Abstract
Cancer is an increasingly frequent malignancy worldwide, and despite the advances in drug development, it is still necessary to develop new plant-derived medicines. Euterpe oleracea (açaí) is abundant in South and Central America and has health benefits due to its high levels of phytochemicals, including lignans and polyphenols. The aim of this review was to systematically describe the safety and antitumor effects of açaí in preclinical models using rodents to provide a more comprehensive assessment of açaí for both therapeutic uses and the development of future clinical studies in cancer. Eligible studies were identified using four international databases (PubMed, Medline, Lilacs and SciELO) from their inception date through December 2017. The included studies were analyzed with methodological rigor (QATRS) to enable better quality control for these experimental studies. Sixty publications were identified in the databases, but only 9 articles were eligible: 6 evaluated the pharmacological effects of açaí in animal models of cancer (1 model each of esophageal cancer, urothelial cancer, melanoma and Walker-256 tumor and 2 models of colon cancer), and 3 were toxicological assays using preclinical models with rodents. Overall, 747 animals were analyzed. On a QATRS score scale of 0–20, the quality of the studies ranged from 16 to 20 points. Pulp was the main fraction of açaí administered, and an oral administration route was most common. The açaí dosage administered by gavage ranged from 30 mg/kg to 40,000 mg/kg, and açaí fed in the diet accounted for 2.5% to 5% of the diet. The anticarcinogenic and chemopreventive activities of açaí were observed in all experimental models of cancer and reduced the incidence, tumor cell proliferation, multiplicity and size of the tumors due to the antiinflammatory, antiproliferative and proapoptotic properties of açaí. No genotoxic effects were observed after açaí administration. The results of this review suggest that açaí is safe and can be used as a chemoprotective agent against cancer development. Açaí therapy may be a novel strategy for treating cancer.
Introduction
The use of natural products as medicines accounts for approximately 30% of the currently available drugs [1], and in some therapeutic areas, the amount of plant-derived medicines reaches 60% [2,3]. Brazil has the greatest amount of biodiversity in the world and plays an important role in the area of natural bioactive compounds by contributing natural products to design new clinical medicines [1,4]. Thus, there has been growing research aimed at establishing the therapeutic potential of natural products against several diseases.
Euterpe oleracea Mart. is a member of the family Arecaceae and is a typical palm of the rainforest in the Amazon region, in the states of the northern region of Brazil, including Guianas, Colombia, Ecuador, and Venezuela [5]. The fruit, popularly known as “açaí”, weighs approximately 2 g, and the color of the mature fruit is dark purple [6]. Açaí is a traditional food in many regions of Brazil [7,8], and its consumption has increased significantly over the last several years, not only in Brazil but also in Europe and the USA, where the fruit gained popularity after being promoted as a “super fruit” [9]. Currently, due to the health benefits and therapeutic potential of açaí, locally grown açaí are increasingly exported around the world as energy drinks [6,10], “functional foods” [7,8], cosmetics and pharmaceutical products [9]. Açaí pulp is composed of approximately 48% lipids, 13% protein, 8% amino acids, 25% total sugars and minor compounds such as fiber and vitamins (A, B1, B2, B3, C and E) [8,11,12]. Moreover, it is rich in several phytochemicals, including lignans, phenolic compounds (anthocyanins, proanthocyanidins and other flavonoids) and resveratrol, in low concentrations [8,11,12]. The seeds of açaí possess the highest concentration of polyphenols (28.3%), followed by the whole fruit (25.5%) and the bark (15.7%) [13].
The pharmacological effects of açaí are associated with its chemical composition, particularly the presence of bioactive substances, such as phenolics, flavonoids and anthocyanins [14–17]. To date, açaí has been shown to have pharmacological properties including antiinflammatory, antioxidant, cardioprotective and anticancer activities [1,7–9,18,19]. Furthermore, açaí was not shown to be genotoxic in vitro and in vivo studies conducted, in cultured human lymphocytes and hepatoma cell lines [20], in rodents [21] and in humans [22].
The aim of this review was to systematically describe the safety and antitumor effects of açaí in preclinical models using rodents, to provide a comprehensive assessment of açaí for therapeutic use. Preclinical studies using rodents were evaluated to investigate whether the current knowledge supports cancer clinical trials with açaí.
Methods
Search strategy
A careful literature search was performed to identify publications that studied the use of E. oleracea extract in experimental animal models of cancer and/or evaluated the safety/toxicity of açaí in animal models. Studies were identified by searching the electronic databases: PubMed, Medline-Bireme, Lilacs and SciELO from their inception date through December 2017 (S1 Table). The search terms were as follows: (“Euterpe oleracea” AND cancer treatment) OR (“Euterpe oleracea” AND cancer animal model) OR (Açaí AND cancer treatment) OR (Açaí AND cancer animal model) AND (“Euterpe oleracea” AND toxicity) OR (Açaí AND toxicity). The search was performed without restrictions on the language or year of publication. Two reviewers (KCRB and JA-P) selected the qualified studies independently by browsing the titles, abstracts or full texts based on the eligibility criteria. The duplicates were removed. The eligible articles were separated for analysis of the study methodology and results (S1 Table). Any disagreements were resolved by discussion with two additional reviewers (DEM and JAP).
Inclusion and exclusion criteria
Articles were included if the following criteria were met: (1) evaluated the pharmacological effect of açaí in animal models of cancer and/or (2) performed toxicological analyzes after açaí administration in experimental animal models. Articles were excluded if the following criteria were met: (1) were reviews of literature; (2) did not analyze the use of açaí in vivo; (3) did not use the order Rodentia; (4) did not evaluate the toxicological effects of açaí administration in vivo; and (5) used only in vitro experimental models.
Data extraction
Three investigators (KCRB, JA-P and JAP) independently conducted the extraction of details from each study including the following: (1) basic information, including the publication year, the first author's name, the type of animals, the sex, the in vivo model and the experimental interventions; (2) basic information about the açaí treatment, including the fraction and origin of E. oleracea, dose, administration route, posology, diluents and treatment groups; and (3) outcome measures used to evaluate E. oleracea extract, therapeutic indications (pharmacodynamic), açaí signaling pathways and safety evaluations. When a single publication included studies with animals, posology or types of interventions that were different, these data were extracted and considered as independent experiments. Any disagreements regarding the extracted data were resolved by discussion with an additional reviewer (DEM).
Quality assessment
For assessment of quality, two independent reviewers (KCRB and JA-P) used a quality rating scale as an animal/tissue research scale (QATRS). The QATRS is a 20-point scaled evaluation chart that was designed based on randomization, blinding, the similarity of the animal/tissue model to human applications, standardization and the reliability of the measurement techniques, management of study withdrawals, and appropriateness of the statistical methods [23]. Any disagreements were resolved by discussion with two additional reviewers (DEM and JAP).
