Abstract
Patients with early estrogen receptor-positive breast cancer are at continuous risk of relapse even after more than 10 years of follow up. Currently, no biomarker that identifies patients for early versus late recurrence, or one that selects patients or tumors for longer versus shorter durations of endocrine therapy (ET) is available and a crucial question is how to properly select patients who could be spared extended ET or those who require it. In the last 20 years more than 40,000 women were enrolled in randomized trials to answer the question of optimal duration of ET. According to the results of these studies extended adjuvant ET is more effective than standard 5 years of adjuvant ET. Extended ET in patients who remain premenopausal after 5 years of adjuvant tamoxifen is still tamoxifen for another 5 years. Extended ET with aromatase inhibitors (AIs) should be offered to postmenopausal women with substantial residual risk of relapse after completing 5 years of tamoxifen therapy. Extension of AI treatment to 10 years resulted in significantly better 5-year disease-free survival including disease recurrence local/distant or the occurrence of contralateral breast cancer events. Currently, new therapeutic targets are under investigation, but the beneficial effect of prolonged treatment for high-risk patients, identified by using multigenomic tests, remains unclear. Thus, further studies need to be performed to confirm the advantage of extended adjuvant ET in selected patients.
Keywords: aromatase inhibitor, breast cancer, gene-expression profiling, hormone receptor-positive breast cancer, hormone therapy, luminal breast cancer, molecular testing, tamoxifen
Introduction
Patients with breast cancer are at continuous risk of relapse. Timing and pattern of breast cancer recurrence varies considerably and is influenced by classic prognostic factors and by adjuvant treatment strategies.1,2
Recurrences continue to occur later in follow up (e.g. years 5–10),1 especially in patients with estrogen receptor (ER)-positive disease, and more than two-thirds of deaths occur beyond 5 years from diagnosis,3 suggesting a potential mechanism of cell dormancy for a protracted period of time, despite adjuvant therapies.4 Independent genetic and epigenetic traits may arise and drive the recurrences that were not present in the original primary tumors.5
The proportion of patients carrying dormant cells may be even higher, as in some patients these cells never progress to become macrometastases during the patient’s lifetime, and even if they do, the resultant slowly growing metastases may remain asymptomatic and undetected.4
The recent Early Breast Cancer Trialists’ Collaborative Group meta-analysis after 5 years of adjuvant endocrine therapy (ET) reported the influence of various characteristics of the original tumor on the 20-year incidence of breast cancer outcomes. The 88 trials analyzed involved 62,923 women and among those with ER-positive, early stage breast cancer who were scheduled to receive only 5 years of adjuvant ET, distant recurrences occurred at a steady rate for at least another 15 years after the end of the 5-year treatment period. Throughout this time, the original nodal status and tumor diameter remained remarkably strong determinants of the annual recurrence rate.6
Results from the International Breast Cancer Study Group trials I–V after 24 years of follow up were in line with these findings showing that patients with ER-positive tumors continued to have a higher risk of relapse, including distant metastases, during years 5–25, reinforcing the need for long-term clinical follow up to understand the pattern of recurrence of breast cancer and confirm that micrometastases are completely eradicated.7 In particular, the population of ER-positive patients maintained a significant risk of relapse even after more than 10 years of follow up.
Nowadays considerable efforts are spent in identifying areas where optimal care may be achieved with ‘less’ or ‘more’ treatment and the needs of a specific patient may be better defined through consideration of subset analyses or individualized approaches to care, in order to decide whether to escalate or de-escalate treatment.
To date, there is no biomarker that identifies patients for early versus late recurrence, or one that selects patients or tumors for longer versus shorter durations of ET. As a result, a crucial question to be answered in the near future is how to select appropriately patients who could be spared extended ET or those who require it.
Trials investigating ET duration
Tamoxifen
Large randomized clinical trials have been conducted to evaluate the role of extended ET with the primary goal of preventing or at least delaying distant relapses (Table 1). The rationale for these trials was based on the known natural history of breast cancer with an annual rate of death of approximately 5% for at least 15 years, even after 5 years of tamoxifen therapy.8
Table 1.
Trials on extended endocrine treatment.
| Trial | Population | Menopausal status | Follow-up years | Previous treatment | Extended treatment | HR disease-free survival (p) | HR overall survival (p) |
|---|---|---|---|---|---|---|---|
| National Surgical Adjuvant Breast and Bowel Project B-14 (NSABP-B14), | 1172 ER+/N- | Pre- and post- | 7 | TAM | TAM | 1.3 (p = 0.03) | NR (p = 0.07) |
| Adjuvant Tamoxifen: Longer Against Shorter (ATLAS) | 6846 ER+/N any | Pre- and post- | 8 | TAM | TAM | 0.84 (p = 0.002) | 0.71 (p = 0.01) |
| aTTom | 6953 ER+ (39%)/N any |
Pre- and post- | 9 | TAM | TAM | 0.86 (p = 0.003) | 0.91 (p = NS) |
| MA.17 | 5187 ER+/any N | Post- | 5.4 | TAM | LET | 0.52 (p < 0.001) | 0.61 (p < 0.001) |
| Austrian Breast and Colorectal Study Group (ABCSG) 6a | 586 ER+/any N |
Post- | 5.2 | TAM | ANA (3 years) | 0.62 (p = 0.031) | 0.89 |
| NSABP B-33 | 1598 ER+/any N |
Post- | 2.5 | TAM | EXE | 0.68 (p = 0.07) | NS |
| SOLE | 4884 ER+/N+ |
Post- | 5 | Any ET | LET cont. versus int. | 1.08 (p = 0.31) | 0.85 (p = 0.16) |
| DATA | 1912 ER+/any N |
Post- | 4.1 | TAM 2–3 years | ANA 6 years versus 3 years | 0.79* (p = 0.7) | 0.91* (p = 0.60) |
| NSABP B-42 | 3966 ER+/any N |
Post- | 6.9 | AI or TAM/AI | LET | 0.85 (p = 0.048)# | 1.15 (p = 0.22) |
| IDEAL | 1824 ER+/any N |
Post- | 6.6 | Any ET | LET 5 years versus 2.5 years | 0.92 (p = 0.49) | 1.04 (p = 0.79) |
| ABCSG-16 | 3484 ER+/any N |
Post- | 8.8 | Any ET | ANA 2 years versus 5 years | 1.007 (p = 0.925) | NS |
| MA. 17R | 1918 ER+/any N |
Post- | 6.3 | TAM | LET | 0.66 (p = 0.01) | 0.97 (p = 0.83) |
Adapted disease-free survival; adapted overall survival.
