Abstract
Objective
This study measured a variety of within-exposure fear changes and tested the relationship of each with treatment outcomes in exposure therapy.
Method
We coded 459 videotaped exposure tasks from 111 participants in three clinical trials for pediatric OCD (POTS trials). Within exposures, fear level was observed continuously and alongside exposure process. Fear change metrics of interest were selected for relevance to mechanistic theory. Fear decreases were classified by function; non-habituation decreases were associated with observed non-learning processes (e.g., avoidance), whereas habituation decreases appeared to result from an internal and indirect process. Outcomes were post-treatment change in symptom severity, global improvement, and treatment response.
Results
Greater cumulative habituation across treatment was associated with larger reductions in symptom severity, greater global improvement, and increased odds of treatment response. Fear activation, fear variability, and non-habituation fear decreases did not predict any outcomes. Exploratory analyses examined fear changes during habituation and non-habituation exposures; higher peak fear during non-habituation exposures was associated with attenuated global improvement.
Conclusions
Habituation is conceptually consistent with multiple mechanistic theories and should continue to be investigated as a practical marker of initial extinction learning and possible moderator of the relationship between fear activation and outcome. Results support the importance of functional and frequent fear measurement during exposures, and discussion considers implications of these findings for future studies aiming to understand learning during exposure and improve exposure delivery.
Keywords: Exposure therapy, functional analysis, habituation, OCD, mechanism
Introduction
Exposure-based therapies are among the most well-tested and efficacious mental health treatments, with large effect sizes for treating Obsessive-Compulsive Disorder (OCD) and anxiety disorders across the lifespan (Freeman et al., 2014; Higa-McMillan, Francis, Rith-Najarian, & Chorpita, 2016; Hofmann & Smits, 2008). Exposure is the most common practice element in treatment protocols for anxiety (Chorpita & Daleiden, 2009), and there is strong evidence that it is a necessary and primary component of these treatment packages (e.g., Ale, McCarthy, Rothschild, & Whiteside, 2015). Despite clear efficacy, exposure-based cognitive-behavioral therapy (CBT) has not outperformed usual care in effectiveness studies (Weisz et al., 2013). Although there are many barriers present in practice settings, decreased quality of exposure may explain some of the science-to-service gap. When learning CBT for anxiety, community therapists have had difficulty reaching criteria for adherence and competence (Beidas, Barmish, & Kendall, 2009), and this effect is particularly pronounced with exposure (McLeod et al., 2017). Even with intensive training, therapists report exposure as the least sustained of all CBT practice elements, describing decreased confidence about its effectiveness over time (Chu et al., 2015). This raises the possibility that reduced quality leads therapists to observe limited effect in practice and subsequently discontinue the use of exposure. Thus, therapist training approaches that specifically target key elements of exposure quality along with quantity could potentially improve both clinical outcomes and sustainability in practice settings.
The ability to practically measure when the mechanism of exposure has been engaged could help therapists improve quality by providing a proximal therapeutic goal and guiding the use of relevant prescribed/proscribed behaviors (i.e. what the therapist should and should not do). These behaviors could be targeted with training that includes individual feedback about progress toward the mechanistic goal, facilitating therapist awareness and specific actions when course-correction is needed (e.g., Herschell, Kolko, Baumann, & Davis, 2010). Despite these potential advantages, there are no known measures of exposure mechanism that consistently predict clinical outcomes, and none that hold promise for feasible use in training and practice.
Fear change as a practical measure of mechanism
Broadly, the behavioral theory underlying exposure suggests that it works by facilitating fear extinction learning. Specific mechanistic models differ as to the precise nature of that learning, but most outline changes in acute fear during exposures that signal and/or facilitate learning (Craske, Treanor, Conway, Zbozinek, & Vervliet, 2014). Exposure is also used to elicit other forms of acute distress during anxiety and/or OCD treatment (e.g., disgust, incompleteness, intolerance of uncertainty), which we include in this paper under the term ‘fear.’ Assessment of fear change is commonly used within exposure tasks as part of clinical treatment and may be practically advantageous for identifying mechanism engagement in training and practice. Specifically, three within-exposure fear change constructs are theorized to have mechanistic implications: fear activation, fear reduction (or habituation), and fear variability. According to Emotional Processing Theory (EPT), fear activation ‘calls up’ or activates the individual’s association of a stimulus with a set of fear responses and/or feared consequences, and habituation, a form of fear reduction, signals disruption of those associations and facilitates extinction learning (Foa & Kozak, 1986). Inhibitory Learning Theory (ILT) describes fear variability as an important source of context variation that improves later retrieval of the non-threat associations learned during exposure (Craske et al., 2014). However, these within-exposure fear change constructs have inconsistently predicted clinical outcomes. Fear activation and fear reduction have been studied most commonly, with mixed results (Craske et al., 2008; Rupp, Doebler, Ehring, & Vossbeck-Elsebusch, 2017). Fear variability has shown somewhat more consistent relationships with outcome, though it has inconsistently changed outcomes as intended when directly manipulated, and has rarely been studied in diagnostic samples or in the context of clinical treatment (Jacoby & Abramowitz, 2016; Weisman & Rodebaugh, 2018).
Fear Change Metrics
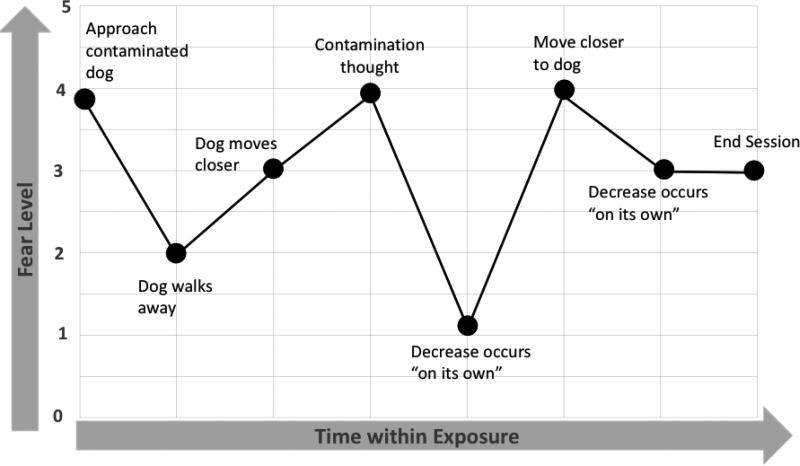
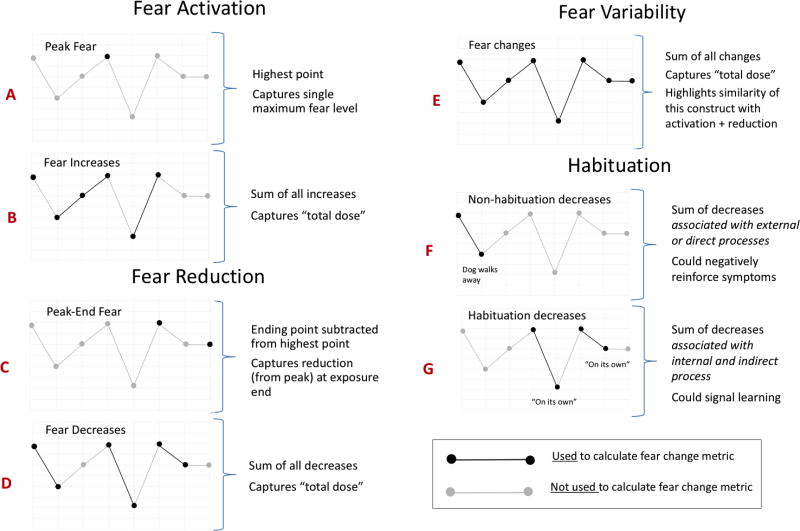
One source of discrepant findings could be the way fear change constructs are sampled and calculated, or their metrics. To illustrate this complex issue clearly, we present a clinical example that pairs the process of an exposure with its corresponding fear trajectory (presented together in Figure 1). We then consider the conceptual implications of different fear change metrics for this example trajectory using a series of graphs (Figure 2). Each graph in figure 2 is labeled with a letter (A–G); these letters link concepts discussed in the text to the graph that illustrates the corresponding metric.
Figure 1. Example exposure: Fear trajectory.
Example Exposure. The chosen exposure task is to stand approximately two feet away from the family dog. As the exposure begins, the patient approaches the contaminated dog with initial observed fear of 4/5. After several minutes, the dog walks away from the patient (attempting to sniff around the office), the patient’s perception of immediate risk lessens, and fear decreases accordingly to 2/5. The therapist shortens the leash and the dog moves closer, with fear increase to 3/5. The patient then has a new contamination thought, becoming concerned that the leash may have brushed his arm inadvertently— and fear increases to 4/5. The patient remains engaged in the exposure even with this thought, and fear eventually decreases on its own to 1/5. The patient then agrees to move closer to the dog by about one foot, and fear increases again to 4/5. Over the next few minutes, fear decreases to 3/5, and then they run out of time in the session. The patient leaves with instructions to resist cleaning his arm or any other contaminated areas for the remainder of the day
Figure 2.
Example exposure: Fear change metrics
Fear activation is usually calculated as the peak fear level during an exposure (graph A in Figure 2; Craske et al., 2008). However, peak fear could miss important instances of fear activation (planned or inadvertent) at other times during exposure. The cumulative sum of all fear increases could be a more appropriate metric if we wish to capture the total ‘dose’ (i.e. every occurrence; graph B). A similar issue is seen with fear reduction in the example exposure. Although usually calculated as ending fear subtracted from peak fear (graph C; Craske et al., 2008), the cumulative sum of all instances of fear decrease (graph D) may better reflect total dose and additionally avoid ‘false negatives’ by including time points other than exposure end. Applying similar logic for calculating fear variability (i.e. cumulative sum of all instances of fear change) demonstrates how this metric would be identical to the combined metrics for fear activation + fear reduction (graph E) and underscores the potential similarity of fear change constructs across ILT and EPT mechanistic models.
