Abstract
Background
We aimed to examine short- and long-term mortality in a mixed population of patients with interstitial lung disease (ILD) with acute respiratory failure, and to identify those at lower vs higher risk of in-hospital death.
Methods
We conducted a single-center retrospective cohort study of 126 consecutive adults with ILD admitted to an ICU for respiratory failure at a tertiary care hospital between 2010 and 2014 and who did not undergo lung transplantation during their hospitalization. We examined associations of ICU-day 1 characteristics with in-hospital and 1-year mortality, using Poisson regression, and examined survival using Kaplan-Meier curves. We created a risk score for in-hospital mortality, using a model developed with penalized regression.
Results
In-hospital mortality was 66%, and 1-year mortality was 80%. Those with connective tissue disease-related ILD had better short-term and long-term mortality compared with unclassifiable ILD (adjusted relative risk, 0.6; 95% CI, 0.3-0.9; and relative risk, 0.6; 95% CI, 0.4-0.9, respectively). Our prediction model includes male sex, interstitial pulmonary fibrosis diagnosis, use of invasive mechanical ventilation and/or extracorporeal life support, no ambulation within 24 h of ICU admission, BMI, and Simplified Acute Physiology Score-II. The optimism-corrected C-statistic was 0.73, and model calibration was excellent (P = .99). In-hospital mortality rates for the low-, moderate-, and high-risk groups were 33%, 65%, and 96%, respectively.
Conclusions
We created a risk score that classifies patients with ILD with acute respiratory failure from low to high risk for in-hospital mortality. The score could aid providers in counseling these patients and their families.
Key Words: idiopathic interstitial pneumonia, idiopathic pulmonary fibrosis, intensive care, interstitial lung disease, respiratory failure
Abbreviations: CTD-ILD, connective tissue disease-related interstitial lung disease; ECLS, extracorporeal life support; ILD, interstitial lung disease; IMV, invasive mechanical ventilation; IPF, idiopathic pulmonary fibrosis; IQR, interquartile range; LASSO, least absolute shrinkage and selection operator; NIPPV, noninvasive positive-pressure ventilation; RR, risk ratio; SAPS-II, Simplified Acute Physiology Score-II; uILD, unclassifiable interstitial lung disease
The interstitial lung diseases (ILDs) are a family of diseases defined by alveolar injury, inflammation, and/or fibrosis.1 For those with idiopathic pulmonary fibrosis (IPF), long-term outcomes are poor, with a median survival time of 3.8 years after diagnosis.2 For those with ILDs related to connective tissue disease (CTD) such as rheumatoid arthritis, systemic sclerosis, or myositis, long-term outcomes are more favorable, possibly due to earlier detection, the greater number of nonprogressive forms, or response to immunosuppressive and antiinflammatory treatment.3, 4, 5, 6 While many adults with IPF and other fibrotic ILDs experience either slow progression or stability punctuated by periods of decline, approximately 10% per year experience an “acute exacerbation,” which can lead to acute respiratory failure requiring admission to an ICU.7
Several studies describe a poor prognosis for patients with IPF who receive invasive mechanical ventilation (IMV), reporting in-hospital mortality rates of 73% to 100%.8, 9, 10, 11, 12, 13, 14 Critically ill adults with CTD-ILD and acute respiratory failure have reported outcomes that are similar to those with IPF,15, 16, 17 although select studies suggest a better prognosis for CTD-ILD and drug-induced ILDs.18, 19 Even if patients with ILD initially survive an exacerbation causing acute respiratory failure, their lung function decline may never recover, putting them at higher risk of death after hospital discharge.20
Given these poor outcomes, when patients with ILD have acute respiratory failure, physicians, patients, and their caregivers must often quickly decide whether to provide invasive support, such as IMV or extracorporeal life support (ECLS), or to recommend palliative and end-of-life care. Although outcomes of critically ill adults with ILD are previously described, no studies have examined short- and long-term outcomes across ILD subtypes. Furthermore, there are no methods to differentiate risk of dying in the hospital for this population. Our first goal was to describe short- and long-term outcomes among a variety of critically ill patients with ILD. Our second goal was to develop a clinical prediction model and risk score using characteristics measured during the first day of ICU admission in order to identify those at lower risk of death for whom aggressive therapy may be warranted, and those at a higher risk of death for whom palliative and end-of-life care may be recommended.
Materials and Methods
Study Design, Setting, and Participants
We performed a retrospective cohort study. There were 162 consecutive adults with physician-diagnosed ILD admitted to the New York-Presbyterian/Columbia University Medical Center’s medical ICUs between January 1, 2010 and December 31, 2014. We excluded those who underwent lung transplantation during their hospital admission (n = 25) and those who were admitted to the ICU for causes other than acute respiratory failure (n = 12), leaving 126 participants for inclusion in our study. We included those listed for lung transplantation, but excluded those who ultimately underwent lung transplantation to allow our results to inform the answer to the question “What is the likelihood my patient will survive this hospitalization if they do not undergo lung transplantation?” This study was approved by the Columbia University Institutional Review Board (IRB: AAAO8353).
