Abstract
Homotypic fusion of yeast vacuoles requires a regulated sequence of events. During priming, Sec18p disassembles cis-SNARE complexes. The HOPS complex, which is initially associated with the cis-SNARE complex, then mediates tethering. Finally, SNAREs assemble into trans-complexes before the membranes fuse. The t-SNARE of the vacuole, Vam3p, plays a central role in the coordination of these processes. We deleted the N-terminal region of Vam3p to analyze the role of this domain in membrane fusion. The truncated protein (Vam3ΔN) is sorted normally to the vacuole and is functional, because the vacuolar morphology is unaltered in this strain. However, in vitro vacuole fusion is strongly reduced due to the following reasons: Assembly, as well as disassembly of the cis-SNARE complex is more efficient on Vam3ΔN vacuoles; however, the HOPS complex is not associated well with the Vam3ΔN cis-complex. Thus, primed SNAREs from Vam3ΔN vacuoles cannot participate efficiently in the reaction because trans-SNARE pairing is substantially reduced. We conclude that the N-terminus of Vam3p is required for coordination of priming and docking during homotypic vacuole fusion.
INTRODUCTION
Transport between organelles is mediated by vesicles that bud from a donor membrane, are transported to and finally fuse with their target membrane (Rothman and Wieland, 1996; Mellman and Warren, 2000). In recent years, conserved sets of membrane proteins designated as SNAREs (soluble NSF attachment protein receptors) were identified in all eucaryotes as key players in docking and fusion (Rothman, 1994; Jahn and Südhof, 1999; Lin and Scheller, 2000). They have characteristic coiled-coil domains, which tightly interact in a parallel four-helix bundle (Katz et al., 1998; Poirier et al., 1998; Sutton et al., 1998; Weimbs et al., 1998). Initially, SNAREs are found in cis-complexes on both vesicle and target membranes (Walch-Solimena et al., 1995; Otto et al., 1997; Holthuis et al., 1998; Ungermann et al., 1998a). After disassembly by the ATPase NSF and its cofactor α-SNAP, SNAREs on opposing membranes interact in a trans-complex (Ungermann et al., 1998b; Weber et al., 1998). This process is considered to be a central event in membrane fusion (Hanson et al., 1997; Chen et al., 1999). Because different intracellular compartments have distinct SNARE proteins in their membranes, it is believed that their distinct interactions also account for the specificity of intracellular membrane traffic (McNew et al., 2000; Scales et al., 2000). Furthermore, in an in vitro liposome fusion assay, it was shown that isolated SNAREs are able to fuse lipid bilayers, suggesting that these proteins directly mediate fusion (Weber et al., 1998).
The in vitro homotypic fusion of yeast vacuoles represents an ideal system to study membrane fusion and the role of SNARE proteins in the context of an authentic organelle fusion reaction (Nichols et al., 1997; Wickner and Haas, 2000). The vacuolar SNAREs Vam3p, Nyv1p, Vti1p, Ykt6p, and Vam7p are initially found in a cis-complex on the isolated organelle (Ungermann et al., 1999). This complex is dissociated through the action of Sec18p, Sec17p, and LMA1 (Mayer et al., 1996; Ungermann et al., 1998a; Xu et al., 1998). After this priming step, docking is initiated by the interaction of the Rab protein Ypt7p with Vam7p and the homotypic fusion and protein sorting (HOPS) complex (Price et al., 2000a; Seals et al., 2000; Ungermann et al., 2000). The HOPS complex, consists of Vam2p and Vam6p (Price et al., 2000a) and of the class C Vps proteins Vps11p, Vps16p, Vps18p, and Vps33p (Rieder and Emr, 1997; Seals et al., 2000; Wurmser et al., 2000). Formation of a trans-SNARE complex triggers downstream reactions that require Ca2+, calmodulin, protein phosphatase 1, and the VO ATPase proteolipid to mediate complete membrane fusion (Peters and Mayer, 1998; Peters et al., 1999, 2001).
The t-SNARE that is structurally best characterized is the neuronal syntaxin 1a. It consists of three interaction domains: the transmembrane anchor that can interact with synaptobrevin (Laage et al., 2000), the C-terminal helix 3 (H3), which contains the coiled–coil region and provides the platform for SNARE complex assembly (Kee et al., 1995; Sutton et al., 1998; Jahn and Südhof, 1999), and the N-terminal helices A, B, and C (HABC), which independently fold into a three-helix-bundle (Fernandez et al., 1998). Biochemical studies have shown that the N-terminal domain of syntaxin can bind to its C-terminal domain, resulting in a closed conformation that can act as an inhibitor of SNARE complex formation (Dulubova et al., 1999; Misura et al., 2000). Furthermore, Sec1 proteins can interact with syntaxin-like t-SNAREs by recognizing the closed conformation (Jahn and Südhof, 1999). Even though the function is still not understood, the interaction of Sec1 proteins and t-SNAREs appears to be essential for fusion and in most cases seems to involve the N-terminal domain (Banta et al., 1990; Ossig et al., 1991; Wu et al., 1999; Jahn, 2000; Verhage et al., 2000; see discussion).
The yeast vacuolar t-SNARE Vam3p is structurally related to syntaxin, but does not adopt a closed conformation (Dulubova et al., 2001). We prepared mutant yeast strains lacking the N-terminal domain of Vam3p to monitor the interactions and to characterize the function of this domain during the fusion of intact organelles. Truncation of the N-terminus leads to significantly reduced vacuole fusion. Although priming occurs more efficiently in the absence of the N-terminus, trans-SNARE complex formation is less than half of that of the wild-type Vam3p. We observe that HOPS/Vps33p is not recruited efficiently to the cis-complex in the absence of the N-terminal domain of Vam3p, suggesting that this domain is required to ensure the robust transition from priming to docking.
MATERIALS AND METHODS
Reagents and Yeast Strains
Antibodies against Vam3p and Vti1p were increased in New Zealand white rabbits, α-Vam3p by injecting recombinant full-length His6-tagged Vam3p, α-Vti1p with a GST-fusion protein containing the complete cytoplasmic domain of Vti1p (von Mollard et al., 1997; Ungermann et al., 1998b)
Deletion mutants of VAM3 were introduced into yeast strains BJ3505 (MATa pep4::HIS3 prb1-Δ1.6RHIS3 lys-208 trp1-Δ101 ura3-52 gal2 can) and DKY6281 (MATa leu2-3 leu2-112 ura3-52 his3-Δ200 trp1 Δ901 lys2-Δ901 lys2-801 suc2-Δ9 pho8::TRP1) by transformation and loop in-loop out at the chromosomal VAM3 locus of the plasmid pRS406 Vam3ΔN containing a URA3-marker and encoding the N-terminal deleted Vam3p. Ura+ transformants were selected. Ura− clones that were generated in a second selection with 5-fluoroorotic acid were then tested for loss of the wild-type VAM3 sequence by PCR and the size shift by immunoblotting (Ungermann et al., 1999). The strains BJ3505 VPS33::ProtA and BJ3505 VAM3ΔN VPS33::ProtA were created by transformation of a PCR fragment containing the sequence encoding Protein A and a kanMX6 selection marker with flanking regions of the VPS33 3′-region and the downstream sequence of the terminator (Knop et al., 1999). Colonies that grew on YPD+Geneticin plates were restreaked and analyzed for the Protein A tag by PCR and immunoblotting.
Vacuole Fusion
Vacuole fusion is measured by a biochemical complementation assay (Conradt et al., 1992; Haas, 1995). Vacuoles from DKY6281 have normal proteases but lack the membrane protein alkaline phosphatase. Vacuoles from BJ3505 accumulate alkaline phosphatase in the unprocessed and catalytically inactive “pro” form because of the deletion of the gene encoding the protease Pep4p. Incubation of a mixture of these vacuoles in reaction buffer at 26°C in the presence of cytosol and ATP leads to fusion, content mixing, and processing of proalkaline phosphatase by Pep4p. The active alkaline phosphatase is measured in a colorimetric assay at the end of the fusion reaction.