Fear Reduction vs. Habituation Metrics
EPT originally used the term ‘habituation’ to describe a form of fear reduction that accompanies exposure learning (e.g., Foa and Kozak, 1986). Though habituation has become somewhat synonymous with fear reduction in the exposure literature, it most precisely implicates a subset of fear reduction (i.e. fear reduction associated with learning). To capture this form specifically, it could be helpful to observe how fear reduction ‘maps on’ to concurrent exposure process, similar to the way our example fear trajectory ‘maps on’ to exposure process in figure 1. This may aid in identifying instances of fear reduction that appear functionally linked to an observed non-learning process versus those that appear to arise indirectly through internal processing. The latter would more plausibly signal learning and is thus consistent with the original use of the term habituation in EPT.
Non-learning causes of fear reduction are many, and include external events or actions (e.g., change in the exposure stimulus, introduction of safety signals, presence of distractors, accommodation, avoidance, escape, rituals) and direct internal actions intended to reduce fear (e.g., distraction, fear/thought suppression, mental rituals, focus on anticipated escape, mental disengagement). All of these events and actions are generally thought to interfere with learning and thus proscribed in exposure practice (e.g., Weisman & Rodebaugh, 2018). However, they occur commonly during exposures in clinical treatment, where the learning context is less tightly controlled than in laboratory paradigms. This is illustrated in the example exposure, where the first instance of fear reduction occurs after the dog walks away—an external event that functions as inadvertent stimulus withdrawal and is most consistent with a non-learning process (non-habituation; graph F in figure 2). In two later instances, fear reduction seems to occur ‘on its own’ and can be categorized as appearing both indirect and internal (graph G). Thus, measures that use a functional approach to separate habituation vs. non-habituation forms of fear reduction and capture all instances of fear change could improve the accuracy and conceptual relevance of fear change metrics for informing real-world treatment.
Summary and Current Study
Fear changes within exposures could be a practical way to detect mechanism engagement and facilitate therapist training but have inconsistently predicted clinical outcome in prior studies—potentially due to use of metrics that imprecisely ‘map on’ to learning. Metrics that incorporate all instances of fear change and use functional analysis to distinguish between habituation and non-habituation forms of fear reduction may be most accurate but have not previously been explicated or tested. In general, few studies have examined multiple types of within-exposure fear change over a course of treatment, and to our knowledge, none have done so in a large clinical trial sample. This study aimed to address these gaps. We completed microanalytic observational coding (Exposure Process Coding System; EPCS; Benito, Conelea, Garcia, & Freeman, 2012) of videotaped exposures for 111 youth with OCD who received exposure-based CBT in one of three randomized, controlled trials (Franklin et al., 2011; Freeman et al., 2014; Pediatric OCD Treatment Study (POTS) Team, 2004). Primary outcomes in the current study were assessed via independent evaluator (IE) at baseline and/or post-treatment and included reduction in OCD symptom severity, global improvement, and treatment response. Specifically, we aimed to examine relationships with outcome for metrics based on all instances of fear change (functionally-defined habituation, non-habituation fear decreases, fear increases, and fear changes; Aim 1), common existing fear change metrics (peak fear, peak-end fear, and exposures ending at zero fear; Aim 2), and the number and duration of exposures (Aim 3). To understand whether the occurrence of habituation is relevant for understanding other fear change metrics, we also explored whether each metric was associated with outcome when occurring with and without habituation in the same exposure (Aim 4).
Method
Original Treatment Trials
Participants in the current study were randomized to receive exposure-based Cognitive Behavioral Therapy (CBT) for OCD, with or without a Selective Reuptake Inhibitor (SRI), in one of three Pediatric OCD Treatment Study (POTS) trials. Treatment occurred at three sites: University of Pennsylvania (Penn), Duke University, or Brown University. All procedures for the original trials and for the current study were approved by relevant IRBs, and all participants provided informed consent; results are found in the original trial publications as cited below. In POTS I (POTS Team, 2004), 112 youth ages 7–17 were randomized to receive CBT alone, medication management (MM) alone, CBT+MM, or pill placebo. In POTS II (Franklin et al., 2011), 124 youth ages 7–17, considered to be partial SRI responders, were randomized to receive MM alone, MM plus instruction in CBT (MM + iCBT), or MM+CBT. In POTS Jr (Freeman et al., 2014), 127 youth ages 5–8 were randomized to receive CBT or relaxation therapy (RT).
Exposures
Trained study therapists administered exposures using published CBT treatment manuals (March & Mulle, 1998; Freeman & Garcia, 2008) to which they were highly adherent (Franklin et al., 2011; Freeman et al., 2014; POTS Team, 2004). These manuals detail treatment ingredients including exposure (11 sessions in POTS I and II and 8 sessions in POTS Jr.), but delivery approach was not explicitly described in the manuals (e.g., exposure duration, intensity, rationale, therapist behaviors during exposure, SUDS sampling frequency).
Participants in the Current Sample
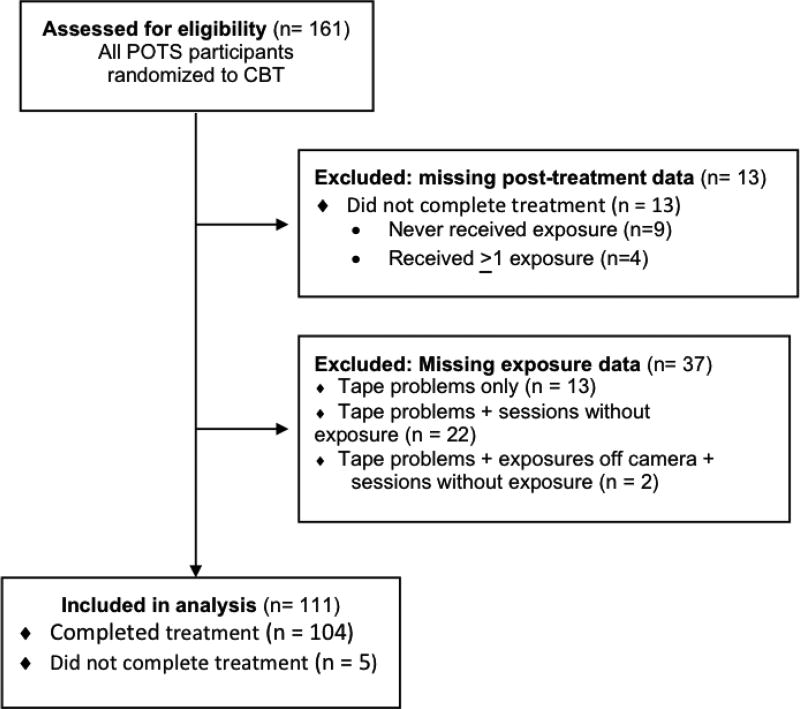
POTS trial participants randomized to a treatment arm containing CBT (N = 161) and completing post-treatment assessment (N = 148) were eligible for the current study. Of these, 111 had at least one video available for EPCS coding that included a visible and audible exposure (figure 3); this final participant sample is representative of original trial participants for baseline symptom severity, age, gender, and medication status (SRI versus none), but with significant differences in proportion of EPCS-coded participants by study and site (Table 1).
Figure 3.
Eligibility Criteria and Included Participants
Table 1.
Characteristics of participants meeting inclusion criteria
| Randomized to CBT N = 161 |
Completed Assessments N = 148a |
Video Available (Current Sample) N = 111 |
||
|---|---|---|---|---|
| Study | POTS I | 34.8% | 34.0%a | 24.1% |
| POTS II | 26.1% | 25.9% | 27.7% | |
| POTS Jr. | 39.1% | 40.1% | 48.2% | |
|
| ||||
| Site | Brown | 26.1% | 25.9% | 33.0% |
| Duke | 35.4% | 39.5% | 38.4% | |
| Penn | 38.5% | 34.7 % a | 28.6% | |
|
| ||||
| Medication Status | SRI | 44.1% | 43.5% | 41.1% |
| No medications | 55.9% | 56.5% | 58.9% | |
|
| ||||
| Gender | Male | 42.2% | 42.9% | 44.6% |
| Female | 57.8% | 57.1% | 55.4% | |
|
| ||||
| Race/Ethnicity | White | 93.2% | 93.2% | 91.2% |
| Black | 2.5% | 2.7% | 3.5% | |
| Other Race | 3.7% | 4.1% | 4.4% | |
| Hispanic | 4.3% | 4.1% | 5.3% | |
|
| ||||
| Comorbidity | Anxiety | 55.9% | 54.7% | 58.4% |
| Externalizing | 23.0% | 23.6% | 26.4% | |
| Tics | 6.2% | 6.8% | 8.0% | |
| Mood | 6.2% | 5.4% | 5.3% | |
|
| ||||
| Age | M = 10.21 | M = 10.26 | M = 10.17 | |
| (SD = 3.26) | (SD = 3.32) | (SD = 3.41) | ||
|
| ||||
| Baseline Severity (CYBOCS) | M = 25.14 | M = 25.06 | M = 25.15 | |
| (SD = 4.48) | (SD = 4.55) | (SD = 4.67) | ||
Sample with completed assessments vs. sample with video available have different proportion of POTS I participants with video available (Χ2 (2) = 21.83, p < .05) and different proportion of Penn participants with video available (Χ2 (2) = 27.00, p < .05)
Videotapes
Session videos were selected with the goal of equally sampling early, middle, and late protocol exposures for each participant. The final sample included 459 exposures sampled from 48.0% of all possible protocol exposure sessions, and equally represent early (32.0%), middle (32.0%), and late (36.0%) sessions. Videos were excluded due to technological problems, damage, or when they could not be located (31.3%), when exposures occurred off camera (e.g., out of the office; 7.4%), or when the sampling goal (having early, middle, and late sessions coded) was met for a given participant (16.3%). Videos were included in ‘total dose’ calculations but were not coded with EPCS when a session was viewed but an exposure did not occur during the session (19.0%). Compared with other sites, Penn had a higher proportion of sampled videos with technological problems, that were damaged, or that could not be located (55.5% at Penn, 16.0% at Duke, and 10.2% at Brown; Χ2 = 217.55, p < .05), resulting in fewer participants in the current sample (Table 1). Compared with other studies, POTS I had a higher proportion of sampled sessions without exposures (39.0% in POTS I, 20.0% in POTS II, and 11.0% in POTS Jr, Χ2 = 47.55, p < .05), resulting in fewer participants with EPCS coding in the current sample. Mean number of sessions included for each participant was 5.03 (SD = 2.50), and mean number of exposures coded with EPCS for each participant was 4.00 (SD = 3.08).
