Abstract
Chronic active B cell receptor (BCR) signaling, a hallmark of the activated B cell-like (ABC) subtype of diffuse large B cell lymphoma (DLBCL), engages the CARD11-MALT1-BCL10 (CBM) adapter complex to activate IκB kinase (IKK) and the classical NF-κB pathway. Here we show that the CBM complex includes the E3 ubiquitin ligases cIAP1 and cIAP2, which are essential mediators of BCR-dependent NF-κB activity in ABC DLBCL. cIAP1/2 attach K63-linked polyubiquitin chains on themselves and on BCL10, resulting in the recruitment of IKK and the linear ubiquitin chain ligase LUBAC, which is essential for IKK activation. SMAC mimetics target cIAP1/2 for destruction, and consequently suppress NF-κB and selectively kill BCR-dependent ABC DLBCL lines, supporting their clinical evaluation in patients with ABC DLBCL.
Introduction
The diffuse large B cell lymphoma (DLBCL) diagnostic category encompasses two molecular subtypes – activated B cell-like (ABC) and germinal center B cell-like (GCB) – that are histologically indistinguishable but differ profoundly in their gene expression phenotypes and in the oncogenic genomic variants that they acquire (Shaffer et al., 2012). The ABC subtype was so-named because its gene expression profile resembles that of normal B cells in which the B cell receptor (BCR) has been engaged by antigen (Alizadeh et al., 2000). The vast majority of ABC DLBCL primary tumors and cell lines have a gene expression signature that reflects NF-κB pathway activation, suggesting that this pathway is central to the pathogenesis of this cancer type (Davis et al., 2001). Indeed, functional and structural genomic studies have revealed the molecular mechanisms underlying constitutive NF-κB activity in ABC DLBCL, highlighting the key role of chronic active B cell receptor (BCR) signaling and oncogenic MYD88 mutations (Davis et al., 2010; Lenz et al., 2008a; Ngo et al., 2011). These molecular insights have led to advances in the treatment of ABC DLBCL, including the effectiveness of ibrutinib, a Bruton’s tyrosine kinase (BTK) inhibitor that prevents BCR signaling from engaging the NF-κB pathway (Wilson et al., 2015). The success of ibrutinib in producing remissions in ABC DLBCL prompted us to search for additional molecular targets in the BCR pathway that are amenable to therapeutic attack.
Protein ubiquitination regulates NF-κB activation at multiple levels within the various signaling cascades that engage this survival pathway (Chen, 2012). In particular, the K63-linked and linear polyubiquitin chains modulate signaling networks and are required for efficient and fine-tuned NF-κB induction in response to various stimuli (Walczak et al., 2012). The linear ubiquitin chain assembly complex (LUBAC), which joins ubiquitin moieties in a head to tail fashion, is required for NF-κB-dependent survival of ABC DLBCL (Yang et al., 2014). In the chronic active BCR signaling that characterizes ABC DLBCL, LUBAC associates with the CARD11-BCL10-MALT1 (CBM) signaling complex, where it attaches linear polyubiquitin to the NEMO (IKKγ) subunit of IκB kinase (IKK), the key regulatory kinase in the NF-κB pathway. However, the mechanism by which the CBM complex recruits and regulates LUBAC function is unknown.
K63-linked ubiquitination controls many aspects of NF-κB signaling by creating binding platforms for proteins that have ubiquitin binding domains that recognize K63 linkages, such as the LUBAC subunit RNF31 and NEMO (Fujita et al., 2014; Gerlach et al., 2011; Ikeda et al., 2011; Kensche et al., 2012). The cellular inhibitor of apoptosis 1 and 2 (cIAP1 and cIAP2) proteins are E3 ubiquitin ligases that facilitate the attachment of both K63-linked and K48-linked polyubiquitin chains to target proteins (Liston et al., 1996). cIAP1 and cIAP2 (cIAP1/2) belong to the inhibitor of apoptosis protein (IAP) family owing to their three amino-terminal IAP repeat domains, which facilitate binding to caspases and other proteins (Roy et al., 1997; Samuel et al., 2006). In addition, cIAP1/2 have carboxy-terminal RING finger domains that mediate their E3 ubiquitin ligase activities (Yang et al., 2000). Although cIAP1/2 can bind to caspases, they are poor caspase inhibitors (Eckelman and Salvesen, 2006). Instead, cIAP1/2 regulate various signal transduction pathways, including the activation of NF-κB in response to various stimuli (Chu et al., 1997; Park et al., 2004; Rothe et al., 1995; Samuel et al., 2006; Shu et al., 1996; Varfolomeev et al., 2007; Vince et al., 2007; Wang et al., 1998).
Initial evidence that cIAP1/2 regulate the classical NF-κB pathway arose from studies of signaling by tumor necrosis factor α (TNFα) (Micheau and Tschopp, 2003; Rothe et al., 1995). Following TNF receptor I engagement by TNFα, multiple signaling proteins are recruited to the receptor, including cIAP1/2. The E3 ligase activity of cIAP1/2 is thereby stimulated, leading to K63-linked polyubiquitination of cIAP1/2 themselves and the adapter RIP1. K63-linked polyubiquitin chains are recognized by the ubiquitin binding domains of various signaling mediators, including the TAB1/2-TAK1 complex and LUBAC (Bertrand et al., 2008; Haas et al., 2009; Varfolomeev et al., 2008).
A second function of cIAP1/2 is to restrain the alternative NF-κB pathway, which was uncovered during the genetic analysis of multiple myeloma (Annunziata et al., 2007; Keats et al., 2007). Subsequent mechanistic investigations revealed that a protein complex consisting of cIAP1/2, TRAF2 and TRAF3 functions in the cytosol to degrade the kinase NIK, an activator of the alternative NF-κB pathway (Vallabhapurapu et al., 2008; Zarnegar et al., 2008b). Currently model, cIAP1/2 are believed to mediate K48-polyubiquitination and degradation of NIK since deletion of cIAP1/2 results in NIK accumulation. Ligand engagement by certain TNF family receptors recruits cIAP1/2-TRAF2-TRAF3 complex to the receptor, causing cIAP1/2 to instead target TRAF2 and TRAF3 for destruction, thereby stabilizing NIK and activating NF-κB. Deletion of both cIAP1 and cIAP2 in mouse B cells results in expansion of the peripheral B cell pool, apparently due to constitutive activation of the alternative NF-κB pathway (Gardam et al., 2011). Deletion and/or mutation of TRAF3 occurs in ~15% of ABC DLBCL tumors and contributes to alternative NF-κB activation in these cases (Zhang et al., 2015).
A role for cIAP1/2 in B cells participating in adaptive immune responses was revealed by mice lacking expression of cIAP1 and cIAP2 in B cells, which were significantly impaired in their ability to generate a germinal center immune response to a T cell-dependent antigen (Gardam et al., 2011). While this phenotype was ascribed to a defect in CD40 signaling in B cells, the possibility that BCR signaling might be impaired was not tested. Following BCR and T cell receptor (TCR) crosslinking, BCL10 acquires K63-linked polyubiquitin (Satpathy et al., 2015; Wu and Ashwell, 2008), but the responsible K63 ubiquitin ligase has not been reported. In addition, BCL10 is modified with linear polyubiquitin following BCR engagement (Satpathy et al., 2015), which is presumed to be a consequence of LUBAC association with the CBM complex (Yang et al., 2014). Ubiquitination of BCL10 is necessary to efficiently recruit IKK to the CBM complex and activate NF-κB following TCR engagement (Wu and Ashwell, 2008), but the role of BCL10 ubiquitination in BCR-dependent NF-κB activation has not been investigated.
Pharmaceutical efforts to inhibit IAP proteins were inspired by structural and functional analysis of second mitochondria-derived activator of caspases (SMAC), a small protein that is released from mitochondria during apoptosis and inhibits IAP proteins by binding to their BIR domains (Fulda and Vucic, 2012). Small molecules that function like SMAC (SMAC mimetics) have a range of specificities for IAP family members, with some tailored to block XIAP engagement of caspases while others have more specificity for cIAP1/2. Monomeric cIAP1/2 exist in an autoinhibited state in which the RING domain interacts with the BIR3 domain. Certain SMAC mimetics bind to the BIR3 domain, thereby releasing the RING domain to dimerize and become an active E3 ligase, ultimately leading to intense K48-linked autoubiquitination and destruction of the protein (Dueber et al., 2011). One such SMAC mimetic under clinical investigation is birinapant (TL32711), a dimeric molecule with two cIAP1/2 binding moieties that promotes cIAP1/2 degradation and inhibits NF-κB activation by TNFα in various tumor cell models, thereby promoting tumor cell death (Allensworth et al., 2013; Benetatos et al., 2014; Condon et al., 2014; Krepler et al., 2013).
The role of K63-linked polyubiquitination in pro-survival oncogenic signaling in ABC DLBCL has not been explored in detail. We hypothesized that K63-linked ubiquitination by cIAP1/2 had an important role in ABC DLBCL biology and that cIAP1/2 antagonists may be effective therapeutic agents in this disease.
Results
Frequent copy number gains of the BIRC2/BIRC3 locus in ABC DLBCL
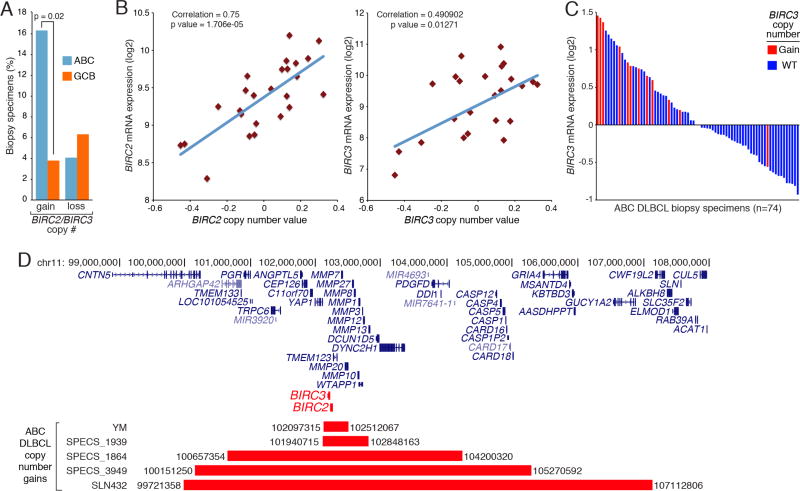
The genes encoding cIAP1 and cIAP2, BIRC2 and BIRC3 respectively, are situated next to each other on human chromosome 11. In an array-based comparative genomic hybridization (aCGH) dataset from DLBCL tumors (Scholtysik et al., 2015), gain/amplification of the genomic region encompassing BIRC2 and BIRC3 was significantly more common in ABC DLBCL (8/49; 16.33%) than in GCB DLBCL (3/79, 3.8%) (Figure 1A). By contrast, both ABC and GCB DLBCL tumors had comparably low frequencies of copy number loss of the BIRC2/BIRC3 locus (4.1% vs. 6.3%, p=0.7071). BIRC2 and BIRC3 expression levels correlated with DLBCL copy number (Figure 1B). In an independent set of ABC DLBCL (Lenz et al., 2008b), those with BIRC2/BIRC3 copy number gains had high relative BIRC3 mRNA levels (Figure 1C) and real-time PCR (Figure S1). A majority (8/11) of ABC DLBCL cases with BIRC2/BIRC3 copy number gains had either a MYD88 or CD79B mutation, while none had mutations affecting CARD11 or A20, which regulate downstream BCR and MYD88 signaling (Table S1). While most of the BIRC2/BIRC3 locus copy number gains encompassed many megabases, aCGH revealed that some were more focal (Figure 1D). One ABC DLBCL cell line, YM, has a copy number gain that involving BIRC2 and BIRC3 and only 3 other genes, which is notable since YM cells depend on cIAP1 and cIAP2 for survival (see below). These genetic findings prompted us to explore the role of cIAP1/2 in ABC DLBCL biology.