Yeast cells from 1 liter logarithmically growing culture were spheroblasted with recombinant lyticase and lysed by addition of DEAE dextran and heat shock as described (Haas, 1995). Vacuoles were purified by flotation in a discontinuous Ficoll gradient. The isolation procedure enriches vacuoles 45- to 50-fold with respect to total cell protein in the gradient. Only trace amounts of ER and cytosol are recovered by the procedure (Haas, 1995). Vacuoles were used immediately after isolation. The standard fusion reaction (30 μl) contained 3 μg of each vacuole type (BJ3505 and DKY6281) in reaction buffer (10 mM PIPES/KOH, pH 6.8, 200 mM sorbitol, 150 mM KCl, 0.5 mM MgCl2, 0.5 mM MnCl2), 0.5 mM ATP, 3 mg/ml cytosol, 3.5 U/ml creatine kinase, 20 mM creatine phosphate, and a protease inhibitor cocktail (PIC; Xu and Wickner, 1996) containing 7.5 μM pefabloc SC, 7.5 ng/ml leupeptin, 3.75 μM o-phenanthroline, and 37.5 ng/ml pepstatin. For preclustering, vacuoles were centrifuged for 4 min at 10,000 × g and briefly resuspended in reaction buffer. To reduce proteolysis in the coimmunoprecipitation experiments, only the protease A–deficient BJ3505 vacuoles were analyzed.
Immunoprecipitation
After the reaction, vacuoles were pelleted (8000 × g, 5 min, 4°C), washed with 500 μl of reaction buffer, and reisolated as before. Vacuoles were detergent-solubilized by the addition of 1 ml of 1% NP-40, 125 mM NaCl, 10 mM Tris/HCl, pH 7.4, 0.5× PIC, and 1 mM PMSF and incubated on a nutator at 4°C for 10 min. Nonsolubilized material was removed by centrifugation (20,000 × g, 10 min, 4°C). A fraction (5%) of the clarified supernatant was removed, and proteins were precipitated by the addition of TCA (13% vol/vol). The remaining detergent extract was added to Protein A–Sepharose beads containing the coupled antibodies (Ungermann et al., 1999) and incubated on a nutator at 4°C for 2 h or overnight. The beads were reisolated by brief centrifugation and were washed three times with 1 ml of lysis buffer containing 0.1% NP-40 for 10 min. Retained proteins were eluted by the addition of 1 ml of 0.1 M glycine/HCl, pH 2.5, 0.025% NP-40. Proteins were precipitated by TCA, washed with 1 ml of ice cold 100% acetone, briefly dried, and dissolved in SDS-sample buffer. Analysis of protein complexes was done by SDS-PAGE and Western blotting.
Protein A Purification
Protein A–tagged Vps33p was purified with IgG-Sepharose FastFlow (Amersham-Pharmacia, Freiburg, Germany). Vacuoles from the respective strains (300 μg) were pelleted by centrifugation (10000 × g, 10 min), solubilized in 2 ml of 0.1% TX100, 50 mM NaCl, 20 mM HEPES/KOH, pH 7.9, 0.5× PIC, and 1 mM PMSF, and incubated on a nutator at 4°C for 20 min. Nonsolubilized material was removed by centrifugation (20,000 × g, 10 min, 4°C). The supernatant was loaded on a Qiagen (Hilden, Germany) 5 ml polypropylene column and incubated with 100 μl of IgG-Sepharose for 2 h on a nutator. The column was drained by gravity and washed three times with 10 mM HEPES/KOH, pH 7.9, 50 mM NaCl and once with 10 mM HEPES/KOH, pH 7.9, 100 mM NaCl. Protein was eluted with 1 ml 0.1 M glycine, pH 2.6, and precipitated with TCA (as described above).
Analysis of trans-SNARE Complexes
Trans-SNARE pairing was analyzed with vacuoles prepared from DKY6281 vam3Δ, BJ3505 nyv1Δ, and BJ3505 nyv1Δ VAM3ΔN. Vacuoles (120 μg each) were mixed in a 750 μl reaction with cytosol and ATP and incubated for 45 min at 26°C. A 30-μl aliquot was used to measure alkaline phosphatase activity. Vacuoles were collected by centrifugation (16000 × g, 4°C, 10 min), washed with 10 mM PIPES/KOH, pH 6.8, 200 mM sorbitol, and solubilized with 1 ml 20 mM Tris/Cl, pH 7.4, 0.1% Triton X-100, 150 mM NaCl for 10 min, and Nyv1p was coimmunoprecipitated with α-Vam3p antibodies as described (Ungermann et al., 1998b).
RESULTS
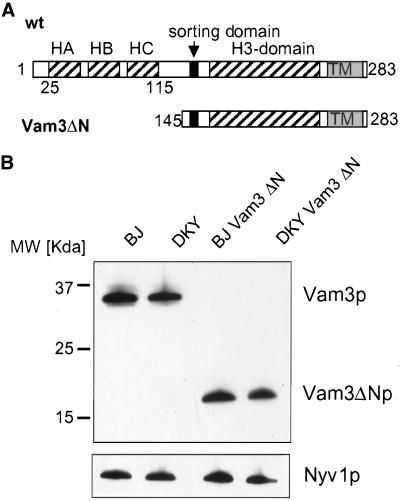
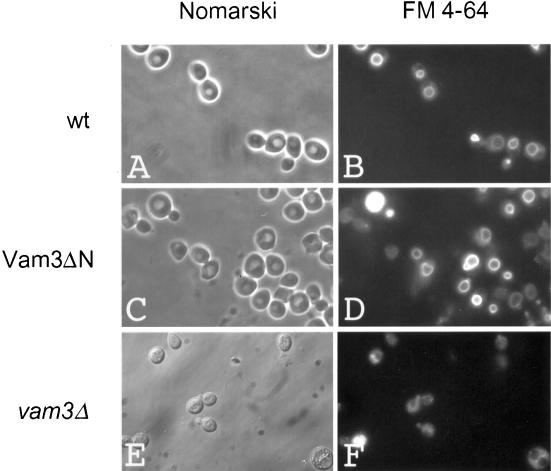
Although the function of the H3 coiled-coil domain of t-SNAREs is well described, the role of the N-terminus has been a matter of debate. To study the function of the N-terminal domain in membrane fusion, we deleted amino acids 1–145 of Vam3p, preserving the complete SNARE domain, the transmembrane domain, and the di-leucine sorting signal from N154 to L160 (Darsow et al., 1998), resulting in Vam3ΔN (Figure 1A). We introduced the truncated gene by homologous recombination into our tester strains, where it replaced the wild-type gene and was under the control of the original promoter (see MATERIALS AND METHODS). As a first test for functionality, we analyzed vacuolar morphology of these strains by labeling the cells with the lipophilic dye FM4–64 (Vida and Emr, 1995). Missorting or complete absence of Vam3p results in severe fragmentation of vacuoles (Figure 2, E and F; Darsow et al., 1997; Nichols et al., 1997). In contrast to this, vacuoles of the strain expressing Vam3ΔN were similar to vacuoles from the wild-type strain (Figure 2, C and D, and A and B, respectively), indicating that the truncated protein is properly sorted and functional in homotypic fusion. Furthermore, the N-terminal deleted Vam3p is found on the vacuoles at a level comparable to the wild-type protein (Figure 1B). Also, sorting of vacuolar alkaline phosphatase was unaltered in the mutant strain (our unpublished observations).
Figure 1.
Deletion of the N-terminal domain of Vam3p. (A) Domain structure of wild-type Vam3p according to coiled-coil prediction (SwissProt Expasy “coils”) and the NMR structure (Dulubova et al., 2001). The approximate sites of coiled-coil domains are indicated by striped boxes, transmembrane domains (TM) by gray boxes, and the sorting domain (amino acids 154–160) by black boxes. Vam3ΔN is deleted from amino acids 1–145. (B) Expression and sorting of the truncated Vam3p. Vacuoles were prepared as described and a fraction (10 μg) of each wild-type and mutant tester strain was analyzed by SDS-PAGE followed by Western blotting. Immunoblots were decorated with antibodies to Vam3p and Nyv1p.
Figure 2.
Vacuolar morphology is unaltered in the Vam3ΔN mutant. Wild-type or mutant BJ3505 strains were incubated with 10 μM of the dye FM4–64 (Molecular Probes, Eugene, OR) for 20 min at 30°C in YPD. Cells were centrifuged briefly (1 min at 5000 × g), washed twice with YPD medium, and chased for 15 min at 30°C (Vida and Emr, 1995). Stained vacuoles were analyzed in a standard fluorescence microscope. (A and B) BJ3505 wild-type cells; (C and D) BJ3505 VAM3ΔN cells; (E and F) BJ3505 vam3Δ cells as a negative control.