Outcome Measures and Procedures
Outcome measures were administered in the POTS trials by Independent Evaluators (IEs) who were trained to a reliable standard on each measure, blind to treatment condition, and not otherwise involved in the study. All measures were administered at baseline (week 0) and/or post-treatment (week 12 in POTS I and II; week 14 in POTS Jr). OCD symptom severity. The children’s Yale Brown Obsessive Compulsive Scale (CY-BOCS; Scahill et al., 1997) is the gold-standard measure of pediatric OCD severity, with total scores ranging from 0 (no illness) to 40 (extreme severity). The CY-BOCS has excellent psychometric properties, including sensitivity to change in treatment (Scahill et al., 1997). CY-BOCS change from baseline to post-treatment, where lower (negative) values indicate greater symptom reduction, is a primary outcome variable in the current study. Global improvement. The Clinical Global Impression-Improvement scale (CGI; Guy, 1976) is a brief measure of global improvement, rated on a 1 (very much improved) to 7 (very much worse), and has excellent psychometric properties, including sensitivity to change in treatment. Post-treatment CGI score, where lower values indicate greater improvement, is a primary outcome variable in the current study. Treatment response. Participants were classified as treatment responders when having CY-BOCS change ≥30% from baseline to post-treatment and a post-treatment CGI score ≥ 2 (indicating “much improved” or “very much improved”). This method of calculating treatment response was used in POTS Jr. (Freeman et al., 2014), and incorporates dimensional symptom reduction as well as clinically meaningful global change. Treatment response is a primary outcome variable in the current study.
Exposure Process Coding Procedures
EPCS is a microanalytic coding system designed to measure observable therapeutic process with relevance to behavioral theory during exposure. Iterative EPCS development included initial pilot testing in a separate sample of young children with OCD (N = 18), demonstrating good inter-coder reliability and initial predictive validity with treatment outcome (Benito et al., 2012). This pilot revealed that naturalistic SUDS sampling may be too infrequent to sufficiently to capture many fear changes and that fear reduction might often be the result of observed non-learning processes such as accommodation or avoidance; therefore, EPCS was subsequently revised to include observer-rated fear (adapted from Chu, Crocco, et al., 2015) and functionally-defined habituation.
Prior to coding, videos were screened for presence of exposure. When an exposure was present, visible, and audible, EPCS coding was then completed using Noldus Observer software (Noldus XT v. 9.0, 2009), which links coded observations to video and generates a timestamp for each variable. Coders first marked the start and stop of each exposure. Exposure start occurred when at least one of the following was observed: therapist statement that an exposure was starting, clear presentation of an exposure stimulus, or at least two SUDS ratings or other therapist assessment of difficulty regarding a present task. Exposure stop occurred when any of the following were observed: therapist statement that exposure was over, withdrawal of the exposure stimulus (without subsequent reintroduction), or change in session focus without reference to an ongoing exposure task. Number and duration of exposures were calculated using exposure start and stop timestamps. Seventy-three sessions included more than one coded exposure (19.7%); EPCS coding was completed for each exposure task and later combined to create session- and participant-level metrics (see ‘Estimated Total Dose of Fear Change Metrics,’ below).
Codes of interest in the current study (described in the next sections) include patient-rated SUDS, observer-rated fear change, and observer-rated habituation. EPCS codes other events during exposure tasks (e.g., therapist behaviors); these were not used in the current study. Inter-coder reliability, calculated within each exposure (N = 79 double-coded exposures), was conducted in SPSS using ICC(2) and is described for each code of interest, below.
Observer-rated fear
EPCS samples observer-rated fear continuously, starting at the onset of the exposure for the duration of the exposure task. Fear is rated on a 0 to 5 scale, where 0 indicates no fear (no verbal or behavioral evidence of fear) and 5 indicates maximum fear (patient verbally and/or behaviorally expresses highest possible levels of fear and/or appears very uncomfortable, with indicators that may include high levels of stereotypic movement, anxious verbalizations, sweating, crying, or refusals). Inter-coder reliability was good to excellent in this sample for each observed fear level (levels 0–5; ICC range .72-.98). Observer-rated fear was used to calculate the cumulative sum of fear decreases, fear increases, and fear changes, as well as peak fear, peak minus end fear, and the number of exposures ending in zero fear (Table 2).
Table 2.
Fear change metrics and parallel constructs in the current study
| Current Study Metric | Construct | Operational Definition |
|---|---|---|
| Non-Continuous Metrics | ||
|
| ||
| Peak – End Fear | Fear reduction | End fear subtracted from highest fear during the exposure |
| Peak Fear | Fear activation | Highest fear change observed during the exposure |
| Exposure ending at zero fear | Fear reduction | Exposure ended with fear change of zero (yes/no) |
|
| ||
| Continuous Metrics | ||
|
| ||
| Habituation | Habituation | Cumulative sum of fear decreases throughout exposure that were not observed to be the function of a direct or external process (i.e. appear internal and indirect) |
| Non-habituation fear decreases | Fear reduction | Cumulative sum of fear decreases throughout exposure that were observed to be the function of a direct or external process |
| Fear Increases | Fear activation | Cumulative sum of fear increases throughout exposure |
| Fear Changes | Fear variability | Cumulative sum of all fear increases and decreases throughout exposure |
Observer-rated habituation
For each instance of fear decrease, the coder judged whether it was functionally linked to an observed non-learning process that was either external or direct (e.g., rituals, avoidance, accommodation, reduction of task difficulty, change in exposure stimulus, distraction). Although internal processes that directly reduce fear are not observable, these were also included in coder judgment of habituation when verbalized (e.g., stated attempts to ‘make’ fear reduce, focus on anticipation of exposure end, use of mental distraction or rituals). In the absence of the above events, fear decreases were judged to be habituation. Inter-coder reliability in this sample was excellent for judgment of habituation (ICC = .87). This variable was used to calculate the cumulative sum of habituation and to determine whether a given exposure included habituation at any time (habituation exposure) or did not (non-habituation exposure).
Child-rated subjective units of distress (SUDS)
During the exposure, any instance of child-reported SUDS was recorded by the coder on a scale of 0 (no anxiety) to 10 (maximum anxiety). Inter-coder reliability in this sample was excellent for each SUDS level (levels 0–10; ICC range .96-.99). Consistent with results from EPCS pilot testing (Benito et al., 2012), naturalistic SUDS sampling frequency in the present sample was highly variable (22.0% of exposures without SUDS ratings, 10.2% of exposures with one SUDS rating; M = 5.34, SD = 7.22). This precluded accurate calculation of fear change metrics using SUDS; however, SUDS ratings were used when available to provide evidence of construct validity for observer fear ratings. Within each exposure, metrics calculated using coder ratings had large and significant relationships with parallel metrics using SUDS ratings (peak fear, r = .51, p < .05; ending fear, r = .50, p < .05; peak minus end fear, r = .47, p < .05). Comparison of correlation size using Fisher’s r to z transformation revealed that these were significantly larger than all other correlations between non-parallel metrics (e.g., peak fear with end fear; Z range = 2.62–9.27, ps < .05), providing evidence of construct validity.
Coder training
EPCS coders completed initial training to criterion, including guided reading of the EPCS manual, observing trained coders, coding with supervision of trained coders, and independent coding of training videos to criterion (K or ICC > .80 on all fear change metrics). Ongoing training included weekly meetings to discuss EPCS implementation and prevent coder drift, double-coding a minimum of 10% of videos for reliability, and review of an additional 10% of videos by the first author. EPCS coders included four bachelor’s level research assistants and one post-doctoral fellow.
Statistical Analysis
Estimated total dose of fear change metrics
We estimated participant-level total dose (i.e. summed within participant and across treatment) for each metric of interest by first calculating the participant-level rate per session, using all EPCS coded and session viewing data (sessions not EPCS coded because an exposure was not completed contributed a zero value). When more than one exposure occurred in a session, these values were first summed across exposures tasks to create one session-level value. The participant-level total dose was calculated as the product of the rate and the number of protocol exposure sessions attended during treatment.
