by music, and will be held on an airy, well-lighted, and well-equipped gym. Qualified and certificated trainers will carefully supervise every training session, and they will work with groups of no more than three persons to ensure that the participants will do the exercises correctly, and at a correct intensity. The training specialist and other study staff will constantly support the participants.
2.6. Exercise program rationale
Since there is no information regarding the ideal exercise model to induce higher levels of the S-Klotho plasma levels, FIT-AGEING will apply different exercise methods. These methods will be (i) PAR [[40], [41], [42], [43]], (ii) HIIT, and (iii) WB-EMS.
One of the most important aims of the study is to compare various exercise intensity levels (moderate vs. high intensity) to test if higher intensity levels provide more benefits despite the application of a lower training volume.
The trial length will be twelve weeks based on (i) the results of a previous study [30] and (ii) taking into account that the substantial physiological adaptations occur within the first 3–6 months of exercise [40]. We will provide no dietary prescriptions or instructions to the participants in the control and exercise groups. The participants will be asked to maintain their dietary habits during the intervention period.
2.6.1. PAR training
2.6.1.1. Volume
Given the importance of the transferability of results to the general population in terms of time, intensity, and frequency, the volume of PAR will be based on the minimum physical activity recommended by the World Health Organization (150 min/week at moderate intensity).
2.6.1.2. Intensity
Physical activity at moderate intensity is recommended to sedentary people by important health institutions to obtain health benefits [41,42,44]. Physical activity at 60% of the heart rate reserve produces significant physiological adaptations in sedentary adults [41,42,44]. For this reason, the intensity selected for PAR aerobic training is 60–65% of the heart rate reserve (HRres). One-repetition maximum (1RM) is the maximum amount of force that can be generated in one maximal contraction, and it is used to determine the intensity of resistance training [45]. The World Health Organization recommends an intensity of 40–50% of 1RM to improve muscle strength, and to increase muscle mass in sedentary persons which have never done strength training [43]. Therefore, the intensity selected for this training modality is 40–50% of 1RM. We will also consider other variables that influence strength training, such as eccentric-isometric-concentric speed ratio, recovery time, and range of motion [46].
2.6.1.3. Frequency
Considering several studies that have compared the effects of different training frequencies on physical fitness as a health marker, the World Health Organization recommends a dose of 3 or 4 days/week [44]. Because the lack of time is one of the most important causes of participant dropout, we have determined that the PAR group will train 3 days/week, the minimum frequency recommended. Resistance training will be performed on 2 of these 3 days/week. In addition, the participants will be advised to refrain from training for a minimum of 24 h and ideally 48 h. The participants will be phoned if they do not meet the weekly recommendations.
2.6.1.4. Type of exercise
The exercises programmed for the aerobic training section will be treadmill, cycle-ergometer, and elliptical ergometer. For the resistance training section, we will include weight-bearing and guided pneumatic machines, involving the major upper and lower body muscle groups [43].
2.6.1.5. Training load variation
We consider that participants may not be immediately capable of meeting the volume and intensity dose required. Therefore, we have proposed a gradual progression to control the exercise dose (see Table 2) based on a previous RCT [37].
Table 2.
PAR training periodization.
| PHASES | WEEKS | AEROBIC TRAINING |
RESISTANCE TRAINING |
||||
|---|---|---|---|---|---|---|---|
| Volume (min) | Intensity (%HRres) | Intensity (%RM) | Type of exercise | Training stimulus | |||
| FAMILIARIZATION | WEEK 1 | 75 | 60 | Weight-bearing and elastic band | Movement pattern and global movements | Movement pattern | Compensatory training |
| WEEK 2 | 105 | 60 | |||||
| PHASE I | WEEK 3 | 120 | 60 | RM ASSESTMENT | |||
| WEEK 4 | 150 | 60 | 50 | Exercises involving major muscle groups | Initial adaptations to resistance training | Compensatory training | |
| WEEK 5 | 150 | 60 | 50 | ||||
| WEEK 6 | 150 | 60 | 50 | ||||
| WEEK 7 | 150 | 60 | 50 | ||||
| PHASE II | WEEK 8 | 120 | 60 | RM ASSESTMENT | |||
| WEEK 9 | 150 | 60 | 50 | Exercises involving major muscle groups | Session type A: mechanical tension and muscle damage |
Session type B: Metabolic Stress |
|
| WEEK 10 | 150 | 60 | 50 | ||||
| WEEK 11 | 150 | 60 | 50 | ||||
| WEEK 12 | 150 | 60 | 50 | ||||
PAR: Physical Activity Recommendations for adults proposed by the World Health Organization, HRres: Heart Rate Reserve, RM: Repetition Maximum.
The participants will begin with an aerobic dose of 75 min/week at 60%HRres. It will progressive increase 30 min/week, and the participants will achieve 150 min/week on the 4th week. Regarding resistance training, the participants will perform a 2-week familiarization phase; they will learn the movement patterns which are based on resistance exercises of a specific training program (dead lift, squat, horizontal, and vertical push-pull, etc.). In addition, the participants will do compensatory exercises to improve core competency and joints stabilization, in order to avoid injuries.
It is well known that, as the fitness level of participants increases, aerobic and strength load should be higher. Aerobic training intensity will increase, since it is necessary to rise the previously established intensity to maintain a specific percentage of HRres, when the physical fitness is increased. In addition, we will measure the RM of all exercises on the first week of each phase in order to adjust the resistance training load. On the other hand, it is important to consider that the session organization determines different physiological adaptations in terms of muscle hypertrophy (metabolic stress, muscle damage etc.) [46]. Due to the fact that the best training stimulus to induce higher levels of the S-Klotho plasma levels is unknown, we will include different types of sessions during each training phase.
2.6.1.6. Training periodization
The training periodization is shown in Table 2. It is divided into two different phases, and its duration will be of 5 weeks, starting with a familiarization phase. The training program structure is based on other randomized controlled trials, which had the aim to meet the physical activity recommendations for adults suggested by World Health Organization [37,41].
FAMILIARIZATION PHASE: This phase will last two weeks. The principal aim of this phase will be to learn the main movement patterns (squat, hinge, bridge, and horizontal and vertical pulls and push) and to improve many physical fitness components such as cardiorespiratory fitness, core stability, joint stabilizing muscles, balance, and flexibility. These sessions will prepare the participants for the 1RM evaluation.
PHASE 1: The participants will perform two combined sessions (aerobic and resistance training) and only one aerobic training session in phase 1. The aerobic training volume will be 150 min/week (except in RM weeks, with a duration of 120 min/week) and the aerobic training intensity will be 60%HRres in all cases. The resistance training will include exercises involving the major muscle groups and principal movement patterns (squat, bench press, dead lift, lateral pull down …) and compensatory exercises.
Combined sessions will have type I structure (see Table 3) which alternate 4 resistance exercises involving the major muscle groups, 2 core stability exercises, and 2 compensatory exercises with 10-min sets of aerobic training.
Table 3.