p value did not reach statistical significance level of 0.0418.
AI, aromatase inhibitor; ANA, anastrozole; ER, estrogen receptor; ET, endocrine therapy; EXE, exemestane; HR, hazard ratio; LET, letrozole; NS, not significant; TAM, tamoxifen.
The National Surgical Adjuvant Breast and Bowel Project B-14 (NSABP-B14),9 aTTom trial,10 and the Adjuvant Tamoxifen: Longer Against Shorter (ATLAS) trial11 were the three main prospective trials evaluating the role of extended tamoxifen treatment and included the largest number of patients. They had a similar design: after 5 years of treatment with tamoxifen, patients were randomized to additional tamoxifen. ET with tamoxifen significantly reduced breast cancer recurrence rates and mortality in the ER-positive subgroup of patients. This effect was mainly seen after the first decade (hazard ratio [HR] 0.75, 95% confidence interval [CI] 0.62–0.90). Previous trials of 5 years of tamoxifen have shown a carryover benefit more than 10 years after discontinuation.12 Thus, the benefit of continuing tamoxifen for a further 5 years belongs to the carryover benefit from the first 5 years and to the concurrent benefit of a further 5 years of tamoxifen. Overall the global benefit translates into a total relapse risk reduction of 39% (p < 0.0001) and a risk reduction of breast cancer mortality of 36% (p < 0.0001). After completion of 10 years of treatment, this estimated risk was reduced by 30% for relapse (two-sided p = 0.01) and 48% for mortality (two sided p < 0.0001), continuing for at least 5 years.13
A meta-analysis in unselected patients included data from the three above mentioned trials plus two additional smaller trials: the Scottish Adjuvant Tamoxifen Trial (342 patients)14 and the Eastern Cooperative Oncology Group (ECOG) adjuvant trials E4181/ES181 (193 patients).15 The total number of patients was 21,554, the majority being postmenopausal (87%). Among all randomized patients, extended adjuvant tamoxifen was not associated with a significant reduction in the odds of breast cancer recurrence (odds ratio [OR] 0.89, 95% CI 0.76–1.05; p = 0.17). Patients with lymph node-positive breast cancer derived some reduction in recurrence although the long-term effects of this on all-cause death remained unclear.16 The meta-analysis had some limitations, as the trials included had different follow-up times, some had a short follow-up time (i.e. less than 10 years), there was no access to ER status in a large proportion of patients, and were not derived from individual patient-level data.
Aromatase inhibitors
MA.17 was the first, large, randomized, double-blind, placebo-controlled phase III study investigating the role of extended adjuvant therapy with letrozole following completion of around 5 years of standard tamoxifen in postmenopausal women with hormone receptor (HR)-positive early stage breast cancer.17 The first interim analyses of the trial results after a median follow up of 2.5 years confirmed that letrozole significantly reduced the risk of recurrent breast cancer (42%), regardless of the patient’s nodal status or receipt of prior chemotherapy, and significantly reduced the risk of distant metastasis (40%). Importantly, letrozole as extended adjuvant therapy achieved a significant improvement in overall survival (OS) in women with node-positive disease. Mortality was reduced by 39% among the approximately 2500 women with node-positive disease randomized in the study.18 Notably, Goss and colleagues found an absolute improvement in disease-free survival (DFS) at 4 years in the premenopausal subset of patients (those who started as premenopausal), 9.7% compared with 3.4% in those postmenopausal from the start.19 Remarkably, in this analysis, premenopausal women with node-negative disease experienced 100% DFS on extended letrozole.
Other two smaller trials, the NSABP-B3320 and the Austrian Breast and Colorectal Study Group (ABCSG) trial 6a,21 also confirmed the efficacy of extending aromatase inhibitor (AI) treatment beyond the standard 5 years of tamoxifen therapy.
Conversely, the phase III randomized, double-blind clinical trial, NSABP B-42, presented at the 2016 San Antonio Breast Cancer Symposium, did not show a similar benefit for extending adjuvant ET beyond 5 years. Median follow-up time from randomization for the 3923 patients included in the analyses was 6.9 years.22 Extended letrozole therapy resulted in a nonsignificant 15% reduction in the risk of a DFS event, which included a recurrence of the original breast cancer, cancer in the opposite breast, a nonbreast malignancy, or death from any cause prior to the occurrence of one of the other DFS events. Extended letrozole therapy did not improve OS. However, a statistically significant improvement in breast cancer-free interval was noted, with a 29% reduction in the risk of breast cancer recurrence or cancer in the opposite breast as a first event. In addition, a 28% statistically significant reduction in the cumulative risk of distant recurrence was observed.