Figure 1. Copy Number gains of BIRC2/3 loci in ABC DLBCL biopsy specimens.
(A) Frequency of BIRC2/BIRC3 copy number changes in ABC and GCB DLBCL cases by aCGH. (B) Correlation between BIRC2 (left) or BIRC3 (right) mRNA levels with gene copy number in DLBCL. (C) BIRC3 mRNA levels in 74 ABC DLBCL biopsy specimens. (D) Chromosomal regions containing BIRC2 and BIRC3 with focal copy number gain in ABC DLBCL samples. See also Figure S1 and Table S1.
cIAP1/2 contribute to classical NF-κB activity in ABC DLBCL
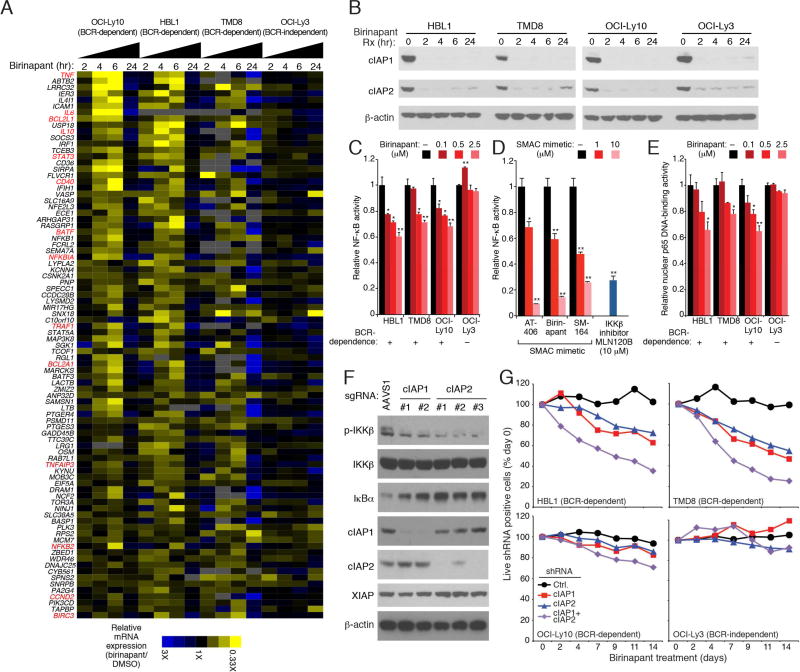
To examine the oncogenic role of cIAP1/2 in ABC DLBCL, we used SMAC mimetics to reduce their expression ABC DLBCL lines. All three SMAC mimetics tested – AT-146, birinapant and SM-164 –reduced the protein levels of cIAP1 and cIAP2 in each ABC DLBCL line (Figure S2A). To gain insight into which biological processes were regulated by cIAP1/2 in ABC DLBCL, we compared the gene expression changes caused by birinapant with a database of gene expression signatures that reflect regulatory processes in normal and malignant blood cells (Shaffer et al., 2006). The genes downregulated by birinapant overlapped significantly with signatures reflecting NF-κB signaling in ABC DLBCL (Table S2, Figure 2A). NF-κB signaling genes were downregulated in three BCR-dependent ABC DLBCL lines (OCI-Ly10, TMD8 and HBL1), but not in the BCR-independent ABC DLBCL line OCI-Ly3. Expression of NF-κB signature genes decreased maximally after 6 hours of birinapant treatment but then rebounded at 24 hours, which may be partially explained by a modest rise in cIAP2 protein levels at 24 hours (Figure 2B). However, this rebound in NF-κB target gene expression may also be explained by effects of cIAP1/2 depletion on the alternative NF-κB pathway (see below).
Figure 2. cIAP1/2 contribute to classic NF-κB activation pathway in ABC DLBCL.
(A) mRNA levels of NF-κB-10 signature genes (Shaffer et al., 2006) in the indicated ABC DLBCL lines treated with birinapant (2.5 µM) relative to DMSO-treated cells. (B) Immunoblot analysis of the indicated proteins in whole cell lysates of ABC DLBCL lines treated with birinapant (2.5 µM) for the indicated times. (C) Relative activity of an NF-κB-dependent luciferase reporter in ABC DLBCL lines after treatment with the indicated birinapant concentrations for 24 hours. (D) Relative activity of an NF-κB-dependent luciferase reporter in the HBL1 line upon treatment of indicated drugs for 24 hours. (E) ELISA measurement of relative NF-κB p65 DNA-binding activity in nuclear extracts from ABC DLBCL lines treated with the indicated birinapant concentrations for 24 hours. (F) Cell lysates from HBL1 cells transduced with lentiviruses expressing the indicated sgRNAs, puromycin selected (5 days) and further cultured (11 days) were immunoblotted for the indicated proteins. (G) The fraction of viable shRNA-expressing cells relative to the total live cell fraction at the indicated times following induction of the indicated shRNAs, normalized to day 0 values. All error bars denote SEM of triplicates. p values (Student’s t test) compare treatment groups with the DMSO control; * p < 0.05; ** p < 0.01. See also Figure S2 and Table S2.
In three BCR-dependent ABC DLBCL lines, but not in the BCR-independent OCI-Ly3 line, birinapant inhibited NF-κB activity in a dose-dependent manner (Figure 2C and Figure S2B). Three structurally distinct SMAC mimetics inhibited the NF-κB reporter and were as effective as MLN120B, a selective IKKβ inhibitor (Lam et al., 2005) (Figure 2D). Treatment of three BCR-dependent ABC DLBCL lines with birinapant decreased the nuclear abundance of NF-κB dimers containing the p65 subunit, indicating that cIAP1/2 contribute to classical NF-κB activity in these cells (Figure 2E).
We next used the CRISPR gene targeting system (Cong et al., 2013; Mali et al., 2013) to probe the function of cIAP1 and cIAP2 genetically. Small guide RNAs (sgRNAs) targeting the cIAP1 and cIAP2 coding regions were coexpressed in HBL1 ABC DLBCL cells with the endonuclease Cas9, as described (Wang et al., 2014). cIAP1 and cIAP2 sgRNAs effectively reduced or eliminated expression of their targets after 16 days, while a control sgRNA had no effect (Figure 2F). Inactivation of cIAP1 or cIAP2 decreased phosphorylation of IKKβ and stabilized the IKK substrate IκBα, indicating lower activity of the classical NF-κB pathway (Figure 2F). Similarly, knockdown of cIAP1 or cIAP2 using small hairpin RNAs (shRNAs) was toxic for BCR-dependent ABC DLBCL lines but not for the BCR-independent OCI-Ly3 line (Figure 2G, Figure S2C). Depletion of both ubiquitin ligases was more toxic than depletion of either alone, indicating that they function in a partially redundant fashion. The gene signatures that were downregulated upon knockdown of cIAP1 and cIAP2 overlapped with those altered by birinapant, many of which reflect NF-κB signaling (Table S2, Figure S2D). Together, these genetic data indicate that cIAP1/2 participate in NF-κB activation in ABC DLBCL.
cIAP1/2 contribute to chronic active B cell receptor signaling in ABC DLBCL
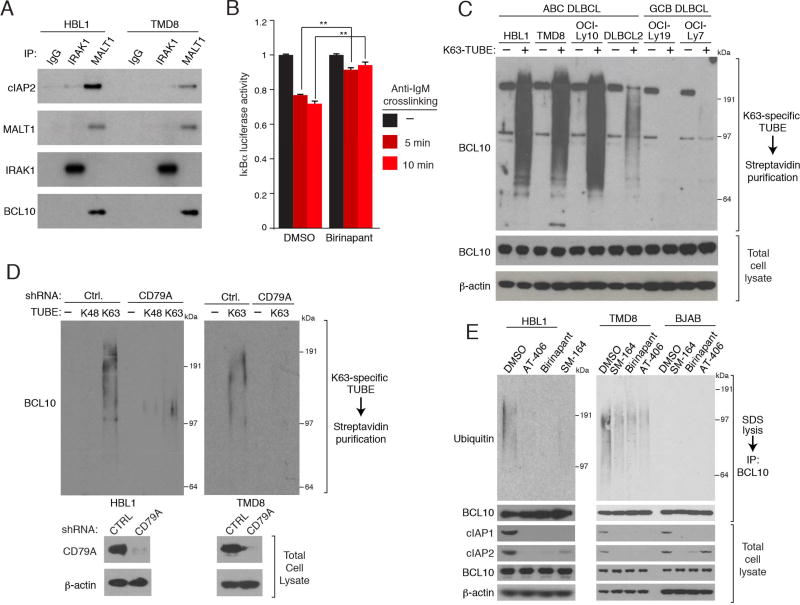
We next investigated the role of cIAP1/2 in the BCR and MYD88 pathways, both of which govern NF-κB activity in ABC DLBCL. cIAP2 co-immunoprecipitated with the MALT1 subunit of the CBM complex in two ABC DLBCL lines, suggesting a role for cIAP2 in BCR signaling (Figure 3A). By contrast, little if any association between cIAP2 and IRAK1, which mediates MYD88 signaling in these ABC DLBCL lines, was detected. To test whether cIAP1/2 contribute to BCR-dependent IKK activity, we engineered TMD8 cells to express an IKK reporter consisting of the IKK substrate IκBα fused to luciferase (Lam et al., 2005). Anti-IgM-mediated crosslinking of the BCR on the surface of these cells decreased IκBα-luciferase levels within 5–10 minutes, indicating an increase in IKK activity, but birinapant treatment prevented this IKK activation (Figure 3B). Thus, cIAP1/2 are integral components of the CBM complex that are required for optimal BCR-dependent IKK activation.
