Abstract
Asymmetric C–H metalation has recently emerged as a promising approach for developing enantioselective C–H activation reactions. However, this approach is typically limited to 5- and 6-membered cyclometalation, thereby preventing the asymmetric functionalization of C–H bonds at positions remote to existing functional groups. Herein, we report the realization of enantioselective remote meta-C–H arylation of benzylamines, as well as arylation and alkylation of homobenzylamines. The desymmetrization and kinetic resolution are achieved using an achiral ligand and a catalytic amount of chiral transient mediator that relays an initial ortho-C–H activation to the meta position. The same chiral transient mediator affords comparable enantioselectivities with different classes of substrates containing either neutral σ-donor or anionic coordinating groups. This relay strategy could provide an alternative means to remote chiral induction, one of the most challenging problems in asymmetric catalysis.
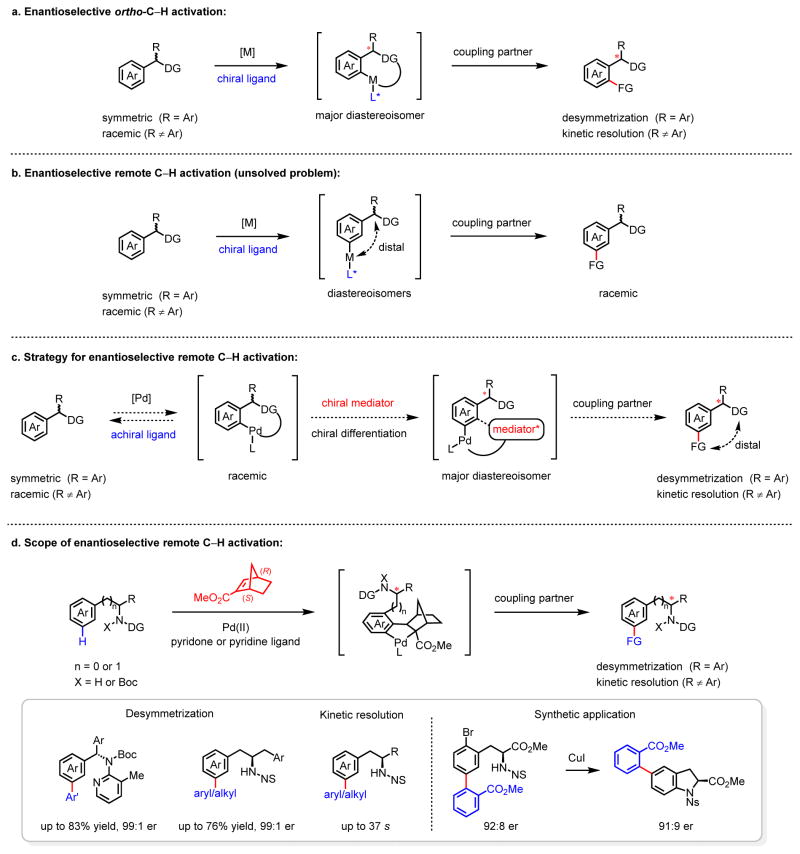
Enantioselective C–H activation reactions via asymmetric metalation have the potential to open a new avenue for constructing chiral molecules owing to the diverse reactivity of chiral carbon–metal bonds1–2. Efforts to achieve this goal have been met with tremendous difficulties due to the lack of appropriate catalytic redox manifolds and conceptual bases for effective chiral ligand design. Enantioselective activation of C(sp2)–H bonds has played an important role in developing chiral ligands and understanding chiral induction in the metalation of C–H bonds. For example, an early example of Ru(0)-catalyzed atropselective alkylation of 2-arylpyridine afforded only 49% ee3. In the past decade, the extensive search for suitable metal catalysts, chiral ligands and catalytic cycles that can achieve highly enantioselective C–H activation reactions has led to the finding that a Pd(II) catalyst bound to a chiral mono-protected amino acid ligand (MPAA) enables highly enantioselective C–H coupling via a Pd(II)/Pd(0) catalytic cycle4–6, as well as C–H iodination and oxidation involving Pd(II)/Pd(IV) catalysis7–8 (Figure 1a). In addition to point desymmetrizations, this class of chiral ligand is also competent in planar and axial desymmetrizations, as demonstrated by multiple research groups9–12. Pd(0)-catalyzed intramolecular vinylation and arylation of C(sp2)–H bonds has also been rendered enantioselective by using chiral phosphine ligands13–14. Recently, chiral rhodium15–18 and iridium19 catalysts have been developed to enable enantioselective C(sp2)–H activation; however, these reactions are largely limited to either intramolecular silylations or pyridine-directed C–H olefinations. Hitherto, transition metal-catalyzed enantioselective remote meta-C(sp2)–H activation has not been reported.20–23 Asymmetric metalation of remote sp2-C–H bonds presents a distinct challenge: the metal insertion event occurs too far from the carbon that becomes the chiral center. Remote C–H functionalization directed by a nitrile-template has been developed recently;22 however, the control of the stereochemistry through a conformationally flexible macro-cyclic transition state remains to be demonstrated (Figure 1b). In general, remote chiral induction remains one of the most challenging problems in asymmetric catalysis.24–25
Figure 1. Enantioselective C(sp2)–H activation.
