Abstract
The Million Hearts Initiative has a goal of preventing 1 million heart attacks and strokes—the leading causes of mortality—through several public health and healthcare strategies by 2017. The American Heart Association and American College of Cardiology support the program. The Cardiovascular Risk Reduction Model was developed by Million Hearts and the Center for Medicare & Medicaid Services as a strategy to asses a value-based payment approach toward reduction in 10-year predicted risk of atherosclerotic cardiovascular disease (ASCVD) by implementing cardiovascular preventive strategies to manage the “ABCS” (aspirin therapy in appropriate patients, blood pressure control, cholesterol management, and smoking cessation).
The purpose of this special report is to describe the development and intended use of the Million Hearts Longitudinal ASCVD Risk Assessment Tool. The Million Hearts Tool reinforces and builds on the “2013 ACC/AHA Guideline on the Assessment of Cardiovascular Risk” by allowing clinicians to estimate baseline and updated 10-year ASCVD risk estimates for primary prevention patients adhering to the appropriate ABCS over time, alone or in combination. The tool provides updated risk estimates based on evidence from high-quality systematic reviews and meta-analyses of the ABCS therapies. This novel approach to personalized estimation of benefits from risk-reducing therapies in primary prevention may help target therapies to those in whom they will provide the greatest benefit, and serves as the basis for a Center for Medicare & Medicaid Services program designed to evaluate the Million Hearts Cardiovascular Risk Reduction Model.
Keywords: cardiovascular diseases, stroke, myocardial infarction, prevention, population, mortality, morbidity
Atherosclerotic cardiovascular disease (ASCVD) remains the leading cause of mortality and major morbidity in the United States, particularly among older Americans.1 The incidence of ASCVD events increases dramatically with each decade of life after 45 years of age in all sex and racial/ethnic groups. Most heart attacks and strokes occur in older adults as a result of cumulative exposure to preventable or modifiable causal risk factors that arise from adverse environmental conditions and behavioral/lifestyle patterns, including elevated blood pressure, adverse atherogenic blood lipid levels, diabetes mellitus, and tobacco use. Primordial prevention—preventing the development of these adverse risk factors in the first place—represents a promising and essential strategy for future control of ASCVD in the United States.2 However, a substantial amount of risk is already present among older Americans, who will require primary preventive interventions using evidence-based therapies to reduce their ASCVD risk.
THE MILLION HEARTS INITIATIVE: PREVENTING 1 MILLION HEART ATTACKS AND STROKES
In 2012, the US Department of Health and Human Services (HHS) initiated Million Hearts, a national public-private initiative with an ambitious goal of preventing 1 million heart attacks and strokes by 2017.3 The Centers for Disease Control and Prevention and the Centers for Medicare & Medicaid Services (CMS) co-lead the initiative on behalf of HHS. The American Heart Association (AHA) and American College of Cardiology (ACC) have enthusiastically supported the program.
Million Hearts aims to prevent heart attacks and strokes by pursuing a number of public health and healthcare strategies, outlined in Table 1.4 Central to the implementation of these strategies is management of the “ABCS”—aspirin therapy in appropriate patients, blood pressure control, cholesterol management, and smoking cessation. Identification of individuals at high risk for ASCVD, in whom application of these evidence-based therapies would have the greatest benefit, and improving adherence to these therapies once prescribed are essential for achieving these goals.
Table 1.
Strategies of the Million Hearts initiative to prevent 1 million heart attacks and strokes.4
|
|
|
|
|
ABCS indicates aspirin therapy in appropriate patients, blood pressure control, cholesterol management, and smoking cessation.
In support of Million Hearts, the Center for Medicare & Medicaid Innovation (CMMI) of the CMS recently announced its plans to perform a large cluster randomized payment model test of value-based payment designed to determine whether financially rewarding reductions in 10-year predicted risk for ASCVD across a physician’s patient population is an effective model to reduce the burden of heart attack and stroke.5 This Cardiovascular Risk Reduction Model will represent the largest test of value-based prevention payment conducted by CMS. As described by Sanghavi and Conway:
Medicare beneficiaries will be encouraged to “know [their] numbers,” share decision making with their physicians, and choose from a menu of options (for example, controlling blood pressure…, taking daily aspirin, or eliminating tobacco use) tailored to the patient’s readiness. The model’s value-based payment design will reward not specific blood pressure values or cholesterol target numbers but rather reduction in predicted risk of myocardial infarction and stroke. On the payment side, clinicians will be rewarded on a sliding scale tiered by absolute risk reduction across their entire high-risk patient panel, which increases incentives for health management of entire cohorts of patients. An additional benefit is that overtreatment of individuals at low risk could be minimized because overtreatment is not rewarded significantly.5
To conduct the test of the Cardiovascular Risk Reduction Model, CMS solicited the creation of the Million Hearts Longitudinal ASCVD Risk Assessment Tool, an innovative tool that predicts baseline 10-year ASCVD risk, projects changes in ASCVD risk that would be expected with initiation of and adherence to evidence-based therapies, and incorporates individual patient responses to these therapies over time to allow for dynamic, longitudinal ASCVD risk prediction. The model was developed by CMMI in collaboration with a research and development team from the CMS Alliance to Modernize Healthcare (CAMH), a federally funded research and development center (FFRDC) operated by the The MITRE Corporation for HHS. In this special report, we describe the development of the Longitudinal ASCVD Risk Estimator that was designed to support the overarching Million Hearts Cardiovascular Risk Reduction Model to be tested, and its application in clinical practice.
RATIONALE
Primary Prevention of ASCVD
Effective strategies exist for the primary prevention of ASCVD,6 but they are currently underutilized or not applied to the appropriate spectrum of patients, with clear undertreatment of higher-risk patient groups for whom efficacy has been demonstrated.1,7,8 Aspirin prevents ASCVD in certain groups, but its use must be weighed against the risk for major bleeding.9–11 Decades of research have demonstrated the benefits of blood pressure–lowering medications among individuals with elevated blood pressure, including among those with modest elevations in blood pressure but elevated global ASCVD risk.12–16 Likewise, in the past 20 years, statin medications have emerged as safe and highly effective medications for ASCVD primary prevention among essentially all groups at higher risk for ASCVD,17–20 with the exception of those on hemodialysis. Tobacco cessation substantially reduces ASCVD risk, and effective drugs and behavioral interventions can improve rates of smoking cessation.21
The “2013 ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults”22 identified 4 groups of patients for whom net clinical benefit of statin therapy has been demonstrated in randomized clinical trials: (1) patients with clinical ASCVD; (2) individuals with low-density lipoprotein (LDL)-cholesterol ≥190 mg/dL not attributable to secondary causes; (3) patients aged 40 to 75 years with diabetes mellitus and LDL-cholesterol 70 to 189 mg/dL; and (4) patients aged 40 to ≥75 years with estimated 10-year risk for ASCVD 7.5%. The last group represents those patients who need primary prevention of ASCVD as a result of elevated risk attributable to combinations of risk factors in the context of age, sex, and race/ethnicity. Despite the demonstrated benefits of pharmacological interventions (the ABCS), these therapies are often underutilized in populations with existing ASCVD23,24 as well as among higher-risk individuals eligible for primary prevention of ASCVD events.8
Current Paradigm for ASCVD Primary Prevention
The current paradigm for decision-making in the primary prevention of ASCVD is that the intensity of prevention efforts should be calibrated to the absolute ASCVD risk. This paradigm was promulgated in 1996,25 operationalized in the Third Adult Treatment Panel cholesterol guidelines (ATP III) in 200126 and 2004,27 and endorsed by the 2013 ACC/AHA guidelines on risk assessment28 and cholesterol management.22 Under this paradigm, individuals at low absolute predicted risk for ASCVD in the near term (<10 years) are recommended for all appropriate lifestyle modifications (eg, tobacco cessation, dietary and physical activity improvements), whereas those at higher predicted risk should receive lifestyle counseling as well as consideration for immediate drug therapy (eg, aspirin, blood pressure–lowering therapies, and statins) to reduce risk. Central to the approach to primary prevention in the 2013 ACC/AHA cholesterol guidelines is shared decision-making between the patient and clinician that begins with estimation of the patient’s absolute 10-year ASCVD risk, incorporates individual factors that may revise the risk estimate (up or down), considers the expected benefits and potential harms that would be expected from statin therapy, and includes patient preferences.22
The approach to basing decision-making on absolute ASCVD risk has been adopted widely by US and international guidelines and professional societies and permits more efficient and appropriate selection of patients for pharmacological treatment compared with usual care.29–31 Use of the 2013 ACC/AHA risk-based approach would prevent more events and treat fewer patients than an approach based solely on inclusion/exclusion criteria from randomized clinical trials, or a hybrid approach based on risk and the trials’ inclusion/exclusion criteria.32 A recent simulation suggested the use of individualized statin benefit estimation for younger, lower-risk individuals.33 Although current US hypertension guidelines recommend drug therapy solely based on blood pressure levels, studies also suggest that selecting patients by absolute predicted short-term ASCVD risk would be a more efficient means of determining benefit from antihypertensive therapy.29,31 Likewise, given the known risks for major bleeding associated with aspirin therapy, guidelines have adopted an approach that compares the expected benefit from aspirin in reducing heart attack and stroke balanced against the absolute risk for bleeding, to understand the net clinical benefit of aspirin.9,11
Estimating Baseline ASCVD Risk in Primary Prevention
A critical component of the approach to ASCVD risk reduction in the 2013 ACC/AHA22,28 and other guidelines is the estimation of the 10-year risk for preventable ASCVD events (including coronary death, nonfatal myocardial infarction, and fatal or nonfatal stroke) for individual patients, followed by application of evidence-based thresholds to determine eligibility for drug therapy. The 2013 ACC/AHA risk assessment guidelines developed new multivariable equations (the “Pooled Cohort Equations”) to provide sex- and race-specific estimates of ASCVD risk for black and Non-Hispanic white American men and women based on age, total and high-density lipoprotein (HDL)-cholesterol levels, systolic blood pressure (SBP), antihypertensive treatment use, diabetes mellitus history, and current smoking status. These equations were developed specifically to provide sex- and race-specific estimates for the first time, and to include strokes in addition to CHD in the outcome, which allows for better representation of preventable risk among women and blacks. Other outcomes, such as elective revascularization procedures, were not included because of their poor correlation with patient characteristics and extreme geographic variation in rates.28
The 2013 Pooled Cohort Equations for estimating ASCVD risk in the US population were derived from 5 community- and population-based studies, including the Framingham Heart Study, Framingham Offspring Study, ARIC (Atherosclerosis Risk in Communities) study, CHS (Cardiovascular Health Study), and CARDIA (Coronary Artery Risk Development in Young Adults) study. Data from ~25,000 individuals aged 40 to 79 years were available.28 To date, these equations have been evaluated in the derivation cohorts and validated in several external cohorts. Some have noted a mismatch between predicted risk from the equations and observed risk during follow-up; overestimation of risk by the equations tends to be observed in selected healthy volunteer cohorts who tend to have higher socioeconomic status and/or who were intensively treated with preventive therapies after inception that would alter the natural history of ASCVD.34–36 Others have noted underestimation of risk by the Pooled Cohort Equations in patient groups with inflammatory conditions, such as HIV.37 In contrast, the equations have demonstrated good discrimination and excellent calibration around decision thresholds in cohorts that are population-based and broadly representative of the United States, specifically including Medicare-eligible participants.38 In the REGARDS cohort (REasons for Geographic And Racial Differences in Stroke), which includes community-dwelling individuals from all 48 contiguous Unites States and large numbers of black and white participants, the Pooled Cohort Equations were overall well-calibrated, including among Medicare participants. Among groups at the highest predicted risk levels, the Pooled Cohort Equations predicted somewhat higher ASCVD rates than were observed, likely attributable to use of preventive therapies during follow-up.38 Clinicians therefore should be aware that the Pooled Cohort Equations, like all such risk assessment tools, estimate risk for patient groups and perform best in patient samples that resemble the derivation populations.
