Abstract
The Hv1 proton channel shares striking structural homology with fourth transmembrane helical segment-type voltage-sensor (VS) domains but manifests distinctive functional properties, including a proton-selective “aqueous” conductance and allosteric control of voltage-dependent gating by changes in the transmembrane pH gradient. The mechanisms responsible for Hv1’s functional properties remain poorly understood, in part because methods for measuring gating currents that directly report VS activation have not yet been described. Here, we describe an approach that allows robust and reproducible measurement of gating-associated charge movements in Hv1. Gating currents reveal that VS activation and proton-selective aqueous conductance opening are thermodynamically distinct steps in the Hv1 activation pathway and show that pH changes directly alter VS activation. The availability of an assay for gating currents in Hv1 may aid future efforts to elucidate the molecular mechanisms of gating cooperativity, pH-dependent modulation, and H+ selectivity in a model VS domain protein.
Introduction
The voltage-gated proton channel Hv1 is a member of a large superfamily of voltage-sensor- (VS) domain-containing proteins that function as voltage-gated ion channels (VGCs) and voltage-sensitive lipid phosphatases (VSPs) (1, 2, 3, 4). Despite lacking a canonical ion-channel-pore domain, Hv1 mediates an activated-state H+-selective “aqueous” conductance (GAQ) that is believed to utilize a water-wire pathway for H+ transfer within the hydrated VS-domain central crevice (1, 2, 5, 6, 7, 8, 9). Although residues that alter ion selectivity in Hv1 have been identified (10, 11), it remains unclear why GAQ is observed in Hv1 but not related VS domains (5, 6). New methods that isolate VS activation from GAQ opening are therefore needed.
A generally accepted model of VS activation posits that changes in membrane potential act on gating charges to drive conformational rearrangement of the fourth transmembrane helical segment (S4) in the VS domain (12). The phenomenological similarities between time- and voltage-dependent gating in dimeric Hv1 channels and the gating of cation currents through the pore domains of tetrameric VGCs strongly suggest that VS activation operates by a similar mechanism (1, 2, 9, 13, 14, 15). However, Hv1 also manifests biophysical features, such as allosteric control of voltage-dependent gating by changes in the transmembrane pH gradient (ΔpH = pHI − pHO), that are evidently unique among VGCs and VSPs (1, 2, 7, 9, 16, 17). The structural and mechanistic bases for functional differences between Hv1 and related VS-domain-containing proteins are incompletely understood.
In VGCs and VSPs, depolarization-dependent S4 movement reorganizes protein-associated charges within the electrical field, producing a capacitive gating-associated current (IG) that is integrated in time to yield the total “on” gating charge (QON) (18). Under appropriate experimental conditions (i.e., when permeant ions are removed or an inactivating pore mutation is introduced), IG can be directly measured using voltage-clamp electrophysiology (18, 19, 20). In VGCs, the shapes and positions of the gating charge versus voltage (Q-V) and conductance versus voltage (G-V) relations are different, indicating that they represent thermodynamically distinct events in the channel activation pathway (18, 21). Studies have begun to elucidate the molecular mechanisms of “electromechanical” coupling in tetrameric VGCs (21, 22, 23, 24, 25), but it remains unclear whether a similar phenomenon occurs in Hv1 channels. To directly address this question, gating current measurements are needed. However, the intrinsic GAQ-mediated H+ current in Hv1 is likely to confound efforts to record IG in isolation.
Compared to ionic currents, gating-current amplitudes are small; removal of the permeant ion or pore block thus serves to reduce or eliminate ionic current and experimentally isolate IG (18, 19, 20). Abolishing GAQ-mediated current in Hv1 by ion substitution would likely require that the recording-solution pH be raised (i.e., [H+] lowered) beyond the range that is permissive for voltage-clamp electrophysiology in biological membranes, limiting the utility of this approach. N214R (N4R) and N214K substitutions were previously shown to attenuate steady-state outward currents carried by GAQ, but inward “tail” currents (ITAIL) remain measurable (7, 9, 26). Introducing N4R into the background of R205H (R1H) permits isolation of the resting-state H+ “shuttle” conductance (GSH) without substantially perturbing GAQ gating (9), suggesting that N214R could also be useful for revealing IG in Hv1. Here, we show that incorporating N214R into the background of rapidly activating Hv1 mutants (W207A or R205A) enables direct measurement of gating currents in human Hv1 channels expressed in mammalian cells.
Materials and Methods
Cell culture and electrophysiology
Wild-type (WT) or mutant human Hv1 cDNA (NM_032369) in the pcDNA5/FRT/TO vector was used to create isogenic tetracycline-inducible FlpIn293-TREx stable cell lines as previously described (9). Cells were plated onto glass coverslips, and expression of mutant Hv1 proteins was induced by addition of tetracycline (1.0 μg/mL) to the culture medium 24–72 h before electrophysiology. Whole-cell currents were measured at 22–24°C using an A-M Systems model 2400 amplifier (A-M Systems, Sequim, WA) as described previously (9). Currents are elicited by voltage-step protocols from a holding potential (VHOLD) to the prepulse step potential (VSTEP) for the indicated time and subsequently to the test potential (VTEST; typically +100 mV). Data were low-pass filtered at 2–5 kHz and digitized at 10–20 kHz using a National Instruments USB-5251 DAQ (National Instruments, Austin, TX) interfaced to a PC computer running a custom LabVIEW 7-based data acquisition and amplifier control program (C. A. Villalba-Galea; details and software distribution available on request). Data were analyzed using Clampfit9 (Molecular Devices, San Jose, CA) and Origin 8.1 (OriginLab, Northampton, MA). The standard intracellular and extracellular solutions contained the following (in mM): 100 Bis (2-hydroxyethyl)amino-tris(hydroxymethyl) methane (Bis-Tris), 1 ethylene glycol tetraacetic acid (EGTA), and 8 HCl; pH was adjusted to 6.5 and a final osmolality of 310–320 mOsm by the addition of tetramethylammonium hydroxide (TMAOH) and methanesulfonic acid (HMeSO3). pHO-dependent gating was measured in bath solutions containing 100 mM 2-(N-morpholino)ethanesulfonic acid (MES, pH 5.5) in place of Bis-Tris, as previously described (7). Series resistance is routinely compensated ∼80%, and liquid-junction potential corrections are not applied.
Data analysis
Unless otherwise indicated, data represent means ± standard error (SE) of values measured in n cells. ISTEP represents the peak current during steps to the indicated potential (VSTEP). The activated state “aqueous” conductance in Hv1 (GAQ) was calculated from GAQ = ISTEP/V-EREV, where EREV is the zero-current potential determined from inspection of the ISTEP-V relation. Tail current (ITAIL) amplitude is measured by fitting the decaying current to a monoexponential function of the form ITAIL = I0 + Ae−V/t (where I0 is the minimal current after decay of ITAIL, A is current amplitude, V is membrane potential, and t is time) and extrapolating fits to the instant the voltage was changed. Leak currents were subjected to offline linear subtraction. To estimate voltage-dependent gating parameters, ITAIL-V relations are fitted to a Boltzmann function of the following form: , where V0.5 is the voltage at which 50% of the maximal current is reached, dx is a slope factor, and ITAIL max and ITAIL min represent the maximal and minimal tail-current amplitudes, respectively. GAQ-V relations are fitted to a single Boltzmann of the form , where V0.5, dx, GAQ max, and GAQ min have the same meanings as defined for ITAIL. In some cases, effective gating valence (zG) was calculated from Boltzmann fits, where zG = RT/F × dx, where F, R, and T have their usual meanings (i.e., RT/F = 25.3 mV at 20°C, the approximate temperature at which experiments were conducted). Curve fits reported in figure legends represent the best fit to the mean data; Table 1 reports mean ± SE of fitted or calculated values determined separately in the indicated number of individual experiments.
Table 1.
