Levels of ultraviolet B radiation (UVBR), a potent immunosuppressant, have increased in parts of the world due to anthropogenic thinning of the Earth’s protective ozone layer. However, little is known about how UVBR influences amphibian immune function, or if increased UVBR exposure can contribute to disease emergence in susceptible species.
Keywords: adaptive, Batrachochytrium dendrobatidis, chytridiomycosis, embryo, innate, larvae
Abstract
Amphibian populations the world over are under threat of extinction, with as many as 40% of assessed species listed as threatened under IUCN Red List criteria (a significantly higher proportion than other vertebrate group). Amongst the key threats to amphibian species is the emergence of novel infectious diseases, which have been implicated in the catastrophic amphibian population declines and extinctions seen in many parts of the world. The recent emergence of these diseases coincides with increased ambient levels of ultraviolet B radiation (UVBR) due to anthropogenic thinning of the Earth’s protective ozone layer, raising questions about potential interactions between UVBR exposure and disease in amphibians. While reasonably well documented in other vertebrate groups (particularly mammals), the immunosuppressive capacity of UVBR and the potential for it to influence disease outcomes has been largely overlooked in amphibians. Herein, we review the evidence for UVBR-associated immune system disruption in amphibians and identify a number of direct and indirect pathways through which UVBR may influence immune function and disease susceptibility in amphibians. By exploring the physiological mechanisms through which UVBR may affect host immune function, we demonstrate how ambient UVBR could increase amphibian susceptibility to disease. We conclude by discussing the potential implications of elevated UVBR for inter and intraspecific differences in disease dynamics and discuss how future research in this field may be directed to improve our understanding of the role that UVBR plays in amphibian immune function.
Introduction
Amphibians are currently the vertebrate taxon most threatened with extinction (Houlahan et al., 2000; Hof et al., 2011; IUCN, 2016). Global amphibian numbers have undergone substantial declines over the last four decades, with between 40 and 50% of examined species listed as at least ‘Near Threatened’ by the International Union for the Conservation of Nature (IUCN) Red List of Threatened Species (IUCN, 2016). A major contributor to recent amphibian declines was determined following the discovery of the novel fungal pathogens Batrachochytrium dendrobatidis (Bd) in 1998 (Berger et al., 1998) and Batrachochytrium salamandrivorans (BSal) (Martel et al., 2013). Both pathogens cause the deadly amphibian disease chytridiomycosis (Berger et al., 1998; Longcore et al., 1999). Since its identification almost 20 years ago, Bd has been recorded in over 500 amphibian species worldwide and has been implicated in the decline or extinction of several hundred of these (Berger et al., 1998; Lips et al., 2006; Cheng et al., 2011; Van Rooij et al., 2015). In addition to Bd and Bsal, several other diseases caused by amphibian pathogens (Ranaviruses and trematode parasites) have recently increased in prevalence. Ranaviruses have now been detected in more than 70 amphibian species in 20 countries (Miller et al., 2011; Gray and Chinchar, 2015), making them the second most common infectious agent of amphibians after Bd (Chen and Robert, 2011). Similarly, trematode infections caused by the parasite Riberioa ondatrae have increased in North American amphibians to such an extent that they have contributed significantly to recent local population declines (reviewed by Rohr et al., 2009).
An apparent rise in the number of disease-related amphibian declines has prompted questions as to why such a trend should be occurring now (Hof et al., 2011; Blaustein et al., 2012; James et al., 2015; Kolby and Daszak, 2016). Increased contact between wildlife, humans and domestic animals has undoubtedly amplified the exposure of wildlife to novel, potentially pathogenic organisms (Daszak et al., 2000). However, the consequences of a novel host–pathogen interaction depend not only on the specific physical/physiological characteristics of the host and pathogen, but also on how these are shaped by their interactions with the surrounding environment (Daszak et al., 2001; Blaustein et al., 2012). Environmental conditions not only affect pathogen growth, transmissibility and pathogenicity but also influence host behaviour, immune function and pathogen exposure regimes (Fisman, 2007). Host immune function is sensitive to a range of biotic and abiotic environmental variables like temperature (Engelsma et al., 2003; Raffel et al., 2006; Ndong et al., 2007; Barber et al., 2016), habitat quality (Cary et al., 2014; Katzenback et al., 2014; Krynak et al., 2015; Makrinos and Bowden, 2016), nutritional status (Venesky et al., 2012), competition (Groner et al., 2014) and importantly, solar UVB radiation (Kripke et al., 1992; Lahnsteiner et al., 2011; Debecker et al., 2015; Abu Bakar et al., 2016).
Ultraviolet B radiation radiation (UVBR) forms a part of the solar electromagnetic spectrum (wavelength range 280–320 nm). Although the majority of solar UVBR reaching the outer atmosphere is absorbed by stratospheric ozone, a small amount does reach the Earth’s surface (van der Leun, 2004). Spatial and temporal variations in ozone thickness influence UVBR levels at the Earth’s surface. Other factors, such as solar angle and proximity, cloud cover, surface reflectance (albedo) and altitude, also influence terrestrial UVBR levels (Xenopoulos and Schindler, 2001). While UVBR can penetrate into aquatic environments, surface waves, reflectivity and levels of dissolved organic carbon (DOC) significantly affect the transmission of UVBR and the depth to which it can influence aquatic systems (Xenopoulos and Schindler, 2001).
UVBR is a powerful natural stressor because it can interact with a range of biological molecules and is capable of causing extensive cellular and molecular (DNA and protein) damage (Diffey, 1991). At the organismal level, UVBR exposure can adversely impact survival, growth rates, developmental trajectories, locomotion and predator avoidance. In amphibians, embryonic and larval stages are particularly sensitive to UVBR. These life stages are at increased risk from UVBR as they are often exposed to direct sunlight, are most commonly found during spring and summer (when UV levels are highest) and/or have a limited capacity to avoid UVBR exposure (Blaustein and Belden, 2003). UVBR can have a variety of effects on embryonic and larval amphibians including increased mortality (e.g. Crump et al., 1999; Belden et al., 2003; Alton et al., 2010), decreased growth and impaired development (e.g. Belden and Blaustein, 2002; Calfee et al., 2006), developmental abnormalities (e.g. van Uitregt et al., 2007; Romansic et al., 2009) and reduced locomotor performance and altered behaviour (e.g. Kats et al., 2000; van Uitregt et al., 2007). UVBR can also modulate the effects of other stressors (both biotic and abiotic) on amphibians (reviewed by Alton and Franklin, 2017). However, not all species respond to UVBR in the same way (Licht and Grant, 1997). Responses to UVBR can vary with geographic and seasonal exposure patterns (Peterson et al., 2002), as well as with the capacity to avoid and/or repair DNA damage (e.g. Hansen et al., 2002; Blaustein et al., 2004; Palen et al., 2005; Thurman et al., 2014).
Studies on non-amphibian taxa show UVBR is both genotoxic and a powerful modulator of immune function, with studies of fish and mammal species showing increased incidence of, and susceptibility to, disease when exposed to sublethal doses (Salo et al., 2000; de Gruijl, 2008; Blount and Pike, 2012; Siroski et al., 2012; Ullrich and Byrne, 2012). In comparison with other vertebrate taxa, the impacts of UVBR on amphibian immune function and disease are poorly documented and the role of UVBR in disease-related mortality and decline of amphibian populations remains conjectural. There are, however, a number of lines of evidence to suggest that disease-related amphibian declines in some areas may be linked to increased UVBR exposure. For example, many Bd-related amphibian declines have occurred in montane environments (Lips, 1998; Middleton et al., 2001; Bosch et al., 2007; Kriger and Hero, 2008; Rohr and Raffel, 2010; Walker et al., 2010) where ambient UVBR levels are significantly higher than at lower altitudes because there is less atmospheric filtration of UVBR (UV levels increase by 10–12% for every 1000 m of elevation) (Madronich et al., 1998). In addition, many declines attributable to disease (e.g. declines in eastern Australia and South America during the late 1970s and 1980s) coincide spatially and/or temporally with increases in UVBR associated with stratospheric ozone depletion (Berger et al., 1998; Lips, 1998; Lips et al., 2006).
UVBR-induced immunosuppression was suggested as a potential factor contributing to amphibian declines as far back as 1993 (Carey, 1993). However, the impacts of UVBR exposure on amphibian immune function and disease susceptibility have been little studied since, and much remains unknown about whether changes in global UVBR levels have contributed to recent disease-associated amphibian declines. In the relatively small number of studies that have attempted to address this issue, results have been conflicting and/or inconclusive. For example, Garcia et al. (2006) found that exposure of juvenile (post-metamorphic) frogs of three species (Rana cascadae, Bufo boreas and Hyla regilla) to low levels of UVBR for 3 days did not increase susceptibility to Bd or subsequent mortality. Likewise, Searle et al. (2010) found no increase in mortality rates or in the infectiousness of Bd when Rana cascade larvae were exposed to UVBR and Bd simultaneously. UVBR exposure has also been linked to reduced Bd susceptibility in larvae of Bufo bufo (Ortiz-Santaliestra et al., 2011) but increased Bd susceptibility in green tree frog (Litoria caerulea) larvae (Cramp and Franklin, preliminary unpublished observations). Differences in the source of the UVBR (natural or artificial), UVBR dose, the timeframe of exposure, life history stage at the time of exposure and species influence amphibian UVBR exposure outcomes (Licht and Grant, 1997; Alton and Franklin, 2017). Experimental differences across existing studies may also contribute to the lack of consensus on the role that UVBR might play in Bd susceptibility. Although Bd epidemiology is undeniably complex, the links between chytridiomycosis and elevation/altitude, with increased prevalence/severity of chytridiomycosis at higher altitude sites, are clear. With UVBR levels increasing with altitude (Madronich et al., 1998), it is conceivable that the greater prevalence of this and other diseases at high altitude may be related to increased UVBR exposure. Currently, our capacity to understand the relationship between UVBR and disease outcomes is significantly hampered by the lack of understanding of the fundamental way(s) in which UVBR can interact with, or modulate, amphibian immune function.
Pathways to impact: mechanisms through which UVBR can influence amphibian immune function
The vertebrate immune system is a suite of complex, interrelated morphological, physiological, cellular and chemical facets that animals use to determine self from non-self (Schulenburg et al., 2009). Immune defences can be classified as innate or adaptive; innate immune defences are considered the ‘first line of defence’ and provide rapid, non-specific protection against a variety of potential pathogens. The innate immune system includes physical barriers like the skin which impedes the movement of microbes into the body, mucus which often contains a variety of antimicrobial substances and the hosts’ own commensal microbiome with which a potential pathogen must successfully outcompete in order to become an established infection. The innate immune system also includes cell-mediated defences in the form of white blood cells and chemical cascades like complement and lysozyme. The acquired or adaptive immune system is a set of immune responses highly specific to the pathogen that induced them and includes the production of antibodies against foreign antigens and the retention of immunological memory. The functions of the vertebrate immune system can be affected by exposure to a range of environmental factors and through the effects of other (competing) physiological processes.
