Abstract
Glioblastoma is the most common adult central nervous system malignancy and carries a poor prognosis. Disease progression and recurrence after chemoradiotherapy are assessed via serial magnetic resonance imaging sequences. T2-weighted fluid-attenuated inversion recovery (FLAIR) signal is presumed to represent edema containing microscopic cancer infiltration. Here we assessed the prognostic impact of computerized volumetry of FLAIR signal in the peri-treatment setting for glioblastoma. We analyzed pre- and posttreatment FLAIR sequences of 40 patients treated at the Columbia University Medical Center between 2011 and 2014, excluding those without high-quality FLAIR imaging within 2 weeks before treatment and 60 to 180 days afterward. We manually contoured regions of FLAIR hyperintensity as per Radiation Therapy Oncology Group guidelines and calculated the volumes of nonenhancing tumor burden. At the time of this study, all but 1 patient had died. Pre- and posttreatment FLAIR volumes were assessed for correlation to overall and progression-free survival. Larger post-treatment FLAIR volumes from sequences taken between 60 and 180 days after conclusion of chemoradiotherapy were negatively correlated with overall survival (P = .048 on Pearson's correlation and P = .017 and P = .043 on univariable and multivariable Cox regression analyses, respectively) and progression-free survival (P = .002 on Pearson's correlation and P = < .001 and P = < .001 on univariable and multivariable Cox regression analyses). This study suggests that higher FLAIR volumes in the 2- to 6-month posttreatment window are associated with worsened survival.
Keywords: glioblastoma, radiation, FLAIR, MRI, brain cancer
Introduction
Glioblastoma (GBM) is the most common primary malignant neoplasm of the central nervous system with an annual incidence in the United States of 4–5 per 100,000 (1). The standard of care includes a maximal safe surgical resection and adjuvant radiotherapy with concurrent and continued adjuvant temozolomide chemotherapy (2). Despite aggressive trimodality therapy, patients invariably recur with a median interval to disease progression of ∼8 months and a 5-year overall survival (OS) rate of <10% (3).
After completing radiation therapy, patients are monitored closely, typically with serial magnetic resonance imaging (MRI) and routine clinical assessment (4). Disease progression is diagnosed radiographically and/or in the context of clinical symptoms or declining performance status. The MacDonald criteria, introduced in 1990, provided an objective methodology for tumor response assessment using changes in tumor area derived from maximal bidimensional measurements of enhancing regions on T1-weighted imaging with gadolinium. In addition, corticosteroid use and performance status were also considered in these criteria (5). In 2010, the Response Assessment in Neuro-Oncology Working Group (RANO) proposed new criteria for response assessment following chemoradiotherapy (CRT) to address the limitations of the MacDonald criteria, including the problems of pseudoprogression and nonenhancing tumor progression (6). Therefore, the criteria for disease progression differ depending on the time interval from initial treatment, with 12 weeks as a discriminator to account for pseudoprogression. In addition, nonenhancing tumor shown by increased T2-weighted Fluid-Attenuated Inversion Recovery (FLAIR) signal was included in the evaluation for disease progression.
In addition to post-treatment follow-up, FLAIR signal is an integral component of radiation therapy (RT) planning for GBM. Clinical target volumes in radiation treatment include T1 and T2/FLAIR hyperintensities along with the resection cavity and a 2-cm margin (7). Given the importance placed upon FLAIR signal in radiation treatment and in post-treatment surveillance imaging, many studies have attempted to correlate FLAIR volume with important clinical variables such as OS and progression-free survival (PFS) in the posttreatment setting. Results of these studies have varied, and despite RANO recommendations encouraging its inclusion, the significance and prognostic value of FLAIR signal have not yet been clearly shown in tumor response assessment of GBM (8–12).
The purpose of the current study was to test the hypothesis that the volume of hyperintense FLAIR signal correlates with meaningful clinical outcomes, particularly OS and PFS. Earlier studies have relied on ellipsoid and spheroid estimates of FLAIR volume, generating crude approximations of tumor burden (13). The RANO report itself, in 2010, admitted a technological shortcoming in reference to more objective quantifications of FLAIR volumes (6). In contrast to these previous limitations, our method of manual segmentation followed by computerized volumetry simultaneously reduces user bias and improves reproducibility (14, 15).