Similarly, the approach of the 2013 ACC/AHA cholesterol and risk assessment guidelines is substantially better than the older ATP III approach in sensitivity for detecting which patients will experience myocardial infarction39 and in identifying patients with a significant burden of coronary atherosclerosis.40–42 The current approach also has an acceptable number-needed-to-treat to prevent a ASCVD event43 and is cost-effective by current standards.44 It is estimated that the 2013 ACC/AHA guideline approach to cholesterol reduction could reduce the number of ASCVD events in the United States by 475,000 in the next decade, compared with the ATP III guideline approach, most of which would occur in older individuals who are at highest risk for ASCVD.45 Full implementation of the 2013 ACC/AHA cholesterol guidelines approach in all untreated, statin-eligible adults could achieve 78% of the Healthy People 2020 ASCVD prevention goal, with most of the benefits accruing to older, higher-risk individuals.46
Estimating Potential Benefits of ASCVD Risk-Reducing Therapies in Individual Patients
After estimating the baseline ASCVD risk, clinicians and patients currently have few tools to help them select and prioritize the most important or effective therapies to reduce ASCVD risk in the near term. As noted, the 2013 Pooled Cohort Equations predict the absolute 10-year risk for an incident ASCVD event if an individual with that profile at baseline were not treated with additional preventive therapies (ie, their “natural history”). It may be tempting to use these equations also as a means of estimating the benefits of interventions, by updating risk factor levels in the same equations. For example, one could attempt to provide an updated 10-year ASCVD risk by incorporating data on a patient’s new (presumably lower) blood pressure level after initiating antihypertensive therapy.
However, estimating the effect of a given therapy on updated 10-year risk simply by changing a risk factor level or removing adverse risk factors in the Pooled Cohort Equations (or any other similar risk prediction equations) provides inaccurate results. For example, a 70-year-old black man who does not have diabetes mellitus and is not a current smoker, with a total cholesterol of 200 mg/dL and HDL-cholesterol of 50 mg/dL and an untreated SBP of 150 mm Hg, has a 10-year predicted ASCVD risk of 15.3%, per the Pooled Cohort Equations. Using the Pooled Cohort Equations to estimate the effect of initiating antihypertensive therapy with a reduction of SBP to 130 mm Hg on therapy, one would get a counterintuitive and inappropriate result: the updated 10-year risk estimate of the patient would now be higher, at 19.5%. Although we know from clinical trial data that such antihypertensive therapy would in fact lower his risk for heart attack and stroke, the equations are not designed to reflect this change in an individual. Rather, the “updated” risk estimate reflects the natural history of a differentindividual who was treated for hypertension at some point before age 70 years and therefore is at higher risk because of longer burden and severity of elevated blood pressure. Thus, antihypertensive therapy use carries a positive coefficient in risk prediction models (indicating higher risk for those on therapy), because lowering blood pressure using medications does not reduce ASCVD risk to the same level as an individual who always had the lower blood pressure level in the absence of medications.47
Rationale for the Million Hearts Longitudinal ASCVD Risk Assessment Tool
In developing the Million Hearts Model to estimate the effect on change in ASCVD risk with evidence-based therapies, we judged that the optimal approach is to estimate the 10-year predicted “natural history” risk of ASCVD, and then to simulate the expected average risk reduction associated with a given therapeutic intervention. In addition, we judged that it would be useful to assess the updated risk for ASCVD at a follow-up visit in a patient who has adopted a risk-reducing therapy (eg, blood pressure–lowering or statin medications) based on the actual observed change in the blood pressure or LDL-cholesterol, or the institution of aspirin or cessation of smoking. Our objective was to create an innovative tool that will assist policymakers, clinicians, and patients to estimate the expected effects of different preventive interventions, used singly and jointly, on ASCVD risk reduction in the Medicare population. The specific intent was to provide this tool as a basis for estimating projected/expected ASCVD risk reduction and actual/achieved risk reduction as a means for the CMMI to estimate physician performance in reducing ASCVD risk among high-risk Medicare beneficiaries.
A secondary goal was to create a tool applicable to a wider age range, including younger patients. Previous attempts have not been based on a systematic evidence review to determine the beneficial and harmful effects of the interventions, have not incorporated measures of variance in their estimates, and have not used the current US risk assessment paradigm based on the Pooled Cohort Equations.28 We therefore developed the novel Million Hearts Longitudinal ASCVD Risk Assessment Tool specifically for the CMMI Medicare program and describe its background and use in the text that follows. Use of the tool in other patient groups and clinical settings could be considered in the future if appropriate validation studies suggest its utility.
METHODS OF TOOL DEVELOPMENT
State of the Science and Gaps in Knowledge
The effects of some of the Million Hearts ABCS interventions on fatal and nonfatal ASCVD outcomes have been estimated previously through individual systematic reviews. However, to our knowledge, there are no reports that have summarized the effects of these interventions in primary prevention using a rigorous, transparent search, with comparison, evaluation, and synthesis of systematic reviews through what is known as an overview of systematic reviews.
Furthermore, because some individuals with increased risk for ASCVD are eligible for multiple treatments, it was important to attempt to quantify the effects of concomitant preventive treatments. We may assume that the effects of multiple treatments are simply additive because most randomized controlled trials of aspirin, blood pressure–lowering therapies, and statins have included individuals who are on additional risk-reducing drug therapy. However, there exists the possibility of interaction effects that might be negative or positive. A positive interaction indicates effects of multiple preventive interventions that exceed the additive estimate (ie, the whole greater than the sum of the parts). Conversely, a negative interaction could occur if the effects of multiple interventions are less than expected from simple addition. A number of scenarios could lead to negative interactions, such as floor effects, in which there is a minimum event rate associated with risk factors below which interventions cannot further lower risk, or less-than-anticipated reductions in risk from aspirin in the setting of concomitant tobacco cessation or initiation of cholesterol-lowering therapy. The potential for interactions requires a detailed evidence review to incorporate these effects into models that attempt to estimate the impact of risk modification from simultaneous interventions.
The website QIntervention 2014 (qintervention.org)48 aims to provide information to clinicians and patients on changes in ASCVD risk based on individual or concomitant interventions with blood pressure–lowering therapy, statin therapy, and tobacco cessation. However, the meta-analysis used to inform the effect estimate of statins has not been updated in >5 years, the website does not currently incorporate the effect of aspirin, and the effect of blood pressure–lowering treatment is erroneously47 estimated to be the same as the risk based on the same blood pressure levels without treatment. These features limit its usefulness.
Regarding the effects of combinations of interventions, some might argue that these were quantified in trials such as Steno-2.49,50 However, in addition to recommendations for more intensive drug therapy with aspirin, statin, and ACE-inhibitor use, Steno-2 included a behavioral component and more frequent office visits as components of a complex intervention.51 Combination drug therapy trials with either a factorial design (eg, the ASCOT-LLA [Anglo-Scandinavian Cardiac Outcomes Trial – Lipid Lowering Arm])52 or fixed-dose combination therapy trials53 may be better suited to address this question of whether concomitant therapy with a combination of aspirin, blood pressure–lowering therapies, statins, or all of these leads to effects similar to those that would be predicted based on the individual drug effects. We also judged that there were undoubtedly other studies that inform the interaction of multiple interventions that could be identified with a systematic review approach.
To address these gaps in knowledge, we leveraged the infrastructure of the Cochrane Heart Group United States Satellite to derive the best available quantitative evidence on the expected effects of risk-reducing therapies (the ABCS) for ASCVD. We then combined these results with the predicted baseline risks for patients derived from the 2013 Pooled Cohort Equations to estimate projected and achieved ASCVD risk reduction benefits over time.
Systematic Review of ABCS Therapies in ASCVD Risk Reduction
The detailed methods and results of the systematic review have been published.6 Briefly, we used standardized methods outlined in the Cochrane Handbook of Systematic Reviews54 (www.handbook.cochrane.org) for the performance of an overview of systematic reviews for each of the following interventions for primary ASCVD prevention: aspirin, blood pressure–lowering therapy, statins, and pharmacologic tobacco cessation strategies. Of note, we focused specifically on statins for cholesterol management because of the extensive evidence base and endorsement of statins by numerous guidelines as the primary means for management of LDL-cholesterol to reduce ASCVD risk.22,55
We created separate protocols and search strategies for each overview and published each protocol on PROSPERO (International Prospective Register of Systematic Reviews, http://www.crd.york.ac.uk/PROSPERO/; Registration No. CRD42015023444). We selected systematic reviews of randomized clinical trials involving patients without prevalent ASCVD to derive treatment effect estimates that applied to a primary prevention population. We compared the effects of each intervention reported in the original systematic reviews, assessed the methodological quality of each systematic review using the AMSTAR tool (Assessment of Multiple SysTemAtic Reviews),56 and assessed the overall quality of evidence using the GRADE methodology (Grading of Recommendations Assessment, Development and Evaluation).57
As described below, all available data from clinical trials have reported only the intermediate end point of successful tobacco cessation/abstinence with no trials powered to detect differences in clinical outcomes. Although this is an important outcome, it did not address the needs of the tool. We therefore performed a separate literature search to estimate the ASCVD risk reduction achieved with tobacco cessation. By consensus, and in consultation with CMMI and CAMH, we selected 2 articles58,59 that used the most rigorous methodology to estimate longitudinal risk reduction for stroke and myocardial infarction associated with tobacco cessation. Notably, it was also not feasible to estimate the effects of lifestyle interventions on ASCVD event reduction in the near term. The widely differing approaches and scope of lifestyle interventions made them difficult to compare, or replicate, and data on hard outcomes for ASCVD events are sparse, as indicated in the AHA/ACC 2013 lifestyle management guidelines.60 Nonetheless, the importance of background and ongoing therapeutic lifestyle change is acknowledged, as all clinical trials of drug therapy for ASCVD risk reduction have been performed in the context of advice for lifestyle changes. Further, changes in risk factor levels ascribed to behavior changes will be captured through updated risk factor estimates in the risk assessment tool.
Systematic Review of Combination Preventive Therapies
To compare the effect of concomitant treatment against additive treatment, we performed a systematic review of randomized clinical trials including individuals who did not have ASCVD, with or without diabetes mellitus.6 We searched for trials that had assessed the effects on ASCVD risk of concomitant drug treatment (compared with single component treatment alone) using any one of the following combinations: aspirin + blood pressure–lowering therapy; aspirin + statin therapy; blood pressure–lowering + statin therapy; or aspirin + blood pressure–lowering therapy + statin therapy.
To compare the effects of concomitant treatment with the predicted, additive effects from individual treatments, we used data derived from both systematic reviews, including subgroup comparisons, where available. Based on our findings (detailed below), there were few trials that directly addressed this question, but we found no evidence for either a positive or negative interaction. Observational studies were not considered because of their inherent increased risks of bias, particularly when evaluating the effects of interventions. An alternative methodology to evaluate the potential effect modification of concomitant treatment would be to perform meta-regressions of systematic reviews on aspirin, blood pressure–lowering, and cholesterol–lowering therapy, where the presence or absence of concomitant therapy would serve as the explanatory variable. This approach was rejected because of the expected instability of such estimates.
Development of Million Hearts Longitudinal ASCVD Risk Assessment Tool
We created a spreadsheet-based tool that allows users to enter data on risk factor levels at a baseline visit, and that provides an age-, sex-, and race-specific 10-year ASCVD risk estimate for men and women based on the 2013 Pooled Cohort Equations.28 Using an iterative development process, and with frequent consultation with the CAMH and CMMI teams as well as other contractors, we developed the means for estimating the projected/expected average risk reduction conferred by any of the 4 Million Hearts “ABCS” interventions. This prospective risk reduction (as determined by the systematic reviews described previously) was represented as an updated projected 10-year ASCVD risk after:
Initiation or continuation of aspirin alone;
Initiation or intensification of blood pressure–lowering therapy alone;
Initiation of moderate-intensity statin, or intensification from moderate-intensity to high-intensity statin alone;
Achievement of successful tobacco cessation alone; or
All possible combinations of the above therapies (as relevant, see below).