a, Enantioselective ortho-C–H activation. b, Enantioselective remote C–H activation (unsolved problem). c, Strategy for enantioselective remote C–H activation. d, Scope of enantioselective remote C–H activation.
Here we report a strategy using a catalytic amount of chiral norbornene as a transient mediator to achieve enantioselective remote C–H activation (Figure 1c). The norbornene plays a dual role: to relay C–H activation from the ortho position to the remote meta position26–30; and to achieve chiral differentiation of the racemic ortho-C–H palladation intermediates, generated by an achiral catalyst, to ensure the formation of the enantio-enriched product. This approach has been successfully implemented in enantioselective remote meta-C–H activation reactions of benzyl and homobenzylamines, both of which are prevalent motifs in bioactive natural products, drug compounds, and chiral organic catalysts (Figure 1d). The chiral norbornene methyl (1S,4R)-bicyclo[2.2.1]hept-2-ene-2-carboxylate ((+)-NBE-CO2Me) serves as a versatile chiral transient mediator (CTM) for both X- and L-type substrates. Kinetic resolution of homobenzylamines was also achieved using the same CTM to broaden the scope of the substrates.
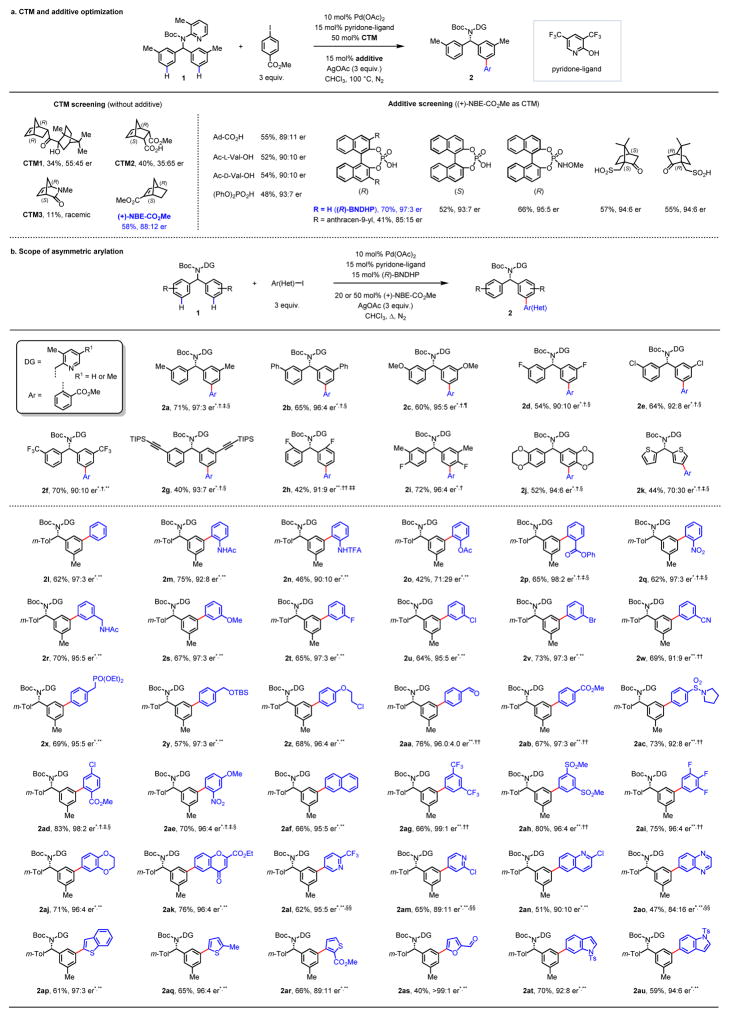
Our experimental design was based on a previous finding that norbornene can intercept the ortho-palladation intermediate via migratory insertion and subsequently effect meta-C–H functionalizations26–28. This reaction mechanism guided us to hypothesize that a chiral, enantioenriched norbornene might be able to differentiate the racemic ortho-palladate intermediate during the alkene insertion, or the subsequent alkene insertion intermediate (from the meta-C–H palladation step). We selected diarylmethylamine 131, which contains a σ-donor coordinating group, as the model substrate to investigate the potential asymmetric arylation using chiral norbornene mediators (Figure 2a). While the chiral bicyclo[2.2.1]heptene derivatives CTM1 and CTM2 were effective mediators, both gave negligible enantioselectivity. Likewise, the lactam CTM3 failed to display any degree of stereoinduction. The use of chiral (+)-NBE-CO2Me as the transient mediator led to a dramatic improvement in enantioselectivity to 88:12 (see Supplementary Information for optimization). While neither inorganic base nor carboxylic acid additives were effective, the phosphoric acid (PhO)2PO2H increased the er to 93:7. Pleasingly, (R)-BNDHP produced a 70% yield and an excellent