The phase III DATA study randomly assigned 1912 postmenopausal women who had received 2–3 years of adjuvant tamoxifen to either 3 years or 6 years of anastrozole. The primary endpoint was adapted DFS (aDFS), defined as DFS starting 3 years after randomization. The study was designed to detect an increase in the aDFS with 6 years of anastrozole versus 3 years corresponding with a HR of 0.60. Instead, the HR was 0.79 (p = 0.07). The 5-year aDFS was 83.1% for patients receiving an additional 6 years of anastrozole and 79.4% for those receiving 3 years. In a subgroup analysis that was not prespecified, extended anastrozole showed a significant effect on aDFS in patients with ER-positive and progesterone receptor-positive, HER2-negative, node-negative disease, and who had received neoadjuvant chemotherapy. For patients with ⩾pT2, node-positive and all of the above-mentioned factors, a further increase in the 5-year aDFS was observed when randomized to 6 years of anastrozole treatment (86.0% versus 75.9%, HR 0.58 (0.39–0.89); p = 0.01). In conclusion, results of the DATA trial do not support extended adjuvant therapy for all postmenopausal patients with HR-positive breast cancer, whereas there is a selected group of patients that seems to benefit.23
The randomized phase III IDEAL trial compared a total of 7.5 years versus 10 years of ET. Patients received 5 years of adjuvant tamoxifen (10%), AI (30%), or tamoxifen followed by an AI (60%). The 1824 patients were randomized to letrozole for 2.5 years or 5 more years of extended therapy. The 5-year DFS rate was 82% in patients receiving 2.5 more years of treatment and 83.4% for those receiving an additional 5 years (HR = 0.92; p = 0.49). OS and distant metastasis-free interval were not different between both groups. A reduction in occurrence of second primary breast cancer was observed with 5 years of treatment (HR 0.39). Subgroup analysis did not identify patients who benefited from 5 years of extended therapy.24
Results from the ABCSG-16 phase III trial were presented at the 2017 San Antonio Breast Cancer Symposium. From February 2004 to June 2010, 3484 postmenopausal women with HR+ early stage breast cancer were enrolled into this trial. The patients received an initial 5 years of adjuvant ET, and were randomly assigned to 2 years (n = 377) or 5 years (n = 380) of extended adjuvant therapy. About 50% of the enrolled patients were initially treated with tamoxifen and the remainder with AIs or any sequence of tamoxifen and AI. At 10 years from randomization, the DFS rates were 71.1% for the 2-year group versus 70.3% for the 5-year group (HR 0.997, 95% CI 0.86–1.15; p = 0.982). At 10 years following randomization, 85.3% of patients in the 2-year arm were alive versus 84.9% of those in the 5-year arm (HR 1.007; p = 0.947). There was no difference between an additional 2 years and 5 years of anastrozole in terms of OS, time to contralateral breast cancer, and time to second primary cancer, all of which were secondary endpoints in the trial.25
Recent published results of the SOLE trial showed that in postmenopausal women with HR-positive breast cancer, extended use of intermittent letrozole did not improve DFS compared with continuous use of letrozole. After a median follow up of 60 months, DFS was 85.8% (95% CI 84.2–87.2) in the intermittent letrozole group compared with 87.5% (86.0–88.8) in the continuous letrozole group (HR 1.08, 95% CI 0.93–1.26; p = 0.31). Adverse events were reported as expected and were similar between the two groups. The results of the SOLE trial supported the safety and feasibility of temporary treatment breaks in selected patients who might require them because of side effects during extended ET. The magnitude of the beneficial effect of extended letrozole use in postmenopausal women who had previously received an AI during the first 5 years is low and should be weighed against the side effects.26
The MA.17R trial, a double-blind, placebo-controlled trial, included 1918 patients who had received AI for a total of 4.5–6 years. In most cases, AI therapy was preceded by tamoxifen treatment and 20.7% of the patients received the AI as single adjuvant ET. Patients were further randomized to either 5 years of letrozole or placebo. After a median follow up of 6.3 years, the 5-year DFS was significantly longer (95%) for patients receiving letrozole compared with those receiving placebo (91%). The 5-year OS did not differ significantly between patient groups (93% versus 94%; p = 0.83). Women in the extended letrozole group had a 34% lower risk of breast cancer recurrence (HR 0.66 [0.48–0.91; p = 0.01]). The annual incidence of contralateral breast cancer was lower in the letrozole group than in the placebo group (0.21% versus 0.49%), indicating a breast cancer prevention effect. Overall, there were no significant differences in either overall quality of life or menopause-specific quality of life between women treated with letrozole for 5 years and those receiving placebo.27 Following the MA.17 trial demonstrating that 5 years of letrozole improved DFS in breast cancer patients after 5 years of tamoxifen therapy, a question remained: might longer treatment be better?
