Abstract
Efforts to stem the global rise in obesity have been minimally effective, perhaps in part because our understanding of the psychological and behavioral drivers of obesity is limited. It is well established that stimuli that are paired with palatable foods can powerfully influence food-seeking and feeding behaviors. However, how consumption of sugary, fatty “junk-foods” affects these motivational responses to food cues is poorly understood. Here, we determined the effects of short- and long-term “junk-food” consumption on the expression of cue potentiated feeding and conditioned food cup approach to Pavlovian conditioned stimuli (CS). Further, to determine the degree to which effects of “junk-food” were selective to Pavlovian motivational processes, we varied the predictive validity of the CS by including training groups conditioned with unique CS-US contingencies ranging from −1.0 to +1.0. “Junk-food” did not enhance cue potentiated feeding in any group, but expression of this potentiation effect varied with the CS-US contingency independent of diet. In contrast, “junk-food” consistently enhanced conditioned approach to the food cup; this effect was dependent on the previously established CS-US contingency. That is, consumption of “junk-food” following training enhanced approach to the food cup only in response to CSs with previously positive CS-US contingencies. This was accompanied by reduced motivation for the US itself. Together these data show that “junk-food” consumption selectively enhances incentive motivational responses to previously established food CSs, without altering cue potentiated feeding induced by these same CSs, and in the absence of enhanced motivation for food itself.
Keywords: obesity, incentive motivation, food cues, striatum, behavior, Pavlovian conditioning
1. Introduction
World-wide obesity rates have steadily increased over the past half century (W.H.O, 2017). Efforts to stem the tide of this trend have been minimally effective, perhaps in part because our understanding of the psychological and behavioral drivers of obesity is limited (Chan & Woo, 2010). One potentially important psychological contributor to the development of obesity is the influence that Pavlovian conditioned stimuli (CSs) exert on craving, food-seeking, and consummatory behaviors (Dagher, 2009; Berridge et al., 2010; Chan & Woo, 2010). For example, repeated pairings of an initially neutral CS with an unconditioned stimulus (US) food reward, results in the acquisition of an association between the CS and the food US (CS-US association). Once these associations are established, mere presentation of the CS elicits conditioned reflexes and behavioral responses appropriate to the nature of the initial US (Pavlov, 1927). Conditioned reflexes manifest as physiological changes, such as increased salivation, whereas conditioned responses manifest as changes in appetitive behaviors, such as increased approach toward the site of expected US delivery. In addition to acquiring predictive significance, CSs can also acquire strong motivational valence across conditioning. The motivational influence of CSs can trigger enhanced ‘cravings’, promote instrumental food-seeking behaviors (Pavlovian-to-instrumental transfer), and ultimately increase the amount of food consumed (Weingarten, 1983; Birch et al., 1989; Rogers & Hill, 1989; Pandit et al., 2012; Blechert et al., 2016).
Enhanced cue-triggered motivation for food has been implicated as a factor in the development of human obesity (Dagher, 2009; Berridge et al., 2010). For example, in humans the scent and sight of foods elicit increases in heart rate, blood pressure, skin conductance, salivation, and subjective ratings of craving and hunger. These responses occur in healthy weight individuals, but are enhanced in obese subjects and those identified as binge eaters (Nederkoorn et al., 2000; Udo et al., 2014; Wolz et al., 2017). Similarly, presentation of food CSs increases food consumption in healthy weight individuals, an effect that is also enhanced in obese subjects (Birch et al., 1989; Wolz et al., 2017). Furthermore, several brain imaging studies in healthy, overweight, and obese subjects have found that food cues elicit activations in corticolimbic regions, and that the magnitude of these effects is enhanced in overweight and obese populations (Rothemund et al., 2007; Stoeckel et al., 2008; Bruce et al., 2010; Yokum et al., 2014). Although basal differences in neurobehavioral responsivity to food cues likely contribute to weight gain (Demos et al., 2012; Murdaugh et al., 2012; Yokum et al., 2014; Robinson et al., 2015; Derman & Ferrario, 2018), determining how consumption of sugary, fatty foods may impact these motivational responses to food cues is critical for understanding the mechanisms driving food-seeking and over-eating.
While diet composition has an obvious and direct influence on fat accumulation and metabolism, diets high in fats and sugars also have wide-ranging effects on the function of corticolimbic regions that may render individuals more susceptible to the motivational influence of food cues (Counotte et al., 2014; Robinson et al., 2015; Liu et al., 2016; Brown et al., 2017). However, only a few preclinical studies have examined how sugar- and fat-rich diets affect motivational responses to Pavlovian cues. One such study found that obesity resulting from consumption of a sugary, fatty “junk-food” diet enhanced willingness to work for a previously learned food CS (i.e., conditioned reinforcement; Robinson et al., 2015). Similarly, another recent study found that consumption of a 60% high-fat diet enhanced instrumental responding in the ‘incubation of craving’ model in outbred rats (Dingess et al., 2017). Together these data suggest that consumption of obesogenic diets may enhance conditioned motivational responses to food CSs. However, whether these effects are related to the predictive validity of a given CS and how these diet manipulations may affect different aspects of cue-triggered motivation are poorly understood. Therefore here, we determined the effects of both, short- and-long term “junk-food” consumption on the expression of conditioned approach and cue potentiated feeding.
Cue potentiated feeding is a well-established procedure that tests the ability of a learned food-predictive CS to spur on food intake in the face of selective satiation (Weingarten, 1983; Reppucci & Petrovich, 2012). Additionally, the nature of this testing procedure also provides information about conditioned approach to the site of US delivery. Thus, here were assessed the impact of “junk-food” consumption of cue-induced consummatory and conditioned appetitive responses. Furthermore, in order to determine whether effects of “junk-food” were specific to Pavlovian motivational processes, we systematically varied the predictive validity of the CS across distinct training groups by varying the CS-US contingencies between groups. If “junk-food” consumption imparts a change in behavior that is specific to Pavlovian motivation, then effects of “junk-food” should be found in response to CSs with positive predictive validity, but not to CSs with neutral or negative predictive validity.
While the precise neurobiological underpinnings of conditioned approach and cue potentiated feeding are not fully understood, they have been shown to depend upon corticolimbic circuitry and on dopaminergic and glutamatergic transmission in these regions (Petrovich et al., 2007; Blaiss & Janak, 2009; McDannald et al., 2013; Cole et al., 2015; Keefer & Petrovich, 2017). Given that consumption of sugar- and fat-rich diets enhance function within these circuits (Baladi et al., 2015; Peng et al., 2015; Robinson et al., 2015; Ferrario et al., 2016; Oginsky et al., 2016), we predicted that “junk-food” consumption would enhance conditioned approach and cue potentiated feeding. Moreover, if this diet manipulation selectively affects Pavlovian motivational processes, then we would expect behavioral differences would manifest only in groups trained with a strong positively predictive CS-US relationship.
2. General Materials and Methods
2.1. Subjects
Adult male Sprague Dawley rats (total N=139) purchased from Harlan Laboratories (now Envigo) were used. Rats were pair housed and maintained on a reverse light-dark schedule (12/12), where all behavioral experiments were performed during the dark phase. Rats were 70 days old at the start of each experiment. All procedures were approved by The University of Michigan Institutional Animal Care and Use Committee. Additional details for all procedures and housing can be found at: https://sites.google.com/a/umich.edu/ferrario-lab-public-protocols/.
2.2. Pavlovian Conditioning
Prior to training, rats (Experiment 1, n=80; Experiment 2, n=59) were food restricted to 85–90% of their free-feeding bodyweight and maintained at this weight for the duration of training. All training and testing was conducted in standard Med Associate operant chambers. After reaching their target weight range, rats underwent 2 food cup training sessions (1/day) in which 20 food pellets (US; 45mg Bioserv #F0021; 0.75 protein, 0.5 fat, 2.36 carbohydrate kCal/g) were delivered into the food cup on a variable time (VT) schedule of 60 seconds (range, 30”–90”). Next, rats underwent Pavlovian conditioning in 8 daily sessions. Here we describe the general procedure and followed by specific details about the relationship between CS-US presentations for Experiment 1 and 2 in section 3.1 and 3.2, respectively. Each session consisted of four presentations of a 2-minute noise conditioned stimulus (CS) with an average inter-trial-interval (ITI) of 5 minutes (range 3’–7’). Each session lasted ~28 minutes and rats received a total of 16 pellet deliveries per session. Food cup entries during the CS and ITI periods were recorded throughout each session.
