Abstract
Alpha-1 antitrypsin deficiency (AATD) is characterized by low serum levels of or dysfunctional alpha-1 proteinase inhibitor. In the lung parenchyma, this results in a loss of protection against the activity of serine proteases, particularly neutrophil elastase. The resultant imbalance in protease and antiprotease activity leads to an increased risk for the development of early-onset emphysema and COPD. As in traditional smoke-related COPD, the assessment of the severity and disease progression of lung disease in AATD is conventionally based on lung function; however, pulmonary function tests are unable to discriminate between emphysema and airways disease, the two hallmark pathologic features of COPD. CT imaging has been used as a tool to further characterize lung structure and evaluate therapeutic interventions in AATD-related COPD. Moreover, recent advances in quantitative CT have significantly improved our assessment of the lung architecture, which has provided investigators and clinicians with a more detailed evaluation of the extent and severity of emphysema and airways disease in AATD. In addition, serial CT imaging measures are becoming increasingly important, as they provide a tool to monitor emphysema progression. This review describes the principles of CT technology and the role of CT imaging in assessing pulmonary disease progression in AATD, including the effect of therapeutic interventions.
Key Words: alpha-1 antitrypsin, alpha-1 antitrypsin deficiency, alpha-1 proteinase inhibitor, COPD, CT, emphysema
Abbreviations: 2-D, two-dimensional; 3-D, three-dimensional; A1-PI, alpha-1 proteinase inhibitor; AATD, alpha-1 antitrypsin deficiency; FRC, functional residual capacity; HU, Hounsfield unit; PD15, 15th percentile density; TLC, total lung capacity; VI, voxel index
Alpha-1 antitrypsin deficiency (AATD) is the most recognized genetic disorder associated with an increased risk of COPD. The main feature of AATD is low serum levels of alpha-1 proteinase inhibitor (A1-PI), which results in a loss of protection in the lung parenchyma against the activity of proteases such as neutrophil elastase, proteinase-3, and cathepsin G.1 The resulting protease-antiprotease imbalance, along with the loss of important antiapoptotic and immunomodulatory functions of A1-PI, leads to an increased risk for the development of early-onset emphysema, particularly in the presence of environmental exposures such as tobacco smoking.2 Compared with smoking-related COPD, typical patients with AATD-related COPD present at a younger age (before 45 years) and have panlobular emphysema with lower lobe predominance. However, the pattern observed in up to one-third of patients with AATD is predominantly centrilobular with an upper lobe distribution due to their exposure to tobacco smoke.3, 4
As noted for COPD in general, the assessment of AATD-related COPD severity and disease progression is conventionally based on spirometry.5 Measures such as the FEV1 have been classically used to quantify the degree of airflow obstruction and loss of ventilatory function.6 However, pulmonary function tests are unable to discriminate emphysema from airways disease, the two hallmark pathologic features of COPD.7 For this reason, in recent years tools such as CT scanning have been used to further characterize the lung structure and evaluate the impact of therapeutic interventions in AATD-related COPD. This article reviews the principles of CT technology, the utility of CT scanning in the assessment of emphysema in COPD, and its emerging role as an outcome in therapeutic trials in AATD-related COPD.
Principles of CT Scan Technology
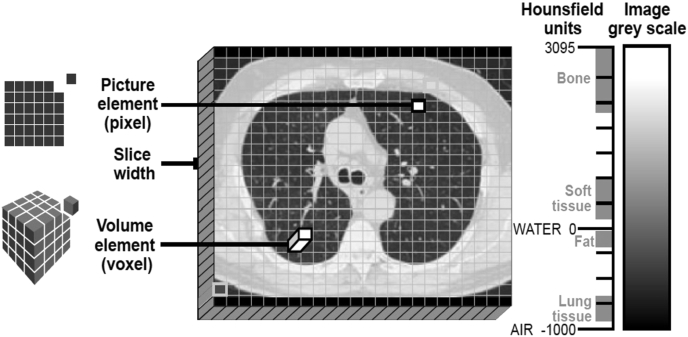
CT was first introduced more than 40 years ago.8 Briefly, conventional scanners consist of a narrow beam of X-rays positioned opposite a row of detectors, and assembled in a circular arrangement that rotates around the axis of the patient to produce a transverse planar image.9 The attenuation of the X-ray beams is measured as they pass through tissues of varying densities, allowing the construction of a two-dimensional (2-D) axial image. Axial images are composed of pixels, which are representative of the tissue density of the region scanned. Pixel means “picture element” and relates to the smallest square unit of a digital image, typically arranged in a 2-D grid. The detail (resolution) of an image is dependent on the number of pixels: the more pixels present, the more closely the image resembles the tissue being scanned (Fig 1). With the use of computer software, multiple axial, sagittal, and coronal images can be stacked on top of each other and assembled into a 3-D picture, which is composed of voxels, of the scanned tissue. A voxel is the “volume element” and is the building block from which the larger 3-D images are formed (Fig 1). In CT scans, a voxel value represents the X-ray attenuation of the tissue; the attenuation value is expressed in Hounsfield units (HU),9 and provides an indication of the density of the scanned tissue. Tissue density is measured on an arbitrary scale (range, –1,000 to +1,000 HU) based on attenuation of the water (0 HU) and air (–1,000 HU; minimum HU value) (Fig 1).
Figure 1.
Visual depiction of the Hounsfield scale, representing the radiodensities of various tissues/materials.