Results
Study selection
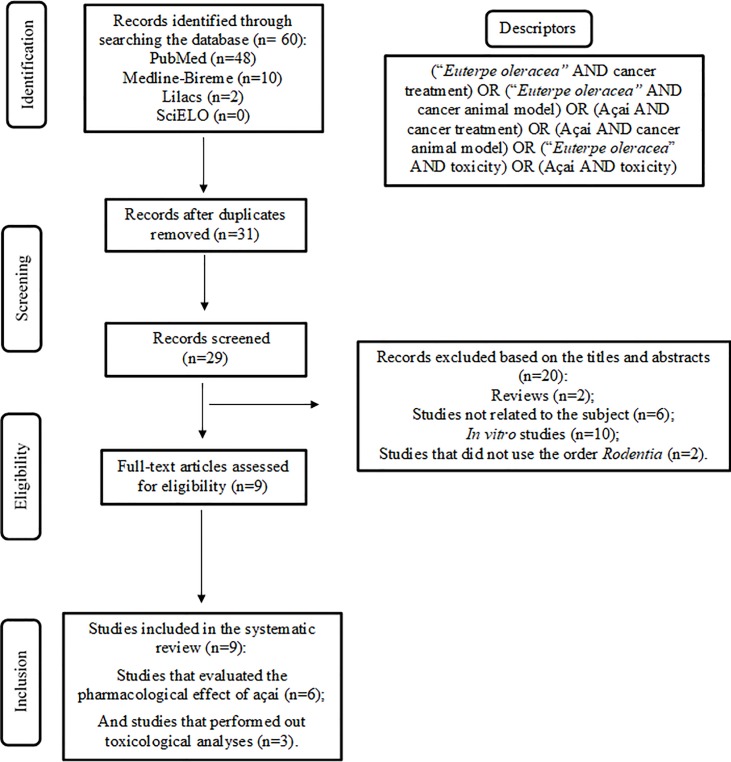
A flowchart of the articles that were included in the review is illustrated in Fig 1. A total of 60 publications were identified in the databases; however, 31 were duplicate articles. Among the 29 articles selected, 20 were excluded based on the titles and abstracts because they did not meet the inclusion criteria: 2 were literature reviews [7,24]; 6 did not study açaí in an animal model of cancer and/or did not perform a toxicological analysis [25–30]; 10 were in vitro studies [13,20,31–38]; and 2 did not use the order Rodentia [21,39]. After reading the full texts 9 articles were included for their critical evaluations of the safety and effectiveness of açaí in animal experimental models [40–48].
Fig 1. Flowchart of the study selection and inclusion in the review.
Characteristics of the experimental models
The articles included were analyzed with a critical appraisal tool (QATRS), which allowed for improved quality control of the experimental studies in animal performed independently (see methods). QATRS scores ranged from 0 to 20, and the quality of the studies ranged from 16 to 20 points (Table 1). Among the 9 studies that were included, 6 evaluated the pharmacological effects of açaí in experimental models of cancer, including esophageal [40], urothelial [41], and colon cancer [42,43], and melanoma [44] and Walker-256 tumors [45], and 3 performed toxicological analyses of açaí in experimental models [46–48]. For the interventions used in the experimental models, 4 studies used chemically induced cancer models [40,41,43], 2 used inoculation of tumor cells [44,45], and 3 used models with DNA damage induced by a chemotherapeutic agent [46–48]. The studies involved 2 species and 6 varieties of rodents: C57BL/6 mice [44], F344 rats [40], Wistar rats [42,45,47,48], Swiss mice [41,46], ICR mice [43] and Balb/c mice [48] (Table 1).
Table 1. Basic information on the in vivo experimental models used to test the effects of E. oleracea.
| Model | Animals | Interventions | Referencesa | QATRS |
|---|---|---|---|---|
| Cancer | Male F344 rats | Esophageal carcinogenesis induced by NMBA | Stoner et al. 2010 | 16 |
| Male Swiss mice | Urothelial carcinogenesis induced by BBN and MNU | Fragoso et al. 2012 | 20 | |
| Male Wistar rats | Colon carcinogenesis induced by DMH | Fragoso et al. 2013 | 20 | |
| Male Wistar rats | Anorexia-cachexia syndrome induced by Walker-256 tumor | Nascimento et al. 2016 | 16 | |
| Male ICR mice | Colon carcinogenesis induced by AOM and DSS | Choi et al. 2017 | 16 | |
| Female C57BL/6 mice | Melanoma induced by transplantation of B16F10 cells | Monge-Fuentes et al. 2017 | 18 | |
| Toxicity | Male Swiss mice | DNA damage induced by doxorubicin | Ribeiro et al. 2010 | 18 |
| Male Wistar rats | DNA damage induced by doxorubicin | Marques et al. 2016 | 18 | |
| BALB/c mice | DNA damage induced by cyclophosphamide | Schauss et al. 2010a | 18 | |
| Wistar rats | Acute and subchronic oral toxicity study | Schauss et al. 2010a | 18 |
AOM = azoxymethane; BBN = N-butyl-N-(4-hydroxybutyl)-nitrosamine; B16F10 = melanoma cell lines; DSS = dextran sulfate sodium; DMH = 1,2-dimethylhydrazine; ICR = International Cancer Research; MNU = N-methyl-N-nitrosourea; NMBA = N-nitrosomethylbenzylamine.
aA reference can have more than one model of disease.
Açaí information
Table 2 shows the basic information about the açaí extract used in the experimental models. The most commonly used açaí fraction was the pulp [40–43,46], followed by the juice [48], oil [44,47] and seeds [45]. Seven studies mentioned the açaí origin, and all of the açaí extracts were from Brazil [40–43,45–47]. The main administration route of açaí was oral; 4 studies administered açaí by gavage [45–48], and 4 studies administered açaí as part of the diet [40–43]. The dosage ranged from 30 mg/kg to 40,000 mg/kg in studies that administered açaí by gavage and was administered as a single dose or as 1 daily dose for 90 consecutive days; in the studies that administered açaí as part of the diet 2.5% to 5% açaí supplementation was provided in the diet for 10 to 35 weeks (Table 2). In addition, Schauss and colleagues used oral and intraperitoneal administration of açaí at a dose of 0.1mg/0.15mL (daily dose during 7 consecutive days) to assess the possible genotoxic effects of açaí using BALB/c mice [48], and Monge-Fuentes and colleagues used 50 mg/mL of açaí administered intratumorally in an experimental model of melanoma [44]. The results regarding the therapeutic indications, effects and safety of açaí in experimental models are summarized in Table 3.
Table 2. Basic information regarding the E. oleracea extract used in the in vivo experimental models.
| Fraction | Origin of açaí | Dosing | Diluent and placebo | Administration | Posology | Reference |
|---|---|---|---|---|---|---|
| Juiceb | Not mentioned | 0.1 mg/0.15mL | Saline | Oral (gavage) and IP | 1 daily dose over 7 days | Schauss et al. 2010a |
| Not mentioned | 5,000 and 20,000 mg/kg | Not mentioned | Oral (gavage) | Single dose | Schauss et al. 2010a | |
| Not mentioned | 10,000; 20,000 and 40,000 mg/kg | Saline | Oral (gavage) | 1 daily dose over 90 days | Schauss et al. 2010a | |
| Oil | Brazil (Amapá) | 30, 100 and 300 mg/kg | Tween | Oral (gavage) | 1 daily dose over 14 days | Marques et al. 2016 |
| Not mentioned | 50 mg/mL | PBS | Intratumoral | Five applications within 15 days (1, 4, 7, 10 and 13 days) |
Monge-Fuentes et al. 2017 | |
| Pulp | Brazil | 5% | AIN diet | Oral (diet) | 35 weeks | Stoner et al. 2010 |
| Brazil (SP) | 3,330; 10,000 and 16,670 mg/kg | Saline | Oral (gavage) | Single dose | Ribeiro et al. 2010a | |
| Brazil (SP) | 3,330; 10,000 and 16,670 mg/kg | Distilled water | Oral (gavage) | 1 daily dose over 14 days | Ribeiro et al. 2010a | |
| Brazil (Pará) | 2.5% and 5% | Standard diet | Oral (diet) | 10 weeks | Fragoso et al. 2012 and 2013a | |
| Brazil (Pará) | 5% | Standard diet | Oral (diet) | 20 weeks | Fragoso et al. 2013a | |
| Brazil (Pará) | 2.5% and 5% | Diet formulatedc | Oral (diet) | 14 weeks | Choi et al. 2017 | |
| Seed | Brazil | 100 and 200 mg/mL | Ethanol-water | Oral (gavage) | 1 daily dose over 14 days | Nascimento et al. 2016 |
AIN = American Institute of Nutrition; IP = intraperitoneal; SP = São Paulo; PBS = Phosphate buffered saline.
a A reference can have different methods of administration of açaí.
bJuice of MonaVie Active® = In addition to açaí, contains lesser amounts of 19 fruits and berries.
cA cereal-based commercial diet for mice formulated by the Orient Bio Group (Seongnam, Korea).