Measurements
Data were extracted from the electronic medical record and combined with mortality data from the Social Security Death Index file. We assessed predictor variables that would be reliably recorded during the first 24 h of ICU admission. These variables included age, sex, BMI, smoking status, Simplified Acute Physiology Score (SAPS-II), laboratory values including arterial blood gas results, albumin, creatinine, bilirubin, and platelets, whether the patient ambulated on the first day of ICU admission, whether the patient had a do-not-resuscitate preference on the first day of ICU admission, and use of either invasive mechanical support (defined as IMV and/or ECLS), noninvasive positive-pressure ventilation (NIPPV), or only a high-flow nasal cannula or supplemental oxygen masks. Our ICUs have physical therapists who every day seek to ambulate ICU patients who are deemed safe for early mobilization. Since 64% of participants were missing a CT scan within 72 h of ICU admission, 36% were missing an echocardiogram within 72 h of ICU admission, and 55% did not undergo pulmonary function tests within 6 months of hospital admission, we did not use these variables for prediction modeling.
One author (D. J. L.) systematically reviewed all clinical data for each study participant including the electronic medical record, blood work, pulmonary function tests within 6 months of admission when available (45% of cases), CT images when available (86% of cases), and pathology reports when available (56% of cases). A diagnosis of IPF was made according to the 2011 ATS/ERS/JRS/ALAT IPF guidelines.21 A diagnosis of CTD-ILD was made when the patient had an autoimmune disease known to cause interstitial lung disease (ILD), based on guideline criteria.22, 23, 24, 25, 26, 27, 28 A diagnosis of unclassifiable ILD (uILD) was made when there was insufficient information to make a specific ILD diagnosis, which is in accordance with the revised ATS/ERS ILD classification guidelines.29 See e-Appendix 1 for further details.
We categorized ILD diagnoses into four groups: IPF, CTD-ILD, “other” ILDs, and uILDs. “Other” ILDs included specific ILD subtype diagnoses that were too few in number to comprise their own category for comparison. Our primary outcome was in-hospital mortality, and our secondary outcome was 1-year survival. Participants not reported as deceased in the Social Security Death Index were censored on earlier of the last known follow-up date or August 31, 2015.
Statistical Analysis
Continuous variables are presented as median with interquartile range (IQR). Categorical variables are summarized as frequencies and percentages. We used Kaplan-Meier curves and log-rank tests to examine unadjusted survival after ICU admission. We used Poisson regression with robust variance estimation to examine associations between ILD diagnosis and both in-hospital and 1-year mortality with and without adjustment for age, sex, and SAPS-II, which we purposefully selected for inclusion in the models because we considered them to be potential confounders. We performed similar analyses examining the association between invasive mechanical support and mortality. uILD was used as the reference category since it was the most common diagnosis.
We constructed a clinical prediction model using least absolute shrinkage and selection operator (LASSO)-penalized logistic regression analysis, using the leave-one-out cross-validation method to optimize lambda.30 Lambda is a tuning parameter used in LASSO to systematically penalize coefficients of predictor variables to shrink to zero so that only the most predictive variables remain in the model. We employed leave-one-out cross-validation, a method in which each iteration fits a model to n − 1 samples of the data set and evaluates it on the single, remaining data point. This method demonstrates improved effectiveness for small sample sizes compared with 10-fold cross-validation, as it is thought to provide a more reliable estimate of the fit.31, 32
We included demographic and clinical characteristics that we considered relevant to in-hospital mortality selected within the first 24 h of ICU admission: age, sex, a diagnosis of IPF, BMI, serum albumin, platelet count, SAPS-II, patient preferences for resuscitation on ICU-day 1, and whether they ambulated on ICU-day 1. We used generalized additive models to evaluate continuous covariates for nonlinear associations with in-hospital mortality (all were linear). In order to create a point score for risk, we first categorized SAPS-II, and categorized BMI by World Health Organization criteria.33 To calculate the points for our score system, we multiplied each regression coefficient in the final model by 2 and rounded to the nearest integer. This is an established algorithm that has been used previously.34, 35, 36 We classified the scores into low-risk, moderate-risk, and high-risk categories that maximized the observed differences in in-hospital mortality. We assessed the discrimination of the risk score by examining the area under the receiver operating characteristic curve, and tested the model’s calibration by using the Hosmer-Lemeshow goodness of fit χ2 test statistic for 10 equally sized groups. To correct for optimism and to internally validate the model, we repeatedly fit the model with 100 bootstrap samples to calculate the average area under the receiver operating characteristic curve.37 We calculated in-hospital and 1-year mortality for each risk category. We compared Kaplan-Meier curves of each risk category, using the log-rank test. There were no missing covariate data. We performed statistical analysis with Stata 14.1 (StataCorp) and R version 3.4.1.
Results
Participant Characteristics
Of the 126 study participants, 90% had received a preexisting diagnosis of ILD and 10% had received a diagnosis of ILD on hospital admission. There were 21 participants (17%) who were listed for lung transplantation at any point during the ICU stay. The median age was 61 years (IQR, 54-69); 60 (48%) were female; and 67 (53%) were ever-smokers. The median BMI was 26 (IQR, 22-30). The median SAPS-II was 31 (IQR, 26-39), representing a median predicted mortality of approximately 10%.
There were 15 participants (12%) with IPF, 23 (18%) with CTD-ILD, and 36 (29%) with “other” ILDs (Table 1). Fifty-two participants (41%) had uILD, largely due to insufficient clinical information available at the time of ICU admission to determine a more specific diagnosis.
Table 1.