Methodology
All analyses were performed in compliance with our institutional review board guidelines, and consent was waived based on the retrospective nature of the study. We searched our departmental database for patients treated between January 2011 and February 2014 with standard-of-care therapy at the Columbia University Medical Center for a new diagnosis of histologically confirmed GBM. To be included, patients must have undergone T2/FLAIR MRI acquisition within 2 weeks before adjuvant treatment was initiated, as well as between 60 and 180 days after conclusion of RT. Because distinguishing between progression and pseudoprogression using MRI is not possible, and RANO criteria recommend a waiting period posttreatment before changes in management can be made (6), our study set a minimum wait period of 60 days after conclusion of CRT. All imaging sequences were reviewed manually and were excluded if any data were of poor quality (ie, motion-degraded) by a user who was blinded to the patient's clinical data, purpose, or results of this study. Patients with multiple GBM foci were similarly excluded. For patients with multiple FLAIR sequences within the 2- to 6-month window posttreatment, the earliest available images were used.
The radiation protocol for 28 patients consisted of fractionated linear accelerator-based irradiation Monday through Friday for a period of 6 weeks, with patients receiving 2 Gy per fraction for a total of 60 Gy. All patients received concomitant temozolomide (75 mg/m2/d, 7 d/wk) during radiotherapy followed by adjuvant temozolomide for up to 6 cycles (150–200 mg/m2/d for 5-day during, each 28-day cycle). Regions of FLAIR hyperintensity were contoured by a single experimenter adhering to The Radiation Therapy Oncology Group guidelines and blinded to the OS results. Functional MRI of the Brain Software Library (FSL) was used to perform segmentation, and the volumes were calculated with MATLAB software.
A hypofractionated protocol, consisting of 2.67 Gy per fraction for total dose of 40 Gy over 3 weeks, was prescribed to 12 patients. The hypofractionated protocol was reserved for elderly patients given its shorter treatment period; in accordance with other published data, an earlier study evaluating outcomes of patients at our own institution yielded no significant difference in OS between standard and hypofractionated treatment regimens when adjusted for age and concomitant chemotherapy (16).
OS was computed from the start of RT to date of death either known by Columbia University Medical Center or from internet searches of publicly available death data; OS was defined as all-cause mortality. Any patient lost to follow-up or for whom follow-up indicated that death was imminent but a specific date could not be identified was censored at the date of last confirmed contact; in this manner, 11/40 patients were censored. PFS was computed from the start of RT to the date of primary disease progression confirmed by an independent neuroradiologist and immediately followed by a change in management, typically bevacizumab per standard-of-care guidelines; 4/40 patients were censored because death occurred before confirmed progression of disease.
Statistical analysis of volumetric parameters and other relevant parameters used Pearson correlation and Cox proportional hazards model. Statistical significance was P ≤ .05, and all statistical calculations and graphs were performed using SPSS version 24.
Results
In total, 40 patients (females, 16; males, 24; median age, 62.5 years at diagnosis) were included in the final analysis (Table 1). On the basis of the operative report, surgical procedures were defined as biopsy, subtotal resection, or total resection. The median time period between surgery and treatment onset was 29 days. At the time of this study, 1/40 patients remained alive. The median OS of the cohort is 457 days, and the median PFS is 176 days.
Table 1.
Patient Characteristics
| Total | 40 |
| Gender | |
| Female | 16 (40.0%) |
| Male | 24 (60.0%) |
| Median age at diagnosis | 62.5 (range 16–85) |
| Alive at study time | 1 (2.5%) |
| Median overall survival | 457 days (range 119–1372) |
| Median progression-free survival | 176 days (range 42–835) |
| Ethnicity | |
| White | 28 (70%) |
| Hispanic | 9 (22.5%) |
| Asian | 2 (5%) |
| Black | 1 (2.5%) |
| Surgery | |
| Biopsy only | 2 (5%) |
| Subtotal resection | 33 (82.5% |
| Total resection | 5 (12.5%) |
| Radiotherapy dose | |
| 60 Gy | 28 (70%) |
| 40 Gy | 12 (30%) |
| Median FLAIR volumes | |
| PreRT | 35.957 cm3 (range 3.150–144.629) |
| PostRT | 29.143 cm3 (range 0.297–175.641) |
| MGMT status | |
| Methylated | 10 (25%) |
| Unmethylated | 16 (40%) |
| Unknown | 14 (35%) |
| IDH-1 status | |
| Mutated | 3 (7.5%) |
| Unmutated | 36 (90%) |
| Unknown | 1 (2.5%) |
Four ethnicities (Latino, Asian, black and white) were represented in the patient population, with whites and Latinos comprising 92.5% of the cohort. An earlier study at our institution yielded no significance in OS between these four racial groups after treatment for GBM (17).