Table 3. Results of cancer treatments and safety evaluations of E. oleracea extract in animal models.
| References | Therapeutic indication | Action of açaí | Unchanged parameters | Effects of açaí |
|---|---|---|---|---|
| Stoner et al. 2010 | Chemopreventive | ↓ incidence, multiplicity and inflammatory cytokines; ↑ serum antioxidant capacity and IFNγ |
Body weight, food consumption, pro and antiinflammatory |
Inhibits esophageal tumorigenesis progression |
| Fragoso et al. 2012 | Chemopreventive (anticarcinogenic) |
↓ incidence, multiplicity, tumor cell proliferation, urothelial preneoplastic lesions, p63 and PCNA expression and DNA damage | Body weight, food consumption, bladder and kidney weight, kidney biochemical markers, cytoplasmatic and nuclear β-catenin expression | Inhibits urothelial bladder carcinogenesis |
| Fragoso et al. 2013 | Chemopreventive | ↓ invasiveness, multiplicity and growth of tumor, cell proliferation and cleaved caspase-3, number of aberrant crypts | Body weight, food consumption, β-catenin expression and toxicity | Inhibits colon carcinogenesis |
| Nascimento et al. 2016 | Anticarcinogenic | ↓ tumor, muscle total protein; ↑ oxidative stress in cerebral cortex |
Liver protein, oxidative stress in muscle and liver | Reduces Walker-256 tumor |
| Choi et al. 2017 | Anticarcinogenic | ↓ incidence, multiplicity and tumor, cell proliferation, proinflammatory cytokines and COX-2; ↑ cleaved-caspase-3 expression. |
Not mentioned | Inhibits colon carcinogenesis |
| Monge-Fuentes et al. 2017 | Anticarcinogenic (Photodynamic) | ↓ tumor, liver and spleen weight; ↑ necrosis; Differences in body weight |
Toxicity of the kidneys and lungs | Reduces melanoma carcinogenesis (photosensitizer) |
| Ribeiro et al. 2010 | Protective effects | ↓ MNPCE and DXR-induced genotoxicity in bone marrow or liver and kidney cells |
PCE, DNA damage and genotoxic effects | Reduction in DNA damage induced by DXR |
| Schauss et al. 2010 | Not mentioned | Not mentioned | Body weight, food consumption, mortality, organ weights, ophthalmology, urinalysis, hematological and biochemical parameters, and genotoxicity | Negative mutagenic effects |
| Marques et al. 2016 | Not mentioned | ↑ cell viability | DNA damage, clastogenic and aneugenic effect | Negative genotoxicity effects |
COX-2 = cyclooxygenase 2; DXR = antitumoral agent doxorubicin; IFNγ = interferon gamma; MNPCE = number of micronucleated peripheral blood polychromatic erythrocytes cells; PCE = peripheral blood polychromatic erythrocytes cells; PCNA = proliferating cell nuclear antigen.
Safety of açaí
The absence of toxicity of açaí was reported in 6 studies after testing açaí in experimental models [41,42,44,46–48], and no significant differences in animal body weight or food consumption were reported in 4 studies [40–42,48]. DNA damage induced by antitumor medication was evaluated in 3 studies, and no genotoxic effects were observed after açaí administration by gavage [46–48] (Table 3).
Using a micronucleus test and a comet assay, Ribeiro and colleagues reported no differences between the control and açaí groups in bone marrow and peripheral blood cells polychromatic erythrocytes, and in liver and kidney cells, thus demonstrating the absence of genotoxic effects of açaí. In addition, açaí reduced DNA damage induced by doxorubicin (DXR), suggesting a protective role in human health [46]. In a study done by Schauss and colleagues, açaí did not cause mutagenic effects, as demonstrated by a bacterial reverse mutation assay, a chromosomal aberration assay, a mammalian cell mutation assay and an in vivo micronucleus study [48]. In the same way, Marques and colleagues evaluated the genotoxic potential of açaí in rat cells. The authors used a comet assay and a micronucleus test and showed that on both cytogenetic tests, no significant genotoxic effects were observed at the three tested dosages of açaí [47].
Antitumoral effects of açaí
The anticarcinogenic and chemopreventive activities of açaí, as evidenced by reductions in the incidence of tumors, tumor cell proliferation, and multiplicity and size of tumors, were observed in all the experimental models of cancer [40–45] (Table 3).
Stoner and colleagues reported that açaí was effective at inhibiting the progression of esophageal tumorigenesis, reducing the levels of the serum cytokines (IL-5 and IL-8), and increasing serum antioxidant capacity and interferon-gamma (IFNγ) levels [40]. By contrast, the esophageal tumor size and serum levels of IL-1β, IL-4, IL-13 and tumor necrosis factor-alpha (TNF-α) were not significantly affected by adding açaí to the diet for 35 weeks [40].
Fragoso and colleagues reported that açaí was effective at inhibiting urinary bladder carcinogenesis, reducing DNA damage, and reducing the expression of p63 and proliferating cell nuclear antigen (PCNA) [41]. However, altered cytoplasmatic and nuclear β-catenin were not significantly affected by adding açaí to the diet for 10 weeks [41].
Two studies reported that açaí was effective at inhibiting colon carcinogenesis induced by 1,2-dimethylhydrazine (DMH) in Wistar rats [42] and azoxymethane (AOM) with dextran sulfate sodium (DSS) in ICR mice [43]. Nevertheless, the opposite results were observed with regard to cleaved caspase-3 expression after supplementation with 2.5% and 5% of açaí in the diet for 10 [42], 14 [43] or 20 weeks [42]. Despite the discrepancies between these studies, the quality evaluation of the results of the articles showed good quality QATRS (16/20 and 20/20, respectively) [42,43]. Moreover, Choi and colleagues reported that açaí treatment down-regulated myeloperoxidase (MPO) and proinflammatory cytokines (TNF-α, IL-1β and IL-6), inhibited cyclooxygenase 2 (COX-2), PCNA and Bcl-2, and increased Bad and cleaved caspase-3 expression in an experimental model of cancer colon [43].
Monge-Fuentes and colleagues reported that açaí was an effective photosensitizer because it reduced melanoma carcinogenesis by increaseing the necrotic tissue per tumor area after 5 applications of intratumoral açaí during a period of 15 days [44].
Nascimento and colleagues reported an anticarcinogenic effect (tumor diameter and weight) of açaí in anorexia-cachexia syndrome induced by Walker-256 tumors due to the antioxidant activity of açaí after 1 daily dose of açaí over 14 consecutive days [45].
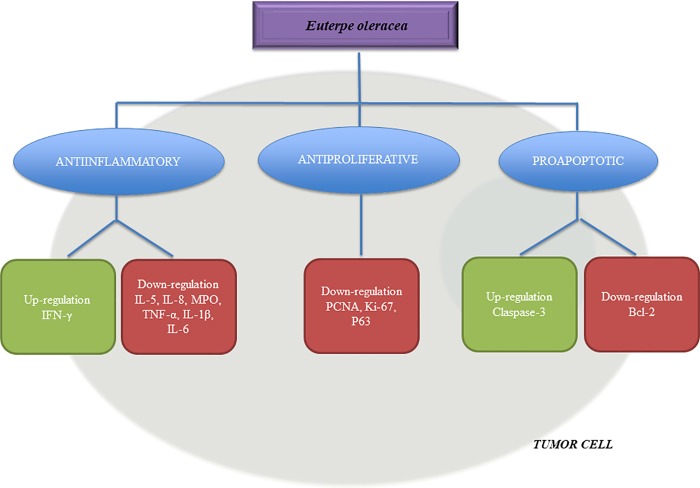
Finally, based on the results of this review study, we created a schematic representation of the effects of açaí in tumor cells (Fig 2). Açaí showed antitumoral functions due to its antiinflammatory, antiproliferative and proapoptotic properties.