Combined training session in the PAR training program.
| Session Type I |
Session Type II |
||||||
|---|---|---|---|---|---|---|---|
| EXERCISE | SETS | VOLUME | INTENSITY | EXERCISE | SETS | VOLUME | INTENSITY |
| WARM-UP |
WARM-UP |
||||||
| Aerobic Warm-up | 1 | 5 min | 60% HRres | Aerobic Warm-up | 1 | 5 min | 60% HRres |
| Dynamic Warm-up |
1 |
5 min |
Dynamic Warm-up |
1 |
5 min |
||
|
MAIN PART |
MAIN PART |
||||||
| Aerobic I | 1 | 10 min | 60% HRres | Aerobic I | 1 | 10 min | 60% HRres |
| Resistance Exercise I | 1 | 10 reps | 40–50% RM | Aerobic II | 1 | 10 min | 60% HRres |
| Resistance Exercise II | 1 | 10 reps | 40–50% RM | Resistance Exercise I | 1 | 10 reps | 40–50% RM |
| Resistance Exercise III | 1 | 10 reps | 40–50% RM | Resistance Exercise V | 1 | 10 reps | 40–50% RM |
| Resistance Exercise IV | 1 | 10 reps | 40–50% RM | Resistance Exercise II | 1 | 10 reps | 40–50% RM |
| Aerobic II | 1 | 10 min | 60% HRres | Resistance Exercise VI | 1 | 10 reps | 40–50% RM |
| Resistance Exercise I | 1 | 10 reps | 40–50% RM | Resistance Exercise III | 1 | 10 reps | 40–50% RM |
| Resistance Exercise II | 1 | 10 reps | 40–50% RM | Resistance Exercise VI | 1 | 10 reps | 40–50% RM |
| Resistance Exercise III | 1 | 10 reps | 40–50% RM | Resistance Exercise IV | 1 | 10 reps | 40–50% RM |
| Resistance Exercise IV | 1 | 10 reps | 40–50% RM | Resistance Exercise VIII | 1 | 10 reps | 40–50% RM |
| Aerobic III | 1 | 10 min | 60% HRres | Aerobic III | 1 | 10 min | 60% HRres |
| Resistance Exercise I | 1 | 10 reps | 40–50% RM | Aerobic IV | 1 | 10 min | 60% HRres |
| Resistance Exercise II | 1 | 10 reps | 40–50% RM | COOL-DOWN | 1 | 5 min | |
| Resistance Exercise III | 1 | 10 reps | 40–50% RM | ||||
| Resistance Exercise IV | 1 | 10 reps | 40–50% RM | ||||
| Aerobic IV | 1 | 10 min | 60% HRres | ||||
| Resistance Exercise I | 1 | 10 reps | 40–50% RM | ||||
| Resistance Exercise II | 1 | 10 reps | 40–50% RM | ||||
| Resistance Exercise III | 1 | 10 reps | 40–50% RM | ||||
| Resistance Exercise IV | 1 | 10 reps | 40–50% RM | ||||
| COOL-DOWN | 1 | 5 min | |||||
PAR: Physical Activity Recommendations for adults proposed by the World Health Organization, HRres: Heart Rate Reserve, RM: Repetition Maximum, Reps: Repetitions, Min: minutes.
PHASE 2: In this case, the combined sessions will be different in order to provide a different resistance training stimulus [41,46,47]. The combined session will be divided into type I session (which focuses on mechanical tension and muscle damage) and type II session (which focuses on metabolic stress) [46,47]. Both sessions will include similar exercises to those reported in phase 1, as well as several exercises which involve small muscle groups (lateral raises, French press, or lateral raises).
2.6.1.7. Training sessions
The participants should complete a total of 60 min of aerobic exercise in non-combined sessions (only aerobic exercises). These sessions will start with a dynamic standardized warm-up, including several muscle activation exercises. In addition, aerobic sessions will include compensatory exercises.
All combined training sessions will begin like a non-combined session. After the warm-up, an aerobic exercise will be carried out on 10-min sets, alternating with resistance exercises (depending on the session [see Table 3]). The participants will have the possibility to change the ergometer in different 10-min aerobic sets (treadmill, elliptical, or cycle-ergometer).
In all cases, the training session will end with a cooling-down protocol (active global stretching); the participants will complete 5 anterior or posterior chain exercises.
2.6.2. HIIT training
HIIT describes physical exercise characterized by short and intermittent efforts of vigorous activity, interspersed with resting periods at passive or low-intensity exercises. There are many HIIT protocols, and the specific physiological adaptations induced by this training modality are related to exercise stimulus, (i.e. the intensity, duration, or number of intervals performed), as well as the duration and activity patterns during recovery [48,49]. The energy expenditure at HIIT (more intensity and low volume) is the same (or even higher in some cases) as moderate intensity exercise. However, HIIT physiological and health-related markers are better in healthy and diseased populations [[49], [50], [51]]. These findings are important from a public health perspective, because the ‘lack of time’ is one of the most common problems to do exercise.
2.6.2.1. Volume
The volume in HIIT (40–65 min/week at high intensity) will be smaller than the minimum physical activity recommended by the World Health Organization (75 min/week at vigorous intensity).
2.6.2.2. Intensity
The HIIT intensity is based on scientific evidence [48,49,52,53]. HIIT participants will perform two different complementary protocols: (i) HIIT with long intervals (Type A session), with an intensity of >95% maximum oxygen uptake (VO2max) and (ii) HIIT with short intervals (Type B session), with an intensity of >120% VO2max (>90% HRres or <9 Rating of Perceived Exertion {0–10 RPE scale} [54]). The intensity will progressively increase after the familiarization phase.
2.6.2.3. Frequency
Traditionally, HIIT has been recommended 3 times/week [52,53]. However, considering the age of the participants (45–65 years old) and their training level (sedentary), we have decided to reduce the training frequency (twice per week), since this population needs a 72-h rest after a HIIT session [55].
2.6.2.4. Type of exercise
The exercises programmed for HIIT with long intervals (type A session) are walking on the treadmill with personalized slopes. For the HIIT with short intervals (type B session), the participants will perform eight weight-bearing exercises in circuit form, (i.e. squat, dead lift, high knees up, high heels up, push up, horizontal row, lateral plank, and frontal plank).
2.6.2.5. Training load variation
We consider that participants will not be immediately capable of meeting the volume and intensity dose required; therefore, we propose a gradual progression to control the exercise dose.
The participant will start with a dose of <40 min/week at 80%–90% VO2max in type A and type B sessions (HIIT familiarization phase). It will progressive increase to 50 min/week at >95% in type A session and 120% VO2 max in type B session (HIIT phase I) and to 65 min/week at >95% in type A session and 120% VO2 max in type B session (HIIT phase II).
2.6.2.6. Training periodization
The training periodization is shown in Table 4. It is divided into three phases: (1) HIIT familiarization phase, (2) HIIT phase I, (3) HIIT phase II.
Table 4.
HIIT training periodization.
