Abstract
Objectives
We sought to describe the characteristics of adult patients with bronchiectasis enrolled in the US Bronchiectasis Research Registry (BRR).
Methods
The BRR is a database of patients with non-cystic-fibrosis bronchiectasis (NCFB) enrolled at 13 sites in the United States. Baseline demographic, spirometric, imaging, microbiological, and therapeutic data were entered into a central Internet-based database. Patients were subsequently analyzed by the presence of NTM.
Results
We enrolled 1,826 patients between 2008 and 2014. Patients were predominantly women (79%), white (89%), and never smokers (60%), with a mean age of 64 ± 14 years. Sixty-three percent of the patients had a history of NTM disease or NTM isolated at baseline evaluation for entry into the BRR. Patients with NTM were older, predominantly women, and had bronchiectasis diagnosed at a later age than those without NTM. Gastroesophageal reflux disease (GERD) was more common in those with NTM, whereas asthma, primary immunodeficiency, and primary ciliary dyskinesia were more common in those without NTM. Fifty-one percent of patients had spirometric evidence of airflow obstruction. Patients with NTM were more likely to have diffusely dilated airways and tree-in-bud abnormalities. Pseudomonas and Staphylococcus aureus isolates were cultured less commonly in patients with NTM. Bronchial hygiene measures were used more often in those with NTM, whereas antibiotics used for exacerbations, rotating oral antibiotics, steroid use, and inhaled bronchodilators were more commonly used in those without NTM.
Conclusions
Adult patients with bronchiectasis enrolled in the US BRR are described, with differences noted in demographic, radiographic, microbiological, and treatment variables based on stratification of the presence of NTM.
Key words: airways, bronchiectasis, nontuberculous mycobacteria, Pseudomonas, registry
Abbreviations: AFB, acid-fast bacillus; BRR, Bronchiectasis Research Registry; CF, cystic fibrosis; DCC, data coordinating center; GERD, gastroesophageal reflux; LUL, left upper lobe; NCFB, non-cystic-fibrosis bronchiectasis; NTM, nontuberculous mycobacteria; PEP, positive expiratory pressure; RML, right middle lobe; RUL, right upper lobe
FOR EDITORIAL COMMENT SEE PAGE 953
Bronchiectasis is a chronic lung disease characterized by dilatation of airways, with injury to the bronchial walls due to recurrent infection and inflammation. It is typically distinguished by whether or not the patient has underlying cystic fibrosis (CF). Adult non-CF bronchiectasis (NCFB) is heterogeneous and has numerous causes.1, 2, 3, 4, 5 Idiopathic bronchiectasis and infection-related bronchiectasis represent the majority of adult cases of NCFB in most series.4, 5, 6, 7, 8 The prevalence of NCFB appears to be increasing in the United States. Seitz et al9 analyzed a 5% sample of Medicare Part B beneficiaries and reported that from 2000 to 2007, the prevalence increased 8.74% annually. In addition, the prevalence of NCFB increases substantially with aging.10 Furthermore, NCFB imposes a significant financial burden on patients and the US health-care system, with annual costs approximating $630 million.10
Despite the significant morbidity of NCFB and significant financial burden, there are limited data regarding the characteristics of patients with NCFB in the United States. To better define the characteristics of patients with NCFB and provide a resource for clinical trials and other research, the Bronchiectasis Research Registry (BRR) was established within the COPD Foundation in 2008. The registry includes 13 sites across the country where patients are enrolled through a centralized database. As such, the BRR is not a tool to generate population-based prevalence data. To our knowledge, this is the first report describing the US BRR cohort.
Methods
The BRR is a centralized database of patients with bronchiectasis identified at 13 clinical sites throughout the United States (e-Appendix 1). Adult patients with a physician-established diagnosis of bronchiectasis were eligible for inclusion. The institutional review board of each participating site approved the study, as did an administrative institutional review board for the data collecting center (DCC). After providing informed consent, medical records were queried by a study coordinator or principal investigator using standardized recording forms. For purposes of this report, NCFB is heretofore labeled as bronchiectasis. The exclusion of patients with primary CF was established based on clinical history, previous sweat chloride test results, genetic testing results, or a combination, at the time of enrollment. Exacerbations were recorded based on historical information. Data were entered through a centralized Internet-based entry system at the University of North Carolina. Study coordinators received training from the DCC. Quality control occurred in real time, as the data management system incorporated expected range checks. The BRR is sponsored by the COPD Foundation.
Spirometry
Spirometric results measured closest to the time of enrollment were abstracted from patient records. Spirometric results were considered normal when the FEV1/FVC was ≥ 0.70 and the FVC and FEV1 were > 80% of predicted. Airflow obstruction was defined as FEV1/FVC < 0.70 and was defined as mild, moderate, severe, and very severe obstruction with a FEV1 of ≥ 80% predicted, ≥ 50% and < 80%, ≥ 30% and < 50%, and < 30% of predicted, respectively.11 Patients in whom the FEV1/FVC was > 0.70 and the FVC was < 80% were labeled as having restriction. A bronchodilator response was considered present when the FEV1 or FVC improved ≥ 12% after bronchodilator use.
Chest Imaging
Chest CT scans were read by principal investigators or site radiologists.
Microbiological Evaluation
A maximum of three respiratory culture results for bacteria, fungi, and mycobacteria (total of a maximum of nine culture results) were abstracted between the 2 years prior to and 90 days after enrollment, which was defined as the baseline period. We recorded positive culture results during the baseline period, and we subsequently stratified these results based on patients’ nontuberculous mycobacteria (NTM) status. For the purposes of this analysis, we defined patients with NTM as those with either a reported history of pulmonary NTM disease prior to enrollment or those with one or more NTM isolates in respiratory specimen cultures within the baseline period, or both.
