Abstract
Objectives
Anger expression is assumed to have mostly negative health effects. Yet, evidence is mixed on how anger expression influences African Americans’ cardiovascular health. The present research aimed to clarify this link by examining moderating effects of chronic discrimination on the relationship between anger expression and cardiovascular risk among African Americans in experimental (Study 1) and epidemiological (Study 2) studies.
Methods
Study 1 examined how African Americans’ trait anger expression was linked to (a) physiologic reactivity to acute social rejection during an interracial encounter (Session 1) and (b) total/HDL cholesterol assessed two months later (Session 2). Study 2 examined the relationship between anger expression and total/HDL cholesterol with a larger sample of African Americans from the Midlife in the United States (MIDUS) survey. Both studies examined perceptions of chronic discrimination as a moderator of the relationships between anger expression and biological responses.
Results
In Study 1 higher anger expression was associated with quicker cortisol recovery and greater testosterone reactivity following outgroup social rejection in Session 1 and lower total/HDL cholesterol in Session 2. Study 2 replicated the relationship between anger expression and lower total/HDL cholesterol and further showed that this relationship was unique to the expressive aspect of anger. Importantly, in both studies, these potentially beneficial effects of anger expression were evident among individuals with lower perceptions of chronic discrimination.
Conclusions
These findings suggest that anger expression, when coupled with low levels of chronic discrimination, is associated with adaptive patterns of physiologic responses among African Americans. (246 words; 250 max)
Keywords: African Americans, anger expression, chronic discrimination, cardiovascular health
Anger expression is assumed to be primarily an unhealthy response with possible negative health consequences. Since the days of the Greek physician Galen (A.D. 131-201), it has been proposed that expression of anger or hostility may increase risks for a variety of health problems, including hypertension and coronary heart disease. In support of this view, a large number of studies found positive associations between anger expression and increased risk for cardiovascular diseases (see Chida & Steptoe, 2009 for a meta-analysis). These results are further complemented by longitudinal evidence, showing that the propensity toward anger expression increases the likelihood of cardiovascular malfunctions later in life (Everson, Goldberg, Kaplan, Julkunen, & Salonen, 1998; Kawachi, Sparrow, Spiro, Vokonas, & Weiss, 1996). However, this literature rests, in large part, on Western, mostly European or European American populations. Relatively fewer investigations have examined the health effects of anger expression among African Americans, and moreover, they provide mixed evidence; some studies report detrimental health effects of anger expression (Dorr, Brosschot, Sollers, & Thayer, 2007; Harburg, Gleiberman, Russell, & Cooper, 1991), whereas others document salubrious effects (Armstead, Lawler, Gorden, Cross, & Gibbons, 1989; Jorgensen, Johnson, Kolodziej, & Schreer, 1996), or no effect at all (Tomfohr, Pung, & Dimsdale, 2016).
One possibility that might account for this heterogeneity in the literature is that the association between anger expression and health is not invariant across contexts, but instead may depend on socio-cultural conditions to which individuals are chronically exposed (Boylan & Ryff, 2013; Eng, Fitzmaurice, Kubzansky, Rimm, & Kawachi, 2003; Kitayama et al., 2015). Yet, much of the prior literature has examined the anger expression-health link among African Americans without consideration of socio-cultural contexts where anger expression occurs. The present research aims to fill this gap by examining exposure to chronic discrimination as one such contextual factor that could moderate the relationships between anger expression and short-term and long-term cardiovascular risk among African Americans.
Anger Expression and Health: Context Matters
Growing evidence suggests that the health effects of anger expression are often context-dependent. One consistent pattern that emerged from these studies is that anger expression is not detrimental to all, but can be more toxic to those who are exposed to adverse life conditions. For example, Merjonen et al. (2008) tested childhood socioeconomic status (SES) as a proxy of such adversity and found that higher trait anger was associated with increased risk of atherosclerosis, only among young adults with lower childhood-SES. Similarly, anger expression is associated with increased risk of acute coronary heart disease (de Leon, 1992; Mittleman, 1997) and elevated levels of pro-inflammatory cytokines (Boylan & Ryff, 2013), among those with low educational attainment. Importantly, Beatty and Matthews (2009) suggest that these moderating effects, previously identified among Europeans or European Americans, may generalize to African Americans by showing that trait anger predicted blood pressure more strongly among those living in lower SES neighborhoods.
Notably, increasing evidence further suggests that anger expression can be beneficial for some people who are not exposed to stressful environments and thus have relatively greater access to economic and psychosocial resources. For example, one prospective study found that anger expression predicted lower risk of stroke and nonfatal myocardial infraction two years later among highly educated, male health professionals (Eng et al., 2003). Similarly, among middle-aged white-collar men, higher anger expression predicted decreased risk of coronary heart disease eight years later, whereas there was no such benefit of anger expression among their blue-collar counterparts (Haynes, Feinleib, & Kannel, 1980).
Taken together, these findings suggest that whether anger expression is a health risk or benefit may critically depend on social conditions to which people are chronically exposed. Greater expression of anger is likely to be detrimental to individuals who are already at risk, such as those with low SES, as these individuals may be exposed to more situations that elicit anger and thus the frequency of anger expression can serve as a proxy for their exposure to anger-including life difficulties. Moreover, these individuals may have fewer resources to cope with potentially adverse consequences of expressing anger (Gallo & Matthews, 2003). In contrast, anger expression may be health-protective or even health-promoting for those who are not exposed to such adversities and thus have greater access to psychosocial resources and economic privileges (Eng et al., 2003; Kitayama et al., 2015). However, these studies exclusively focused on SES as a proxy of these life conditions, and thus, it remains unknown whether the moderating effects identified above would generalize to other types of life challenges or adversities that characterize the lives of many African Americans. The present research aims to address this issue by examining chronic discrimination as one such type of adversity. Chronic discrimination can create a barrier in attaining SES for members of minority groups by seriously limiting their access to educational and employment opportunities, and thus, it could be argued that the effects of discrimination and SES may not be independent (Forman, Williams, & Jackson, 1997). Yet, evidence exists that SES is often inversely related to experiences of discrimination (Sigelman & Welch, 1991), thereby suggesting that they may present different life challenges to African Americans.
Perceptions of Chronic Discrimination as a Moderator
Racial discrimination is one of the most salient stressors African Americans encounter in daily life (R. Clark, Anderson, Clark, & Williams, 1999). Experiences of discrimination are more common among African Americans than other ethnic groups (Sternthal, Slopen, & Williams, 2011), and moreover, discriminatory experiences result in more deleterious psychological consequences for African Americans, such as a reduced sense of control and mastery (Feagin, 1991; Valentine, Silver, & Twigg, 1999) and increased feelings of distress (Broman, Mavaddat, & Hsu, 2000). Evidence also suggests that perceived discrimination is a serious health risk, which exacerbates poor physical and mental health by activating multiple stress pathways (R. Clark et al., 1999; Harrell, 2000). For example, as compared to European Americans, African Americans show higher levels of allostatic load—i.e., cumulative wear and tear on physiological systems (Geronimus, Hicken, Keene, & Bound, 2006), possibly caused by repeated exposure to structural adversities such as discrimination (i.e., weathering hypothesis; Geronimus, 1992). Importantly, anger expression is one of the primary emotional responses following experiences of discrimination (Brondolo, Brady Ver Halen, Pencille, Beatty, & Contrada, 2009), and yet, health correlates of anger expression and chronic discrimination are typically examined in separate literatures (see R. Clark, 2006 for a notable exception). By bridging these separate bodies of literature, we examined the interactive effects of anger expression and chronic discrimination on cardiovascular risk, guided by two predictions.
First, consistent with the past literature that anger expression may be more harmful to those who are already at risk (Beatty & Matthews, 2009; Boylan & Ryff, 2013), we predicted that the health-compromising effects of anger would be more pronounced for African Americans with higher perceptions of chronic discrimination because these individuals may have less coping resources available to them to buffer against maladaptive health effects typically linked to hostility or anger, such as lower levels of self-efficacy and reduced sense of mastery or control (Feagin, 1991; Valentine et al., 1999). This prediction is further motivated by a recent study suggesting that those who use anger primarily as a means to vent frustrations are more likely to have compromised health because expressing anger may serve as an index of the degree to which these individuals are exposed to frustration-inducing life difficulties (Kitayama et al., 2015). Given that repeated experiences of discrimination decrease a sense of control while evoking strong feelings of frustration (Feagin, 1991; Valentine et al., 1999), anger may be construed more as a means to vent frustrations for African Americans who are exposed to chronic discrimination. Accordingly, for these individuals, anger expression could be associated with elevated cardiovascular risk, possibly via the accumulated burden of physiologic stress reactivity, as the frequency of anger expression in this context is likely to reflect repeated experiences of frustration resulting from discriminatory experiences.
In contrast, we expected that the relationship between anger expression and elevated cardiovascular risk would be either attenuated (de Leon, 1992; Mittleman, 1997) or even reversed (Eng et al., 2003; Haynes et al., 1980) among African Americans with lower perceptions of chronic discrimination. Evidence suggests that when people use anger adaptively, either in a constructive way with a motivation to resolve or improve the situation (Davidson, MacGregor, Stuhr, Dixon, & MacLean, 2000), or as a form of social communication to signal dominance and power (Kitayama et al., 2015; Park et al., 2013), this can be linked to salubrious health effects. These adaptive functions of anger may be more readily available for those who have greater psychosocial resources such as a higher sense of personal efficacy or control over their environments. Consistent with this view, a sense of control has been identified as a strong predictor of the use of active, problem-focused (vs. emotion-focused) coping styles (Menaghan, 1982; Ross & Mirowsky, 1989). Insofar as African Americans with low (vs. high) levels of discrimination have higher sense of control and efficacy, it could then be argued that these individuals may be enabled to use the adaptive functions of anger more. For these individuals, a discriminatory event may not be perceived as a stable and enduring source of stress, but instead as a temporary problem that can be overcome with coping efforts. Thus, if anger expression is repeatedly used as an active coping strategy to deal with the controllable social stressors, this may eventually be linked to better cardiovascular health, as the frequency of anger expression in this context is likely to reflect a sense of efficacy or control as well as other favorable life conditions that enable African Americans to use anger in a more adaptive way.
Present Research
We tested these predictions using two studies with complementary research designs—one experimental (Study 1) and one epidemiological (Study 2).
Study 1 examined the relationship between trait anger expression and two indices of cardiovascular risk among African Americans in two sessions; (a) physiologic stress reactivity (Session 1) and (b) the ratio of total-to-HDL cholesterol (i.e., total/HDL cholesterol) (Session 2). In Session 1, African Americans (n = 53) interacted with a European American, same-sex stranger who provided them with negative social feedback, while their cardiovascular and neuroendocrine responses were measured. Approximately two months later, a subsample of participants (n = 20) participated in Session 2 for a blood draw from which we assayed their cholesterol levels.
Study 2 aimed to test the generalizability of the Study 1 finding with a larger sample with more diverse demographic characteristics. It examined the relationship between anger expression and total/HDL cholesterol among African Americans (n = 233) from the Midlife in the United States (MIDUS) survey, after adjusting for a variety of confounding factors that could influence the link between anger expression and lipids profiles, including age, gender, education, and health status. Study 2 further examined whether the hypothesized moderating effect of chronic discrimination is specific to the expressive aspect of anger or extends to other aspects of anger by testing three other related anger constructs (i.e., experience, suppression, and control of anger).
