Abstract
Introduction
We aimed to investigate the effects of intake of yogurt containing Bifidobacterium longum (BB536-y) and fructo-oligosaccharides (FOS) in preventing colorectal carcinogenesis in healthy subjects, and the preventive effects of short-chain fatty acids (SCFA), whose production was enhanced by the intake of BB536-y and FOS, in human colon cancer cell lines.
Materials and methods
The subjects were 27 healthy persons who were divided into a group taking yogurt containing BB536 (BB536-y group; n = 14) and a group taking yogurt containing BB536 and FOS (BB536-y with FOS group; n = 13) once a day for 5 weeks. The feces were sampled before and after the intake to analyze the amount of SCFA in the feces and the profile of intestinal flora, such as putrefactive bacteria and Bacteroides fragilis enterotoxin (ETBF). Subsequently, human colon cancer cell lines (DLD-1 cells, WirDr cells) were cultured in the presence of SCFA (butyric acid, isobutyric acid, acetic acid) in order to evaluate the cell growth-inhibitory activity of SCFA (WST-8 assay) by calculating the IC50 value from the dose-response curve.
Results
Intake of BB536-y increased the total amount of SCFA in the feces and significantly suppressed the detection rate of ETBF and growth of putrefactive bacteria. Intake of BB536-y with FOS was associated with a higher Bifidobacterium detection rate than that of BB536-y alone. The contents of butyric acid, isobutyric acid, and acetic acid, namely, of SCFA, were also decreased. Analysis of the results of culture of DLD-1 cells and WirDr cells in the presence of butyric acid, isobutyric acid, and acetic acid revealed that each of the substances showed significant cell growth-inhibitory activity, with the activity being the highest for butyric acid, followed by that for isobutyric acid and acetic acid.
Conclusion
These findings suggest that intake of both BB536-y and BB536-y with FOS prevents colorectal carcinogenesis.
How to cite this article: Ohara T, Suzutani T. Intake of Bifidobacterium longum and Fructo-oligosaccharides prevents Colorectal Carcinogenesis. Euroasian J Hepato-Gastroenterol 2018;8(1):11-17.
Keywords: Colorectal carcinoma, Gastroenterology, Intestinal microbiome, Prebiotics, Probiotics, Short-chain fatty acids.
INTRODUCTION
Patients with colorectal cancer have been reported to show decreased production of SCFA and increased production of putrefactive bacteria, and ETBF in the large bowel, which are attracting attention as risk factors for colorectal carcinogenesis.1-3 The probiotics BB536 has the effect of improving intestinal inflammation and eliminating ETBF.4 Fructo-oligosaccharides have been reported as prebiotics, with the potential to increase the intestinal density of Bifidobacteria.5 Short-chain fatty acids generated by the intestinal flora have been reported to have the effect of inhibiting the production of putrefactive bacteria by acidification of the intestinal environment, and thereby of potentially controlling colon carcinogenesis.1
In this study, in order to investigate the effects of intake of BB536-y and FOS in preventing colorectal carcinogenesis, we examined the amounts of putrefactive bacteria, ETBF, and SCFA in fecal samples obtained from the BB536-y and BB536-y with FOS intake groups, and conducted culture of colon cancer cell lines in the presence of SCFA to study the cell-inhibitory effects of SCFA.
MATERIALS AND METHODS
The subjects were 27 healthy persons (mean age: 60.2 years) who were divided into a group taking yogurt containing BB536 alone (BB536-y group; n = 14) and a group taking yogurt containing BB536-y and FOS (BB536 with FOS group; n = 13). The BB536-y or BB536-y with FOS (6 gm/day), depending on the group, was given to the subjects once a day for 5 weeks. The feces were sampled before and after the intake to analyze the amounts and composition of the SCFA, the profile of the intestinal flora, including the amounts of putrefactive bacteria and ETBF production (Fig. 1, Study 1).