At present, most CT scans are performed with spiral/helical scanners. These scanners rotate continuously in one direction with the patient moving continuously through the scanner during the tube rotation. In addition to reducing the scan time, this allows scanning of a volume of tissue, rather than single discrete slices.9, 10 Further advances were made with the introduction of multislice scanners, which contain up to eight rows of detectors and have faster tube rotation times, thus providing faster coverage of a given volume of tissue.10 The biggest advantage of spiral and multislice scanners is their ability to image large anatomic volumes (eg, the entire thorax) during a single breath-hold.11 Acquisition of the entire tissue volume allows the density of 3-D voxels, instead of 2-D pixels, to be calculated. Thus, 3-D reconstructions can be created and lung volumes can be measured more accurately.11 Alternatively, smaller volumes of tissue can be examined using much thinner slices, usually around 1 mm.10
CT Densitometry and Assessment of Emphysema
The distribution of emphysema can be determined either by qualitative, visual assessment of scanned images by a radiologist or by quantitative assessment by CT imaging.12, 13, 14 Although visual scoring has been shown to correlate with corresponding pathologic data,15, 16, 17, 18 it can be subject to high intra- and interobserver variability, with subjective assessment by the readers shown to both under- and overestimate the extent of disease.12, 17, 19 Nevertheless, for the clinical diagnosis of emphysema visual inspection by a radiologist is usually sufficient. The Fleischner Society standardized the description of emphysema patterns determined visually.20
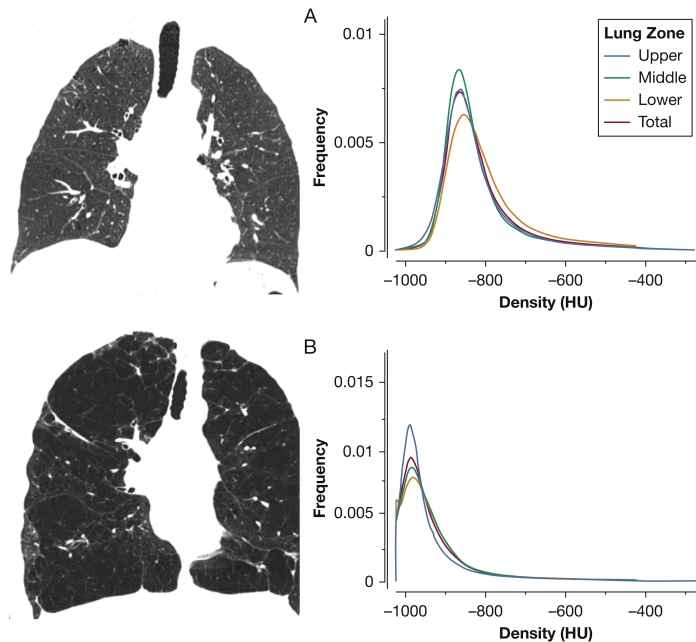
A more precise, reader-independent estimate of the extent and severity of disease can be provided by quantitative CT scanning.12, 19 Specific software is available that can automatically recognize the lungs, trace their contours, and determine histograms of lung attenuation values, which are used to distinguish nonemphysematous and emphysematous lung tissue.21 In healthy lung, attenuation values are most frequently distributed between –750 and –850 HU, with a mean attenuation at –789 HU. In contrast, in an emphysematous lung an abnormal accumulation of air contributes to decreased lung density, and the distribution curve is shifted toward lower attenuation levels (Fig 2). Earlier studies have shown that low attenuation values reflect regions of emphysematous destruction with good visual12 and pathologic scoring correlations.16, 22, 23
Figure 2.
CT densitometric measures of emphysema. A, Coronal slice of a normal lung and the distribution of Hounsfield units (HU). Note that the mean HU values for various lung zones (blue, green, and yellow lines) and whole lung (red) are approximately –850. B, Coronal slice of an emphysematous lung. Note that the HU values are now shifted toward –1,000 HU, the density of air.
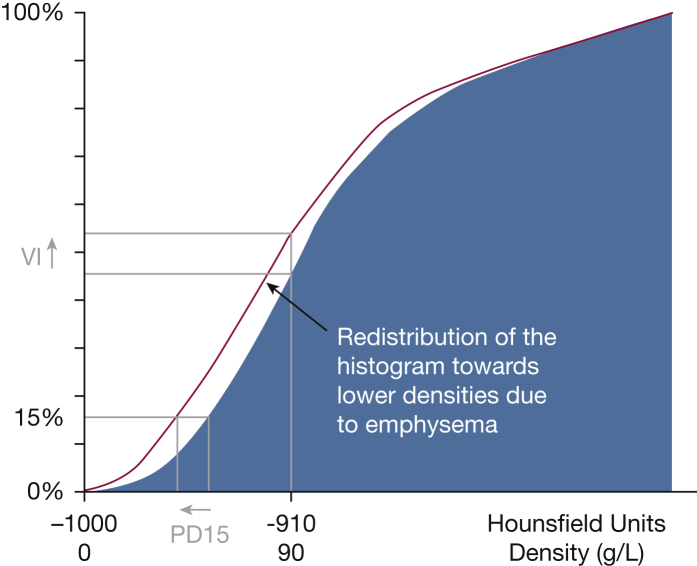
The burden of emphysema on CT is usually expressed as the percentage of low-attenuation areas below a given threshold, with greater values indicating more emphysema. Thresholds below –910, –950, and –960 HU are typically used and correlated with pathologic emphysema.24, 25, 26 The first cutoff point is the most accepted to define mild emphysema, while the last two are used to define severe emphysema. Large non-AATD COPD clinical studies such as COPDGene (COPD Genetic Epidemiology),27 SPIROMICS (Subpopulations and Intermediate Outcome Measures in COPD Study),28 and ECLIPSE (Evaluation of COPD Longitudinally to Identify Predictive Surrogate End-points)29 have mostly used –950 HU as their threshold value to define emphysema. This threshold method is also known as the voxel index (VI).