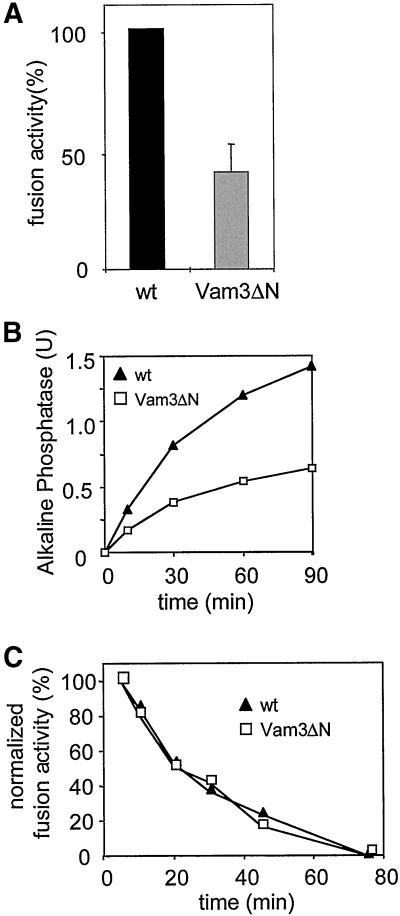
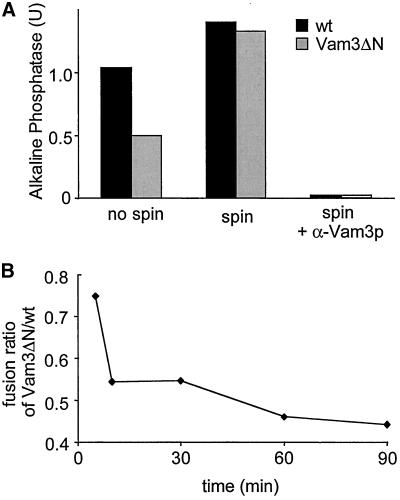
Homotypic fusion of vacuolar vesicles can be reconstituted in vitro by incubating isolated vacuoles in the presence of ATP and cytosol (Conradt et al., 1992; Haas et al., 1994; see MATERIALS AND METHODS). We used this assay to map the stage at which the N-terminus might function during membrane fusion. To our surprise, and in contrast to recent findings by Wang et al. (2001), we observed a pronounced decrease in fusion activity of vacuoles prepared from the mutant strain, indicating that the N-terminal domain is required for efficient fusion (Figure 3A). Relative to wild-type control, diminished fusion was observed consistently over a period of 90 min (Figure 3B). Extending the standard incubation time from 90 to 240 min did not increase the overall fusion of the Vam3ΔN vacuoles (our unpublished observations). This may be due to consumption or decay of critical components over time (Figure 3C and our unpublished observations). Furthermore, we found that the N-terminus does not function to inactivate Vam3p, which has been postulated for syntaxin (Jahn and Südhof, 1999). When vacuoles from both tester strains were incubated separately and mixed at different time points, the fusion signal declined equally in the wild-type and the mutant strain (Figure 3C).
Figure 3.
Decreased fusion of Vam3ΔN vacuoles. (A) Fusion activity of Vam3ΔN- compared with wild-type-vacuoles. Standard fusion reactions with cytosol and ATP were incubated for 90 min at 26°C, and alkaline phosphatase activity was determined as described in MATERIALS AND METHODS. To allow comparison of independent experiments, wild-type vacuole fusion was set to 100%. Fusion of Vam3ΔN vacuoles was reduced to 40 ± 11% (mean ± SEM, n = 14). (B) Time course of the fusion reaction. Standard fusion reactions (150 μl) containing wild-type or Vam3ΔN tester vacuoles were started with cytosol and ATP. At the indicated time points a 30-μl aliquot was removed and placed on ice to stop the reaction. Fusion activity was measured after 90 min. A representative experiment is shown. (C) Loss of fusion competence over time. Wild-type or mutant BJ3505 and DKY6281 vacuoles were separately incubated with cytosol and ATP at 26°C. At the indicated time points 20 μl of BJ3505 and DKY6281 vacuoles were mixed and incubated for an additional 70 min. Then fusion activity was assayed.
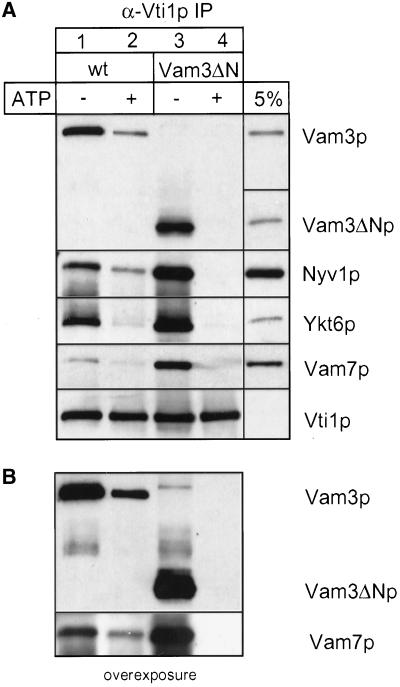
We therefore used established assays (Wickner and Haas, 2000) to investigate the function of the amino terminus of Vam3p and to understand why fusion is reduced in its absence. Homotypic vacuole fusion can be separated into distinct subreactions termed priming, docking, and fusion (Wickner and Haas, 2000). We asked if Vam3ΔN vacuoles were defective at any of those stages. First, we analyzed whether the deletion of the N-terminus of Vam3p alters the composition of the cis-SNARE or its disassembly during priming. Vacuoles were pretreated with or without ATP, and SNARE complexes were isolated from detergent extracts by coimmunoprecipitation with antibodies against the SNARE Vti1p (Figure 4). Indeed, wild-type as well as truncated Vam3p is in a cis-SNARE complex with the SNAREs, Vti1p, Nyv1p, Ykt6p, and Vam7p, in the absence of ATP. However, in all experiments, two to three times more SNAREs were found in precipitations from Vam3ΔN vacuoles than in wild-type (Figure 4A, lane 1 vs. 3). Furthermore, priming, as measured by the ATP-dependent disassembly of the cis-SNARE complex, was much more efficient on Vam3ΔN vacuoles (Figure 4B; lane 2 vs. 4), suggesting that SNARE complexes containing Vam3ΔN are either less stable or more accessible to Sec18p than those with wild-type Vam3p. Thus, removal of the N-terminus of Vam3p obviously alters the dynamics of the SNARE complex.
Figure 4.
Vam3ΔN assembles into cis-SNARE complexes. Wild-type or mutant vacuoles (60 μg each) were incubated in reaction buffer with cytosol, with or without ATP at 26°C. After 10 min the vacuoles were collected by centrifugation. Solubilization and immunoprecipitation was performed as described in MATERIALS AND METHODS. Immunoprecipitation was done with a polyclonal anti-Vti1p antiserum covalently coupled to Protein A–Sepharose beads (Ungermann et al., 1999). Proteins were eluted with 0.1 M glycine, pH 2.6, precipitated with TCA, separated on 15% SDS-polyacrylamide gels, and detected with the indicated antibodies (A). A fraction of the wild-type detergent extract (5%; for Vam3p, Vam7p, Vti1p, and Ykt6p) as well as a fraction of the mutant extract (Vam3ΔN) was precipitated and loaded as a controls. The amount of coprecipitated SNAREs was quantified densitometrically (NIH image 1.6): wild-type − ATP, 100 ± 16; wild-type + ATP, 46 ± 10; Vam3ΔN − ATP, 225 ± 14; Vam3ΔN + ATP, 24 ± 4 (mean density after background subtraction in arbitrary units ± SEM, n = 4). (B) An overexposure of the immunoblots decorated with anti-Vam3p and anti-Vam7p.