enantioselectivity (97:3 er). Bulky substituents (like anthracenyl group) on the phosphoric acid proved detrimental to the reaction. To investigate the role of the chiral norbornene and phosphoric acid, we performed a number of control experiments. The use of racemic NBE-CO2Me and (R)-BNDHP gave poor enantioselectivity (57:43). The use of (S)-BNDHP, (R)-phosphorylimide or chiral camphorsulfonic acid in combination with (+)-NBE-CO2Me led to a slight drop in yield and enantioselectivity. These combined experiments suggest that (+)-NBE-CO2Me is responsible for the chiral induction and the chiral phosphoric acid has a minor beneficial effect.
Figure 2. Enantioselective meta-C–H arylation of diarylmethylamines.
a, CTM and additive optimization. Reaction conditions: 10 mol% Pd(OAc)2, 15 mol% pyridone-ligand, 50 mol% (+)-NBE-CO2Me, 15 mol% additive, 3 equiv. methyl 4-iodobenzoate, 3 equiv. AgOAc, CHCl3, 100 °C. For each entry number (in bold), data are reported as NMR yield. b, Scope of asymmetric arylation. Reaction conditions: 10 mol% Pd(OAc)2, 15 mol% pyridone-ligand, 20 mol% (+)-NBE-CO2Me, 15 mol% (R)-BNDHP, 3 equiv. Ar–I, 3 equiv. AgOAc, CHCl3, 100 °C. *R1 = Me. †15 mol% (PhO)2PO2H. ‡1.5 equiv. Ar–I, 2 equiv. AgOAc. §80 °C. ¶60 °C. **50 mol% (+)-NBE-CO2Me. ††R1 = H. ‡‡20 mol% Pd(OAc)2, 30 mol% pyridone-ligand, 30 mol% (R)-BNDHP. §§15 mol% Pd(OAc)2, 23 mol% pyridone-ligand. For each entry number (in bold), data are reported as isolated yield. The absolute configuration of 2ah was determined by X-ray crystallography. DG, directing group; Ar, aryl group; m-Tol, meta-tolyl group. Reducing the catalyst loading to 5 mol% produced comparable results for substrate 1a′ (see Table S6 in Supplementary Information).
Using the optimized conditions, we then tested the substrate scope with methyl 2-iodobenzoate (Figure 2b). Electron-neutral (2a, 2b), electron-donating (2c) and electron-withdrawing (2d to 2f) substituents were all well tolerated, providing enantioselectivities of up to 97:3. Substrate 1g bearing an alkyne group afforded a lower yield due to the instability of the C–C triple bond under the standard conditions. Substituents at the ortho-position (2h) reduced the reactivity via a presumed steric effect, but high enantioselectivity (91:9 er) was preserved. Bis-substituted arenes 2i and 2j, which contain heterocycles, were also compatible and afford consistently high enantioselectivity. The 5-membered heterocycle 2k gave lower selectivity, probably due to a coordinative effect.