We conducted extensive sensitivity analyses to include all possible therapy combinations and account for all plausible scenarios of risk factor changes. To avoid overestimation of the potential effects of therapies in combination, as described below, we included “floor” values of absolute risk, below which projected risks with interventions cannot go, which were calculated based on the predicted risk of individuals with untreated optimal levels of all risk factors at the same age, sex and race.
We also created an additional feature to allow for estimation of updated 10-year risk for ASCVD based on a given patient’s baseline risk and achieved change in risk factor levels at follow-up. The data from the systematic literature reviews were used to provide estimates of ASCVD risk reduction associated with observed adherence to aspirin therapy, absolute change in SBP, absolute change in LDL-cholesterol, and successful tobacco cessation over different durations. Again, extensive iterative testing was performed to consider all possible scenarios and combinations of risk factor levels at follow-up (including both improvement and worsening of risk factor levels), with extensive input from the CMMI and CAMH teams as well as other contractors. We judged that both “floor” and “ceiling” values were needed to reflect plausible thresholds below (or above) which improvements (or worsening) in risk factors would not be associated with additional changes in ASCVD risk. Floor values were applied as described previously. Ceiling values were applied when, at a follow-up visit, a patient had a new risk factor (eg, had started smoking) or worsened levels of a risk factor (eg, higher level of SBP). In these cases, the updated risk value could not be higher than the ceiling of the risk of someone who had always been a smoker (or had that blood pressure value) at the same age and risk factor levels.
THE MILLION HEARTS LONGITUDINAL ASCVD RISK ASSESSMENT TOOL
Effects of Risk-Reducing Therapies
The results of the systematic reviews, which were performed to inform the tool, have been previously published.6 Briefly, from 1,967 identified reports, 35 systematic reviews of randomized clinical trials were identified, including 15 reviews of aspirin, 4 of blood pressure–lowering therapy, 12 of statins, and 4 of tobacco cessation drugs. Methodological quality varied, but 30 were judged to be of sufficient quality based on AMSTAR ratings. Using the highest quality evidence, the effects aspirin, blood pressure–lowering therapy, statins, and smoking cessation on ASCVD risk are summarized in Table 2.
Table 2.
Results of systematic review with relative risk estimates for ASCVD risk reduction.6
| Therapy | Estimated RR for ASCVD Events (95% CI) | Quality of Evidence* | Comment |
|---|---|---|---|
| Aspirin | 0.90 (0.85–0.96) | High | Increased risk for major bleeding (RR, 1.54; 95% CI, 1.30–1.82) |
| Blood pressure– lowering† | CHD: 0.84 (0.79–0.90) overall; 0.79 (0.72–0.86) per 10 mm Hg reduction in SBP | High | Adverse effects poorly reported |
| Stroke: 0.64 (0.56–0.73) overall; 0.54 (0.45–0.65) per 10 mm Hg reduction in SBP | High | ||
| Cholesterol-lowering (statin) | 0.75 (0.70–0.81) overall ; 0.75 (0.70–0.80) per 1 mmol/L [38.7 mg/dL] reduction in LDL- cholesterol | High | No increased risk for adverse effects overall (RR, 1.00; 95% CI, 0.97–1.03) |
| Smoking cessation‡ | 0.73 overall; 0.85 at 1 y (>6–18 mo follow up); 0.73 at 2 y (>18–30 mo); 0.62 at 3 y (>30–42 mo); 0.53 at 4 y (>42 mo) | Not graded | Adverse effects poorly reported |
High quality of evidence indicates that further research is unlikely to change our confidence in the estimate of effect.
Aggregate relative risks for all ASCVD (CHD plus stroke) with blood pressure–lowering are 0.73 overall and 0.65 per 10 mm Hg reduction in SBP.
Effects on ASCVD events poorly reported. Therefore these effect estimates were derived from Lee et al.58,59
ASCVD indicates atherosclerotic cardiovascular disease; CI, confidence interval; CHD, coronary heart disease; RR, relative risk; and SBP, systolic blood pressure.
High-quality evidence indicated that, compared with placebo, use of aspirin reduced the risks for ASCVD (relative risk [RR], 0.90; 95% confidence interval [CI], 0.85–0.96). Of note, risk reduction was similar for men and women, but effects were greater for CHD reduction in men and stroke reduction in women.6
Blood pressure–lowering therapy was associated with a 16% reduction in CHD events (RR, 0.84; 95% CI, 0.79–0.90), and a 36% reduction in stroke (RR, 0.64; 95% CI, 0.56–0.73) with relatively modest decreases in blood pressure (6/3 mm Hg) associated with treatment.6 These data were used to scale the achieved observed risk reductions for SBP level at the follow-up visit, with an expected RR of 0.73 for initiation of blood pressure–lowering therapy overall, and a RR of 0.65 per 10 mm Hg actual SBP lowering.
High-quality evidence for statins revealed a 25% reduction in major ASCVD events (RR, 0.75; 95% CI, 0.70–0.81), and reductions in fatal and nonfatal CHD and stroke events. The mean difference in LDL-cholesterol associated with use of a statin was 1.00 mmol/L (95% CI, 0.85–1.16) or 38.7 mg/dL (95% CI, 32.9–44.9).6 Treatment effects standardized per 1 mmol/L reduction in LDL-cholesterol were also reported by the Cholesterol Treatment Trialists for major vascular events (RR, 0.75; 95% CI, 0.70–0.80).17,18 Therefore, these values were used to scale the achieved observed risk reductions for LDL-cholesterol levels at the follow-up visit.
Tobacco cessation drugs increased the odds of continued abstinence at 6 months (odds ratio range, 1.82 [95% CI, 1.60–2.06] to 2.88 [95% CI, 2.40–3.47]), but direct effects on ASCVD events were poorly reported.6 We therefore performed a separate literature review and, after consultation within the team and discussion with biostatistical and content experts, as well as with CAMH and CMMI, we elected to use the studies by Lee et al58,59 to estimate time-dependent effects of successful smoking cessation on heart attack and stroke risk reduction up to 4 years after baseline. As a result, the combined estimates for risk reduction associated with smoking cessation are 15% at 1 year (>6–18 months’ follow-up), 27% at 2 years (>18–30 months), 38% at 3 years (>30–42 months), and 47% at 4 years (>42 months). Because the risk reduction estimates for aspirin, blood pressure–lowering, and statins were based on trials with typical follow-up exceeding 2 years, we used the 2-year estimate for smoking cessation as the basis for prospective risk reduction estimation.
Aspirin increased the risk for major bleeding (RR, 1.54; 95% CI, 1.30–1.82), and statins did not increase overall risk for adverse effects (RR, 1.00; 95% CI, 0.97–1.03). Adverse effects of blood pressure–lowering therapy and tobacco cessation drugs were not possible to characterize systematically because they are inconsistently reported in the trials and poorly reported among systematic reviews including people without established vascular disease.6
In our systematic review evaluating potential interaction with combination therapy, we identified 4 factorial, randomized controlled clinical trials61–64 including 21,358 participants that evaluated these combinations:
Blood pressure–lowering therapy + aspirin versus blood pressure–lowering therapy;
Blood pressure–lowering therapy + statin versus blood pressure–lowering therapy;
Blood pressure–lowering therapy + statin versus statin.
We found no evidence of an interaction among these combinations in terms of effects on ASCVD events, blood pressure, or lipid levels, but power to detect differences was limited because of small sample sizes or low event rates. Three of 4 studies had a high risk of bias across at least 1 domain. We also separately examined the ASCOT-LLA trial results,52 which did not meet inclusion criteria for our systematic review (≥10% of participants in the trial had prevalent vascular disease), and found no evidence of treatment interaction. Overall, there does not appear to be evidence of any interaction effect of combination therapy for the primary prevention of ASCVD, but we rated the quality of evidence as low, downgrading because of study limitations and imprecision. Data published subsequent to our systematic review search from the HOPE-3 trial (Heart Outcomes Prevention Evaluation) appear consistent with simple additive effects and lack of interaction with combination blood pressure–lowering and statin therapy.15
Million Hearts Longitudinal ASCVD Risk Assessment Tool
The Million Hearts tool is intended to identify candidates for primary prevention therapies and assist in their management. Use of the tool occurs in 3 steps: (1) estimating the baseline 10-year risk for ASCVD; (2) considering the potential benefits of risk-reducing therapies for a given patient in the context of a patient-clinician discussion and shared decision-making; and (3) assessing the updated ASCVD risk based on the response to therapy. The User’s Guide (Appendix) provides detailed instructions on use of the Million Hearts tool. An example of use of the tool for a patient is provided in Figure 1.
Figure 1.
Patient Scenario
Step 1: Estimating the Baseline 10-Year Risk
On the first tab of the spreadsheet, the baseline 10-year ASCVD risk estimate is calculated using the ACC/AHA 2013 Pooled Cohort Equations,28 which provide sex- and race--specific 10-year estimates of ASCVD risk. The Pooled Cohort Equations were developed from samples of black and non-Hispanic white men and women who were 40 to 79 years of age, apparently healthy, and free of a previous history of nonfatal myocardial infarction (recognized or unrecognized), stroke, heart failure, percutaneous coronary intervention, coronary artery bypass surgery, or current atrial fibrillation. Patients with end-stage renal disease were not included in the derivation sample; such patients require individualized care with respect to use of aspirin and blood pressure–lowering therapies, and data on use of statin medications in patients with end-stage renal disease do not indicate overall benefit. For some patients with symptomatic or advanced heart failure, similar considerations and individualized decision-making may be necessary. However, recent data reinforce the importance of ASCVD risk-reducing therapies even among patients with heart failure of ischemic etiology.20 Therefore, the Million Hearts Longitudinal ASCVD Risk Assessment Tool is intended for use in a broad population aged 40 to 79 years and eligible for primary prevention of ASCVD, with the exclusions noted previously. For older (and younger) individuals, guidelines recommend individualized care decisions. Patients with LDL-cholesterol of at least 190 mg/dL may have familial hypercholesterolemia and should be evaluated and considered for statin therapy regardless of age and estimated 10-year ASCVD risk.
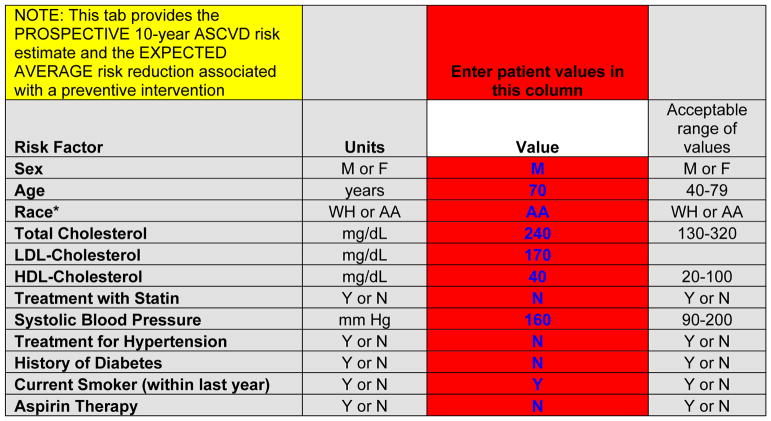
The patient’s 10-year ASCVD risk is estimated by entering data in the red cells (Figure 2) on the patient’s sex, age, race/ethnicity, total and HDL-cholesterol levels, SBP, treatment for hypertension, history of diabetes mellitus (ever) and current smoking status (within the past year). Of note, additional data (LDL-cholesterol levels, statin treatment, aspirin therapy) are also collected during this phase to provide context for the projected risk reduction and to provide baseline values for updating ASCVD risk at follow-up visits.
Figure 2.
Data entry for estimation of 10-year risk for ASCVD at a baseline visit, for the example provided in the patient scenario (Figure 1)
AA indicates African American, or black; ASCVD, atherosclerotic cardiovascular disease; HDL, high-density lipoprotein; LDL, low-density lipoprotein; and WH, non-Hispanic white.
