Abstract
Alzheimer's and Parkinson's diseases are the most common neurodegenerative diseases. They are characterized by protein aggregates and so can be considered as prion-like disease. The major components of these deposits are amyloid peptide and tau for Alzheimer's disease, α-synuclein and synphilin-1 for Parkinson's disease. Drugs currently proposed to treat these pathologies do not prevent neurodegenerative processes and are mainly symptomatic therapies. Molecules inducing inhibition of aggregation or disaggregation of these proteins could have beneficial effects, especially if they have other beneficial effects for these diseases. Thus, several natural polyphenols, which have antioxidative, anti-inflammatory and neuroprotective properties, have been largely studied, for their effects on protein aggregates found in these diseases, notably in vitro. In this article, we propose to review the significant papers concerning the role of polyphenols on aggregation and disaggregation of amyloid peptide, tau, α-synuclein, synphilin-1, suggesting that these compounds could be useful in the treatments in Alzheimer's and Parkinson's diseases.
Keywords: natural polyphenols, protein aggregates, Alzheimer's disease, amyloid peptide, amyloid plaques, hyperphosphorylated tau, Parkinson's disease, a-synuclein, synphilin-1
Introduction
General presentation of polyphenols
In plants, polyphenols (or phenolic compounds) play an essential role, in protection from ultraviolet radiation and against aggression by pathogens or predators, contribute to their colour and flavour and facilitate growth and reproduction. To date, more than 8000 natural polyphenols have been identified in plants (Pandey and Rizvi, 2009). They may be grouped into classes according to the shared structural characteristics of their carbon skeletons. The main classes include phenolic acids and derivatives (hydroxybenzoic and hydroxycinnamic), flavonoids (flavanols, flavonols, flavones, flavanones, isoflavones, chalcones, anthocyanins), tannins (condensed or hydrolysable), stilbenes, lignans, coumarins, lignins (Naczk and Shahidi, 2006). Polyphenols share one common feature: an aromatic ring with at least one hydroxyl substituent. However, they vary greatly in their complexity from phenols to the highly polymerized tannins. They occur predominantly as conjugates with one or more sugars residues generally linked to hydroxyl groups or, less frequently, aromatic carbon atoms (Pandey and Rizvi, 2009). The principle sugar residue is glucose, while others (e.g., galactose, rhamnose, xylose or arabinose) are also encountered (Bravo, 1998). When ingested, polyphenols enter the digestive system primarily in form of glycosides, although some aglycones may be present. Before absorption, these compounds must be hydrolysed by endogenous enzymes (Pandey and Rizvi, 2009). This metabolism should be kept in mind when interpreting results, since the forms reaching the blood and tissues are different from those present in food: the most common polyphenols in our diet are not necessarily those showing highest concentration of active metabolites in target tissues (Pandey and Rizvi, 2009).
Biological activities and health implications of polyphenols
In the last decade of 20th century, the major researches focused on antioxidant activity of polyphenols due to their properties to scavenge free radical such as reactive oxygen species or hydroxyl radicals, which are produced in living organisms. Due to their unpaired electron, free radicals are very reactive species and their overproduction can cause damage to all biological macromolecules (DNA, proteins, lipids), resulting in cell alteration. Polyphenols can also act as antioxidants through their capacity to chelate metal ions such as iron. Some of them have antioxidant activity stronger than the reference water soluble vitamin E analogue Trolox, (Fauconneau et al., 1997). There are increasing evidences that polyphenols may protect cell constituents against oxidative damage and limit various disease associated with oxidative stress. Number of in vitro and in vivo studies have demonstrated that polyphenols intake limits the incidence of coronary heart diseases, in particular atherosclerosis in which low density lipoprotein (LDL) oxidation play a key mechanism in the development of this pathology. Likewise, studies provide evidence for a protective role of a diet rich in polyphenols against chronic diseases including cancers (Anantharaju et al., 2016) and diabetes (Jung et al., 2007). In more recent works, polyphenols may provide protection in neurodegenerative disorders, such as Alzheimer's disease (AD) and Parkinson's disease (PD) (Figure 1).
Figure 1.
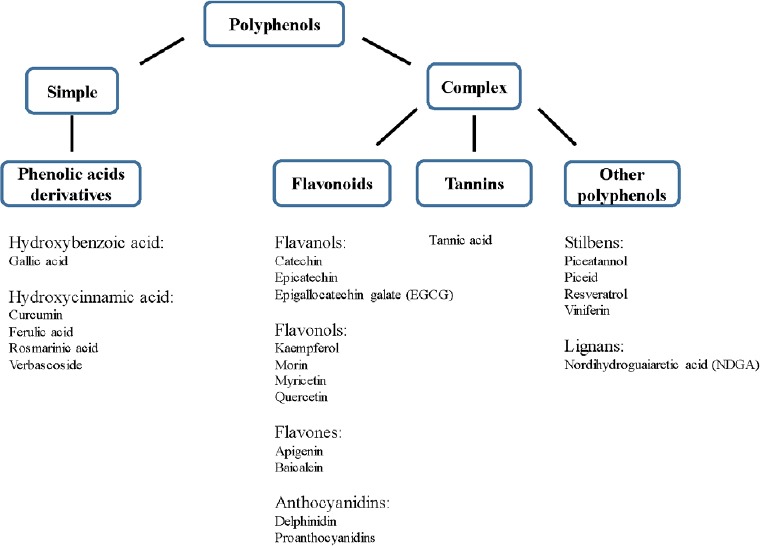
Classification of representative natural polyphenols reported in this article as modulating protein aggregates in Alzheimer's disease and Parkinson's disease.
AD and PD
AD and PD are the most common neurodegenerative disorders. For demographic reasons, these two diseases affect an increasing percentage of population. The common molecular mechanism observed in these neurodegenerative diseases is the formation of protein aggregates (Hashimoto et al., 2003). The ubiquitin-proteasome system cannot remove these aggregates not only because they are in excessive quantities but also because these proteins are misfolded, have excessive sizes and consequently present resistance to degradation. Indeed, aggregation and accumulation of these misfolded proteins in the central nervous system is due to a result of changes in the native proteins conformation and is consequent to aberrant production or overexpression of specific proteins, leading to progressive neurological impairment and neuronal dysfunction observed in AD (Hashimoto et al., 2003; Gadad et al., 2011) and in PD (Guerrero et al., 2013). Autophagy, as a lysosomal pathway, could play an important role in preventing the accumulation of abnormal proteins (Kroemer and Levine, 2008). However, defects of autophagy and accumulation of protein aggregates are observed in neurodegenerative diseases such as AD (Lee et al., 2010; Gusdon et al., 2012; Francois et al., 2014) and PD (Wu et al., 2011; Lynch-Day et al., 2012).
