Abstract
The widely expressed transmembrane glycoprotein, cluster of differentiation 36 (CD36), a scavenger receptor class B protein (SR-B2), serves many functions in lipid metabolism and signaling. Here, we review CD36’s role in facilitating cellular long-chain fatty acid uptake across the plasma membrane, particularly in heart and skeletal muscles. CD36 acts in concert with other membrane proteins, such as peripheral plasma membrane fatty acid-binding protein, and is an intracellular docking site for cytoplasmic fatty acid-binding protein. The cellular fatty-acid uptake rate is governed primarily by the presence of CD36 at the cell surface, which is regulated by the subcellular vesicular recycling of CD36 from endosomes to the plasma membrane. CD36 has been implicated in dysregulated fatty acid and lipid metabolism in pathophysiological conditions, particularly in high-fat diet-induced insulin resistance and diabetic cardiomyopathy. Current research is exploring signaling pathways and vesicular trafficking routes involving CD36 to identify metabolic targets to manipulate the cellular utilization of fatty acids. Because of its rate-controlling function in the use of fatty acids in the heart and muscle, CD36 would be a preferable target to protect myocytes against lipotoxicity. Despite a poor understanding of its mechanism of action, CD36 has emerged as a pivotal membrane protein involved in whole-body lipid homeostasis.
Keywords: cluster of differentiation 36, scavenger receptor B2, heart, muscle
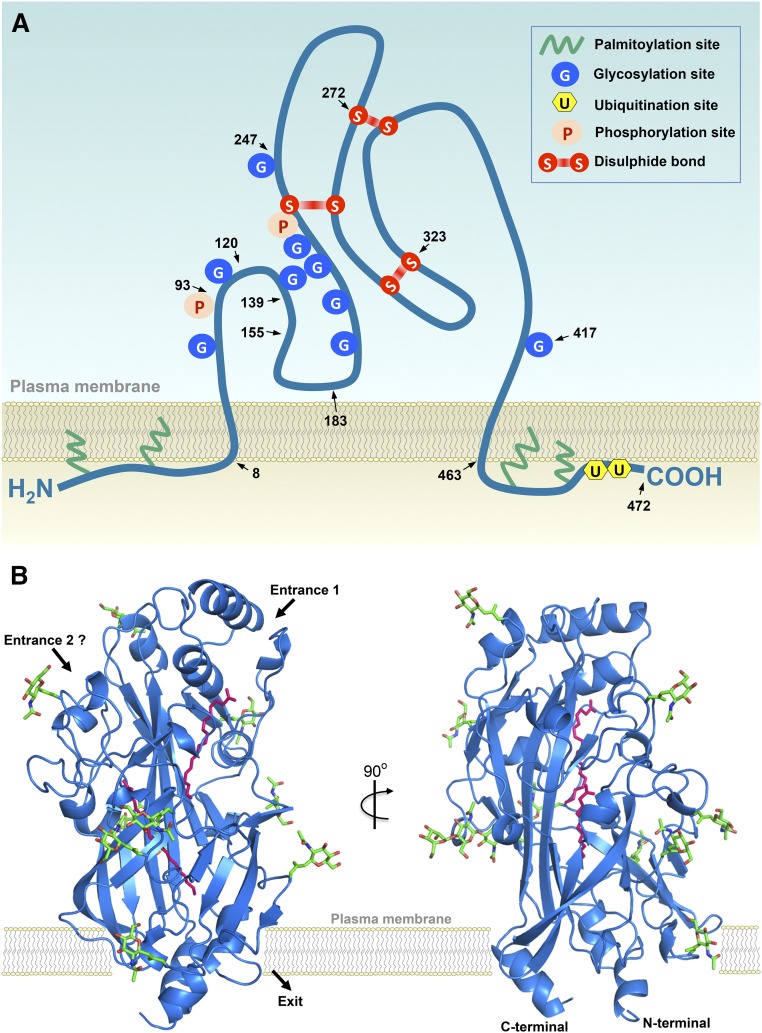
Cluster of differentiation 36 (CD36) was first described in 1977 as glycoprotein IV (GP IIIb or GP IV), the fourth major band observed upon SDS-polyacrylamide gel electrophoresis of human platelet membranes (1). Ten years later, the protein was shown to be identical to the antigen recognized by monoclonal antibody OKM5, a marker for monocytes and macrophages and designated as the leukocyte differentiation antigen CD36 (2). In that same year, CD36 was reported to be the cellular receptor for thrombospondin (3), implicating a role of CD36 in irreversible platelet aggregation, as well as in the adherence of erythrocytes infected with the malaria parasite, Plasmodium falciparum, to the endothelium (4). Subsequently, it was discovered that CD36 is a macrophage receptor for oxidized LDL (oxLDL), thereby establishing its role as a scavenger receptor (5), and that CD36 acts as facilitator of membrane fatty acid transport (6). CD36 is currently known to be a member of a superfamily of scavenger receptor proteins (class B), which also includes scavenger receptor (SR)-B1 and lysosomal integral membrane protein-2 (LIMP-2). These three proteins share their structure, which comprises two transmembrane domains, a relatively large extracellular domain, and both the amino and carboxyl termini located within the cytoplasm (7). As a result, CD36 is now officially designated as SR-B2 (8). Human CD36 has recently been crystallized (9). The structure and proposed membrane topology of CD36 are schematically depicted in Fig. 1.
Fig. 1.
Structure and membrane topology of glycoprotein CD36. A: Two-dimensional structure. Schematic presentation of CD36 (SR-B2) showing the two transmembrane domains. The large extracellular loop has nine observed N-linked glycosylation sites (positions 79, 102, 134, 205, 220, 231, 235, 247, and 417) and two phosphorylation sites (positions 92 and 237), bringing the total molecular mass to about 88 kDa. Disulfide bonds between extracellular cysteines are present between amino acid residues 243–311, 272–333, and 313–323. The small cytoplasmic tails contain the NH2 and COOH termini and each is palmitoylated, most likely to anchor the protein in the membrane. In addition, the COOH terminus contains two ubiquitination sites. The region formed by residues 93–120 was identified as the thrombospondin binding site that by residues 120–155 was mapped for oxLDL, while residues 139–183 form a multiligand binding site. The carboxyl head group of long-chain fatty acids interacts with residue Lys164. Arrowheads and numbers indicate the approximate positions of amino acid residues. Reproduced and updated from (18) with permission. B: Three-dimensional structure. The ribbon diagram shows the structure of CD36 in blue with the nine N-linked glycosylation sites and associated sugars in green. Two palmitic acid molecules, bound in the central cavity of the protein, are shown as pink sticks. The putative entrances for fatty acids to enter this central cavity and the putative exit are indicated by black arrows, with entrance 1 being more likely than entrance 2. Importantly, the positioning of CD36 with respect to the membrane and its putative interactions with phospholipids and other membrane constituents have not yet been delineated in detail and, therefore, are only vaguely indicated. Reproduced and adapted from (9) with permission.
CD36 is not expressed ubiquitously, yet is present in a variety of mammalian cell types, including hematopoietic cells (platelets, monocytes, macrophages), endothelial cells, specialized epithelial cells in the breast and eye, enterocytes, and insulin-responsive cells, such as adipocytes, and cardiac and skeletal myocytes. This expression pattern is paralleled by a similar broad number of functions, which are, however, mostly related to the regulation of lipid metabolism and innate immunity. Specifically, CD36 is involved in inflammatory responses and atherothrombotic diseases, intestinal fat absorption, lipid storage in adipose tissue and lipid utilization by cardiac and skeletal muscle, metabolic disorders, such as obesity and diabetes, and Alzheimer’s disease [reviewed in (10)]. Interestingly, CD36 is also expressed in human taste bud cells where it functions as a fatty acid sensor acting in oral fat perception and digestive anticipation (11, 12).