Genomic tools to predict late recurrences
A first step to an individualized extended ET for preventing late metastases is to identify women at risk and to understand the underlying biology. Clinical factors such as increased tumor size and nodal positivity have been shown to be associated with late relapse.28 In particular, tumors with node-positive disease remain at sufficiently high risk, even after 5 years of ET, to justify extension of ET. As already mentioned in the MA.17 trial, the greatest benefit of extended letrozole therapy was seen in the node-positive subgroup of patients.17
Gene-expression profiling represents a next step to individualized breast cancer management. It might help to predict the risk of relapse in the individual patient. The prognostic value of the oncotype DX recurrence score (RS) for late/distant recurrence (>5 years) was first evaluated in the Anastrozole or Tamoxifen Alone or Combined (ATAC) trial (transATAC).29 In this analysis, oncotype DX was not prognostic for late/distant relapse after adjustment for classical clinical–pathological factors. A more recent analysis of the NSABP B-14 trial showed that oncotype DX RS was prognostic for late/distant recurrence in patients with higher quantitative estrogen receptor expression (ESR1) mRNA level,30 with a low risk of distant recurrence in years 6–10 for patients with high ESR1 mRNA and low RS (6.8%). The results suggested that patients with intermediate and high RS with higher ESR1 levels at initial diagnosis might benefit from extended adjuvant therapy and provide further evidence that ESR1 signaling is biologically involved in late metastasis.
The Breast Cancer Index (BCI) consists of two independent markers: HOXB13/IL17BR (H/I) gene-expression ratio and molecular-grade index (MGI) (a set of cell cycle-related genes). BCI has been shown to predict the 10-year distant recurrence rate in ER-positive, node-negative patients. In the MA.17 trial it was found that BCI and H/I but not MGI were predictive of late relapse and HOXB13 expression at diagnosis was predictive of benefit from extended letrozole therapy.31 High H/I was statistically significantly associated with a decrease in late recurrence in patients receiving extended letrozole therapy. When adjusted to classical clinical–pathological factors, high H/I remained statistically significantly associated with patient benefit from letrozole. In addition, the BCI was evaluated in the transATAC study cohort for the prognostication of distant recurrence in a late period of follow up.32 It was shown that the BCI as a continuous score was an independent strong factor for late/distant recurrence in women with node-negative disease, as it identified patients at sufficiently low risk of late/distant relapse that could avoid extended ET.
The PAM50 is another prognostic multigene test. Based on the PAM50 gene analysis, the PAM50 risk of recurrence (ROR) score can be calculated and patients can be assigned to defined ROR-based risk groups. The usefulness of this test was investigated in 1246 patients in the ABCSG-8 trial.33 Depending on their ROR score, patients were assigned to a low, intermediate, or high-risk group. Between years 5 and 15, the absolute risk of distant recurrence was 17.5% in the high-risk group and 2.4% in the low-risk group (HR 6.90 [1.89–11.87]; p < 0.001), respectively.
EndoPredict was evaluated in the transATAC cohort of patients for the prediction of late/ distant relapses.34 These findings confirmed that EPclin might identify a subgroup of patients with HR-positive, HER2-negative disease irrespective of nodal status with a low-risk of late/distant recurrence. Proliferation genes analyzed in this cohort added independent prognostic information to all clinical parameters included in the model for the prediction of early recurrences (0–5 years). In fact, a high expression of genes, thought to contribute to cell-cycle progression, was significantly associated with higher rates of distant metastasis during the first 5 years but no longer showed a significant additional prognostic performance during the timespan thereafter. In contrast, genes associated with ER signaling were not significantly associated with early metastases but showed additional prognostic information in the second time interval. The EPclin score identified a low-risk group of women accounting for 64% of patients after 5 years of follow up. These patients have an absolute risk of distant metastasis of 1.8% between 5 years and 10 years of follow up and might be sufficiently treated with 5 years of adjuvant ET.34
Most of the prognostic multigene tests rely heavily on genes associated with cell-cycle progression and/or proliferation. Not surprisingly, these signatures failed to identify late events.35 For instance, the oncotype DX assay failed to identify late/distant metastases in the transATAC trial.31 In contrast, the BCI was found to be a significant prognostic factor in the same trial.31 BCI combines genes that are associated with proliferation and ER signaling.36
Although a large amount of data was produced using the genomic tests to identify patients who might relapse late, no prospective data exist and we do not have yet sufficient information to justify the routine use of these tests. Currently, none of the main international guidelines including the American Society of Clinical Oncology, European Society of Medical Oncology, and the St. Gallen Consensus Statement recommends the use of multigene assays for the prognostication of late/ distant recurrence.