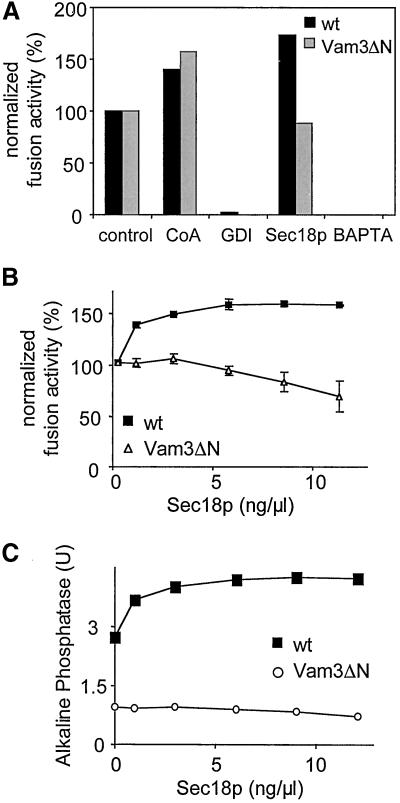
Next we asked if the observed alteration in the cis-SNARE complex would influence the sensitivity of the vacuoles to known inhibitors and activators (Figure 5). Both types of vacuoles were equally sensitive to Gdi1p, a docking inhibitor (Haas et al., 1995), as well as the fusion inhibitor BAPTA (Peters and Mayer, 1998) and were similarly stimulated by coenzyme A (CoA; Haas and Wickner, 1996; Veit et al., 2001). Interestingly, addition of Sec18p, which enhances priming and thus fusion of wild-type vacuoles by ∼150%, had virtually no stimulatory effect on the fusion of Vam3ΔN vacuoles (Figure 5, B and C). Sec18p promotes disassembly of cis-SNARE complexes (Mayer et al., 1996; Ungermann et al., 1998a), resulting most likely in more activated SNAREs that can participate in the docking step. If added in excess, Sec18p can also inhibit fusion, probably by continuously activating SNAREs and delaying their entry into the reaction (Ungermann et al., 1998b). Because SNARE complexes containing Vam3ΔN disassemble more easily than those containing wild-type Vam3p (Figure 4), Sec18p cannot stimulate, but rather inhibits fusion (Figure 5B; Ungermann et al., 1998b). It is possible that Sec18p associates more tightly with the cis-SNARE complex of Vam3ΔN vacuoles, an explanation that is suggested by preliminary experiments (our unpublished observations).
Figure 5.
Effect of fusion inhibitors and activators. (A) Standard fusion reactions (30 μl) containing cytosol and ATP were incubated at 26°C for 90 min with or without the indicated inhibitors (64 ng/μl Gdi1p [GDI]; 10 mM BAPTA) or activators (10 μM CoA; 5 ng/μl Sec18p). To compare the results obtained for wild-type (black bars) and Vam3ΔN (gray bars) vacuoles, the fusion measured for control conditions of both strains was set to 100%. (B and C) Effect of Sec18p on the fusion reaction. Purified recombinant His6-Sec18p (Haas and Wickner, 1996) was added at the indicated concentrations to 30 μl fusion reactions to stimulate priming. The reaction mixture was incubated at 26°C for 90 min and then fusion was measured. (A and C) Representative experiments; (B) average values of three independent experiments ± SEM expressed as percentage.
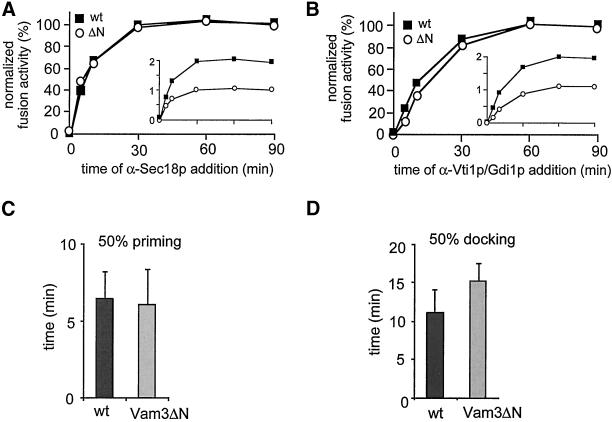
The stages of vacuole fusion can be kinetically separated in a time-of-addition experiment (Mayer et al., 1996). We questioned whether the fast disassembly of the SNARE complex of Vam3ΔN influences any of these stages. A standard reaction was started in the presence of cytosol and ATP. At each time point, aliquots were withdrawn and incubated for the remaining time in the presence of the indicated inhibitors (Figure 6). To directly compare the inhibition kinetics of wild-type and Vam3ΔN vacuoles (as shown in the inlets), the values were normalized by setting the units of fusion obtained at 90 min to 100% (Figure 6, A and B). The priming step, as monitored by the acquisition of resistance to antibodies to Sec18p, was not altered by the removal of the amino-terminus of Vam3p (Figure 6A), even though priming was very efficient (Figure 4). However, resistance to docking inhibitors such as Gdi1p or antibodies against Vti1p was moderately delayed when wild-type and Vam3ΔN vacuoles were compared (Figure 6B). Statistical analysis of the time at which 50% of total fusion in the presence of priming and docking inhibitors was reached confirmed that docking, but not priming was indeed significantly slower (Figure 6, D and F). This suggests a role of the N-terminal domain in the coordination of docking.
Figure 6.
Vam3ΔN vacuoles show a delay in docking. Standard fusion reactions (200 μl), containing wild-type or Vam3ΔN vacuoles, were incubated in the presence of cytosol and ATP; at the indicated time points a 30-μl aliquot was mixed with an inhibitor and incubation at 26°C was continued for a total of 90 min. Concentration of antibodies to Sec18p and Vti1p IgGs was 0.1 μg/μl, Gdi1p was used at 64 μg/ml. (A) Sensitivity to α-Sec18p of wild-type and Vam3ΔN vacuoles. Priming kinetics obtained were normalized by setting fusion at 90 min to 100%. An inlet shows the alkaline phosphatase units before normalization. Average values of nine independent experiments are shown. (B) Sensitivity to α-Vti1p or Gdi1p of wild-type and Vam3ΔN vacuoles. Average values of 20 independent experiments are shown. (C) Time point, where fusion inhibition by α-Sec18 is 50%. Wild-type 6.5 ± 1.7 min (SEM), n = 9; Vam3ΔN 6.2 ± 2.2 min (SEM), n = 9. Difference not significant (Student's t test = 0.4). (D) Time point, where fusion inhibition by α-Vti1 or Gdi1p is 50%. Wild-type 11.5 ± 2.9 min (SEM), n = 20; Vam3ΔN 15.3 ± 2.4 min (SEM), n = 20. Difference is highly significant (Student's t test < 0.00001).
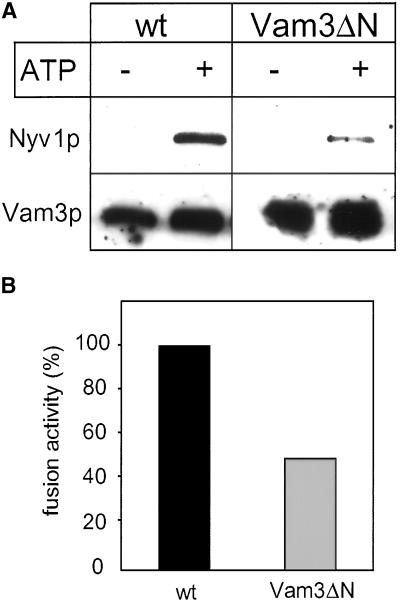
It seemed unlikely that this moderate delay in docking could alone account for the drastic effect seen in the fusion reaction (Figure 3). Therefore, we compared the amount of trans-SNARE complexes that form during a fusion reaction in the presence of the wild-type Vam3p or the truncated protein. We deleted the v-SNARE Nyv1p in both strains, isolated the vacuoles, and fused them with vacuoles derived from a strain that lacks Vam3p (DKY6281 vam3Δ). Trans-complexes between Nyv1p and Vam3p only form if vacuoles dock in an ATP-dependent manner (Ungermann et al., 1998b), as analyzed by coimmunoprecipitation of Nyv1p with an antibody against Vam3p. Nyv1p interacts with Vam3p only after priming with ATP (Figure 7A). Complex formation as well as fusion activity is strongly diminished if the truncated Vam3p is present on the tester vacuoles (Figure 7), showing that a decrease in fusion directly correlates with reduced trans-SNARE pairing. We consider the slight docking delay (Figure 6) unlikely to be responsible for this strong effect. Taken together, the data suggest that even though a high percentage of SNAREs is available for the reaction after priming (Figure 4), only a few enter the right pathway.