Treatment
Treatment information was abstracted across several domains, including the use of antibiotics, corticosteroids, or bronchodilators; medication for acid suppression; mucus-active agents; and measures to enhance bronchial hygiene. Categories of antibiotic use included antibiotics for exacerbation only, any suppressive antibiotic, rotation of oral suppressive antibiotics, or inhaled suppressive antibiotics. Measures to improve bronchial hygiene were defined as any nonpharmacologic measure to improve bronchial hygiene.
Statistical Analysis
The analysis population consisted of patients enrolled in the BRR as of July 1, 2014. Demographic and physical characteristics, medical history, respiratory symptoms, imaging findings, spirometric findings, microbiological culture results, and therapies administered were summarized for all participants, with subsequent stratification based on NTM status, as already described. χ2 tests for categorical data and t tests for continuous data were used to compare patients with NTM and those without NTM for the subset of variables for which clinically meaningful relationships were hypothesized. These comparisons were considered exploratory, and no adjustment for potential confounding variables or multiple comparisons were made.
Results
Demographic and Baseline Characteristics
A total of 1,941 patients were enrolled in the BRR as of July 1, 2014. One hundred fifteen patients were subsequently excluded from analysis due to withdrawal of consent (19 patients), diagnosis of NTM without bronchiectasis (11 patients), missing identification of sex (24 patients), or missing NTM status (61 patients). The evaluable 1,826 patients with bronchiectasis enrolled between 2008 and 2014 were then categorized based on NTM status. Baseline information at the time of enrollment is detailed in Table 1. The study population was predominantly composed of women (79%) and non-Hispanic white patients (89%). The mean age was 64 ± 14 years, with a diagnosis of bronchiectasis in most patients (77%) occurring between the ages of 50 and 79 years. Forty-seven percent had commercial insurance coverage and 47% had Medicare or Medicaid. Sixty percent were never smokers and 68% had a prior history of pneumonia. Three percent had primary ciliary dyskinesia, 3% had pectus excavatum, and 1% had HIV infection.
Table 1.
Demographics and Clinical Characteristics of Patients With Bronchiectasisa
| Characteristic | Data Available (No.) | Overall (N = 1,826) | NTM (n = 1,158) | No NTM (n = 668) | P Valueb |
|---|---|---|---|---|---|
| Sex, No. (%) | 1,826 | ||||
| Female | 1,439 (79) | 964 (83) | 475 (71) | < .01 | |
| Age, mean ± SD, y | 1,823 | 64 ± 14 | 66 ± 12 | 61 ± 17 | < .01 |
| Age at diagnosis, mean ± SD, y | 1,456 | 57 ± 17 | 59 ± 15 | 53 ± 19 | < .01 |
| Race/ethnicity, No. (%) | 1,709 | ||||
| Non-Hispanic white | 1,514 (89) | 1,003 (91) | 511 (85) | < .01 | |
| Non-Hispanic black | 34 (2) | 7 (1) | 27 (4) | ||
| Hispanic | 73 (4) | 41 (4) | 32 (5) | ||
| Asian | 60 (4) | 41 (4) | 19 (3) | ||
| Other | 28 (2) | 16 (1) | 12 (2) | ||
| Primary insurance, No. (%) | 1,684 | ||||
| Commercial | 794 (47) | 504 (48) | 290 (46) | < .01 | |
| Medicaid and other state programs | 49 (3) | 24 (2) | 25 (4) | ||
| Medicare | 749 (44) | 485 (46) | 264 (42) | ||
| No insurance | 18 (1) | 9 (1) | 9 (1) | ||
| Other (including Tricare) | 74 (4) | 29 (3) | 45 (7) | ||
| BMI, mean ± SD, kg/m2 | 1,812 | 23.2 ± 5.7 | 22.5 ± 5.5 | 24.3 ± 5.8 | < .01 |
| q1, q3, | 19.9, 25.1 | 19.7, 24.3 | 20.3, 26.8 | ||
| Smoking, No. (%) | 1,815 | ||||
| Never | 1,094 (60) | 686 (60) | 408 (61) | .74 | |
| Former | 693 (38) | 447 (39) | 246 (37) | ||
| Current | 28 (2) | 18 (2) | 10 (2) | ||
| Chest wall deformity, No. (%) | 1,731 | ||||
| None | 1,657 (96) | 1,038 (96) | 619 (96) | .02 | |
| Pectus excavatum | 56 (3) | 39 (4) | 17 (3) | ||
| Other | 18 (1) | 6 (1) | 12 (2) | ||
| Otitis or rhinosinusitis, No. (%) | 1,562 | ||||
| Yes | 388 (25) | 222 (23) | 166 (29) | < .01 | |
| Comorbidities, No. (%) | |||||
| History of pneumonia | 1,745 | 1,187 (68) | 758 (69) | 429 (67) | .45 |
| COPD | 1,778 | 350 (20) | 217 (19) | 133 (20) | .60 |
| Asthma | 1,783 | 515 (29) | 298 (26) | 217 (33) | < .01 |
| GERD | 1,789 | 841 (47) | 577 (51) | 264 (40) | < .01 |
| Rheumatologic disease | 1,775 | 142 (8) | 87 (8) | 55 (8) | .60 |
| Chronic ulcerative colitis or Crohn’s disease | 1,795 | 47 (3) | 26 (2) | 21 (3) | .25 |
| Primary immunodeficiency | 1,776 | 89 (5) | 44 (4) | 45 (7) | < .01 |
| Primary ciliary dyskinesia | 1,791 | 52 (3) | 20 (2) | 32 (5) | < .01 |
| Prior tuberculosis, No. (%) | 1,781 | ||||
| Yes | 70 (4) | 50 (4) | 20 (3) | .14 | |
| History of pulmonary exacerbation in the past 2 y, No. (%) | 1,754 | 1,124 (64) | 687 (62) | 437 (68) | .01 |
| No. of pulmonary exacerbations in the past 2 y, No. (%) | 992 | 3.0 ± 2.8 | 2.7 ± 2.3 | 3.4 ± 3.3 | < .01 |
GERD = gastroesophageal reflux disease; NTM = nontuberculous mycobacteria.