Figure 3. cIAP1/2 participate in chronic active BCR signaling in ABC DLBCL.
(A) Immunoprecipitation (IP) from ABC DLBCL lines using the indicated antibodies were immunoblotted for the indicated proteins. (B) Relative luciferase activity of an IκBα-luciferase fusion protein in TMD8 cells treated with birinapant (5µM) for 6 hours and then with an anti-IgM antibody (10 µg/ml) for the indicated times. Reporter activity was normalized to values from DMSO-treated samples at time zero. Error bars denote SEM of triplicates. p values (Student’s t test) compare treatment groups with the DMSO control; ** p < 0.01. (C) SDS lysates of the indicated lines were subjected to biotin-labeled K63-specific TUBE binding, streptavidin purification. TUBE-purified proteins or total lysates were analyzed by immunoblotting. (D) ABC DLBCL lines were induced to express a CD79A or control (Ctrl.) shRNA. SDS lysates were mixed with biotin-labeled, K63-specific or K48-specific TUBEs followed by streptavidin purification. TUBE-purified proteins or total lysates were analyzed by immunoblotting. (E) SDS (1%) lysates prepared from DLBCL lines treated with the indicated SMAC mimetics (5 µM) for 24 hours, diluted and subjected to anti-BCL10 IP. IPs or total lysates were analyzed by immunoblotting. See also Figure S3.
Many components of the CBM complex – including TRAF6, MALT1 and BCL10 – have been suggested to undergo K63-specific ubiquitination in normal T lymphocytes upon TCR stimulation (Oeckinghaus et al., 2007; Sun et al., 2004; Wu and Ashwell, 2008), but the ubiquitination of these proteins have not been carefully evaluated in DLBCL lines. To address this issue, we used biotin-labeled, K63-specific Tandem Ubiquitin Binding Entities (K63-TUBE), which can selectively enrich for polyubiquitinated proteins that have K63-linkages rather than K48-linkages (Silva et al., 2015). K63-TUBE-mediated pull down in ABC DLBCL lines enriched for ubiquitinated BCL10, which ran as a broad smear in immunoblots, but this was not observed in GCB DLBCL lines (Figure 3C). Inhibition of chronic active BCR signaling by knockdown of the BCR subunit CD79A substantially reduced the levels of K63-ubiquitinated BCL10 in ABC DLBCL lines (Figure 3D). Likewise, SMAC mimetic-mediated depletion of cIAP1 or cIAP2 decreased ubiquitinated BCL10 in BCR-dependent ABC DLBCL lines but not in GCB DLBCL lines (Figure 3E). Depletion of cIAP1/2 had no effect on BCL10 polyubiquitination in OCI-Ly3 cells, which has an activating CARD11 mutation (Figure S3A), which fits with previous evidence activating CARD11 mutants can induce BCL10 ubiquitination (Chan et al., 2013). Indeed, ectopic expression of a constitutively active CARD11 mutant in ABC DLBCL cells with a WT CARD11 locus counteracted birinapant toxicity, leading to selective outgrowth of these transduced cells (Figure S3B).
In contrast to the pronounced K63-ubiquitination of BCL10 in ABC DLBCL lines, little if any K48-ubiqutination of BCL10 could be detected using a K48-specific TUBE (Figure 3D), and BCL10 protein stability was unchanged following depletion of cIAP1/2 with birinapant (Figure S3C). Thus, cIAP1/2 act downstream of BCR signaling to modify BCL10 with K63-linked polyubiquitin in ABC DLBCL.
cIAP1/2-dependent BCL10 ubiquitination promotes IKK recruitment to the CBM complex
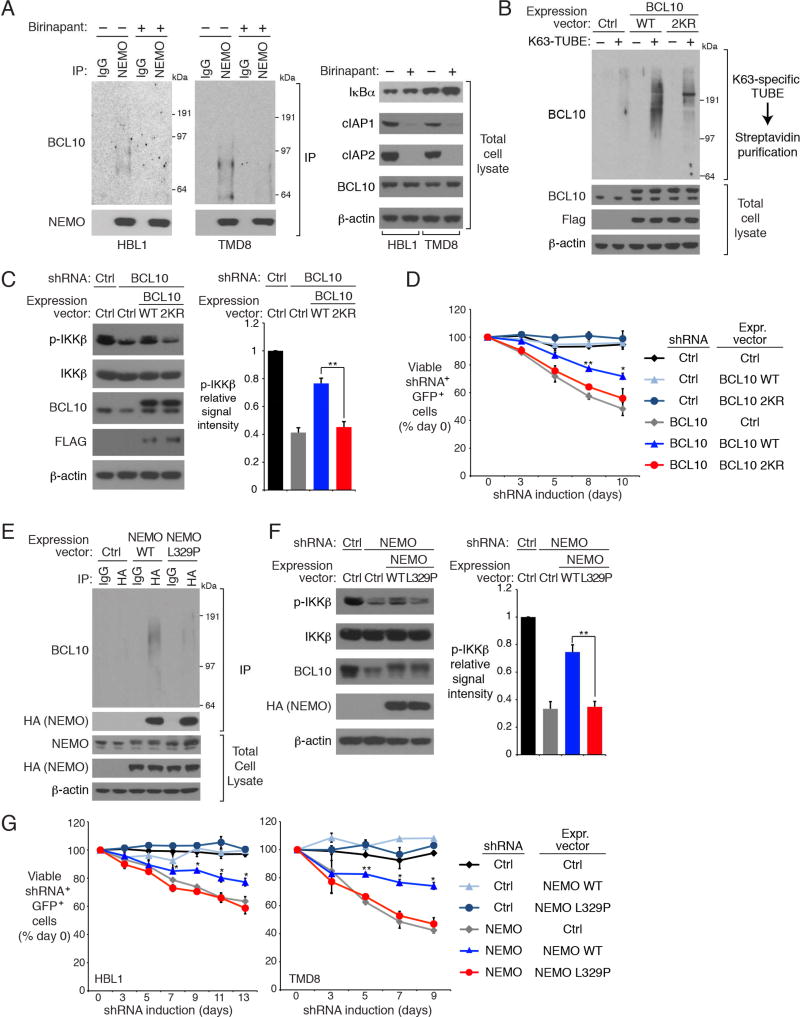
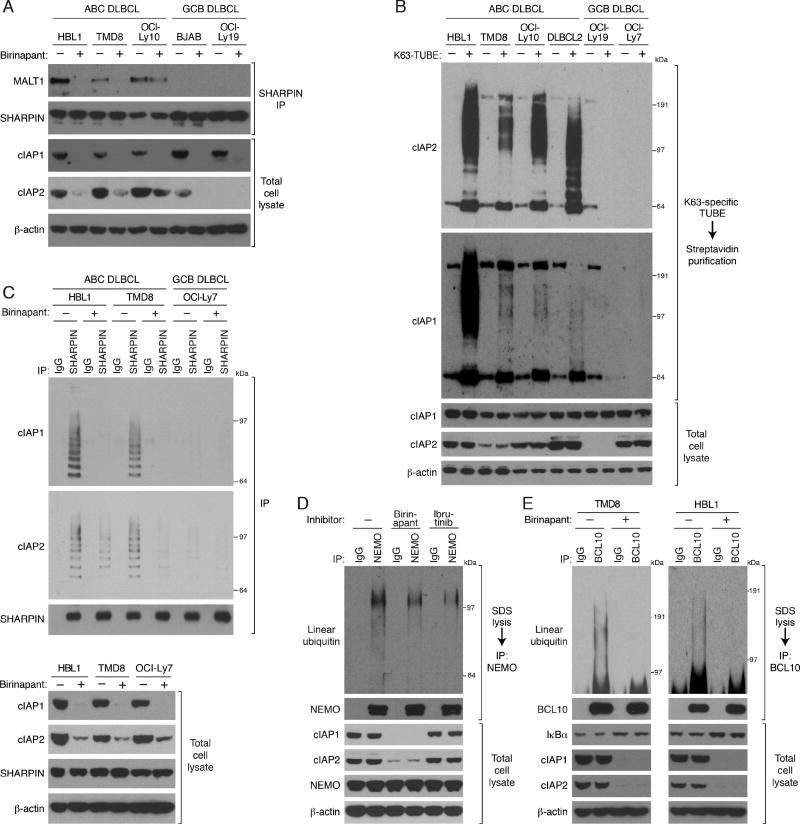
Following TCR signaling, K63-polyubiquitination of BCL10 is recognized by the UBAN ubiquitin-binding domain of the IKK NEMO subunit, resulting in the recruitment of IKK to the CBM complex (Wu and Ashwell, 2008). In ABC DLBCL cell lines, ubiquitinated BCL10 constitutively associated with NEMO, which was abrogated by treatment with SMAC mimetics (Figure 4A, Figure S4A). Likewise, the constitutive binding of NEMO to MALT1, another component in CBM complex, was also inhibited by birinapant treatment of ABC DLBCL lines (Figure S4B). Thus, K63-polyubiquitination by cIAP1/2 is essential for IKK recruitment to the CBM complex in ABC DLBCL.
Figure 4. cIAP1/2 is required for BCL10 binding to IKK NEMO in ABC DLBCL.
(A) NEMO IPs or total lysates from HBL1 and TMD8 cells treated with birinapant (5 µM) for 24 hours were immunoblotted for the indicated proteins. (B) Cell lysates of HBL1 cells expressing WT or 2KR mutant BCL10 were subjected to biotin-labeled K63-specific TUBE binding and streptavidin purification, and analyzed by immunoblotting. (C) Control or BCL10 shRNAs were inducibly expressed in HBL1 cells that had been transduced with WT or 2KR BCL10, or with a control vector. Lysates were analyzed by immunoblotting for the indicated proteins. Relative phospho- (p-) IKKβ levels were quantified by densitometry (right). Error bars denote SEM. p values (Student’s t test) compare the BCL10 WT and 2KR rescue groups. (D) HBL1 cells transduced with WT or 2KR BCL10, or with a control vector, were induced to express control or BCL10 shRNAs along with GFP. The fraction of viable, GFP+/shRNA+ cells relative to the live cell fraction is plotted at the indicated times following shRNA induction, normalized to day 0 values. Error bars denote SEM. p values (Student’s t test) compare the BCL10 WT and 2KR rescue groups at the indicated time points. (E) Anti-HA IPs or total lysates of HBL1 cells expressing the indicated HA epitope-tagged WT or L329P mutant NEMO were analyzed by immunoblotting for the indicated proteins. (F) Control or NEMO shRNAs were inducibly expressed in HBL1 cells that had been transduced with WT or L329P NEMO, or with an empty vector. Lysates were analyzed by immunoblotting for the indicated proteins. Relative p-IKKβ levels were quantified by densitometry. Error bars denote SEM. p values (Student’s t test) compare the NEMO WT and L329P rescue groups. (G) Cells transduced with WT or L329P NEMO, or with a control vector, were induced to express control or NEMO shRNAs along with GFP. The fraction of viable, GFP+/shRNA+ cells relative to the live cell fraction is plotted at the indicated times following shRNA induction, normalized to day 0 values. Error bars denote SEM. p values (Student’s t test) compare the NEMO WT and L329P rescue groups at the indicated time points; * p <0.05; ** p < 0.01. See also Figure S4.