| HIIT Familiarization phase | ||||||||
|---|---|---|---|---|---|---|---|---|
| Week | 1 | 2 | 3 | 4 | ||||
| Session (type) | 1 (A) | 2 (B) | 3 (A) | 4 (B) | 5 (A) | 6 (B) | 7 (A) | 8 (B) |
| Exercises | 1 (Tr) | 8 × 2 = 16 (W-B) | 1 (Tr) | 8 × 2 = 16 (W-B) | 1 (Tr) | 8 × 2 = 16 (W-B) | 1 (Tr) | 8 × 2 = 16 (W-B) |
| Volume | 12 min | 16 min | 14 min | 21 min | 16 min | 16 min | 18 min | 21 min |
| Intensity | 80%VO2m | 80%VO2m | 80%VO2m | 80%VO2m | 90%VO2m | 90%VO2m | 90%VO2m | 90%VO2m |
| Sets | 6 | 2 | 7 | 2 | 8 | 2 | 9 | 2 |
| Set duration | 4 min | 8 min | 4 min | 10.5 min | 4 min | 8 min | 4 min | 10.5 min |
| Work exercise | 2 min | 15 Sec | 2 min | 20 Sec | 2 min | 15 Sec | 2 min | 20 Sec |
| Rest exercise | 2 min (pass) | 15 Sec | 2 min (pass) | 20 Sec | 2 min (pass) | 15 Sec | 2 min (pass) | 20 Sec |
| Rest between sets |
– |
5 min (60% VO2m) |
– |
5 min (60% VO2m) |
– |
5 min (60% VO2m) |
– |
5 min (60% VO2m) |
|
HIIT PHASE I | ||||||||
| Week | 5 | 6 | 7 | 8 | ||||
| Session (type) | 9 (A) | 10 (B) | 11 (A) | 12 (B) | 13 (A) | 14 (B) | 15 (A) | 16 (B) |
| Exercises | 1 (Tr) | 8 × 2 = 16 (W-B) | 1 (Tr) | 8 × 2 = 16 (W-B) | 1 (Tr) | 8 × 2 = 16 (W-B) | 1 (Tr) | 8 × 2 = 16 (W-B) |
| Volume | 16 min | 16 min | 18 min | 21 min | 20 min | 27 min | 20 min | 32 min |
| Intensity | >95% VO2m | 120%VO2m | >95% VO2m |
120%VO2m | >95% VO2m |
120%VO2m | >95% VO2m |
120%VO2m |
| Sets | 8 | 2 | 9 | 2 | 10 | 2 | 10 | 2 |
| Set duration | 4 min | 8 min | 4 min | 10.5 min | 4 min | 13.5 min | 4 min | 16 min |
| Work exercise | 2 min | 15 Sec | 2 min | 20 Sec | 2 min | 25 Sec | 2 min | 30 Sec |
| Rest exercise | 2 min (pass) | 15 Sec | 2 min (pass) | 20 Sec | 2 min (pass) | 25 Sec | 2 min (pass) | 30 Sec |
| Rest between sets |
– |
5 min (60% VO2m) |
– |
5 min (60% VO2m) |
– |
5 min (60% VO2m) |
– |
5 min (60% VO2m) |
|
HIIT PHASE II | ||||||||
| Week | 9 | 10 | 11 | 12 | ||||
| Session (type) | 17 (A) | 18 (B) | 19 (A) | 20 (B) | 21 (A) | 22 (B) | 23 (A) | 24 (B) |
| Exercises | 1 (Tr) | 8 × 2 = 16 (W-B) | 1 (Tr) | 8 × 2 = 16 (W-B) | 1 (Tr) | 8 × 2 = 16 (W-B) | 1 (Tr) | 8 × 2 = 16 (W-B) |
| Volume | 18 min | 24 min | 21 min | 31.5 min | 24 min | 40.5 min | 24 min | 40.5 min |
| Intensity | >95% VO2m |
120%VO2m | >95% VO2m |
120%VO2m | >95% VO2m |
120%VO2m | >95% VO2m |
120%VO2m |
| Sets | 6 | 3 | 7 | 3 | 8 | 3 | 8 | 3 |
| Set duration | 4 min | 8 min | 4 min | 10.5 min | 4 min | 13.5 min | 4 min | 13.5 min |
| Work exercise | 3 min | 15 Sec | 3 min | 20 Sec | 3 min | 25 Sec | 3 min | 25 Sec |
| Rest exercise | 2 min (pass) | 15 Sec | 2 min (pass) | 20 Sec | 2 min (pass) | 25 Sec | 2 min (pass) | 25 Sec |
| Rest between sets | – | 5 min (60% VO2m) | – | 5 min (60% VO2m) | – | 5 min (60% VO2m) | – | 5 min (60% VO2m) |
Type A; High Intensity Interval Training on the treadmill with individual slopes, Type B; High Intensity Interval Power Training (weight-bearing exercises), Tr; Treadmill, W-B; Weight-Bearing exercises, VO2m; Maximum oxygen uptake, Min; minutes, Sec; seconds, Pas; Passive.
HIIT FAMILIARIZATION PHASE: This phase will last 4 weeks. The participants will carry out 2 types of sessions each week, type A and type B. The intensity selected for the first and the second week will be 80% VO2max. The intensity and the volume will be higher in the third and fourth week. In session type A, the participants will complete 6–9 sets of 4 min (2 min work/2 min rest) with a maximal duration of 18 min/session. In session type B, the participants will complete 2 sets (8–9.5 min) of 16 exercises (15–20 s work/15–20 s rest) with an active rest of 5 min at 60% VO2max and a maximal duration of 19 min/session.
HIIT PHASE I: The participants will do two different sessions as in the familiarization phase. The intensity will be >95%VO2max in type A session, and >120%VO2max in type B session. The training volume will be less than 50 min/week. In session type A, the participants will complete 8–10 sets of 4 min (2 min work/2 min rest) with a maximal duration of 20 min/session. In session type B, the participants will complete 2 sets (8–12.5 min of duration) of 16 exercises (15–30 s work/15–30 s rest) with an active rest of 5 min at 60% VO2max and a maximal duration of 25 min/session.
HIIT PHASE II: Sessions will follow the same structure than HIIT PHASE I. However, training volume will be more than 50 min/week but less than 65 min/week. In session type A, the participants will complete 6–8 sets of 5 min (3 min work/2 min rest) with a maximal duration of 24 min/session (intensity >95% VO2max). In session type B, the participants will complete 3 sets (8–12.5 min duration) of 16 exercises (15–30 s work/15–30 s rest) with an active rest of 5 min–60% VO2max and a maximal duration of 37 min/session (intensity >120% VO2max). The exercises in session type B can be modified in order to increase their difficulty (add external load, increase range of motion, add instability, etc.) because it will be expected that physical fitness increases as a training adaptation.
2.6.2.7. Training sessions
Type A session: It will start with a dynamic standardized warm-up, including several muscle activation exercises followed by 5 min of aerobic exercise on the treadmill at 60% VO2max. After the warm-up, the participants will complete several treadmill sets following the established parameters previously described.
Type B session: It will start with a dynamic standardized warm-up. The participants will perform eight weight-bearing exercises (in circuit form) twice per set with an active rest (walking at 60%VO2max) following the periodization previously established.
In all cases, the training session will end with the same cooling-down protocol described in PAR.
2.6.3. WB-EMS training
WB-EMS is becoming increasingly popular as a novel training technology. WB-EMS is able to simultaneously stimulate up to 14–18 regions or 8–12 different muscle groups with up to 2.800 cm2 electrode area [56]. Very few studies have determined the influence of WB-EMS on ageing, physical fitness, body composition, and physiological parameters in sedentary healthy adults [[57], [58], [59]], and its effects are controversial [60,61]. Therefore, it is essential to follow the scientific recommendations related to WB-EMS to avoid possible health problems [62,63] produced by the irresponsible use of this technology [58,59,63].
The WB-EMS training program will follow the same structure as the HIIT intervention in terms of volume, intensity, frequency, type of exercise, training load variation, training periodization, and training session. However, electrical impulses will be included in order to assess whether the WB-EMS training will produce an extra effect compared with the HIIT program.
2.6.3.1. Electrical parameters
Given that the participants have never done WB-EMS, we decided to establish a progressive and gradual WB-EMS training periodization in order to avoid possible dangerous health consequences, such as increased creatine kinase levels and rhabdomyolysis [60,61].
The electrical parameters progression is shown in Table 5. Using the WB-EMS devices from Wiemspro® (Malaga, Spain), bipolar, symmetrical, and rectangular electric pulse will be applied. The periodization of electric parameters can be seen in Table 5.
Table 5.
