Abstract
Background
Lymphangioleiomyomatosis (LAM) is a destructive lung disease of women caused by proliferation of neoplastic-like LAM cells, with mutations in the TSC1/2 tumor suppressor genes. Based on case reports, levels of cancer antigen 125 (CA-125), an ovarian cancer biomarker, can be elevated in patients with LAM. We hypothesized that elevated serum CA-125 levels seen in some patients with LAM were due to LAM, not other malignancies, and might respond to sirolimus treatment.
Methods
Serum CA-125 levels were measured for 241 patients at each visit. Medical records were reviewed for co-morbidities, disease progression, and response to sirolimus treatment. CA-125 expression in LAM cells was determined by using immunohistochemical analysis.
Results
Almost 25% of patients with LAM had at least one elevated serum CA-125 measurement. Higher serum CA-125 levels correlated with lower FEV1, premenopausal status, and pleural effusion in a multivariate model (each P < .001). Serum CA-125 levels decreased following sirolimus treatment (P = .002). CA-125 and α-smooth muscle actin were co-expressed in LAM lung nodules.
Conclusions
Higher serum CA-125 levels were associated with pleural effusions and reduced pulmonary function and were decreased with sirolimus therapy. LAM cells express CA-125. Some elevated serum CA-125 levels may reflect serosal membrane involvement.
Key Words: CA-125, lymphangioleiomyomatosis, mTOR, pleural effusion, tuberous sclerosis
Abbreviations: α-SMA, α-smooth muscle actin; AML, angiomyolipoma; CA-125, cancer antigen 125; Dlco, diffusing capacity for carbon monoxide; LAM, lymphangioleiomyomatosis; LLM, lymphangioleiomyoma; MMP, matrix metalloproteinase; mTOR, mechanistic (mammalian) target of rapamycin; mTORC1, mechanistic (mammalian) target of rapamycin complex 1; MUC16, mucin 16; TSC, tuberous sclerosis complex; VEGF-D, vascular endothelial growth factor D
FOR EDITORIAL COMMENT, SEE PAGE 298
Lymphangioleiomyomatosis (LAM), a multi-system, low-grade neoplasm primarily found in women, is characterized by cystic lung destruction with impairment of pulmonary function, abdominal tumors, and lymphatic abnormalities.1, 2 LAM may be sporadic or associated with tuberous sclerosis complex (TSC). LAM lung lesions include neoplastic smooth muscle-like LAM cells with mutations in TSC1 (hamartin) and/or TSC2 (tuberin) genes. Hamartin and tuberin form a complex that is a negative regulator of the mechanistic target of rapamycin (mTOR).3 mTOR is found in two complexes: mTORC1 and mTORC2. mTORC1 coordinates growth factor signals and nutrient availability affecting cell proliferation, cell size, autophagy, and other critical biological processes. Dysfunction of the TSC1/TSC2 complex results in constitutive activation of mTORC1, resulting in abnormal growth of LAM cells.4 Sirolimus, an immunosuppressive agent, binds to mTOR in the presence of FK506-binding protein 1A, 12 kDa and inhibits the mTORC1 pathway.5 In LAM, sirolimus is effective in stabilizing lung function,6 reducing the size of angiomyolipomas (AMLs),7 and reducing or resolving chylothoraces, chylous infiltration of the lung parenchyma, and lymphangioleiomyomas (LLMs).8, 9
Cancer antigen 125 (CA-125) was identified as overexpressed in ovarian cancer.10 CA-125 is a type I transmembrane protein11 expressed on the epithelial surface lining of multiple organs, including the respiratory tract.12 As a major component of mucus,13 CA-125 protects the surface lining of internal organs from pathogens14, 15 and serves as a biomarker and survival predictor in ovarian cancer.10 A chemotherapeutic response in ovarian cancer is associated with a decrease in serum CA-125 levels.16 Abnormally high expression of serum CA-125 is also seen in breast,17 lung,18 uterine,19 and gastric cancer.20
We have noticed high levels of serum CA-125 in patients with LAM during standard clinical testing. Because CA-125 has been used as a marker of ovarian cancer and because the patient study population was predominantly female, high levels of serum CA-125 may be a source of concern to patients with LAM and their physicians. Serum CA-125 levels may also be of clinical utility for monitoring LAM diagnosis, progression, or treatment. We hypothesized that serum CA-125 levels might be elevated and associated with disease progression in patients with LAM and that sirolimus treatment could decrease CA-125 levels.
Patients and Methods
Patient Cohort and Statistical Analysis
Research subjects were enrolled in the LAM protocol (95-H-0186; approved by the institutional review board of the National Heart, Lung, and Blood Institute) and provided written informed consent. Diagnoses of LAM in 241 patients were confirmed by histopathologic findings or the presence of cystic disease by CT scan, plus TSC, AMLs, high vascular endothelial growth factor D (VEGF-D) levels, and/or LLMs or chylous effusions. Patients were excluded if their medical records revealed other malignancies or ovarian cysts. CA-125 serum levels were measured in a Clinical Laboratory Improvement Amendments–approved laboratory, using the Abbott AxSYM automated serological analyzer or Immulite analyzer (Siemens); levels > 34 U/mL were considered elevated. Values determined by Immulite were converted to AxSYM-compatible values. Patient records were reviewed for clinical phenotypes. Pulmonary function testing was performed as described.21 VEGF-D testing was performed at the Translational Core Laboratories, Cincinnati Children’s Hospital.