AD and PD share some neuropathological features with prion diseases and consequently these neurodegenerative diseases are considered as prion-like diseases. They involve proteins able to aggregate, β-amyloid (Aβ) peptide, tau, α-synuclein (α-syn) and synphilin-1, following conformational changes (Toni et al., 2017). Moreover, metal ions can directly bind to them, enhancing aggregates formation.
AD is the most prevalent neurodegenerative disease. It accounts for an estimated 60% to 80% of all dementias (Alzheimer's Association, 2017), which concerns more than 36 million people in the Word and the number of affected people is expected to be more than 115 million in 2050 (Alzheimer's Disease International, 2015). The estimated prevalence exceeds 10% for people over 65 years old (Alzheimer's Association, 2017). Extracellular senile plaques, constituted by deposition of aggregated Aβ peptides and intraneuronal neurofibrillary tangles (NFTs) composed by accumulation of hyperphosphorylated tau protein are two of the major histopathological lesions leading to the progression of the pathogenesis in this disease. Aβ is mainly produced by amyloidogenic metabolism of amyloid precursor protein, by the sequential action of β- and γ-secretases, leading to the liberation of peptide between 39 and 42 amino-acid residues. This last undergoes conformation modifications, acquires a β-sheet structure and so becomes prone to aggregation (Greenwald and Riek, 2010, 2012). Tau is a soluble microtubule protein present in neuronal cells that plays a dominant role in axonal growth and neuronal development by stabilizing the micro-tubular assembly (Mietelska-Porowska et al., 2014). Under pathological conditions, up regulation of kinases (Dolan and Johnson, 2010) and down regulation of phosphatases (Liu et al., 2005) result in hyperphosphorylation of tau protein, leading to double helical insoluble filaments and tangled clumps NFTs. These last form inside the neuronal cell body during the progression of AD and are relatively insoluble protein complexes (Iqbal et al., 2014).
PD is a progressive motor disease and is the second most common neurodegenerative disease after AD. It affects close to 5% of people over 65 years old (Wirdefeldt et al., 2011). It is characterized by the progressive loss of dopaminergic neurons from the substantia nigra region of the brain, with some surviving nigral dopaminergic neurons containing cytosolic filamentous inclusions known as Lewy bodies (LBs) and Lewy neurites (LNs). These toxic proteins are due to abnormal protein folding and endoplasmic reticulum stress and there are many, such as α-syn (Irizarry et al., 1998; Spillantini et al., 1998) and synphilin-1 (Wakabayashi et al., 2000). α-syn, a major fibrillar component of LBs and LNs, is a presynaptic neuronal protein. Its precise function is not well known but it may play a role in signals transmission and in the regulation of the dopamine. In physiological conditions, α-syn is soluble. However, under pathological conditions, it undergoes abnormal conformation, becomes insoluble and leads to toxic aggregates. Synphilin-1 is an α-syn-interacting protein also present in the LBs, which promotes inclusion formation (Xie et al., 2010).
Polyphenols Effects on Aggregates in Alzheimer's and Parkinson's Prion-Like Diseases
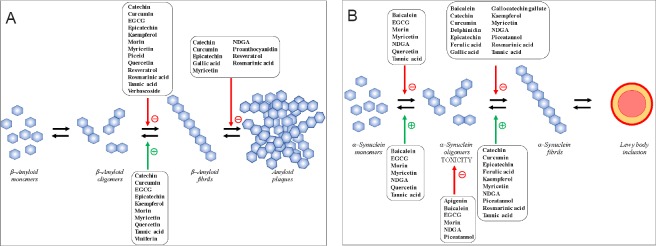
Polyphenols are described to have beneficial effects on prion-like diseases (Additional Table 1 (554.1KB, pdf) and Figure 2). Indeed, PAQUID study described that consumption of high levels of flavonoid polyphenols decrease the risk of dementia by 50% (Commenges et al., 2000). Many of them decrease amyloid, tau, α-syn and synphilin-1 deposits, by inhibition of their formation or by disaggregation of them (Stefani and Rigacci, 2014; Sivanesam and Andersen, 2016; Nabavi et al., 2017; Velander et al., 2017; Habtemariam, 2018). These properties are mediated by direct interaction with proteins or by interaction with metal ions promoting aggregation. Indeed, polyphenols are able to bind and chelate many different bivalent metals, such as Cu2+, Zn2+ and Fe2+, involved in amyloid aggregation (Singh et al., 2008; Mandel et al., 2011). Since soluble amyloid oligomers of Aβ, α-syn and tau appear to have structural features in common (Kayed et al., 2003), a number of compounds that inhibited the assembly of Aβ, α-syn and tau may recognise and interact with this common structure.
Figure 2.
Main effects of cited polyphenols modulating protein aggregates in Alzheimer's disease (A) and Parkinson's disease (B).
EGCG: Epigallocatechin 3-gallate; NDGA: nordihydroguaiaretic acid.
Natural polyphenols effects in Alzheimer's disease (AD) and Parkinson's disease (PD): in vitro and in vivo studies cited in the paper.
Polyphenols and AD
Polyphenols and β-amyloid peptide
Effects of numerous natural polyphenols on in vitro β-amyloid peptide aggregation/disaggregation and on amyloid deposits formation in vivo are studied and described (Figure 2A and Additional Table 1 (554.1KB, pdf) ). Many polyphenols, such as wine-or olive tree-related polyphenols, tannic acid, curcumin, and EGCG, were described to inhibit in vitro β-amyloid aggregation in fibrils and disaggregation of them (Velander et al., 2017). These properties are mediated by interaction with metal ions promoting aggregation or by direct interaction with amyloid peptide but also by direct interaction of them with polyphenols, notably by interfering with β-sheets (Toni et al., 2017).
Thus, Ono et al. (2003) showed by fluorescence spectroscopic analysis with thioflavin T and electron microscopy that, in vitro, wine-related polyphenols (myricetin, morin, quercetin, kaempferol (+)-catechin and (–)-epicatechin) dose-dependently inhibited formation and extension of fibrillary Aβ (fAβ) from Aβ1–40 and Aβ1–42. Moreover, these polyphenols dose-dependently destabilized preformed fAβs, with overall activity of the molecules examined in the order of: myricetin = morin = quercetin > kaempferol > (+)-catechin = (–)-epicatechin. The same authors demonstrated similar effects for tannic acid on the formation, extension and destabilization of fAβ (Ono et al., 2004). Rivière et al. (2007) examined the effects of stilbenes on Aβ25–35 fibril formation, using UV-visible measurements and electron microscopy. The inhibitory properties of resveratrol, piceid, resveratrol diglucoside, piceatannol, astringin and viniferin were characterized and compared. Resveratrol and piceid were shown to be the most efficient to inhibit Aβ polymerization (Rivière et al., 2007). The ability of multiple polyphenolic glycosides and their esterified derivative to interact with metal ions and metal-free/-associated Aβ, and further control both metal-free and metal-induced Aβ aggregation was investigated through western blot assay, transmission electron microscopy, ultraviolet (UV)-visible spectroscopy, fluorescence spectroscopy, and nuclear magnetic resonance (NMR) spectroscopy by Korshavn et al. (2015) The cytotoxicity of the compounds and their ability to mitigate the toxicity induced by both metal-free and metal-bound Aβ were also examined. Thus, verbascoside, natural product present in olive tree and its esterified derivative was shown to regulate the aggregation and cytotoxicity of metal-free and/or metal-associated Aβ to different extents (Korshavn et al., 2015). A recent study demonstrated that trans ε-viniferin disaggregated Aβ1–42 with better efficiency than resveratrol (Vion et al., 2017).
