Abstract
Performance fatigability is characterized as an acute decline in motor performance caused by an exercise-induced reduction in force or power of the involved muscles. Multiple mechanisms contribute to performance fatigability and originate from neural and muscular processes, with the task demands dictating the mechanisms. This review highlights that (1) inadequate activation of the motoneuron pool can contribute to performance fatigability, and (2) the demands of the task and the physiological characteristics of the population assessed, dictate fatigability and the involved mechanisms. Examples of task and population differences in fatigability highlighted in this review include contraction intensity and velocity, stability and support provided to the fatiguing limb, sex differences, and aging. A future challenge is to define specific mechanisms of fatigability and to translate these findings to real-world performance and exercise training in healthy and clinical populations across the life span.
Human performance can be limited during athletic endeavors, exercise training, ergonomic tasks, and daily activities by fatigue that develop in the neuromuscular system. This fatigue is often referred to as muscle fatigue but is also referred to as performance fatigability, and is defined as an acute exercise–induced reduction in force and power output of the involved muscles that dictate performance (Gandevia 2001; Kent-Braun et al. 2012; Enoka and Duchateau 2016). Fatigability during exercise, however, is often accompanied by increased perceptions of fatigue and afferent inputs that may contribute to impaired performance (Enoka and Duchateau 2016; Taylor et al. 2016). Fatigability of muscles not only limits performance acutely but, in the long term, can be used to enhance performance. In combination with adequate dosage and recovery, fatigability causes acute overload of the neuromuscular system, which, in the long term, is necessary for neuromuscular adaptions and improved performance such as increased muscle strength and endurance.
Fatigability of performance occurs during both maximal- and submaximal-effort motor tasks, and is often quantified as a reduction in the maximal voluntary contraction (MVC) force of the involved muscles. Fatigability can develop despite successful performance of a submaximal effort task. This is illustrated with the reduction MVC force when assessed intermittently during submaximal contractions (Bigland-Ritchie et al. 1986a; Hunter et al. 2004c; Smith et al. 2007). If the submaximal task is performed for an extended duration, failure of the task will occur because the force or power requirements for successful performance become greater than the maximal strength or power capable of being exerted (Hunter et al. 2004d). Thus, the time to failure of a submaximal task can also be used to quantify fatigability of a muscle group and may more closely represent the activation and metabolic requirements of the muscles during performance of daily tasks (Enoka and Duchateau 2008).
Although motor performance is ultimately limited by the output of the active muscle(s), multiple mechanisms contribute to performance fatigability and they originate from both neural and muscular processes (Fig. 1). This means that fatigability depends on the capacity of the nervous system to provide adequate activation as well as the contractile function of the involved muscles (Enoka and Duchateau 2016). Some of the potential sites and processes of limitation are identified in Figure 1. Although there are physiological processes that limit force and power generation during a fatiguing task, some physiological adjustments are compensatory as redundant physiological systems and feedback loops attempt to maintain homeostasis (e.g., group III/IV afferent feedback from the muscles to the nervous system). Hence, identifying the primary mechanisms for the reduction in human performance during fatiguing exercise can be challenging. One approach has been to determine the task dependency of fatigability by assessing the time to failure of a submaximal task and determining the physiological adjustments when the task conditions are altered (Hunter et al. 2004d).
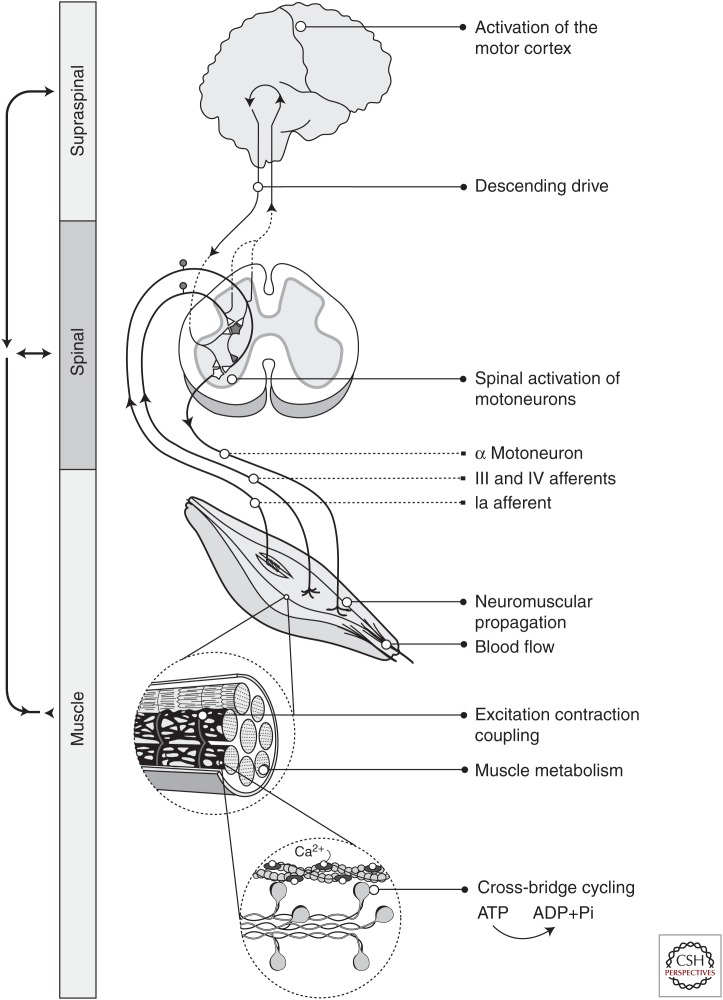
Figure 1.
Sites of fatigue. Schematic of the sites and processes that lead to force and power generation at the muscle and can contribute to performance fatigability. Processes include activation of the motor cortex, descending drive, spinal activation of the motoneuron pool, neuromuscular propagation across the neuromuscular junction, muscle perfusion (blood flow) and processes at the muscle fiber including excitation–contraction coupling, metabolism, and cross-bridge kinetics. Shown within the spinal cord schematic are selected excitatory and inhibitory pathways to the spinal cord that have been found to modulate the output of the motoneuron pool during fatiguing contractions. Motoneurons receive inhibitory inputs from a variety of sources that are mediated through inhibitory interneurons (represented as the shaded neuron and synapse in the circuitry). However, the dominant inhibitory effect from group III–IV afferents during fatiguing contractions involves a reduction in excitatory input by presynaptic inhibition of the group Ia afferents (Hunter et al. 2004d). Note that excitatory pathways are shown as white triangles and inhibitory pathways as the dark-shaded triangles.
This review presents evidence that (1) neural mechanisms contribute to performance fatigability, and (2) performance fatigability and the involved mechanisms are specific to the task demands, muscle group, and the population being tested, including the age and sex of the individual. Following is a discussion on the neural mechanisms and some of the techniques used to expose those mechanisms that contribute to fatigability. In general, muscular mechanisms of fatigability will not be discussed and they are covered more thoroughly in Cheng et al. (2017).
NEURAL MECHANISMS OF PERFORMANCE FATIGABILITY
Performance fatigability is often attributed to contractile processes (Kent-Braun et al. 2012; Debold et al. 2016); however, reductions in voluntary activation (neural drive) can play a significant role in the decline of force and power during fatiguing exercise even in healthy and motivated populations (Gandevia 2001; Enoka et al. 2011). A loss of voluntary activation of the involved muscles can account for as much as 25% of the reductions in maximal force during single-limb exercise (Gandevia 2001). Whole-body exercise also results in large reductions in voluntary activation and maximal force of the relevant muscles such as that seen after long duration exercise (e.g., ultramarathon) (Millet et al. 2002, 2003) and short duration high-intensity exercise (e.g., rowing) (Husmann et al. 2016).
There are many potential sites within the central nervous system that can limit activation during fatiguing exercise and ultimately motor output from the muscle. The motoneuron pool receives inputs from descending efferent pathways, spinal interneurons, and peripheral afferent feedback (e.g., Ia afferents; group III and IV afferents) (Fig. 1), which are then integrated within the spinal cord and can alter voluntary drive (Gandevia 2001; Enoka et al. 2011; Taylor et al. 2016). The motor output is also influenced by the intrinsic properties of the motoneurons themselves (e.g., persistent inward currents) and neuromodulators (e.g., serotonin and norepinephrine) (Taylor et al. 2016). Thus, a change in efferent inputs, afferent feedback, and the intrinsic properties of the motoneuron can alter the strength and rate of voluntary contraction. Identifying the rate-limiting mechanism(s) for the decline in power or force during fatiguing exercise, however, can be difficult because some of the physiological adjustments that accompany fatigability are compensatory rather than limiting, particularly during submaximal tasks (Taylor et al. 2016).
There are various techniques and approaches that have been used to identify mechanisms within the nervous system that contribute to fatigability. A common technique to determine the level of neural drive (voluntary activation) is stimulation of the motor nerve or motor cortex during an MVC, and the subsequent measurement of the superimposed twitch force (Gandevia et al. 1996; Gandevia 2001; Todd et al. 2016). For example, extra force evoked at the motor nerve by the electrically stimulated contraction during the maximal effort indicates that either the motor units were not all recruited voluntarily or that they were discharging at rates that were not high enough to produce full fusion of force during the MVC (Merton 1954; Belanger and McComas 1981; Herbert and Gandevia 1999). During or after a fatiguing task, an increase (growth) in the amplitude of the superimposed twitch force that is elicited during a MVC implies a failure of voluntary drive at one or more sites proximal to the site of stimulation. An acute loss of voluntary activation has been termed central fatigue, indicating that a loss of MVC force is, in part, caused by fatigue within the central nervous system (Gandevia 2001). Stimulation of the motor cortex (transcranial magnetic stimulation [TMS]), allows detection of the fatigue occurring upstream of the motor cortex that limits neural drive (Todd et al. 2016). These stimulation techniques of voluntary activation, however, are limited to select muscle groups mostly during maximal efforts (Gandevia 2001; Taylor 2009; Todd et al. 2016).
The electromyography (EMG) signal during voluntary contractions and in response to stimulation at various sites of the neuromuscular system can provide information about the net excitability in the central nervous system (Dideriksen et al. 2011; McNeil et al. 2013). For example, during sustained MVCs, the global EMG activity declines (Bigland-Ritchie et al. 1983b) because of less responsive motoneurons, a reduction in descending drive, and less afferent feedback from the muscle spindles (Taylor et al. 2016). During submaximal fatiguing contractions, the global EMG activity increases primarily because of an increase in motor unit recruitment and altered discharge rates in response to increased descending drive as the active muscle fibers become progressively fatigued and spinal excitability declines (Lippold et al. 1960; Fuglevand et al. 1993; Sogaard et al. 2006; McNeil et al. 2011). Altered single motor unit behavior (recruitment and discharge rate) during fatiguing contractions is able to be detected with intramuscular EMG recordings (Bigland-Ritchie et al. 1983a; Carpentier et al. 2001; Riley et al. 2008) and more recently with noninvasive surface multichannel array electrodes (Duchateau and Enoka 2011; Farina et al. 2016).
Corticospinal and motoneuronal excitability can be determined by assessing the change in the EMG response to stimulation during or after fatiguing exercise (Taylor et al. 1996; Sacco et al. 1997; Sidhu et al. 2009). Common sites of stimulation are the motor cortex (motor-evoked potential [MEP]), the cervicomedullary junction (cerviomedullary-evoked potential [CMEP]), motor nerve (muscle compound action potential [M wave], V wave, and F wave) (Taylor et al. 1996; Taylor and Gandevia 2008; Gruet et al. 2013; McNeil et al. 2013), and afferent nerves (Hoffman reflex [H reflex]) (Duchateau et al. 2002; Levenez et al. 2008; McNeil et al. 2013). These are some of the techniques used to show altered excitability within the central nervous system during single-limb fatiguing exercise (Taylor and Gandevia 2008) and during locomotor exercise (Sidhu et al. 2013). In addition, brain imaging such as functional magnetic resonance imaging (fMRI) during fatiguing exercise shows that cortical and subcortical areas of the brain can increase or decrease in activation during fatiguing tasks and at task failure (Liu et al. 2003; Benwell et al. 2007; van Duinen et al. 2007; Hou et al. 2016).
