Abstract
Background
The treatment of chronic hypersensitivity pneumonitis (cHP) often includes systemic oral corticosteroids, but the optimal pharmacologic management remains unclear. The morbidity associated with prednisone has motivated the search for alternative therapies. We aimed to determine the effect of treatment with mycophenolate mofetil (MMF) or azathioprine (AZA) on lung function in patients with cHP.
Methods
Patients with cHP treated with either MMF or AZA were retrospectively identified from four interstitial lung disease centers. Change in lung function before and after treatment initiation was analyzed using linear mixed-effects modeling (LMM), adjusting for age, sex, smoking history, and prednisone use.
Results
Seventy patients were included: 51 were treated with MMF and 19 with AZA. Median follow-up after treatment initiation was 11 months. Prior to treatment initiation, FVC and diffusion capacity of the lung for carbon monoxide (Dlco) % predicted were declining at a mean rate of 0.12% (P < .001) and 0.10% (P < .001) per month, respectively. Treatment with either MMF or AZA was not associated with improved FVC (0.5% at 1 year; P = .46) but was associated with a statistically significant improvement in Dlco of 4.2% (P < .001) after 1 year of treatment. Results were similar in the subgroup of patients treated with MMF for 1 year; the FVC increased nonsignificantly by 1.3% (P = .103) and Dlco increased by 3.9% (P < .001).
Conclusions
Treatment with MMF or AZA is associated with improvements in Dlco in patients with cHP. Prospective randomized trials are needed to validate their effectiveness for cHP.
Key Words: azathioprine, hypersensitivity pneumonitis, interstitial lung disease, mycophenolate mofetil
Abbreviations: AZA, azathioprine; cHP, chronic hypersensitivity pneumonitis; Dlco, diffusion capacity of the lung for carbon monoxide; HP, hypersensitivity pneumonitis; ILD, interstitial lung disease; LMM, linear mixed-effects model; MMF, mycophenolate mofetil; PFT, pulmonary function test; UBC, University of British Columbia; UCalgary, University of Calgary; UCSF, University of California, San Francisco
Hypersensitivity pneumonitis (HP) is an immune-mediated interstitial lung disease (ILD) resulting from exposure to a sensitizing antigen.1 HP is classically categorized as acute, subacute, or chronic according to its clinical presentation.2 Chronic hypersensitivity pneumonitis (cHP), typically defined by the presence of radiographic or histologic fibrosis, or both,3, 4 has an insidious onset over a period of months to years and can be challenging to differentiate from other forms of chronic ILD.5, 6, 7
The cornerstone of treatment for HP is antigen remediation, although corticosteroids are also commonly used. Previous prospective studies in patients with acute farmer’s lung demonstrated that a short course of corticosteroids can hasten resolution of respiratory symptoms and improve the diffusing capacity of the lung for carbon monoxide (Dlco) after 1 month of therapy.8, 9, 10 Similar to acute and subacute HP, patients with cHP are often managed with corticosteroids, despite a lack of evidence supporting their efficacy.3, 4 The challenges of identifying an inciting antigen, the morbidity associated with corticosteroids, and the often progressive course of cHP has motivated the search for alternative approaches to therapy, with steroid-sparing immunosuppressive agents proposed as a potential alternative.4
Mycophenolate mofetil (MMF) and azathioprine (AZA) are cell-cycle inhibitors used in the treatment of many inflammatory ILDs such as those related to connective tissue disease.11, 12, 13 There are anecdotal reports of these drugs being used for the treatment of cHP despite a lack of evidence supporting their efficacy in this ILD subtype. In this study, we aimed to evaluate the effects of MMF or AZA on lung function in patients with cHP by comparing lung function trajectories before and after the onset of therapy and to assess the tolerability of these agents in this patient population.