In this review, we will summarize our current understanding of the role of CD36 in cellular (long-chain) fatty acid utilization, focusing on the regulation of fatty acid uptake in heart and muscle, the role of CD36 in the pathogenesis of muscular insulin resistance and type 2 diabetes, and the application of CD36 as a target to manipulate cellular fatty acid utilization.
CD36 FACILITATES CELLULAR LONG-CHAIN FATTY ACID UTILIZATION
Discovery of CD36 as putative fatty acid translocase
From the 1980s onwards, there has been a dispute on the mechanism and regulation of the uptake of (long-chain) fatty acids by parenchymal cells, in particular with respect to the involvement, or not, of membrane-associated proteins in this process (13). While the lipophilic nature of the plasma membrane would allow the rapid passage of fatty acids, from a physiological perspective such free movement of fatty acids in or out of cells without control at the membrane would be undesirable and could seriously hamper coordination of intracellular fatty acid availability with changing metabolic needs (14).
In a search for membrane-associated fatty acid transporters, Abumrad and colleagues studied fatty acid uptake into isolated rat adipocytes. Prior incubation of the cells with diisothiocyanodisulfonic acid or with sulfo-N-succinimidyl derivates of long-chain fatty acids (in particular oleate) led to a marked (about 70%) and irreversible inhibition of the rate of fatty acid uptake (15, 16). Both inhibitors reacted covalently with a membrane protein of about 85–88 kDa, suggesting this protein to be involved in membrane permeation of (long-chain) fatty acids. Subsequent cloning of this protein, then referred to as “putative fatty acid translocase” (FAT), revealed it to be the rat homolog of the glycoprotein, CD36 (6). The role of FAT/CD36 in facilitating fatty acid uptake by adipocytes was further supported by a superimposed time course of its expression and of oleate uptake rate during adipose differentiation, and by a parallel induction of its expression and oleate transport in preadipocytes following treatment with the glucocorticoid, dexamethasone (6). Soon thereafter, a similar role was revealed for CD36 in fatty acid metabolism in cardiac and skeletal muscle (17).
CD36 is the predominant protein facilitating fatty acid uptake
Several other groups also reported on the identification of peripheral and integral membrane proteins putatively involved in the cellular uptake of fatty acids. These include plasma membrane fatty acid-binding protein (FABPpm; 40–43 kDa), fatty acid transport proteins 1–6 (FATP1–6; 63 kDa), and caveolin-1 (21–24 kDa) [for review see (18, 19)]. Of these, the FATPs were found to be enzymes, i.e., acyl-CoA synthetases, functioning in cellular fatty acid uptake by converting incoming fatty acids directly into their acyl-CoA ester, resulting in so-called metabolic trapping of fatty acids (19). However, CD36 appears to be the predominant membrane protein facilitating fatty acid transport, at least in adipocytes, enterocytes, cardiac myocytes, and skeletal myocytes (20–22). For instance, in contracting isolated cardiomyocytes, CD36 was found to contribute approximately 70% to the rate of fatty acid uptake (23).
Convincing evidence for a role of CD36 in cellular fatty acid uptake was obtained when studying mice with a targeted deletion of CD36. Compared with wild-type mice, CD36 knockout mice showed reduced fatty acid uptake rates in isolated cardiomyocytes (24), and in vivo in heart (−50% to −80%), skeletal muscle (−40% to −75%), and adipose tissue (−60% to −70%), but not in liver (20). Accordingly, in liver, CD36 expression is relatively low (21), but can be highly upregulated under hyperlipidemic conditions to contribute to the onset of hepatic steatosis (25). For CD36 regulation in liver, the reader is referred to other reviews, e.g., (26, 27). These reductions in fatty acid uptake in CD36 knockout mice also contributed to altered rates of fatty acid metabolism, especially with regard to fatty acid oxidation in working hearts and skeletal muscles (22). Similarly, in a group of 47 patients carrying various single nucleotide polymorphisms in the CD36 gene, Tanaka et al. (28) observed virtually absent fatty acid uptake in vivo in the heart, but no changes in liver. These polymorphisms included insertions of a premature stop codon to result in a truncated protein that is degraded.
With respect to nomenclature, it should be mentioned that, for convenience, these proteins commonly are referred to as “fatty acid transporters,” despite the remaining uncertainty as to the exact mechanism by which any of these proteins participate in the fatty acid transport process within the plasma membrane. After all, these proteins merely share the feature of facilitating, not necessarily transporting, the transmembrane translocation of (long-chain) fatty acids.
Molecular mechanism of cellular fatty acid uptake
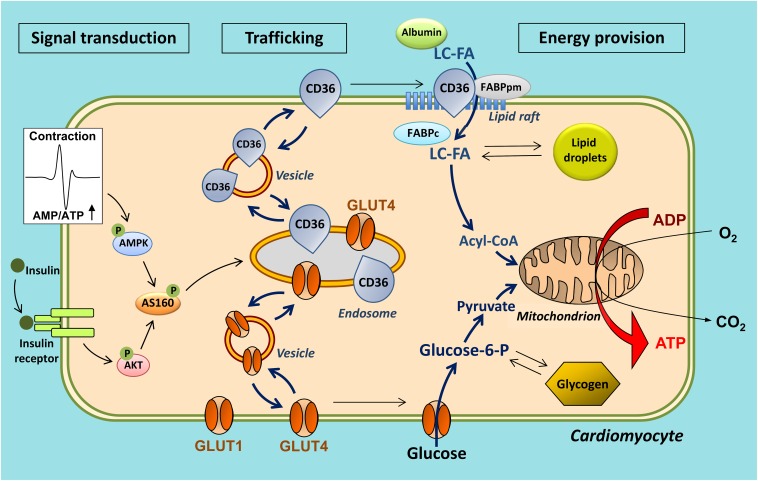
Insight into the molecular mechanism by which fatty acids traverse the plasma membrane to enter the soluble cytoplasm has increased markedly in the last decade, especially with respect to the involvement of CD36 [reviewed in (14)]. At the extracellular site, CD36 presumably functions as an acceptor for fatty acids to promote the partitioning of the fatty acids and their delivery to the outer leaflet of the lipid bilayer, most likely in plasma membrane lipid rafts (29) (adsorption step) (Fig. 1, Fig. 2). Subsequently, the fatty acids make their way from the outer to the inner leaflet of the membrane, a process referred to as “flip-flop” (the polar carboxyl group of the fatty acid moves through the bilayer interior and repositions at the opposite interface) (translocation step). This latter process occurs very fast and would not need assistance from membrane proteins (30). At the inner side of the membrane, the fatty acids move into the aqueous phase to bind to cytoplasmic FABP (FABPc) (desorption step). Desorption from the membrane has been suggested to be the rate-limiting step of overall transmembrane transport (30). At the intracellular side, CD36 may facilitate the transport by providing a docking site for FABPc (31) or for enzymes that act on fatty acids, such as acyl-CoA synthetase (32). In line with this, intracellularly, the presence of FABPc is required for proper functioning of CD36, as it was reported that transfection of CD36 in a rat heart muscle cell line (H9c2) devoid of FABPc did not result in increased rates of fatty acid uptake (33). Taken together, CD36 is viewed to function in sequestering fatty acids in the membrane, and helping to organize them within specific membrane domains (presumably lipid rafts) in order to make the fatty acids readily available for subsequent aqueous transport and/or enzymic conversion (Fig. 2). Interestingly, there is evidence to suggest that at the extracellular side, CD36 displays protein-protein interaction with FABPpm (34), possibly indicating that clusters of membrane proteins function in facilitating and modulating cellular fatty acid uptake. A concerted action among these various proteins may allow a fine-tuning of fatty acid transport so as to have this substrate readily available for efficient intracellular utilization (18).