In addition, emphysema can be expressed as a percentile density (PD). For example, a 15th percentile density (PD15) indicates the HU value below which the lower 15% of all voxels are distributed. This approach has been typically used in AATD studies, and in one such study it was concluded that any percentile in the 10th to 30th range (corresponding to attenuation values between –950 and –890 HU) is sensitive enough to assess emphysema progression.30 Some investigators have found that compared with the VI, PD15 is a more consistent measure of lung density change across a wide range of physiologic impairments (Fig 3)31; for this reason, PD15 has been widely adopted as a standard to assess the extent and progression of emphysema. In addition, since lung attenuation values (in Hounsfield units) can be converted to lung tissue density values (g/L) by adding 1,000 to the Hounsfield units,32 PD15 values can be used to provide a more graphical description of lung tissue loss. For example, a PD15 value of –950 HU equals a lung density of 50 g/L, meaning that 15% of the pixels/voxels have a density value below 50 g/L. The lower the PD15 values in grams per liter (ie, closer to 0), the more emphysema is present. As a reference, the PD15 (adjusted for total lung capacity, TLC) found in nonsmoking subjects is around 80 g/L, while values of 67, 52, and 29 g/L correspond to mild, moderate, and severe emphysema, respectively (our unpublished data). These values might vary across CT and software manufacturers.
Figure 3.
Cumulative frequency distribution histogram of lung density at the 15th percentile (PD15) and voxel index (VI) defining emphysema as the percentage of low-attenuation areas below –950 HU (y axis).
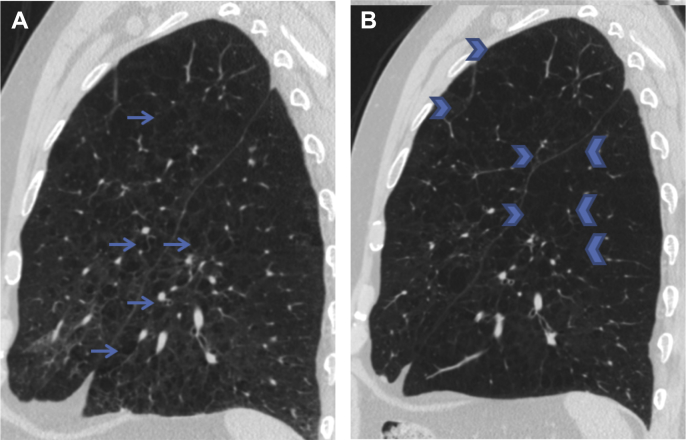
An important physiologic confounder to consistent CT lung density measurements is the variability caused by lung volume,33 which is dependent on the patients’ inspiratory level during CT scan acquisition and their disease state. The lung is a distensible organ that when fully inflated contains mostly air, and lung density at full inspiration is lower when compared with a scan taken at full expiration. Progressive hyperinflation may also contribute to the loss of lung density associated with AATD-related emphysema.34 Therefore, correcting for lung volume is crucial to the accuracy of CT lung density measurements, in particular when serial assessments are to be made.35, 36, 37 Two methods for lung volume correction have been explored. Although both methods improve the reproducibility of CT densitometry, statistical adjustment (using total lung volume as a covariate) has been reported to be more sensitive than physiologic adjustment (adjusting the scan to a ratio of total lung volume divided by the predicted TLC).38 In the future, densitometry tools that include technology to correct for different inspiratory volumes will become more widely available, enabling physicians to obtain quantitative CT densitometry results for individual follow-up of patients (Fig 4). In addition, other factors that can affect lung density need to be accounted for. Conditions such as interstitial lung disease, pulmonary edema, and current smoking status can increase lung density measures, while smoking cessation and the presence of lung cysts may have the opposite effect.
Figure 4.
CT sagittal slices from a smoker, showing progression of emphysema. A, Emphysema in both the left upper and lower lobes is seen (arrows) on baseline CT scan (mean whole-lung emphysema, 18.7%; lung density at 15th percentile, 40.2 g/L). B, Progression of the emphysematous destruction of the parenchyma is seen 5 years later (some areas are marked with arrowheads) (mean whole-lung emphysema, 32.3%; lung density at 15th percentile, 27.3 g/L). Case from the COPDGene Study.
CT Measures of Emphysema and Clinical Outcomes
In smoking- and AATD-related COPD, CT quantification of emphysema has been shown to correlate with symptoms, airflow obstruction, higher lung volumes, a lower gas transfer coefficient, poorer gas exchange, and higher blood leukocyte counts.39, 40, 41, 42 A greater burden of emphysema on CT imaging is associated with poorer health status43 and lower exercise capacity.44 The presence of emphysema on CT scans also indicates increased risk of death,45 in particular when CT density progression is noted in lower lung zones in patients with AATD with moderate-to-severe lung function impairment.46 In fact, CT measures of emphysema (ie, VI –910 HU) were more strongly associated with mortality than FEV1.45 CT-defined emphysema has also been related to several biomarkers associated with smoking-related COPD, including neutrophil elastase, soluble receptor for advanced glycation end-product, surfactant protein D, intercellular adhesion molecule 1, chemokine ligand 20, and biomarkers of extracellular matrix degradation,29, 47, 48, 49 highlighting its close relation to the pathophysiology of the disease. All these associations raise the value of CT quantification of emphysema as part of the multidimensional evaluation of patients with AATD, and as an important parameter to assess in therapeutic trials.