Percentages and other descriptive statistics calculated after excluding participants with missing data from the column total. Less than 1% of participants had missing data for all items except the following: age at diagnosis (30%), race/ethnicity (6%), primary insurance (8%), chest wall deformity (5%), history of pneumonia (4%), otitis or rhinosinusitis (14%), respiratory distress at birth (17%), COPD (3%), asthma (2%), GERD (2%), rheumatologic disease (3%), chronic ulcerative colitis or Crohn’s disease (2%), primary immunodeficiency (3%), primary ciliary dyskinesia (2%), and prior tuberculosis (2%).
P values for categorical variables are from χ2 tests, and from t tests for continuous variables comparing patients with NTM vs patients without NTM.
As also shown in Table 1, 63% (1,158 of 1,826) had coexistent NTM. Patients with NTM compared with patients without NTM were older, diagnosed with bronchiectasis at a later age, predominantly women, and had a lower BMI. Gastroesophageal reflux disease (GERD) was present more frequently in those with NTM, whereas asthma, primary ciliary dyskinesia, and immunodeficiency were more common in those without NTM.
Exacerbations were reported at baseline in 64% of patients within the preceding 2 years. Patients with NTM had fewer exacerbations (2.7 ± 2.3) during the prior 2 years than did those without NTM (3.4 ± 3.3; P < .01) (Table 1).
Table 2 summarizes respiratory symptoms. The most common symptoms included cough (73%) that was productive (53%), dyspnea (64%), and fatigue (50%). Fatigue and hemoptysis were more common in those with NTM, whereas cough was more common in those without NTM. Sweat chloride testing and CF nasal potential difference measurements were performed in 12% and 1.9% of patients, respectively; the results were abnormal in 9% and 11%, respectively (data not shown).
Table 2.
Symptoms in Patients With Bronchiectasis by NTM Statusa
| Symptom | Data Available (No.) | Overall (N = 1,826) | NTM (n = 1,158) | No NTM (n = 668) | P Valueb |
|---|---|---|---|---|---|
| Fatigue, No. (%) | 1,770 | ||||
| Yes | 886 (50) | 591 (53) | 295 (46) | < .01 | |
| Daily bouts of coughing, No. (%) | 1,804 | ||||
| Yes, any | 1,314 (73) | 825 (72) | 489 (74) | .32 | |
| Daily productive cough, No. (%) | 1,788 | ||||
| Yes, productive cough | 951 (53) | 568 (50) | 383 (59) | < .01 | |
| Hemoptysis, No. (%) | 175 | ||||
| Yes | 409 (23) | 283 (25) | 126 (19) | < .01 | |
| Dyspnea, No. (%) | 1,442 | ||||
| No, not at rest or when active | 663 (46) | 420 (46) | 243 (46) | .98 | |
| Yes, only when active | 779 (54) | 493 (54) | 286 (54) |
See Table 1 legend for expansion of abbreviations.
Percentages and other descriptive statistics calculated after excluding participants with missing data from the column total. Less than 1% of participants had missing data for all items except the following: undue fatigue (3%), daily bouts of coughing (1%), daily productive cough (2%), hemoptysis (3%), and dyspnea (21%).
P values for categorical variables are from χ2 tests and from t tests for continuous variables comparing patients with NTM vs patients without NTM.
Spirometry
Eighty-five percent of patients had spirometric results reported. No significant difference was observed between patients with NTM and patients without NTM (Table 3). Twenty-six percent had normal spirometric results. Fifty-one percent of patients had obstruction. Three-quarters of patients with obstruction (76%) fell into the mild to moderate category. Twenty percent of patients had suggestive restrictive impairment. Only 5% of patients had a response to aerosol bronchodilators.
Table 3.
Spirometric Test Results for Patients With Bronchiectasis
| Results | Data Available (No.) | Overall (N = 1,826) | NTM (n = 1,158) | No NTM (n = 668) | P Valuea |
|---|---|---|---|---|---|
| Prebronchodilator findings, No. (%)b | 1,552 | ||||
| FEV1/FVC ≥ 0.70, FVC ≥ 0.80, and FEV1 ≥ 0.80 (normal) | 399 (26) | 252 (26) | 147 (26) | ||
| FEV1/FVC ≥ 0.70, FVC ≥ 0.80, and FEV1 < 0.80 (nearly normal) | 363 (23) | 229 (23) | 134 (24) | ||
| Any obstruction | 790 (51) | 502 (51) | 208 (51) | .86 | |
| Mild or moderate obstruction | 555 (36) | 366 (37) | 189 (33) | .11 | |
| Severe or very severe obstruction | 235 (15) | 136 (14) | 99 (17) | .06 | |
| Restriction | 317 (20) | 200 (20) | 117 (21) | .92 | |
| Postbronchodilator findings, No. (%)c | 963 | ||||
| FVC or FEV1 improved ≥ 12% | 47 (5) | 33 (5) | 14 (4) |
See Table 1 legend for expansion of abbreviations.
P values for categorical variables are from χ2 tests and from t tests for continuous variables comparing patients with NTM and patients without NTM.
Includes participants with prebronchodilator FEV1, FVC, and FEV1/FVC measurements. Any obstruction is defined as FEV1/FVC < .70. Mild or moderate obstruction combines the mild and moderate groups defined in text. Severe or very severe obstruction combines the severe and very severe groups defined in text. Restricted is defined as FEV1/FVC ≥ 0.70 and FVC < 0.80.