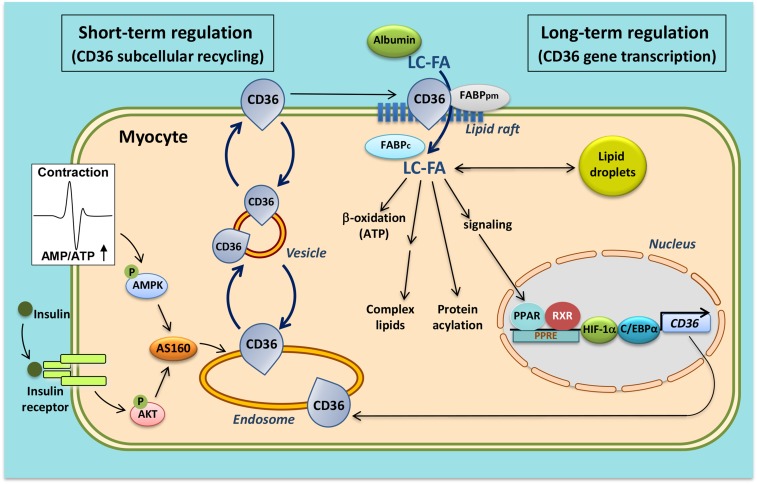
Fig. 2.
Schematic presentation of both the facilitatory and regulatory roles of CD36/SR-B2 in (long-chain) fatty acid uptake into cardiac and skeletal myocytes. At sarcolemmal lipid rafts, CD36, most likely in interaction with the peripheral membrane protein, FABPpm, and, at the intracellular site, with FABPc, facilitates the entry of fatty acids into the cell (middle part of figure). Short-term regulation (i.e., minutes) of the rate of cellular fatty acid uptake occurs by reversible intracellular recycling (vesicular transport) of CD36 from an endosomal storage compartment to the sarcolemma, which is triggered by either changes in the frequency of muscle contraction or by plasma insulin. These latter triggers are mediated by the AMP-activated kinase (AMPK) and insulin signaling cascades, respectively, whose signals converge at AS160 (left part of figure). Long-term regulation of cellular fatty acid uptake occurs via changes in CD36 gene transcription, mediated, among others, by fatty acid-induced PPAR activation, HIF-1, and C/EBPα (right part of figure). See text for further explanation. RXR, retinoid X receptor.
Recent crystallization studies have shed more light on the mechanism of fatty acid transport by CD36. First, LIMP-2 was crystallized, and based on the close similarity with CD36, it was deduced that CD36 possesses a cavity running through the entire length of the protein through which the fatty acids can be transferred to the lipid bilayer (7). This would then provide the molecular basis for the adsorption step. Further support for such a cavity comes from crystallization of CD36 when bound to fatty acids (Fig. 1B) (9). The cavity was shown to contain up to two fatty acids at a time, thereby indicating that CD36 truly acts as a fatty acid transporter whereby fatty acids pass through the CD6 ectodomain to be exposed to the plasma membrane surface (Fig. 1B). These crystallization studies add powerful support to the fatty acid transport function of CD36.
Whether CD36 would preferentially bind specific (long-chain) fatty acid types has remained elusive. This is due, at least in part, to the complexity of appropriate experimental approaches, which would need to consider, among others, differences among fatty acid types in aqueous solubility and differences in interaction with soluble proteins (such as albumin) and with biological membranes. Of note, there is circumstantial evidence that the cellular uptake of fatty acid species from marine oils, in particular EPA (20:5 n-3) and DHA (22:6 n-3), is also facilitated by CD36 (35, 36), suggesting that the beneficial health effects of n-3 long-chain polyunsaturated fatty acids are dependent on the sarcolemmal presence and proper functioning of CD36.
Recycling of CD36 between endosomes and the plasma membrane
The observation that CD36 is not only present on the cell membrane but also in intracellular compartments, notably endosomes, has triggered a series of studies, which revealed that CD36 is not merely a facilitator of transmembrane fatty acid transport, but in fact serves a pivotal role as regulator of the rate of cellular fatty acid uptake. Thus, it was disclosed that regulation of fatty acid transport occurs by the reversible translocation of CD36 from endosomes to the plasma membrane to increase fatty acid uptake. For instance, in both cardiac and skeletal muscle, the portion of CD36 that is stored in endosomes is estimated to be approximately 50% (37, 38). Either an increase in muscle contraction or the presence of insulin each stimulate, within a few minutes, the translocation of CD36 from the endosomal compartment to the sarcolemma upon which the fatty acid uptake rate increases up to 2-fold (37–39). The process of contraction-induced CD36 translocation is mediated by AMP-activated kinase (39). Insulin-induced translocation is mediated by phosphoinositide-3-kinase (38). Insulin and contraction signaling operate independently to induce CD36 translocation, but converge at the level of the Rab GTPase-activating protein, AS160, via an inactivating phosphorylation. This leads to disinhibition of Rab 8a, which is then allowed to use the accelerated GDP/GTP cycling for the benefit of CD36 translocation (40). Remarkably, this mechanism of regulation of cellular fatty acid uptake by recycling of CD36 is very similar to the well-known regulation of cellular glucose uptake, which, in cardiac and skeletal muscle, involves the translocation of glucose transporter-4 (GLUT4) from an intracellular storage depot to the sarcolemma (41). Upon increased muscle contraction or insulin stimulation, both CD36 and GLUT4 are recruited to the sarcolemma within the same time frame resulting in increased uptake rates for both fatty acids and glucose (18) (Fig. 3). CD36 recycling has also been confirmed to occur in human skeletal muscle (42, 43) and in adipose tissue (44).
Fig. 3.
Scheme illustrating the similarity in regulation of CD36-mediated long-chain fatty acid (LC-FA) uptake and GLUT4-mediated glucose uptake by cardiomyocytes. In response to contractile activity or stimulation with insulin, both the fatty acid transporter, CD36 (also designated SR-B2), and the glucose transporter, GLUT4, translocate to the sarcolemma to increase fatty acid and glucose uptake, respectively. Note that CD36 and GLUT4 may be recruited from distinct stores within the endosomal compartment. The glucose transporter, GLUT1, is constitutively expressed in the sarcolemma and contributes up to 25% of glucose uptake. For clarity, the putative intracellular recycling of other fatty acid transporters (FABPpm and selected FATPs) in fatty acid uptake is not shown. Adapted from (18).
Interestingly, it has been observed that CD36 may translocate to the plasma membrane without a concomitant change in fatty acid uptake, suggesting that translocation by itself is not sufficient for increasing fatty acid uptake and that other factors are required for full “activity” of CD36 at the cell surface (45, 46). This “insufficient” translocation is induced by acute increases in [Ca2+]i, and might serve to prepare the heart for increased fatty acid uptake during physiological contraction by inducing transporter translocation (46).
Current research is aimed at further deciphering the signaling pathways, vesicular trafficking routes, and cytoskeletal network proteins that control the short-term subcellular recycling of CD36, especially in relation to that of GLUT4. The outcome of such research would be to identify target proteins that could specifically increase, or decrease, fatty acid uptake without impairing glucose uptake, which could be beneficial for treatment of cardiac metabolic diseases [explained in more detail in other reviews, e.g., (47, 48)]. For instance, it has been reported that, in cardiac muscle, specific vesicle-associated membrane proteins (VAMPs) are necessary for both CD36 and GLUT4 translocation, while one isoform (VAMP4) is specifically involved in CD36 traffic and another isoform (VAMP7) specifically in GLUT4 traffic (49).
CD36 is also subject to various posttranslational modifications, which may be involved in its translocation. Although the four intracellular cysteines of CD36 offer bona fide palmitoylation sites, it was reported that palmitoylation at these sites is not involved in regulation of CD36 translocation (50). CD36 has also been proposed as a target of O-linked N-acetylglucosamine (O-GlcNac), the modification of which was observed to induce translocation of CD36 to cardiac sarcolemma and subsequently increase both the rate of myocellular fatty acid uptake and fatty acid oxidation (51).