2.3. Junk-Food Diet
After initial Pavlovian conditioning, rats were placed on ad lib chow (Test Diet, 5001; 4.5% fat, 23% protein, 48.7% carbohydrates; 4 kcal/g) for 1 day, and then assigned to chow (Chow) or “Junk-Food” (JF) groups counterbalanced by weight and behavior during Pavlovian training. The “JF” diet is a mash consisting of Chips Ahoy! chocolate chip cookies (260g), Frito Lays potato chips (80g), Jif peanut butter (260g), Nestle Nesquik chocolate powder (260g), Test Diet, 5001 (400g) and water (355ml; 19.6% fat, 14% protein, 58% carbohydrates; 4.5 kcal/g). Bodyweights and food intake (per cage) were monitored for the remainder of the experiment.
2.4. Cue Potentiated Feeding and Conditioned Approach Testing
Testing for cue potentiated feeding was adapted from existing procedures (Galarce et al., 2007; Cole et al., 2015). Rats were given free access to the same Bioserv food US used in initial training for one hour prior to testing. Next, rats were placed in the operant boxes and food intake was recorded during two distinct 5 minute phases of testing: baseline (BL) and CS presentation (CS test). In each of these phases, a pre-weighed portion (~2–2.5g) of pellets was placed directly into the food cup without obscuring the infrared beam used to detect food cup entries. During the BL test, there were no CS presentations and rats were free to eat from the food cup during the 5 minute period. The rats were then briefly removed from the chamber, the remaining pellets were collected, and a fresh pre-weighed portion of pellets was placed in the food cup (~2–2.5g). Rats were then returned to the chamber for the CS test (5 minutes). During this test, rats were given two 90 second CS presentations, the first occurred 30 seconds into testing, and the 2nd occurred 60 seconds after the first presentation; an additional 30 seconds of post-CS responding was recorded after the final CS presentation. Our testing procedure was adapted from Galarce et al (2007) which used a 5 minute test with 2 CS presentations. However, here we shortened the duration of CS to 90 seconds in order to enhance our ability to observe conditioned approach, while maintaining a 5 min total testing time. After testing, rats were then returned to their home cage and the pellets remaining in the food cup were collected and weighed. Consumption was determined by measuring the difference in weight of the pellets remaining from pellets provided during each phase of testing. In addition, food cup entries were measured throughout.
2.5. Extinction and Reinstatement Test
During testing borderline effects of JF consumption were found in the Zero Contingency and 50% Positive Contingency groups, which led us to probe this effect further using an extinction/reinstatement protocol. Here, rats underwent 4 days of extinction training; this was identical to their original Pavlovian conditioning, except no USs were delivered. Next, they were tested for reinstatement of conditioned approach to the food cup. In this test, rats received one CS-US pairing at the start of the session (reinstatement of CS-US association), followed by 4 presentations of the CS alone. Food cup entries were recorded throughout extinction and reinstatement testing.
2.6. Progressive Ratio Testing
Testing under progressive ratio conditions was used to determine whether JF consumption altered motivation for the US. Rats were trained on a Fixed Ratio-1 (FR1) to press an active lever to earn the same Bioserv pellet US from Pavlovian conditioning (3, 20-min sessions; 1 session/day). Following FR1 training, rats were tested on a progressive ratio (PR) schedule in which the ratio of lever presses required to earn a pellet was progressively increased using the following formula: response ratio [=5e(US delivery × 0.2)]−5 (as in Richardson & Roberts, 1995). The PR test sessions closed after either 30 minutes passed without any active lever response or the total session length reached 3 hours. Lever presses, food cup entries, and the final ratio achieved (i.e., breakpoint) were recorded.
2.7. Statistics
Statistical analyses were performed using Graphpad Prism (Version 7.0c) and included: unpaired and paired t-tests, and one-way and two-way RM ANOVAs. Sidak’s and Dennett’s multiple comparisons were used for post-hoc and planned comparisons.
3. Design of individual experiments
3.1. Experiment 1. Effects of Limited Junk-Food Consumption
In Experiment 1, four separate groups of rats were trained, each with a distinct CS-US contingency. The purpose here was to determine whether subsequent consumption of a JF diet resulted in effects specific to the initial predictive nature of the CS, or general effects that were not unique to prior learning. Therefore, during Pavlovian conditioning we systematically varied the predictive validity of CS [p(US|CS)-p(US|ITI)] between groups from 100% negative to 100% positive. Specifically, in the 100% Negative Contingency group (n=18), the predictive validity of the CS was set to −1.0, thus US deliveries occurred only during the ITI and never during the CS. In the Zero Contingency group (n=20), the predictive validity of the CS was set to 0.0, where US deliveries occurred with equal probability during both the CS and the ITI period. In the 50% Positive Contingency group (n=20), the predictive validity of the CS was set to +0.5, with US deliveries occurring on half the CS trials and never during the ITI. Finally, for the 100% Positive Contingency group (n=42), the predictive validity of the CS was set to +1.0, such that USs were delivered during every CS trial and never during the ITI. In each of these training groups US deliveries were presented on a VT schedule within the appropriate delivery windows. (See Rescorla, 1968 for discussion of the effects of CS-US contingency on conditioned responding.) Following Pavlovian conditioning, half the rats in each training contingency group were given free access to chow or JF in their home cage (as described above, section 2.3) for 14 days before testing. During this period, food intake and bodyweight were recorded at least three times per week.
During testing, we found trends for increases in conditioned approach in JF vs. Chow treated rats in the Zero Contingency and 50% Positive Contingency trained groups, which led us to further probe this effect using an extinction reinstatement protocol in these groups as described above (section 3.4). In addition, potential effects of JF on motivation for the US were evaluated in these same rats using progressive ratio testing (section 3.5).
3.2. Experiment 2: Effects of Prolonged Junk-food Consumption and Junk-food Deprivation
For Experiment 2, two groups of rats underwent Pavlovian training: a 100% Negative Contingency group (n=18) and a 100% Positive Contingency group (n=41) as described above (section 3.1). Briefly, the 100% Negative contingency group only received pellets during the ITI and the 100% Positive Contingency group only received pellets in the presence of the CS. We then assigned rats to one of three diet manipulation groups: Ad lib chow (Chow; 100% negative n=6, 100% Positive n=10), continuous junk-food (JF; 100% negative n=6, 100% Positive n=16) and junk-food deprivation (JF-Dep; 100% negative n=6, 100% Positive n=16) groups. Rats in the continuous JF group (JF) were given ad lib access to junk-food in their home cage for a total of 45 days. Rats in the JF Deprivation group (JF-Dep) received ad lib access to junk-food in the home cage for 30 days before junk-food was removed and replaced by free access to lab chow for 14 additional days. Importantly, groups were counterbalanced for performance during initial acquisition and weight. In addition, JF and JF-Dep groups were counterbalanced for food intake and weight gain across the first 30 days of diet access. Forty-five days following the final day of Pavlovian conditioning session and the start of diet exposure, rats were tested for cue potentiated feeding and conditioned approach as described above (section 2.4). Thus, for rats in the JD-Dep group, testing occurred 14 days after their last day on the junk-food diet.