The distribution of CA-125 values was skewed to the right (with some very high measurements); to minimize the effect of these high numbers, the CA-125 data were log-transformed. All measurements from multiple visits per subject were considered. Mixed effects models were used to identify factors associated with CA-125 levels. Variables identified in a univariate analysis as significantly associated with CA-125 at P < .10 were considered for inclusion in the multivariate model. Variables included age, time of follow-up from initial visit, FEV1, diffusing capacity for carbon monoxide (Dlco), AML, menopausal status, TSC diagnosis, lymphatic involvement (ie, ascites, pleural effusions, adenopathy, chylous effusions, and/or LLMs), and pleural effusions. Variables with P values ≤ .05 remained in the model. SAS version 9.3 (SAS Institute, Inc) was used for all analyses.
Immunostaining of LAM Lung Tissue
Formalin-fixed, paraffin-embedded lung tissue sections were immunostained with the monoclonal antibody HMB45 (1:50; Dako) and/or anti-CA-125 antibodies (1:200; Santa Cruz Biotechnology) using an alkaline phosphatase procedure (Vectastain ABC-AP; Vector Laboratories) or hydrogen peroxidase/diaminobenzidine kit (Spring Bioscience), following manufacturer’s directions.
Frozen or formalin-fixed, paraffin-embedded LAM lung tissue sections were immunostained by using an indirect fluorescent immunostaining technique as described previously.22 Anti-CA-125 (1:50) and anti-α-smooth muscle actin (α-SMA) (1:100; Abcam) antibodies were paired with Texas Red anti-mouse IgG (1:200; Vector Laboratories) or sheep polyclonal anti-rabbit IgG fluorescein isothiocyanate (1:200; Abcam Inc), respectively. Sections were stained with 4′,6-diamidino-2-phenylindole. A Zeiss LSM 510 META laser scanning confocal microscope equipped with 405 diode, argon, and helium-neon laser sources, using a Plan Neofluar 40X oil objective, was used to inspect the lung sections.
Results
Serum CA-125 and Clinical Correlations
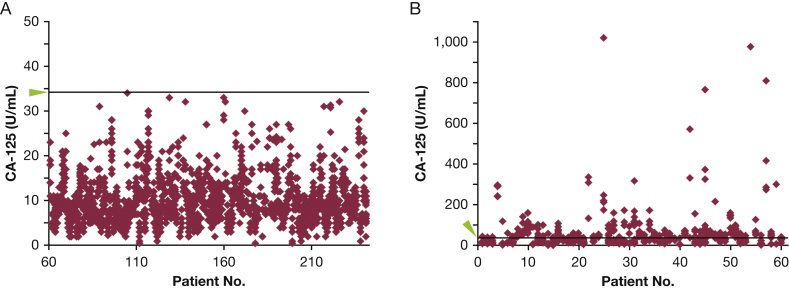
We examined levels of CA-125 in serum from patients with LAM. Because serum CA-125 levels are known to be elevated in cancers, patients who had malignancies such as breast,17 lung,18 uterine,19 ovarian,10 and gastric cancer were excluded from the study cohort.20 Also excluded were measurements from patients while taking sirolimus (so as not to confound the initial study with effects of the drug). CA-125 values were in the normal range (≤ 34 U/mL) for 181 of 241 patients (75.1%) for all visits (Fig 1A). Sixty patients (24.9%) had at least one above-normal serum CA-125 measurement (>34 U/mL) (Fig 1B), and 17 of these 60 patients had above-normal serum CA-125 levels at every visit. Univariate analysis, examining CA-125 levels as a continuous variable, revealed a correlation between higher serum CA-125 levels and decreased pulmonary function (percent predicted FEV1: P < .001; Dlco: P < .001). Patients with LAM and higher serum CA-125 levels were younger and premenopausal (P = .044 and P = .001, respectively). Some associations were maintained in the multivariate analysis (FEV1: P < .001; menopause: P < .001) (Fig 2A, Table 1). Percent predicted Dlco was also a significant predictor of CA-125 levels in the multivariate model (P = .022), but the presence of Dlco as a co-predictor decreased the effect of FEV1 (P = .005), and the model performance did not improve with its addition.
Figure 1.
A, B, Distribution of serum CA-125 levels for (A) patients (numbers 61-241) who never had an above-normal level of CA-125 (> 34 U/mL) or (B) for patients (numbers 1-60) who had at least one above-normal level of CA-125. Each diamond represents the level of serum CA-125 at a different visit. The green arrowhead and line mark the threshold level of 34 U/mL. CA-125 = cancer antigen 125.
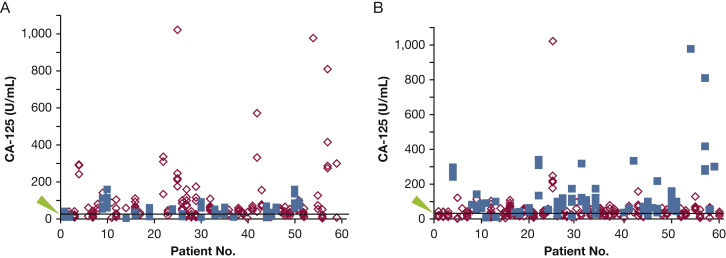
Figure 2.
A, B, Serum CA-125 levels are associated with (A) menopausal status (P < .001) and (B) pleural effusions (P < .001). Each diamond or square represents the level of serum CA-125 at a visit. A, Blue squares are levels in postmenopausal patients, and red diamonds are levels in premenopausal patients. B, Blue squares are levels in patients with pleural effusions, and red diamonds are levels in patients without pleural effusions. The arrowhead and line mark the threshold level of 34 U/mL. See Figure 1 legend for expansion of abbreviation.
Table 1.
