Abstract
Introduction
Hepatocellular carcinoma (HCC) has a close relationship with lipid metabolism. Peroxisome proliferator-activated receptor α (PPARα) plays a crucial role in the regulation of fatty acid oxidation in the liver. However, the role of PPARα in HCC remains unclear.
Methods
A total of 804 HCC specimens were collected to construct a tissue microarray and for immunohistochemical analysis. The relationship between PPARα expression and clinical features of HCC patients was analyzed. Kaplan–Meier analysis was conducted to assess the prognostic value of PPARα expression levels.
Results
The expression of PPARα in HCC was noticeably decreased in HCC tissues. HCC patients with high levels of PPARα expression in cytoplasm had smaller tumors (P=0.027), less vascular invasion (P=0.049), and a higher proportion of complete involucrum (P=0.038). Kaplan–Meier analysis showed that HCC patients with low PPARα expression in the cytoplasm had significantly worse outcomes in terms of overall survival (P<0.001), disease-free survival (P=0.024), and the probability of recurrence (P=0.037). Similarly, overall survival was significantly shorter in HCC patients with negative PPARα expression in the nucleus (P=0.034). Multivariate Cox analyses indicated that tumor size (P=0.001), TNM stage (P<0.001), vascular invasion (P<0.001), and PPARα expression in the cytoplasm (P<0.001) were found to be independent prognostic variables for overall survival.
Conclusion
Our data revealed that PPARα expression was decreased in HCC samples. High PPARα expression was correlated with longer survival times in HCC patients, and served as an independent factor for better outcomes. Our study therefore provides a promising biomarker for prognostic prediction and a potential therapeutic target for HCC.
Keywords: peroxisome proliferator-activated receptors α, lipid metabolism, hepatocellular carcinoma, prognostic biomarker
Introduction
Hepatocellular carcinoma (HCC) is the third most frequent cause of cancer death worldwide.1 Due to the extensive heterogeneity in clinical presentation and tumor biology, the classification for HCC therapy is complicated.2,3 One of the heterogeneities of HCC is tumor metabolic reprogramming. Although most tumors share common metabolic transformations like aerobic glycolysis,4 the metabolic phenotypes of cancer cells are highly diverse because of the tumor microenvironment. For example, primary ovarian cancer cells have highly activated lipogenesis in order to supply the lipid required for uncontrolled cell proliferation. However, when ovarian cancer metastasizes to omental fat that contains a microenvironment abundant in adipocytes, the cancer cells are metabolically reprogrammed to favor more lipid oxidation using adipocyte-derived fatty acids.5 In HCC, due to the high rate of nutrient consumption and lack of vasculature, HCC cells frequently experience a stressful metabolic microenvironment, which is characterized by oxygen and nutrient deficiency.6 There may therefore be adaptive metabolic reprogramming that develops within HCC cells that allows them to cope with the stressful metabolic microenvironment. A study conducted by Wang et al revealed that HCC cells display distinct lipid levels, which are positively correlated with HCC cell survival in stressful metabolic microenvironments.7 In addition, acetyl-coenzyme A carboxylase alpha plays an important role in HCC cells by promoting fatty acid synthesis and thereby increasing the lipid content of these cells.
Another important approach to increasing the lipid content in HCC cells, in addition to increasing fatty acid synthesis, is to inhibit fatty acid oxidation. Previous studies have confirmed that peroxisome proliferator-activated receptors (PPARs) play a crucial role in regulating fatty acid oxidation and the upstream rate-limiting enzyme of fatty acid oxidation, carnitine palmitoyltransferase 1.8 Despite similar structures, the three PPAR isotypes α, β, and γ vary greatly in their tissue distribution, pharmacology, type of endogenous ligand, and biological effects. Especially in the liver, PPARα acts as a master regulator of liver metabolism. PPARα-regulated processes are thought to be involved in all liver diseases.9 Owing to the importance of PPARα in metabolism, many studies have examined the role of PPARα in tumorigenesis, with some studies implicating it in the promotion and development of cancer, with others presenting evidence for an anti-tumorigenic role.10 PPARα activation increases proliferation in breast cancer cell lines and in a renal cell carcinoma cell line,11,12 and its persistent activation causes liver cancer in rodents, whereas PPARα null mice have been shown to be resistant to the hepatocarcinogenic effects of PPARα agonists.13 Despite these findings, the role of PPARα as either a tumor suppressor or inducer in HCC remains unclear, and may differ in different species.
In this study, we investigated the role of PPARα in human HCC. We showed that PPARα expression was positively correlated with overall survival in patients with HCC, and therefore it provides a promising biomarker for prognostic prediction and is a potential therapeutic target for the clinical management of HCC.
Subjects and methods
Subjects
A total of 804 paraffin-embedded HCC specimens collected between January 2000 and December 2010 were obtained from the archives of the Department of Pathology of Sun Yat-sen University Cancer Center. None of the patients received any chemotherapy or radiotherapy prior to surgery. The follow-up period was defined as the interval from the date of surgery to the date of death or the last follow-up. This study was approved by the Institute Research Medical Ethics Committee of Sun Yat-sen University Cancer Center. All tissues were anonymous and the requirement of obtaining informed consent was waived by the Institute Research Medical Ethics Committee of Sun Yat-sen University Cancer Center.