Types of Interstitial Lung Disease in 126 Adults Admitted to the ICU With Acute Respiratory Failure
| Type of Interstitial Lung Disease | Participants [No. (%)] |
|---|---|
| Idiopathic pulmonary fibrosis | 15 (11.9) |
| Connective tissue disease | 23 (18.3) |
| Rheumatoid arthritis | 7 (5.6) |
| Systemic sclerosis | 9 (7.1) |
| Systemic lupus erythematosus | 1 (0.8) |
| Primary Sjögren syndrome | 1 (0.8) |
| Myositis | 4 (3.2) |
| Mixed connective tissue disease | 1 (0.8) |
| Other interstitial lung disease | 36 (28.6) |
| Idiopathic nonspecific idiopathic pneumonia | 11 (8.7) |
| Hypersensitivity pneumonitis | 13 (10.3) |
| Vasculitis | 1 (0.8) |
| Chronic eosinophilic pneumonia | 1 (0.8) |
| Chemotherapy/radiation therapy | 2 (1.6) |
| Combined pulmonary fibrosis and emphysema | 5 (4.0) |
| Idiopathic pleuroparenchymal fibroelastosis | 1 (0.8) |
| Familial pulmonary fibrosis | 1 (0.8) |
| Inflammatory bowel disease related | 1 (0.8) |
| Unclassifiable idiopathic interstitial pneumoniaa | 52 (41.3) |
Based on American Thoracic Society/European Respiratory Society definition of idiopathic interstitial pneumonia with either inadequate or discordant clinical, radiologic, and pathologic data to support a specific interstitial lung disease subtype diagnosis.
There were 19 participants (15%) who received high-flow nasal cannula oxygen and oxygen masks only, 27 (21%) who received NIPPV and supplemental oxygen only, and 80 (64%) who received IMV during the first 24 h of ICU admission. Specifically, 79 participants received only IMV, 1 received only ECLS, and 13 received both. Baseline characteristics, including SAPS-II and use of IMV and NIPPV, were largely similar across ILD categories (Table 2). However, those with IPF tended to be older than those with CTD-ILD, and were more frequently male smokers.
Table 2.
Baseline Characteristics of Adults With Interstitial Disease and Acute Respiratory Failure
| Variable | IPF (n = 15) | CTD (n = 23) | Other ILD (n = 36) | Unclassifiable ILD (n = 52) |
|---|---|---|---|---|
| Baseline characteristics | ||||
| Age, median (IQR), y | 65 (59-69) | 58 (44-68) | 61 (50.5-66) | 62.5 (55.5-72.5) |
| Male, No. (%) | 12 (80) | 8 (34.8) | 20 (55.6) | 26 (50) |
| BMI, median (IQR), kg/m2 | 24.7 (22.3-28.4) | 25.1 (23-29.2) | 26.1 (22.9-29.3) | 26.9 (20.6-30.2) |
| Ever-smoker, No. (%) | 13 (86.7) | 10 (43.5) | 21 (58.3) | 23 (44.2) |
| Supplemental oxygen use, No. (%) | 13 (86.7) | 17 (73.9) | 27 (75) | 32 (61.5) |
| Supplemental oxygen flow rate, median (IQR), L/min | 5 (3-8) | 3 (0-3) | 3 (0.5-4) | 2 (0-4) |
| In-hospital characteristics | ||||
| SAPS-II, median (IQR) | 33 (27-38) | 32 (24-42) | 30.5 (24-37.5) | 31.5 (26.5-42) |
| NIPPV only, No. (%) | 2 (13.3) | 5 (21.7) | 8 (22.2) | 12 (23.1) |
| Invasive device within 24 h of admission | 12 (80) | 13 (56.5) | 22 (61.1) | 33 (63.5) |
| Ambulation within 24 h of admission, No. (%) | 4 (27) | 8 (34.8) | 9 (25) | 14 (26.9) |
| Admission laboratory values, median (IQR) | ||||
| pH | 7.44 (7.40-7.47) | 7.38 (7.30-7.42) | 7.44 (7.36-7.47) | 7.40 (7.32-7.45) |
| Partial pressure of carbon dioxide | 38 (33-59) | 45 (38-60) | 44.5 (39-53.5) | 46.5 (38.5-61.5) |
| White blood cell count | 10.4 (6.8-13.7) | 12.6 (9.8-17.2) | 12.9 (10.1-18.6) | 15.5 (12.4-17.3) |
| Platelets | 195 (164-238) | 301 (210-246) | 235.5 (161.5-317) | 217 (155-298) |
| Serum creatinine | 0.65 (0.56-1.38) | 0.93 (0.68-1.62) | 0.79 (0.56-0.94) | 0.84 (0.62-1.3) |
| Serum bicarbonate | 26 (23-34) | 24 (19-28) | 28 (24-32) | 27 (23.5-31) |
| Serum albumin | 3.1 (2.7-3.8) | 3.2 (2.8-3.6) | 3.4 (3.2-3.7) | 3.2 (2.8-3.6) |
| Serum total bilirubin | 0.7 (0.5-0.9) | 0.6 (0.4-0.9) | 0.6 (0.4-0.95) | 0.65 (0.5-0.95) |
| Do not resuscitate order on admission, No. (%) | 4 (26.7) | 3 (13) | 2 (5.6) | 10 (19.2) |
| Listed for lung transplant during ICU stay, No. (%) | 6 (40) | 2 (8.7) | 9 (25) | 4 (7.7) |
| ICU length of stay, d | 8 (4-12) | 9 (6-15) | 6 (4-9.5) | 4.5 (3-12) |
| Hospital length of stay, d | 9 (5-14) | 19 (9-32) | 13 (6.5-22.5) | 9 (13-17.5) |
Data are expressed as No. of patients (%) and median (IQR). CTD = connective tissue disease; ILD = interstitial lung disease; IPF = idiopathic pulmonary fibrosis; IQR = interquartile range; NIPPV = noninvasive positive pressure ventilation; SAPS = Simplified Acute Physiology Score.