Figure 7.
Vam3ΔN vacuoles form less trans-SNARE complexes. (A) Coimmunoprecipitation of Nyv1p with anti-Vam3p. Vacuoles from BJ3505 nyv1Δ or BJ3505 nyv1Δ VAM3ΔN were fused with DKY6281 vam3Δ as described in MATERIALS AND METHODS. After the incubation vacuoles were collected by centrifugation, solubilized and immunoprecipitated with α-Vam3p. Eluted proteins were separated by SDS-PAGE and detected by Western blotting with antibodies to Nyv1p and Vam3p. The amount of coprecipitated SNAREs was quantified densitometrically (NIH image 1.6): wild-type, 100 ± 10; Vam3ΔN, 34 ± 16 (mean density after background subtraction in arbitrary units ± SEM, n = 3). (B) A 30 μl aliquot was removed from the identical fusion reaction described in (A) and incubated for 90 min at 26°C to measure fusion activity. Fusion of BJ3505 nyv1Δ with DKY6281 vam3Δ (black bar) was set to 100%.
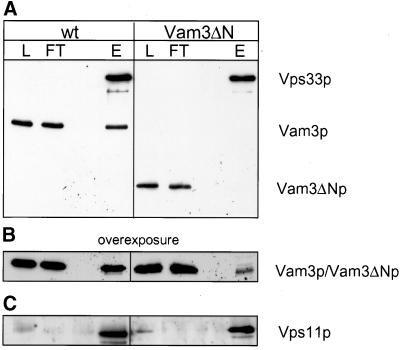
This lack of coordination between priming and docking suggested that the N-terminus of Vam3p might be necessary to recruit tethering factors to the cis-SNARE complex. We previously reported that the HOPS complex is associated with the SNARE complex on isolated vacuoles (Price et al., 2000a). HOPS is released from the cis-SNARE complex upon priming and interacts with Ypt7p to initiate vacuole tethering (Price et al., 2000a). We therefore asked whether SNARE complexes containing Vam3ΔN are able to bind HOPS. Vps33p, one component of the HOPS complex and a Sec1 homologue, was C-terminally tagged with Protein A in both strains. We then purified the tagged Vps33p via IgG Sepharose from vacuolar detergent extracts and probed for bound Vam3p by Western blotting. Although full-length Vam3p copurifies well with Vps33p, little Vam3ΔN was bound to Vps33 (Figure 8A). Only after overexposure did a small fraction of Vam3ΔN become visible (Figure 8B). The integrity of HOPS was maintained during the purification, because Vps11p, another member of the HOPS complex (Sato et al., 2000; Seals et al., 2000), was in a complex with Vps33p in both strains (Figure 8C). Thus, the N-terminal domain of Vam3p is essential to efficiently recruit HOPS/Vps33p to the cis-SNARE complex.
Figure 8.
Efficient recruitment of HOPS/Vps33p to Vam3p depends on the N-terminal domain. Vacuoles (300 μg) prepared from BJ VPS33:: ProtA or BJ VAM3ΔN VPS33:: ProtA strains, were solubilized, and Protein A–tagged Vps33p was purified as described in MATERIALS AND METHODS. Fractions of the Protein-A-purification were precipitated with TCA, separated on a 15% SDS gel and blotted onto nitrocellulose. The Western blot probed with antibodies against Vam3p (A and B) or Vps11 (C). L = 1% of total protein loaded on the column; FT = 1% of the flow-through; E = 30% of the eluate. The left panel shows the isolation from vacuoles with full length Vam3p and the right panel the isolation from Vam3ΔN vacuoles (The high-molecular-weight band is Vps33p, which was detected by the secondary antibody because of the Protein A–tag).
The reduced recruitment of HOPS to the cis-SNARE complex could explain the uncoordinated transition from priming to tethering. We asked if we could support this transition and promote fusion of Vam3ΔN vacuoles by enhancing physical contact between the vacuoles. We therefore preclustered wild-type and Vam3ΔN vacuoles by centrifugation, briefly resuspended them in reaction buffer containing ATP and cytosol, and incubated them for 90 min. Strikingly, this precentrifugation step only slightly stimulated fusion of wild-type vacuoles, but supported Vam3ΔN vacuoles such that both vacuole types showed virtually the same fusion activity (Figure 9A). Fusion was authentic, because it was dependent on ATP and sensitive to antibodies against Vam3p (Figure 9A). We expected that this rescue could only function early in the reaction, because it was initiated by vacuole priming. This is indeed the case. Figure 9B shows the ratio of fusion between Vam3ΔN and wild-type vacuoles. Centrifugation can rescue the mutant only when performed at early time points in the reaction (Figure 9B). This suggests that increased physical contact can overcome the docking defect of Vam3ΔN vacuoles. We also tested whether we could rescue the trans-SNARE pairing by preclustering the vacuoles. However, mutant vacuoles with wild-type Vam3p as well as with Vam3ΔN were too sensitive and did not show any activity after a precentrifugation step (our unpublished observations).
Figure 9.
Preclustering rescues the reduced fusion of Vam3ΔN vacuoles. (A) Vacuoles prepared from wild-type or Vam3ΔN tester strains were incubated in a standard fusion reaction with cytosol and ATP at 26°C. Alternatively, tester vacuoles were mixed and centrifuged for 4 min at 9000 × g at 4°C, the supernatant was removed and the vacuoles were briefly resuspended in reaction buffer containing cytosol and ATP and incubated for 90 min at 26°C. Where indicated, IgGs to Vam3p (0.1 μg/μl) were added to the reaction. Fusion activity of wild-type vacuoles was set to 100%. (B) Time course of preclustering by centrifugation. Standard fusion reactions with wild-type or Vam3ΔN vacuoles where started by incubation at 26°C, at the indicated time points an aliquot was withdrawn, centrifuged for 4 min, 9000 × g, 4°C and resuspended, incubation at 26°C was continued to a total of 90 min. To illustrate the time-dependent effect of centrifugation, the ratio of fusion signals obtained for Vam3ΔN versus wild-type vacuoles is shown.
In sum, the N-terminal domain of Vam3p is preserving the physical interaction of the SNAREs with the HOPS complex within the cis-SNARE complex, thereby ensuring a coordinated transition from priming to tethering.
DISCUSSION
The role of the amino terminus of t-SNAREs has been analyzed before with purified proteins (Fernandez et al., 1998; Parlati et al., 1999; Misura et al., 2000; Munson et al., 2000). With the use of vacuole fusion as a model system, we were able to show that the N-terminus of Vam3p acts as a central domain by 1) recruiting the HOPS complex to the cis-SNARE complex and 2) by allowing coordinated docking. In the absence of this domain only a fraction of the vacuoles enters the right pathway, as shown by the reduced trans-SNARE pairing and fusion. This suggests that the N-terminus is needed for the processes and interactions that coordinate docking before membrane fusion.
The deletion of the N-terminal region of Vam3p obviously alters the composition and function of the SNARE complex, whereas transport to the vacuole and the basic interaction with the other SNAREs on the vacuole (Vti1p, Ykt6p, Vam7p, and Nyv1p) was unaffected. Cis-SNARE complexes precipitated from mutant strains contained substantially more SNAREs than those isolated from wild-type vacuoles (Figure 4). Removal of the N-terminus may reduce steric hindrance and could allow for a better assembly of the SNARE complex. In reverse, the lack of this domain may permit a more efficient access of Sec18/17p, which is suggested by preliminary experiments (our unpublished observations). Both explanations would be consistent with our finding that complexes containing Vam3ΔN are more abundant and disassemble more efficiently. In agreement with this, stimulation of SNARE disassembly by addition of Sec18p has no stimulatory effect on the fusion of Vam3ΔN vacuoles, but boosts the fusion of wild-type vacuoles (see Figure 5). Even though more SNARE complexes are found in cis and are primed, fusion is reduced, indicating a lack in coordination of the reaction.
A recent study addressing the role of the N-terminus of Vam3p reported no alteration of SNARE complexes on Vam3ΔN vacuoles (Wang et al., 2001). However, it is possible that this can only be seen if the complex is precipitated with antibodies to a well-accessible vacuolar SNARE, because the SNARE complex becomes unstable during purification (unpublished observations). Nevertheless, our observation correlates with in vitro studies of isolated exocytotic SNARE complexes. Here, deleting the N-terminal region of either syntaxin or yeast Sso1 allowed faster or more efficient assembly of SNARE complexes (Nicholson et al., 1998; Parlati et al., 1999; Munson et al., 2000).
