Abstract
Background
The emergence of central sleep apnea (CSA) during positive airway pressure (PAP) therapy has been observed clinically in approximately 10% of obstructive sleep apnea titration studies. This study assessed a PAP database to investigate trajectories of treatment-emergent CSA during continuous PAP (CPAP) therapy.
Methods
U.S. telemonitoring device data were analyzed for the presence/absence of emergent CSA at baseline (week 1) and week 13. Defined groups were as follows: obstructive sleep apnea (average central apnea index [CAI] < 5/h in week 1, < 5/h in week 13); transient CSA (CAI ≥ 5/h in week 1, < 5/h in week 13); persistent CSA (CAI ≥ 5/h in week 1, ≥ 5/h in week 13); emergent CSA (CAI < 5/h in week 1, ≥ 5/h in week 13).
Results
Patients (133,006) used CPAP for ≥ 90 days and had ≥ 1 day with use of ≥ 1 h in week 1 and week 13. The proportion of patients with CSA in week 1 or week 13 was 3.5%; of these, CSA was transient, persistent, or emergent in 55.1%, 25.2%, and 19.7%, respectively. Patients with vs without treatment-emergent CSA were older, had higher residual apnea-hypopnea index and CAI at week 13, and more leaks (all P < .001). Patients with any treatment-emergent CSA were at higher risk of therapy termination vs those who did not develop CSA (all P < .001).
Conclusions
Our study identified a variety of CSA trajectories during CPAP therapy, identifying several different clinical phenotypes. Identification of treatment-emergent CSA by telemonitoring could facilitate early intervention to reduce the risk of therapy discontinuation and shift to more efficient ventilator modalities.
Key Words: central sleep apnea, CPAP, telemonitoring
Abbreviations: AHI, apnea-hypopnea index; ASV, adaptive servo-ventilation; CAI, central apnea index; CSA, central sleep apnea; FOT, forced oscillation technique; HI, hypopnea index; OAI, obstructive apnea index; PAP, positive airway pressure therapy; PSG, polysomnography; SDB, sleep-disordered breathing
In some patients with obstructive sleep apnea (OSA) it is now well known that central sleep apnea (CSA) may emerge or persist during CPAP therapy after elimination of obstructive events.1 This phenomenon has been referred to by a variety of terms, including complex sleep apnea, but was recognized in the third edition of the International Classification of Sleep Disorders and called “treatment-emergent CSA.”2 Treatment-emergent CSA has also been included in a European Respiratory Society Task Force document on nocturnal central breathing disturbances.3
A number of studies have evaluated the prevalence and natural course of treatment-emergent CSA.1, 4, 5, 6, 7, 8, 9 Reported prevalence rates range from 0.56% to 20.3%.1, 4, 5, 6, 7, 8, 9, 10 This large variation can be attributed to many methodologic and biologic factors, including patient demographic and clinical characteristics such as body mass index, sex, age, and comorbidities, and procedure-related factors like split-night titration and titration in the supine position.4, 8, 10, 11 In addition, sample sizes in existing studies have been generally small. Of note, the trajectories of treatment-emergent CSA appear to be a dynamic process, with central apneas being transient in some patients, persistent in others, and developing later after CPAP initiation in another subgroup.8 Therefore, the timing and absence of multiple/repeated assessments could influence reported prevalence rates.
Patients with unresolved persistent or treatment-emergent CSA during CPAP could be at risk of poor compliance and/or therapy termination.5, 12 Therefore, it is clinically important to be able to identify which subgroup of patients with OSA are at risk of developing CSA during CPAP therapy so that appropriate intervention is provided to ensure ongoing device use or to select a more suitable treatment option. Although some data on risk factors for treatment-emergent CSA are available,4, 8, 10, 11 there is still no consensus and the pathogenesis of treatment-emergent CSA during CPAP therapy remains unclear.
In this study, we use “big data” for the first time to determine the trajectories of treatment-emergent CSA during CPAP therapy.
Methods
Data Inclusion
A U.S. positive airway pressure (PAP) device telemonitoring database (AirView; ResMed) was queried. The study was exempt from ethics review given that the database is deidentified (an institutional review board waiver was provided). Patients and session records satisfying the following criteria were included: random sample representing 30% of database population; patients registered in the United States; first device mode was fixed-level CPAP or automatically adjusting CPAP; use of an AirSense/AirCurve 10 device (ResMed) reporting apnea-hypopnea index (AHI)/central apnea index (CAI)13; therapy start date from January 1 to 2, October 2015; CPAP therapy continued for ≥ 90 days; ≥ 1 day with device usage of ≥ 1 h in both week 1 and week 13 of therapy; valid data entry (age plausible [< 123 years] and valid data blocks [synchronized]). The PAP devices used differentiate between central and obstructive apneas using forced oscillation techniques (FOTs) to determine airway patency.13 Central apneas are scored when upper airway resistance is low and obstructive apneas are scored when the resistance is high.