The amphibian immune system is similar to that of most other vertebrates in that it incorporates both innate and adaptive immune pathways (Carey et al., 1999). In amphibians, as in other vertebrate taxa, the immune defences of early life (embryonic and larval) stages are less well developed than those of adult frogs. Some embryonic immune defences are, nevertheless, established very early (Rollins-Smith, 1998) with egg membranes forming a direct barrier against pathogen entry (Robert and Ohta, 2009). There is also some evidence that maternal antibodies are passed into the eggs during vitellogeneis providing additional protection during embryonic development (Poorten and Kuhn, 2009). Initial adaptive immunological competence is achieved approximately 2 weeks post-fertilization in Xenopus laevis larvae with the development of the principle lymphoid tissues, the spleen and thymus (Robert and Ohta, 2009). Larval adaptive immune function is comparatively less robust than that of adult amphibians, with larval amphibians having fewer lymphocytes and reduced antibody diversity than adults (Rollins-Smith, 2017). During metamorphosis, hormonal changes result in a sharp reduction in larval lymphocyte abundance (immunosuppression) as the immune system is reorganized from the larval type to the adult type to prevent the larval immune system from attacking the newly formed adult tissues (Rollins-Smith, 1998; Robert and Ohta, 2009). Following metamorphosis, immune function is gradually restored, however, mature immune function may not be achieved for up to a year post-metamorphosis (Rollins-Smith, 1998, 2017). Taken together, the larval and juvenile life history stages are comparatively most at risk from disease or infection because of the immaturity of the immune system. It is not surprising then that many amphibian diseases are most problematic for embryonic, larval and juvenile life stages (Gray et al., 2009; Miller et al., 2011; Langhammer et al., 2014).
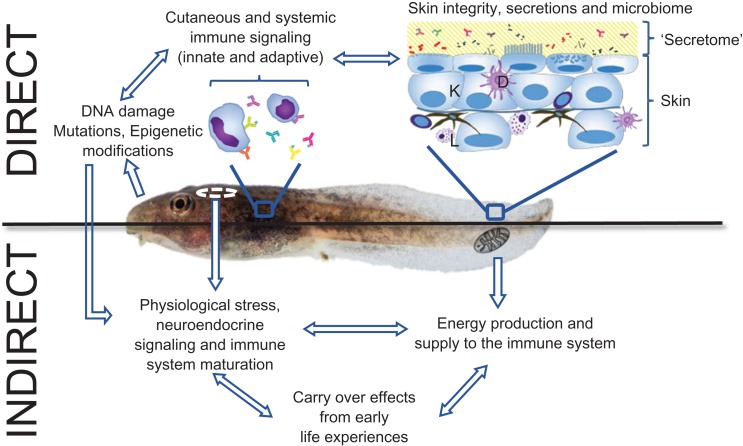
Embryonic and larval amphibians are more likely to be exposed to UVBR than adult stages, although several declining anuran and urodele species (i.e. Taudactylus eungellensis and Taudactylus acutirostris, several Atelopus spp. and a number of aquatic salamander species) also have diurnal juvenile and adult stages for which UVBR exposure may be harmful. In the subsequent sections of this review, we will explore how UVBR exposure influences immune defences early in development (embryonic, larval and juvenile). We will show that UVBR can act directly on components of the innate and adaptive immune systems, as well as indirectly influencing immune function through impacts on other physiological systems or by influencing the capacity of other environmental stressors to affect immune function (Figure 1). In doing so, we review not only studies examining the impacts of UVBR on amphibian species directly but also relevant studies on other taxa where data for amphibians is lacking. In reviewing the evidence for potential effects of UVBR on amphibian immune function, we also identify gaps in our understanding of how UVBR, through its impact on immune function, may contribute to disease processes in amphibians.
Figure 1:
Schematic representation of the potential direct and indirect pathways through which UVBR can influence immune function in amphibian early life stages. UVBR can directly kill and damage cells in the outer skin layers, disrupting the physical integrity of the skin, compromising the function of cutaneous dendritic cells (D) and leucocytes (L), and triggering exposed keratinocytes (K) to release a cascade of immunosuppressive molecules that inhibit innate and adaptive immune functions of the systemic immune system. UVBR can disrupt the innate immune function of the cutaneous ‘secretome’ (mucus, antimicrobial peptides, complement, lysozyme, etc.) and influence the composition of the host microbiome. Indirectly, UVBR may influence immune function via its impact on interrelated physiological systems. For example, UVBR can affect immune function by disrupting energy production and/or distribution pathways, by influencing neuroendocrine signalling pathways controlling immune system maturation or inducing a physiological stress response (involving glucocorticoids). UVRB-associated damage to DNA and other biomolecules may also have a lasting impact on immune function by influencing gene expression patterns in subsequent life stages. Image: Cameron Baker©.
Direct modulation of immune system function by UVBR
Skin integrity
The barrier function of epithelia is an integral component of the innate immune system of all animals and serves as the ‘first line of defence’ against potential microbial invasion. Damage to the integrity of the skin compromises the barrier role of the skin and increases the capacity for pathogens to enter the tissues and cause infection. In most vertebrates, UVBR is absorbed by the epidermis and often does not penetrate into the deeper skin layers (Biniek et al., 2012). However, larval amphibian skin is potentially highly sensitive to UVBR, since their protective pigment (melanin) layers lie largely beneath the epidermis within the dermis (Fox, 1986). In most taxa, including amphibians, acute responses to elevated UVBR can include sunburn (Blazer et al., 1997; Little and Fabacher, 2003), melanogenesis (skin darkening), lesions on the dorsal skin and eyes (McFadzen et al., 2000; Zamzow, 2004; Kazerouni and Khodabandeh, 2010), necrosis and sloughing of the skin (Noceda et al., 1997; Abedi et al., 2015), a reduction in epidermal strength (Biniek et al., 2012), and epidermal hyperplasia and oedema (Matsumura and Ananthaswamy, 2004). Larvae of two frog species, H. regilla and Rana aurora, experienced both skin damage and lens opacity following exposure to elevated levels of artificial UVBR (Flamarique et al., 2000). Subsequent fungal infections in areas of UV-related skin damage have been reported (Flamarique et al., 2000; Little and Fabacher, 2003), indicating that UVBR exposure can compromise the immunological barrier function of amphibian skin. Whether these results apply to natural exposure regimes is presently unclear as the aforementioned studies were undertaken under laboratory conditions using artificial light sources.
While exposure to elevated UVBR can sometimes lead to obvious outward signs of skin damage, sub-erythemal exposures can also lead to levels of barrier dysfunction that can precede infection by secondary fungal pathogens (Nowak, 1999). Since sub-erythemal skin damage is difficult to determine macroscopically, it may go unnoticed in wild amphibian studies where levels of embryonic and larval mortality are naturally very high anyway. If, like in other taxa, sub-erythemal skin damage increases the risk of pathogen infections in early amphibian life stages, further work is needed to better understand how UVBR exposure regimes influence infection outcomes. Moreover, exposure ‘thresholds’ are likely to vary across species and populations with the capacity of animals to behaviourally and/or morphologically limit their exposure to, or tolerate, UVBR in their environment. Similarly, since UVBR levels vary temporally and spatially, exposure regimes (fluctuating vs acute exposures) may also affect skin responses within and across life history stages. We, therefore, need to examine the possibility that early UVBR-associated skin damage may have long-term consequences for cutaneous immune function in later life history stages and appreciate that the acute and long-term effects of UVBR on skin function are likely to vary both between and within species.
Skin secretions
Skin secretions form a vital component of the innate immune system of all animals. Secretions function as both a physical barrier to pathogens, by trapping and preventing their establishment on the skin surface, and as a chemical barrier, containing a variety of antimicrobial proteins and peptides like proteases, lectins and lysozyme, which actively kill microbes on contact (Ellis, 2001; Zasloff, 2002; Magnadottir, 2006). Embryonic and larval amphibians have several types mucus- and peptide-secreting cells in the integument (Altig and McDiarmid, 1999) that produce substances with well-defined immunoprotective functions (Woodhams et al., 2016). Damage to this layer can directly increase the risk of pathogen infection (Kanno et al., 1989; Magarinos et al., 1995; Zamzow and Siebeck, 2006). In some reef fish, cutaneous mucus also (indirectly) influences the innate immune defences (i.e. the barrier function of skin) by reducing the penetrance of UVBR through to the more UVBR-sensitive skin layers (Zamzow and Losey, 2002; Zamzow, 2004; Zamzow and Siebeck, 2006; Eckes et al., 2008); whether larval amphibian mucus has a similar photoprotective function is largely unknown. Notably, UVBR exposure can reduce the abundance and distribution of mucus-secreting cells in fish larvae (Kaweewat and Hofer, 1997; McFadzen et al., 2000; Kazerouni and Khodabandeh, 2010; Abedi et al., 2015). It is currently uncertain if UVBR exposure can influence mucus production in amphibians. The loss of mucus-producing cells may be particularly problematic for embryonic and larval animals whose adaptive immune systems are underdeveloped relative to adult frogs (Rollins-Smith, 1998).
Non-mucus skin secretions are an important component of the innate immune system of larval, juvenile and adult amphibians (Woodhams et al., 2007, 2016). Antimicrobial peptides (AMPs) have been shown to be a particularly important constituent of non-mucus skin secretions, since many are effective against cutaneous pathogens like Bd (Rollins-Smith, 2009). Indeed, the diversity and abundance of cutaneous AMPs is thought to be a major factor contributing to the differential susceptibility of frog species to Bd (Rollins-Smith, 2009). Abiotic stressors such as pollutants and low environmental temperatures can differentially influence the production, composition and release of AMPs from skin glands (Davidson et al., 2007). However, little is known about the capacity for UVBR exposure to directly influence the production and/or composition of cutaneous AMP in larval amphibians or for early UVBR exposures to affect AMP production or composition in subsequent life history stages.
As far as we could determine, no studies have explored the hypothesis that early UVBR exposure can disrupt the physiochemical properties of embryonic and larval amphibian skin. Laboratory studies are required to establish a cause-and-effect relationship between UVBR exposure and skin secretion properties. If such a relationship can be established, it will be important to ascertain how the timing, dose and intensity of UVBR exposure might affect the subsequent composition of the cutaneous ‘secretome’ or influence the subsequent development of cutaneous mucus and glandular tissues.
