Abstract
Introduction: In the recent decades, starch has been modified using different methods for the various forms of applications. Some new starch derivatives were prepared through a simple and convenient method in the grafting of amino acids: L-alanine, L-leucine and L-phenyl alanine to starch.
Methods: First, the amine groups of amino acids were protected using phthalic anhydride then the acidic side of amino acids were activated with chlorination using thionyl chloride, and the resultant acid chlorides were reacted with starch in aqueous media at room temperature.
Results: Performing the various spectroscopy experiments on the obtained compounds showed that the new derivative of starch has been formed. The structure of all synthesized materials was determined and confirmed using common spectroscopy methods and their thermal behavior was examined using DSC experiment.
Conclusion: New amino acid derivatives of starch and their nanocarriers successfully prepared through a simple and convenient method. The size of nanocarriers evaluated using DLS and TEM experiments. The spherical shape of particles shows that nanocarriers have been formed and the size of these particles are approximately 92, 137 and 97 nm. Performing the wettability test determined that all the resulted materials are soluble in water. Nanocarriers of the obtained modified starches were prepared using dialysis method and naproxen was utilized as a model drug molecule. The drug release dynamics in buffered solution were studied and investigation of the drug release mechanism showed that in case of L-alanine- and L-phenylalanine-modified starches, drug release followed the Fickian diffusion with a slight deviation.
Keywords: Amino acid, Drug delivery, Starch, Nanocarrier
Introduction
Starch is considered as a natural polysaccharide and an abundant and renewable compound which is often modified in order to improve its industrial use.1 Over the last few decades, starch has been modified using different methods for the various forms of applications.2 It is said that the attributes of modified starch usually depend on the substituent’s nature and the degree of substitution (DS). In this way, starch has been modified with natural products such as betaine through an ester bond.3 Applying starch because of its low costs, abundant availability, renewability and no toxicity has been of most interest in recent years, Researchers have conducted an experiment in which gelatinized potato starch has been reacted with acyl chlorides.4 Some studies showed that starch nanocrystals are modified using fatty acids in aqueous media instead of using organic solvents.5,6 Wang and Xie7 suggested that cationic cornstarch derivatives could be synthesized through the reaction of cornstarch with glycidyltrimethylammonium chloride with a high DS. Indeed a new cationic starch was synthesized through the Mannich reaction between cornstarch and hydroxymethyl dimethylamine hydrochloride.8
A lot of carboxylic groups were grafted carboxymethyl starch (CMS) to prepare useful biopolymer-based materials.9 Further, modification of cellulose, vinyl saccharides, and starch in various ways and applications has received considerable attention.10-13
Antimicrobial synthetic macromolecules have diverse applications in medical, food packaging and hygienic industries. Amino acids have gained great interest in these applications. Polysaccharides such as chitin and chitosan have successfully modified with peptides14-17 and amino acids for different applications.18-20 Starch-co-polyacrylamid modified with amino acid and their biological activities were studied for their antimicrobial activities using cut plug method.21 The properties of conjugated lysine and polylysine with starch have also been studied.22
Recently, a strategy has been reported for the preparation of multifunctional nanocarriers based on the biodegradable/biocompatible polymers for the active targeting and drug delivery.23
Chitosan grafted amino acid derivatives were synthesized in order to develop a gene delivery with specifies of biocompatible, safe and effective vector.24
The reason for using polysaccharides as stimuli-responsive nanosystems is their intrinsic structure and characteristics and nowadays several kinds of polysaccharides have been used in order to develop the sensitive nanomaterials for therapy, diagnosis and their combination.25
Recently, the synthesis of new nanoparticle formulation of starch grafted lactic acid included montmorillonite (MMT) and their application as nanocarrier drug delivery system has been reported.26,27
Also, a facile procedure for the preparation of Fe3O4 magnetic nanoparticles coated with starch-g-poly(methyl methacrylate-co-PEG-acrylamide) has been developed28 and a stimuli-responsive polymeric nanosystem has been synthesized for the theranostic applications.29
As another example of the application of anticancer drug delivery agent, it could be referred to bio-dendrimeric β-CD-(spacer-β-CD) supramolecule which was synthesized through click reaction.30
In other researches, a different kind of nanocarriers has prepared for drug delivery applications.31-33
In this work, the synthesis of some new derivatives of starch with various amino acids using a simple and convenient method is reported. As obtained results showed that the modified polysaccharide cause the resultant compounds to be suitable for drug delivery systems due to biodegradability and their great wettability. Based on the prepared compounds, their related nanocarriers were prepared using dialysis method to form a new class of hydrophilic nanocarriers, naproxen has been utilized as drug molecule and their release dynamics in the PBS solution were investigated.
Materials and Methods
Materials
Starch has been purchased from Merck containing (20%-25%) amylose; for removing the moisture, it was heated up to 110°C for 10 hours, L-phenylalanine, L-leucine, L-alanine, thionyl chloride and hydrazine was purchased from Merck. Phthalic anhydride was also purchased from Merck and was recrystallized from chloroform.