Each session record contained five types of data: (1) patient demographic data, (2) treatment usage, (3) clinical metrics including statistical summary (eg, median, 95th percentile) of leak and pressure (for automatic pressure modes), (4) respiratory events (residual AHI, apnea index, hypopnea index [HI], obstructive apnea index [OAI], CAI, unknown apnea index), and (5) pressure settings (for fixed-pressure modes). For the purposes of this analysis, CPAP refers to both fixed-level and automatically adjusting CPAP. For patients using automatic CPAP, 95th percentile pressure data were regarded as equivalent to the pressure setting for fixed CPAP.
Assessments
Patients were grouped into one of four different categories based on average CAI in week 1 and week 13: OSA (average CAI < 5/h in both week 1 and week 13); transient CSA (average CAI ≥ 5/h in week 1, < 5/h in week 13); persistent CSA (average CAI ≥ 5/h in both week 1 and week 13); emergent CSA (average CAI < 5/h in week 1, ≥ 5/h in week 13). In addition, we also performed a post hoc analysis looking at the influence of more severe sleep-disordered breathing (SDB) on treatment-emergent CSA in these four different groups in patients with residual AHI ≥ 15/h on CPAP, as described in a recent task force recommendation.3
Age, average residual AHI/CAI, average pressure, and leak in the first 90 days were compared between the four patient subgroups. In addition, device usage hours and rates of CPAP therapy termination were determined and compared between patient subgroups. Therapy termination or a dropout event was defined as a patient’s having zero usage on CPAP mode for 30 consecutive days or switching to another therapy mode. Continuation of CPAP therapy was assumed as long as a dropout event did not occur.
Statistical Analysis
All statistical comparisons were performed with R software (version 3.3.1). Treatment usage was imputed as zero if it was missing for a particular night. For other data fields, including clinical metrics and respiratory events, missing data were not imputed and not included in calculations of mean values, standard deviations and proportions. Analysis of variance or a t test was used for comparison of normally distributed continuous variable (eg, age), depending on the number of groups for comparison. The Kruskal-Wallis rank sum test was used for comparisons of nonnormally distributed continuous variables (including AHI, CAI, pressure, leak). Categorical variables (including first CPAP mode, AHI/CAI/pressure/leak groups) were compared by χ2 test. A P value ≤ 0.05 was considered statistically significant. A multivariable Cox regression model with adjustment for covariates (including age, average 95th percentile CPAP level, and average 95th percentile leak in the first 90 days) was used to generate survival curves showing the likelihood of ongoing CPAP usage after 90 days in the various patient subgroups.
Results
Study Population
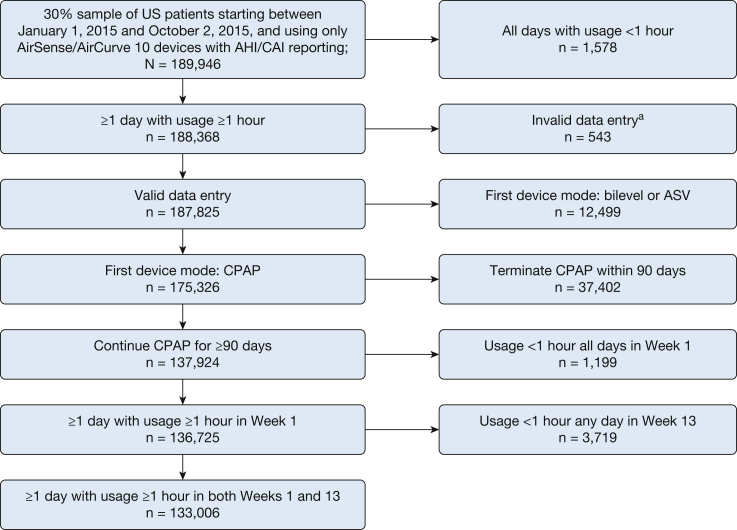
In the 30% random sample of US patients who started PAP therapy in the assessment window, 189,946 patients were treated with a CPAP device with AHI/CAI reporting. Of the eligible CPAP-treated patients, 133,006 had ≥ 90 days on CPAP and ≥ 1 day with use ≥ 1 h in both week 1 and week 13 (Table 1). A flow diagram showing patient identification and selection is shown in Figure 1. The proportion of patient sessions with missing data in at least one data field was 1.2%.
Table 1.
Demographic Data and Device Usage Characteristics of Patients Using CPAP for ≥ 90 Days, and ≥ 1 Day With Device Usage of ≥ 1 h in Both Week 1 and Week 13
| Demographic Data/Device Usage Data | Patients (n = 133,006) |
|---|---|
| Age, y | 56.4 ± 13.8 |
| Age distribution, y | |
| ≤ 40 | 17,389 (13.1) |
| 41-50 | 25,450 (19.1) |
| 51-60 | 35,620 (26.8) |
| 61-70 | 34,298 (25.8) |
| 71-80 | 16,739 (12.6) |
| > 80 | 3,510 (2.6) |
| Average usage (days 1-90), h/d | 6.0 ± 1.9 |
| Average daily CPAP level (days 1-90), mm Hg | 11.0 ± 2.8 |
| Average daily median leak (days 1-90), L/min | 6.0 ± 9.4 |
Values represent means ± standard deviation, or No. of patients (%).