The techniques summarized above have been used in humans and in combination with unique experimental strategies to show altered voluntary activation and excitability of the central nervous system during fatiguing exercise. Some of the unique approaches include ischemia to enhance afferent feedback (Bigland-Ritchie et al. 1986b; Taylor et al. 1996; Butler et al. 2003; Kennedy et al. 2013), spinal blocks to reduce afferent feedback during or at termination of fatiguing exercise (Amann et al. 2009; Sidhu et al. 2014; Blain et al. 2016), pairing of various stimulations to determine potential sites of fatigue such as the spinal cord (McNeil et al. 2009, 2011), intracortical facilitation and intracortical inhibition (Hunter et al. 2016a), pharmacological manipulation of neurotransmitters such as dopamine, serotonin, and norepinephrine (Wilson and Maughan 1992; Watson et al. 2005; Roelands and Meeusen 2010; Klass et al. 2012), imposition of a cognitive challenge before or during a fatiguing exercise (Marcora et al. 2009; Yoon et al. 2009b; Keller-Ross et al. 2014), and altering the environmental conditions, including temperature (Todd et al. 2005) and inspired oxygen concentration (Goodall et al. 2010, 2012).
In general, the approaches and studies highlighted above provide evidence that (1) fatiguing exercise is often limited within the central nervous system resulting in a decrease in the activation signal to the muscle, (2) the contribution of neural mechanism can vary considerably as the task demands and environments change, and (3) the ability to drive skeletal muscle during a maximal task may not represent the limitations in activation during submaximal tasks and that are often performed during daily tasks and whole-body exercise. Thus, although both neural and muscular mechanisms contribute to exercise-induced reductions in force or power, a loss in voluntary activation can account for up to 25%–30% of performance fatigability (Gandevia 2001; Millet et al. 2002).
FATIGABILITY IS SPECIFIC TO THE TASK DEMANDS AND POPULATION
Fatigability is specific to the task demands because varying the task requirements will stress different sites or change the rate of stress of a given site within the neuromuscular system (Enoka and Stuart 1992; Bigland-Ritchie et al. 1995). Consequently, the mechanisms of fatigability and the rate that they limit performance vary with the task requirements. Performance fatigability, for example, can vary according to the contraction type, speed and intensity, the involved muscle groups, environmental conditions and state of arousal, and support provided to a limb.
Task dependency of fatigability is obvious when we consider the time to failure of an isometric contraction performed with a single limb at several different intensities altering the rate of motor unit activation and metabolic demands on the contractile proteins (Fuglevand et al. 1993; Taylor et al. 1996; Burnley et al. 2012). Similarly, the time to failure of dynamic exercise at a given power (e.g., cycling) will decrease as the intensity and power requirements increase (Vanhatalo et al. 2016; Sundberg et al. 2017), resulting in a hyperbolic relation between the intensity and the time to failure of the dynamic task (Fig. 2A) (Poole et al. 2016). Thus, intensity of contraction is an important task variable that influences fatigability because the metabolic demands and rate of motor unit activation is greater during high- compared with low-intensity tasks.
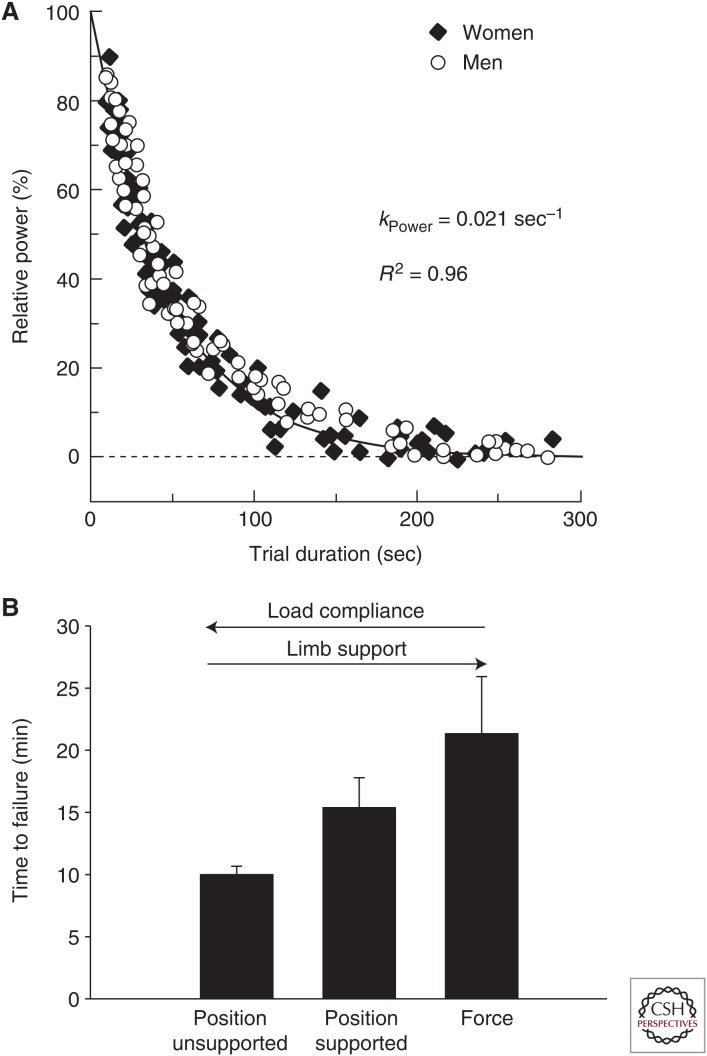
Figure 2.
Task dependency of performance fatigability. (A) Power-task duration relation for cycling in young men and women. Shown is the hyperbolic relation for cycling between power (relative to maximum power achieved during a 3-sec test at 80 rpm) and time to failure (trial duration) for multiple cycling tests performed at various power outputs. Over several days of testing, active young men (n = 7) and women (n = 7) performed trials to obtain the peak 3-sec power output (100%) followed by 11 to 14 constant-load tests to elicit failure between 3 and 300 sec. The time constant for the power-duration relationships was 0.0207/sec (R2 = 0.96) so that the greater the relative power the shorter the time to failure. The time constant and shape of the hyperbolic relation did not differ between the sexes. (Figure and data adapted with permission from Sundberg et al. 2017.) (B) Force and position task. Time to failure (mean ± SEM) of sustained isometric submaximal contractions performed with the ankle dorsiflexor muscles for a force task and two positions tasks that differed in load compliance and foot support. Data are extracted from two studies performed on a total of 23 young (18–30 yr) men (n = 12) and women (n = 11) (Hunter et al. 2008b; Yoon et al. 2009a). The subjects were seated with knee at 90 deg of flexion, the ankle in neutral position (0 deg dorsiflexion) and with a load supported at the forefoot equivalent to 20% of maximal voluntary contraction (MVC). The load compliance and support provided to the foot varied between the three tasks: There was no support provided under the foot for the “position unsupported” task, a position task with foot support (position supported) that allowed one degree of freedom of movement at the ankle in the sagittal plane, and a force task (force) in which the forefoot was rigidly attached to a force transducer.
In addition to many other task variables such as the muscle group (e.g., Frey Law and Avin 2010; Senefeld et al. 2013), contraction type (Pasquet et al. 2000; Madeleine et al. 2002; Baudry et al. 2007), and contraction velocity (Callahan and Kent-Braun 2011; Dalton et al. 2012; Yoon et al. 2013), there can also be differences in fatigability between populations, including men versus women, young versus old adults, and healthy versus clinical populations (Kent-Braun et al. 2012; Hunter 2016a,b). Differences in fatigability between populations occur because characteristics of the neuromuscular system that are common to a group of individuals will impact the physiological adjustments and rate-limiting mechanisms during a fatiguing task. Following are examples of how the task or population characteristics can influence the mechanisms and magnitude of fatigability, with an emphasis on the ability of the nervous system to provide adequate activation to the muscle.
FORCE AND POSITION TASK
A compelling example of how neural mechanisms can limit performance of a submaximal task is shown with the comparison of the time to failure of two isometric fatiguing tasks, the “force task” and the “position task.” These tasks differ in the support provided to the limb and the load compliance (Hunter et al. 2002, 2008b; Maluf et al. 2005; Klass et al. 2008b). The “force task” involves restraint of the limb to a force transducer while the individual sustains a force at a given load (e.g., 20% of MVC) with minimal compliance and maximal external limb support. The “position task” involves supporting an equivalent inertial load (e.g., a load equivalent to 20% MVC) with volitional muscle force while the subject maintains position of the limb with minimal external limb support. Thus, the load compliance for the position task is much greater than for the force task (Enoka et al. 2011). Despite a similar external load, the time to task failure for the position task is usually briefer than the force task for a range of muscle groups, including the elbow flexors, knee extensors, finger abductor, and ankle dorsiflexors (Hunter et al. 2002, 2008b; Maluf et al. 2005; Klass et al. 2008b; Rudroff et al. 2010, 2011). Figure 2B shows that the time to task failure for a force task was longer for the ankle dorsiflexor muscles (less fatigable) compared with a position task that varied in external foot support and load compliance (Hunter et al. 2008b; Yoon et al. 2009a).
The differential inputs received by the motoneuron pool during a force and position task are associated with the difference in fatigability between the tasks (i.e., the briefer time to failure for the position task). The physiological adjustments are typically more rapid during the position task than the force task, including a greater rate of increase in the mean arterial pressure, heart rate, and perceived exertion (Hunter et al. 2002, 2005b, 2008b; Rudroff et al. 2011). These data indicate greater excitation to the motoneuron pool during the position task (Rudroff et al. 2005). Thus, the rate of increase in EMG during a position task is usually greater than for a force task (Hunter et al. 2008b) because of a more rapid recruitment of motor units during the position task (Mottram et al. 2005; Baudry et al. 2009).
Accordingly, the time course of the H reflex declined more rapidly during the position task compared with the force task despite similar levels of fatigue in the muscle (quantified with the twitch contraction) and similar magnitudes of supraspinal fatigue (assessed with TMS) (Klass et al. 2008b). Additional experiments showed that the heteronymous monosynaptic Ia facilitation was greater and Ia presynaptic inhibition was reduced during the early stages of a position task compared with the force task (Baudry and Enoka 2009; Baudry et al. 2010). The heightened sensitivity of the stretch reflex during the position task to maintain a compliant load (position task) appears to lead to greater activation of gamma motoneurons and increased excitation of muscle spindles, increased Ia afferent feedback to correct deviations of limb position, and differential modulation of presynaptic Ia inhibition of the position task than the force task (Enoka et al. 2011). Thus, spinal mechanisms involving depressed synaptic input from Ia afferent feedback and a reduction the capacity of the central nervous system to provide adequate excitation to the motoneuron pool appear to be responsible for the briefer time to failure for the more compliant load in the position task compared with the force task.