We next surveyed the scope of aryl iodides (Figure 2b). The scope of ortho-substituted aryl iodides (2m to 2q) is broad, providing enantioselectivity in up to 97:3 er. Electronically diverse meta-substituted aryl iodides (2r to 2w) were tolerated, and produced enantiomeric ratios greater than 95:5 (2s to 2v). The scope of para-substituted aryl iodides is also broad, and electronic factors do not affect the enantioselectivity (2x to 2ac). Multi-substituted aryl iodides (2ad to 2ai) were also tolerated under the conditions, and the enantiomeric ratios all exceeded 95:5. The above scope of various aryl iodides features either halogens (as in 2t to 2v) or reactive groups (NHAc in 2m and 2r, phosphonate moiety in 2x, alkyl chloride 2z, and aldehyde in 2aa) that can serve as useful synthetic handles for subsequent chemical manipulation. Importantly, heterocyclic aryl iodides also proved successful in the reaction (2aj to 2au). Pyridine, quinoline, quinazoline, furan, thiophene, and indole derivatives were all suitable coupling partners, providing good to high enantioselectivities. The simple removal of directing group was demonstrated with product 2ae (see Supplementary Information for details).
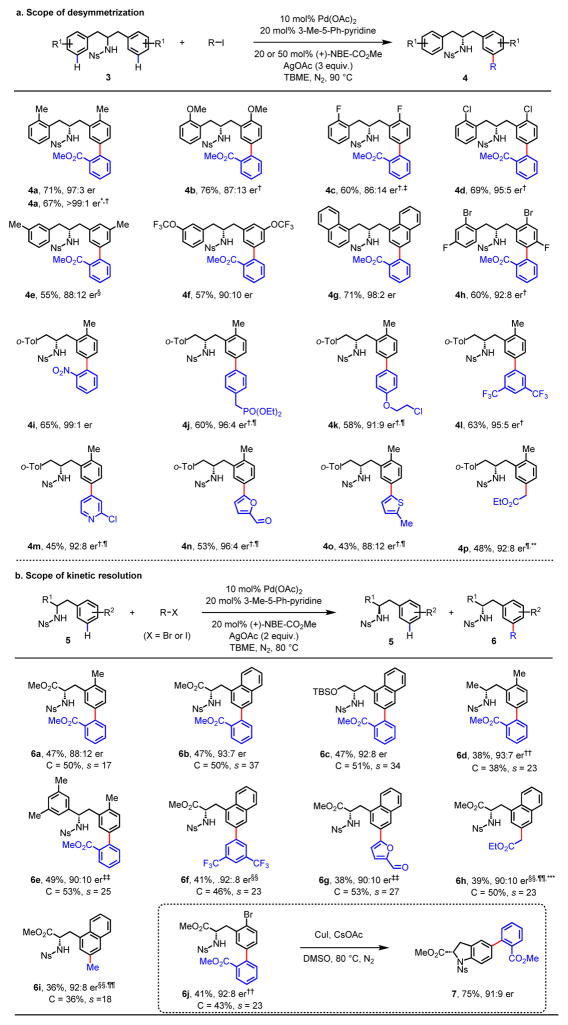
We then investigated asymmetric meta-C–H activation of homobenzylamines32. Until now, no ortho-C–H activation method has achieved the construction of a homobenzylic chiral center. Considering that the challenge might be overcome by the CTM strategy, we tested different mediators and found that (+)-NBE-CO2Me remained the most effective one, providing a 97:3 enantioselectivity with nosyl-protected homobenzylamine 3a, which contains an anionic coordinating group (see Supplementary Information for details). Notably, the use of a nosyl-protected (Ns) amino moiety as the directing group represents a practical advantage in synthetic applications. Further investigation revealed that this method covers a broad range of symmetric homobenzylamines (Figure 3a). Ortho-, meta-, and para-substituents were all well-tolerated, and provided the desired products (4a to 4f, 4h) in good to high enantioselectivities. Moreover, both naphthalene (4g) and the bis-substituted arene (4h) were amenable to the standard conditions.