Quantitative CT Measurement of Emphysema to Assess the Therapeutic Effect of A1-PI Therapy in AATD
Early work demonstrated that serial CT scans expressed as PD15 were highly reproducible (intraclass coefficient of variation, 0.96).50 In addition, it was demonstrated that CT lung densitometry was more sensitive than other measurements of emphysema progression, and that the changes in CT lung density were related to changes in lung function, providing the foundation to use this imaging tool as an endpoint for therapeutic interventions in AATD.38 COPD progresses slowly with high variability in FEV1 decline; therefore, detecting a significant decline in FEV1 would require the enrollment of hundreds to thousands of patients in a clinical trial and several years of follow-up.51 On the basis of the above information, instead of FEV1, investigators have used CT measures of emphysema as an endpoint in AATD clinical trials with relatively smaller sample sizes and shorter follow-up times.
To date, CT-measured lung density has been used as an outcome measure to assess the therapeutic effect of A1-PI augmentation therapy on the progression of AATD-related emphysema in three randomized placebo-controlled trials (Table 1). First, the Danish-Dutch trial assessed 56 ex-smokers with AATD and moderate emphysema. CT measurements were performed at close to functional residual capacity (FRC) and at 75% of TLC.52 Subsequently, the EXACTLE (Exacerbations and CT Scan as Lung Endpoints) trial assessed 77 patients with AATD, using CT measurements taken at TLC.38 Although the results of both studies were indicative of slower rates of lung density loss in patients treated with A1-PI therapy, the difference (vs placebo) was not statistically significant (except for one specific analytical method applied in EXACTLE). However, the trend toward a favorable effect of treatment in these underpowered studies was suggestive of protection against loss of lung tissue.38, 52 In fact, a pooled analysis of the two studies supported the efficacy of A1-PI therapy to significantly reduce the decline in lung density in patients with AATD.53
Table 1.
Randomized Trials with Alpha-1 Proteinase Inhibitor Augmentation Therapy That Used Lung Density Changes at TLC as an Outcome
| Population (No.) |
Treatment |
Annual Lung Density Decline Measured at TLC (g/L) |
||||||
|---|---|---|---|---|---|---|---|---|
| Study | A1-PI | Placebo | A1-PI | Duration | A1-PI | Placebo | Difference | P Value |
| Dirksen et al52/1999 | 28 | 28 | 250 mg/kg every 4 weeks | 3 y | 1.5 ± 0.41 | 2.57 ± 0.41 | 1.07 ± 0.58 (P = .07) | .007 |
| Dirksen et al38/2009a: Exacerbations and CT Scan as Lung Endpoints (EXACTLE) trial | 36 | 35 | 60 mg/kg weekly | 2 y | 2.82 ± 5.0 | 4.20 ± 3.44 | 0.85 (–0.065 to 1.778) (analysis method 1) | .068 |
| 2.38 ± 5.25 | 3.81 ± 4.06 | 0.70 (–0.028 to 1.427) (analysis method 2) | .059 | |||||
| 3.38 ± 4.62 | 4.82 ± 3.81 | 1.59 (–0.220 to 3.412) (analysis method 3) | .084 | |||||
| 2.89 ± 4.73 | 4.12 ± 4.14 | 1.47 (0.009 to 2.935) (analysis method 4) | .049 | |||||
| Chapman et al54/2015: Randomized, Placebo-Controlled Trial of Augmentation Therapy in Alpha-1 Proteinase Inhibitor Deficiency (RAPID-RCT) | 93 | 87 | 60 mg/kg weekly | 2 y | 1.45 ± 0.23 | 2.19 ± 0.25 | 0.74 (0.06 to 1.42) | .03 |
A1-PI = alpha-1 proteinase inhibitor; TLC = total lung capacity.
This study reported four different adjustments for evaluation of lung density.
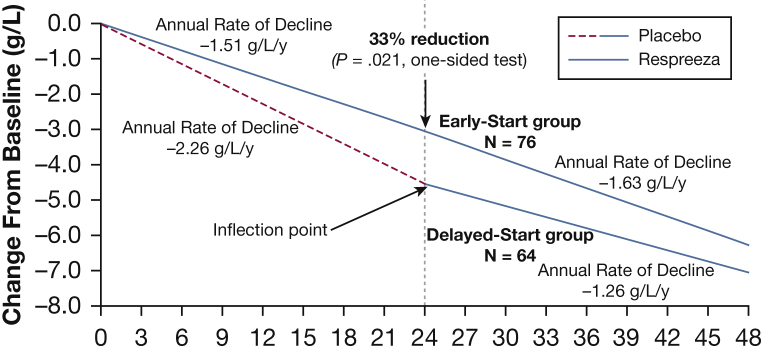
The RAPID-RCT (Randomized, placebo-controlled trial of augmentation therapy in Alpha-1 Proteinase Inhibitor Deficiency) and RAPID-OLE (RAPID Open Label Extension) trials are the largest clinical programs assessing A1-PI therapy completed to date.54, 55 In RAPID-RCT, patients with AATD (n = 180) were randomized to A1-PI therapy (60 mg/kg/wk) or placebo for 2 years. Primary endpoints were CT PD15 measured at TLC, FRC, and TLC + FRC combined. Although the loss of CT lung density measured at FRC and at combined TLC + FRC was not significantly different between the groups, measures of CT lung density at TLC significantly slowed the progression of emphysema compared with placebo (mean annual rate of lung density loss, −1.45 vs −2.19 g/L/y, respectively; P = .03). This beneficial effect of A1-PI therapy was further confirmed in the RAPID-OLE trial, in which all participants received A1-PI regardless of whether they were randomized to A1-PI or placebo in the original trial (RAPID-RCT). The mean reduction in the annual rate of lung density between baseline and month 24 was significantly lower in the early-start group (which received active therapy throughout), compared with the delayed-start group (which received placebo in the original trial; −1.51 vs −2.26 g/L/y; P = .021) (Fig 5).55 This represents a 33% reduction in the annual rate of lung density decline; an absolute reduction of 0.75 g/L/y in favor of A1-PI therapy. Furthermore, following the switch from placebo to active therapy in the delayed-start group, a statistically significant reduction in the annual rate of lung density decline was established (a difference of 0.52 g/L/y; P = .001).55 The lung density loss that occurred in the delayed-start group prior to receiving A1-PI therapy was not regained, suggesting a disease-modifying effect of treatment in patients with AATD.55 In addition, changes in PD15 at TLC were weakly but significantly related to changes in percent predicted FEV1 during the 4-year follow-up. Results from both the RAPID and EXACTLE trials show that the therapeutic effect was more prominent in the basal region of the lung, thus highlighting that CT densitometry is a technically feasible outcome measure to assess the efficacy of emphysema-modifying therapies. Specifically for AATD, the use of this technology highlights the importance of early institution of A1-PI augmentation therapy to impede the loss of lung tissue.