Rate-governing step in cellular fatty acid utilization
With respect to the rate-limiting kinetic step in the overall fatty acid uptake process in the heart, earlier work had suggested that the mitochondrial enzyme, carnitine palmitoyltransferase-1 (CPT-1), serves an overall rate-limiting role in cardiac fatty acid utilization (52). However, more recent studies have questioned such a role for CPT-1 [reviewed in (22)]. For instance, partial inhibition of CPT-1 activity in vivo in rats through administration of etomoxir resulted in a 44% reduced cardiac CPT-1 activity, but failed to alter the rates of fatty acid uptake and oxidation (53). Thus, CPT-1 does not act as a major rate-controlling site in total cardiac fatty acid flux. Instead, cellular fatty acid uptake (i.e., trans-sarcolemmal transport) might be the primary site for regulation of fatty acid flux (22), while CPT-1 acts as a regulatory site for mitochondrial β-oxidation.
Transcriptional control of CD36 expression
The occurrence of CD36 in various tissues follows its role as facilitator of fatty acid transport in tissues with a high capacity for fatty acid metabolism, and fluctuates depending on developmental, hormonal, and environmental conditions. CD36 expression is high in segments of the intestine where most lipid absorption occurs, in adipose tissue in which fatty acids are stored in neutral lipids, and in cardiac and (oxidative) skeletal muscle in which fatty acids constitute the main substrate for energy production. CD36 is also found in endothelial cells, lung pneumocytes, platelets, and macrophages, whereby its expression likewise has been associated with fatty acid uptake and to binding of oxLDLs.
The expression of CD36 is regulated by agonists of the nuclear PPARs in a tissue-specific manner. PPARs are a family of transcription factors activated by a diversity of molecules, including fatty acids and fatty acid metabolites, and regulate the transcription of a large variety of genes implicated in metabolism, inflammation, proliferation, and differentiation in different cell types (54). While PPARα is expressed mostly in tissues exhibiting high rates of fatty acid oxidation (liver, cardiac and skeletal muscle, kidney, and brown adipose tissue) and regulates the expression of proteins involved in fatty acid catabolism, PPARγ is expressed predominantly in white adipose tissue and is a key regulator of adipogenesis. The third family member, PPARβ/δ, shows a more widespread tissue distribution. In skeletal muscle, its expression is upregulated during fasting and endurance exercise (54). PPARs can directly regulate the transcription of target genes by forming a heterodimer with the retinoid X receptor and binding to so-called PPAR response element (PPRE) sequences in the promotor- or transcribed-regions of target genes through DNA binding domains (54). The CD36 gene in mice (55) and humans (56) has been shown to possess one or more functional PPREs close to the transcription start site. Furthermore, in the distal promotor regions, there are additional species-specific PPREs (57). Yet, CD36 expression patterns appear very similar between species, suggesting redundancy in these PPREs (57).
Fatty acids, especially long-chain fatty acids, are considered to be the natural ligands for PPARs (58). In addition, various fatty acid metabolites, such as acyl-CoA esters, oxidized fatty acids, and eicosanoids, also activate PPARs (59). Activation of PPARα in heart and PPARβ/δ in skeletal muscle results in the coordinated increased expression of various proteins and enzymes involved in mitochondrial β-oxidation of fatty acids, including an upregulation of proteins involved in cellular fatty acid uptake, i.e., CD36 and FABPc. In this way, by facilitating the entry and thus promoting the intracellular availability of fatty acids, CD36 positively influences its own de novo synthesis (feed-forward cycle) (Fig. 2). In cardiac muscle, the pivotal roles of both CD36 and the nuclear receptor, PPARα, in governing cardiac fatty acid utilization are evident from the observations that: i) PPARα-null mice display a decreased capacity of cardiac fatty acid utilization (60, 61); ii) cardiac-specific overexpression of PPARα in mice results in enhanced fatty acid uptake and utilization (62); while iii) deletion of CD36 in PPARα-overexpressing mice prevented an increase in cardiac fatty acid utilization (63).
Besides PPARs, other transcriptional activators have also been implicated in the regulation of CD36 expression, including CCAAT/enhancer-binding protein-α and hypoxia-inducible factor-1 [for review see (64)]. Yet further research is needed to elucidate how these (and other) factors coordinate CD36 expression in a condition- and tissue-specific manner.
ROLE OF CD36 IN THE PATHOGENESIS OF MUSCULAR INSULIN RESISTANCE
Permanent relocation of CD36 initiates lipid oversupply
Several chronic diseases are characterized by a perturbed fatty acid or lipid metabolism. For instance, obesity is associated with disturbances in the control of glucose and lipid metabolism resulting in an atherogenic plasma lipid profile and deleterious triacylglycerol accumulation in non-adipose tissues like liver, heart, and muscle (ectopic lipid accumulation). Consequently, obese individuals are at high risk of developing insulin resistance, which may further lead to type 2 diabetes and cardiovascular complications, such as acute myocardial infarction and diabetic cardiomyopathy (65, 66). Because of its key role in the alterations in lipid metabolism upon fatty acid oversupply, CD36 has been implicated in the etiology of obesity-induced or high-fat diet-induced ectopic lipid accumulation and insulin resistance, and has been suggested as a target for therapeutic intervention (18, 67).
The molecular mechanism whereby high-fat diet induces lipid accumulation in cardiac and skeletal muscle, and subsequently insulin resistance and contractile dysfunction, has been unraveled in much detail and shows a key role for CD36. Chronic oversupply of fatty acids to the heart or muscle triggers changes in the subcellular recycling of CD36, resulting in a permanent relocation of this transporter from endosomes to the sarcolemma. At least in rats, this change is very rapid, i.e., within 2–3 days after the start of high-fat feeding, and is accompanied by a concomitant increase in the rate of myocellular fatty acid uptake (Fig. 4) (68). Fatty acids then not only become the main metabolic substrate for energy production, but also lead to excess intramyocellular formation of triacylglycerols, diacylglycerols, and ceramides. These latter two fatty acid metabolites inhibit insulin signaling with the main consequence being an impairment of the translocation of the glucose transporter, GLUT4, from endosomes to the sarcolemma. The latter causes a lowered glucose uptake rate and a decreased incorporation of glucose into glycogen. At that stage, the heart or muscle has become insulin resistant and shows impaired contractile function (22, 68). In conclusion, CD36 plays an essential role already in an early stage of the development of insulin resistance due to lipid overload. Indeed, specific inhibition of CD36 by sulfo-N-succinimidyl-oleate (69) or deletion of CD36 (70) protects against high-fat diet-induced insulin resistance and cardiac contractile dysfunction.
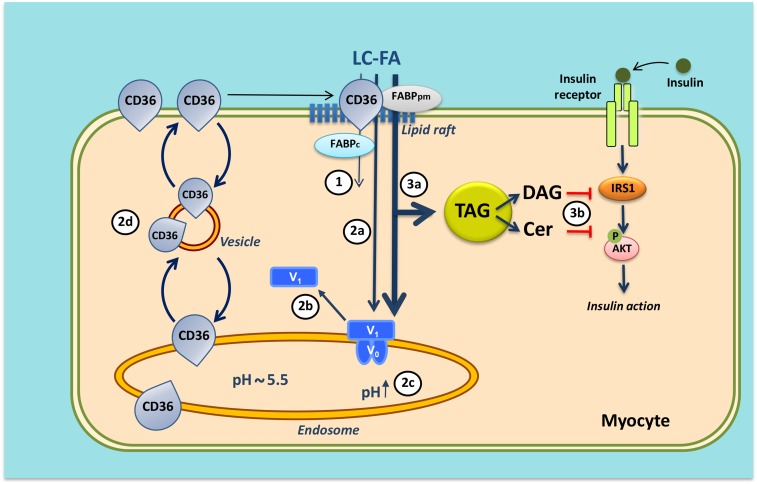
Fig. 4.