Preparation of protected amino acids
3.00 g (1.66 ×10-2 mol) phthalic anhydride, 1.47 g L-alanine (same mol) and 20 mL of acetic acid were added to a 100 mL flask and the mixture was refluxed for about 12 hours. Solvent (acetic acid) was removed in reduced pressure and 100 mL of water and 5 mL of concentrated and cold HCl was added to obtain solid, the precipitate was filtered and washed with water for several times and it was dried. The obtained product (imide acid) recrystallized from water/methanol to give the white crystals. L-phenylalanine and L-leucine were also protected using this method and the same molar ratio as alanine.34
Chlorination of amino acids
About 1.00 g of the obtained product (4.56 ×10-1 mol of imide acid) and an excess amount of thionyl chloride (10 mL) was added to 50 mL flask and refluxed for about 6 hours. The unreacted thionyl chloride was removed; n-hexane (20 mL) was added, and after stirring for 1 hour, it was removed via distillation; then the product was collected.
Preparation of starch grafted amino acids (starch-g-amino acids)
Starch was dispersed in alkali water at room temperature and stirred for 1 hour; 0.5 mol of chlorinated amino acid was dissolved in dry acetonitrile and added to the mixture drop-wise; after stirring for about 20 minutes, 150 mL of methanol was added for precipitation of the product. After filtration, it was dried in vacuum and kept in desiccate.35
Deprotection of amino acids
Deprotection of amino groups was carried out using hydrazine in H2O as a solvent. This procedure was investigated through 1H NMR, and when the chemical shifts in the aromatic area are emitted, it shows that the deprotection has completely done.
Preparation of nanocarriers and drug loading
Some starch-g-amino acid nanocarriers prepared through dialysis method without using any surfactant. Thirty milligrams of starch-g-amino acid was added to 10 mL of (DMSO) and 10 mg of naproxen as a model drug was added. The resultant sample was dialyzed with a dialysis membrane (12000 g/mol) and against water. At first, the medium was replaced once in the first hour and then it was replaced every 5 hours. The dialysis continued for 24 hours.
The resulting suspension was freeze-dried with Christ alpha freeze dryer (1-4 LSC). Starch nanocarriers cooled at -70°C for 24 hours. For identifying the loaded drug, the freeze-dried samples (naproxen loaded starch nanocarriers) were stirred vigorously in methanol for 3 hours and sonicated (about 15 minutes), then centrifuged. The concentration of drug was measured with Shimadzu spectrophotometer (Shimadzu Corp., Kyoto, Japan) at 232 nm.
Drug release studies
Drug release dynamics was studied in pH 7.4, for 10 hours. All nanocarriers were dissolved in phosphate buffer solution and poured in dialysis bag. The dialysis bag was soaked in 50 mL of buffer and it was stirred; temperature was fixed at 37°C and in different intervals, 4 mL of sample was taken out and replaced with the same amount of buffer (at the same temperature). The samples absorption values were measured at 232 nm. The related concentrations were calculated using calibration profiles and release % was determined.
Measurements
FT-IR spectrometer (Bruker-Tensor 27) was used for FT-IR analysis. Starch and KBr were mixed at the ratio of 5/200 mg. All samples were dissolved at 60°C in DMSO-d6 and the 1H NMR spectra were obtained using Bruker 400 MHz LEO 440i microscope was used for SEM images. The operating voltage was 10 kV. Samples were gold coated for 15 seconds. TEM micrographs were obtained using LEO 906 (80 v) and preserved on Kodak film. The size of nanocarriers and their morphology were evaluated with TEM after freeze drying. The DSC thermograms were obtained by Linseis DSC L 63/45 calorimeter (Vielitzerstr, Germany) and the rate of heating was 10°C/min. The size of nanocarriers was determined by measuring the angle of scattered light. The nanocarriers were passed through the laser beam inside a SALD-2101 (Shimadzu Corp., Kyoto, Japan) in order to measure their size. This technique measures bulk materials continuously across a size ranging from 10 nm to 3 mm. Particle size was measured through a sample solution prepared by the dialysis method (concentration: 0.2 wt. %).
Results
Esterification of starch
The modified starch derivatives were synthesized by the chloride derivatives of amino acid in a heterogeneous condition, through the chemical grafting. The reaction pathway was shown in Scheme 1. Water is an inexpensive and non-toxic solvent which makes this method appealing and easy to use.
Scheme 1.
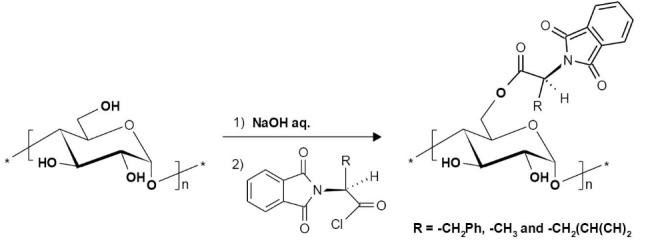
Synthesis rout for grafting amino acids to starch.