O6-methylguanine DNA methyltransferase (MGMT) promoter methylation status was known in 26 patients; 38% of this group were methylated. IDH-1 mutation status was known in 39 patients; all but 3 in this group were wild-type.
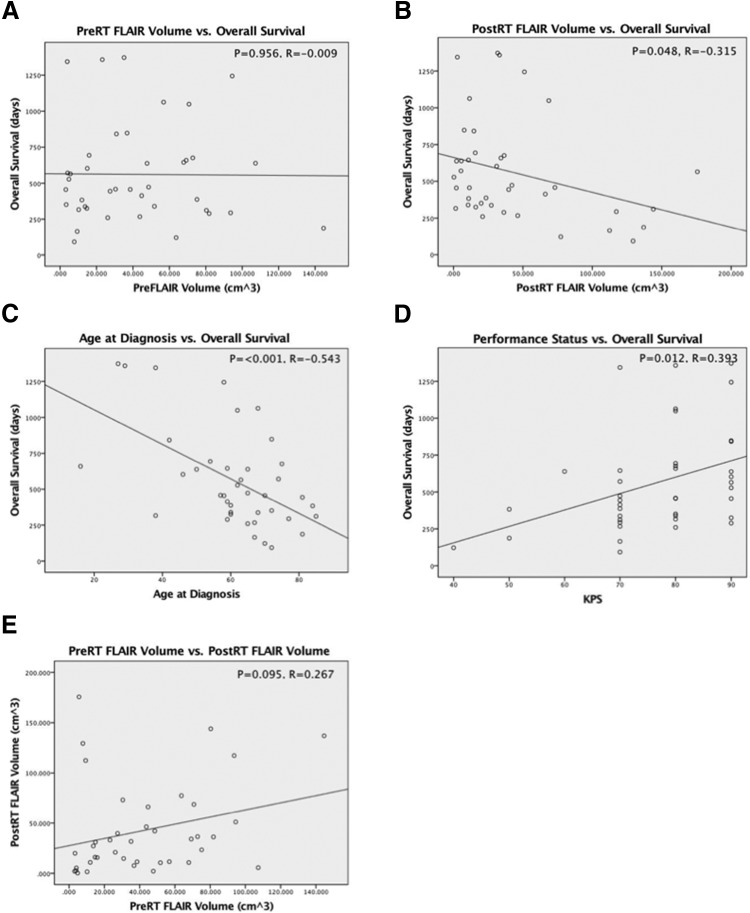
For the 80 pre- and posttreatment FLAIR sequences analyzed, the median pre-RT volume was 35.975 cm3, and the median post-RT volume was 29.143 cm3. Pearson bivariate correlation was performed for all patients; for censored patients, the date of last visit was used as a survival endpoint. Figure 1 displays the results of the regression analyses for OS. There was no significant correlation between the volume of FLAIR signal pretreatment and OS (P = .956, R = −0.009). A statistically significant correlation was found between the volume of posttreatment FLAIR signal and OS (P = .048, R = −0.315); the negative slope of the regression line indicates worse OS with increased post-RT FLAIR volume in the 2- to 6-month posttreatment window. Pearson correlation of patient age at the time of diagnosis yielded a statistically significant result when matched with OS (P = < .001, R = −0.543), with the negative slope of the regression line indicating worse survival with advanced age. A significant positive correlation was seen between Karnofsky performance status (KPS) and improved survival (P = .012, R = 0.393). There was a trend that did not reach significance between pre- and posttreatment FLAIR volumes (P = .095, R = 0.267).
Figure 1.
Pretreatment fluid-attenuated inversion recovery (FLAIR) volume was not correlated with overall survival (OS) (A). Posttreatment FLAIR volume was significantly correlated with OS and with a moderate negative Pearson coefficient (B). Patient age at diagnosis was significantly correlated with OS and with a moderate negative Pearson coefficient (C). Performance status was significantly correlated with OS and with a moderate positive Pearson coefficient (D). Pre- and posttreatment FLAIR volumes displayed a trend toward positive correlation that did not reach statistical significance (E).