Fig. 3. Enantioselective meta-C–H activation of homobenzylamines.
a, Scope of desymmetrization. Reaction conditions: 10 mol% Pd(OAc)2, 20 mol% 3-methyl-5-phenylpyridine, 20 mol% (+)-NBE-CO2Me, 3 equiv. R–I, 3 equiv. AgOAc, TBME, 90 °C. *Methyl 2-bromobenzoate (as arylation reagent). †50 mol% (+)-NBE-CO2Me. ‡15 mol% Pd(OAc)2, 30 mol% 3-methyl-5-phenylpyridine. §1.4 equiv. Ar–I. ¶15 mol% Pd(OAc)2, 30 mol% 4-acetylpyridine (as ligand). **1.5 equiv. (+)-NBE-CO2Me, DCM (as solvent). b, Scope of kinetic resolution. Reaction conditions: 10 mol% Pd(OAc)2, 20 mol% 3-methyl-5-phenylpyridine, 20 mol% (+)-NBE-CO2Me, 0.5 equiv. R–X, 2 equiv. AgOAc, TBME, 80 °C. ††1.5 equiv. R–X. ‡‡1 equiv. R–X. §§3 equiv. R–X, 3 equiv. AgOAc. ¶¶20 mol% 4-acetylpyridine, DCM. ***50 mol% (+)-NBE-CO2Me. For each entry number (in bold), data are reported as isolated yield. The absolute configurations of 4m and 4n were determined by X-ray crystallography. TBME, tert-butylmethylether; o-Tol, ortho-tolyl group; DCM, dichloromethane. Reducing the catalyst loading to 5 mol% produced comparable results for substrate 3a (see Table S10 in Supplementary Information).
The reaction tolerated electronically diverse aryl iodides (4i to 4l) and provided enantioselectivities in up to 99:1 er. Heterocyclic aryl iodides such as pyridine, furan, and thiophene (4m to 4o) were also successful. In addition to aryl iodides, 2-bromobenzoate was also tested and provided moderate yield and excellent enantioselectivity (4a, >99:1 er). Likewise, the aliphatic coupling partner iodoacetate gave product 4p with 92:8 er.
In addition to desymmetrization, kinetic resolution of secondary amines was also realized (Figure 3b). (+)-NBE-CO2Me successfully resolved a racemic mixture of amino esters providing meta-arylated products and recovered starting materials in good enantioselectivity with 2-bromo-benzoate (6a, 6b). These meta-arylated phenylalanines are central motifs in a family of bioactive natural products33. Alkyl- and aryl-substituted homobenzylamines (6c to 6e) were also compatible with this system. Moreover, simple arene (6f), heterocycle (6g) and alkyl groups (6h, 6i) were all enantioselectively installed at the meta-position by this method. To showcase the synthetic utility of this reaction, a copper-catalyzed intramolecular amination of 6j yielded chiral indoline 7 with the 5-position substituted, a core structure of chemotherapy medication Vincristine.
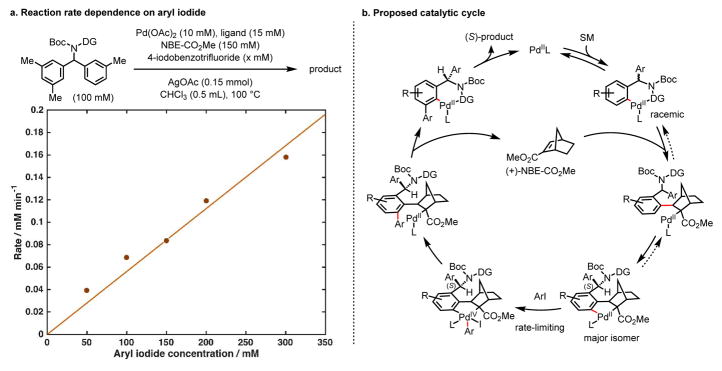
Mechanistic studies of our previous enantioselective C–H activation reactions have established the asymmetric C–H metalation step as the enantio-determining step34–35. Since the chiral mediator (+)-NBE-CO2Me) is not involved in the ortho-C–H activation step, chiral differentiation should occur in one of the subsequent steps. A number of mechanistic experiments were thus carried out to provide further insights (see Supplementary Information for details). First, kinetic isotope effects (KIE) of 1.03 and 1.33 for the ortho- and meta-deuterated substrates respectively indicated that the C–H activation steps are likely not rate-limiting. First-order rate dependence observed for aryl iodide points to the oxidative addition of aryl iodide as rate-limiting (Figure 4a).36 Second, substantial hydrogen/deuterium exchange at the ortho-position and the lack of such exchange at the meta-position suggests that the ortho-C–H activation step is fast and reversible while the meta-C–H activation is not readily reversible on the reaction time scale. These combined data suggest that the chiral differentiation by the chiral norbornene occurs at either the norbornene insertion into the ortho-palladation intermediate or subsequent meta-C–H activation step (Figure 4b).
Figure 4. Mechanistic studies.
a, Reaction rate dependence on aryl iodide. b, Proposed catalytic cycle.