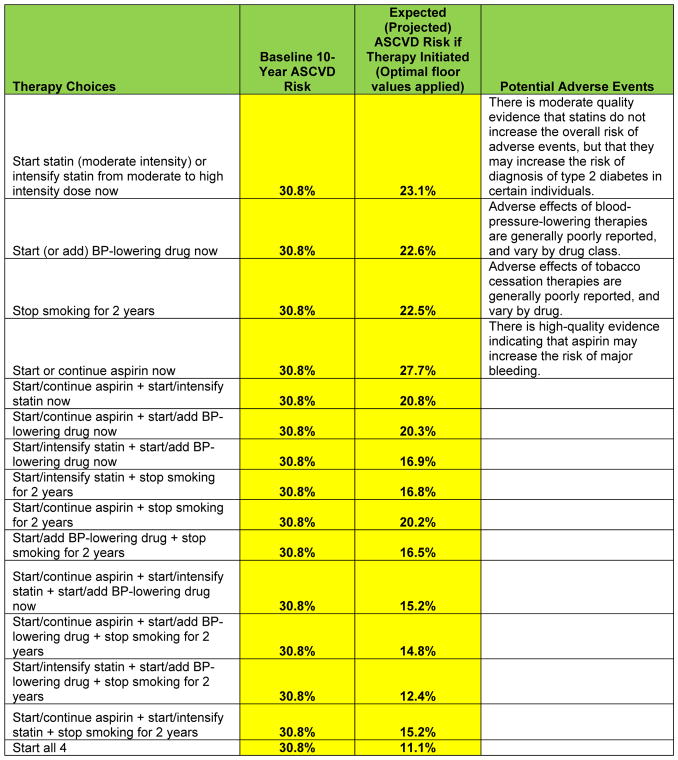
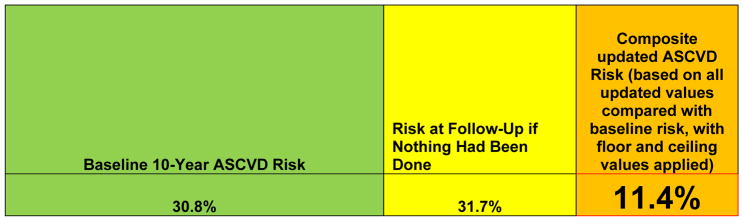
After entering the baseline risk factor levels, the user is presented with the baseline 10-year ASCVD risk estimate (Figure 3). Of note, this is a sex- and race--specific estimate for a black or non-Hispanic white man or woman. For individuals of other race/ethnic groups, the relevant sex-specific equation for non-Hispanic whites is used, as recommended by the guidelines. These estimates may overestimate risk somewhat in Asian/Pacific Islander-East Asian Americans and Hispanic/Latino Americans, especially Mexican-Americans; they may underestimate risk somewhat in API-South Asian Americans, Asian/Pacific Islander-Other groups, Puerto-Ricans, and in American Indians/Alaska Natives. The calibration for individuals in “Other/Mixed Race” race/ethnic groups is uncertain. In the patient scenario in Figure 1, the 10-year risk estimate is 30.8% for a 70-year-old black man who is a current smoker with no history of diabetes mellitus, with an untreated total cholesterol of 240 mg/dL and HDL-cholesterol of 40 mg/dL, and an untreated SBP of 160 mm Hg. This should be interpreted as follows: if we had 100 patients like this man, then we estimate that 31 of them will have or die from a heart attack or stroke in the next 10 years.
Figure 3.
Baseline 10-year ASCVD risk estimate for the patient scenario, and projected ASCVD risk if a given therapy or combination of therapies is used
ASCVD indicates atherosclerotic cardiovascular disease; and BP, blood pressure.
Step 2: Considering Potential Benefits of Therapies, Alone and in Combination
In addition to the baseline 10-year ASCVD risk estimate, on the first tab of the spreadsheet users will see the projected 10-year ASCVD risk that would be associated with institution of specific preventive therapies (Figures 1 and 3) as designated for each row. These estimates are a function of the baseline predicted 10-year risk from for the patient and the expected average relative risk reduction associated with a given therapy experienced by participants in randomized clinical trials, using the data described previously from the systematic reviews. Of note, the risk reduction estimates come from randomized controlled trials that have tended to last for 3 to 5 years, so it is assumed that patients who would start a medication would stay on it for at least that period of time, and adhere to the therapy similarly to participants in the trials. Logic models have been applied so that patients cannot receive an expected risk reduction for therapies that are not relevant. For example, if a patient is already a nonsmoker, users will see “NA” (not applicable) appear in rows where “stop smoking” would be a strategy. Similarly, for patients with estimated 10-year ASCVD risk below 5%, users will see “NA” appear in rows where statin use or intensification would be added, given that the guidelines do not recommend consideration of statin therapy for this group. Statin use at baseline does not affect the baseline 10-year risk estimate—the on-treatment values of total cholesterol and HDL-cholesterol should be used for the risk estimate, and credit for further potential reduction in LDL-cholesterol is only given if the patient is on a moderate dose statin and could escalate to a high-intensity dose to further lower their LDL-cholesterol.
For patients already taking blood pressure–lowering drugs, one can estimate the expected effect of additional medication. However, if a patient has a baseline SBP <120 mm Hg, or <130 mm Hg with diabetes mellitus, no additional benefit of SBP lowering is assumed, and addition of blood pressure–lowering drugs results in a response of “NA.” For patients with estimated 10-year ASCVD risk below 10%, users will see “NA” appear in rows where aspirin would be started because guidelines do not typically recommend consideration of aspirin therapy for patients with 10-year risk <10%. If the patient is taking aspirin therapy at baseline, the expected risk reduction for continuing aspirin is assumed to be the same as starting aspirin de novo, because it is uncertain how to quantify any increase in risk associated with aspirin cessation.
Additive effects of medications in combination are assumed for these and all other calculations of expected or updated risk. Finally, the values of projected ASCVD risk in the setting of therapies are subject to a floor effect, meaning that expected ASCVD risks that are reported with initiation/addition of preventive therapy cannot be lower than the predicted 10-year ASCVD risk for someone with the same age and race/ethnicity category who has an optimal risk factor profile (total cholesterol, 170 mg/dL; HDL-cholesterol, 60 mg/d; SBP, 110 mm Hg; nonsmoker; non-diabetic; and no blood pressure–lowering drugs). Users are also provided with statements regarding possible adverse effects of medications.
These data are intended for use to guide the patient-clinician discussion recommended in the 2013 ACC/AHA cholesterol guidelines.22 This patient-clinician discussion was recommended because the guideline panel wished to avoid automatic assignment (or nonassignment) of a statin to a patient simply because of their 10-year risk estimate, without an important discussion to add clinician judgment and patient preferences to the final decision, especially in primary prevention. Using the Million Hearts tool, the patient and clinician can see the projected absolute risk reduction associated with initiation and continuation of each therapy, or combinations of therapies, and weigh this in the context of other considerations, including patient preferences for taking medications, potential adverse drug reactions or interactions, and where they see the most “bang for the buck.”
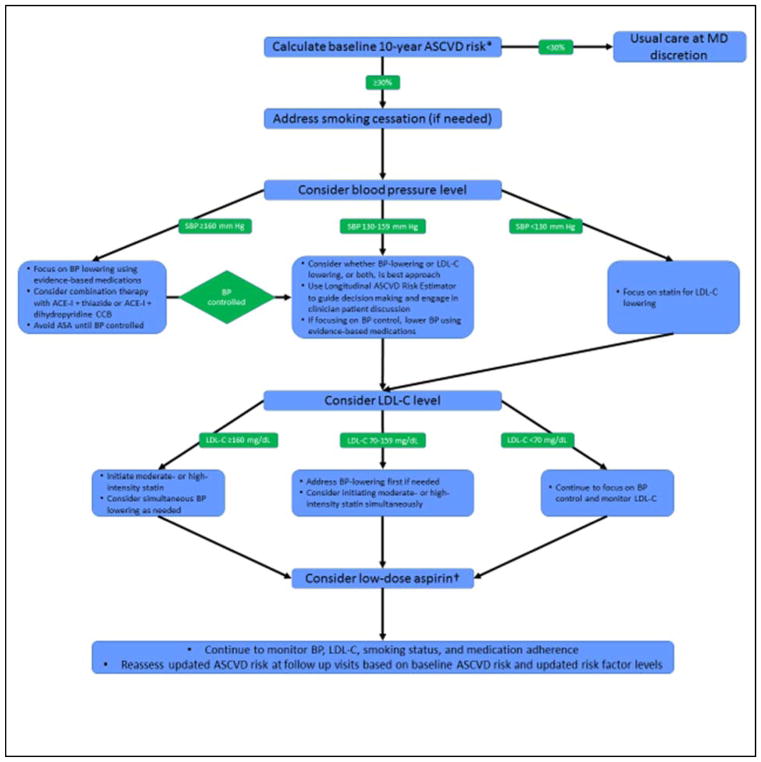
It may be difficult to assign priority to treatment strategies when considering multiple risk-reducing therapies. Figure 4 provides a suggested algorithm th at may be used by clinicians and patients in the CMMI program in addition to the Longitudinal ASCVD Risk Assessment Tool if there is uncertainty regarding which approach should be considered first. After assessment of 10-year risk for eligible patients, counseling and efforts aimed at smoking cessation should be considered for all current smokers. Next, clinicians may consider blood pressure level and focus particularly on controlling patients with significantly elevated SBP (≥160 mm Hg). When blood pressure levels are lower, the relative benefits of statins or antihypertensive therapy, or both, should be considered. Finally, clinicians and patients may wish to consider low-dose aspirin therapy. The suggested clinical algorithm in Figure 4 can be informed by the results of the Million Hearts tool with regard to expected risk reductions from the various therapies, but it should not replace clinical judgment.
Figure 4.
Suggested clinical algorithm for prioritizing decisions regarding preventive therapies in the Million Hearts Cardiovascular Risk Reduction Model
*Patients with clinical atherosclerotic cardiovascular disease or LDL-C 190 mg/dL should be treated with high-intensity (or maximally tolerated) statin.
†Use USPSTF recommendations and consider the potential risk for major bleeding when considering use of aspirin.
ACE-I indicates angiotensin-converting-enzyme inhibitor; ASA, aspirin; ASCVD, atherosclerotic cardiovascular disease; BP, blood pressure; LDL-C, low-density lipoprotein–cholesterol; MD, physician; and SBP, systolic blood pressure.
Step 3: Assessing the Updated ASCVD Risk at a Follow-Up Visit Based on the Response to Therapy
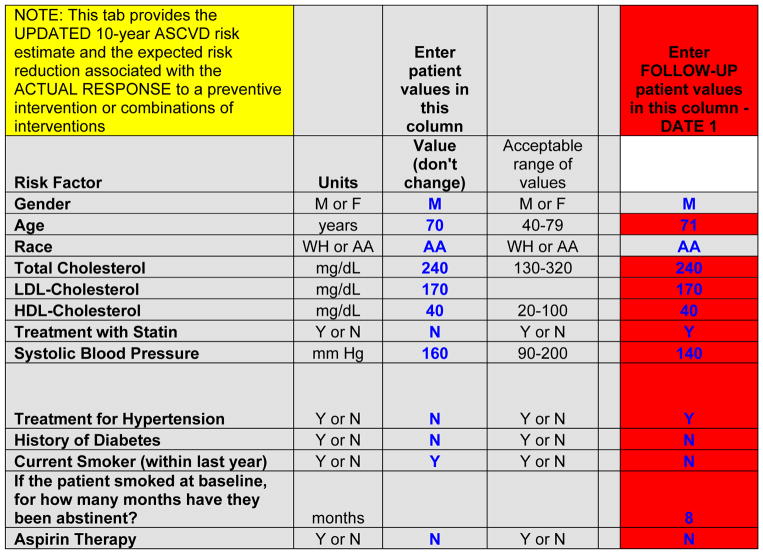
On a second tab, the spreadsheet provides 10-year ASCVD risk information and the potential for ASCVD risk reduction with selected preventive therapies for a provider and patient at a “follow-up” visit (Figures 1 and 5). The values from the baseline visit are carried forward from the values entered on the first tab of the spreadsheet. At the follow-up visit, users enter values only in the red cells. Continuous variables may have increased or decreased in the interim, and some categorical values may have changed: smokers may have become nonsmokers; nonsmokers may have initiated/resumed smoking; non–aspirin users may have started aspirin; aspirin users may have stopped; patients without diabetes mellitus may have developed diabetes mellitus; and patients may have started antihypertensive therapy. As noted below, individuals with diabetes mellitus at baseline are treated as still having diabetes mellitus, and those who required antihypertensive therapy at baseline are assumed to still require it.