Electrical parameters in the WB-EMS training periodization.
| FAMILIARIZATION PHASE | ||||||||
|---|---|---|---|---|---|---|---|---|
| Week | 1 | 2 | 3 | 4 | ||||
| Session (type) | 1 (A) | 2 (B) | 3 (A) | 4 (B) | 5 (A) | 6 (B) | 7 (A) | 8 (B) |
| Frequency | 15 Hz | 35 Hz | 15 Hz | 35 Hz | 15 Hz | 40 Hz | 15 Hz | 40 Hz |
| Intensity | 100 mA | 80 mA | 100 mA | 80 mA | 100 mA | 80 mA | 100 mA | 80 mA |
| RPE impulse (0–10) | 5–6 | 5–6 | 6–7 | 6–7 | 7–8 | 7–8 | 7–8 | 7–8 |
| Duty cycle |
99% (59¨:1¨) |
50% (15¨:15¨) |
99% (59¨:1¨) |
57% (20¨:15¨) |
99% (59¨:1¨) |
50% (15¨:15¨) |
99% (59¨:1¨) |
57% (20¨:15¨) |
|
HIIT PHASE I | ||||||||
| Week | 5 | 6 | 7 | 8 | ||||
| Session (type) | 9 (A) | 10 (B) | 11 (A) | 12 (B) | 13 (A) | 14 (B) | 15 (A) | 16 (B) |
| Frequency | 20 Hz | 45 Hz | 20 Hz | 45 Hz | 20 Hz | 50 Hz | 20 Hz | 55 Hz |
| Intensity | 100 mA | 80 mA | 100 mA | 80 mA | 100 mA | 80 mA | 100 mA | 80 mA |
| RPE impulse (0–10) | 7–8 | 7–8 | 7–8 | 7–8 | 7–8 | 7–8 | 7–8 | 7–8 |
| Duty cycle |
99% (59¨:1¨) |
50% (15¨:15¨) |
99% (59¨:1¨) |
57% (20¨:15¨) |
99% (59¨:1¨) |
63% (25¨:15¨) |
99% (59¨:1¨) |
67% (30¨:15¨) |
|
HIIT PHASE II | ||||||||
| Week | 9 | 10 | 11 | 12 | ||||
| Session (type) | 17 (A) | 18 (B) | 19 (A) | 20 (B) | 21 (A) | 22 (B) | 23 (A) | 24 (B) |
| Frequency | 25 Hz | 60 Hz | 20 Hz | 65 Hz | 20 Hz | 70 Hz | 20 Hz | 75 Hz |
| Intensity | 100 mA | 80 mA | 100 mA | 80 mA | 100 mA | 80 mA | 100 mA | 80 mA |
| RPE impulse (0–10) | 8–9 | 8–9 | 8–9 | 8–9 | 8–9 | 8–9 | 8–9 | 8–9 |
| Duty cycle | 99% (59¨:1¨) | 50% (15¨:15¨) | 99% (59¨:1¨) | 57% (20¨:15¨) | 99% (59¨:1¨) | 63% (25¨:15¨) | 99% (59¨:1¨) | 63% (25¨:15¨) |
Type A; High Intensity Interval Training on the treadmill with individual slopes, Type B; High Intensity Interval Power Training (weight-bearing exercises), Hz; Hertz, mA; milliamps.
The typical frequency used in WB-EMS studies has been 85 Hz [[56], [57], [58], [59],63]. However, our participants will be sedentary adults aged 45–65, and it has been shown that ageing is associated with a muscle mass decrease, especially type II fibers, and this decrease in muscle tissue begins around the age of 50 and dramatically increases beyond the age of 60 [64,65]. In addition, it is well-known that the ideal frequency to recruit type I fibers is 7–33 Hz [66]. Therefore, we will apply a frequency of 15–33 Hz in our type A session (aerobic exercise). On the other hand, we will apply a frequency of 35–75 Hz in the type B session (resistance exercises) because, in this case, it will be active type II fibers and the optimal frequency is 35–100 Hz [66].
INTENSITY: The intensity applied in our intervention program will be 80–100 mA, following the scientific guidelines established in local electrostimulation in order to improve fitness and body composition (>50 mA) [66]. The impulse intensity was individually adapted in accordance with the participants in order to generate similar values of rate of perceived exertion (RPE) than other WB-EMS studies (49, 54–59) using the Borg CR-10 Scale “5” of “9” [67].
IMPULSE WIDTH: The scientific recommendations regarding this matter range between 200 and 400 μsec. We adjusted this parameter in relation to the body segment: thigh zone (400 μsec), glute zone (350 μsec), abdominal zone (300 μsec), dorsal zone (250 μsec), cervical (200 μsec), chest zone (200 μsec), and arm zone (200 μsec) [66].
DUTY CYCLE: The stimulation ratio (duty cycle) is defined as ratio of on-time to the total cycle time (% duty cycle = 100/[total time/on-time]). A duty cycle of 50–70% will be used [[57], [58], [59],68]. We have programmed a duty cycle of 50–67% in type B (resistance training) session following scientific evidence, but duty cycle in type A session (aerobic exercise) will be 99% because the frequency will be low and the work time will be of 3 min maximum.
2.7. Control group
We will provide general advice to the control group participants though an information meeting presided by a graduate in Sport Sciences. They will be instructed to maintain their lifestyle.
2.8. Outcome variables
The primary outcome of our study is the S-Klotho plasma levels. The secondary outcome variables include physical fitness components, body composition and anthropometric measurements, energy expenditure and nutrients oxidation, heart rate variability (HRV), health blood biomarkers, free-living physical activity, cognitive variables, health-related quality of life, dietary habits, and cardiovascular disease risk factors (secondary outcomes) in sedentary healthy adults.
The baseline measurement will be organized on 4 days:
Day 1: Medical examination (anamnesis, blood pressure assessment …) and fasting blood measurements.
Day 2: HRV during 15 min, basal metabolic rate measure by indirect calorimetry during 30 min, body composition by Dual Energy X-ray Absorptiometry scan (DXA) and anthropometric measurements, and maximal fat oxidation during an incremental treadmill protocol with a gas analyzer. All tests will be conducted under fasting conditions.
Day 3: Isokinetic dynamometry strength test, cognition test and a questionnaire battery test which includes the ALPHA – Questionnaire Environment Perception, the Beck Depression Inventory (BDI-II), the Sedentary Behavior, the Physical Activity Attitude Questionnaire (C-AAF), Par-Q & You, the Health Questionnaire SF-36, the Socio-demographic data, the HOPE assessment, the Physical Fitness Condition Scale-International (IFIS), the Pittsburgh Sleep quality, the Revised Life Orientation Test (LOTR), the Satisfaction With Life Scale (SWLS), the Trait-Meta Mood Scale (TMMS-24), the Positive and Negative Affect Schedule (PANAS), PREDIMED questionnaire, and the Sexual Desire Questionnaire.
Day 4: Muscle strength test (manual isometric dynamometry and core resistance stability), simple and discriminative reaction test and maximum exercise test on a treadmill (h/p cosmos, Italy) with a gas analyzer, electrocardiogram, and blood pressure control. All tests will be supervised by a graduate in sport sciences, and a sport medicine doctor.
We will use accelerometers to objectively measure physical activity, sleep quality, and sleep volume. Finally, we will control the dietary intake by three 24 h recalls and by a food frequency questionnaire.
2.8.1. Primary outcome: S-Klotho plasma levels
We will collect blood samples from the antecubital vein after 12 h of fasting. The S-Klotho plasma levels will be measured by ELISA using a soluble α-klotho ELISA assay kit (Demeditec, Kiel, Germany). The kit is a non-competitive solid-phase sandwich ELISA that uses two types of highly specific antibodies (purified mouse anti-human Klotho IgG). The optical density is measured at a wavelength of 450 nm ± 2 nm and a standard curve is generated using known antigen concentrations. All participants will be requested to abstain from drugs and/or caffeine, to eat a standardized dinner before sampling, and to avoid any physical activity of moderate intensity (24 h before) and/or vigorous intensity (48 h before).
We will also measure ageing markers such as a general biochemical profile, red cells profile, immunological blood profile, brain-derived neurotrophic factor, IGF-1, insulin, growth hormone, testosterone, free-testosterone, cortisol, dehydroepiandrosterone, and D hormone. The ELISA kits and spectrophotometry will be utilized to perform these analyses.
2.8.2. Secondary outcome
2.8.2.1. Physical fitness
Cardiorespiratory fitness will be measured through a maximum treadmill test (h/p cosmos, Italy) applying the modified Balke protocol [69], which has been widely used and validated [37,[70], [71], [72]]. We will also measure the O2 uptake and CO2 production with the breath by breath gas analyzer (CPX Ultima CardiO2, Medical Graphics Corp, St Paul, USA) calibrated with known gas mixtures and environmental air immediately before the test. Consistently across each trial, the participants will be strongly encouraged to invest maximum effort. The criteria for achieving VO2max will be respiratory exchange ratio ≥1.1, a plateau in VO2 (change of <100 ml/min in the last three consecutive 10 s stage), and a heart rate within 10 beats/min of the age-predicted maximal heart rate (208–0.7∗age) [73]. We will include a second and constant work rate test performed at 110% of the work rate achieved after the modified Balke protocol test to resolve the classic V̇O2-work rate plateau, which is the unambiguous validation of VO2max [74]. The exercise electrocardiogram will be continuously monitored.