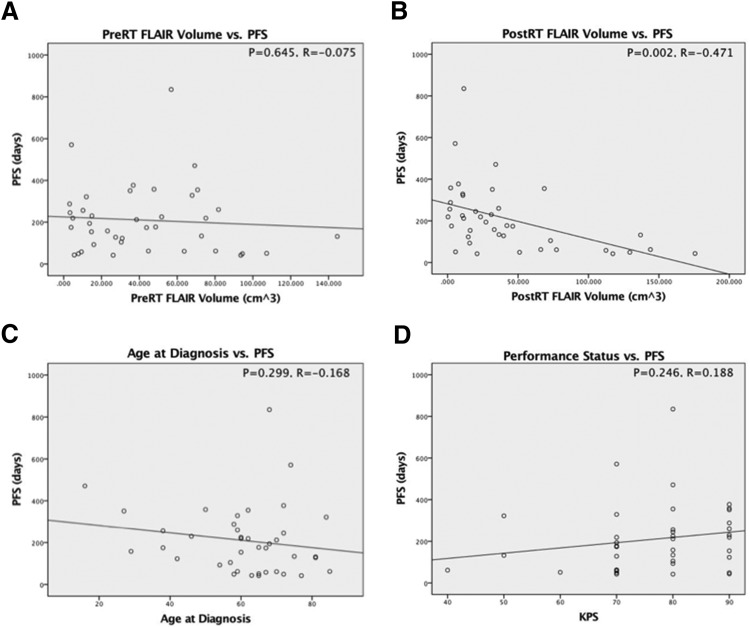
Figure 2 displays the results of the regression analysis for PFS. There was no significant correlation between the volume of FLAIR signal pretreatment and PFS (P = .645, R = −0.075). A statistically significant correlation was found between the volume of posttreatment FLAIR signal and PFS (P = .002, R = −0.471); the negative slope of the regression line indicates worse PFS with increased post-RT FLAIR volume in the 2- to 6-month posttreatment window. Neither Pearson correlation of patient age at the time of diagnosis nor KPS was significant with PFS (P = .299, R = −0.168 and P = .246, R = 0.188, respectively) (Figure 3).
Figure 2.
Pretreatment FLAIR volume was not correlated with progression-free survival (PFS) (A). Posttreatment FLAIR volume was significantly correlated with PFS and with a moderate negative Pearson coefficient (B). Patient age at diagnosis was not correlated with PFS (C). Karnofsky performance status (KPS) was not correlated with PFS (D).
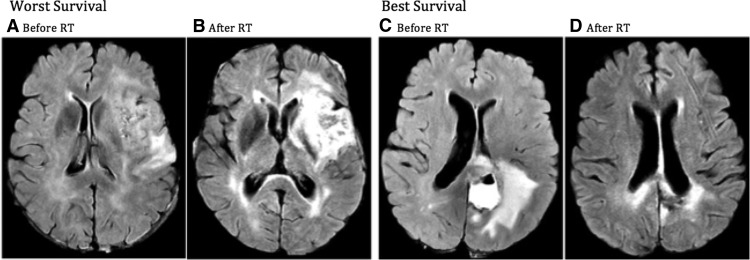
Figure 3.
Worst survival: representative patient who had the lowest survival in the cohort (119 days). The patient had a moderate degree of FLAIR hyperintensity before beginning chemoradiation (A) that was dramatically increased at 71 days after treatment (B).
Best survival: representative patient who had the best survival in the cohort (1372 days). The patient had a dominant FLAIR signal prior to beginning chemoradiation (C) that nearly resolved at the 96-day time point (D).
Univariable and multivariable analyses were performed with Cox proportional hazards model, with the latter using age at diagnosis, pre-RT FLAIR volume, and post-RT FLAIR volume as covariates (Tables 2 and 3). Post-RT FLAIR volume reached statistical significance for OS in both uni- and multivariable analyses (P = .017 and P = .043, respectively), with hazard ratios of 1.010 and 1.008, respectively. Post-RT FLAIR volume also reached statistical significance for PFS in both uni- and multivariable analysis (P = < .001 and P = < .001, respectively), with hazard ratios of 1.020 and 1.026, respectively. Age at diagnosis reached statistical significance for OS in both uni- and multivariable analyses (P = .013 and P = .024, respectively), although not for PFS.