4. Results Experiment 1: Effects of CS-US contingency and 14 days of junk-food exposure
4.1. Pavlovian Conditioning
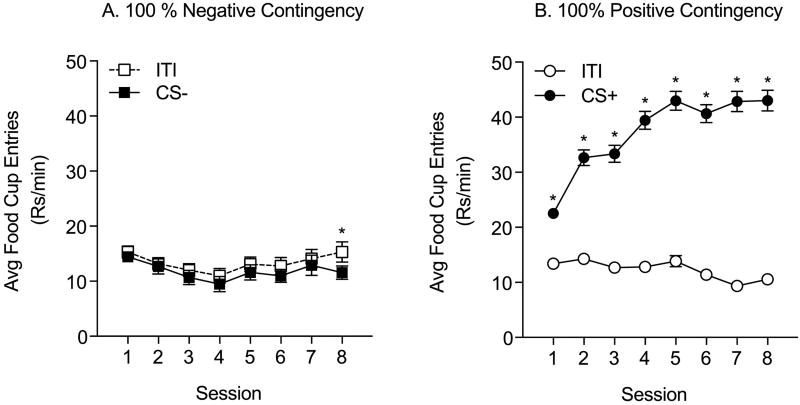
For Experiment 1, 80 male Sprague Dawley rats were food restricted and then trained. The average weight prior to food restriction was 297.2g ± 2.01g. Fig 1 shows the rate of food cup entries during Pavlovian conditioning. (Note that food cup responses were taken across the entire session and include responding in the presence of the US). In the 100% Negative Contingency group (n=18), food cup entries during the ITI increased across sessions relative to entries during the CS− (Fig 1A: two-way RM ANOVA; main effect of phase, F(1,17)=13.91, p<0.01; main effect of session, F(7,119)=7.26, p<0.01; phase by session interaction F(7,119)=4.31, p<0.01), consistent with pellet delivery during the ITI period. In the Zero Contingency group (n=20), food cup entries were slightly, though significantly greater during CS0 presentations than during the ITI, and the magnitude of this effect was fairly stable across training (Fig 1B: two-way RM ANOVA; main effect of phase, F(1,19)=120.5, p<0.01; main effect of session, F(7,133)=7.26, p<0.01; phase by session interaction, F(7,133)=4.8, p<0.01). In the 50% Positive contingency group (n=20) the rate of food cup entries during CS+ presentation increased significantly across sessions compared to the ITI (Fig 1C: two-way RM ANOVA; main effect of phase, F(1,19)=399.9, p<0.01; main effect of session, F(7,133)=22.15, p<0.01; phase by session interaction, F(7,133)=38.8, p<0.01). Likewise in the 100% Positive contingency group (n=42) food cup entries during the CS+ relative to entries during the ITI increased significantly across training (Fig 1D: two-way RM ANOVA; main effect of phase, F(1,41)=582.1, p<0.01; main effect of session, F(7,287)=12.15, p<0.01; phase by session interaction, F(7,133)=63.7, p<0.01). Although measured in the presence of US deliveries, the progressive increases in the magnitude of responding during the CS period are consistent with the development of conditioned approach to the CS+ in the Positive Contingency groups. In addition to these within-subject assessments, we also compared the rate of food cup entries during the ITI and CS across all the training groups. As expected, the rate of food cup entries during CS presentations varied significantly by training contingency (Fig 1E: two-way RM ANOVA; main effect of group, F(3,96)=108.4, p<0.01; main effect of session, F(7,672)=16.28, p<0.01). Specifically, the rate of responding during the CS period was greatest in the 100% Positive Contingency group, whereas the rate in the 50% Positive Contingency group was greater than in the Zero and 100% Negative Contingency groups, and lastly the rate in the Zero Contingency group was greater than in the 100% Negative Contingency group. These group effects became more pronounced across sessions (Fig 1E: two-way RM ANOVA; group × session interaction, F(21,672)=16.75, p<0.01).
Figure 1.
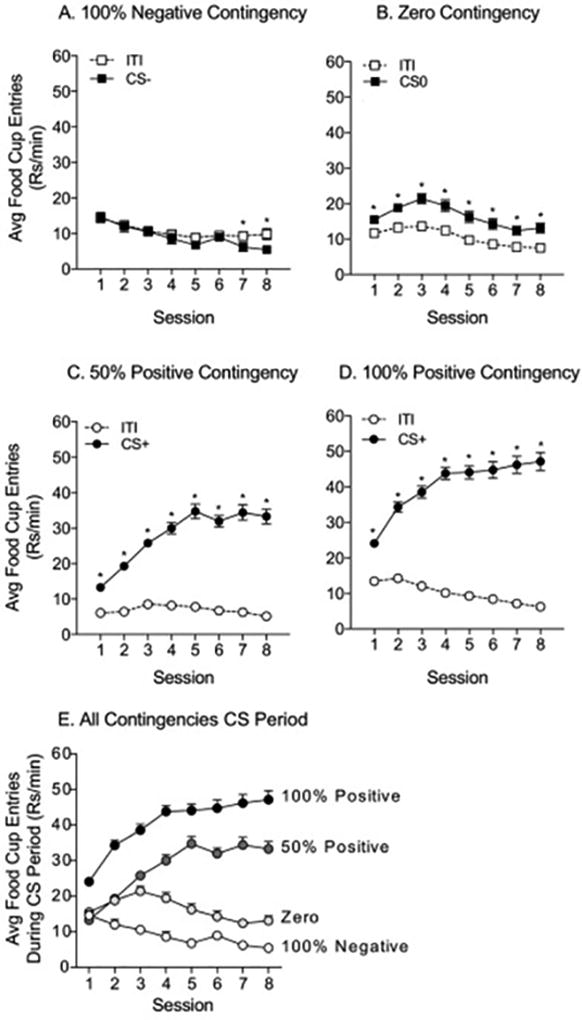
Pavlovian Conditioning, Experiment 1: Average food cup entries during the ITI and CS presentations across conditioning sessions are shown for each contingency group (±SEM). A) 100% Negative Contingency: pellets given during ITI, but never during CS presentations (CS−). By the end of conditioning, entrances into the food cup were greater during the ITI than CS period. B) Zero Contingency: pellets given with equal probability during the ITI and the CS period (CS0). Food cup entries were higher during the CS0 throughout conditioning. C) 50% Positive Contingency: pellets given on 50% of the CS presentations (CS+) and never during the ITI. The rate of entries during the CS increased across sessions. D) 100% Positive Contingency: pellets given with every CS presentation (CS+) and never during the ITI. The rate of entries during the CS increased across sessions, while entries during the ITI decreased. E) Average food cup entries during the CS across conditioning sessions for each contingency group (±SEM). The rate of responding systematically increases as the predictive validity of the CS increases. * = post-hoc comparisons, ITI vs. CS period, p<0.05.
The result above, include responding that occurred in the presence of the US, and thus are not a direct measure of conditioned approach per se. Therefore, we also evaluated the latency to approach the food cup following CS onset during the last 4 sessions of Pavlovian training. As expected, the latency to enter the food cup following CS onset was significantly greater in the 100% Negative Contingency group relative to all other groups, but did not differ between the remaining groups (Supplemental Fig 1: two-way RM ANOVA; main effect of group, F(3,96)=44.46, p<0.01; main effect of session, F(3,288)=4.14, p<0.01 ; significant group by session interaction, F(9,288)=4.06, p<0.01).
4.2. Post-Training Junk-food Consumption
Following training, rats were relieved from food restriction and split into Chow and JF treatment group matched for weight and behavior during conditioning (for behavioral counterbalancing see Supplemental Fig 2A–D: two-way RM ANOVA; no effects of future diet treatment Chow vs. JF groups: 100% Negative Contingency: p=0.44; Zero Contingency: p=0.49; 50% Positive Contingency: p=0.75; 100% Positive Contingency: p=0.24). Food intake for both Chow and JF groups began relatively high, but then dropped over time (Supplemental Fig 2E: two-way RM ANOVA; main effect of day, F(13,394)=53.54, p<0.01). This is likely due to a refeeding effect following chronic food restriction during training. In addition, the JF group consumed significantly more calories compared to Chow group across all days (Supplemental Fig 2E: two-way RM ANOVA; main effect of diet, F(1,38)=45.63, p<0.01; significant day by group interaction, F(13,494)=6.45, p<0.01).