Fig 2. Schematic representation of the effects of açaí on tumor cells.
Açaí was shown to have antitumoral functions due its antiinflammatory, antiproliferative and proapoptotic properties.
Discussion
Research on the pharmacological effects of natural products for the treatment of several diseases has significantly increased in the last decades. In this sense, açaí has been marketed as a dietary food supplement because of its health benefits due to its high levels of phytochemicals, including lignans and polyphenols. Studies have demonstrated that açaí has biological effects, such as antioxidant, antiinflammatory, antiproliferative, antinociceptive and antitumorigenic activities [13,16,17,19,49–55].
To the best of our knowledge, 15 clinical trials with açaí was carried [17–19,22,56–66], however none of these studies evaluated the effect of açaí in the cancer treatment. The aim of this review was critically to evaluate the existence of scientific data about the safety and antitumor effects of açaí in preclinical models using rodents, to support cancer clinical trials. The human health benefits of açaí was improvements in antioxidant benefit [17–19,56,61,62,64,65]; cardiovascular health [17,22,56] with beneficial action in hemodynamic [58,60] and metabolic parameters [18,22]; modulation of inflammation [58] and reduction global pain [62]; reduces muscle stress [18,57] and improves effort tolerance in elite athletes [57]; besides to be safe and effective as contrast agent for magnetic resonance imaging [59,63,66]. Due to the important nutritional properties for human benefits and therapeutic potential, the açaí became relevant functional foods.
As far as we know, the present work is the first review to focus on the antitumorigenic and toxicological effects of açaí in preclinical trials using rodents. Overall, nine studies were included in this review [40–48]. Although we conducted a thorough literature search, using four international databases, one limitation is that our conclusions may be narrow due to the lack of availability of published articles and because all of the included studies were published in English. In spite of the small number of studies found, we assessed them with a range of methodological rigor in accordance with the QATRS, which encompasses various aspects that enable better quality control for these experimental studies [23]. A strong point of our review is that all the included studies had good quality as assessed by the QATRS score (all had a score greater than or equal to 16/20). A total of 747 animals of the order Rodentia were analyzed. The results indicated that açaí has a chemopreventive effect (anticancer) by inhibiting tumor growth and leads to a reduction in tumor size, suggesting antiproliferative, pro-apoptotic and anti-inflammatory activity [40–45]. In addition, the toxicological studies showed that açaí did not cause DNA damage or genotoxic or mutagenic effects in the evaluated animals, suggesting that it is safe for clinical testing [46–48].
Most of the studies found that açaí significantly decreased tumor incidence or tumorigenic inhibition and prevented DNA damage without causing genotoxic effects when it was administered orally (in the diet or by gavage). These results suggest that the oral route is a good choice for evaluation of the effects of açaí in humans clinical studies since this is an easy and safe route of administration. It should be noted that the significant results found with the oral administration of açaí have also been described in other diseases, such as obesity and hepatic steatosis [67], endometriosis [55], renovascular hypertension [68] and neuropathic pain [53]. The articles included in this review described different doses of açaí that were administered orally by gavage (range 30 mg/kg to 40,000 mg/kg) [45–48]. However, Marques and colleagues observed that at an açaí dose of 300 mg/kg, a few animals showed signs of toxicity (diarrhea and bristling of the hair), which is why they did not test higher doses [47]. Recently, our group reported that a dose of açaí of 200 mg/kg administered by gavage for 30 consecutive days had efficacy in suppressing endometriotic lesions in a Sprague-Dawley rat model without any signs of toxicity [55]. Although considered a benign disease, endometriosis frequently presents with characteristics of malignancy [69]. Therefore, we suggest that an açaí dose of 200 mg/kg is safe for preclinical testing and is a promising novel pharmacological treatment for cancer due to its anticarcinogenic and chemopreventive effects.
With regard to the ability of açaí to inhibit carcinogenesis, and the incidence and multiplicity of tumors in experimental models of cancer using rodents, in vitro studies also showed that açaí decreased cell viability, suppressed proliferation and induced apoptosis, suggesting the anticancer and antioxidant activity of açaí against C-6 rat brain glioma cells [49], MCF-7 breast cancer cells [13,38] and colon cancer cells [34]. These results suggest that açaí contains phytochemicals that can be used as natural chemopreventive agents [13,40,42].
A large number of studies have shown the importance of chronic exposure to proinflammatory cytokines in tumorigenesis [70–72]. The results of this review show that açaí acts in the inflammatory processes involved in induced-cancer in animals by decreasing the levels of IL-1β, IL-5, IL-6, IL-8, COX-2, TNF-α and MPO and increasing the levels of IFN-γ [40,43]. An in vitro study of polymorphonuclear cells showed a reduction in the IL-8 levels that was associated with the decreasing inflammatory conditions after açaí treatment [64]. Xie and colleagues evaluated flavonoids isolated from açaí pulp and observed a reduction in serum levels, gene expression and protein levels of both the cytokines TNF-α and IL-6 in the resident macrophages cells [73]. Açaí was also able to prevent increases in the levels of IL-1β and TNF-α in the brain tissues of a CCI4 experimental model [27]. In addition, açaí reduced the COX-2 expression and PGE2 levels in an experimental model of endometriosis [55] and reduced the MPO levels in a rat renal ischemia/reperfusion model [74].
As a result of this review, it was possible to identify the antiproliferative pathways by which açaí acts by reducing PCNA, Ki-67 and p63 [41–43]. These proteins are involved in tumor development, survival and metastasis of different tumors [75–77]. In addition, the anti-apoptotic proteins Bcl-2 was also reduced after açaí treatment in animals with induced-cancer [43], in agreement with a study of human colon cancer cells in which the proapoptotic activities of polyphenolics from açaí were described [34]. Polyphenolics may regulate distinct steps of the apoptotic process and/or the expression of regulatory proteins, such as the downregulation of Bcl-2 and cleavage of caspase-3 [78,79]. Açaí polyphenolics were previously described to have proapoptotic and antiproliferative activities in leukemia cancer cells through caspase-3 activation [80]. Surprisingly, as a result of this review, it was possible to identify the discrepancies in the levels of cleaved caspase-3 in colon carcinogenesis induced after açaí treatment [41,43]. Choi and colleagues observed that açaí increased the cleaved caspase-3 levels in the supernatants of colon strips [43], but Fragoso and colleagues described the opposite results using immunohistochemical techniques in colon tumor tissues [41].
Another specie from Brazilian Euterpe, o Euterpe edulis, has been studied because has important nutritional properties for human health. E. edulis Mart., commonly known as juçara or jussara and açaí-do-sol, is a native tree of the Atlantic Forest and has similar nutritional properties of açaí [81], however açaí has twice of the polyphenols concentration [82]. Recently, a review described 25 articles about the phytochemical characterization and biological activities of juçara [81]. Nevertheless, none of these studies evaluated the effect of E. edulis in the cancer treatment and two studies described the safety evaluation of E. edulis, however with controversial results. Barros Freitas et al, 2017 showed juçara prevent the oxidative damage resulting from the cafeteria diet and no evidenced signs of lipid peroxidation in renal or in cardiac tissue in Wistar rats [82]. On the other hand, Felzenszwald et al., 2013, demonstreted E. edulis was able to induce mutagenicity and clastogenic/aneugenic effects in Wistar rats [25].