In both studies, we examined perceptions of chronic discrimination as a moderator of the relationships between anger expression and cardiovascular risk factors.
Study 1
Study 1 consisted of two sessions. First, we conducted a lab experiment (Session 1) to examine how African Americans’ trait anger expression was linked to physiologic stress reactivity following an acute experience of social rejection during an interracial encounter and whether this relationship was moderated by perceived levels of chronic discrimination. Building on growing evidence that autonomic and neuroendocrine responses to acute stressors are strongly linked to cardiovascular biomarkers and disease risk (Aschbacher et al., 2008; Aschbacher et al., 2013; Carroll, Lovallo, & Phillips, 2009), we assessed physiologic stress responses of African Americans while they interacted with a newly acquainted European American, same-sex stranger (i.e., confederate), who provided them with negative interpersonal feedback. Social rejection from a European American partner is often construed as discrimination by racial minority group members (Crocker, Voelkl, Testa, & Major, 1991; Mendes, Major, McCoy, & Blascovich, 2008). For example, when African Americans are rejected by a European American partner, they are more likely to attribute the rejection to discrimination compared to when European Americans are rejected by a different-race partner (Mendes et al., 2008). We examined whether African Americans’ anger expression would be linked to distinct patterns of physiologic stress reactivity following outgroup social rejection depending on their perceptions of chronic discrimination.
If African Americans who perceive higher chronic discrimination were more vulnerable to health-compromising effects of anger expression, their trait anger expression might be linked to less adaptive physiologic stress responses following outgroup rejection, compared to those with lower perceptions of discrimination. Our analysis focused on three physiologic responses that could index adaptive patterns of stress reactivity—(a) cardiac vagal withdrawal, (b) cortisol recovery, and (c) testosterone reactivity. These responses were assessed while African Americans performed a cooperative task with their European American partner, immediately after they received negative social feedback from this partner.
First, cardiac vagal withdrawal, measured as reductions in respiratory sinus arrhythmia (RSA) in response to environmental demands, has been suggested to reflect an individual’s active coping in response to stressors or an increased attentional focus to the environment (Gentzler, Santucci, Kovacs, & Fox, 2009; Muhtadie, Koslov, Akinola, & Mendes, 2015; Porges, 1995). For example, according to Porges’ polyvagal theory (Porges, 1995; Porges, Doussard-Roosevelt, Portales, & Greenspan, 1996), the evolution of the vagal system enabled mammals to flexibly respond to changing environmental signals, either by increasing vagal activity in response to relaxation cues or by withdrawing vagal influences in response to environmental challenges. In particular, greater vagal withdrawal in response to challenges is considered adaptive as it enables people to meet the situational demands more effectively by enhancing their attentional focus to relevant stimuli. If anger expression impairs stress regulation and disrupts attentional focus during the cooperative task immediately following social rejection, this would then be reflected as a reduced vagal withdrawal. We thus predicted that African Americans with higher perceptions of discrimination would show less vagal withdrawal (i.e., less of a decrease in RSA reactivity), whereas those with lower perceptions of it would show greater vagal withdrawal, possibly reflecting their enhanced task engagement and better self-regulation during the cooperative task.
Second, if anger expression results in impaired regulation of stress responses following social rejection particularly among African Americans with high levels of discrimination, this might be related to a delay in physiologic recovery from the stressor (Epel, McEwen, & Ickovics, 2010). We thus predicted that African Americans with higher trait anger expression would show slower cortisol recovery following social rejection, especially for those with higher perceptions of discrimination. In contrast, higher anger expression might be linked to quicker cortisol recovery among those who perceive less discrimination.
Third, recent evidence suggests that one potential pathway through which anger expression could be associated with better health is via dominance display (Kitayama et al., 2015; Park & Kitayama, 2017). When people feel a desire to aggress against others to establish dominance and if they are enabled to express anger with this intention, this form of anger expression may be experienced as empowering, thereby potentially related to salubrious health effects. We explored whether this adaptive function of anger is more readily available for those with low (vs. high) levels of discrimination by assessing neuroendocrine responses associated with a tendency to establish dominance relationships—i.e., testosterone reactivity. Increasing evidence suggests that transient changes in endogenous testosterone levels are associated with greater engagement in aggressive and competitive behaviors motivated by a desire to establish or display dominance (see Carré & Olmstead, 2015; Eisenegger, Haushofer, & Fehr, 2011 for reviews). For example, testosterone reactivity following a competitive task is associated with an increase in dominance motivation and greater willingness to engage in a subsequent competition (Carré & McCormick, 2008; Mehta & Josephs, 2006). We thus examined whether African Americans’ anger expression would be linked to heightened testosterone reactivity following social rejection, possibly indexing their desire to aggress against their partner with an intention to display dominance, particularly among those with less discrimination.
In addition to assessing physiologic reactivity, we also examined how anger expression was linked to a biomarker of cardiovascular risk among a random subsample of participants who completed a blood draw approximately two months later (Session 2). We assayed the serum for total/HDL cholesterol as an index of coronary heart risk based on growing evidence that this cholesterol profile is a predictor of the vulnerability to a variety of heart and vascular conditions such as stroke, atherosclerosis, and arterial hypertension (Castelli, 1996; Kannel, Vasan, Keyes, Sullivan, & Robins, 2008; Kinosian, Glick, Preiss, & Puder, 1995). Paralleling our predictions for physiologic reactivity, we predicted that for those who perceive more discrimination, anger expression would be linked to increased total/HDL cholesterol while this relationship would be attenuated or reversed for those with less discrimination.
Finally, as an exploratory step, we examined whether and how the physiologic changes measured during the lab session (Session 1) would be associated with participants’ cholesterol levels we assessed two months later (Session 2) as an initial step in understanding physiological channels through which anger expression might affect cardiovascular risk.
Method
Participants
One hundred and six African Americans (61 females; Mage = 25.31, SDage = 4.83) were recruited via flyers and internet postings in San Francisco, CA. No mention of race was included in the postings, but we did target geographic areas and websites that reflected a larger proportion of African Americans. Prior to participation, we excluded participants for medical or psychiatric conditions that might influence physiologic responses, including (a) current or past self-reported history of psychiatric disorder, (b) significant medical illness (e.g., heart disease, hypertension), (c) pregnancy, and (d) stage II obesity (Body-mass-index; BMI > 35). Those who passed the screening were invited to the lab to complete a two-hour experiment in exchange for $67. Approximately two months later, a subset of these participants (n = 36; 33.97%) participated in an additional session for a blood draw (18 females, Mage = 25.97, SDage = 5.07) (see Aschbacher et al., 2016 for procedure). They were additionally compensated $50. The study was approved by the Institutional Review Board of the University of California, San Francisco.
Session 1: Lab Experiment
Pre-lab online survey
Before coming to the lab, participants completed a series of questionnaires online, which included measures of anger expression and chronic discrimination (see Measures section below), along with other measures administered either as filler or exploratory purposes.1
Consenting and baseline measures
After completing the online survey, participants were scheduled for a two-hour lab visit. To minimize the effects of circadian fluctuations in testosterone and cortisol levels (Touitou & Haus, 2000), participants were scheduled between 12:00pm and 5:30pm. After providing informed consent, participants were asked to provide a 1.5 mL saliva sample from which their baseline testosterone and cortisol were assayed (baseline saliva assessment). Participants were told to passively drool saliva into a sterile polypropylene microtubule.
Immediately following the baseline saliva assessment, participants received an experimental manipulation that was unrelated to the hypothesis of the present study. Specifically, one half of the participants were given intranasal oxytocin and the other half were given placebo (Park, Flores, Woolley, & Mendes, 2016). We do not discuss the results from this manipulation as it is not relevant to our research question and this variable did not interact with the social rejection manipulation (see below) to predict any of the data we report in this study. Nonetheless, we adjusted for the effects of this manipulation by controlling for this factor in our analysis (see Data Attrition and Analytic Strategy section below).
Next, an experimenter applied physiological sensors to obtain electrocardiographic (ECG) signals while participants sat quietly for a 5-minute baseline physiological recording.
Social rejection manipulation
To induce feelings of social rejection, we used the same protocol from a previous study examining cardiovascular responses to discrimination (Mendes et al., 2008). Participants were told that they would interact with another “participant,” who was in a different room. In reality, the other participant was one of the research assistants (i.e., a European American who was the same-sex and similar age as the participant) who was trained to act interested but neutral throughout the interaction with participants.2 The participant and the confederate were able to see and hear each other over large television monitors (42”) through an audiovisual connection made between the two rooms. After brief introductions, they were given instructions for the upcoming speech task; the participant was “randomly assigned” to give a speech on the topic of “why I make a good friend” for 3 minutes while the confederate evaluated the speech. After providing instructions, the experimenter turned off the television in the participant’s room so that this would not be a distraction during the speech, although the participant was told that the connection was still on and their partner could see and hear their speech. The participant was then left alone to prepare the speech silently for 1 minute, after which they delivered the speech for 3 minutes.
After the speech, the experimenter returned to the room and asked the participant to answer several questions about the speech task. The participant was then asked to click the “SEND/RECEIVE” button on the computer screen to exchange their answers with their partner’s responses, which included the partner’s evaluation form. Following Mendes et al. (2008), we presented five statements on the evaluation form with the partner’s ostensible ratings on each statement on a scale of −4 to +4 (“I would like to work at the same business or job as my partner,” “I would like to work closely on a project or team with my partner,” “I would like to get to know my partner better,” “I would enjoy being neighbors with my partner,” and “I would like to be close friends with my partner”). To induce feelings of social rejection, we provided negative feedback to half of the participants—i.e., ratings of 0 for the first three statements and −1, and −2 for the fourth, and fifth, respectively. In contrast, the other half of the participants received positive feedback with favorable ratings—i.e., ratings of +3 for the first two statements and +4 for the remaining three. Both the experimenters and confederates were kept blind not only to the type of the feedback but also to the fact that we provided any feedback to the participants. The study director (i.e., the second author) was the only study personnel who knew this manipulation was included.
In-person interaction
After participants reviewed the evaluation form, the experimenter moved the confederate into the participant’s room so that they could perform two interactive tasks together, during which we measured participants’ physiological responses to compute an index of RSA reactivity. The participant and the confederate first engaged in a cooperative task, based on the game of taboo. In this task, each player alternated providing clues for target words for 2 minutes without using any of the five “taboo” words listed on their prompt cards. After the dyad performed the taboo game for 8 minutes, they performed another interactive task (i.e., a tactile finger-spelling task) for 3 minutes, which we included for an exploratory purpose (see Online Supplementary Materials for the results from this task).
After the completion of the tasks, the confederate was moved out of the room and the participant was asked to provide second (post-interaction) and third (recovery) saliva samples, approximately 18 and 33 minutes following the onset of the in-person interaction, respectively. Finally, the experimenter removed physiological sensors, probed for suspicion, and debriefed participants.
See Figure 1 for the timeline of the Session 1 procedure.
Figure 1.