Fig. 1:
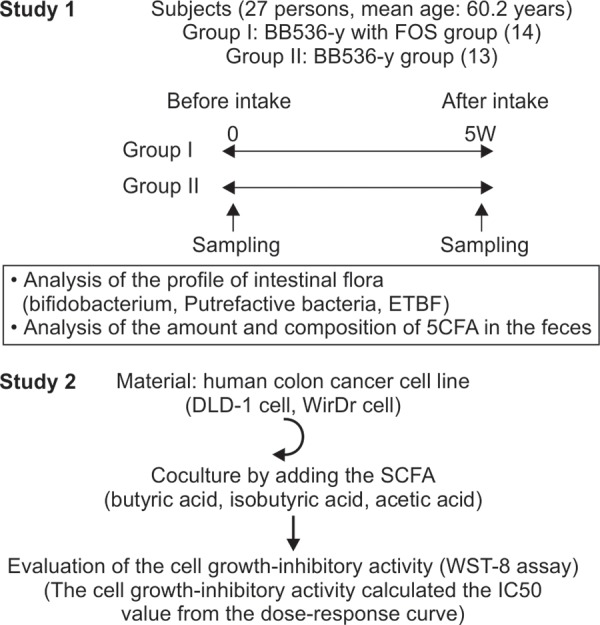
Experimental procedure
Microbial Community, SCFA, and pH Analysis
One gram of each fecal sample was placed immediately after collection in 9 mL of anaerobic diluent in a test tube, which was then tightly stoppered and stored at -80°C until the assay. Analysis of enteric bacterial flora was carried out in accordance with Mitsuoka’s method, but without using the plate-in-bottle technique, and the bacterial count per gram of wet stool specimen was expressed as the log-transformed value. Samples for measurement of the SCFA and pH were taken from the frozen fecal samples. After thawing, 0.5 gm of feces was diluted 5-fold with sterile distilled water and homogenized using a beads shocker (Yasui Kikai Corporation, Osaka, Japan) (2,500 rpm, 4°C, 2 min). The pH value was measured using a semiconducting electrode (ISFET pH meter KS701, Shindengen Electric Manufacturing Co., Ltd. Tokyo, Japan). One milliliter of the homogenate was transferred to a microcentrifuge tube. After centrifugation at 15,000g for 10 minutes at 4°C, 500 μL of supernatant was taken, and then 100 μL of 50 mM NaOH and 500 μL of chloroform were added into the supernatant. After centrifugation at 15,000g for 10 minutes at 4°C, 450 μL of the upper layer was collected and stored overnight at -80°C. Samples were defrosted and recentrifuged at 15,000g for 10 minutes at 4°C, and the supernatant (200 μL) was then filtered through a cellulose acetate filter (0.20 μm; DISMIC-13cp, Tokyo Roshi Kaisha, Ltd., Tokyo, Japan) (ADVANTEC). This supernatant was used to analyze the SCFA and other organic acids by high-performance liquid chromatography (HPLC). Eight organic acids were measured by HPLC (JASCO, Tokyo, Japan) equipped with two ShodexRSpak KC-811 columns (8 mm × 30 cm long; Showa Denko K.K., Tokyo, Japan), and a guard column (ShodexRSpak KC-G, Showa Denko K.K., Tokyo, Japan). The mobile phase used was 5% acetonitrile in 3 mM HClO4; the flow rate was 1.0 mL/min, and the column temperature was 55°C. The postcolumn reaction solution was composed of 0.2 mM bromothymol blue and 1.5 mM Na2HPO4/12H2O. The reaction solution flow rate was 1.5 mL/min. The detector was a multiwavelength detector set for detection at 430 nm (MD-1510; JASCO, Tokyo, Japan).
In vitro Assessment of the Cell Growth-inhibitory Effect of SCFA (Butyric Acid, Isobutyric Acid, and Acetic Acid) against Human Colon Cancer Cells
Colon cancer cell lines (DLD-1, WirDr) were cultured in RPMI 1640, DMEN (containing 25 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) supplemented with 10% fetal bovine serum, 1% penicillin, and streptomycin, and maintained at 37°C in a humidified atmosphere containing 50 mL/L CO2. The cultured cell concentration was adjusted to 2 × 106 cells per well (96-well plate), and Dulbecco’s phosphate-buffered saline was used as a vehicle for the test substance. As for changes in the pH of the cell culture medium by the addition of SCFA, it was ascertained that the pH of the cell culture medium was maintained at a constant value between 7 and 8 even after the addition of high concentrations of the test substances and that there was no intertest substance difference in the effects on the pH, and the influence of SCFA addition was examined by setting 11 graded concentrations (50 mM to 300 μM) of isobutyric acid and butyric acid and also 11 graded concentrations (250 to 1.5 mM) of acetic acid by serial dilution.