Includes only participants with both prebronchodilator and postbronchodilator FEV1 and FVC measurements.
Chest CT
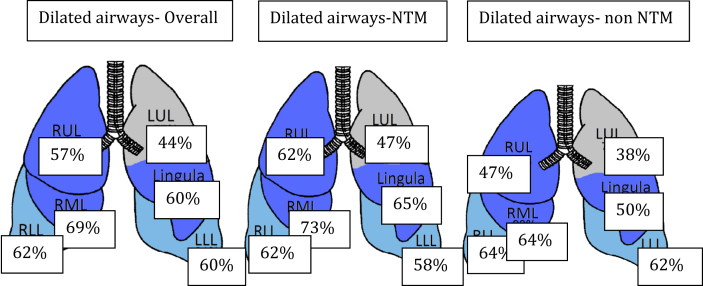
CT scans were available for analysis from 1,553 patients (85%). The right middle lobe (RML) (69%) was the most commonly involved lobe, whereas the upper division of the left upper lobe (LUL) (44%) was the least commonly involved. RML, lingular, right upper lobe (RUL), and LUL airway dilation was more common in those with NTM (Fig 1). A single lobe was involved in only 11% of patients and occurred more commonly in those without NTM (Table 4). Sixty percent of patients had tree-in-bud infiltrates, with involvement of all lobes including a higher percentage of tree-in bud infiltrates in patients with NTM. Mucoid impaction within the RML, lingula, and RUL was more common in patients with NTM (data not shown). In general, patients with NTM were more likely to have dilated airways, thickened walls, or mucoid impaction within the upper lobes, lingula, and middle lobes.
Figure 1.
All lobes were involved; the RML (69%) was involved most and the upper division of the LUL (44%) was involved least. Except for the lower lobes, the other lobes were involved to a greater extent in NTM than in subjects without NTM. LUL =left upper lobe; NTM = nontuberculous mycobacteria; RLL = right lower lobe; RML = right middle lobe; RUL = right upper lobe.
Table 4.
Chest CT Imaging Findings in Patents With Bronchiectasis
| Chest CT Finding | Data Available (No.) | Overall (N = 1,826) | NTM (n = 1,158) | No NTM (n = 668) | P Valuea |
|---|---|---|---|---|---|
| Dilated airways, No. (%) | |||||
| None indicatedb | 1,553 | 105 (7) | 72 (7) | 33 (6) | .18 |
| Left upper lobe (upper division) | 1,390 | 607 (44) | 413 (47) | 194 (38) | < .01 |
| Lingula | 1,392 | 832 (60) | 573 (65) | 259 (50) | < .01 |
| Left lower lobe | 1,389 | 830 (60) | 510 (58) | 320 (62) | .13 |
| Right upper lobe | 1,392 | 787 (57) | 544 (62) | 243 (47) | < .01 |
| Right middle lobe | 1,398 | 969 (69) | 639 (73) | 330 (64) | < .01 |
| Right lower lobe | 1,388 | 867 (62) | 536 (62) | 331 (64) | .36 |
| Only 1 of the above sites | 1,419 | 152 (11) | 82 (9) | 70 (13) | < .01 |
| 2-3 of the above sites | 1,419 | 479 (34) | 276 (31) | 203 (39) | |
| > 3 of the above sites | 1,419 | 683 (48) | 464 (52) | 219 (42) | |
| Tree-in-bud infiltrates, No. (%) | |||||
| None indicated | 1,542 | 610 (40) | 315 (32) | 295 (52) | < .01 |
| Left upper lobe (upper division) | 1,465 | 366 (25) | 279 (30) | 87 (16) | < .01 |
| Lingula | 1,467 | 528 (36) | 394 (43) | 134 (24) | < .01 |
| Left lower lobe | 1,472 | 556 (38) | 388 (42) | 168 (31) | < .01 |
| Right upper lobe | 1,474 | 520 (35) | 397 (43) | 123 (22) | < .01 |
| Right middle lobe | 1,464 | 581 (40) | 419 (46) | 162 (30) | < .01 |
| Right lower lobe | 1,472 | 611 (42) | 426 (46) | 185 (34) | < .01 |
| Only 1 of the above sites | 1,491 | 119 (8) | 78 (8) | 41 (7) | < .01 |
| 2-3 of the above sites | 1,491 | 331 (22) | 222 (24) | 109 (20) | |
| > 3 of the above sites | 1,491 | 431 (29) | 324 (35) | 107 (19) | |
| Any dilated airways, thickened airway walls, or mucoid impaction, No. (%) | |||||
| None indicated | 1,577 | 40 (3) | 26 (3) | 14 (2) | .77 |
| Left upper lobe (upper division) | 1,380 | 713 (52) | 482 (56) | 231 (45) | < .01 |
| Lingula | 1,380 | 926 (67) | 634 (73) | 292 (58) | < .01 |
| Left lower lobe | 1,399 | 965 (69) | 585 (67) | 380 (73) | .02 |
| Right upper lobe | 1,393 | 899 (65) | 613 (70) | 286 (55) | < .01 |
| Right middle lobe | 1,397 | 1,062 (76) | 698 (79) | 364 (71) | < .01 |
| Right lower lobe | 1,396 | 1,004 (72) | 618 (70) | 386 (74) | .12 |
| Only 1 of the above sites | 1,470 | 135 (9) | 84 (9) | 51 (9) | < .01 |
| 2-3 of the above sites | 1,470 | 491 (33) | 280 (30) | 211 (39) | |
| > 3 of the above sites | 1,470 | 804 (55) | 537 (58) | 267 (49) |
See Table 1 legend for expansion of abbreviations.
P values for categorical variables are from χ2 tests and from t tests for continuous variables comparing patients with NTM vs patients without NTM.