These effects can be partially explained by interaction of polyphenols with amyloid peptide. Thus, a non-covalent complex between ε-viniferin glucoside and Aβ was observed by electrospray ionization mass spectrometry (Richard et al., 2011). Moreover, in a recent review, rosmarinic acid was described to also directly interact with Aβ1–42 (Taguchi et al., 2017). These molecules interfere with β-sheet to exert their activity (Francioso et al., 2015). But polyphenols effects on metal ions contribute also to their properties. An assay using 2-(5-Bromo-2-pyridylazo)-5-[N-propyl-N-(3-sulfopropyl)amino]phenol disodium salt dihydrate (5-Br-PAPS) allowing the examination of Zn(II) and Cu(II) complexation showed that gallic acid, EGCG, and curcumin are multifunctional agents (Chan et al., 2016).
Many in vivo studies demonstrated in different AD models that polyphenols inhibited amyloid deposits in brain mice. Thus, it was shown that curcumin, injected peripherally into aged APPsweTg2576 mice crossed the blood brain barrier (BBB) and bound to amyloid plaques, reducing amyloid levels and plaque formation decisively (Yang et al., 2005). Another study, using in vivo multiphoton microscopy, demonstrated that curcumin crossed the BBB and labeled senile plaques and cerebrovascular amyloid angiopathy in another model of AD, APP(Swe)/PS1dE9 mice. Moreover, systemic treatment on these mice with curcumin for 7 days cleared and reduced existing plaques (Garcia-Alloza et al., 2007). When these same mice were fed during 6 months, between 3 and 9 months of age, with polyphenol-rich grape seed extract, containing gallic acid, catechin, epicatechin and proanthocyanidins or with curcumin, a decrease of Aβ deposition was observed (Wang et al., 2009). In the same way, when APPsweTg2576 were fed with myricetin, nordihydroguaiaretic acid (NDGA) or rosmarinic acid for 10 months from the age of 5 months, amyloid plaques in mice brains were significantly decreased (Hamaguchi et al., 2009). Resveratrol administrated by food at AD Tg19959 mice during 45 days also diminished plaque formation in a region specific manner, in medial cortex, striatum and hypothalamus (Karuppagounder et al., 2009).
Polyphenols and tau
Some polyphenols were also shown to inhibit phosphorylation and aggregation of tau. For example, EGCG was described to bind tau in this phosphorylation site with high affinity and to modify tridimensional structure of tau, leading to inhibition of its aggregation (Gueroux et al., 2017). A recent study showed that, by direct interaction with tau, rosmarinic acid prevented β-sheet assembly (Cornejo et al., 2017). Grape seed-derived polyphenol extract seems to potentially interfere with the assembly of tau peptides into neurotoxic aggregates (Wang et al., 2010). Same authors showed that oral administration of this extract significantly decreased the development of AD type tau neuropathology in the brain of Thy-1 mutated human tau (TMHT) mouse model of AD through mechanisms associated with attenuation of extracellular signal-receptor kinase 1/2 signaling in the brain (Wang et al., 2010). Moreover, polyphenols induce disaggregation of aggregated tau (Duff et al., 2010) and modify ultrastructure of paired helical filaments isolated from AD brains, decreasing enlargement of filaments (Ksiezak-Reding et al., 2012). Both daily intraperitoneal injections between 12 and 14 months and oral treatment between 8 and 14 months by green tea EGCG induced decrease of insoluble hyperphosphorylated tau in brain of APPswe Tg2576 mice (Rezai-Zadeh et al., 2005, 2008). Moreover, a systemic treatment with curcumin during 7 days, partially restored distorted neurites in APP(Swe)/PS1dE9 mice (Garcia-Alloza et al., 2007).
Polyphenols and PD
Polyphenols and α-syn
In vitro, α-syn has been shown to form amyloid fibrils and these fibrils have been detected in patient with PD in the form of plaques in the brain. However, autopsies have shown that there is no correlation between the amount of fibrils and the severity of PD (Goldberg and Lansbury, 2000). A prominent hypothesis is that the soluble β-oligomers which are formed prior to the formation of mature fibrils are the cause of cytotoxicity, probably by membrane pore formation (Sivanesam and Andersen, 2016). Therefore, the prevention of protein aggregation and oligomerization is an attractive strategy in combating the neurodegeneration because it acts on the very beginning of the proposed cellular pathway leading to cell death. In order to modulate amyloidogenesis pathways, common strategies are (i) to prevent α-syn from forming toxic oligomers, (ii) to increase the amyloidogenesis so that α-syn spends very little time in the presumably toxic oligomers state, and (iii) to direct α-syn to form off-pathway non¬toxic aggregates (Sivanesam and Andersen, 2016).