Vacuoles with Vam3ΔN recruit less HOPS to the cis-SNARE complex (Figure 8), even though the amount of Vps33p on the vacuole, which is one of the subunits of the complex, is not altered (our unpublished observations). HOPS is required for the initial contact of vacuoles, termed tethering, where it associates with Ypt7p upon release from the SNARE complex (Price et al., 2000a, 2000b; Sato et al., 2000; Seals et al., 2000). Emr and colleagues suggested that HOPS exclusively interacts with the disassembled Vam3p after priming (Sato et al., 2000) and serves as a chaperone for trans-SNARE pairing. This is possible and supported by our observations that lack of the N-terminus of Vam3p results in less trans-SNARE pairs and slightly delayed docking (Figures 6D and 7A). We think, however, that the main interaction between Vam3p—and here in particular the N-terminus—and HOPS is required in the context of the cis-SNARE complex (Figure 8A). This may occur during the reformation of the cis-SNARE complex. From recent experiments it appears that cis-SNARE complexes are not necessarily a result of a previous fusion event. Vacuoles containing Vam3p with mutations in the coiled-coil domain still have cis-SNARE complexes, but do not show fusion (Wang et al., 2001). Vam3p may be involved in the recruitment of HOPS while it is not yet complexed with other SNAREs, which would explain the contradictory results. Furthermore, it was reported that Vps33p, added as a lysate from a Vps33p overproducing yeast strain, was binding equally efficient to recombinant Vam3p with or without the N-terminus, although the coiled-coil domain was essential for this interaction (Dulubova et al., 2001). It is difficult to compare this observation with ours, because the interaction of HOPS and Vam3p most likely does not reflect the recruitment of HOPS to the cis-SNARE complex. The interaction of Vam3p with HOPS requires Vps18 (Sato et al., 2000). It is possible that only the monomeric Vps33p was binding to the recombinant Vam3p because it was overexpressed in yeast and may not have been part of the HOPS complex. It will therefore be important to analyze the assembly of the cis-SNARE complex in greater detail to evaluate these discrepancies.
Our data suggest that the Vam3ΔN cis-SNARE complex assembles and disassembles more efficiently, but because the interaction with HOPS is strongly reduced, only a fraction of primed SNAREs is able to pair in a trans-configuration, leading to fusion. Presumably the remaining SNAREs eventually assemble back in cis. Preclustering overcomes the necessity to physically tether the vacuoles and may allow the mutant SNAREs to bypass the coordinated HOPS requirement, although a function of HOPS during the fusion of Vam3ΔN is very likely.
The N-terminus of syntaxins/t-SNAREs has attracted much interest, mainly because of the interaction with Sec1p and its ability to fold back onto the coiled-coil domain (Kee et al., 1995; Fernandez et al., 1998; Nicholson et al., 1998; Munson et al., 2000; Misura et al., 2000; Yang et al., 2000). Recently, it was reported that the requirement for a cofactor of Munc18/nSec1p, Munc13, can be bypassed if a mutant syntaxin with a constitutively open conformation is expressed in Caenorhabditis elegans (Richmond et al., 2001). Such a bypass has not been reported for Munc18 itself; in fact, neurons from munc18 null mice do not show any synaptic exocytosis (Verhage et al., 2000), suggesting that Sec1 proteins are essential in exocytosis. Even though there is a basic interaction of t-SNAREs with Sec1 proteins, there is also a lot of variability of this theme, as shown by a few examples: Novick and coworkers found that the yeast exocytic t-SNARE Sso1p only interacts with Sec1p when assembled into SNARE complexes (Carr et al., 1999), whereas syntaxin binds the neuronal Sec1p in a stoichimetric 1:1 complex (Hata et al., 1993; Misura et al., 2000). Bryant and James (2001) recently showed that the yeast t-SNARE of the late Golgi, Tlg2p, interacts specifically with the Sec1 homolog Vps45p via its N-terminus, although it is not clear if this occurs in the context of the SNARE complex. Here, Vps45p function is essential to stabilize Tlg2p. In contrast to the wild-type Tlg2p, the truncated, but nonfunctional Tlg2p forms SNARE complexes even in the absence of Vps45p, suggesting that the Sec1 protein stabilizes the t-SNARE by binding to its N-terminus (Bryant and James, 2001). The Sec1p-homologue required for vacuole fusion, Vps33p, is part of the HOPS complex, which interacts with the cis-SNARE complex implying that it also binds to assembled SNAREs and not to uncomplexed Vam3p (Price et al., 2000a). Deletion of the N-terminal domain leads to a drastic decrease in binding to the HOPS complex in vivo (Figure 8), indicating that this domain is involved in the interaction.
The variety of the folding of the N-termini of t-SNAREs and the context in which Sec1-like proteins interact with them makes it challenging to derive common principles. All seem to interact with t-SNAREs either in a complex with other SNAREs such as Sec1p or Vps33p or in a dimeric complex such as Munc18 (Hata et al., 1993). This interaction occurs in part via the N-terminus, demonstrated by functional assays or direct binding studies (this study; Kee et al., 1995; Bryant and James, 2001). Furthermore, Sec1 proteins are observed in tethering complexes (Burd et al., 1997; Tall et al., 1999; Price et al., 2000a; Sato et al., 2000; Wurmser et al., 2000). They may function as a chaperone for the t-SNARE as suggested by Bryant and James (2001), and they might act as late as the final fusion step as described by Grote et al. (2000). Although much of this remains to be resolved, our results allow a clear assignment of the N-terminus of Vam3p through the analysis of an authentic fusion reaction. Our data suggest that although the C-terminal H3 domain of Vam3p is mainly required for direct “SNARE” interactions, the N-terminal domain is needed for interacting with SNARE effectors and regulators before and during the tethering and docking steps.
ACKNOWLEDGMENTS
We thank Bill Wickner for generously providing antibodies and reagents, and Dieter Langosch, Uta Ungermann, Walter Nickel, and members of the Ungermann laboratory for critically reading the manuscript. This work was supported by a grant of the Deutsche Forschungsgemeinschaft (UN111/2-1; Nachwuchsgruppen in den Biowissenschaften).