Figure 5.
Data entry for estimation of updated 10-year risk for ASCVD at a follow-up visit, for the example provided in the patient scenario (Figure 1).
AA indicates African American, or black; ASCVD, atherosclerotic cardiovascular disease; HDL, high-density lipoprotein; LDL, low-density lipoprotein; and WH, non-Hispanic white.
After entering all of these values, users are presented with 3 different 10-year ASCVD risk estimates (Figure 6). The first is the baseline 10-year ASCVD risk estimate, which is the same as that provided on the first tab of the spreadsheet, from the patient’s age and risk factor values at the baseline visit. An updated 10-year ASCVD risk estimate represents what would have happened if nothing had been done after the baseline visit, providing the risk at the follow-up age that would have been present using the baseline risk factor values, if no interventions had been performed. Similar caveats apply to this risk estimate for sex/race-ethnic groups as noted previously. Finally, users will see the actual updated 10-year ASCVD risk associated with this patient’s status at follow-up. This value is a function of the baseline risk, the follow-up age, and the interim change in therapies and risk factor levels, which are themselves a function of response to therapy and adherence. The duration of smoking cessation (if relevant) and the measured changes in the follow-up values of LDL-cholesterol and SBP determine the updated actual risk estimate. For example, the risk associated with a 1 mmol/L (~38.7 mg/dL) change in the value of LDL-cholesterol was 0.75 from the systematic reviews cited previously. The tool uses the actual change in LDL-cholesterol relative to (scaled to) the reported risk reduction. Thus, if a patient achieved a 0.5 mmol/L reduction (~19 mg/dL) in LDL-cholesterol, the risk reduction would be (0.75)0.5, equal to 0.87. If the patient achieved a 2 mmol/L reduction in LDL-cholesterol (~78 mmol/L), the risk reduction would be (0.75)2, equal to 0.56.
Figure 6.
Ten-year ASCVD risk values for use at a follow-up visit: 10-year ASCVD risk estimate at baseline visit for the patient scenario in Figure 1, 10-year ASCVD risk at follow-up if nothing had been done in the interim, and updated 10-year ASCVD risk based on patient’s current age, baseline risk, and achieved risk factor values at follow-up visit.
ASCVD indicates atherosclerotic cardiovascular disease.
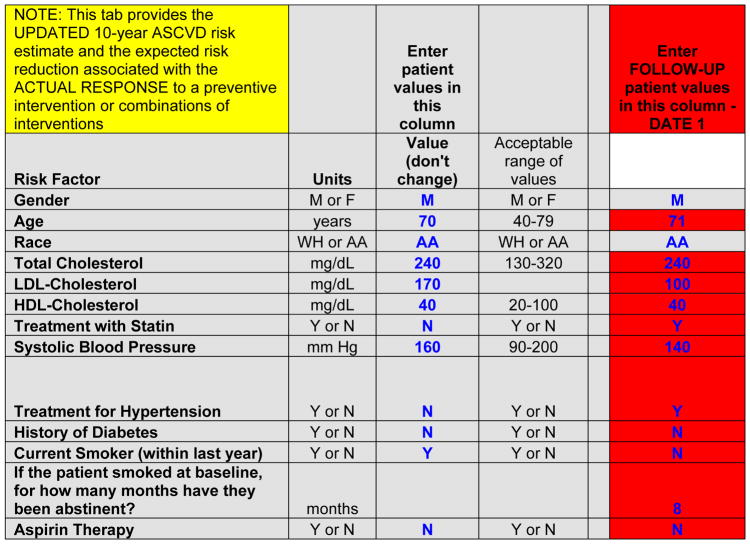
Follow-up aspirin use/nonuse also influences the updated risk estimate. Likewise, incident diabetes mellitus or resumption/initiation of smoking would adversely affect the follow-up risk estimate. The values for the actual updated 10-year ASCVD risk estimate have floor and ceiling values applied. The floor value is calculated as the predicted 10-year ASCVD risk for someone with optimal risk factor levels at the follow-up age. The ceiling value is the predicted 10-year ASCVD risk calculated from the actual updated risk factor profile, including use of medications. To estimate the potential risk reduction that can be obtained with additional interventions being considered during the follow-up visit, users can modify the values of risk factors and treatments in the red cells (Figures 7 and 8) to obtain new risk estimates representing responses to new future treatment scenarios.
Figure 7.
Data entry for estimation of future 10-year risk for ASCVD after a follow-up visit if a statin was started, with reduction of LDL-cholesterol to 100 mg/dL, for the example provided in the patient scenario in Figure 1
AA indicates African American, or black; ASCVD, atherosclerotic cardiovascular disease; HDL, high-density lipoprotein; LDL, low-density lipoprotein; and WH, non-Hispanic white.
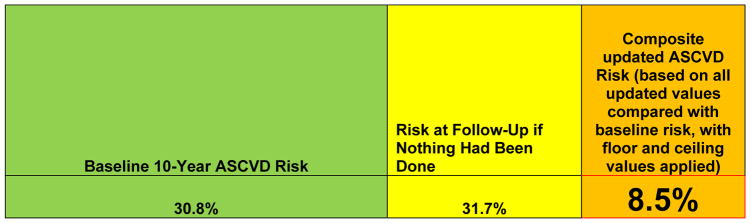
Figure 8.
Ten-year ASCVD risk values for use at a follow-up visit: 10-year ASCVD risk estimate at baseline visit for the patient scenario in Figure 1, 10-year ASCVD risk at follow-up if nothing had been done in the interim, and updated 10-year ASCVD risk based on patient’s current age, baseline risk, and future projected risk factor values after initiation of a statin and continuation of current therapies.
ASCVD indicates atherosclerotic cardiovascular disease.
RECOMMENDATIONS FOR USE IN CLINICAL PRACTICE
Appropriate therapeutic lifestyle changes are recommended for all individuals, regardless of ASCVD risk. For example, heart healthy eating patterns (eg, DASH and Mediterranean-style eating patterns) can further improve adverse risk factor levels and reduce cardiovascular events.60,65 Participation in recommended amounts of physical activity has numerous cardiovascular health benefits and is associated with reduced risk and improved health-related outcomes.60,66 Maintenance of appropriate body mass index, and weight loss for those in whom it is indicated, can contribute significantly to ASCVD risk factor control as well.60,67 Tobacco cessation is urged for current tobacco users at all ages regardless of ASCVD risk, given the numerous adverse health consequences of smoking and strong relationship between tobacco use and ASCVD events.
In selected individuals at higher ASCVD risk, evidence-based approaches using medications including aspirin, blood pressure–lowering medications, cholesterol-lowering medications are appropriate in addition to tobacco cessation strategies among those who use tobacco. In general, the higher the absolute risk for ASCVD, the greater will be the absolute benefit from these medications, with a correspondingly greater net clinical benefit. Net clinical benefit can be defined as the potential for absolute risk reduction (events avoided) compared with the absolute potential for significant adverse events from taking a medication. It can be represented by a comparison of the number-needed-to-treat less the number-needed-to-harm for a given medication, which must be interpreted using absolute risks and expected risk reductions. The Million Hearts tool provides baseline assessments of 10-year ASCVD risk and projected risk reductions from institution of evidence-based medications that can be used as part of the guideline-recommended, patient-clinician discussion of risks and benefits from therapy.22 The tool also provides updated estimates of ASCVD risk based on individual patient response to therapies at follow up. Figure 1 with the patient scenario provides an example of how the Million Hearts Longitudinal ASCVD Risk Assessment Tool may be used to facilitate risk reduction in the clinical setting. The spreadsheet version of the tool is the original format, but is not the final user interface. An online version of this tool that was developed specifically for CMS and participating medical practices will be assessed in high-risk primary prevention Medicare patients as part of the Million Hearts Cardiovascular Risk Reduction Model. In addition, the ACC plans development of an app for mobile devices and an interactive web-based tool. Future work should assess the tool in other patient groups and clinical settings prior to its widespread application.
POTENTIAL LIMITATIONS
There are a number of potential limitations to the approach used to develop this tool. First, although the Pooled Cohort Equations are well calibrated for the broad US Medicare population of blacks and non-Hispanic whites,38 data are limited on their performance in other racial/ethnic groups. The 2013 ACC/AHA risk assessment guideline made weaker recommendations for these other groups, indicating that use of the equations for whites of the same sex seemed more appropriate than use of the equations for blacks, but that the non-Hispanic white equations would be expected to overestimate true risk in Hispanic-Americans (other than Puerto Ricans) and East Asian-Americans, and would be likely underestimate risk in Puerto Ricans, Native American, and South Asian-American groups.
Age is the most important correlate of ASCVD events, thus age is a major driver of the Pooled Cohort Equations risk. Therefore, even individuals with optimal levels of risk factors may have elevated 10-year risk estimates simply on the basis of their age. Chronological age represents time of exposure to risk factors; older people with optimal levels of all risk factors may, in fact, not have as high of a 10-year risk as the equations would predict. After age 65 years, the prevalence of all optimal risk factor levels, and 10-year risk estimates <5%, is low in the United States.28,45,68–70 This reality is unfortunate from a public health perspective, yet may reduce the effect of this potential limitation in our estimates. The significance of this concern is limited in the context of the proposed use of this tool for the Million Hearts Cardiovascular Risk Reduction Model, which will focus on very high-risk individuals (10-year predicted ASCVD risk >30%). Age alone cannot produce such high-risk estimates without significant concomitant risk factor burden.
The Pooled Cohort Equations provide a probability of disease; they do not provide prognostic certainty. They should be interpreted by a clinician or patient as in the following example. For a patient with specified risk factor levels, assume that the 10-year risk estimate for ASCVD is 20%. The correct interpretation of this result is not that an individual is at 20% risk, but rather that if there were 100 such individuals, by the end of 10 years, one would expect that 20 of them, or 1 in 5, would have a major coronary or stroke event. Whether the individual patient in question would be 1 of the 20 affected or 1 of the 80 unaffected during that time cannot be discerned from the risk estimate. However, as discussed in detail in the 2013 ACC/AHA cholesterol guidelines, there is evidence for net clinical benefit (ie, likelihood that more events will be prevented than adverse events caused) for statins down to as low as ~7.5% 10-year ASCVD risk for using high-intensity statins and even lower for using moderate-intensity statins. Cost-effectiveness data44 further support these treatment thresholds, which were based on event rates in the control groups of statin primary prevention trials.
The Million Hearts Tool provides an “updated” 10-year risk estimate that is based on both the baseline risk and the expected benefit from a preventive intervention (ie, aspirin, blood pressure–lowering therapy, statin, or tobacco cessation, or combinations thereof). We applied the best available evidence of effect size for each of the interventions from the medical literature. However, in doing so, we are applying a group mean effect of treatment to an individual patient scenario, with uncertainty in the revised estimate, because of, heterogeneity in response to treatment. We note that this issue is relevant to all evidence-based interventions in clinical practice.71 We have improved the confidence of the updated on-treatment risk estimate by scaling the risk reduction to the amount of change in SBP or LDL-cholesterol or duration of smoking cessation. It is likely that the group-level, that is, practice-level, estimates of changes in risk will be more accurate and precise than individual-based estimates of changes in risk. It is also likely that the imprecision of the updated risk estimates has limited impact in individuals at very high predicted risk. As the baseline 10-year risk is lower and approaches values <10%, the imprecision may be more meaningful.