Cost, benefits and adherence to ET
For premenopausal women undergoing extended ET the long-term costs and consequences of premature menopause from ovarian function suppression (OFS) are unknown, but could be estimated through a cost-effectiveness analysis performed with a Markov chain Monte Carlo simulation model. In a hypothetical cohort of premenopausal women with ER-positive early breast cancer the costs and benefits of three extended endocrine strategies were estimated: (a) no further treatment; (b) tamoxifen for 5 years (extended tamoxifen); or (c) OFS/AI for 5 years. Effectiveness was measured in years of life-expectancy gain. The simulation covered a 40-year period. Extended tamoxifen yielded a higher average life-expectancy gain than OFS/AI (17.31 years versus 17.06 years) at lower average cost (US$3550 versus US$14,312). For 18,000 premenopausal ER-positive women, the simulation estimated 13,236, 12,557, and 11,338 deaths with no further treatment, extended tamoxifen, and OFS/AI, respectively, but an additional 1897 deaths from OFS, for a total of 13,235 deaths associated with OFS/AI. After 24.6 years of follow up, more women are expected to die from OFS/AI than extended tamoxifen. For premenopausal women with ER-positive cancer who have completed adjuvant tamoxifen, another 5 years of tamoxifen is the preferable extended ET strategy.37
For postmenopausal women undergoing extended ET, letrozole was more cost effective in node-positive patients than in node-negative patients (Can$26,553 versus Can$46,049 per quality-adjusted life year [QALY]). Results were robust to variations in age, healthcare costs, and utilities.38 Extended AI and extended tamoxifen result in improved QALYs and lower healthcare costs versus standard tamoxifen. Extended AI results in the greatest improvement in QALYs and is the most cost-effective treatment alternative despite its higher drug costs.39
The risks of side effects such as bone fracture for AIs, pulmonary embolus, and endometrial cancer for women with a uterus while on tamoxifen increase with longer treatment, although the absolute risk of death from them is low (< 0.5%).10,11,18 Cumulative toxicity of AIs compared with tamoxifen was investigated in a systematic review and meta-analysis comprising 7 trials and 30,023 patients.40 Longer AI therapy was associated with an increased risk of bone fractures (OR = 1.47), cardiovascular disease (OR = 1.26), and hypercholesterolemia (OR = 3.14), while longer AI therapy had a decreased risk of venous thrombosis (OR = 0.55) and endometrial cancer (OR = 0.34) compared with tamoxifen.
Adherence to ET may represent an issue; in fact only about 50% of women successfully complete 5 years therapy. It has been reported that 50% of patients discontinue treatment with tamoxifen or an AI within 3 years.41 Factors associated with nonadherence include low recurrence risk perception, side effects, age extremes, medication cost, suboptimal patient–physician communication, and lack of social support. Nonadherence appears to have an effect on the prognosis and only a few studies have investigated predictive factors for it.42,43
Recently, the Evaluation of Therapy Management and Patient Compliance in Postmenopausal Hormone Receptor-positive Breast Cancer Patients Receiving Letrozole Treatment (EvAluate-TM) study prospectively investigated treatment persistence on adjuvant letrozole in a population of 3941 postmenopausal women with early breast cancer. The main factors influencing premature treatment discontinuation were older age (HR 1.02/year), comorbidities (HR 1.06/comorbidity), low body mass index, and lower tumor grade (HR 0.85/grade unit). Authors concluded that older, multimorbid patients with low tumor grade and low body mass index are at the greatest risk for treatment discontinuation and might benefit from compliance and support programs promoting compliance.44
Optimal duration of ET
According to the results of the studies described above, there is evidence that extended adjuvant ET is more effective than standard 5 years of adjuvant ET. Figure 1 summarizes the evidence from the main clinical studies and indicates the optimal duration of ET in different patient settings, while considering the limitations that may come from clinical practice, patient preference, and possible comorbidities. Based on the MA.17 trial results, ‘perimenopausal’ women that become postmenopausal after initial chemotherapy or during tamoxifen therapy should be encouraged to continue with extended ET with AIs. The current option for extended ET in patients who remain premenopausal after 5 years of adjuvant tamoxifen treatment is tamoxifen for another 5 years, based on the ATLAS and aTTom trials. Pooled analysis of these results have confirmed that 10 years of adjuvant tamoxifen compared with no ET reduces breast cancer deaths by 33% in the first 10 years, and by a further 25% beyond 10 years.10 St Gallen panelists in 2017 recommended that premenopausal women who are at high risk for recurrence and have concluded 5 years of tamoxifen should extend ET to 10 years.44 Results from the recent Suppression of Ovarian Function Trial (SOFT) and Tamoxifen and Exemestane Trial (TEXT) recommend OFS for high-risk premenopausal patients in the first 5 years of ET, in combination with tamoxifen or exemestane46,47 (e.g. younger patients and/or with node involvement). Unfortunately, we do not have any data on extended ET after 5 years of either AI or an AI plus OFS therapy, especially in the premenopausal setting. In postmenopausal women AIs after 5 years of tamoxifen led to significant improvement in DFS and in OS improvement in the high-risk, node-positive subset of patients.48 Therefore, extended ET with AIs should be offered to postmenopausal women with substantial residual risk of relapse after completing 5 years of tamoxifen therapy.19 Results from the MA.17R trial support extended treatment of AI up to 10 years for a specific subset of patients with high residual risk of late relapse, although no OS benefit was seen.27 Extension of AIs to 2–3 years compared with 5–6 years was also recently investigated by the IDEAL,24 DATA,23 and ABCSG-1625 trials but none of these trials showed an overall advantage for a longer duration of AI treatment, although a longer duration of AI could be discussed for selected high-risk patients, according to the results of a subgroup analysis from the DATA trial.23
Figure 1.