SEX DIFFERENCES IN PERFORMANCE FATIGABILITY
Performance fatigability can differ between men and women for fatiguing isometric and dynamic tasks (Hunter 2016a,b). Although men are often stronger and more powerful than women for a range of muscle groups, men are usually more fatigable for isometric tasks and some slow-to-moderate velocity dynamic tasks when performed at the same intensity of contraction as women (Fig. 3). Similarly, men were more powerful than women during maximal sprint cycling but were more fatigable for men during multiple sprints, although the sex difference in power reduction was minimized for a subgroup of men and women who were matched for mechanical work (Billaut and Bishop 2012). After long-duration running (110 km), men also have shown greater reductions in MVC force of the knee extensor muscles than women competitors (Temesi et al. 2015). These sex differences in fatigability occur because the differences between men and women in anatomy, physiology, strength, and power can alter the rate that fatigue develops at sites along the neuromuscular system. Furthermore, as discussed below, the sex difference in fatigability and the responsible mechanism are dependent on the details and demands of the task (Hunter 2009, 2014).
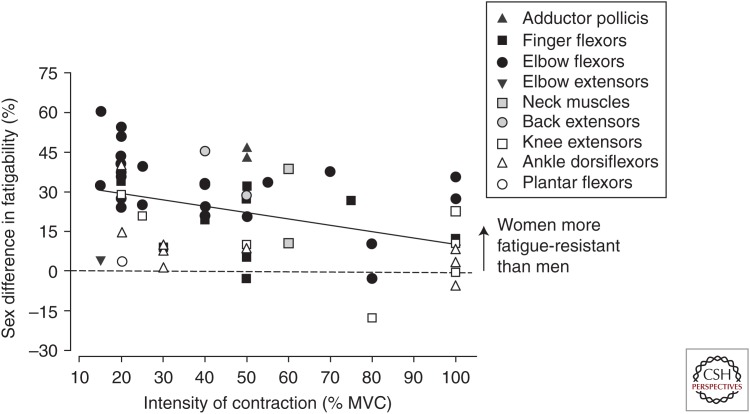
Figure 3.
Sex differences in fatigability for isometric voluntary contractions. Represented are mean data from 46 isometric contraction studies (intermittent and sustained) that assessed fatigability of men and women. Plotted is the percentage sex difference in fatigability in each study, calculated as the mean difference in fatigability between men and women as a percent of the women’s value. The fatigability values used for the calculation were either the fatigue index or time to task failure for the sustained or intermittent isometric fatiguing contractions. The x-axis represents the contraction intensity (percentage of maximal voluntary contraction [MVC]) at which the fatiguing contractions were performed. Upper limb muscles are represented in closed symbols and lower limb muscles in open symbols. Back and neck muscles are represented as gray symbols. Most data points are above the line, indicating that women were less fatigable than men for many of the muscle groups. There was a significant negative relation between the relative contraction intensity and the magnitude of the sex difference for the isometric contractions when all muscle groups were included (r2 = 0.19). (From Hunter 2016a; reprinted, with permission.)
In general, mechanisms for sex-based differences in fatigability include skeletal muscle metabolism and contractile properties (Esbjornsson et al. 1993; Russ et al. 2005; Hunter et al. 2006a; Billaut and Bishop 2009), muscle perfusion more so during submaximal sustained isometric contractions (Hunter 2016a), and voluntary activation for select tasks and muscle groups (Russ and Kent-Braun 2003; Martin and Rattey 2007). In whole muscle, women typically possess a greater proportional area of fibers that possess type I myosin heavy chain (MHC) isoforms, which rely more on oxidative metabolism and are relatively more fatigueresistant than MHC type II fibers (Hunter 2016a). The fibers of men and women do not appear to differ in the amount of force able to be produced per cross-sectional area (specific tension) (Trappe et al. 2003; Krivickas et al. 2006), and so the sexes primarily vary in strength, MHC content, and metabolic characteristics of the whole muscle owing to the different numbers and size of the fibers. Thus, during fatiguing exercise, men showed greater acidosis and peak glycolytic rates of whole muscle compared with women, and women showed greater oxidative capacity than men (Fig. 4) (Esbjornsson et al. 1993; Russ et al. 2005).
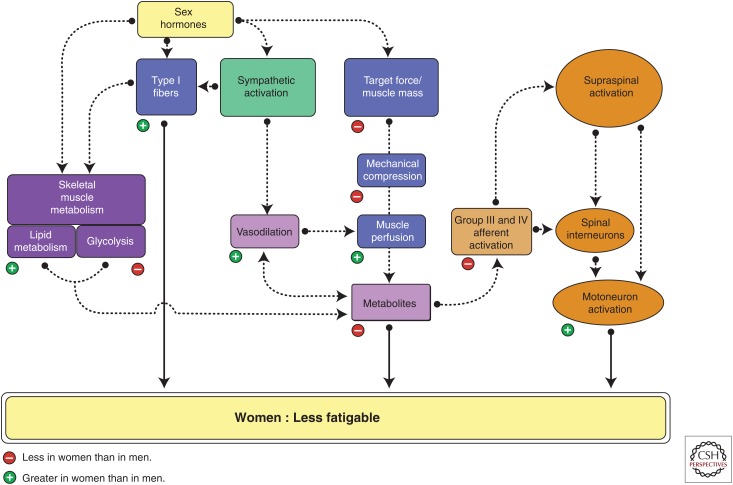
Figure 4.
Sex differences in fatigability. Shown are potential mechanisms that may contribute to the sex differences in fatigability and when women are less fatigable than men during fatiguing contractions. Mechanisms in the muscle, central nervous system, and feedback between them are featured. The contribution of potential mechanisms, however, can vary with the task demands, environmental conditions, and muscle group. A negative sign indicates that the physiological variable or process is less in women than men and, conversely, a positive sign indicates that it is greater in women than men. (From Hunter 2014; adapted, with permission.)
The mechanisms for the sex differences in fatigability also can include activation of the motoneuron pool from cortical and subcortical regions. Differences between men and women in synaptic inputs to the motoneuron pool during fatiguing contractions is likely affected by differential activation of metabolically sensitive small afferent fibers in the muscle (Fig. 4). For example, reductions in voluntary activation were greater in men than women for isometric fatiguing contractions during lower limb exercise (Russ and Kent-Braun 2003; Martin and Rattey 2007). The greater loss of neural drive in the men during these high-intensity contractions was probably attributable to a larger accumulation of metabolites in the active skeletal muscle. Group III and IV muscle afferents are sensitive to ischemia and metabolite accumulation (Martin et al. 2008; Kaufman 2011; Murphy et al. 2011) associated with fatigue and pain (Taylor et al. 2016). At the spinal cord, group III and IV afferents excite or inhibit motoneurons, and this may depend on the muscle group (extensors vs. flexors) and whether the excitation is from pain or metabolites (Martin et al. 2006, 2008). Excitation of the group III and IV afferents, however, appears to result in a net decrease of motor unit discharge rates and reduced voluntary activation (e.g., Woods et al. 1987; Martin et al. 2006, 2008; Dideriksen et al. 2010; Amann 2012; Rossman et al. 2012; Kennedy et al. 2013, 2015). The primary mechanism(s) for the sex differences in fatigability, however, is not always easy to identify because of the feedback loop between muscle and the central nervous system (Fig. 1).
The reduction in voluntary activation during a fatiguing task, however, can differ between men and women for different muscle groups. At baseline (before fatiguing exercise), voluntary activation is typically similar for men and women for most muscle groups (Russ and Kent-Braun 2003; Hunter et al. 2006a; Keller et al. 2011). For the elbow flexor muscles, voluntary activation can be significantly reduced in both men and women for both isometric and slow dynamic fatiguing contractions, and typically there is no sex difference in the reduction of voluntary activation (Hunter et al. 2006a; Yoon et al. 2007, 2015; Keller et al. 2011). However, this is in contrast to studies that have shown a larger reduction in voluntary activation for men than women during fatiguing exercise of the lower limb muscles coupled with greater fatigability of the men (Russ and Kent-Braun 2003; Martin and Rattey 2007). This sex difference in voluntary activation likely involved greater activation and inhibitory greater feedback from metabolically sensitive group III and IV fibers.
The above discussion illustrates that the sex difference in fatigability and the responsible mechanism are specific to the task demands and the involved muscles. There are a number of task variables that, when altered, potentially change the mechanisms and magnitude of the sex difference in performance fatigability, with some of the known variables summarized in Table 1. The sex difference in fatigability is task-specific because when the requirements of the task are altered the neuromuscular sites of fatigue can differ or be stressed at different rates for men and women (Hunter 2009). For example, the sex difference in the elbow flexor muscles is typically greater than for the ankle dorsiflexor muscles (Avin et al. 2010). There are also no apparent sex differences in fatigability for the elbow extensor muscles during low-intensity isometric contractions (Dearth et al. 2010), although large sex differences are observed for the elbow flexor muscles during sustained and intermittent tasks (Sato and Ohashi 1989; Hunter and Enoka 2001; Hunter et al. 2004c, 2009). The greater fatigability of men compared with women for isometric contractions with the elbow flexor muscles is, in part, because of contractile mechanisms and sometimes muscle perfusion during low-to-moderate intensity sustained contractions when men are stronger than women (Hunter and Enoka 2001; Hunter et al. 2004b, 2006a,b; Keller et al. 2011). Strength-related differences in muscle perfusion cannot account for the greater fatigue resistance of women than men during intermittent contractions when the sexes are closely matched for strength (Hunter et al. 2004c).
Table 1.
Task variables that influence the sex differences in fatigability
| Variables | Fatigability | Examples of supporting studies |
|---|---|---|
| Intensity of contraction | ||
| Low intensity | M > F | Hunter and Enoka 2001; Yoon et al. 2007; Keller et al. 2011 |
| Low intensity strength matched | M = F | Hunter et al. 2004b, 2006b |
| Moderate intensity | M > F | Fulco et al. 1999; Hunter et al. 2004c, 2009 |
| High intensity | M > F | Russ and Kent-Braun 2003; Hunter et al. 2006a; Martin and Rattey 2007 |
| M = F | Maughan et al. 1986; Yoon et al. 2007 | |
| Muscle group (isometric only) | ||
| Adductor pollicis | M > F | Fulco et al. 1999 |
| Elbow flexors | M > F | Hunter et al. 2004c; Avin et al. 2010; Keller et al. 2011 |
| Finger flexors (handgrip) | M > F | West et al. 1995; Hunter et al. 2006b |
| Elbow extensors | M = F | Dearth et al. 2010 |
| Trunk flexors | M = F | Deering et al. 2017 |
| Trunk extensors | M > F | Clark et al. 2003; Lariviere et al. 2006 |
| Knee extensors | M > F | Clark et al. 2005 |
| Ankle dorsiflexors | M > F | Russ and Kent-Braun 2003 |
| M = F | Kent-Braun et al. 2002; Hunter et al. 2008b; Avin et al. 2010 | |
| Velocity of contraction | ||
| Slow velocity concentric | M > F | Yoon et al. 2015 |
| Moderate velocity concentric | M > F | Pincivero et al. 2003 |
| High velocity concentric | M = F | Senefeld et al. 2013 |
| Slow to moderate eccentric | M = F | Hubal et al. 2008; Lee et al. 2017 |
| F > M | Sewright et al. 2008; Power et al. 2013 | |
| Cognitive challenge imposed | ||
| Elbow flexors (isometric) | F increase fatigability; M minimal change | Yoon et al. 2009b; Keller-Ross et al. 2014; Pereira et al. 2015 |
| Ankle dorsiflexors (isometric) | F and M no change | Vanden Noven et al. 2014 |
Shown are task variables that are known to alter the magnitude of the sex difference in performance fatigability during single-limb exercise, along with examples of how that variable can change and the general findings to date. Examples of supporting studies are cited in the last column although the list is not exhaustive.
F, Females; M, males.
M > F, Males are more fatigable than females.
F > M, Females are more fatigable than males.
M = F, Females are similar in fatigability.
