Abstract
Strokes involving the artery of Percheron (AOP), an anatomic variant of thalamic vascular supply, are rare. Little is known about the inpatient hospital course for these patients. We retrospectively identified consecutive patients with AOP in their medical charts from a university-based tertiary care hospital from January 1, 2000, to August 15, 2017. A chart review identified demographics, transfer status, in-hospital versus community onset of stroke, emergency medical services (EMS) use, presenting signs/symptoms, time to radiologic diagnosis (from time of presentation to tertiary care hospital or from time of initial symptom onset in an already hospitalized patient), tissue plasminogen activator (tPA) use, intensive care unit (ICU) stays, intubation, length of stay (LOS), and discharge location. After radiologic inclusion/exclusion criteria were applied, 12 patients were included in the study. There were 7 men and 5 women, and the mean age (SD) was 68 (15). Seven were transfers, and 4 had an in-hospital stroke. Of the 8 community-onset strokes, 7 utilized EMS. Mental status changes occurred in 11 of 12 and ocular disturbances in all patients. Time to radiologic diagnosis averaged 1.9 (median = 1.1) days. One patient received tPA. Eight received care in the ICU. Four were intubated. Average LOS was 8.3 days. Four were discharged home, 3 entered inpatient rehabilitation facilities, and 5 entered skilled nursing facilities. In-hospital stroke status further complicates the already challenging diagnosis of AOP infarct, and clinicians must maintain a high suspicion for this rare stroke in order to quickly diagnose and intervene.
Keywords: artery of Percheron, stroke, bilateral thalamic infarct, hospital course
Introduction
One variant of the posterior circulation of the brain, the artery of Percheron (AOP), consists of a single vessel supplying the bilateral thalami. Infarcts in this vessel are uncommon1,2 and present with a challenging clinical picture, including mental status changes and eye movement pathologies. The AOP strokes are thus difficult to recognize and may carry significant morbidity and mortality compared to other forms of stroke.3 In the era of stroke thrombolysis and embolectomy, prompt recognition of this stroke syndrome could greatly improve survival and functional recovery.
Both the clinical symptoms and radiologic features of AOP infarct have been relatively well defined.4-6 However, there are limited data on metrics of inpatient clinical course (eg, length of stay [LOS]) in AOP infarcts. This case series will explore these metrics in a retrospective sample of AOP infarcts. Additionally, it will examine characteristics of initial stroke presentation, including percentage of in-hospital strokes versus community-onset strokes, as in-hospital strokes have been previously shown to add to the challenge of inpatient stroke care and to adversely affect outcomes,7 which is particularly salient in an already rare and complex stroke presentation.
Methods
Radiologic Inclusion and Exclusion Criteria
We retrospectively identified consecutive patients from a university-based tertiary care hospital using a search function within the electronic medical record system to identify patients with AOP in their radiology reports and/or progress notes in the period from January 1, 2000, to August 15, 2017. Charts were reviewed for documentation of probable acute AOP ischemic infarction based on characteristic magnetic resonance diffusion-weighted imaging changes in the bilateral thalami (Figure 1). Patients were excluded if other imaging modalities, that is, computed tomography angiogram or venogram, suggested a more likely etiology, such as venous sinus thrombosis.
Figure 1.
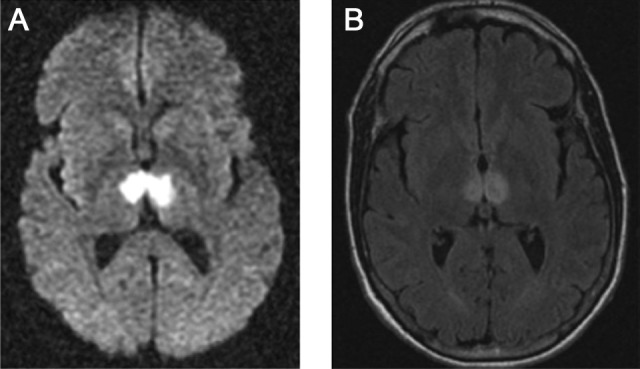
Representative radiologic appearance of artery of Percheron infarction showing signal changes in the anteromedial thalami bilaterally. A, Restriction on magnetic resonance (MR) diffusion-weighted imaging (DWI). B, Signal hyperintensities on fluid-attenuated inversion recovery (FLAIR) MR imaging.