The cutaneous microbiome
The microbiome of the amphibian integument plays an important role in host immunity and disease progression (Longo et al., 2015; Rebollar et al., 2016). As with all organisms, the ‘external’ surfaces of amphibians are colonized by a large and diverse array of microbiota (bacteria, fungi, viruses and mites), most of which are commensal or transient and cause no harm at all to the host. A subset of these microbes produce metabolites that actively discourage establishment of potential pathogens, thereby contributing to disease resistance (Patra et al., 2016). The host’s microbiome also provides a highly competitive microenvironment in which a potential pathogen must outcompete the commensal microbiota in order to become established. The composition of the host microbiome can determine the outcome of infection by pathogens, with recent studies showing variation in host susceptibility to Bd is linked to the presence/absence of certain bacteria on the host’s integument (Briggs et al., 2010; Jani et al., 2017).
Given the links between Bd infection outcomes and microbiome composition, there is considerable interest in understanding those factors that may regulate or disturb amphibian microbiomes. Like all ecosystems, host-associated microbiomes are sensitive to environmental disturbances. Aquatic pH, salinity and temperature have been identified as primary regulators of host- and non-host-associated microbial communities (Fierer and Jackson, 2006; Lozupone and Knight, 2007; Costello et al., 2009; Kueneman et al., 2014). Solar UVBR has well-documented antimicrobial properties, capable of affecting both commensal and pathogenic organisms (Faergemann and Larko, 1987; Dotterud et al., 2008; Wang et al., 2012). Recent work in humans suggests that UVB radiation can modulate aspects of the host microbiomes more broadly, and in doing so, influence disease development and outcomes (Patra et al., 2016). However, the role that UVBR may play in the regulation of innate immune function in amphibians, through its impact on host microbiome composition remains largely unknown. In the only study to date that has addressed this question in amphibians, pond shading had no impact on larval microbiome composition in Rana catesbeiana (Krynak et al., 2015), suggesting that UVBR may not influence the structure of the microbiome in some aquatic amphibians. However, since UVBR penetrance into aquatic systems is dependent on the amount of dissolved organic matter in the water, pond shading levels and solar angle (Xenopoulos and Schindler, 2001), further work is needed to determine if aquatic microbiomes can actually be influenced by natural levels of UVBR in other environs where UVBR penetrance is higher (e.g. at high altitude, clear stream sites).
Cutaneous and systemic immune signalling
The cutaneous immune system comprises interconnected innate and adaptive cellular networks involved in localized and systemic immune responses to potential pathogens (Mann et al., 2012). In vertebrate skin, keratinocytes in the epidermis produce a complex array of AMPs, pro-inflammatory cytokines and chemokines in response to the activation of pathogen recognition receptors on their cells’ surfaces (Mann et al., 2012). In addition to these cells, antigen-presenting dendritic cells (Langerhans cells) actively patrol the integument for would-be pathogens. These cells perform a vital role bridging the cutaneous innate and adaptive immune systems: they can recognize a diverse array of pathogen- and self-associated molecules and can initiate the appropriate immune or tolerogenic responses (Sleijffers et al., 2004; Mann et al., 2012).
The effects of UVBR exposure on cutaneous immune signalling in amphibians is largely unexplored; however, data from mammalian models show that UVBR exposure can induce local and systemic immunosuppression by reducing cutaneous dentritic cell abundance, and by reducing their capacity to recognize antigens and induce an adaptive immune response (Kripke, 2013). In addition, UVBR exposure induces the secretion of immunosuppressive cytokines (including interleukin-10) by keratinocytes, which suppresses other local and systemic immune responses (Elmets et al., 1983; Beissert and Schwarz, 1999) and impedes DNA damage repair mechanisms (Sreevidya et al., 2008). While data relating to non-mammalian taxa are limited, studies on fish suggest a common action of UVBR on elements of the systemic immune system. In some fish species, UVBR exposure correlates with a reduction in the abundance of circulating peripheral lymphocytes (Jokinen et al., 2000, 2001; Markkula et al., 2005, 2007, 2009), with a reduced functional capacity of white blood cells to respond to stimulation (Salo et al., 1998; Markkula et al., 2009) and with lower blood immunoglobulin levels (Jokinen et al., 2001, 2008, 2011; Markkula et al., 2005).
Importantly, local and systemic immunomodulation by UVBR exposure has been directly linked to a wide range of mammalian pathogen infection outcomes (reviewed by Sleijffers et al., 2004). Similarly in fish, UVBR can negatively affect pathogen infection rates even when UVBR doses are not sufficient to cause overt skin damage (Markkula et al., 2007; Cramp et al., 2014). Moreover, the effects of UVBR on infection susceptibility can manifest after just a single exposure and can persist for several weeks (Jeevan and Kripke, 1989). The effects of UVBR on cutaneous immune function and systemic signalling in amphibians (larval or adult) are largely unknown. However, given the similarly of the amphibian immune system to that of other vertebrates (Carey et al., 1999), it is not unreasonable to expect that similar responses to UVBR might occur.
Indirect modulation of immune function by UVBR
Physiological stress, neuroendocrine signalling and immune system maturation
UVBR can exert its influence on immune function through its capacity to influence a wide range of endocrine functions. Hormones mediate many of the mechanisms that underpin phenotypic responses to environmental stimuli (Gilbert and Epel, 2008); in particular, hormones of the systemic stress axis (hypothalamic–pituitary–adrenal in mammals and birds, or hypothalamic–pituitary–intrarenal axis in fish, amphibians and reptiles) are principle mediators of physiological responses to environmental stimuli (Denver, 2009). These hormones include glucocorticoids (corticosterone and cortisol) released from the adrenal (intrarenal) tissues during stressful events, which function to rapidly mobilize energy supplies by reducing the energy supplied to non-vital physiological processes. While beneficial in the short-term, chronically elevated glucocorticoid levels can be maladaptive, suppressing immune function and increasing susceptibility to disease (Wedemeyer, 1970; Snieszko, 1974; Barton and Iwama, 1991; Engelsma et al., 2003; Murray and Peeler, 2005; Bowden, 2008; Tort, 2011). Chronically elevated glucocorticoid levels may increase pathogen susceptibility in amphibians (Belden and Kiesecker, 2005). Biotic and abiotic environmental stressors such as overcrowding, acidification, food deprivation, predation, temperature stress and pollution can elicit an increase in corticosterone levels in amphibian larvae (e.g. Belden et al., 2010; Searle et al., 2010; Crespi and Warne, 2013), while UVBR exposure can increase circulating levels of glucocorticoids in some fish species (Markkula et al., 2007). Whether UVBR exposure is capable of elevating glucocorticoid levels in amphibians, however, remains largely unexplored. In the only study to date, Rana cascadae larvae reared under ambient solar UVBR levels showed a slight, but non-significant, increase in corticosterone levels after 7 and 42 days of exposure (Belden et al., 2003). Further work is needed to determine if solar UVBR can induce a chronic stress response in other amphibian species, and if this may contribute to immunosuppression leading to increased pathogen susceptibility.
In amphibians, thyroid hormones (THs), along with glucocorticoids, play a major role in the timing and control of tissue development and metamorphosis (Denver, 2009; Croteau et al., 2010). Changes in the levels of these hormones in response to environmental stressors can, therefore, influence growth rates, tissue development and metamorphic timing (Denver, 2009). UVBR exposure has been shown to impede larval development and reduce deiodinase 2 expression in some tissues of Rana pipiens tadpoles and (Croteau et al., 2009) suggesting that UVBR exposure may impair development via its influence on the thyroid axis. Environmental factors that influence neurohormonal pathways are likely to influence a number of physiological systems, including the transition from the larval- to adult-type immune system (Rollins-Smith and Smits, 2004). Indeed, maturation of the larval immune system is highly dependent on both TH and glucocorticoid levels (Rollins-Smith and Blair, 1990; Rollins-Smith, 1998). Conceivably then, larval UVBR exposure may disrupt both the glucocorticoid- and TH-signalling pathways to impair immune system development. While this question is yet to be explicitly explored, Ceccato et al. (2016) showed that juvenile frogs reared as larvae under moderate levels of UVBR, had lower levels of circulating leucocytes and reduced antigen swelling responses suggesting a delayed transition from larval to adult phenotype. Recently metamorphosed frogs are at greater risk from pathogens because of the relative immaturity of the immune system (Rollins-Smith, 2017), so there is potential for early UVBR exposure to compound this risk by delaying the development of the adult-type immune system. The role that the glucocorticoid or TH axes played in these responses remains to be determined.
Energy production and supply to the immune system
Immune responses and resistance to pathogens and parasites are physiologically demanding (Demas et al., 1997; Martin et al., 2003; Hawley and Altizer, 2011). Energetic costs associated with UVBR exposure (including UVBR damage repair or avoidance mechanisms) may result in trade-offs, reducing an animal’s capacity to mount an effective immune response. Consistent with this view, studies of amphibian larvae have shown that energetic costs associated with repair or avoidance of UVBR damage may be significant, compromising larval growth and foraging opportunities (van de Mortel and Buttemer, 1998; Alton et al., 2010, 2011). Direct UVBR-induced damage to biomolecules within mitochondria (e.g. ROS damage) may also compromise energy production (Cramp et al., 2014). Studies of amphibian larvae have also shown that increased cutaneous melanin production in response to UVBR (Bancroft, 2007) may limit the amount of energy available for growth and, potentially, immune function as well—a view supported by studies of damselflies showing a trade-off between melanin production and immune function (Debecker et al., 2015). Whether this trade-off in energy investment influences the outcome of a pathogen challenge, however, remains to be determined.
Carryover effects from early life experiences
The effects of UVBR on amphibian immune function may not manifest immediately, which may mask the potential risk from this stressor. Juvenile amphibian immune function can be significantly influenced by environmental stressors experienced during the larval period, including pond drying and dietary stress (Gervasi and Foufopoulos, 2008; Venesky et al., 2012; Krynak et al., 2015); however, relatively little is known about how early UVBR exposure might influence long-term immune function in amphibians. UVBR has been linked to long-term effects on immune function though direct DNA damage (mutations) as well as through epigenetic processes in mammals (e.g. Gronniger et al., 2010; Shen et al., 2017). For instance, the development of skin cancer in humans follows UVBR-induced immunosuppression that can occur many decades after the causative exposure (Elmets et al., 2014). Early development is a particularly vulnerable stage for mutagenic and epigenetic modifications, since it is a period of rapid cell replication (Perera and Herbstman, 2011). Given that early life stages are most likely to experience UVBR, our group has been exploring the capacity for early UVBR exposure to have a delayed effect on immune function in amphibians. We have found that juvenile frogs, exposed to elevated UVBR as larvae, have reduced antigen responses, reduced white blood cell counts (Ceccato et al., 2016) and are more susceptible to Bd than those exposed to low/no UVBR as larvae. These findings indicate that early developmental exposure to UVBR has long-term ramifications for amphibian immune function and disease susceptibility. Further work is required to understand the mechanistic basis for the latent effects of UVBR exposure on amphibians, and how UVBR dose, intensity and the timing of exposure can influence latent immunosuppression.