Effects of natural polyphenols on α-syn aggregation/disaggregation are studied and described (Figure 2B and Additional Table 1 (554.1KB, pdf) ). Using fluorescence spectroscopy with thioflavin S and electron microscopy, Ono and Yamada (2006) examined the effects of 13 antioxidants on the formation and destabilization of preformed α-syn fibrils. Their anti fibrillogenic and fibril-destabilizing activity was in the order: tannic acid = nordihydroguaiaretic acid = curcumin = rosmarinic acid = myricetin > kaempferol = ferulic acid > catechin = epi-catechin. Thus, they concluded that these antioxidants could prevent the development of α-synucleinopathies, not only through scavenging reactive oxygen species, but also through directly inhibiting the deposition of fibrils in the brain. Masuda et al. (2006) tested 39 polyphenols and 14 from them are strong inhibitors of α-syn filament assembly. Baicalein, delphinidin, gallocatechin gallate, and rosmarinic acid seem to be of particular interest for their properties to inhibit α-syn filament formation. They formed soluble, non cytotoxic (on human neurons SH-SY5Y cells), oligomeric α-syn, probably by binding to the C-terminal region, suggesting that this may be the mechanism by which filament formation is inhibited. 48 different flavonoids belonging to several classes were tested by Meng et al. (2010) for their ability to inhibit the aggregation of α-syn by stabilizing non-pathogenic protein conformation. Majority of the flavonoids inhibit α-syn polymerization either delaying or completely abolishing fibril formation and disaggregate the preformed fibrils into monomer and non¬pathogenic oligomers. Molecule such as baicalein tightly bind to the protein and greatly stabilize its native unfold conformation. The mechanism of the strong inhibition is mostly due to the formation of Schiff base (Meng et al., 2009). Hong et al. (2008) specified that baicalein-stabilized oligomers are β-sheet-enriched according to Circular Discroism and Fourier Transform Infra Red analysis. They did not form fibrils even after very prolonged incubation. Oligomers were extremely stable. These baicalein-stabilized oligomers, being added to the solution of the aggregating α-syn, were able to noticeably inhibit its fibrillation. Using confocal single-molecule fluorescence spectroscopy, Caruana et al. (2011) used the structural diversity of 14 polyphenols to define key molecular scaffolds most effective in inhibiting oligomer formation by α-syn and disaggregating pre-formed oligomers. They found that baicalein, EGCG, nordihydroguaiaretic acid, morin, myricetin, quercetin and tannic acid have strong effects. They conclude that the best candidate required: (1) aromatic recognition elements that would allow non-covalent binding to the α-syn monomer/oligomer and (2) hydroxyl groups (especially the presence of three > two > one –OH group on the same ring structure) that would hinder the progress of the self-assembly process and/or destabilize its structure. In another work, Caruana confirmed the ability of α-syn oligomers to permeabilise phospholipids membranes, potentially via pore-forming mechanism resembling the mechanism of neuronal toxicity in vivo (Caruana et al., 2012). They found that a group of small-molecule polyphenols (Nordihydroguaiaretic acid, Morin, Baicalein and Apigenin) strongly protect against membrane perturbation induced by aggregated wild-type and mutant (A30P, A53T) α-syn.
Some studies focused more specifically on one polyphenol and drew attention. Curcumin has been extensively studied on different models of neurodegenerative diseases and the results suggested that it could be of interest in the treatment of these pathologies. However, the potential efficacy of curcumin is limited owing to its poor potency and bioavailability. For this reason, Ahsan et al. (2015) screened different curcumin derivatives and they found that curcumin pyrazole derivatives inhibit α-syn aggregation and reduce α-syn associated neurotoxicity by employing several biophysical, imaging techniques, dot blot and cell based assays. EGCG bind to the natively unfolded polypeptides, forming complexes and preventing their conversion into toxic, on-pathway aggregation intermediates. The stimulation of this off-pathway led to the reduced cytotoxicity (Ehrnhoefer et al., 2008). It has also been suggested that EGCG is capable of binding to the oligomeric state of α-syn, destabilizing it and preventing it from interacting with membranes that would ultimately lead to cytotoxicity (Lorenzen et al., 2014). In a recent study, Yang et al. (2017) showed that EGCG exhibited its protective effect against α-syn mediated cytotoxicity, not only by producing the off-pathway compact oligomers, but also by facilitating the conversion of “active” oligomers into fibrils, thus accelerating the removal of active oligomers which could exert the membrane disruption and subsequent cellular degeneration. Gallic acid potently inhibited the formation of fibrils of α-syn and reduced the rate of formation of oligomers (Liu et al., 2014). It binds to soluble, non-toxic oligomers and stabilize their structure. Additionally, by using structure activity relationship, data obtained from 14 structurally similar benzoic acid derivatives showed that the inhibition of α-syn fibrillation was related to the number of hydroxyl moieties and their position on the phenyl ring (Ardah et al., 2014). Piceatannol inhibited the formation of α-syn fibrils and was able to destabilize preformed filaments. Furthermore, it protected PC12 (neuron like) cells against α-syn-induced toxicity (Temsamani et al., 2016). Albani et al. (2009) showed that resveratrol pre-treatment protect SK-N-BE cells from the toxicity arising from aggregation-prone protein (α-syn(A30P), mutated α-syn in familial Parkinsonism).
In animal models of PD, several studies clearly demonstrated that various polyphenols possess neuroprotective effects, but no study provided information on interaction between polyphenols and α-syn and on fibrils formation.
Polyphenols and synphilin-1
Few studies described the role of polyphenols in aggregation of synphilin-1. However, Pal et al demonstrated that a curcumin analogue, the 3,5-bis(2-flurobenzylidene)piperidin-4-one induced a marked decrease in synphilin-1 aggregation in the dopaminergic SHSY-5Y cells (Pal et al., 2011). This curcumin analog prevents this aggregation by preventing covalent modifications and by maintaining the expression of the protein disulfide isomerase.
Conclusion
This paper reviews the significant papers that demonstrated beneficial effects of natural polyphenols against protein aggregates found in AD an PD. These properties can be explained by the capacity of polyphenols to inhibit aggregation of major components of pathological aggregates, i.e., Aβ in amyloid plaques, tau in NTF, α-syn and synphilin-1 in LB and LNs and to partially disaggregate them. Many mechanisms are implicated, such as direct interaction of polyphenols with β-sheets of these proteins, leading to inhibition of abnormal conformational changes or hyperphosphorylation, but also by interaction with metal ions promoting aggregation. These effects are particularly interesting because polyphenols possess other beneficial properties, such as antioxidative, anti-inflammatory, pro-autophagic and neuroprotective activities. These properties, by themselves, may participate in the reduction in protein aggregation and deposition, without a direct effect of polyphenols on the process of protein aggregation and deposition. As multi-targeting drugs, polyphenols may lead to the development of therapeutic agents that could prove useful in combating AD and PD. However, it is difficult to predict nontoxic efficient doses for in vivo studies and clinical trials, because there is not necessarily a conversion relationship between in vitro and in vivo doses, notably because in vivo experiments are influenced by complex factors such as neuroendocrine system and immunity (Chen et al., 2018). Moreover, the doses between humans and animals are usually converted by body surface area calculation method, but this result may be not reliable because of the differences in drug metabolism between these two species.
To our knowledge, no clinical trial studied the role of polyphenols in the formation of protein aggregates in prion-like diseases. The only clinical studies investigating polyphenols effects in neurodegenerative diseases evaluated their role to prevent or treat cognitive impairment associated with neurodegeneration. Such, clinical trials using only resveratrol and curcumin showed their efficacy to preserve or restore cognitive function (Mazzanti and Di Giacomo, 2016), but these beneficial effects are not necessary be explained only by their action on aggregates. It would be necessary to evaluate beneficial effects of the other polyphenols on major symptoms in AD and PD, i.e., cognitive decline in AD and motor disorders in PD and to clarify relation between the role of polyphenols on protein aggregates and their symptomatic effects.
Additional file:
Additional Table 1 (554.1KB, pdf) : Natural polyphenols effects in Alzheimer's disease (AD) and Parkinson's disease (PD): in vitro and in vivo studies cited in the paper.
Acknowledgments
We thank the association “France Alzheimer Vienne” for its constant encouragement.