Clinical Characteristics of Patients With LAM, From Visits When They Were Not Taking Sirolimus, Divided According to Categories of Serum CA-125 Levels
| Characteristic | All Normal CA-125 Levels | At Least One Above-Normal CA-125 Level | At Least Two Above-Normal CA-125 Levels | All Above-Normal CA-125 Levels |
|---|---|---|---|---|
| Visits | 8.6 ± 0.4 (1-22) [181] | 9.0 ± 0.6 (1-20) [60] | 8.9 ± 0.8 (3-20) [40] | 6.5 ± 1.1 (1-17) [17] |
| Initial age, y | 45.3 ± 0.7 (21-76) [181] | 41.0 ± 1.1 (25-63) [60] | 42.8 ± 1.2 (25-63) [40] | 39.6 ± 2.0 (25-57) [17] |
| Initial FEV1 (percent predicted) | 80.2 ± 1.9 (20-133) [175] | 70.9 ± 3.5 (19-116) [56] | 67.4 ± 4.2 (19-115) [36] | 68.1 ± 5.8 (35-115) [15] |
| Initial Dlco (percent predicted) | 77.7 ± 1.9 (25-138) [174] | 64.8 ± 3.3 (22-116) [56] | 61.7 ± 4.1 (23-116) [36] | 59.4 ± 6.0 (30-107) [15] |
| Final age, y | 51.4 ± 0.7 (24-79) [181] | 46.5 ± 1.3 (26-71) [60] | 48.2 ± 1.5 (26-71) [40] | 43.3 ± 2.5 (26-62) [17] |
| Final FEV1 (percent predicted) | 69.9 ± 2.2 (13-134) [161] | 62.5 ± 3.5 (22-134) [57] | 64.1 ± 4.1 (22-109) [37] | 63.3 ± 6.2 (22-109) [17] |
| Final Dlco (percent predicted) | 66.1 ± 2.1 (6-120) [152] | 53.8 ± 3.3 (21-121) [56] | 54.4 ± 3.9 (24-111) [37] | 50.7 ± 5.1 (26-101) [17] |
| Lymphatic involvementa,b | ||||
| No | 111 (61.3) | 16 (26.7) | 7 (17.5) | 1 (5.9) |
| Yes | 70 (38.7) | 44 (73.3) | 33 (82.5) | 16 (94.1) |
| Pleural effusionb,c | ||||
| No | 171 (95.5)c | 30 (50.0) | 16 (40.0) | 2 (11.8) |
| Yes | 8 (4.5) | 30 (50.0) | 24 (60.0) | 15 (88.2) |
| TSC | ||||
| No | 146 (80.7) | 47 (78.3) | 34 (85.0) | 14 (82.4) |
| Yes | 35 (19.3) | 13 (21.7) | 6 (15.0) | 3 (17.6) |
| AMLb | ||||
| No | 90 (49.7) | 30 (50.0) | 22 (55.0) | 9 (52.9) |
| Yes | 91 (50.3) | 30 (50.0) | 18 (45.0) | 8 (47.1) |
Data are presented as average ± SEM (range) [No.] or No. (%). In a univariate analysis, with cancer antigen 125 (CA-125) as a continuous variable, higher serum CA-125 levels were significantly associated with lower percent predicted FEV1 (P < .001) and diffusing capacity for carbon monoxide (Dlco) (P < .001), younger age (P < .044), premenopause (P = .001), lymphatic involvement (P < .001), and pleural effusions (P < .001). Tuberous sclerosis complex (TSC) and the presence of angiomyolipomas (AMLs) were not significantly associated with serum CA-125 levels. After multivariate analysis considering the variables associated with CA-125 levels, higher CA-125 levels were significantly associated with lower percent predicted FEV1 (P < .001), premenopause (P < .001), and a history of pleural effusion (P < .001). Percent predicted Dlco was also a significant predictor of CA-125 level in the multivariate model (P = .022), but its presence in the model reduced the effect of FEV1 (P = .005). Correlations were found across the whole study population; patients in this table were grouped into categories for illustrative purposes only.
Lymphatic involvement includes ascites, pleural effusions, adenopathy, chylous effusions, and/or lymphangioleiomyomas.
Data indicate a history of lymphatic involvement, pleural effusions, and/or AML.
Data on pleural effusion history are unavailable for two patients.
Univariate analysis determined that the presence of TSC in 48 patients with LAM had no effect on serum CA-125 level (Table 1). The presence of AMLs was also not associated with CA-125 serum levels. Univariate analysis revealed that higher serum levels of CA-125 were associated with lymphatic involvement, as defined by ascites, pleural effusions, adenopathy, chylous effusions, and/or LLMs, in patients with LAM (P < .001) and with pleural effusions alone (P < .001); pleural effusions remained a predictive factor of CA-125 levels in the multivariate analysis (P < .001) (Fig 2B). Of the 17 patients with above-normal serum CA-125 levels at every visit, 15 (88.2%) had a history of pleural effusions. Chest radiographs or CT scans were available for 77 of the visits by these 15 patients, and 59 (76.6%) of the scans revealed the presence of pleural effusions at the same visit the above-normal serum CA-125 levels were detected.
Serum CA-125 Levels in Therapy
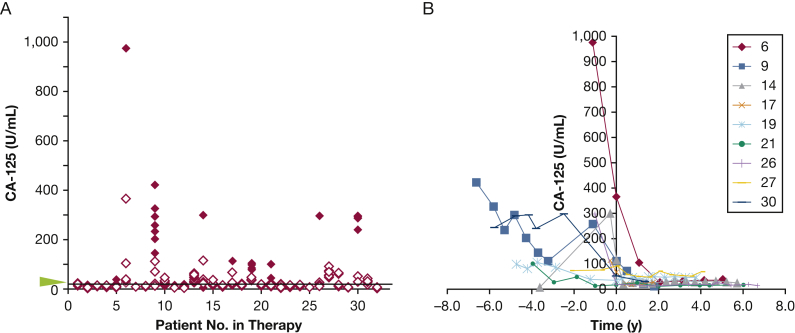
Thirty-two patients with LAM were undergoing sirolimus treatment. Serum CA-125 levels were measured in patients who were taking sirolimus for at least two consecutive visits and with at least one visit prior to initiation of sirolimus therapy. Patients were followed an average of 4.4 years prior to sirolimus treatment and an average of 3.4 years (range, 6 months-9 years) on treatment. Data showed that treatment with sirolimus was associated with decreased serum CA-125 levels (P = .002) (Fig 3). Of the 32 patients taking sirolimus, 17 never had above-normal levels of CA-125, either prior to or following treatment. Nine patients had many visits with CA-125 levels > 65 U/mL; CA-125 levels in these patients decreased with sirolimus treatment but did not necessarily fall to < 34 U/mL. Interestingly, all nine patients had chylous pleural effusions that diminished or resolved with sirolimus treatment.