Mortality by ILD Diagnosis and Use of Invasive Mechanical Support
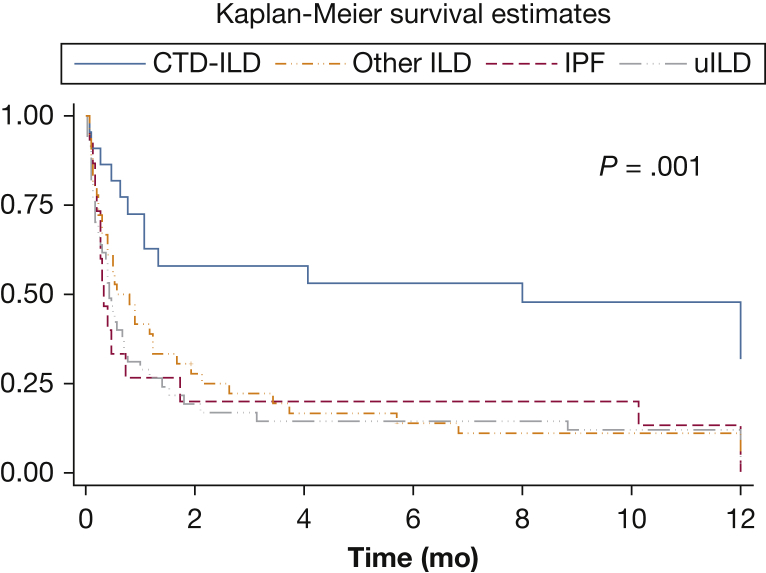
A total of 83 participants (66%) died in hospital; 72 (87%) died within 30 days of hospital admission, 10 (12%) died between 30 and 90 days of hospital admission, and one died 103 days after hospital admission. A total of 101 (80%) died within 1 year of being hospitalized. Unadjusted survival by each ILD category is shown in Figure 1 (log-rank P = .001). The in-hospital mortality rates for IPF, CTD-ILD, “other” ILD, and uILD groups were 80%, 39%, 69%, and 71%, respectively. One-year mortality rates for IPF, CTD-ILD, “other” ILD, and uILD groups were 87%, 52%, 89% and 85%, respectively (Table 3). Those with CTD-ILD had lower in-hospital mortality than those with uILD (adjusted risk ratio [RR], 0.6; 95% CI, 0.3-0.9; P = .02) and a lower 1-year risk of death (adjusted RR, 0.6; 95% CI, 0.4-0.9; P = .02) (Table 3).
Figure 1.
Kaplan-Meier 1-year survival curves from the time of hospitalization for acute respiratory failure for categories of interstitial lung disease. CTD-ILD = connective tissue disease-related ILD; ILD = interstitial lung disease; IPF = idiopathic pulmonary fibrosis; uILD = unclassifiable ILD. P value is for the log-rank test.
Table 3.
In-Hospital and 1-Year Mortality of Adults With Interstitial Lung Disease and Acute Respiratory Failure
| Variable | All | IPF | CTD-ILD | Other ILD | uILD |
|---|---|---|---|---|---|
| Participants, No. | 126 | 15 | 23 | 36 | 52 |
| In-hospital mortality | |||||
| Deaths, No. | 83 | 12 | 9 | 25 | 37 |
| Mortality, % | 66 | 80 | 39 | 69 | 71 |
| Unadjusted mortality risk ratio (95% CI) | 1.1 (0.8-1.5) | 0.5 (0.3-0.9) | 1.0 (0.7-1.3) | Ref | |
| Adjusted mortality risk ratio (95% CI) | 1.1 (0.8-1.5) | 0.6 (0.3-0.9) | 1.0 (0.8-1.3) | Ref | |
| One-year mortality | |||||
| Deaths, No. | 101 | 13 | 12 | 32 | 44 |
| Mortality, % | 80 | 87 | 52 | 89 | 85 |
| Unadjusted mortality risk ratio (95% CI) | 1.0 (0.8-1.3) | 0.6 (0.4-0.9) | 1.1 (0.9-1.2) | Ref | |
| Adjusted mortality risk ratio (95% CI) | 1.0 (0.8-1.2) | 0.6 (0.4-0.9) | 1.0 (0.9-1.2) | Ref |
Adjusted risk ratios are adjusted for age, sex, and SAPS-II score. CTD-ILD = connective tissue disease-interstitial lung disease; ILD = interstitial lung disease; IPF = idiopathic pulmonary fibrosis; uILD = unclassifiable interstitial lung disease.
Of the 80 participants who received invasive mechanical support, 75% died in the hospital, compared with only 50% of those who did not receive invasive mechanical support (adjusted RR, 1.5; 95% CI, 1.1-2.0; P = .01). At 1 year, there was no discernible difference in mortality between those who did and did not receive invasive mechanical support (adjusted RR, 1.2; 95% CI, 0.97-1.4; P = .1).