Methods
Study Population
The study cohort included patients from four different centers with specialized ILD clinics: University of California, San Francisco (UCSF), University of British Columbia (UBC), Centre Hospitalier de l’Université de Montréal (CHUM), and University of Calgary (UCalgary). Patients were identified from prospective ILD databases at UCSF and UBC and from retrospective review of medical records from the ILD clinics at CHUM and UCalgary. Patients were included if they had a multidisciplinary team diagnosis of cHP, received treatment with either MMF or AZA for this diagnosis, and had a least one set of pulmonary function tests (PFTs) performed before and after treatment initiation. The diagnosis of cHP was established locally in each center after discussion and integration of the clinical, radiologic, and pathologic findings in a multidisciplinary conference, which is consistent with prior reports.3, 14, 15 Local institutional review boards at each center approved this project (UCSF Institutional Human Subject Review Committee (10-01592), UBC Research Ethics Board (H15-01958), Comité d’éthique de la recherche du CHUM (2016-6066, CE15.194-CA), and UCalgary Research Ethics Board (REB15-1682_REN).
Clinical data extracted from the medical record included age, sex, smoking history, identification of a causative antigen by clinical history, treatment history (including doses and dates of initiation and discontinuation of medications), tolerability, and pulmonary function test results before and after the introduction of MMF or AZA.
Statistical Analysis
Longitudinal trajectories of FVC % predicted and Dlco % predicted before and after treatment initiation were the primary outcomes. We used linear mixed-effects models (LMMs) to evaluate the change in FVC % predicted and Dlco % predicted, positing piecewise linear trajectories in time, with change points at the initiation of treatment with MMF or AZA. We also assessed for the presence of an immediate change in FVC % predicted and Dlco % predicted at initiation. We analyzed the entire cohort as a whole and then repeated the analyses for different subgroups according to the type of medication (MMF or AZA). All models were adjusted for potential confounders: age, sex, smoking history, and prednisone use, the latter as a time-varying covariate. In addition, we evaluated for the presence of a potential interaction between treatment and clinical center. Patients who were switched from one drug to another were used in the analysis of the effect of the initial drug provided that they used it for at least 3 months; otherwise, their data were used to estimate the effect of the second drug. Based on the LMM for each treatment, we estimated the effect of treatment on change in lung function after 1 year of treatment as the net difference in the observed change and the counterfactual change that would have been expected had treatment not been initiated. Specifically, this net treatment effect was calculated as the change in monthly rate of change at initiation multiplied by 12 months. To illustrate these analyses, we plotted the actual and counterfactual trajectories of the mean, centered on month of treatment initiation. Stata, version 14 (Stata Corp LP) was used for all statistical analyses.
Results
Cohort Formation and Study Population
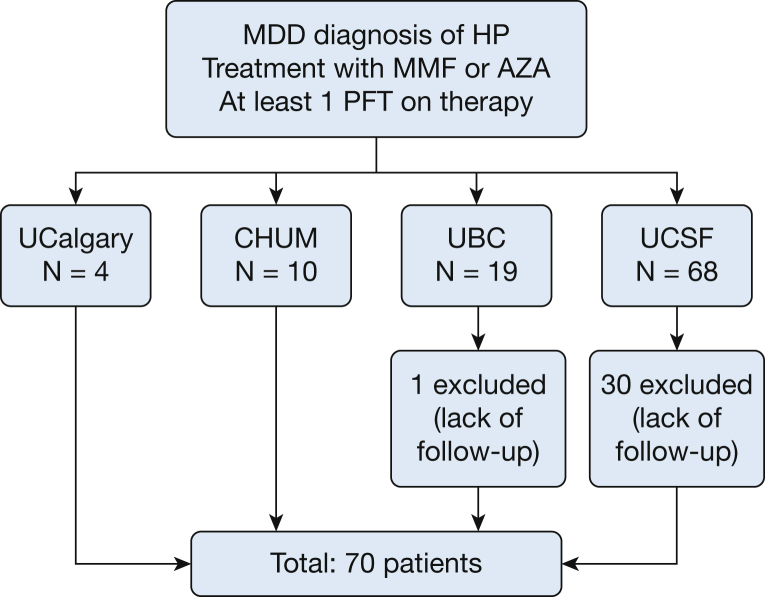
One hundred one patients with a diagnosis of cHP treated with MMF or AZA were identified from the four ILD centers. Of these patients, 31 were excluded (30 from UCSF and one from UBC) due to lack of follow-up data after the initiation of therapy; 70 patients were included in the final cohort (Fig 1). Patients included in the study were younger and more likely to have undergone a surgical lung biopsy than those who were excluded for lack of follow-up. Other baseline characteristics were similar between the two groups (e-Table 1). In addition, overall survival was comparable in both groups (log-rank P = .327). Patient baseline characteristics are presented in Table 1. The majority of subjects were men and never smokers. A high proportion underwent surgical lung biopsy (78.6%), and all patients without histopathologic confirmation had been exposed to an identified causative antigen.