In summary, remote enantioselective C–H activation reactions were realized by relaying ortho-C–H activation to remote meta-C–H activation using a chiral norbornene as the mediator. The chiral amplification is achieved by fast reversible non-asymmetric C–H activation followed by enantioselective norbornene insertion or meta-C–H activation. This approach is compatible with substrates containing either neutral σ-donor or anionic coordinating groups.
Methods Summary
General procedure for the Pd/(+)-NBE-CO2Me catalyzed enantioselective meta-C–H activation
Arene (0.10 mmol, 1.0 equiv), Pd(OAc)2 (2.2 mg, 10 μmol, 10 mol%), Ligand (15 mol% or 20 mol%), hydrogenphosphate (15 mol% for benzylamine substrate), aryl iodide (0.3 mmol, 3.0 equiv.), and AgOAc (50 mg, 0.30 mmol, 3.0 equiv.) were added into a 2-dram reaction vial. Solvent and (+)-NBE-CO2Me (20 mol% or 50 mol%) were added to the mixture. The vial was flushed with N2, and capped. The reaction mixture was then stirred at the given temperature for 12 to 24 hours. After cooling to room temperature, the mixture was filtered through Celite and eluted with EtOAc. The filtrate was evaporated under reduced pressure. Purification by preparative TLC chromatography afforded the desired product. Full experimental details and characterization of new compounds can be found in the Supplementary Information.
Supplementary Material
Acknowledgments
We gratefully acknowledge The Scripps Research Institute, the NIH ((National Institute of General Medical Sciences grant 2R01GM102265) for their financial support. Y. S. thanks Jiangsu Overseas Research & Training Program for University Prominent Young & Middle-aged Teachers and Presidents.
Footnotes
Supplementary Information is available in the online version of the paper.
Author Contributions J.-Q. Y. and H. S. conceived the concept. H. S. developed the enantioselective remote C–H activation. H. S. and A. N. H. performed the mechanistic study. H. S., A. N. H., Y. S., and Q. S. prepared reaction substrates. J.-Q. Y. directed the project.
The authors declare no competing financial interests.
Readers are welcome to comment on the online version of this article.
Data Availability The data supporting the findings of this study are available within the article and its Supplementary Information files. Metrical parameters for the structure of 2ah, 4m, and 4n (see Supplementary Information) are available free of charge from the Cambridge Crystallographic Data Centre (https://www.ccdc.cam.ac.uk/) under reference number CCDC-1586807, CCDC-1586808, and CCDC-1586809, respectively.
References
- 1.Giri R, Shi BF, Engle KM, Maugel N, Yu JQ. Transition metal-catalyzed C–H activation reactions: diastereoselectivity and enantioselectivity. Chem Soc Rev. 2009;38:3242–3272. doi: 10.1039/b816707a. [DOI] [PubMed] [Google Scholar]
- 2.Newton CG, Wang SG, Oliveira CC, Cramer N. Catalytic enantioselective transformations involving C–H bond cleavage by transition-metal complexes. Chem Rev. 2017;117:8908–8976. doi: 10.1021/acs.chemrev.6b00692. [DOI] [PubMed] [Google Scholar]
- 3.Kakiuchi F, Gendre PL, Yamada A, Ohtaki H, Murai S. Atropselective alkylation of biaryl compounds by means of transition metal-catalyzed C–H/olefin coupling. Tetrahedron: Asymmetry. 2000;11:2647–2651. [Google Scholar]
- 4.Shi BF, Maugel N, Zhang YH, Yu JQ. PdII-catalyzed enantioselective activation of C(sp2)–H and C(sp3)–H bonds using monoprotected amino acids as chiral ligands. Angew Chem Int Ed. 2008;47:4882–4886. doi: 10.1002/anie.200801030. [DOI] [PubMed] [Google Scholar]