Interactions with other environmental stressors
Like most animals, amphibian immune function can be affected, directly and indirectly, by a range of environmental stressors including temperature (Raffel et al., 2006), diet quality (Venesky et al., 2012), aquatic pH (Krynak et al., 2015), predator stress (e.g. Groner et al., 2013), social stress (Groner et al., 2014; Burraco and Gomez-Mestre, 2016; Aspbury et al., 2017) and chemical contaminants (e.g. Cary et al., 2014; Sifkarovski et al., 2014). UVBR can interact significantly with many of these and other environmental stressors to alter their effects on amphibian physiology (Bancroft et al., 2008; Alton and Franklin, 2017). One particularly well-studied interaction is between UVBR and environmental temperature. In amphibians, exposure to elevated UVBR exacerbates the negative effects of low temperatures on survival, growth and performance (Grant and Licht, 1995; Broomhall et al., 2000; van Uitregt et al., 2007). UVBR compounds the effects of low temperatures by inducing DNA damage, which is slow to be repaired because DNA repair processes are thermally sensitive (Lamare et al., 2006). We have recently found that enzymatic DNA repair rates are ~50% slower at 20°C than they are at 30°C in early Limnodynastes peronii larvae (unpublished data). The accumulation of UVBR-associated DNA damage underpins the immunosuppressive nature of UVBR in mammals (Kripke, 1981), but it is unclear if the same is true for amphibians. Conceivably, cool temperatures may impair DNA repair mechanisms, resulting in the accumulation of UVBR-associated damage that triggers immunosuppression. If this is the case, thermal effects on UVBR-associated DNA repair rates may explain some disease-related amphibian declines at high elevation where UVBR levels are naturally high and temperatures are low (van Uitregt et al., 2007). However, further work is needed to build the mechanistic link between UVBR exposure, low temperature and disease susceptibility across species and populations.
Conclusions and future directions
In this review, we have explored the potential for one changing environmental variable, UVBR, to influence amphibian fitness through its capacity to exert direct and indirect effects on immune function. While relatively little is known about how UVBR can influence amphibian immune function, a large body of literature from studies of other taxa strongly supports the idea that UVBR exposure may increase the susceptibility of amphibians to disease. The potential immunosuppressive effects of UVBR, however, have been largely overlooked in studies investigating the cause(s) of disease-related amphibian population declines. Understanding the role that elevated UVBR levels may have played and may continue to play, in the emergence or exacerbation of amphibian diseases is, therefore, important.
Anthropogenic increases in solar UVBR exposure correlate positively with patterns of enigmatic amphibian decline in many parts of the world (Kiesecker et al., 2001; Middleton et al., 2001). While a novel pathogen (Bd) was subsequently shown to be the proximate cause of many of these declines, the possibility that increased UVBR may have contributed to the emergence and epidemiology of the disease has remained largely unexplored. Elevated UVBR has been largely neglected from major environmental models of Bd-related declines because of a lack of data on natural UVBR levels in the amphibian–pathogen microenvironment. UVBR levels are difficult to accurately quantify and can vary enormously, over both space and time, as a consequence of solar distance and angle, vegetation cover, cloud thickness and levels of dissolved organic matter in water (Xenopoulos and Schindler, 2001). UVBR levels also co-vary with other changing environmental factors such as temperature, cloud cover and rainfall patterns. Temperature and rainfall significantly influence amphibian behaviour as well as incident UVBR levels, so disentangling the potential negative effects of increased UVBR exposure from the effects of other, co-varying environmental factors, has been, and remains, a complex challenge.
Satellite-based monitoring of global ozone levels and incident UVBR provide a relatively coarse measure of exposure risk and can be used to monitor changes in UVBR levels. Satellite-based UVR data have been used in a small number of studies to model Bd infection patterns over a relatively broad spatial scale (the Iberian Peninsula) (Walker et al., 2010; Ortiz-Santaliestra et al., 2011). While the data indicated that Bd presence in the study populations was not predicted by any one environmental factor, the conditional prevalence of infection was weakly, negatively correlated with solar radiation levels (Walker et al., 2010). However, the authors stress caution in the interpretation of these findings, mainly because of the incongruity of scale between the coarse climatic statistics used in these studies and the actual microenvironments occupied by both the hosts and the pathogen (Walker et al., 2010). In order to understand if/how UVBR may impact amphibian health and disease processes and to mitigate potential risks for highly threatened species or insurance populations, we need a better understanding of how amphibians interact with UVBR in their environment. Future studies need to consider realistic measures of UVBR in the immediate environment where animals are most likely to be exposed and consider both spatial and temporal variation in UVBR exposure patterns. Likewise, further work is needed to understand how amphibians behaviourally influence individual exposure histories through their choice of oviposition site (e.g. Palen et al., 2005; Thurman et al., 2014) and/or through avoidance or thermoregulatory activities.
Following on from this, the question of ‘how much UVBR is too much’ is key to assessing whether anthropogenic increases in UVBR have contributed to some disease-associated amphibian declines. Amphibians, as a group, occupy a diverse array of environments and may experience a wide range of UVBR levels: how much UVBR is too much is likely to be highly species, population and life-stage specific. For instance, species or populations naturally living in high altitude, open canopy and/or low DOC environments typically experience higher levels of UVBR than fossorial, or fully nocturnal species. Moreover, exposure to UVBR only becomes problematic when an animal’s capacity to respond to, or repair, induced damage is exceeded. While amphibians employ a range of defences against UVBR, their capacity to invoke these can differ enormously across species, populations and life history stages (van de Mortel and Buttemer, 1998; Belden et al., 2003; Palen et al., 2005). Therefore, the threshold level for UVBR exposure above which repair/avoidance strategies are ineffective is likely to be highly context (species, life history stage, population, etc.) specific. Future work is needed to assess whether UVBR exposure thresholds exist for immunosuppression. Thresholds for UVBR-associated immunosuppression are likely to be different for embryos vs larvae and adults and differ across species and/or populations. Understanding the factors that influence the performance of morphological and molecular defence mechanisms, and the plasticity within repair and defence mechanisms to contend with diverse and variable UVBR levels is needed. Likewise, the capacity for larvae to detect and behaviourally modulate their exposure to UVBR needs to be more widely explored.
Ecoimmunological studies often measure immune system responses to environmental stimuli as a proxy for individual fitness (Downs and Stewart, 2014). However, increasingly, studies that employ single immune assays, like white blood cell counts or lectin-induced swelling assays, have been criticized for being overly simplistic or overinterpreted due to the challenge of linking immune measures to actual pathogen susceptibility, resistance or recovery (Hawley and Altizer, 2011). There is also often a lack of correlation among immune assays within single individuals or species (Hawley and Altizer, 2011). Attempts to link host susceptibility to pathogens with responses to non-pathogenic immune assays are hampered by a lack of understanding about which immune parameters will best predict pathogen susceptibility. Moreover, different pathogens may differentially interact with the immune system, and so markers that may predict outcomes for one pathogen, may bear no relevance to infection outcomes for another pathogen. Consequently, it is important that future studies of UVBR on amphibian immune function examine a suite of traits that reflect the diverse ways that the immune system may to respond to a challenge, both within and across life stages, including by directly measuring pathogen susceptibility, infection intensity and immune responses to antigenic challenges.
Immune function is a key individual-level trait that influences populations because it directly affects the survival outcome of a pathogen challenge (Downs and Stewart, 2014). However, within a community, variability in host immune function can promote selection and the evolution of resistance to infection (Lazzaro and Little, 2009). Variability in the responses of immune system components to the environment can promote polymorphism within a population; environmental heterogeneity can then maintain genetic variation in immunity by promoting alternative phenotypes over space and time (Lazzaro and Little, 2009). Given that UVBR exposure history likely differs significantly within and between populations, UVBR may differentially influence selection pressure on amphibian immune function genes, which in turn may shape differences in disease prevalence and infection outcome. Relatively fast-paced changes in UVBR levels may have placed additional pressure on populations, as the rate and magnitude of change could have exceeded the capacity for evolutionary mechanisms to maintain genetic heterogeneity in immune traits. This may have rendered some amphibian populations or species more susceptible to novel pathogens and contributed to the catastrophic declines that followed. Analysis of the way that UVBR can shape genetic heterogeneity within and between species and populations would inform our understanding of how UVBR may influence the evolution of disease resistant or susceptible phenotypes.
Historically, the primary role of the immune system has been regarded as the detection and elimination of pathogens (i.e. ‘resistance’). While in this review we have focused on the potential for UVBR to disrupt amphibian resistance pathways, tolerance of pathogens is increasingly recognised as an important immune strategy for managing host–pathogen interactions (Medzhitov et al., 2012). Tolerance can be defined as responses that limit the negative effects of the infection on the host without influencing the pathogen burden. Resistance-based immune responses are energetically costly and often damaging to the host; tolerance of a pathogen may allow hosts to maximize their fitness by preventing costly immunopathological responses (Medzhitov et al., 2012). The concept of tolerance is relatively new to ecoimmunology and the mechanisms underpinning it are poorly understood. Conceivably though, UVBR exposure may shape disease dynamics within populations by reducing the capacity of hosts to tolerate otherwise manageable microbial interactions. Conversely, UVBR has been shown to impair some resistance pathways, increasing pathogen/allergen tolerance, termed ‘photo-tolerance’ (Spellman et al., 1984; de Gruijl, 2008). While pathogen tolerance may be an effective strategy to minimize the negative effects of a pathogen on an individual, tolerant hosts can also serve as highly infectious vectors of disease (Medzhitov et al., 2012). This has potentially significant implications for the spread of pathogens within and between populations. Several amphibian species, including the American bullfrog (Lithobates catesbeianus) and the clicking froglet (Crinnia signifera), have been identified as highly virulent vectors of Bd capable of harbouring and spreading the fungus to more susceptible species in their environments (Schloegel et al., 2012; Brannelly et al., 2018). Further work is required to determine if UVBR exposure regimes can influence pathogen tolerance in amphibians and if this may contribute to inter and intraspecific differences in susceptibility to pathogens like Bd.