Figure 1.
Cohort formation. AZA = azathioprine; CHUM = Centre Hospitalier de l’Université de Montréal; HP = hypersensitivity pneumonitis; MMD = multidisciplinary discussion; MMF = mycophenolate mofetil; PFT = pulmonary function test; UBC = University of British Columbia; UCalgary = University of Calgary; UCSF = University of California, San Francisco.
Table 1.
Patient Characteristics
| Characteristic | (N = 70) |
|---|---|
| Mean age (SD), y | 60.5 (11.3) |
| Male, No. (%) | 39 (55.7) |
| Ever smoker, No. (%) | 24 (34.3) |
| Surgical lung biopsy, No. (%) | 55 (78.6) |
| Exposure, No. (%) | |
| Mold | 20 (28.6) |
| Bird | 11 (15.7) |
| Down feather products | 6 (8.6) |
| Othera | 4 (5.7) |
| Unknown | 29 (41.4) |
| Pulmonary function at MMF or AZA initiation | |
| Mean FVC % predicted (SD) | 65.2 (18.0) |
| Mean Dlco % predicted (SD) | 49.8 (15.2) |
| MMF, No. (%) | 51 (72.9) |
| Daily dose, range, mg | 1,000-3,000 |
| AZA, No. (%) | 19 (27.1) |
| Daily dose, range, mg | 100-150 |
| Mean dose (SD), mg | 114.9 (23.1) |
| Prednisone, No. (%) | 59 (84.3) |
| Dose, range, mg | 10-30 |
| Patients treated with prednisone prior to MMF or AZA initiation, No. (%) | 14 (23.0) |
| Prednisone dose at MMF or AZA initiation, mg | 12.33 (13.99) |
| Prednisone dose at 6 mo after MMF or AZA initiation, mg | 3.75 (5.25) |
AZA = azathioprine; Dlco = diffusing capacity for carbon monoxide; MMF = mycophenolate mofetil.
Other exposures included swamp cooler (n = 1), farming products (n = 1), and hot tub (n = 2).
Fifty-one patients (72.9%) were treated with MMF, 19 patients (27.1%) were treated with AZA, and five patients (7%) received AZA briefly before switching to MMF. Median follow-up duration after treatment initiation was 11 months (interquartile range, 7.1-14.9). Patients had PFT results available for a median of 19.8 months before the initiation of MMF or AZA (interquartile range, 5.6-48.24). On average, patients had 5.61 FVC measurements and 5.04 measurements of Dlco prior to MMF or AZA initiation and 2.8 FVC measurements and 2.55 measurements of Dlco after MMF or AZA initiation. The majority of patients (84%) also received corticosteroids at a mean dose of 12.3 mg/d at the time of MMF or AZA initiation.