Of the 15 lysine residues in BCL10, K31 and K63 are apparent attachment sites for K63-polyubiquitin in TCR signaling (Wu and Ashwell, 2008). To probe the function of BCL10 ubiquitination in ABC DLBCL cells, we ectopically expressed the WT BCL10 or the BCL10 2KR mutant in which both K31 and K63 were changed to arginine., BCL10 enriched using the K63-specific TUBE from cells transduced with WT BCL10 ran as a polyubiquitinated protein smear, which was significantly reduced in cells expressing BCL10 2KR (Figure 4B). To assess the contribution of BCL10 ubiquitination to NF-κB activity and viability in ABC DLBCL, we knocked down the endogenous BCL10 using an shRNA targeting the BCL10 3’ untranslated region and ectopically expressed WT or 2KR BCL10 coding regions. BCL10 knockdown decreased IKKβ phosphorylation, which was restored by WT but not 2KR BCL10 (Figure 4C). Similarly, the toxicity of BCL10 knockdown in ABC DLBCL cells was reversed by ectopic provision of WT but not 2KR BCL10 (Figure 4D).
To further assess the impact of BCL10 polyubiquitination on NF-κB activation in ABC DLBCL, we engineered HBL1 cells to express either WT IKK NEMO or the L329P mutant that abrogates ubiquitin binding by the NEMO UBAN domain (Wu et al., 2006). Polyubiquitinated BCL10 was co-immunoprecipitated with WT NEMO but not with the L329P mutant (Figure 4E). NF-κB pathway activity, as judged by IKKβ phosphorylation, was decreased by NEMO knockdown, which could be reversed by ectopic expression of WT but not L329P NEMO (Figure 4F). Likewise, the toxicity of NEMO knockdown for ABC DLBCL lines was mitigated by re-expression of WT but not L329P NEMO (Figure 4G). Overall, our data support a model that cIAP1/2-dependent polyubiquitination of BCL10 promotes IKK recruitment to the CBM complex in a fashion that depends on ubiquitin binding by IKK NEMO.
LUBAC recruitment to the CBM complex in ABC DLBCL depends on cIAP1/2
In ABC DLBCL lines with chronic active BCR signaling, LUBAC associates constitutively with the CBM complex, where it recognizes and linear-ubiquitinates IKK NEMO (Yang et al., 2014). The LUBAC subunit SHARPIN associated with the CBM component MALT1 in BCR-dependent ABC DLBCL lines but not in GCB DLBCL lines, but treatment with SMAC mimetics reduced this association (Figure 5A and Figure S5A). The zinc finger domain of the LUBAC subunit RNF31 binds preferentially to K63-polyubiquitin chains, which is essential for LUBAC recruitment to receptor complexes (Gerlach et al., 2011; Haas et al., 2009; Ikeda et al., 2011). The SHARPIN-MALT1 association was equivalent in cells expressing either WT or 2KR BCL10, suggesting that BCL10 ubiquitination by cIAP1/2 is not necessary for LUBAC recruitment (Figure S5B). We therefore tested whether LUBAC might directly interact with K63-polyubiquitin chains formed on cIAP1/2 by autoubiquitination (Blankenship et al., 2009). Using a K63-specific TUBE assay, we observed abundant K63-polyubiquitination of cIAP1 and cIAP2 in ABC but not GCB DLBCL lines (Figure 5B). By co-immunoprecipitation, SHARPIN associated with ubiquitinated cIAP1 and cIAP2 in two ABC DLBCL lines but not in a GCB DLBCL line, and this association was prevented by SMAC mimetics (Figure 5C and Figure S5C), suggesting that autoubiquitination of cIAP1/2 could be responsible for LUBAC recruitment to the CBM complex.
Figure 5. LUBAC recruitment to the CBM complex in ABC DLBCL depends on cIAP1/2.
(A) SHARPIN IPs or total lysates of the indicated DLBCL lines treated with birinapant (5 µM) for 24 hours were immunoblotted for the indicated proteins. (B) Total SDS lysates or after biotin-labeled K63-specific TUBE binding and streptavidin purification from the indicated DLBCL lines were analyzed by immunoblotting. (C) SHARPIN IPs of DLBCL lines treated with birinapant (5 µM) for 24 hours or total lysates were immunoblotted for the indicated proteins. (D) Lysates prepared using 1% SDS from HBL1 cells treated with birinapant (5 µM) or ibrutinib (10 nM) for 24 hours were diluted and then subjected to IP. IPs or total lysates were immunoblotted for the indicated proteins. (E) Lysates prepared using 1% SDS from ABC DLBCL cells treated with birinapant (5 µM) for 24 hours were diluted and subjected to IP. IPs or total lysates were immunoblotted for the indicated proteins. See also Figure S5.
Given the role of cIAP1/2 in recruiting LUBAC to the CBM complex, we assessed the influence of birinapant on linear ubiquitination of NEMO (Figure 5D). Birinapant partially inhibited linear ubiquitination of NEMO in HBL1 cells, as did treatment with the BTK inhibitor ibrutinib, which inhibits CBM activity. The residual NEMO ubiquitination may be mediated by MYD88 since LUBAC also associates with the mutant MYD88 signaling complex in ABC DLBCL cells (Yang et al., 2014). A recent study revealed that BCL10 is modified by linear polyubiquitin in a mouse B cell line following BCR crosslinking (Satpathy et al., 2015). BCL10 was also linear-ubiquitinated in ABC DLBCL lines, and SMAC mimetics decreased this modification, in keeping with their ability to block LUBAC recruitment (Figure 5E and Figure S5D). Linear ubiquitin chains could either attach to BCL10 directly or contribute to branched polyubiquitin chains that are built upon K63-linked chains attached to BCL10, as described during IL-1 signaling (Emmerich et al., 2013). In summary, cIAP1/2-mediated polyubiquitination is required for the recruitment of LUBAC to the CBM complex and the linear ubiquitination of NEMO and BCL10.
Role of cIAP1/2 in alternative NF-κB pathway activation in ABC DLBCL
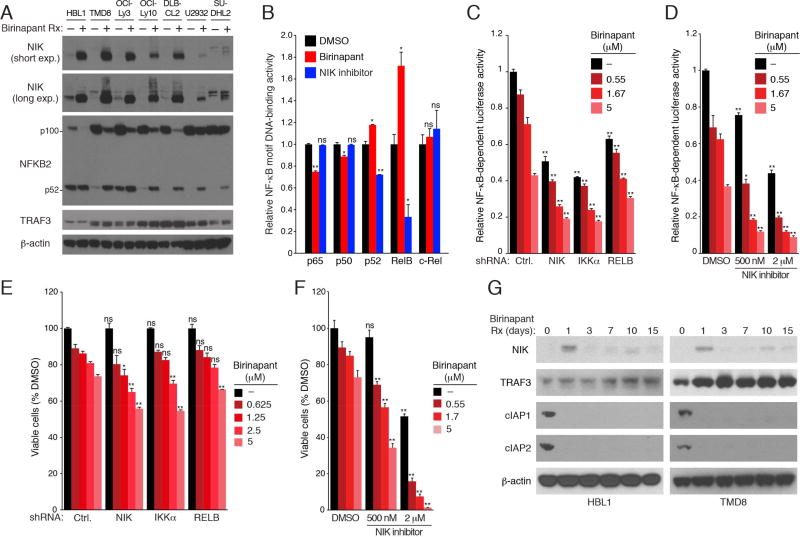
In normal B cells, cIAP1/2 promote the K48-polyubiquitination and destruction of NIK, a kinase that activates the alternative NF-κB pathway (Vallabhapurapu et al., 2008; Zarnegar et al., 2008a). Consequently, loss of cIAP1/2 leads to NIK stabilization, IKKα activation, proteolytic processing of NFKB2 from its p100 isoform to its p52 isoform, and nuclear accumulation of the alternative NF-κB heterodimer p52/RelB. In untreated ABC DLBCL lines, we observed little NIK protein expression, but 6-hour treatment with birinapant caused NIK to accumulate and induced the processing of NFKB2 p100 into p52 (Figure 6A). Treatment of HBL1 cells for one day with birinapant decreased nuclear NF-κB binding activity involving the p65 and p50 subunits, consistent with the role of cIAP1/2 in promoting IKKβ activation of the classical NF-κB pathway (Figure 6B). In contrast, birinapant treatment increased NF-κB motif binding by p52 and RelB, consistent with NIK stabilization and activation of the alternative NF-κB pathway (Figure 6B). Treatment with a s NIK inhibitor decreased p52 and RelB DNA binding (Figure 6B), suggesting that HBL1 cells have a basal degree of alternative NF-κB activation, a view supported by their low NIK protein levels and basal processing of NFKB2 into the p52 isoform (Figure 6A). Knockdown of NIK, IKKα, or RELB as well as treatment with a NIK inhibitor decreased the NF-κB pathway activity in HBL1 cells (Figures 6C, 6D). Addition of birinapant further decreased NF-κB activity, consistent with combined inhibition of the classical and alternative NF-κB pathways (Figure 6C, 6D). Accordingly, birinapant had more toxicity for HBL1 cells when the alternative NF-κB pathway was simultaneously inhibited by NIK, IKKα or RELB shRNAs or by the NIK inhibitor (Figure 6E, 6F).
Figure 6. Negative regulation of alternative NF-κB activity by cIAP1/2 in ABC DLBCL.
(A) Lysates of ABC DLBCL lines treated with birinapant (5 µM) for 6 hours analyzed by immunoblotting for the indicated proteins. (B) NF-κB DNA-binding activity utilizing the indicated NF-κB subunits determined in nuclear extracts of HBL1 cells treated with birinapant (5 µM) or a NIK inhibitor (1 µM) for 24 hours, relative to DMSO-treated cells. (C) Relative activity of an NF-κB-dependent luciferase reporter in HBL1 cells expressing the indicated shRNAs for 2 days followed by birinapant treatment for 24 hours. (D) Relative activity of an NF-κB-dependent luciferase reporter in HBL1 cells following treatment (24 hr) with the indicated concentrations of birinapant +/− a NIK inhibitor. (E) Relative numbers of viable HBL1 cells that were induced to express the indicated shRNAs for 2 days followed by treatment with the indicated birinapant concentrations for 4 days. (F) Relative viability of HBL1 cells following treatment (4 days) with the indicated concentrations of birinapant +/− a NIK inhibitor. (G) Lysates of HBL1 or TMD8 cells treated with birinapant (2.5 µM) for the indicated times analyzed by immunoblotting for the indicated proteins. Error bars denote SEM of triplicates. p values (Student’s t test) compare treatment groups with the DMSO control in (B); the control and experimental shRNA groups at the same birinapant concentrations in (C and E); the DMSO and NIK inhibitor groups at the same birinapant concentrations in (D and F); ns: no statistical difference; * p < 0.05; ** p < 0.01.