Global UVBR levels have increased by between 2 and 6% since the middle of the last century (Lemus-Deschamps and Makin, 2012) and are expected to remain significantly elevated for much of this century (Ball et al., 2018; Montzka et al., 2018). Elevated UVBR levels correlate broadly with patterns of disease-related amphibian biodiversity loss, particularly at high altitude (Kiesecker et al., 2001; Middleton et al., 2001), yet potential causal links between UVBR exposure and amphibian disease remain relatively unexplored. UVBR is a powerful immunosuppressant in other animals and likely affects amphibians in the same way. However, further work is needed to establish the mechanistic link between UVBR, immune function and disease susceptibility in amphibians. By taking a physiological approach to understanding the mechanisms through which the environment shapes amphibian immune function, we can better predict how environmental change is likely to influence disease dynamics with and between species and populations. Physiological studies are uniquely positioned in this regard, because environmental effects on physiology can be experimentally tested. Therefore, unlike correlative studies, empirical physiological studies can provide the compelling evidence needed to establish direct cause-and-effect (Carey, 2005; Cooke et al., 2013). The diversity of ways in which the environment can shape population and species persistence via impacts on individual physiology, highlights the importance of taking a holistic and integrative approach to address ecoimmunological problems in a rapidly changing world. We have identified a number of direct and indirect pathways through which elevated UVBR could shape individual immune function and influence disease processes in amphibians, which we hope may guide future research in this field. Currently, our understanding of how UVBR and disease influence amphibians is restricted to a relatively small number of ‘model’ species. This represents a critical knowledge gap which may bias our responses to population declines in under-represented species. There remains an urgent need for fundamental data on how the environment shapes amphibian immune responses across orders, species, populations and life stages, and how this influences their capacity to resist pathogen infections.
Acknowledgements
The authors would like to thank Ed Meyer, Lesley Alton, Michel Ohmer, Kathleen Doody, Niclas Lundsgaard, Samuel Morrison and three anonymous referees for discussions and/or comments on earlier versions of the manuscript who have assisted in forming this review. We would also like to acknowledge the organising committee of the Conservation Physiology session at the Society of Experimental Biology Annual Main Meeting, Brighton 2016, for the invitation to present this material at the meeting.
Funding
This work was supported by an Australian Research Council grant to CEF (DP140102773) and a Society for Experimental Biology grant to RLC.
References
- Abedi S, Sharifpour I, Mozanzadeh MT, Zorriehzahra J, Khodabandeh S, Gisbert E (2015) A histological and ultrastructural study of the skin of rainbow trout (Oncorhynchus mykiss) alevins exposed to different levels of ultraviolet B radiation. J Photoch Photobio B 147: 56–62. [DOI] [PubMed] [Google Scholar]
- Abu Bakar A, Bower DS, Stockwell MP, Clulow S, Clulow J, Mahony MJ (2016) Susceptibility to disease varies with ontogeny and immunocompetence in a threatened amphibian. Oecologia 181: 997–1009. [DOI] [PubMed] [Google Scholar]
- Altig R, McDiarmid RW (1999) Chapter 3. Body plan: development and morphology In McDiarmid RW, Altig R, eds, Tadpoles: The Biology of Anuran Larvae. The University of Chicago Press, Chicago and London, pp 24–51. [Google Scholar]
- Alton LA, Wilson RS, Franklin CE (2010) Risk of predation enhances the lethal effects of UV-B in amphibians. Global Change Biol 16: 538–545. [Google Scholar]
- Alton LA, White CR, Wilson RS, Franklin CE (2011) The energetic cost of exposure to UV radiation for tadpoles is greater when they live with predators. Funct Ecol 26: 93–103. [Google Scholar]
- Alton LA, Franklin CE (2017) Drivers of amphibian declines: effects of ultraviolet radiation and interactions with other environmental factors. Clim Change Resp 4: 6. [Google Scholar]
- Aspbury AS, Grayson KL, Fantaye S, Nichols I, Myers-Burton M, Ortiz-Mangual X, Gabor CR (2017) The association between male-biased sex ratio and indicators of stress in red-spotted newts. Physiol Behav 173: 156–162. [DOI] [PubMed] [Google Scholar]
- Ball WT, Alsing J, Mortlock DJ, Staehelin J, Haigh JD, Peter T, Tummon F, Stübi R, Stenke A, Anderson J, et al. (2018) Evidence for a continuous decline in lower stratospheric ozone offsetting ozone layer recovery. Atmos Chem Phys 18: 1379–1394. [Google Scholar]
- Bancroft BA. (2007) Ultraviolet radiation as an environmental stressor of amphibians. PhD Dissertation. Oregon State University, Oregon, USA. http://ir.library.oregonstate.edu/concern/graduate_thesis_or_dissertations/gm80j076n.
- Bancroft BA, Baker NJ, Blaustein AR (2008) A meta-analysis of the effects of ultraviolet B radiation and its synergistic interactions with pH, contaminants, and disease on amphibian survival. Conserv Biol 22: 987–996. [DOI] [PubMed] [Google Scholar]
- Barber I, Berkhout BW, Ismail Z (2016) Thermal change and the dynamics of multi-host parasite life cycles in aquatic ecosystems. Integr Comp Biol 56: 561–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton BA, Iwama GK (1991) Physiological changes in fish from stress in aquaculture with emphasis on the response and effects of corticosteroids. Annu Rev Fish Dis 1: 3–26. [Google Scholar]
- Beissert S, Schwarz T (1999) Mechanisms involved in ultraviolet light induced immunosuppression. J Invest Dermatol 4: 61–64. [DOI] [PubMed] [Google Scholar]
- Belden LK, Blaustein AR (2002) Exposure of red-legged frog embryos to ambient UV-B radiation in the field negatively affects larval growth and development. Oecologia 130: 551–554. [DOI] [PubMed] [Google Scholar]
- Belden LK, Moore IT, Mason RT, Wingfield JC, Blaustein AR (2003) Survival, the hormonal stress response and UV-B avoidance in Cascades Frog tadpoles (Rana cascadae) exposed to UV-B radiation. Funct Ecol 17: 409–416. [Google Scholar]
- Belden LK, Wingfield JC, Kiesecker JM (2010) Variation in the hormonal stress response among larvae of three amphibian species. J Exp Zool Part A 313: 524–531. [DOI] [PubMed] [Google Scholar]
- Berger L, Speare R, Daszak P, Green DE, Cunningham AA, Goggin CL, Slocombe R, Ragan MA, Hyati AD, McDonald KR et al. (1998) Chytridiomycosis causes amphibian mortality associated with population declines in the rain forests of Australia and Central America. P Natl Acad Sci—Biol 95: 9031–9036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biniek K, Levi K, Dauskardt RH (2012) Solar UV radiation reduces the barrier function of human skin. P Natl Acad Sci—Biol 109: 17111–17116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein AR, Belden LK (2003) Amphibian defenses against ultraviolet-B radiation. Evol Dev 5: 89–97. [DOI] [PubMed] [Google Scholar]
- Blaustein AR, Han B, Fasy B, Romansic J, Scheessele EA, Anthony RG, Marco A, Chivers DP, Belden LK, Kiesecker JM, et al. (2004) Variable breeding phenology affects the exposure of amphibian embryos to ultraviolet radiation and optical characteristics of natural waters protect amphibians from UV-B in the US Pacific Northwest: comment. Ecology 85: 1747–1754. [Google Scholar]
- Blaustein AR, Gervasi SS, Johnson PTJ, Hoverman JT, Belden LK, Bradley PW, Xie GY (2012) Ecophysiology meets conservation: understanding the role of disease in amphibian population declines. Philos T R Soc B 367: 1688–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blazer VS, Fabacher DL, Little EE, Ewing MS, Kocan KM (1997) Effects of ultraviolet-B radiation on fish: histologic comparison of a UVB-sensitive and a UVB-tolerant species. J Aquat Anim Health 9: 132–143. [Google Scholar]
- Blount JD, Pike TW (2012) Deleterious effects of light exposure on immunity and sexual coloration in birds. Funct Ecol 26: 37–45. [Google Scholar]
- Bosch J, Carrascal LM, Durán L, Walker S, Fisher MC (2007) Climate change and outbreaks of amphibian chytridiomycosis in a montane area of Central Spain; is there a link? P Roy Soc B-Biol Sci 274: 253–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowden TJ. (2008) Modulation of the immune system of fish by their environment. Fish Shellfish Immunol 25: 373–383. [DOI] [PubMed] [Google Scholar]
- Brannelly LA, Webb RJ, Hunter DA, Clemann N, Howard K, Skerratt LF, Berger L, Scheele BC (2018) Non-declining amphibians can be important reservoir hosts for amphibian chytrid fungus. Anim Conserv 21: 91–101. [Google Scholar]
- Briggs CJ, Knapp RA, Vredenburg VT (2010) Enzootic and epizootic dynamics of the chytrid fungal pathogen of amphibians. Proc Natl Acad Sci U S A 107(21): 9695–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broomhall SD, Osborne WS, Cunningham RB (2000) Comparative effects of ambient ultraviolet-B radiation on two sympatric species of Australian frogs. Conserv Biol 14: 420–427. [Google Scholar]
- Burraco P, Gomez-Mestre I (2016) Physiological stress responses in amphibian larvae to multiple stressors reveal marked anthropogenic effects even below lethal levels. Physiol Biochem Zool 89: 462–472. [DOI] [PubMed] [Google Scholar]
- Calfee RD, Bridges CM, Little EE (2006) Sensitivity of two Salamander (Ambystoma) species to ultraviolet radiation. J Herpetol 40: 35–42. [Google Scholar]