Longitudinal Change in Lung Function
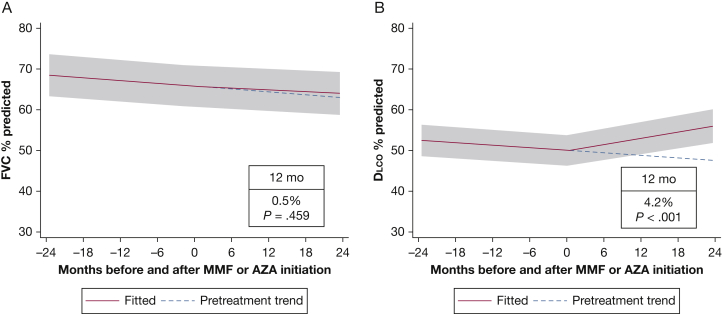
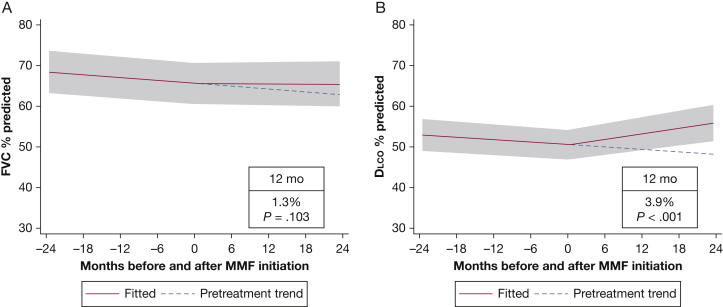
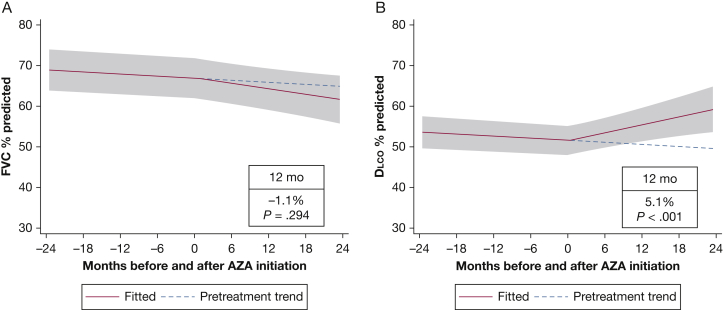
The sample of 70 patients contributed 589 observations of FVC % predicted and 532 observations of Dlco % predicted to the analysis. Before MMF or AZA initiation, FVC % predicted and Dlco % predicted were declining at a rate of 0.12% (P < .001) and 0.10% (P < .001) per month, respectively. Patients receiving prednisone before the initiation of MMF or AZA experienced the same decline in both FVC % predicted and Dlco % predicted as the overall population. For the entire cohort, FVC % predicted did not increase significantly after 1 year of treatment (mean increase, 0.5%; 95% CI, –0.9 to 1.9; P = .46) (Fig 2A). In contrast, the Dlco % predicted increased significantly after 1 year of treatment (mean increase, 4.2%; 95% CI, 2.6-5.9; P < .001) (Fig 2B). In this cohort, FVC % predicted increased by 5% and 10%, respectively, in 17 patients (24%) and 9 patients (13%) after MMF or AZA initiation. Gas exchange (Dlco % predicted) improved by 5% in 23 patients (33%) and by 10% in 14 patients (20%) receiving therapy compared with PFT results obtained before therapy. In the MMF subgroup, FVC % predicted did not improve significantly after 1 year (mean increase, 1.3%; 95% CI, –0.3 to 2.8; P = .103), but Dlco % predicted demonstrated improvement (mean increase at 1 year, 3.9%; 95% CI, 2.1-5.6; P < .001) (Fig 3). Similarly, in the subgroup of patients treated with AZA, there was no significant change in FVC % predicted after initiation of therapy, but the Dlco % predicted improved significantly (Fig 4). There was no statistically significant interaction between treatment and centers or between treatment and the method used to identify study patients (prospective database vs retrospective chart review). At 6 months after the initiation of MMF or AZA, 42% of patients were receiving prednisone at an average dose of 3.75 mg.
Figure 2.