The Million Hearts Tool focuses on the quantitative changes in risk that may occur with institution of drug therapies. The evidence base for these data is the clinical trials in which background advice for therapeutic lifestyle change has also been provided to participants. Whereas lifestyle improvement is a critical feature of ASCVD prevention, the complex interventions on diet, physical activity, weight and other factors that have been studied to date are heterogeneous and quantification of those results is beyond the scope of current efforts. Nonetheless, adopting therapeutic lifestyle change is likely beneficial largely through its impact on risk factors such as lipids and blood pressure, which are represented in the updated risk estimates provided by the tool.
During iterative testing and evaluation by an external group, concerns were raised regarding the amount of risk reduction associated with lowering of SBP. In multiple sensitivity scenarios, patients who achieve large amounts of SBP reduction (especially if >20 mm Hg) have substantial reductions in risk and frequently reach the floor value given that there is an additional risk reduction of 35% for each 10 mm Hg lowering of SBP. Accordingly, we performed a further systematic review and reexamined relevant data72–77 and also examined recent evidence synthesis analyses in the context of the recent SPRINT (Systolic Blood Pressure Intervention Trial) and HOPE-3 trials.14,15 Discussion was also undertaken to understand the potential contribution of regression-dilution bias to large risk reductions. After deliberation, we continued the current framework, with floor values for the amount of absolute risk reduction, and no additional reduction for SBP lowering <130 mm Hg for those with diabetes mellitus or <120 mm Hg for those without diabetes mellitus.
Finally, we have considered whether there are floor effects to preventive therapies and ceiling effects after adverse changes in risk factor levels. Extensive prior research has defined an optimal risk profile that is associated with extremely low lifetime risks for development of ASCVD in middle-aged and older individuals.68,78,79 During our testing of the tool, we have demonstrated that it is possible that individuals with elevated predicted 10-year risks for whom we estimate updated predicted risks after institution of preventive therapy could have updated estimated risks that are lower than risk estimates for those with a truly optimal lifelong risk factor profile. This estimated outcome seems unlikely; published data from the National Heart, Lung, and Blood Institute–funded CARDIA and MESA (Multi-Ethnic Study of Atherosclerosis) cohorts demonstrate that treatment of elevated risk factor levels (eg, blood pressure) to optimal levels does not reduce risk to the same level as someone who always had optimal levels.47 Therefore, we have included floor effects for the projected or updated ASCVD risk estimates. Likewise, for instances in which patients return at a follow-up visit with elevations (rather than improvements) in SBP or LDL-cholesterol compared with their baseline values, or with incident diabetes mellitus or cigarette smoking in the interim, we included ceiling effects in the updated risk estimates to avoid overestimation of risk in these scenarios.
CONCLUSIONS AND IMPLICATIONS
The Million Hearts initiative is designed to reduce the burden of ASCVD in the United States and aims to prevent 1 million heart attacks and strokes by 2017. A key focus of this initiative is implementation of the ABCS in appropriate individuals at elevated risk. Substantial observational data support the current paradigm in primary prevention of ASCVD that the intensity of prevention efforts should match the absolute risk of the patient, with drug therapy reserved for those at higher predicted risk, in whom net clinical benefit will be greater.
In concert with Million Hearts, CMMI aims to assess whether incentivizing physicians to reduce ASCVD risk among Medicare beneficiaries at high predicted risk will result in lower rates of heart attacks and strokes. To support the proposed Million Hearts Cardiovascular Risk Reduction Model testing this hypothesis, we have developed the Longitudinal ASCVD Risk Assessment Tool. The tool provides a baseline 10-year ASCVD risk estimate for black and non-Hispanic white men and women. The tool also provides projected values of risk reduction that would be associated with institution of ABCS therapies alone or in combination; these estimates are based on the best available evidence from formal, high-quality systematic reviews and meta-analyses. The projected risk reductions that are presented by the tool are based on average responses to the therapies, and are intended to guide decision-making around what preventive therapies to pursue (in the context of therapeutic lifestyle change) while considering net clinical benefit in the course of the guideline-recommended, patient-clinician discussion. Finally, the tool also provides updated 10-year ASCVD risk estimates, to be calculated at a follow-up visit, which represents a more personalized updated risk estimate that reflects the actual response of a given patient, incorporating their individual changes in risk factor levels. This approach to personalized estimation of benefits from risk-reducing therapies may represent the next wave in clinical practice to help target therapies to those in whom they will provide the greatest benefit.
The Million Hearts Tool has been developed to assist clinicians and patients to understand risk, to monitor patients’ risks over time, and to quantify potential benefits of preventive therapies based on high-quality evidence. It can also assist CMMI and clinical practices in monitoring risk in patient cohorts over time. Whereas it requires further assessment and validation in diverse clinical populations and scenarios, its implementation in the Million Hearts Cardiovascular Risk Reduction Model proposed by CMMI for high-risk Medicare patients will be an important scientific investigation of the current paradigm for ASCVD prevention.
Writing Group Disclosures
| Writing Group Member |
Employment | Research Grant | Other Research Support |
Speakers’ Bureau/ Honoraria |
Expert Witness |
Ownership Interest |
Consultant/ Advisory Board |
Other |
|---|---|---|---|---|---|---|---|---|
| Donald M. Lloyd-Jones | Northwestern University | Center for Medicare and Medicaid Innovation* | None | None | None | None | None | None |
| David C. Goff, Jr. | Colorado School of Public Health | None | None | None | None | None | None | None |
| Martha Gulati | University of Arizona | None | None | None | None | None | None | None |
| Mark D. Huffman | Northwestern University Preventive Medicine | National Cancer Institute†; NHLBI†; National Institute of Biomedical Imaging and Bioengineering*; Center for Medicare and Medicaid Innovation/The MITRE Corporation†; JR Alberts Foundation (FoodSwitch USA)*; World Heart Federation† | None | None | None | None | None | None |
| Kunal N. Karmali | Northwestern University Preventive Medicine | None | None | None | None | None | None | None |
| Frederick A. Masoudi | University of Colorado & Colorado Cardiovascular Outcomes Consortium | None | None | None | None | None | None | None |
| Colleen Pelser | The MITRE Corp. | None | None | None | None | None | None | None |
| Darshak M. Sanghavi | Centers for Medicare and Medicaid Services | None | None | None | None | None | None | None |
| Janet S. Wright | CDC/CMMI Million Hearts CMS Innovations Center | None | None | None | None | None | None | None |
This table represents the relationships of writing group members that may be perceived as actual or reasonably perceived conflicts of interest as reported on the Disclosure Questionnaire, which all members of the writing group are required to complete and submit. A relationship is considered to be “significant” if (a) the person receives $10,000 or more during any 12-month period, or 5% or more of the person’s gross income; or (b) the person owns 5% or more of the voting stock or share of the entity, or owns $10,000 or more of the fair market value of the entity. A relationship is considered to be “modest” if it is less than “significant” under the preceding definition.
Modest.
Significant.
Reviewer Disclosures
| Reviewer | Employment | Research Grant |
Other Research Support |
Speakers’ Bureau/ Honoraria |
Expert Witness |
Ownership Interest |
Consultant/ Advisory Board |
Other |
|---|---|---|---|---|---|---|---|---|
| Roger S. Blumenthal | Johns Hopkins University | None | None | None | None | None | None | None |
| Karen L. Furie | Rhode Island Hospital | None | None | None | None | None | None | None |
| Neil J. Stone | Northwestern University | None | None | None | None | None | None | None |
| Salim S. Virani | VA Medical Center Health Services Research and Development Center for Innovations, Baylor College of Medicine | None | None | None | None | None | None | None |
This table represents the relationships of reviewers that may be perceived as actual or reasonably perceived conflicts of interest as reported on the Disclosure Questionnaire, which all reviewers are required to complete and submit. A relationship is considered to be “significant” if (a) the person receives $10,000 or more during any 12-month period, or 5% or more of the person’s gross income; or (b) the person owns 5% or more of the voting stock or share of the entity, or owns $10,000 or more of the fair market value of the entity. A relationship is considered to be “modest” if it is less than “significant” under the preceding definition.
Footnotes
The American Heart Association and the American College of Cardiology make every effort to avoid any actual or potential conflicts of interest that may arise as a result of an outside relationship or a personal, professional, or business interest of a member of the writing panel. Specifically, all members of the writing group are required to complete and submit a Disclosure Questionnaire showing all such relationships that might be perceived as real or potential conflicts of interest.