The importance of the cortical activity in limiting performance during submaximal tasks of men and women is seen when a cognitive challenge is performed before fatiguing exercise (Bray et al. 2008; Marcora et al. 2009; Pageaux et al. 2013) and also when it is imposed during a fatiguing contraction (Yoon et al. 2009b; Keller-Ross et al. 2014; Pereira et al. 2015). Imposing a cognitive challenge is another task variable that can alter the fatigability of men and women differently (Table 1). For example, during control conditions without a cognitive challenge imposed, women were able to sustain an isometric fatiguing contraction at 20% MVC with the elbow flexor muscles longer than men (e.g., Hunter and Enoka 2001; Keller et al. 2011). The time to failure, however, was reduced when the cognitive challenge of counting backward by a two-digit number (e.g., 13 from a four-digit number) was imposed during the fatiguing contraction (Yoon et al. 2009b; Keller-Ross et al. 2014; Pereira et al. 2015). The reduction in time to failure (increased fatigability) was much greater for the women than the men despite similar decreases in MVC force at the end of the fatiguing contraction and a similar reduction in voluntary activation upstream of the motor cortex (assessed with TMS) (Keller-Ross et al. 2014). These differences in fatigability with a cognitive challenge and the interaction with the sex of the individual were not observed in the ankle dorsiflexor muscles (Vanden Noven et al. 2014), reinforcing that the sex difference in fatigability can differ across muscle groups. The primary mechanisms for the altered sex difference in fatigability with the cognitive challenge in the elbow flexors are not fully understood. These examples, however, indicate that greater cortical inputs during a fatiguing task or depletion of cognitive resources before a fatiguing task can increase the rate that fatigue develops in the neuromuscular system for men and women, and for some muscle groups such as the elbow flexors alter the sex difference in fatigability.
Although the sex difference for isometric fatiguing tasks is relatively robust for many muscle groups (Fig. 3), the sex difference is largely diminished for fast-velocity single-limb contractions (Senefeld et al. 2013; Hunter 2016b). For example, the reduction in power with a load equivalent to 20% MVC was similar for men and women (both young and old adults) who performed repeated fast velocity contractions with the elbow flexor and knee extensor muscles (Senefeld et al. 2013, 2016). How the sex difference in fatigability during dynamic exercise varies across different contraction intensities and interacts with contraction velocity is not known. Interestingly, for lower limb cycling, the time constant that describes the power–duration relationships across intensities above an individual’s critical power was similar for trained young men and women (Fig. 2A) (Sundberg et al. 2017). The power–time relationship and critical power is dependent on the metabolic responses and MHC composition in the muscle (Poole et al. 2016; Vanhatalo et al. 2016). The effect of sex on fatigability around the critical power thresholds is relatively unexplored and presents with many opportunities for future studies across a range of tasks.
AGE DIFFERENCES IN PERFORMANCE FATIGABILITY
Age-related changes within the neuromuscular system can alter the rate at which a site within the neuromuscular system is stressed during repeated or sustained activation, often resulting in differences in fatigability between young and old adults. Compared with young adults, some of the changes in the neuromuscular system of old adults (> 60 yr) include neuronal atrophy throughout the central nervous system (Ward 2006; Clark and Taylor 2011), a net loss of motor units (Campbell et al. 1973; Tomlinson and Irving 1977), degradation of the neuromuscular junction (Hepple and Rice 2016), decreased muscle mass (sarcopenia) because of a reduction in the number and size of the surviving motor units (Lexell et al. 1988), a shift in the composition of MHC within fibers to a smaller proportional area of muscle that expresses MHC IIa isoforms in old muscle compared with young (Larsson and Karlsson 1978; Larsson et al. 1979; Hunter et al. 1999; Purves-Smith et al. 2014), and slowed calcium kinetics and reduced efficiency of the contractile and structural proteins of the muscle fiber (for review, see Hunter et al. 2016b). Specific tension of single fibers (force/fiber cross-sectional area) may be reduced with aging in individuals ∼70–73 yr compared with young adults (Larsson et al. 1997; D’Antona et al. 2003; Lamboley et al. 2015; Power et al. 2016) and even in very old master’s athletes (∼80 yr) (Power et al. 2016). Some studies, however, indicate specific tension is preserved with aging especially when young and old adults are matched for physical activity levels (Trappe et al. 2003; Venturelli et al. 2015; for review, see Hunter et al. 2016b). These age-related changes tend to accelerate into very old age (> 80 yr) but may also be exacerbated by other factors such as disuse, genetics, nutrition (decreased total caloric and protein intake), altered hormonal status, inflammatory mediators, and factors resulting in altered protein synthesis and sarcopenia (Doherty 2003; Degens and Korhonen 2012). Furthermore, the age-related reductions in muscle function and skeletal muscle fiber characteristics are not typically uniform across the different muscles with greater reductions in strength, power, and muscle cross-sectional area in the lower limb such as the knee extensor muscles compared with the elbow flexor and handgrip muscles (Hunter et al. 2000; Raj et al. 2010; Venturelli et al. 2014, 2015). Thus, some old adults have experienced larger alterations within the neuromuscular system compared with others of the same age and sex so that the between-subject variability in motor function including fatigability is typically greater among old adults than young adults (Rantanen et al. 1998; Christou 2011; Degens and Korhonen 2012; Vanden Noven et al. 2014; Hunter et al. 2016b).
Old adults also tend to show greater variability during performance of a motor task than young (Enoka et al. 2003; Hunter et al. 2016b), including repeated activation during a fatiguing task (Kent-Braun et al. 2014; Senefeld et al. 2016). Thus, the within-subject variability of repeated activation is larger with aging, particularly those who are >80 yr, frail, and inactive (Hunter et al. 2008a; Kent-Braun et al. 2014; Vanden Noven et al. 2014; Senefeld et al. 2016). The mechanisms for the large age-related variability in motor output during fatiguing exercise are not known but are consistent with increased variability of voluntary activation during repeated MVCs (Hunter et al. 2016b), suggesting that neural mechanisms may be responsible. Certainly, the large between- and within-subject variability among old adults for strength and fatiguing performance can make the comparison of young and old adults challenging, particularly in studies of cross-sectional design. However, it is also clear that advanced age is often accompanied by lower predictability of performance during fatiguing motor tasks of an already compromised neuromuscular system.
Consequently, when young and old adults perform fatiguing contractions, their muscles fatigue at different rates to that of young adults, although the underlying mechanisms depend on task demands (Hunter et al. 2016b). Healthy old adults (∼60–75 yr) are typically less fatigable than young for maximal and submaximal isometric fatiguing contractions performed at the same relative intensity of contraction. This decreased fatigability is shown even when young and old adults are matched for strength (Hunter et al. 2005a) and also occurs for both men and women (Hunter et al. 2004a; Christie et al. 2011; Yoon et al. 2012). For both men and women, the mechanism for the age difference in fatigability is associated with a more fatigue-resistant muscle that accompanies aging (Kent-Braun 2009; Hunter et al. 2016b). During isometric contractions, old muscle shows less reliance on glycolytic metabolism compared with young, and a lower accumulation of inorganic phosphate and hydrogen ions that often interfere with force production (Lanza et al. 2007; Kent-Braun 2009; Callahan et al. 2016). Consistent with these findings, old adults show a larger proportional area of fibers expressing MHC I isoforms (Larsson et al. 1979; Lexell et al. 1988; Hunter et al. 1999; Purves-Smith et al. 2014) and slower contractile properties (Vandervoort and McComas 1986; Narici et al. 1991; Hunter et al. 2008a; Callahan and Kent-Braun 2011; Molenaar et al. 2013; Yoon et al. 2013).
Voluntary activation and cortical inputs can also affect the age difference in fatigability and recovery of force after isometric fatiguing contractions. For example, imposition of a cognitive challenge during performance of an isometric contraction increased fatigability (decreased time to task failure) in old men and women (Pereira et al. 2015). Imposition of a cognitive challenge also resulted in increased variability of motor output during an isometric contraction seen as larger fluctuations in force and lower predictability of the time to task failure for old adults compared with the young adults (Vanden Noven et al. 2014; Pereira et al. 2015). Furthermore, reductions in voluntary activation and motor unit discharge rates in the muscles of old adults compared with young adults contributed to a slower and delayed recovery of force after an isometric fatiguing contractions (Hunter et al. 2008a; Dalton et al. 2010a; Yoon et al. 2012).
In contrast to fatigability of isometric tasks and slow dynamic contraction tasks, old adults are generally more fatigable than young adults for fatiguing tasks involving fast-velocity contractions. For slow-to-moderate velocity contractions (∼<180 deg/sec) with the elbow flexor and knee extensor muscles (Callahan et al. 2009; Dalton et al. 2012; Yoon et al. 2013), old adults were less fatigable than young adults. However, old adults were more fatigable than young adults for high-velocity concentric contractions with the elbow flexor, ankle dorsiflexor, plantar flexor, and knee extensor muscles (McNeil and Rice 2007; Dalton et al. 2010b; Callahan and Kent-Braun 2011; Senefeld et al. 2016; Wallace et al. 2016).
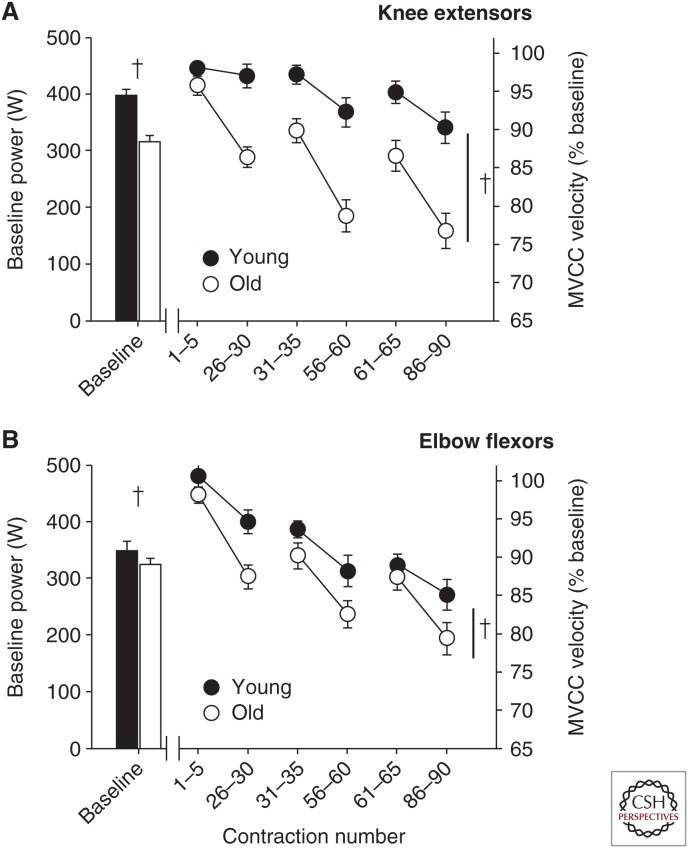
The age differences in fatigability and the altered relationship with contraction velocity depends on the muscle groups and the age of the individual. For fast velocity contractions with a load equivalent to 20% of MVC, old adults (∼71 yr) were more fatigable than young adults (∼21 yr) (Senefeld et al. 2016). The age difference in the reduction in power during the fatiguing task, however, was greater for the knee extensor muscles (∼36%) than the elbow flexor muscles (∼10%) (Fig. 5) (Senefeld et al. 2016). These results are consistent with the greater age-associated reduction in strength, power, and cross-sectional area observed in lower limb muscles possibly mediated, in part, by disuse (Hunter et al. 2000; Raj et al. 2010; Venturelli et al. 2014, 2015). There were no sex differences in fatigability of the fast contractions for the young or the old adults in this study for either muscle group (Senefeld et al. 2016). The mechanisms for the muscle- and velocity-dependent age difference in fatigability appears to involve contractile mechanisms associated with a slower shortening velocity that occurs with advanced age (Baudry et al. 2007; Callahan and Kent-Braun 2011; Yoon et al. 2013). Thus, the slower shortening velocity of the muscle fibers of old adults (Larsson et al. 1997) and a net loss of motor units, especially high-threshold type II motor units, may contribute to an increase in fatigability during high-velocity fatiguing tasks. Furthermore, old adults have shown less optimal activation with advanced age when rapid activation is required (Klass et al. 2008a; Wallace et al. 2016), so that both neural and muscular mechanisms likely contribute to the greater fatigability with aging during high-velocity dynamic contractions.