Characterization of starch-g-amino acid
FT-IR
The modified starches FT-IR spectra before their deprotection were compared as shown in Fig. 1. As Fig. 1 demonstrates, the major characteristic peaks are the bending and stretching vibration of OH group of starch, which occurs around 1650 and 3276 cm-1, respectively.
Fig. 1.
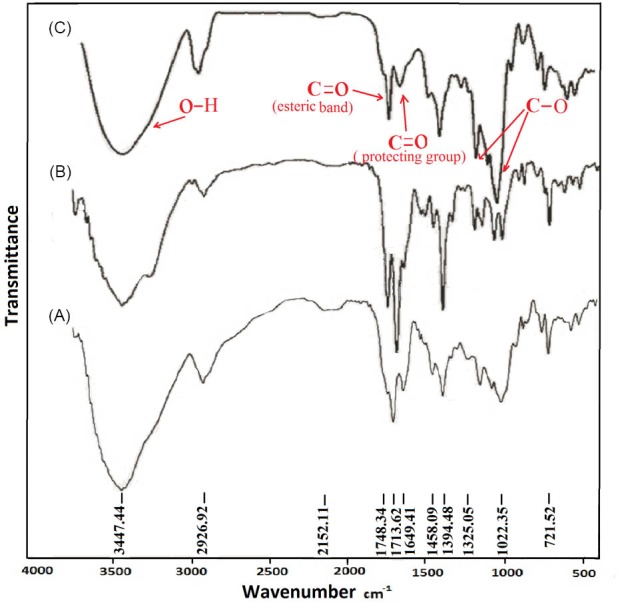
FT-IR spectra of starch modified with: L-alanine (A), L-leucine (B) and L-phenyl alanine (C).
In the anhydrous glucose ring, the C-O-H and C-O-C group, the stretching vibration of C-O bonding appeared at 1150, 1077 and 990 cm-1, respectively.
The vibration of C-O-C ring in starch appeared at 760 cm-1. Fig. 1A–C exhibits both amino acid and starch characteristics combination. The presence of carbonyl absorption band could confirm the formation of amino acid grafted starch. The band in 1710 cm is related to the carbonyl moiety in phthalimide protecting group. The bands appear at 1742, 1739 and 1740 cm-1 are the absorption of carbonyl groups in phenylalanine, alanine and leucine grafted starch respectively, which shows that the esterification has been completed.
1HNMR analysis
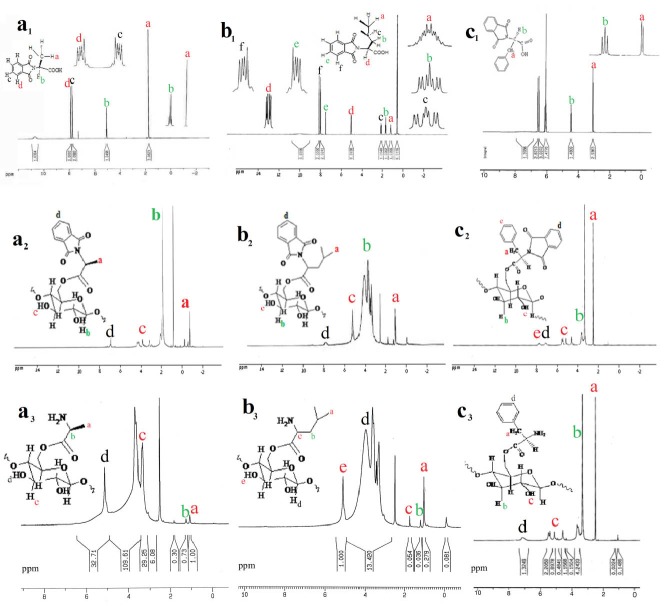
The 1HNMR spectra of protected amino acids presented and 1HNMR spectra for starch-g-(L-Phenyl alanine), L-leucine and L-alanine before deprotection of amino acids are shown in Fig. 2(a1-c1) and Fig. 2(a2-c2) , respectively. Chemical shifts between 2.5-5 ppm corresponding to the hydrogens in the backbone of starch. As seen in the starch-g-alanine spectrum ( Fig. 2a2 ), signals in chemical shifts 1 ppm and 1.5 ppm are corresponding to the hydrogen of -CH3 group and hydrogen linked to the chiral center. The chemical shifts in 1 ppm are related to the hydrogens in aliphatic group starch-g-leucine as shown in Fig. 2b2 , and chemical shifts in 7.5 ppm are corresponding to the hydrogens of the phenyl group in starch-g-phenylalanine (see Fig. 2c2 ). The signals for aromatic hydrogens are related to the protecting group in all three spectra. After deprotection of amino acids, aromatic hydrogens disappeared for the protecting group of phthalimide (see Fig. 2a3-2c3 ).
Fig. 2.
1H NMR spectra of protected amino acids (a1-c1): L-alanine protected phthalimide (a1), L-leucine protected phthalimide (b1), L-phenyl alanine protected phthalimide (c1); starch-g-amino acid before deprotection (a2-c2): starch-g-(L-alanine) (a2), starch-g-(L-leucine) (b2) and starch-g-(L-phenyl alanine) (c2) and starch-g-amino acids after deprotection (a3-c3): starch-g-(L-alanine) (a3), starch-g(-L-leucine) (b3) and starch-g-(L-phenyl alanine) (c3).