Figure 3.
A, B, Levels of serum CA-125 in patients taking sirolimus. A, Solid red diamonds represent levels of CA-125 prior to sirolimus treatment, and empty red diamonds represent levels following sirolimus treatment. Although a decrease in serum CA-125 levels is associated with sirolimus treatment (P = .002), of the 32 patients taking sirolimus, 17 had normal serum CA-125 levels both prior to and following treatment. Nine patients had many visits with serum CA-125 levels > 65 U/mL prior to and/or following treatment. The arrowhead and line mark the threshold level of 34 U/mL. B, Serum CA-125 levels in these nine patients over time prior to and following the start of sirolimus treatment. See Figure 1 legend for expansion of abbreviation.
CA-125 and VEGF-D in Therapy
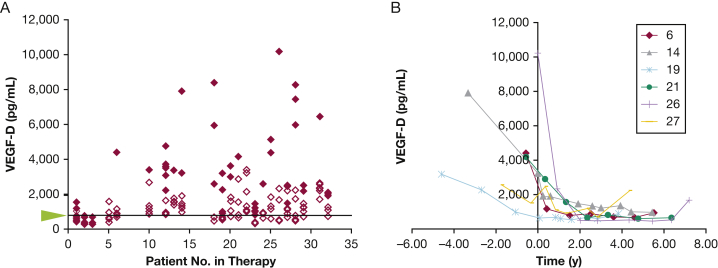
VEGF-D is a biomarker used for the diagnosis of LAM; serum levels > 800 pg/mL, along with cystic changes in the lung, are diagnostic for LAM.23 Serum VEGF-D is also a marker of response to sirolimus.24 Of the 32 aforementioned patients taking sirolimus, serum VEGF-D levels were measured in 23 (Fig 4). Two patients never had VEGF-D levels greater than the diagnostic threshold of 800 pg/mL, and 12 never had CA-125 levels greater than the cutoff of 34 U/mL. Figure 4 also shows levels of VEGF-D over time with sirolimus treatment for six of the nine patients with pleural effusions and higher levels of CA-125. Serum VEGF-D levels seem to be a more informative marker than serum CA-125 levels.
Figure 4.
A, B, Levels of serum VEGF-D in patients taking sirolimus. A, Solid red diamonds represent levels of VEGF-D prior to sirolimus treatment, and empty red diamonds represent levels following sirolimus treatment. The arrowhead and line mark the threshold level of 800 pg/mL. B, Serum VEGF-D levels in patients over time prior to and following the start of sirolimus treatment. VEGF-D = vascular endothelial growth factor D.
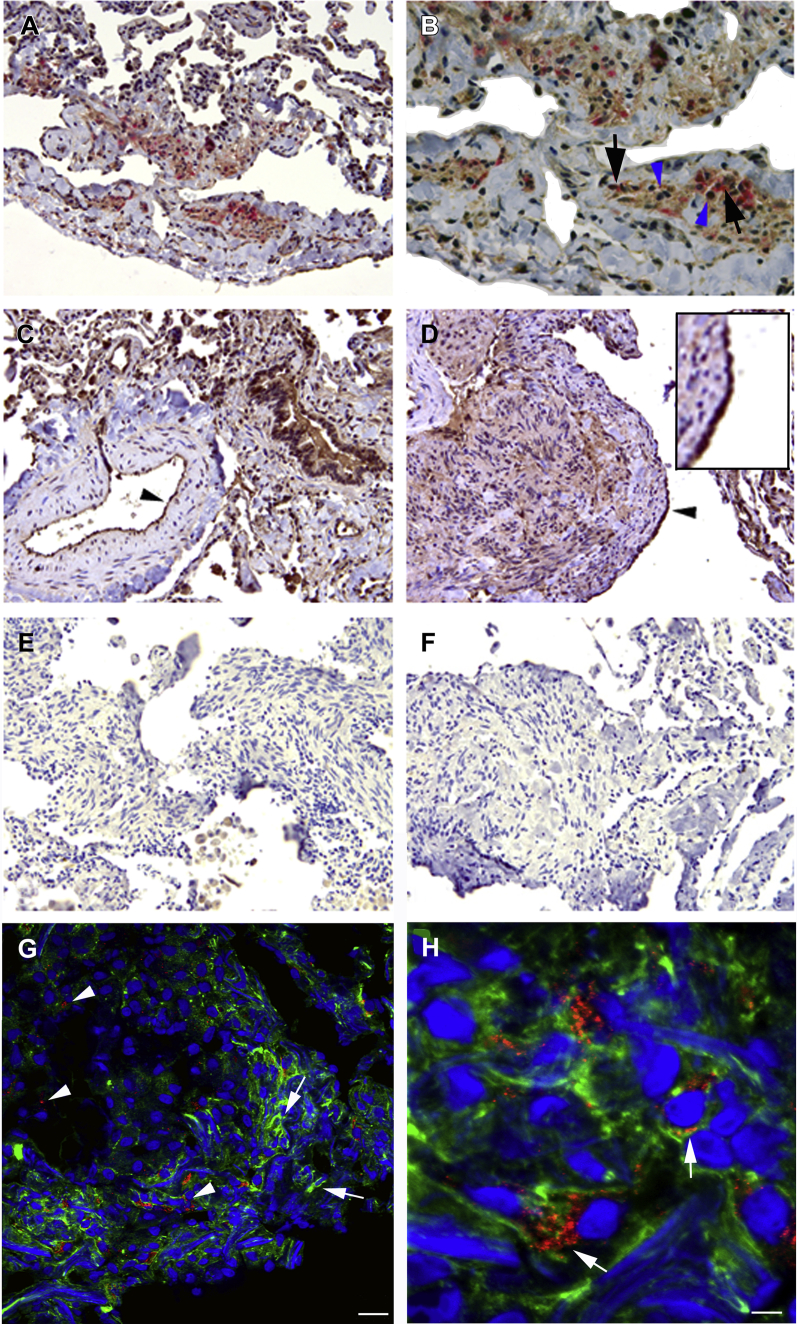
CA-125 Expression in LAM Nodules
Immunohistochemical analysis of LAM lung tissue showed CA-125 within the LAM lung nodule adjacent to cells reactive to HMB45, a LAM cell marker25 (Figs 5A, 5B), and in the bronchial epithelial cells in “normal” tissue areas of LAM lung (Fig 5C). CA-125 expression was evident in hyperplastic type II pneumocytes (Fig 5D) in contrast to peritumoral lung.26 Figures 5E and 5F show negative controls. The close association of CA-125 expression with HMB45-positive cells prompted us to investigate the existence of LAM cells reactive to both markers.