Similar to the experiment conducted in a previous study,6 human colon cancer cell lines (DLD-1, WirDr) were cultured in the presence of SCFA (butyric acid, isobutyric acid, acetic acid) and the IC50 values were determined to evaluate the cell growth-inhibitory activity of SCFA (Fig. 1, Study 2).
The cell growth-inhibitory activity was quantified by counting using a WST-8 assay kit (Dojindo Molecular Technologies, Inc., Japan), by measuring the spectropho-tometric absorbance (450 nm) of water-soluble formazan formed via reduction by intracellular dehydrogenase. The IC50 value, representing the test substance concentration, producing a 50% inhibition of cell growth, was calculated from the dose-response curve constructed by plotting the cell count of the control culture against the test substance concentration.
Statistical Analysis
Data are presented as means of n experiments with the standard deviation (mean ± SD) and the standard error (mean ± SE). Statistical data processing was performed with XLSTAT (http://WWW.xlstat.com) using Student’s t-test. The p-values of less than 0.05 were considered as indicative of statistical significance.
RESULTS
Both the BB536-y and BB536y with FOS intake groups showed an increase in the total amount of SCFA and suppression of the growth of putrefactive bacteria in the feces; ETBF could also hardly be detected in the feces in either group (Tables 1 and 2).
Table 1: Results of fecal bacteria before and after intake
| Group | Before intake | 5 weeks | |||||
| I | Total bacteria | 10.25 ± 0.60a | 10.53 ± 0.81 | ||||
| Bifidobacterium | 9.44 ± 0.11 | 9.80 ± 0.44b | |||||
| Frequency of occurrence (%) | (9.7) | (30.6)* | |||||
| Clostridium perfringens | 7.42 ± 0.38 | 5.60 ± 0.65* | |||||
| Frequency of occurrence (%) | (13.2) | (12.6) | |||||
| ETBF | 7.36 ± 0.85 | 1.90 ± 0.33* | |||||
| II | Total bacteria | 10.35 ± 0.50 | 10.55 ± 0.61 | ||||
| Bifidobacterium | 9.33 ± 0.21 | 9.74 ± 0.64 | |||||
| Frequency of occurrence (%) | (9.6) | (30.2)* | |||||
| C. perfringens | 7.40 ± 0.28 | 5.59 ± 0.45* | |||||
| Frequency of occurrence (%) | (13.1) | (12.5) | |||||
| ETBF | 7.40 ± 0.79 | 1.80 ± 0.40* |
aBacteria counts are expressed as mean ± SD of log 10 number per gram wet feces; bFigures in parenthesis are frequency of occurrence (%); Statistically significant at *p < 0.05 levels.
Table 2: Results of fecal putrefactive products before and after intake
| Group | Products | Before intake | 5 weeks | ||||
| I | Ammonia | 52.20 ± 1.88a | 44.20 ± 2.61* | ||||
| Phenol | 3.20 ± 6.66 | 2.15 ± 5.50 | |||||
| p-Cresol | 112.22 ± 60.70 | 98.50 ± 53.86 | |||||
| Indole | 12.01 ± 6.67 | 11.06 ± 7.00 | |||||
| Skatole | 2.10 ± 2.01 | 2.20 ± 2.40 | |||||
| Sulfide | 18.36 ± 5.52 | 12.60 ± 6.22* | |||||
| II | Ammonia | 53.20 ± 1.78 | 43.20 ± 2.7i* | ||||
| Phenol | 3.29 ± 6.10 | 2.25 ± 6.50 | |||||
| p-Cresol | 114.20 = 63.20 | 99.51 ± 57.96 | |||||
| Indole | 12.51 ± 7.67 | 11.12 ± 7.10 | |||||
| Skatole | 2.20 ± 2.11 | 2.20 ± 2.00 | |||||
| Sulfide | 13.86 ± 6.12 | 12.57 ± 6.00* |
aBacteria counts are expressed as mean ± SD of log 10 number per gram wet feces; Statistically significant at *p < 0.05 levels.
The Bifidobacterium detection rate was higher in the BB536-y with FOS group than in the BB536-y group (Table 1).
The butyric acid and isobutyric acid (SCFA) contents were significantly increased in the fecal samples; the acetic acid content was also increased, although the increase was not statistically significant (Table 3).