Figure 5.
Estimated lung density decline (adjusted lung density at 15th percentile at total lung capacity) over 48 months, using data from the mixed-effects regression model applied to each trial separately (the Randomized, Placebo-Controlled Trial of Augmentation Therapy in Alpha-1 Proteinase Inhibitor Deficiency [RAPID-RCT] and RAPID Open Label Extension [RAPID-OLE] trial intention-to-treat population).
Quantitative CT Assessment of Other Lung Structures
Quantitative CT scanning has also been used to assess airways and vascular disease, as well as extrapulmonary manifestations of smoking- and AATD-related COPD. For example, in an AATD cohort, greater airway wall thickening measured as wall area percent was related to lower expiratory airflows, and the strength of this relationship increased from proximal to more peripheral airways.56 Further work has revealed the concomitant presence of clinically significant bronchiectasis, which may occur in up to 27% of individuals with AATD-related COPD.13 Patients with non-AATD COPD and bronchiectasis detected by CT imaging have higher mortality, higher rates of pulmonary inflammation, and longer recovery times from exacerbations.57, 58 Lung quantitative imaging has also been used to characterize the intraparenchymal vasculature and extraparenchymal main vessels in smokers with and without COPD. For instance, increased pulmonary vascular pruning on noncontrast CT imaging is related to lower lung function and less exercise capacity.59 Similarly, an increased ratio of the diameters of the pulmonary artery to the aorta predicts future exacerbations.60 In addition, in patients without AATD, chest CT measurements of extrapulmonary tissues such as muscle and fat are related to COPD outcomes. The area of the pectoralis muscles, measured on a single-axial slice, is more strongly related to lung function and exercise capacity than body mass index.61 Furthermore, increased visceral adipose tissue content is associated with increased odds of myocardial infarction in patients with smoking-related COPD.62 Thus, these extrapulmonary CT measurements can be applicable in patients with AATD.
Future Perspectives
CT imaging assessment of emphysema has been validated against pathology, lung function, and health status, and is now accepted as a valid endpoint to assess the efficacy of A1-PI therapy. However, several practical issues exist regarding its applicability for routine clinical practice, including availability of specific software and the expertise required for analysis and interpretation of data. In addition, there is a need for standardization and validation of the technology/methodology, including efforts to assess the nonbiologic variations in lung density across CT scanners and software from different manufacturers, in order to allow more reproducible measurements across centers in large trials.63 There are also concerns regarding radiation dose, and advances are being made in exploring low-dose scan protocols to minimize radiation exposure. Furthermore, as the distribution of AATD-related emphysema is not uniform, targeted sampling of specific lung regions may be more appropriate for mapping AATD disease progression in the lung.34 It is possible that in the future, CT measures could predict which subjects with AATD will most likely benefit from augmentation therapy.
Concluding Comments
The evidence presented in this review suggests that CT scanning is a useful imaging tool to phenotype and measure interventions in AATD. CT lung density seems to be a clinically relevant outcome for emphysema trials. Because some caveats remain, more research is needed before it can be used routinely in clinical practice.
Acknowledgments
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: M. A. C. has received grant support from CSL Behring for a PI-initiated trial and participates in research trials sponsored by CSL Behring, Kamada, and Grifols. A. A. D. has received speaker fees from Novartis, Inc.
Other contributions: Editorial assistance was provided by Meridian HealthComms, funded by CSL Behring.