Clinical Data
A retrospective clinical chart review was performed to identify age, sex, race/ethnicity, and risk factors. Risk factors included hypertension, hyperlipidemia, overweightness/obesity, diabetes mellitus, tobacco use, carotid stenosis, history of stroke/transient ischemic attack (TIA), atrial fibrillation or other arrhythmia, coronary artery disease, valvular heart disease, patent foramen ovale, myocardial infarction, atrial septal defect, family history of stroke/TIA, and hypercoagulable state. Transfer status and reason for transfer were recorded. Community-onset versus in-hospital stroke status was recorded, and principal hospital problem was collected for the latter patients. Utilization of emergency medical services (EMS) was recorded for community-onset strokes.
Based on the classification system established by Arauz et al, signs and symptoms during initial clinical presentation were sorted into the following categories8,9: mental status disturbance (MSD), ocular disturbance (OD), language disturbance (LD) or speech disturbance (SD), motor disturbance (MD), cerebellar disturbance (CD), and others. The MSD was graded on a continuum from delirium to coma. The OD included vertical gaze palsy with or without diplopia secondary to skew deviation, anisocoria, or other pupillary abnormalities. The LD included aphasia as well as other linguistic difficulty, such as, anomia, while SD included dysarthria. The MD included any paresis or plegia of the face or upper or lower extremities. The CD included any evidence of dysmetria or ataxia. Other signs included any return of primitive reflexes, such as, Babinski, or anything previously unspecified.
Admission National Institutes of Health Stroke Scale (NIHSS) scores were abstracted from charts where available. If prospective scores were not documented within a chart, a score was retrospectively calculated in the manner previously described by Williams et al10 based on the earliest available note written by a neurologic team. Time to radiologic diagnosis of AOP infarct (from time of presentation to tertiary care hospital or from time of initial symptom onset in an already hospitalized patient) was calculated. The following other data were recorded: treatment with thrombolysis or embolectomy, utilization of intensive care unit (ICU) services, intubation, tracheostomy or percutaneous endoscopic gastrostomy (PEG) tube use; LOS; residual symptoms at the time of discharge; and discharge location. Descriptive statistics were used to describe the patients with stroke. Our university’s institutional review board approved this study. A waiver of informed consent was granted.
Results
Patient Demographics, Risk Factors, Transfer Status, and In-Hospital Versus Community-Onset Stroke Status
Forty-two patients had mention of AOP within their charts. After inclusion/exclusion criteria were applied, 12 patients were found to have an acute AOP infarct. Patient demographics, risk factors, transfer status, and in-hospital versus community-onset stroke status can be found in Table 1. There were 7 men and 5 women, and the mean age (SD) was 68 (15). Seven patients were transferred from outside hospitals. Six patients were transferred for further specialized evaluation of stroke symptoms that had been recognized by the outside institutions. The seventh transfer patient’s initial principal hospital problem was non-ST-segment elevation myocardial infarction with complete heart block, for which she was transferred. Four patients experienced in-hospital strokes, and 8 had community-onset strokes (including both transfer and nontransfer patients). Seven of the 8 community-onset strokes were transported by EMS.
Table 1.
Patient Demographics, Risk Factors, Transfer Status, and In-Hospital Versus Community-Onset Stroke Status.