Fitted and Calculated Gating Parameters
| QON | ITAIL | ITAIL-QON | ||||||
|---|---|---|---|---|---|---|---|---|
| Construct | Parameter | Mean | SE | n | Mean | SE | n | Difference |
| VM (mV) | −38.9 | 0.8 | 6 | −31.6 | 0.8 | 6 | 7.3a | |
| W207A-N214R | V0.5 (mV) | −40.0 | 1.1 | 6 | −31.4 | 0.6 | 6 | 8.6b |
| zG (e0) | 2.80 | 0.06 | 6 | 3.21 | 0.11 | 6 | 0.41NS | |
| VM (mV) | −24.1 | 0.3 | 12 | −11.3 | 0.4 | 12 | 12.8c | |
| W207A-N214R-ΔC | V0.5 (mV) | −24.6 | 0.3 | 12 | −15.7 | 0.4 | 12 | 8.9d |
| zG (e0) | 1.75e | 0.01 | 12 | 1.52f | 0.02 | 12 | −0.23g | |
| VM (mV) | 17.8 | 0.8 | 3 | 25.7 | 1.6 | 3 | 8.0NS | |
| R205A-N214R | V0.5 (mV) | 25.1 | 2.3 | 3 | 26.4 | 1.3 | 3 | 1.3NS |
| zG (e0) | 2.14h | 0.12 | 3 | 1.89i | 0.07 | 3 | −0.25NS | |
Data represent calculated VM or Boltzmann-fitted V0.5 and zδ parameters for QON-V and ITAIL-V relations measured at pHO 6.5 in the indicated number of cells.
W207A-N214R: ITAIL VM vs. QON VM, p = 0.03.
W207A-N214R: ITAIL V0.5 vs. QON V0.5, p = 0.02.
W207A-N214R-ΔC: ITAIL VM vs. QON VM, p = 1.6 × 10−7.
W207A-N214R-ΔC: ITAIL V0.5 vs. QON V0.5, p = 2.2 × 10−5.
QONzG: W207A-N214R vs. W207A-N214R-ΔC, p = 2.3 × 10−7.
ITAILzG: W207A-N214R vs. W207A-N214R-ΔC, p = 5.6 × 10−7.
W207A-N214R-ΔC: ITAILzG vs. QONzG, p = 0.01.
QONzG: W207A-N214R vs. R205A-N214R, p = 0.01.
ITAILzG: W207A-N214R vs. R205A-N214R, p = 0.02.
The GAQ activation time course (see Fig. 1, A and B) is fitted to a single exponential function of the form , where I is the measured ITAIL at time t and Imax is ITAIL at t = ∞, d is the delay, and τ is the activation time constant (i.e., τACT). GAQ activation in W207A-N214R-ΔC (see Fig. S3) is fitted to a monoexponential function of the form . The fitted exponential time course is then scaled to the amplitude of the steady-state ISTEP at each voltage (i.e., ITAIL max = ISTEP) and subtracted from the measured ISTEP to yield transient outward IG at each VSTEP. Channel number (N) is calculated by dividing the total gating charge (QON) by the unitary gating valences (zδ = 6 e0 for full-length Hv1 channels and zδ = 3 e0 for ΔC truncated channels) estimated from variance analysis (27). Unitary conductance (γAQ) is calculated from the slope of a plot of GAQ max (determined from ITAIL) versus N (i.e., GAQ max = N × γAQ). The “on” gating charge (QON) is determined by the trapezoidal integration of IG.
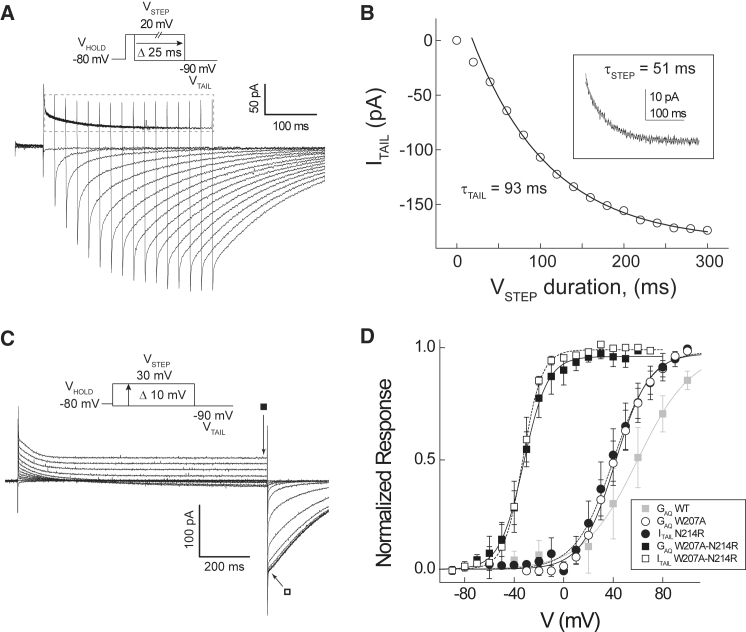
Figure 1.
GAQ-V and ITAIL-V relations in Hv1 W207A-N214R. (A) Representative currents elicited by the indicated voltage step protocol (inset: VHOLD = −80 mV; VSTEP = +20, 0–300 ms duration in 25 ms increments; VTAIL = −90 mV) in a cell expressing in W207A-N214R are shown. Current measured during the 300-ms-duration VSTEP is magnified in (B, inset). (B) ITAIL amplitude (current shown in (C)) is plotted as a function of VSTEP duration. The solid black line represents a fit of the data to a single exponential decay function (τTAIL = 93 ms) after a delay (18 ms). The inset shows current elicited by VSTEP = +20 mV, duration 300 ms; the solid gray line represents a fit of the data to a single exponential decay function (τSTEP = 51 ms). (C) Representative currents elicited by the indicated voltage protocol (inset: VHOLD = −80 mV; VSTEP = −90 to +30 mV in 10-mV increments, 1-s duration; VTAIL = −90 mV) in a cell expressing W207A-N214R are shown. Symbols indicate times at which ISTEP (filled square) and ITAIL (open square) are measured. (D) Steady-state currents measured at the end of VSTEP (ISTEP) are used to calculate GAQ (see Materials and Methods), and data are normalized to their respective maxima in each cell to generate GAQ-V relations for WT (filled gray squares; n = 4), W207A (open circles; n = 4), and W207A-N214R (filled squares; n = 7). Mean normalized ITAIL-V relations are shown for N214R (filled circles; n = 4) and W207A-N214R (open squares; n = 9). Solid lines indicate Boltzmann fits to the mean GAQ-V (WT, gray line: V0.5 = +59.7 mV, zG = 1.2 e0; W207A: V0.5 = +41.9 mV, zG = 1.9 e0; W207A-N214R: V0.5 = −32.3 mV, zG = 2.6 e0) and dashed lines indicate Boltzmann fits to mean ITAIL-V (N214R: V0.5 = +40.1 mV, zG = 1.7 e0; W207A-N214R: V0.5 = −33.0 mV, zG = 3.3 e0) relations.
Requests for biological materials (i.e., cDNA constructs used for this study) should be directed to the corresponding author. Additional information and requests for software used for integration of gating currents may be directed to Carlos A. Villalba-Galea (cvillalbagalea@pacific.edu).
Results
To measure gating-charge movement in Hv1, we hypothesized that it would be necessary to identify a mutant channel in which 1) the time course of GAQ activation is greatly accelerated and 2) current carried by GAQ is greatly diminished. A mutation of a conserved Trp residue in the S4 helix of Hv1 (W207) is reported to greatly speed the time course of GAQ activation (28). Mutations at R205 (R1) also exhibit rapid GAQ activation by membrane depolarization (1, 2, 7, 9, 27, 29), but the effects of this and other S4 Arg mutations to decrease the apparent gating-charge valence (zG) (27) represent a potential limitation for their use in measuring gating current. Introducing an additional Arg residue into the S4 helix of Hv1 (N214R) was previously shown to block outward steady-state currents carried by GAQ (7, 9, 26). We therefore combined W207A and N214R mutations (W207A-N214R) and induced expression of mutant channels in a modified HEK-293 cell line by addition of tetracycline to the culture medium (see Materials and Methods).