Participant met the inclusion criteria for bronchiectasis, but site with dilated airways not identified.
Microbiological Evaluation
Of 1,826 evaluable patients, 1,645 (90%) had at least one type of culture performed during the baseline period. This included 1,314 patients (72%) with one or more acid-fast bacillus (AFB) cultures, 1,406 (77%) with one or more bacterial cultures, and 1,087 patients (60%) with one or more fungal cultures performed. For patients with AFB cultures, 484 (37%) isolated Mycobacterium avium complex, 130 (10%) isolated M. abscessus/chelonae, and 90 (8%) isolated other mycobacterial or Nocardia species (Table 5). Of those with bacterial cultures, 470 (33%) isolated Pseudomonas species and 170 (12%) isolated S. aureus. A variety of other bacterial pathogens were reported. Among those with fungal cultures, Aspergillus species were most commonly isolated.
Table 5.
Microbiological Results for Patients With Bronchiectasis
| Microbiological Result | Data Available (No.) | Overall (N = 1,826) | NTM (n = 1,158) | No NTM (n = 668) | P Valuea |
|---|---|---|---|---|---|
| Bacterial culture findings, No. (%) | |||||
| No growth in any culture | 1,406 | 93 (7) | 68 (8) | 25 (5) | .06 |
| Oropharyngeal flora | 1,406 | 1,037 (74) | 669 (74) | 368 (73) | .57 |
| Haemophilus influenzae | 1,406 | 116 (8) | 72 (8) | 44 (9) | .64 |
| Streptococcus pneumoniae | 1,406 | 49 (3) | 26 (3) | 23 (5) | .10 |
| Staphylococcus aureusb | 1,406 | 170 (12) | 92 (10) | 78 (15) | < .01 |
| Pseudomonas aeruginosac | 1,406 | 470 (33) | 270 (30) | 200 (40) | < .01 |
| Stenotrophomonas maltophilia | 1,406 | 76 (5) | 54 (6) | 22 (4) | .19 |
| Klebsiella pneumoniae | 1,406 | 35 (2) | 24 (3) | 11 (2) | .58 |
| Moraxella catarrhalis | 1,406 | 20 (1) | 9 (1) | 11 (2) | .10 |
| Achromobacter | 1,406 | 15 (1) | 9 (1) | 6 (1) | .79 |
| Alcaligenes | 1,406 | 13 (1) | 5 (1) | 8 (2) | .08 |
| Serratia marcescens | 1,406 | 30 (2) | 25 (3) | 5 (1) | .03 |
| Burkholderia species | 1,406 | 5 (0) | 3 (0) | 2 (0) | 1.00 |
| Mycobacterial smear/culture | |||||
| AFB smear positive | 1,314 | 319 (24) | 302 (33) | 17 (4) | < .01 |
| Growth in any culture | 1,314 | 657 (50) | 653 (71) | 4 (1) | < .01 |
| Mycobacterium avium complex | 1,314 | 484 (37) | 484 (52) | 0 | |
| Mycobacterium abscessus/chelonaed | 1,314 | 130 (10) | 130 (14) | 0 | |
| Mycobacterium kansasii | 1,314 | 8 (1) | 8 (1) | 0 | |
| Mycobacterium gordonae | 1,314 | 37 (3) | 37 (4) | 0 | |
| Other mycobacterial species | 1,314 | 36 (3) | 36 (4) | 0 | |
| Nocardia | 1,314 | 9 (1) | 8 (1) | 1 (0) | |
| Fungal culture findings, No. (%) | |||||
| No growth in any culture | 1,087 | 534 (49) | 364 (48) | 170 (53) | .10 |
| Aspergillus speciese | 1,087 | 211 (19) | 159 (21) | 52 (16) | .08 |
| Scedosporium apiospermumf | 1,087 | 34 (3) | 28 (4) | 6 (2) | .18 |
| Other fungal species | 1,087 | 392 (36) | 284 (37) | 108 (34) | .28 |
| Summary of lower respiratory culture findings, No. (%) | 1,645 | ||||
| No growth in any culture | 136 (8) | 85 (8) | 51 (9) | ||
| Multiple pathogens isolated | 1,050 (64) | 736 (67) | 314 (58) |
AFB = acid-fast bacillus; MRSA = methicillin-resistant Staphylococcus aureus. See Table 1 legend for expansion of other abbreviations.
P values for categorical variables are from Fisher’s exact tests when counts are < 10 and from χ2 tests otherwise, comparing patients with NTM vs patients without NTM.
Includes 30 reported as methicillin sensitive, 58 as methicillin resistant, and 3 coded as “MRSA” from open-ended responses.
Includes 52 reported as none mucoid, 174 as at least 1 mucoid, and 17 coded as “Pseudomonas” from open-ended responses.
Includes 4 reported in open-ended findings that were coded as Mycobacterium chelonae and 9 reported as Mycobacterium massiliense.
Includes 56 responses to open-ended findings that were coded as Aspergillus not otherwise speciated.
Includes 6 responses to open-ended findings that were coded as Scedosporium not otherwise speciated.
Although isolation of Pseudomonas species was common among the entire cohort, it was significantly less common among patients with NTM (n = 270 [30%]) vs patients without NTM (n = 200 [40%; P < .01]. Similarly, S. aureus was also less common among patients with NTM, occurring in 92 patients with NTM (10%) and 78 patients without NTM (15%), respectively (P < .01). No significant difference in Aspergillus isolation was identified between NTM (n = 159 [21%]) and non-NTM groups (n = 52 [16%]; P = . 08).