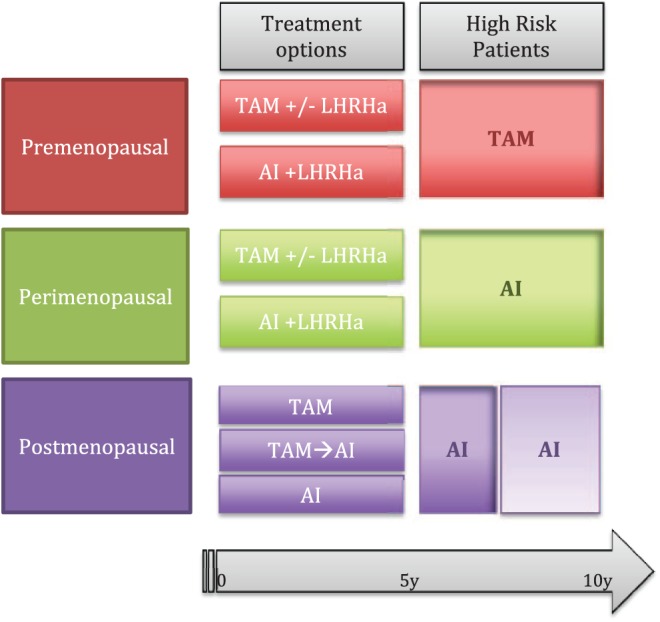
Optimal duration of endocrine therapy (ET).
The figure summarizes the evidence derived from clinical studies: the optimal duration of ET in different patient settings. Treatment options are those currently available for the first 5 years. High-risk patients are those who might require an extended treatment and their characteristics are defined in the text.
AI, aromatase inhibitor; LHRHa, luteinizing hormone-releasing hormone agonist; TAM, tamoxifen.
Patients who do not fall into the high-risk categories, those with small, low-proliferating tumors without the involvement of the axillary lymph nodes, according to current evidence could benefit from 5 years of ET, without the need for extended therapy.
Conclusion
The first study aiming to investigate extended adjuvant therapy in women with endocrine-responsive breast cancer began almost 20 years ago. During these years, nearly 40,000 women have been enrolled in randomized clinical trials with the aim of answering this question. Nowadays, although many aspects have certainly been clarified, we still do not know precisely how to select women who should be treated with extended therapy and those who require short-term therapy or even no treatment. As previously illustrated, there are some patients who have tumors and characteristics with little evidence supporting the use of prolonged adjuvant ET. Many of these do very well with tamoxifen alone for up to 5 years and this might be the only economically viable alternative in many circumstances.
There is also a consistent group of patients, either pre- or postmenopausal, who will benefit from extended ET. This group of patients includes those who had a tumor for which they received chemotherapy, or had axillary lymphnode involvement, or the tumor had biological characteristics that made them fall into categories considered at risk.
Although some of these clinical and biological aspects have been clarified by clinical studies, there are still patients for whom it is unclear whether they can benefit from extensive ET.
Currently, new therapeutic targets are under investigation aiming to either kill awoken dormant cells or keep them permanently dormant.49 As recently reported in clinical phase III trials, new compounds like cyclin-dependent kinases play an important role in cell-cycle progression and targeting these kinases led to a cell-cycle block and improvement in the outcome of ER-positive breast cancer subtypes. Based on this, the CDK4/6 inhibitors seem to be promising agents and, among them, 2 years of adjuvant palbociclib, in combination with ET, is currently being tested in the ongoing phase III PALbociclib CoLlaborative Adjuvant Study (PALLAS) trial. Results of this trial will inform the need for further extended treatment in high-risk pre- and postmenopausal ER-positive early breast cancer patients.
The beneficial effect of prolonged treatment for high-risk patients, identified by using multigenomic tests, remains unclear. Thus, further studies need to be performed to confirm the advantage and proper timing of extended adjuvant ET in a selected population.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare no conflicts of interest in preparing this article.
Contributor Information
Elisabetta Munzone, Division of Medical Senology, European Institute of Oncology, Milan, Italy.
Marco Colleoni, Division of Medical Senology, European Institute of Oncology, Via Ripamonti, 435, Milano 20141, Italy.
References
- 1. Pagani O, Price KN, Gelber RD, et al. Patterns of recurrence of early breast cancer according to estrogen receptor status: a therapeutic target for a quarter of a century. Breast Cancer Res Treat 2009; 117: 319–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Goldhirsch A, Gelber RD, Price KN, et al. Effect of systemic adjuvant treatment on first sites of breast cancer relapse. Lancet 1994; 343: 377–381. [DOI] [PubMed] [Google Scholar]
- 3. Saphner T, Tormey DC, Gray R. Annual hazard rates of recurrence for breast cancer after primary therapy. J Clin Oncol 1996; 14: 2738–2746. [DOI] [PubMed] [Google Scholar]
- 4. Zhang XH-F, Giuliano M, Trivedi MV, et al. Metastasis dormancy in estrogen receptor-positive breast cancer. Clin Cancer Res 2013; 19: 6389–6397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yates LR, Knappskog S, Wedge D, et al. Genomic evolution of breast cancer metastasis and relapse. Cancer Cell 2017; 32: 169–184.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pan H, Gray R, Braybrooke J, et al. 20-year risks of breast-cancer recurrence after stopping endocrine therapy at 5 years. N Engl J Med 2017; 377: 1836–1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Colleoni M, Sun Z, Price KN, et al. Annual hazard rates of recurrence for breast cancer during 24 years of follow-up: results from the international breast cancer study group trials I to V. J Clin Oncol 2016; 34: 927–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Saphner T, Tormey DC, Gray R. Annual hazard rates of recurrence for breast cancer after primary therapy. J Clin Oncol 1996; 14: 2738–2746. [DOI] [PubMed] [Google Scholar]