Mixed-effects model estimates for FVC % predicted and Dlco % predicted before and after initiation of mycophenolate or azathioprine. The gray shading indicates the 95% CI. Dlco = diffusion capacity of the lung for carbon monoxide. See Figure 1 legend for expansion of other abbreviations.
Figure 3.
Mixed-effects model estimates for FVC % predicted and Dlco % predicted before and after initiation of mycophenolate. The gray shading indicates the 95% CI. See Figure 1 and 2 legends for expansion of abbreviations.
Figure 4.
Mixed-effects model estimates for FVC % predicted and Dlco % predicted before and after initiation of azathioprine. The gray shading indicates the 95% CI. See Figure 1 and 2 legends for expansion of abbreviations.
Tolerability of MMF and AZA
MMF and AZA were well tolerated in the majority of subjects (Table 2), with 10 patients (14.3%) reporting adverse effects. GI symptoms were the most common adverse effects for both medications. The dose of MMF needed to be reduced in three of 70 patients (4.3%). In addition, MMF was discontinued in two patients for transaminitis (n = 1) and diarrhea (n = 1). Finally, AZA was discontinued in two patients for transaminitis (n = 1) and diarrhea (n = 1), and five patients were switched from AZA to MMF.
Table 2.
Tolerability of Mycophenolate and Azathioprine
| Variable | Complete Cohort (N = 70) | MMF (n = 51) | AZA (n = 19) |
|---|---|---|---|
| Reported side effects, No. (%) | 10 (14.3) | 7 (13.7) | 3 (15.8) |
| Nausea | 3 (4.3) | 3 (5.8) | 0 |
| Diarrhea | 2 (2.9) | 1 (1.9) | 1 (5.3) |
| Transaminitis | 2 (2.9) | 1 (1.9) | 1 (5.3) |
| GI upset | 1 (1.4) | 1 (1.9) | 0 |
| Bloating | 1 (1.4) | 1 (1.9) | 0 |
| Fatigue | 1 (1.4) | 0 | 1 (5.3) |
| Management of side effect, No. (%) | |||
| Dose reduction | 3 (4.3) | 3 (5.8) | 0 |
| Drug discontinuation | 2 (2.9) | 2 (5.8) | 2 (10.5) |
See Table 1 legend for expansion of abbreviations.
Discussion
In this multicenter retrospective study, we demonstrated that treatment of cHP with MMF or AZA is associated with improved gas exchange and a reduction in prednisone dose. MMF and AZA appeared well tolerated, with low rates of medication discontinuation and similar adverse effects compared with previous reports.11, 13, 16 To our knowledge, this is the first study to describe the effects of MMF or AZA on the clinical course of cHP.
There is a pressing need to identify alternative therapeutic approaches for cHP, as the established approach of antigen removal and corticosteroid use is often ineffective or impractical. Antigen removal is recommended as an initial therapeutic step in all patients; however, this is not always possible given that up to 60% of patients with cHP are antigen indeterminate.3, 4, 17 Even when an antigen has been identified, removal can be challenging when it requires removal of a pet or moving from a family home. Moreover, it is not uncommon for patients with cHP to have progressive disease despite presumed exposure remediation. The use of corticosteroids in patients with cHP has been adopted from studies demonstrating short-term efficacy in patients with acute farmer’s lung8, 9, 10; however, there have been no prospective studies evaluating the efficacy, safety, or tolerability of corticosteroids for cHP. In our study, there was no difference in the trajectory of lung function decline between patients who were treated with prednisone before the initiation of MMR or AZA and those who were not. This raises important questions as to the efficacy of steroid therapy in patients with cHP, with additional studies needed to address this question properly. Our typical practice has been to taper the prednisone dose shortly after initiation of either MMF or AZA, using intermittent clinical assessments to inform response to therapy. The lack of a standardized protocol in this retrospective study limits a better understanding of the impact of steroid tapering on pulmonary function in cHP.