Abbreviations used:
- HOPS
homotypic fusion and protein sorting
- SNARE
SNAP (soluble NSF attachment protein) receptor
REFERENCES
- Banta LM, Vida TA, Herman PK, Emr SD. Characterization of yeast Vps33p, a protein required for vacuolar protein sorting and vacuole biogenesis. Mol Cell Biol. 1990;10:4638–4649. doi: 10.1128/mcb.10.9.4638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant NJ, James DE. Vps45p stabilizes the syntaxin homologue Tlg2p and positively regulates SNARE complex formation. EMBO J. 2001;20:3380–3388. doi: 10.1093/emboj/20.13.3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burd CG, Peterson M, Cowles CR, Emr SD. A novel Sec18p/NSF-dependent complex required for Golgi-to-endosome transport in yeast. Mol Biol Cell. 1997;8:1089–1104. doi: 10.1091/mbc.8.6.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr CM, Grote E, Munson M, Hughson FM, Novick PJ. Sec1p binds to SNARE complexes and concentrates at sites of secretion. J Cell Biol. 1999;146:333–344. doi: 10.1083/jcb.146.2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YA, Scales SJ, Patel SM, Doung YC, Scheller RH. SNARE complex formation is triggered by Ca2+ and drives membrane fusion. Cell. 1999;97:165–174. doi: 10.1016/s0092-8674(00)80727-8. [DOI] [PubMed] [Google Scholar]
- Conradt B, Shaw J, Vida T, Emr S, Wickner W. In vitro reactions of vacuole inheritance in Saccharomyces cerevisiae. J Cell Biol. 1992;119:1469–1479. doi: 10.1083/jcb.119.6.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darsow T, Rieder SE, Emr SD. A multispecificity syntaxin homologue, Vam3p, essential for autophagic and biosynthetic protein transport to the vacuole. J Cell Biol. 1997;138:517–529. doi: 10.1083/jcb.138.3.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darsow T, Burd CG, Emr SD. Acidic di-leucine motif essential for AP-3-dependent sorting and restriction of the functional specificity of the Vam3p vacuolar t-SNARE. J Cell Biol. 1998;142:913–922. doi: 10.1083/jcb.142.4.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulubova I, Sugita S, Hill S, Hosaka M, Fernandez I, Südhof TC, Rizo J. A conformational switch in syntaxin during exocytosis: role of munc18. EMBO J. 1999;18:4372–4382. doi: 10.1093/emboj/18.16.4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulubova I, Yamaguchi T, Wang Y, Südhof TC, Rizo J. Vam3p structure reveals conserved and divergent properties of syntaxins. Nat Struct Biol. 2001;8:258–264. doi: 10.1038/85012. [DOI] [PubMed] [Google Scholar]
- Fernandez I, Ubach J, Dulubova I, Zhang X, Südhof TC, Rizo J. Three-dimensional structure of an evolutionarily conserved N-terminal domain of syntaxin 1A. Cell. 1998;94:841–849. doi: 10.1016/s0092-8674(00)81742-0. [DOI] [PubMed] [Google Scholar]
- Grote E, Carr CM, Novick PJ. Ordering the final events in yeast exocytosis. J Cell Biol. 2000;151:439–452. doi: 10.1083/jcb.151.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas A. A quantitative assay to measure homotypic vacuole fusion in vitro. Methods Cell Sci. 1995;17:283–294. [Google Scholar]
- Haas A, Conradt B, Wickner W. G-protein ligands inhibit in vitro reactions of vacuole inheritance. J Cell Biol. 1994;126:87–97. doi: 10.1083/jcb.126.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas A, Scheglmann D, Lazar T, Gallwitz D, Wickner W. The GTPase Ypt7p of Saccharomyces cerevisiae is required on both partner vacuoles for the homotypic fusion step of vacuole inheritance. EMBO J. 1995;14:5258–5270. doi: 10.1002/j.1460-2075.1995.tb00210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas A, Wickner W. Homotypic vacuole fusion requires Sec17p (yeast α-SNAP) and Sec18p (yeast NSF) EMBO J. 1996;15:3296–3305. [PMC free article] [PubMed] [Google Scholar]
- Hanson PI, Heuser JE, Jahn R. Neurotransmitter release—four years of SNARE complexes. Curr Opin Neurobiol. 1997;7:310–315. doi: 10.1016/s0959-4388(97)80057-8. [DOI] [PubMed] [Google Scholar]
- Hata Y, Slaughter CA, Südhof TC. Synaptic vesicle fusion complex contains unc-18 homologue bound to syntaxin. Nature. 1993;366:347–351. doi: 10.1038/366347a0. [DOI] [PubMed] [Google Scholar]
- Holthuis JC, Nichols BJ, Dhruvakumar S, Pelham HR. Two syntaxin homologues in the TGN/endosomal system of yeast. EMBO J. 1998;17:113–126. doi: 10.1093/emboj/17.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn R, Südhof TC. Membrane fusion and exocytosis. Annu Rev Biochem. 1999;68:863–911. doi: 10.1146/annurev.biochem.68.1.863. [DOI] [PubMed] [Google Scholar]
- Jahn R. Sec1/Munc18 proteins: mediators of membrane fusion moving to center stage. Neuron. 2000;27:201–204. doi: 10.1016/s0896-6273(00)00029-5. [DOI] [PubMed] [Google Scholar]
- Katz L, Hanson PI, Heuser JE, Brennwald P. Genetic and morphological analyses reveal a critical interaction between the C-termini of two SNARE proteins and a parallel four helical arrangement for the exocytic SNARE complex. EMBO J. 1998;17:6200–6209. doi: 10.1093/emboj/17.21.6200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kee Y, Lin RC, Hsu S-C, Scheller RH. Distinct domains of syntaxin are required for synaptic vesicle fusion complex formation and dissociation. Neuron. 1995;14:991–998. doi: 10.1016/0896-6273(95)90337-2. [DOI] [PubMed] [Google Scholar]
- Knop M, Siegers K, Pereira G, Zachariae W, Winsor B, Nasmyth K, Schiebel E. Epitope tagging of yeast genes using a PCR-based strategy: more tags and improved practical routines. Yeast. 1999;15:963–972. doi: 10.1002/(SICI)1097-0061(199907)15:10B<963::AID-YEA399>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Laage R, Rohde J, Brosig B, Langosch D. A conserved membrane-spanning amino acid motif drives homomeric and supports heteromeric assembly of presynaptic SNARE proteins. J Biol Chem. 2000;275:17481–17487. doi: 10.1074/jbc.M910092199. [DOI] [PubMed] [Google Scholar]
- Lin RC, Scheller RH. Mechanisms of synaptic vesicle exocytosis. Annu Rev Cell Dev Biol. 2000;16:19–49. doi: 10.1146/annurev.cellbio.16.1.19. [DOI] [PubMed] [Google Scholar]
- Mayer A, Wickner W, Haas A. Sec18p (NSF)-driven release of Sec17p (α-SNAP) can precede docking and fusion of yeast vacuoles. Cell. 1996;85:83–94. doi: 10.1016/s0092-8674(00)81084-3. [DOI] [PubMed] [Google Scholar]
- McNew JA, Parlati F, Fukuda R, Johnston RJ, Paz K, Paumet F, Sollner TH, Rothman JE. Compartmental specificity of cellular membrane fusion encoded in SNARE proteins. Nature. 2000;407:153–159. doi: 10.1038/35025000. [DOI] [PubMed] [Google Scholar]
- Mellman I, Warren G. The road taken: past and future foundations of membrane traffic. Cell. 2000;100:99–112. doi: 10.1016/s0092-8674(00)81687-6. [DOI] [PubMed] [Google Scholar]
- Misura KM, Scheller RH, Weis WI. Three-dimensional structure of the neuronal-Sec1-syntaxin 1a complex. Nature. 2000;404:355–362. doi: 10.1038/35006120. [DOI] [PubMed] [Google Scholar]
- Munson M, Chen X, Cocina AE, Schultz SM, Hughson FM. Interactions within the yeast t-SNARE Sso1p that control SNARE complex assembly. Nat Struct Biol. 2000;7:894–902. doi: 10.1038/79659. [DOI] [PubMed] [Google Scholar]
- Nichols BJ, Ungermann C, Pelham HRB, Wickner WT, Haas A. Homotypic vacuolar fusion mediated by t- and v-SNAREs. Nature. 1997;387:199–202. doi: 10.1038/387199a0. [DOI] [PubMed] [Google Scholar]
- Nicholson KL, Munson M, Miller RB, Filip TJ, Fairman R, Hughson FM. Regulation of SNARE complex assembly by an N-terminal domain of the t- SNARE Sso1p. Nat Struct Biol. 1998;5:793–802. doi: 10.1038/1834. [DOI] [PubMed] [Google Scholar]
- Ossig R, Dascher C, Trepte HH, Schmitt HD, Gallwitz D. The yeast SLY gene products, suppressors of defects in the essential GTP-binding Ypt1 protein, may act in endoplasmic reticulum-to-Golgi transport. Mol Cell Biol. 1991;11:2980–2993. doi: 10.1128/mcb.11.6.2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto H, Hanson PI, Jahn R. Assembly and disassembly of a ternary complex of synaptobrevin, syntaxin, and SNAP-25 in the membrane of synaptic vesicles. Proc Natl Acad Sci USA. 1997;94:6197–6201. doi: 10.1073/pnas.94.12.6197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parlati F, Weber T, McNew JA, Westermann B, Söllner TH, Rothman JE. Rapid and efficient fusion of phospholipid vesicles by the alpha-helical core of a SNARE complex in the absence of an N-terminal regulatory domain. Proc Natl Acad Sci USA. 1999;96:12565–12570. doi: 10.1073/pnas.96.22.12565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters C, Mayer A. Ca2+/calmodulin signals the completion of docking and triggers a late step of vacuole fusion. Nature. 1998;396:575–80. doi: 10.1038/25133. [DOI] [PubMed] [Google Scholar]