Tissue microarray (TMA) construction and immunohistochemistry (IHC)
The TMA slides included 804 HCC tissues along with their adjacent normal tissues. Using a tissue array instrument (Minicore; Excilone, Elancourt, France), each tissue core was punched (diameter: 0.6 mm) from the marked areas and re-embedded. All specimens were fixed in 4% paraformaldehyde in 0.1 M phosphate buffer for 24 hours and embedded in paraffin wax. Following this, the paraffin-embedded HCC sections were sliced into 4-μm slices and mounted onto glass slides. After dewaxing, the slides were treated with 3% hydrogen peroxide in methanol and blocked using a biotin blocking kit (Dako Denmark A/S, Glostrup, Denmark). After blocking, the slides were incubated with a PPARα antibody (ab8934, 1:1000; Abcam, Cambridge, UK) overnight in a moist chamber at 4°C. After washing three times in PBS, the slides were incubated with biotinylated goat anti-mouse antibodies for 1 hour. The slides were then stained with 3,3′-diaminobenzidine tetrahydrochloride. Finally, the slides were counterstained with Mayer’s hematoxylin and observed under a microscope.
The level of PPARα protein expression was determined by semiquantitative IHC detection. Intensity was scored according to the standard: “0” (negative staining); “1” (weak staining); “2” (moderate staining); and “3” (strong staining). The final score was calculated by multiplying the percentage of positive expression by the intensity score. The scores were independently determined by two pathologists. The median IHC score was chosen as the cut off value for defining high and low expression.
Statistical analysis
Statistical analysis was performed using SPSS (version 16.0; SPSS Inc, Chicago, IL, USA). Student’s t-test and Pearson’s χ2 test, or Fisher’s exact test, were chosen for examining the correlations between PPARα expression level and the clinical and pathological variables. Survival curves were constructed using the Kaplan–Meier method (log-rank test). A multivariate Cox proportional hazards regression model was used to evaluate the independence of PPARα in predicting outcomes. Differences were defined as significant for P-values <0.05.
Results
Expression of PPARα in HCC TMA tissues
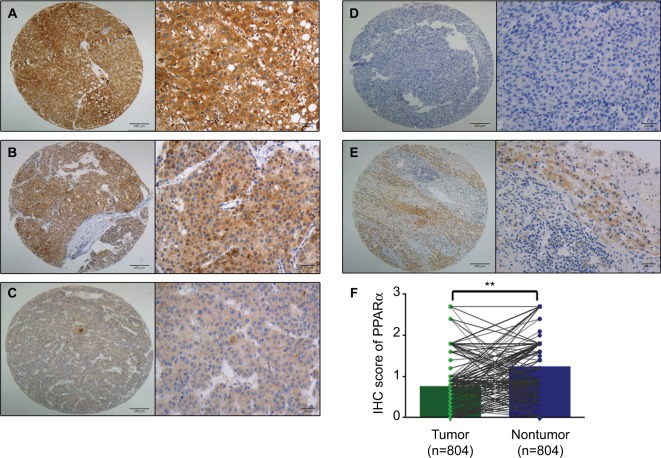
First, paraffin-embedded HCC tissues were collected to detect PPARα expression (n=804). PPARα was mainly expressed in the cytoplasm of HCC liver cancer cells, with only a small fraction of cells showing expression in the nucleus. The PPARα IHC score for HCC tissue was 0.74±0.61, being significantly lower than normal liver tissue which had a score of 1.22±0.65 (P<0.001, Figure 1).
Figure 1.
PPARα is mainly expressed in the cytoplasm with only a few tissues showing nuclear expression.
Notes: Representative images of PPARα (cytoplasm) expression in HCC tissues with strong (A), moderate (B), weak (C), and negative (D) expression are shown. Representative images of PPARα expression in a nontumor sample are also shown (E) (left panel: magnification ×100; right panel: magnification ×400). PPARα expression is decreased in HCC tissues compared with the corresponding nontumor tissue as assessed by IHC (**P<0.001) (F).
Abbreviations: PPARα, peroxisome proliferator-activated receptor α; HCC, hepatocellular carcinoma; IHC, immunohistochemistry.
Association of cytoplasmic PPARα with HCC clinical features
To determine the potential clinical significance of PPARα in HCC, the relationship between PPARα and the clinical features of HCC patients was evaluated. Using the median IHC score in the tumor tissue (IHC score 0.9), a high level of PPARα expression was found in 15.3% (123/804) of cases. HCC patients with high levels of PPARα expression had smaller tumor sizes (P=0.027), less vascular invasion (P=0.049), and a higher proportion of complete involucrum (P=0.038), as shown in Table 1. Since HCC is a male-dominant liver disease, we also compared the expression of PPARα in male and female. However, we did not observe a significant difference of PPARα expression between male and female (0.75±0.62 vs 0.69±0.56, P=0.42).
Table 1.