Although not completely understood, the pathogenesis of cHP is believed to involve an inflammatory response mediated by immune complexes and T lymphocytes.1, 18 The proposed role of B cells in the pathogenesis of cHP is indirectly supported by a recent case report and small case series (n = 6) suggesting that rituximab is an effective rescue therapy in selected patients with HP that is refractory to other systemic immune suppressants.19, 20 MMF, a prodrug of mycophenolic acid, exerts its immunosuppressive effect by inhibiting inosine monophosphate dehydrogenase, which is uniquely involved in the proliferation of T and B lymphocytes.21 AZA is a purine analog that blocks the pathway of purine synthesis, therefore limiting the proliferation of lymphocytes and other cell types.22 Both MMF and AZA have a potential role in cHP given what is known about the biological characteristics of this disease; however, the precise cellular mechanisms underlying the development and progression of cHP are not well characterized, and future work is needed to enable targeted therapeutic approaches for this disease. It is not entirely clear why treatment with MMF or AZA resulted in improvements in gas transfer but not FVC. Given their mechanisms of action, these therapies may primarily target areas of active inflammation, subsequently improving gas exchange as measured by Dlco. It is not expected that the fibrosis associated with cHP would be reversed with either MMF or AZA, which may account for the lack of improvement in FVC. Recent data suggest that MMF may improve gas transfer in scleroderma-associated ILD.13 Additionally, MMF may affect pulmonary vascular remodeling, based on animal studies in pulmonary arterial hypertension.23 Further research is needed to better characterize the physiological effects of MMF or AZA in fibrotic lung diseases.
The results of this study are strengthened by the inclusion of a large number of well-characterized patients with cHP from four ILD centers in multiple countries. In addition, we used a robust statistical approach of linear mixed modeling to measure the effect of MMF or AZA on lung function in patients with cHP, rather than simply comparing the mean lung function before and after treatment. This statistical approach allowed us to account for the variability in timing of medication initiation, number of FVC and Dlco assessments, and duration of follow-up for each patient. However, there are several limitations to this study. First, all patients were from academic centers, thus potentially limiting the generalizability of our results. However, we found few differences in the patients who returned for follow-up compared with those who were subsequently managed in the community. Second, given the retrospective design, there was no systematic monitoring of therapy, medication dosage, adverse effects, or prednisone use; therefore, we cannot define these measures with further granularity to ensure patient compliance or the presence of minor unreported adverse effects. We were unable to assess important clinical outcomes such as mortality, changes seen on radiography, functional capacity, dyspnea, or quality of life, and these factors should be measured in future studies. There may also be selection bias, as some patients from the UCSF cohort were excluded due to lack of follow-up data. Third, we assumed piecewise linear change in lung function, which may not be accurate for all patients. In addition, we cannot rule out confounding by unmeasured factors, potentially including levels of exposure, that may vary over time within patients. Most importantly, our study lacks comparison with an untreated control group due to the small numbers of such patients in our cohorts and the likely severe bias of confounding by indication that would be present in such a comparison. Thus, we elected to estimate treatment effects using a pre/post quasi-experimental design.
In summary, we showed that treatment with MMF or AZA is associated with improved gas transfer and is generally well tolerated in patients with cHP. Both medications could be promising treatment options for long-term therapy for cHP; however, prospective clinical trials are desperately needed.