Table 2.
Cox Proportional Hazards Model to Assess Effects on OS
| Covariate | Univariable Analysis |
Multivariable Analysis |
||||||
|---|---|---|---|---|---|---|---|---|
| Coefficient (B) | HR [expB] | 95% CI | P Value | Coefficient (B) | HR [expB] | 95% CI | P Value | |
| Age | 0.037 | 1.038 | 1.008–1.068 | .013 | 0.035 | 1.036 | 1.005–1.068 | .024 |
| KPS | −0.029 | 0.972 | 0.935–1.010 | .149 | N/A | |||
| Gender | ||||||||
| Female | 0.187 | 1.206 | 0.562–2.587 | .631 | N/A | |||
| Male | (reference) | |||||||
| FLAIR volume | ||||||||
| Pre-RT | 0.005 | 1.005 | 0.993–1.018 | .414 | 0.001 | 1.000 | 0.987–1.013 | .986 |
| Post-RT | 0.010 | 1.010 | 1.002–1.018 | .017 | 0.008 | 1.008 | 1.001–1.016 | .043 |
| % Change | 0.001 | 1.000 | 1.000–1.001 | .297 | N/A | |||
| MGMT status | ||||||||
| Unmethylated | 0.383 | 1.467 | 0.540–3.984 | .453 | N/A | |||
| Methylated | (reference) | |||||||
| IDH-1 status | ||||||||
| Unmutated | 1.714 | 5.553 | 0.736–41.879 | .096 | N/A | |||
| Mutated | (reference) | |||||||
| RT dose | ||||||||
| 40 Gy | 0.794 | 2.212 | 0.922–5.306 | .075 | N/A | |||
| 60 Gy | (reference) | |||||||
| Extent of resection | ||||||||
| Biopsy only | 0.792 | 2.209 | 0.305–16.002 | .433 | N/A | |||
| Subtotal | 0.748 | 2.112 | 0.495–9.008 | .312 | ||||
| Total | (reference) | |||||||
Note: Data in bold represent statistical significance (P < .05).
Abbreviations: OS, overall survival; KPS, Karnofsky performance status; HR, hazard ratio; CI, confidence interval.
Table 3.
Cox Proportional Hazards Model to Assess Effects on PFS
| Covariate | Univariable Analysis |
Multivariable Analysis |
||||||
|---|---|---|---|---|---|---|---|---|
| Coefficient (B) | HR [expB] | 95% CI | P Value | Coefficient (B) | HR [expB] | 95% CI | P Value | |
| Age | 0.008 | 1.008 | 0.988–1.028 | .431 | 0.002 | 1.002 | 0.983–1.021 | .849 |
| KPS | −0.017 | 0.983 | 0.956–1.012 | .254 | N/A | |||
| Gender | ||||||||
| Female | 0.113 | 1.120 | 0.565–2.220 | .746 | N/A | |||
| Male | (reference) | |||||||
| FLAIR volume | ||||||||
| Pre-RT | 0.001 | 1.001 | 0.990–1.013 | .811 | −0.012 | 0.988 | 0.976–0.999 | .042 |
| Post-RT | 0.020 | 1.020 | 1.011–1.029 | <.001 | 0.026 | 1.026 | 1.015–1.038 | <.001 |
| % Change | 0.001 | 1.001 | 1.001–1.002 | .001 | N/A | |||
| MGMT status | ||||||||
| Unmethylated | −0.170 | 0.844 | 0.367–1.941 | .690 | N/A | |||
| Methylated | (reference) | |||||||
| IDH-1 status | ||||||||
| Unmutated | 0.189 | 1.208 | 0.287–5.084 | .796 | N/A | |||
| Mutated | (reference) | |||||||
| RT dose | ||||||||
| 40 Gy | 0.261 | 1.298 | 0.619–2.719 | .490 | N/A | |||
| 60 Gy | (reference) | |||||||
| Extent of resection | ||||||||
| Biopsy only | −0.523 | 0.593 | 0.108–3.257 | .633 | N/A | |||
| Subtotal | 0.171 | 1.186 | 0.409–3.444 | .754 | ||||
| Total | (reference) | |||||||
Note: Data in bold represent statistical significance (P < .05).