Figure 5.
Age differences in fatigability with dynamic contractions. Peak power with a load equivalent to 20% maximal voluntary isometric contraction (MVIC) torque at baseline (in Watts [W], left y-axis) and during 90 maximal-effort velocity contractions (normalized to baseline [%], right y-axis) for the knee extensor (A), and elbow flexor (B) muscles. The absolute power (W) was the average of six contractions performed before the fatiguing task by 35 young adults (21.0 ± 2.6 yr; 16 men and 19 women) and 32 old adults (71.3 ± 6.3 yr; 18 men and 14 women). Participants performed 90 maximal-effort, fast, concentric, isotonic contractions (one contraction/3 sec, three sets of 30 contractions separated by ∼10 sec) with a 20% MVIC load with the elbow flexor and knee extensor muscles on separate days. Shown are means (±SEM) of five contractions at the start and end of the three sets of contractions and normalized to the baseline values. Old adults had greater reductions in power than young adults with a larger age difference for the knee extensor (36% age difference) than the elbow flexor muscles (10% age difference) (age difference is shown by a daggar, P < 0.05). MVCC, Maximal voluntary concentric contraction. (Data adapted from Senefeld et al. 2016.)
Performance fatigability is also greater with very advanced age (>∼75–80 yr), especially in lower limb muscles. During a dynamic fatiguing task with the ankle dorsiflexor muscles, very old men (∼84 yr) showed greater reductions in power (fatigability) than young men (∼24 yr), but there were minimal differences in the reductions in power production between the old men (∼64 yr) and young men (McNeil and Rice 2007). Furthermore, the ankle dorsiflexor muscles of old adults aged ∼77 yr were more fatigable than young (∼31 yr) for repeated maximal concentric and eccentric contractions performed at 50 deg/sec−1 (Baudry et al. 2007). It is not clear, however, if this increased fatigability in the very old adults (∼77 yr) was because of advanced age or was unique to the ankle dorsiflexor muscles at slower velocities.
The age-related fatigue resistance during isometric tasks in adults <75 yr discussed previously, appears to shift with advanced age because old adults >75 yr were more fatigable for a sustained isometric fatiguing contraction for the ankle dorsiflexor muscles (Justice et al. 2014). The underlying cause for this shift in the difference in fatigability between the old and very old adults is not clear but is consistent with the general demise of the aging neuromuscular system that accelerates after ∼75 yr (Hunter et al. 2016b). Cross-sectional studies show that maximal strength and power decline at much greater rates in people >∼75 yr (Lindle et al. 1997; Hunter et al. 2000; Metter et al. 2004; Reid and Fielding 2012). Thus, there may be a threshold around ∼75 yr in which accelerated declines in motor function occur, and this includes altered fatigability during both isometric and dynamic fatiguing contractions especially in the lower limb muscles. However, there remains a limited understanding of the mechanisms for the age difference in fatigability with very advanced age, particularly in women, and also the associations between fatigability, functional performance, frailty, and disability.
CONCLUSIONS
Performance fatigability and the responsible mechanisms are dictated by the task demands, muscle group, and the characteristics of the population assessed. Some of the variables known to influence fatigability of limb muscle groups include the degree of limb support and load compliance, contraction type, contraction intensity and velocity, cognitive demand required during a fatiguing task, and the sex and age of the individual. Although motor output ultimately occurs at the muscle, neural mechanisms often contribute to performance fatigability because the activation signal is not adequate to optimally activate all of the available motor units for the given task demands. However, the contribution of neural mechanisms is dictated by the task demands and population characteristics. The challenge is to define specific mechanisms of task dependency of fatigability and test their relevance in different types of real-world activities when performed by men and women across the life span. Determining these mechanisms will also have relevance to vulnerable populations, such as those with chronic disease who are often limited by fatiguing daily functional activities.
Footnotes
Editors: Juleen R. Zierath, Michael J. Joyner, and John A. Hawley
Additional Perspectives on The Biology of Exercise available at www.perspectivesinmedicine.org
REFERENCES
*Reference is also in this collection.
- Amann M. 2012. Significance of group III and IV muscle afferents for the endurance exercising human. Clin Exp Pharmacol Physiol 39: 831–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann M, Proctor LT, Sebranek JJ, Pegelow DF, Dempsey JA. 2009. Opioid-mediated muscle afferents inhibit central motor drive and limit peripheral muscle fatigue development in humans. J Physiol 587: 271–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avin KG, Naughton MR, Ford BW, Moore HE, Monitto-Webber MN, Stark AM, Gentile AJ, Law LA. 2010. Sex differences in fatigue resistance are muscle group dependent. Med Sci Sports Exerc 42: 1943–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudry S, Enoka RM. 2009. Influence of load type on presynaptic modulation of Ia afferent input onto two synergist muscles. Exp Brain Res 199: 83–88. [DOI] [PubMed] [Google Scholar]
- Baudry S, Klass M, Pasquet B, Duchateau J. 2007. Age-related fatigability of the ankle dorsiflexor muscles during concentric and eccentric contractions. Eur J Appl Physiol 100: 515–525. [DOI] [PubMed] [Google Scholar]
- Baudry S, Rudroff T, Pierpoint LA, Enoka RM. 2009. Load type influences motor unit recruitment in biceps brachii during a sustained contraction. J Neurophysiol 102: 1725–1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudry S, Maerz AH, Enoka RM. 2010. Presynaptic modulation of Ia afferents in young and old adults when performing force and position control. J Neurophysiol 103: 623–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belanger AY, McComas AJ. 1981. Extent of motor unit activation during effort. J Appl Physiol Respir Environ Exerc Physiol 51: 1131–1135. [DOI] [PubMed] [Google Scholar]
- Benwell NM, Mastaglia FL, Thickbroom GW. 2007. Changes in the functional MR signal in motor and non-motor areas during intermittent fatiguing hand exercise. Exp Brain Res 182: 93–97. [DOI] [PubMed] [Google Scholar]
- Bigland-Ritchie B, Johansson R, Lippold OC, Smith S, Woods JJ. 1983a. Changes in motoneurone firing rates during sustained maximal voluntary contractions. J Physiol 340: 335–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigland-Ritchie B, Johansson R, Lippold OC, Woods JJ. 1983b. Contractile speed and EMG changes during fatigue of sustained maximal voluntary contractions. J Neurophysiol 50: 313–324. [DOI] [PubMed] [Google Scholar]
- Bigland-Ritchie B, Furbush F, Woods JJ. 1986a. Fatigue of intermittent submaximal voluntary contractions: Central and peripheral factors. J Appl Physiol (1985) 61: 421–429. [DOI] [PubMed] [Google Scholar]
- Bigland-Ritchie BR, Dawson NJ, Johansson RS, Lippold OC. 1986b. Reflex origin for the slowing of motoneurone firing rates in fatigue of human voluntary contractions. J Physiol 379: 451–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigland-Ritchie B, Rice CL, Garland SJ, Walsh ML. 1995. Task-dependent factors in fatigue of human voluntary contractions. Adv Exp Med Biol 384: 361–380. [DOI] [PubMed] [Google Scholar]
- Billaut F, Bishop D. 2009. Muscle fatigue in males and females during multiple-sprint exercise. Sports Med 39: 257–278. [DOI] [PubMed] [Google Scholar]
- Billaut F, Bishop DJ. 2012. Mechanical work accounts for sex differences in fatigue during repeated sprints. Eur J Appl Physiol 112: 1429–1436. [DOI] [PubMed] [Google Scholar]
- Blain GM, Mangum TS, Sidhu SK, Weavil JC, Hureau TJ, Jessop JE, Bledsoe AD, Richardson RS, Amann M. 2016. Group III/IV muscle afferents limit the intramuscular metabolic perturbation during whole body exercise in humans. J Physiol 594: 5303–5315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray SR, Martin Ginis KA, Hicks AL, Woodgate J. 2008. Effects of self-regulatory strength depletion on muscular performance and EMG activation. Psychophysiology 45: 337–343. [DOI] [PubMed] [Google Scholar]
- Burnley M, Vanhatalo A, Jones AM. 2012. Distinct profiles of neuromuscular fatigue during muscle contractions below and above the critical torque in humans. J Appl Physiol (1985) 113: 215–223. [DOI] [PubMed] [Google Scholar]
- Butler JE, Taylor JL, Gandevia SC. 2003. Responses of human motoneurons to corticospinal stimulation during maximal voluntary contractions and ischemia. J Neurosci 23: 10224–10230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan DM, Kent-Braun JA. 2011. Effect of old age on human skeletal muscle force-velocity and fatigue properties. J Appl Physiol 111: 1345–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan DM, Foulis SA, Kent-Braun JA. 2009. Age-related fatigue resistance in the knee extensor muscles is specific to contraction mode. Muscle Nerve 39: 692–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan DM, Umberger BR, Kent JA. 2016. Mechanisms of in vivo muscle fatigue in humans: Investigating age-related fatigue resistance with a computational model. J Physiol 594: 3407–3421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell MJ, McComas AJ, Petito F. 1973. Physiological changes in ageing muscles. J Neurol Neurosurg Psychiatry 36: 174–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpentier A, Duchateau J, Hainaut K. 2001. Motor unit behaviour and contractile changes during fatigue in the human first dorsal interosseus. J Physiol 534: 903–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Cheng AJ, Place N, Westerblad H. 2017. Molecular basis for exercise-induced fatigue: The importance of strictly controlled cellular Ca2+ handling. Cold Spring Harb Perspect Med 10.1101/cshperspect.a29710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie A, Snook EM, Kent-Braun JA. 2011. Systematic review and meta-analysis of skeletal muscle fatigue in old age. Med Sci Sports Exerc 43: 568–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christou EA. 2011. Aging and variability of voluntary contractions. Exerc Sport Sci Rev 39: 77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark BC, Taylor JL. 2011. Age-related changes in motor cortical properties and voluntary activation of skeletal muscle. Curr Aging Sci 4: 192–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark BC, Manini TM, The DJ, Doldo NA, Ploutz-Snyder LL. 2003. Gender differences in skeletal muscle fatigability are related to contraction type and EMG spectral compression. J Appl Physiol 94: 2263–2272. [DOI] [PubMed] [Google Scholar]