DSC analysis
The DSC thermograms for native starch and starch derivatives were obtained. DSC results for the native starch also amino acid-modified starches are shown in Table 1. As shown, the peaks in 112-240°C are related to the crystalline phase of native starch and above 240°C decomposition occurs. Starch derivatives do not show a discrete signal in DSC thermo-grams. That approves the amino acids crystalline phase. The peak around 112°C-240°C in starch derivatives is due to the crystalline phase of the starch, which indicate that the crystalline phase was conserved in the alkaline media during the esterification of starch. The DSC results are in accord with previous reports in the literature.5,6
Table 1. Thermal analysis data from DSC thermo grams, DS values and the related Zeta potentials for nanocarriers .
| St- derivative | T onset | T offset | T Max | DS | Zeta potential(mV) |
| Starch SS | 40.6 | 160.3 | 125.1 | - | -35 |
| St-alanine | 53.2 | 160.9 | 112.1 | 0.34 | -15 |
| St-leucine | 99.2 | 186.4 | 147.2 | 0.30 | -19 |
| St-phenyl alanine | 45.3 | 136.8 | 93.4 | 0.46 | -10 |
DS: degree of substitution.
Wettability test
The wettability is defined as the solvent’s tendency to spread on the polymer’s surface in the attendance of one or more solvent that is immiscible. For determining the chemical, physical and a number of polymer’s functional properties, the wettability is considered as one of the essential parameters. The wettability can be studied by a simple test qualitatively in a way that, the obtained products mix with two solvents (immiscible and having different polarity) for observing the affinity of these substances. In our tests, chloroform and distilled water were chosen as solvents, the bottom phase owned to chloroform (d = 1.47). and the upper one to the water (d = 1). In the reference test equivalent amount of native starch was added to the mixture. Obviously, due to the high affinity of native starch to water, it remains in the water and cannot immigrate into chloroform. When we shake the container, starch remains in the water again (Fig. 3A). Hence, we conclude that native starch has a high affinity for return water as a polar solvent. Another test carried out with starch derivatives by adding the starch derivatives to the mixture of solvents and then shake it, we noticeably observed that these derivatives do not immigrate into chloroform (Fig. 3B), signifying that the nature of derivatives is polar.
Fig. 3.
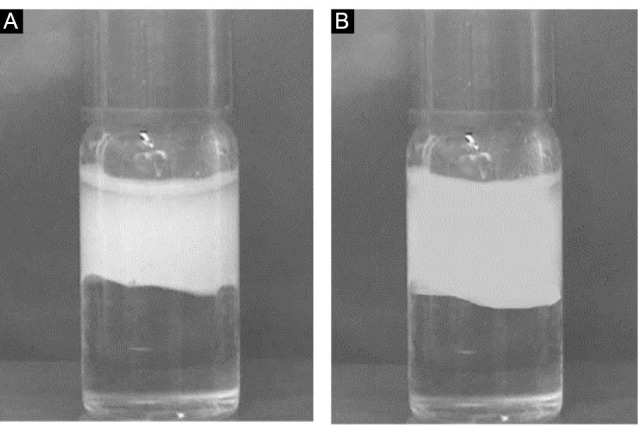
Wettability tests of native starch (A) and starch derivatives of amino acids (B).
Determination of DS
DS value of starch derivatives has defined as the number of hydroxyl (-OH) groups that are substituted per D-glucopyranosyl unite. For the 100 units of glucose in the chain of starch, the DS is considered the average numbers of hydroxyl groups. Meanwhile, the DS can be calculated using integrity of related signals in 1HNMR spectra. Otherwise, in a way, DS value is calculated using the intensity of protons peaks related to an amino acid which is divided to the intensity of 7 the number of protons in one glucose unit via equation (1):
DS = ISignal/n/IAGU/7 (1)
In this calculations, n is the number of protons in amino acids, and AGU is the number of glucose units. The obtained results are presented in Table 1.
Characterization of nanocarriers
Size and morphological evaluation of the nanocarriers
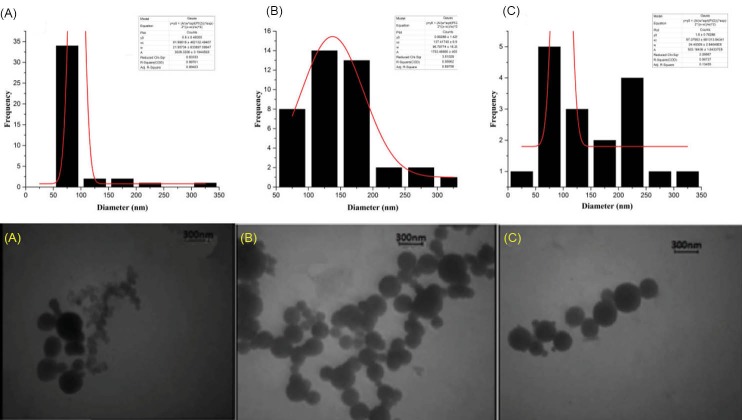
Transmission electron microscopy was operated for considering the morphological and size changes for nanocarriers of starch-g-amino acid (Fig. 4) and image software was used to obtain the average particle size distribution that is shown above the related sample. The spherical shape of particles shows that nanocarriers have been formed and the size of these particles are approximately 92, 137 and 97 nm for nanocarriers of starch-g-(L-alanine) (Fig. 4A), Starch-g-(L-phenyl alanine) (Fig. 4B) and starch-g-(L-leucine) (Fig. 4C), respectively.