Figure 1.
Flow diagram. AHI = apnea-hypopnea index; ASV = adaptive servo-ventilation; CAI = central apnea index. aInvalid data entry = age implausible (n = 497), or received data and session date not synchronized (n = 46).
CSA Occurrence During CPAP
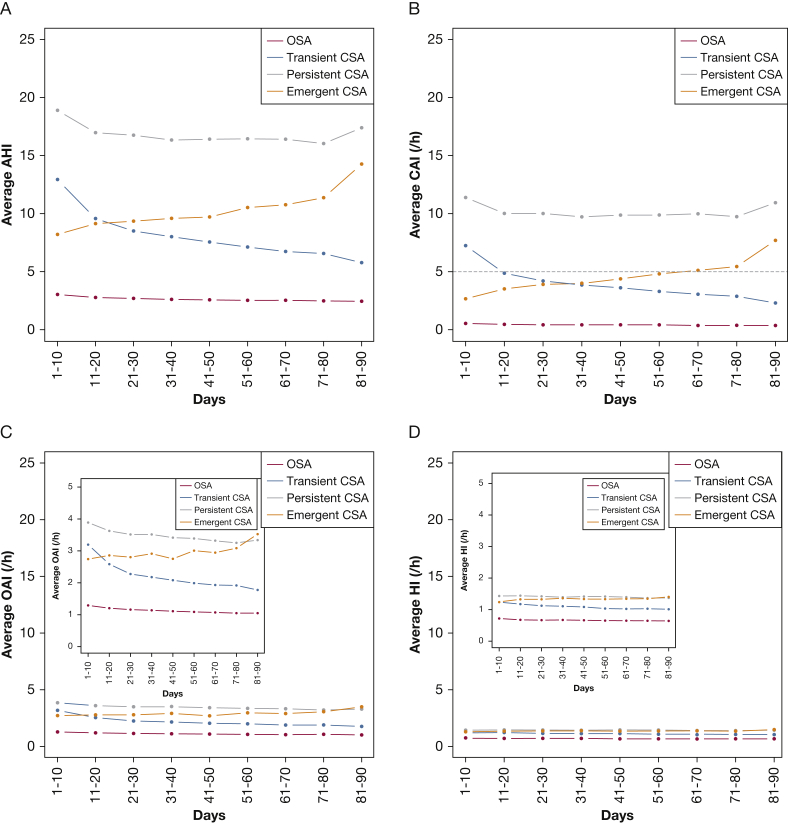
Overall, 4,621 of 133,006 patients (3.5%) had CSA at week 1 or week 13 of CPAP therapy. Of these, 2,547 patients (55.1%) had transient CSA, 912 had emergent CSA (19.7%), and 1,162 (25.2%) had persistent CSA. There were a number of statistically significant differences between patients in the various subgroups (Table 2). Patients with CSA during CPAP therapy were older, had higher residual AHI and CAI, and were less likely to achieve an average AHI < 15/h or CAI < 5/h in the first 90 days; patients with emergent CSA also had significantly higher leak during CPAP in the first 90 days (Table 2). Consistent with the different phenotype definitions, patients with persistent CSA had persistently higher residual AHI and CAI values over time, those with transient or emergent CSA showed decreasing or increasing AHI and CAI over time, while AHI values were consistently < 5/h in patients with OSA (Fig 2). Average residual OAI and HI values remained below 5/h for all groups at all times (Fig 2). Similar trends were observed when day-by-day values of AHI and CAI over the first 2 weeks of therapy were analyzed (e-Fig 1).
Table 2.