Treatment
Therapies for bronchiectasis were reported in 1,826 patients (Table 6). Forty-one percent (727 of 1,764) of patients reported antibiotic use for exacerbations only. Any suppressive antibiotic use was noted in 694 of 1,775 patients (39%), with aerosol antibiotics reported in 178 of 1,759 (10%). Compared with patients without NTM, patients with NTM used antibiotics for exacerbations only less often (36% vs 51%; P < .01) but used any suppressive antibiotic (43% vs 32%; P < .01) more often. There was no difference in aerosol antibiotic use. Seven percent of patients (125 of 1,771) used rotating oral antibiotics, and this was less common in those with NTM (6% vs 9%; P < .01).
Table 6.
Therapies Reported for Patients With Bronchiectasisa
| Therapy | Data Available (No.) | Overall (N = 1,826) | NTM (n = 1,158) | No NTM (n = 668) | P Valueb NTM vs No NTM |
|---|---|---|---|---|---|
| Antibiotic use, No. (%) | |||||
| Antibiotics for acute exacerbations only | 1,764 | 727 (41) | 402 (36) | 325 (50) | < .01 |
| Any suppressive antibiotic | 1,775 | 694 (39) | 491 (43) | 203 (32) | < .01 |
| Rotating oral suppressive antibiotics | 1,771 | 125 (7) | 64 (6) | 61 (9) | < .01 |
| Inhaled suppressive antibiotics | 1,759 | 178 (10) | 113 (10) | 65 (10) | .98 |
| Use of other therapies, No. (%) | |||||
| Inhaled steroid | 1,794 | 696 (39) | 403 (35) | 293 (45) | < .01 |
| Any oral steroid | 1,789 | 237 (13) | 112 (10) | 125 (19) | < .01 |
| Inhaled bronchodilator | 1,798 | 1,098 (61) | 638 (56) | 460 (70) | < .01 |
| Medication for gastric acid suppression | 1,786 | 667 (37) | 432 (38) | 235 (36) | .43 |
| Mucus-active agent | 1,784 | 424 (24) | 252 (22) | 172 (26) | .04 |
| Measures to improve bronchial hygiene, No. (%) | |||||
| Yes | 1,730 | 965 (56) | 642 (59) | 323 (50) | < .01 |
| Chest percussion/postural drainage | 1,711 | 279 (16) | 200 (19) | 79 (12) | < .01 |
| Flutter or positive expiratory pressure valve | 1,719 | 825 (48) | 568 (52) | 257 (40) | < .01 |
| High-frequency chest oscillation | 1,716 | 252 (15) | 142 (13) | 110 (17) | .02 |
See Table 1 legend for expansion of abbreviation.
Percentages and other descriptive statistics calculated after excluding participants with missing data from the column total. Less than 1% of participants had missing data for all items except the following: antibiotics for acute exacerbations only (3%), any suppressive antibiotic (3%), rotating oral suppressive antibiotics (3%), aerosol suppressive antibiotics (4%), inhaled steroid (2%), any oral steroid (2%), inhaled bronchodilator (2%), medication for gastric acid suppression (2%), mucolytic agent (2%), measure to improve bronchial hygiene (5%), chest percussion/postdrainage (6%), uses flutter or Acapella valve (6%), uses high frequency chest oscillation (6%).
P value is from χ2 test comparing patients with NTM vs patients without NTM.
Inhaled steroids were used almost three times more commonly than were oral steroids (39% vs 13%). Inhaled bronchodilators were used in 61% of patients. Inhaled vs oral steroids were used less commonly in those with NTM compared with those without NTM (35% vs 45%; P < .01, and 10% vs 19%; P < .01, respectively), as was the use of inhaled bronchodilators (56% vs 70%; P < .01). Medication for acid suppression was used in slightly greater than one-third of patients (37%); 86% of such substances were proton pump inhibitors (data not shown). No difference in the use of medication for acid suppression was reported in those with and those without NTM. Mucus-active agents were used in 24% of patients and included hypertonic saline in 76% of those using mucus-active agents (data not shown). These agents were used slightly more commonly in those without NTM.
Nonpharmacologic measures to improve bronchial hygiene were used in 56% of patients, including 48% of patients (825 of 1,719) using a flutter or positive expiratory pressure (PEP) valve. The overall use of chest percussion/postural drainage and high-frequency chest oscillation was similar at 16% and 15%, respectively. Those with NTM were more likely to use bronchial hygiene, chest percussion, or a flutter or PEP valve compared with those without NTM (59% vs 50%; P < .01; 19% vs 12%; P < .01; or 52% vs 40%; P < .01, respectively).
Discussion
This first report from the BRR describes the largest US cohort of patients with bronchiectasis to date. The registry has prospectively enrolled > 1,900 patients with NFCB, 1,826 of whom were evaluable. Most are non-Hispanic white women and lifelong nonsmokers. In this cohort, a large proportion of the patients had a history of NTM disease or had NTM isolated at their baseline evaluation. Although we identified important differences in patients with and those without NTM, it should be recognized that this registry was developed as a bronchiectasis registry. As such, NTM lung-disease-specific data domains appropriate for a specific NTM lung disease registry were not collected. Nonetheless, our findings are in agreement with published data reporting that most patients with idiopathic bronchiectasis are female nonsmokers.4, 5, 6, 7, 8 In addition, we report that those with NTM and bronchiectasis are more likely to be women, are less likely to have Pseudomonas isolated in sputum, and are older at the time of diagnosis than those without NTM.
In agreement with previous studies, we also describe a broad spectrum of comorbidities associated with bronchiectasis.1, 2, 3, 7, 8 Patients with asthma, primary immunodeficiency, and primary ciliary dyskinesia were less likely to have NTM, whereas those with GERD were more likely to have NTM. Prior investigations have demonstrated coexistent NTM lung disease and GERD.12, 13
In this study, more than one-half of the subjects had evidence of airflow obstruction. Interestingly, one-fifth of the patients also had suggestion of restriction on spirometry. Worse lung function in bronchiectasis is associated with more involvement of bronchiectasis on CT scans, the presence of Pseudomonas species,14, 15, 16, 17, 18 and the presence of COPD.19 Response to bronchodilator use was documented in 5% of subjects, which is lower than has been reported previously.20
There appeared to be no difference in spirometric results between those with NTM and those without in this cohort of patients, a finding that has not been well described previously.