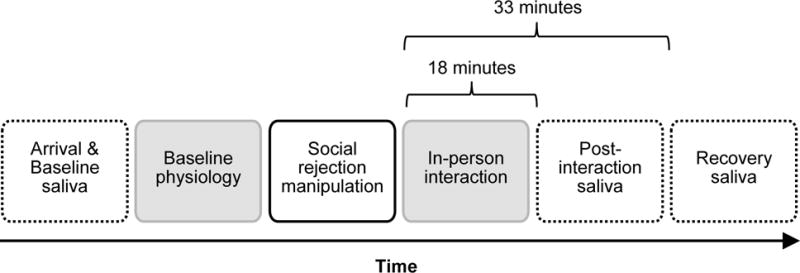
Overview of the Session 1 procedure in Study 1. Dashed outlines indicate the times when saliva samples were collected. Baseline saliva sample was obtained right after consenting and post-interaction and recovery saliva samples were obtained 18 min and 33 min following the onset of the in-person interaction, respectively. Cortisol recovery was calculated by subtracting the final cortisol level from the second cortisol level (post-interaction minus recovery). Testosterone reactivity was computed by subtracting the first testosterone level from the third testosterone level (recovery minus baseline). Gray boxes indicate the times when electrocardiographic (ECG) signals were obtained. RSA reactivity was calculated based on the ECG data by subtracting RSA scores during the last minute of participants’ baseline data from the corresponding scores obtained during the first minute of the in-person interaction. A lower number on this reactivity index indicates greater vagal withdrawal.
Session 2: Blood Draw
Following the lab session, some participants were contacted to determine if they were interested in providing a blood sample. A subsample of 36 participants was scheduled to provide a fasting morning blood sample at the Clinical Research Center approximately two months after the initial visit.3 Women were tested during the follicular phase of the menstrual cycle to minimize the potential effect of hormonal levels on plasma lipids and lipoproteins (Ginsberg et al., 1995). Participants were asked to refrain from exercise or caffeine intake on the morning of the draw. After participants had rested for 15 minutes, a trained research nurse drew blood. Within 30 minutes, whole blood collected into sodium heparin tubes was processed to isolate serum for blood labs. Blood samples were assayed for total and HDL cholesterol and the ratio was calculated (total/HDL cholesterol) as an index of cardiovascular risk (see Aschbacher et al., 2016). The subsample did not significantly differ from the full sample on demographic variables such as age and gender.
Measures
Self-report measures
Anger expression was assessed with the 8-item anger-out subscale of the State-Trait Anger Expression Inventory (STAXI; Spielberger, 1996), one of the most widely used measures of individuals’ general tendency to express anger, which has been shown to reliably predict a variety of health outcomes across different populations, including African Americans (e.g., Engebretson, Matthews, & Scheier, 1989; Finney, Stoney, & Engebretson, 2002). Participants rated their agreement with each item using a 4-point scale (1 = strongly disagree, 4 = strongly agree) (e.g., Much of the time I feel like expressing my anger; α = .77, M = 16.32, SD = 4.13).
Perceptions of chronic discrimination were assessed with the 20-item Daily Life Experiences subscale of the Racial and Life Experiences Scales (RaLES; Harrell, 1997). This subscale is a validated measure of the perception of daily experiences of race-based micro-aggressions among African Americans (e.g., Ong & Edwards, 2008; Seaton, Yip, & Sellers, 2009). Participants rated how often (0 = never, 5 = once a week or more) during the past year they experienced discriminatory events because of their race (e.g., being ignored, overlooked, or not given service [in a restaurant, store, etc.], being accused of something or treated suspiciously; α = .93, M = .64, SD = .69).
Cardiovascular responses
Cardiac vagal responses were measured continuously during the study using ECG obtained from two Ag/AgCl electrodes placed in a modified Lead II configuration (right upper chest, left lower rib), interfaced with Biopac MP150 data acquisition system (Goleta, CA). The ECG data were scored offline using HRV module from Mindware Technologies (Mindware Technologies, Gahanna, OH; HRV 3.0), which estimates RSA in accordance with the recommendations of the Society for Psychophysiological Research Committee on heart rate variability (HRV) (Berntson et al., 1997). Trained research assistants visually inspected the digitized ECG signal in each minute bin and edited incorrectly identified R spikes after removing artifacts. Following Berntson, Cacioppo, & Quigley (1993), we applied a 4 Hz time series to interpolate the interbeat interval (IBI) time series and used a second-order polynomial to minimize nonstationary trends. The residual series were then tapered with a Hanning window and spectral-power valued were determined with Fast Fourier Transform. The integral power within the respiration frequency band (.12 to .4 Hz) was used as an indicator of RSA for each minute.
RSA reactivity values were calculated by subtracting RSA scores during the last minute of baseline from the RSA scores obtained during the first minute of the in-person interaction. We chose the first minute of the in-person interaction because we expected that participants would experience highest levels of physiologic reactivity when they had to encounter and interact with an out-group member who had just rejected them (see Koslov, Mendes, Pajtas, & Pizzagalli, 2011; Mendes & Koslov, 2013 for a similar approach). A lower number on this reactivity index indicates greater cardiac vagal withdrawal during the interaction relative to the baseline, which has been associated with better regulation of stress responses and enhanced cognitive control and engagement during active tasks (Gentzler et al., 2009; Muhtadie et al., 2015; Porges, 1995).
Neuroendocrine responses
Immediately following the lab session, saliva samples were frozen at −80 °C until shipped on dry ice to be assayed. Samples were assayed for cortisol and testosterone concentrations with a time-resolved immunoassay with fluorescence detention. The samples were assayed twice and the inter-assay coefficients of variation (CV) were 6.11%, 5.30%, and 4.43% for baseline, post-interaction, and recovery cortisol, respectively, and were 6.00%, 5.22%, and 6.22% for baseline, post-interaction, and recovery testosterone, respectively. The averaged data of the two assays were used for the analysis. Since the cortisol and testosterone values did not follow normal distribution at all three time points, they were square-root transformed.
Cortisol recovery following social rejection was computed by subtracting recovery cortisol levels from post-interaction cortisol levels (post-interaction minus recovery; see Shapero, Abramson, & Alloy, 2015). We used this index to examine patterns of recovery from the stressor (Epel et al., 2010). Testosterone reactivity was characterized as the difference score between baseline and recovery testosterone levels to reflect the extent to which participants maintained elevated levels of testosterone following the social rejection manipulation compared to their baseline levels (recovery minus baseline; see Carré, Campbell, Lozoya, Goetz, & Welker, 2013). Building on prior evidence that increases in testosterone levels in response to situational demands is associated with increased motivation and/or feelings of power, competitiveness, and dominance (Carré & Olmstead, 2015; Eisenegger et al., 2011), we used testosterone reactivity as a neuroendocrine index of a desire to establish or display dominance.4
See Table 1 for descriptive statistics and intercorrelations among key variables.
Table 1.
Descriptive statistics and intercorrelations among key variables in Study 1
| N | M | SD | 1 | 2 | 3 | 4 | 5 | 6 | |
|---|---|---|---|---|---|---|---|---|---|
| 1. Anger expression | 51 | 16.32 | 4.13 | — | 0.11 | 0.07 | 0.03 | 0.08 | −0.40† |
| 2. Chronic discrimination | 51 | 0.64 | 0.69 | — | 0.02 | −0.27* | 0.08 | −0.15 | |
| 3. RSA reactivity | 50 | −0.70 | 1.55 | — | −0.45*** | 0.03 | 0.12 | ||
| 4. Cortisol recovery | 53 | 0.07 | 0.22 | — | −0.12 | −0.01 | |||
| 5. Testosterone reactivity | 53 | −0.65 | 2.04 | — | −0.44*** | ||||
| 6. Total/HDL cholesterol | 20 | 2.75 | 0.62 | — |
Note. RSA reactivity was calculated by subtracting RSA scores during the last minute of participants’ baseline from the corresponding scores obtained during the first minute of the in-person interaction. Cortisol recovery was calculated by subtracting the final cortisol level from the second cortisol level (post-interaction minus recovery). Testosterone reactivity was computed by subtracting the first testosterone level from the third testosterone level (recovery minus baseline).
p ≤ .10.
p ≤ .05.
p ≤ .001.
Results
Data Attrition and Analytic Strategy
Since the primary focus of this study was to examine African Americans’ physiologic responses to acute social rejection, the current analysis focused on participants who were assigned to the negative feedback condition (n = 54). Nevertheless, when we analyzed the data from participants in the positive feedback condition (n = 52), the effect of anger expression and/or chronic discrimination was not significant on any of the outcome variables we assessed (ps > .119), consistent with previous data showing different physiologic reactions to inter-racial rejection vs. acceptance (Mendes et al., 2008). We additionally excluded one participant who was suspicious about the authenticity of her “partner” and believed that the partner was a confederate. This left 53 African Americans with analyzable data (31 females; Mage = 25.83, SDage = 4.92). Cholesterol data were available for 20 (37.70%) of these participants (7 females; Mage = 26.85, SDage = 5.12).
As noted earlier, the study involved another manipulation that was unrelated to the hypothesis of the present study—i.e., whether participants were given intranasal oxytocin or placebo in Session 1. This manipulation did not interact with the effects of anger expression and/or chronic discrimination to influence any of the outcome variables, but we ran all analyses on the Session 1 data, controlling for this condition effect. To adjust for gender difference in testosterone responses (Archer, 2006), we additionally controlled for gender in the analysis of testosterone reactivity.5
To examine whether chronic discrimination moderated the effects of anger expression on outcome variables we assessed during the lab experiment (RSA reactivity, cortisol recovery, and testosterone reactivity) and the blood draw session (total/HDL cholesterol), we performed a multiple regression analysis on each outcome variable with anger expression, chronic discrimination, and the interaction between the two as predictor variables. Specifically, we entered anger expression and chronic discrimination as predictors of each outcome variable along with control variable(s) in Model 1. We then tested the Anger expression x Chronic discrimination interaction in Model 2, computed after centering both variables (J. Cohen & Cohen, 1983). We used centered variables for both main effects and interaction effects. The results of these analyses are summarized in Table 2.
Table 2.
Unstandardized regression coefficients in predicting each outcome variable as a function of anger expression and chronic discrimination (Model 1) and the interaction between these variables (Model 2) in Study 1.
| A: RSA reactivity | Model 1 All main effects (ΔR2 = 0.019) |
Model 2 + 2-way interaction (ΔR2 = 0.104*) |
||||
|---|---|---|---|---|---|---|
| b | t(44) | sr2 | b | t(43) | sr2 | |
| Intranasal spray | 0.371 | 0.776 | 0.013 | 0.876 | 1.722† | 0.061 |
| Anger expression | 0.018 | 0.294 | 0.002 | 0.020 | 0.357 | 0.003 |
| Chronic discrimination | 0.021 | 0.064 | < 0.001 | −0.086 | −0.264 | 0.001 |
| Anger x Discrimination | 0.174 | 2.257* | 0.104 | |||
| B: Cortisol recovery | Model 1 All main effects (ΔR2 = 0.109) |
Model 2 + 2-way interaction (ΔR2 = 0.129**) |
||||
|---|---|---|---|---|---|---|
| b | t(47) | sr2 | b | t(46) | sr2 | |
| Intranasal spray | −0.081 | −1.287 | 0.031 | −0.164 | −2.482* | 0.102 |
| Anger expression | 0.005 | 0.630 | 0.008 | 0.005 | 0.722 | 0.009 |
| Chronic discrimination | −0.089 | −1.981* | 0.075 | −0.074 | −1.744† | 0.050 |
| Anger x Discrimination | −0.028 | −2.790** | 0.129 | |||
| C: Testosterone reactivity | Model 1 All main effects (ΔR2 = 0.077) |
Model 2 + 2-way interaction (ΔR2 = 0.124*) |
||||
|---|---|---|---|---|---|---|
| b | t(46) | sr2 | b | t(45) | sr2 | |
| Intranasal spray | 1.061 | 1.756† | 0.062 | 0.327 | 0.517 | 0.005 |
| Gender | 0.057 | 0.092 | < 0.001 | −0.020 | −0.034 | < 0.001 |
| Anger expression | 0.018 | 0.245 | 0.001 | 0.020 | 0.286 | 0.001 |
| Chronic discrimination | 0.222 | 0.506 | 0.005 | 0.347 | 0.837 | 0.013 |
| Anger x Discrimination | −0.256 | −2.646* | 0.124 | |||
| D: Total/HDL cholesterol | Model 1 All main effects (ΔR2 = 0.159) |
Model 2 + 2-way interaction (ΔR2 = 0.249*) |
||||
|---|---|---|---|---|---|---|
| b | t(17) | sr2 | b | t(16) | sr2 | |
| Anger expression | −0.054 | −1.664 | 0.137 | 0.005 | 0.147 | 0.001 |
| Chronic discrimination | −0.005 | −0.023 | < 0.001 | −0.632 | −2.083* | 0.161 |
| Anger x Discrimination | 0.127 | 2.594* | 0.249 | |||
Note. Anger x Discrimination indicates the Anger expression x Chronic discrimination interaction effect. Intranasal spray indicates oxytocin vs. placebo conditions.
p ≤ .10.
p ≤ .05.
p ≤ .01.