Demographic Data and Device Usage Characteristics of Various Patient Subgroups, Based on the Presence of CSA During CPAP Therapy
| Demographic Data/Device Usage Data | OSA (n = 128,385) |
Transient CSA (n = 2,547) |
Persistent CSA (n = 1,162) |
Emergent CSA (n = 912) |
P Value |
|---|---|---|---|---|---|
| Age, y | 56.2 ± 13.8a, b, c | 59.2 ± 13.7a,d,e | 62.4 ± 13.5b,d | 62.4 ± 13.0c,e | < .001 |
| CPAP mode during first 90 d, No. (%) | |||||
| Automatic pressure | 60,212 (46.9)a, b, c | 1,348 (52.9)a,d,e | 570 (49.0)b,d,f | 428 (46.9)c,e,f | < .001 |
| Fixed pressure | 61,166 (47.6) | 884 (34.7) | 402 (34.6) | 376 (41.2) | |
| Both | 7,007 (5.5) | 315 (12.4) | 190 (16.4) | 108 (11.8) | |
| Respiratory parameters in the first 90 d | |||||
| AHI, /h | 2.6 ± 3.1a, b, c | 8.1 ± 5.0a,d,e | 16.9 ± 10.0b,d,f | 10.2 ± 6.7c,e,f | < .001 |
| CAI, /h | 0.4 ± 0.7a, b, c | 3.9 ± 2.5a,d,e | 10.2 ± 6.3b,d,f | 4.6 ± 2.8c,e,f | < .001 |
| Average median pressure, cm H2O | 9.8 ± 2.7a | 9.7 ± 2.5a | 9.8 ± 2.4 | 9.8 ± 2.6 | .157 |
| Average 95th percentile pressure, cm H2O | 11.0 ± 2.8a,b | 11.2 ± 2.5a | 11.2 ± 2.5b | 11.1 ± 2.8 | < .001 |
| Average median leak, L/min | 6.0 ± 9.4b,c | 5.3 ± 6.8e | 5.4 ± 6.5b,f | 6.4 ± 7.3c,e,f | < .001 |
| Average 95th percentile leak, L/min | 21.6 ± 18.3b,c | 21.2 ± 16.0d,e | 22.1 ± 15.3b,d,f | 23.5 ± 15.9c,e,f | < .001 |
P values reported in the right-hand column of the table are those for testing the null hypothesis that all groups follow the same distribution; a P value < .05 indicates that there is a statistically significant difference across groups. To examine such differences further, pairwise tests between specific groups were conducted, with results shown as superscripts in the table. Values represent means ± standard deviation, or No. of patients (%). CAI = central apnea index; CSA = central sleep apnea.
Pairwise significance: P < .05, transient CSA vs OSA.
Pairwise significance: P < .05, persistent CSA vs OSA.
Pairwise significance: P < .05, emergent CSA vs OSA.
Pairwise significance: P < .05, persistent CSA vs transient CSA.
Pairwise significance: P < .05, emergent CSA vs transient CSA.
Pairwise significance: P < .05, emergent CSA vs persistent CSA.
Figure 2.
Residual (A) apnea-hypopnea index, (B) central apnea index, (C) obstructive apnea index, and (D) hypopnea index over time in patient subgroups during continuous positive airway pressure therapy. CSA = central sleep apnea; HI = hypopnea index; OAI = obstructive apnea index. See Figure 1 legend for expansion of other abbreviations.
CPAP Usage and Treatment Termination
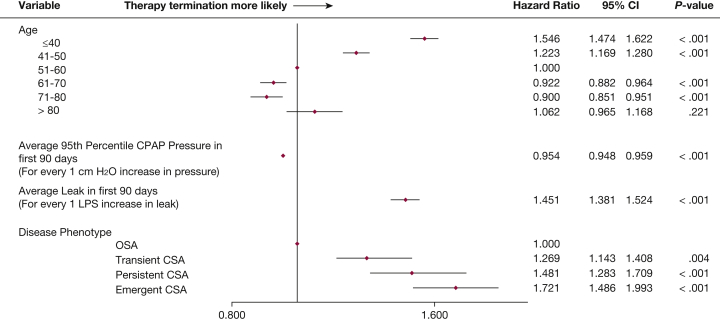
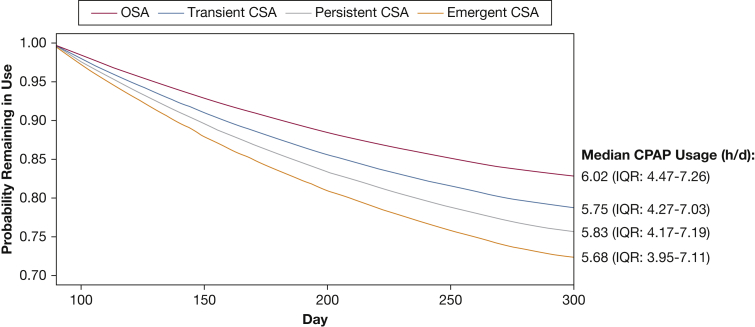
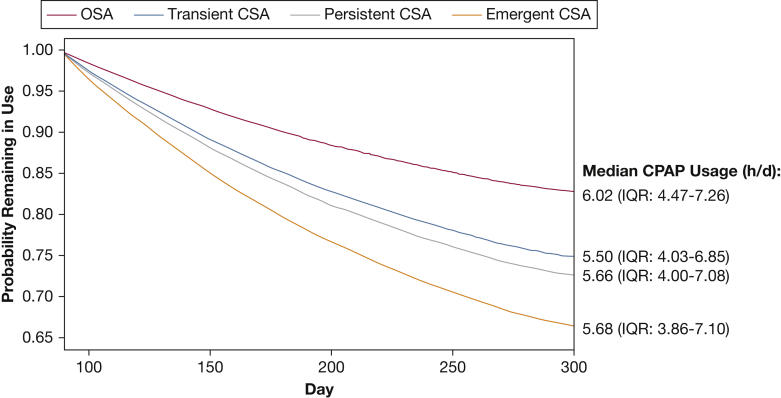
Average daily usage hours in the first 90 days were lower in those who did vs did not develop CSA during CPAP therapy: mean 5.97 h/d (95% CI, 5.96-5.98) in those without CSA, 5.75 h/d (5.68-5.83) in those with transient CSA, 5.87 h/d (5.75-5.99) in those with persistent CSA, and 5.66 h/d (5.52-5.80) in patients with emergent CSA. Patients with any CSA during CPAP were significantly more likely to terminate therapy after 90 days compared with those who did not develop CSA (Fig 3). Younger patients (≤ 50 years) and those with more leak were also significantly more likely to stop CPAP therapy (Fig 3). Compared with the OSA group, patients with any CSA during CPAP were less likely to continue using therapy (Fig 4). The estimated probability of continuing CPAP therapy on day 300 was 83% for the OSA group, and 79%, 76%, and 72% for the transient CSA, persistent CSA and emergent CSA groups, respectively.