We will conduct isokinetic strength tests using a Gymnex Iso-2 dynamometer (EASYTECH s.r.l., Italy), calibrated following the product instructions before the data collection. The knee extensor muscles will be tested concentrically and eccentrically at 180° and 60° s-1. The upper members, hips, and shoulders will be stabilized with safety belts. The rotational axis of the dynamometer will be aligned with the right lateral femoral condyle. The force pad will be placed 3–4 cm above the medial malleolus. The knee extension will start with a 90º-joint angle and it will end at 170°. The subject will be instructed to sub maximally flex and extend the knee five times, and then complete three maximal repetitions. We will allow the participants a 1-min rest between submaximal and maximal trials, and 5 min between 180° and 60° s-1, following a scientific validated protocol [75]. The peak torque will be determined as the single repetition with the highest muscular force output (Nm). The participants will be encouraged by the trainer during the test, and the same trainer-researcher will conduct all the isokinetic testings.
We will measure the handgrip strength using a digital dynamometer (TKK 5101 Grip-D; Takey, Tokyo, Japan). It will be measured following the procedures described elsewhere [76].
We will evaluate the core resistance stability using a standard protocol described by McGill et al. [77], which has been extensively used in scientific studies [78,79]. This methodology includes the Biering-Sorensen extensor endurance test, flexor endurance test (60°), frontal plank test, and the side bridge test.
2.8.2.2. Body composition and anthropometric measurements
The body weight, height, hip circumference, and waist circumference will be determined following the recommended standardization procedures from the International Society for the Advancement of Kinanthropometry (ISAK). We will also evaluate the fat mass, fat free mass, lean body mass, visceral adipose tissue, and bone mineral density by conducting a DXA scan (HOLOGIC, Wi).
2.8.2.3. Energy expenditure and nutrients oxidation
We will evaluate the basal metabolic rate by indirect calorimetry with a breath by breath gas analyzer (CPX Ultima CardiO2, Medical Graphics Corp, St Paul, USA). The participants will be requested to attend the center in the post-absorptive condition (12–14 h fasting), abstain from drugs and/or caffeine, eat an established dinner before sampling, avoid physical activity of moderate intensity (24 h before) and/or vigorous intensity (48 h before). They will lie down on a bed, in a quiet environment. Calorimetric measures will follow the scientific accepted standard to ensure the validity of the tests [80,81].
A standardized treadmill protocol test will be used to measure maximal fat oxidation [82]. More specifically, the test will start at 3.5 km h−1 and at a gradient of 0% during 3 min. The speed will then increase until reaching the maximal speed that the participant can comfortably maintain without running. The grade will increase by 2% every 3 min until a respiratory quotient of 1 is reached. After that, the speed will decrease until 4 km*h −1, and the grade will be 0% during 5 min (active recovery). The respiratory gas measurements will continuously be collected using a metabolic cart (CPX Ultima CardiO2, Medical Graphics Corp, St Paul, USA). Furthermore, the heart rate and rating of the perceived exertion (RPE) record will be measured throughout the whole test.
2.8.2.4. Heart rate variability (HRV)
The assessment of HRV will be carried out in a supine position before the basal metabolic rate test. Polar RS800CX (Polar Electro, Kempele, Finland) will be used to evaluate this parameter (R-R series active). The participants will be informed that their heart activity will be recorded during 10 min, and they will be instructed not to talk or move excessively, and to relax as much as possible. The participants will be in a supine position during 5 min prior to the start of the recording. We will use the Kubios HRV software (University of Eastern Finland, Kuopio, Finland) to process the HRV [83].
2.8.2.5. Physical activity
The amount of physical activity and sleep will be measured by accelerometry (ActiSleep, Actigraph, Pensacola, Florida, USA). The participants will wear two accelerometers (non-dominant wrist and right hip) during 7 consecutive days for 24 h to assess physical activity volume and intensity and sleep quantity and quality.
2.8.2.6. Cognitive performance and reaction time
Cognitive deterioration is one of the most apprehensive aspects of ageing, and it has been shown that exercise contributes to maintaining cognitive and brain function [84]. We will evaluate cognitive functions through simple and discriminative reaction time (Vienna Test System, Moedling, Austria) and the Wechsler Adult Intelligence Scale test battery (WAIS-III), which has been applied in several scientific studies [85].
2.8.2.7. Dietary assessment
We will conduct a dietary assessment at the baseline and after the intervention. Three 24-h dietary recalls (1 during the weekend). We will also administer the participants a food frequency questionnaire. All data will be processed by the dietetic software EVALFINUT® (Ibero-American Foundation of Nutrition, Spain).
3. Potential impact of FIT-AGEING
‘Health, demographic change, and wellbeing’ is one of the main challenges of Horizon 2020. FIT-AGEING contributes to the improvement of our understanding of the causes and mechanisms underlying health, healthy ageing, and disease, which is the principal topic that Research & Innovation has supported (€2 billion). Previous studies have shown that health disabilities (diabetes, osteoporosis, sarcopenic obesity, etc.) could be attributed exclusively to age [[86], [87], [88]].
The expression of the α-Klotho gene is positively correlated with a longer life spam, and the lack of the α-Klotho gene expression is related to ageing phenotypes such as artheriosclerosis, decreased bone mineral density, sarcopenia, skin atrophy, and impaired cognition [89]. Similar anti-ageing effects have also been ascribed to exercise and physical activity [20]. Therefore, the α-Klotho and physical activity are factors that may promote upgrading capacities of the elderly and their relation has been shown in scientific studies [32]. However, the mechanism that may mediate these effects is currently unknown.
FIT-AGEING will study the effect of different exercise modalities on the S-Klotho plasma levels during 3 months (our principal outcome) for the first time in humans. In addition, FIT-AGEING will also measure other important health-related variables that may be related to the ageing process. Our study integrates physiology, biochemical parameters, and preventive medicine in order to investigate how physical exercise can induce an increase of the S-Klotho plasma levels in adults.
The findings from FIT-AGEING could be significant implications for our understanding of exercise and its protective effects against the ageing process and ageing-related diseases.