Fig. 4.
TEM images for nanocarriers of starch-g-(L-alanine) (A), starch-g-(L-phenyl alanine) (B) and of starch-g-(L-leucine) (C).
Particle size analysis
The size of nanocarriers was evaluated by the laser diffraction particle size analyzer. The particle’s diameter is the size of the sphere (minimum surface area) per unit volume. The particle size results for nanocarriers of starch-g-amino acids were presented in Fig. 5. The medium volumetric diameters of prepared nanocarriers containing the drug, for the starch modified L-phenylalanine (b), L-leucine(c) and L-alanine (a) were 0.282 µm, 0.520 µm, and 0.282 µm, respectively that is most probably due to aggregation in aqueous media.
Fig. 5.
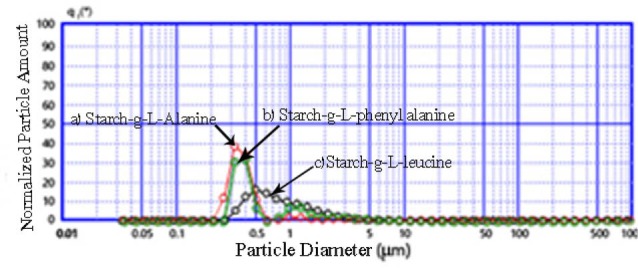
Particle size distribution of nanocarriers: starch-g-(L-alanine) (a), starch-g-(L-phenyl alanine) (b) and starch-g-(L-leucine) (c).
Also, SEM images are presented in Fig. 6. These images show that after freeze-drying the shape of nanocarriers was preserved and the surface of starch was changed.
Fig. 6.

The scanning electron microscopy images for nanocarriers of Starch-g-(L-alanine) (A), nanocarriers of starch-g-(L-phenyl alanine) (B) and nanocarriers of Starch-g-(L-leucine) (C).
Fig. 6A-C are related to nanocarriers of starch-g-(L-alanine), L-leucine and L-phenylalanine, respectively.
Zeta potential of nanocarriers
Zeta potential is originated from the surface charge that could provide information about the electrical interface between the interspersed particles. It is defined as the potential of electrokinetic at the border of the hydrodynamic shear plane of a charged particle that is neighboring to a surface of solid exposed to a liquid. Zeta potential has measured with electrophoresis, wherein the particle immigrates in an external field. Hence, the particle immigrates jointly with the ions in the Stern layer, so Zeta potential can be considered as this boundary layer’s outside potential (that is called the shear plane, too) and can determine the speed of particle movement.36 The measurement of Zeta potential (ζ) is not only for estimating of colloidal stability. Besides it is applied for the rapid estimation of the DS, too.37 Zeta potentials of nanocarriers are shown in Table 1, which indicates their negative potential. There is a relation between the zeta potential and the DS value. For the starch-g-(L-phenyl alanine) with having highest DS value, the measured negative potential has been significantly decreased in comparison to other amino acids.
Drug release studies
Calculating the amount of the trapped and released drug molecule
The amount and efficiency of the trapped drug are calculated with two procedures. In the first method, the amount of the trapped drug was measured using the weight of the nanocarrier before and after complexation. The difference between the measured weights gave the amount of the trapped drug. In the second method, UV spectrophotometer was used.
Drug release dynamics for nanocarriers are presented in Fig. 7. As Fig. 7A shows a controlled release in the case of starch-g-(L-alanine) nanocarrier and after the release of about 9% of the drug. Fig. 7B and 7C present the drug release from starch-g-(L-phenyl alanine) and starch-g-(L-leucine), in this case after the release of 20% of drug we have the controlled release.
Fig. 7.
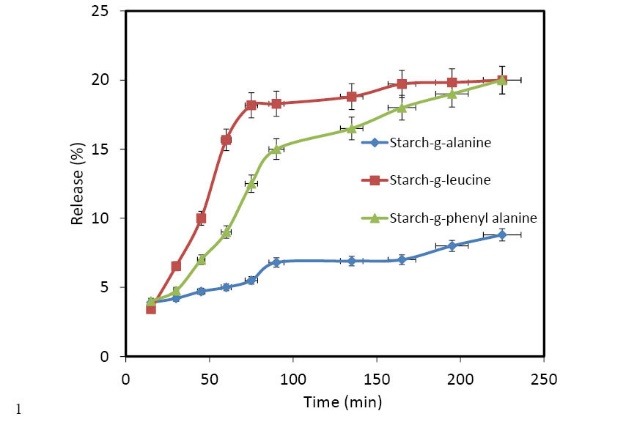
Cumulative drug release (%) of nanocarriers: starch-g- (L-alanine), starch-g-(L-phenylalanine) and starch-g-(L-leucine) (c).