While treatment with birinapant for one day stabilized NIK in two ABC DLBCL lines, NIK levels dropped unexpectedly over the next 2 weeks of treatment, despite addition of fresh birinapant every 3 days (Figure 6G). Interestingly, TRAF3 levels increased during prolonged birinapant treatment, which could contribute to the fall of NIK levels over time (see Discussion) and are consistent with reports of cIAP1/2-mediated K48-polyubiquitination and degradation of TRAF3 (Figure 6G) (Vallabhapurapu et al., 2008; Zarnegar et al., 2008b). Thus, at early time points, the decrease in classical NF-κB activity caused by birinapant is counterbalanced by an increase in alternative NF-κB activity, but at later time points, birinapant primarily decreases classical NF-κB activity.
Birinapant is selectively toxic for BCR-dependent ABC DLBCL models
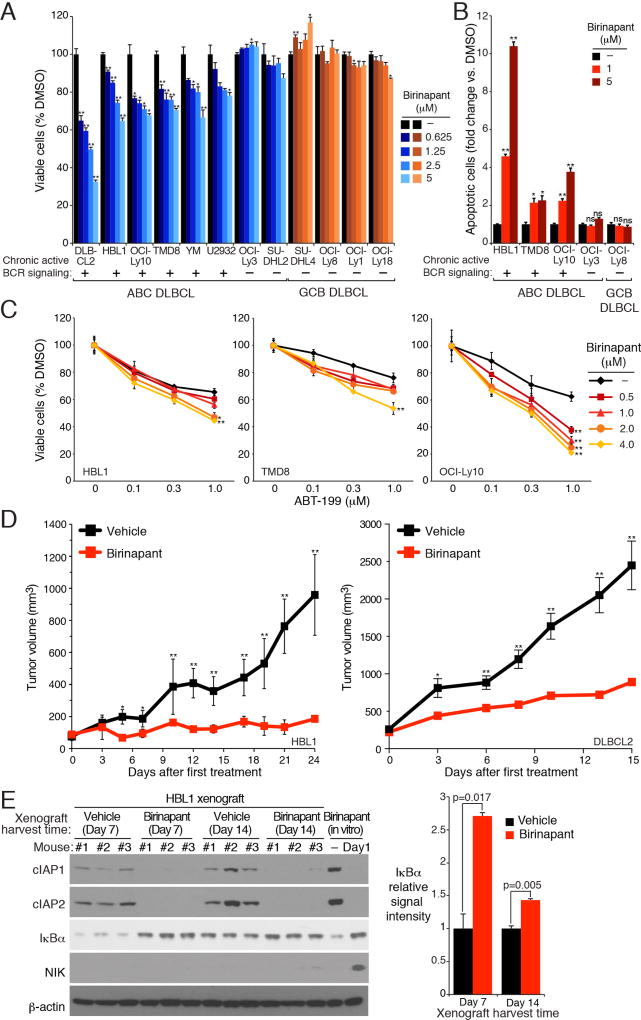
To investigate the therapeutic potential of SMAC mimetics in DLBCL, we determined the viability of ABC and GCB DLBCL lines after treatment with birinapant for 5 days (Figure 7A and Figure S6A). In all 6 ABC DLBCL lines with chronic active BCR signaling, birinapant was toxic in a dose-dependent manner. By contrast, birinapant had little if any toxicity for 2 BCR-independent ABC DLBCL lines or for 7 GCB DLBCL lines. Birinapant treatment induced apoptotic cell death in BCR-dependent ABC DLBCL lines, as measured by cleavage of both PARP and Caspase 3 (Figure 7B). Two other SMAC mimetics, AT-406 and SM-164, exhibited similar efficacy and specificity in killing BCR-dependent ABC DLBCL lines (Figures S6B, S6C).
Figure 7. Selective toxicity of birinapant for BCR-dependent ABC DLBCL lines.
(A) Viability of the indicated DLBCL lines treated with birinapant at the indicated concentrations for 5 days, normalized to DMSO-treated cells. (B) Relative apoptotic cells in DLBCL lines treated with birinapant (2 days) at the indicated concentrations, normalized to DMSO-treated cells. (C) Viability of ABC DLBCL lines after treatment (4 days) with the indicated concentrations of birinapant, ABT-199, or both. Data are normalized to DMSO-treated cells, and then to cells treated with birinapant alone. (D) NOD/SCID mice bearing HBL1 (left) and DLBCL2 (right) xenografts were treated with birinapant or vehicle. Tumor growth was measured as a function of tumor volume. Error bars denote SEM of n=3 (HBL1) or n=5 (DLBCL2). (E) HBL1 cells were established and treated as in D. Cell lysates from malignant cells purified from tumors were immunoblotted for the indicated protein. Relative IκBα levels were quantified by densitometry (right). Error bars denote SEM of triplicates. p values (Student’s t test) compare the vehicle and birinapant groups in (A, B and E); or the ABT-199-only group with the birinapant plus ABT-199 group at the highest ABT-199 concentration (C); * p < 0.05; ** p < 0.01. See also Figures S6 and S7.
We next investigated whether birinapant would combine favorably with other drugs that inhibit oncogenic survival mechanisms in ABC DLBCL. The anti-apoptotic protein BCL2 is highly expressed in ABC DLBCL and BCL2 inhibition by H3-mimetics is toxic to ABC DLBCL lines (Mathews Griner et al., 2014). Since birinapant as a single agent induced apoptotic cell death in ABC DLBCL cells (Figure 7B), we combined it with the BH3-mimetic ABT-199 and observed synergistic effects on the viability of three BCR-dependent ABC DLBCL lines (Figure 7C). Since the viability data in these plots were normalized to remove the effect of birinapant as a single agent, the left shift of the toxicity curves indicates more than additive (i.e. synergistic) toxicity of the drug combination, a view further confirmed by a formal mathematical algorithm (Greco et al., 1990) (Figure S6D). By contrast, birinapant did not synergize with the BTK inhibitor ibrutinib, which is understandable since these two drugs target closely related nodes within the BCR pathway (Figure S6E).
Finally, we evaluated the ability of birinapant to retard growth of ABC DLBCL xenografts. We established ABC DLBCL xenograft mouse models using the HBL1 and DLBCL2 cell lines. In both models, oral treatment with birinapant significantly slowed tumor growth (Figure 7D and Figure S7A), and reduced cIAP1 and cIAP2 protein levels (Figure 7E and Figure S7B). NIK protein levels in HBL1 xenografts after 1 or 2 weeks of treatment were substantially lower than in HBL1 cells treated in vitro with birinapant for 1 day (Figure 7E), in keeping with the drop in NIK protein levels observed after prolonged in vitro treatment with birinapant (Figure 6G). Accordingly, IκBα accumulation and IKKβ phosphorylation reduction were observed in the HBL1 xenografts at the treatment endpoint, (Figures 7E, S7C), indicating NF-κB pathway inhibition. At the doses used, birinapant was well tolerated by mice, with no change in body weight or hematologic parameters (Figures S7D, S7E, and S7F).
Discussion
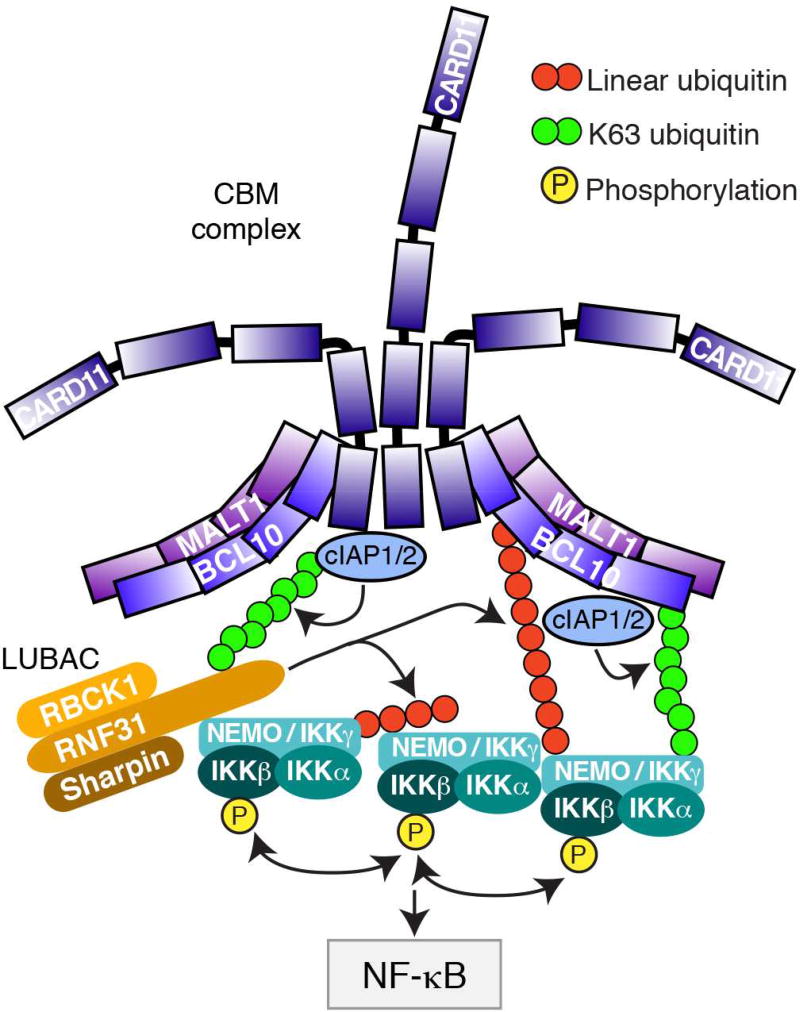
We describe a role for the E3 ubiquitin ligases cIAP1 and cIAP2 in the oncogenic BCR signaling that characterizes ABC DLBCL, and provide a strategy to exploit this knowledge therapeutically using SMAC mimetics. Based on our functional and biochemical analysis of cIAP1/2 action in ABC DLBCL, we propose a working model for CBM-dependent NF-κB activation involving these ubiquitin ligases (Figure 8). cIAP1/2 reside in the CBM complex in ABC DLBCL and are heavily modified by K63-polyubiquitin chains, presumably due to auto-ubiquitination. cIAP1/2 recruit the LUBAC ubiquitin ligase to the CBM complex, presumably because K63-polyubiquitin chains are recognized by the NZF1 ubiquitin binding domain of the LUBAC RNF31 subunit (Fujita et al., 2014; Gerlach et al., 2011; Ikeda et al., 2011). Separately, cIAP1/2 are necessary for IKK recruitment to the CBM complex in a fashion that depends on BCL10 ubiquitination. BCL10 is modified by both K63-polyubiquitin and linear polyubiquitination in ABC DLBCL, and both modifications depend on cIAP1/2. The recruitment of IKK NEMO depends on its UBAN ubiquitin-binding domain, which binds both K63- and linear polyubiquitin chains with different relative affinities (Kensche et al., 2012; Ngadjeua et al., 2013). Conceivably, the initial recruitment of IKK to the CBM complex may depend on its interaction with K63-ubiquitinated BCL10, while IKK retention in the CBM complex may be fostered by linear ubiquitination of BCL10 subsequent to LUBAC recruitment to the CBM complex. As a consequence of these several biochemical activities, cIAP1/2 contribute significantly to BCR-dependent activation of NF-κB in ABC DLBCL, accounting for the selective toxicity of SMAC mimetics in BCR-dependent ABC DLBCL lines.