Acknowledgments
The study is supported by the Spanish Ministry of Education (FPU14/04172 and FPU15/03960). The study was partially supported by the University of Granada, Plan Propio de Investigación 2016, Excellence actions: Units of Excellence; Unit of Excellence on Exercise and Health (UCEES). The authors would like to thank all the participants that take part of the study for their time and effort. We are grateful to Jonatan R. Ruiz for his constructive scientific discussions, and to Ms. Carmen Sainz Quinn for assistance with the English language. This study is part of a Ph.D. Thesis conducted in the Biomedicine Doctoral Studies of the University of Granada, Spain.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.conctc.2018.05.013.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Wallace R.G., Twomey L.C., Custaud M.-A., Turner J., Moyna N., Cummins P.M., Murphy R.P. The role of epigenetics in cardiovascular health and aging: a focus on physical activity and nutrition. Mech. Ageing Dev. 2017 doi: 10.1016/j.mad.2017.11.013. [DOI] [PubMed] [Google Scholar]
- 2.Murray C.J.L., Barber R.M., Lopez A.D., Vos T. Global, regional, and national disability-adjusted life years (DALYs) for 306 diseases and injuries and healthy life expectancy (HALE) for 188 countries, 1990-2013: quantifying the epidemiological transition. Lancet. 2015;386:2145–2191. doi: 10.1016/S0140-6736(15)61340-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilmoth J. Demography of longevity: past, present, and future trends. Exp. Gerontol. 2000;35:1111–1129. doi: 10.1016/s0531-5565(00)00194-7. [DOI] [PubMed] [Google Scholar]
- 4.Kingsley D. Aging and health care costs: narrative versus reality. Poverty & Public Policy. 2015;7:3–21. [Google Scholar]
- 5.Lowsky D.J., Olshansky S.J., Bhattacharya J., Goldman D.P. Heterogeneity in healthy aging. J. Gerontol. A. Biol. Sci. Med. Sci. 2014;69:640–649. doi: 10.1093/gerona/glt162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mora J.C., Valencia W.M. Exercise and older adults. Clin. Geriatr. Med. 2018;34:145–162. doi: 10.1016/j.cger.2017.08.007. [DOI] [PubMed] [Google Scholar]
- 7.Kim J.-H., Hwang K.-H., Park K.-S., Kong I.D., Cha S.-K. Biological role of anti-aging protein Klotho. J. Lifestyle Med. 2015;5:1–6. doi: 10.15280/jlm.2015.5.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuro-o M., Matsumura Y., Aizawa H., Kawaguchi H., Suga T., Utsugi T., Iwasaki H. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- 9.Hu M., Shi M., Zhang J., Pastor J., Nakatani T., Lanske B., Moe O. Klotho: a novel phosphaturic substance acting as an autocrine enzyme in the renal proximal tubule. Faseb. J. 2010;24:3438–3450. doi: 10.1096/fj.10-154765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu J., Shi M., Zhang J., Quiñones H., Griffith C., Kuro-o M. Klotho deficiency causes vascular calcification in chronic kidney disease. J. Am. Soc. Nephrol. 2011;22:124–136. doi: 10.1681/ASN.2009121311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cha S.K., Hu M.C., Kurosu H., Kuro-o M., Moe O. Huang, Regulation of renal outer medullary potassium channel and renal K+ excretion by Klotho. Mol. Pharmacol. 2009;76:38–46. doi: 10.1124/mol.109.055780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim J.-H., Hwang K.-H., Park K.-S., Kong I., Cha S.-K. Biological role of anti-aging protein klotho. J Lifestyle Med. 2015;5:1–6. doi: 10.15280/jlm.2015.5.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamamoto M., Clark J., Pastor J.V., Gurnani P., Nandi A., Kurosu H. Regulation of oxidative stress by the anti-aging hormone klotho. Biol. Chem. 2005;280:3829–3834. doi: 10.1074/jbc.M509039200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kurosu H., Yamamoto M., Clark J.D., Pastor J.V., Nandi A., Gurnani P., Shimomura I. Suppression of aging in mice by the hormone Klotho. Science. 2005;30:1829–1833. doi: 10.1126/science.1112766. (80) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doi S., Zou Y., Togao O., Pastor J.V., John G.B., Wang L., Takahashi M. Klotho inhibits transforming growth factor-β1 (TGF-β1) signaling and suppresses renal fibrosis and cancer metastasis in mice. J. Biol. Chem. 2011;286:8655–8665. doi: 10.1074/jbc.M110.174037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Segawa H., Yamanaka S., Ohno Y., Onitsuka A., Shiozawa K., Aranami F., Furutani J., Tomoe Y., Ito M., Kuwahata M., Imura A., Nabeshima Y., Miyamoto K. Correlation between hyperphosphatemia and type II Na-Pi cotransporter activity in klotho mice. Am. J. Physiol. Ren. Physiol. 2007;292:F769–F779. doi: 10.1152/ajprenal.00248.2006. [DOI] [PubMed] [Google Scholar]
- 17.Myers J., McAuley P., Lavie C., Despres J., Arena R., Kokkinos P. Physical activity and cardiorespiratory fitness as major markers of cardiovascular risk: their independent and interwoven importance to health status. Prog. Cardiovasc. Dis. 2015;57:306–314. doi: 10.1016/j.pcad.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 18.Lee M., Artero E.G., Sui X., Blair S. Review: mortality trends in the general population: the importance of cardiorespiratory fitness. J. Psychopharmacol. 2010;24:27–35. doi: 10.1177/1359786810382057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Volaklis K., Halle M. Meisinger, Muscular strength as a strong predictor of mortality: a narrative review. Eur. J. Intern. Med. 2015;26:303–310. doi: 10.1016/j.ejim.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 20.Castillo-Garzón M., Ruiz J., Ortega F., Gutiérrez A. Anti-aging therapy through fitness enhancement. Clin. Interv. Aging. 2006;1:213–220. doi: 10.2147/ciia.2006.1.3.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kodama S., Saito K., Tanaka S., Maki M., Yachi Y. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: a meta-analysis. Jama. 2009;301:2024–2035. doi: 10.1001/jama.2009.681. [DOI] [PubMed] [Google Scholar]
- 22.Vrachimis A., Hadjicharalambous M., Tyler C. The effect of circuit training on resting heart rate variability, cardiovascular disease risk factors and physical fitness in healthy untrained adults. Health (Irvine. Calif) 2016;8:144–148. [Google Scholar]
- 23.Schjerve I., Tyldum G.A., Tjønna A.E., Stølen T., Loennechen J.P., Hansen H. Both aerobic endurance and strength training programmes improve cardiovascular health in obese adults. Clin. Sci. 2008;115:283–293. doi: 10.1042/CS20070332. [DOI] [PubMed] [Google Scholar]
- 24.Cartee G., Hepple R.T., Bamman M.M., Zierath J. Exercise promotes healthy aging of skeletal muscle. Cell Metabol. 2016;23:1034–1047. doi: 10.1016/j.cmet.2016.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fritzen A., Madsen A.B., Kleinert M., Treebak J.T., Lundsgaard A.M., Jensen T.E., Frøsig C. Regulation of autophagy in human skeletal muscle: effects of exercise, exercise training and insulin stimulation. J. Physiol. 2016;594:745–761. doi: 10.1113/JP271405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tanimura T., Aoi W., Takanami Y., Kawai Y., Mizushima K., Naito Y., Yoshikawa T. Acute exercise increases fibroblast growth factor 21 in metabolic organs and circulation. Phys. Rep. 2016;4:128–138. doi: 10.14814/phy2.12828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Phelps M., Pettan-Brewer C., Ladiges W., Yablonka-Reuveni Z. Decline in muscle strength and running endurance in klotho deficient C57BL/6 mice. Biogerontology. 2013;14:729–739. doi: 10.1007/s10522-013-9447-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mostafidi E., Moeen A., Nasri H., Hagjo A., Ardalan M., Ghorbani-Hagjo A., Ardalan M. Serum klotho levels in trained athletes. Nephro-Urol. Mon. 2016;8:1. doi: 10.5812/numonthly.30245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Santos-Dias A., MacKenzie B., Oliveira-Junior M., Moyses R., Consolim-Colombo F., Vieira R. Longevity protein klotho is induced by a single bout of exercise. Br. J. Sports Med. 2016;1:1. doi: 10.1136/bjsports-2016-096139. [DOI] [PubMed] [Google Scholar]