| Pt | Age (year)/Sex/Ethnicity/Race | Risk Factors | Transfer Pt? | Reason for Transfer | In-Hospital Stroke | Principal Hospital Problem |
|---|---|---|---|---|---|---|
| 1 | 52/F/non-Hispanic white | HTN, HLD, PFO, obesity | Y | Stroke | N | NA |
| 2 | 55/M/non-Hispanic white | HLD, HTN, FH stroke, tobacco use, PFO | Y | Stroke | N | NA |
| 3 | 67/M/non-Hispanic white | HTN, HLD, DM, carotid stenosis, obesity | Y | Stroke | N | NA |
| 4 | 71/M/non-Hispanic white | HTN, HLD, PFO, overweight | Y | Stroke | N | NA |
| 5 | 71/M/non-Hispanic white | HLD, DM, A-fib, CAD | Y | Stroke | N | NA |
| 6 | 81/M/non-Hispanic white | HTN, HLD, CHF, CAD, SSS s/p pacemaker, A-fib, valvular disease | Y | Stroke | N | NA |
| 7 | 89/F/white | CAD, past MI and TIA, A-fib, FH stroke | Y | NSTEMI/complete heart block | Y | NSTEMI |
| 8 | 57/F/non-Hispanic white | HTN, HLD, DM, obesity | N | NA | Y | Pituitary adenoma resection |
| 9 | 76/M/non-Hispanic Asian | HTN, HLD, A-fib, FH stroke, past TIA and stroke | N | NA | Y | Acute rehab s/p MCA stroke |
| 10 | 89/F/non-Hispanic white | HTN, AS, CAD, past MI, A-fib | N | NA | Y | TAVR |
| 11 | 43/F/non-Hispanic white | Tobacco use, PFO, ASD | N | NA | N | NA |
| 12 | 62/M/non-Hispanic white | PFO, hypercoagulable state (Celiac disease) | N | NA | N | NA |
Abbreviations: A-fib, atrial fibrillation; AS, aortic stenosis; ASD, atrial septal defect; CAD, coronary artery disease; CHF: congestive heart failure; DM, diabetes mellitus; F, female; FH, family history; HLD, hyperlipidemia; HTN, hypertension; M, male; MCA, middle cerebral artery; MI, myocardial infarction; N, no; NA, not applicable; NSTEMI, non-ST-segment elevation myocardial infarction; PFO, patent foramen ovale; Pt, patient; rehab, rehabilitation; s/p, status post; SSS, sick sinus syndrome; TAVR, transcatheter aortic valve replacement; TIA, transient ischemic attack; Y, yes.
Presenting Symptoms and Characteristics of Inpatient Hospital Stay
Eleven patients had mental status changes ranging from delirium to coma. Ocular disturbances were present in all patients, with the vast majority (10/12) experiencing vertical gaze palsy. The LD or SD was also present in a majority (7/12), with dysarthria as the most frequent finding. Six patients had prospective admission NIHSS scores recorded in their charts, 5 were retrospectively calculated, and 1 patient did not have a medical note authored by a neurology team and consequently had no NIHSS score. Average initial NIHSS score was 7.3. Time to radiologic diagnosis averaged approximately 1.9 days (median = 1.1 days). Only 1 patient received tissue plasminogen activator (tPA). Eight patients spent time in the ICU (average ICU stay = 2.9 days, range = 1 night to 8 days). Four patients were intubated transiently (average intubation time = 3.7 days). None of the patients required tracheostomy, but 2 patients had PEG tubes placed. The average LOS was 8.3 days (range: 3-14 days). There were no in-hospital deaths. Eleven patients had residual neurologic deficits at the time of discharge, whereas 1 recovered completely after receiving tPA. Four patients were discharged to home, 3 to an inpatient rehabilitation facility, and 5 to skilled nursing facilities (Table 2).
Table 2.
Presenting Symptoms and Characteristics of Inpatient Hospital Stay.