Figure 5.
A-H, Immunoreactivity of anti-CA-125 antibodies in lymphangioleiomyomatosis (LAM) lung tissue sections. A, Anti-CA-125 antibodies (brown) and HMB45 (red) reactivity in cells within a LAM lung nodule. B, Higher magnification of graphic A showed cells within the LAM nodule reactive to anti-CA-125 antibodies (blue arrowheads) adjacent to cells reactive to HMB45 (black arrows). C, Bronchial cells reacted with anti-CA-125 antibodies. Cells lining an artery reacted positively to anti-CA-125 antibodies in normal areas of LAM lung (black arrowhead). D, Type II pneumocytes, lining the surface of the LAM lung nodule, were reactive to anti-CA-125 antibodies (arrowhead and insert). Negative controls (omitting primary antibody) were consistently negative. E, Rabbit, hydrogen peroxidase/diaminobenzidine; F, mouse, alkaline phosphatase/vector red. Magnification: A, C, and D: 20x; B: 40x; E and F: 10x. G, Indirect fluorescent immunochemistry illustrated abundant and diffuse expression of alpha-smooth muscle actin (green, illustrated with arrows), and moderate reactivity with anti-CA-125 antibodies (red, arrowheads) in cells located within a LAM lung nodule. H, In a higher magnification of graphic G, cells react to both anti-CA-125 and anti-a-smooth muscle actin antibodies (merge yellow-orange; arrows). 20-µm sections. See Figure 1 legend for expansion of abbreviation.
For fluorescence microscopy, we chose anti-α-SMA antibodies to identify LAM lung nodules. Although HMB45 reacts mainly with the epithelioid cells of the LAM nodule, α-SMA is present in both epithelioid and spindle-shaped LAM cells.27 Confocal analysis revealed cells in the LAM lung nodule that expressed both CA-125 and α-SMA, although the proteins appear to have different intracellular distributions (Figs 5G, 5H). CA-125 appears concentrated in a perinuclear region, and α-SMA is a more diffuse protein. Therefore, LAM cells express CA-125.
Discussion
We found that cells in the LAM lung nodule express CA-125 and that serum levels of CA-125 are above normal in approximately 25% of patients with LAM. CA-125 was identified as an antigen recognized by an antibody (OC125) raised against an ovarian cancer cell line.10 This antigen is part of the mucin 16 (MUC16), a large transmembrane glycoprotein expressed by normal epithelial cells (reviewed by Felder et al28). MUC16 can be released from the cell surface by cleavage with neutrophil elastase, matrix metalloproteinase (MMP)-7, and MMP-9. All of these proteases may be elevated in LAM and/or present in the LAM lung nodule,29, 30, 31 and this scenario may contribute to high serum CA-125 levels. MUC16, due to its large size, may be able to prevent interactions between tumor cells expressing high levels of MUC16 and natural killer cells that would otherwise lyse the tumor cells.28 MUC16 also binds to two proteins known to promote tumor metastasis, mesothelin and galectin-1. In ovarian cancer, mesothelin binding to MUC16 mediates metastasis to the peritoneum by initiating ovarian tumor cell attachment to the mesothelial epithelium.32 In pancreatic cancer cells, co-expression of MUC16 and mesothelin upregulates MMP-7, promoting cell motility and invasion.33 MUC16 binding to galectin-1 facilitates the export of galectin-1 to the cell surface,34 where it mediates migration and invasion through hypoxia-inducible factor 1α-mTOR signaling in renal cell carcinoma cell lines.35 Thus, CA-125 could play a role in LAM cell dissemination.
Ninety-nine percent of healthy volunteers have serum CA-125 levels < 35 U/mL. Although 5% of patients with nonmalignant conditions and 28% with nonovarian malignancies have above-normal serum CA-125 levels, only 50% of patients with early-stage ovarian cancer have high levels of serum CA-125.36 Therefore, CA-125 has both poor specificity and sensitivity for detecting ovarian cancer. CA-125 may be more useful as a biomarker for ovarian cancer prognosis and for monitoring response to therapy.