Figure 8. Model of cIAP1/2 action in the CBM complex.
The cartoon depicts a working model of how cIAP1/2 K63-ubiquitination controls the recruitment of LUBAC and IKK to the CBM complex, thereby activating IKK activation and increasing NF-κB levels in ABC DLBCL.
Copy number analysis of DLBCL tumors revealed gain or amplification of the BIRC2/BIRC3 locus in ~16% of ABC but not GCB DLBCL tumors, providing genetic evidence to support an essential role for cIAP1/2 in chronic active BCR signaling in ABC DLBCL. Interestingly, inactivating mutations in BIRC3 are recurrent in chronic lymphocytic leukemia (CLL, ~9% of cases), mantle cell lymphoma (MCL, ~6–10% of cases) and splenic marginal zone lymphoma (~5% of cases), and lead to the alternative NF-κB activation (Bea et al., 2013; Puente et al., 2015; Rahal et al., 2014; Rossi et al., 2012). Inactivating mutations of BIRC3 have not been reported in genomic analysis of DLBCL (Lohr et al., 2012; Morin et al., 2011; Pasqualucci et al., 2011; Zhang et al., 2013), which is consistent with our evidence that cIAP2 is required for BCR-dependent NF-κB activation in ABC DLBCL. In CLL and MCL cases with BIRC3 mutations, the BIRC2 locus is invariably unmutated, raising the possibility that cIAP1 could maintain BCR signaling in these cases.
During prolonged birinapant treatment over several days, NIK levels dropped and alternative NF-κB activity waned, suggesting that a negative feedback loop was triggered. During this extended time course, TRAF3 protein levels rose, in keeping with previous studies showing that cIAP1/2 mediate the degradation of TRAF3 (Vallabhapurapu et al., 2008; Zarnegar et al., 2008b). The rise in TRAF3 could conceivably be responsible for the fall in NIK levels, since birinapant-treated cells have residual cIAP1/2 that could work with TRAF3 and TRAF2 to degrade NIK.
Previous studies of SMAC mimetics in cancer have emphasized their ability to sensitize cancer cells to pro-apoptotic stimuli and to switch the response of cancer cells to TNFα from survival to death (Fulda and Vucic, 2012). By contrast, SMAC mimetics target chronic active BCR signaling in ABC DLBCL by eliminating cIAP1/2, which we have shown are intrinsic components of the CBM complex that are necessary for BCR-dependent NF-κB activation. From this perspective, SMAC mimetics could be more effective against ABC DLBCL than against solid tumors because they directly target an essential oncogenic pathway in ABC DLBCL. Based on our mechanistic and functional studies, clinical evaluation of SMAC mimetics in DLBCL should proceed selectively in the ABC DLBCL subtype, both as a single agent and in combination with other drugs that inhibit pro-survival pathways in ABC DLBCL, such as the BCL2 inhibitor ABT-199.
Experimental Procedures
(See Supplemental Experimental Procedures for details)
Patient samples
All samples were studied according to a protocol approved by the National Cancer Institute Institutional Review Board.
BIRC2 and BIRC3 copy number analysis
Affymetrix 250k-Sty SNP array data was downloaded from the GEO database (http://www.ncbi.nlm.nih.gov/geo/) using accession number GSE57612. Gains and losses were determined by the GLAD method, implemented in R. Affymetrix SNP 6.0 data were analyzed using the Aroma algorithm and segments assigned to copy categories as described (Lenz et al., 2008b). Differences in BIRC2/BIRC3 copy number prevalence between ABC and GCB DLBCL was tested using Fisher’s Exact test.
Cell Culture
Cell lines for inducible shRNA-mediated knockdown were modified to express an ecotropic retroviral receptor and a fusion protein of the Tet repressor and the blasticidin resistance gene, as described previously (Ngo et al., 2006). For CRISPR-Cas9 knockdown, cells were transduced with lentiviruses expressing Cas9 and an sgRNA (lentiCRISPR), as described (Shalem et al., 2014).
Tumor model and therapy study
Animal experiments were approved by the National Cancer Institute Animal Care and Use Committee (NCI ACUC) and were performed in accordance with NCI ACUC guidelines. Human ABC DLBCL xenograft models were established by subcutaneous injection of lymphoma cell lines into nonobese diabetic/severe combined immunodeficient (NOD/SCID) mice. For biochemical analysis, xenografts were harvested 24 hours after final dosing with birinapant or vehicle, and lymphoma cells were purified from mouse cells using the MACS Mouse Cell Depletion Kit (Miltenyi Biotec).
Supplementary Material
Significance.
The present study uncovers a role for the ubiquitin ligases cIAP1 and cIAP2 in the BCR signaling pathway as resident components of the CBM complex. SMAC mimetics, which potently reduce the abundance of cIAP1 and cIAP2, have displayed an acceptable safety profile in clinical trials but have yet to show significant activity against solid tumors. We propose that ABC DLBCL is a superior clinical setting in which to demonstrate the efficacy of SMAC mimetics since cIAP1 and cIAP2 are integral components of a key oncogenic pathway in this lymphoma subtype.
Highlights.
-
>
cIAP1/2 are functional E3 ligases in ABC DLBCL that promote NF-κB activation.
-
>
cIAP1/2 reside in the CBM complex and deposit K63-polyubiquitin chains there.
-
>
cIAP1/2 help to recruit LUBAC and IκB kinase to the CBM complex.
-
>
SMAC-mimetics degrade cIAP1/2 and block growth of BCR-dependent ABC DLBCL xenografts.
Yang et al. show that cIAP1 and cIAP2 mediate K63-ubiquitination of BCL10, thus are essential for B cell receptor (BCR)-dependent NF-κB activity in ABC subtype of diffuse large B cell lymphoma (DLBCL). SMAC mimetics target cIAP1/2 for destruction, selectively killing BCR-dependent ABC DLBCL cells.
Acknowledgments
Grant Support
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research. R.S. was supported by the Dr. Mildred Scheel Stiftung für Krebsforschung (Deutsche Krebshilfe). D.W. is the Philip O'Bryan Montgomery, Jr., MD, Fellow of the Damon Runyon Cancer Research Foundation (DRG-2208-14).
The authors thank the patients for their participation. We wish to thank Kathleen Meyer for her assistance with GEO submissions, and the members of the Coriell Genotyping and Microarray Center for their assistance with Affymetrix SNP6.0 array. This study was conducted under the auspices of the Lymphoma/Leukemia Molecular Profiling Project (LLMPP).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Accession number:
Genomic data has been submitted to the GEO database (http://www.ncbi.nlm.nih.gov/geo/) with the accession numbers GSE73281 and GSE73639.
Author Contributions
Y.Y., and L.M.S. designed and oversaw the project. Y.Y., P.K., A.L.S., R.S., and R.M.Y performed experiments and collected data. Y.Y., P.K., A.L.S., and L.M.R. analyzed and interpreted the data. A.L.S. analyzed Affymetrix microarray data. X.L., D.W.H., D.W., and G.W.W analyzed aCGH and Affymetrix SNP6.0 data. H.M.Y., M.N., M.C., Y.Y., H.Z., X.Y., and W.X., provided technical support and critical materials. W.C.C., E.S.J., R.D.G., E.C., A.R., G.O., J.D., and L.R., provided tumor samples. Y.Y., and L.M.S. wrote the manuscript.