Session 1: Physiologic Responses to Acute Social Rejection
We observed a consistent pattern of moderating effects of chronic discrimination on the relationships between anger expression and three physiologic changes following social rejection: RSA reactivity, testosterone reactivity, and cortisol recovery.
First, there was a significant Anger expression x Chronic discrimination interaction on RSA reactivity, b = .174, 95% Confidence Interval (CI95) = [.019, .330], t(43) = 2.26, p = .029. As Figure 2-A illustrates, among African Americans who perceived more discrimination, there was a non-significant tendency that higher trait anger expression was linked to less vagal withdrawal, b = .143, CI95 = [−.018, .304], t(43) = 1.80, p = .079, possibly reflecting their disengagement from the cooperative task following social rejection. In contrast, anger expression did not predict RSA reactivity among those with less discrimination, b = −.102, CI95 = [−.260, .055], t(43) = −1.31, p = .196.
Figure 2.
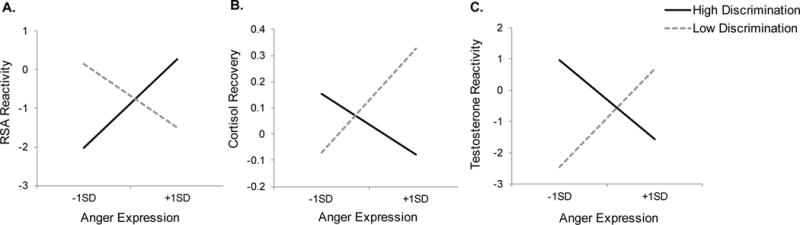
The relationships between African Americans’ anger expression and (A) RSA reactivity, (B) cortisol recovery, and (C) testosterone reactivity following social rejection by a European American interaction partner as a function of perceived chronic discrimination (high vs. low) in Study 1. Dotted lines indicate individuals who perceived low levels of chronic discrimination (−1 SD from the mean) and solid lines indicate those who perceived high levels of chronic discrimination (+1 SD from the mean). Intranasal spray (oxytocin vs. placebo) was controlled for all analyses and gender was additionally controlled for the analysis on testosterone reactivity.
The Anger expression x Chronic discrimination interaction was also significant on cortisol recovery, b = −.028, CI95 = [−.049, −.008], t(46) = −2.79, p = .008. Among African Americans who perceived less discrimination, greater anger expression was associated with faster cortisol recovery following social rejection, b = .025, CI95 = [.004, .045], t(46) = 2.45, p = .018 (see Figure 2-B). However, anger expression did not predict cortisol recovery among those with higher perceptions of discrimination, b = −.015, CI95 = [−.035, .006], t(46) = −1.45, p = .153.
A similar interaction pattern was observed for testosterone reactivity, b = −.256, CI95 = [−452, −.061], t(45) = −2.65, p = .011. As Figure 2-C displays, for African Americans who perceived less discrimination, their anger expression was associated with greater testosterone reactivity following social rejection, b = .197, CI95 = [.002, .392], t(45) = 2.04, p = .047. In contrast, there was no such relationship among those with higher discrimination, b = −.158, CI95 = [−.351, .035], t(45) = −1.65, p = .107.
Session 2: Total/HDL Cholesterol
The Anger expression x Chronic discrimination was also significant on total/HDL cholesterol, b = .127, CI95 = [.023, .231], t(16) = 2.59, p = .020. Anger expression was associated with lower total/HDL cholesterol for African Americans who perceived less discrimination, b = −.107, CI95 = [−.181, −.034], t(16) = −3.09 p = .007. In contrast, the relationship between the two variables was negligible for those with higher perceptions of discrimination, b = .071, CI95 = [−.047, .189], t(16) = 1.28, p = .220.
Correlation Analyses
Next, we examined correlations among the biological responses obtained across two sessions (see Table 1). Among the Session 1 variables, there was a negative relationship between RSA reactivity and cortisol recovery, r(50) = −.45, p = .001, indicating that African Americans who displayed greater decreases in RSA reactivity (i.e., more vagal withdrawal) during the cooperative task showed a faster cortisol recovery following the in-person interaction. Additionally, we examined associations between physiologic responses and total/HDL cholesterol and found that African Americans who showed stronger testosterone reactivity following social rejection displayed lower total/HDL cholesterol two months later, r(20) = −.44, p = .053. None of the other variables predicted total/HDL cholesterol, rs < .12, ps > .644.
Discussion
Study 1 examined how African Americans’ general tendency to express anger is associated with acute physiologic responses following outgroup social rejection and whether this association varies by their perceived levels of racial discrimination. Three key findings emerged.
First, we found consistent patterns of the moderation effects of chronic discrimination on the link between anger expression and cardiovascular risk. These effects were primarily driven by African Americans who perceived less discrimination. Compared to those higher in perceived discrimination, those lower in discrimination showed greater anger expression linked to more adaptive patterns of physiologic reactivity following social rejection, such as sharper declines in cortisol recovery and larger testosterone reactivity. For these individuals, anger expression was also linked to lower total/HDL cholesterol two months later, thereby demonstrating potentially beneficial health effects of anger expression.
Moreover, when we examined the entire sample regardless of their levels of chronic discrimination, we found a negative correlation between testosterone reactivity and total/HDL cholesterol, indicating that greater testosterone reactivity during the inter-racial interaction predicted lower total/HDL cholesterol two months later. This finding suggests that a desire to establish dominance, possibly indexed by elevated testosterone levels (Eisenegger et al., 2011; Mazur & Booth, 1998), might be one of the pathways through which anger expression is linked to protective effects on cardiovascular health (Kitayama et al., 2015; Park & Kitayama, 2017).
One unexpected finding that emerged was that there was no clear evidence suggesting that anger expression was detrimental to those who are already at risk—i.e., African Americans who perceived experiencing high levels of chronic discrimination. For these individuals, the relationships between anger expression and both short-term (stress reactivity) and long-term (cholesterol profiles) cardiovascular risk factors were negligible except that they displayed a marginal tendency to disengage from the cooperative task following social rejection, indexed by less vagal withdrawal. The disengagement, in turn, was associated with slower recovery from the stressor, as evidenced by the negative correlation between RSA reactivity and cortisol recovery.
There are two plausible explanations for the null effects of anger expression among those with higher discrimination. First, it is possible that we did not have enough power to detect more robust effects because of our relatively small sample size. Another possibility is that that perceived discrimination was generally low for our African American participants, who were recruited from San Francisco, CA, one of the most diverse cities in the U.S. (Centner, 2008) (i.e., a floor effect). To address these issues, Study 2 tested a larger sample of African Americans from the MIDUS survey (n = 233), a majority of whom were recruited from Milwaukee, WI, one of the most racially segregated cities in the U.S. (Tolan & Glauber, 2010).
Study 2
Study 2 had three goals. First, we wanted to replicate the association between higher anger expression and lower total/HDL cholesterol, observed in Study 1, with a larger and more diverse sample of African Americans after adjusting for potential confounding variables such as age, gender, and education, and health status of participants.
Second, anger expression is often correlated with other related constructs, such as the extent to which people experience, suppress, or control anger (Kitayama et al., 2015). Thus, Study 1 alone cannot address whether the moderation effects we found are specific to the expressive aspect of anger, or would extend to other related facets. Thus, in Study 2 we additionally tested three other anger constructs, including experience, suppression, and control of anger.
Third, the discrimination measure we used in Study 1 assessed perceptions of daily experiences of discrimination, primarily in the context of interpersonal interactions (e.g., being ignored in a restaurant). Although this measure is widely used, it fails to capture other, more severe forms of race-based aggressions, based on structural or institutional discrimination. We thus used a more inclusive measure of discrimination in Study 2 that can tap both major and minor discriminatory experiences based on three distinct types of discrimination—(a) major lifetime discrimination, (b) daily discrimination, and (c) chronic job discrimination. In addition, to examine whether the hypothesized moderation effect is specific to discrimination-related stressors or extends to other challenges people face in life, we also tested global stress appraisals, assessed with the Perceived Stress Scale (PSS; S. Cohen, Kamarck, & Mermelstein, 1983) as another moderator in our exploratory analysis.
Method
Participants
Participants were a subset from the MIDUS survey sample. The first wave of MIDUS (MIDUS I) was conducted in 1995 with a representative sample of English-speaking adults residing in the contiguous 48 states (aged 25–74), recruited via random digit dialing. A subset of MIDUS I participants completed a follow-up survey in 2004 (MIDUS II; retention rate = 75%). In addition to the national sample, 592 African Americans in Milwaukee, WI were also recruited during the second wave. The current analysis focused on the African American subsample of the MIDUS II participants who attended an additional overnight session for biomarker data collection at one of three General Clinical Research Centers (Madison, WI, Washington, DC, or Los Angeles, CA). The final sample included 233 African Americans (n = 32 from the national sample and n = 201 from the Milwaukee sample; 157 females, Mage = 53.59, SDage = 10.41).
Measures
Cholesterol
Frozen serum and plasma samples were shipped to Meriter Labs (Madison, WI), where total and HDL cholesterol were assayed using a Cobas Integar analyzer (Roche Diagnostics, Indianapolis, IN). Since the total/HDL cholesterol values were positively skewed, they were log-transformed.
Anger expression vs. other aspects of anger
As in Study 1, anger expression was assessed with the anger-out subscale of the STAXI (Spielberger, 1996). Participants indicated how often (1 = almost never, 4 = almost always) they express angry feelings through verbally or physically aggressive behaviors when they feel furious and angry (e.g., I slam doors, I strike out at what infuriates me; α = .81, M = 13.52, SD = 3.94). In addition, three relevant constructs of anger were assessed, including (a) anger suppression, (b) anger control, and (c) anger experience. Anger suppression and anger control were assessed, respectively, with the 8-item anger-in (e.g., I keep things in, I withdraw from people; α = .84, M = 14.84, SD = 4.63) and the 4-item anger-control (e.g., I control my temper, I keep my cool; α = .67, M = 9.16, SD = 2.44) subscales of the STAXI. Anger experience was assessed with a 1-item rating of anger participants reported to have felt during the past 30 days (1 = none of the time, 5 = all of the time; M = 3.54, SD = 1.22), which was included in the extended version of the Positive Affect and Negative Affect Schedule (PANAS; Watson, Clark, & Tellegen, 1988).