Table 3: Results of fecal SCFA before and after intake
| Group | Fatty acid | Before intake | 5 weeks | ||||
| I | Lactic acid | 1.42 ± 0.30a | 1.90 ± 2.77 | ||||
| Propionic acid | 2.20 ± 0.50 | 2.37 ± 0.69 | |||||
| Formic acid | 2.18 ± 1.41 | 2.32 ± 1.19 | |||||
| Acetic acid | 4.30 ± 1.00 | 4.33 ± 1.50 | |||||
| Butyric acid | 1.58 ± 0.38 | 2.19 ± 0.80* | |||||
| Isobutyric acid | 5.98 ± 2.30 | 8.01 ± 4.40* | |||||
| Valeric acid | 0.47 ± 0.38 | 0.61 ± 0.38 | |||||
| Isovaleric acid | 3.65 ± 1.30 | 4.48 ± 2.33 | |||||
| Total SCFA | 21.70 ± 1.02 | 26.10 ± 1.80 | |||||
| II | Lactic acid | 1.40 ± 0.40 | 1.91 ± 2.66 | ||||
| Propionic acid | 2.19 ± 0.30 | 2.36 ± 0.70 | |||||
| Formic acid | 2.17 ± 1.38 | 2.33 ± 1.20 | |||||
| Acetic acid | 4.32 ± 1.10 | 4.34 ± 1.60 | |||||
| Butyric acid | 1.58 ± 0.38 | 2.21 ± 0.50* | |||||
| Isobutyric acid | 5.85 ± 2.30 | 8.11 ± 4.80* | |||||
| Valeric acid | 0.50 ± 0.36 | 0.62 ± 0.32 | |||||
| Isovaleric acid | 3.77 ± 1.50 | 4.51 ± 2.43 | |||||
| Total SCFA | 22.80 ± 1.11 | 27.19 ± 1.81 |
aBacteria counts are expressed as mean ± SD of log 10 number per gram wet feces; Statistically significant at *p < 0.05 levels.
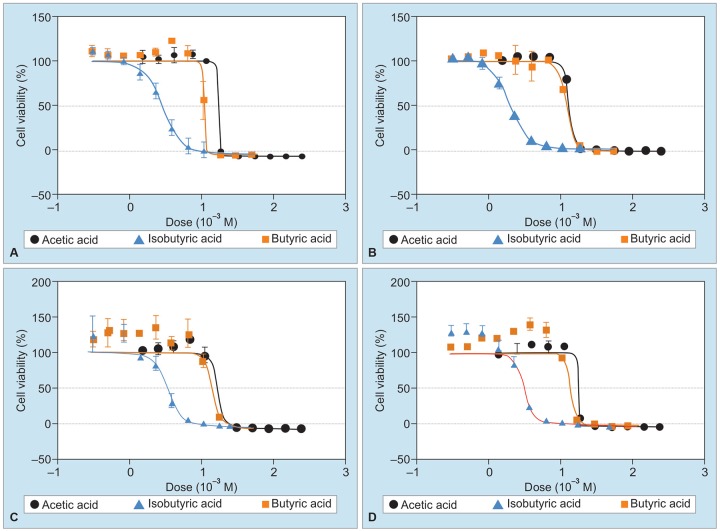
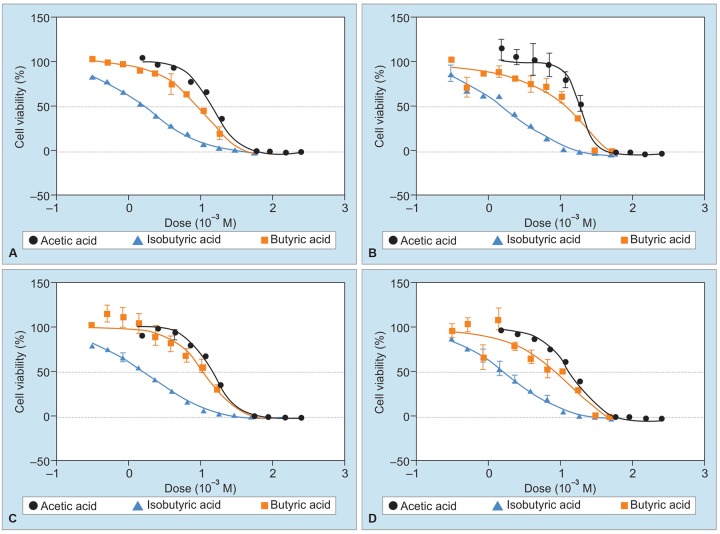
In the culture systems in which DLD-1 cells and WirDr cells were cultured in the presence of butyric acid, isobutyric acid, and acetic acid, each of the substances was found to exert significant cell growth-inhibitory activity, with the IC50 value being the highest for butyric acid, followed by that for isobutyric acid and acetic acid, in that order (Graphs 1, 2 and Table 4).