4.3. Effects of CS-US Contingency and Junk-food on Cue Potentiated Feeding and Conditioned Approach
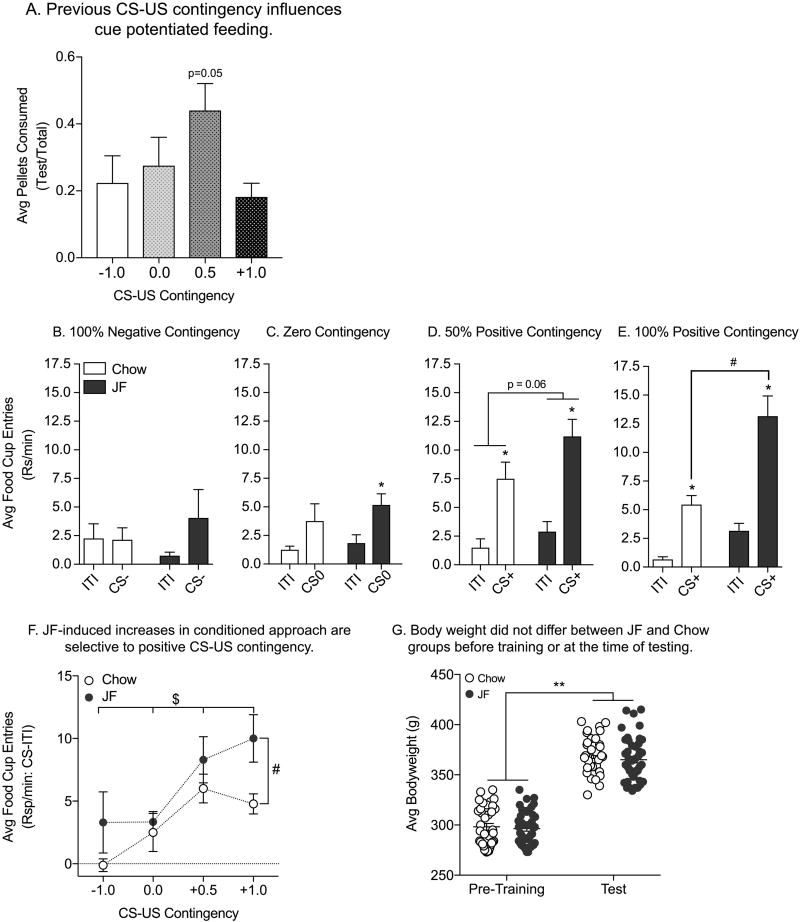
After 14 days on their respective diets, rats were selectively satiated on the US from training (Bioserv pellets) for one hour and then tested for cue potentiated feeding and conditioned approach in the operant chambers. To evaluate the effect of CS presentations on pellet consumption we calculated the proportion of pellets consumed during the CS test relative to the baseline period (CS test /[baseline + CS test]). Cue potentiated feeding differed by training contingency, but did not differ between diet treatment groups (Supplemental Fig 3C: two-way ANOVA; main effect of training, F(3,71)=2.91, p=0.04; no effect of diet, p=.53; no diet by training interaction, p=0.60). Therefore, we collapsed across Chow and JF groups and determined the relationship between initial CS-US contingency and cue potentiated feeding. We found that only the 50% Positive Contingency group exhibited significantly increased pellet consumption in the presence of the CS compared to the 100% Negative Contingency group (Fig 2A: one-way ANOVA; main effect of training, F(3,74)=2.71, p=0.05; Dennett’s, q(74)=2.43, p=0.05). Comparisons here were made back to the 100% Negative Contingency, rather than the Zero Contingency group, because rats trained in the Zero Contingency groups acquired low levels of conditioned approach (see Fig 1B and 2C).
Figure 2.
Test for Cue Potentiated Feeding and Conditioned Approach: A) Average proportion of pellet consumption during CS presentations (±SEM). No effects of JF were found, so data are collapsed across JF and Chow groups (see Supplemental Fig 3 for Chow vs. JF comparisons). Modest cue potentiated feeding was found only in the 50% Positive Contingency group (+0.5) compared to the 100% Negative Contingency group (−1.0). B–E) Average rate of food cup entries (±SEM) during the ITI and CS periods. B) In the Negative Contingency groups, rates of food cup entries were similar during the ITI and CS− period, and there was no effect of JF. C) In the Zero Contingency groups, CS0 presentation resulted in modest increases in food cup entries relative to the ITI in the JF but not Chow group. D) In the 50% Positive Contingency groups, the CS+ elicited conditioned approach in both JF and Chow groups, with significantly greater rates of food cup entries during the CS vs. ITI. In addition, responding was modestly enhanced in the JF vs. Chow group. E) In the 100% Positive Contingency groups, the CS+ elicited conditioned approach in both groups, and this effect was enhanced in the JF vs. Chow group. F) Summary of conditioned approach in chow and JF groups across CS-US contingency. Conditioned approach varied as a function of CS-US contingency in both groups and JF consumption enhanced conditioned approach to the 100% Positive CS+. G) Average body weight (±SEM) for Chow and JF groups. Weight did not differ between groups either prior to initial conditioning, nor at the time of testing. All rats gained significant weight between pre-training and testing. * = planned comparisons, CS vs. ITI, p<0.05; # = main effect of diet, p<0.05; $ = main effect of training; ** = main effect of time, p<0.05.
We next determined whether food cup entries during CS presentation varied as a function of previous training contingency and JF consumption. JF consumption significantly increased conditioned approach relative to Chow groups in rats initially trained with a 50% or 100% Positive CS-US contingency. Specifically, in the 100% Negative Contingency groups, the rate of food cup entries did not differ between the ITI and the CS, nor was there an effect of diet on this behavior (Fig 2B: two-way RM ANOVA; no effect of phase, p=0.23; no effect of diet, p=0.91; no phase by diet interaction, p=0.20). We also performed planned comparisons between the CS and ITI response rates for each diet treatment alone and neither treatment group showed greater responding during the CS vs. ITI (Fig 2B: Sidak’s, ITI vs. CS, Chow: p=0.99; JF: p=0.17). In the Zero Contingency groups overall rates of food cup entries did not differ by diet, however the rate of food cup entries was slightly higher during CS presentations than during the ITI (Fig 2C: two-way RM ANOVA; no effect of diet, p=0.39; main effect of phase, F(1,18)=13.2, p<0.01; no phase by diet interaction, p=0.61). However, additional planned comparisons of responding during the ITI vs. CS in each treatment group revealed that this effect was driven primarily by the behavior of the JF treatment group (Fig 2C: Sidak’s, ITI vs. CS, Chow: p=0.12; JF: t(18)=3.28, p<0.01). Overall, conditioned approach behavior was not dramatically different between JF and Chow treatment groups that previously received 100% Negative or Zero Contingency Pavlovian training.