Association between PPARα expression in the cytoplasm and the clinical features of hepatocellular carcinoma
| Variable | PPARa in cytoplasm
|
P-value | |
|---|---|---|---|
| High expression | Low expression | ||
| Sample size | 123 | 681 | |
| Age, years | 50.60±11.76 | 48.56±11.94 | 0.081 |
| Gender | 0.498 | ||
| Male | 111 (13.8%) | 600 (74.6%) | |
| Female | 12 (1.5%) | 81 (10.1%) | |
| HBsAg | 0.693 | ||
| Positive | 104 (12.9%) | 566 (70.4%) | |
| Negative | 19 (2.4%) | 115 (14.3%) | |
| AFP, ng/mL | 0.394 | ||
| <20 | 31 (3.9%) | 148 (18.4%) | |
| ≥20 | 92 (11.4%) | 533 (66.3%) | |
| Cirrhosis | 0.619 | ||
| Yes | 102 (12.7%) | 551 (68.6%) | |
| No | 21 (2.6%) | 129 (16.1%) | |
| Tumor size, cm | 0.027 | ||
| <5 | 40 (4.9%) | 158 (19.7%) | |
| ≥5 | 83 (10.3%) | 523 (65.1%) | |
| Tumor multiplicity | 0.927 | ||
| Single | 81 (10.1%) | 451 (56.1%) | |
| Multiple | 42 (5.2%) | 230 (28.6%) | |
| Differentiation | 0.390 | ||
| Well-moderate | 13 (1.6%) | 56 (6.9%) | |
| Poor-undifferentiated | 110 (13.7%) | 625 (77.8%) | |
| TNM stage | 0.790 | ||
| I–II | 50 (6.2%) | 286 (35.6%) | |
| III–IV | 73 (9.1%) | 395 (49.1%) | |
| Vascular invasion | 0.049 | ||
| Yes | 108 (13.5%) | 546 (67.9%) | |
| No | 15 (1.9%) | 134 (16.7%) | |
| Involucrum | 0.038 | ||
| Complete | 62 (7.7%) | 274 (34.2%) | |
| Incomplete | 61 (7.6%) | 405 (50.5%) | |
| Lymph node metastasis | 0.097 | ||
| Positive | 3 (0.4%) | 42 (5.2%) | |
| Negative | 120 (14.9%) | 638 (79.5%) | |
| Distant metastasis | 0.050 | ||
| Positive | 6 (0.8%) | 72 (9.0%) | |
| Negative | 116 (14.5%) | 604 (75.7%) | |
Abbreviations: PPARα, peroxisome proliferator-activated receptor α; HBsAg, hepatitis B virus surface antigen; AFP, α-fetoprotein.
Association of PPARα expression in the nucleus and clinical features in HCC
There were only 24 tissues among the 804 samples that showed positive PPARα expression in the nucleus. Accordingly, we also compared tissues with negative and positive PPARα expression in the nucleus. As shown in Table 2, data showed that HCC patients with negative PPARα expression in the nucleus often had larger tumors (P=0.014). Among the HCC patients with positive PPARα expression in the nucleus, a significantly lower proportion had poor undifferentiated tumors (P<0.001), were TNM stage III–IV (P=0.036) and had an incomplete involucrum (P=0.004). We also compared the PPARα between virus-induced HCC and non-virus-induced HCC. We found no difference of PPARα expression between them either in the cytoplasm (0.71±0.55 vs 0.75±0.63, P=0.47) or in the nuclei (0.01±0.08 vs 0.01±0.07, P=0.53).
Table 2.
Association between PPARα expression in the nucleus and the clinical features of hepatocellular carcinoma
| Variable | PPARa in nucleus
|
P-value | |
|---|---|---|---|
| Positive expression | Negative expression | ||
| Sample size | 24 | 780 | |
| Age, years | 51.17±11.54 | 48.80±11.93 | 0.340 |
| Gender | 0.250 | ||
| Male | 23 (2.9%) | 688 (85.6%) | |
| Female | 1 (1.1%) | 92 (11.4%) | |
| HBsAg | 0.578 | ||
| Positive | 19 (2.4%) | 651 (80.9%) | |
| Negative | 5 (0.6%) | 129 (16.1%) | |
| AFP, ng/mL | 0.744 | ||
| <20 | 6 (0.8%) | 607 (75.5%) | |
| ≥20 | 18 (2.2%) | 173 (21.5%) | |
| Cirrhosis | 0.420 | ||
| Yes | 18 (2.2%) | 635 (79.0%) | |
| No | 6 (0.8%) | 144 (18.0%) | |
| Tumor size, cm | 0.014 | ||
| <5 | 11 (1.4%) | 187 (23.2%) | |
| ≥5 | 13 (1.6%) | 593 (73.8%) | |
| Tumor multiplicity | 0.071 | ||
| Single | 20 (2.5%) | 512 (63.7%) | |
| Multiple | 4 (0.5%) | 268 (33.3%) | |
| Differentiation | <0.001 | ||
| Well-moderate | 7 (0.9%) | 62 (7.7%) | |
| Poor-undifferentiated | 17 (2.1%) | 718 (89.3%) | |
| TNM stage | 0.036 | ||
| I–II | 15 (1.9%) | 320 (39.8%) | |
| III–IV | 9 (1.1%) | 460 (57.2%) | |
| Vascular invasion | 0.066 | ||
| Yes | 1 (0.1%) | 148 (18.4%) | |
| No | 23 (2.9%) | 631 (78.6%) | |
| Involucrum | 0.004 | ||
| Complete | 17 (2.1%) | 319 (39.8%) | |
| Incomplete | 7 (0.9%) | 459 (57.2%) | |
| Lymph node metastasis | 0.226 | ||
| Positive | 0 (0%) | 45 (5.6%) | |
| Negative | 24 (3.0%) | 734 (91.4%) | |
| Distant metastasis | 0.102 | ||
| Positive | 0 (0%) | 78 (9.8%) | |
| Negative | 24 (3.0%) | 696 (87.2%) | |
Abbreviations: PPARα, peroxisome proliferator-activated receptor α; HBsAg, hepatitis B virus surface antigen; AFP, α-fetoprotein.