W207A-N214R alters GAQ activation kinetics and steady-state voltage-dependent gating
We previously reported that nontransfected cells and cells that were not exposed to tetracycline do not exhibit voltage-dependent currents under the recording conditions used here (7). However, robust voltage-gated currents are measured under whole-cell voltage clamp in cells expressing N-terminally tagged EGFP-hHv1 W207A-N214R (Fig. 1 A). Increasing the duration of the voltage step (VSTEP) to +20 mV causes the amplitude of the subsequent, inwardly directed tail current (ITAIL) measured at −90 mV to increase and eventually plateau (Fig. 1, A and B). Peak ITAIL rises sigmoidally with VSTEP duration and is fitted to a single-exponential function after a delay (Fig. 1 B), similar to WT Hv1 channels (1, 2, 28, 30). The fitted time constant for ITAIL activation (τTAIL = 93 ms) is substantially faster than WT Hv1 but slower than W207A (28). Unlike W207A and WT Hv1, which pass outward GAQ-mediated currents that display sigmoid activation kinetics during a fixed voltage step (1, 2, 28), W207A-N214R exhibits a complex biphasic time course (Fig. 1 A). A rapidly activating transient outward current decays to an apparent steady state with a fitted time course (τSTEP = 51 ms) that is almost two times faster than ITAIL (Fig. 1 B). The kinetic difference between ISTEP and ITAIL activation time courses suggests that the transient-current decay may report a conformational change that precedes the opening of GAQ.
Next, we sought to establish experimental conditions under which IG and ITAIL are measured under apparent steady-state conditions. QON and ITAIL are each time and voltage dependent (Fig. S1, A–D), and Boltzmann fits to the data show that the fitted V0.5 and slope factors (apparent zG) also depend on the duration of the preceding voltage step (Fig. S1, E and F). A voltage step (VSTEP, before ITAIL measurement) or prepulse (VPP, before QON measurement) of 1-s duration is evidently sufficient to achieve apparent equilibrium conditions, because a longer voltage pulse elicits no additional changes in either the fitted V0.5 or zG (Fig. S1, E and F). We therefore measure ISTEP at the end of a 1-s depolarization and calculate conductance to determine the GAQ-V relation; ITAIL is measured after a subsequent hyperpolarizing step as described previously (1, 9).
As expected, the ITAIL-V and GAQ-V relations in W207A-N214R are highly similar, indicating that they report the same conformational transition, namely, GAQ activation. Compared to WT Hv1 and W207A and N214R single mutants, GAQ gating is shifted toward negative potentials in W207A-N214R (Fig. 1 D). The fitted midpoint (V0.5) of the GAQ-V and ITAIL-V relations in W207A-N214R are ∼90 mV more negative than WT Hv1 and ∼70 mV more negative than either W207A or N214R (Fig. 1 D). Interestingly, the slopes of Boltzmann fits to the GAQ-V and ITAIL-V relations are shallower in WT Hv1 than in W207A, N214R, or W207A-N214R mutant channels (Fig. 1 D). The similarity in zG calculated from Boltzmann fits suggests that the kinetically fast mutant channels tested here are likely to move a similar quantity of gating charge during VS activation. Although measurement of the limiting slope suggests that zG = ∼6 e0 in WT Ciona intestinalis Hv1, indicating that GAQ-V and ITAIL-V relations may significantly underestimate zG (27), zG estimates in mutant channels measured here may nonetheless be usefully compared (Table 1).
Gating currents in Hv1 W207A-N214R
Because GAQ is not substantially open at early times after depolarization, we hypothesize that rapidly activating transient outward currents could represent IG in Hv1. As in WT Hv1, the kinetics of GAQ opening in W207A-N214R are both time and voltage dependent (Fig. S1, A and B). The decay of the transient outward current measured at various potentials is also evidently voltage dependent (Fig. 2 A). Although it should be possible to subtract the GAQ-mediated component of current from the aggregate ISTEP to yield IG in isolation (Fig. 1, A and B), the time course of GAQ activation follows a complex sigmoidal time course (31). Determining the amplitude of H+-current ISTEP contributed by GAQ at each time and voltage depends on accurate kinetic modeling of the activation pathway, which is impractical in W207A-N214R because outward H+ currents are blocked. Instead, we opt to record transient outward currents at a fixed potential (i.e., VSTEP = +100 mV) after stepping through a range of prepulse voltages (VPP). A step to +100 mV from a large negative VPP (i.e., −120 mV), at which the open probability for GAQ is evidently close to its minimum (Fig. 1 D), elicits a large transient outward current with a complex rising and falling kinetic profile (Fig. 2 A). The initial spike of outward current seen in Fig. 2 A is attributed to incomplete compensation of the membrane capacitance and is not analyzed further here. As expected, the amplitude of the transient outward current measured at +100 mV saturates at both positive and negative VPP (Fig. 2 A), suggesting that it represents an authentic IG in Hv1.
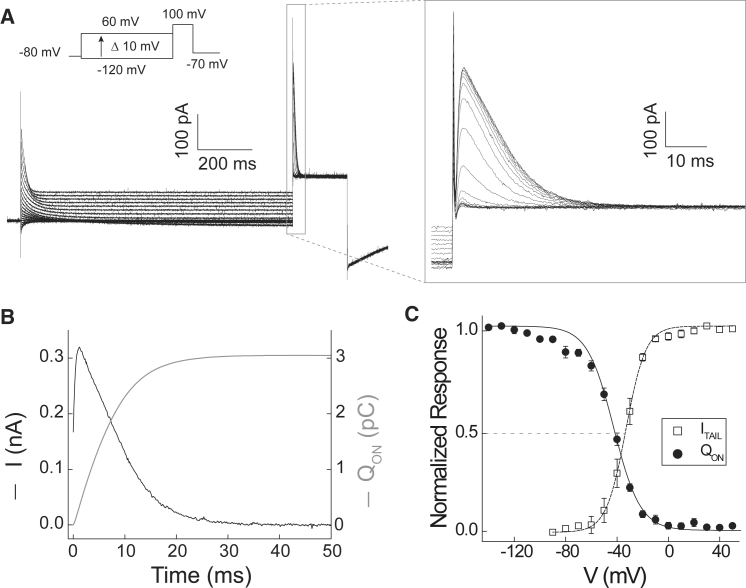
Figure 2.
Integration of transient outward current reveals “on” gating charge (QON) movement. (A) Representative currents elicited by the indicated voltage protocol (VHOLD = −80 mV; VPP = −120 to +60 mV in 10-mV increments, 1-s duration; VSTEP = +100 mV, 200-ms duration; VTAIL = −70 mV) are shown. Transient outward currents at VSTEP = +100 mV within the dashed box are magnified in the boxed inset. Steady-state currents measured at the end of VPP are attributed to GAQ (see also Figs. S3 and S4). (B) The transient outward current (VPP = −100 mV, VSTEP = +100 mV, black line) is plotted together with its time integral (QON, gray line). The timescale is adjusted to reflect the rise in transient outward current after a brief initial current that we attribute to uncompensated capacitance. In this example, gating current integration begins 1.75 ms after the VSTEP voltage command is applied. (C) QON (filled circles) measured at VSTEP = +100 mV (VPP = −120 mV, 1000 ms duration) is normalized to its maximum in each cell and mean data (n = 9 cells) are plotted in function of VPP. The mean ITAIL-V relation (open circles; data from Fig. 1B) is replotted for comparison. Lines represent fits to Boltzmann functions (QON, solid line: V0.5 = −42.5 mV; zG = 2.5 e0; ITAIL, dashed line: V0.5 = −33.0 mV, zG = 3.3 e0). Median voltages (VM) calculated for the normalized mean QON-V and ITAIL-V relations shown here are VM = −44.9 mV and VM = −33.2 mV, respectively.