Perceptions of chronic discrimination
Perceptions of chronic discrimination were assessed as a composite of (a) major lifetime discrimination, (b) daily discrimination, and (c) chronic job discrimination. Major lifetime discrimination was assessed based on the number of major discriminatory events participants reportedly have experienced during their lifetime (11 items; e.g., hassled by police, denied a bank loan; M = 3.04, SD = 2.84). To measure daily discrimination, participants rated how often (1 = never, 4 = often) they experience nine types of discrimination in daily life (e.g., You are treated with less courtesy than other people, People act as if they think you are not smart; α = .78, M = 14.97, SD = 6.58). Participants also rated how often (1 = never, 5 = once a week or more) they experience discrimination in their job setting (6-items; e.g., How often are you watched more closely than other workers?, How often do you think you are unfairly given the jobs that no one else wanted to do?; α = .83, M = 11.87, SD = 5.61) to conceptualize chronic job discrimination (Williams, Yu, Jackson, & Anderson, 1997). Following a procedure from Slopen et al. (2012), the responses from these measures were collapsed to form a single index of chronic discrimination after standardizing each index.
We also assessed participants’ general appraisals of stress with the 10-item PSS (S. Cohen et al., 1983). Participants indicated how often (1 = never, 5 = very often) they experienced various forms of stress during the past month (e.g., found that you could not cope with all the things that you had to do, felt nervous and “stressed”; α = .83, M = 24.82, SD = 6.55).
Control variables
The analysis controlled for several variables that could potentially confound the relationship between anger expression and cholesterol levels, including demographic variables such as age, gender, and educational attainment (1 = 8th grade, junior high school, 12 = Ph.D. or other professional degree) as well as health status of the respondents indexed by BMI, chronic health conditions, and cholesterol medication usage. Chronic health conditions were assessed with the number of health problems participants self-reportedly experienced in the past 12 months (maximum of 30; e.g., diabetes, asthma; M = 3.24, SD = 2.90). Cholesterol medication usage was categorized as whether participants took any medication to treat their cholesterol abnormalities during the past 30 days (0 = no, 1 = yes). Since BMI scores were positively skewed, they were log-transformed.
See Table 3 for descriptive statistics and intercorrelations among key variables.
Table 3.
Descriptive statistics and intercorrelations among key variables in Study 2
| N | M | SD | 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. Anger expression | 230 | 13.52 | 3.94 | — | 0.50*** | −0.13* | −0.18** | 0.09 | 0.27*** | −0.04 |
| 2. Anger suppression | 230 | 14.84 | 4.63 | — | −0.10 | −0.14* | 0.08 | 0.54*** | −0.01 | |
| 3. Anger control | 231 | 9.16 | 2.44 | — | −0.00 | −0.02 | −0.36*** | −0.14* | ||
| 4. Anger experience | 233 | 3.54 | 1.22 | — | −0.13† | −0.09 | −0.01 | |||
| 5. Chronic discrimination | 233 | 0.58 | 1.13 | — | 0.14* | 0.20** | ||||
| 6. Perceived stress | 230 | 24.82 | 6.55 | — | 0.10 | |||||
| 7. Total/HDL cholesterol | 230 | 0.50 | 0.15 | — |
p ≤ .10.
p ≤ .05.
p ≤ .10.
p ≤ .001.
Results
We performed a two-step multiple regression analysis. In Step 1, main effects of anger expression and perceptions of chronic discrimination were entered along with the control variables. Step 2 tested the two-way interaction between anger expression and chronic discrimination. See Table 4 for the results of this analysis.
Table 4.
Unstandardized regression coefficients in predicting total/HDL cholesterol as a function of anger expression and chronic discrimination (Model 1) and the interaction between these variables (Model 2) in Study 2.
| Model 1 All main effects (ΔR2 = 0.122***) |
Model 2 + 2-way interaction (ΔR2 = 0.016*) |
|||||
|---|---|---|---|---|---|---|
| b | t(213) | sr2 | b | t(212) | sr2 | |
| Gender | −0.033 | −1.538 | 0.010 | −0.034 | −1.615 | 0.011 |
| Age | −0.001 | −0.810 | 0.003 | −0.001 | −0.844 | 0.003 |
| Educational attainment | −0.003 | −0.903 | 0.003 | −0.005 | −1.184 | 0.006 |
| Body-mass-index (BMI) | 0.352 | 3.848*** | 0.061 | 0.367 | 4.028*** | 0.066 |
| Chronic health conditions | 0.001 | 0.415 | 0.001 | 0.001 | 0.293 | < 0.001 |
| Cholesterol medication usage | −0.022 | −0.836 | 0.003 | −0.021 | −0.790 | 0.003 |
| Anger expression | −0.003 | −1.283 | 0.007 | −0.003 | −1.246 | 0.006 |
| Chronic discrimination | 0.021 | 2.314* | 0.022 | 0.019 | 2.026* | 0.017 |
| Anger x Discrimination | 0.004 | 1.993* | 0.016 | |||
Note. Anger x Discrimination indicates the Anger expression x Chronic discrimination interaction effect.
p ≤ .05.
p ≤ .001.
This analysis yielded a main effect of chronic discrimination, indicating that African Americans who perceived greater discrimination showed higher total/HDL cholesterol compared to those who perceived less discrimination, b = .019, CI95 = [.001, .037], t(212) = 2.03, p = .044. The main effect of anger expression was not significant, b = −.003, CI95 = [−.008, .002], t(212) = −1.25, p = .214, but there emerged a significant Anger expression x Chronic discrimination interaction, b = .004, CI95 = [.000, .007], t(212) = 1.99, p = .048. Replicating Study 1 and as shown in Figure 3, anger expression was linked to lower total/HDL cholesterol among those who reported experiencing less discrimination, b = −.007, CI95 = [−.014, −.001], t(212) = −2.27, p = .024. In contrast, anger expression was not related to total/HDL cholesterol for those who perceived greater discrimination, b = .001, CI95 = [−.005, .007], t(212) = .26, p = .795.
Figure 3.
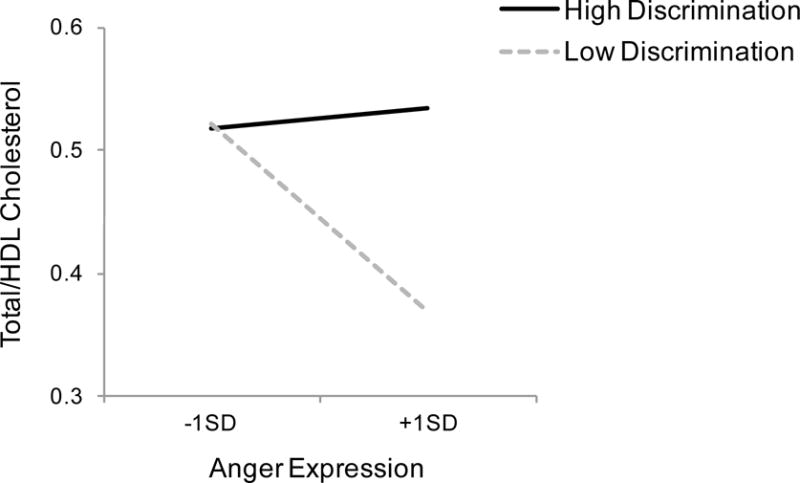
The relationship between anger expression and the total-to-HDL cholesterol ratio (total/HDL cholesterol) as a function of their perceived levels of chronic discrimination (high vs. low) in Study 2. Dotted line indicates individuals who perceived low levels of chronic discrimination (−1 SD from the mean) and solid line indicates those who perceived high levels of chronic discrimination (+1 SD from the mean).
Subsequent analyses confirmed that this pattern of results was unique to the expressive aspect of anger. The critical two-way interaction involving anger and chronic discrimination was not significant with other indices of anger, including anger suppression, b = .003, CI95 = [−.001, .006], t(212) = 1.52, p = .129, anger control, b = −.001, CI95 = [−.008, .006], t(212) = −.39, p = .697, and anger experience, b < .001, CI95 = [−.014, .015], t(212) = .05, p = .957. Moreover, when we tested global stress appraisals as a moderator instead of chronic discrimination, the two-way interaction was not significant, b < −.001, CI95 = [−.001, .001], t(212) = −.35, p = .730, indicating that this finding was specific to discrimination-related stressors.
Discussion
Study 2 replicated and extended Study 1 in three ways. First, replicating the Study 1 finding, the relationship between anger expression and total/HDL cholesterol was significantly moderated by chronic discrimination among a large population of African Americans from the MIDUS survey. This moderation effect persisted even after we adjusted for various potentially confounding factors, such as demographic variables and health status.
Moreover, a series of additional analyses confirmed that our results are specific to anger expression. When we tested three related constructs (i.e., experience, suppression, and control of anger), none of these variables interacted with chronic discrimination in predicting total/HDL cholesterol. Our analysis further highlights the specificity of discrimination-specific life stressors as a moderator of anger as global stress appraisals did not influence the anger expression-cholesterol link.
As shown in Study 1, the moderation effect was driven by African Americans who perceived less discrimination, whose anger expression was associated with healthier cholesterol concentrations. In contrast to our initial prediction, however, we did not find any evidence that anger expression undermines cardiovascular health among those with chronic exposure to discriminatory environments. Notably, a majority of our sample in Study 2 was from Milwaukee, WI, who have been reported to experience higher levels of discrimination compared to overall MIDUS national sample (Slopen et al., 2012; Slopen et al., 2010). This suggests that the null effect of anger expression we observed in Study 1 was less likely due to a lack of power or our focus on the unrepresentative sample of African Americans from a highly racially diverse urban area who may experience generally low levels of discrimination-related stress.
General Discussion
Anger and hostility resulting from race-based stressors are considered risk factors contributing to racial disparities in cardiovascular health (Dressler, Oths, & Gravlee, 2005). Yet, our findings across two studies—one experimental (Study 1) and one epidemiologic (Study 2)— suggest that anger, at least its expressive facet, might at times be linked to adaptive patterns of cardiovascular responses among African Americans under certain socio-cultural conditions. We examined chronic discrimination as one such condition that was hypothesized to moderate the relationships between anger expression and short-term and long-term cardiovascular risk factors.
Chronic Discrimination as a Contextual Moderator
We found across two studies that the relationships between anger expression and two indices of cardiovascular risk—physiologic reactivity (Study 1) and total/HDL cholesterol (Studies 1 and 2)—were significantly moderated by African Americans’ perceived levels of chronic discrimination; these effects were primarily driven by those with lower perceptions of discrimination. For these individuals, higher expression of anger was associated with more adaptive patterns of physiologic responses following outgroup social rejection in Study 1, including faster recovery from the stressor indexed by a steeper decline in cortisol responses (Epel et al., 2010) and a heightened sense of dominance indexed by elevated testosterone reactivity (Eisenegger et al., 2011; Mazur & Booth, 1998). Moreover, paralleling these results, higher anger expression was linked to healthier cholesterol levels two months later among a subsample of participants. Study 2 replicated this relationship with a larger sample of African Americans from the MIDUS survey, while controlling for a number of variables known to be associated with cholesterol concentrations, and further demonstrated that this pattern was unique to the expressive aspect of anger.