- Carey C. (1993) Hypothesis concerning the causes of the disappearance of boreal toads from the mountains of Colorado. Conserv Biol 7: 355–362. [Google Scholar]
- Carey C, Cohen N, Rollins-Smith L (1999) Amphibian declines: an immunological perspective. Dev Comp Immunol 23: 459–472. [DOI] [PubMed] [Google Scholar]
- Carey C. (2005) How physiological methods and concepts can be useful in conservation biology. Integr Comp Biol 45: 4–11. [DOI] [PubMed] [Google Scholar]
- Cary TL, Ortiz-Santaliestra ME, Karasov WH (2014) Immunomodulation in post-metamorphic Northern Leopard frogs, Lithobates pipiens, following larval exposure to polybrominated diphenyl ether. Environ Sci Technol 48: 5910–5919. [DOI] [PubMed] [Google Scholar]
- Ceccato E, Cramp RL, Seebacher F, Franklin CE (2016) Early exposure to ultraviolet-B radiation decreases immune function later in life. Cons Physiol 4(1): cow037 doi:010.1093/conphys/cow1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Robert J (2011) Antiviral immunity in amphibians. Viruses 3: 2065–2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng TL, Rovito SM, Wake DB, Vredenburg VT (2011) Coincident mass extirpation of neotropical amphibians with the emergence of the infectious fungal pathogen Batrachochytrium dendrobatidis. Proc Natl Acad Sci—Biol 108: 9502–9507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke SJ, Sack L, Franklin CE, Farrell AP, Beardall J, Wikelski M, Chown SL (2013) What is conservation physiology? Perspectives on an increasingly integrated and essential science. Cons Physiol 1: cot001–cot001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello EK, Lauber CL, Hamady M, Fierer N, Gordon JI, Knight R (2009) Bacterial community variation in human body habitats across space and time. Science 326: 1694–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramp RL, Reid S, Seebacher F, Franklin CE (2014) Synergistic interaction between UVB radiation and temperature increases susceptibility to parasitic infection in a fish. Biol Lett 10(9): 20140449 Doi:10.1098/Rsbl.2014.0449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespi EJ, Warne RW (2013) Environmental conditions experienced during the tadpole stage alter post-metamorphic glucocorticoid response to stress in an amphibian. Integr Comp Biol 53: 989–1001. [DOI] [PubMed] [Google Scholar]
- Croteau MC, Davidson M, Duarte-Guterman P, Wade M, Popesku JT, Wiens S, Lean DRS, Trudeau VL (2009) Assessment of thyroid system disruption in Rana pipiens tadpoles chronically exposed to UVB radiation and 4-tert-octylphenol. Aquat Toxicol 95: 81–92. [DOI] [PubMed] [Google Scholar]
- Croteau MC, Duarte-Guterman P, Lean DR, Trudeau VL (2010) Preexposure to ultraviolet B radiation and 4-tert-octylphenol affects the response of Rana pipiens tadpoles to 3,5,3′-triiodothyronine. Environ Toxicol Chem 29: 1804–1815. [DOI] [PubMed] [Google Scholar]
- Crump D, Berrill M, Coulson D, Lean D, McGillivray L, Smith A (1999) Sensitivity of amphibian embryos, tadpoles, and larvae to enhanced UV-B radiation in natural pond conditions. Can J Zool 77: 1956–1966. [Google Scholar]
- Daszak P, Cunningham AA, Hyatt AD (2000) Wildlife ecology—emerging infectious diseases of wildlife—threats to biodiversity and human health. Science 287: 443–449. [DOI] [PubMed] [Google Scholar]
- Daszak P, Cunningham AA, Hyatt AD (2001) Anthropogenic environmental change and the emergence of infectious diseases in wildlife. Acta Trop 78: 103–116. [DOI] [PubMed] [Google Scholar]
- Davidson C, Benard MF, Shaffer HB, Parker JM, O’Leary C, Conlon JM, Rollins-Smith LA (2007) Effects of chytrid and carbaryl exposure on survival, growth and skin peptide defenses in foothill yellow-legged frogs. Environ Sci Technol 41: 1771–1776. [DOI] [PubMed] [Google Scholar]
- de Gruijl FR. (2008) UV-induced immunosuppression in the balance. Photochem Photobiol 84: 2–9. [DOI] [PubMed] [Google Scholar]
- Debecker S, Sommaruga R, Maes T, Stoks R (2015) Larval UV exposure impairs adult immune function through a trade-off with larval investment in cuticular melanin. Funct Ecol 29: 1292–1299. [Google Scholar]
- Demas GE, Chefer V, Talan MI, Nelson RJ (1997) Metabolic costs of mounting an antigen-stimulated immune response in adult and aged C57BL/6J mice. Am J Physiol-Reg I 273: R1631–R1637. [DOI] [PubMed] [Google Scholar]
- Denver RJ. (2009) Stress hormones mediate environment-genotype interactions during amphibian development. Gen Comp Endocrinol 164: 20–31. [DOI] [PubMed] [Google Scholar]
- Diffey BL. (1991) Solar ultraviolet radiation effects on biological systems. Phys Med Biol 36: 299–328. [DOI] [PubMed] [Google Scholar]
- Dotterud LK, Wilsgaard T, Vorland LH, Falk ES (2008) The effect of UVB radiation on skin microbiota in patients with atopic dermatitis and healthy controls. Int J Circumpol Heal 67: 254–260. [DOI] [PubMed] [Google Scholar]
- Downs CJ, Stewart KM (2014) A primer in ecoimmunology and immunology for wildlife research and management. Calif Fish Game 100: 371–395. [Google Scholar]
- Eckes MJ, Siebeck UE, Dove S, Grutter AS (2008) Ultraviolet sunscreens in reef fish mucus. Mar Ecol Prog Ser 353: 203–211. [Google Scholar]
- Ellis AE. (2001) Innate host defense mechanisms of fish against viruses and bacteria. Dev Comp Immunol 25: 827–839. [DOI] [PubMed] [Google Scholar]
- Elmets CA, Bergstresser PR, Tigelaar RE, Wood PJ, Streilein JW (1983) Analysis of the mechanism of unresponsiveness produced by haptens painted on skin exposed to low-dose ultraviolet-radiation. J Exp Med 158: 781–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmets CA, Cala CM, Xu H (2014) Photoimmunology. Dermatol Clin 32: 277–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelsma MY, Hougee S, Nap D, Hofenk M, Rombout JHWM, van Muiswinkel WB, Lidy Verburg-van Kemenade BM (2003) Multiple acute temperature stress affects leucocyte populations and antibody responses in common carp. Cyprinus carpio L. Fish Shellfish Immunol 15: 397–410. [DOI] [PubMed] [Google Scholar]
- Faergemann J, Larko O (1987) The effect of UV-light on human skin microorganisms. Acta Derm Venereol 67: 69–72. [PubMed] [Google Scholar]
- Fierer N, Jackson RB (2006) The diversity and biogeography of soil bacterial communities. Proc Natl Acad Sci—Biol 103: 626–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisman DN. (2007) Seasonality of infectious diseases. Annu Rev Public Health 28: 127–143. [DOI] [PubMed] [Google Scholar]
- Flamarique IN, Ovaska K, Davis TM (2000) UV-B induced damage to the skin and ocular system of amphibians. Biol Bull 199: 187–188. [DOI] [PubMed] [Google Scholar]
- Fox H. (1986) Chapter 5 epidermis In Bereiter-Hahn J, Richards KS, Matoltsy AG, eds. Biology of the Integument 2 Vertebrates. Springer, Berlin Heidelberg, pp 78–110. [Google Scholar]
- Garcia TS, Romansic JM, Blaustein AR (2006) Survival of three species of anuran metamorphs exposed to UV-B radiation and the pathogenic fungus Batrachochytrium dendrobatidis. Dis Aquat Org 72: 163–169. [DOI] [PubMed] [Google Scholar]
- Gervasi SS, Foufopoulos J (2008) Costs of plasticity: responses to desiccation decrease post-metamorphic immune function in a pond-breeding amphibian. Funct Ecol 22: 100–108. [Google Scholar]
- Gilbert SF, Epel D (2008) Ecological Developmental Biology: Integrating Epigenetics, Medicine and Evolution. Sinauer Associates, Inc, Sunderland, Massachusetts. [Google Scholar]
- Grant KP, Licht LE (1995) Effects of UV radiation on life history stages of anurans from Ontario, Canada. Can J Zool 73: 2292–2301. [Google Scholar]
- Gray MJ, Miller DL, Hoverman JT (2009) Ecology and pathology of amphibian ranaviruses. Dis Aquat Org 87: 243–266. [DOI] [PubMed] [Google Scholar]
- Gray MJ, Chinchar VG (2015) Ranaviruses: Lethal Pathogens of Ectothermic Vertebrates. Springer Cham Heidelberg, New York, Dordrecht, London: 10.1007/978-3-319-13755-1. Open Access eBook. [DOI] [Google Scholar]
- Groner ML, Buck JC, Gervasi S, Blaustein AR, Reinert LK, Rollins-Smith LA, Bier ME, Hempel J, Relyea RA (2013) Larval exposure to predator cues alters immune function and response to a fungal pathogen in post-metamorphic wood frogs. Ecol Appl 23: 1443–1454. [DOI] [PubMed] [Google Scholar]
- Groner ML, Rollins-Smith LA, Reinert LK, Hempel J, Bier ME, Relyea RA (2014) Interactive effects of competition and predator cues on immune responses of leopard frogs at metamorphosis. J Exp Biol 217: 351–358. [DOI] [PubMed] [Google Scholar]
- Gronniger E, Weber B, Heil O, Peters N, Stab F, Wenck H, Korn B, Winnefeld M, Lyko F (2010) Aging and chronic sun exposure cause distinct epigenetic changes in human skin. PLoS Genet 6: e1000971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen LJ, Fabacher DL, Calfee R (2002) The role of the egg jelly coat in protecting Hyla regilla and Bufo canorus embryos from ultraviolet B radiation during development. Environ Sci Pollut R 9: 412–416. [DOI] [PubMed] [Google Scholar]
- Hawley DM, Altizer SM (2011) Disease ecology meets ecological immunology: understanding the links between organismal immunity and infection dynamics in natural populations. Funct Ecol 25: 48–60. [Google Scholar]
- Hof C, Araujo MB, Jetz W, Rahbek C (2011) Additive threats from pathogens, climate and land-use change for global amphibian diversity. Nature 480: 516–519. [DOI] [PubMed] [Google Scholar]
- Houlahan JE, Findlay CS, Schmidt BR, Meyer AH, Kuzmin SL (2000) Quantitative evidence for global amphibian population declines. Nature 404: 752–755. [DOI] [PubMed] [Google Scholar]
- IUCN (2016) The IUCN Red List of Threatened Species. Version 2016-3. <http://www.iucnredlist.org>. Downloaded on 19 December 2016.