Figure 3.
Forest plot showing risk of therapy termination in the various patient subgroups. LPS = liters per second. See Figure 2 legend for expansion of other abbreviation.
Figure 4.
Multivariate Cox model survival curve for continued use of CPAP after 90 days. IQR = interquartile range. See Figure 2 legend for expansion of other abbreviation.
Post hoc Analysis
A total of 1,538 of 129,923 patients (1.2%) had more severe SDB with a residual AHI of ≥ 15/h and CSA at week 1 or week 13 of CPAP therapy. Of these, 805 patients (52.3%) had transient CSA, 336 had emergent CSA (21.9%), and 397 (25.8%) had persistent CSA. The overall rate of treatment-emergent CSA during CPAP was lower than that for the overall study population (3.5%), but the proportion of patients with each type of CSA-on-CPAP was almost the same, and similar between-group differences in patient characteristics were seen (e-Table 1). Trends for residual AHI, CAI, OAI, and HI (e-Fig 2) mirrored those seen in the main analysis, as did predictors of therapy termination (e-Fig 3). Consistent with the overall analysis, patients with a residual AHI ≥ 15/h and any CSA during CPAP were also more likely to terminate therapy than were members of the OSA group (Fig 5). The estimated probabilities of continuing CPAP on day 300 in the post hoc analysis were 75%, 73%, and 66% for patients with a residual AHI ≥ 15/h and transient, persistent, and emergent CSA, respectively. In addition, more severe SDB was associated with a higher risk of therapy termination compared with the overall analysis (reduction in the probability of continuing CPAP therapy in the CSA groups vs OSA of approximately 8% to 17% compared with 4% to 11%, respectively).
Figure 5.
Multivariate Cox model survival curve for continued use of CPAP after 90 days in the post hoc analysis (patients with baseline apnea-hypopnea index ≥ 15/h). See Figure 2 and 4 legends for expansion of abbreviations.
Discussion
Using a large population-based sample of CPAP device data, we have highlighted the dynamic nature of CSA occurring during CPAP therapy, as has been suggested previously.8, 9 We have also shown that patients with treatment-emergent CSA during CPAP, including those with more severe SDB, have lower device usage and are more likely to terminate therapy. In addition, older age is associated with the development of CSA.
Our study differed from previous research because it introduced 1-week assessment windows and repeated measures based on real-life telemonitoring data, rather than undertaking a single assessment “snapshot” with polysomnography (PSG), and defined three different categories of treatment-emergent CSA and described their trajectories over time. The use of 1-week assessment windows instead of single “snapshots” of CAI on day 1 and day 90 for patient phenotyping was undertaken to address the variability of telemonitoring data. Looking at the data, it was observed that by examining CAI only on day 1 and day 90, the variance in CAI readings was relatively large for CSA phenotype groups even within weeks. This process may result in unreliable classification of CSA phenotype due to irregular readings captured by chance. In addition, CSA occurring during CPAP therapy has been shown to be highly variable over time.5, 9, 14 Thus, a weekly average of CAI over the first week and last week of the 90-day (13-week) period was used to classify CSA phenotype better. We chose the 90-day period because the majority of patients with transient treatment-emergent CSA during CPAP show resolution of central apneas within 8 weeks to 3 months.5, 15 We included patients with use of ≥ 1 h for ≥ 1 day to exclude as few patients as possible in order to minimize selection biases, while ensuring that we could reliably assess AHI/CAI over almost one complete sleep cycle (60-90 min). The inclusion of a full sleep cycle was based on evidence that the number of respiratory events varies between sleep stages.16
Previously reported prevalence rates for treatment-emergent CSA range from 0.56% to 20.3%.5, 7, 9, 11, 17 In our study, the proportion of patients identified who had treatment-emergent CSA at week 1 or week 13 of CPAP therapy was at the lower end of that reported range (3.5%). There are a number of potential explanations for this finding. First, prevalence data from most of the existing studies5, 7, 11, 17 were based on evaluations during CPAP titration using PSG, whereas our assessment calculated weekly averages after CPAP titration had been completed. Also, in our study it is possible that patients showing CPAP-emergent CSA during PSG-guided device titration had already been switched to adaptive servo-ventilation (ASV), reducing the CSA prevalence in our assessments. Second, existing studies included much smaller and more defined patient populations, including those with well-controlled OSA and comorbidities such as heart failure. In contrast, the present study used a large sample of real-life population-based data. PSG factors such as baseline AHI, sample size, and type of sleep study conducted (full night titration vs. split night) likely influence reported prevalence rates of CSA during CPAP therapy.8