Prediction Model for In-Hospital Mortality
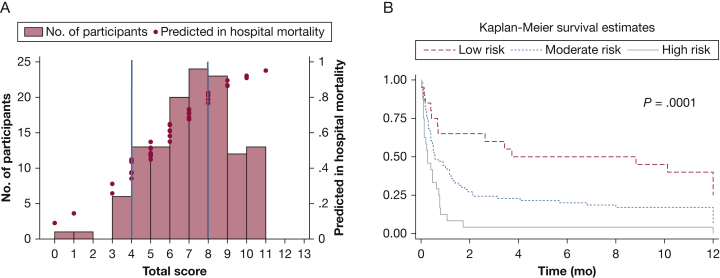
After LASSO-penalized regression, our model included six variables: male sex, IPF diagnosis, BMI, use of invasive mechanical support (vs NIPPV or supplemental oxygen), no ambulation within 24 h of admission, and SAPS-II. With LASSO, the coefficients for age, albumin, platelets, and do-not-resuscitate preference shrank to zero and were dropped from the model. The integer point score system calculated from the final model regression coefficients ranged from 0 to 13 points (e-Table 1). Based on the calculated score, participants were classified into three risk categories: low risk (0-4 points), moderate risk (5-8 points), and high risk (9-13 points) (Table 4). Corresponding in-hospital mortality rates for each group were 33%, 65%, and 96%, respectively (Table 5). The optimism-corrected C-statistic was 0.73, and the model calibration was excellent (goodness of fit P = .99). The frequency distribution of patients by predicted risk of in-hospital mortality based on the risk score is shown in Figure 2A. One-year survival also varied by risk category (P = .0001) with 57%, 81%, and 96% dying by 1 year in the low-, moderate-, and high-risk categories (Fig 2B). Among all of those who survived to hospital discharge, 18 (42%) died within 1 year.
Table 4.
Prediction Model for Patients With a Diagnosis of Interstitial Lung Disease Admitted to an ICU
| Predictor | Points |
|---|---|
| Male sex | 2 |
| Idiopathic pulmonary fibrosis diagnosis | 1 |
| BMI | |
| ≤25 | 0 |
| 26-30 | 1 |
| 31-35 | 2 |
| > 35 | 3 |
| Mechanical ventilation or extracorporeal life support | 2 |
| No ambulation | 1 |
| Simplified Acute Physiology Score II | |
| ≤20 | 0 |
| 21-30 | 3 |
| > 30 | 4 |
| Total points | 13 |
Risk category: Low risk (0-4 points); moderate risk (5-8 points); high risk (9-13 points).
Table 5.
Mortality Risk Categories Using the Prediction Model for Patients With a Diagnosis of Interstitial Lung Disease Admitted to an ICU
| Risk Category |
|||
|---|---|---|---|
| Low (0-4 Points) | Moderate (5-8 Points) | High (9-13 Points) | |
| Participants, No. (%) | 21 (17) | 80 (63) | 25 (20) |
| In-hospital deaths (in-hospital mortality), No. (%) | 7 (33) | 52 (65) | 24 (96) |
| Posthospitalization 1-y deaths among hospital survivors (1-y mortality among hospital survivors), No. (%) | 5 (36) | 13 (46) | 0 (0) |
| One-year deaths (cumulative 1-y mortality), No. (%) | 12 (57) | 65 (81) | 24 (96) |
Figure 2.
A, Frequency distribution of patients by predicted risk of in-hospital mortality based on the low-, moderate-, and high-risk score. B, Kaplan-Meier 1-year survival curves from the time of hospitalization for acute respiratory failure by low-, medium-, and high-risk categories. P value is for the log-rank test.
Discussion
In the largest cohort study to date of critically ill patients with ILD with acute respiratory failure,8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18 we found that a diagnosis of CTD-ILD was independently associated with a substantially lower risk of death, both in the short and long term, compared with patients with other ILDs. We also created the first risk prediction tool for adult patients with ILD with acute respiratory failure, and were able to identify a subgroup with a relatively low risk of in-hospital death (33%). Characteristics that may contribute to a lower risk of in-hospital death include female sex, an ILD diagnosis other than IPF, use of only noninvasive means of oxygen supplementation, nonobese BMI, early ambulation, and low SAPS-II score.
Risk prediction models for noncritically ill patients with chronic ILDs have proven useful in both clinical and research arenas.35, 36, 38, 39, 40, 41, 42 While prior studies of mechanically ventilated patients with IPF have reported high in-hospital mortality and recommend that palliative and end-of-life care be offered to these patients,8, 10 critically ill patients with ILD have been found to receive cardiopulmonary resuscitation more often and are less likely to have documented prognostic discussions.43 These studies suggest that risk stratification of critically ill patients with ILD is needed in order to better tailor expectations and care plans. While our model should not be used as an adjunct to clinical decision-making until it is externally validated, it could potentially increase physicians’ confidence in discussing prognosis and recommending continued critical care vs prioritizing palliative care and limiting life-sustaining treatments. For example, we identified 20% of our cohort as “high risk” with an observed 96% in-hospital mortality. This extremely high in-hospital mortality may help facilitate palliative and end-of-life care discussions between health care providers and the families of these patients. Alternatively, our novel finding of a subgroup of critically ill patients with ILD at relatively low risk of in-hospital mortality (33%) suggests that at least a time-limited trial of critical care may be worth pursuing in these select patients.
There are several strengths to how we created our prediction model. First, we included patients with a diagnosis of ILD, who were admitted to an ICU and who do not undergo lung transplantation during the index hospitalization, regardless of lung transplant listing status. Those with a realistic chance of lung transplantation arguably do not need risk stratification since they will initially receive supportive critical care. If lung transplantation is not likely or not offered, our model may inform physicians’ decisions to recommend further critical care vs palliative and end-of-life care. Second, each variable in our model can be easily and readily assessed on the first day of ICU admission. Third, we considered not only patient demographics and clinical characteristics that often comprise ICU prediction models, but considered patients’ ability to ambulate during the first day of ICU admission, and preference for resuscitation in the first 24 h of ICU admission. Functional status and preferences for life-sustaining therapies are increasingly recognized to be strong predictors of both short- and longer-term outcomes in critically ill patients.44, 45 A do-not-resuscitate order during the first day of ICU admission was dropped from the final model using LASSO, but this could be explained by the observation that most patients and caregivers elected a full code status during an initial time-limited trial of critical care. Third, LASSO is considered to be more unbiased than stepwise regression in fitting a parsimonious model, and is an important way to minimize overfitting in small data sets.46 LASSO has been widely applied in analysis with a large number of candidate predictors, as, for example, in genetics research, and more recently in identifying predictors of outcomes for survivors of acute respiratory distress syndrome.47 Fourth, the discrimination and calibration of the model and the simple point score system are good.