Acknowledgments
Author contributions: J. M. takes responsibility for the content of the manuscript, including the data and analysis and is guarantor of this paper. J. M., K. A. J., H. R. C., C. J. R., and B. L. contributed to the conception and design, acquisition of data, and analysis and interpretation of the data. J. M., J. J. S., A. F., and H. R. C. contributed to the analysis and interpretation of the data. C. A., B., M. E., K. D. J., C. D. F., H. M., and B-P. D. contributed to the acquisition of the data. E. V. and P. J. W contributed to the analysis and interpretation of the data. All authors revised the manuscript for important intellectual content and provided final approval of the version to be published.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: K. A. J. reports personal fees from Hoffman-La Roche and Boehringer Ingelheim outside of the submitted work. H. R. C. reports personal fees from AstraZeneca/Medimmune, Bayer, Biogen, Boehringer Ingelheim, Genentech/Roche, Genoa, Gilead, GlaxoSmithKline, Mesoblast, Moerae Matrix, Pharmakea, Promedior, Prometic, Pulmatrix, Unity, Aeolus, aTry Pharmaceuticals, Grunenthal, UCB Celltech, GBT, and Veracyte outside of the submitted work. C. J. R. reports grants and personal fees from Boehringer Ingelheim and Hoffmann La Roche outside the submitted work. None declared (J. M., E. V., C. A., B. M. E., K. D. J., C. D. F., H. M., B. P. D., P. J. W., B. L.).
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Other contributions: The authors thank the physician members and staff of the UCSF Interstitial Lung Disease Program, the University of British Columbia, the Centre Hospitalier de l’Université de Montréal and the University of Calgary for their assistance in identifying and recruiting patients for this study.
Additional information: The e-Table can be found in the Supplemental Materials section of the online article.
Footnotes
Drs Morisset and Johannson contributed equally to this manuscript.
FUNDING/SUPPORT: This work was supported by the Nina Ireland Program for Lung Health.
Supplementary Data
References
- 1.Selman M., Pardo A., King T.E., Jr. Hypersensitivity pneumonitis: insights in diagnosis and pathobiology. Am J Respir Crit Care Med. 2012;186(4):314–324. doi: 10.1164/rccm.201203-0513CI. [DOI] [PubMed] [Google Scholar]
- 2.Richerson H.B., Bernstein I.L., Fink J.N. Guidelines for the clinical evaluation of hypersensitivity pneumonitis. Report of the Subcommittee on Hypersensitivity Pneumonitis. J Allergy Clin Immunol. 1989;84(5 Pt 2):839–844. doi: 10.1016/0091-6749(89)90349-7. [DOI] [PubMed] [Google Scholar]
- 3.Mooney J.J., Elicker B.M., Urbania T.H. Radiographic fibrosis score predicts survival in hypersensitivity pneumonitis. Chest. 2013;144(2):586–592. doi: 10.1378/chest.12-2623. [DOI] [PubMed] [Google Scholar]
- 4.Fernandez Perez E.R., Swigris J.J., Forssen A.V. Identifying an inciting antigen is associated with improved survival in patients with chronic hypersensitivity pneumonitis. Chest. 2013;144(5):1644–1651. doi: 10.1378/chest.12-2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raghu G., Collard H.R., Egan J.J. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183(6):788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morell F., Villar A., Montero M.A. Chronic hypersensitivity pneumonitis in patients diagnosed with idiopathic pulmonary fibrosis: a prospective case-cohort study. Lancet Respir Med. 2013;1(9):685–694. doi: 10.1016/S2213-2600(13)70191-7. [DOI] [PubMed] [Google Scholar]
- 7.Silva C.I., Muller N.L., Lynch D.A. Chronic hypersensitivity pneumonitis: differentiation from idiopathic pulmonary fibrosis and nonspecific interstitial pneumonia by using thin-section CT. Radiology. 2008;246(1):288–297. doi: 10.1148/radiol.2453061881. [DOI] [PubMed] [Google Scholar]