There are relatively few studies that have investigated the trajectory of treatment-emergent CSA over time, and this is a major strength of our data. Cassel et al9 used PSG to determine the presence of CSA during the first night with stable CPAP and again after 3 months and found a reduction in the rate of CSA over time (from 12.2% at baseline to 6.9% at 3 months). Other studies have also shown that treatment-emergent CSA is not persistent in many patients.5, 6, 15 These results are consistent with our findings showing that treatment-emergent CPAP is not stable and instead varies over time. Transient CSA was the most common form of treatment-emergent CSA in our study (55%), suggesting that a single assessment does not provide an accurate indication of which patients will have persistent CSA during CPAP (25% of those in our study). Furthermore, some patients may not show CSA initially but develop it over time (characterized by the patients with emergent CSA in our study). A European Respiratory Society Task Force recently defined two different phenotypes for CSA emerging during CPAP therapy.3 Their definition of “treatment-emergent CSA” is similar to the group we defined as “transient CSA,” being patients who have little CSA at baseline, and develop CSA during CPAP, which then resolves with continued CPAP use. Patients with CSA emerging and persisting during CPAP were labeled as “treatment-persistent” by the ERS Task Force and as “persistent” in our study. Overall, available data highlight the importance of considering treatment-emergent CSA as a dynamic phenomenon, and classifying patients according to different phenotypes to ensure the most appropriate ongoing management.
This finding is particularly relevant in light of our findings that patients who develop CSA during CPAP have lower device usage in the first 90 days and are more likely to terminate therapy after 90 days. The risk of treatment termination was significantly increased in all patients with vs without treatment-emergent CSA but was highest in those with emergent CSA. This was even more pronounced in patients with a higher residual AHI on CPAP. Regular monitoring based on telemonitoring data could facilitate identification of patients with treatment-emergent CSA, classification of their phenotype, and intervention to maintain or improve CPAP compliance. Alternatively, switching to another PAP device may be warranted, such as ASV, which has been shown to be effective in treating treatment-emergent CSA.18, 19, 20 Current recommendations by a European task force suggest a switch to ASV in patients with treatment-emergent CSA during CPAP who have an AHI ≥ 15/h.3
In terms of risk factors for treatment-emergent CSA, the following have previously been suggested as being important: high AHI, CAI, and arousal index at baseline; high CPAP levels; older age; male sex; low body mass index; and the presence of heart failure or ischemic heart disease.8 In our analyses, older age, higher residual AHI and CAI, and higher leak were possible risk factors for treatment-emergent CSA, similar to what has been reported in a prospective PSG study.9 Our data do not allow us to determine whether the greater leak (mouth and mask) seen in the emergent CSA group played a role in the development of central apneas, but this assertion is theoretically possible given that greater leak could lead to more sleep disruption (or carbon dioxide clearance) that, in turn, might contribute to more central apneas.21
We did find a statistically significant difference in CPAP level between patients with and without treatment-emergent CSA, but this was numerically small and unlikely to be of clinical significance.
An important strength of this study was its ability to provide a detailed and comprehensive picture of the natural course of treatment-emergent CSA during CPAP therapy compared with current data. This is because patients had ongoing monitoring over time and are representative of those treated in clinical practice. In addition, the telemonitoring strategy used transmitted data on a daily basis and recordings took place in a home setting where patients’ natural sleep was minimally disturbed compared with PSG-based controlled studies during CPAP titration. Furthermore, the large and unselected patient population provides the study with good external validity.
Although it permits evaluation of a large data set, there are some limitations to conducting scientific research based on databases created for administrative, rather than scientific, purposes. These include the availability of limited baseline clinical and demographic data, and no information about the severity or type of SDB, CPAP titration, and comorbidities. While we assume that all patients included in the analysis were using CPAP to treat OSA, this assumption may not always have been the case. Use of device-generated data transmitted by telemonitoring means that data quality may be lower than that provided by PSG-based studies (although we tried to find stable estimates by calculating weekly averages). Another possible limitation is the use of FOT to classify apneas as obstructive or central. While FOT is a valid method for distinguishing between obstructive and central events,22 it operates on the assumption that a closed or open airway indicates obstructive or central apnea, respectively. However, it has been shown that the airway might passively close at the end of some central events,23 meaning that FOT could marginally underestimate the number of CSA events. In addition, it is not possible to differentiate between central events occurring during sleep vs periods of wakefulness and therefore some “awake” events might have been included. This might result in underestimation of the severity of CSA because device-derived recording time could be longer than actual sleep time. However, repeated assessment every night reduces this concern. The inclusion of all data for patients who had ≥ 1 day with CPAP usage of ≥ 1 h in week 1 and week 13 also has the potential to influence our results, possibly underestimating the rate and impact of treatment-emergent CSA because CAI readings would be lower in patients with short device usage. Regarding risk factors, it is possible that these could be confounded by factors about which we have no information, such as OSA severity, comorbidities, and concomitant medication.