Graphs 1A to D:
Volume response curves of the cell growth-inhibitory activity in DLD-1 cell
Graphs 2A to D:
Volume response curves of the cell growth-inhibitory activity in WirDr cell
Table 4: Results of the cell growth-inhibitory activity after adding SCFA to colon cancer cell line
| IC50 (mM, mean ± SEM, n = 4) | |||||
| DLD-1 cell | WirDr cell | ||||
| Isobutyric acid | 12.9 ± 0.80 | 10.2 ± 0.77 | |||
| Butyric acid | 2.89 ± 0.29 | 1.59 ± 0.04 | |||
| Acetic acid | 16.6 ± 1.10 | 15.1 ± 1.25 | |||
DISCUSSION
In recent years, it has been reported that the enteric bacterial flora (intestinal microbiota) is involved in the control of the host immune functions and synthesis of glucides and lipids, in the prevention of carcinogenesis.7-9 Control of the enteric bacterial floral balance (intestinal milieu) leads to prevention of various diseases by control of the underlying pathophysiological processes. Functional foods, such as probiotics, prebiotics, and synbiotics have received considerable attention as foodstuffs with the potential to dramatically improve the intestinal milieu.10,11 Prebiotics are sparingly digestible saccharides that take part in the growth of probiotics in the intestine by being taken up by probiotic bacterial species, while synbiotics are food ingredients containing probiotics and prebiotics in synergistic combinations.
Genetic carcinogenesis (familial aggregation carci-nogenesis) accounts for about 20 to 30% of all cases of colorectal cancer, while the remaining approximately 70 to 80% of cases arise as a result of so-called sporadic colorectal carcinogenesis, in which lifestyle/environmental factors, such as diet, physical exercise, and luxury grocery items play a major role.12-14 In regard to the diet, diminished dietary fiber intake results in a delay in the transit time of the contents of the large intestine by inducing increases in the fecal fatty acid and free fatty acid levels and reducing the fecal bulk. Consequent worsening of the intestinal milieu causes an increase in the growth of noxious bacteria and of bile acids in the intestine. The World Cancer Research Fund survey report on the relationship of diet, nutrition, and physical activities with colorectal carcinogenesis identified consumption of red meat and processed meat, habitual alcohol drinking, obesity, and a tall habitus as significant risk factors for colorectal carcinogenesis.15 The report also identified daily physical activity, and consumption of foods containing dietary fiber, milk, and calcium as positive preventive factors.16-18 Dairy products are rich in calcium content, and the incidence of colorectal cancer is known to be low in districts with high consumption levels of dairy products. It has also been reported that production of putrefactive bacteria in the intestines is suppressed in subjects with high intake of dairy products as compared with that in subjects with lesser intake of dairy products, which suggests that alterations in the intestinal milieu may have some impact on colorectal carcinogenesis.1,19,20 Two reports, referred to below, have been published so far on the relationship of the intestinal bacterial flora with colorectal carcinogenesis. According to the first, increased growth of Clostridium perfringens in the feces and an alkalotic stool pH were evident in patients with colorectal cancer, in whom facilitation of intestinal noxious bacteria and depression of intestinal peristalsis were noted.3 Depression of natural killer (NK) cell activity and decreased SCFA production were also evident in patients with colorectal cancer, which could be thought to suppress apoptosis and inhibit bacterial growth.15-22 Furthermore, it has been reported that bu-tyrate prevents colorectal carcinogenesis by controlling histone deacetylase.23,24 The mechanism of the antitu-mor action of SCFA diverges into many branches,25-27 and further examination is necessary. According to the other report, a study in an animal model of colon cancer revealed an increase in the level of ETBF in the feces, which caused persistent chronic inflammation due to destruction of the intestinal GAP junction with evidence of accelerated expression of such transcription factors as STAT-3, which has an antiapoptotic effect.2,3
On the contrary, it has been reported that ingestion of a probiotics (Lactobacillus gasseri) produced dramatic improvement of the intestinal milieu in normal subjects.1 This implies that the ingestion of the probiotics resulted in a conversion of the pH of the intestinal contents to an acidotic pH range, thereby leading to suppression of the growth of putrefactive bacteria and increased SCFA production in the intestines. The report further described an augmentation of the host NK cell activity in response to ingestion of the probiotics.1 The study also demonstrated that augmentation of the NK cell activity served to inhibit tumor cell growth, and that SCFA inhibited cell growth via Wnt signaling.28-32
There was no significant difference in the intestinal milieu-improving effect between a group of healthy subjects receiving BB536-y, i.e., yogurt containing Bifidobacterium longum (probiotics) alone and a group of healthy subjects receiving FOS, a sparingly digestible saccharide, administered in combination with BB536-y (probiotics) in the present study. In both groups, a shift of the fecal pH to the acidotic range, inhibition of putrefactive bacterial proliferation, and increased production of SCFA in the feces, and augmentation of NK cell activity were observed, reflecting a dramatic improvement of the intestinal milieu. Increase in the contents of SCFA, particularly those of butyric acid and isobutyric acid, was noted. Butyric acid and isobutyric acid, in particular, among the SCFA, have been reported to exert an inhibitory effect on colorectal carcinogenesis. The present results suggest that colorectal carcinogenesis can be inhibited by ingestion of functional foods, such as probiotics and prebiotics.