This document was approved by the American Heart Association Science Advisory and Coordinating Committee on September 30, 2016, and the American Heart Association Executive Committee on October 17, 2016, and by the American College of Cardiology Board of Trustees on October 7, 2016.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jimenez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER, 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB on behalf of the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2016 update: a report from the American Heart Association. Circulation. 2016;133:e38–e360. doi: 10.1161/CIR.0000000000000350. [published correction appears in Circulation 2016;133:e599] [DOI] [PubMed] [Google Scholar]
- 2.Weintraub WS, Daniels SR, Burke LE, Franklin BA, Goff DC, Jr, Hayman LL, Lloyd-Jones D, Pandey DK, Sanchez EJ, Schram AP, Whitsel LP on behalf of the American Heart Association Advocacy Coordinating Committee; Council on Cardiovascular Disease in the Young; Council on the Kidney in Cardiovascular Disease; Council on Epidemiology and Prevention; Council on Cardiovascular Nursing; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Clinical Cardiology; and Stroke Council. Value of primordial and primary prevention for cardiovascular disease: a policy statement from the American Heart Association. Circulation. 2011;124:967–990. doi: 10.1161/CIR.0b013e3182285a81. [DOI] [PubMed] [Google Scholar]
- 3.Frieden TR, Berwick DM. The “Million Hearts” initiative--preventing heart attacks and strokes. N Engl J Med. 2011;365:e27. doi: 10.1056/NEJMp1110421. [DOI] [PubMed] [Google Scholar]
- 4. [Accessed August 1, 2016];Million Hearts website. Available at: http://millionhearts.hhs.gov/
- 5.Sanghavi DM, Conway PH. Paying for prevention: a novel test of Medicare value-based payment for cardiovascular risk reduction. JAMA. 2015;314:123–124. doi: 10.1001/jama.2015.6681. [DOI] [PubMed] [Google Scholar]
- 6.Karmali KN, Lloyd-Jones DM, Berendsen MA, Goff DC, Jr, Sanghavi DM, Brown NC, Korenovska L, Huffman MD. Drugs for primary prevention of atherosclerotic cardiovascular disease: an overview of systematic reviews. JAMA Cardiol. 2016;1:341–349. doi: 10.1001/jamacardio.2016.0218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gillespie CD, Hurvitz KA. Prevalence of hypertension and controlled hypertension—United States, 2007–2010. MMWR Morb Mortal Weekly Rep. 2013;62:144–148. [PubMed] [Google Scholar]
- 8.Mercado C, DeSimone AK, Odom E, Gillespie C, Ayala C, Loustalot F. Prevalence of cholesterol treatment eligibility and medication use among adults—United States, 2005–2012. MMWR Morbid Mortal Weekly Rep. 2015;64:1305–1311. doi: 10.15585/mmwr.mm6447a1. [DOI] [PubMed] [Google Scholar]
- 9.Aspirin for the prevention of cardiovascular disease: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;150:396–404. doi: 10.7326/0003-4819-150-6-200903170-00008. [DOI] [PubMed] [Google Scholar]
- 10.Wolff T, Miller T, Ko S. Aspirin for the primary prevention of cardiovascular events: an update of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2009;150:405–410. doi: 10.7326/0003-4819-150-6-200903170-00009. [DOI] [PubMed] [Google Scholar]
- 11.Bibbins-Domingo K. Aspirin use for the primary prevention of cardiovascular disease and colorectal cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2016;164:836–845. doi: 10.7326/M16-0577. [DOI] [PubMed] [Google Scholar]
- 12.Neal B, Macmahon S, Chapman N Blood Pressure Lowering Treatment Trialists’ Collaboration. Effects of ACE inhibitors, calcium antagonists, and other blood-pressure-lowering drugs: results of prospectively designed overviews of randomised trials. Lancet. 2000;356:1955–1964. doi: 10.1016/s0140-6736(00)03307-9. [DOI] [PubMed] [Google Scholar]
- 13.Blood Pressure Lowering Treatment Trialists’ Collaboration. Sundström J, Arima H, Woodward M, Jackson R, Karmali K, Lloyd-Jones D, Baigent C, Emberson J, Rahimi K, MacMahon S, Patel A, Perkovic V, Turnbull F, Neal B. Blood pressure-lowering treatment based on cardiovascular risk: a meta-analysis of individual patient data. Lancet. 2014;384:591–598. doi: 10.1016/S0140-6736(14)61212-5. [DOI] [PubMed] [Google Scholar]
- 14.SPRINT Research Group. Wright JT, Jr, Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, Reboussin DM, Rahman M, Oparil S, Lewis CE, Kimmel PL, Johnson KC, Goff DC, Jr, Fine LJ, Cutler JA, Cushman WC, Cheung AK, Ambrosius WT. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103–2116. doi: 10.1056/NEJMoa1511939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lonn EM, Bosch J, López-Jaramillo P, Zhu J, Liu L, Pais P, Diaz R, Xavier D, Sliwa K, Dans A, Avezum A, Piegas LS, Keltai K, Keltai M, Chazova I, Peters RJG, Held C, Yusoff K, Lewis BS, Jansky P, Parkhomenko A, Khunti K, Toff WD, Reid CM, Varigos J, Leiter LA, Molina DI, McKelvie R, Pogue J, Wilkinson J, Jung H, Dagenais G, Yusuf S. Blood-pressure Lowering in intermediate-risk persons without cardiovascular disease. N Engl J Med. 2016;374:2009–2020. doi: 10.1056/NEJMoa1600175. [DOI] [PubMed] [Google Scholar]
- 16.Sundstrom J, Arima H, Jackson R, Turnbull F, Rahimi K, Chalmers J, Woodward M, Neal B Blood Pressure Lowering Treatment Trialists’ Collaboration. Effects of blood pressure reduction in mild hypertension: a systematic review and meta-analysis. Ann Intern Med. 2015;162:184–191. doi: 10.7326/M14-0773. [DOI] [PubMed] [Google Scholar]
- 17.Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, Pollicino C, Kirby A, Sourjina T, Peto R, Collins R, Simes R Cholesterol Treatment Trialists’ (CTT) Collaborators. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366:1267–1278. doi: 10.1016/S0140-6736(05)67394-1. [DOI] [PubMed] [Google Scholar]
- 18.Cholesterol Treatment Trialists’ (CTT) Collaborators. Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, Bhala N, Peto R, Barnes EH, Keech A, Simes J, Collins R. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376:1670–1681. doi: 10.1016/S0140-6736(10)61350-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cholesterol Treatment Trialists’ (CTT) Collaborators. Mihaylova B, Emberson J, Blackwell L, Keech A, Simes J, Barnes EH, Voysey M, Gray A, Collins R, Baigent C. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet. 2012;380:581–590. doi: 10.1016/S0140-6736(12)60367-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feinstein MJ, Jhund P, Kang J, Ning H, Maggioni A, Wikstrand J, Kjekshus J, Tavazzi L, McMurray J, Lloyd-Jones DM. Do statins reduce the risk of myocardial infarction in patients with heart failure? A pooled individual-level reanalysis of CORONA and GISSI-HF. Eur J Heart Fail. 2015;17:434–441. doi: 10.1002/ejhf.247. [DOI] [PubMed] [Google Scholar]
- 21.Cahill K, Stevens S, Perera R, Lancaster T. Pharmacological interventions for smoking cessation: an overview and network meta-analysis. Cochrane Database Syst Rev. 2013;(5):CD009329. doi: 10.1002/14651858.CD009329.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd-Jones DM, McBride P, Schwartz JS, Shero ST, Smith SC, Jr, Watson K, Wilson PWF. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines [published corrections appear in Circulation. 2014;129(suppl 2):S46–S48 and Circulation. 2015;132:e396] Circulation. 2014;129(suppl 2):S1–S45. doi: 10.1161/01.cir.0000437738.63853.7a. [DOI] [PubMed] [Google Scholar]
- 23.Shah NS, Huffman MD, Ning H, Lloyd-Jones DM. Trends in vascular risk factor treatment and control in US stroke survivors: the National Health and Nutrition Examination Surveys (1999–2010) Circ Cardiovasc Qual Outcomes. 2013;6:270–277. doi: 10.1161/CIRCOUTCOMES.113.000112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shah NS, Huffman MD, Ning H, Lloyd-Jones DM. Trends in myocardial infarction secondary prevention: The National Health and Nutrition Examination Surveys (NHANES), 1999–2012. J Am Heart Assoc. 2015;4:e001709. doi: 10.1161/JAHA.114.001709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matching the Intensity of Risk Factor Management with the Hazard for Coronary Disease Events. J Am Coll Cardiol; 27th Bethesda Conference; September 14–15, 1995; 1996. pp. 957–1047. [PubMed] [Google Scholar]
- 26.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 27.Grundy SM, Cleeman JI, Merz CN, Brewer HB, Jr, Clark LT, Hunninghake DB, Pasternak RC, Smith SC, Jr, Stone NJ National Heart, Lung, and Blood Institute; American College of Cardiology Foundation; American Heart Association. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2004;110:227–239. doi: 10.1161/01.CIR.0000133317.49796.0E. [DOI] [PubMed] [Google Scholar]
- 28.Goff DC, Jr, Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB, Sr, Gibbons R, Greenland P, Lackland DT, Levy D, O’Donnell CJ, Robinson JG, Schwartz JS, Shero ST, Smith SC, Jr, Sorlie P, Stone NJ, Wilson PWF. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines [published correction appears in Circulation. 2014;129(suppl 2):S74–S75] Circulation. 2014;129(suppl 2):S49–S73. doi: 10.1161/01.cir.0000437741.48606.98. [DOI] [PubMed] [Google Scholar]
- 29.Eddy DM, Adler J, Patterson B, Lucas D, Smith KA, Morris M. Individualized guidelines: the potential for increasing quality and reducing costs. Ann Intern Med. 2011;154:627–634. doi: 10.7326/0003-4819-154-9-201105030-00008. [DOI] [PubMed] [Google Scholar]
- 30.Hayward RA, Krumholz HM, Zulman DM, Timbie JW, Vijan S. Optimizing statin treatment for primary prevention of coronary artery disease. Ann Intern Med. 2010;152:69–77. doi: 10.7326/0003-4819-152-2-201001190-00004. [DOI] [PubMed] [Google Scholar]
- 31.Sussman J, Vijan S, Hayward R. Using benefit-based tailored treatment to improve the use of antihypertensive medications. Circulation. 2013;128:2309–2317. doi: 10.1161/CIRCULATIONAHA.113.002290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mortensen MB, Afzal S, Nordestgaard BG, Falk E. Primary prevention with statins: ACC/AHA risk-based approach versus trial-based approaches to guide statin therapy. J Am Coll Cardiol. 2015;66:2699–2709. doi: 10.1016/j.jacc.2015.09.089. [DOI] [PubMed] [Google Scholar]
- 33.Thanassoulis G, Williams K, Altobelli KK, Pencina MJ, Cannon CP, Sniderman AD. Individualized statin benefit for determining statin eligibility in the primary prevention of cardiovascular disease. Circulation. 2016;133:1574–1581. doi: 10.1161/CIRCULATIONAHA.115.018383. [DOI] [PubMed] [Google Scholar]
- 34.Cook NR, Ridker P. Further insight into the cardiovascular risk calculator: the roles of statins, revascularizations, and underascertainment in Thewomen’s health study. JAMA Intern Med. 2014;174:1964–1971. doi: 10.1001/jamainternmed.2014.5336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.DeFilippis AP, Young R, Carrubba CJ, McEvoy JW, Budoff MJ, Blumenthal RS, Kronmal RA, McClelland RL, Nasir K, Blaha MJ. An analysis of calibration and discrimination among multiple cardiovascular risk scores in a modern multiethnic cohort. Ann Intern Med. 2015;162:266–275. doi: 10.7326/M14-1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.DeFilippis AP, Young R, McEvoy JW, Michos ED, Sandfort V, Kronmal RA, McClelland RL, Blaha MJ. Risk score overestimation: the impact of individual cardiovascular risk factors and preventive therapies on the performance of the American Heart Association-American College of Cardiology-Atherosclerotic Cardiovascular Disease risk score in a modern multi-ethnic cohort. Eur Heart J. 2016 Jul 19; doi: 10.1093/eurheartj/ehw301. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Feinstein MJ, Nance RM, Drozd DR, Ning H, Delaney JA, Heckbert SR, Budoff MJ, Mathews WC, Kitahata MM, Saag MS, Eron JJ, Moore RD, Achenbach CJ, Lloyd-Jones DM, Crane HM. Assessing and refining myocardial infarction risk estimation among patients with human immunodeficiency virus. JAMA Cardiol. 2016 doi: 10.1001/jamacardio.2016.4494. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Muntner P, Colantonio LD, Cushman M, Goff DC, Howard G, Howard VJ, Kissela B, Levitan EB, Lloyd-Jones DM, Safford MM. Validation of the atherosclerotic cardiovascular disease pooled cohort risk equations. JAMA. 2014;311:1406–1415. doi: 10.1001/jama.2014.2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mortensen MB, Falk E. Real-life evaluation of European and American high-risk strategies for primary prevention of cardiovascular disease in patients with first myocardial infarction. BMJ Open. 2014;4:e005991. doi: 10.1136/bmjopen-2014-005991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Johnson KM, Dowe DA. Accuracy of statin assignment using the 2013 AHA/ACC cholesterol guideline versus the 2001 NCEP ATP III guideline: Correlation with atherosclerotic plaque imaging. J Am Coll Cardiol. 2014;64:910–919. doi: 10.1016/j.jacc.2014.05.056. [DOI] [PubMed] [Google Scholar]
- 41.Pursnani A, Massaro JM, D’Agostino RB, Sr, O’Donnell CJ, Hoffmann U. Guideline-based statin eligibility, coronary artery calcification, and cardiovascular events. JAMA. 2015;314:134–141. doi: 10.1001/jama.2015.7515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cho YK, Jung CH, Kang YM, Hwang JY, Kim EH, Yang DH, Kang JW, Park JY, Kim HK, Lee WJ. 2013 ACC/AHA Cholesterol Guideline Versus 2004 NCEP ATP III Guideline in the prediction of coronary artery calcification progression in a Korean population. J Am Heart Assoc. 2016;5:e003410. doi: 10.1161/JAHA.116.003410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Paixao AR, Ayers CR, Berry JD, de Lemos JA, Khera A. Atherosclerotic cardiovascular disease prevention: a comparison between the third adult treatment panel and the new 2013 Treatment of Blood Cholesterol Guidelines. Circ Cardiovasc Qual Outcomes. 2014;7:778–779. doi: 10.1161/CIRCOUTCOMES.114.001139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pandya A, Sy S, Cho S, Weinstein MC, Gaziano TA. Cost-effectiveness of 10-year risk thresholds for initiation of statin therapy for primary prevention of cardiovascular disease. JAMA. 2015;314:142–150. doi: 10.1001/jama.2015.6822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pencina MJ, Navar-Boggan AM, D’Agostino RB, Sr, Williams K, Neely B, Sniderman AD, Peterson ED. Application of new cholesterol guidelines to a population-based sample. N Engl J Med. 2014;370:1422–1431. doi: 10.1056/NEJMoa1315665. [DOI] [PubMed] [Google Scholar]
- 46.Egan BM, Li J, White K, Fleming DO, Connell K, Hernandez GT, Jones DW, Ferdinand KC, Sinopoli A. 2013 ACC/AHA Cholesterol Guideline and Implications for Healthy People 2020 Cardiovascular Disease Prevention Goals. J Am Heart Assoc. 2016;5:e003558. doi: 10.1161/JAHA.116.003558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu K, Colangelo LA, Daviglus ML, Goff DC, Pletcher M, Schreiner PJ, Sibley CT, Burke GL, Post WS, Michos ED, Lloyd-Jones DM. Can antihypertensive treatment restore the risk of cardiovascular disease to ideal levels?: The Coronary Artery Risk Development in Young Adults (CARDIA) Study and the Multi-Ethnic Study of Atherosclerosis (MESA) J Am Heart Assoc. 2015;4:e002275. doi: 10.1161/JAHA.115.002275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.QIntervention. [Accessed August 1, 2016];2014 Available at: http://qintervention.org.