- Peters C, Andrews PD, Stark MJ, Cesaro-Tadic S, Glatz A, Podtelejnikov A, Mann M, Mayer A. Control of the terminal step of intracellular membrane fusion by protein phosphatase 1. Science. 1999;285:1084–1087. doi: 10.1126/science.285.5430.1084. [DOI] [PubMed] [Google Scholar]
- Peter C, Bayer MJ, Buhler S, Andersen JS, Mann M, Mayer A. Trans complex formation by proteolipid channels in the terminal phase of membrane fusion. Nature. 2001;409:581–588. doi: 10.1038/35054500. [DOI] [PubMed] [Google Scholar]
- Poirier MA, Xiao WZ, Macosko JC, Chan C, Shin YK, Bennett MK. The synaptic SNARE complex is a parallel four-stranded helical bundle. Nat Struct Biol. 1998;5:765–769. doi: 10.1038/1799. [DOI] [PubMed] [Google Scholar]
- Price A, Seals D, Wickner W, Ungermann C. The docking stage of yeast vacuole fusion requires the transfer of proteins from a cis-SNARE complex to a Rab/Ypt protein. J Cell Biol. 2000a;148:1231–1238. doi: 10.1083/jcb.148.6.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price A, Wickner W, Ungermann C. Proteins needed for vesicle budding from the Golgi complex are also required for the docking step of homotypic vacuole fusion. J Cell Biol. 2000b;148:1223–1229. doi: 10.1083/jcb.148.6.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond JE, Weimer RM, Jorgensen EM. An open form of syntaxin bypasses the requirement of UNC-13 in vesicle priming. Nature. 2001;412:338–341. doi: 10.1038/35085583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieder SE, Emr SD. A novel ring finger protein complex essential for a late step in protein transport to the yeast vacuole. Mol Biol Cell. 1997;8:2307–2327. doi: 10.1091/mbc.8.11.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman JE. Mechanisms of intracellular protein transport. Nature. 1994;372:55–63. doi: 10.1038/372055a0. [DOI] [PubMed] [Google Scholar]
- Rothman JE, Wieland FT. Protein sorting by transport vesicles. Science. 1996;272:227–234. doi: 10.1126/science.272.5259.227. [DOI] [PubMed] [Google Scholar]
- Sato TK, Rehling P, Peterson MR, Emr SD. Class C Vps protein complex regulates vacuolar SNARE pairing, and is required for vesicle docking/fusion. Mol Cell. 2000;6:661–671. doi: 10.1016/s1097-2765(00)00064-2. [DOI] [PubMed] [Google Scholar]
- Scales SJ, Chen YA, Yoo BY, Patel SM, Doung YC, Scheller RH. SNAREs contribute to the specificity of membrane fusion. Neuron. 2000;26:457–464. doi: 10.1016/s0896-6273(00)81177-0. [DOI] [PubMed] [Google Scholar]
- Seals DF, Eitzen G, Margolis N, Wickner WT, Price A. A Ypt/Rab effector complex containing the Sec1 homolog Vps33p is required for homotypic vacuole fusion. Proc Natl Acad Sci USA. 2000;97:9402–9407. doi: 10.1073/pnas.97.17.9402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton RB, Fasshauer D, Jahn R, Brünger AT. Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4 A resolution. Nature. 1998;395:347–353. doi: 10.1038/26412. [DOI] [PubMed] [Google Scholar]
- Tall GG, Hama H, DeWald DB, Horazdovsky BF. The phosphatidylinositol 3-phosphate binding protein Vac1p interacts with a Rab GTPase and a Sec1p homologue to facilitate vesicle-mediated vacuolar protein sorting. Mol Biol Cell. 1999;10:1873–1889. doi: 10.1091/mbc.10.6.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungermann C, Nichols BJ, Pelham HR, Wickner W. A vacuolar v-t-SNARE complex, the predominant form in vivo and on isolated vacuoles, is disassembled and activated for docking and fusion. J Cell Biol. 1998a;140:61–69. doi: 10.1083/jcb.140.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungermann C, Sato K, Wickner W. Defining the functions of trans-SNARE pairs. Nature. 1998b;396:543–548. doi: 10.1038/25069. [DOI] [PubMed] [Google Scholar]
- Ungermann C, von Mollard GF, Jensen ON, Margolis N, Stevens TH, Wickner W. Three v-SNAREs and two t-SNAREs, present in a pentameric cis-SNARE complex on isolated vacuoles, are essential for homotypic fusion. J Cell Biol. 1999;145:1435–1442. doi: 10.1083/jcb.145.7.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungermann C, Price A, Wickner W. A new role for a SNARE protein as a regulator of the Ypt7/Rab-dependent stage of docking. Proc Natl Acad Sci USA. 2000;97:8889–8891. doi: 10.1073/pnas.160269997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veit M, Laage R, Dietrich L, Wang L, Ungermann C. Vac8p release from the SNARE complex and its palmitoylation are coupled and essential for vacuole fusion. EMBO J. 2001;20:3145–3155. doi: 10.1093/emboj/20.12.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhage M, Maia AS, Plomp JJ, Brussaard AB, Heeroma JH, Vermeer H, Toonen RF, Hammer R E, van den Berg T K, Missler M, et al. Synaptic assembly of the brain in the absence of neurotransmitter secretion. Science. 2000;287:864–869. doi: 10.1126/science.287.5454.864. [DOI] [PubMed] [Google Scholar]
- Vida TA, Emr SD. A new vital stain for visualizing vacuolar membrane dynamics and endocytosis in yeast. J Cell Biol. 1995;128:779–792. doi: 10.1083/jcb.128.5.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Mollard GF, Nothwehr SF, Stevens TH. The yeast v-SNARE Vtilp mediates two vesicle transport pathways through interactions with the t-SNAREs Sed5p and Pep12p. J Cell Biol. 1997;137:1511–1524. doi: 10.1083/jcb.137.7.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walch-Solimena C, Blasi J, Edelmann L, Chapman ER, von Mollard GF, Jahn R. The t-SNAREs syntaxin 1 and SNAP-25 are present on organelles that participate in synaptic vesicle recycling. J Cell Biol. 1995;128:637–645. doi: 10.1083/jcb.128.4.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Dulubova I, Rizo J, Südhof TC. Functional analysis of conserved structural elements in yeast syntaxin Vam3p. J Biol Chem. 2001;276:28598–28605. doi: 10.1074/jbc.M101644200. [DOI] [PubMed] [Google Scholar]
- Weber T, Zemelman BV, McNew JA, Westermann B, Gmachl M, Parlati F, Söllner TH, Rothman JE. SNAREpins: minimal machinery for membrane fusion. Cell. 1998;92:759–772. doi: 10.1016/s0092-8674(00)81404-x. [DOI] [PubMed] [Google Scholar]
- Weimbs T, Mostov K, Low SH, Hofmann K. A model for structural similarity between different SNARE complexes based on sequence relationships. Trends Cell Biol. 1998;8:260–262. doi: 10.1016/s0962-8924(98)01285-9. [DOI] [PubMed] [Google Scholar]
- Wickner W, Haas A. Yeast homotypic vacuole fusion: a window on organelle trafficking mechanisms. Annu Rev Biochem. 2000;69:247–275. doi: 10.1146/annurev.biochem.69.1.247. [DOI] [PubMed] [Google Scholar]
- Wu MN, Fergestad T, Lloyd TE, He Y, Broadie K, Bellen HJ. Syntaxin 1A interacts with multiple exocytic proteins to regulate neurotransmitter release in vivo. Neuron. 1999;23:593–605. doi: 10.1016/s0896-6273(00)80811-9. [DOI] [PubMed] [Google Scholar]
- Wurmser AE, Sato TK, Emr SD. New component of the vacuolar class C-Vps complex couples nucleotide exchange on the Ypt7p GTPase to SNARE-dependent docking and fusion. J Cell Biol. 2000;151:551–562. doi: 10.1083/jcb.151.3.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Wickner W. Thioredoxin is required for vacuole inheritance in Saccharomyces cerevisiae. J Cell Biol. 1996;132:787–794. doi: 10.1083/jcb.132.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Sato K, Wickner W. LMA1 binds to vacuoles at Sec18p (NSF), transfers upon ATP hydrolysis to a t-SNARE (Vam3p) complex, and is released during fusion. Cell. 1998;93:1125–1134. doi: 10.1016/s0092-8674(00)81457-9. [DOI] [PubMed] [Google Scholar]
- Yang B, Steegmaier M, Gonzalez LC, Jr, Scheller RH. nSec1 binds a closed conformation of syntaxin1A. J Cell Biol. 2000;148:247–252. doi: 10.1083/jcb.148.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]