References
- 1.Fregonese L., Stolk J. Hereditary alpha-1-antitrypsin deficiency and its clinical consequences. Orphanet J Rare Dis. 2008;3:16. doi: 10.1186/1750-1172-3-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Janciauskiene S.M., Bals R., Koczulla R., Vogelmeier C., Kohnlein T., Welte T. The discovery of α1-antitrypsin and its role in health and disease. Respir Med. 2011;105(8):1129–1139. doi: 10.1016/j.rmed.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 3.Holme J., Stockley R.A. CT scan appearance, densitometry, and health status in protease inhibitor SZ α1-antitrypsin deficiency. Chest. 2009;136(5):1284–1290. doi: 10.1378/chest.09-0057. [DOI] [PubMed] [Google Scholar]
- 4.Parr D.G., Stoel B.C., Stolk J., Stockley R.A. Pattern of emphysema distribution in α1-antitrypsin deficiency influences lung function impairment. Am J Respir Crit Care Med. 2004;170(11):1172–1178. doi: 10.1164/rccm.200406-761OC. [DOI] [PubMed] [Google Scholar]
- 5.Vogelmeier C.F., Criner G.J., Martinez F.J. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease: 2017 report. GOLD Executive Summary. Am J Respir Crit Care Med. 2017;195(5):557–582. doi: 10.1164/rccm.201701-0218PP. [DOI] [PubMed] [Google Scholar]
- 6.Pauwels R.A., Buist A.S., Calverley P.M., Jenkins C.R., Hurd S.S. GOLD Scientific Committee. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) workshop summary. Am J Respir Crit Care Med. 2001;163(5):1256–1276. doi: 10.1164/ajrccm.163.5.2101039. [DOI] [PubMed] [Google Scholar]
- 7.Coxson H.O., Leipsic J., Parraga G., Sin D.D. Using pulmonary imaging to move chronic obstructive pulmonary disease beyond FEV1. Am J Respir Crit Care Med. 2014;190(2):135–144. doi: 10.1164/rccm.201402-0256PP. [DOI] [PubMed] [Google Scholar]
- 8.Hounsfield G.N. Computerized transverse axial scanning (tomography). I. Description of system. 1973. Br J Radiol. 1995;68(815):H166–H172. [PubMed] [Google Scholar]
- 9.Milne S., King G.G. Advanced imaging in COPD: insights into pulmonary pathophysiology. J Thorac Dis. 2014;6(11):1570–1585. doi: 10.3978/j.issn.2072-1439.2014.11.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garvey C.J., Hanlon R. Computed tomography in clinical practice. BMJ. 2002;324(7345):1077–1080. doi: 10.1136/bmj.324.7345.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choromańska A., Macura K.J. Role of computed tomography in quantitative assessment of emphysema. Pol J Radiol. 2012;77(1):28–36. doi: 10.12659/pjr.882578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bankier A.A., De Maertelaer V., Keyzer C., Gevenois P.A. Pulmonary emphysema: subjective visual grading versus objective quantification with macroscopic morphometry and thin-section CT densitometry. Radiology. 1999;211(3):851–858. doi: 10.1148/radiology.211.3.r99jn05851. [DOI] [PubMed] [Google Scholar]
- 13.Parr D.G., Guest P.G., Reynolds J.H., Dowson L.J., Stockley R.A. Prevalence and impact of bronchiectasis in α1-antitrypsin deficiency. Am J Respir Crit Care Med. 2007;176(12):1215–1221. doi: 10.1164/rccm.200703-489OC. [DOI] [PubMed] [Google Scholar]
- 14.Lynch D.A., Murphy J.R., Crapo J.D. A combined pulmonary-radiology workshop for visual evaluation of COPD: study design, chest CT findings and concordance with quantitative evaluation. COPD. 2012;9(2):151–159. doi: 10.3109/15412555.2012.654923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bergin C., Muller N., Nichols D.M. The diagnosis of emphysema: a computed tomographic-pathologic correlation. Am Rev Respir Dis. 1986;133(4):541–546. doi: 10.1164/arrd.1986.133.4.541. [DOI] [PubMed] [Google Scholar]
- 16.Hruban R.H., Meziane M.A., Zerhouni E.A. High resolution computed tomography of inflation-fixed lungs: pathologic-radiologic correlation of centrilobular emphysema. Am Rev Respir Dis. 1987;136(4):935–940. doi: 10.1164/ajrccm/136.4.935. [DOI] [PubMed] [Google Scholar]
- 17.Miller R.R., Muller N.L., Vedal S., Morrison N.J., Staples C.A. Limitations of computed tomography in the assessment of emphysema. Am Rev Respir Dis. 1989;139(4):980–983. doi: 10.1164/ajrccm/139.4.980. [DOI] [PubMed] [Google Scholar]
- 18.Kuwano K., Matsuba K., Ikeda T. The diagnosis of mild emphysema: correlation of computed tomography and pathology scores. Am Rev Respir Dis. 1990;141(1):169–178. doi: 10.1164/ajrccm/141.1.169. [DOI] [PubMed] [Google Scholar]
- 19.Cavigli E., Camiciottoli G., Diciotti S. Whole-lung densitometry versus visual assessment of emphysema. Eur Radiol. 2009;19(7):1686–1692. doi: 10.1007/s00330-009-1320-y. [DOI] [PubMed] [Google Scholar]
- 20.Lynch D.A., Austin J.H., Hogg J.C. CT-definable subtypes of chronic obstructive pulmonary disease: a statement of the Fleischner Society. Radiology. 2015;277(1):192–205. doi: 10.1148/radiol.2015141579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wielputz M.O., Bardarova D., Weinheimer O. Variation of densitometry on computed tomography in COPD: influence of different software tools. PLoS One. 2014;9(11):e112898. doi: 10.1371/journal.pone.0112898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coddington R., Mera S.L., Goddard P.R., Bradfield J.W. Pathological evaluation of computed tomography images of lungs. J Clin Pathol. 1982;35(5):536–540. doi: 10.1136/jcp.35.5.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gould G.A., MacNee W., McLean A. CT measurements of lung density in life can quantitate distal airspace enlargement: an essential defining feature of human emphysema. Am Rev Respir Dis. 1988;137(2):380–392. doi: 10.1164/ajrccm/137.2.380. [DOI] [PubMed] [Google Scholar]