Toxicity data are decisive for evaluating the safety of natural products for clinical treatment because these data investigate the potential for mutagenicity, genotoxicity, clastogenicity and aneugenicity [83]. The toxicological studies included in this review showed that açaí is non-toxic [42,44,46–48], has no genotoxic or mutagenic effects, and has a protective effect on DNA damage caused by antitumoral agents [46–48]. Similarly, Santos and colleagues showed that antioxidant compounds prevented the induction of DNA damage induced by DXR [84]. On the other hand, açaí showed mutagenic effects when assayed in high concentrations in eukaryotic cells of Saccharomyces cerevisiae yeast; however, there is a low mutagenic risk for humans because the tested concentrations were significantly elevated [31]. Since only 3 studies investigated the genetic toxicity of açaí in preclinical trials of rodents [46–48], future research is needed to better understand the efficacy of açaí because its antimutagenic and antioxidant activities may prevent DNA damage and thus improve human health.
Conclusions
The results of this review suggest that açaí is safe and can be used as a chemoprotective agent against cancer by exhibiting antiinflammatory, antioxidant, antiproliferative, and proapoptotic properties. Further studies on the functional relevance of açaí are necessary to build a database that can be used in future clinical investigations aimed at discovering antitumor agents.
Supporting information
(PDF)
(PDF)
Acknowledgments
The authors thank the support of Dr. Roberto Soares de Moura in important intellectual discussion about açaí.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was supported by the Brazilian agency Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro – FAPERJ (JAP and DEM), Fundação Ary Frauzino – Oncobiologia (DEM) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – CAPES (JA-P and KCRB).
References
- 1.Dutra RC, Campos MM, Santos AR, Calixto JB. Medicinal plants in Brazil: Pharmacological studies, drug discovery, challenges and perspectives. Pharmacol Res. 2016;112:4–29. doi: 10.1016/j.phrs.2016.01.021 [DOI] [PubMed] [Google Scholar]
- 2.Mishra BB, Tiwari VK. Natural products: an evolving role in future drug discovery. Eur J Med Chem. 2011;46(10):4769–807. doi: 10.1016/j.ejmech.2011.07.057 [DOI] [PubMed] [Google Scholar]
- 3.Newman DJ, Cragg GM. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J Nat Prod. 2012;75(3):311–35. doi: 10.1021/np200906s [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Valli M, dos Santos RN, Figueira LD, Nakajima CH, Castro-Gamboa I, Andricopulo AD, et al. Development of a natural products database from the biodiversity of Brazil. J Nat Prod.2013;76(3):439–44. doi: 10.1021/np3006875 [DOI] [PubMed] [Google Scholar]
- 5.E Souza BSF, Carvalho HO, Ferreira IM, da Cunha EL, Barros AS, Taglialegna T, et al. Effect of the treatment with Euterpe oleracea Mart. oil in rats with Triton-induced dyslipidemia. Biomed pharmacother. 2017;90:542–7. doi: 10.1016/j.biopha.2017.04.005 [DOI] [PubMed] [Google Scholar]
- 6.de Sousa MO, Souza e Silva L, de Brito Magalhães CL, de Figueiredo BB, Costa DC, Silva ME, et al. The hypocholesterolemic activity of açaí (Euterpe oleracea Mart.) is mediated by the enhanced expression of the ATP-binding cassette, subfamily G transporters 5 and 8 and low-density lipoprotein receptor genes in the rat. Nutr Res. 2012;32(12):976–84. doi: 10.1016/j.nutres.2012.10.001 [DOI] [PubMed] [Google Scholar]
- 7.Kinghorn AD, Chai HB, Sung CK, Keller WJ. The classical drug discovery approach to defining bioactive constituents of botanicals. Fitoterapia. 2011;82(1):71–9. doi: 10.1016/j.fitote.2010.08.015 [DOI] [PubMed] [Google Scholar]
- 8.Ulbricht C, Brigham A, Burke D, Costa D, Giese N, Iovin R, et al. An evidence-based systematic review of açaí (Euterpe oleracea) by the natural standard research collaboration. J Diet Suppl. 2012;9(2):128–47. doi: 10.3109/19390211.2012.686347 [DOI] [PubMed] [Google Scholar]
- 9.de Moura RS, Resende ÂC. Cardiovascular and metabolic effects of açaí, an amazon plant. J Cardiovasc Pharmacol. 2016;68(1):19–26. doi: 10.1097/FJC.0000000000000347 [DOI] [PubMed] [Google Scholar]
- 10.Yamaguchi KK, Pereira LF, Lamarão CV, Lima ES, da Veiga-Junior VF. Amazon açaí: Chemistry and biological activities: A review. Food Chem. 2015;179:137–51. doi: 10.1016/j.foodchem.2015.01.055 [DOI] [PubMed] [Google Scholar]
- 11.Schauss AG, Wu X, Prior RL, Ou B, Huang D, Owens J, et al. Antioxidant capacity and other bioactivities of the freeze-dried amazonian palm berry, Euterpe oleraceae Mart. (Açaí). J Agric Food Chem. 2006;54(22):8604–10. doi: 10.1021/jf0609779 [DOI] [PubMed] [Google Scholar]
- 12.Heinrich M, Dhanji T, Casselman I. Açaí (Euterpe oleracea Mart.)—A phytochemical and pharmacological assessment of the species’ health claims. Phytochemistry Letters. 2011;4(1):10–21. doi: 10.1016/j.phytol.2010.11.005 [Google Scholar]
- 13.Silva DF, Vidal FC, Santos D, Costa MC, Morgado-Díaz JA, do Desterro Soares Brandão Nascimento M, et al. Cytotoxic effects of Euterpe oleracea Mart. in malignant cell lines. BMC Complement Altern Med. 2014;14:175 doi: 10.1186/1472-6882-14-175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Del Pozo-Insfran D, Brenes CH, Talcott ST. Phytochemical composition and pigment stability of açaí (Euterpe oleracea Mart.). J Agric Food Chem. 2004;52(6):1539–45. doi: 10.1021/jf035189n [DOI] [PubMed] [Google Scholar]
- 15.Rodrigues RB, Lichtenthaler R, Zimmermann BF, Papagionnopoulos M, Fabricius H, Marx F, et al. Total oxidant scavenging capacity of Euterpe oleracea Mart. (Açaí) seeds and identification of their polyphenolic compounds. J Agric Food Chem. 2006;54(12):4162–7. doi: 10.1021/jf058169p [DOI] [PubMed] [Google Scholar]
- 16.Moura RS, Ferreira TS, Lopes AA, Pires KM, Nesi RT, Resende AC, et al. Effects of Euterpe oleracea Mart. (Açaí) extract in acute lung inflammation induced by cigarette smoke in the mouse. Phytomedicine. 2012;19(3–4):262–9. doi: 10.1016/j.phymed.2011.11.004 [DOI] [PubMed] [Google Scholar]
- 17.Alqurashi RM, Galante LA, Rowland IR, Spencer JP, Commane DM. Consuption of a flavonoid-rich açaí meal is associated with acute improvements in vascular function and a reduction in total oxidative status in healthy overweight men. Am J Clin Nutr. 2016:104(5):1227–35. doi: 10.3945/ajcn.115.128728 [DOI] [PubMed] [Google Scholar]