Integrating IG yields a direct measure of the gating charge generated by functional Hv1 channels in the plasma membrane (QON). As expected, QON rises exponentially and saturates in time, as illustrated in a representative experiment in which ISTEP is measured at +100 mV (Fig. 2 B). Integrating ISTEP over a range of VPP yields a highly nonlinear QON-V relation that clearly saturates at large positive and negative potentials (Figs. 2 C and S1). QON is maximal after VPP to hyperpolarizing voltages that are expected to drive occupancy of resting-state VS conformations and QON falls toward zero as the driving force for gating-charge translocation decreases (Figs. 2 C and S1). To compare ITAIL-V and QON-V gating parameters, we initially compared Boltzmann fits of the data (Fig. S1). Surprisingly, V0.5 for the QON-V relation is consistently 8–10 mV more negative than the ITAIL-V relation (Figs. 2 C and S1; Table 1), suggesting that gating-charge movement and GAQ opening represent thermodynamically distinct transitions in the Hv1 activation pathway.
In contrast to our expectations, the slopes of Boltzmann functions fitted to QON-V relations are evidently shallower than ITAIL-V relations (Figs. 1 D and 2 C; Table 1). Previous studies in both Hv1 and tetrameric VGCs show that Boltzmann fits to ITAIL-V or G-V relations typically yield smaller estimates of zG than limiting-slope or Q-V relations (1, 2, 27, 32, 33, 34). Closer inspection reveals that although the ITAIL-V relations are well described by single Boltzmann distributions (Fig. 1 D), the QON-V relation characteristically deviates from the Boltzmann fit between −100 and −70 mV (Fig. 2 C). The data therefore suggest that gating-charge movement in Hv1 requires more than one transition, similar to tetrameric VGCs for which complex Q-V relations are not described by simple Boltzmann distributions and fitted-slope values often appear to underestimate zG (14, 20, 21, 35, 36, 37, 38). We therefore calculated the median voltages (VM) for QON-V and ITAIL-V relations to compare thermodynamic gating parameters in a model-independent fashion (38). Similar to V0.5, we find that VM is consistently ∼10 mV more negative for QON than for ITAIL (Table 1), indicating that GAQ opening requires an additional gating transition.
Estimating channel number and unitary conductance from Hv1 gating currents
A straightforward expectation is that QON and ITAIL similarly depend on the number of functional channels (N) in the plasma membrane; maximal QON (QON max) is therefore expected to be tightly correlated with maximal ITAIL amplitude (ITAIL max). If QON = N × zG × PACT, where PACT is the probability that zG has been moved (i.e., the VS domain is activated), and ITAIL max = N × i × POPEN, where i is the unitary current and POPEN is the probability that GAQ is open, the slope of the QON max vs. ITAIL max relation thus reports charge movement per “conducting unit” (i.e., QON max/ITAIL max = zG/i). Consistent with expectations, the plot of QON max vs. ITAIL max is well fitted to a straight line with slope = 5.9 pC/nA (Fig. 3 A). Limiting-slope analysis of GAQ-V relations provides an independent estimate of the gating valence (zG = 6 e0/conducting unit) in WT, presumably dimeric C. intestinalis Hv1 channels (27), allowing us to calculate N from the QON max data. To accurately measure GAQ, each cell must express ∼0.5 × 106 W207A-N214R conducting units, and QON measurements are ideally measured in cells that appear to express >2 × 106 channels (Fig. 3 B). Using the zG-values determined from our Boltzmann fits of QON-V or ITAIL-V relations (Table 1) would approximately double the number of channels needed to generate the measured current or charge movement. Alternatively, if zG-values reported for C. intestinalis Hv1 underestimate the actual gating valence, we might overestimate N somewhat. Given that even highly similar channels like Kv1.2 and Drosophila Shaker can manifest dramatically different gating valences (39), some caution is warranted in the interpretation of absolute channel numbers reported here. Nonetheless, our data suggest that mutant Hv1 channels are highly overexpressed in our studies, and robust protein expression is probably an important prerequisite for IG measurement in Hv1.
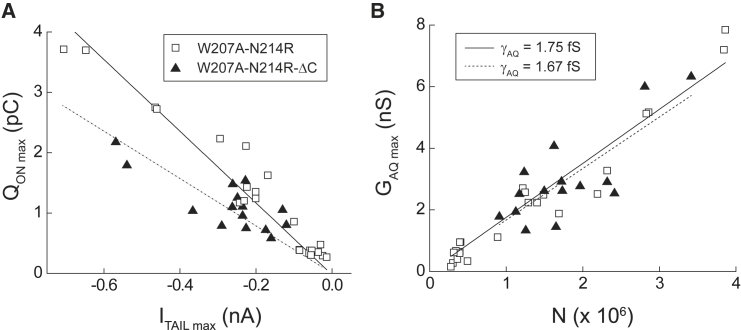
Figure 3.
Estimating unitary conductance in Hv1 W207A-N214R. (A) Plots of maximal QON (QON max) versus maximal ITAIL (ITAIL max) measured in individual cells expressing W207A-N214R (open squares) or W207A-N214R-ΔC (filled triangles) are shown. Mean (± SE) QON max = 1.25 ± 0.22 pC, and ITAIL max = 197 ± 40 pA (n = 24 cells). Lines indicate linear fits of the data (W207A-N214, solid line: slope = 5.9 pC/nA; R2 = 0.97; W207A-N214R-ΔC, dashed line: slope = 3.9 pC/nA; R2 = 0.87. (B) The apparent number of channels (N) is calculated from the total charge (QON max) divided by the previously reported (27) gating valences for full-length (squares; zG = 6 e0) or truncated (triangles; zG = 3 e0) channels’ limiting-slope analyses. Linear fits of maximal GAQ (GAQ max) determined from Boltzmann fits to GAQ-V relations in each cell (see Fig. 1D) versus calculated channel number yield estimates of the GAQ unitary conductances for W207A-N214R (solid line: γAQ = 1.75 fS) and W207A-N214R-ΔC (dashed line: γAQ = 1.67 fS). To see this figure in color, go online.
Standard ion channel theory posits that GAQ max = N × γAQ × POPEN (where POPEN is the probability of GAQ being open), so γAQ can readily be calculated from the slope of the GAQ max vs. N plot (Fig. 3 B). Because N214R blocks GAQ-mediated current by occupying a site at the intracellular entrance to the GAQ-meditated H+ transfer pathway (5, 7, 9, 26), we calculated γAQ from ITAIL measured at negative voltages at which the block is less evident. However, the estimated unitary conductance in W207A-N214R (γAQ = 1.75 fS) is still ∼20-fold smaller than a previous estimate based on variance analyses of native voltage-gated H+ currents in human eosinophils (γ = 36 fS) measured under similar experimental conditions (40). Our data are consistent with a previous study showing that N214R exerts a pronounced blocking effect on outward H+ transfer (9) but suggest that both outward and inward H+ currents are smaller in W207A-N214R than in WT Hv1.
Effect of mutations on gating-charge movement in Hv1
The effects of charge-neutralizing mutations indicate that conserved S4 Arg residues function as gating charges in Hv1 and other VS domain-containing proteins. We therefore combined N214R with a second-site mutation (R205A) that eliminates the most extracellular Arg in S4 (R1) and measured gating currents as described earlier. R205A-N214R exhibits rapid GAQ activation kinetics, prominent GAQ-mediated tail currents at negative voltages, and transient outward gating currents that are similar to W207A-N214R (Fig. S2, A and B). Although the steady-state ISTEP attributed to GAQ appears to be larger in R205A-N214R than W207A-N214R, the GAQ contribution is readily subtracted to yield transient outward currents that can be integrated to estimate QON (Fig. S2, A and C). The positions of the R205A-N214R ITAIL-V and QON-V relations are shifted positively by ∼57 mV compared to W207A-N214R, consistent with previous reports showing that S4 mutations have dramatic effects on gating kinetics and the steady-state thermodynamic properties but nonetheless retain most or all of the essential biophysical properties of voltage-gated H+ channels (1, 2, 9, 28). The fitted V0.5-values for QON-V and ITAIL-V relations in R205A-N214R are not significantly different, but like in W207A-N214R, VM-values are separated by 8 mV (Fig. S2 C; Table 1). zG-values estimated from Boltzmann fits to QON-V (zG = 2.1 ± 0.1 e0) and ITAIL-V (zG = 1.9 ± 0.1 e0) relations are 0.7 e0 and 1.3 e0 smaller, respectively, in R205A-N214R than W207A-N214R (Table 1).