Predictors with zero total counts
EPCS coding was continuous within the sampling window (i.e. within coded exposures) but could not be completed for all exposures as described above, resulting in intermittent sampling across treatment. Such sampling limits the ability to detect when a zero count represents the true absence of an event versus failure to observe the event during the sampling window (Suen & Ary, 2014). Therefore, for metrics where zero scores represent failure to observe an event for a participant (vs. those representing observed values of zero, such as a mean peak fear of zero), we determined whether participants with zero scores had outcome means that did not line up with the remainder of the function, but a range covering the entire sample—and could therefore include a mixture of true zeros and sampling artifact. The analytic strategy described below reflects a parsing out of participants with zero and nonzero count-based predictor scores, along with hypothesis testing to determine significant discontinuity of outcome means. This is most easily visualized in Figures 4 and 5, where participants with zero scores are presented in red (dark grey) and the remainder of the function in blue (light grey). This approach to modeling zero count independent variables follows the theory underlying widely used zero-inflated count models for dependent variables (i.e. that excess zeros are the result of a separate process and should be modeled separately; Hilbe, 2011).
Analytic approach
The primary analyses were conducted using Generalized Linear Mixed Models (GLMMs) in SAS version 9.4 (SAS Institute, Cary, NC, 2003) using PROC GLIMMIX. A random intercept for study (POTS I, POTS II, or POTS Jr.) was included in most models (GLMM), except for those in which study was of interest and treated as a fixed effect, in which case there were no random effects. Distributions and link functions were chosen based on theory (e.g. Poisson for count) and examination of model residuals (e.g. heterogeneity of variance across linear predictions). The nature of the distribution of the predictor was also examined in relationship to the dependent metric. When preliminary inspection of model plots overlaying individual points revealed deviations from homoscedasticity or normality, we first applied square root, followed by log transformations until deviations were corrected (zero counts were assigned a proxy value, but parameterized separately from the main function). We separately modeled the fixed effects of each fear change metric on: a) CYBOCS change from baseline to post-treatment (Gaussian), b) CGI-I at post-treatment (binomial), and c) responder status at post-treatment (binary). The relationship between predictor and outcome was tested using significance of nonzero slope parameters (Holm-adjusted p-values across the three treatment outcomes). Slope estimates were based on transformed predictors and are not directly interpretable in original units. Additionally, in models parameterizing zero counts separately from non-zero counts, the linear predictor at the lowest non-zero count was compared to the mean of the zero counts as a way to test whether zero counts could contain a mixture of true zeros and sampling artifact.
Results
Fear Change Metrics in All Exposures
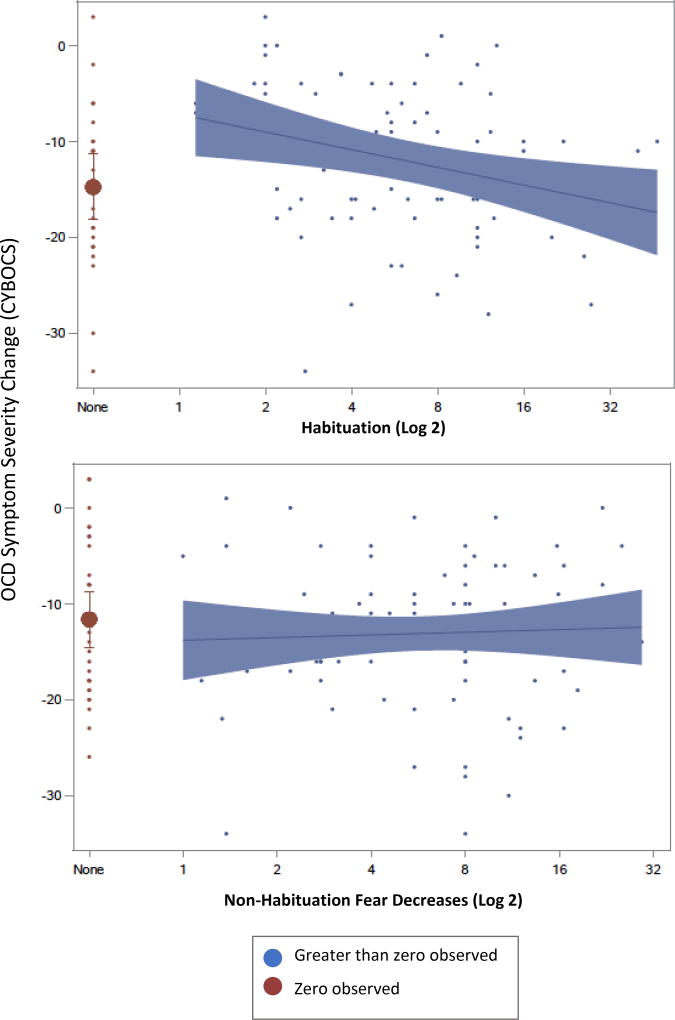
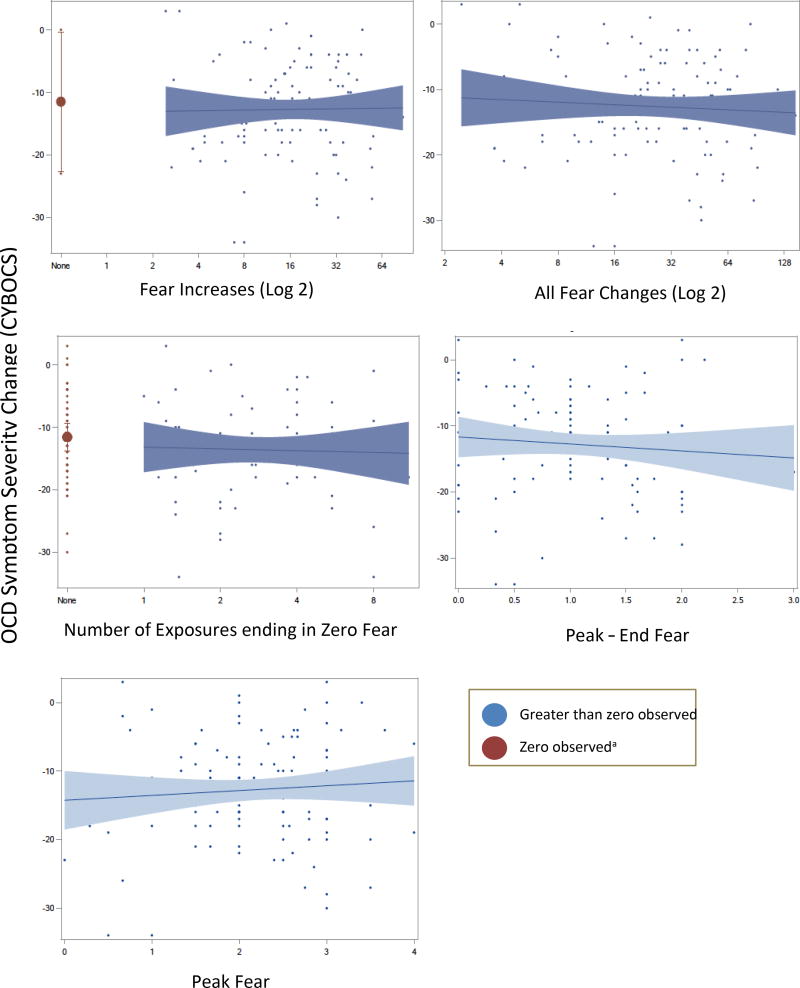
We examined the relationship of fear change metrics (definitions in Table 2) with treatment outcomes; descriptive statistics for each are presented in Table 3. Results are stratified by zero observed versus greater than zero observed as described above. Cumulative sum of habituation significantly predicted change in symptom severity, global improvement, and responder status such that more habituation was related to improved outcomes (Figure 4 and Table 4). By contrast, non-habituation fear decreases did not predict any outcomes (Figure 4 and Table 4). Outcome means for participants with zero observed habituation were significantly discontinuous versus the remainder of the function. For participants with zero observed total dose of habituation, predicted outcomes (based on the function for the remainder of the sample) were significantly different from actual outcomes, indicating poor fit of the function in this subgroup. This was found for all outcomes: CYBOCS change score = 9.41 (SE = 3.06, t(103) = 3.07, p < .05, 95% CI [3.33, 15.48], CGI score = 0.98 (SE = 0.45, t(102) = 2.18, p < .05, 95% CI [0.09, 1.88], and odds of treatment response = −2.23 (SE = 0.93, t(102) = −2.39, p < .05, 95% CI [−4.07, −0.38]). Outcome scores covered the same range as the remainder of the function, suggesting that participants with zero habituation scores could contain a mix of true zeros and sampling artifact (Figure 4). No other fear change metrics significantly predicted treatment outcomes, and no significant differences emerged for zero observed scores of other fear change metrics (Figure 5). Results for all models are presented in Table 4.
Table 3.
Participant-level fear change descriptive statisticsa
| N = 0b | M | SD | Min-Max | |
|---|---|---|---|---|
| Habituation (est. cumulative sum) | 25 | 7.13 | 8.79 | 0–46.75 |
| Non-habituation decreases (est. cumulative sum) | 30 | 6.75 | 8.16 | 0–56.00 |
| Increases (est. cumulative sum) | 2 | 21.79 | 16.64 | 0–88.00 |
| Changes (est. cumulative sum) | 1 | 35.67 | 28.44 | 0–146.67 |
| Exposures Ending at zero (est. total count) | 51 | 1.94 | 2.64 | 0–16.00 |
| Peak – End (mean per exposure) | 11 | 1.08 | .67 | 0–3.00 |
| Peak (mean per exposure) | 1 | 2.21 | .84 | 0–4.00 |
| Number of exposures (est. total count) | -- | 10.05 | 6.02 | 1–38.50 |
| Duration of exposures (est. total minutes) | -- | 84.38 | 58.28 | 3.12–319.15 |
Within participants and across treatment; presented in original units where fear change is rated on a 0 (none) to 5 (maximum) scale
Number of participants with a zero value for the indicated metric
Figure 4.