This cohort includes descriptive high-resolution CT imaging findings in those with and those without NTM. Both diseases involve multiple lobes, although NTM-associated bronchiectasis involves upper lobe and middle lobe distribution more than non-NTM bronchiectasis does, as has been noted by others.21, 22, 23, 24
Treatments for bronchiectasis varied widely within both groups of patients. Antibiotic use was common and nearly evenly split between antibiotics used for acute exacerbations only and suppressive antibiotics. Antibiotics for acute exacerbations only were more commonly used for those without NTM, and suppressive antibiotics were used more frequently in those with NTM. Designation of antibiotics used for NTM vs bronchiectasis was not specified. A relatively small percentage of patients with bronchiectasis used aerosol antibiotics, likely reflecting the lack of positive clinical trials in this population and the period of enrollment. Moreover, current practice patterns in the participating centers reflect the lack of data to support rotating oral antibiotics, which is similar to recommendations in published guidelines.25
Even though there is a paucity of data to support its use, bronchodilator use was noted in more than one-half of patients with bronchiectasis and was more commonly used in those without NTM. Given the central role of mucociliary clearance and bronchial hygiene in the management of bronchiectasis, it is surprising that slightly more than one-half of patients used some measure to improve bronchial hygiene; the majority used a flutter or PEP valve. Consistent use of bronchial hygiene is in alignment with published literature and was used more often by those with NTM than by those without NTM.2, 4, 25, 26
There are several limitations to our study. Because this study describes a cohort of patients enrolled from tertiary referral institutions with interest in NTM lung disease, the demographic information described is potentially biased, including overrepresentation of patients with NTM. Moreover, there was a predominance of geographic groupings of participating sites in the eastern United States. The presence or absence of coexistent illnesses was based on history. It is difficult to ascertain if GERD or coexistent obstructive lung disease, or both, was truly present in conjunction with bronchiectasis or had been ascribed based on compatible symptoms or spirometric findings, or both.
In conclusion, the BRR has enrolled 1,826 evaluable patients with bronchiectasis from 13 sites across the United States. Despite baseline characteristics of the study population sharing phenotypic similarities, this study notes significant differences in patient groups with and without the presence of NTM.
Acknowledgements
Author contributions: T. R. A. had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. A. E. O., A. B., K. N. O., K. L. W., M. L. A. D., M. J., E. E., D. G., M. K., M. M., M. S., B. T., G. Tino, G. Turino, B. C., and C. L. D. contributed substantially to the study design, data analysis and interpretation, and writing of the manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: T. R. A. has participated in clinical trials sponsored by Bayer, Aradigm, and Insmed but has not received any personal or research support. A. E. O. is a principal investigator and received grant support for clinical trials for Aradigm, Bayer, and Insmed and is a consultant for Bayer, Novartis, and Xellia Pharmaceuticals. A. B. is a consultant and principal investigator for a clinical research study for Bayer. K. N. O.’s employer, NIAID, had a Cooperative Research and Development Agreement with Insmed. K. L. W. has received grant support from Insmed and is a consultant for Bayer. D. G. has received support as a consultant for Aradigm/Grifols. M. M. participated in and received support for clinical trials for Aradigm and Bayer and is a consultant for Aradigm/Grifols. M. S. has received grant support for clinical trials with Aradigm, Insmed, Gilead, Pharmaxis, and JHP Pharmaceuticals and is a consultant for Insmed. G. Tino has been a consultant for Aradigm /Grifols and Bayer. C. L. D. has received research support from Insmed. None declared (L. A. D., M. J., E. E., M. K., B. T., G. Turino, and B. C.).
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript; although sponsor's administrative support by Delia Prieto and Elisha Malanga was invaluable.
Other contributions: The BRR is sponsored by the COPD Foundation, a 501 (c) (3) nonprofit organization. The Bronchiectasis Research Consortium would like to acknowledge the support of the Richard H. Scarborough Bronchiectasis Research Fund, the Anna-Maria and Stephen Kellen Foundation, the Bronchiectasis Research Registry Industry Advisory Committee (Aradigm Corp, Bayer HealthCare Inc., Grifols Inc., and Insmed Inc.), and the staff of the COPD Foundation for their commitment to the success of the Registry. It should also be noted that this work would not have been possible without the comprehensive chart reviews and recording of data by dedicated research coordinators at each of the participating sites.
Additional information: The e-Appendix can be found in the Supplemental Materials section of the online article.
Footnotes
FUNDING/SUPPORT: This study was funded by the COPD Foundation and, in part (K. N. O.), by the Intramural Research Program of the National Heart, Lung, and Blood Institute, National Institutes of Health.