To determine whether C-terminal deletion alters zG, we next measured gating currents in W207A-N214R-ΔC. Limiting-slope analyses indicate that zG is ∼50% smaller in Hv1-ΔC (zG ≈ 3 e0) than full-length channels, possibly because the conducting unit is a monomer rather than a dimer (14, 27). If monomeric Hv1-ΔC channels function similarly to dimeric WT channels, zG/i is not expected to be different. However, we find that the slope of the QON max/ITAIL max relation is significantly smaller in W207A-N214R-ΔC than it is in full-length W207A-N214R channels (Fig. 3 A). We observe a similar decrease in the apparent zG/i ratio in R205A-N214R (Fig. S2 D), consistent with the hypothesis that R1 neutralization decreases the total gating-charge valence (1, 2, 9, 27). The effect of ΔC on QON max/ITAIL max therefore suggests that C-terminal truncation alters a unitary channel property (zG and/or i) in addition to its effects on dimer stability and gating cooperativity.
Using the gating valence previously determined for C. intestinalis Hv1-ΔC channels (zG = 3 e0/conducting unit) (27), we estimated that the unitary conductances in W207A-N214-ΔC (γAQ = 1.67 fS) and R205A-N214R (γAQ = 2.21 fS) are not significantly different from W207A-N214R (γAQ = 1.75 fS; Figs. 3 B and S1 E). This result is consistent with an earlier study showing that the two pores in dimeric Hv1 channels are independent and each contributes equally to the total current (41). A straightforward interpretation of the data is that the reduction in QON max/ITAIL max ratio, and thus zG/i, observed in R1- and ΔC-mutant channels is mainly due to a decrease in gating valence. Consistent with this interpretation, we find that, like R205A-N214R, the Boltzmann slopes of both the QON-V and ITAIL-V relations are shallower in W207A-N214R-ΔC than in W207A-N214R (Table 1). In ΔC channels, apparent zG from the QON-V (zG = 1.8 ± 0.1 e0) and ITAIL-V (zG = 1.5 ± 0.1 e0) are 0.9 and 1.8 e0 smaller, respectively, than W207A-N214R (Table 1).
In addition to the effects of ΔC on steady-state properties of unitary Hv1 channels, C-terminal truncation apparently reduces complexity in the Hv1 activation pathway, as evidenced by a switch from sigmoid (WT) to exponential (ΔC) GAQ activation time courses (14, 26). We also find that although the time course for ITAIL activation in W207A-N214R is sigmoidal (Fig. 1 B), ITAIL evidently rises exponentially in W207A-N214R-ΔC (Fig. S3, A–C). We therefore scaled single-exponential fits of the ITAIL time course to the amplitude of the current at the end of each ISTEP (Fig. S3 B) and then subtracted the GAQ-mediated component of current at each voltage from the aggregate ISTEP. Integrating the GAQ-subtracted transient currents measured at a range of VSTEP yields a family of transient currents with the expected voltage and time dependence for gating current (Fig. S3 D). V0.5 for the QON-V relation in W207A-N214R-ΔC, which is well fitted to a single Boltzmann distribution, is shifted positively by ∼+15 mV compared to W207A-N214R (Fig. S3 E; Table 1). Consistent with expectations for a gating current, the τDEACT-V relation of the GAQ-subtracted transient current is bell-shaped, and the peak is located close to the fitted V0.5 (Fig. S3, E and F). Consistent with the previously noted differences in the QON max/ITAIL max relations forW207A-N214R and W207A-N214R-ΔC, we find that the mean Boltzmann slope of QON-V relations measured at +100 mV are shallower in W207A-N214R-ΔC (zG = 1.75 ± 0.01 e0) than W207A-N214R (zG = 2.80 ± 0.06 e0, p < 0.01; Table 1). The mean Boltzmann slope of the W207A-N214R ITAIL-V relation (zG = 3.21 ± 0.11 e0) is also steeper than W207A-N214R-ΔC (zG = 1.52 ± 0.02 e0, p < 0.01; Table 1). In summary, gating currents suggest that both R1 neutralization and C-terminal truncation decrease the gating valence but do not appreciably alter unitary conductance.
Gating-charge movement in W207A-N214R is sensitive to changes in pHO
To determine whether W207A-N214R exhibits the hallmark sensitivity to changes in the pH gradient that is characteristic of Hv1 channel gating (1, 2, 17), we simultaneously measured ITAIL and IG at pHO 6.5 and pHO 5.5 (Figs. 4 A and S4, A and B). GAQ begins to activate at voltages that are negative to the Nernst potential for H+ (EH+), and W207A-N214R mediates steady-state inward currents that are distinct from WT Hv1 (Fig. S4, A and B). Similar to WT Hv1, however, lowering pHO in the physiological range (i.e., pHO < 8) shifts both the position of the GAQ-V relation and the ISTEP reversal potential (ΔEREV = 49.7 ± 4.1 mV, n = 5 cells; see also Fig. S4). ΔEREV measured between pHO 6.5 and pHO 5.5 (pHI 6.5) is close to the Nernst prediction for a H+-selective channel (ΔEH+ = 56 mV at 20°C), suggesting that W207A-N214R remains reasonably H+-selective under our recording conditions, consistent with a previous study of W207 single-mutant channels (28). As expected from the effect of extracellular acidification on the driving force for H+ current, ISTEP and ITAIL amplitudes are altered by pHO changes (Fig. S4, A and B); lowering pHO also speeds ITAIL decay in W207A-N214R (Fig. S4, A and B), consistent with previous reports (1, 9, 17, 28).
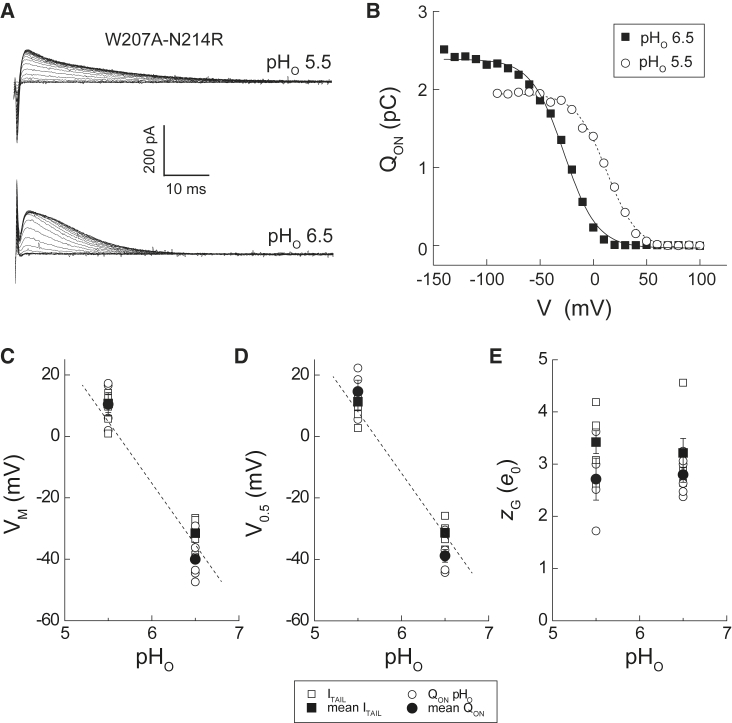
Figure 4.
pHO dependence of QON-V and ITAIL-V relations in W207A-N214R. (A) Representative outward transient current traces recorded at pHO 5.5 (top) or pHO 6.5 (bottom) in a cell expressing W207A-N214R are shown. Currents at +100 mV are elicited by a series of voltage steps between −140 and +100 mV (10-mV increments, duration = 200 ms; data not shown). (B) Representative QON-V relations are generated by integrating the currents (data shown in (A): open circles, pHO 5.5; filled squares, pHO 6.5). Lines represent Boltzmann fits to the data (dashed line, pHO 5.5: QON max = 1.9 pS, V0.5 = 11.8 mV, zG = 1.83 e0; solid line, pHO 6.5: QON max = 2.4 pS, V0.5 = −27.7 mV, zG = 1.70 e0). (C–E) VM (C), V0.5 (D), and zG (E) parameters determined for ITAIL-V (squares) and QON-V relations (circles) at pHO 6.5 or pHO 5.5 are shown. Mean values are indicated by filled symbols, and data from individual experiments are indicated by open symbols. Dashed lines in (C and D) indicate slope = −40 mV/pH unit. To see this figure in color, go online.