However, the important issue of human enteric bacterial flora varying among individuals needs to be borne in mind in the context of the results of this study.
These differences among individual persons may be attributable to differences in the intestinal commensal bacterial species (organic acid-utilizing organisms). In other words, probiotics take part in the production of SCFA from lactic acid in the intestinal tract via commensalism with intestinal commensal bacteria, and that the intestinal commensal bacterial species vary among individuals, resulting in differences in the compositions of the SCFA produced. Eventually, the effects of ingestion of functional foods, such as prebiotics, probiotics, and synbiotics vary among individuals, posing a major problem in the clinical application of these foods.
Major bacterial species currently used as probiotics are species of Bifidobacterium and Lactobacillus. Many problems remain to be resolved yet in relation to future clinical application of probiotics, and conducting large-scale clinical trials, examination of the behavior of probiotics in the intestinal tract, and identification of intestinal commensal bacteria that produce SCFA via commensalism with particular probiotic bacterial species are needed. Also, the development of new sparingly digestible saccharides (prebiotics) involved in probiotic bacterial growth, and of probiotic bacterial species and synbiotics, which specifically produce beneficial SCFA is needed. We believe that the present report will undoubtedly serve as a foundation for the development of new strategies for preventing colorectal carcinogenesis.
Footnotes
Source of support: Nil
Conflict of interest: None
REFERENCES
- 1.Ohara T, Yoshino K, Kitajima M. Possibility of preventing colorectal carcinogenesis with probiotics. Hepatogas-troenterology. 2010 Nov-Dec;57(104):1411–1415. [PubMed] [Google Scholar]
- 2.Toprak NU, Yagci A, Gulluoglu BM, Akin ML, Demirkalem P, Celenk T, Soyletir G. A possible role of Bacteroides fragilis enterotoxin in the aetiology of colorectal cancer. Clin Microbiol Infect. 2006 Aug;12(8):782–786. doi: 10.1111/j.1469-0691.2006.01494.x. [DOI] [PubMed] [Google Scholar]
- 3.Wu S, Rhee KJ, Albesiano E, Rabizadeh S, Wu X, Yen HR, Huso DL, Brancati FL, Wick E, McAllister F et al. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat Med. 2009 Sep;15(9):1016–1022. doi: 10.1038/nm.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Odamaki T, Sugahara H, Yonezawa S, Yaeshima T, Iwatsuki K, Tanabe S, Tominaga T, Togashi H, Benno Y, Xiao JZ. Effect of the oral intake of yogurt containing Bifidobacterium longum BB536 on the cell numbers of enterotoxigenic Bacteroides fragilis in microbiota. Anaerobe. 2012 Feb;18(1):14–18. doi: 10.1016/j.anaerobe.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 5.Terpend K, Possemiers S, Daguet D, Marzorati M. Arabino-galactan and fructo-oligosaccharides have a different fermentation profile in the Simulator of the Human Intestinal Microbial Ecosystem (SHIME®) Environ Microbiol Rep. 2013 Aug;5(4):595–603. doi: 10.1111/1758-2229.12056. [DOI] [PubMed] [Google Scholar]