Footnotes
Conflicts of interest: The authors declare no competing financial and scientific interests.
Financial support: None.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer review report:
Reviewer: Xavier d’Anglemont de Tassigny, Hospital Universitario Virgen del Rocio, Spain.
Comments to authors: The paper provides an updated overview of the effect of polyphenol on protein aggregation in the two major neurodegeneration diseases. It fairly sounds like a catalog but will definitely help other researchers in the field to look for the cited references. The authors have made a considerable effort to gather a collection of recent studies favoring the use of polyphenols as a potential treatments to prevent the protein aggregates in AD and PD.
References
- Ahsan N, Mishra S, Jain MK, Surolia A, Gupta S. Curcumin Pyrazole and its derivative (N-(3-Nitrophenylpyrazole) Curcumin inhibit aggregation, disrupt fibrils and modulate toxicity of wild type and mutant alpha-synuclein. Sci Rep. 2015;5:9862. doi: 10.1038/srep09862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albani D, Polito L, Batelli S, De Mauro S, Fracasso C, Martelli G, Colombo L, Manzoni C, Salmona M, Caccia S, Negro A, Forloni G. The SIRT1 activator resveratrol protects SK-N-BE cells from oxidative stress and against toxicity caused by alpha-synuclein or amyloid-beta (1-42) peptide. J Neurochem. 2009;110:1445–1456. doi: 10.1111/j.1471-4159.2009.06228.x. [DOI] [PubMed] [Google Scholar]
- Alzheimer's Disease International. World Alzheimer Report 2015. The Global Impact of Dementia. An analysis of prevalence, incid ence, cost and trends. Alzheimer's Disease International (ADI) 2015 [Google Scholar]
- Alzheimer’s Association. 2017 Alzheimer's disease facts and figures. Alzheimers Dement. 2017;13:325–373. [Google Scholar]
- Anantharaju PG, Gowda PC, Vimalambike MG, Madhunapantula SV. An overview on the role of dietary phenolics for the treatment of cancers. Nutr J. 2016;15:99. doi: 10.1186/s12937-016-0217-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardah MT, Paleologou KE, Lv G, Abul Khair SB, Kazim AS, Minhas ST, Al-Tel TH, Al-Hayani AA, Haque ME, Eliezer D, El-Agnaf OM. Structure activity relationship of phenolic acid inhibitors of alpha-synuclein fibril formation and toxicity. Front Aging Neurosci. 2014;6:197. doi: 10.3389/fnagi.2014.00197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo L. Polyphenols: chemistry, dietary sources, metabolism, and nutritional significance. Nutr Rev. 1998;56:317–333. doi: 10.1111/j.1753-4887.1998.tb01670.x. [DOI] [PubMed] [Google Scholar]
- Caruana M, Hogen T, Levin J, Hillmer A, Giese A, Vassallo N. Inhibition and disaggregation of alpha-synuclein oligomers by natural polyphenolic compounds. FEBS Lett. 2011;585:1113–1120. doi: 10.1016/j.febslet.2011.03.046. [DOI] [PubMed] [Google Scholar]
- Caruana M, Neuner J, Hogen T, Schmidt F, Kamp F, Scerri C, Giese A, Vassallo N. Polyphenolic compounds are novel protective agents against lipid membrane damage by alpha-synuclein aggregates in vitro. Biochim Biophys Acta. 2012;1818:2502–2510. doi: 10.1016/j.bbamem.2012.05.019. [DOI] [PubMed] [Google Scholar]
- Chan S, Kantham S, Rao VM, Palanivelu MK, Pham HL, Shaw PN, McGeary RP, Ross BP. Metal chelation, radical scavenging and inhibition of Aβ42.fibrillation by food constituents in relation to Alzheimer's disease. Food Chem. 2016;199:185–194. doi: 10.1016/j.foodchem.2015.11.118. [DOI] [PubMed] [Google Scholar]
- Chen SQ, Wang ZS, Ma YX, Zhang W, Lu JL, Liang YR, Zheng XQ. Neuroprotective effects and mechanisms of tea bioactive components in neurodegenerative diseases. Molecules. 2018;23:E512. doi: 10.3390/molecules23030512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commenges D, Scotet V, Renaud S, Jacqmin-Gadda H, Barberger-Gateau P, Dartigues JF. Intake of flavonoids and risk of dementia. Eur J Epidemiol. 2000;16:357–363. doi: 10.1023/a:1007614613771. [DOI] [PubMed] [Google Scholar]
- Cornejo A, Aguilar Sandoval F, Caballero L, Machuca L, Munoz P, Caballero J, Perry G, Ardiles A, Areche C, Melo F. Rosmarinic acid prevents fibrillization and diminishes vibrational modes associated to beta sheet in tau protein linked to Alzheimer's disease. J Enzyme Inhib Med Chem. 2017;32:945–953. doi: 10.1080/14756366.2017.1347783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan PJ, Johnson GV. The role of tau kinases in Alzheimer's disease. Curr Opin Drug Discov Devel. 2010;13:595–603. [PMC free article] [PubMed] [Google Scholar]
- Dong W, Wang R. Effects of resveratrol-induced cellular autophagy in control of neurodegenerative diseases. Yao Xue Xue Bao. 2016;51:18–22. [PubMed] [Google Scholar]
- Duff K, Kuret J, Congdon EE. Disaggregation of tau as a therapeutic approach to tauopathies. Curr Alzheimer Res. 2010;7:235–240. doi: 10.2174/156720510791050885. [DOI] [PubMed] [Google Scholar]
- Ehrnhoefer DE, Bieschke J, Boeddrich A, Herbst M, Masino L, Lurz R, Engemann S, Pastore A, Wanker EE. EGCG redirects amyloidogenic polypeptides into unstructured, off-pathway oligomers. Nat Struct Mol Biol. 2008;15:558–566. doi: 10.1038/nsmb.1437. [DOI] [PubMed] [Google Scholar]
- Fauconneau B, Waffo-Teguo P, Huguet F, Barrier L, Decendit A, Merillon JM. Comparative study of radical scavenger and antioxidant properties of phenolic compounds from Vitis vinifera cell cultures using in vitro tests. Life Sci. 1997;61:2103–2110. doi: 10.1016/s0024-3205(97)00883-7. [DOI] [PubMed] [Google Scholar]
- Francioso A, Punzi P, Boffi A, Lori C, Martire S, Giordano C, D’Erme M, Mosca L. β-sheet interfering molecules acting against β-amyloid aggregation and fibrillogenesis. Bioorg Med Chem. 2015;23:1671–1683. doi: 10.1016/j.bmc.2015.02.041. [DOI] [PubMed] [Google Scholar]