| Pt | MSD | OD | LD/SD | MD | CD | Others | Initial NIHSS | aTime to Radiologic Diagnosis | LOS | ICU LOS | Intubation | Symptoms at Hospital D/C | Discharge Location |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Comatose | VGP, anisocoria, diplopia, right III palsy | N | N | N | L Babinski, unsteady | 1 | 1 day, 9 hours, 46 minutes | 4 days | None | N | Ocular and balance symptoms | Home |
| 2 | Alert | VGP, anisocoria, diplopia | Dysarthria | Y | Ataxia | L Babinski, unsteady | 4 | Missing | 3 days | 1 night | N | Diplopia | Home |
| 3 | Obtunded | VGP, diplopia, left VI palsy | Dysarthria | N | N | N | 3 | 11 hours, 12 minutes | 13 days | None | N | Improving mental status, diplopia, gait instability | SNF |
| 4 | Stuporous | VGP, anisocoria, diplopia | Dysarthria | N | N | R pronator drift | 6 | 5 hours, 56 minutes | 5 days | None | At OSH | Diplopia | Home |
| 5 | Delirious | VGP | Dysarthria | N | N | R pronator drift, bilateral Babinski | 6 | 4 days, 14 hours, 43 minutes | 10 days | 2 days | N | Dysarthria, VGP | SNF |
| 6 | Obtunded | VGP | Dysarthria | N | N | Vertigo, bilateral Babinski, unsteady, dysphagia | 9 | 4 days, 4 hours, 43 minutes | 14 days | 8 days | 5 days | VGP | IRF |
| 7 | Stuporous | Pupils unreactive | N | N | N | Unsteady, incontinence | Missing | 2 days, 4 hours | 6 days | None | N | Gait instability | SNF |
| 8 | Delirious | VGP, anisocoria, diplopia | Dysarthria, anomia | Y | N | R pronator drift | 2 | 11 hours, 14 minutes | 8 days | 3 days | N | Diplopia | SNF |
| 9 | Stuporous | VGP, pupils unreactive | N | Y | N | Vertigo | 9 | 17 hours, 43 minutes | 13 days | 4 days | 4 days | Weakness, VGP | IRF |
| 10 | Obtunded | VGP, diplopia | N | Y | N | Bilateral Babinski | 1 | 4 days, 21 hours, 56 minutes | 7 days | 2 days | N | Diplopia | SNF |
| 11 | Stuporous | Anisocoria | Dysarthria | Y | N | Bilateral Babinski, clonus | 16 | 3 hours, 35 minutes | 5 days | 1 day | N | None | Home |
| 12 | Obtunded | VGP | N | N | N | R Babinski, dysphagia | 23 | 1 day, 1 hour, 52 minutes | 11 days | 3 days | 2 days | Improving mental status, VGP, paucity of speech | IRF |
Abbreviations: CD, cerebellar disturbance; D/C, discharge; ICU, intensive care unit; IRF, inpatient rehabilitation facility; L, left; LD, language disturbance; LOS, length of stay; MD, motor disturbance; MSD, mental status disturbance; N, no; NIHSS, National Institutes of Health Stroke Scale; OSH, outside hospital; OD, ocular disturbance; Pt, patient; R, right; SD, speech disturbance; SNF, skilled nursing facility; VGP, vertical gaze palsy; Y, yes.
aTime to radiologic diagnosis defined as follows: duration of time from arrival at our hospital to radiologic diagnosis of artery of Percheron infarct by our medical staff. If patient was already in hospital for another reason, the clock started at the onset of stroke symptoms.
Discussion
In this retrospective case series of patients with radiologically confirmed diagnosis of AOP, we found that almost two-thirds of the patients were transferred from another hospital and one-third were in-hospital strokes. The relatively high proportion of transfer patients likely limited the number of patients receiving acute stroke treatment, for example, tPA, at our institution. The patients presented here also had a disproportionately high number of in-hospital strokes, compared with the 2.2% to 17% of all-comer ischemic strokes as previously reported by Cumbler, who described the unique challenges of in-hospital stroke including increased comorbidities, distinct risk factors, and greater number of mimics on the differential diagnosis.7 This makes an already difficult diagnosis even more complicated. The AOP infarct is a challenging diagnosis that requires a heightened clinical suspicion for recognition.
The clinical syndrome associated with an AOP territory infarct is relatively well defined and includes features such as mental status changes, vertical gaze paresis, and memory deficits.11 While presenting symptoms in our patients were largely classical for this rare clinical syndrome, these symptoms are not necessarily typical for stroke at large. In addition, many other neurologic disorders (including Wernicke-Korsakoff syndrome, subarachnoid hemorrhage, toxic-metabolic encephalopathy, and focal dyscognitive seizure) have overlapping symptoms. For instance, acute mental status changes without apparent motor deficits are characteristic of subarachnoid hemorrhage, toxic-metabolic encephalopathy, and focal dyscognitive seizures. Memory dysfunction plus confabulation or perseveration can occur in conjunction with eye movement pathologies in AOP infarct,12 which makes differentiation from Wernicke-Korsakoff syndrome particularly difficult. The AOP infarct is thus characterized by both nonspecific symptoms and infrequent presentation, which problematizes even the most astute physician’s efforts to efficiently diagnose it.