- 5.Shi BF, Zhang YH, Lam JK, Wang DH, Yu JQ. Pd(II)-catalyzed enantioselective C–H olefination of diphenylacetic acids. J Am Chem Soc. 2010;132:460–461. doi: 10.1021/ja909571z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Du ZJ, Guan J, Wu GJ, Xu P, Gao LX, Han FS. Pd(II)-catalyzed enantioselective synthesis of P-stereogenic phosphinamides via desymmetric C–H arylation. J Am Chem Soc. 2015;137:632–635. doi: 10.1021/ja512029x. [DOI] [PubMed] [Google Scholar]
- 7.Chu L, Xiao KJ, Yu JQ. Room-temperature enantioselective C–H iodination via kinetic resolution. Science. 2014;346:451–455. doi: 10.1126/science.1258538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng XF, Li Y, Su YM, Yin F, Wang JY, Sheng J, Vora HU, Wang XS, Yu JQ. Pd(II)-catalyzed enantioselective C–H activation/C–O bond formation: Synthesis of chiral benzofuranones. J Am Chem Soc. 2013;135:1236–1239. doi: 10.1021/ja311259x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao DW, Shi YC, Gu Q, Zhao ZL, You SL. Enantioselective synthesis of planar chiral ferrocenes via palladium-catalyzed direct coupling with arylboronic acids. J Am Chem Soc. 2013;135:86–89. doi: 10.1021/ja311082u. [DOI] [PubMed] [Google Scholar]
- 10.Pi C, Li Y, Cui XL, Zhang H, Han YB, Wu YJ. Redox of ferrocene controlled asymmetric dehydrogenative Heck reaction via palladium-catalyzed dual C–H bond activation. Chem Sci. 2013;4:2675–2679. [Google Scholar]
- 11.Pi C, Cui XL, Liu XY, Guo MX, Zhang HY, Wu YJ. Synthesis of ferrocene derivatives with planar chirality via palladium-catalyzed enantioselective C–H bond activation. Org Lett. 2014;16:5164–5167. doi: 10.1021/ol502509f. [DOI] [PubMed] [Google Scholar]
- 12.Gao DW, Gu Q, You SL. Pd(II)-catalyzed intermolecular direct C–H bond iodination: an efficient approach toward the synthesis of axially chiral compounds via kinetic resolution. ACS Catal. 2014;4:2741–2745. [Google Scholar]
- 13.Albicker MR, Cramer N. Enantioselective palladium-catalyzed direct arylations at ambient temperature: access to indanes with quaternary stereocenters. Angew Chem, Int Ed. 2009;48:9139–9142. doi: 10.1002/anie.200905060. [DOI] [PubMed] [Google Scholar]
- 14.Shintani R, Otomo H, Ota K, Hayashi T. Palladium-catalyzed asymmetric synthesis of silicon-stereogenic dibenzosiloles via enantioselective C–H bond functionalization. J Am Chem Soc. 2012;134:7305–7308. doi: 10.1021/ja302278s. [DOI] [PubMed] [Google Scholar]
- 15.Kuninobu Y, Yamauchi K, Tamura N, Seiki T, Takai K. Rhodium-catalyzed asymmetric synthesis of spirosilabifluorene derivatives. Angew Chem, Int Ed. 2013;52:1520–1522. doi: 10.1002/anie.201207723. [DOI] [PubMed] [Google Scholar]
- 16.Shibata T, Shizuno T, Sasaki T. Enantioselective synthesis of planar-chiral benzosiloloferrocenes by Rh-catalyzed intramolecular C–H silylation. Chem Commun. 2015;51:7802–7804. doi: 10.1039/c5cc00723b. [DOI] [PubMed] [Google Scholar]
- 17.Lee T, Wilson TW, Berg R, Ryberg P, Hartwig JF. Rhodium-catalyzed enantioselective silylation of arene C–H bonds: desymmetrization of diarylmethanols. J Am Chem Soc. 2015;137:6742–6745. doi: 10.1021/jacs.5b03091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun Y, Cramer N. Rhodium(III)-catalyzed enantiotopic C–H activation enables access to P-chiral cyclic phosphinamides. Angew Chem, Int Ed. 2017;56:364–367. doi: 10.1002/anie.201606637. [DOI] [PubMed] [Google Scholar]
- 19.Shibata T, Shizuno T. Iridium-catalyzed enantioselective C–H alkylation of ferrocenes with alkenes using chiral diene ligands. Angew Chem, Int Ed. 2014;53:5410–5413. doi: 10.1002/anie.201402518. [DOI] [PubMed] [Google Scholar]
- 20.Saidi O, Marafie J, Ledger AEW, Liu PM, Mahon MF, Kociok-Köhn G, Whittlesey MK, Frost CG. Ruthenium-catalyzed meta sulfonation of 2-phenylpyridines. J Am Chem Soc. 2011;133:19298–19301. doi: 10.1021/ja208286b. [DOI] [PubMed] [Google Scholar]