Mechanism of lipid-induced v-ATPase inhibition and loss of insulin sensitivity in cardiac myocytes upon lipid over-supply (1). When the supply of long-chain fatty acids (LC-FA) is low, CD36 is primarily found in endosomes. Furthermore, the v-ATPase V0 subcomplex, which is integral to the endosomal membrane, is assembled with the cytosolic V1 subcomplex, allowing for acidification of the endosomal lumen. In this situation, the available LC-FA is metabolized to meet the immediate energy demand of the myocyte. Elevated extracellular LC-FA supply triggers a series of events: increased CD36-mediated LC-FA uptake results in elevated intramyocellular LC-FA levels causing the V1 subcomplex to dissociate (circled 2a); the V1 subcomplex is shifted toward the cytoplasm (circled 2b); the disassembly of v-ATPase leads to endosomal alkalinization (circled 2c); and increased endosomal pH triggers the translocation of CD36 vesicles to the sarcolemma (circled 2d). Upon chronic lipid over-supply, where LC-FA uptake surpasses the metabolic needs, further processes are set in motion: the lipid-induced increase in sarcolemmal CD36 feeds forward to further LC-FA uptake and progressive lipid storage [triacylglycerol (TAG)] (circled 3a); and excess formation of diacylglycerol (DAG) and ceramides (Cer) culminates in impairment of the insulin signaling cascade and, therefore, loss of insulin sensitivity (circled 3b). Modified from (71).
Molecular mechanism underlying permanent CD36 relocation
It has not yet been elucidated why increased fatty acid delivery to heart or muscle results in an imbalance in the subcellular recycling of CD36, with most of the protein permanently residing on the sarcolemma so that the rate of incoming fatty acids is not tuned anymore to the metabolic needs of the myocyte. Recent studies, however, suggest that increased CD36 translocation to the sarcolemma is caused by changes in the properties of the endosomes, which form the subcellular storage compartment of CD36 (see the section on CD36 recycling). The endosomes are acidic organelles, but lose their acidification upon lipid oversupply due to inhibition of the proton pumping activity of vacuolar-type H+-ATPase (v-ATPase) (71). Hence, endosomal alkalinization was observed in rats fed a high-fat diet and appeared as an early lipid-induced event preceding the onset of insulin resistance (Fig. 4).
v-ATPase is a multimeric protein complex consisting of an integral membrane sub-complex (V0), encompassing the proton channel, and a peripheral sub-complex (V1), containing the ATP-binding pocket (72). The mechanism of lipid-induced v-ATPase inhibition involves disassembly of V1 from V0, and subsequent migration of V1 into the soluble cytoplasm (Fig. 4) (71). These new insights suggest that reassembly of v-ATPase, leading to its reactivation, could be an effective approach to retain CD36 intracellularly and treat lipid-induced diabetic cardiomyopathy.
CONCLUDING REMARKS AND FUTURE PERSPECTIVES
CD36 has emerged as a multi-functional protein involved in multiple homeostatic and pathological processes. In this review, we focused on its role in cellular fatty acid uptake and utilization, especially in cardiac and skeletal muscle, to conclude that CD36 serves to facilitate fatty acid uptake across the plasma membrane by offering an extracellular lipophilic tunnel through which fatty acids are guided to enter the membrane bilayer, and that CD36 is subject to dynamic subcellular recycling that permits adjustment of the presence of CD36 in the membrane to tune the uptake of fatty acids to the metabolic needs of the cell. Taken together, CD36 is pivotal for governing the rate of cellular fatty acid uptake.
At the protein level, the precise mechanism of action of CD36 has not yet been fully elucidated. For instance, besides the already mentioned palmitoylation and O-GlcNAcylation, CD36 also undergoes other posttranslational modifications, such as phosphorylation, glycosylation, and ubiquitination [reviewed in (73)]. Phosphorylation [ecto-phosphorylation at positions Thr92 and Ser237 (74)] and ubiquitination likely do not play a role in heart and muscle (73), but may be important in other tissues (75). Glycosylation is a permanent modification and is involved in proper folding of CD36 (76). In addition, as mentioned, the role of putatively interacting proteins and/or factors that may influence the functioning of CD36 is still elusive. Finally, to what extent the various ligands compete for binding to CD36 needs to be investigated.
Based on its rate-controlling function in the utilization of fatty acids by heart and muscle, CD36 would be the preferable target to protect myocytes, and perhaps other CD36-expressing cells, against lipotoxicity during periods of lipid overexposure. Such an approach would require specifically targeting the fatty acid-binding function of CD36 without disturbing its other physiological functions. Indeed, selective targeting seems feasible, as it was found with isolated rat cardiomyocytes that sulfo-N-succinimidyl oleate does block CD36-mediated palmitate uptake, while both hexarelin, a synthetic growth hormone releasing peptide that binds to CD36, and EP 80317, a CD36-selective ligand that inhibits cholesterol transport, each had no effect on palmitate uptake (46). Still, intervening in the CD36 subcellular recycling machinery is expected to allow a more specific approach because, in general, the proteins involved in vesicular trafficking occur in tissue-specific types.
A final interesting direction of CD36 research is the field of cancer and metastasis, as there is emerging evidence that, in certain types of cancer, CD36 expression drives tumor progression by increasing the availability of fatty acids and lipids [e.g., see (77, 78)]. In addition, in a recent study, Pascual et al. (79) observed that a subpopulation of cancer cells with unique metastasis-initiating potential could be marked by CD36, highlighting a key role of lipid metabolism in metastatic colonization.
Acknowledgments
The authors would like to thank their collaborators and previous and current laboratory members for stimulating discussions, in particular Arend Bonen (University of Guelph, Guelph, Canada), Nada Abumrad (Washington University, St. Louis, MO), Jim Hamilton (Boston University, Boston, MA), Jason Dyck (University of Alberta, Edmonton, Alberta, Canada), Lisa Heather (University of Oxford, Oxford, UK), Miranda Nabben, Dietbert Neumann, Dipanjan Chanda, and Ilvy Geraets. The authors also thank Dr. Matthew Higgins (University of Oxford, Oxford, UK) for discussions on the CD36 structure and his help with the preparation of Fig. 1.