References
- Alizadeh AA, Eisen MB, Davis RE, Ma C, Lossos IS, Rosenwald A, Boldrick JC, Sabet H, Tran T, Yu X, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000;403:503–511. doi: 10.1038/35000501. [DOI] [PubMed] [Google Scholar]
- Allensworth JL, Sauer SJ, Lyerly HK, Morse MA, Devi GR. Smac mimetic Birinapant induces apoptosis and enhances TRAIL potency in inflammatory breast cancer cells in an IAP-dependent and TNF-alpha-independent mechanism. Breast cancer research and treatment. 2013;137:359–371. doi: 10.1007/s10549-012-2352-6. [DOI] [PubMed] [Google Scholar]
- Annunziata CM, Davis RE, Demchenko Y, Bellamy W, Gabrea A, Zhan F, Lenz G, Hanamura I, Wright G, Xiao W, et al. Frequent engagement of the classical and alternative NF-kappaB pathways by diverse genetic abnormalities in multiple myeloma. Cancer Cell. 2007;12:115–130. doi: 10.1016/j.ccr.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bea S, Valdes-Mas R, Navarro A, Salaverria I, Martin-Garcia D, Jares P, Gine E, Pinyol M, Royo C, Nadeu F, et al. Landscape of somatic mutations and clonal evolution in mantle cell lymphoma. Proc Natl Acad Sci U S A. 2013;110:18250–18255. doi: 10.1073/pnas.1314608110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benetatos CA, Mitsuuchi Y, Burns JM, Neiman EM, Condon SM, Yu G, Seipel ME, Kapoor GS, Laporte MG, Rippin SR, et al. Birinapant (TL32711), a bivalent SMAC mimetic, targets TRAF2-associated cIAPs, abrogates TNF-induced NF-kappaB activation, and is active in patient-derived xenograft models. Mol Cancer Ther. 2014;13:867–879. doi: 10.1158/1535-7163.MCT-13-0798. [DOI] [PubMed] [Google Scholar]
- Bertrand MJ, Milutinovic S, Dickson KM, Ho WC, Boudreault A, Durkin J, Gillard JW, Jaquith JB, Morris SJ, Barker PA. cIAP1 and cIAP2 facilitate cancer cell survival by functioning as E3 ligases that promote RIP1 ubiquitination. Mol Cell. 2008;30:689–700. doi: 10.1016/j.molcel.2008.05.014. [DOI] [PubMed] [Google Scholar]
- Blankenship JW, Varfolomeev E, Goncharov T, Fedorova AV, Kirkpatrick DS, Izrael-Tomasevic A, Phu L, Arnott D, Aghajan M, Zobel K, et al. Ubiquitin binding modulates IAP antagonist-stimulated proteasomal degradation of c-IAP1 and c-IAP2(1) Biochem J. 2009;417:149–160. doi: 10.1042/BJ20081885. [DOI] [PubMed] [Google Scholar]
- Chan W, Schaffer TB, Pomerantz JL. A quantitative signaling screen identifies CARD11 mutations in the CARD and LATCH domains that induce Bcl10 ubiquitination and human lymphoma cell survival. Mol Cell Biol. 2013;33:429–443. doi: 10.1128/MCB.00850-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZJ. Ubiquitination in signaling to and activation of IKK. Immunological reviews. 2012;246:95–106. doi: 10.1111/j.1600-065X.2012.01108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu ZL, McKinsey TA, Liu L, Gentry JJ, Malim MH, Ballard DW. Suppression of tumor necrosis factor-induced cell death by inhibitor of apoptosis c-IAP2 is under NF-kappaB control. Proc Natl Acad Sci U S A. 1997;94:10057–10062. doi: 10.1073/pnas.94.19.10057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condon SM, Mitsuuchi Y, Deng Y, LaPorte MG, Rippin SR, Haimowitz T, Alexander MD, Kumar PT, Hendi MS, Lee YH, et al. Birinapant, a smac-mimetic with improved tolerability for the treatment of solid tumors and hematological malignancies. Journal of medicinal chemistry. 2014;57:3666–3677. doi: 10.1021/jm500176w. [DOI] [PubMed] [Google Scholar]
- Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, Hsu PD, Wu X, Jiang W, Marraffini LA, Zhang F. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339:819–823. doi: 10.1126/science.1231143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis RE, Brown KD, Siebenlist U, Staudt LM. Constitutive nuclear factor kappa B activity is required for survival of activated B Cell-like diffuse large B cell lymphoma cells. J Exp Med. 2001;194:1861–1874. doi: 10.1084/jem.194.12.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis RE, Ngo VN, Lenz G, Tolar P, Young RM, Romesser PB, Kohlhammer H, Lamy L, Zhao H, Yang Y, et al. Chronic active B-cell-receptor signalling in diffuse large B-cell lymphoma. Nature. 2010;463:88–92. doi: 10.1038/nature08638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dueber EC, Schoeffler AJ, Lingel A, Elliott JM, Fedorova AV, Giannetti AM, Zobel K, Maurer B, Varfolomeev E, Wu P, et al. Antagonists induce a conformational change in cIAP1 that promotes autoubiquitination. Science. 2011;334:376–380. doi: 10.1126/science.1207862. [DOI] [PubMed] [Google Scholar]
- Eckelman BP, Salvesen GS. The human anti-apoptotic proteins cIAP1 and cIAP2 bind but do not inhibit caspases. J Biol Chem. 2006;281:3254–3260. doi: 10.1074/jbc.M510863200. [DOI] [PubMed] [Google Scholar]
- Emmerich CH, Ordureau A, Strickson S, Arthur JS, Pedrioli PG, Komander D, Cohen P. Activation of the canonical IKK complex by K63/M1-linked hybrid ubiquitin chains. Proc Natl Acad Sci U S A. 2013;110:15247–15252. doi: 10.1073/pnas.1314715110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita H, Rahighi S, Akita M, Kato R, Sasaki Y, Wakatsuki S, Iwai K. Mechanism underlying IkappaB kinase activation mediated by the linear ubiquitin chain assembly complex. Mol Cell Biol. 2014;34:1322–1335. doi: 10.1128/MCB.01538-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulda S, Vucic D. Targeting IAP proteins for therapeutic intervention in cancer. Nat Rev Drug Discov. 2012;11:109–124. doi: 10.1038/nrd3627. [DOI] [PubMed] [Google Scholar]
- Gardam S, Turner VM, Anderton H, Limaye S, Basten A, Koentgen F, Vaux DL, Silke J, Brink R. Deletion of cIAP1 and cIAP2 in murine B lymphocytes constitutively activates cell survival pathways and inactivates the germinal center response. Blood. 2011 doi: 10.1182/blood-2010-10-312793. [DOI] [PubMed] [Google Scholar]
- Gerlach B, Cordier SM, Schmukle AC, Emmerich CH, Rieser E, Haas TL, Webb AI, Rickard JA, Anderton H, Wong WW, et al. Linear ubiquitination prevents inflammation and regulates immune signalling. Nature. 2011;471:591–596. doi: 10.1038/nature09816. [DOI] [PubMed] [Google Scholar]
- Greco WR, Park HS, Rustum YM. Application of a new approach for the quantitation of drug synergism to the combination of cis-diamminedichloroplatinum and 1-beta-D-arabinofuranosylcytosine. Cancer Res. 1990;50:5318–5327. [PubMed] [Google Scholar]
- Haas TL, Emmerich CH, Gerlach B, Schmukle AC, Cordier SM, Rieser E, Feltham R, Vince J, Warnken U, Wenger T, et al. Recruitment of the linear ubiquitin chain assembly complex stabilizes the TNF-R1 signaling complex and is required for TNF-mediated gene induction. Mol Cell. 2009;36:831–844. doi: 10.1016/j.molcel.2009.10.013. [DOI] [PubMed] [Google Scholar]
- Ikeda F, Deribe YL, Skanland SS, Stieglitz B, Grabbe C, Franz-Wachtel M, van Wijk SJ, Goswami P, Nagy V, Terzic J, et al. SHARPIN forms a linear ubiquitin ligase complex regulating NF-kappaB activity and apoptosis. Nature. 2011;471:637–641. doi: 10.1038/nature09814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keats JJ, Fonseca R, Chesi M, Schop R, Baker A, Chng WJ, Van Wier S, Tiedemann R, Shi CX, Sebag M, et al. Promiscuous mutations activate the noncanonical NF-kappaB pathway in multiple myeloma. Cancer Cell. 2007;12:131–144. doi: 10.1016/j.ccr.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kensche T, Tokunaga F, Ikeda F, Goto E, Iwai K, Dikic I. Analysis of nuclear factor-kappaB (NF-kappaB) essential modulator (NEMO) binding to linear and lysine-linked ubiquitin chains and its role in the activation of NF-kappaB. J Biol Chem. 2012;287:23626–23634. doi: 10.1074/jbc.M112.347195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krepler C, Chunduru SK, Halloran MB, He X, Xiao M, Vultur A, Villanueva J, Mitsuuchi Y, Neiman EM, Benetatos C, et al. The novel SMAC mimetic birinapant exhibits potent activity against human melanoma cells. Clin Cancer Res. 2013;19:1784–1794. doi: 10.1158/1078-0432.CCR-12-2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam LT, Davis RE, Pierce J, Hepperle M, Xu Y, Hottelet M, Nong Y, Wen D, Adams J, Dang L, Staudt LM. Small molecule inhibitors of IkappaB kinase are selectively toxic for subgroups of diffuse large B-cell lymphoma defined by gene expression profiling. Clin Cancer Res. 2005;11:28–40. [PubMed] [Google Scholar]
- Lenz G, Davis RE, Ngo VN, Lam L, George TC, Wright GW, Dave SS, Zhao H, Xu W, Rosenwald A, et al. Oncogenic CARD11 mutations in human diffuse large B cell lymphoma. Science. 2008a;319:1676–1679. doi: 10.1126/science.1153629. [DOI] [PubMed] [Google Scholar]
- Lenz G, Wright GW, Emre NC, Kohlhammer H, Dave SS, Davis RE, Carty S, Lam LT, Shaffer AL, Xiao W, et al. Molecular subtypes of diffuse large B-cell lymphoma arise by distinct genetic pathways. Proc Natl Acad Sci U S A. 2008b;105:13520–13525. doi: 10.1073/pnas.0804295105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston P, Roy N, Tamai K, Lefebvre C, Baird S, Cherton-Horvat G, Farahani R, McLean M, Ikeda JE, MacKenzie A, Korneluk RG. Suppression of apoptosis in mammalian cells by NAIP and a related family of IAP genes. Nature. 1996;379:349–353. doi: 10.1038/379349a0. [DOI] [PubMed] [Google Scholar]
- Lohr JG, Stojanov P, Lawrence MS, Auclair D, Chapuy B, Sougnez C, Cruz-Gordillo P, Knoechel B, Asmann YW, Slager SL, et al. Discovery and prioritization of somatic mutations in diffuse large B-cell lymphoma (DLBCL) by whole-exome sequencing. Proc Natl Acad Sci U S A. 2012;109:3879–3884. doi: 10.1073/pnas.1121343109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mali P, Yang L, Esvelt KM, Aach J, Guell M, DiCarlo JE, Norville JE, Church GM. RNA-guided human genome engineering via Cas9. Science. 2013;339:823–826. doi: 10.1126/science.1232033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews Griner LA, Guha R, Shinn P, Young RM, Keller JM, Liu D, Goldlust IS, Yasgar A, McKnight C, Boxer MB, et al. High-throughput combinatorial screening identifies drugs that cooperate with ibrutinib to kill activated B-cell-like diffuse large B-cell lymphoma cells. Proc Natl Acad Sci U S A. 2014;111:2349–2354. doi: 10.1073/pnas.1311846111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micheau O, Tschopp J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell. 2003;114:181–190. doi: 10.1016/s0092-8674(03)00521-x. [DOI] [PubMed] [Google Scholar]