- James TY, Toledo LF, Rodder D, Leite DD, Belasen AM, Betancourt-Roman CM, Jenkinson TS, Soto-Azat C, Lambertini C, Longo AV, et al. (2015) Disentangling host, pathogen, and environmental determinants of a recently emerged wildlife disease: lessons from the first 15 years of amphibian chytridiomycosis research. Ecol Evol 5: 4079–4097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jani AJ, Knapp RA, Briggs CJ (2017) Epidemic and endemic pathogen dynamics correspond to distinct host population microbiomes at a landscape scale. Proc. R. Soc. B 284: 20170944 10.1098/rspb.2017.0944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeevan A, Kripke ML (1989) Effect of a single exposure to ultraviolet radiation on Mycobacterium bovis Bacillus Calmette-Guerin infection in mice. J Immunol 143: 2837–2843. [PubMed] [Google Scholar]
- Jokinen EI, Salo HM, Markkula SE, Aaltonen TM, Immonen AK (2000) Effects of ultraviolet light on immune parameters of the roach. Toxicol Lett 112: 303–310. [DOI] [PubMed] [Google Scholar]
- Jokinen EI, Salo HM, Markkula SE, Immonen AK, Aaltonen TM (2001) Ultraviolet B irradiation modulates the immune system of fish (Rutilus rutilus, Cyprinidae) part III: lymphocytes. Photochem Photobiol 73: 505–512. [DOI] [PubMed] [Google Scholar]
- Jokinen IE, Markkula ES, Salo HM, Kuhn P, Nikoskelainen S, Arts MT, Browman HI (2008) Exposure to increased ambient ultraviolet B radiation has negative effects on growth, condition and immune function of juvenile Atlantic salmon (Salmo salar). Photochem Photobiol 84: 1265–1271. [DOI] [PubMed] [Google Scholar]
- Jokinen IE, Salo HM, Markkula E, Rikalainen K, Arts MT, Browman HI (2011) Additive effects of enhanced ambient ultraviolet B radiation and increased temperature on immune function, growth and physiological condition of juvenile (parr) Atlantic Salmon, Salmo salar. Fish Shellfish Immunol 30: 102–108. [DOI] [PubMed] [Google Scholar]
- Kanno T, Nakai T, Muroga K (1989) Mode of transmission of vibriosis among ayu Plecoglossus altivelis. J Aquat Anim Health 1: 2–6. [Google Scholar]
- Kats LB, Kiesecker JM, Chivers DP, Blaustein AR (2000) Effects of UV-B radiation on anti-predator behavior in three species of amphibians. Ethology 106: 921–931. [Google Scholar]
- Katzenback BA, Holden HA, Falardeau J, Childers C, Hadj-Moussa H, Avis TJ, Storey KB (2014) Regulation of the Rana sylvatica brevinin-1SY antimicrobial peptide during development and in dorsal and ventral skin in response to freezing, anoxia and dehydration. J Exp Biol 217: 1392–1401. [DOI] [PubMed] [Google Scholar]
- Kaweewat K, Hofer R (1997) Effect of UV-B radiation on goblet cells in the skin of different fish species. J Photochem Photobiol B: Biol 41: 222–226. [Google Scholar]
- Kazerouni EG, Khodabandeh S (2010) Effects of ultraviolet radiation on skin structure and ultrastructure in Caspian Sea Salmon, Salmo trutta caspius, during alevin stage. Toxicol Environ Chem 92: 903–914. [Google Scholar]
- Kiesecker JM, Blaustein AR, Belden LK (2001) Complex causes of amphibian population declines. Nature 410: 681–684. [DOI] [PubMed] [Google Scholar]
- Kolby JE, Daszak P (2016) The emerging amphibian fungal disease, chytridiomycosis: a key example of the global phenomenon of wildlife emerging infectious diseases. Microbiol Spectr 4(3), 10.1128/microbiolspec.EI10-0004-2015. [DOI] [PubMed] [Google Scholar]
- Kriger KM, Hero J-M (2008) Altitudinal distribution of chytrid (Batrachochytrium dendrobatidis) infection in subtropical Australian frogs. Austral Ecol 33: 1022–1032. [Google Scholar]
- Kripke ML. (1981) Immunologic mechanisms in UV radiation carcinogenesis. Adv Cancer Res 34: 69–106. [DOI] [PubMed] [Google Scholar]
- Kripke ML, Cox PA, Alas LG, Yarosh DB (1992) Pyrimidine dimers in DNA initiate systemic immunosuppression in UV-irradiated mice. Proc Natl Acad Sci—Biol 89: 7516–7520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kripke ML. (2013) Reflections on the field of photoimmunology. J Invest Dermatol 133: 27–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krynak KL, Burke DJ, Benard MF (2015) Larval environment alters amphibian immune defenses differentially across life stages and populations. PLoS One 10: e0130383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kueneman JG, Parfrey LW, Woodhams DC, Archer HM, Knight R, McKenzie VJ (2014) The amphibian skin-associated microbiome across species, space and life history stages. Mol Ecol 23: 1238–1250. [DOI] [PubMed] [Google Scholar]
- Lahnsteiner F, Haunschmid R, Mansour N (2011) Possible reasons for late summer brown trout (Salmo trutta Linnaeus 1758) mortality in Austrian prealpine river systems. J Appl Ichthyol 27: 83–93. [Google Scholar]
- Lamare MD, Barker MF, Lesser MP, Marshall C (2006) DNA photorepair in echinoid embryos: effects of temperature on repair rate in Antarctic and non-Antarctic species. J Exp Biol 209: 5017–5028. [DOI] [PubMed] [Google Scholar]
- Langhammer PF, Burrowes PA, Lips KR, Bryant AB, Collins JP (2014) Susceptibility to the amphibian chytrid fungus varies with ontogeny in the direct developing frog, Eleutherodactylus coqui. J Wildl Dis 50: 438–446. [DOI] [PubMed] [Google Scholar]
- Lazzaro BP, Little TJ (2009) Immunity in a variable world. Philos T R Soc B 364: 15–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemus-Deschamps L, Makin JK (2012) Fifty years of changes in UV Index and implications for skin cancer in Australia. Int J Biometeorol 56: 727–735. [DOI] [PubMed] [Google Scholar]
- Licht LE, Grant KP (1997) The effects of ultraviolet radiation on the biology of amphibians. Am Zool 37: 137–145. [Google Scholar]
- Lips KR. (1998) Decline of a tropical montane amphibian fauna. Conserv Biol 12: 106–117. [Google Scholar]
- Lips KR, Brem F, Brenes R, Reeve JD, Alford RA, Voyles J, Carey C, Livo L, Pessier AP, Collins JP (2006) Emerging infectious disease and the loss of biodiversity in a Neotropical amphibian community. Proc Natl Acad Sci USA 103(9): 3165–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little EE, Fabacher DF (2003) UVR-induced injuries in freshwater vertebrates In Helbling EW, Zagarese HE, eds. UV Effects in Aquatic Organisms and Ecosystems. R Soc Chem, Cambridge, pp 431–454. [Google Scholar]
- Longcore JE, Pessier AP, Nichols DK (1999) Batrachochytrium dendrobatidis gen et sp nov, a chytrid pathogenic to amphibians. Mycologia 91: 219–227. [Google Scholar]
- Longo AV, Savage AE, Hewson I, Zamudio KR (2015) Seasonal and ontogenetic variation of skin microbial communities and relationships to natural disease dynamics in declining amphibians. Roy Soc Open Sci 2(7): 140377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozupone CA, Knight R (2007) Global patterns in bacterial diversity. Proc Natl Acad Sci—Biol 104: 11436–11440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madronich S, McKenzie RL, Bjorn LO, Caldwell MM (1998) Changes in biologically active ultraviolet radiation reaching the Earth’s surface. J Photoch Photobio B 46: 5–19. [DOI] [PubMed] [Google Scholar]
- Magarinos B, Pazos F, Santos Y, Romalde JL, Toranzo AE (1995) Response of Pasteurella piscicida and Flexibacter maritimus to skin mucus of marine fish. Dis Aquat Org 21: 103–108. [Google Scholar]
- Magnadottir B. (2006) Innate immunity of fish (overview). Fish Shellfish Immunol 20: 137–151. [DOI] [PubMed] [Google Scholar]
- Makrinos DL, Bowden TJ (2016) Natural environmental impacts on teleost immune function. Fish Shellfish Immunol 53: 50–57. [DOI] [PubMed] [Google Scholar]
- Mann ER, Smith KM, Bernardo D, Al-Hassi HO, Knight SC, Hart AL (2012) Review: skin and the immune system. J Clin Exp Dermatol Res S2: 003. [Google Scholar]
- Markkula E, Salo HM, Rikalainen K, Jokinen IE (2009) Long-term UVB irradiation affects the immune functions of carp (Cyprinus carpio) and rainbow trout (Oncorhynchus mykiss). Photochem Photobiol 85: 347–352. [DOI] [PubMed] [Google Scholar]
- Markkula SE, Salo HM, Immonen AK, Jokinen EI (2005) Effects of short- and long-term ultraviolet b irradiation on the immune system of the common carp (Cyprinus carpio). Photochem Photobiol 81: 595–602. [DOI] [PubMed] [Google Scholar]
- Markkula SE, Karvonen A, Salo H, Valtonen ET, Jokinen EI (2007) Ultraviolet B irradiation affects resistance of rainbow trout (Oncorhynchus mykiss) against bacterium Yersinia ruckeri and trematode Diplostomum spathaceum. Photochem Photobiol 83: 1263–1269. [DOI] [PubMed] [Google Scholar]
- Martel A, Spitzen-van der Sluijs A, Blooi M, Bert W, Ducatelle R, Fisher MC, Woeltjes A, Bosman W, Chiers K, Bossuyt F, et al. (2013) Batrachochytrium salamandrivorans sp. nov. causes lethal chytridiomycosis in amphibians. Proc Natl Acad Sci—Biol 110: 15325–15329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin LB, Scheuerlein A, Wikelski M (2003) Immune activity elevates energy expenditure of house sparrows: a link between direct and indirect costs? Proc Roy Soc B-Biol Sci 270: 153–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura Y, Ananthaswamy HN (2004) Toxic effects of ultraviolet radiation on the skin. Toxicol Appl Pharmacol 195: 298–308. [DOI] [PubMed] [Google Scholar]
- McFadzen I, Baynes S, Hallam J, Beesley A, Lowe D (2000) Histopathology of the skin of UV-B irradiated sole (Solea solea) and turbot (Scophthalmus maximus) larvae. Mar Environ Res 50: 273–277. [DOI] [PubMed] [Google Scholar]
- Medzhitov R, Schneider DS, Soares MP (2012) Disease tolerance as a defense strategy. Science 335: 936–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton EM, Herman JR, Celarier EA, Wilkinson JW, Carey C, Rusin RJ (2001) Evaluating ultraviolet radiation exposure with satellite data at sites of amphibian declines in Central and South America. Conserv Biol 15: 914–929. [Google Scholar]
- Miller D, Gray M, Storfer A (2011) Ecopathology of Ranaviruses infecting amphibians. Viruses 3: 2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montzka SA, Dutton GS, Yu P, Ray E, Portmann RW, Daniel JS, Kuijpers L, Hall BD, Mondeel D, Siso C, et al. (. 2018) An unexpected and persistent increase in global emissions of ozone-depleting CFC-11. Nature 557: 413–417. [DOI] [PubMed] [Google Scholar]
- Murray AG, Peeler EJ (2005) A framework for understanding the potential for emerging diseases in aquaculture. Prev Vet Med 67: 223–235. [DOI] [PubMed] [Google Scholar]