Our study has several limitations. Like prior cohort studies of critically ill adult patients with ILD,8, 9, 10, 11, 12, 14, 15, 16, 17, 18, 19 our data originate from a single academic center using a retrospective study design. We used vital statistics to determine 1-mortality for hospital survivors, and did not ascertain their potential cause of death. Further, measurements important to determining mortality risk may have been excluded, such as pulmonary function tests, CT scans, and echocardiography findings. However, these measurements were deliberately omitted from our model as they are not reliably available on admission, negating the purpose of a simple prediction tool, and prone to inaccuracies when recording retrospectively. Our effect estimates for the use of IMV or ECLS reflect the risk of mortality only among those who did not receive a lung transplant and therefore should not be considered reflective of outcomes for patients who undergo IMV or ECLS as a potential bridge to lung transplantation. Finally, although we performed internal validation with correction for optimism, our model is not externally validated. A prospective study using data from multiple centers with larger sample sizes of critically ill ILD populations is needed to validate the accuracy of our risk classification system prior to use in clinical practice.
Conclusion
We created a risk prediction model for adults with physician-diagnosed ILD admitted to the ICU for acute respiratory failure and who did not undergo lung transplantation. While our model still requires external validation, it suggests that a number of these patients are at lower risk of dying in hospital, and that initial aggressive care may therefore be reasonable. For high-risk patients, earlier palliative care involvement and emphasis on end-of-life care may be appropriate.
Acknowledgments
Author contributions: M. R. B. takes responsibility for the content of the manuscript, including the data and analysis. W. D. G., D. J. L., M. Ba., N. M. P., and M. R. B. contributed to study concept and design; W. D. G., M. Bi., A. J., M. R. B., and D. J. L. contributed to data collection and analysis; W. D. G. drafted the manuscript; D. J. L., N. M. P., M. Bi., A. J., D. B., M. Ba., and M. R. B. contributed to critical appraisal of the manuscript. All authors approved the submission of this version for publication.
Financial/nonfinancial disclosure: D. J. L. has served as a consultant for Roche, Veracyte, Fibrogen, Global Blood Therapeutics, Sanofi Genzyme, Boehringer-Ingelheim and Philips Respironics. D. B. is currently on the medical advisory boards of ALung Technologies and Kadence. None declared (W. D. G., M. Bi., A. J., N. P., M. Ba., M. R. B.).
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Additional information: The e-Appendix and e-Table can be found in the Supplemental Materials section of the online article.
Footnotes
FUNDING/SUPPORT: This study was supported by Grant K23 AG045560 and a Columbia University Aging Center Faculty Research Fellowship (M. R. B.); by Grants K24 HL115354 and R01 HL103676 (D. J. L.); and by Grant UL1 TR001873.
Drs Bacchetta and Baldwin are co-senior authors of this article.
Supplementary Data
References
- 1.Rosas I.O., Dellaripa P.F., Lederer D.J., Khanna D., Young L.R., Martinez F.J. Interstitial lung disease: NHLBI Workshop on the Primary Prevention of Chronic Lung Diseases. Ann Am Thorac Soc. 2014;11(suppl 3):S169–S177. doi: 10.1513/AnnalsATS.201312-429LD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raghu G., Chen S.Y., Yeh W.S. Idiopathic pulmonary fibrosis in US Medicare beneficiaries aged 65 years and older: incidence, prevalence, and survival, 2001-11. Lancet Respir Med. 2014;2(7):566–572. doi: 10.1016/S2213-2600(14)70101-8. [DOI] [PubMed] [Google Scholar]
- 3.Bouros D., Wells A.U., Nicholson A.G. Histopathologic subsets of fibrosing alveolitis in patients with systemic sclerosis and their relationship to outcome. Am J Respir Crit Care Med. 2002;165(12):1581–1586. doi: 10.1164/rccm.2106012. [DOI] [PubMed] [Google Scholar]
- 4.Fischer A., Swigris J.J., Groshong S.D. Clinically significant interstitial lung disease in limited scleroderma: histopathology, clinical features, and survival. Chest. 2008;134(3):601–605. doi: 10.1378/chest.08-0053. [DOI] [PubMed] [Google Scholar]
- 5.Lee H.K., Kim D.S., Yoo B. Histopathologic pattern and clinical features of rheumatoid arthritis-associated interstitial lung disease. Chest. 2005;127(6):2019–2027. doi: 10.1378/chest.127.6.2019. [DOI] [PubMed] [Google Scholar]
- 6.Douglas W.W., Tazelaar H.D., Hartman T.E. Polymyositis-dermatomyositis-associated interstitial lung disease. Am J Respir Crit Care Med. 2001;164(7):1182–1185. doi: 10.1164/ajrccm.164.7.2103110. [DOI] [PubMed] [Google Scholar]
- 7.Collard H.R., Ryerson C.J., Corte T.J. Acute exacerbation of idiopathic pulmonary fibrosis: an international working group report. Am J Respir Crit Care Med. 2016;194(3):265–275. doi: 10.1164/rccm.201604-0801CI. [DOI] [PubMed] [Google Scholar]
- 8.Blivet S., Philit F., Sab J.M. Outcome of patients with idiopathic pulmonary fibrosis admitted to the ICU for respiratory failure. Chest. 2001;120(1):209–212. doi: 10.1378/chest.120.1.209. [DOI] [PubMed] [Google Scholar]