Association of PPARα expression with clinical outcomes in HCC patients
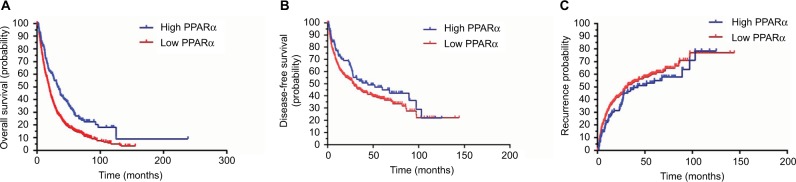
To determine the prognostic impact of PPARα expression on HCC patients, we conducted a Kaplan–Meier survival analysis using data from the 804 HCC patients enrolled in the study. In HCC patients with low PPARα expression in the cytoplasm, the Kaplan–Meier analysis revealed that the patients had significantly worse outcomes in terms of overall survival (P<0.001). Similar trends were observed for disease-free survival and the probability of recurrence, which showed that HCC patients with low PPARα expression in the cytoplasm had significantly worse outcomes than those with high PPARα expression in the cytoplasm, with worse disease-free survival (P=0.024), and a higher probability of recurrence (P=0.037), as shown in Figure 2.
Figure 2.
Low PPARα (cytoplasm) expression is correlated with an unfavorable prognosis in 804 HCC patients.
Notes: Kaplan–Meier analysis shows significant differences in overall survival between postoperative HCC patients with high and low PPARα (cytoplasm) expression (A). A similar trend was observed in HCC patients with high and low PPARα (cytoplasm) expression comparing disease-free survival (B) and the probability of recurrence (C).
Abbreviations: PPARα, peroxisome proliferator-activated receptor α; HCC, hepatocellular carcinoma.
We also conducted a Kaplan–Meier survival analysis to explore the differences in prognosis for HCC patients with both positive and negative PPARα expression in the nucleus. The data indicated that HCC patients with negative PPARα expression in the nucleus always had poorer outcomes, as shown in Figure 3. The overall survival was significantly shorter in HCC patients with negative PPARα expression in the nucleus (P=0.034). However, we did not find a prognostic impact of PPARα expression in the nucleus in HCC patients with respect to disease-free survival and the probability of recurrence (data not shown).
Figure 3.
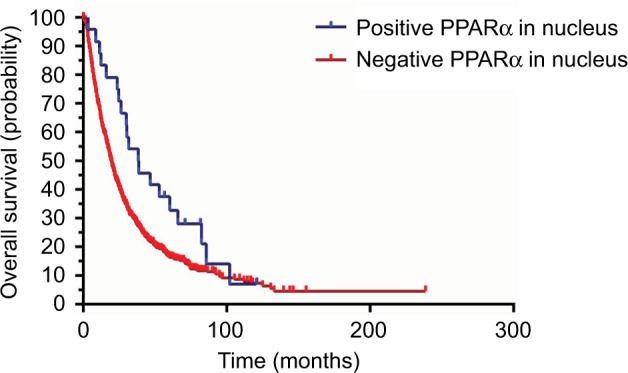
Positive PPARα (nucleus) expression is correlated with favorable prognosis in HCC patients. Kaplan–Meier analysis shows significant differences in overall survival between HCC patients with positive and negative PPARα (nucleus) expression. However, there was no significant difference observed with positive and negative PPARα (nucleus) expression comparing disease-free survival and the probability of recurrence.
Abbreviations: PPARα, peroxisome proliferator-activated receptor α; HCC, hepatocellular carcinoma.
Univariate and multivariate analyses of prognostic variables in HCC
To evaluate whether PPARα expression was an independent risk factor for outcomes in HCC, both univariate and multivariate analyses were conducted. Age, serum α-fetoprotein level, tumor size, tumor multiplicity, tumor differentiation, TNM stage, vascular invasion, involucrum, and PPARα expression in the cytoplasm and nucleus were all shown to be prognostic variables for overall survival in HCC patients. In the multivariate analysis, only tumor size (P=0.001), TNM stage (P<0.001), vascular invasion (P<0.001), and PPARα expression in the cytoplasm (P<0.001) were found to be independent prognostic variables for overall survival (Table 3).
Table 3.
Univariate and multivariate analyses of prognostic variables for overall survival
| Variables | Univariate analysis
|
Multivariate analysis
|
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Age, years | 0.991 | 0.985–0.997 | 0.004 | |||
| Gender | 0.872 | 0.684–1.112 | 0.268 | |||
| HBsAg | 1.177 | 0.957–1.447 | 0.123 | |||
| AFP | 1.252 | 1.046–1.499 | 0.014 | |||
| Cirrhosis | 0.974 | 0.799–1.187 | 0.794 | |||
| Tumor size, cm | 1.641 | 1.368–1.969 | <0.001 | 1.382 | 1.146–1.666 | 0.001 |
| Tumor multiplicity | 1.634 | 1.395–1.915 | <0.001 | |||
| Differentiation | 1.625 | 1.238–2.134 | <0.001 | |||
| TNM | 2.074 | 1.771–2.429 | <0.001 | 1.774 | 1.497–2.193 | <0.001 |
| Vascular invasion | 2.594 | 2.146–3.136 | <0.001 | 1.785 | 1.453–2.193 | <0.001 |
| Involucrum | 1.373 | 1.176–1.603 | <0.001 | 1.189 | 1.013–1.395 | 0.034 |
| PPARα (cytoplasm) | 1.593 | 1.276–1.989 | <0.001 | 1.595 | 1.274–1.997 | <0.001 |
| PPARα (nucleus) | 1.612 | 1.033–2.517 | 0.036 | |||
Abbreviations: HR, hazard ratio; PPARα, peroxisome proliferator-activated receptor α; HBsAg, hepatitis B virus surface antigen; AFP, α-fetoprotein.