- 21.Hofmann N, Ackermann L. meta-Selective C–H bond alkylation with secondary alkyl halides. J Am Chem Soc. 2013;135:5877–5884. doi: 10.1021/ja401466y. [DOI] [PubMed] [Google Scholar]
- 22.Leow D, Li G, Mei TS, Yu JQ. Activation of remote meta-C–H bonds assisted by an end-on template. Nature. 2012;486:518–522. doi: 10.1038/nature11158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Phipps RJ, Gaunt MJ. A meta-selective copper-catalyzed C–H bond arylation. Science. 2009;323:1593–1597. doi: 10.1126/science.1169975. [DOI] [PubMed] [Google Scholar]
- 24.Clayden J, Lund A, Vallverdú L, Helliwell M. Ultra-remote stereocontrol by conformational communication of information along a carbon chain. Nature. 2004;431:966–971. doi: 10.1038/nature02933. [DOI] [PubMed] [Google Scholar]
- 25.Hurtley AE, Stone EA, Metrano AJ, Miller SJ. Desymmetrization of diarylmethylamido bis(phenols) through peptide-catalyzed bromination: enantiodivergence as a consequence of a 2 amu alteration at an achiral residue within the catalyst. J Org Chem. 2017;82:11326–11336. doi: 10.1021/acs.joc.7b02339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang XC, Gong W, Fang LZ, Zhu RY, Li S, Engle KM, Yu J-Q. Ligand-enabled meta-C–H activation using a transient mediator. Nature. 2015;519:334–338. doi: 10.1038/nature14214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dong Z, Wang J, Dong G. Simple amine-directed meta-selective C–H arylation via Pd/norbornene catalysis. J Am Chem Soc. 2015;137:5887–5890. doi: 10.1021/jacs.5b02809. [DOI] [PubMed] [Google Scholar]
- 28.Shen PX, Wang XC, Wang P, Zhu RY, Yu JQ. Ligand-enabled meta-C–H alkylation and arylation using a modified norbornene. J Am Chem Soc. 2015;137:11574–11577. doi: 10.1021/jacs.5b08914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ye J, Lautens M. Palladium-catalysed norbornene-mediated C–H functionalization of arenes. Nature Chem. 2015;7:863–870. doi: 10.1038/nchem.2372. [DOI] [PubMed] [Google Scholar]
- 30.Della Ca’ N, Fontana M, Motti E, Catellani M. Pd/Norbornene: A winning combination for selective aromatic functionalization via C–H bond activation. Acc Chem Res. 2016;49:1389–1400. doi: 10.1021/acs.accounts.6b00165. [DOI] [PubMed] [Google Scholar]
- 31.Wang P, Farmer ME, Yu JQ. Ligand-promoted meta-C–H functionalization of benzylamines. Angew Chem Int Ed. 2017;56:5125–5129. doi: 10.1002/anie.201701803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ding Q, Ye S, Cheng G, Wang P, Farmer ME, Yu JQ. Ligand-enabled meta-selective C–H arylation of nosyl-protected phenethylamines, benzylamines, and 2-aryl anilines. J Am Chem Soc. 2017;139:417–425. doi: 10.1021/jacs.6b11097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Albrecht BK, Williams RM. A concise, total synthesis of the TMC-95A/B proteasome inhibitors. Proc Nat Acad Sci U S A. 2004;101:11949–11954. doi: 10.1073/pnas.0308432101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Musaev DG, Kaledin A, Shi BF, Yu JQ. Key mechanistic features of enantioselective C–H bond activation reactions catalyzed by [(chiral mono-N-protected amino acid)–Pd(II)] complexes. J Am Chem Soc. 2012;134:1690–1698. doi: 10.1021/ja208661v. [DOI] [PubMed] [Google Scholar]
- 35.Plata RE, Hill DE, Haines BE, Musaev DG, Chu L, Hickey DP, Sigman MS, Yu JQ, Blackmond DG. A role for Pd(IV) in catalytic enantioselective C–H functionalization with monoprotected amino acid ligands under mild conditions. J Am Chem Soc. 2017;139:9238–9245. doi: 10.1021/jacs.7b03716. [DOI] [PubMed] [Google Scholar]
- 36.Jiao L, Herdtweck E, Bach T. Pd(II)-catalyzed regioselective 2-alkylation of indoles via a norbornene-mediated C–H activation: mechanism and applications. J Am Chem Soc. 2012;134:14563–14572. doi: 10.1021/ja3058138. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.