- 9.Mollica C., Paone G., Conti V. Mechanical ventilation in patients with end-stage idiopathic pulmonary fibrosis. Respiration. 2010;79(3):209–215. doi: 10.1159/000225932. [DOI] [PubMed] [Google Scholar]
- 10.Saydain G., Islam A., Afessa B., Ryu J.H., Scott J.P., Peters S.G. Outcome of patients with idiopathic pulmonary fibrosis admitted to the intensive care unit. Am J Respir Crit Care Med. 2002;166(6):839–842. doi: 10.1164/rccm.2104038. [DOI] [PubMed] [Google Scholar]
- 11.Fumeaux T., Rothmeier C., Jolliet P. Outcome of mechanical ventilation for acute respiratory failure in patients with pulmonary fibrosis. Intensive Care Med. 2001;27(12):1868–1874. doi: 10.1007/s00134-001-1150-0. [DOI] [PubMed] [Google Scholar]
- 12.Stern J.B., Mal H., Groussard O. Prognosis of patients with advanced idiopathic pulmonary fibrosis requiring mechanical ventilation for acute respiratory failure. Chest. 2001;120(1):213–219. doi: 10.1378/chest.120.1.213. [DOI] [PubMed] [Google Scholar]
- 13.Gaudry S., Vincent F., Rabbat A. Invasive mechanical ventilation in patients with fibrosing interstitial pneumonia. J Thorac Cardiovasc Surg. 2014;147(1):47–53. doi: 10.1016/j.jtcvs.2013.06.039. [DOI] [PubMed] [Google Scholar]
- 14.Al-Hameed F.M., Sharma S. Outcome of patients admitted to the intensive care unit for acute exacerbation of idiopathic pulmonary fibrosis. Can Respir J. 2004;11(2):117–122. doi: 10.1155/2004/379723. [DOI] [PubMed] [Google Scholar]
- 15.Tachikawa R., Tomii K., Ueda H. Clinical features and outcome of acute exacerbation of interstitial pneumonia: collagen vascular diseases-related versus idiopathic. Respiration. 2012;83(1):20–27. doi: 10.1159/000329893. [DOI] [PubMed] [Google Scholar]
- 16.Suda T., Kaida Y., Nakamura Y. Acute exacerbation of interstitial pneumonia associated with collagen vascular diseases. Respir Med. 2009;103(6):846–853. doi: 10.1016/j.rmed.2008.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park I.N., Kim D.S., Shim T.S. Acute exacerbation of interstitial pneumonia other than idiopathic pulmonary fibrosis. Chest. 2007;132(1):214–220. doi: 10.1378/chest.07-0323. [DOI] [PubMed] [Google Scholar]
- 18.Lee J., Yim J.J., Yang S.C. Outcome of patients with connective tissue disease requiring intensive care for respiratory failure. Rheumatol Int. 2012;32(11):3353–3358. doi: 10.1007/s00296-011-2158-6. [DOI] [PubMed] [Google Scholar]
- 19.Vial-Dupuy A., Sanchez O., Douvry B. Outcome of patients with interstitial lung disease admitted to the intensive care unit. Sarcoidosis Vasc Diffuse Lung Dis. 2013;30(2):134–142. [PubMed] [Google Scholar]
- 20.Paterniti M.O., Bi Y., Rekic D., Wang Y., Karimi-Shah B.A., Chowdhury B.A. Acute exacerbation and decline in forced vital capacity are associated with increased mortality in idiopathic pulmonary fibrosis. Ann Am Thorac Soc. 2017;14(9):1395–1402. doi: 10.1513/AnnalsATS.201606-458OC. [DOI] [PubMed] [Google Scholar]
- 21.Raghu G., Collard H.R., Egan J.J. ATS/ERS/JRS/ALAT Committee on Idiopathic Pulmonary Fibrosis. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183(6):788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vij R., Strek M.E. Diagnosis and treatment of connective tissue disease-associated interstitial lung disease. Chest. 2013;143(3):814–824. doi: 10.1378/chest.12-0741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aletaha D., Neogi T., Silman A.J. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62(9):2569–2581. doi: 10.1002/art.27584. [DOI] [PubMed] [Google Scholar]
- 24.Masi A.T., Subcommittee for Scleroderma Criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee Preliminary criteria for the classification of systemic sclerosis (scleroderma) Arthritis Rheum. 1980;23(5):581–590. doi: 10.1002/art.1780230510. [DOI] [PubMed] [Google Scholar]
- 25.Petri M., Orbai A.M., Alarcon G.S. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012;64(8):2677–2686. doi: 10.1002/art.34473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vitali C., Bombardieri S., Jonsson R. Classification criteria for Sjogren’s syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis. 2002;61(6):554–558. doi: 10.1136/ard.61.6.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koreeda Y., Higashimoto I., Yamamoto M. Clinical and pathological findings of interstitial lung disease patients with anti-aminoacyl-tRNA synthetase autoantibodies. Intern Med. 2010;49(5):361–369. doi: 10.2169/internalmedicine.49.2889. [DOI] [PubMed] [Google Scholar]
- 28.Tani C., Carli L., Vagnani S. The diagnosis and classification of mixed connective tissue disease. J Autoimmun. 2014;48-49:46–49. doi: 10.1016/j.jaut.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 29.Travis W.D., Costabel U., Hansell D.M. An official American Thoracic Society/European Respiratory Society statement: update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med. 2013;188(6):733–748. doi: 10.1164/rccm.201308-1483ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Genders TSS, Coles A, Hoffmann U, et al; CAD Consortium and the PROMISE Investigators. The external validity of prediction models for the diagnosis of obstructive coronary artery disease in patients with stable chest pain: insights from the PROMISE Trial [published online ahead of print June 9, 2017]. JACC Cardiovasc Imaging. https://doi.org/10.1016/j.jcmg.2017.02.020.