The most vital reason for timely recognition of AOP infarct is intervention with tPA. While 1 patient who received tPA made a dramatic recovery, the other 11 patients had persistent neurologic deficits at the time of discharge from the hospital, and only a third were discharged directly home. Furthermore, the mean LOS in our cohort was 8.3 (median = 7.5) days, compared to a previously reported average of 5.3 days for all-comer strokes.13 These measures underscore the significant morbidity secondary to AOP infarct and need for prompt recognition. The average duration of 1.9 days to radiologic diagnosis suggests that significant improvements could be made to the process, but the current study lacks the precision to identify where the greatest delays occur. A further limitation is that we started with medical charts mentioning the AOP. We do not know how many strokes were not recognized as AOP infarct or used other terminology. These limitations largely derive from our study’s retrospective nature. In addition, comparison of data from our AOP infarct cohort to national data for stroke at large is made difficult by our study’s small sample size. Despite these limitations, this case series serves as a reminder to physicians both of the complexity of AOP infarcts and to include this rare diagnosis on the differential for mental status changes.
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Carrera E, Michel P, Bogousslavsky J. Anteromedian, central, and posterolateral infarcts of the thalamus: three variant types. Stroke. 2004;35(12):2826–2831. [DOI] [PubMed] [Google Scholar]
- 2. Pezzini A, Zotto ED, Archetti S, et al. Thalamic infarcts in young adults: relationship between clinical-topographic features and pathogenesis. Eur Neurol. 2002;47(1):30–36. [DOI] [PubMed] [Google Scholar]
- 3. Gentilini M, De Renzi E, Crisi G. Bilateral paramedian thalamic artery infarcts: report of eight cases. J Neurol Neurosurg Psychiatry. 1987;50(7):900–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lazzaro NA, Wright B, Castillo M, et al. Artery of Percheron infarction: imaging patterns and clinical spectrum. AJNR Am J Neuroradiol. 2010;31(7):1283–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kumral E, Evyapan D, Balkir K, et al. Bilateral thalamic infarction. Clinical, etiological and MRI correlates. Acta Neurol Scand. 2001;103(1):35–42. [DOI] [PubMed] [Google Scholar]
- 6. Ben Slamia L, Jemaa HB, Benammou S, Tlili-Graiess K. Occlusion of the artery of Percheron: clinical and neuroimaging correlation. J Neuroradiol. 2008;35(4):244–245. [DOI] [PubMed] [Google Scholar]
- 7. Cumbler E. In-hospital ischemic stroke. Neurohospitalist. 2015;5(3):173–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Arauz A, Patiño-Rodríguez HM, Vargas-González JC, et al. Clinical spectrum of artery of Percheron infarct: clinical-radiological correlations. J Stroke Cerebrovasc Dis. 2014;23(5):1083–1088. [DOI] [PubMed] [Google Scholar]
- 9. de la Cruz-Cosme C, Marquez-Martinez M, Aguilar-Cuevas R, Romero-Acebal M, Valdivielso-Felices P. Percheron artery syndrome: variability in presentation and differential diagnosis. Rev Neurol. 2011;53(4):193–200. [PubMed] [Google Scholar]
- 10. Williams LS, Yilmaz EY, Lopez-Yunez AM. Retrospective assessment of initial stroke severity with the NIH Stroke Scale. Stroke. 2000;31(4):858–862. [DOI] [PubMed] [Google Scholar]
- 11. Jiménez Caballero PE. Bilateral paramedian thalamic artery infarcts: report of 10 cases. J Stroke Cerebrovasc Dis. 2010;19(4):283–289. [DOI] [PubMed] [Google Scholar]
- 12. Reilly M, Connolly S, Stack J, et al. Bilateral paramedian thalamic infarction: a distinct but poorly recognized stroke syndrome. Q J Med. 1992;82(297):63–70. [PubMed] [Google Scholar]
- 13. Hall MJ, Levant S, DeFrances CJ. Hospitalization for stroke in U.S. Hospitals, 1989-2009. NCHS Data Brief. May 2012;95:1–8. [PubMed] [Google Scholar]