Conclusions
The emergence of CSA during CPAP therapy is highly variable for the first 90 days of treatment. There are a variety of different patterns of emerging CSA during CPAP therapy, identifying several different patient phenotypes. These findings highlight the importance of regular and ongoing monitoring of patients during CPAP therapy, rather than basing clinical decisions on a single assessment at an arbitrary time point. Older patients, and those with higher residual AHI and leak, appear to be more at risk of emergent CSA during CPAP. Further research is warranted on the role that CSA plays in OSA management. Studies should be dedicated to specific management pathways after early identification by telemonitoring. Also, how different PAP modalities impact central events and how they might be used to optimize SDB treatment outcomes remain to be evaluated.
Acknowledgments
Author contributions: D. L. and H. W. are guarantors of the paper, and take responsibility for the integrity of the work as a whole, from inception to published article. The first draft of the manuscript was prepared by all of the authors, who had unrestricted access to the data, with the assistance of an independent medical writer funded by ResMed. The manuscript was reviewed and edited by all of the authors. All of the authors made the decision to submit the manuscript for publication and assume responsibility for the accuracy and completeness of the analyses and for the fidelity of this report to the trial protocol.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: P. A. C. holds an endowed academic chair at the University of Sydney, created by funding from ResMed Inc. His department receives research support (equipment) from ResMed, SomnoMed, and Zephyr Sleep Technologies. He has been a consultant/advisor for Zephyr Sleep Technologies, Novo Nordisk, and Fisher and Paykel Healthcare. J. L. P. reports grants from ResMed, during the conduct of the study; grants and personal fees from ResMed, grants and personal fees from Philips, grants from Fisher and Paykel, grants and personal fees from Sefam, grants from AstraZeneca, grants and personal fees from Agiradom, personal fees from Elevie, grants and personal fees from Vitalaire, personal fees from Boehringer, outside the submitted work. D. L., J. A., A. B., and S. S. are all employees of ResMed. H. W. was employed by ResMed during the conduct of the study; he has also received lecture fees from Vital Air, Boehringer Ingelheim, and ResMed, and research support from ResMed. A. M. is an Officer of the American Thoracic Society and has relinquished all outside personal income since 2012; ResMed provided a philanthropic donation to UC San Diego in support of a sleep center.
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Other contributions: The authors thank Gibson Gay, MSc, and Yang Yan, MSc, for assistance with the analysis. Medical writing support was provided by Nicola Ryan, BSc, independent medical writer, funded by ResMed.
Additional information: The e-Figures and e-Table can be found in the Supplemental Materials section of the online article.
Footnotes
FUNDING/SUPPORT: This work was supported by ResMed Ltd. J. L. P. was partly supported by the French National Research Agency in the framework of the “Investissements d’Avenir” program [ANR-15-IDEX-02].
Supplementary Data
References
- 1.Westhoff M., Arzt M., Litterst P. Prevalence and treatment of central sleep apnoea emerging after initiation of continuous positive airway pressure in patients with obstructive sleep apnoea without evidence of heart failure. Sleep Breath. 2012;16(1):71–78. doi: 10.1007/s11325-011-0486-0. [DOI] [PubMed] [Google Scholar]
- 2.American Academy of Sleep Medicine . 3rd ed. American Academy of Sleep Medicine; Darien, IL: 2014. International Classification of Sleep Disorders. [Google Scholar]
- 3.Randerath W., Verbraecken J., Andreas S. Definition, discrimination, diagnosis and treatment of central breathing disorders during sleep. Eur Respir J. 2017;49(1):1600959. doi: 10.1183/13993003.00959-2016. [DOI] [PubMed] [Google Scholar]
- 4.Verbraecken J. Complex sleep apnoea syndrome. Breathe. 2013;9(5):373–380. [Google Scholar]
- 5.Javaheri S., Smith J., Chung E. The prevalence and natural history of complex sleep apnea. J Clin Sleep Med. 2009;5(3):205–211. [PMC free article] [PubMed] [Google Scholar]