- 9. Fisher B, Dignam J, Bryant J, et al. Five versus more than five years of tamoxifen for lymph node-negative breast cancer: updated findings from the National Surgical Adjuvant Breast and Bowel Project B-14 randomized trial. J Natl Cancer Inst 2001; 93: 684–690. [DOI] [PubMed] [Google Scholar]
- 10. Gray RG, Rea D, Handley K, et al. aTTom: long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years in 6,953 women with early breast cancer. ASCO Meet Abstr 2013; 31(Suppl. 18): 5. [Google Scholar]
- 11. Davies C, Pan H, Godwin J, et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet 2013; 381: 805–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Davies C, Godwin J, Gray R, et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet 2011; 378: 771–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schiavon G, Smith IE. Status of adjuvant endocrine therapy for breast cancer. Breast Cancer Res. 2014; 16: 206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stewart HJ, Prescott RJ, Forrest APM. Scottish adjuvant tamoxifen trial: a randomized study updated to 15 years. J Natl Cancer Inst 2001; 93: 456–462. [DOI] [PubMed] [Google Scholar]
- 15. Tormey DC, Gray R, Falkson HC. Postchemotherapy adjuvant tamoxifen therapy beyond five years in patients with lymph node-positive breast cancer. Eastern Cooperative Oncology Group. J Natl Cancer Inst 1996; 88: 1828–1833. [DOI] [PubMed] [Google Scholar]
- 16. Al-Mubarak M, Tibau A, Templeton AJ, et al. Extended adjuvant tamoxifen for early breast cancer: a meta-analysis. PLoS One 2014; 9: e88238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Goss PE, Ingle JN, Martino S, et al. A randomized trial of letrozole in postmenopausal women after five years of tamoxifen therapy for early-stage breast cancer. N Engl J Med 2003; 349: 1793–1802. [DOI] [PubMed] [Google Scholar]
- 18. Goss PE, Ingle JN, Martino S, et al. Randomized trial of letrozole following tamoxifen as extended adjuvant therapy in receptor-positive breast cancer: updated findings from NCIC CTG MA.17. J Natl Cancer Inst 2005; 97: 1262–1271. [DOI] [PubMed] [Google Scholar]
- 19. Goss PE, Ingle JN, Martino S, et al. Impact of premenopausal status at breast cancer diagnosis in women entered on the placebo-controlled NCIC CTG MA17 trial of extended adjuvant letrozole. Ann Oncol 2013; 24: 355–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mamounas EP, Jeong JH, Lawrence Wickerham D, et al. Benefit from exemestane as extended adjuvant therapy after 5 years of adjuvant tamoxifen: intention-to-treat analysis of the national surgical adjuvant breast and bowel project B-33 trial. J Clin Oncol 2008; 26: 1965–1971. [DOI] [PubMed] [Google Scholar]
- 21. Jakesz R, Greil R, Gnant M, et al. Extended adjuvant therapy with anastrozole among postmenopausal breast cancer patients: results from the randomized Austrian breast and colorectal cancer study group trial 6a. J Natl Cancer Inst 2007; 99: 1845–1853. [DOI] [PubMed] [Google Scholar]
- 22. Mamounas EP, Bandos H, Lembersky BC, et al. A randomized, double-blinded, placebo-controlled clinical trial of extended adjuvant endocrine therapy (tx) with letrozole (L) in postmenopausal women with hormone-receptor (+) breast cancer (BC) who have completed previous adjuvant tx with an aromatase inhibitor (AI). Presented at 2016 San Antonio Breast Cancer Symposium, December 6–10, 2016; San Antonio, TX. Abstract S1–05. [Google Scholar]
- 23. Tjan-Heijnen V, van Hellemond I, Peer P, et al. First results from the multicenter phase III DATA study comparing 3 versus 6 years of anastrozole after 2–3 years of tamoxifen in postmenopausal women with hormone receptor-positive early breast cancer. Cancer Res 2017; 77(Suppl. 4): abstract S1–S03. [Google Scholar]
- 24. Blok EJ, Kroep JR, Meershoek-Klein Kranenbarg E, et al. Optimal duration of extended adjuvant endocrine therapy for early breast cancer; results of the IDEAL trial (BOOG 2006–05). J Natl Cancer Inst 2017; 110 DOI: 10.1093/jnci/djx134. [DOI] [PubMed] [Google Scholar]
- 25. Gnant M, Steger G, Greil R, et al. A prospective randomized multi-center phase-III trial of additional 2 versus additional 5 years of anastrozole after initial 5 years of adjuvant endocrine therapy – results from 3,484 postmenopausal women in the ABCSG-16 trial. Presented at 2017 San Antonio Breast Cancer Symposium, December 5–9, 2017; San Antonio, TX Abstract GS3–01. http://www.onclive.com/conference-coverage/sabcs-2017/no-difference-in-overall-survival-with-shorter-extended-ai-therapy (accessed 15 January 2018). [Google Scholar]
- 26. Colleoni M, Luo W, Karlsson P, et al. Extended adjuvant intermittent letrozole versus continuous letrozole in postmenopausal women with breast cancer (SOLE): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. Epub ahead of print 17 November 2017. DOI: 10.1016/S1470-2045(17)30715-5. [DOI] [PubMed] [Google Scholar]
- 27. Goss PE, Ingle JN, Pritchard KI, et al. Extending aromatase-inhibitor adjuvant therapy to 10 years. N Engl J Med 2016; 375: 209–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kennecke HF, Olivotto IA, Speers C, et al. Late risk of relapse and mortality among postmenopausal women with estrogen responsive early breast cancer after 5 years of tamoxifen. Ann Oncol 2007; 18: 45–51. [DOI] [PubMed] [Google Scholar]