- Francois A, Rioux Bilan A, Quellard N, Fernandez B, Janet T, Chassaing D, Paccalin M, Terro F, Page G. Longitudinal follow-up of autophagy and inflammation in brain of APPswePS1dE9 transgenic mice. J Neuroinflammation. 2014;11:139. doi: 10.1186/s12974-014-0139-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadad BS, Britton GB, Rao KS. Targeting oligomers in neurodegenerative disorders: lessons from alpha-synuclein, tau, and amyloid-beta peptide. J Alzheimers Dis 24 Suppl. 2011;2:223–232. doi: 10.3233/JAD-2011-110182. [DOI] [PubMed] [Google Scholar]
- Garcia-Alloza M, Borrelli LA, Rozkalne A, Hyman BT, Bacskai BJ. Curcumin labels amyloid pathology in vivo, disrupts existing plaques, and partially restores distorted neurites in an Alzheimer mouse model. J Neurochem. 2007;102:1095–1104. doi: 10.1111/j.1471-4159.2007.04613.x. [DOI] [PubMed] [Google Scholar]
- Goldberg MS, Lansbury PT Jr. Is there a cause-and-effect relationship between alpha¬synuclein fibrillization and Parkinson's disease? Nat Cell Biol. 2000;2:E115–119. doi: 10.1038/35017124. [DOI] [PubMed] [Google Scholar]
- Greenwald J, Riek R. Biology of amyloid: structure, function, and regulation. Structure. 2010;18:1244–1260. doi: 10.1016/j.str.2010.08.009. [DOI] [PubMed] [Google Scholar]
- Greenwald J, Riek R. On the possible amyloid origin of protein folds. J Mol Biol. 2012;421:417–426. doi: 10.1016/j.jmb.2012.04.015. [DOI] [PubMed] [Google Scholar]
- Gueroux M, Fleau C, Slozeck M, Laguerre M, Pianet I. Epigallocatechin 3-gallate as an inhibitor of tau phosphorylation and aggregation: a molecular and structural insight. J Prev Alzheimers Dis. 2017;4:218–225. doi: 10.14283/jpad.2017.35. [DOI] [PubMed] [Google Scholar]
- Guerrero E, Vasudevaraju P, Hegde ML, Britton GB, Rao KS. Recent advances in alpha¬synuclein functions, advanced glycation, and toxicity: implications for Parkinson's disease. Mol Neurobiol. 2013;47:525–536. doi: 10.1007/s12035-012-8328-z. [DOI] [PubMed] [Google Scholar]
- Gusdon AM, Zhu J, Van Houten B, Chu CT. ATP13A2 regulates mitochondrial bioenergetics through macroautophagy. Neurobiol Dis. 2012;45:962–972. doi: 10.1016/j.nbd.2011.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habtemariam S. Molecular pharmacology of rosmarinic and salvianolic acids: potential seeds for Alzheimer's and vascular dementia drugs. Int J Mol Sci. 2018;19:E458. doi: 10.3390/ijms19020458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamaguchi T, Ono K, Murase A, Yamada M. Phenolic compounds prevent Alzheimer's pathology through different effects on the amyloid-beta aggregation pathway. Am J Pathol. 2009;175:2557–2565. doi: 10.2353/ajpath.2009.090417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto M, Rockenstein E, Crews L, Masliah E. Role of protein aggregation in mitochondrial dysfunction and neurodegeneration in Alzheimer's and Parkinson's diseases. Neuromolecular Med. 2003;4:21–36. doi: 10.1385/NMM:4:1-2:21. [DOI] [PubMed] [Google Scholar]
- Hong DP, Fink AL, Uversky VN. Structural characteristics of alpha-synuclein oligomers stabilized by the flavonoid baicalein. J Mol Biol. 2008;383:214–223. doi: 10.1016/j.jmb.2008.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal K, Liu F, Gong CX. Alzheimer disease therapeutics: focus on the disease and not just plaques and tangles. Biochem Pharmacol. 2014;88:631–639. doi: 10.1016/j.bcp.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irizarry MC, Growdon W, Gomez-Isla T, Newell K, George JM, Clayton DF, Hyman BT. Nigral and cortical Lewy bodies and dystrophic nigral neurites in Parkinson's disease and cortical Lewy body disease contain alpha-synuclein immunoreactivity. J Neuropathol Exp Neurol. 1998;57:334–337. doi: 10.1097/00005072-199804000-00005. [DOI] [PubMed] [Google Scholar]
- Jung EH, Kim SR, Hwang IK, Ha TY. Hypoglycemic effects of a phenolic acid fraction of rice bran and ferulic acid in C57BL/KsJ-db/db mice. J Agric Food Chem. 2007;55:9800–9804. doi: 10.1021/jf0714463. [DOI] [PubMed] [Google Scholar]
- Karuppagounder SS, Pinto JT, Xu H, Chen HL, Beal MF, Gibson GE. Dietary supplementation with resveratrol reduces plaque pathology in a transgenic model of Alzheimer's disease. Neurochem Int. 2009;54:111–118. doi: 10.1016/j.neuint.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayed R, Head E, Thompson JL, McIntire TM, Milton SC, Cotman CW, Glabe CG. Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science. 2003;300:486–489. doi: 10.1126/science.1079469. [DOI] [PubMed] [Google Scholar]
- Korshavn KJ, Jang M, Kwak YJ, Kochi A, Vertuani S, Bhunia A, Manfredini S, Ramamoorthy A, Lim MH. Reactivity of metal-free and metal-associated amyloid-beta with glycosylated polyphenols and their esterified derivatives. Sci Rep. 2015;5:17842. doi: 10.1038/srep17842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroemer G, Levine B. Autophagic cell death: the story of a misnomer. Nat Rev Mol Cell Biol. 2008;9:1004–1010. doi: 10.1038/nrm2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ksiezak-Reding H, Ho L, Santa-Maria I, Diaz-Ruiz C, Wang J, Pasinetti GM. Ultrastructural alterations of Alzheimer's disease paired helical filaments by grape seed-derived polyphenols. Neurobiol Aging. 2012;33:1427–1439. doi: 10.1016/j.neurobiolaging.2010.11.006. [DOI] [PubMed] [Google Scholar]