- 30.Matsubara T., Miyaki A., Akazawa N., Choi Y., Ra S.-G., Tanahashi K., Kumagai H., Oikawa S., Maeda S. Aerobic exercise training increases plasma Klotho levels and reduces arterial stiffness in postmenopausal women. Am. J. Physiol. Heart Circ. Physiol. 2014;306:H348–H355. doi: 10.1152/ajpheart.00429.2013. [DOI] [PubMed] [Google Scholar]
- 31.Ahima R. Connecting obesity, aging and diabetes. Nat. Med. 2009;15:996–997. doi: 10.1038/nm0909-996. [DOI] [PubMed] [Google Scholar]
- 32.Saghiv M., Goldhammer E., Sagiv M., Ben-Sira D. Effects of aerobic exercise training on S-Klotho in young and elderly. Jpn. J. Physiol. 2015;1:1–2. [Google Scholar]
- 33.Schulz D., Grimes K.F. Generation of allocation sequences in randomised trials: chance, not choice. Lancet. 2002;359:515–519. doi: 10.1016/S0140-6736(02)07683-3. [DOI] [PubMed] [Google Scholar]
- 34.Hawkins D. Chapmanan; London: 1980. Identification of Outliers. [Google Scholar]
- 35.Chow S., Wang H., Shao J. CRC; 2007. Sample Size Calculations in Clinical Research. [Google Scholar]
- 36.Ruiz J.R., Perales M., Pelaez M., Lopez C., Lucia A., Barakat R. Supervised exercise-based intervention to prevent excessive gestational weight gain: a randomized controlled trial. Mayo Clin. Proc. 2013;88:1388–1397. doi: 10.1016/j.mayocp.2013.07.020. [DOI] [PubMed] [Google Scholar]
- 37.Sanchez-Delgado G., Martinez-Tellez B., Olza J., Aguilera C.M., Labayen I., Ortega F.B., Ruiz J. Activating brown adipose tissue through exercise (ACTIBATE) in young adults: rationale, design and methodology. Contemp. Clin. Trials. 2015;45:416–425. doi: 10.1016/j.cct.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 38.Holm S. A simple sequentially rejective multiple test procedure. Scand. J. Stat. 1979;1:65–70. [Google Scholar]
- 39.Hollis F., Campbell S. What is meant by intention to treat analysis? Survey of published randomised controlled trials. BMJ. 1999;319:374–670. doi: 10.1136/bmj.319.7211.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pedersen B., Saltin B.K. Evidence for prescribing exercise as therapy in chronic disease. Scand. J. Med. Sci. Sports. 2006;16:3–63. doi: 10.1111/j.1600-0838.2006.00520.x. [DOI] [PubMed] [Google Scholar]
- 41.WHO . World Health Organ publications; Geneva, Switzerland: 2010. Global Recommendations on Physical Activity for Health. [PubMed] [Google Scholar]
- 42.US . 2016. Department of Health and Human Services. Physical Activity Guidelines. [Google Scholar]
- 43.ACSM American College of Sports Medicine position stand. Progression models in resistance training for healthy adults. Med. Sci. Sports Exerc. 2009;41:687–708. doi: 10.1249/MSS.0b013e3181915670. [DOI] [PubMed] [Google Scholar]
- 44.Garber C.E., Blissmer B., Deschenes M., Franklin B.A., Lamonte M.J., Lee I.M., Swain D. American college of sports medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med. Sci. Sports Exerc. 2011;43:1334–1359. doi: 10.1249/MSS.0b013e318213fefb. [DOI] [PubMed] [Google Scholar]
- 45.Marchese A., Hill A.R. Pearson; Sydney (Australia): 2011. The essential guide to fitness: for the fitness instructor. [Google Scholar]
- 46.Schoenfeld B. The mechanisms of muscle hypertrophy and their application to resistance training. J. Strength Condit Res. 2010;24:2857–2872. doi: 10.1519/JSC.0b013e3181e840f3. [DOI] [PubMed] [Google Scholar]
- 47.Schoenfeld B.J., Ogborn D., Krieger J. Effects of resistance training frequency on measures of muscle hypertrophy: a systematic review and meta-analysis. Sports Med. 2016;1:1–9. doi: 10.1007/s40279-016-0543-8. [DOI] [PubMed] [Google Scholar]
- 48.Hwang C.L., Yoo J.K., Kim H.K., Hwang M.H., Handberg E.M., Petersen J.W., Christou D. Novel all-extremity high-intensity interval training improves aerobic fitness, cardiac function and insulin resistance in healthy older adults. Exp. Gerontol. 2016;82:112–119. doi: 10.1016/j.exger.2016.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gibala M.J., Little J.P., MacDonald M.J., Hawley J. Physiological adaptations to low-volume, high-intensity interval training in health and disease. J. Physiol. 2012;590:1077–1084. doi: 10.1113/jphysiol.2011.224725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ulbrich A., Angarten V.G., Netto A.S., Sties S.W., Bündchen D.C., de Mara L.S., de Carvalho T. Comparative effects of high intensity interval training versus moderate intensity continuous training on quality of life in patients with heart failure: study protocol for a randomized controlled trial. Clin. Trials Regul. Sci. Cardiol. 2016;13:21–28. [Google Scholar]
- 51.Hwang C.L., Chou C.H., Wu Y. Effect of aerobic interval training on exercise capacity and metabolic risk factors in people with cardiometabolic disorders: a meta-analysis. J Cardiopulm Rehabil Prev. 2011;31 doi: 10.1097/HCR.0b013e31822f16cb. [DOI] [PubMed] [Google Scholar]
- 52.Buchheit M., Laursen P. High-intensity interval training, solutions to the programming puzzle. Sports Med. 2013;43:313–338. doi: 10.1007/s40279-013-0029-x. [DOI] [PubMed] [Google Scholar]
- 53.Lunt H., Draper N., Marshall H.C., Logan F.J., Hamlin M.J., Shearman J.P., Frampton C. High intensity interval training in a real world setting: a randomized controlled feasibility study in overweight inactive adults, measuring change in maximal oxygen uptake. PLoS One. 2014;9 doi: 10.1371/journal.pone.0083256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Borg G.A. Psychophysical bases of perceived exertion. Med. Sci. Sports Exerc. 1982;14:377–381. [PubMed] [Google Scholar]
- 55.Herbert P., Grace F.M., Sculthorpe N. Exercising caution: prolonged recovery to a single session of high intensity interval training in older men. J Am Gerontol Soc. 2015;1:13–19. doi: 10.1111/jgs.13365. [DOI] [PubMed] [Google Scholar]
- 56.Filipovic A., Kleinöder H., Plück D., Hollmann W., Bloch W., Grau M. Influence of whole-body electrostimulation on human red blood cell deformability. J. Strength Condit Res. 2015;29:2570–2578. doi: 10.1519/JSC.0000000000000916. [DOI] [PubMed] [Google Scholar]
- 57.Kemmler W., Von-Stengel S., Schwarz J., Mayhew J. Effect of whole-body electromyostimulation on energy expenditure during exercise. J. Strength Condit Res. 2012;26:240–245. doi: 10.1519/JSC.0b013e31821a3a11. [DOI] [PubMed] [Google Scholar]
- 58.Kemmler W., Schliffka R., Mayhew J., von Stengel S. Effects of whole-body electromyostimulation on resting metabolic rate, body composition, and maximum strength in postmenopausal women: the training and electrostimulation trial. J. Strength Condit Res. 2010;24:1880–1887. doi: 10.1519/JSC.0b013e3181ddaeee. [DOI] [PubMed] [Google Scholar]