- Ndong D, Chen YY, Lin YH, Vaseeharan B, Chen JC (2007) The immune response of tilapia Oreochromis mossambicus and its susceptibility to Streptococcus iniae under stress in low and high temperatures. Fish Shellfish Immunol 22: 686–694. [DOI] [PubMed] [Google Scholar]
- Noceda C, Sierra SG, Martinez JL (1997) Histopathology of UV-B irradiated brown trout Salmo trutta skin. Dis Aquat Org 31: 103–108. [Google Scholar]
- Nowak BF. (1999) Significance of environmental factors in aetiology of skin diseases of teleost fish. Bull Eur Assoc Fish Pathol 19: 290–292. [Google Scholar]
- Ortiz-Santaliestra ME, Fisher MC, Fernandez-Beaskoetxea S, Fernandez-Beneitez MJ, Bosch J (2011) Ambient ultraviolet B radiation and prevalence of infection by Batrachochytrium dendrobatidis in two amphibian species. Conserv Biol 25(5): 975–82. [DOI] [PubMed] [Google Scholar]
- Palen WJ, Williamson CE, Clauser AA, Schindler DE (2005) Impact of UV-B exposure on amphibian embryos: linking species physiology and oviposition behaviour. Proc Roy Soc B-Biol Sci 272: 1227–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patra V, Byrne SN, Wolf P (2016) The skin microbiome: is it affected by UV-induced immune suppression? Front Microbiol 7: 1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera F, Herbstman J (2011) Prenatal environmental exposures, epigenetics, and disease. Reprod Toxicol 31: 363–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson GS, Johnson LB, Axler RP, Diamond SA (2002) Assessment of the risk of solar ultraviolet radiation to amphibians. II. In situ characterization of exposure in amphibian habitats. Environ Sci Technol 36: 2859–2865. [DOI] [PubMed] [Google Scholar]
- Poorten TJ, Kuhn RE (2009) Maternal transfer of antibodies to eggs in Xenopus laevis. Dev Comp Immunol 33: 171–175. [DOI] [PubMed] [Google Scholar]
- Raffel TR, Rohr JR, Kiesecker JM, Hudson PJ (2006) Negative effects of changing temperature on amphibian immunity under field conditions. Funct Ecol 20: 819–828. [Google Scholar]
- Rebollar EA, Hughey MC, Medina D, Harris RN, Ibanez R, Belden LK (2016) Skin bacterial diversity of Panamanian frogs is associated with host susceptibility and presence of Batrachochytrium dendrobatidis. ISME J 10: 1682–1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert J, Ohta Y (2009) Comparative and developmental study of the immune system in Xenopus. Dev Dyn 238: 1249–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohr JR, Raffel TR, Sessions SK (2009) Digenetic trematodes and their relationship to amphibian declines and deformities In Heatwole H, Wilkinson JW, eds. Amphibian Biology, Vol 8. Amphibian Decline: Diseases, Parasites, Maladies, and Pollution. Surrey Beatty & Sons, Chipping Norton, Australia, pp 3067–3088. [Google Scholar]
- Rohr JR, Raffel TR (2010) Linking global climate and temperature variability to widespread amphibian declines putatively caused by disease. Proc Natl Acad Sci—Biol 107: 8269–8274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollins-Smith L, Smits JEG (2004) Amphibian models and approaches to immunotoxicology In Tryphonas H, Fournier M, Blakley BR, Smits J, Brousseau P, eds. Investigative Immunotoxicology. Taylor & Francis Group, Boca Raton, London, New York, Singapore, pp 77–90. [Google Scholar]
- Rollins-Smith LA, Blair P (1990) Expression of class II major histocompatibility complex antigens on adult T cells in Xenopus is metamorphosis-dependent. Dev Immunol 1: 97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollins-Smith LA. (1998) Metamorphosis and the amphibian immune system. Immunol Rev 166: 221–230. [DOI] [PubMed] [Google Scholar]
- Rollins-Smith LA. (2009) The role of amphibian antimicrobial peptides in protection of amphibians from pathogens linked to global amphibian declines. Biochim Biophys Acta 1788: 1593–1599. [DOI] [PubMed] [Google Scholar]
- Rollins-Smith LA. (2017) Amphibian immunity–stress, disease, and climate change. Dev Comp Immunol 66: 111–119. [DOI] [PubMed] [Google Scholar]
- Romansic JM, Waggener AA, Bancroft BA, Blaustein AR (2009) Influence of ultraviolet-B radiation on growth, prevalence of deformities, and susceptibility to predation in Cascades frog (Rana cascadae) larvae. Hydrobiologia 624: 219–233. [Google Scholar]
- Salo HM, Aaltonen TM, Markkula SE, Jokinen EI (1998) Ultraviolet B irradiation modulates the immune system of fish (Rutilus rutilus, Cyprinidae). I. Phagocytes. Photochem Photobiol 67: 433–437. [PubMed] [Google Scholar]
- Salo HM, Jokinen EI, Markkula SE, Aaltonen TM, Penttila HT (2000) Comparative effects of UVA and UVB irradiation on the immune system of fish. J Photochem Photobiol B Biol 56: 154–162. [DOI] [PubMed] [Google Scholar]
- Schloegel LM, Toledo LF, Longcore JE, Greenspan SE, Vieira CA, Lee M, Zhao S, Wangen C, Ferreira CM, Hipolito M, et al. (2012) Novel, panzootic and hybrid genotypes of amphibian chytridiomycosis associated with the bullfrog trade. Mol Ecol 21: 5162–77. [DOI] [PubMed] [Google Scholar]
- Schulenburg H, Kurtz J, Moret Y, Siva-Jothy MT (2009) Introduction. Ecological immunology. Philos Trans R Soc Lond B Biol Sci 364: 3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Searle CL, Belden LK, Bancroft BA, Han BA, Biga LM, Blaustein AR (2010) Experimental examination of the effects of ultraviolet-B radiation in combination with other stressors on frog larvae. Oecologia 162: 237–245. [DOI] [PubMed] [Google Scholar]
- Shen Y, Stanislauskas M, Li G, Zheng D, Liu L (2017) Epigenetic and genetic dissections of UV-induced global gene dysregulation in skin cells through multi-omics analyses. Sci Rep 7: 42646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sifkarovski J, Grayfer L, Andino FD, Lawrence BP, Robert J (2014) Negative effects of low dose atrazine exposure on the development of effective immunity to FV3 in Xenopus laevis. Dev Comp Immunol 47: 52–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siroski PA, Poletta GL, Fernandez L, Ortega HH, Merchant ME (2012) Ultraviolet Radiation on innate immunity and growth of broad-snouted Caiman (Caiman latirostris): implications for facilities design. Zoo Biol 31: 523–533. [DOI] [PubMed] [Google Scholar]
- Sleijffers A, Garssen J, Vos JG, Loveren H (2004) Ultraviolet light and resistance to infectious diseases. J Immunotoxicol 1: 3–14. [DOI] [PubMed] [Google Scholar]
- Snieszko SF. (1974) The effects of environmental stress on outbreaks of infectious diseases of fishes. J Fish Biol 6: 197–208. [Google Scholar]
- Spellman CW, Anderson WL, Bernhard EJ, Tomasi TB (1984) Suppression of antibody responses to topically applied antigens by ultraviolet light irradiation—induction of phototolerance. J Exp Med 160: 1891–1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreevidya CS, Khaskhely NM, Fukunaga A, Khaskina P, Ullrich SE (2008) Inhibition of photocarcinogenesis by platelet-activating factor or serotonin receptor antagonists. Cancer Res 68: 3978–3984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurman LL, Garcia TS, Hoffman PD (2014) Elevational differences in trait response to UV-B radiation by long-toed salamander populations. Oecologia 175: 835–845. [DOI] [PubMed] [Google Scholar]
- Tort L. (2011) Stress and immune modulation in fish. Dev Comp Immunol 35: 1366–1375. [DOI] [PubMed] [Google Scholar]
- Ullrich SE, Byrne SN (2012) The immunologic revolution: photoimmunology. J Invest Dermatol 132: 896–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Mortel TF, Buttemer WA (1998) Avoidance of ultraviolet-B radiation in frogs and tadpoles of the species Litoria aurea, L. dentata and L. peronii. Proc Linnean Soc NSW 119: 173–179. [Google Scholar]
- van der Leun JC. (2004) The ozone layer. Photodermatol Photoimmunol Photomed 20: 159–162. [DOI] [PubMed] [Google Scholar]
- Van Rooij P, Martel A, Haesebrouck F, Pasmans F (2015) Amphibian chytridiomycosis: a review with focus on fungus-host interactions. Vet Res 46: 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Uitregt VO, Wilson RS, Franklin CE (2007) Cooler temperatures increase sensitivity to ultraviolet B radiation in embryos and larvae of the frog Limnodynastes peronii. Global Change Biol 13: 1114–1121. [Google Scholar]
- Venesky MD, Wilcoxen TE, Rensel MA, Rollins-Smith L, Kerby JL, Parris MJ (2012) Dietary protein restriction impairs growth, immunity, and disease resistance in southern leopard frog tadpoles. Oecologia 169: 23–31. [DOI] [PubMed] [Google Scholar]
- Walker SF, Bosch J, Gomez V, Garner TW, Cunningham AA, Schmeller DS, Ninyerola M, Henk DA, Ginestet C, Arthur CP, et al. (2010) Factors driving pathogenicity vs. prevalence of amphibian panzootic chytridiomycosis in Iberia. Ecol Lett 13: 372–382. [DOI] [PubMed] [Google Scholar]
- Wang Y, Zhu W, Shu M, Jiang Y, Gallo RL, Liu Y-T, Huang C-M (2012) The response of human skin commensal bacteria as a reflection of UV radiation: UV-B decreases porphyrin production. PLoS One 7: e47798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedemeyer G. (1970) The role of stress in the disease resistance of fishes. Am Fish Sot Spec Pub 5: 30–35. [Google Scholar]
- Woodhams DC, Ardipradja K, Alford RA, Marantelli G, Reinert LK, Rollins-Smith LA (2007) Resistance to chytridiomycosis varies among amphibian species and is correlated with skin peptide defenses. Anim Conserv 10: 409–417. [Google Scholar]
- Woodhams DC, Bell SC, Bigler L, Caprioli RM, Chaurand P, Lam BA, Reinert LK, Stalder U, Vazquez VM, Schliep K, et al. (2016) Life history linked to immune investment in developing amphibians. Cons Physiol 4: cow025–cow025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xenopoulos MA, Schindler DW (2001) Physical factors determining ultraviolet radiation flux into ecosystems In Cockell CS, Blaustein AR, eds. Ecosystems, Evolution, and Ultraviolet Radiation. Springer-Verlag, New York, pp 36–62. [Google Scholar]
- Zamzow JP. (2004) Effects of diet, ultraviolet exposure, and gender on the ultraviolet absorbance of fish mucus and ocular structures. Mar Biol 144: 1057–1064. [Google Scholar]
- Zamzow JP, Losey GS (2002) Ultraviolet radiation absorbance by coral reef fish mucus: photo-protection and visual communication. Environ Biol Fishes 63: 41–47. [Google Scholar]
- Zamzow JP, Siebeck UE (2006) Ultraviolet absorbance of the mucus of a tropical damselfish: effects of ontogeny, captivity and disease. J Fish Biol 69: 1583–1594. [Google Scholar]
- Zasloff M. (2002) Antimicrobial peptides of multicellular organisms. Nature 415: 389–395. [DOI] [PubMed] [Google Scholar]