Behavior differed dramatically in the Positive Contingency groups. Specifically, the 50% Positive Contingency groups showed clear conditioned approach, making significantly more food cup entries during the CS vs. ITI phase of testing (Fig 2D: two-way RM ANOVA; main effect of phase, F(1,18)=43.4, p<0.01). In addition, there was a modest effect of diet, such that responding in the JF treated group was elevated compared to the Chow group (Fig 2D: main effect of diet, F(1,18)=3.99, p=0.06; no phase by diet interaction, p=0.30). Similarly, the 100% Positive Contingency groups also showed strong conditioned approach (Fig 2E: two-way RM ANOVA; main effect of phase, F(1,26)=40.77, p<0.01). Furthermore, this effect was strongly enhanced in JF vs. Chow groups (Fig 2E: two-way RM ANOVA; main effect of diet, F(1,26)=19.64, p<0.01; significant phase by diet interaction, F(1,26)=5.08, p=0.03). Post hoc analysis of the phase by diet interaction revealed that JF selectively increased entries in the presence of the CS, whereas ITI rates did not differ (Fig 2E: Sidak’s, Chow vs. JF, ITI: p=0.25; CS: t(52)=4.72, p<0.01). A summary of the relationship between training and diet treatment on conditioned approach is shown in Fig 2F. This illustrates the progressive increase of conditioned approach behavior as a function of CS-US contingency in both groups. In addition, JF treated rats exhibit stronger rates of responding and this effect is most pronounced with the fully predictive CS (Fig 2F: two-way RM ANOVA; main effect of diet, F(1,72)=6.28, p=0.01; main effect of CS-US contingency, F(3,72)=6.20, p<0.01; no diet by CS-US interaction p=0.50). Importantly, bodyweights did not differ between Chow and JF groups prior to initial training or on the day of testing, although bodyweights did increase across time, consistent with normal growth (Fig 2G: two-way RM ANOVA; no effect of diet treatment group, p=0.48; main effect of time, F(1,78)=2096, p<0.01; no diet by time interaction, p=0.52). Thus, effects of JF on conditioned approach described above occurred in the absence of any significant weight differences between Chow and JF groups. In summary, cue potentiated feeding varied by initial Pavlovian training contingencies, but was not strongly affected by diet. In contrast, there was a positive relationship between the expression of conditioned approach and the strength of initial CS-US relationships which was enhanced by JF consumption only in those rats that had received 50% or 100% Positive Contingency training.
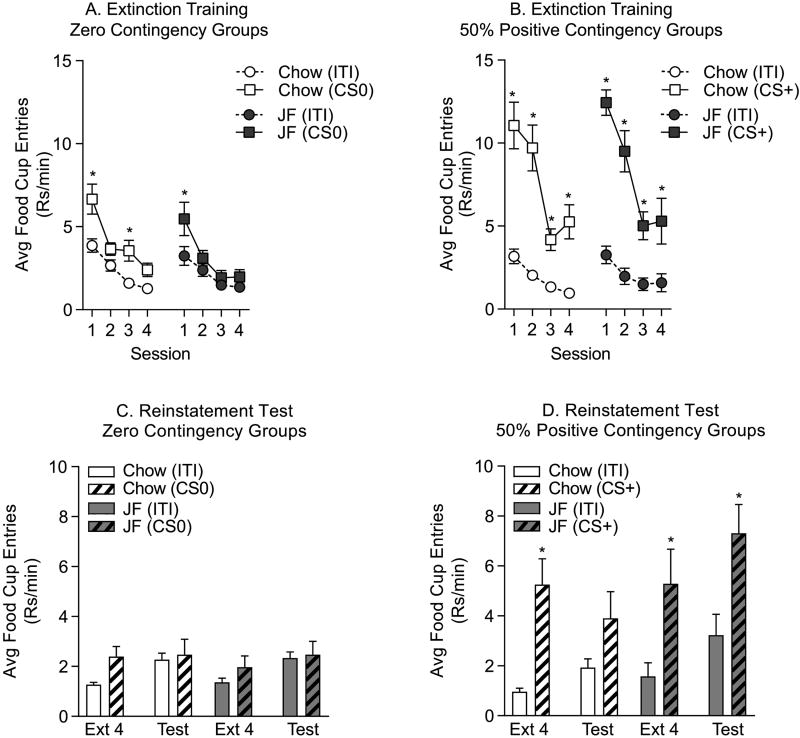
4.4. Effects of Junk-food on Extinction and Reinstatement Testing
After the testing described above, the same rats from the Zero and 50% Positive Contingency groups then underwent 4 sessions of extinction training followed by reinstatement testing in order to further probe the trends toward an effect of JF on conditioned approach outside of the cue potentiated feeding testing procedure. As expected, in the Zero Contingency groups, food cup entries during the CS and during the ITI were relatively low and were reduced even further by extinction training (Fig 3A: two-way RM ANOVA; Chow group, main effect of session, F(3,21)=14.88, p<0.01; main effect of phase, F(1,7)=45.7, p<0.01; no session by phase interaction, p=0.12; JF group, main effect session, F(3,33)=14.05, p<0.01; main effect of phase, F(1,11)=10.53, p<0.01; no session by phase interaction, p=0.10). In addition, behavior between Chow and JF groups was similar across extinction training (Fig 3A: two-way RM ANOVA; ITI: no effect of diet, p=0.55; no diet by session interaction, p=0.67; CS: no effect of diet, p=0.12; no diet by session interaction, p=0.71). In addition, planned comparisons between CS and ITI responding, revealed that by the final session CS responding was not different than ITI for both groups (Sidak’s, ITI vs. CS, Chow group: p=0.21; JF group: p=0.72).
Figure 3.
Extinction Training and Reinstatement Testing. A) Average rate of food cup entries (±SEM) during the ITI and CS0 period during extinction training for Zero Contingency groups. Both Chow (open symbols) and JF (closed symbols) groups decreased responding across the four extinction sessions. In the Chow group, differential responding during the CS0 vs. ITI persisted slightly longer than the JF group, but by the fourth session there were no differences in the rate of responding during the CS and ITI. B) Average rate of food cup entries (±SEM) during the ITI and CS+ period during extinction training for 50% Positive Contingency groups. Chow and JF groups significantly reduced the rate of food cup entries during CS+ presentations across extinction. The degree of extinction achieved by the fourth session was similar between Chow and JF groups. C) Average rate of food cup entries (±SEM) during the final extinction session and during reinstatement testing (i.e., following one CS-US pairing) in the Zero Contingency groups. Conditioned approach was not observed during reinstatement testing in either the Chow or JF groups with prior zero contingency training. D) Average rate of food cup entries (±SEM) during the final extinction session and during reinstatement testing in the 50% Partial Contingency groups. One CS-US pairing did not fully reinstate responding in either Chow or JF groups, although conditioned approach was absent in the Chow group, but maintained in the JF group during reinstatement testing. * = planned comparisons, CS vs. ITI, p<0.05
In the 50% Positive Contingency groups responding to the CS dropped significantly over the four sessions of extinction, and again there were no differences between Chow and JF treatment groups (Fig 3B: two-way RM ANOVA; Chow group, main effect of session, F(3,27)=37.46, p<0.01; main effect of phase, F(1,9)=37.62, p<0.01; significant phase by session interaction, F(3,27)=10.04, p<0.01; JF group, main effect of session, F(3,27)=20.69, p<0.01; main effect of phase, F(1,9)=171.1, p<0.01; significant phase by session interaction, F(3,27)=8.11, p<0.01; Fig 3B: two-way RM ANOVA; ITI, no effect of diet, p=0.70; no diet by session interaction, p=0.36; CS, p=0.68; no diet by session interaction, p=0.77). Importantly, conditioned approach behavior did not fully extinguish in the 50% Positive Contingency groups (Fig 3B: Sidak’s, ITI vs. CS, Chow group: t(27)=5.43, p<0.01; JF group: t(27)=3.75, p<0.01). This was deliberate, as we chose to implement limited extinction training, so as not to completely abolish the CS-US association (see Delamater et al., 2017 for discussion).
Twenty-four hours after the last extinction session, rats were brought back to the operant boxes and given one paired presentation of the CS and US at the start of test session followed by 4 presentations of the CS alone. As expected, in the Zero Contingency group one CS-US pairing was not sufficient to enhance food cup entries during the CS relative to the ITI, nor did behavior differ between diet treatment groups, although ITI responding did increase following extinction training (Fig 3C: two-way RM ANOVA; main effect of phase, F(3,54)=4.42, p=0.01; no effect of diet, p=0.87; no phase by diet interaction, p=0.87). In the 50% Positive Contingency groups, one CS-US pairing was not sufficient to increase conditioned approach behavior above levels reached on the final day of extinction (i.e., to induce reinstatement; Fig 3D: two-way RM ANOVA; main effect of phase, F(3,54)=19.44, p<0.01; no effect of diet, p=0.19; diet by phase interaction, F(3,54)=2.38, p=0.08). However, planned comparisons examining responding during the ITI vs. CS revealed that during testing conditioned approach was absent in the Chow group, but was maintained in the JF group (Fig 3D: Reinstatement Test: Sidak’s CS vs. ITI; Chow Group: p=0.24; JF Group: t(54)=4.28, p<0.01). Thus, although we did not observe a true reinstatement effect in either group, JF experienced rats nonetheless maintained conditioned approach during the reinstatement test, whereas the Chow group did not. This is consistent with conditioned approach behavior during the cue potentiated feeding test and suggests that JF not only enhances the magnitude, but may also enhance the persistence of conditioned approach.