Footnotes
Abbreviations:
- CD36
- cluster of differentiation 36
- CPT-1
- carnitine palmitoyltransferase-1
- FABPc
- cytoplasmic fatty acid-binding protein
- FABPpm
- plasma membrane fatty acid-binding protein
- FAT
- fatty acid translocase (putative) (CD36/SR-B2)
- FATP
- fatty acid transport protein
- GLUT4
- glucose transporter-4
- oxLDL
- oxidized LDL
- PPRE
- PPAR response element
- SR
- scavenger receptor
- VAMP
- vesicle-associated membrane protein
- v-ATPase
- vacuolar-type H+-ATPase
REFERENCES
- 1.Clemetson K. J., Pfueller S. T., Luscher E. F., and Jenkins C. S. P.. 1977. Isolation of the membrane glycoproteins of human platelets by lectin affinity chromatography. Biochim. Biophys. Acta. 464: 493–508. [DOI] [PubMed] [Google Scholar]
- 2.Shaw S. 1987. Characterization of human leukocyte differentiation antigens. Immunol. Today. 8: 1–3. [DOI] [PubMed] [Google Scholar]
- 3.Asch A. S., Barnwell J., Silverstein R. L., and Nachman R. L.. 1987. Isolation of the thrombospondin membrane receptor. J. Clin. Invest. 79: 1054–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ockenhouse C. F., and Chulay J. D.. 1988. Plasmodium falciparum sequestration: OKM5 antigen (CD36) mediates cytoadherence of parasitized erythrocytes to a myelomonocytic cell line. J. Infect. Dis. 157: 584–588. [DOI] [PubMed] [Google Scholar]
- 5.Endemann G., Stanton L. W., Madden K. S., Bryant C. M., White R. T., and Protter A. A.. 1993. CD36 is a receptor for oxidized low density lipoprotein. J. Biol. Chem. 268: 11811–11816. [PubMed] [Google Scholar]
- 6.Abumrad N. A., El-Maghrabi M. R., Amri E. Z., Lopez E., and Grimaldi P. A.. 1993. Cloning of a rat adipocyte membrane protein implicated in binding or transport of long-chain fatty acids that is induced during preadipocyte differentiation. Homology with human CD36. J. Biol. Chem. 268: 17665–17668. [PubMed] [Google Scholar]
- 7.Neculai D., Schwake M., Ravichandran M., Zunke F., Collins R. F., Peters J., Neculai M., Plumb J., Loppnau P., Pizarro J. C., et al. . 2013. Structure of LIMP-2 provides functional insights with implications for SR-BI and CD36. Nature. 504: 172–176. [DOI] [PubMed] [Google Scholar]
- 8.Prabhudas M., Bowdish D., Drickamer K., Febbraio M., Herz J., Kobzik L., Krieger M., Loike J., Means T. K., Moestrup S. K., et al. . 2014. Standardizing scavenger receptor nomenclature. J. Immunol. 192: 1997–2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hsieh F. L., Turner L., Bolla J. R., Robinson C. V., Lavstsen T., and Higgins M. K.. 2016. The structural basis for CD36 binding by the malaria parasite. Nat. Commun. 7: 12837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Silverstein R. L., and Febbraio M.. 2009. CD36, a scavenger receptor involved in immunity, metabolism, angiogenesis, and behavior. Sci. Signal. 2: re3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laugerette F., Passilly-Degrace P., Patris B., Niot I., Febbraio M., Montmayeur J. P., and Besnard P.. 2005. CD36 involvement in orosensory detection of dietary lipids, spontaneous fat preference, and digestive secretions. J. Clin. Invest. 115: 3177–3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Niot I., and Besnard P.. 2017. Appetite control by the tongue-gut axis and evaluation of the role of CD36/SR-B2. Biochimie. 136: 27–32. [DOI] [PubMed] [Google Scholar]
- 13.Bonen A., Chabowksi A., Luiken J. J. F. P., and Glatz J. F. C.. 2007. Mechanisms and regulation of protein-mediated cellular fatty acid uptake: Molecular, biochemical and physiological evidence. Physiology (Bethesda). 22: 15–29. [DOI] [PubMed] [Google Scholar]
- 14.Glatz J. F. C., and Luiken J. J. F. P.. 2014. Control of myocardial fatty acid uptake. In Cardiac Energy Metabolism in Health and Disease (Advances in Biochemistry in Health and Disease). G. D. Lopaschuk and N. S. Dhalla, editors. Springer Science Inc., New York. 49–67. [Google Scholar]
- 15.Harmon C. M., Luce P., Beth A. H., and Abumrad N. A.. 1991. Labeling of adipocyte membranes by sulfo-N-succinimidyl derivatives of long-chain fatty acids: Inhibition of fatty acid transport. J. Membr. Biol. 121: 261–268. [DOI] [PubMed] [Google Scholar]
- 16.Harmon C. M., and Abumrad N. A.. 1993. Binding of sulfosuccinimidyl fatty acids to adipocyte membrane proteins: Isolation and amino-terminal sequence of an 88-kD protein implicated in transport of long-chain fatty acids. J. Membr. Biol. 133: 43–49. [DOI] [PubMed] [Google Scholar]
- 17.Van Nieuwenhoven F. A., Verstijnen C. P., Abumrad N. A., Willemsen P. H., Van Eys G. J., Van der Vusse G. J., and Glatz J. F.. 1995. Putative membrane fatty acid translocase and cytoplasmic fatty acid-binding protein are co-expressed in rat heart and skeletal muscles. Biochem. Biophys. Res. Commun. 207: 747–752. [DOI] [PubMed] [Google Scholar]
- 18.Glatz J. F. C., Luiken J. J. F. P., and Bonen A.. 2010. Membrane fatty acid transporters as regulators of lipid metabolism: Implications for metabolic disease. Physiol. Rev. 90: 367–417. [DOI] [PubMed] [Google Scholar]
- 19.Kazantzis M., and Stahl A.. 2012. Fatty acid transport proteins, implications in physiology and diseases. Biochim. Biophys. Acta. 1821: 852–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abumrad N. A., and Goldberg I. J.. 2016. CD36 actions in the heart: lipids, calcium, inflammation, repair and more? Biochim. Biophys. Acta. 1861: 1442–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim T. T., and Dyck J. R. B.. 2016. The role of CD36 in the regulation of myocardial lipid metabolism. Biochim. Biophys. Acta. 1861: 1450–1460. [DOI] [PubMed] [Google Scholar]
- 22.Glatz J. F. C., Nabben M., Heather L. C., Bonen A., and Luiken J. J. F. P.. 2016. Regulation of the subcellular trafficking of CD36, a major determinant of cardiac fatty acid utilization. Biochim. Biophys. Acta. 1861: 1461–1471. [DOI] [PubMed] [Google Scholar]
- 23.Luiken J. J., Willems J., van der Vusse G. J., and Glatz J. F.. 2001. Electrostimulation enhances FAT/CD36-mediated long-chain fatty acid uptake by isolated rat cardiac myocytes. Am. J. Physiol. Endocrinol. Metab. 281: E704–E712. [DOI] [PubMed] [Google Scholar]
- 24.Habets D. D., Coumans W. A., Voshol P. J., den Boer M. A., Febbraio M., Bonen A., Glatz J. F., and Luiken J. J.. 2007. AMPK-mediated increase in myocardial long-chain fatty acid uptake critically depends on sarcolemmal CD36. Biochem. Biophys. Res. Commun. 355: 204–210. [DOI] [PubMed] [Google Scholar]
- 25.Memon R. A., Fuller J., Moser A. H., Smith P. J., Grunfeld C., and Feingold K. R.. 1999. Regulation of putative fatty acid transporters and acyl-CoA synthetase in liver and adipose tissue in ob/ob mice. Diabetes. 48: 121–127. [DOI] [PubMed] [Google Scholar]
- 26.Lee J. H., Zhou J., and Xie W.. 2008. PXR and LXR in hepatic steatosis: a new dog and an old dog with new tricks. Mol. Pharm. 5: 60–66. [DOI] [PubMed] [Google Scholar]
- 27.He J., Lee J. H., Febbraio M., and Xie W.. 2011. The emerging roles of fatty acid translocase/CD36 and the aryl hydrocarbon receptor in fatty liver disease. Exp. Biol. Med. (Maywood). 236: 1116–1121. [DOI] [PubMed] [Google Scholar]