Subgroup analyses of prognostic value of PPARα expression in the cytoplasm in HCC
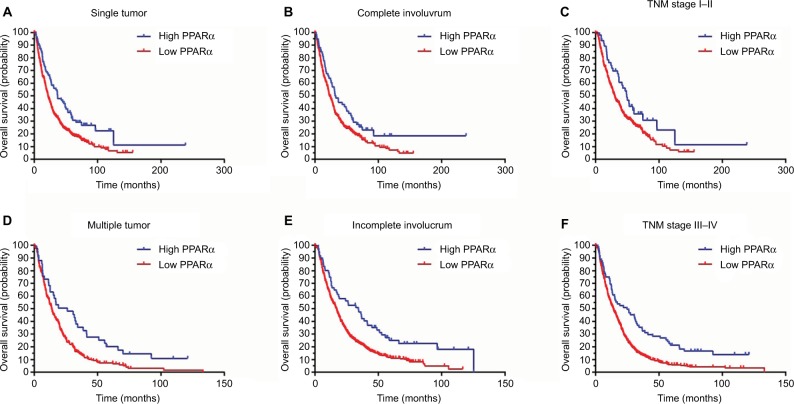
A stratified survival analysis was also conducted to further reveal the prognostic significance of PPARα expression in the cytoplasm among HCC patients. The Kaplan–Meier survival analysis showed that PPARα expression in the cytoplasm was associated with overall survival in both single and multiple HCCs (single HCCs: P=0.001, multiple HCCs: P=0.003), in complete and incomplete involucrum HCCs (complete involucrum HCCs: P=0.027, incomplete involucrum HCCs: P<0.001), and in TNM stage I–II HCCs and III–IV HCCs (TNM stage I–II HCCs: P=0.021, TNM stage III–IV HCCs: P<0.001), as shown in Figure 4.
Figure 4.
Low PPARα (cytoplasm) expression is associated with unfavorable outcomes in subgroups of HCC patients.
Notes: A stratified survival analysis was conducted for PPARα (cytoplasm) expression and unfavorable outcomes in single (A) and multinodular HCC (D), in HCCs with complete (B) and incomplete involucrum (E), in HCCs with TNM stage I–II (C) and stage III–IV (F).
Abbreviations: PPARα, peroxisome proliferator-activated receptor α; HCC, hepatocellular carcinoma.
Discussion
HCC is an end-stage liver disease with chronic viral infection accounting for most of the HCC etiology worldwide, especially in Asia.14–16 Recently, the rate of nutrient-associated HCC such as non-alcohol fatty liver disease-induced HCC has been found to be increasing.17,18 In addition, the reprogramming of energy metabolism is one of the hallmarks of cancer.19 Increasing evidence suggests that solid tumors might be also reliant on non-glucose carbon sources.19 Cancer cells show increased expression of enzymes involved in de novo fatty acid synthesis, which is very important for cellular biosynthesis such as in cell membranes.20 The more lipid content in tumor cells, the higher the invasiveness.21 However, to increase lipid content for cellular biosynthesis, inhibition of lipid oxidation is a potential approach besides increasing de novo lipogenesis. PPARα plays the central role in the lipid oxidation pathway in hepatocytes. Hence, we evaluated the expression of PPARα in HCC and explored the relationship of PPARα with HCC prognosis.
The PPAR family belongs to the nuclear hormone receptor superfamily. Like other nuclear hormone receptors, PPARs have four functional domains: amino-terminal domain, DNA binding domain, transcriptional activity regulation domains, and ligand binding domains.22,23 PPAR is located in the cytoplasm and when activated by ligands, it transforms into a heterodimer with retinoic X receptors (RXRs) or glucocorticoid receptor and then enters the nucleus and binds to the peroxisome proliferator response element to activate target gene transcription.22,24,25 Peroxisome proliferator response element is a specific DNA sequence located upstream of PPAR target genes. PPARα is a member of the PPAR family, which contains three members: PPARα, PPARδ, and PPARγ. As the first protein identified in the PPAR family, PPARα is mostly expressed in the liver and brown adipose tissue, followed by heart, kidney, and skeletal muscle, where it plays an important role in lipid metabolism. Previous reports have shown that PPARα plays an important role in regulating cell proliferation and maintaining the metabolic balance in cells, regulating tumorigenesis, as well as other cell biology processes.23 However, there are controversial data surrounding the role of PPARα in tumorigenesis.
In colon cancer, it is well-established that sustained chronic colon inflammation can promote the occurrence of colon cancer. One study reported that increased PPARα expression could inhibit the expression of pro-inflammatory cytokines such as interleukin-17 and interferons γ, which implies an anti-tumorigenic effect of PPARα in colon cancer.26 In breast cancer, one study found lack of PPARα expression in patients with basal-like breast cancer.27 In human breast cancer (MCF-7) and human cervical cancer (A2780) cell lines, PPARα reduces the expression of VEGF by promoting the ubiquitination of hypoxia inducible factor alpha.28 On the other hand, studies have also revealed the tumorigenic impact of PPARα in breast cancer. PPARα can be activated by leukotrienes produced by regulatory B cells and in this way promote cancer metastasis.29 Activating PPARα can also lead to the upregulation of the expression of cyclin E and the promotion of breast cancer proliferation.30 The role of PPARα in cancer is still unknown and requires further exploration. It is possible that PPARα activation could reduce or promote tumorigenesis, depending on the type of tissue and different PPARα ligands. Especially in HCC, since PPARα is primarily expressed in liver tissue, the role of PPARα in HCC tumorigenesis is still unknown. Activation of PPARα has effects on fatty acid catabolism, hepatocyte proliferation, hepatomegaly, and is closely related to the occurrence of HCC.31 However, fenofibrate, an oral agonist of PPARα, can inhibit the proliferation of Huh7 cells, an HCC cell line, by inhibiting the Akt pathway.32 It is also well-known that sustained activation of PPARα induces HCC in rodents.33
In this study, we found that the expression of PPARα in HCC tissue was significantly lower than in normal liver tissue. Interestingly, we found that PPARα is mainly expressed in the cytoplasm of liver cancer cells, with only a small fraction of cancer cells expressing PPARα in the nucleus. In the cytoplasm, following activation by ligands such as fatty acids, PPARα forms a heterodimer after which it translocates from the cytoplasm to the nucleus,34 where the heterodimer acts as a transcription factor by binding to peroxisome proliferator response elements located upstream of target genes, thus activating target gene transcription. In this way, PPARα regulates lipid oxidation, inflammation, and immune-related gene expression.34 Based on the results of our study, we believe that PPARα activation is inhibited in HCC cells. The mechanism underlying this may be that inhibition of PPARα activation will inhibit fatty acid oxidation, thereby increasing the intracellular lipid content, which can then be used to synthesize important cellular constituents such as cell membranes, or for use as an energy store to deal with metabolic stress, as has previously been reported.7 However, this hypothesis needs to be explored further.