Change in Symptom Severity as a function of Habituation and Non-Habituation Fear Decreases
Table 4.
Participant-level EPCS-rated fear change metrics predicting treatment outcome
| OCD Symptom SeverityΔa | Global Improvementb | Treatment Responsec | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fear change Metric (slope) | Estimate | SE | t(df) | 95% CI | Estimate | SE | t(df) | 95% CI | Estimate | SE | t(df) | 95% CI |
| Habituation† | −1.85 | 0.69 | −2.69(103) | −3.21, −0.48 | −0.28 | 0.11 | −2.64(103) | −0.49, −0.07 | 0.54 | 0.22 | 2.44(102) | 0.10,0.98 |
| Non-habituation fear decreases | −0.19 | 0.76 | −0.24(103) | −1.69, 1.32 | 0.01 | 0.11 | 0.09(102) | −0.23, 0.23 | −0.02 | 0.22 | −0.09(102) | −0.45, 0.41 |
| Fear increases | 0.11 | 0.67 | 0.17(103) | −1.23, 1.44 | −0.01 | 0.09 | −0.11(102) | −0.20,0.18 | −0.05 | 0.19 | −0.23(102) | −0.43, 0.34 |
| Fear changes | −0.39 | 0.61 | −0.64(103) | −1.60,0.82 | −0.11 | 0.09 | −1.21(102) | −0.27, 0.07 | 0.10 | 0.17 | 0.61(102) | −0.24, 0.44 |
| Exposures ending at zero fear | −0.29 | 1.18 | −0.24(103) | −2.62, 2.05 | −0.23 | 0.18 | −1.23(102) | −0.59,0.14 | 0.09 | 0.36 | 0.24(102) | −0.62, 0.79 |
| Peak – End fear | −1.06 | 1.23 | −0.86(97) | −3.50, 1.39 | −0.12 | 0.18 | −0.68(96) | −0.47, 0.47 | 0.33 | 0.35 | 0.94(96) | −0.37, 1.03 |
| Peak fear | 0.71 | 0.92 | 0.77(104) | −1.11,2.53 | 0.20 | 0.14 | 1.46(103) | −0.08, 0.55 | −0.33 | 0.27 | −1.22(103) | −0.87, 0.42 |
| Number exposures | −1.23 | 0.96 | −1.28(102) | −3.15,0.68 | −0.28 | 0.14 | −2.03(101) | −0.55, −0.01 | 0.36 | 0.27 | 1.35(101) | −0.17,0.90 |
| Duration exposures | −0.55 | 0.72 | −0.77(102) | −1.97,0.87 | −0.13 | 0.10 | −1.29(101) | −0.33, 0.07 | 0.21 | 0.20 | 1.09(101) | −0.17,0.60 |
p < .05 (Holm-adjusted across outcomes);
Significant difference between model predicted and actual values for participants with predictor value of zero;
CYBOCS change baseline to post-treatment (lower values indicate greater symptom reduction);
CGI-I post-treatment (lower values indicate greater global improvement);
Treatment Responder (higher values indicate greater odds of treatment response)
Figure 5. Change in Symptom Severity as a function of Fear Change Metrics.
aParticipants with zero observed scores were modeled separately for metrics based on counts (Fear increases, sum of all fear changes, exposures ending in zero fear) but not for metrics where zero values were observed (peak fear, peak-end fear). One participant had zero fear changes and was removed from that analysis for model convergence
Zero observed vs. > 0 observed habituation groups
We conducted a series of follow-up analyses to determine whether systematic differences between zero observed and greater than zero observed habituation groups could account for the discontinuous function. These analyses used GLM (binary logit model predicting group status) to examine group differences in gender, age, study, site, baseline symptom severity, and medication status; there were no significant differences between the groups, ps > .05. We similarly examined whether these groups differed as a function of EPCS sampling; odds of having zero observed habituations increased with a smaller number of EPCS coded exposures (Χ2 = 6.66, p < .05, OR = 0.68, 95% CI [0.50, 0.91). Given remaining aims related to exploring the relationship of fear change metrics separately in habituation and non-habituation exposures, all subsequent analyses in the present study were also modeled parameterizing those with zero habituations separately. This was accomplished by including a main effect for a dummy coding of zero habituation exposures and an interaction term for this dummy variable and the primary predictor.
SRI status
We conducted a second series of follow-up analyses to explore whether medication status related to fear change metrics. Due to design differences across studies with respect to medication use, these analyses used GLM to examine study by treatment group differences for each metric; there were no significant differences between groups, ps > .05.
Number and Duration of Exposures
We examined whether number and duration of exposures across treatment predicted outcomes (Aim 3). When predicting global improvement, there was a significant interaction for zero versus greater than zero habituation groups (F(101) = 6.94, p < .05), with the direction of slopes suggesting that more exposures were associated with greater global improvement among participants with observed habituation (Table 4), but with reduced global improvement among those with zero observed habituation (Estimate = 0.48, SE = 0.26, t (101) = 1.88, 95% CI [−0.03, 0.99]). However, these slopes were not significant in either group (ps > .05). Total number and duration of exposures did not predict any other outcomes, and there were no significant group interactions in these models (ps > .05).
Fear Change Metrics during Habituation and Non-habituation Exposures
We first calculated exposure-level descriptive statistics to characterize features of habituation and non-habituation exposures. To explore whether fear change metrics relate to patient outcome only when occurring with habituation or without habituation, we conducted participant-level analyses for the following predictor variables occurring during an exposure in which any habituation occurred (habituation exposure) and separately for those during an exposure in which no habituation occurred (non-habituation exposure; Aim 4): fear increases, fear changes, number of exposures ending in zero fear, peak fear, and peak minus end fear. Exposure-level Descriptive Statistics. When compared with non-habituation exposures (N = 241), habituation exposures (N = 218) were characterized by a higher cumulative sum of fear increases and changes, larger reduction from peak to ending fear, higher peak fear, and lower cumulative sum of non-habituation fear decrease (Table 5); there was not a significant difference in the proportion ending with zero fear, p > .05. Participant-level Analyses: Exposures with Habituation. Because participants with zero observed habituations did not have any habituation exposures by definition, they were not included in these analyses, and analyses were therefore not stratified. There were no significant relationships of any fear change metrics with treatment outcome (ps > .05) during exposures with habituation (Table 6). Participant-level Analyses: Exposures without habituation. Most participants with at least one habituation across treatment also had some number of individual exposures that did not contain habituation; therefore, these and participants with zero observed habituation are included in the following analyses and results are stratified. Results for those with greater than zero habituations are presented in Table 6. Results for peak fear: Higher peak fear during exposures without habituation predicted reduced global improvement among participants with at least one observed habituation (Table 6); there was a significant interaction by group (F(74) = 4.84, p < .05), but the effect among participants with zero observed habituations was not significant (t (74) = −1.24, p > .05). Peak fear did not significantly predict other outcomes (ps > .05). Results for number of exposures: When predicting global improvement, there was a significant group interaction, F(74) = 5.47, p < .05. Opposite direction of effects by group suggests that more exposures related to attenuated global improvement among participants with zero observed habituation (Estimate = 0.48, SE = 0.26, t(101) = 1.88, 95% CI [−0.03, 0.99]), but related to greater global improvement among those with greater than zero habituation observed (Table 6). However, slope estimates were not significant in either group. Number of exposures did not significantly predict other outcomes (ps > .05). For all other predictors (fear changes, exposures ending at zero fear, peak-end fear, and duration of exposures) main effects and interactions were not significant for any outcomes (ps > .05).
Table 5.
Exposures with and without habituation: Exposure-level descriptive fear change statisticsa and comparison
| Habituation Exposuresb | Non- Habituation Exposuresc | Comparison | ||||||
|---|---|---|---|---|---|---|---|---|
| M | SD | Min-Max | M | SD | Min-Max | t(df) | 95% CI (difference) |
|
| Habituation (sum) | 1.89 | 1.32 | 0–9 | -- | -- | -- | -- | -- |
| Non-habituation decreases (sum) | 0.67 | 1.24 | 0–8 | 0.98 | 1.45 | 0–7 | 2.44(457) | 0.60, 0.56 |
| Increases (sum) | 3.54 | 1.98 | 0–12 | 2.12 | 1.98 | 1–9 | −7.63(457) | −1.78, −1.05 |
| Changes (sum) | 6.10 | 3.27 | 1–23 | 3.11 | 3.28 | 0–15 | −9.14 (457) | −3.63, −2.35 |
| Peak – End | 1.59 | 0.77 | 0–4 | 0.58 | 0.81 | 0–4 | −13.55(449) | −1.15, −0.86 |
| Peak | 2.73 | 0.89 | 1–5 | 1.91 | 1.36 | 0–5 | −7.51(449) | −1.03, −0.61 |
| Duration (minutes) | 11.96 | 8.01 | 1.05–42.93 | 9.55 | 8.07 | 0.32–46.83 | −3.21(457) | −3.89, −0.94 |
p < 05
Original units, fear change rated on a 0 (none) to 5 (maximum) scale;
N = 218 habituation exposures;
N = 241 exposures without habituation
Table 6.