Discussion
Starch as a natural and abundant polysaccharide component is considered for drug delivery in the colon. Introducing natural components such as amino acids in starch backbone causes the resulted derivatives of starch to be highly soluble in polar solvents. Performing different experiments using FTIR, 1HNMR, DSC proved the formation of these desired derivatives. Because of water solubility of derivatives, it seems that the nanocarriers formation is probably due to their self-assembly. From a comparison of diagrams related to the particle size distribution of starch-g-amino acids as presented in Fig. 5 and nanocarriers have been formed through self-assembly. It seems that the formation of the micellar structure was not possible because of the lack of hydrophobicity in the resulted system. However, modification of starch with components such as amino acids was the aim of this work to find which of these modified compounds would show better-controlled release in pH 7.4.
It was found that the release of naproxen from nanocarriers depends on different factors such as biodegradability of polymer, the grafted groups in the starch backbone, the size of the drug, concentration of drug, pH, temperature, amphiphilicity of drug and the interactions between polymer and drug. And as shown in Fig. 7, in the case of starch-g-leucine the controlled release curve is most suitable. L-leucine has more aliphatic groups and the amphoteric groups can cause better loading of naproxen and due to this reason, the slow and gentle slope release dynamic is observed. Diffusion mechanism of the drug from nanocarriers: Case II, Non-Fickian and Fickian, diffusion calculated using the following equation (2):
Mt/M∞=ktn
Mt/M∞ is drug’s fractional release time t, “K” is system’s constant and “n”: the mechanism of diffusion characteristics. For Case II, Fickian and Non-Fickian diffusion “n” is 1, 0.5 and 0.5-1.0, respectively.38
Consideration of drug release mechanism shows that in the case of starch-g-(L-alanine) and L-phenyl alanine drug release follows Fickian diffusion with the slight deviation (see Table 2).
Table 2. The calculated values of n, K and R2 .
| Nanocarrier | n | K | R 2 |
| Starch-g-alnine | 0.473 | 1.19 | o.985 |
| St-g-phenyl alanine | 0.45 | 1.099 | o.937 |
| St-g-leucine | 0.67 | 1.64 | 0.943 |
Conclusion
The nanocarrier of amino acid derivatives with starch was prepared in aqueous media in a simple and convenient way. Nanocarriers of derivatives were obtained using dialysis method and naproxen as a model drug. The Size of the nanocarriers was studied using particle size and TEM. The spherical shape of particles shows that nanocarriers have been formed and the size of these particles are approximately 92, 137 and 97 nm. The SEM studies showed that the surface modification has been successfully performed and the size of nanocarriers has been reduced. Drug release investigation was carried out in pH 7.4 for 10 hours and the mechanism of release was studied. The investigation of the drug release mechanism showed that in case of L-alanine- and L-phenylalanine-modified starches drug release follows the Fickian diffusion with a slight deviation.
Competing interests
There is not any competing interest.
Ethical approval
There is not any ethical issue in this work.
Acknowledgments
We take great pleasure to thank the University of Tabriz and Research Center for Pharmaceutical Nanotechnology (RCPN) of Tabriz University of Medical Science for their support of this work. We also thank Mr. H. Hashemi for his help in figures adjusting.
Research Highlights
What is current knowledge?
√ The modification of starch-co-polyacrylamid with different amino acids and investigation of their antimicrobical activities have been previously reported.
What is new here?
√ In this work, we synthesized starch-g-amino acids in aqueous media as a system for drug delivery applications.
√ In this work, we synthesized starch-g-amino acids in aqueous media as a system for drug delivery applications.