In the present study, we found that almost 25% of patients with LAM had at least one above-normal serum CA-125 level over multiple visits (Fig 1). Seventeen patients had above-normal CA-125 levels at all visits, with 15 having a history of pleural effusions and demonstration of pleural effusions according to scans in 76.6% of these visits. Similarly, nine patients receiving sirolimus treatment had pleural effusions that diminished or resolved with treatment, along with decreases in serum CA-125 levels. Serum CA-125 levels were associated with FEV1, menopause status, and pleural effusions in a multivariate analysis. However, the CA-125 association with FEV1 was not a causative one. In an alternative analysis in which FEV1 was considered as an outcome, pleural effusions were a significant predictor of FEV1 (P = .036; adjusted for initial FEV1 value), whereas CA-125 levels were not (P = .165; adjusted for initial FEV1).
CA-125 is detected in pleura, peritoneum, and pericardium epithelia. A study of patients with chronic renal failure divided the subjects into those with and those without fluid in serosal cavities. Those patients with serosal fluid had markedly higher serum levels of CA-125 than those without serosal fluid, leading to the conclusion that serum CA-125 could be a biomarker for irritation of the pericardium, peritoneum, or pleura.37 This conclusion was also reached by studies examining patients with ovarian cancer with pleural effusions38 or ascites,39 a patient with lymphoma and ascites,40 a patient with sarcoidosis and ascites,41 and patients with liver cirrhosis39, 42 or hepatocellular carcinoma with ascites.39 It is likely that the elevated serum CA-125 levels seen in the patients with LAM with pleural effusions are attributable to irritation of the pleura itself. Because sirolimus has been shown to reduce or resolve pleural effusions,8, 43 a decrease in serum CA-125 levels for patients taking sirolimus may be due to its effect on the pleura and pleural effusions. Although the involvement of the pleura may account for some of the high levels of CA-125, two of the 17 patients with high serum CA-125 levels at every visit had no evidence of pleural effusions, and of the 60 patients with at least one elevated CA-125 level, 30 (50.0%) had no history of pleural effusions. In these cases, the LAM nodule may be the source of CA-125.
Serum VEGF-D is the best biomarker to date for diagnosis of LAM and to follow LAM progression and treatment.24 Interestingly, VEGF-D levels associate with lymphatic involvement (LLMs and adenopathy)44 similar to the association of CA-125 with lymphatic involvement. In the present study, we found that although CA-125 levels were associated with disease progression and treatment, the percentage of patients with elevated CA-125 levels was probably not large enough to be a more useful biomarker than VEGF-D. It is important to note, however, that high levels of CA-125 can be detected in patients with LAM, especially those with pleural effusions, and that these levels do not necessarily indicate the presence of other cancers such as ovarian cancer.
Conclusions
We explored the clinical utility of CA-125 as a biomarker in LAM and examined the molecule in LAM tissues and disease. We found high levels of serum CA-125 in almost 25% of patients with LAM. High levels of CA-125 were associated with worse percent predicted FEV1 and the presence of pleural effusions. Levels of serum CA-125 decrease with sirolimus treatment, perhaps reflecting both a direct effect on the LAM nodule, which expresses CA-125, and an indirect effect on pleural effusions.
Acknowledgments
Author contributions: J. M. serves as the guarantor of the paper and takes responsibility for the integrity of the work as a whole, from inception to published article. J. M., C. G., G. P-R., and W. S. provided the conceptual framework and drafted the manuscript. C. G. and G. P-R. performed experiments and, with W. S., analyzed the data. M. H. recruited patients, and M. H. and P. J.-W. oversaw sample collection. B. G. contributed samples. M. S. performed the statistical analysis. All authors reviewed the final version of the manuscript and gave their approval.
Financial/nonfinancial disclosures: None declared.
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Other contributions: The authors thank Martha Vaughan, MD (Cardiovascular and Pulmonary Branch, National Heart, Lung, and Blood Institute [NHLBI], National Institutes for Health [NIH]) for helpful discussions and critical review of the manuscript; Christian Combs, PhD, and Daniela Malide, MD, PhD, of the Light Microscopy Core Facility (NHLBI, NIH); and Zu-Xi Yu, MD, PhD, of the Pathology Core Facility (NHLBI, NIH) for help with immunostaining. They also appreciate the assistance of the LAM Foundation and the Tuberous Sclerosis Alliance in recruiting patients for the studies.
Footnotes
FUNDING/SUPPORT: The study was supported by the Intramural Research Program, the National Institutes of Health, the National Heart, Lung, and Blood Institute, and the National Human Genome Research Institute.