Abbreviations: PFS, progression-free survival; KPS, Karnofsky performance status; HR, hazard ratio; CI, confidence interval
Pre-RT FLAIR volume on multivariable analysis and the percent change in FLAIR volume from pre- to post-RT on univariable analysis showed a significant association with PFS (P = .042 and P = .001, respectively), although not with OS. Patient gender and radiotherapy dose (60 Gy vs 40 Gy) were also not associated with OS or PFS on univariable analysis, although the latter showed a trend toward association with OS (P = .075).
In total, 26 patients had known MGMT promoter status; there was no significant association between MGMT and OS/PFS within this cohort. Patients with IDH-1 mutations showed a trend toward increased OS (P = .096), although not significant. Although extent of tumor resection is a well-established predictor for OS, no significant association between extent of resection and OS/PFS was determined within this patient cohort (18).
Discussion
Hyperintensity on FLAIR sequences is presumed to represent edema due to microscopic cancer infiltration (19), and regions of FLAIR signal are considered as essential elements of treatment planning. Many studies have sought to correlate patient outcomes and tumor response on the basis of FLAIR signal alone, and the results have been mixed. Despite the importance of FLAIR signal in radiotherapy planning, in modern clinical practice, performance status and patient-reported symptoms are the most indicative of neurological decline and disease progression. Our study sought to evaluate the prognostic value of FLAIR signal in the context of GBM in the pre- and posttreatment setting. Our results also showed a statistically significant negative correlation between FLAIR volume 2–6 months after CRT and both OS and PFS.
The historical practice of geometric approximations of tumor burden has been increasingly vilified in recent years. Iliadis et al. evaluated preoperative FLAIR volumes for survival prognosis in 50 patients with GBM and compared geometric approximations with computerized volumetry. The conclusion was that geometric models tend to overestimate FLAIR volume and should not be used (13). Zhang et al. proposed a pretreatment volume ratio of FLAIR to gross tumor burden as a significant predictor of survival, but listed its volume acquisition method (ie, ellipsoid approximation) as a limitation in accuracy (9). In a seminal study assessing reliability, precision, and ease of use of computerized volumetry among doctors, students, and volunteers, Huber et al. determined that changes in FLAIR volume in particular had the fewest precision errors among an already reliable set of tested parameters (14). The results of our study mirror those of an Israeli study published in 2016, in which pretreatment FLAIR volume was not associated with OS, whereas 3-month post-treatment volumes were significantly correlated with OS (20). We posit that there are inconsistencies in the findings of other studies owing to variations in analytic technique and reliance, by some, on geometric models to estimate FLAIR volume. Although these approaches avoid subjectivity, our analysis was blinded to clinical data and relied on a standard FLAIR contouring technique used for RT planning.
Our study showed no correlation between pre- and posttreatment FLAIR volumes. This lack of coherence could be explained by the acute effects of both ionizing radiation and chemotherapy leading to alterations in the volume and exact composition of FLAIR hyperintense regions. It is for these reasons that pseudoprogression confounds analyses in the immediate post-treatment setting. We accounted for potential radiographic pseudoprogression by setting a minimum waiting period of 60 days following CRT, a time frame similar to the 12 weeks proposed by the RANO criteria. Again, disagreements in the published literature may be explained by the time points analyzed and the influence of pseudoprogression on the results.
Our study also determined a significant correlation between OS and both patient age at diagnosis and KPS; these findings are in congruence with well-established prognostic thought and are nonmodifiable risk factors. They do, therefore, lend further credence to the validity of this patient cohort within the context of the larger patient population.
Although selection bias is a common risk in retrospective studies, we minimized this risk by including all patients with GBM treated at our institution over the aforementioned time period who had high-quality pre- and posttreatment FLAIR sequences; the latter occurring 60–180 days after CRT. Although our study was limited by the small patient population, the findings are made robust by inclusion of only high-quality imaging data and selection of time points that limit the effects of pseudoprogression. As with any study evaluating nonenhancing tumor using T2/FLAIR signal, our study was also limited by inherent difficulties and inaccuracy of determining the exact extent of nonenhancing tumor, as surrounding peritumoral edema and postradiation white matter changes have similar appearance on FLAIR sequences (21). Another limitation is the omission of concomitant steroid usage during and after treatment; these data were not available for the majority of patients. Steroids, such as dexamethasone, inhibit both phospholipase A2, as well as histamine release from mast cells, leading to reduced edema and therefore FLAIR signal (22, 23). It is thus warranted that future studies control for steroid usage and possibly include dosage as a covariate in multivariable analyses. Incidentally, no patient received antiangiogenic agents such as bevacizumab during the periods in which imaging data were collected.