- Lee JH, Moon JH, Kim SW, Jeong JK, Nazim UM, Lee YJ, Seol JW, Park SY. EGCG-mediated autophagy flux has a neuroprotection effect via a class III histone deacetylase in primary neuron cells. Oncotarget. 2015;6:9701–9717. doi: 10.18632/oncotarget.3832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Yu WH, Kumar A, Lee S, Mohan PS, Peterhoff CM, Wolfe DM, Martinez-Vicente M, Massey AC, Sovak G, Uchiyama Y, Westaway D, Cuervo AM, Nixon RA. Lysosomal proteolysis and autophagy require presenilin 1 and are disrupted by Alzheimer-related PS1 mutations. Cell. 2010;141:1146–1158. doi: 10.1016/j.cell.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin TK, Chen SD, Chuang YC, Lin HY, Huang CR, Chuang JH, Wang PW, Huang ST, Tiao MM, Chen JB, Liou CW. Resveratrol partially prevents rotenone-induced neurotoxicity in dopaminergic SH-SY5Y cells through induction of heme oxygenase-1 dependent autophagy. Int J Mol Sci. 2014;15:1625–1646. doi: 10.3390/ijms15011625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Iqbal K, Grundke-Iqbal I, Rossie S, Gong CX. Dephosphorylation of tau by protein phosphatase 5: impairment in Alzheimer's disease. J Biol Chem. 2005;280:1790–1796. doi: 10.1074/jbc.M410775200. [DOI] [PubMed] [Google Scholar]
- Liu Y, Carver JA, Calabrese AN, Pukala TL. Gallic acid interacts with alpha-synuclein to prevent the structural collapse necessary for its aggregation. Biochim Biophys Acta. 2014;1844:1481–1485. doi: 10.1016/j.bbapap.2014.04.013. [DOI] [PubMed] [Google Scholar]
- Lorenzen N, Nielsen SB, Yoshimura Y, Vad BS, Andersen CB, Betzer C, Kaspersen JD, Christiansen G, Pedersen JS, Jensen PH, Mulder FA, Otzen DE. How epigallocatechin gallate can inhibit alpha-synuclein oligomer toxicity in vitro. J Biol Chem. 2014;289:21299–21310. doi: 10.1074/jbc.M114.554667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch-Day MA, Mao K, Wang K, Zhao M, Klionsky DJ. The role of autophagy in Parkinson's disease. Cold Spring Harb Perspect Med. 2012;2:a009357. doi: 10.1101/cshperspect.a009357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel SA, Amit T, Weinreb O, Youdim MB. Understanding the broad-spectrum neuroprotective action profile of green tea polyphenols in aging and neurodegenerative diseases. J Alzheimers Dis. 2011;25:187–208. doi: 10.3233/JAD-2011-101803. [DOI] [PubMed] [Google Scholar]
- Masuda M, Suzuki N, Taniguchi S, Oikawa T, Nonaka T, Iwatsubo T, Hisanaga S, Goedert M, Hasegawa M. Small molecule inhibitors of alpha-synuclein filament assembly. Biochemistry. 2006;45:6085–6094. doi: 10.1021/bi0600749. [DOI] [PubMed] [Google Scholar]
- Mazzanti G, Di Giacomo S. Curcumin and resveratrol in the management of cognitive disorders: What is the clinical evidence? Molecules. 2016;21:E1243. doi: 10.3390/molecules21091243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X, Munishkina LA, Fink AL, Uversky VN. Molecular mechanisms underlying the flavonoid-induced inhibition of alpha-synuclein fibrillation. Biochemistry. 2009;48:8206–8224. doi: 10.1021/bi900506b. [DOI] [PubMed] [Google Scholar]
- Meng X, Munishkina LA, Fink AL, Uversky VN. Effects of various flavonoids on the alpha-synuclein fibrillation process. Parkinsons Dis. 2010;2010:650794. doi: 10.4061/2010/650794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mietelska-Porowska A, Wasik U, Goras M, Filipek A, Niewiadomska G. Tau protein modifications and interactions: their role in function and dysfunction. Int J Mol Sci. 2014;15:4671–4713. doi: 10.3390/ijms15034671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabavi SF, Sureda A, Dehpour AR, Shirooie S, Silva AS, Devi KP, Ahmed T, Ishaq N, Hashim R, Sobarzo-Sanchez E, Daglia M, Braidy N, Volpicella M, Vacca RA, Nabavi SM. Regulation of autophagy by polyphenols: Paving the road for treatment of neurodegeneration. Biotechnol Adv. 2017 doi: 10.1016/j.biotechadv.2017.12.001. doi: 10.1016/j.biotechadv.2017.12.001. [DOI] [PubMed] [Google Scholar]
- Naczk M, Shahidi F. Phenolics in cereals, fruits and vegetables: occurrence, extraction and analysis. J Pharm Biomed Anal. 2006;41:1523–1542. doi: 10.1016/j.jpba.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Ono K, Yamada M. Antioxidant compounds have potent anti-fibrillogenic and fibril-destabilizing effects for alpha-synuclein fibrils in vitro. J Neurochem. 2006;97:105–115. doi: 10.1111/j.1471-4159.2006.03707.x. [DOI] [PubMed] [Google Scholar]
- Ono K, Hasegawa K, Naiki H, Yamada M. Anti-amyloidogenic activity of tannic acid and its activity to destabilize Alzheimer's beta-amyloid fibrils in vitro. Biochim Biophys Acta. 2004;1690:193–202. doi: 10.1016/j.bbadis.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Ono K, Yoshiike Y, Takashima A, Hasegawa K, Naiki H, Yamada M. Potent anti¬amyloidogenic and fibril-destabilizing effects of polyphenols in vitro: implications for the prevention and therapeutics of Alzheimer's disease. J Neurochem. 2003;87:172–181. doi: 10.1046/j.1471-4159.2003.01976.x. [DOI] [PubMed] [Google Scholar]
- Pal R, Miranda M, Narayan M. Nitrosative stress-induced Parkinsonian Lewy-like aggregates prevented through polyphenolic phytochemical analog intervention. Biochem Biophys Res Commun. 2011;404:324–329. doi: 10.1016/j.bbrc.2010.11.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey KB, Rizvi SI. Plant polyphenols as dietary antioxidants in human health and disease. Oxid Med Cell Longev. 2009;2:270–278. doi: 10.4161/oxim.2.5.9498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezai-Zadeh K, Arendash GW, Hou H, Fernandez F, Jensen M, Runfeldt M, Shytle RD, Tan J. Green tea epigallocatechin-3-gallate (EGCG) reduces beta-amyloid mediated cognitive impairment and modulates tau pathology in Alzheimer transgenic mice. Brain Res. 2008;1214:177–187. doi: 10.1016/j.brainres.2008.02.107. [DOI] [PubMed] [Google Scholar]
- Rezai-Zadeh K, Shytle D, Sun N, Mori T, Hou H, Jeanniton D, Ehrhart J, Townsend K, Zeng J, Morgan D, Hardy J, Town T, Tan J. Green tea epigallocatechin-3-gallate (EGCG) modulates amyloid precursor protein cleavage and reduces cerebral amyloidosis in Alzheimer transgenic mice. J Neurosci. 2005;25:8807–8814. doi: 10.1523/JNEUROSCI.1521-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard T, Poupard P, Nassra M, Papastamoulis Y, Iglesias ML, Krisa S, Waffo-Teguo P, Merillon JM, Monti JP. Protective effect of epsilon-viniferin on beta-amyloid peptide aggregation investigated by electrospray ionization mass spectrometry. Bioorg Med Chem. 2011;19:3152–3155. doi: 10.1016/j.bmc.2011.04.001. [DOI] [PubMed] [Google Scholar]