- 18.Sadowska-Krepa E, Klapcinska B, Podgórski T, Szade B, Tyl K, Hadzik A. Effects of supplementation with açaí (Euterpe oleracea Mart.) berry-based juice blend on the blood antioxidant defence capacity and lipid profile in junior hurdlers. A pilot study. Biol Sport. 2015;32(2):161–8. doi: 10.5604/20831862.1144419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barbosa PO, Pala D, Silva CT, de Souza MO, do Amaral JF, Vieira RA, et al. Açaí (Euterpe oleracea Mart.) pulp dietary intake improves cellular antioxidant enzymes and biomarkers of serum in healthy women. Nutrition. 2016;32(6):674–80. doi: 10.1016/j.nut.2015.12.030 [DOI] [PubMed] [Google Scholar]
- 20.Marques ES, Tsuboy MSF, Carvalho JCT, Rosa PCP, Perazzo FF, Gaivão IOM, et al. First cytotoxic, genotoxic, and antigenotoxic assessment of Euterpe oleracea fruit oil (açaí) in cultured human cells. Genet Mol Res. 2017;16(3). doi: 10.4238/gmr16039700 [DOI] [PubMed] [Google Scholar]
- 21.Caiado RR, Peris CS, Lima-Filho AAS, Urushima JGP, Novais E, Badaró E, et al. Retinal toxicity of açaí fruit (Euterpe Oleracea) dye concentrations in rabbits: Basic principles of a new dye for chromovitrectomy in humans. Curr Eye Res. 2017;42(8):1185–93. doi: 10.1080/02713683.2017.1297995 [DOI] [PubMed] [Google Scholar]
- 22.Udani JK, Singh BB, Singh VJ, Barrett ML. Effects of açaí (Euterpe oleracea Mart.) berry preparation on metabolic parameters in a healthy overweight population: a pilot study. Nutr J. 2011;10:45 doi: 10.1186/1475-2891-10-45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bashardoust Tajali S, Macdermid JC, Houghton P, Grewal R. Effects of low power laser irradiation on bone healing in animals: a meta-analysis. J Orthop Surg Res. 2010;5(1):1 doi: 10.1186/1749-799X-5-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schreckinger ME, Lotton J, Lila MA, de Mejia EG. Berries from South America: a comprehensive review on chemistry, health potential, and commercialization. J Med Food. 2010;13(2):233–46. doi: 10.1089/jmf.2009.0233 [DOI] [PubMed] [Google Scholar]
- 25.Felzenszwalb I, da Costa Marques MR, Mazzei JL, Aiub CA. Toxicological evaluation of Euterpe edulis: a potential superfruit to be considered. Food Chem Toxicol. 2013;58:536–44. doi: 10.1016/j.fct.2013.05.029 [DOI] [PubMed] [Google Scholar]
- 26.Kim YS, Jung H, Zerin T, Song HY. Protein profiling of paraquat-exposed rat lungs following treatment with açaí (Euterpe oleracea Mart.) berry extract. Mol Med Rep. 2013;7(3):881–6. doi: 10.3892/mmr.2013.1259 [DOI] [PubMed] [Google Scholar]
- 27.de Souza Machado F, Kuo J, Wohlenberg MF, da Rocha Frusciante M, Freitas M, Oliveira AS, et al. Subchronic treatment with açaí frozen pulp prevents the brain oxidative damage in rats with acute liver failure. Metab Brain Dis. 2016;31(6):1427–34. doi: 10.1007/s11011-016-9873-3 [DOI] [PubMed] [Google Scholar]
- 28.Kowar M, Friedrich C, Jacobs AH. [Pregabalin as a rare cause of liver disease]. Dtsch Med Wochenschr. 2015;140(23);1759–60. doi: 10.1055/s-0041-105987 [DOI] [PubMed] [Google Scholar]
- 29.Leba LJ, Brunschwig C, Saout M, Martial K, Bereau D, Robinson JC. Oenocarpus bacaba and Oenocarpus bataua leaflets and roots: A new source of antioxidant compounds. Int J Mol Sci. 2016;17(7). doi: 10.3390/ijms17071014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brasil A, Rocha FAF, Gomes BD, Oliveira KRM, de Carvalho TS, Batista EJO, et al. Diet enriched with the Amazon fruit açaí (Euterpe oleracea) prevents electrophysiological deficits and oxidative stress induced by methyl-mercury in the rat retina. Nutr Neurosci. 2017;20(5);265–72. doi: 10.1080/1028415X.2015.1119378 [DOI] [PubMed] [Google Scholar]
- 31.Spada PD, de Souza GG, Bortolini GV, Henriques JA, Salvador M. Antioxidant, mutagenic, and antimutagenic activity of frozen fruits. J Med Food. 2008;11(1):144–51. doi: 10.1089/jmf.2007.598 [DOI] [PubMed] [Google Scholar]
- 32.Silva D. Analysis of cytotoxicity of fruit extract juçara (Euterpe oleracea mart) of Maranhão in human malignant cells. Tese. Universidade do Estado do Rio de Janeiro, 2013.
- 33.Wong DY, Musgrave IF, Harvey BS, Smid SD. Açaí (Euterpe oleraceae Mart.) berry extract exerts neuroprotective effects against β-amyloid exposure in vitro. Neurosci Lett. 2013;556:221–6. doi: 10.1016/j.neulet.2013.10.027 [DOI] [PubMed] [Google Scholar]
- 34.Dias MM, Noratto G, Martino HS, Arbizu S, Peluzio M do C, Talcott S, et al. Pro-apoptotic activities of polyphenolics from açaí (Euterpe oleracea Martius) in human SW-480 colon cancer cells. Nutr Cancer. 2014;66(8):1394–405. doi: 10.1080/01635581.2014.956252 [DOI] [PubMed] [Google Scholar]
- 35.Brito C, Stavroullakis AT, Ferreira AC, Li K, Oliveira T, Nogueira-Filho G, et al. Extract of açaí-berry inhibits osteoclast differentiation and activity. Arch Oral Biol. 2016;68:29–34. doi: 10.1016/j.archoralbio.2016.03.016 [DOI] [PubMed] [Google Scholar]
- 36.Machado AK, Andreazza AC, da Silva TM, Boligon AA, do Nascimento V, Scola G, et al. Neuroprotective effects of açaí (Euterpe oleracea Mart.) against rotenone in vitro exposure. Oxid Med Cell Longev. 2016;2016:8940850 doi: 10.1155/2016/8940850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brito C, Stavroullakis A, Oliveira T, Prakki A. Cytotoxicity and potential anti-inflammatory activity of velutin on RAW 264.7 cell line differentiation: Implications in periodontal bone loss. Arch Oral Biol. 2017;83:348–56. doi: 10.1016/j.archoralbio.2017.09.001 [DOI] [PubMed] [Google Scholar]
- 38.Freitas DDS, Morgado-Díaz JA, Gehren AS, Vidal FCB, Fernandes RMT, Romão W, et al. Cytotoxic analysis and chemical characterization of fractions of the hydroalcoholic extract of the Euterpe oleracea Mart. seed in the MCF-7 cell line. J Pharm Pharmacol. 2017;69(6);714–21. doi: 10.1111/jphp.12679 [DOI] [PubMed] [Google Scholar]
- 39.Vrailas-Mortimer A, Gomez R, Dowse H, Sanyal S. A survey of the protective effects of some commercially available antioxidant suppllements in genetically and chemically induced models of oxidative stress in Drosophila melanogaster. Exp Gerontol. 2012;47(9):712–22. doi: 10.1016/j.exger.2012.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stoner GD, Wang LS, Seguin C, Rocha C, Stoner K, Chiu S, et al. Multiple berry types prevent N-nitrosomethylbenzylamine-induced esophageal cancer in rats. Pharm Res. 2010;27(6);1138–45. doi: 10.1007/s11095-010-0102-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fragoso MF, Prado MG, Barbosa L, Rocha NS, Barbisan LF. Inhibition of mouse urinary bladder carcinogenesis by açaí fruit (Euterpe oleraceae Martius) intake. Plant Foods Hum Nutr. 2012;67(3):235–41. doi: 10.1007/s11130-012-0308-y [DOI] [PubMed] [Google Scholar]