In contrast, we did not find any strong evidence linking anger expression to increased cardiovascular risk among African Americans who perceived greater chronic discrimination. There emerged one marginal tendency that anger expression was linked to less vagal withdrawal in Study 1, possibly reflecting their disengagement or impaired stress regulation following social rejection (Gentzler et al., 2009; Muhtadie et al., 2015; Porges, 1995). However, the effects of anger expression on other biological outcomes were statistically trivial in both studies. Given prior research suggesting that anger can be more toxic to individuals with life adversities, such as those with lower SES (Beatty & Matthews, 2009; de Leon, 1992), it seems puzzling that we did not observe any such effect among African American participants. We speculate that the discrepancy between previous studies and our findings could be due to the types of adversities. Previous studies suggest that people with lower SES may be more vulnerable to anger expression because of their relative lack of coping resources (Gallo & Matthews, 2003). However, our analysis suggests that anger expression may not be maladaptive in the context of discrimination. An awareness of racism might be protective for individuals in less advantaged environments (Sellers, Smith, Shelton, Rowley, & Chavous, 1998), insofar as they are equipped with coping mechanisms to deal with these situations. Anger expression or any type of expressive behaviors that involve confrontations or fighting back might serve as effective strategies to deal with racism in such environments. Consistent with this view, growing evidence suggests that African Americans who use confrontational strategies to deal with racism, which require overt expression of feelings or actions (e.g., speaking up or trying to change things) experience better health compared to those who use more defensive, passive coping strategies that do not involve expressive behaviors (e.g., avoiding it or keeping to yourself; A. J. Thomas, Witherspoon, & Speight, 2008; Utsey, Ponterotto, Reynolds, & Cancelli, 2000). This direct, and thus more active form of anger reaction in response to discrimination may then offset potentially maladaptive health effects typically linked to hostility or anger. This idea must be tested in future research by examining whether anger expression produces different health outcomes depending on its intended use as an active, confrontational strategy as opposed to a passive, defensive one.
How Can Anger Expression be Healthy?
An important question raised by this work concerns the mechanisms driving divergent health effects of anger expression. One possible explanation comes from a recent theory in social psychology highlighting two prominent functions of anger—(a) venting frustration and (b) displaying dominance (Kitayama et al., 2015; Park & Kitayama, 2017; Park et al., 2013). A central thesis of this theory is that anger expression should produce different health consequences depending on which form of anger predominates in a given environment. Specifically, in contexts where the vented-frustration form of anger is more likely to occur, anger expression may be linked to compromised health because the frequency of expressing anger in these contexts is likely to reflect the extent to which an expresser is exposed to frustration-inducing life difficulties, such as limited access to social and material resources. In contrast, in contexts where the dominance-display function of anger is more likely, anger expression might be linked to better health as the frequency of anger expression in these contexts is likely to reflect the degree to which the expresser is exposed to more favorable life conditions, such as greater access to social and economic privileges.
Our analysis suggests a possibility that chronic discrimination may serve as a critical contextual moderator that can determine the availability of the dominance-display function of anger among African Americans. By expressing anger, some individuals might be able to restore a sense of control, maintain self-respect, and compensate for the status challenge following frustrating experiences, through a display of dominance and power (Crick, Casas, & Mosher, 1997; Dépret & Fiske, 1993; Henry, 2009). This act of dominance display is likely reinforced more in African American cultural contexts where masculinity identities such as toughness, power, and aggressiveness are considered important (A. Thomas, Hammond, & Kohn-Wood, 2015). Our data suggest that this adaptive function of anger may be more readily available for those who do not perceive themselves as being exposed to high levels of discrimination, and thus have some control over their environments. When repeatedly used as an effective intimidation strategy to deal with the “controllable” discrimination (M. S. Clark, Pataki, & Carver, 1996; Jones & Pittman, 1982), this beneficial aspect of anger may eventually lead to better cardiovascular health, as it is likely to index the degree to which people experience dominance, a sense of control, and interpersonal effectiveness in a given environment.
Initial evidence supporting this interpretation comes from the correlation we found between testosterone reactivity and cholesterol levels in Study 1. Regardless of perceptions of chronic discrimination, African Americans who exhibited greater testosterone reactivity following social rejection showed lower total/HDL cholesterol two months later. Coupled with emerging evidence that higher testosterone levels are associated with reduced cardiovascular risk (Khaw et al., 2007), this finding hints at the possibility that feelings of dominance might be an important mechanism through which anger expression exerts protective effects on cardiovascular risk. Future research would benefit from a careful assessment of this idea by directly assessing dominance as well as other potential pathways linking anger expression to better health. For example, it has been suggested that if one expresses anger with a motivation to resolve a problem in a constructive way, this could bring out protective health effects (Davidson et al., 2000). One future extension will be to examine in what conditions this adaptive function of anger is made more available among African Americans.
Limitations and Future Directions
Several shortcomings of the current work should be noted. First, our analysis is based on cross-sectional data, which limits the conclusion that anger expressions leads to different biological outcomes, rather than the reverse. A longitudinal extension of this work is necessary to establish the causal relationship between the two variables. Second, we focused on a single biomarker of cholesterol concentrations to assess cardiovascular risk. Although total/HDL cholesterol is one of the widely-studied biomarkers of coronary heart risk (Castelli, 1996; Kannel et al., 2008), it is important to examine a broader range of health indices related to cardiovascular risk. Third, our work only tested a self-report measure of anger expression. Although evidence exists that self-reported levels of anger expression are reliably associated with a variety of health conditions across different populations (e.g., Eng et al., 2003; Engebretson et al., 1989; Everson et al., 1998), future research would benefit by testing behavioral indices of anger expression. Relatedly, we examined individuals’ general tendency to express anger instead of specifying the contexts where they express it. Future research is necessary to examine how anger expression, assessed in the context of discrimination, influences health. Finally, our analyses in Study 1, especially on the cholesterol data, are based on a small sample. Although our analyses in this study resulted in the interaction effects between two key predictors with medium-to-large effect sizes (with sr2 ranging from .10 to .25), caution is due when interpreting these findings given the small sample size.
Concluding Remarks
The present research suggests that among African Americans anger expression can have divergent effects on cardiovascular risk factors, depending on perceptions of chronic discrimination. Our findings challenge the prevalent assumption that anger is uniformly bad for health (see also Consedine, Magai, & Horton, 2005; Consedine et al., 2006; Kitayama et al., 2015; Park & Kitayama, 2017). Rather, they suggest that anger may be beneficial at times, depending on the extent to which African Americans perceive their environments as discriminatory, thereby highlighting the importance of taking into account socio-cultural conditions individuals are chronically situated in to achieve better understanding of biological pathways of health.
Supplementary Material
Acknowledgments
This research was supported by grants from the National Science Foundation (BCS1430799) and the National Institute of Mental Health (T32 MH019391).
Footnotes
A complete list of the measures can be viewed in Online Supplementary Materials.
We used a total of 23 confederates for this study (11 males and 12 females). The large number of confederates was due to the length of time this study took to complete—almost two years. Controlling for confederates did not substantially alter any of the results we report in this study.
We had Session 2 two months later, not immediately following Session 1, because we wanted enough time to pass so that the affective responses from the lab task were not re-experienced during the blood draw visit, but also did not want the second session to be too distant from the first one to minimize the effects of having noise or other intervening factors (that could occur during the interval between two sessions) that might affect the data from Session 2.
Anger expression and/or chronic discrimination did not influence other indices of neuroendocrine responses following social rejection, including cortisol reactivity (recovery minus baseline) and testosterone recovery (post-interaction minus recovery), ts < │−1.40│, ps > .168.
When we used testosterone responses standardized within gender, the results did not change substantially. The Anger expression x Chronic discrimination remained significant, b = −.128, CI95 = [−.228, −.028], t(46) = −2.58, p = .013, and the gender-adjusted testosterone scores marginally predicted total/HDL cholesterol, r(20) = −.42, p = .064.
References
- Adler NE, Epel ES, Castellazzo G, Ickovics JR. Relationship of subjective and objective social status with psychological and physiological functioning: preliminary data in healthy white women. Health Psychology. 2000;23:586–592. doi: 10.1037//0278-6133.19.6.586. [DOI] [PubMed] [Google Scholar]
- Archer J. Testosterone and human aggression: an evaluation of the challenge hypothesis. Neuroscience & Biobehavioral Reviews. 2006;30(3):319–345. doi: 10.1016/j.neubiorev.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Armstead CA, Lawler KA, Gorden G, Cross J, Gibbons J. Relationship of racial stressors to blood pressure responses and anger expression in Black college students. Health Psychology. 1989;8(5):541–556. doi: 10.1037/0278-6133.8.5.541. [DOI] [PubMed] [Google Scholar]