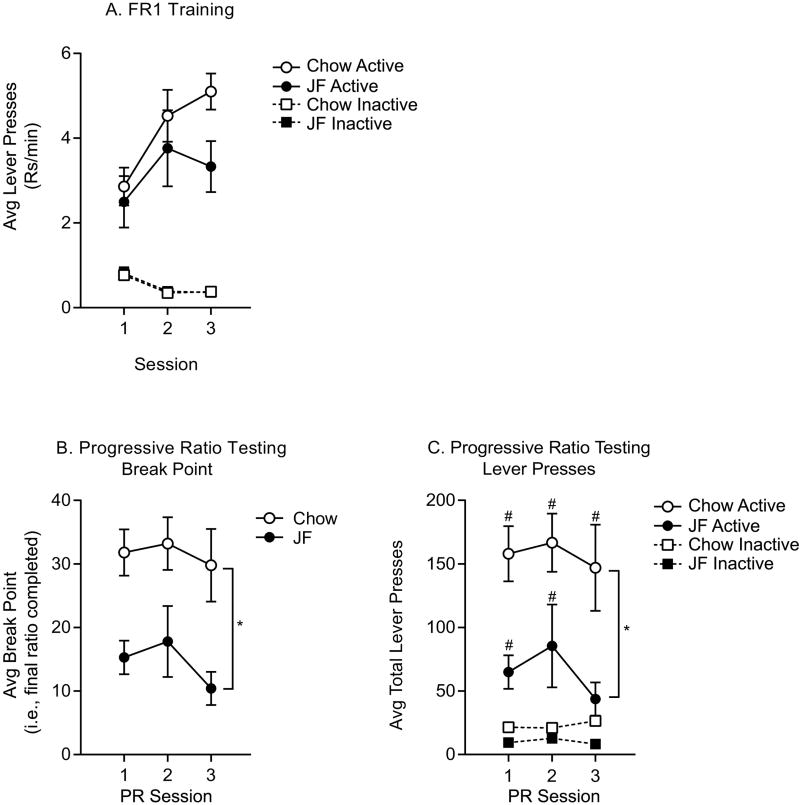
4.5. Effects of Junk-food on Instrumental Responding for the US
Data above show that JF consumption enhances conditioned responding to food cues, but we had not examined the effect of this diet on motivation for the US itself. Therefore, rats from the 50% Positive Contingency groups were used to examine instrumental responding for the US. Across three sessions of FR1 training, Chow and JF groups increased their rates of active lever responding while decreasing their inactive lever responding (Fig 4A: three-way RM ANOVA; main effect of lever, F(1,108)=153.9, p<0.01; no effect of session, p=0.14; session by lever interaction, F(2,108)=6.28, p<0.01). Active lever responding in the Chow group was modestly elevated by the end of training compared to the JF group (Fig 4A: three-way RM ANOVA; main effect of diet, F(1,108)=3.43, p=0.07; main effect of lever, F(1,108)=153.90, p<0.01; lever by diet interaction, F(1,108)=3.73, p=0.06; no session by diet interaction, p=0.48; no session × lever × diet interaction, p=0.54). Next, rats were shifted to a progressive ratio schedule to determine if JF treatment altered the willingness to work for US deliveries. The Chow group achieved significantly higher breakpoints across three sessions compared to the JF group (Fig 4B: two-way RM ANOVA; main effect of diet, F(1,18)=12.17, p<0.01; no effect of session, p=0.21; no diet × session interaction, p=0.79). In concordance with this result, total active lever responses were greater in the Chow group than in the JF group (Fig 4C: three-way RM ANOVA; main effect of diet, F(1,108)=27.17, p<0.01; main effect of lever, F(1,108)=87.26, p<0.01; no effect of session, p=0.48; diet by lever interaction, F(1,108)=15.57, p<0.01; no diet by session interaction, p=0.81; no session by lever interaction, p=0.45; no session × lever × diet interaction, p=0.97). Moreover, planned comparisons between active v inactive lever responding revealed that, repeated progressive ratio testing in the JF group resulted in a loss of preferential active lever responding on the third test, whereas in the Chow group active lever responding remained significantly higher than inactive responding throughout testing (Fig 4C: Sidak’s, active vs. inactive, JF group: p>0.10; Chow group: t(108)=4.87, p<0.01). Thus, although incentive motivational responses to the CS were enhanced by JF consumption, the willingness to work for the US itself was reduced. This result is consistent with well-established behavioral and neural dissociations between preparatory and consummatory aspects of feeding (see also discussion, section 6).
Figure 4.
Instrumental Training and Progressive Ratio Testing. A) Average active and inactive lever presses (±SEM) during FR1 training. Both groups preferentially responded on the active vs. inactive lever, and behavior was similar between Chow and JF groups. B) Average break point (±SEM). The final ratio completed (i.e., break point) was significantly higher in the Chow vs. JF group. C) Average total active and inactive lever presses (±SEM) during progressive ratio testing. In concordance with the breakpoints, active lever pressing was significantly higher in the Chow vs. JF group. Moreover, the Chow group preferentially engaged the active lever across testing, whereas the JF group lost preference for the active lever by the third test. * = main effect of diet, p<0.05; # = planned comparisons, active vs. inactive levers, p<0.05.
5. Results Experiment 2: Effects of prolonged junk-food exposure, with and without junk-food deprivation
The purpose of this experiment was to determine whether prolonging the JF consumption period, with and without a JF deprivation period, would produce enhancements in cue potentiated feeding and/or conditioned approach.
5.1. Pavlovian Conditioning
Rats were trained either in the 100% Negative Contingency (n=18) or the 100% Positive Contingency (n=42) using the same procedure as in Experiment 1 above (average bodyweight: 340g ± 2.53g). As expected, in the 100% Negative Contingency group food cup entries were slightly higher during ITI than during the CS− (Fig 5A: two-way RM ANOVA; 100% Negative Contingency group, main effect of phase, F(1,17)=41.07, p<0.01; main effect of session, F(7,119)=2.24, p=0.04; no phase by session interaction, p=0.13); whereas in the 100% Positive Contingency group, food cup entries were greater during the CS+ than during the ITI, with this difference increasing across conditioning sessions (Fig 5B: two-way RM ANOVA; 100% Positive Contingency group, main effect of phase, F(1,41)=821.9, p<0.01; main effect of session, F(7,287)=19.7, p<0.01; phase by session interaction, F(7,287)=75.51, p<0.01).
Figure 5.
Pavlovian Conditioning, Experiment 2: Average food cup entries during the ITI and CS presentations across conditioning sessions are shown for each contingency group (±SEM). A) 100% Negative Contingency: By the end of training, the rates of food cup entries during the ITI were modestly, though significantly higher than during the CS−. B) 100% Positive Contingency: The rate of food cup entries was greater during CS+ vs. ITI presentations, and this difference increased significantly across sessions. * = post-hoc comparisons, ITI vs. CS, p<0.05.