- 31.Hastie T., Tibshurani R., Friedman J. Springer; New York: 2001. The Elements of Statistical Learning, 1st ed. Springer Series in Statistics. [Google Scholar]
- 32.Evgeniou T., Pontil M., Elisseeff A. Leave one out error, stability, and generalization of voting combinations of classifiers. Machine Learning. 2004;55(1):71–97. [Google Scholar]
- 33.World Health Organization . World Health Organization; Geneva, Switzerland: 2000. Obesity: Preventing and Managing the Global Epidemic. Report of a WHO Consultation (WHO Technical Report Series 894) [PubMed] [Google Scholar]
- 34.Mehta H.B., Mehta V., Girman C.J., Adhikari D., Johnson M.L. Regression coefficient-based scoring system should be used to assign weights to the risk index. J Clin Epidemiol. 2016;79:22–28. doi: 10.1016/j.jclinepi.2016.03.031. [DOI] [PubMed] [Google Scholar]
- 35.Ley B., Ryerson C.J., Vittinghoff E. A multidimensional index and staging system for idiopathic pulmonary fibrosis. Ann Intern Med. 2012;156(10):684–691. doi: 10.7326/0003-4819-156-10-201205150-00004. [DOI] [PubMed] [Google Scholar]
- 36.Morisset J., Vittinghoff E., Elicker B.M. Mortality risk prediction in scleroderma-related interstitial lung disease: the SADL model. Chest. 2017;152(5):999–1007. doi: 10.1016/j.chest.2017.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Steyerberg E.W., Harrell F.E., Jr., Borsboom G.J., Eijkemans M.J., Vergouwe Y., Habbema J.D. Internal validation of predictive models: efficiency of some procedures for logistic regression analysis. J Clin Epidemiol. 2001;54(8):774–781. doi: 10.1016/s0895-4356(01)00341-9. [DOI] [PubMed] [Google Scholar]
- 38.King T.E., Jr., Tooze J.A., Schwarz M.I., Brown K.R., Cherniack R.M. Predicting survival in idiopathic pulmonary fibrosis: scoring system and survival model. Am J Respir Crit Care Med. 2001;164(7):1171–1181. doi: 10.1164/ajrccm.164.7.2003140. [DOI] [PubMed] [Google Scholar]
- 39.Wells A.U., Desai S.R., Rubens M.B. Idiopathic pulmonary fibrosis: a composite physiologic index derived from disease extent observed by computed tomography. Am J Respir Crit Care Med. 2003;167(7):962–969. doi: 10.1164/rccm.2111053. [DOI] [PubMed] [Google Scholar]
- 40.du Bois R.M., Weycker D., Albera C. Ascertainment of individual risk of mortality for patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2011;184(4):459–466. doi: 10.1164/rccm.201011-1790OC. [DOI] [PubMed] [Google Scholar]
- 41.Goh N.S., Desai S.R., Veeraraghavan S. Interstitial lung disease in systemic sclerosis: a simple staging system. Am J Respir Crit Care Med. 2008;177(11):1248–1254. doi: 10.1164/rccm.200706-877OC. [DOI] [PubMed] [Google Scholar]
- 42.Ryerson C.J., Vittinghoff E., Ley B. Predicting survival across chronic interstitial lung disease: the ILD-GAP model. Chest. 2014;145(4):723–728. doi: 10.1378/chest.13-1474. [DOI] [PubMed] [Google Scholar]
- 43.Brown C.E., Engelberg R.A., Nielsen E.L., Curtis J.R. Palliative care for patients dying in the intensive care unit with chronic lung disease compared with metastatic cancer. Ann Am Thorac Soc. 2016;13(5):684–689. doi: 10.1513/AnnalsATS.201510-667OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baldwin M.R., Narain W.R., Wunsch H. A prognostic model for 6-month mortality in elderly survivors of critical illness. Chest. 2013;143(4):910–919. doi: 10.1378/chest.12-1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Walkey A.J., Barnato A.E., Wiener R.S., Nallamothu B.K. Accounting for patient preferences regarding life-sustaining treatment in evaluations of medical effectiveness and quality. Am J Respir Crit Care Med. 2017;196(8):958–963. doi: 10.1164/rccm.201701-0165CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wolfe R., Abramson M.J. Modern statistical methods in respiratory medicine. Respirology. 2014;19(1):9–13. doi: 10.1111/resp.12223. [DOI] [PubMed] [Google Scholar]
- 47.Brown S.M., Wilson E., Presson A.P. Predictors of 6-month health utility outcomes in survivors of acute respiratory distress syndrome. Thorax. 2017;72(4):311–317. doi: 10.1136/thoraxjnl-2016-208560. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.