- 24.Gevenois P.A., de Maertelaer V., De Vuyst P., Zanen J., Yernault J.C. Comparison of computed density and macroscopic morphometry in pulmonary emphysema. Am J Respir Crit Care Med. 1995;152(2):653–657. doi: 10.1164/ajrccm.152.2.7633722. [DOI] [PubMed] [Google Scholar]
- 25.Muller N.L., Staples C.A., Miller R.R., Abboud R.T. “Density mask”: an objective method to quantitate emphysema using computed tomography. Chest. 1988;94(4):782–787. doi: 10.1378/chest.94.4.782. [DOI] [PubMed] [Google Scholar]
- 26.Madani A., Zanen J., de Maertelaer V., Gevenois P.A. Pulmonary emphysema: objective quantification at multi-detector row CT—comparison with macroscopic and microscopic morphometry. Radiology. 2006;238(3):1036–1043. doi: 10.1148/radiol.2382042196. [DOI] [PubMed] [Google Scholar]
- 27.Martinez C.H., Chen Y.H., Westgate P.M. Relationship between quantitative CT metrics and health status and BODE in chronic obstructive pulmonary disease. Thorax. 2012;67(5):399–406. doi: 10.1136/thoraxjnl-2011-201185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Woodruff P.G., Barr R.G., Bleecker E. Clinical significance of symptoms in smokers with preserved pulmonary function. N Engl J Med. 2016;374(19):1811–1821. doi: 10.1056/NEJMoa1505971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coxson H.O., Dirksen A., Edwards L.D. The presence and progression of emphysema in COPD as determined by CT scanning and biomarker expression: a prospective analysis from the ECLIPSE study. Lancet Respir Med. 2013;1(2):129–136. doi: 10.1016/S2213-2600(13)70006-7. [DOI] [PubMed] [Google Scholar]
- 30.Dirksen A., Friis M., Olesen K.P., Skovgaard L.T., Sorensen K. Progress of emphysema in severe alpha 1-antitrypsin deficiency as assessed by annual CT. Acta Radiol. 1997;38(5):826–832. doi: 10.1080/02841859709172418. [DOI] [PubMed] [Google Scholar]
- 31.Parr D.G., Stoel B.C., Stolk J., Stockley R.A. Validation of computed tomographic lung densitometry for monitoring emphysema in α1-antitrypsin deficiency. Thorax. 2006;61(6):485–490. doi: 10.1136/thx.2005.054890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thomsen L.H., Shaker S.B., Dirksen A. Correlation between emphysema and lung function in healthy smokers and smokers with COPD. Chronic Obstr Pulm Dis. 2015;2(3):204–213. doi: 10.15326/jcopdf.2.3.2014.0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parr D.G., Dirksen A., Piitulainen E., Deng C., Wencker M., Stockley R.A. Exploring the optimum approach to the use of CT densitometry in a randomised placebo-controlled study of augmentation therapy in alpha 1-antitrypsin deficiency. Respir Res. 2009;10:75. doi: 10.1186/1465-9921-10-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parr D.G., Sevenoaks M., Deng C., Stoel B.C., Stockley R.A. Detection of emphysema progression in alpha 1-antitrypsin deficiency using CT densitometry: methodological advances. Respir Res. 2008;9:21. doi: 10.1186/1465-9921-9-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stoel B.C., Putter H., Bakker M.E. Volume correction in computed tomography densitometry for follow-up studies on pulmonary emphysema. Proc Am Thorac Soc. 2008;5(9):919–924. doi: 10.1513/pats.200804-040QC. [DOI] [PubMed] [Google Scholar]
- 36.Papandrinopoulou D., Tzouda V., Tsoukalas G. Lung compliance and chronic obstructive pulmonary disease. Pulm Med. 2012;2012:542769. doi: 10.1155/2012/542769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schiska A. The role of computed tomography in the diagnosis and treatment of emphysema. Radiol Technol. 2016;87(3):340–343. [PubMed] [Google Scholar]
- 38.Dirksen A., Piitulainen E., Parr D.G. Exploring the role of CT densitometry: a randomised study of augmentation therapy in α1-antitrypsin deficiency. Eur Respir J. 2009;33(6):1345–1353. doi: 10.1183/09031936.00159408. [DOI] [PubMed] [Google Scholar]
- 39.Subramanian D.R., Gupta S., Burggraf D. Emphysema- and airway-dominant COPD phenotypes defined by standardised quantitative computed tomography. Eur Respir J. 2016;48(1):92–103. doi: 10.1183/13993003.01878-2015. [DOI] [PubMed] [Google Scholar]
- 40.Grydeland T.B., Dirksen A., Coxson H.O. Quantitative computed tomography measures of emphysema and airway wall thickness are related to respiratory symptoms. Am J Respir Crit Care Med. 2010;181(4):353–359. doi: 10.1164/rccm.200907-1008OC. [DOI] [PubMed] [Google Scholar]
- 41.Takahashi M., Yamada G., Koba H., Takahashi H. Computed tomography-based centrilobular emphysema subtypes relate with pulmonary function. Open Respir Med J. 2013;7:54–59. doi: 10.2174/1874306401307010054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang G., Wang L., Ma Z., Zhang C., Deng K. Quantitative emphysema assessment of pulmonary function impairment by computed tomography in chronic obstructive pulmonary disease. J Comput Assist Tomogr. 2015;39(2):171–175. doi: 10.1097/RCT.0000000000000187. [DOI] [PubMed] [Google Scholar]
- 43.Dowson L.J., Guest P.J., Stockley R.A. Longitudinal changes in physiological, radiological, and health status measurements in α1-antitrypsin deficiency and factors associated with decline. Am J Respir Crit Care Med. 2001;164(10):1805–1809. doi: 10.1164/ajrccm.164.10.2106036. [DOI] [PubMed] [Google Scholar]