- 42.Fragoso MF, Romualdo GR, Ribeiro DA, Barbisan LF. Açaí (Euterpe oleracea Mart.) feeding attenuates dimethylhydrazine-induced rat colon carcinogenesis. Food Chem Toxicol. 2013;58:68–76. doi: 10.1016/j.fct.2013.04.011 [DOI] [PubMed] [Google Scholar]
- 43.Choi YJ, Choi YJ, Kim N, Nam RH, Lee S, Lee HS, et al. Açaí berries inhibit colon tumorigenesis in azoxymethane/dextran sulfate sodium-treated mice. Gut Liver. 2017;11(2);243–52. doi: 10.5009/gnl16068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Monge-Fuentes V, Muehlmann LA, Longo JP, Silva JR, Fascineli ML, de Souza P, et al. Photodynamic therapy mediated by açaí oil (Euterpe oleracea Martius) in nanoemulsion: A potential treatment for melanoma. J Photochem Photobiol. 2017;166:301–10. doi: 10.1016/j.jphotobiol.2016.12.002 [DOI] [PubMed] [Google Scholar]
- 45.Nascimento VH, Lima CD, Paixão JT, Freitas JJ, Kietzer KS. Antioxidant effects of açaí seed (Euterpe oleracea) in anorexia-cachexia syndrome induced by Walker-256 tumor. Acta Cir Bras. 2016;31(9):597–601. doi: 10.1590/S0102-865020160090000004 [DOI] [PubMed] [Google Scholar]
- 46.Ribeiro JC, Antunes LM, Aissa AF, Darin JD, de Rosso VV, Mercadante AZ, et al. Evaluation of the genotoxic and antigenotoxic effects after acute and subacute treatments with açaí pulp (Euterpe oleracea Mart.) on mice using the erythrocytes micronucleus test and the comet assay. Mutat Res. 2010;695(1–2):22–8. doi: 10.1016/j.mrgentox.2009.10.009 [DOI] [PubMed] [Google Scholar]
- 47.Marques ES, Froder JG, Carvalho JC, Rosa PC, Perazzo FF, Maistro EL. Evaluation of the genotoxicity of Euterpe oleraceae Mart. (Arecaceae) fruit oil (açaí), in mammalian cells in vivo. Food Chem Toxicol. 2016;93:13–9. doi: 10.1016/j.fct.2016.04.018 [DOI] [PubMed] [Google Scholar]
- 48.Schauss AG, Clewell A, Balogh L, Szakonyi IP, Financsek I, Horváth J, et al. Safety evaluation of an açaí-fortified fruit and berry functional juice beverage (MonaVie Active((R)). Toxicology. 2010;278(1):46–54. doi: 10.1016/j.tox.2010.04.017 [DOI] [PubMed] [Google Scholar]
- 49.Hogan S, Chung H, Zhang L, Li J, Lee Y, Dai Y, et al. Antiproliferative and antioxidant properties of anthocyanin-rich extract from açaí. Food Chem 2010;118(2):208–14. doi: 10.1016/j.foodchem.2009.04.099. [Google Scholar]
- 50.Matheus ME, Oliveira SBF, Silveira CS, Rodrigues VP, de Souza Menezes F, Fernandes PD. Inhibitory effects of Euterpe oleraceae Mart. on nitric oxide production and iNOS expression. J Ethnopharmacol. 2006;107(2):291–6. doi: 10.1016/j.jep.2006.03.010 [DOI] [PubMed] [Google Scholar]
- 51.Schauss AG, Wu X, Prior RL, Ou B, Patel D, Huang D, et al. Phytochemical and nutrient composition of the freeze-dried amazonian palm berry, Euterpe Oleraceae Mart. (Açaí). J Agric Food Chem. 2006;54(22):8598–603. doi: 10.1021/jf060976g [DOI] [PubMed] [Google Scholar]
- 52.de Moura RS, Pires KM, Santos Ferreira T, Lopes AA, Nesi RT, Resende AC, et al. Addition of açaí (Euterpe oleracea) to cigarettes has a protective effect against emphysema in mice. Food Chem Toxicol. 2011;49(4):855–63. doi: 10.1016/j.fct.2010.12.007 [DOI] [PubMed] [Google Scholar]
- 53.Sudo RT, Neto ML, Monteiro CE, Amaral RV, Resende ÂC, Souza PJ, et al. Antinociceptive effects of hydroalcoholic extract from Euterpe oleracea Mart. (Açaí) in a rodent model of acute and neuropathic pain. BMC Complement Altern Med. 2015;15(208). doi: 10.1186/s12906-015-0724-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Poulose SM, Fischer DR, Larson J, Bielinski DF, Rimando AM, Carey AN, et al. Anthocyanin-rich açaí (Euterpe oleracea Mart.) fruit pulp fractions attenuate inflammatory stress signaling in mouse brain BV-2 microglial cells. J Agric Food Chem. 2012;60(4):1084–93. doi: 10.1021/jf203989k [DOI] [PubMed] [Google Scholar]
- 55.Machado DE, Rodrigues-Baptista KC, Alessandra-Perini J, Soares de Moura R, Santos TA, Pereira KG, et al. Euterpe Oleracea extract (Açaí) is a promising novel pharmacological therapeutic treatment for experimental endometriosis. PLoS One. 2016;11(11):e0166059 doi: 10.1371/journal.pone.0166059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pala D, Barbosa PO, Silva CT, de Souza MO, Freitas FR, Volp ACP, et al. Açaí (Euterpe oleracea Mart.) dietary intake plasma lipids, apolipoproteins, cholesteryl ester transfer to high-density lipoprotein and redox metabolism: A prospective study in women. Clin Nutri. 2017;37(2):618–23. doi: 10.1016/j.clnu.2017.02.001 [DOI] [PubMed] [Google Scholar]
- 57.Carvalho-Peixoto J, Moura MR, Cunha FA, Lollo PC, Monteiro WD, Carvalho LM, et al. Consumption of açaí (Euterpe oleracea Mart.) functional beverage reduces muscle stress and improves effort tolerance in elite athletes: a randomized controlled intervention study. Appl Physiol Nutr Metab. 2015;40(7)725–33. doi: 10.1139/apnm-2014-0518 [DOI] [PubMed] [Google Scholar]
- 58.Pereira IS, Moreira Cançado Mascarenhas Pontes TC, Lima Vieira RA, de Freitas Folly GA, Cacilda Silva F, Pereira de Oliveira FL, et al. The consumption of açaí pulp changes the concentrations of plasminogen activator inhibitor-1 and epidermal growth factor (EGF) in apparently healthy women. Nutr Hosp. 2015;32(2):931–45. doi: 10.3305/nh.2015.32.2.9135 [DOI] [PubMed] [Google Scholar]
- 59.Bittman ME, Callahan MJ. The effective use of açaí juice, blueberry juice and pineapple juice as negative contrast agents for magnetic resonance cholangiopancreatography in children. Pediatr Radiol. 2014; 44(7):883–7. doi: 10.1007/s00247-014-2884-5 [DOI] [PubMed] [Google Scholar]
- 60.Gale AM, Kaur R, Baker WL. Hemodynamic and electrocardiographic effects of açaí berry in healthy volunteers: a randomized controlled trial. Int J Cardiol. 2014;174(2):421–3. doi: 10.1016/j.ijcard.2014.04.036 [DOI] [PubMed] [Google Scholar]
- 61.Ellinger S, Gordon A, Kurten M, Jungfer E, Zimmermann BF, Zur B. Bolus consumption of a specifically designed fruit juice rich in anthocyanins and ascorbic acid did not influence markers of antioxidative defense in healthy humans. J Agric Food Chem. 2012;60(45):11292:300. doi: 10.1021/jf300719t [DOI] [PubMed] [Google Scholar]