Based on the results of our study, we found that a high level of PPARα expression in the cytoplasm was associated with smaller tumor sizes, less vascular invasion, and a higher proportion of complete involucrum. Similarly, positive PPARα expression in the nucleus was often accompanied with a smaller tumor size and a significantly lower proportion of poor, undifferentiated tumors, tumors at TNM stage III–IV, and an incomplete involucrum. Previous studies have reported that activating PPARα can inhibit cancer cell growth and reduce vessel formation.28,35 In HCC, PPARα may play a similar role in reducing HCC cell proliferation and vessel formation, since our data indicated that high PPARα expression in the cytoplasm and positive PPARα expression in the nucleus are accompanied by smaller tumor sizes and less vascular invasion. The specific role of PPARα in the development of HCC needs to be studied further to confirm this hypothesis.
The prognostic implications of PPARα expression in HCC have not been previously reported. In our study, PPARα was identified as an independent factor for overall survival in a large cohort of 804 patients with HCC. Patients with high levels of PPARα expression usually survived for a longer period. These data suggest that PPARα expression has clinical implications in predicting outcomes in HCC patients. PPARα is a major regulator of lipid metabolism in the liver; the relationship between liver fat content and PPARα expression needs to be further explored. In addition, in our study, only PPARα expression in HCC cell cytoplasm was prognostic for disease-free survival and the probability of recurrence. To further confirm the prognostic effect of PPARα expression in the nucleus for disease-free survival and the probability of recurrence among HCC patients, further study will be required involving more patients positive for PPARα expression in the HCC cell nucleus.
Retinoids and their receptors have a close relationship with PPAR.36,37 RXRs and retinoic acid receptors (RAR) are two main nuclear receptors binding with retinoids. Retinoids are a group of structural and functional analogs of vitamin A and play an important role in the regulation of cell proliferation and differentiation.38,39 Both RXR and RAR are composed of three subtypes, α, β, and γ. RAR binds with 9-cis retinoic acid and all-trans-retinoic acid while RXR only binds with 9-cis retinoic acid. A study reported that RXRα was decreased not only in HCC, but also in the early stage of liver carcinogenesis.40,41 In addition, studies have also revealed that hepatocarcinogenesis is accompanied by the accumulation of a phosphorylated form of RXRα, which is an inactive form of RXRα and abolishes its ability to form heterodimers with other nuclear receptors.42 Evidence focusing on the relationship between RAR and HCC is still limited. Studies show that RARb is a tumor suppressor gene.43 Enhanced RARβ expression correlates with the growth inhibitory effect of cancer cells and the absence of RARβ expression is accompanied with tumor progression.44 In addition, overexpression RARβ by vector induce drug sensitivity of tumor cells and suppresses proliferation.45 However, the specific role of RXR subtypes and RAR subtypes in HCC still needs further exploration.
In summary, our data demonstrate a role for PPARα in the development of HCC. Data reveal that PPARα expression is decreased in HCC samples and, unlike PPARα in rodent liver, PPARα in human HCC may have an antitumor effect. Increases in PPARα expression were significantly correlated with improved tumor differentiation and less vascular invasion. High PPARα expression was correlated with longer survival times in HCC patients and served as an independent factor for better outcomes. Collectively, our data suggest that PPARα is a promising biomarker for the prognosis of patients with HCC.