- 59.Kemmler W., Teschler M., Weißenfels A., Bebenek M., Fröhlich M., Kohl M., von Stengel S. Effects of whole-body electromyostimulation versus high-intensity resistance exercise on body composition and strength: a randomized controlled study. Complement. Altern. Med. 2016;1:1–9. doi: 10.1155/2016/9236809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kemmler W., Teschler M., Bebenek M., Von Stengel S. (Very) high Creatinkinase concentration after exertional whole-body electromyostimulation application: health risks and longitudinal adaptations. Wien Med. Wochenschr. 2015;165:427–435. doi: 10.1007/s10354-015-0394-1. [DOI] [PubMed] [Google Scholar]
- 61.Finsterer J., Stöllberger C. Severe rhabdomyolysis after MIHA-bodytec® electrostimulation with previous mild hyper-CK-emia and non-compaction. Int. J. Cardiol. 2015;180:100–102. doi: 10.1016/j.ijcard.2014.11.148. [DOI] [PubMed] [Google Scholar]
- 62.Malnick S., Band Y., Alin P., Maffiuletti N. It's time to regulate the use of whole body electrical stimulation. BMJ. 2016;352:1693. doi: 10.1136/bmj.i1693. [DOI] [PubMed] [Google Scholar]
- 63.Kemmler W., Froehlich M., Stengel V., Kleinöder H. Whole-body electromyostimulation–the need for common sense! Rationale and guideline for a safe and effective training. Dtsch. Z. Sportmed. 2016;67 [Google Scholar]
- 64.Verdijk L.B., Koopman R., Schaart G., Meijer K., Savelberg H.H., Loon L. Satellite cell content is specifically reduced in type II skeletal muscle fibers in the elderly. Am. J. Physiol. Metab. 2007;292:151–157. doi: 10.1152/ajpendo.00278.2006. [DOI] [PubMed] [Google Scholar]
- 65.Deschenes M. Effects of aging on muscle fibre type and size. Sports Med. 2004;34:809–824. doi: 10.2165/00007256-200434120-00002. [DOI] [PubMed] [Google Scholar]
- 66.Filipovic A., Kleinöder H., Dörmann U., Mester J. Electromyostimulation—a systematic review of the influence of training regimens and stimulation parameters on effectiveness in electromyostimulation training of selected strength parameters. J. Strength Condit Res. 2011;25:3218–3230. doi: 10.1519/JSC.0b013e318212e3ce. [DOI] [PubMed] [Google Scholar]
- 67.Borg A., Kaijser E. A comparison between three rating scales for perceived exertion and two different work tests. Scand. J. Med. Sci. Sports. 2006;16:57–69. doi: 10.1111/j.1600-0838.2005.00448.x. [DOI] [PubMed] [Google Scholar]
- 68.Von-Stengel S., Bebenek M., Engelke K., Wolfgang K. Whole-body electromyostimulation to fight osteopenia in elderly females: the randomized controlled training and electrostimulation trial (TEST-III) J. Osteoporos. 2015;11:1–7. doi: 10.1155/2015/643520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Balke R., Ware B. An experimental study of physical fitness of Air Force personnel. United States Armed Forces Med. Journal. 1959;10:675–688. [PubMed] [Google Scholar]
- 70.Wei M., Kampert J.B., Barlow C.E., Nichaman M.Z., Gibbons L.W., Paffenbarger R.S., Blair S. Relationship between low cardiorespiratory fitness and mortality in normal-weight, overweight, and obese men. Jama. 1999;282:1547–1553. doi: 10.1001/jama.282.16.1547. [DOI] [PubMed] [Google Scholar]
- 71.Sui X., LaMonte M.J., Laditka N., Hardin J.W., Chase N., Hooker S.P., Blair S. Cardiorespiratory fitness and adiposity as mortality predictors in older adults. J. Am. Med. Assoc. 2007;298:2507–2516. doi: 10.1001/jama.298.21.2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ortega F.B., Lee D.C., Katzmarzyk T., Ruiz J.R., Sui X., Church T.S., Blair S. The intriguing metabolically healthy but obese phenotype: cardiovascular prognosis and role of fitness. Eur. Heart J. 2013;34:389–397. doi: 10.1093/eurheartj/ehs174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pallarés J., Morán-Navarro R. Methodological approach to the cardiorespiratory endurance training. J. Sport Heal. Res. 2012;4:119–136. [Google Scholar]
- 74.Poole D.C., Jones A.M. Measurement of the maximum oxygen uptake V̇O2max: V̇O2peak is no longer acceptable. J. Appl. Physiol. 2017;122:997–1002. doi: 10.1152/japplphysiol.01063.2016. [DOI] [PubMed] [Google Scholar]
- 75.Artero E.G., Espada-Fuentes J.C., Argüelles-Cienfuegos J., Román A., Gómez-López P.J., Gutiérrez A. Effects of whole-body vibration and resistance training on knee extensors muscular performance. Eur. J. Appl. Physiol. 2012;112:1371–1378. doi: 10.1007/s00421-011-2091-0. [DOI] [PubMed] [Google Scholar]
- 76.Ruiz-Ruiz J., Mesa J., Gutiérrez A., Castillo M. Hand size influences optimal grip span in women but not in men. J. Hand Surg. Am. 2002;27:897–901. doi: 10.1053/jhsu.2002.34315. [DOI] [PubMed] [Google Scholar]
- 77.McGill S., Childs A., Liebenson C. Endurance times for low back stabilization exercises: clinical targets for testing and training from a normal database. Arch. Phys. Med. Rehabil. 1999;80:941–944. doi: 10.1016/s0003-9993(99)90087-4. [DOI] [PubMed] [Google Scholar]
- 78.Shamsi M.B., Rezaei M., Zamanlou M., Sadeghi M., Pourahmadi M. Does core stability exercise improve lumbopelvic stability (through endurance tests) more than general exercise in chronic low back pain? A quasi-randomized controlled trial. Physiother. Theory Pract. 2016;32:171–178. doi: 10.3109/09593985.2015.1117550. [DOI] [PubMed] [Google Scholar]
- 79.Willson J., Dougherty C.P., Ireland M.L., Davis I. Core stability and its relationship to lower extremity function and injury. J. Am. Acad. Orthop. Surg. 2005;13:316–325. doi: 10.5435/00124635-200509000-00005. [DOI] [PubMed] [Google Scholar]
- 80.Fullmer S., Benson-Davies S., Earthman C.P., Frankenfield D.C., Gradwell E., Lee P., Trabulsi J. Evidence analysis library review of best practices for performing indirect calorimetry in healthy and Non-critically ill individuals. J. Acad. Nutr. Diet. 2015;115:1417–1446. doi: 10.1016/j.jand.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 81.Compher C., Frankenfield D., Keim N., Roth-Yousey L. Best practice methods to apply to measurement of resting metabolic rate in adults: a systematic review. J. Am. Diet Assoc. 2006;106:881–903. doi: 10.1016/j.jada.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 82.Achten M., Gleeson A.E., Jeukendrup A. Determination of the exercise intensity that elicits maximal fat oxidation. Med. Sci. Sports Exerc. 2002;34:92–97. doi: 10.1097/00005768-200201000-00015. [DOI] [PubMed] [Google Scholar]
- 83.Tarvainen M.P., Niskanen P., Lipponen J.A., Ranta-Aho P.O., Karjalainen P. Kubios HRV–heart rate variability analysis software. Comput. Meth. Progr. Biomed. 2014;113:210–220. doi: 10.1016/j.cmpb.2013.07.024. [DOI] [PubMed] [Google Scholar]
- 84.John N.A., Saranya K., Dhanalakshmi Y., John J. Aging-mediated neuromuscular instability and delayed choice reaction time. Int. J. Med. Sci. Publ. Health. 2016;5 [Google Scholar]
- 85.Bright P., Hale E., Gooch V.J., Myhill T., van der Linde I. The national adult reading test: restandardisation against the wechsler adult intelligence scale. Neuropsychol. Rehabil. 2016;1:1–9. doi: 10.1080/09602011.2016.1231121. [DOI] [PubMed] [Google Scholar]
- 86.Tian Y., Xu S. Association of sarcopenic obesity with the risk of all-cause mortality: a meta-analysis of prospective cohort studies. Geriatr. Gerontol. Int. 2016;16:155–166. doi: 10.1111/ggi.12579. [DOI] [PubMed] [Google Scholar]
- 87.Strawbridge W.J., Wallhagen M., Cohen R. Successful aging and well-being: self-rated compared with rowe and kahn. Gerontol. 2002;42:727–733. doi: 10.1093/geront/42.6.727. [DOI] [PubMed] [Google Scholar]
- 88.Rowe R., Kahn J. Human aging: usual and successful. Science. 1987;237:143–149. doi: 10.1126/science.3299702. [DOI] [PubMed] [Google Scholar]
- 89.Kuro-o M. Klotho. Pflügers Arch. J. Physiol. 2010;459:333–343. doi: 10.1007/s00424-009-0722-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.