5.2. Post-Training Junk-food Consumption and Deprivation
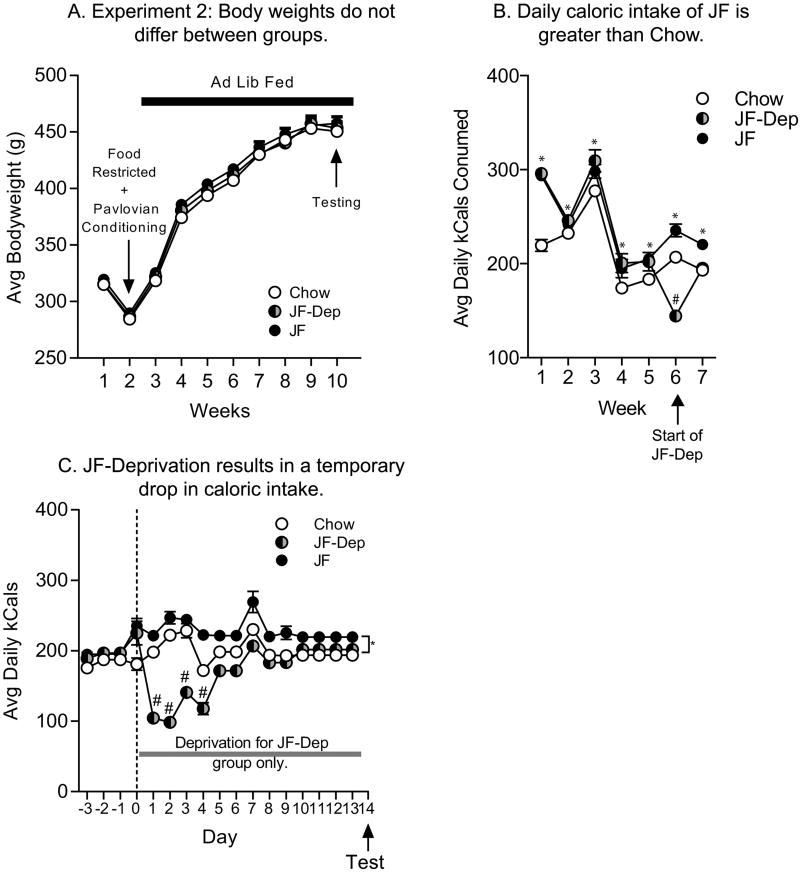
Following initial training, rats were relieved from food restriction and assigned to either Chow (n=15), JF (n=22) or JF-Dep (n=22) diet treatment groups, counterbalanced for behavior during Pavlovian conditioning and weight. (For behavioral counterbalancing of these diet treatment groups see Supplemental Fig 5: two-way RM ANOVA; no effects of future diet treatment 100% Negative Contingency group: p=0.23; 100% Positive Contingency group: p=0.73). Bodyweights in the Chow, JF Dep, and JF groups did not differ at any point throughout study (Fig 6A: two-way RM ANOVA; no effect of diet, p=0.44; no diet by time interaction, p=0.96). Despite the lack of differences in body weight, daily kCal consumption differed between treatment groups. Specifically, during the first 4 weeks of treatment, caloric intake per cage was significantly higher in the JF and JD-Dep groups relative to the Chow group, and consumption was similar between JF and JF-Dep groups (Fig 6B: two-way RM ANOVA; main effect of diet treatment, F(6,162)=192.9, p<0.01; main effect of time, F(2,27)=11.09, p<0.01; diet by time interaction F(12,162)=21.31, p<0.01). When rats in the JF-Dep group were put back on a chow only diet, caloric intake dropped dramatically in the first week and was significantly lower in the JD-Dep group compared to both Chow and JF groups (Fig 6B, Week 6: Sidak’s: JF-Dep vs. JF, t(189)=9.97, p<0.01; JF-Dep vs. Chow, t(189)=2.86, p<0.01). Examination of daily consumption during the JF-deprivation window is shown in Fig 6C. The reduction in caloric intake in the JF-Dep group was immediately apparent after the removal from JF diet and persisted for 4 days (Fig 6C: two-way RM ANOVA; main effect of diet treatment, F(2,27)=27.72, p<0.01; diet by time interaction F(32,432)=24.06, p<0.01). After this time, caloric intake between the Chow and JF-Dep groups did not differ (Fig 6C: Day 5, Sidak’s: JF-Dep vs, p<0.31).
Figure 6.
Body Weight and Caloric Intake Across Time. A) Average weight (± SEM) prior to and after lifting food restriction. Bodyweights did not differ between the groups at any point in the experiment. B) Average daily caloric intake per cage per week (± SEM) across the ad lib feeding period. Food intake started off high and dropped across time in all the groups. This is likely due to a refeeding effect in response to the previous food-restriction period. In addition, caloric intake of JF was greater than that of Chow. JF was available ad lib during weeks 1–5 for both JF and JF-Dep groups. On week 6, JF was removed and replaced by lab chow for rats in the JF-Dep group. Caloric intake of the JF-Dep dropped significantly below both Chow and JF groups in the week following JF deprivation. Chow intake in the JF-Dep then rose to levels comparable to the Chow group the following week. C) Average daily caloric intake per cage per day (± SEM). In the JF-Dep group, as soon as JF was replaced by chow (day 0), daily caloric intake dropped dramatically to below that of both JF and Chow groups. This self-imposed suppression of feeding slowly abated over time and returned to levels similar to the Chow group by day 5 of JF deprivation. * = post-hoc comparisons, JF vs. Chow, p<0.05; # = post-hoc comparisons, JF-Dep vs. Chow, p<0.05.
5.3. Effects of Prolonged Junk-Food, With and Without Junk-Food Deprivation, on Cue Potentiated Feeding and Conditioned Approach
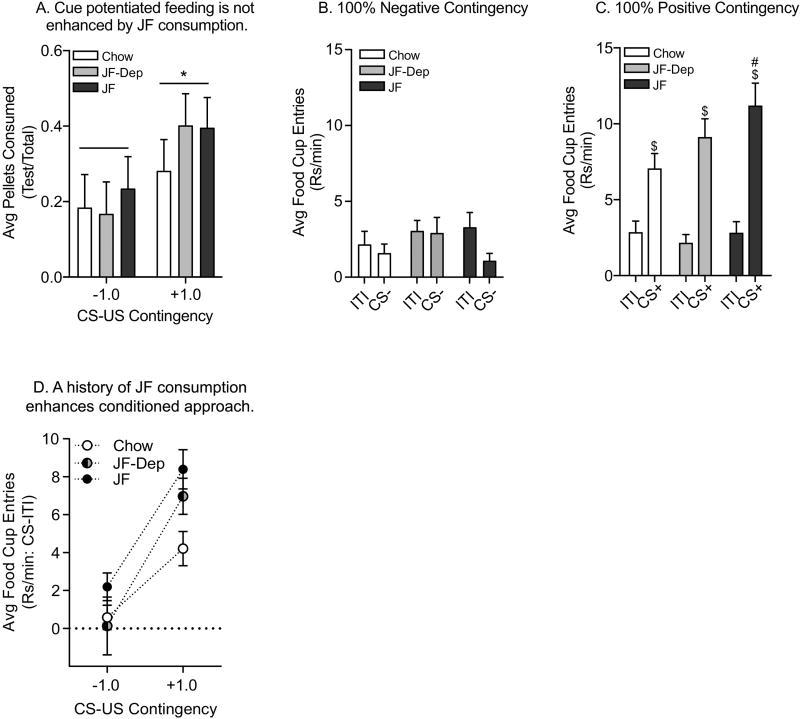
Chow, JF and JF-Dep groups were then tested for cue potentiated feeding and conditioned approach as described above. Briefly, rats were selectively sated on Bioserv pellets for one hour followed by baseline (BL) and CS presentation (CS test) in the operant chambers. Again, we did not find any effect of diet on cue potentiated feeding (Fig 7A: two-way ANOVA; no effect of diet, p=0.72; no diet by training interaction, p=0.78). However, in this cohort tested 45 days after the final Pavlovian conditioning session, presentation of the CS significantly increased food intake in the 100% Positive Contingency groups (Fig 7A: main effect of training, F(1,54)=4.00, p=0.05).
Figure 7.