- Morin RD, Mendez-Lago M, Mungall AJ, Goya R, Mungall KL, Corbett RD, Johnson NA, Severson TM, Chiu R, Field M, et al. Frequent mutation of histone-modifying genes in non-Hodgkin lymphoma. Nature. 2011;476:298–303. doi: 10.1038/nature10351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngadjeua F, Chiaravalli J, Traincard F, Raynal B, Fontan E, Agou F. Two-sided ubiquitin binding of NF-kappaB essential modulator (NEMO) zinc finger unveiled by a mutation associated with anhidrotic ectodermal dysplasia with immunodeficiency syndrome. J Biol Chem. 2013;288:33722–33737. doi: 10.1074/jbc.M113.483305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngo VN, Davis RE, Lamy L, Yu X, Zhao H, Lenz G, Lam LT, Dave S, Yang L, Powell J, Staudt LM. A loss-of-function RNA interference screen for molecular targets in cancer. Nature. 2006;441:106–110. doi: 10.1038/nature04687. [DOI] [PubMed] [Google Scholar]
- Ngo VN, Young RM, Schmitz R, Jhavar S, Xiao W, Lim KH, Kohlhammer H, Xu W, Yang Y, Zhao H, et al. Oncogenically active MYD88 mutations in human lymphoma. Nature. 2011;470:115–119. doi: 10.1038/nature09671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oeckinghaus A, Wegener E, Welteke V, Ferch U, Arslan SC, Ruland J, Scheidereit C, Krappmann D. Malt1 ubiquitination triggers NF-kappaB signaling upon T-cell activation. Embo J. 2007;26:4634–4645. doi: 10.1038/sj.emboj.7601897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SM, Yoon JB, Lee TH. Receptor interacting protein is ubiquitinated by cellular inhibitor of apoptosis proteins (c-IAP1 and c-IAP2) in vitro. FEBS Lett. 2004;566:151–156. doi: 10.1016/j.febslet.2004.04.021. [DOI] [PubMed] [Google Scholar]
- Pasqualucci L, Trifonov V, Fabbri G, Ma J, Rossi D, Chiarenza A, Wells VA, Grunn A, Messina M, Elliot O, et al. Analysis of the coding genome of diffuse large B-cell lymphoma. Nat Genet. 2011;43:830–837. doi: 10.1038/ng.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puente XS, Bea S, Valdes-Mas R, Villamor N, Gutierrez-Abril J, Martin-Subero JI, Munar M, Rubio-Perez C, Jares P, Aymerich M, et al. Non-coding recurrent mutations in chronic lymphocytic leukaemia. Nature. 2015 doi: 10.1038/nature14666. [DOI] [PubMed] [Google Scholar]
- Rahal R, Frick M, Romero R, Korn JM, Kridel R, Chan FC, Meissner B, Bhang HE, Ruddy D, Kauffmann A, et al. Pharmacological and genomic profiling identifies NF-kappaB-targeted treatment strategies for mantle cell lymphoma. Nat Med. 2014;20:87–92. doi: 10.1038/nm.3435. [DOI] [PubMed] [Google Scholar]
- Rossi D, Trifonov V, Fangazio M, Bruscaggin A, Rasi S, Spina V, Monti S, Vaisitti T, Arruga F, Fama R, et al. The coding genome of splenic marginal zone lymphoma: activation of NOTCH2 and other pathways regulating marginal zone development. J Exp Med. 2012;209:1537–1551. doi: 10.1084/jem.20120904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothe M, Pan MG, Henzel WJ, Ayres TM, Goeddel DV. The TNFR2-TRAF signaling complex contains two novel proteins related to baculoviral inhibitor of apoptosis proteins. Cell. 1995;83:1243–1252. doi: 10.1016/0092-8674(95)90149-3. [DOI] [PubMed] [Google Scholar]
- Roy N, Deveraux QL, Takahashi R, Salvesen GS, Reed JC. The c-IAP-1 and c-IAP-2 proteins are direct inhibitors of specific caspases. EMBO J. 1997;16:6914–6925. doi: 10.1093/emboj/16.23.6914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel T, Welsh K, Lober T, Togo SH, Zapata JM, Reed JC. Distinct BIR domains of cIAP1 mediate binding to and ubiquitination of tumor necrosis factor receptor-associated factor 2 and second mitochondrial activator of caspases. J Biol Chem. 2006;281:1080–1090. doi: 10.1074/jbc.M509381200. [DOI] [PubMed] [Google Scholar]
- Satpathy S, Wagner SA, Beli P, Gupta R, Kristiansen TA, Malinova D, Francavilla C, Tolar P, Bishop GA, Hostager BS, Choudhary C. Systems-wide analysis of BCR signalosomes and downstream phosphorylation and ubiquitylation. Molecular systems biology. 2015;11:810. doi: 10.15252/msb.20145880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholtysik R, Kreuz M, Hummel M, Rosolowski M, Szczepanowski M, Klapper W, Loeffler M, Trumper L, Siebert R, Kuppers R Molecular Mechanisms in Malignant Lymphomas Network Project of the Deutsche, K. Characterization of genomic imbalances in diffuse large B-cell lymphoma by detailed SNP-chip analysis. Int J Cancer. 2015;136:1033–1042. doi: 10.1002/ijc.29072. [DOI] [PubMed] [Google Scholar]
- Shaffer AL, 3rd, Young RM, Staudt LM. Pathogenesis of human B cell lymphomas. Annu Rev Immunol. 2012;30:565–610. doi: 10.1146/annurev-immunol-020711-075027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer AL, Wright G, Yang L, Powell J, Ngo V, Lamy L, Lam LT, Davis RE, Staudt LM. A library of gene expression signatures to illuminate normal and pathological lymphoid biology. Immunological reviews. 2006;210:67–85. doi: 10.1111/j.0105-2896.2006.00373.x. [DOI] [PubMed] [Google Scholar]
- Shalem O, Sanjana NE, Hartenian E, Shi X, Scott DA, Mikkelsen TS, Heckl D, Ebert BL, Root DE, Doench JG, Zhang F. Genome-scale CRISPR-Cas9 knockout screening in human cells. Science. 2014;343:84–87. doi: 10.1126/science.1247005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu HB, Takeuchi M, Goeddel DV. The tumor necrosis factor receptor 2 signal transducers TRAF2 and c-IAP1 are components of the tumor necrosis factor receptor 1 signaling complex. Proc Natl Acad Sci U S A. 1996;93:13973–13978. doi: 10.1073/pnas.93.24.13973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva GM, Finley D, Vogel C. K63 polyubiquitination is a new modulator of the oxidative stress response. Nat Struct Mol Biol. 2015;22:116–123. doi: 10.1038/nsmb.2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Deng L, Ea CK, Xia ZP, Chen ZJ. The TRAF6 ubiquitin ligase and TAK1 kinase mediate IKK activation by BCL10 and MALT1 in T lymphocytes. Mol Cell. 2004;14:289–301. doi: 10.1016/s1097-2765(04)00236-9. [DOI] [PubMed] [Google Scholar]
- Vallabhapurapu S, Matsuzawa A, Zhang W, Tseng PH, Keats JJ, Wang H, Vignali DA, Bergsagel PL, Karin M. Nonredundant and complementary functions of TRAF2 and TRAF3 in a ubiquitination cascade that activates NIK-dependent alternative NF-kappaB signaling. Nat Immunol. 2008;9:1364–1370. doi: 10.1038/ni.1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varfolomeev E, Blankenship JW, Wayson SM, Fedorova AV, Kayagaki N, Garg P, Zobel K, Dynek JN, Elliott LO, Wallweber HJ, et al. IAP antagonists induce autoubiquitination of c-IAPs, NF-kappaB activation, and TNFalpha-dependent apoptosis. Cell. 2007;131:669–681. doi: 10.1016/j.cell.2007.10.030. [DOI] [PubMed] [Google Scholar]
- Varfolomeev E, Goncharov T, Fedorova AV, Dynek JN, Zobel K, Deshayes K, Fairbrother WJ, Vucic D. c-IAP1 and c-IAP2 are critical mediators of tumor necrosis factor alpha (TNFalpha)-induced NF-kappaB activation. J Biol Chem. 2008;283:24295–24299. doi: 10.1074/jbc.C800128200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vince JE, Wong WW, Khan N, Feltham R, Chau D, Ahmed AU, Benetatos CA, Chunduru SK, Condon SM, McKinlay M, et al. IAP antagonists target cIAP1 to induce TNFalpha-dependent apoptosis. Cell. 2007;131:682–693. doi: 10.1016/j.cell.2007.10.037. [DOI] [PubMed] [Google Scholar]
- Walczak H, Iwai K, Dikic I. Generation and physiological roles of linear ubiquitin chains. BMC Biol. 2012;10:23. doi: 10.1186/1741-7007-10-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CY, Mayo MW, Korneluk RG, Goeddel DV, Baldwin AS., Jr NF-kappaB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science. 1998;281:1680–1683. doi: 10.1126/science.281.5383.1680. [DOI] [PubMed] [Google Scholar]
- Wang T, Wei JJ, Sabatini DM, Lander ES. Genetic screens in human cells using the CRISPR-Cas9 system. Science. 2014;343:80–84. doi: 10.1126/science.1246981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson WH, Young RM, Schmitz R, Yang Y, Pittaluga S, Wright G, Lih CJ, Williams PM, Shaffer AL, Gerecitano J, et al. Targeting B cell receptor signaling with ibrutinib in diffuse large B cell lymphoma. Nat Med. 2015;21:922–926. doi: 10.1038/nm.3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CJ, Ashwell JD. NEMO recognition of ubiquitinated Bcl10 is required for T cell receptor-mediated NF-kappaB activation. Proc Natl Acad Sci U S A. 2008;105:3023–3028. doi: 10.1073/pnas.0712313105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CJ, Conze DB, Li T, Srinivasula SM, Ashwell JD. Sensing of Lys 63-linked polyubiquitination by NEMO is a key event in NF-kappaB activation [corrected] Nat Cell Biol. 2006;8:398–406. doi: 10.1038/ncb1384. [DOI] [PubMed] [Google Scholar]
- Yang Y, Fang S, Jensen JP, Weissman AM, Ashwell JD. Ubiquitin protein ligase activity of IAPs and their degradation in proteasomes in response to apoptotic stimuli. Science. 2000;288:874–877. doi: 10.1126/science.288.5467.874. [DOI] [PubMed] [Google Scholar]
- Yang Y, Schmitz R, Mitala J, Whiting A, Xiao W, Ceribelli M, Wright GW, Zhao H, Yang Y, Xu W, et al. Essential role of the linear ubiquitin chain assembly complex in lymphoma revealed by rare germline polymorphisms. Cancer Discov. 2014;4:480–493. doi: 10.1158/2159-8290.CD-13-0915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarnegar B, Yamazaki S, He JQ, Cheng G. Control of canonical NF-kappaB activation through the NIK-IKK complex pathway. Proc Natl Acad Sci U S A. 2008a;105:3503–3508. doi: 10.1073/pnas.0707959105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarnegar BJ, Wang Y, Mahoney DJ, Dempsey PW, Cheung HH, He J, Shiba T, Yang X, Yeh WC, Mak TW, et al. Noncanonical NF-kappaB activation requires coordinated assembly of a regulatory complex of the adaptors cIAP1, cIAP2, TRAF2 and TRAF3 and the kinase NIK. Nat Immunol. 2008b;9:1371–1378. doi: 10.1038/ni.1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Calado DP, Wang Z, Frohler S, Kochert K, Qian Y, Koralov SB, Schmidt-Supprian M, Sasaki Y, Unitt C, et al. An oncogenic role for alternative NF-kappaB signaling in DLBCL revealed upon deregulated BCL6 expression. Cell reports. 2015;11:715–726. doi: 10.1016/j.celrep.2015.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Grubor V, Love CL, Banerjee A, Richards KL, Mieczkowski PA, Dunphy C, Choi W, Au WY, Srivastava G, et al. Genetic heterogeneity of diffuse large B-cell lymphoma. Proc Natl Acad Sci U S A. 2013;110:1398–1403. doi: 10.1073/pnas.1205299110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.