- Aschbacher K, Derakhshandeh R, Flores AJ, Narayan S, Mendes WB, Springer ML. Circulating angiogenic cell function is inhibited by cortisol in vitro and associated with psychological stress and cortisol in vivo. Psychoneuroendocrinology. 2016;67:216–223. doi: 10.1016/j.psyneuen.2016.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschbacher K, Mills PJ, von Kanel R, Hong S, Mausbach BT, Roepke SK, Grant I. Effects of depressive and anxious symptoms on norepinephrine and platelet P-selectin responses to acute psychological stress among elderly caregivers. Brain, behavior, and immunity. 2008;22(4):493–502. doi: 10.1016/j.bbi.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschbacher K, O’Donovan A, Wolkowitz OM, Dhabhar FS, Su Y, Epel E. Good stress, bad stress and oxidative stress: insights from anticipatory cortisol reactivity. Psychoneuroendocrinology. 2013;38(9):1698–1708. doi: 10.1016/j.psyneuen.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty DL, Matthews KA. Unfair treatment and trait anger in relation to nighttime ambulatory blood pressure in African American and white adolescents. Psychosomatic Medicine. 2009;71(8):813–820. doi: 10.1097/PSY.0b013e3181b3b6f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA. Beck Anxiety Inventory Manual. San Antonio, TX: Psychological Corporation; 1993. [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Archives of General Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Cacioppo JT, Quigley KS. Respiratory sinus arrhythmia: Autonomic origins, physiological mechanisms, and psychophysiological implications. Psychophysiology. 1993;30(2):183–196. doi: 10.1111/j.1469-8986.1993.tb01731.x. [DOI] [PubMed] [Google Scholar]
- Berntson GG, J TB, Eckberg DL, Grossman P, Kaufmann PG, Malik M, Van Der Molen MW. Heart rate variability: Origins, methods, and interpretive caveats. Psychophysiology. 1997;34(6):623–648. doi: 10.1111/j.1469-8986.1997.tb02140.x. [DOI] [PubMed] [Google Scholar]
- Boylan JM, Ryff CD. Varieties of anger and the inverse link between education and inflammation: Toward an integrative framework. Psychosomatic Medicine. 2013;75(6):566–574. doi: 10.1097/PSY.0b013e31829683bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broman CL, Mavaddat R, Hsu SY. The experience and consequences of perceived racial discrimination: A study of African Americans. Journal of Black Psychology. 2000;26(2):165–180. doi: 10.1177/0095798400026002003. [DOI] [Google Scholar]
- Brondolo E, Brady Ver Halen N, Pencille M, Beatty D, Contrada RJ. Coping with racism: a selective review of the literature and a theoretical and methodological critique. Journal of Behavioral Medicine. 2009;32(1):64–88. doi: 10.1007/s10865-008-9193-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Research. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Carré JM, Campbell JA, Lozoya E, Goetz SM, Welker KM. Changes in testosterone mediate the effect of winning on subsequent aggressive behaviour. Psychoneuroendocrinology. 2013;38(10):2034–2041. doi: 10.1016/j.psyneuen.2013.03.008. [DOI] [PubMed] [Google Scholar]
- Carré JM, McCormick CM. Aggressive behavior and change in salivary testosterone concentrations predict willingness to engage in a competitive task. Hormones and Behavior. 2008;54(3):403–409. doi: 10.1016/j.yhbeh.2008.04.008. [DOI] [PubMed] [Google Scholar]
- Carré JM, Olmstead NA. Social neuroendocrinology of human aggression: examining the role of competition-induced testosterone dynamics. Neuroscience. 2015;286:171–186. doi: 10.1016/j.neuroscience.2014.11.029. [DOI] [PubMed] [Google Scholar]
- Carroll D, Lovallo WR, Phillips AC. Are large physiological reactions to acute psychological stress always bad for health? Social and Personality Psychology Compass. 2009;3(5):725–743. doi: 10.1111/j.1751-9004.2009.00205.x. [DOI] [Google Scholar]
- Castelli WP. Lipids, risk factors and ischaemic heart disease. Atherosclerosis. 1996;124:S1–S9. doi: 10.1016/0021-9150(96)05851-0. [DOI] [PubMed] [Google Scholar]
- Centner R. Places of privileged consumption practices: Spatial capital, the dot-com habitus, and San Francisco’s internet boom. City & Community. 2008;7(3):193–223. doi: 10.1111/j.1540-6040.2008.00258.x. [DOI] [Google Scholar]
- Chida Y, Steptoe A. The association of anger and hostility with future coronary heart disease: a meta-analytic review of prospective evidence. Journal of the American College of Cardiology. 2009;53(11):936–946. doi: 10.1016/j.jacc.2008.11.044. [DOI] [PubMed] [Google Scholar]
- Clark MS, Pataki SP, Carver VH. Some thoughts and findings on self-presentation of emotions in relationships. In: Fletcher GJO, Fitness J, editors. Knowledge structures in close relationships: A social psychological approach. Mahwah, NJ: Erlbaum; 1996. pp. 247–274. [Google Scholar]
- Clark R. Interactive but not direct effects of perceived racism and trait anger predict resting systolic and diastolic blood pressure in black adolescents. Health Psychology. 2006;25(5):580–585. doi: 10.1037/0278-6133.25.5.580. [DOI] [PubMed] [Google Scholar]
- Clark R, Anderson NB, Clark VR, Williams DR. Racism as a stressor for African Americans: A biopsychosocial model. American Psychologist. 1999;54(10):805–816. doi: 10.1037/0003-066x.54.10.805. [DOI] [PubMed] [Google Scholar]
- Cohen J, Cohen P. Applied multiple regression/correlation analysis for the behavioral sciences. Hillsdale, NJ: Erlbaum; 1983. [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. Journal of Health and Social Behavior. 1983;24:385–396. [PubMed] [Google Scholar]
- Consedine NS, Magai C, Horton D. Ethnic variation in the impact of emotion and emotion regulation on health: A replication and extension. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2005;60(4):P165–P173. doi: 10.1093/geronb/60.4.P165. [DOI] [PubMed] [Google Scholar]
- Consedine NS, Magai C, Kudadjie-Gyamfi EK, Longfellow JK, Ungar TM, King AR. Stress versus discrete negative emotions in the prediction of physical complaints: does predictive utility vary across ethnic groups? Cultural Diversity and Ethnic Minority Psychology. 2006;12(3):541–557. doi: 10.1037/1099-9809.12.3.541. [DOI] [PubMed] [Google Scholar]
- Crick NR, Casas JF, Mosher M. Relational and overt aggression in preschool. Developmental Psychology. 1997;33(4):579–588. doi: 10.1037/0012-1649.33.4.579. [DOI] [PubMed] [Google Scholar]
- Crocker J, Voelkl K, Testa M, Major B. Social stigma: The affective consequences of attributional ambiguity. Journal of Personality and Social Psychology. 1991;60:218–228. doi: 10.1037//0022-3514.64.1.60. [DOI] [PubMed] [Google Scholar]
- Davidson K, MacGregor MW, Stuhr J, Dixon K, MacLean D. Constructive anger verbal behavior predicts blood pressure in a population-based sample. Health Psychology. 2000;19(1):55–64. doi: 10.1037/0278-6133.19.1.55. [DOI] [PubMed] [Google Scholar]
- de Leon CFM. Anger and impatience/irritability in patients of low socioeconomic status with acute coronary heart disease. Journal of Behavioral Medicine. 1992;15(3):273–284. doi: 10.1007/bf00845356. [DOI] [PubMed] [Google Scholar]
- Dépret E, Fiske ST. Social cognition and power: Some cognitive consequences of social structure as a source of control deprivation. In: Weary G, Gleicher F, Marsh KL, editors. Control motivation and social cognition. New York: Springer; 1993. pp. 176–202. [Google Scholar]
- Dorr N, Brosschot JF, Sollers JJ, 3rd, Thayer JF. Damned if you do, damned if you don’t: the differential effect of expression and inhibition of anger on cardiovascular recovery in black and white males. International Journal of Psychophysiology. 2007;66(2):125–134. doi: 10.1016/j.ijpsycho.2007.03.022. [DOI] [PubMed] [Google Scholar]
- Downey G, Feldman SI. Implications of rejection sensitivity for intimate relationships. Journal of Personality and Social Psychology. 1996;70(6):1327–1343. doi: 10.1037//0022-3514.70.6.1327. [DOI] [PubMed] [Google Scholar]
- Dressler WW, Oths KS, Gravlee CC. Race and ethnicity in public research: Models to Explain Health Disparities. Annual Review of Anthropology. 2005;34(1):231–252. doi: 10.1146/annurev.anthro.34.081804.120505. [DOI] [Google Scholar]
- Eisenegger C, Haushofer J, Fehr E. The role of testosterone in social interaction. Trends in Cognitive Sciences. 2011;15(6):263–271. doi: 10.1016/j.tics.2011.04.008. [DOI] [PubMed] [Google Scholar]
- Eng PM, Fitzmaurice G, Kubzansky LD, Rimm EB, Kawachi I. Anger expression and risk of stroke and coronary heart disease among male health professionals. Psychosomatic Medicine. 2003;65(1):100–110. doi: 10.1097/01.psy.0000040949.22044.c6. [DOI] [PubMed] [Google Scholar]
- Engebretson TO, Matthews KA, Scheier MF. Relations between anger expression and cardiovascular reactivity: Reconciling inconsistent findings through a matching hypothesis. Journal of Personality and Social Psychology. 1989;57(3):513–521. doi: 10.1037/0022-3514.57.3.513. [DOI] [PubMed] [Google Scholar]
- Epel ES, McEwen BS, Ickovics JR. Embodying psychological thriving: Physical thriving in response to stress. Journal of Social Issues. 2010;54(2):301–322. doi: 10.1111/j.1540-4560.1998.tb01220.x. [DOI] [Google Scholar]
- Everson SA, Goldberg DE, Kaplan GA, Julkunen J, Salonen JT. Anger expression and incident hypertension. Psychosomatic Medicine. 1998;60(6):730–735. doi: 10.1097/00006842-199811000-00014. [DOI] [PubMed] [Google Scholar]
- Feagin JR. The continuing significance of race: Antiblack discrimination in public places. American Sociological Review. 1991;56(1):101–116. doi: 10.2307/2095676. [DOI] [Google Scholar]
- Finney ML, Stoney CM, Engebretson TO. Hostility and anger expression in African American and European American men is associated with cardiovascular and lipid reactivity. Psychophysiology. 2002;39(3):340–349. doi: 10.1017/s0048577201393101. [DOI] [PubMed] [Google Scholar]
- Forman TA, Williams DR, Jackson JS. Race, place, and discrimination. Perspectives on Social Problems. 1997;9:231–261. [Google Scholar]
- Gallo LC, Matthews KA. Understanding the association between socioeconomic status and physical health: Do negative emotions play a role? Psychological Bulletin. 2003;129(1):10–51. doi: 10.1037/0033-2909.129.1.10. [DOI] [PubMed] [Google Scholar]
- Gentzler AL, Santucci AK, Kovacs M, Fox NA. Respiratory sinus arrhythmia reactivity predicts emotion regulation and depressive symptoms in at-risk and control children. Biological Psychology. 2009;82(2):156–163. doi: 10.1016/j.biopsycho.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geronimus AT. The weathering hypothesis and the health of African-American women and infants: evidence and speculations. Ethnicity Disease. 1992;2:207–221. [PubMed] [Google Scholar]
- Geronimus AT, Hicken M, Keene D, Bound J. “Weathering” and age patterns of allostatic load scores among blacks and whites in the United States. American Journal of Public Health. 2006;96(5):826–833. doi: 10.2105/AJPH.2004.060749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg HN, Karmally W, Siddiqui M, Holleran S, Tall AR, Blaner WS, Ramakrishnan R. Increases in dietary cholesterol are associated with modest increases in both LDL and HDL cholesterol in healthy young women. Arteriosclerosis, Thrombosis, and Vascular Biology. 1995;15(2):169–178. doi: 10.1161/01.atv.15.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harburg E, Gleiberman L, Russell M, Cooper ML. Anger-coping styles and blood pressure in black and white males: Buffalo, New York. Psychosomatic Medicine. 1991;53(2):153–164. doi: 10.1097/00006842-199103000-00005. [DOI] [PubMed] [Google Scholar]
- Harrell SP. The Racism and Life Experience Scales (RaLES): Self-administration version 1997 [Google Scholar]
- Harrell SP. A multidimensional conceptualization of racism-related stress: Implications for the well-being of people of color. American Journal of Orthopsychiatry. 2000;70(1):42–57. doi: 10.1037/h0087722. [DOI] [PubMed] [Google Scholar]
- Haynes S, Feinleib M, Kannel WB. The relationship of psychosocial factors to coronary heart disease in the Framingham Study. Iii. Eight-year incidence of coronary heart disease. American Journal of Epidemiology. 1980;111(1):37–58. doi: 10.1093/oxfordjournals.aje.a112873. [DOI] [PubMed] [Google Scholar]
- Henry PJ. Low-status compensation: A theory for understanding the role of status in cultures of honor. Journal of Personality and Social Psychology. 2009;97:451–466. doi: 10.1037/a0015476. [DOI] [PubMed] [Google Scholar]
- Islam MR, Hewstone M. Dimensions of contact as predictors of intergroup anxiety, perceived out-group variability, and out-group attitude: An integrative model. Personality and Social Psychology Bulletin. 1993;19(6):700–710. doi: 10.1177/0146167293196005. [DOI] [Google Scholar]