- Rivière C, Richard T, Quentin L, Krisa S, Merillon JM, Monti JP. Inhibitory activity of stilbenes on Alzheimer's beta-amyloid fibrils in vitro. Bioorg Med Chem. 2007;15:1160–1167. doi: 10.1016/j.bmc.2006.09.069. [DOI] [PubMed] [Google Scholar]
- Singh M, Arseneault M, Sanderson T, Murthy V, Ramassamy C. Challenges for research on polyphenols from foods in Alzheimer's disease: bioavailability, metabolism, and cellular and molecular mechanisms. J Agric Food Chem. 2008;56:4855–4873. doi: 10.1021/jf0735073. [DOI] [PubMed] [Google Scholar]
- Sivanesam K, Andersen NH. Modulating the amyloidogenesis of α-synuclein. Curr Neuropharmacol. 2016;14:226–237. doi: 10.2174/1570159X13666151030103153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spillantini MG, Crowther RA, Jakes R, Hasegawa M, Goedert M. alpha-Synuclein in filamentous inclusions of Lewy bodies from Parkinson's disease and dementia with Lewy bodies. Proc Natl Acad Sci U S A. 1998;95:6469–6473. doi: 10.1073/pnas.95.11.6469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefani M, Rigacci S. Beneficial properties of natural phenols: highlight on protection against pathological conditions associated with amyloid aggregation. Biofactors. 2014;40:482–493. doi: 10.1002/biof.1171. [DOI] [PubMed] [Google Scholar]
- Taguchi R, Hatayama K, Takahashi T, Hayashi T, Sato Y, Sato D, Ohta K, Nakano H, Seki C, Endo Y, Tokuraku K, Uwai K. Structure-activity relations of rosmarinic acid derivatives for the amyloid beta aggregation inhibition and antioxidant properties. Eur J Med Chem. 2017;138:1066–1075. doi: 10.1016/j.ejmech.2017.07.026. [DOI] [PubMed] [Google Scholar]
- Temsamani H, Krisa S, Decossas-Mendoza M, Lambert O, Merillon JM, Richard T. Piceatannol and other wine stilbenes: a pool of inhibitors against α-synuclein aggregation and cytotoxicity. Nutrients. 2016;8:E367. doi: 10.3390/nu8060367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toni M, Massimino ML, De Mario A, Angiulli E, Spisni E. Metal dyshomeostasis and their pathological role in prion and prion-like diseases: the basis for a nutritional approach. Front Neurosci. 2017;11:3. doi: 10.3389/fnins.2017.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velander P, Wu L, Henderson F, Zhang S, Bevan DR, Xu B. Natural product-based amyloid inhibitors. Biochem Pharmacol. 2017;139:40–55. doi: 10.1016/j.bcp.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vion E, Page G, Bourdeaud E, Paccalin M, Guillard J, Rioux Bilan A. Trans ε-viniferin is an amyloid-β disaggregating and anti-inflammatory drug in a mouse primary cellular model of Alzheimer's disease. Mol Cell Neurosci. 2017;88:1–6. doi: 10.1016/j.mcn.2017.12.003. [DOI] [PubMed] [Google Scholar]
- Wakabayashi K, Engelender S, Yoshimoto M, Tsuji S, Ross CA, Takahashi H. Synphilin-1 is present in Lewy bodies in Parkinson's disease. Ann Neurol. 2000;47:521–523. [PubMed] [Google Scholar]
- Wang J, Santa-Maria I, Ho L, Ksiezak-Reding H, Ono K, Teplow DB, Pasinetti GM. Grape derived polyphenols attenuate tau neuropathology in a mouse model of Alzheimer's disease. J Alzheimers Dis. 2010;22:653–661. doi: 10.3233/JAD-2010-101074. [DOI] [PubMed] [Google Scholar]
- Wang J, Liu Y, Li XH, Zeng XC, Li J, Zhou J, Xiao B, Hu K. Curcumin protects neuronal cells against status-epilepticus-induced hippocampal damage through induction of autophagy and inhibition of necroptosis. Can J Physiol Pharmacol. 2017;95:501–509. doi: 10.1139/cjpp-2016-0154. [DOI] [PubMed] [Google Scholar]
- Wang SF, Wu MY, Cai CZ, Li M, Lu JH. Autophagy modulators from traditional Chinese medicine: Mechanisms and therapeutic potentials for cancer and neurodegenerative diseases. J Ethnopharmacol. 2016;194:861–876. doi: 10.1016/j.jep.2016.10.069. [DOI] [PubMed] [Google Scholar]
- Wang YJ, Thomas P, Zhong JH, Bi FF, Kosaraju S, Pollard A, Fenech M, Zhou XF. Consumption of grape seed extract prevents amyloid-beta deposition and attenuates inflammation in brain of an Alzheimer's disease mouse. Neurotox Res. 2009;15:3–14. doi: 10.1007/s12640-009-9000-x. [DOI] [PubMed] [Google Scholar]
- Wirdefeldt K, Adami HO, Cole P, Trichopoulos D, Mandel J. Epidemiology and etiology of Parkinson's disease: a review of the evidence. Eur J Epidemiol 26 Suppl. 2011;1:S1–58. doi: 10.1007/s10654-011-9581-6. [DOI] [PubMed] [Google Scholar]
- Wu Y, Li X, Zhu JX, Xie W, Le W, Fan Z, Jankovic J, Pan T. Resveratrol-activated AMPK/SIRT1/autophagy in cellular models of Parkinson's disease. Neurosignals. 2011;19:163–174. doi: 10.1159/000328516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie YY, Zhou CJ, Zhou ZR, Hong J, Che MX, Fu QS, Song AX, Lin DH, Hu HY. Interaction with synphilin-1 promotes inclusion formation of alpha-synuclein: mechanistic insights and pathological implication. FASEB J. 2010;24:196–205. doi: 10.1096/fj.09-133082. [DOI] [PubMed] [Google Scholar]
- Yang F, Lim GP, Begum AN, Ubeda OJ, Simmons MR, Ambegaokar SS, Chen PP, Kayed R, Glabe CG, Frautschy SA, Cole GM. Curcumin inhibits formation of amyloid beta oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J Biol Chem. 2005;280:5892–5901. doi: 10.1074/jbc.M404751200. [DOI] [PubMed] [Google Scholar]
- Yang JE, Rhoo KY, Lee S, Lee JT, Park JH, Bhak G, Paik SR. EGCG-mediated protection of the membrane disruption and cytotoxicity caused by the ‘active oligomer’ of alpha-synuclein. Sci Rep. 2017;7:17945. doi: 10.1038/s41598-017-18349-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Natural polyphenols effects in Alzheimer's disease (AD) and Parkinson's disease (PD): in vitro and in vivo studies cited in the paper.