- Clark BC, Collier SR, Manini TM, Ploutz-Snyder LL. 2005. Sex differences in muscle fatigability and activation patterns of the human quadriceps femoris. Eur J Appl Physiol 94: 196–206. [DOI] [PubMed] [Google Scholar]
- Dalton BH, Harwood B, Davidson AW, Rice CL. 2010a. Recovery of motoneuron output is delayed in old men following high-intensity fatigue. J Neurophysiol 103: 977–985. [DOI] [PubMed] [Google Scholar]
- Dalton BH, Power GA, Vandervoort AA, Rice CL. 2010b. Power loss is greater in old men than young men during fast plantar flexion contractions. J Appl Physiol 109: 1441–1447. [DOI] [PubMed] [Google Scholar]
- Dalton BH, Power GA, Vandervoort AA, Rice CL. 2012. The age-related slowing of voluntary shortening velocity exacerbates power loss during repeated fast knee extensions. Exp Gerontol 47: 85–92. [DOI] [PubMed] [Google Scholar]
- D’Antona G, Pellegrino MA, Adami R, Rossi R, Carlizzi CN, Canepari M, Saltin B, Bottinelli R. 2003. The effect of ageing and immobilization on structure and function of human skeletal muscle fibres. J Physiol 552: 499–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dearth DJ, Umbel J, Hoffman RL, Russ DW, Wilson TE, Clark BC. 2010. Men and women exhibit a similar time to task failure for a sustained, submaximal elbow extensor contraction. Eur J Appl Physiol 108: 1089–1098. [DOI] [PubMed] [Google Scholar]
- Debold EP, Fitts RH, Sundberg CW, Nosek TM. 2016. Muscle fatigue from the perspective of a single crossbridge. Med Sci Sports Exerc 48: 2270–2280. [DOI] [PubMed] [Google Scholar]
- Deering RE, Senefeld JW, Pashibin T, Neumann DA, Hunter SK. 2017. Muscle function and fatigability of trunk flexors in males and females. Biol Sex Differ 10.1186/s13293-017-0133-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degens H, Korhonen MT. 2012. Factors contributing to the variability in muscle ageing. Maturitas 73: 197–201. [DOI] [PubMed] [Google Scholar]
- Dideriksen JL, Farina D, Baekgaard M, Enoka RM. 2010. An integrative model of motor unit activity during sustained submaximal contractions. J Appl Physiol 108: 1550–1562. [DOI] [PubMed] [Google Scholar]
- Dideriksen JL, Enoka RM, Farina D. 2011. Neuromuscular adjustments that constrain submaximal EMG amplitude at task failure of sustained isometric contractions. J Appl Physiol 111: 485–494. [DOI] [PubMed] [Google Scholar]
- Doherty TJ. 2003. Invited review: Aging and sarcopenia. J Appl Physiol 95: 1717–1727. [DOI] [PubMed] [Google Scholar]
- Duchateau J, Enoka RM. 2011. Human motor unit recordings: Origins and insight into the integrated motor system. Brain Res 1409: 42–61. [DOI] [PubMed] [Google Scholar]
- Duchateau J, Balestra C, Carpentier A, Hainaut K. 2002. Reflex regulation during sustained and intermittent submaximal contractions in humans. J Physiol 541: 959–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoka RM, Duchateau J. 2008. Muscle fatigue: What, why and how it influences muscle function. J Physiol 586: 11–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoka RM, Duchateau J. 2016. Translating fatigue to human performance. Med Sci Sports Exerc 48: 2228–2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoka RM, Stuart DG. 1992. Neurobiology of muscle fatigue. J Appl Physiol 72: 1631–1648. [DOI] [PubMed] [Google Scholar]
- Enoka RM, Christou EA, Hunter SK, Kornatz KW, Semmler JG, Taylor AM, Tracy BL. 2003. Mechanisms that contribute to differences in motor performance between young and old adults. J Electromyogr Kinesiol 13: 1–12. [DOI] [PubMed] [Google Scholar]
- Enoka RM, Baudry S, Rudroff T, Farina D, Klass M, Duchateau J. 2011. Unraveling the neurophysiology of muscle fatigue. J Electromyogr Kinesiol 21: 208–219. [DOI] [PubMed] [Google Scholar]
- Esbjornsson M, Sylven C, Holm I, Jansson E. 1993. Fast twitch fibres may predict anaerobic performance in both females and males. Int J Sports Med 14: 257–263. [DOI] [PubMed] [Google Scholar]
- Farina D, Negro F, Muceli S, Enoka RM. 2016. Principles of motor unit physiology evolve with advances in technology. Physiology 31: 83–94. [DOI] [PubMed] [Google Scholar]
- Frey Law LA, Avin KG. 2010. Endurance time is joint-specific: A modelling and meta-analysis investigation. Ergonomics 53: 109–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuglevand AJ, Zackowski KM, Huey KA, Enoka RM. 1993. Impairment of neuromuscular propagation during human fatiguing contractions at submaximal forces. J Physiol 460: 549–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulco CS, Rock PB, Muza SR, Lammi E, Cymerman A, Butterfield G, Moore LG, Braun B, Lewis SF. 1999. Slower fatigue and faster recovery of the adductor pollicis muscle in women matched for strength with men. Acta Physiol Scand 167: 233–239. [DOI] [PubMed] [Google Scholar]
- Gandevia SC. 2001. Spinal and supraspinal factors in human muscle fatigue. Physiol Rev 81: 1725–1789. [DOI] [PubMed] [Google Scholar]
- Gandevia SC, Allen GM, Butler JE, Taylor JL. 1996. Supraspinal factors in human muscle fatigue: Evidence for suboptimal output from the motor cortex. J Physiol 490: 529–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodall S, Ross EZ, Romer LM. 2010. Effect of graded hypoxia on supraspinal contributions to fatigue with unilateral knee-extensor contractions. J Appl Physiol 109: 1842–1851. [DOI] [PubMed] [Google Scholar]
- Goodall S, Gonzalez-Alonso J, Ali L, Ross EZ, Romer LM. 2012. Supraspinal fatigue after normoxic and hypoxic exercise in humans. J Physiol 590: 2767–2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruet M, Temesi J, Rupp T, Levy P, Millet GY, Verges S. 2013. Stimulation of the motor cortex and corticospinal tract to assess human muscle fatigue. Neuroscience 231: 384–399. [DOI] [PubMed] [Google Scholar]
- Hepple RT, Rice CL. 2016. Innervation and neuromuscular control in ageing skeletal muscle. J Physiol 594: 1965–1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert RD, Gandevia SC. 1999. Twitch interpolation in human muscles: Mechanisms and implications for measurement of voluntary activation. J Neurophysiol 82: 2271–2283. [DOI] [PubMed] [Google Scholar]
- Hou LJ, Song Z, Pan ZJ, Cheng JL, Yu Y, Wang J. 2016. Decreased activation of subcortical brain areas in the motor fatigue state: An fMRI study. Front Psychol 7: 1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubal MJ, Rubinstein SR, Clarkson PM. 2008. Muscle function in men and women during maximal eccentric exercise. J Strength Cond Res 22: 1332–1338. [DOI] [PubMed] [Google Scholar]
- Hunter SK. 2009. Sex differences and mechanisms of task-specific muscle fatigue. Exerc Sport Sci Rev 37: 113–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter SK. 2014. Sex differences in human fatigability: Mechanisms and insight to physiological responses. Acta Physiol (Oxf) 210: 768–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter SK. 2016a. The relevance of sex differences in performance fatigability. Med Sci Sports Exerc 48: 2247–2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter SK. 2016b. Sex differences in fatigability of dynamic contractions. Exp Physiol 101: 250–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter SK, Enoka RM. 2001. Sex differences in the fatigability of arm muscles depends on absolute force during isometric contractions. J Appl Physiol 91: 2686–2694. [DOI] [PubMed] [Google Scholar]
- Hunter SK, Thompson MW, Ruell PA, Harmer AR, Thom JM, Gwinn TH, Adams RD. 1999. Human skeletal sarcoplasmic reticulum Ca2+ uptake and muscle function with aging and strength training. J Appl Physiol 86: 1858–1865. [DOI] [PubMed] [Google Scholar]
- Hunter SK, Thompson MW, Adams RD. 2000. Relationships among age-associated strength changes and physical activity level, limb dominance, and muscle group in women. J Gerontol A Biol Sci Med Sci 55: B264–B273. [DOI] [PubMed] [Google Scholar]
- Hunter SK, Ryan DL, Ortega JD, Enoka RM. 2002. Task differences with the same load torque alter the endurance time of submaximal fatiguing contractions in humans. J Neurophysiol 88: 3087–3096. [DOI] [PubMed] [Google Scholar]
- Hunter SK, Critchlow A, Enoka RM. 2004a. Influence of aging on sex differences in muscle fatigability. J Appl Physiol 97: 1723–1732. [DOI] [PubMed] [Google Scholar]
- Hunter SK, Critchlow A, Shin IS, Enoka RM. 2004b. Fatigability of the elbow flexor muscles for a sustained submaximal contraction is similar in men and women matched for strength. J Appl Physiol 96: 195–202. [DOI] [PubMed] [Google Scholar]
- Hunter SK, Critchlow A, Shin IS, Enoka RM. 2004c. Men are more fatigable than strength-matched women when performing intermittent submaximal contractions. J Appl Physiol 96: 2125–2132. [DOI] [PubMed] [Google Scholar]
- Hunter SK, Duchateau J, Enoka RM. 2004d. Muscle fatigue and the mechanisms of task failure. Exerc Sport Sci Rev 32: 44–49. [DOI] [PubMed] [Google Scholar]
- Hunter SK, Critchlow A, Enoka RM. 2005a. Muscle endurance is greater for old men compared with strength-matched young men. J Appl Physiol 99: 890–897. [DOI] [PubMed] [Google Scholar]
- Hunter SK, Rochette L, Critchlow A, Enoka RM. 2005b. Time to task failure differs with load type when old adults perform a submaximal fatiguing contraction. Muscle Nerve 31: 730–740. [DOI] [PubMed] [Google Scholar]
- Hunter SK, Butler JE, Todd G, Gandevia SC, Taylor JL. 2006a. Supraspinal fatigue does not explain the sex difference in muscle fatigue of maximal contractions. J Appl Physiol (1985) 101: 1036–1044. [DOI] [PubMed] [Google Scholar]
- Hunter SK, Schletty JM, Schlachter KM, Griffith EE, Polichnowski AJ, Ng AV. 2006b. Active hyperemia and vascular conductance differ between men and women for an isometric fatiguing contraction. J Appl Physiol 101: 140–150. [DOI] [PubMed] [Google Scholar]
- Hunter SK, Todd G, Butler JE, Gandevia SC, Taylor JL. 2008a. Recovery from supraspinal fatigue is slowed in old adults after fatiguing maximal isometric contractions. J Appl Physiol 105: 1199–1209. [DOI] [PubMed] [Google Scholar]
- Hunter SK, Yoon T, Farinella J, Griffith EE, Ng AV. 2008b. Time to task failure and muscle activation vary with load type for a submaximal fatiguing contraction with the lower leg. J Appl Physiol 105: 463–472. [DOI] [PubMed] [Google Scholar]
- Hunter SK, Griffith EE, Schlachter KM, Kufahl TD. 2009. Sex differences in time to task failure and blood flow for an intermittent isometric fatiguing contraction. Muscle Nerve 39: 42–53. [DOI] [PubMed] [Google Scholar]
- Hunter SK, McNeil CJ, Butler JE, Gandevia SC, Taylor JL. 2016a. Short-interval cortical inhibition and intracortical facilitation during submaximal voluntary contractions changes with fatigue. Exp Brain Res 234: 2541–2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter SK, Pereira HM, Keenan KG. 2016b. The aging neuromuscular system and motor performance. J Appl Physiol (1985) 121: 982–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husmann F, Gube M, Felser S, Weippert M, Mau-Moeller A, Bruhn S, Behrens M. 2016. Central factors contribute to knee extensor strength loss after 2000-m rowing in elite male and female rowers. Med Sci Sports Exerc 49: 440–449. [DOI] [PubMed] [Google Scholar]