We demonstrate here for the first time, to our knowledge, that gating-charge movement in Hv1 is also pH sensitive. First, we show that the rate of IG decay at +100 mV is faster at pHO 6.5 compared to pHO 5.5 (Fig. 4 A), indicating that the “on” gating charge moves faster at more alkaline pH. Raising pHO reciprocally slows the time course of ITAIL decay at −90 mV (Figs. 4 A and S4, A and B). The kinetic effects of pHO changes on IG and ITAIL in W207A-N214R are consistent with the effect of pHO changes on the GAQ activation time course in WT Hv1, in which extracellular alkalization both speeds opening and slows closing (1, 2, 17, 42). Consistent with the effects of pHO changes on the GAQ-V relation, V0.5 and VM-values determined for QON-V relations at pHO 6.5 and pHO 5.5 are displaced by ∼40 mV (Fig. 4, B–D). The magnitude of the pH-dependent shift in gating-charge movement in W207A-N214R is therefore comparable to the response measured in WT and other Hv1 mutant channels, including the W207A single mutant (7, 28). In contrast to V0.5 and VM, zG-values estimated from the slopes of Boltzmann fits to QON-V and ITAIL-V relations are not significantly different at pHO 6.5 and pHO 5.5 (Fig. 4, B and E; Table 1). Finally, we find that QON max is smaller at pHO 5.5 than at pHO 6.5 (Fig. 4 B). On average, the quantity of gating charge moved at pHO 5.5 is 29% smaller than at pHO 6.5 (QON max pH5.5/QON max pH 6.5 = 0.71 ± 0.05; mean ± SE, n = 5 cells, p = 0.02 by Student’s paired t-test).
Discussion
Although Hv1 shares structural and functional homology with other voltage-dependent cation channels and phosphatases, its VS domain is evidently unique in mediating an intrinsic, activated-state “aqueous” H+ conductance (1, 2, 7). GAQ gating in Hv1 is also strongly modulated by changes in ΔpH (1, 2, 17), but the molecular mechanism(s) responsible for pH-dependent gating remain unknown (7, 28). Strikingly, neutralization of candidate ionizable residues in Hv1 is insufficient to abrogate either pHO-dependent gating or H+-selective ion permeation (5, 6, 7), but neutralization of H168 was shown to attenuate sensitivity to changes in pHI (16). So far, only one nonfunctional mutant Hv1 channel (D112V) has been described (11), but it remains unclear whether this mutation exerts its effect by 1) causing a reorganization of the H+ transfer pathway to block GAQ-mediated H+ transfer or 2) preventing a voltage-dependent conformational rearrangement that is necessary for GAQ opening. To understand the mechanisms of VS activation and GAQ gating independently from H+ transfer, new experimental tools are needed.
Here, we show that for the first time, to our knowledge, gating currents associated with the movement of protein-associated charge can be measured in mutant Hv1 channels. We attribute the success of our experimental strategy to three factors: 1) high expression of mutant proteins driven in stable, tetracycline-inducible cell lines; 2) incorporation of mutations (W207A or R205A) that dramatically speed the normally slow GAQ activation kinetics in mammalian Hv1 channels; and 3) partial block of GAQ-mediated ionic current by N214R. Together, these experimental manipulations reveal the existence of transient currents with gating properties that are distinct from the ionic H+ current. IG directly reports VS activation, and its integral yields the quantity of charge moved, which is directly proportional to GAQ. IG measurements in the Hv1 mutants tested here exhibit all the expected features of authentic gating currents, including time- and voltage-dependent gating and saturable dependence on membrane potential. One limitation of our study is that we have so far been unable to cleanly separate the relative contributions of GAQ and the “off” gating charge (QOFF) to the measured ITAIL, and the expectation that QON = QOFF remains to be demonstrated. Additional studies are thus needed to identify mutant Hv1 channels in which inwardly directed H+ currents carried by GAQ are selectively blocked.
Hv1 gating currents reveal several previously unknown biophysical properties of Hv1 channels. First, the QON-V relation is nonidentical to the GAQ-V (or ITAIL-V) relation, indicating that GAQ opening requires a thermodynamic transition that is distinct from VS activation per se. “Electromechanical” coupling between VS activation and pore opening is a well-established phenomenon in tetrameric voltage-gated cation channels (18, 21, 22, 23, 25) but has not been described for Hv1, which lacks the canonical pore domain and instead utilizes the VS domain for both gating and H+-selective ion conduction (1, 2). Our results are consistent with the possibility that a conformational rearrangement that follows VS activation (i.e., the movement of gating charge in the transmembrane electrical field) is necessary to open the H+ transfer pathway. Gating hysteresis measured in the related VSP from C. intestinalis (CiVSP) is attributed to a voltage-independent transition (43), and evidence for a similar step in the Hv1 activation pathway has also been described previously (31). The ability to measure gating currents in Hv1 may be useful for dissecting the molecular bases of gating hysteresis and thermodynamic coupling of VS activation and GAQ-mediated “gating pore” opening in a simple model protein.
The shape of the QON-V relation appears to reveal gating complexity that has not been previously measured in Hv1 channels. Although both resting-state currents in the R1H mutant (9) and fluorescence signals measured under voltage clamp (29) suggest that VS activation in Hv1 does not proceed in a single gating transition, we previously lacked an experimental approach to directly measure how changes in membrane potential drive gating-charge movement in Hv1. Combined with simultaneous measurements of ionic current and an estimate of the elementary gating valence (zG), gating-charge measurements provide new insight into the unitary conductance of the GAQ pathway and the absolute number of functional Hv1 channel subunits in the membrane. Previously, N and γAQ have been amenable to estimation using current-variance analysis (40). As expected from previous studies (1, 27, 44, 45), neutralization of a highly conserved S4 Arg side chain in Hv1 (R205) decreases the quantity of gating charge per conducting unit (zG/i). Unexpectedly, however, we find that C-terminal truncation also appears to decrease zG/i in Hv1 W207A-N214R, suggesting that in addition to its role in stabilizing dimerization (41, 46, 47), the C-terminus may intimately interact with the voltage-dependent gating machinery. Having the ability to both measure gating current and estimate zG from the limiting slope on the same protein will enable direct measurement of zG and γAQ, allowing researchers to experimentally test hypotheses about VS activation, pH-dependent gating, and H+ conduction mechanisms in Hv1 that have so far remained mysterious.
pH-dependent gating, which is manifested by an ∼40 mV/pH unit shift in the position of the GAQ-V relation, is one of the hallmark biophysical features of Hv1 channels (1, 2, 7, 17). Both VM and V0.5-values determined for ITAIL-V relations are similarly pH-dependent (Fig. 4), indicating that pHO sensitivity is not perturbed in W207A-N214R, at least under the experimental conditions tested here. At physiological pH (i.e., pH < 8), the W207A single mutant is also sensitive to changes in pHI (28), suggesting that W207A-N214R probably also senses and transduces changes in ΔpH. pHO sensitivity in W207A-N214R therefore appears to be similar to WT Hv1 and a large number of other mutants reported previously (1, 2, 7, 11, 17, 28). This conclusion is reinforced by the observation that apparent zG estimated from Boltzmann fits to the ITAIL-V and QON-V relations is not discernably pH-sensitive between pHO 5.5 and pH 6.5O (Fig. 4 B).