- 6.Kuzniar T.J., Pusalavidyasagar S., Gay P.C., Morgenthaler T.I. Natural course of complex sleep apnea: a retrospective study. Sleep Breath. 2008;12(2):135–139. doi: 10.1007/s11325-007-0140-z. [DOI] [PubMed] [Google Scholar]
- 7.Endo Y., Suzuki M., Inoue Y. Prevalence of complex sleep apnea among Japanese patients with sleep apnea syndrome. Tohoku J Exp Med. 2008;215(4):349–354. doi: 10.1620/tjem.215.349. [DOI] [PubMed] [Google Scholar]
- 8.Nigam G., Pathak C., Riaz M. A systematic review on treatment-emergent central sleep apnea. Ann Thorac Med. 2016;11(3):202–210. doi: 10.4103/1817-1737.185761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cassel W., Canisius S., Becker H.F. A prospective polysomnographic study on the evolution of complex sleep apnoea. Eur Respir J. 2011;38(2):329–337. doi: 10.1183/09031936.00162009. [DOI] [PubMed] [Google Scholar]
- 10.Khan M.T., Franco R.A. Complex sleep apnea syndrome. Sleep Disord. 2014;2014:798487. doi: 10.1155/2014/798487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morgenthaler T.I., Kagramanov V., Hanak V., Decker P.A. Complex sleep apnea syndrome: is it a unique clinical syndrome? Sleep. 2006;29(9):1203–1208. doi: 10.1093/sleep/29.9.1203. [DOI] [PubMed] [Google Scholar]
- 12.Mulgrew A.T., Lawati N.A., Ayas N.T. Residual sleep apnea on polysomnography after 3 months of CPAP therapy: clinical implications, predictors and patterns. Sleep Med. 2010;11(2):119–125. doi: 10.1016/j.sleep.2009.05.017. [DOI] [PubMed] [Google Scholar]
- 13.Weinreich G., Wang Y., Armitstead J., Bateman P., Richards G., Teschler H. Accuracy of AHI detection of the S9 AutoSet APAP device. Am J Respir Crit Care Med. 2013;187(Suppl.):A3747. [Google Scholar]
- 14.Ryan C.M., Floras J.S., Logan A.G. Shift in sleep apnoea type in heart failure patients in the CANPAP trial. Eur Respir J. 2010;35(3):592–597. doi: 10.1183/09031936.00070509. [DOI] [PubMed] [Google Scholar]
- 15.Dernaika T., Tawk M., Nazir S., Younis W., Kinasewitz G.T. The significance and outcome of continuous positive airway pressure-related central sleep apnea during split-night sleep studies. Chest. 2007;132(1):81–87. doi: 10.1378/chest.06-2562. [DOI] [PubMed] [Google Scholar]
- 16.Liu Y., Su C., Liu R. NREM-AHI greater than REM-AHI versus REM-AHI greater than NREM-AHI in patients with obstructive sleep apnea: clinical and polysomnographic features. Sleep Breath. 2011;15(3):463–470. doi: 10.1007/s11325-010-0358-z. [DOI] [PubMed] [Google Scholar]
- 17.Lehman S., Antic N.A., Thompson C., Catcheside P.G., Mercer J., McEvoy R.D. Central sleep apnea on commencement of continuous positive airway pressure in patients with a primary diagnosis of obstructive sleep apnea-hypopnea. J Clin Sleep Med. 2007;3(5):462–466. [PMC free article] [PubMed] [Google Scholar]
- 18.Allam J.S., Olson E.J., Gay P.C., Morgenthaler T.I. Efficacy of adaptive servoventilation in treatment of complex and central sleep apnea syndromes. Chest. 2007;132(6):1839–1846. doi: 10.1378/chest.07-1715. [DOI] [PubMed] [Google Scholar]
- 19.Brown S.E., Mosko S.S., Davis J.A., Pierce R.A., Godfrey-Pixton T.V. A retrospective case series of adaptive servoventilation for complex sleep apnea. J Clin Sleep Med. 2011;7(2):187–195. [PMC free article] [PubMed] [Google Scholar]
- 20.Morgenthaler T.I., Kuzniar T.J., Wolfe L.F., Willes L., McLain W.C., III, Goldberg R. The complex sleep apnea resolution study: a prospective randomized controlled trial of continuous positive airway pressure versus adaptive servoventilation therapy. Sleep. 2014;37(5):927–934. doi: 10.5665/sleep.3662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Montesi S.B., Bakker J.P., Macdonald M. Air leak during CPAP titration as a risk factor for central apnea. J Clin Sleep Med. 2013;9(11):1187–1191. doi: 10.5664/jcsm.3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Badia J.R., Farre R.O., John Kimoff R. Clinical application of the forced oscillation technique for CPAP titration in the sleep apnea/hypopnea syndrome. Am J Respir Crit Care Med. 1999;160(5 Pt 1):1550–1554. doi: 10.1164/ajrccm.160.5.9902085. [DOI] [PubMed] [Google Scholar]
- 23.Badr M.S., Toiber F., Skatrud J.B., Dempsey J. Pharyngeal narrowing/occlusion during central sleep apnea. J Appl Physiol. 1995;78:1806–1815. doi: 10.1152/jappl.1995.78.5.1806. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.