Participant-level EPCS-rated fear change metrics occurring during habituation and non-habituation exposures: Exploratory relationships with outcome
| OCD Symptom SeverityΔa | Global Improvementb | Treatment Responsec | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fear Change Metric (slope) | Estimate | SE | t(df) | 95% CI | Estimate | SE | t(df) | 95% CI | Estimate | SE | t(df) | 95% CI |
| Habituation Exposuresd | ||||||||||||
|
| ||||||||||||
| Fear increases | −1.15 | 0.61 | −1.89(82) | −2.35, 0.06 | −0.15 | 0.09 | −1.69(82) | −0.33, 0.03 | 0.33 | 0.18 | 1.81(82) | −0.03, 0.69 |
| Fear changes | −1.14 | 0.63 | −1.82(82) | −2.39, 0.11 | −0.15 | 0.10 | −1.55(82) | −0.34, 0.04 | 0.36 | 0.19 | 1.88(82) | −0.02, 0.73 |
| Exposures Ending at zero fear | −1.02 | 0.72 | −1.42(82) | −2.44, 0.41 | −0.21 | 0.12 | −1.79(82) | −0.45, 0.02 | 0.36 | 0.24 | 1.51(82) | −0.11, 0.84 |
| Peak – End fear | −0.50 | 1.26 | −0.40(82) | −3.01, 2.00 | −0.11 | 0.19 | −0.59(82) | −0.48, 0.26 | 0.56 | 0.38 | 1.46(82) | −0.21, 1.32 |
| Peak fear | 0.38 | 1.04 | 0.37(82) | −1.70, 2.46 | 0.12 | 0.15 | 0.75(82) | −0.19, 0.42 | 0.02 | 0.30 | 0.05(82) | −0.59, 0.62 |
| Number habituation exposures | −1.46 | 0.89 | −1.65(82) | −3.22, 0.30 | −0.22 | 0.14 | −1.63(82) | −0.50, 0.05 | 0.35 | 0.27 | 1.29(82) | −0.19, 0.88 |
| Duration habituation exposures | −0.52 | 0.56 | −0.93(82) | −1.64, 0.59 | −0.08 | 0.08 | −0.94(82) | −0.25, 0.09 | 0.23 | 0.16 | 1.39(82) | −0.10, 0.55 |
|
| ||||||||||||
| Non-Habituation Exposurese | ||||||||||||
|
| ||||||||||||
| Fear increases | 1.03 | 0.64 | 1.61(75) | −0.24, 2.30 | 0.22 | 0.10 | 2.15(74) | 0.02, 0.42 | −0.37 | 0.21 | −1.74(74) | −0.79, 0.05 |
| Fear changes | 0.68 | 0.57 | 1.19(74) | −0.46, 1.81 | 0.15 | 0.09 | 1.71(74) | −0.03, 0.32 | −0.23 | 0.17 | −1.34(73) | −0.58, 0.11 |
| Exposures Ending at zero fear | −0.90 | 0.81 | −1.10(75) | −2.52, 0.73 | −0.27 | 0.13 | −2.06(74) | −0.53, −0.01 | 0.34 | 0.25 | 1.38(74) | −0.15, 0.83 |
| Peak – End fear | −2.87 | 1.45 | −1.98(75) | −5.76, 0.01 | −0.22 | 0.22 | −1.00(74) | −0.67, 0.22 | 0.51 | 0.45 | 1.15(74) | −0.37, 1.40 |
| Peak fear‡ | 0.66 | 0.86 | 0.77(75) | −1.05, 2.36 | 0.34 | 0.12 | 2.82(74) | 0.10, 0.57 | −0.51 | 0.24 | −2.09(74) | −0.99, −0.02 |
| Number non-habituation exposures‡ | 0.46 | 1.20 | 0.38(75) | −1.93, 2.84 | −0.17 | 0.17 | −0.98(74) | −0.50, 0.17 | 0.04 | 0.32 | 0.13(74) | −0.60, 0.68 |
| Duration non-habituation exposures | 0.19 | 0.80 | 0.24(75) | −1.40, 1.79 | 0.02 | 0.11 | 0.19(74) | −0.20, 0.24 | −0.04 | 0.21 | −0.16(74) | 0.46, 0.39 |
p < .05 (Holm-adjusted across outcomes);
Significant interaction between zero habituations observed versus greater than zero habituations observed;
CYBOCS change baseline to post-treatment (lower values indicate greater symptom reduction);
CGI-I post-treatment (lower values indicate greater global improvement);
Treatment Responder (higher values indicate greater odds of treatment response);
N = 86 with at least one exposure containing habituation;
N = 81 with at least one exposure that did not contain habituation
Discussion
Fear Change Metrics
Results of this study support within-exposure habituation--fear decreases that appear to occur indirectly through an internal process--as a unique predictor of OCD symptom reduction, global improvement, and treatment response in youth. By contrast, non-habituation fear decreases—those which were functionally linked to an observed non-learning process--did not predict any outcomes. These findings underscore the importance of using functional analysis to identify fear decreases that appear most consistent with learning. Fear reduction that was calculated based on a limited number of instances (peak-end fear) also did not predict outcomes, even during habituation exposures, suggesting that habituation regularly occurs throughout exposure tasks and that sampling only at the end may be insufficient. All other fear change metrics failed to predict outcomes, including indices of fear activation (cumulative sum of fear increases; peak fear), variability (cumulative sum of all fear changes) and overlearning (number of exposures ending in zero fear).
Together, results suggest that functionally-defined habituation within exposure tasks might serve to signal initial extinction learning in exposure therapy. This contrasts the results of prior studies (Jacoby & Abramowitz, 2016; Weisman & Rodebaugh, 2018), a difference that might be explained by methods used in the current study (e.g., treatment-seeking clinical sample, multi-session course of exposure therapy, symptom-specific and global clinical outcomes, new fear change metrics). Future studies should aim to understand how this habituation metric corresponds to indices of within-trial learning during fear extinction paradigms, as well as how it relates to post-exposure phases of extinction learning (i.e. consolidation and later retrieval of extinction memories). Relatedly, present findings could inform future studies of augmentation strategies that enhance consolidation or retrieval (e.g., D-cycloserine, methylene blue); effects of such agents appear to depend upon initial learning within exposures (Telch et al., 2014; Smits et al., 2013). Future studies should also examine the rate and predictive value of this metric among adults, for whom anxiety disorders and OCD are associated with deficits in extinction learning (e.g., Craske et al., 2014; McLaughlin et al., 2015). The nature of extinction learning deficits among youth with these disorders is poorly understood and is a critical area for further investigation, especially in light of evidence that adolescents experience normative impairment in extinction learning (particularly related to context-dependent retrieval of extinction memories, Schnechner et al., 2014). Results of the current study may serve as an initial step towards future work aiming to understand the pathways of extinction learning across development during exposure therapy.
Exposure Dose
The total number and duration of exposures across treatment did not predict any outcomes, which is consistent with results of some prior studies (Hedtke, Kendall, & Tiwari, 2009; Kircanski & Peris, 2015), and may suggest that an individual’s dose of exposure is not best conceptualized as the number or length of exposure tasks. Ultimately, it could be more accurate to consider dose as the degree to which exposures provide a relevant and/or potent learning experience. We measured dose as the cumulative sum of all instances of fear change, yet there may be many ways to conceptualize this and future studies should continue to explore these. We are also careful to note that this study used a clinical trial sample where exposure was the primary treatment ingredient. Recent evidence suggests that more exposure tasks robustly predict improved outcomes when using CBT packages that contain a smaller proportion of sessions with exposure (Peris et al., 2017). Additionally, exposure is used infrequently in practice settings (Whiteside et al., 2016), where increasing the number and/or length of exposures is likely to be an important goal.
Exposures with vs. without habituation
Exploratory findings indicate that fear activation could function differently depending upon whether habituation also occurs during the exposure. Higher peak fear during exposures without habituation predicted poorer global improvement— suggesting that higher fear levels without habituation might produce suboptimal improvement in exposure therapy. This is consistent with some prior studies showing that higher peak fear during exposures predicts higher post-treatment fear (e.g., Kamphius & Telch, 2000), including when models account for fear reduction (e.g., a non-significant trend reported by Wolitzky-Taylor & Telch, 2009) —though it contrasts other previous findings (as reviewed in Craske et al., 2008). However, we note that habituation exposures were characterized by higher peak fear and greater fear increases (vs. exposures without habituation), lending support to the idea that fear activation should be understood alongside habituation. Future studies will need to examine fear activation more closely, with particular focus on how and whether it should be manipulated for optimizing exposure outcome.
Exposure Characteristics
Examining descriptive statistics for fear change metrics in this sample sheds light on the characteristics of clinical trial exposures, which in many ways differ from frequently discussed features of ‘traditional’ exposure (i.e. those in which delivery is presumed to be guided by EPT). Traditional exposure is thought to aim for maximum fear reduction in a gradual and linear manner, and to end after achieving this goal (Craske et al., 2008; Abramowitz & Arch, 2014, Jacoby & Abramowitz, 2016). However, most exposures in our sample included multiple fear increases beyond initial activation (i.e. multiple peaks) and nearly 50% of participants never had any observed exposures ending with zero fear. Overall, only 14.2% of exposures were characterized by a single peak followed by linear fear decrease. These features seem more consistent with newer literature describing exposures guided by ILT (Craske et al., 2014), and have implications for designing studies that test novel ways to improve exposure outcomes. It may be of limited value to test new approaches against comparison conditions that maximize ‘traditional’ exposure characteristics (e.g., gradual and linear fear reduction), to the degree that those characteristics do not represent the exposures that were conducted during original clinical trials. Improving exposure outcomes is critically important, and future studies should aim to accurately understand clinical trial exposures so that we may design comparison conditions against which to test the relative improvement of novel approaches.