- 6.Greening DW, Ji H, Kapp EA, Simpson RJ. Sulindac modulates secreted protein expression from LIM 1215 colon carcinoma cells prior to apoptosis. Biochim Biophys Acta. 2013;1834(11):2293–2307. doi: 10.1016/j.bbapap.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 7.Jensen ML, Thymann T, Cilieborg MS, Lykke M, Molbak L, Jensen BB, Schmidt M, Kelly D, Mulder I, Burrin DG et al. Antibiotics modulate intestinal immunity and prevent necrotizing enterocolitis in preterm neonatal piglets. Am J Physiol Gastrointest Liver Physiol. 2014 Jan;306(1):G59–G71. doi: 10.1152/ajpgi.00213.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Breen DM, Rasmussen BA, Côté CD, Jackson VM, Lam TK. Nutrient-sensing mechanisms in the gut as therapeutic targets for diabetes. Diabetes. 2013 Sep;62(9):3005–3013. doi: 10.2337/db13-0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Myles IA, Fontecilla NM, Janelsins BM, Vithayathil PJ, Segre JA, Datta SK. Parental dietary fat intake alters offspring microbiome and immunity. J Immunol. 2013 Sep;191(6):3200–3209. doi: 10.4049/jimmunol.1301057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mookiah S, Sieo CC, Ramasamy K, Abdullah N, Ho YW. Effects of dietary prebiotics, probiotic and synbiotics on performance, caecal bacterial populations and caecal fermentation concentrations of broiler chickens. J Sci Food Agric. 2014 Jan 30;94(2):341–348. doi: 10.1002/jsfa.6365. [DOI] [PubMed] [Google Scholar]
- 11.Mugambi MN, Musekiwa A, Lombard M, Young T, Blaauw R. Association between funding source, methodological quality and research outcomes in randomized controlled trials of synbiotics, probiotics and prebiotics added to infant formula: a systematic review. BMC Med Res Methodol. 2013 Nov 13;13:137. doi: 10.1186/1471-2288-13-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Percesepe A, Ponz De Leon M. Hereditary factors in tumors of the digestive system. Ann Ist Super Sanita. 1996;32(4):629–642. [PubMed] [Google Scholar]
- 13.Ushio K. Genetic and familial factors in colorectal cancer. Jpn J Clin Oncol. 1985 Apr;15 (Suppl 1):281–298. [PubMed] [Google Scholar]
- 14.Ruder EH, Thiébaut AC, Thompson FE, Potischman N, Subar AF, Park Y, Graubard BI, Hollenbeck AR, Cross AJ. Adolescent and mid-life diet: risk of colorectal cancer in the NIH-AARP Diet and Health Study. Am J Clin Nutr. 2011 Dec;94(6):1607–1619. doi: 10.3945/ajcn.111.020701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Corpet DE. Red meat and colon cancer: should we become vegetarians, or can we make meat safer? Meat Sci. 2011 Nov;89(3):310–316. doi: 10.1016/j.meatsci.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 16.Pierre FH, Martin OC, Santarelli RL, Taché S, Naud N, Guéraud F, Audebert M, Dupuy J, Meunier N, Attaix D et al. Calcium and α-tocopherol suppress cured-meat promotion of chemically induced colon carcinogenesis in rats and reduce associated biomarkers in human volunteers. Am J Clin Nutr. 2013 Nov;98(5):1255–1262. doi: 10.3945/ajcn.113.061069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ukhanova M, Culpepper T, Baer D, Gordon D, Kanahori S, Valentine J, Neu J, Sun Y, Wang X, Mai V. Gut microbiota correlates with energy gain from dietary fibre and appears to be associated with acute and chronic intestinal diseases. Clin Microbiol Infect. 2012 Jul;18 (Suppl 4):62–66. doi: 10.1111/j.1469-0691.2012.03859.x. [DOI] [PubMed] [Google Scholar]
- 18.Solomons NW. Nature’s perfect food revisited: recent insights on milk consumption and chronic disease risk. Nutr Rev. 2002 Jun;60(6):180–182. doi: 10.1301/002966402320243278. [DOI] [PubMed] [Google Scholar]
- 19.Alex S, Lange K, Amolo T, Grinstead JS, Haakonsson AK, Szalowska E, Koppen A, Mudde K, Haenen D, Al-Lahham S et al. Short-chain fatty acids stimulate angiopoietin-like 4 synthesis in human colon adenocarcinoma cells by activating peroxisome proliferator-activated receptor γ. Mol Cell Biol. 2013 Apr;33(7):1303–1316. doi: 10.1128/MCB.00858-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holma R, Korpela R, Sairanen U, Blom M, Rautio M, Poussa T, Saxelin M, Osterlund P. Colonic methane production modifies gastrointestinal toxicity associated with adjuvant 5-fluorouracil chemotherapy for colorectal cancer. J Clin Gastroenterol. 2013 Jan;47(1):45–51. doi: 10.1097/MCG.0b013e3182680201. [DOI] [PubMed] [Google Scholar]