In conclusion, post-treatment FLAIR volume is a significant predictor of OS and PFS in patients with GBM, as determined with multiple statistical methods. Future models should consider this specific time point in the development of more refined prognostic tools.
Acknowledgments
Disclosures: No disclosures to report.
Conflict of Interest: The authors have no conflicts of interest to declare.
Footnotes
- FLAIR
- Fluid-attenuated inversion recovery
- GBM
- Glioblastoma
- MRI
- magnetic resonance imaging
- CRT
- chemoradiotherapy
- RT
- radiation therapy
- OS
- overall survival
- PFS
- progression-free survival
- MGMT
- O6-methylguanine DNA methyltransferase
- KPS
- Karnofsky performance status
References
- 1. Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359(5):492–507. [DOI] [PubMed] [Google Scholar]
- 2. Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B, Belanger K, Hau P, Brandes AA, Gijtenbeek J, Marosi C, Vecht CJ, Mokhtari K, Wesseling P, Villa S, Eisenhauer E, Gorlia T, Weller M, Lacombe D, Cairncross JG, Mirimanoff RO; European Organisation for Research and Treatment of Cancer Brain Tumour and Radiation Oncology Groups; National Cancer Institute of Canada Clinical Trials Group. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–466. [DOI] [PubMed] [Google Scholar]
- 3. Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO; European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 4. van den Bent MJ, Vogelbaum MA, Wen PY, Macdonald DR, Chang SM. End point assessment in gliomas: novel treatments limit usefulness of classical Macdonald's criteria. J Clin Oncol. 2009;27(18):2905–2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Macdonald DR, Cascino TL, Schold SC Jr., Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8(7):1277–1280. [DOI] [PubMed] [Google Scholar]
- 6. Wen PY, Macdonald DR, Reardon DA, Cloughesy TF, Sorensen AG, Galanis E, Degroot J, Wick W, Gilbert MR, Lassman AB, Tsien C, Mikkelsen T, Wong ET, Chamberlain MC, Stupp R, Lamborn KR, Vogelbaum MA, van den Bent MJ, Chang SM. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28(11):1963–1972. [DOI] [PubMed] [Google Scholar]
- 7. Colman H, Berkey BA, Maor MH, Groves MD, Schultz CJ, Vermeulen S, Nelson DF, Mehta MP, Yung WK; Radiation Therapy Oncology Group. Phase II Radiation Therapy Oncology Group trial of conventional radiation therapy followed by treatment with recombinant interferon-beta for supratentorial glioblastoma: results of RTOG 9710. Int J Radiat Oncol Biol Phys. 2006;66(3):818–824. [DOI] [PubMed] [Google Scholar]
- 8. Ellingson BM, Cloughesy TF, Lai A, Nghiemphu PL, Mischel PS, Pope WB. Quantitative volumetric analysis of conventional MRI response in recurrent glioblastoma treated with bevacizumab. Neuro Oncol. 2011;13(4):401–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhang Z, Jiang H, Chen X, Bai J, Cui Y, Ren X, Chen X, Wang J, Zeng W, Lin S. Identifying the survival subtypes of glioblastoma by quantitative volumetric analysis of MRI. J Neurooncol. 2014;119(1):207–214. [DOI] [PubMed] [Google Scholar]
- 10. Gzell CE, Wheeler HR, McCloud P, Kastelan M, Back M. Small increases in enhancement on MRI may predict survival post radiotherapy in patients with glioblastoma. J Neurooncol. 2016;128(1):67–74. [DOI] [PubMed] [Google Scholar]
- 11. Dempsey MF, Condon BR, Hadley DM. Measurement of tumor “size” in recurrent malignant glioma: 1D, 2D, or 3D? AJNR Am J Neuroradiol. 2005;26(4):770–776. [PMC free article] [PubMed] [Google Scholar]