- 29. Sestak I, Dowsett M, Zabaglo L, et al. Factors predicting late recurrence for estrogen receptor-positive breast cancer. J Natl Cancer Inst 2013; 105: 1504–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wolmark N, Mamounas EP, Baehner FL, et al. Prognostic impact of the combination of recurrence score and quantitative estrogen receptor expression (ESR1) on predicting late distant recurrence risk in estrogen receptor-positive breast cancer after 5 years of tamoxifen: results from NRG oncology/nati. J Clin Oncol 2016; 34: 2350–2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sgroi DC, Carney E, Zarrella E, et al. Prediction of late disease recurrence and extended adjuvant letrozole benefit by the HOXB13/IL17BR biomarker. J Natl Cancer Inst 2013; 105: 1036–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sestak I. Identifying biomarkers to select patients with early breast cancer suitable for extended adjuvant endocrine therapy. Breast Care 2017; 12: 146–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Filipits M, Nielsen TO, Rudas M, et al. The PAM50 risk-of-recurrence score predicts risk for late distant recurrence after endocrine therapy in postmenopausal women with endocrine-responsive early breast cancer. Clin Cancer Res 2014; 20: 1298–1305. [DOI] [PubMed] [Google Scholar]
- 34. Dubsky P, Brase JC, Jakesz R, et al. The EndoPredict score provides prognostic information on late distant metastases in ER+/HER2- breast cancer patients. Br J Cancer 2013; 109: 2959–2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Esserman LJ, Moore DH, Tsing PJ, et al. Biologic markers determine both the risk and the timing of recurrence in breast cancer. Breast Cancer Res Treat 2011; 129: 607–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Xiao-Jun M, Salunga R, Dahiya S, et al. A five-gene molecular grade index and HOXB13.IL17BR are complementary prognostic factors in early stage breast cancer. Clin Cancer Res 2008; 14: 2601–2608. [DOI] [PubMed] [Google Scholar]
- 37. Kwon JS, Pansegrau G, Nourmoussavi M, et al. Costs and benefits of extended endocrine strategies for premenopausal breast cancer. J Natl Compr Canc Netw 2017; 15: 1015–1021. [DOI] [PubMed] [Google Scholar]
- 38. Delea TE, Karnon J, Smith RE, et al. Cost-effectiveness of extended adjuvant letrozole therapy after 5 years of adjuvant tamoxifen therapy in postmenopausal women with early-stage breast cancer. Am J Manag Care 2006; 12: 374–386. [PubMed] [Google Scholar]
- 39. Erman A, Nugent A, Amir E, et al. Cost-effectiveness analysis of extended adjuvant endocrine therapy in the treatment of post-menopausal women with hormone receptor positive breast cancer. Breast Cancer Res Treat 2014; 145: 267–279. [DOI] [PubMed] [Google Scholar]
- 40. Amir E, Seruga B, Niraula S, et al. Toxicity of adjuvant endocrine therapy in postmenopausal breast cancer patients: a systematic review and meta-analysis. J Natl Cancer Inst 2011; 103: 1299–1309. [DOI] [PubMed] [Google Scholar]
- 41. Hadji P, Ziller V, Kyvernitakis J, et al. Persistence in patients with breast cancer treated with tamoxifen or aromatase inhibitors: a retrospective database analysis. Breast Cancer Res Treat 2013; 138: 185–191. [DOI] [PubMed] [Google Scholar]
- 42. Chirgwin JH, Giobbie-Hurder A, Coates AS, et al. Treatment adherence and its impact on disease-free survival in the breast international group 1–98 trial of tamoxifen and letrozole, alone and in sequence. J Clin Oncol 2016; 34: 2452–2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hershman DL, Kushi LH, Shao T, et al. Early discontinuation and nonadherence to adjuvant hormonal therapy in a cohort of 8,769 early-stage breast cancer patients. J Clin Oncol 2010; 28: 4120–4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nabieva N, Kellner S, Fehm T, et al. Influence of patient and tumor characteristics on early therapy persistence with letrozole in postmenopausal women with early breast cancer: results of the prospective Evaluate-TM study with 3941 patients. Ann Oncol. 2018; 29(1): 186–192. [DOI] [PubMed] [Google Scholar]
- 45. Curigliano G, Burstein HJ, P Winer E, et al. De-escalating and escalating treatments for early-stage breast cancer: the St. Gallen International Expert Consensus Conference on the primary therapy of early breast cancer 2017. Ann Oncol 2017; 28: 1700–1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pagani O, Regan MM, Walley BA, et al. Adjuvant exemestane with ovarian suppression in premenopausal breast cancer. N Engl J Med 2014; 371: 107–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Francis PA, Regan MM, Fleming GF, et al. Adjuvant ovarian suppression in premenopausal breast cancer. N Engl J Med 2015; 372: 1673. [DOI] [PubMed] [Google Scholar]
- 48. Goss PE, Ingle JN, Pater JL, et al. Late extended adjuvant treatment with letrozole improves outcome in women with early-stage breast cancer who complete 5 years of tamoxifen. J Clin Oncol 2008; 26: 1948–1955. [DOI] [PubMed] [Google Scholar]
- 49. Gomis RR, Gawrzak S. Tumor cell dormancy. Mol Oncol 2017; 11: 62–78. [DOI] [PMC free article] [PubMed] [Google Scholar]