Although Boltzmann fits to the QON-V curves suggest that the unitary gating valence, zG, is insensitive to changes in pHO, caution is warranted in the interpretation of zG-values determined from Boltzmann fits to the data. First, QON-V relations in W207A-N214R are evidently complex (Fig. 2 C), suggesting that gating-charge movement does not proceed in a single gating step. Boltzmann fitting of G-V curves is known to underestimate zG, particularly when VS activation requires multiple state transitions and is therefore not well described by a two-state model (35, 38). Indeed, zG estimated using the limiting-slope method in C. intestinalis Hv1 (∼6 e0) is two to three times larger than values derived from fits to GAQ-V relations in mammalian Hv1 channels (1, 2, 14, 27, 32). We therefore use limiting-slope estimates of zG for the purpose of calculating channel number and unitary conductance in full-length (6 e0; i.e., W207A-N214R) and truncated (3 e0; i.e., W207A-N214R-ΔC) Hv1 channels in this study. Our estimates of N and γAQ would therefore differ by a factor of only ∼2 if values from Boltzmann fits to our data (Table 1) were used.
Although W207A dramatically alters GAQ activation and deactivation kinetics, its effect on estimated unitary conductance was not reported previously (28), and it remains unknown whether mutations of this residue alter the size or structure of the H+ permeation pathway in Hv1. Resting- and activated-state model and x-ray structures predict that the W207 side chain probably faces away from the hydrated VS central crevice (7, 8, 9, 32, 48, 49, 50, 51), suggesting that if W207 mutations alter γAQ, they probably do so indirectly. On the other hand, the basic side chain of N214R was previously shown to occupy a site that can selectively block outwardly directed H+ transfer from an intracellular site that lies within the electrical field (9). Because the γAQ estimated for W207A-N214R (Fig. 3 B) is >10-fold smaller than a previous estimate for WT Hv1 (40), we conclude that the N214R side chain probably reduces the amplitudes of both ISTEP and ITAIL. Stated differently, the positive charge introduced at the R4 position appears to reduce γAQ in a voltage-dependent fashion that is reminiscent of pore block by permeant ions in VGCs (52, 53).
In summary, we report a method for reliably and reproducibly measuring gating currents in mammalian cells by expressing a human Hv1 double mutant that displays fast activation kinetics and selective block of the outwardly directed ionic H+ current. IG in Hv1 displays many of the biophysical features previously reported for other VS-domain proteins, including thermodynamic coupling of VS activation and pore opening. Gating currents also enable new methods for estimating Hv1 unitary conductance and channel number. The ability to directly measure VS activation in Hv1 may be helpful for testing a variety of unresolved hypotheses about the mechanisms of voltage- and pH-dependent gating and H+ conduction in Hv1. Gating currents are also likely to be useful for addressing fundamental questions about VS activation that are common to Hv1, VSPs, and VGCs. For example, it remains unclear why Hv1 channels are evidently unique among VS-domain proteins in mediating an intrinsic, activated-state GAQ. Gating-current measurements should enable experimental separation of mutagenic effects on VS activation versus pore structure and potentially help to clarify why specific mutations (i.e., D112V in human Hv1) render channels nonfunctional, whereas other substitutions at the same position act mainly to erode H+ selectivity (11).
Author Contributions
V.D.L.R. designed and executed experiments, analyzed results, and prepared figures and tables. I.S.R. designed and executed experiments, analyzed results, prepared figures and tables, and wrote the manuscript.
Acknowledgments
The authors wish to thank Carlos A. Villalba-Galea for development of data acquisition and analysis software and for many helpful discussions.
This work was supported by NIH R01GM092908 (I.S.R.) and CONACYT postdoctoral fellowship 251376 (V.D.L.R.).
Editor: Baron Chanda.
Footnotes
Victor De La Rosa’s present address is Department of Cell and Integrative Physiology, University of Texas Health Science Center at San Antonio, San Antonio, Texas
Four figures are available at http://www.biophysj.org/biophysj/supplemental/S0006-3495(18)30583-6.
Supporting Material
References
- 1.Ramsey I.S., Moran M.M., Clapham D.E. A voltage-gated proton-selective channel lacking the pore domain. Nature. 2006;440:1213–1216. doi: 10.1038/nature04700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sasaki M., Takagi M., Okamura Y. A voltage sensor-domain protein is a voltage-gated proton channel. Science. 2006;312:589–592. doi: 10.1126/science.1122352. [DOI] [PubMed] [Google Scholar]
- 3.Murata Y., Iwasaki H., Okamura Y. Phosphoinositide phosphatase activity coupled to an intrinsic voltage sensor. Nature. 2005;435:1239–1243. doi: 10.1038/nature03650. [DOI] [PubMed] [Google Scholar]
- 4.Yu F.H., Catterall W.A. The VGL-chanome: a protein superfamily specialized for electrical signaling and ionic homeostasis. Sci. STKE. 2004;2004:re15. doi: 10.1126/stke.2532004re15. [DOI] [PubMed] [Google Scholar]
- 5.Bennett A.L., Ramsey I.S. CrossTalk opposing view: proton transfer in Hv1 utilizes a water wire, and does not require transient protonation of a conserved aspartate in the S1 transmembrane helix. J. Physiol. 2017;595:6797–6799. doi: 10.1113/JP274553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bennett A.L., Ramsey I.S. Rebuttal from Ashley L. Bennett and Ian Scott Ramsey. J. Physiol. 2017;595:6803. doi: 10.1113/JP274984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramsey I.S., Mokrab Y., Clapham D.E. An aqueous H+ permeation pathway in the voltage-gated proton channel Hv1. Nat. Struct. Mol. Biol. 2010;17:869–875. doi: 10.1038/nsmb.1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wood M.L., Schow E.V., Tobias D.J. Water wires in atomistic models of the Hv1 proton channel. Biochim. Biophys. Acta. 2012;1818:286–293. doi: 10.1016/j.bbamem.2011.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Randolph A.L., Mokrab Y., Ramsey I.S. Proton currents constrain structural models of voltage sensor activation. eLife. 2016;5:e18017. doi: 10.7554/eLife.18017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berger T.K., Isacoff E.Y. The pore of the voltage-gated proton channel. Neuron. 2011;72:991–1000. doi: 10.1016/j.neuron.2011.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Musset B., Smith S.M., DeCoursey T.E. Aspartate 112 is the selectivity filter of the human voltage-gated proton channel. Nature. 2011;480:273–277. doi: 10.1038/nature10557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vargas E., Yarov-Yarovoy V., Roux B. An emerging consensus on voltage-dependent gating from computational modeling and molecular dynamics simulations. J. Gen. Physiol. 2012;140:587–594. doi: 10.1085/jgp.201210873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chanda B., Bezanilla F. A common pathway for charge transport through voltage-sensing domains. Neuron. 2008;57:345–351. doi: 10.1016/j.neuron.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 14.Gonzalez C., Koch H.P., Larsson H.P. Strong cooperativity between subunits in voltage-gated proton channels. Nat. Struct. Mol. Biol. 2010;17:51–56. doi: 10.1038/nsmb.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De La Rosa V., Bennett A.L., Ramsay I.S. Coupling between an electrostatic network and the Zn2+ binding site modulates Hv1 activation. J Gen Physiol. 2018 doi: 10.1085/jgp.201711822. Published online May 9, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cherny V.V., Morgan D., DeCoursey T.E. Histidine168 is crucial for ΔpH-dependent gating of the human voltage-gated proton channel, hHV1. J Gen Physiol. 2018 doi: 10.1085/jgp.201711968. Published online May 9, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cherny V.V., Markin V.S., DeCoursey T.E. The voltage-activated hydrogen ion conductance in rat alveolar epithelial cells is determined by the pH gradient. J. Gen. Physiol. 1995;105:861–896. doi: 10.1085/jgp.105.6.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Armstrong C.M., Bezanilla F. Currents related to movement of the gating particles of the sodium channels. Nature. 1973;242:459–461. doi: 10.1038/242459a0. [DOI] [PubMed] [Google Scholar]