- 12. Radbruch A, Lutz K, Wiestler B, Bäumer P, Heiland S, Wick W, Bendszus M. Relevance of T2 signal changes in the assessment of progression of glioblastoma according to the Response Assessment in Neurooncology criteria. Neuro Oncol. 2012;14(2):222–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Iliadis G, Selviaridis P, Kalogera-Fountzila A, Fragkoulidi A, Baltas D, Tselis N, Chatzisotiriou A, Misailidou D, Zamboglou N, Fountzilas G. The importance of tumor volume in the prognosis of patients with glioblastoma: comparison of computerized volumetry and geometric models. Strahlenther Onkol. 2009;185(11):743–750. [DOI] [PubMed] [Google Scholar]
- 14. Huber T, Alber G, Bette S, Boeckh-Behrens T, Gempt J, Ringel F, Alberts E, Zimmer C, Bauer JS. Reliability of semi-automated segmentations in glioblastoma. Clin Neuroradiol. 2017;27(2):153–161. [DOI] [PubMed] [Google Scholar]
- 15. Chow DS, Qi J, Guo X, Miloushev VZ, Iwamoto FM, Bruce JN, Lassman AB, Schwartz LH, Lignelli A, Zhao B, Filippi CG. Semiautomated volumetric measurement on postcontrast MR imaging for analysis of recurrent and residual disease in glioblastoma multiforme. AJNR Am J Neuroradiol. 2014;35(3):498–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang TJ, Wu CC, Jani A, Estrada J, Ung T, Chow DS, Soun JE, Saad S, Qureshi YH, Gartrell R, Saadatmand HJ, Saraf A, Garrett MD, Grubb C, Isaacson SR, Cheng SK, Sisti MB, Bruce JN, Sheth SA, Lassman AB, Iwamoto FM, McKhann GM 2nd. Hypofractionated radiation therapy versus standard fractionated radiation therapy with concurrent temozolomide in elderly patients with newly diagnosed glioblastoma. Pract Radiat Oncol. 2016;6(5):306–314. [DOI] [PubMed] [Google Scholar]
- 17. Wu CC, Wang TJ, Jani A, Estrada JP, Ung T, Chow DS, Soun JE, Saad S, Qureshi YH, Gartrell R, Saadatmand HJ, Saraf A, Garrett MD, Grubb CS, Isaacson SR, Cheng SK, Sisti MB, Bruce JN, Sheth SA, Lassman AB, McKhann GM 2nd. A modern radiotherapy series of survival in hispanic glioblastoma patients. World Neurosurg. 2016;88:260–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Oppenlander ME, Wolf AB, Snyder LA, Bina R, Wilson JR, Coons SW, Ashby LS, Brachman D, Nakaji P, Porter RW, Smith KA, Spetzler RF, Sanai N. An extent of resection threshold for recurrent glioblastoma and its risk for neurological morbidity. J Neurosurg. 2014;120(4):846–853. [DOI] [PubMed] [Google Scholar]
- 19. Watanabe M, Tanaka R, Takeda N. Magnetic resonance imaging and histopathology of cerebral gliomas. Neuroradiology. 1992;34(6):463–469. [DOI] [PubMed] [Google Scholar]
- 20. Grossman R, Shimony N, Shir D, Gonen T, Sitt R, Kimchi TJ, Harosh CB, Ram Z. Dynamics of FLAIR volume changes in glioblastoma and prediction of survival. Ann Surg Oncol. 2017; 24(3):794–800. [DOI] [PubMed] [Google Scholar]
- 21. Walker AJ, Ruzevick J, Malayeri AA, Rigamonti D, Lim M, Redmond KJ, Kleinberg L. Postradiation imaging changes in the CNS: how can we differentiate between treatment effect and disease progression? Future Oncol. 2014;10(7):1277–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Andrade MV, Hiragun T, Beaven MA. Dexamethasone suppresses antigen-induced activation of phosphatidylinositol 3-kinase and downstream responses in mast cells. J Immunol. 2004;172(12):7254–7262. [DOI] [PubMed] [Google Scholar]
- 23. Flower RJ, Blackwell GJ. Anti-inflammatory steroids induce biosynthesis of a phospholipase A2 inhibitor which prevents prostaglandin generation. Nature. 1979;278(5703):456–459. [DOI] [PubMed] [Google Scholar]