- 8.Monkare S. Influence of corticosteroid treatment on the course of farmer's lung. Eur J Respir Dis. 1983;64(4):283–293. [PubMed] [Google Scholar]
- 9.Kokkarinen J.I., Tukiainen H.O., Terho E.O. Effect of corticosteroid treatment on the recovery of pulmonary function in farmer's lung. Am Rev Respir Dis. 1992;145(1):3–5. doi: 10.1164/ajrccm/145.1.3. [DOI] [PubMed] [Google Scholar]
- 10.Kokkarinen J.I., Tukiainen H.O., Terho E.O. Recovery of pulmonary function in farmer's lung. A five-year follow-up study. Am Rev Respir Dis. 1993;147(4):793–796. doi: 10.1164/ajrccm/147.4.793. [DOI] [PubMed] [Google Scholar]
- 11.Fischer A., Brown K.K., Du Bois R.M. Mycophenolate mofetil improves lung function in connective tissue disease-associated interstitial lung disease. J Rheumatol. 2013;40(5):640–646. doi: 10.3899/jrheum.121043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berezne A., Ranque B., Valeyre D. Therapeutic strategy combining intravenous cyclophosphamide followed by oral azathioprine to treat worsening interstitial lung disease associated with systemic sclerosis: a retrospective multicenter open-label study. J Rheumatol. 2008;35(6):1064–1072. [PubMed] [Google Scholar]
- 13.Tashkin D.P., Roth M.D., Clements P.J. Mycophenolate mofetil versus oral cyclophosphamide in scleroderma-related interstitial lung disease (SLS II): a randomised controlled, double-blind, parallel group trial. Lancet Respir Med. 2016;4(9):708–719. doi: 10.1016/S2213-2600(16)30152-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bradley B., Branley H.M., Egan J.J. Interstitial lung disease guideline: the British Thoracic Society in collaboration with the Thoracic Society of Australia and New Zealand and the Irish Thoracic Society. Thorax. 2008;63(suppl 5):v1–v58. doi: 10.1136/thx.2008.101691. [DOI] [PubMed] [Google Scholar]
- 15.Johannson K.A., Elicker B.M., Vittinghoff E. A diagnostic model for chronic hypersensitivity pneumonitis. Thorax. 2016;71(10):951–954. doi: 10.1136/thoraxjnl-2016-208286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Owen C., Ngian G.S., Elford K. Mycophenolate mofetil is an effective and safe option for the management of systemic sclerosis-associated interstitial lung disease: results from the Australian Scleroderma Cohort Study. Clin Exp Rheumatol. 2016;34(suppl 100[5]):170–176. [PubMed] [Google Scholar]
- 17.Vourlekis J.S., Schwarz M.I., Cherniack R.M. The effect of pulmonary fibrosis on survival in patients with hypersensitivity pneumonitis. Am J Med. 2004;116(10):662–668. doi: 10.1016/j.amjmed.2003.12.030. [DOI] [PubMed] [Google Scholar]
- 18.Lacasse Y., Girard M., Cormier Y. Recent advances in hypersensitivity pneumonitis. Chest. 2012;142(1):208–217. doi: 10.1378/chest.11-2479. [DOI] [PubMed] [Google Scholar]
- 19.Lota H.K., Keir G.J., Hansell D.M. Novel use of rituximab in hypersensitivity pneumonitis refractory to conventional treatment. Thorax. 2013;68(8):780–781. doi: 10.1136/thoraxjnl-2013-203265. [DOI] [PubMed] [Google Scholar]
- 20.Keir G.J., Maher T.M., Ming D. Rituximab in severe, treatment-refractory interstitial lung disease. Respirology. 2014;19(3):353–359. doi: 10.1111/resp.12214. [DOI] [PubMed] [Google Scholar]
- 21.Allison A.C., Eugui E.M. Mycophenolate mofetil and its mechanisms of action. Immunopharmacology. 2000;47(2-3):85–118. doi: 10.1016/s0162-3109(00)00188-0. [DOI] [PubMed] [Google Scholar]
- 22.Maltzman J.S., Koretzky G.A. Azathioprine: old drug, new actions. J Clin Invest. 2003;111(8):1122–1124. doi: 10.1172/JCI18384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zheng Y., Li M., Zhang Y., Shi X., Li L., Jin M. The effects and mechanisms of mycophenolate mofetil on pulmonary arterial hypertension in rats. Rheumatology Int. 2010;30(3):341–348. doi: 10.1007/s00296-009-0966-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.