The discrepancy between exposure characteristics in this sample and ‘traditional’ exposure elements could be explained by several factors. First, it is possible, though seemingly unlikely, that fear changes are primarily driven by individual patient variability and not influenced by exposure delivery. Second, there could be differences in the exposure approaches used among therapists in this sample. Although we have not formally assessed this in the current study, our experience coding these videos suggests this to be true. Despite therapists being highly adherent at the ‘ingredient’ level (e.g., completed an exposure or did not), treatment manuals rarely integrate theoretical principles with procedures, nor do they typically address specific process behaviors (i.e. what to say or do during the exposure). Specific and reproducible therapist behaviors that contribute to exposure quality are difficult to disseminate and may most accurately exist ‘in the heads’ of treatment or theory developers. Broadly speaking, this difficulty may contribute to therapists (and researchers) misunderstanding both the intended therapist behaviors in a given mechanistic model and whether actual therapist behaviors are entirely consistent with what was intended. It will be critical for the field to use clear operational definitions for these so that they might be communicated and reproduced accurately in both research and practice.
Clinical Implications
Habituation ratings in the present study were based on idiosyncratic functional analysis of an individual’s symptoms and each exposure task, which is a core procedure in exposure therapy. Clinicians can use functional analysis in this manner to determine when an instance of fear decrease appears internally-driven and indirect, thus considered habituation. Importantly, clinicians should note these instances as they occur throughout exposure, and not only at the end. Recognizing habituation could aid in early detection of treatment response or be used to inform exposure planning, although we are careful to note that this study did not test these specific uses. These results also provide clinicians with information about the range of fear change processes occurring during exposures for pediatric OCD and describe patterns of these relating to outcome. However, we emphasize that we did not investigate which therapeutic processes or other factors cause fear changes to occur. This warrants considerable attention in future studies before we might draw conclusions about the best way to trigger fear changes in practice or research. As an example, in one analog study, fear variability only predicted outcome after partialling out the effects of its direct manipulation (Kircanski et al., 2012). It is possible that direct attempts to produce some fear changes could inadvertently alter function—for example, by introducing an external cause of fear reduction (e.g., ending exposures at a predetermined time, resulting in anxiety reduction via escape for some trials and/or individuals). It is also possible that optimal fear changes will vary by individual or situation, for example, if increasing fear is prescribed at low or moderate fear levels but proscribed when fear is high or when habituation seems less likely to occur (e.g. with observed difficulty resisting rituals). These examples illustrate the complex task of understanding how fear changes could be manipulated, and to what effect. Future studies will need to answer these questions before making more specific recommendations about changing practice.
Limitations and Future Directions
Observational Measurement
Our goal was to identify a possible marker of exposure learning, yet behavioral observation has limitations for capturing internal change. Additionally, fear measures across different units of analysis (e.g., behavioral, physiological, self-report; National Advisory Mental Health Council, 2016) can relate differentially to outcome. For example, in a recent study of anxious youth receiving CBT, pre-treatment reduction in physiologic arousal during an extinction task predicted later treatment response, yet reduction in youth-reported negative stimulus valence did not (Waters & Pine, 2016). Correlations between observational and self-report fear metrics were large in the current sample, yet far from entirely overlapping. This may relate to coding anchors used in EPCS, which incorporate behavioral elements (e.g., facial expressions, avoidance actions, verbalizations) and physiologic elements (e.g., trembling, sweating). Future studies will need to continue examining the overlap of fear measures across respondents and units of analysis to determine which combination of these best predicts outcomes. Limitations notwithstanding, there are no known measures or methods that directly assess learning, behavioral observation has a high degree of practical utility for clinical application, and present results initially establish the predictive validity of this approach with treatment outcome.
Trial Design
The POTS trials were designed to detect treatment effects and videos were originally collected for randomly sampling therapist adherence rather than coding every exposure. Despite missing video data, the current sample is representative of original trial participant baseline characteristics, but with differences in sample proportions by site and study. We emphasize that our goal was to examine fear change processes in relation to clinical outcome, rather than to account for results of the original trials. Also, each trial was designed to answer different questions about medication use, and this must be considered when interpreting our exploratory finding that medication use does not relate to fear change processes. These results are likely influenced by the fact that most youth on medication in the current sample were participants in POTS II, thus were also ‘partial responders’ seeking treatment even after optimizing medications. Future studies should continue to examine medication status in relation to fear changes during exposure.
OCD Sample
CBT protocols for OCD often emphasize exposure as the primary ‘ingredient,’ making them well-suited for detecting variation within exposures that relates to clinical outcome. However, POTS protocols included other treatment ingredients (e.g., family training, cognitive skills, reward system) and results of this study do not inform the relative contribution of those. Nevertheless, this underscores the potentially important role of processes within exposure that significantly relate to outcome even when other (presumably active) treatment ingredients are present. Additionally, due to symptom heterogeneity in OCD, exposures in this sample likely included a range of targets that could over-represent some non-fear distress constructs (e.g., incompleteness) compared to exposures for anxiety disorders. However, evidence suggests that such constructs also respond to exposure-based treatment (e.g., Coles & Ravid, 2016) and they are commonly targeted during exposures for other anxiety disorders (e.g., disgust in phobias, intolerance of uncertainty and perfection in GAD). Future studies should aim to understand how fear and non-fear distress targets differ with respect to exposure process and outcomes.
Participants with zero habituation
Outcome means for these participants were discontinuous from the remainder of the habituation function, indicating possible artifact related to the limitations of intermittent sampling (across treatment) for capturing the absence of behavioral events (Suen & Ary, 2014). Follow-up analyses support this, showing that having fewer coded exposures was associated with increased likelihood of having zero observed habituations. No other baseline characteristics, treatment variables, or outcome variables were related. Despite this, these participants could differ on some key variable that was not formally assessed. It is also possible that they represent a subset who achieved gains through a distinct exposure mechanism. However, the relationship between number of exposures and global improvement was significantly different for this group, with the direction suggesting that more exposures relate to attenuated global improvement. Although this slope estimate did not reach statistical significance, it suggests that improvements in this subgroup would not have occurred via exposure. More plausibly, results were influenced by other treatment ingredients or by sampling artifact and should thus be interpreted with caution. Nonetheless, we opted to retain these participants in analysis to facilitate discussion of methodological and/or theoretical underpinnings and so that future studies might examine whether similar subgroup findings emerge. Future studies should also be prospectively designed to examine fear change processes and to explore the optimal sampling window and frequency for capturing habituation.
Most importantly, this study was designed to identify specific fear changes that could serve as a proximal and practical marker of mechanism engagement (i.e. learning) during treatment. It was not designed to differentiate among internal learning processes, nor to compare fear change metrics for purposes of demonstrating the superiority of a particular mechanistic model. Although we retained the term habituation to be both parsimonious and consistent with existing mechanistic theory, there are multiple ways this observed change might be conceptualized. It could be viewed as fear reduction occurring through fear toleration (ILT and Acceptance and Commitment Therapy models), as a way to disconfirm beliefs (Cognitive model) or violate expectancy (ILT model) about the permanence of fear, or simply as ensuring that exposures do not end with escape. Ultimately, the operational definition seems most valuable—it is a form of fear reduction that appears to occur through an indirect and internal process.
Overall, present results suggest that observed habituation could signal mechanism engagement in both research and practice settings. However, we did not experimentally manipulate habituation and future studies will be needed to fully test 1) whether and how it can be manipulated, or its ‘levers’, and 2) whether it indeed changes outcomes once successfully manipulated. We also note that the rationale used to frame this paper is in line with our ultimate goal of improving exposure therapy training and practice, yet this study used a clinical trial sample and did not yet seek to understand relevant therapist behaviors or training approaches. Subsequent studies in this experimental therapeutics series hope to: 1) identify specific therapist behaviors that relate to habituation and clinical outcome, 2) use results to develop a practical therapist feedback tool, and 3) test whether adding this tool to gold-standard training can change therapist behaviors during exposures for OCD and anxiety in a practice setting.
Public Health Significance.
This study highlights fear change within exposures as an important process that relates to outcomes in exposure therapy for youth with OCD.
Acknowledgments
This article received funding from the National Institute of Mental Health, R21MH096828, R33MH096828 & R01MH112516, awarded to Dr. Kristen Benito.