- 28.Tanaka T., Nakata T., Oka T., Ogawa T., Okamoto F., Kusaka Y., Sohmiya K., Shimamoto K., and Itakura K.. 2001. Defect in human myocardial long-chain fatty acid uptake is caused by FAT/CD36 mutations. J. Lipid Res. 42: 751–759. [PubMed] [Google Scholar]
- 29.Pohl J., Ring A., Korkmaz U., Ehehalt R., and Stremmel W.. 2005. FAT/CD36-m,ediated long-chain fatty acid uptake in adipocytes requires plasma membrane rafts. Mol. Biol. Cell. 16: 24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hamilton J. A. 2007. New insights into the roles of proteins and lipids in membrane transport of fatty acids. Prostaglandins Leukot. Essent. Fatty Acids. 77: 355–361. [DOI] [PubMed] [Google Scholar]
- 31.Spitsberg V. L., Matitashvili E., and Gorewit R. C.. 1995. Association and coexpression of fatty-acid-binding protein and glycoprotein CD36 in the bovine mammary gland. Eur. J. Biochem. 230: 872–878. [DOI] [PubMed] [Google Scholar]
- 32.Schneider H., Staudacher S., Poppelreuther M., Stremmel W., Ehehalt R., and Füllekrug J.. 2014. Protein mediated fatty acid uptake: synergy between CD36/FAT-facilitated transport and acyl-CoA synthetase-driven metabolism. Arch. Biochem. Biophys. 546: 8–18. [DOI] [PubMed] [Google Scholar]
- 33.Van Nieuwenhoven F. A., Luiken J. J. F. P., De Jong Y. F., Grimaldi P. A., Van der Vusse G. J., and Glatz J. F. C.. 1998. Stable transfection of fatty acid translocase (CD36) in a rat heart muscle cell line (H9c2). J. Lipid Res. 39: 2039–2047. [PubMed] [Google Scholar]
- 34.Chabowski A., Górski J., Luiken J. J. F. P., Glatz J. F. C., and Bonen A.. 2007. Evidence for a concerted action of FAT/CD36 and FABPpm to increase fatty acid transport across the plasma membrane. Prostaglandins Leukot. Essent. Fatty Acids. 77: 345–353. [DOI] [PubMed] [Google Scholar]
- 35.Franekova V., Angin Y., Hoebers N. T., Coumans W. A., Simons P. J., Glatz J. F., Luiken J. J., and Larsen T. S.. 2015. Marine omega-3 fatty acids prevent myocardial insulin resistance and metabolic remodeling as induced experimentally by high insulin exposure. Am. J. Physiol. Cell Physiol. 308: C297–C307. [DOI] [PubMed] [Google Scholar]
- 36.Glatz J. F., and Luiken J. J.. 2015. Fatty acids in cell signaling: historical perspective and future outlook. Prostaglandins Leukot. Essent. Fatty Acids. 92: 57–62. [DOI] [PubMed] [Google Scholar]
- 37.Bonen A., Luiken J. J. F. P., Arumugam Y., Glatz J. F. C., and Tandon N. N.. 2000. Acute regulation of fatty acid uptake involves the cellular redistribution of fatty acid translocase. J. Biol. Chem. 275: 14501–14508. [DOI] [PubMed] [Google Scholar]
- 38.Luiken J. J. F. P., Koonen D. P., Willems J., Zorzano A., Becker C., Fisher Y., Tandon N. N., van der Vusse G. J., Bonen A., and Glatz J. F. C.. 2002. Insulin stimulates long-chain fatty acid utilization by rat cardiac myocytes through cellular redistribution of FAT/CD36. Diabetes. 51: 3113–3119. [DOI] [PubMed] [Google Scholar]
- 39.Luiken J. J. F. P., Coort S. L. M., Willems J., Coumans W. A., Bonen A., van der Vusse G. J., and Glatz J. F. C.. 2003. Contraction-induced fatty acid translocase/CD36 translocation in rat cardiac myocytes is mediated through AMP-activated protein kinase signaling. Diabetes. 52: 1627–1634. [DOI] [PubMed] [Google Scholar]
- 40.Samovski D., Su X., Xu Y., Abumrad N. A., and Stahl P. D.. 2012. Insulin and AMPK regulate FA translocase/CD36 plasma membrane recruitment in cardiomyocytes via Rab GAP AS160 and Rab8a Rab GTPase. J. Lipid Res. 53: 709–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Klip A., Sun Y., Chiu T. T., and Foley K. P.. 2014. Signal transduction meets vesicle traffic: the software and hardware of GLUT4 translocation. Am. J. Physiol. Cell Physiol. 306: C879–C886. [DOI] [PubMed] [Google Scholar]
- 42.Aguer C., Mercier J., Man C. Y., Metz L., Bordenave S., Lambert K., Jean E., Lantier L., Bounoua L., Brun J. F., et al. . 2010. Intramyocellular lipid accumulation is associated with permanent relocation ex vivo and in vitro of fatty acid translocase (FAT)/CD36 in obese patients. Diabetologia. 53: 1151–1163. [DOI] [PubMed] [Google Scholar]
- 43.Aguer C., Foretz M., Lantier L., Hebrard S., Viollet B., Mercier J., and Kitzmann M.. 2011. Increased FAT/CD36 cycling and lipid accumulation in myotubes derived from obese type 2 diabetic patients. PLoS One. 6: e28981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lobo S., and Bernlohr D. A.. 2007. Fatty acid transport in adipocytes and the development of insulin resistance. Novartis Found. Symp. 286: 113–121. [DOI] [PubMed] [Google Scholar]
- 45.Eyre N. S., Cleland L. G., and Mayrhofer G.. 2008. FAT/CD36 expression alone is insufficient to enhance cellular uptake of oleate. Biochem. Biophys. Res. Commun. 370: 404–409. [DOI] [PubMed] [Google Scholar]
- 46.Angin Y, Steinbusch L. K., Simons P. J., Greulich S., Hoebers N. T., Douma K., van Zandvoort M. A., Coumans W. A., Wijnen W., Diamant M., et al. . 2012. CD36 inhibition prevents lipid accumulation and contractile dysfunction in rat cardiomyocytes. Biochem. J. 448: 43–53. [DOI] [PubMed] [Google Scholar]
- 47.Glatz J. F., Bonen A., Ouwens D. M., and Luiken J. J.. 2006. Regulation of sarcolemmal transport of substrates in the healthy and diseased heart. Cardiovasc. Drugs Ther. 20: 471–476. [DOI] [PubMed] [Google Scholar]
- 48.Schwenk R. W., Luiken J. J., Bonen A., and Glatz J. F.. 2008. Regulation of sarcolemmal glucose and fatty acid transporters in cardiac disease. Cardiovasc. Res. 79: 249–258. [DOI] [PubMed] [Google Scholar]
- 49.Schwenk R. W., Dirkx E., Coumans W. A., Bonen A., Klip A., Glatz J. F. C., and Luiken J. J. F. P.. 2010. Requirement for distinct vesicle-associated membrane proteins in insulin- and AMP-activated protein kinase (AMPK)-induced translocation of GLUT4 and CD36 in cultured cardiomyocytes. Diabetologia. 53: 2209–2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van Oort M. M., Drost R., Janßen L., Van Doorn J. M., Kerver J., Van der Horst D. J., Luiken J. J., and Rodenburg K. C.. 2014. Each of the four intracellular cysteines of CD36 is essential for insulin- or AMP-activated protein kinase-induced CD36 translocation. Arch. Physiol. Biochem. 120: 40–49. [DOI] [PubMed] [Google Scholar]
- 51.Laczy B., Fulop N., Onay-Besikci A., Des Rosiers C., and Chatham J. C.. 2011. Acute regulation of cardiac metabolism by the hexosamine biosynthesis pathway and protein O-GlcNAcylation. PLoS One. 6: e18417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Saddik M., Gamble J., Witters L. A., and Lopaschuk G. D.. 1993. Acetyl-CoA carboxylase regulation of fatty acid oxidation in the heart. J. Biol. Chem. 268: 25836–25845.7902355 [Google Scholar]
- 53.Luiken J. J., Niessen H. E., Coort S. L., Hoebers N., Coumans W. A., Schwenk R. W., Bonen A., and Glatz J. F.. 2009. Etomoxir-induced partial carnitine palmitoyltransferase-I (CPT-I) inhibition in vivo does not alter cardiac long-chain fatty acid uptake and oxidation rates. Biochem. J. 419: 447–455. [DOI] [PubMed] [Google Scholar]