- Justice JN, Mani D, Pierpoint LA, Enoka RM. 2014. Fatigability of the dorsiflexors and associations among multiple domains of motor function in young and old adults. Exp Gerontol 55: 92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman MP. 2011. The exercise pressor reflex in animals. Exp Physiol 97: 51–58. [DOI] [PubMed] [Google Scholar]
- Keller ML, Pruse J, Yoon T, Schlinder-Delap B, Harkins A, Hunter SK. 2011. Supraspinal fatigue is similar in men and women for a low-force fatiguing contraction. Med Sci Sports Exerc 43: 1873–1883. [DOI] [PubMed] [Google Scholar]
- Keller-Ross ML, Pereira HM, Pruse J, Yoon T, Schlinder-Delap B, Nielson KA, Hunter SK. 2014. Stressor-induced increase in muscle fatigability of young men and women is predicted by strength but not voluntary activation. J Appl Physiol (1985) 116: 767–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy DS, McNeil CJ, Gandevia SC, Taylor JL. 2013. Firing of antagonist small-diameter muscle afferents reduces voluntary activation and torque of elbow flexors. J Physiol 591: 3591–3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy DS, Fitzpatrick SC, Gandevia SC, Taylor JL. 2015. Fatigue-related firing of muscle nociceptors reduces voluntary activation of ipsilateral but not contralateral lower limb muscles. J Appl Physiol (1985) 118: 408–418. [DOI] [PubMed] [Google Scholar]
- Kent-Braun JA. 2009. Skeletal muscle fatigue in old age: Whose advantage? Exerc Sport Sci Rev 37: 3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent-Braun JA, Ng AV, Doyle JW, Towse TF. 2002. Human skeletal muscle responses vary with age and gender during fatigue due to incremental isometric exercise. J Appl Physiol 93: 1813–1823. [DOI] [PubMed] [Google Scholar]
- Kent-Braun JA, Fitts RH, Christie A. 2012. Skeletal muscle fatigue. Compr Physiol 2: 997–1044. [DOI] [PubMed] [Google Scholar]
- Kent-Braun JA, Callahan DM, Fay JL, Foulis SA, Buonaccorsi JP. 2014. Muscle weakness, fatigue, and torque variability: Effects of age and mobility status. Muscle Nerve 49: 209–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klass M, Baudry S, Duchateau J. 2008a. Age-related decline in rate of torque development is accompanied by lower maximal motor unit discharge frequency during fast contractions. J Appl Physiol 104: 739–746. [DOI] [PubMed] [Google Scholar]
- Klass M, Levenez M, Enoka RM, Duchateau J. 2008b. Spinal mechanisms contribute to differences in the time to failure of submaximal fatiguing contractions performed with different loads. J Neurophysiol 99: 1096–1104. [DOI] [PubMed] [Google Scholar]
- Klass M, Roelands B, Levenez M, Fontenelle V, Pattyn N, Meeusen R, Duchateau J. 2012. Effects of noradrenaline and dopamine on supraspinal fatigue in well-trained men. Med Sci Sports Exerc 44: 2299–2308. [DOI] [PubMed] [Google Scholar]
- Krivickas LS, Fielding RA, Murray A, Callahan D, Johansson A, Dorer DJ, Frontera WR. 2006. Sex differences in single muscle fiber power in older adults. Med Sci Sports Exerc 38: 57–63. [DOI] [PubMed] [Google Scholar]
- Lamboley CR, Wyckelsma VL, Dutka TL, McKenna MJ, Murphy RM, Lamb GD. 2015. Contractile properties and sarcoplasmic reticulum calcium content in type I and type II skeletal muscle fibres in active aged humans. J Physiol 593: 2499–2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanza IR, Larsen RG, Kent-Braun JA. 2007. Effects of old age on human skeletal muscle energetics during fatiguing contractions with and without blood flow. J Physiol 583: 1093–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lariviere C, Gravel D, Gagnon D, Gardiner P, Bertrand Arsenault A, Gaudreault N. 2006. Gender influence on fatigability of back muscles during intermittent isometric contractions: A study of neuromuscular activation patterns. Clin Biomech (Bristol, Avon) 21: 893–904. [DOI] [PubMed] [Google Scholar]
- Larsson L, Karlsson J. 1978. Isometric and dynamic endurance as a function of age and skeletal muscle characteristics. Acta Physiol Scand 104: 129–136. [DOI] [PubMed] [Google Scholar]
- Larsson L, Grimby G, Karlsson J. 1979. Muscle strength and speed of movement in relation to age and muscle morphology. J Appl Physiol Respir Environ Exerc Physiol 46: 451–456. [DOI] [PubMed] [Google Scholar]
- Larsson L, Li X, Frontera WR. 1997. Effects of aging on shortening velocity and myosin isoform composition in single human skeletal muscle cells. Am J Physiol 272: C638–C649. [DOI] [PubMed] [Google Scholar]
- Lee A, Baxter J, Eischer C, Gage M, Hunter SK, Yoon T. 2017. Sex differences in neuromuscular function after repeated eccentric contractions of the knee extensor muscles. Eur J Appl Physiol 10.1007/s00421-017-3599-8. [DOI] [PubMed] [Google Scholar]
- Levenez M, Garland SJ, Klass M, Duchateau J. 2008. Cortical and spinal modulation of antagonist coactivation during a submaximal fatiguing contraction in humans. J Neurophysiol 99: 554–563. [DOI] [PubMed] [Google Scholar]
- Lexell J, Taylor CC, Sjostrom M. 1988. What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year-old men. J Neurol Sci 84: 275–294. [DOI] [PubMed] [Google Scholar]
- Lindle RS, Metter EJ, Lynch NA, Fleg JL, Fozard JL, Tobin J, Roy TA, Hurley BF. 1997. Age and gender comparisons of muscle strength in 654 women and men aged 20–93 yr. J Appl Physiol 83: 1581–1587. [DOI] [PubMed] [Google Scholar]
- Lippold O, Redfearn J, Vuco J. 1960. The electromyography of fatigue. Ergomonics 3: 121–131. [Google Scholar]
- Liu JZ, Shan ZY, Zhang LD, Sahgal V, Brown RW, Yue GH. 2003. Human brain activation during sustained and intermittent submaximal fatigue muscle contractions: An fMRI study. J Neurophysiol 90: 300–312. [DOI] [PubMed] [Google Scholar]
- Madeleine P, Jorgensen LV, Sogaard K, Arendt-Nielsen L, Sjogaard G. 2002. Development of muscle fatigue as assessed by electromyography and mechanomyography during continuous and intermittent low-force contractions: Effects of the feedback mode. Eur J Appl Physiol 87: 28–37. [DOI] [PubMed] [Google Scholar]
- Maluf KS, Shinohara M, Stephenson JL, Enoka RM. 2005. Muscle activation and time to task failure differ with load type and contraction intensity for a human hand muscle. Exp Brain Res 167: 165–177. [DOI] [PubMed] [Google Scholar]
- Marcora SM, Staiano W, Manning V. 2009. Mental fatigue impairs physical performance in humans. J Appl Physiol (1985) 106: 857–864. [DOI] [PubMed] [Google Scholar]
- Martin PG, Rattey J. 2007. Central fatigue explains sex differences in muscle fatigue and contralateral cross-over effects of maximal contractions. Pflugers Arch 454: 957–969. [DOI] [PubMed] [Google Scholar]
- Martin PG, Smith JL, Butler JE, Gandevia SC, Taylor JL. 2006. Fatigue-sensitive afferents inhibit extensor but not flexor motoneurons in humans. J Neurosci 26: 4796–4802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin PG, Weerakkody N, Gandevia SC, Taylor JL. 2008. Group III and IV muscle afferents differentially affect the motor cortex and motoneurones in humans. J Physiol 586: 1277–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maughan RJ, Harmon M, Leiper JB, Sale D, Delman A. 1986. Endurance capacity of untrained males and females in isometric and dynamic muscular contractions. Eur J Appl Physiol 55: 395–400. [DOI] [PubMed] [Google Scholar]
- McNeil CJ, Rice CL. 2007. Fatigability is increased with age during velocity-dependent contractions of the dorsiflexors. J Gerontol A Biol Sci Med Sci 62A: 624–629. [DOI] [PubMed] [Google Scholar]
- McNeil CJ, Martin PG, Gandevia SC, Taylor JL. 2009. The response to paired motor cortical stimuli is abolished at a spinal level during human muscle fatigue. J Physiol 587: 5601–5612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil CJ, Giesebrecht S, Gandevia SC, Taylor JL. 2011. Behaviour of the motoneurone pool in a fatiguing submaximal contraction. J Physiol 589: 3533–3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil CJ, Butler JE, Taylor JL, Gandevia SC. 2013. Testing the excitability of human motoneurons. Front Hum Neurosci 7: 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merton PA. 1954. Voluntary strength and fatigue. J Physiol 123: 553–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metter EJ, Talbot LA, Schrager M, Conwit RA. 2004. Arm-cranking muscle power and arm isometric muscle strength are independent predictors of all-cause mortality in men. J Appl Physiol 96: 814–821. [DOI] [PubMed] [Google Scholar]
- Millet GY, Lepers R, Maffiuletti NA, Babault N, Martin V, Lattier G. 2002. Alterations of neuromuscular function after an ultramarathon. J Appl Physiol 92: 486–492. [DOI] [PubMed] [Google Scholar]
- Millet GY, Martin V, Lattier G, Ballay Y. 2003. Mechanisms contributing to knee extensor strength loss after prolonged running exercise. J Appl Physiol (1985) 94: 193–198. [DOI] [PubMed] [Google Scholar]
- Molenaar JP, McNeil CJ, Bredius MS, Gandevia SC. 2013. Effects of aging and sex on voluntary activation and peak relaxation rate of human elbow flexors studied with motor cortical stimulation. Age (Dordr) 35: 1327–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottram CJ, Jakobi JM, Semmler JG, Enoka RM. 2005. Motor unit activity differs with load type during a fatiguing contraction. J Neurophysiol 93: 1381–1392. [DOI] [PubMed] [Google Scholar]
- Murphy MN, Mizuno M, Mitchell JH, Smith SA. 2011. Cardiovascular regulation by skeletal muscle reflexes in health and disease. Am J Physiol Heart Circ Physiol 301: H1191–H1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narici MV, Bordini M, Cerretelli P. 1991. Effect of aging on human adductor pollicis muscle function. J Appl Physiol 71: 1277–1281. [DOI] [PubMed] [Google Scholar]
- Pageaux B, Marcora SM, Lepers R. 2013. Prolonged mental exertion does not alter neuromuscular function of the knee extensors. Med Sci Sports Exerc 45: 2254–2264. [DOI] [PubMed] [Google Scholar]
- Pasquet B, Carpentier A, Duchateau J, Hainaut K. 2000. Muscle fatigue during concentric and eccentric contractions. Muscle Nerve 23: 1727–1735. [DOI] [PubMed] [Google Scholar]
- Pereira HM, Spears VC, Schlinder-Delap B, Yoon T, Harkins A, Nielson KA, Hoeger Bement M, Hunter SK. 2015. Sex differences in arm muscle fatigability with cognitive demand in older adults. Clin Orthop Relat Res 473: 2568–2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pincivero DM, Gandaio CM, Ito Y. 2003. Gender-specific knee extensor torque, flexor torque, and muscle fatigue responses during maximal effort contractions. Eur J Appl Physiol 89: 134–141. [DOI] [PubMed] [Google Scholar]
- Poole DC, Burnley M, Vanhatalo A, Rossiter HB, Jones AM. 2016. Critical power: An important fatigue threshold in exercise physiology. Med Sci Sports Exerc 48: 2320–2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power GA, Dalton BH, Rice CL, Vandervoort AA. 2013. Peak power is reduced following lengthening contractions despite a maintenance of shortening velocity. Appl Physiol Nutr Metab 38: 1196–1205. [DOI] [PubMed] [Google Scholar]