References
- 1.Richardson S, Gorton L. Characterisation of the substituent distribution of starch and cellulose derivatives. Anal Chim Acta. 2003;497:27–65. doi: 10.1016/j.aca.2003.08.005. [DOI] [Google Scholar]
- 2.Kaur B, Ariffin F, Bhat R, Karim AA. Progress in starch modification in the last decade. Food Hydrocolloid. 2012;26:398–404. doi: 10.1016/j.foodhyd.2011.02.016. [DOI] [Google Scholar]
- 3.Granö H, Yli-Kauhaluoma J, Suortti T, Käki J, Nurmi K. Preparation of starch betainate: a novel cationic starch derivative. Carbohyd Polym. 2000;41:277–83. doi: 10.1016/S0144-8617(99)00146-0. [DOI] [Google Scholar]
- 4.Fang J, Fowler P, Tomkinson J, Hill C. The preparation and characterisation of a series of chemically modified potato starches. Carbohyd Polym. 2002;47:245–52. doi: 10.1016/S0144-8617(01)00187-4. [DOI] [Google Scholar]
- 5.Namazi H, Dadkhah A. Convenient method for preparation of hydrophobically modified starch nanocrystals with using fatty acids. Carbohyd Polym. 2010;79:731–7. doi: 10.1016/j.carbpol.2009.09.033. [DOI] [Google Scholar]
- 6.Namazi H, Fathi F, Dadkhah A. Hydrophobically modified starch using long-chain fatty acids for the preparation of nanosized starch particles. Sci Iran. 2011;18:439–45. doi: 10.1016/j.scient.2011.05.006. [DOI] [Google Scholar]
- 7.Wang Y, Xie W. Synthesis of cationic starch with a high degree of substitution in an ionic liquid. Carbohyd Polym. 2010;80:1172–7. doi: 10.1016/j.carbpol.2010.01.042. [DOI] [Google Scholar]
- 8.Jiang Y, Ju B, Zhang S, Yang J. Preparation and application of a new cationic starch ether–Starch–methylene dimethylamine hydrochloride. Carbohyd Polym. 2010;80:467–73. doi: 10.1016/j.carbpol.2009.12.002. [DOI] [Google Scholar]
- 9.Saboktakin MR, Maharramov A, Ramazanov MA, Mahkam M. Modification of carboxymethyl starch as nano carriers for oral drug delivery. Nat Sci. 2007;5:67. [Google Scholar]
- 10.Namazi H, Jafarirad S. Preparation of the new derivatives of cellulose and oligomeric species of cellulose containing magneson II chromophore. J Appl Polym Sci. 2008;110:4034–9. doi: 10.1002/app.28965. [DOI] [Google Scholar]
- 11.Namazi H, Salimi F. Synthesis and polymerization of vinyl saccharides" as Glycomonomers" bearing 2, 3: 5, 6-Di-O-isopropylidene-α-D-mannofuranose. Iran Polym J. 2011;20:77–86. [Google Scholar]
- 12.Namazi H, Mosadegh M, Dadkhah A. New intercalated layer silicate nanocomposites based on synthesized starch-g-PCL prepared via solution intercalation and in situ polymerization methods: As a comparative study. Carbohyd Polym. 2009;75:665–9. doi: 10.1016/j.carbpol.2008.09.006. [DOI] [Google Scholar]
- 13.Namazi H, Mosadegh M. Preparation and properties of starch/nanosilicate layer/ polycaprolactone composites. J Polym Environ. 2011;19:980–7. doi: 10.1007/s10924011-0366-5. [DOI] [Google Scholar]
- 14.Anghileri A, Lantto R, Kruus K, Arosio C, Freddi G. Tyrosinase-catalyzed grafting of sericin peptides onto chitosan and production of protein–polysaccharide bioconjugates. J Biotechnol. 2007;127:508–19. doi: 10.1016/j.jbiotec.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 15.Chung T-W, Lu Y-F, Wang S-S, Lin Y-S, Chu S-H. Growth of human endothelial cells on photochemically grafted Gly–Arg–Gly–Asp (GRGD) chitosans. Biomaterials. 2002;23:4803–9. doi: 10.1016/S0142-9612(02)00231-4. [DOI] [PubMed] [Google Scholar]
- 16.Aberg CM, Chen T, Olumide A, Raghavan SR, Payne GF. Aberg CM, Chen T, Olumide A, Raghavan SR, Payne GFEnzymatic grafting of peptides from casein hydrolysate to chitosanPotential for value-added byproducts from food-processing wastes. J Agr Food Chem. 2004;52:788–93. doi: 10.1016/j.jbiotec.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 17.Chen T, Embree HD, Wu LQ, Payne GF. In vitro protein–polysaccharide conjugation: Tyrosinase‐catalyzed conjugation of gelatin and chitosan. Biopolymers. 2002;64:292–302. doi: 10.1002/bip.10196. [DOI] [PubMed] [Google Scholar]
- 18.Aiba S-i, Minoura N, Fujiwara Y. Graft copolymerization of amino acids onto partially deacetylated Chitin. Int J Biol Macromol. 1985;7:120–1. doi: 10.1016/0141-8130(85)90042-X. [DOI] [Google Scholar]
- 19.Chi P, Wang J, Liu C. Synthesis and characterization of polycationic chitosan-graft-poly (l-lysine) Mater Lett. 2008;62:147–50. doi: 10.1016/j.matlet.2007.04.117. [DOI] [Google Scholar]
- 20.Casettari L, Vllasaliu D, Lam JK, Soliman M, Illum L. Biomedical applications of amino acid-modified chitosans: A review. Biomaterials. 2012;33:7565–83. doi: 10.1016/j.biomaterials.2012.06.104. [DOI] [PubMed] [Google Scholar]