- 54.Neels J. G., and Grimaldi P. A.. 2014. Physiological functions of peroxisome proliferator-activated receptor β. Physiol. Rev. 94: 795–858. [DOI] [PubMed] [Google Scholar]
- 55.Teboul L., Febbraio M., Gaillard D., Amri E. Z., Silverstein R., and Grimaldi P. A.. 2001. Structural and functional characterization of the mouse fatty acid translocase promoter: activation during adipose differentiation. Biochem. J. 360: 305–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tontonoz P., Nagy L., Alvarez J. G., Thomazy V. A., and Evans R. M.. 1998. PPARgamma promotes monocyte/macrophage differentiation and uptake of oxidized LDL. Cell. 93: 241–252. [DOI] [PubMed] [Google Scholar]
- 57.Mikkelsen T. S., Xu Z., Zhang X., Wang L., Gimble J. M., Lander E. S., and Rosen E. D.. 2010. Comparative epigenomic analysis of murine and human adipogenesis. Cell. 143: 156–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Grimaldi P. A., Teboul L., Gaillard D., Armengod A. V., and Amri E. Z.. 1999. Long chain fatty acids as modulators of gene transcription in preadipose cells. Mol. Cell. Biochem. 192: 63–68. [PubMed] [Google Scholar]
- 59.Georgiadi A., and Kersten S.. 2012. Mechanisms of gene regulation by fatty acids. Adv. Nutr. 3: 127–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Peters J. M., Hennuyer N., Staels B., Fruchart J. C., Fievet C., Gonzalez F. J., and Kelly D. P.. 1997. Alterations in lipoprotein metabolism in peroxisome proliferator-activated receptor α-deficient mice. J. Biol. Chem. 272: 27307–27312. [DOI] [PubMed] [Google Scholar]
- 61.Djouadi F., Weinheimer C. J., Saffitz J. E., Pitchford C., Bastin J., Gonzalez F. J., and Kelly D. P.. 1998. A gender-related defect in lipid metabolism and glucose homeostasis in peroxisome proliferator-activated receptor α -deficient mice. J. Clin. Invest. 102: 1083–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Finck B. N., Lehman J. J., Leone T. C., Welch M. J., Bennett M. J., Kovacs A., Han X., Gross R. W., Kozak R., Lopaschuk G. D., et al. . 2002. The cardiac phenotype induced by PPARalpha overexpression mimics that caused by diabetes mellitus. J. Clin. Invest. 109: 121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang J., Sambandam N., Han X., Gross R. W., Courtois M., Kovacs A., Febbraio M., Finck B. N., and Kelly D. P.. 2007. CD36 deficiency rescues lipotoxic cardiomyopathy. Circ. Res. 100: 1208–1217. [DOI] [PubMed] [Google Scholar]
- 64.Chanda D., Luiken J. J. F. P., and Glatz J. F. C.. 2016. Signaling pathways involved in cardiac energy metabolism. FEBS Lett. 590: 2364–2374. [DOI] [PubMed] [Google Scholar]
- 65.Zlobine I., Gopal K., and Ussher J. R.. 2016. Lipotoxicity in obesity and diabetes-related cardiac dysfunction. Biochim. Biophys. Acta. 1861: 1555–1568. [DOI] [PubMed] [Google Scholar]
- 66.Abdurrachim D., Luiken J. J. F. P., Nicolay K., Glatz J. F. C., Prompers J. J., and Nabben M.. 2015. Good and bad consequences of altered fatty acid metabolism in heart failure: evidence of mouse models. Cardiovasc. Res. 106: 194–205. [DOI] [PubMed] [Google Scholar]
- 67.Liu Y., Neumann D., Glatz J. F., and Luiken J. J.. Molecular mechanism of lipid-induced cardiac insulin resistance and contractile dysfunction. Prostaglandins Leukot. Essent. Fatty Acids. Epub ahead of print. June 13, 2016; doi:10.1016/j.plefa.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 68.Bonen A., Jain S. S., Snook L. A., Han X-X., Yoshida Y., Buddo K. H., Lally J. S., Pask E. D., Paglialunga S., Beaudoin M. S., et al. . 2015. Extremely rapid increase in fatty acid transport and intramyocellular lipid accumulation but markedly delayed insulin resistance after high fat feeding in rats. Diabetologia. 58: 2381–2391. [DOI] [PubMed] [Google Scholar]
- 69.Coort S. L. M., Willems J., Coumans W. A., van der Vusse G. J., Bonen A., Glatz J. F. C., and Luiken J. J. F. P.. 2002. Sulfo-N-succinimidyl esters of long chain fatty acids specifically inhibit fatty acid translocase (FAT/CD36)-mediated cellular fatty acid uptake. Mol. Cell. Biochem. 239: 213–219. [PubMed] [Google Scholar]
- 70.Steinbusch L. K., Luiken J. J., Vlasblom R., Chabowski A., Hoebers N. T., Coumans W. A., Vroegrijk I. O., Voshol P. J., Ouwens D. M., Glatz J. F., et al. . 2011. Absence of fatty acid transporter CD36 protects against Western-type diet-related cardiac dysfunction following pressure overload in mice. Am. J. Physiol. Endocrinol. Metab. 301: E618–E627. [DOI] [PubMed] [Google Scholar]
- 71.Liu Y., Steinbusch L. K. M., Nabben M., Kapsokalyvas D., van Zandvoort M., Schönleitner P., Antoons G., Simons P. J., Coumans W. A., Geomini A., et al. . 2017. Palmitate-induced vacuolar-type H+-ATPase inhibition feeds forward into insulin resistance and contractile dysfunction. Diabetes. 66: 1521–1534. [DOI] [PubMed] [Google Scholar]
- 72.Kane P. M. 2012. Targeting reversible disassembly as a mechanism of controlling V-ATPase activity. Curr. Protein Pept. Sci. 13: 117–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Luiken J. J. F. P., Chanda D., Nabben M., Neumann D., and Glatz J. F. C.. 2016. Post-translational modifications of CD36 (SR-B2): Implications for regulation of myocellular fatty acids uptake. Biochim. Biophys. Acta. 1862: 2253–2258. [DOI] [PubMed] [Google Scholar]
- 74.Asch A. S., Liu I., Briccetti F. M., Barnwell J. W., Kwakye-Berko F., Dokun A., Goldberger J., and Pernambuco M.. 1993. Analysis of CD36 binding domains: ligand specificity controlled by dephosphorylation of an ectodomain. Science. 262: 1436–1440. [DOI] [PubMed] [Google Scholar]
- 75.Smith J., Su X., El-Maghrabi R., Stahl P. D., and Abumrad N. A.. 2008. Opposite regulation of CD36 ubiquitination by fatty acids and insulin: effects on fatty acid uptake. J. Biol. Chem. 283: 13578–13585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hoosdally S. J., Andress E. J., Wooding C., Martin C. A., and Linton K. J.. 2009. The Human Scavenger Receptor CD36: glycosylation status and its role in trafficking and function. J. Biol. Chem. 284: 16277–16288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hale J. S., Otvos B., Sinyuk M., Alvarado A. G., Hitomi M., Stoltz K., Wu Q., Flavahan W., Levison B., Johansen M. L., et al. . 2014. Cancer stem cell-specific scavenger receptor CD36 drives glioblastoma progression. Stem Cells. 32: 1746–1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhao J., Zhi Z., Wang C., Xing H., Song G., Yu X., Zhu Y., Wang X., Zhang X., and Di Y.. 2017. Exogenous lipids promote the growth of breast cancer cells via CD36. Oncol. Rep. 38: 2105–2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pascual G., Avgustinova A., Mejetta S., Martín M., Castellanos A., Attolini C. S., Berenguer A., Prats N., Toll A., Hueto J. A., et al. . 2017. Targeting metastasis-initiating cells through the fatty acid receptor CD36. Nature. 541: 41–45. [DOI] [PubMed] [Google Scholar]