- 62.Jensen GS, Ager DM, Redman KA, Mitzner MA, Benson KF, Schauss AG. Pain reduction and improvement in range of motion after daily consumption of an açaí (Euterpe oleracea Mart.) pulp-fortified polyphenolic-rich fruit and berry juice blend. J Med Food. 2011;14(7–8):702–11. doi: 10.1089/jmf.2010.0150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sanchez TA, Elias J Jr, Colnago LA, de Almeida Troncon LE, de Oliveira RB, Baffa O, et al. Clinical feasibility of Açaí (Euterpe oleracea) pulp as an oral contrast agente for magnetic resonance cholangiopancreatography. J Comput Assist Tomogr. 2009;33(5):666–71. doi: 10.1097/RCT.0b013e31819012a0 [DOI] [PubMed] [Google Scholar]
- 64.Jensen GS, Wu X, Patterson KM, Barnes J, Carter SG, Scherwitz L, et al. In vitro and in vivo antioxidant and anti-inflammatory capacities of an antioxidant-rich fruit and berry juice blend. Results of a pilot and randomized, double-blinded, placebo-controlled, crossover study. J Agric Food Chem. 2008;56(18):8326–33. doi: 10.1021/jf8016157 [DOI] [PubMed] [Google Scholar]
- 65.Mertens-Talcott SU, Rios J, Jilma-Stohlawetz P, Pacheco-Palencia LA, Meibohm B, Talcott ST. Pharmacokinetics of anthocyanins and antioxidant effects after the consumption of anthocyanin-rich acai juice and pulp (Euterpe oleracea Mart.) in human healthy volunteers. J Agric Food Chem. 2008;56(17)7796–802. doi: 10.1021/jf8007037 [DOI] [PubMed] [Google Scholar]
- 66.Córdova-Fraga T, de Araújo DB, Sanchez TA, Elias J Jr, Carneiro AA, Brandt Oliveira R, et al. Euterpe oleracea (Açaí) as an alternative oral contrast agent in MRI of the gastrointestinal system: preliminary results. Magn Reson Imaging. 2004;22(3):389–93. doi: 10.1016/j.mri.2004.01.018 [DOI] [PubMed] [Google Scholar]
- 67.de Oliveira PR, da Costa CA, de Bem GF, Cordeiro VS, Santos IB, de Carvalho LC, et al. Euterpe oleracea Mart.-derived polyphenols protect mice from diet-induced obesity and fatty liver by regulating hepatic lipogenesis and cholesterol excretion. Plos One. 2015;10(12):e0143721 doi: 10.1371/journal.pone.0143721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.da Costa CA, Ognibene DT, Cordeiro VSC, de Bem GF, Santos IB, Soares RA, et al. Effect of Euterpe oleracea Mart. seeds extract on chronic ischemic renal injury in renovascular hypertensive rats. J Med Food. 2017;20(10):1002–10. doi: 10.1089/jmf.2017.0011 [DOI] [PubMed] [Google Scholar]
- 69.Machado DE, Palumbo AJ, Santos JM, Mattos RM, dos Santos TA, Seabra SH, et al. A GFP endometriosis model reveals important morphological characteristics of the angiogenic process that govern benign and malignant diseases. Histol Histopathol. 2014;29(7):903–12. doi: 10.14670/HH-29.903 [DOI] [PubMed] [Google Scholar]
- 70.Becker C, Fantini MC, Schramm C, Lehr C, Wirtz S, Nikolaev A, et al. TGF-beta suppresses tumor progression in colon cancer by inhibition of IL-6 trans-signaling. Immunity. 2004;21(4):491–501. doi: 10.1016/j.immuni.2004.07.020 [DOI] [PubMed] [Google Scholar]
- 71.Popivanova BK, Kitamura K, Wu Y, Kondo T, Kagaya T, Kaneko S, et al. Blocking TNF-α in mice reduces colorectal carcinogenesis associated with chronic colitis. J Clin Invest. 2008;118(2):560–70. doi: 10.1172/JCI32453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Grivennikov S, Karin E, Terzic J, Mucida D, Yu GY, Vallabhapurapu S, et al. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell. 2009;15(2):103–13. doi: 10.1016/j.ccr.2009.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xie C, Kang J, Li Z, Schauss AG, Badger TM, Nagarajan S, et al. The açaí flavonoid velutin is a potent anti-inflammatory agent: blockade of LPS-mediated TNF-α and IL-6 production through inhibiting NF-κB activation and MAPK pathway. J Nutr Biochem, 2012;23(9):1184–91. doi: 10.1016/j.jnutbio.2011.06.013 [DOI] [PubMed] [Google Scholar]
- 74.El Morsy EM, Ahmed MA, Ahmed AA. Attenuation of renal ischemia/reperfusion injury by açaí extract preconditioning in a rat model. Life Sci. 2015;123:123–35. doi: 10.1016/j.lfs.2014.11.013 [DOI] [PubMed] [Google Scholar]
- 75.Guzinska-Ustymowicz K, Pryczynicz A, Kemona A, Czyzewska J. Correlation between proliferation markers: PCNA, Ki-67, MCM-2 and antiapoptotic protein Bcl-2 in colorectal cancer. Anticancer Res. 2009;29(8):3049–52. [PubMed] [Google Scholar]
- 76.Graziano V, de Laurenzi V. Role of p63 in cancer development. Biochim Biophys Acta. 2011;1816(1):57–66. doi: 10.1016/j.bbcan.2011.04.002 [DOI] [PubMed] [Google Scholar]
- 77.Qiu X, Mei J, Yin J, Wang H, Wang J, Xie M. Correlation analysis between expression of PCNA, Ki-67 and COX-2 and X-ray features in mammography in breast cancer. Oncol Lett. 2017;14(3):2912–8. doi: 10.3892/ol.2017.6516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Roy AM, Baliga MS, Katiyar SK. Epigallocatechin-3-gallate induces apoptosis in estrogen receptor-negative human breast carcinoma cells via modulation in protein expression of p53 and Bax and caspase-3 activation. Mol Cancer Ther. 2005;4(1):81–90. [PubMed] [Google Scholar]
- 79.Forester SC, Gu Y, Lambert JD. Inhibition of starch digestion by the green tea polyphenol, (-)-epigallocatechin-3-gallate. Mol Nutr Food Res. 2012;56(11):1647–54. doi: 10.1002/mnfr.201200206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Del Pozo-Insfran D, Percival SS, Talcott ST. Açaí (Euterpe Oleracea Mart.) polyphenolics in their glycoside and aglycone forms induce apoptosis of HL-60 leukemia cells. J Agric Food Chem. 2006;54(4):1222–9. doi: 10.1021/jf052132n [DOI] [PubMed] [Google Scholar]
- 81.Cardoso AL, de Liz S, Rieger DK, Farah ACA, Kunradi Vieira FG, Altenburg de Assis MA, et al. An Update on the Biological Activities of Euterpe edulis (Juçara). Panta Med. 2018. doi: 10.1055/s-0044-101624 [DOI] [PubMed] [Google Scholar]
- 82.de Barros Freitas R, Melato FA, Oliveira JM, Bastos DS, Cardoso RM, Leite JP, et al. Euterpe edulis effects on cardiac and renal tissues of Wistar rats fed with cafeteria diet. Nutr Hosp. 2017;34(1):186–92. doi: 10.20960/nh.996 [DOI] [PubMed] [Google Scholar]
- 83.Rim KT, KIM SJ. A review on mutagenicity testing for hazard classification of chemicals at work: focusing on in vivo micronucleus test for allyl chloride. Saf Health Work. 2015;6(3):184–91. doi: 10.1016/j.shaw.2015.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Santos RA, Takahashi CS. Anticlastogenic and antigenotoxic effects of selenomethionine on doxorubicin-induced damage in vitro in human lymphocytes. Food Chem Toxicol. 2008;46(2):671–7. doi: 10.1016/j.fct.2007.09.090 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.