Acknowledgments
This study was supported by grants from the National Key R&D Program of China (2017YFC1309000), National Natural Science Foundation of China (nos 81572405, 81572406, 81502079, 81602135), and Science and Technology Program of Guangzhou (201707020038).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301–1314. doi: 10.1016/S0140-6736(18)30010-2. [DOI] [PubMed] [Google Scholar]
- 2.Cai SH, Lu SX, Liu LL, Zhang CZ, Yun JP. Increased expression of hepatocyte nuclear factor 4 alpha transcribed by promoter 2 indicates a poor prognosis in hepatocellular carcinoma. Therap Adv Gastroenterol. 2017;10:761–771. doi: 10.1177/1756283X17725998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen SL, Liu LL, Lu SX, et al. HBx-mediated decrease of AIM2 contributes to hepatocellular carcinoma metastasis. Mol Oncol. 2017;11:1225–1240. doi: 10.1002/1878-0261.12090. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 4.Vander HM, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nieman KM, Kenny HA, Penicka CV, et al. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat Med. 2011;17:1498–1503. doi: 10.1038/nm.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hirayama A, Kami K, Sugimoto M, et al. Quantitative metabolome profiling of colon and stomach cancer microenvironment by capillary electrophoresis time-of-flight mass spectrometry. Cancer Res. 2009;69:4918–4925. doi: 10.1158/0008-5472.CAN-08-4806. [DOI] [PubMed] [Google Scholar]
- 7.Wang MD, Wu H, Fu GB, et al. Acetyl-coenzyme A carboxylase alpha promotion of glucose-mediated fatty acid synthesis enhances survival of hepatocellular carcinoma in mice and patients. Hepatology. 2016;63:1272–1286. doi: 10.1002/hep.28415. [DOI] [PubMed] [Google Scholar]
- 8.Chung S, Kim YJ, Yang SJ, Lee Y, Lee M. Nutrigenomic functions of PPARs in obesogenic environments. PPAR Res. 2016;2016:4794576. doi: 10.1155/2016/4794576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mello T, Materozzi M, Galli A. PPARs and mitochondrial metabolism: from NAFLD to HCC. PPAR Res. 2016;2016:7403230. doi: 10.1155/2016/7403230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Youssef J, Badr M. Peroxisome proliferator-activated receptors and cancer: challenges and opportunities. Br J Pharmacol. 2011;164:68–82. doi: 10.1111/j.1476-5381.2011.01383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suchanek KM, May FJ, Robinson JA, et al. Peroxisome proliferator-activated receptor alpha in the human breast cancer cell lines MCF-7 and MDA-MB-231. Mol Carcinog. 2002;34:165–171. doi: 10.1002/mc.10061. [DOI] [PubMed] [Google Scholar]
- 12.Abu AO, Wettersten HI, Weiss RH. Inhibition of PPARα induces cell cycle arrest and apoptosis, and synergizes with glycolysis inhibition in kidney cancer cells. PLoS One. 2013;8:e71115. doi: 10.1371/journal.pone.0071115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peters JM, Cattley RC, Gonzalez FJ. Role of PPAR alpha in the mechanism of action of the nongenotoxic carcinogen and peroxisome proliferator Wy-14,643. Carcinogenesis. 1997;18:2029–2033. doi: 10.1093/carcin/18.11.2029. [DOI] [PubMed] [Google Scholar]
- 14.Cai S, Cao J, Yu T, Xia M, Peng J. Effectiveness of entecavir or telbivudine therapy in patients with chronic hepatitis B virus infection pretreated with interferon compared with de novo therapy with entecavir and telbivudine. Medicine (Baltimore) 2017;96:e7021. doi: 10.1097/MD.0000000000007021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cai S, Yu T, Jiang Y, Zhang Y, Lv F, Peng J. Comparison of entecavir monotherapy and de novo lamivudine and adefovir combination therapy in HBeAg-positive chronic hepatitis B with high viral load: 48-week result. Clin Exp Med. 2016;16:429–436. doi: 10.1007/s10238-015-0373-2. [DOI] [PubMed] [Google Scholar]
- 16.Cai SH, Lv FF, Zhang YH, Jiang YG, Peng J. Dynamic comparison between Daan real-time PCR and Cobas TaqMan for quantification of HBV DNA levels in patients with CHB. BMC Infect Dis. 2014;14:85. doi: 10.1186/1471-2334-14-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ou H, Cai S, Liu Y, Xia M, Peng J. A noninvasive diagnostic model to assess nonalcoholic hepatic steatosis in patients with chronic hepatitis B. Therap Adv Gastroenterol. 2017;10:207–217. doi: 10.1177/1756283X16681707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shaohang C, Zejin O, Duan L, et al. Risk factors associated with liver steatosis and fibrosis in chronic hepatitis B patient with component of metabolic syndrome. United Eur Gastroenterol J. 2018;6:558–566. doi: 10.1177/2050640617751252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pavlova NN, Thompson CB. The emerging hallmarks of cancer metabolism. Cell Metab. 2016;23:27–47. doi: 10.1016/j.cmet.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sunami Y, Rebelo A, Kleeff J. Lipid metabolism and lipid droplets in pancreatic cancer and stellate cells. Cancers (Basel) 2017;10:E3. doi: 10.3390/cancers10010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Z, Kang Y. Lipid metabolism fuels cancer’s spread. Cell Metab. 2017;25:228–230. doi: 10.1016/j.cmet.2017.01.016. [DOI] [PubMed] [Google Scholar]
- 22.Han L, Shen WJ, Bittner S, Kraemer FB, Azhar S. PPARs: regulators of metabolism and as therapeutic targets in cardiovascular disease. Part I: PPAR-alpha. Future Cardiol. 2017;13:259–278. doi: 10.2217/fca-2016-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao J, Yuan S, Jin J, Shi J, Hou Y. PPARα regulates tumor progression, foe or friend? Eur J Pharmacol. 2015;765:560–564. doi: 10.1016/j.ejphar.2015.09.027. [DOI] [PubMed] [Google Scholar]