- 19.Almers W. Observations on intramembrane charge movements in skeletal muscle. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1975;270:507–513. doi: 10.1098/rstb.1975.0027. [DOI] [PubMed] [Google Scholar]
- 20.Perozo E., MacKinnon R., Stefani E. Gating currents from a nonconducting mutant reveal open-closed conformations in Shaker K+ channels. Neuron. 1993;11:353–358. doi: 10.1016/0896-6273(93)90190-3. [DOI] [PubMed] [Google Scholar]
- 21.Ledwell J.L., Aldrich R.W. Mutations in the S4 region isolate the final voltage-dependent cooperative step in potassium channel activation. J. Gen. Physiol. 1999;113:389–414. doi: 10.1085/jgp.113.3.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Long S.B., Campbell E.B., Mackinnon R. Voltage sensor of Kv1.2: structural basis of electromechanical coupling. Science. 2005;309:903–908. doi: 10.1126/science.1116270. [DOI] [PubMed] [Google Scholar]
- 23.Chowdhury S., Haehnel B.M., Chanda B. Interfacial gating triad is crucial for electromechanical transduction in voltage-activated potassium channels. J. Gen. Physiol. 2014;144:457–467. doi: 10.1085/jgp.201411185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fernández-Marino A.I., Harpole T.J., Chanda B. Gating interaction maps reveal a noncanonical electromechanical coupling mode in the Shaker K+ channel. Nat. Struct. Mol. Biol. 2018;25:320–326. doi: 10.1038/s41594-018-0047-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bezanilla F., Perozo E., Stefani E. Gating of Shaker K+ channels: II. The components of gating currents and a model of channel activation. Biophys. J. 1994;66:1011–1021. doi: 10.1016/S0006-3495(94)80882-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sakata S., Kurokawa T., Okamura Y. Functionality of the voltage-gated proton channel truncated in S4. Proc. Natl. Acad. Sci. USA. 2010;107:2313–2318. doi: 10.1073/pnas.0911868107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gonzalez C., Rebolledo S., Larsson H.P. Molecular mechanism of voltage sensing in voltage-gated proton channels. J. Gen. Physiol. 2013;141:275–285. doi: 10.1085/jgp.201210857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cherny V.V., Morgan D., DeCoursey T.E. Tryptophan 207 is crucial to the unique properties of the human voltage-gated proton channel, hHV1. J. Gen. Physiol. 2015;146:343–356. doi: 10.1085/jgp.201511456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qiu F., Rebolledo S., Larsson H.P. Subunit interactions during cooperative opening of voltage-gated proton channels. Neuron. 2013;77:288–298. doi: 10.1016/j.neuron.2012.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DeCoursey T.E. Hydrogen ion currents in rat alveolar epithelial cells. Biophys. J. 1991;60:1243–1253. doi: 10.1016/S0006-3495(91)82158-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Villalba-Galea C.A. Hv1 proton channel opening is preceded by a voltage-independent transition. Biophys. J. 2014;107:1564–1572. doi: 10.1016/j.bpj.2014.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chamberlin A., Qiu F., Larsson H.P. Hydrophobic plug functions as a gate in voltage-gated proton channels. Proc. Natl. Acad. Sci. USA. 2014;111:E273–E282. doi: 10.1073/pnas.1318018111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.DeCoursey T.E., Cherny V.V. Deuterium isotope effects on permeation and gating of proton channels in rat alveolar epithelium. J. Gen. Physiol. 1997;109:415–434. doi: 10.1085/jgp.109.4.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tombola F., Ulbrich M.H., Isacoff E.Y. The opening of the two pores of the Hv1 voltage-gated proton channel is tuned by cooperativity. Nat. Struct. Mol. Biol. 2010;17:44–50. doi: 10.1038/nsmb.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bezanilla F., Villalba-Galea C.A. The gating charge should not be estimated by fitting a two-state model to a Q-V curve. J. Gen. Physiol. 2013;142:575–578. doi: 10.1085/jgp.201311056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tao X., Lee A., MacKinnon R. A gating charge transfer center in voltage sensors. Science. 2010;328:67–73. doi: 10.1126/science.1185954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lacroix J.J., Hyde H.C., Bezanilla F. Moving gating charges through the gating pore in a Kv channel voltage sensor. Proc. Natl. Acad. Sci. USA. 2014;111:E1950–E1959. doi: 10.1073/pnas.1406161111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chowdhury S., Chanda B. Estimating the voltage-dependent free energy change of ion channels using the median voltage for activation. J. Gen. Physiol. 2012;139:3–17. doi: 10.1085/jgp.201110722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ishida I.G., Rangel-Yescas G.E., Islas L.D. Voltage-dependent gating and gating charge measurements in the Kv1.2 potassium channel. J. Gen. Physiol. 2015;145:345–358. doi: 10.1085/jgp.201411300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cherny V.V., Murphy R., DeCoursey T.E. Properties of single voltage-gated proton channels in human eosinophils estimated by noise analysis and by direct measurement. J. Gen. Physiol. 2003;121:615–628. doi: 10.1085/jgp.200308813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tombola F., Ulbrich M.H., Isacoff E.Y. The voltage-gated proton channel Hv1 has two pores, each controlled by one voltage sensor. Neuron. 2008;58:546–556. doi: 10.1016/j.neuron.2008.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Musset B., Cherny V.V., DeCoursey T.E. Detailed comparison of expressed and native voltage-gated proton channel currents. J. Physiol. 2008;586:2477–2486. doi: 10.1113/jphysiol.2007.149427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Villalba-Galea C.A., Sandtner W., Bezanilla F. S4-based voltage sensors have three major conformations. Proc. Natl. Acad. Sci. USA. 2008;105:17600–17607. doi: 10.1073/pnas.0807387105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Seoh S.A., Sigg D., Bezanilla F. Voltage-sensing residues in the S2 and S4 segments of the Shaker K+ channel. Neuron. 1996;16:1159–1167. doi: 10.1016/s0896-6273(00)80142-7. [DOI] [PubMed] [Google Scholar]
- 45.Aggarwal S.K., MacKinnon R. Contribution of the S4 segment to gating charge in the Shaker K+ channel. Neuron. 1996;16:1169–1177. doi: 10.1016/s0896-6273(00)80143-9. [DOI] [PubMed] [Google Scholar]
- 46.Koch H.P., Kurokawa T., Larsson H.P. Multimeric nature of voltage-gated proton channels. Proc. Natl. Acad. Sci. USA. 2008;105:9111–9116. doi: 10.1073/pnas.0801553105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee S.Y., Letts J.A., Mackinnon R. Dimeric subunit stoichiometry of the human voltage-dependent proton channel Hv1. Proc. Natl. Acad. Sci. USA. 2008;105:7692–7695. doi: 10.1073/pnas.0803277105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Okuda H., Yonezawa Y., Fujiwara Y. Direct interaction between the voltage sensors produces cooperative sustained deactivation in voltage-gated H+ channel dimers. J. Biol. Chem. 2016;291:5935–5947. doi: 10.1074/jbc.M115.666834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Takeshita K., Sakata S., Nakagawa A. X-ray crystal structure of voltage-gated proton channel. Nat. Struct. Mol. Biol. 2014;21:352–357. doi: 10.1038/nsmb.2783. [DOI] [PubMed] [Google Scholar]
- 50.Li Q., Shen R., Perozo E. Resting state of the human proton channel dimer in a lipid bilayer. Proc. Natl. Acad. Sci. USA. 2015;112:E5926–E5935. doi: 10.1073/pnas.1515043112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kulleperuma K., Smith S.M., Pomès R. Construction and validation of a homology model of the human voltage-gated proton channel hHV1. J. Gen. Physiol. 2013;141:445–465. doi: 10.1085/jgp.201210856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Woodhull A.M. Ionic blockage of sodium channels in nerve. J. Gen. Physiol. 1973;61:687–708. doi: 10.1085/jgp.61.6.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hille B., Schwarz W. Potassium channels as multi-ion single-file pores. J. Gen. Physiol. 1978;72:409–442. doi: 10.1085/jgp.72.4.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.