- 44.Diaz A.A., Bartholmai B., San Jose Estepar R. Relationship of emphysema and airway disease assessed by CT to exercise capacity in COPD. Respir Med. 2010;104(8):1145–1151. doi: 10.1016/j.rmed.2010.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dawkins P.A., Dowson L.J., Guest P.J., Stockley R.A. Predictors of mortality in α1-antitrypsin deficiency. Thorax. 2003;58(12):1020–1026. doi: 10.1136/thorax.58.12.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Green C.E., Parr D.G., Edgar R.G., Stockley R.A., Turner A.M. Lung density associates with survival in alpha 1 antitrypsin deficient patients. Respir Med. 2016;112:81–87. doi: 10.1016/j.rmed.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 47.Bihlet A.R., Karsdal M.A., Sand J.M. Biomarkers of extracellular matrix turnover are associated with emphysema and eosinophilic-bronchitis in COPD. Respir Res. 2017;18(1):22. doi: 10.1186/s12931-017-0509-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carolan B.J., Hughes G., Morrow J. The association of plasma biomarkers with computed tomography-assessed emphysema phenotypes. Respir Res. 2014;15(1):127. doi: 10.1186/s12931-014-0127-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stolk J., Veldhuisen B., Annovazzi L. Short-term variability of biomarkers of proteinase activity in patients with emphysema associated with type Z alpha-1-antitrypsin deficiency. Respir Res. 2005;6:47. doi: 10.1186/1465-9921-6-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stolk J., Dirksen A., van der Lugt A.A. Repeatability of lung density measurements with low-dose computed tomography in subjects with alpha-1-antitrypsin deficiency-associated emphysema. Invest Radiol. 2001;36(11):648–651. doi: 10.1097/00004424-200111000-00004. [DOI] [PubMed] [Google Scholar]
- 51.Schluchter M.D., Stoller J.K., Barker A.F., Alpha 1-Antitrypsin Deficiency Registry Study Group Feasibility of a clinical trial of augmentation therapy for α1-antitrypsin deficiency. Am J Respir Crit Care Med. 2000;161(3):796–801. doi: 10.1164/ajrccm.161.3.9906011. [DOI] [PubMed] [Google Scholar]
- 52.Dirksen A., Dijkman J.H., Madsen F. A randomized clinical trial of α1-antitrypsin augmentation therapy. Am J Respir Crit Care Med. 1999;160(5):1468–1472. doi: 10.1164/ajrccm.160.5.9901055. [DOI] [PubMed] [Google Scholar]
- 53.Stockley R.A., Parr D.G., Piitulainen E., Stolk J., Stoel B.C., Dirksen A. Therapeutic efficacy of alpha-1 antitrypsin augmentation therapy on the loss of lung tissue: an integrated analysis of 2 randomised clinical trials using computed tomography densitometry. Respir Res. 2010;11:136. doi: 10.1186/1465-9921-11-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chapman K.R., Burdon J.G., Piitulainen E. Intravenous augmentation treatment and lung density in severe α1 antitrypsin deficiency (RAPID): a randomised, double-blind, placebo-controlled trial. Lancet. 2015;386(9991):360–368. doi: 10.1016/S0140-6736(15)60860-1. [DOI] [PubMed] [Google Scholar]
- 55.McElvaney N.G., Burdon J., Holmes M. Long-term efficacy and safety of α1 proteinase inhibitor treatment for emphysema caused by severe α1 antitrypsin deficiency: an open-label extension trial (RAPID-OLE) Lancet Respir Med. 2017;5(1):51–60. doi: 10.1016/S2213-2600(16)30430-1. [DOI] [PubMed] [Google Scholar]
- 56.Yamashiro T., Matsuoka S., Estepar R.S. Quantitative airway assessment on computed tomography in patients with α1-antitrypsin deficiency. COPD. 2009;6(6):468–477. doi: 10.3109/15412550903341521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Martinez-Garcia M.A., de la Rosa Carrillo D., Soler-Cataluna J.J. Prognostic value of bronchiectasis in patients with moderate-to-severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;187(8):823–831. doi: 10.1164/rccm.201208-1518OC. [DOI] [PubMed] [Google Scholar]
- 58.Patel I.S., Vlahos I., Wilkinson T.M. Bronchiectasis, exacerbation indices, and inflammation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2004;170(4):400–407. doi: 10.1164/rccm.200305-648OC. [DOI] [PubMed] [Google Scholar]
- 59.Estepar R.S., Kinney G.L., Black-Shinn J.L. Computed tomographic measures of pulmonary vascular morphology in smokers and their clinical implications. Am J Respir Crit Care Med. 2013;188(2):231–239. doi: 10.1164/rccm.201301-0162OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wells J.M., Washko G.R., Han M.K. Pulmonary arterial enlargement and acute exacerbations of COPD. N Engl J Med. 2012;367(10):913–921. doi: 10.1056/NEJMoa1203830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McDonald M.L., Diaz A.A., Ross J.C. Quantitative computed tomography measures of pectoralis muscle area and disease severity in chronic obstructive pulmonary disease: a cross-sectional study. Ann Am Thorac Soc. 2014;11(3):326–334. doi: 10.1513/AnnalsATS.201307-229OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Diaz A.A., Young T.P., Kurugol S. Abdominal visceral adipose tissue is associated with myocardial infarction in patients with COPD. Chronic Obstr Pulm Dis. 2015;2(1):8–16. doi: 10.15326/jcopdf.2.1.2015.0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen-Mayer H.H., Fuld M.K., Hoppel B. Standardizing CT lung density measure across scanner manufacturers. Med Phys. 2017;44(3):974–985. doi: 10.1002/mp.12087. [DOI] [PMC free article] [PubMed] [Google Scholar]