- 21.Jahns F, Wilhelm A, Jablonowski N, Mothes H, Radeva M, Wolfert A, Greulich KO, Glei M. Butyrate suppresses mRNA increase of osteopontin and cyclooxygenase-2 in human colon tumor tissue. Carcinogenesis. 2011 Jun;32(6):913–920. doi: 10.1093/carcin/bgr061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fung KY, Ooi CC, Zucker MH, Lockett T, Williams DB, Cosgrove LJ, Topping DL. Colorectal carcinogenesis: a cellular response to sustained risk environment. Int J Mol Sci. 2013 Jun 27;14(7):13525–13541. doi: 10.3390/ijms140713525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lazarova DL, Chiaro C, Wong T, Drago E, Rainey A, O’Malley S, Bordonaro M. CBP activity mediates effects of the histone deacetylase inhibitor butyrate on WNT activity and apoptosis in colon cancer cells. J Cancer. 2013 Jul 15;4(6):481–490. doi: 10.7150/jca.6583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kamitani H, Taniura S, Ikawa H, Watanabe T, Kelavkar UP, Eling TE. Expression of 15-lipoxygenase-1 is regulated by histone acetylation in human colorectal carcinoma. Carcinogenesis. 2001 Jan;22(1):187–191. doi: 10.1093/carcin/22.1.187. [DOI] [PubMed] [Google Scholar]
- 25.Worthley DL, Whitehall VL, Le Leu RK, Irahara N, Buttenshaw RL, Mallitt KA, Greco SA, Ramsnes I, Winter J, Hu Y et al. DNA methylation in the rectal mucosa is associated with crypt proliferation and fecal short-chain fatty acids. Dig Dis Sci. 2011 Feb;56(2):387–396. doi: 10.1007/s10620-010-1312-4. [DOI] [PubMed] [Google Scholar]
- 26.Munjal U, Glei M, Pool-Zobel BL, Scharlau D. Fermentation products of inulin-type fructans reduce proliferation and induce apoptosis in human colon tumour cells of different stages of carcinogenesis. Br J Nutr. 2009 Sep;102(5):663–671. doi: 10.1017/S0007114509274770. [DOI] [PubMed] [Google Scholar]
- 27.Bajka BH, Clarke JM, Cobiac L, Topping DL. Butyrylated starch protects colonocyte DNA against dietary protein-induced damage in rats. Carcinogenesis. 2008 Nov;29(11):2169–2174. doi: 10.1093/carcin/bgn173. [DOI] [PubMed] [Google Scholar]
- 28.Rocca YS, Roberti MP, Arriaga JM, Amat M, Bruno L, Pampena MB, Huertas E, Loria FS, Pairola A, Bianchini M et al. Altered phenotype in peripheral blood and tumor-associated NK cells from colorectal cancer patients. Innate Immun. 2013 Feb;19(1):76–85. doi: 10.1177/1753425912453187. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Z, Song Y, Wang XL. Inositol hexaphosphate-induced enhancement of natural killer cell activity correlates with suppression of colon carcinogenesis in rats. World J Gastro- enterol. 2005 Aug 28;11(32):5044–5046. doi: 10.3748/wjg.v11.i32.5044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vannucci L, Fiserova A, Horvath O, Rossmann P, Mosca F, Pospisil M. Cancer evolution and immunity in a rat colorectal carcinogenesis model. Int J Oncol. 2004 Oct;25(4):973–981. [PubMed] [Google Scholar]
- 31.Bordonaro M. Crosstalk between Wnt signaling and RNA processing in colorectal cancer. J Cancer. 2013;4(2):96–103. doi: 10.7150/jca.5470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lazarova DL, Wong T, Chiaro C, Drago E, Bordonaro M. p300 influences butyrate-mediated WNT hyperactivation in colorectal cancer cells. J Cancer. 2013 Jul 18;4(6):491–501. doi: 10.7150/jca.6582. [DOI] [PMC free article] [PubMed] [Google Scholar]