References
- Abramowitz JS, Arch JJ. Strategies for Improving Long-Term Outcomes in Cognitive Behavioral Therapy for Obsessive-Compulsive Disorder: Insights From Learning Theory. Cognitive and Behavioral Practice. 2014;27(1):20–31. [Google Scholar]
- Ale CM, McCarthy DM, Rothschild LM, Whiteside SPH. Components of Cognitive Behavioral Therapy Related to Outcome in Childhood Anxiety Disorders. Clinical Child and Family Psychology Review. 2015;75(3):240–251. doi: 10.1007/s10567-015-0184-8. [DOI] [PubMed] [Google Scholar]
- Beidas RS, Barmish AJ, Kendall PC. Training as usual: Can therapist behavior change after reading a manual and attending a brief workshop on cognitive behavioral therapy for youth anxiety? The Behavior Therapist / AABT. 2009;32(5):97. [Google Scholar]
- Benito KG, Conelea C, Garcia AM, Freeman JB. CBT Specific Process in Exposure-Based Treatments: Initial Examination in a Pediatric OCD Sample. Journal of Obsessive-Compulsive and Related Disorders. 2012;7(2):77–84. doi: 10.1016/j.jocrd.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chorpita BF, Daleiden EL. Mapping evidence-based treatments for children and adolescents: application of the distillation and matching model to 615 treatments from 322 randomized trials. Journal of Consulting and Clinical Psychology. 2009;77(3):566–579. doi: 10.1037/a0014565. [DOI] [PubMed] [Google Scholar]
- Chu BC, Crocco ST, Arnold CC, Brown R, Southam-Gerow MA, Weisz JR. Sustained Implementation of Cognitive-Behavioral Therapy for Youth Anxiety and Depression: Long-term Effects of Structured Training and Consultation on Therapist Practice in the Field. Professional Psychology, Research and Practice. 2015;46(1):70–79. doi: 10.1037/a0038000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles ME, Ravid A. Clinical presentation of not-just right experiences (NJREs) in individuals with OCD: Characteristics and response to treatment. Behaviour Research and Therapy. 2016;87:182–187. doi: 10.1016/j.brat.2016.09.013. [DOI] [PubMed] [Google Scholar]
- Craske MG, Kircanski K, Zelikowsky M, Mystkowski J, Chowdhury N, Baker A. Optimizing inhibitory learning during exposure therapy. Behaviour Research and Therapy. 2008;46(1):5–27. doi: 10.1016/j.brat.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Craske MG, Treanor M, Conway CC, Zbozinek T, Vervliet B. Maximizing exposure therapy: an inhibitory learning approach. Behaviour Research and Therapy. 2014;58:10–23. doi: 10.1016/j.brat.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foa EB, Kozak MJ. Emotional processing of fear: exposure to corrective information. Psychological Bulletin. 1986;99(1):20–35. [PubMed] [Google Scholar]
- Franklin ME, Sapyta J, Freeman JB, Khanna M, Compton S, Almirall D, March JS. Cognitive behavior therapy augmentation of pharmacotherapy in pediatric obsessive-compulsive disorder: the Pediatric OCD Treatment Study II (POTS II) randomized controlled trial. JAMA: The Journal of the American Medical Association. 2011;306(11):1224–1232. doi: 10.1001/jama.2011.1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman J, Sapyta J, Garcia A, Compton S, Khanna M, Flessner C, Franklin M. Family-based treatment of early childhood obsessive-compulsive disorder: the Pediatric Obsessive-Compulsive Disorder Treatment Study for Young Children (POTS Jr)--a randomized clinical trial. JAMA Psychiatry. 2014;71(6):689–698. doi: 10.1001/jamapsychiatry.2014.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedtke KA, Kendall PC, Tiwari S. Safety-seeking and coping behavior during exposure tasks with anxious youth. Journal of Clinical Child and Adolescent Psychology. 2009;38(1):1–15. doi: 10.1080/15374410802581055. [DOI] [PubMed] [Google Scholar]
- Herschell AD, Kolko DJ, Baumann BL, Davis AC. The role of therapist training in the implementation of psychosocial treatments: a review and critique with recommendations. Clinical Psychology Review. 2010;30(4):448–466. doi: 10.1016/j.cpr.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higa-McMillan CK, Francis SE, Rith-Najarian L, Chorpita BF. Evidence Base Update: 50 Years of Research on Treatment for Child and Adolescent Anxiety. Journal of Clinical Child and Adolescent Psychology: The Official Journal for the Society of Clinical Child and Adolescent Psychology, American Psychological Association, Division 53. 2016;45(2):91–113. doi: 10.1080/15374416.2015.1046177. [DOI] [PubMed] [Google Scholar]
- Hilbe JM. Modeling Count Data. International Encyclopedia of Statistical Science. 2011:836–839. [Google Scholar]
- Hofmann SG, Smits JAJ. Cognitive-behavioral therapy for adult anxiety disorders: a meta-analysis of randomized placebo-controlled trials. The Journal of Clinical Psychiatry. 2008;69(4):621–632. doi: 10.4088/jcp.v69n0415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacoby RJ, Abramowitz JS. Inhibitory learning approaches to exposure therapy: A critical review and translation to obsessive-compulsive disorder. Clinical Psychology Review. 2016;49:28–40. doi: 10.1016/j.cpr.2016.07.001. [DOI] [PubMed] [Google Scholar]
- Kamphius JH, Telch MJ. Effects of distraction and guided threat reappraisal on fear reduction during exposure-based treatments for specific fears. Behavior Research and Therapy. 2000;38:1163–1181. doi: 10.1016/s0005-7967(99)00147-3. [DOI] [PubMed] [Google Scholar]
- Kircanski K, Mortazavi A, Castriotta N, Baker AS, Mystkowski JL, Y R, Craske MG. Challenges to the traditional exposure paradigm: variability in exposure therapy for contamination fears. Journal of Behavior Therapy and Experimental Psychiatry. 2012;43(2):745–751. doi: 10.1016/j.jbtep.2011.10.010. [DOI] [PubMed] [Google Scholar]
- Kircanski K, Peris TS. Exposure and response prevention process predicts treatment outcome in youth with OCD. Journal of Abnormal Child Psychology. 2015;43(3):543–552. doi: 10.1007/s10802-014-9917-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- March JS, Mulle K. OCD in Children and Adolescents: A Cognitive-Behavioral Treatment Manual. New York, NY: Guilford Press; 1998. [Google Scholar]
- McLaughlin NA, Strong D, Abrantes A, Garnaat S, Cerny A, O’Connell C, Fadok R, Spofford C, Rasmussen S, Milad MR, Greenberg BD. Extinction retention and fear renewal in a lifetime obsessive-compulsive disorder sample. Behavioral and Brain Research. 2015;280:72–77. doi: 10.1016/j.bbr.2014.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeod BD, Southam-Gerow MA, Jensen-Doss A, Hogue A, Kendall PC, Weisz JR. Benchmarking Treatment Adherence and Therapist Competence in Individual Cognitive-Behavioral Treatment for Youth Anxiety Disorders. Journal of Clinical Child and Adolescent Psychology. 2017:1–13. doi: 10.1080/15374416.2017.1381914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Advisory Mental Health Council Workgroup on Tasks and Measures for Research Domain Criteria. Bethesda, MD: 2016. Behavioral Assessment Methods for RDoC Constructs. [Google Scholar]
- Noldus LPJJ. The Observer: A software system for collection and analysis of observational data. Behavior Research Methods, Instruments, & Computers: A Journal of the Psychonomic Society, Inc. 1991;23(3):415–429. doi: 10.3758/bf03195516. [DOI] [PubMed] [Google Scholar]
- Pediatric OCD Treatment Study (POTS) Team. Cognitive-behavior therapy, sertraline, and their combination for children and adolescents with obsessive-compulsive disorder: the Pediatric OCD Treatment Study (POTS) randomized controlled trial. JAMA: The Journal of the American Medical Association. 2004;292(16):1969–1976. doi: 10.1001/jama.292.16.1969. [DOI] [PubMed] [Google Scholar]
- Peris TS, Caporino NE, O’Rourke S, Kendall PC, Walkup JT, Albano AM, Bergman RL, McCracken JT, Birmaher B, Ginsburg GS, Sakolsky D, Piacentini J, Compton SN. Therapist-Reported Features of Exposure Tasks That Predict Differential Treatment Outcomes for Youth With Anxiety. Journal of the American Academy of Child and Adolescent Psychiatry. 2017;56:1043–1052. doi: 10.1016/j.jaac.2017.10.001. [DOI] [PubMed] [Google Scholar]
- Rupp C, Doebler P, Ehring T, Vossbeck-Elsebusch AN. Emotional Processing Theory Put to Test: A Meta-Analysis on the Association Between Process and Outcome Measures in Exposure Therapy. Clinical Psychology & Psychotherapy. 2017;24(3):697–711. doi: 10.1002/cpp.2039. [DOI] [PubMed] [Google Scholar]
- SAS Institute, Cary, NC. 9.4 [Computer Program] Sas, S; Version, S T A T; 2003. [Google Scholar]
- Scahill L, Riddle MA, McSwiggin-Hardin M, Ort SI, King RA, Goodman WK, Leckman JF. Children’s Yale-Brown Obsessive Compulsive Scale: reliability and validity. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36(6):844–852. doi: 10.1097/00004583-199706000-00023. [DOI] [PubMed] [Google Scholar]
- Shechner T, Hong M, Britton JC, Pine DS, Fox NA, et al. Fear conditioning and extinction across development: Evidence from human studies and animal models. Biological Psychiatry. 2014;100:1–12. doi: 10.1016/j.biopsycho.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits JA, Rosenfield D, Otto MW, Powers MB, Hofmann SG, Telch MJ, Pollack MH, Tart CD. D-cycloserine enhancement of fear extinction is specific to successful exposure sessions: evidence from the treatment of height phobia. Biological Psychiatry. 2013;73:1054–1058. doi: 10.1016/j.biopsych.2012.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suen HK, Ary D. Analyzing Quantitative Behavioral Observation Data. New York, NY: Psychology Press; 2014. [Google Scholar]
- Telch MJ, Bruchey AK, Rosenfield D, Cobb AR, Smits J, Pahl S, Gonzalez-Lima F. Effects of post-session administration of methylene blue on fear extinction and contextual memory in adults with claustrophobia. American Journal of Psychiatry. 2014;171:1091–1098. doi: 10.1176/appi.ajp.2014.13101407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisz JR, Kuppens S, Eckshtain D, Ugueto AM, Hawley KM, Jensen-Doss A. Performance of evidence-based youth psychotherapies compared with usual clinical care: a multilevel meta-analysis. JAMA Psychiatry. 2013;70(7):750–761. doi: 10.1001/jamapsychiatry.2013.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteside SPH, Deacon BJ, Benito K, Stewart E. Factors associated with practitioners’ use of exposure therapy for childhood anxiety disorders. Journal of Anxiety Disorders. 2016;40:29–36. doi: 10.1016/j.janxdis.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters AM, Pine DS. Evaluating differences in Pavlovian fear acquisition and extinction as predictors of outcome from cognitive behavioural therapy for anxious children. The Journal of Child Psychology and Psychiatry. 2016;57:869–876. doi: 10.1111/jcpp.12522. [DOI] [PubMed] [Google Scholar]