References
- 1.Taveira-DaSilva A.M., Moss J. Clinical features, epidemiology, and therapy of lymphangioleiomyomatosis. Clin Epidemiol. 2015;7:249–257. doi: 10.2147/CLEP.S50780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Glasgow C.G., Steagall W.K., Taveira-Dasilva A. Lymphangioleiomyomatosis (LAM): molecular insights lead to targeted therapies. Respir Med. 2010;104(suppl 1):S45–S58. doi: 10.1016/j.rmed.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Laplante M., Sabatini D.M. mTOR signaling in growth control and disease. Cell. 2012;149(2):274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goncharova E.A., Goncharov D.A., Spaits M. Abnormal growth of smooth muscle-like cells in lymphangioleiomyomatosis: role for tumor suppressor TSC2. Am J Respir Cell Mol Biol. 2006;34(5):561–572. doi: 10.1165/rcmb.2005-0300OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benjamin D., Colombi M., Moroni C., Hall M.N. Rapamycin passes the torch: a new generation of mTOR inhibitors. Nat Rev Drug Discov. 2011;10(11):868–880. doi: 10.1038/nrd3531. [DOI] [PubMed] [Google Scholar]
- 6.McCormack F.X., Inoue Y., Moss J. Efficacy and safety of sirolimus in lymphangioleiomyomatosis. N Engl J Med. 2011;364(17):1595–1606. doi: 10.1056/NEJMoa1100391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bissler J.J., McCormack F.X., Young L.R. Sirolimus for angiomyolipoma in tuberous sclerosis complex or lymphangioleiomyomatosis. N Engl J Med. 2008;358(2):140–151. doi: 10.1056/NEJMoa063564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taveira-DaSilva A.M., Hathaway O., Stylianou M., Moss J. Changes in lung function and chylous effusions in patients with lymphangioleiomyomatosis treated with sirolimus. Ann Intern Med. 2011;154(12):797–805. doi: 10.1059/0003-4819-154-12-201106210-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moua T., Olson E.J., St. Jean H.C., Ryu J.H. Resolution of chylous pulmonary congestion and respiratory failure in LAM with sirolimus therapy. Am J Respir Crit Care Med. 2012;186(4):389–390. doi: 10.1164/ajrccm.186.4.389. [DOI] [PubMed] [Google Scholar]
- 10.Bast R.C., Jr., Feeney M., Lazarus H., Nadler L.M., Colvin R.B., Knapp R.C. Reactivity of a monoclonal antibody with human ovarian carcinoma. J Clin Invest. 1981;68(5):1331–1337. doi: 10.1172/JCI110380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haridas D., Ponnusamy M.P., Chugh S., Lakshmanan I., Seshacharyulu P., Batra S.K. MUC16: molecular analysis and its functional implications in benign and malignant conditions. FASEB J. 2014;28(10):4183–4199. doi: 10.1096/fj.14-257352. [DOI] [PubMed] [Google Scholar]
- 12.Gipson I.K., Blalock T., Tisdale A. MUC16 is lost from the uterodome (pinopode) surface of the receptive human endometrium: in vitro evidence that MUC16 is a barrier to trophoblast adherence. Biol Reprod. 2008;78(1):134–142. doi: 10.1095/biolreprod.106.058347. [DOI] [PubMed] [Google Scholar]
- 13.Rachagani S., Torres M.P., Moniaux N., Batra S.K. Current status of mucins in the diagnosis and therapy of cancer. Biofactors. 2009;35(6):509–527. doi: 10.1002/biof.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blalock T.D., Spurr-Michaud S.J., Tisdale A.S. Functions of MUC16 in corneal epithelial cells. Invest Ophthalmol Vis Sci. 2007;48(10):4509–4518. doi: 10.1167/iovs.07-0430. [DOI] [PubMed] [Google Scholar]
- 15.Kesimer M., Scull M., Brighton B. Characterization of exosome-like vesicles released from human tracheobronchial ciliated epithelium: a possible role in innate defense. FASEB J. 2009;23(6):1858–1868. doi: 10.1096/fj.08-119131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gadducci A., Cosio S., Fanucchi A., Negri S., Cristofani R., Genazzani A.R. The predictive and prognostic value of serum CA 125 half-life during paclitaxel/platinum-based chemotherapy in patients with advanced ovarian carcinoma. Gynecol Oncol. 2004;93(1):131–136. doi: 10.1016/j.ygyno.2003.12.043. [DOI] [PubMed] [Google Scholar]
- 17.Zhang S.J., Hu Y., Qian H.L. Expression and significance of ER, PR, VEGF, CA15-3, CA125 and CEA in judging the prognosis of breast cancer. Asian Pac J Cancer Prev. 2013;14(6):3937–3940. doi: 10.7314/apjcp.2013.14.6.3937. [DOI] [PubMed] [Google Scholar]
- 18.Cedres S., Nunez I., Longo M. Serum tumor markers CEA, CYFRA21-1, and CA-125 are associated with worse prognosis in advanced non-small-cell lung cancer (NSCLC) Clin Lung Cancer. 2011;12(3):172–179. doi: 10.1016/j.cllc.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 19.Patsner B., Yim G.W. Predictive value of preoperative serum CA-125 levels in patients with uterine cancer: the Asian experience 2000 to 2012. Obstet Gynecol Sci. 2013;56(5):281–288. doi: 10.5468/ogs.2013.56.5.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Polat E., Duman U., Duman M. Preoperative serum tumor marker levels in gastric cancer. Pak J Med Sci. 2014;30(1):145–149. doi: 10.12669/pjms.301.3968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taveira-DaSilva A.M., Stylianou M.P., Hedin C.J., Hathaway O., Moss J. Decline in lung function in patients with lymphangioleiomyomatosis treated with or without progesterone. Chest. 2004;126(6):1867–1874. doi: 10.1378/chest.126.6.1867. [DOI] [PubMed] [Google Scholar]