- Power GA, Minozzo FC, Spendiff S, Filion ME, Konokhova Y, Purves-Smith MF, Pion C, Aubertin-Leheudre M, Morais JA, Herzog W, et al. 2016. Reduction in single muscle fiber rate of force development with aging is not attenuated in world class older masters athletes. Am J Physiol Cell Physiol 310: C318–C327. [DOI] [PubMed] [Google Scholar]
- Purves-Smith FM, Sgarioto N, Hepple RT. 2014. Fiber typing in aging muscle. Exerc Sport Sci Rev 42: 45–52. [DOI] [PubMed] [Google Scholar]
- Raj IS, Bird SR, Shield AJ. 2010. Aging and the force-velocity relationship of muscles. Exp Gerontol 45: 81–90. [DOI] [PubMed] [Google Scholar]
- Rantanen T, Masaki K, Foley D, Izmirlian G, White L, Guralnik JM. 1998. Grip strength changes over 27 yr in Japanese-American men. J Appl Physiol (1985) 85: 2047–2053. [DOI] [PubMed] [Google Scholar]
- Reid KF, Fielding RA. 2012. Skeletal muscle power: A critical determinant of physical functioning in older adults. Exerc Sport Sci Rev 40: 4–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley ZA, Maerz AH, Litsey JC, Enoka RM. 2008. Motor unit recruitment in human biceps brachii during sustained voluntary contractions. J Physiol 586: 2183–2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelands B, Meeusen R. 2010. Alterations in central fatigue by pharmacological manipulations of neurotransmitters in normal and high ambient temperature. Sports Med 40: 229–246. [DOI] [PubMed] [Google Scholar]
- Rossman MJ, Venturelli M, McDaniel J, Amann M, Richardson RS. 2012. Muscle mass and peripheral fatigue: A potential role for afferent feedback? Acta Physiol (Oxf) 206: 242–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudroff T, Poston B, Shin IS, Bojsen-Moller J, Enoka RM. 2005. Net excitation of the motor unit pool varies with load type during fatiguing contractions. Muscle Nerve 31: 78–87. [DOI] [PubMed] [Google Scholar]
- Rudroff T, Justice JN, Matthews S, Zuo R, Enoka RM. 2010. Muscle activity differs with load compliance during fatiguing contractions with the knee extensor muscles. Exp Brain Res 203: 307–316. [DOI] [PubMed] [Google Scholar]
- Rudroff T, Justice JN, Holmes MR, Matthews SD, Enoka RM. 2011. Muscle activity and time to task failure differ with load compliance and target force for elbow flexor muscles. J Appl Physiol 110: 125–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russ DW, Kent-Braun JA. 2003. Sex differences in human skeletal muscle fatigue are eliminated under ischemic conditions. J Appl Physiol 94: 2414–2422. [DOI] [PubMed] [Google Scholar]
- Russ DW, Lanza IR, Rothman D, Kent-Braun JA. 2005. Sex differences in glycolysis during brief, intense isometric contractions. Muscle Nerve 32: 647–655. [DOI] [PubMed] [Google Scholar]
- Sacco P, Thickbroom GW, Thompson ML, Mastaglia FL. 1997. Changes in corticomotor excitation and inhibition during prolonged submaximal muscle contractions. Muscle Nerve 20: 1158–1166. [DOI] [PubMed] [Google Scholar]
- Sato H, Ohashi J. 1989. Sex differences in static muscular endurance. J Hum Ergol (Tokyo) 18: 53–60. [PubMed] [Google Scholar]
- Senefeld J, Yoon T, Bement MH, Hunter SK. 2013. Fatigue and recovery from dynamic contractions in men and women differ for arm and leg muscles. Muscle Nerve 48: 436–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senefeld J, Yoon T, Hunter SK. 2016. Age differences in fatigability of arm and leg muscles of men and women: Associations with physical function. Exp Gerontol 87: 74–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sewright KA, Hubal MJ, Kearns A, Holbrook MT, Clarkson PM. 2008. Sex differences in response to maximal eccentric exercise. Med Sci Sports Exerc 40: 242–251. [DOI] [PubMed] [Google Scholar]
- Sidhu SK, Bentley DJ, Carroll TJ. 2009. Locomotor exercise induces long-lasting impairments in the capacity of the human motor cortex to voluntarily activate knee extensor muscles. J Appl Physiol 106: 556–565. [DOI] [PubMed] [Google Scholar]
- Sidhu SK, Cresswell AG, Carroll TJ. 2013. Corticospinal responses to sustained locomotor exercises: Moving beyond single-joint studies of central fatigue. Sports Med 43: 437–449. [DOI] [PubMed] [Google Scholar]
- Sidhu SK, Weavil JC, Venturelli M, Garten RS, Rossman MJ, Richardson RS, Gmelch BS, Morgan DE, Amann M. 2014. Spinal μ-opioid receptor-sensitive lower limb muscle afferents determine corticospinal responsiveness and promote central fatigue in upper limb muscle. J Physiol 592: 5011–5024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JL, Martin PG, Gandevia SC, Taylor JL. 2007. Sustained contraction at very low forces produces prominent supraspinal fatigue in human elbow flexor muscles. J Appl Physiol 103: 560–568. [DOI] [PubMed] [Google Scholar]
- Sogaard K, Gandevia SC, Todd G, Petersen NT, Taylor JL. 2006. The effect of sustained low-intensity contractions on supraspinal fatigue in human elbow flexor muscles. J Physiol 573: 511–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundberg CW, Hunter SK, Bundle MW. 2017. Rates of performance loss and neuromuscular activity in men and women during cycling: Evidence for a common metabolic basis of muscle fatigue. J Appl Physiol (1985) 122: 130–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JL. 2009. Point: The interpolated twitch does/does not provide a valid measure of the voluntary activation of muscle. J Appl Physiol (1985) 107: 354–355. [DOI] [PubMed] [Google Scholar]
- Taylor JL, Gandevia SC. 2008. A comparison of central aspects of fatigue in submaximal and maximal voluntary contractions. J Appl Physiol 104: 542–550. [DOI] [PubMed] [Google Scholar]
- Taylor JL, Butler JE, Allen GM, Gandevia SC. 1996. Changes in motor cortical excitability during human muscle fatigue. J Physiol 490: 519–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JL, Amann M, Duchateau J, Meeusen R, Rice CL. 2016. Neural contributions to muscle fatigue: From the brain to the muscle and back again. Med Sci Sports Exerc 48: 2294–2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temesi J, Arnal PJ, Rupp T, Feasson L, Cartier R. Gergele L, Verges S, Martin V, Millet GY. 2015. Are females more resistant to extreme neuromuscular fatigue? Med Sci Sports Exerc 47: 1372–1382. [DOI] [PubMed] [Google Scholar]
- Todd G, Butler JE, Taylor JL, Gandevia SC. 2005. Hyperthermia: A failure of the motor cortex and the muscle. J Physiol 563: 621–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd G, Taylor JL, Gandevia SC. 2016. Measurement of voluntary activation based on transcranial magnetic stimulation over the motor cortex. J Appl Physiol (1985) 121: 678–686. [DOI] [PubMed] [Google Scholar]
- Tomlinson BE, Irving D. 1977. The numbers of limb motor neurons in the human lumbosacral cord throughout life. J Neurol Sci 34: 213–219. [DOI] [PubMed] [Google Scholar]
- Trappe S, Gallagher P, Harber M, Carrithers J, Fluckey J, Trappe T. 2003. Single muscle fibre contractile properties in young and old men and women. J Physiol 552: 47–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanden Noven ML, Pereira HM, Yoon T, Stevens AA, Nielson KA, Hunter SK. 2014. Motor variability during sustained contractions increases with cognitive demand in older adults. Front Aging Neurosci 6: 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandervoort AA, McComas AJ. 1986. Contractile changes in opposing muscles of the human ankle joint with aging. J Appl Physiol 61: 361–367. [DOI] [PubMed] [Google Scholar]
- van Duinen H, Renken R, Maurits N, Zijdewind I. 2007. Effects of motor fatigue on human brain activity, an fMRI study. Neuroimage 35: 1438–1449. [DOI] [PubMed] [Google Scholar]
- Vanhatalo A, Black MI, DiMenna FJ, Blackwell JR, Schmidt JF, Thompson C, Wylie LJ, Mohr M, Bangsbo J, Krustrup P, et al. 2016. The mechanistic bases of the power-time relationship: Muscle metabolic responses and relationships to muscle fibre type. J Physiol 594: 4407–4423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venturelli M, Morgan GR, Donato AJ, Reese V, Bottura R, Tarperi C, Milanese C, Schena F, Reggiani C, Naro F, et al. 2014. Cellular aging of skeletal muscle: Telomeric and free radical evidence that physical inactivity is responsible and not age. Clin Sci (Lond) 127: 415–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venturelli M, Saggin P, Muti E, Naro F, Cancellara L, Toniolo L, Tarperi C, Calabria E, Richardson RS, Reggiani C, et al. 2015. In vivo and in vitro evidence that intrinsic upper- and lower-limb skeletal muscle function is unaffected by ageing and disuse in oldest-old humans. Acta Physiol (Oxf) 215: 58–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace JW, Power GA, Rice CL, Dalton BH. 2016. Time-dependent neuromuscular parameters in the plantar flexors support greater fatigability of old compared with younger males. Exp Gerontol 74: 13–20. [DOI] [PubMed] [Google Scholar]
- Ward NS. 2006. Compensatory mechanisms in the aging motor system. Ageing Res Rev 5: 239–254. [DOI] [PubMed] [Google Scholar]
- Watson P, Hasegawa H, Roelands B, Piacentini MF, Looverie R, Meeusen R. 2005. Acute dopamine/noradrenaline reuptake inhibition enhances human exercise performance in warm, but not temperate conditions. J Physiol 565: 873–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West W, Hicks A, Clements L, Dowling J. 1995. The relationship between voluntary electromyogram, endurance time and intensity of effort in isometric handgrip exercise. Eur J Appl Physiol Occup Physiol 71: 301–305. [DOI] [PubMed] [Google Scholar]
- Wilson WM, Maughan RJ. 1992. Evidence for a possible role of 5-hydroxytryptamine in the genesis of fatigue in man: Administration of paroxetine, a 5-HT re-uptake inhibitor, reduces the capacity to perform prolonged exercise. Exp Physiol 77: 921–924. [DOI] [PubMed] [Google Scholar]
- Woods JJ, Furbush F, Bigland-Ritchie B. 1987. Evidence for a fatigue-induced reflex inhibition of motoneuron firing rates. J Neurophysiol 58: 125–137. [DOI] [PubMed] [Google Scholar]
- Yoon T, Schlinder Delap B, Griffith EE, Hunter SK. 2007. Mechanisms of fatigue differ after low- and high-force fatiguing contractions in men and women. Muscle Nerve 36: 512–524. [DOI] [PubMed] [Google Scholar]
- Yoon T, Hawe R, Hunter SK. 2009a. Variation in limb support influences the time to task failure for a postural contraction. J Mot Behav 41: 393–395. [DOI] [PubMed] [Google Scholar]
- Yoon T, Keller ML, De-Lap BS, Harkins A, Lepers R, Hunter SK. 2009b. Sex differences in response to cognitive stress during a fatiguing contraction. J Appl Physiol 107: 1486–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon T, Schlinder-Delap B, Keller ML, Hunter SK. 2012. Supraspinal fatigue impedes recovery from a low-intensity sustained contraction in old adults. J Appl Physiol 112: 849–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon T, Schlinder-Delap B, Hunter SK. 2013. Fatigability and recovery of arm muscles with advanced age for dynamic and isometric contractions. Exp Gerontol 48: 259–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon T, Doyel R, Widule C, Hunter SK. 2015. Sex differences with aging in the fatigability of dynamic contractions. Exp Gerontol 70: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]