Supplementary Data
References
- 1.Metersky M.L. The initial evaluation of adults with bronchiectasis. Clin Chest. Med. 2012;33:219–231. doi: 10.1016/j.ccm.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 2.McShane P.J., Naureckas E.T., Tino G. Non-cystic fibrosis bronchiectasis. Am J Respir Crit Care Med. 2013;188:647–656. doi: 10.1164/rccm.201303-0411CI. [DOI] [PubMed] [Google Scholar]
- 3.McShane P.J., Naureckas E.T., Strek M.E. Bronchiectasis in a diverse US population: effects of ethnicity on etiology and sputum culture. Chest. 2012;142:159–167. doi: 10.1378/chest.11-1024. [DOI] [PubMed] [Google Scholar]
- 4.O'Donnell A.E. Bronchiectasis. Chest. 2008;134:815–823. doi: 10.1378/chest.08-0776. [DOI] [PubMed] [Google Scholar]
- 5.Lonni S., Chalmers J.D., Goeminne P.C. Etiology of Non-cystic fibrosis bronchiectasis in adults and its correlation to disease severity. Ann Am Thorac Soc. 2015;12:1764–1770. doi: 10.1513/AnnalsATS.201507-472OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barker A.F. Bronchiectasis. N Engl J Med. 2002;346:1383–1393. doi: 10.1056/NEJMra012519. [DOI] [PubMed] [Google Scholar]
- 7.Shoemark A., Ozerovitch L., Wilson R. Aetiology in adult patients with bronchiectasis. Respir. Med. 2007;101:1163–1170. doi: 10.1016/j.rmed.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 8.Pasteur M.C., Helliwell S.M., Houghton S.J. An investigation into causative factors in patients with bronchiectasis. Am J Respir Crit Care Med. 2000;162:1277–1284. doi: 10.1164/ajrccm.162.4.9906120. [DOI] [PubMed] [Google Scholar]
- 9.Seitz A.E., Olivier K.N., Adjemian J. Trends in bronchiectasis among Medicare beneficiaries in the United States, 2000 to 2007. Chest. 2012;142:432–439. doi: 10.1378/chest.11-2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weycker D., Edelsberg J., Oster G. Prevalence and economic burden of bronchiectasis. Clin Pulm Med. 2005;12:205–209. [Google Scholar]
- 11.Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of COPD 2016. http://goldcopd.org/global-strategy-diagnosis-management-prevention-copd-2016/. Accessed February 20, 2017.
- 12.Koh W.J., Lee J.H., Kwon Y.S. Prevalence of gastroesophageal reflux disease in patients with nontuberculous mycobacterial lung disease. Chest. 2007;131:1825–1830. doi: 10.1378/chest.06-2280. [DOI] [PubMed] [Google Scholar]
- 13.Thomson R.M., Armstrong J.G., Looke D.F. Gastroesophageal reflux disease, acid suppression, and Mycobacterium avium complex pulmonary disease. Chest. 2007;131:1166–1172. doi: 10.1378/chest.06-1906. [DOI] [PubMed] [Google Scholar]
- 14.Ho P.L., Chan K.N., Ip M.S. The effect of Pseudomonas aeruginosa infection on clinical parameters in steady-state bronchiectasis. Chest. 1998;114:1594–1598. doi: 10.1378/chest.114.6.1594. [DOI] [PubMed] [Google Scholar]
- 15.Ip M., Lauder I.J., Wong W.Y. Multivariate analysis of factors affecting pulmonary function in bronchiectasis. Respiration. 1993;60:45–50. doi: 10.1159/000196172. [DOI] [PubMed] [Google Scholar]
- 16.Evans S.A., Turner S.M., Bosch B.J. Lung function in bronchiectasis: the influence of Pseudomonas aeruginosa. Eur Respir J. 1996;9:1601–1604. doi: 10.1183/09031936.96.09081601. [DOI] [PubMed] [Google Scholar]
- 17.Ryall B., Davies J.C., Wilson R. Pseudomonas aeruginosa, cyanide accumulation and lung function in CF and non-CF patients with bronchiectasis. Eur Respir J. 2008;32:740–747. doi: 10.1183/09031936.00159607. [DOI] [PubMed] [Google Scholar]
- 18.Davies G., Wells A.U., Doffman S. The effect of Pseudomonas aeruginosa on pulmonary function in patients with bronchiectasis. Eur Respir J. 2006;28:974–979. doi: 10.1183/09031936.06.00074605. [DOI] [PubMed] [Google Scholar]
- 19.Loubeyre P., Paret M., Revel D. Thin-section CT detection of emphysema associated with bronchiectasis and correlation with pulmonary function tests. Chest. 1996;109:360–365. doi: 10.1378/chest.109.2.360. [DOI] [PubMed] [Google Scholar]
- 20.Murphy M.B., Reen D.J., Fitzgerald M.X. Atopy, immunological changes, and respiratory function in bronchiectasis. Thorax. 1984;39:179–184. doi: 10.1136/thx.39.3.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim R.D., Greenberg D.E., Ehrmantraut M.E. Pulmonary nontuberculous mycobacterial disease: prospective study of a distinct preexisting syndrome. Am J Respir Crit Care Med. 2008;178:1066–1074. doi: 10.1164/rccm.200805-686OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lynch D.A., Simone P.M., Fox M.A. CT features of pulmonary Mycobacterium avium complex infection. J Comput Assis Tomogr. 1995;19:353–360. doi: 10.1097/00004728-199505000-00003. [DOI] [PubMed] [Google Scholar]
- 23.Hollings N.P., Wells A.U., Wilson R. Comparative appearances of non-tuberculous mycobacteria species: a CT study. Eur Radiol. 2002;12:2211–2217. doi: 10.1007/s00330-001-1282-1. [DOI] [PubMed] [Google Scholar]
- 24.Reiff D.B., Wells A.U., Carr D.H. CT findings in bronchiectasis: limited value in distinguishing between idiopathic and specific types. AJR Am J Roentgenol. 1995;165:261–267. doi: 10.2214/ajr.165.2.7618537. [DOI] [PubMed] [Google Scholar]
- 25.Pasteur M.C., Bilton D., Hill A.T. British Thoracic Society guideline for non-CF bronchiectasis. Thorax. 2010;65(suppl 1):i1–i58. doi: 10.1136/thx.2010.136119. [DOI] [PubMed] [Google Scholar]
- 26.Murray M.P., Pentland J.L., Hill A.T. A randomised crossover trial of chest physiotherapy in non-cystic fibrosis bronchiectasis. Eur Respir J. 2009;34:1086–1092. doi: 10.1183/09031936.00055509. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.