- 24.Huang JY, Bi Y, Zuo GW, Feng T. Effects of HBx on localization of PPARγ in HepG2 cell line. Acta Academiae Medicinae Militaris Tertiae. 2008;18:1693–1696. [Google Scholar]
- 25.Moran EP, Ma JX. Therapeutic effects of PPAR alpha on neuronal death and microvascular impairment. PPAR Res. 2015;2015:595426. doi: 10.1155/2015/595426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee JW, Bajwa PJ, Carson MJ, et al. Fenofibrate represses interleukin-17 and interferon-gamma expression and improves colitis in interleukin-10-deficient mice. Gastroenterology. 2007;133:108–123. doi: 10.1053/j.gastro.2007.03.113. [DOI] [PubMed] [Google Scholar]
- 27.Baker BG, Ball GR, Rakha EA, et al. Lack of expression of the proteins GMPR2 and PPARα are associated with the basal phenotype and patient outcome in breast cancer. Breast Cancer Res Treat. 2013;137:127–137. doi: 10.1007/s10549-012-2302-3. [DOI] [PubMed] [Google Scholar]
- 28.Zhou J, Zhang S, Xue J, et al. Activation of peroxisome proliferator-activated receptor α (PPARα) suppresses hypoxia-inducible factor-1α (HIF-1α) signaling in cancer cells. J Biol Chem. 2012;287:35161–35169. doi: 10.1074/jbc.M112.367367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wejksza K, Lee-Chang C, Bodogai M, et al. Cancer-produced metabolites of 5-lipoxygenase induce tumor-evoked regulatory B cells via peroxisome proliferator-activated receptor alpha. J Immunol. 2013;190:2575–2584. doi: 10.4049/jimmunol.1201920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang NW, Wu CT, Chen DR, Yeh CY, Lin C. High levels of arachidonic acid and peroxisome proliferator-activated receptor-alpha in breast cancer tissues are associated with promoting cancer cell proliferation. J Nutr Biochem. 2013;24:274–281. doi: 10.1016/j.jnutbio.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 31.Scalera A, Tarantino G. Could metabolic syndrome lead to hepatocarcinoma via non-alcoholic fatty liver disease? World J Gastroenterol. 2014;20:9217–9228. doi: 10.3748/wjg.v20.i28.9217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamasaki D, Kawabe N, Nakamura H, et al. Fenofibrate suppresses growth of the human hepatocellular carcinoma cell via PPARα-independent mechanisms. Eur J Cell Biol. 2011;90:657–664. doi: 10.1016/j.ejcb.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 33.Tanaka N, Moriya K, Kiyosawa K, Koike K, Gonzalez FJ, Aoyama T. PPARα activation is essential for HCV core protein-induced hepatic steatosis and hepatocellular carcinoma in mice. J Clin Invest. 2008;118:683–694. doi: 10.1172/JCI33594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Adeghate E, Adem A, Hasan MY, Tekes K, Kalasz H. Medicinal chemistry and actions of dual and pan PPAR modulators. Open Med Chem J. 2011;5:93–98. doi: 10.2174/1874104501105010093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martinasso G, Oraldi M, Trombetta A, et al. Involvement of PPARs in cell proliferation and apoptosis in human colon cancer specimens and in normal and cancer cell lines. PPAR Res. 2007;2007:93416. doi: 10.1155/2007/93416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wagner CE, Jurutka PW, Marshall PA, Heck MC. Retinoid X receptor selective agonists and their synthetic methods. Curr Top Med Chem. 2017;17:742–767. doi: 10.2174/1568026616666160617091559. [DOI] [PubMed] [Google Scholar]
- 37.Tripathy S, Chapman JD, Han CY, et al. All-trans-retinoic acid enhances mitochondrial function in models of human liver. Mol Pharmacol. 2016;89:560–574. doi: 10.1124/mol.116.103697. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 38.Mark M, Ghyselinck NB, Chambon P. Function of retinoid nuclear receptors: lessons from genetic and pharmacological dissections of the retinoic acid signaling pathway during mouse embryogenesis. Annu Rev Pharmacol Toxicol. 2006;46:451–480. doi: 10.1146/annurev.pharmtox.46.120604.141156. [DOI] [PubMed] [Google Scholar]
- 39.Chambon P. A decade of molecular biology of retinoic acid receptors. FASEB J. 1996;10:940–954. [PubMed] [Google Scholar]
- 40.Hoshikawa Y, Kanki K, Ashla AA, et al. c-Jun N-terminal kinase activation by oxidative stress suppresses retinoid signaling through proteasomal degradation of retinoic acid receptor α protein in hepatic cells. Cancer Sci. 2011;102:934–941. doi: 10.1111/j.1349-7006.2011.01889.x. [DOI] [PubMed] [Google Scholar]
- 41.Matsushima-Nishiwaki R, Shidoji Y, Nishiwaki S, Yamada T, Moriwaki H, Muto Y. Aberrant metabolism of retinoid X receptor proteins in human hepatocellular carcinoma. Mol Cell Endocrinol. 1996;121:179–190. doi: 10.1016/0303-7207(96)03863-4. [DOI] [PubMed] [Google Scholar]
- 42.Yoshimura K, Muto Y, Shimizu M, et al. Phosphorylated retinoid X receptor alpha loses its heterodimeric activity with retinoic acid receptor beta. Cancer Sci. 2007;98:1868–1874. doi: 10.1111/j.1349-7006.2007.00621.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moore DM, Kalvakolanu DV, Lippman SM, et al. Retinoic acid and interferon in human cancer: mechanistic and clinical studies. Semin Hematol. 1994;31:31–37. [PubMed] [Google Scholar]
- 44.Lippman SM, Lotan R. Advances in the development of retinoids as chemopreventive agents. J Nutr. 2000;130:479S–482S. doi: 10.1093/jn/130.2.479S. [DOI] [PubMed] [Google Scholar]
- 45.Sun SY, Lotan R. Retinoids and their receptors in cancer development and chemoprevention. Crit Rev Oncol Hematol. 2002;41:41–55. doi: 10.1016/s1040-8428(01)00144-5. [DOI] [PubMed] [Google Scholar]