- 21.El-Hamshary H, Al-Sigeny S, Ali MM. Synthesis and biological study of some amino acid functionalized starch-graft-polyacrylamide. Carbohyd Polym. 2006;64:282–6. doi: 10.1016/j.carbpol.2005.11.036. [DOI] [Google Scholar]
- 22.Yang W, Hattori M, Kawaguchi T, Takahashi K. Properties of starches conjugated with lysine and poly (lysine) by the Maillard reaction. J Agr Food Chem. 1998;46:442–5. doi: 10.1021/jf970515i. [DOI] [PubMed] [Google Scholar]
- 23.Nicolas J, Mura S, Brambilla D, Mackiewicz N, Couvreur P. Design, functionali zation strategies and biomedical applications of targeted biodegradable/biocompatible polymer-based nanocarriers for drug delivery. Chem Soc Rev. 2013;42:1147–235. doi: 10.1039/C2CS35265F. [DOI] [PubMed] [Google Scholar]
- 24.Layek B, Singh J. Amino acid grafted chitosan for high performance genedelivery: comparison of amino acid hydrophobicity on vector and polyplex characteristics. Biomacromolecules. 2013;14:485–94. doi: 10.1021/bm301720g. [DOI] [PubMed] [Google Scholar]
- 25.Xiao C. Development of Stimuli-Responsive Polysaccharides-based Nanothe ranostics. Curr Nanosci. 2016;12:33–7. doi: 10.2174/1573413711666150706182959. [DOI] [Google Scholar]
- 26.Namazi H, Belali S. Starch‐g‐lactic acid/montmorillonite nanocomposite: Synth esis, characterization and controlled drug release study. Starch‐Stärke. 2015 doi: 10.1002/star.201400226. [DOI] [Google Scholar]
- 27.Narayanan D, Nair S, Menon D. A systematic evaluation of hydroxyethyl starch as a potential nanocarrier for parenteral drug delivery. Int J Biol Macromol. 2015;74:575–84. doi: 10.1016/j.ijbiomac.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 28.Zohreh N, Hosseini SH, Pourjavadi A. Hydrazine-modified starch coated magnetic nanoparticles as an effective pH-responsive nanocarrier for doxorubicin delivery. J Ind Eng Chem. 2016;39:203–9. doi: 10.1016/j.jiec.2016.05.029. [DOI] [Google Scholar]
- 29.Poorgholy N, Massoumi B, Jaymand M. A novel starch-based stimuli-responsive nanosystem for theranostic applications. Int J Biol Macromol. 2017;97:654–61. doi: 10.1016/j.ijbiomac.2017.01.063. [DOI] [PubMed] [Google Scholar]
- 30.Toomari Y, Namazi H. Synthesis of supramolecular biodendrimeric β-CD-(spacer-β-CD) 21 via click reaction and evaluation of its application as anticancer drug delivery agent. International Journal of Polymeric Materials and Polymeric Biomaterials. 2016;65:487–96. doi: 10.1080/00914037.2015.1129960. [DOI] [Google Scholar]
- 31.Mohammadifar E, Adeli M, Kharat AN, Namazi H, Haag R. Stimuli‐Responsive Core Multishell Dendritic Nanocarriers. Macromol Chem Phys. 2017:218. doi: 10.1002/macp.201600525. [DOI] [Google Scholar]
- 32.Namazi H, Motamedi S, Namvari M. Synthesis of new functionalized citric acid-based dendrimers as nanocarrier agents for drug delivery. Bioimpacts. 2011;1:63–9. doi: 10.5681/bi.2011.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Namazi H, Toomari Y, Abbaspour H. Fabrication of triblock ABA type peptide dendrimer based on glutamic acid dimethyl ester and PEG as a potential nano drug delivery agent. Bioimpacts. 2014;4:175. doi: 10.15171/bi.2014.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mallakpour S, Rafiee Z. Safe and fast polyamidation of 5-[4-(2-phthalimidiylpr opanoylamino) benzoylamino] isophthalic acid with aromatic diamines in ionic liquid under microwave irradiation. Polymer. 2008;49:3007–13. doi: 10.1016/j.polymer.2008.05.013. [DOI] [Google Scholar]
- 35.Fang J, Fowler P, Sayers C, Williams P. The chemical modification of a range of starches under aqueous reaction conditions. Carbohyd Polym. 2004;55:283–9. doi: 10.1016/j.carbpol.2003.10.003. [DOI] [Google Scholar]
- 36.Genovese D, Lozano J. The effect of hydrocolloids on the stability and viscosityof cloudy apple juices. Food Hydrocolloid. 2001;15:1–7. doi: 10.1016/S0268-005X(00)00053-9. [DOI] [Google Scholar]
- 37.Wongsagonsup R, Shobsngob S, Oonkhanond B, Varavinit S. Zeta potential (ζ) and pasting properties of phosphorylated or crosslinked rice starches. Starch‐Stärke. 2005;57:32–7. doi: 10.1002/star.200400311. [DOI] [Google Scholar]
- 38.Namazi H, Kanani A. Investigation diffusion mechanism of β-lactam conjugated telechelic polymers of PEG and β-cyclodextrin as the new nanosized drug carrier devices. Carbohyd Polym. 2009;76:46–50. doi: 10.1016/j.carbpol.2008.09.023. [DOI] [Google Scholar]