- 22.Steagall W.K., Pacheco-Rodriguez G., Glasgow C.G. Osteoprotegerin contributes to the metastatic potential of cells with dysfunctional TSC2 tumor suppressor gene. Am J Pathol. 2013;183(3):938–950. doi: 10.1016/j.ajpath.2013.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Young L.R., VanDyke R., Gulleman P.M. Serum vascular endothelial growth factor-D prospectively distinguishes lymphangioleiomyomatosis from other diseases. Chest. 2010;138(3):674–681. doi: 10.1378/chest.10-0573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Young L.R., Lee H.S., Inoue Y. Serum VEGF-D concentration as a biomarker of lymphangioleiomyomatosis severity and treatment response: a prospective analysis of the Multicenter International Lymphangioleiomyomatosis Efficacy of Sirolimus (MILES) trial. Lancet Respir Med. 2013;1(6):445–452. doi: 10.1016/S2213-2600(13)70090-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matsumoto Y., Horiba K., Usuki J., Chu S.C., Ferrans V.J., Moss J. Markers of cell proliferation and expression of melanosomal antigen in lymphangioleiomyomatosis. Am J Respir Cell Mol Biol. 1999;21(3):327–336. doi: 10.1165/ajrcmb.21.3.3693. [DOI] [PubMed] [Google Scholar]
- 26.Nouwen E.J., Pollet D.E., Eerdekens M.W., Hendrix P.G., Briers T.W., De Broe M.E. Immunohistochemical localization of placental alkaline phosphatase, carcinoembryonic antigen, and cancer antigen 125 in normal and neoplastic human lung. Cancer Res. 1986;46(2):866–876. [PubMed] [Google Scholar]
- 27.Valencia J.C., Steagall W.K., Zhang Y. Antibody αPEP13h reacts with lymphangioleiomyomatosis cells in lung nodules. Chest. 2015;147(3):771–777. doi: 10.1378/chest.14-0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Felder M., Kapur A., Gonzalez-Bosquet J. MUC16 (CA125): tumor biomarker to cancer therapy, a work in progress. Molecular Cancer. 2014;13:129–143. doi: 10.1186/1476-4598-13-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prizant H., Taya M., Lerman I. Estrogen maintains myometrial tumors in a lymphangioleiomyomatosis model. Endocr Relat Cancer. 2016;23(4):265–280. doi: 10.1530/ERC-15-0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barnes E.A., Kenerson H.L., Mak B.C., Yeung R.S. The loss of tuberin promotes cell invasion through the β-catenin pathway. Am J Respir Cell Mol Biol. 2010;43(5):617–627. doi: 10.1165/rcmb.2008-0335OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hayashi T., Fleming M.V., Stetler-Stevenson W.G. Immunohistochemical study of matrix metalloproteinases (MMPs) and their tissue inhibitors (TIMPs) in pulmonary lymphangioleiomyomatosis (LAM) Hum Pathol. 1997;28(9):1071–1078. doi: 10.1016/s0046-8177(97)90061-7. [DOI] [PubMed] [Google Scholar]
- 32.Rump A., Morikawa Y., Tanaka M. Binding of ovarian cancer antigen CA125/MUC16 to mesothelin mediates cell adhesion. J Biol Chem. 2004;279(10):9190–9198. doi: 10.1074/jbc.M312372200. [DOI] [PubMed] [Google Scholar]
- 33.Chen S.H., Hung W.C., Wang P., Paul C., Konstantopoulos K. Mesothelin binding to CA125/MUC16 promotes pancreatic cancer cell motility and invasion via MMP-7 activation. Sci Rep. 2013;3:1870. doi: 10.1038/srep01870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seelenmeyer C., Wegehingel S., Lechner J., Nickel W. The cancer antigen CA125 represents a novel counter receptor for galectin-1. J Cell Sci. 2003;116(pt 7):1305–1318. doi: 10.1242/jcs.00312. [DOI] [PubMed] [Google Scholar]
- 35.White N.M., Masui O., Newsted D. Galectin-1 has potential prognostic significance and is implicated in clear cell renal cell carcinoma progression through the HIF/mTOR signaling axis. Br J Cancer. 2014;110(5):1250–1259. doi: 10.1038/bjc.2013.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bottoni P., Scatena R. The role of CA 125 as tumor marker: biochemical and clinical aspects. Adv Exp Med Biol. 2015;867:229–244. doi: 10.1007/978-94-017-7215-0_14. [DOI] [PubMed] [Google Scholar]
- 37.Sevinc A., Buyukberber S., Ramazan S., Kiroglu Y., Turk H.M., Ates M. Elevated serum CA-125 levels in hemodialysis patients with peritoneal, pleural, or pericardial fluids. Gynecol Oncol. 2000;77(2):254–257. doi: 10.1006/gyno.2000.5776. [DOI] [PubMed] [Google Scholar]
- 38.Lindgren J., Kuusela P., Hellstrom P.E., Pettersson T., Klockars M. The ovarian cancer associated antigen CA 125 in patients with pleural effusions. Eur J Cancer Clin Oncol. 1988;24(4):737–739. doi: 10.1016/0277-5379(88)90308-2. [DOI] [PubMed] [Google Scholar]
- 39.Bergmann J.F., Bidart J.M., George M., Beaugrand M., Levy V.G., Bohuon C. Elevation of CA 125 in patients with benign and malignant ascites. Cancer. 1987;59(2):213–217. doi: 10.1002/1097-0142(19870115)59:2<213::aid-cncr2820590206>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 40.Hazarika N., Dhabhar B., Saikia T.K. Highly elevated serum CA 125 in a lady with ascites and retroperitoneal mass—a diagnostic dilemma. JAPI. 2008;56:47–48. [PubMed] [Google Scholar]
- 41.Mak G.Z., Welsh J.H., Chang E., Lathi R.B., Poythress E.L. Chylous ascites caused by sarcoidosis. Hospital Physician. 2002;38(1) 27-32,36. [Google Scholar]
- 42.Zuckerman E., Lanir A., Sabo E. Cancer antigen 125: a sensitive marker of ascites in patients with liver cirrhosis. Am J Gastroenterol. 1999;94(6):1613–1618. doi: 10.1111/j.1572-0241.1999.01152.x. [DOI] [PubMed] [Google Scholar]
- 43.Ando K., Kurihara M., Kataoka H. The efficacy and safety of low-dose sirolimus for treatment of lymphangioleiomyomatosis. Respir Investig. 2013;51(3):175–183. doi: 10.1016/j.resinv.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 44.Glasgow C.G., Avila N.A., Lin J.P., Stylianou M.P., Moss J. Serum vascular endothelial growth factor-D levels in patients with lymphangioleiomyomatosis reflect lymphatic involvement. Chest. 2009;135(5):1293–1300. doi: 10.1378/chest.08-1160. [DOI] [PMC free article] [PubMed] [Google Scholar]