- 49.Gaede P, Lund-Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med. 2008;358:580–591. doi: 10.1056/NEJMoa0706245. [DOI] [PubMed] [Google Scholar]
- 50.Gaede P, Vedel P, Larsen N, Jensen GV, Parving HH, Pedersen O. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med. 2003;348:383–393. doi: 10.1056/NEJMoa021778. [DOI] [PubMed] [Google Scholar]
- 51.Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M Medical Research Council Guidance. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ. 2008;337:a1655. doi: 10.1136/bmj.a1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sever P, Dahlof B, Poulter N, Wedel H, Beevers G, Caulfield M, Collins R, Kjeldsen S, Kristinsson A, McInnes G, Mehlsen J, Nieminem M, O’Brien E, Ostergren J ASCOT Steering Committee Members. Potential synergy between lipid-lowering and blood-pressure-lowering in the Anglo-Scandinavian Cardiac Outcomes Trial. Eur Heart J. 2006;27:2982–2988. doi: 10.1093/eurheartj/ehl403. [DOI] [PubMed] [Google Scholar]
- 53.de Cates AN, Farr MR, Wright N, Jarvis MC, Rees K, Ebrahim S, Huffman MD. Fixed-dose combination therapy for the prevention of cardiovascular disease. Cochrane Database Syst Rev. 2014;(4):CD009868. doi: 10.1002/14651858.CD009868.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cochrane Handbook for Systematic Reviews of Interventions, Version 5.1.0 (updated March 2011) The Cochrane Collaboration; 2011. [Google Scholar]
- 55.Lloyd-Jones DM, Morris PB, Ballantyne CM, Birtcher KK, Daly DD, Jr, DePalma SM, Minissian MB, Orringer CE, Smith SC., Jr 2016 ACC expert consensus decision pathway on the role of non-statin therapies for LDL-cholesterol lowering in the management of atherosclerotic cardiovascular disease risk: a report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2016;68:92–125. doi: 10.1016/j.jacc.2016.03.519. [DOI] [PubMed] [Google Scholar]
- 56.Shea BJ, Grimshaw JM, Wells GA, Boers M, Andersson N, Hamel C, Porter AC, Tugwell P, Moher D, Bouter LM. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol. 2007;7:10. doi: 10.1186/1471-2288-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schunemann HJ GRADE Working Group. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee PN, Fry JS, Hamling JS. Using the negative exponential distribution to quantitatively review the evidence on how rapidly the excess risk of ischaemic heart disease declines following quitting smoking. Regul Toxicol Pharmacol. 2012;64:51–67. doi: 10.1016/j.yrtph.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 59.Lee PN, Fry JS, Thornton AJ. Estimating the decline in excess risk of cerebrovascular disease following quitting smoking—a systematic review based on the negative exponential model. Regul Toxicol Pharmacol. 2014;68:85–95. doi: 10.1016/j.yrtph.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 60.Eckel RH, Jakicic JM, Ard JD, de Jesus JM, Houston Miller N, Hubbard VS, Lee I-M, Lichtenstein AH, Loria CM, Millen BE, Nonas CA, Sacks FM, Smith SC, Jr, Svetkey LP, Wadden TA, Yanovski SZ. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines [published corrections appear in Circulation. 2014;129(suppl 2):S100–S101 and Circulation. 2015;131:e326] Circulation. 2014;129(suppl 2):S76–S99. doi: 10.1161/01.cir.0000437740.48606.d1. [DOI] [PubMed] [Google Scholar]
- 61.Asselbergs FW, Diercks GF, Hillege HL, van Boven AJ, Janssen WM, Voors AA, de Zeeuw D, de Jong PE, van Veldhuisen DJ, van Gilst WH Prevention of Renal and Vascular Endstage Disease Intervention Trial (PREVEND IT) Investigators. Effects of fosinopril and pravastatin on cardiovascular events in subjects with microalbuminuria. Circulation. 2004;110:2809–2816. doi: 10.1161/01.CIR.0000146378.65439.7A. [DOI] [PubMed] [Google Scholar]
- 62.Hansson L, Zanchetti A, Carruthers SG, Dahlof B, Elmfeldt D, Julius S, Menard J, Rahn KH, Wedel H, Westerling S. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. HOT Study Group. Lancet. 1998;351:1755–1762. doi: 10.1016/s0140-6736(98)04311-6. [DOI] [PubMed] [Google Scholar]
- 63.Hedblad B, Wikstrand J, Janzon L, Wedel H, Berglund G. Low-dose metoprolol CR/XL and fluvastatin slow progression of carotid intima-media thickness: Main results from the Beta-Blocker Cholesterol-Lowering Asymptomatic Plaque Study (BCAPS) Circulation. 2001;103:1721–1726. doi: 10.1161/01.cir.103.13.1721. [DOI] [PubMed] [Google Scholar]
- 64.Zanchetti A, Crepaldi G, Bond MG, Gallus G, Veglia F, Mancia G, Ventura A, Baggio G, Sampieri L, Rubba P, Sperti G, Magni A PHYLLIS Investigators. Different effects of antihypertensive regimens based on fosinopril or hydrochlorothiazide with or without lipid lowering by pravastatin on progression of asymptomatic carotid atherosclerosis: principal results of PHYLLIS—a randomized double-blind trial. Stroke. 2004;35:2807–2812. doi: 10.1161/01.STR.0000147041.00840.59. [DOI] [PubMed] [Google Scholar]
- 65.Estruch R, Ros E, Salas-Salvadó J, Covas M-I, Corella D, Arós F, Gómez-Gracia E, Ruiz-Gutiérrez V, Fiol M, Lapetra J, Lamuela-Raventos RM, Serra-Majem L, Pintó X, Basora J, Muñoz MA, Sorlí JV, Martínez JA, Martínez-González MA. Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med. 2013;368:1279–1290. doi: 10.1056/NEJMc1806491. [DOI] [PubMed] [Google Scholar]
- 66.2008 physical activity guidelines for Americans. Washington, DC: US Department of Health and Human Services; 2008. [Google Scholar]
- 67.Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, Hu FB, Hubbard VS, Jakicic JM, Kushner RF, Loria CM, Millen BE, Nonas CA, Pi-Sunyer FX, Stevens J, Stevens VJ, Wadden TA, Wolfe BM, Yanovski SZ. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/ American Heart Association Task Force on Practice Guidelines and The Obesity Society [published correction appears in Circulation. 2014;129(suppl 2):S139–S140] Circulation. 2014;129(suppl 2):S102–S138. doi: 10.1161/01.cir.0000437739.71477.ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF, Arnett DK, Fonarow GC, Ho PM, Lauer MS, Masoudi FA, Robertson RM, Roger V, Schwamm LH, Sorlie P, Yancy CW, Rosamond WD on behalf of the American Heart Association Strategic Planning Task Force and Statistics Committee. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association’s strategic Impact Goal through 2020 and beyond. Circulation. 2010;121:586–613. doi: 10.1161/CIRCULATIONAHA.109.192703. [DOI] [PubMed] [Google Scholar]
- 69.Marma AK, Berry JD, Ning H, Persell SD, Lloyd-Jones DM. Distribution of 10-year and lifetime predicted risks for cardiovascular disease in US adults: findings from the National Health and Nutrition Examination Survey 2003 to 2006. Circ Cardiovasc Qual Outcomes. 2010;3:8–14. doi: 10.1161/CIRCOUTCOMES.109.869727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shay CM, Ning H, Allen NB, Carnethon MR, Chiuve SE, Greenlund KJ, Daviglus ML, Lloyd-Jones DM. Status of cardiovascular health in US adults: prevalence estimates from the National Health and Nutrition Examination Surveys (NHANES) 2003–2008. Circulation. 2012;125:45–56. doi: 10.1161/CIRCULATIONAHA.111.035733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Smith GD. Epidemiology, epigenetics and the ‘Gloomy Prospect’: embracing randomness in population health research and practice. Int J Epidemiol. 2011;40:537–562. doi: 10.1093/ije/dyr117. [DOI] [PubMed] [Google Scholar]
- 72.Ettehad D, Emdin CA, Kiran A, Anderson SG, Callender T, Emberson J, Chalmers J, Rodgers A, Rahimi K. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet. 2016;387:957–67. doi: 10.1016/S0140-6736(15)01225-8. [DOI] [PubMed] [Google Scholar]
- 73.Lv J, Neal B, Ehteshami P, Ninomiya T, Woodward M, Rodgers A, Wang H, MacMahon S, Turnbull F, Hillis G, Chalmers J, Perkovic V. Effects of intensive blood pressure lowering on cardiovascular and renal outcomes: a systematic review and meta-analysis. PLoS Med. 2012;9:e1001293. doi: 10.1371/journal.pmed.1001293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Thomopoulos C, Parati G, Zanchetti A. Effects of blood pressure lowering on outcome incidence in hypertension: 3. Effects in patients at different levels of cardiovascular risk--overview and meta-analyses of randomized trials. J Hypertens. 2014;32:2305–2314. doi: 10.1097/HJH.0000000000000380. [DOI] [PubMed] [Google Scholar]
- 75.Thomopoulos C, Parati G, Zanchetti A. Effects of blood pressure lowering on outcome incidence in hypertension. 1. Overview, meta-analyses, and meta-regression analyses of randomized trials. J Hypertens. 2014;32:2285–2295. doi: 10.1097/HJH.0000000000000378. [DOI] [PubMed] [Google Scholar]
- 76.Thomopoulos C, Parati G, Zanchetti A. Effects of blood pressure lowering on outcome incidence in hypertension: 7. Effects of more vs. less intensive blood pressure lowering and different achieved blood pressure levels - updated overview and meta-analyses of randomized trials. J Hypertens. 2016;34:613–622. doi: 10.1097/HJH.0000000000000881. [DOI] [PubMed] [Google Scholar]
- 77.Xie X, Atkins E, Lv J, Bennett A, Neal B, Ninomiya T, Woodward M, MacMahon S, Turnbull F, Hillis GS, Chalmers J, Mant J, Salam A, Rahimi K, Perkovic V, Rodgers A. Effects of intensive blood pressure lowering on cardiovascular and renal outcomes: updated systematic review and meta-analysis. Lancet. 2016;387:435–443. doi: 10.1016/S0140-6736(15)00805-3. [DOI] [PubMed] [Google Scholar]
- 78.Lloyd-Jones DM, Dyer AR, Wang R, Daviglus ML, Greenland P. Risk factor burden in middle age and lifetime risks for cardiovascular and non-cardiovascular death (Chicago Heart Association Detection Project in Industry) Am J Cardiol. 2007;99:535–540. doi: 10.1016/j.amjcard.2006.09.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lloyd-Jones DM, Leip EP, Larson MG, D’Agostino RB, Beiser A, Wilson PW, Wolf PA, Levy D. Prediction of lifetime risk for cardiovascular disease by risk factor burden at 50 years of age. Circulation. 2006;113:791–798. doi: 10.1161/CIRCULATIONAHA.105.548206. [DOI] [PubMed] [Google Scholar]