- John OP, Srivastava S. The Big-Five trait taxonomy: History, measurement, and theoretical perspectives. In: Pervin LA, John OP, editors. Handbook of personality: Theory and research. Vol. 2. New York: Guilford Press; 1999. pp. 102–138. [Google Scholar]
- Jones EE, Pittman TS. Toward a general theory of strategic self-presentation. In: Suls J, editor. Psychological perspectives on the self. Vol. 1. Hillsdale, NJ: Erlbaum; 1982. pp. 231–262. [Google Scholar]
- Jorgensen RS, Johnson BT, Kolodziej ME, Schreer GE. Elevated blood pressure and personality: A meta-analytic review. Psychological Bulletin. 1996;120(2):293–320. doi: 10.1037/0033-2909.120.2.293. [DOI] [PubMed] [Google Scholar]
- Kannel WB, Vasan RS, Keyes MJ, Sullivan LM, Robins SJ. Usefulness of the triglyceride-high-density lipoprotein versus the cholesterol-high-density lipoprotein ratio for predicting insulin resistance and cardiometabolic risk (from the Framingham Offspring Cohort) American Journal of Cardiology. 2008;101(4):497–501. doi: 10.1016/j.amjcard.2007.09.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawachi I, Sparrow D, Spiro A, Vokonas P, Weiss ST. A prospective study of anger and coronary heart disease: The normative aging study. Circulation. 1996;94(9):2090–2095. doi: 10.1161/01.cir.94.9.2090. [DOI] [PubMed] [Google Scholar]
- Khaw KT, Dowsett M, Folkerd E, Bingham S, Wareham N, Luben R, Day N. Endogenous testosterone and mortality due to all causes, cardiovascular disease, and cancer in men: European prospective investigation into cancer in Norfolk (EPIC-Norfolk) Prospective Population Study. Circulation. 2007;116(23):2694–2701. doi: 10.1161/CIRCULATIONAHA.107.719005. [DOI] [PubMed] [Google Scholar]
- Kinosian B, Glick H, Preiss L, Puder KL. Cholesterol and coronary heart disease: predicting risks in men by changes in levels and ratios. Journal of Investigative Medicine: The Official Publication of the American Federation for Clinical Research. 1995;43(5):443–450. [PubMed] [Google Scholar]
- Kitayama S, Park J, Boylan JM, Miyamoto Y, Levine CS, Markus HR, Ryff CD. Expression of anger and ill health in two cultures: an examination of inflammation and cardiovascular risk. Psychological Science. 2015;26(2):211–220. doi: 10.1177/0956797614561268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koslov K. Dissertation. Harvard University; Cambridge: 2010. Pounding hearts and brittle smiles: The effect of resource depletion on outgroup positivity. [Google Scholar]
- Koslov K, Page-Gould E, Mendes WB. Affect contagion during inter-racial interactions. Harvard University; Cambridge: 2014. [Google Scholar]
- Koslov K, Mendes WB, Pajtas PE, Pizzagalli DA. Asymmetry in resting intracortical activity as a buffer to social threat. Psychological Science. 2011;22(5):641–649. doi: 10.1177/0956797611403156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazur A, Booth A. Testosterone and dominance in men. Behavioral and Brain Sciences. 1998;21(3):353–363. [PubMed] [Google Scholar]
- Mehta PH, Josephs RA. Testosterone change after losing predicts the decision to compete again. Hormones and Behavior. 2006;50(5):684–692. doi: 10.1016/j.yhbeh.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Menaghan EG. Measuring coping effectiveness: A panel analysis of marital problems and coping efforts. Journal of Health and Social Behavior. 1982;23:220–234. [PubMed] [Google Scholar]
- Mendes WB, Koslov K. Brittle smiles: positive biases toward stigmatized and outgroup targets. Journal of Experimental Psychology: General. 2013;142(3):923–933. doi: 10.1037/a0029663. [DOI] [PubMed] [Google Scholar]
- Mendes WB, Major B, McCoy S, Blascovich J. How attributional ambiguity shapes physiological and emotional responses to social rejection and acceptance. Journal of Personality and Social Psychology. 2008;94(2):278–291. doi: 10.1037/0022-3514.94.2.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza-Denton R, Downey G, Purdie VJ, Davis A, Pietrzak J. Sensitivity to status-based rejection: Implications for African American students’ college experience. Journal of Personality and Social Psychology. 2002;83(4):896–918. doi: 10.1037/0022-3514.83.4.896. [DOI] [PubMed] [Google Scholar]
- Merjonen P, Pulkki-Raback L, Puttonen S, Keskivaara P, Juonala M, Telama R, Keltikangas-Jarvinen L. Anger is associated with subclinical atherosclerosis in low SES but not in higher SES men and women. The Cardiovascular Risk in Young Finns Study. Journal of Behavioral Medicine. 2008;31(1):35–44. doi: 10.1007/s10865-007-9131-6. [DOI] [PubMed] [Google Scholar]
- Mittleman MA. Educational attainment, anger, and the risk of triggering myocardial infarction onset. Archives of Internal Medicine. 1997;157(7):769–775. doi: 10.1001/archinte.1997.00440280091008. [DOI] [PubMed] [Google Scholar]
- Muhtadie L, Koslov K, Akinola M, Mendes WB. Vagal flexibility: A physiological predictor of social sensitivity. Journal of Personality and Social Psychology. 2015;109(1):106–120. doi: 10.1037/pspp0000016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong AD, Edwards LM. Positive affect and adjustment to perceived racism. Journal of Social and Clinical Psychology. 2008;27(2):105–126. doi: 10.1521/jscp.2008.27.2.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Flores AJ, Woolley J, Mendes WB. The effects of intranasal oxytocin on African Americans’ responses to outgroup acceptance and rejection. 2016 doi: 10.3389/fpsyg.2022.916305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Kitayama S. Anger expression and health: The cultural moderation hypothesis. In: Ryff CD, Krueger RF, editors. Oxford Handbook of Integrated Health Science. 2017. [Google Scholar]
- Park J, Kitayama S, Markus HR, Coe CL, Miyamoto Y, Karasawa M, Ryff CD. Social status and anger expression: the cultural moderation hypothesis. Emotion. 2013;13(6):1122–1131. doi: 10.1037/a0034273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratto F, Sidanius J, Stallworth LM, Malle BF. Social dominance orientation: A personality variable predicting social and political attitudes. Journal of Personality and Social Psychology. 1994;67(4):741–763. doi: 10.1037/0022-3514.67.4.741. [DOI] [Google Scholar]
- Porges SW. Orienting in a defensive world: Mammalian modifications of our evolutionary heritage. A polyvagal theory. Psychophysiology. 1995;32(4):301–318. doi: 10.1111/j.1469-8986.1995.tb01213.x. [DOI] [PubMed] [Google Scholar]
- Porges SW, Doussard-Roosevelt JA, Portales AL, Greenspan SI. Infant regulation of the vagal “brake” predicts child behavior problems: A psychobiological model of social behavior. Developmental Psychobiology. 1996;29(8):697–712. doi: 10.1002/(sici)1098-2302(199612)29:8<697::aid-dev5>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Ross CE, Mirowsky J. Explaining the social patterns of depression: Control and problem solving-or support and talking? Journal of Health and Social Behavior. 1989;30:206–219. [PubMed] [Google Scholar]
- Seaton EK, Yip T, Sellers RM. A longitudinal examination of racial identity and racial discrimination among African American adolescents. Child Development. 2009;80(2):406–417. doi: 10.1111/j.1467-8624.2009.01268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellers RM, Smith MA, Shelton JN, Rowley SA, Chavous TM. Multidimensional model of racial identity: a reconceptualization of African American racial identity. Personality and Social Psychology Review. 1998;2(1):18–39. doi: 10.1207/s15327957pspr0201_2. [DOI] [PubMed] [Google Scholar]
- Shapero BG, Abramson LY, Alloy LB. Emotional reactivity and internalizing symptoms: Moderating role of emotion regulation. Cognitive Therapy and Research. 2015;40(3):328–340. doi: 10.1007/s10608-015-9722-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheier MF, Carver CS, Bridges MW. Distinguishing optimism from neuroticism (and trait anxiety, self-mastery, and self-esteem): A reevaluation of the Life Orientation Test. Journal of Personality and Social Psychology. 1994;67(6):1063–1078. doi: 10.1037/0022-3514.67.6.1063. [DOI] [PubMed] [Google Scholar]
- Sigelman L, Welch S. Black Americans’ views of racial inequality: The dream deferred. New York: Cambridge University Press; 1991. [Google Scholar]
- Slopen N, Dutra LM, Williams DR, Mujahid MS, Lewis TT, Bennett GG, Albert MA. Psychosocial stressors and cigarette smoking among African American adults in midlife. Nicotine & Tobacco Research. 2012;14(10):1161–1169. doi: 10.1093/ntr/nts011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slopen N, Lewis TT, Gruenewald TL, Mujahid MS, Ryff CD, Albert MA, Williams DR. Early life adversity and inflammation in African Americans and whites in the midlife in the United States survey. Psychosomatic Medicine. 2010;72(7):694–701. doi: 10.1097/PSY.0b013e3181e9c16f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD. State-Trait Anger Expression Inventory: Professional manual. Odessa, FL: John Wiley & Sons, Inc; 1996. [Google Scholar]
- Stern C, West TV. Circumventing anxiety during interpersonal encounters to promote interest in contact: An implementation intention approach. Journal of Experimental Social Psychology. 2014;50:82–93. doi: 10.1016/j.jesp.2013.09.008. [DOI] [Google Scholar]
- Sternthal M, Slopen N, Williams DR. Racial disparities in health: How much does stress really matter? Du Bois Review: Social Science Research on Race. 2011;8:95–113. doi: 10.1017/S1742058X11000087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas A, Hammond WP, Kohn-Wood LP. Chill, be cool man: African American men, identity, coping, and aggressive ideation. Cultural Diversity and Ethnic Minority Psychology. 2015;21(3):369–379. doi: 10.1037/a0037545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas AJ, Witherspoon KM, Speight SL. Gendered racism, psychological distress, and coping styles of African American women. Cultural Diversity and Ethnic Minority Psychology. 2008;14(4):307–314. doi: 10.1037/1099-9809.14.4.307. [DOI] [PubMed] [Google Scholar]
- Tolan T, Glauber B. Milwaukee area tops Brookings segregation study of census data. Journal Sentinel. 2010;14 [Google Scholar]
- Tomfohr LM, Pung MA, Dimsdale JE. Mediators of the relationship between race and allostatic load in African and White Americans. Health Psychology. 2016;35(4):322–332. doi: 10.1037/hea0000251. [DOI] [PubMed] [Google Scholar]
- Touitou Y, Haus E. Alterations with aging of the endocrine and neuroendocrine circadian system in humans. Chronobiology International: The Journal of Biological and Medical Rhythm Research. 2000;17(3):369–390. doi: 10.1081/cbi-100101052. [DOI] [PubMed] [Google Scholar]
- Utsey SO, Ponterotto JG, Reynolds AL, Cancelli AA. Racial discrimination, coping, life satisfaction, and self-esteem among african americans. Journal of Counseling and Development. 2000;78(1):72–80. [Google Scholar]
- Valentine S, Silver L, Twigg N. Locus of control, job satisfaction, and job complexity: The role of perceived race discrimination. Psychological Reports. 1999;84(3c):1267–1273. doi: 10.2466/pr0.1999.84.3c.1267. [DOI] [Google Scholar]
- Watkins PC, Woodward K, Stone T, Kolts RL. Gratitude and happiness: Development of a measure of gratitude, and relationships with subjective well-being. Social Behavior and Personality: An International Journal. 2003;31(5):431–451. doi: 10.2224/sbp.2003.31.5.431. [DOI] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. Journal of Personality and Social Psychology. 1988;54(6):1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Williams DR, Yu Y, Jackson JS, Anderson NB. Racial differences in physical and mental health: Socio-economic status, stress and discrimination. Journal of Health Psychology. 1997;2(3):335–351. doi: 10.1177/135910539700200305. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