Test for Cue Potentiated Feeding and Conditioned Approach, Experiment 2. A) Average proportion of pellet consumption during CS presentation (±SEM). In this cohort, rats in the 100% Positive Contingency groups showed cue potentiated feeding compared to 100% Negative Contingency groups. There was no significant effect of diet on this behavior. B–D) Average rate of food cup entries (±SEM) during the ITI and CS presentations. B). There were no effects of CS or diet manipulation on conditioned approach in the 100% Negative Contingency groups. C) All groups with prior 100% Positive Contingency conditioning showed conditioned approach, with significantly greater magnitude of food cup entries during the CS+ vs. ITI. In addition, the rate of CS+ evoked food cup entries was significantly greater in the JF vs. Chow group. In the JF-Deprivation group, this effect of JF was modest, but not completely absent. D) Summary of conditioned approach in Chow, JF-Dep and JF groups across CS-US contingency (CS-ITI). In the 100% Negative contingency trained groups, CS evoked increases in the rate of food-cup entries did not differ across diet treatment groups (−1.0). In the 100% Positive Contingency trained groups (+1.0), JF enhanced conditioned responding, and this effect of JF was reduced, but not completely absent in the JF-Deprivation group. * = main effect of diet, p<0.05; $ = main effect of phase, CS vs. ITI; # = post-hoc comparisons of CS+, Chow vs. JF, p<0.05.
Food cup entries during testing are shown in Fig 7. Consistent with results from Experiment 1, in the Negative Contingency groups, the rate of food cup entries did not differ between the ITI and the CS, nor was there any effects of diet on this behavior (Fig 7B: two-way RM ANOVA; no effect of phase, p=0.12; no effect of diet, p=0.43; no diet by phase interaction, p=0.37). In contrast, in the 100% Positive Contingency groups all groups entered the food cup more during the CS relative to the ITI and this effect interacted with diet (Fig 7C: two-way RM ANOVA; main effect of phase F(1,39)=120.9, p<0.01; no effect of diet, p=0.31; phase by diet interaction, F(2,39)=3.81, p=0.03). Moreover, post-hoc analysis revealed that food cup entries during the CS were significantly greater in the JF group compared to the Chow group, with no differences in ITI responding (Fig 7C: Sidak’s, Chow vs. JF, CS: t(78)=2.60, p=0.02; ITI: p=1.00). Fig 7D depicts the number of food cup entries above the ITI (i.e., CS-ITI) elicited by the CS in all groups. This illustrates that responding is not altered by diet in the Negative contingency groups, but that JF consumption enhanced conditioned approach in the Positive Contingency groups, even after a period of JF deprivation (Two-tailed t-test: Chow vs. JF-Dep : t(24)=1.96, p=0.06).
In sum, in rats tested 45 days after initial training (Exp. 2), a food CS with 100% predictive validity elicited cue potentiated feeding, whereas this potentiation effect was absent when testing was conducted 14 days after initial training (Exp. 1). Furthermore, results from Experiment 2 replicated JF induced enhancements in conditioned approach observed in Experiment 1. Additionally, the inclusion of the JF-Dep group extended these findings by demonstrating that enhancements in incentive motivation persisted after JF consumption has ceased.
6. Discussion
6.1. Overview
In the current study, we determined how limited and prolonged JF consumption alter cue potentiated feeding and conditioned approach in response to a previously established food paired CS. In addition, to establish the degree to which these behaviors and JF effects are influenced by the predictive validity of the CS, we included groups trained with distinct CS-US contingencies ranging from −1.0 to +1.0. We found that both cue potentiated feeding and conditioned approach varied as a function of CS-US contingency, with greater potentiation of feeding and conditioned approach induced by CSs that are more predictive of US delivery. Moreover, we found that both limited (14 day) and prolonged (45 day) JF consumption enhanced conditioned approach, but not cue potentiated feeding. Furthermore, this effect of JF on behavior was dependent on the previously established CS-US contingency. That is, consumption of JF following training enhanced approach to the food cup only in response to CSs with previously positive CS-US contingencies. This selective effect of JF on incentive motivation was further supported by maintenance of conditioned responding in the JF vs. Chow group following extinction and reinstatement testing. Interestingly, these JF induced increases in incentive motivational responses to the CS occurred alongside reduced motivation for the US itself assessed by progressive ratio testing. Together, these data show that JF consumption in the absence of overt obesity selectively enhances incentive motivational responses to previously established food CSs, without dramatically altering consumption induced by these same CSs. In addition, they extend our understanding of how CS-US contingency influences cue potentiated feeding.
6.2. Effects JF consumption and CS-US contingency on cue potentiated feeding
During initial Pavlovian conditioning, we varied the CS-US contingency from −1.0 to +1.0. We then formed Chow and JF groups counterbalanced by weight and initial performance during conditioning. Rats were tested for cue potentiated feeding and conditioned approach after either 14 (Exp. 1) or 45 (Exp. 2) days of free access to chow or JF, or 30 days of JF followed by 14 days of JF-deprivation (Exp. 2). We did not find any effect of JF on cue potentiated feeding, either when JF was given continuously for 14 or 45 days, or following JF-deprivation. The lack of an effect of JF cannot be explained by differences in the amount of food consumed during selective satiation, as JF and Chow groups ate similar amounts during this period (Supplemental Fig 3A). Moreover, pellet consumption during the 5 minute baseline period, which served as the comparison for determining the cue potentiated feeding effect, was similar between all groups (Supplemental Fig 3B). Thus, the data indicate that all rats ate to a level of similar satiety prior to CS presentation.
To our knowledge this is the first study to examine the effect of CS-US contingency (i.e., the predictive validity of the CS) on cue potentiated feeding. We found that cue potentiated feeding varied as a function of CS-US contingency, with greater potentiation of feeding induced by CSs that are more predictive of US delivery. These data are important because they establish that increased consumption in response to CS presentation is not an all or none phenomenon, but is related to the nature of the relationship between the CS and US. In addition, in rats tested 45 days after initial training (Exp. 2), a food CS with 100% predictive validity elicited cue potentiated feeding, whereas this potentiation effect was absent when testing was conducted 14 days after initial training (Exp. 1). Although speculative, this suggests that the expression of cue potentiated feeding may increase as a function of time since initial Pavlovian training. This phenomenon is reminiscent of ‘incubation of craving’ effects reported for both food and drug reinforcers, in which “cue-triggered reward-seeking” intensifies as a function of time since the last instrumental self-administration session (Lu et al., 2004). However, additional studies directly examining the effect of time off on cue potentiated feeding are needed, as comparisons between day 14 and 45 testing are limited here as these were conducted in completely separate experiments.
The absence of a cue potentiation of feeding effect in the 100% Positive Contingency groups found in Experiment 1 was unexpected. However, it is worth noting that although there is a significant amount of variation in the procedures used to study cue potentiated feeding, most have implemented a within-subjects design that inherently incorporates discrimination learning (e.g., Holland et al., 2002; Galarce et al., 2007). Thus, in previous studies the same rats were trained and tested with a CS+ and a CS−, rather than the between-subjects design used here. Therefore, it is possible that conditioning using a within-subject CS+/CS− discrimination, rather than the between subjects approach used here, may have better engaged sensory specific learning about the CS+ which in turn may have enhanced the consummatory drive acquired by the CS+ (for discussion of enhanced discrimination learning see Delamater, 1998; Delamater, 2012). This raises interesting questions about the impact of initial learning on the expression of cue potentiated feeding that should be addressed in future.
In Experiment 1, the 50%, but not 100% predictive CS resulted in modest cue potentiated feeding effect (Fig 2A, Supplemental Fig 3C). This may have been due to the amplification of incentive salience induced by uncertainty of the partially predictive CS. Indeed, uncertainty enhances specific aspects of incentive salience of Pavlovian conditioned stimuli (Boakes, 1977; Anselme et al., 2013). While the process mediating the attribution of incentive motivational properties to CSs may be distinct from the mechanism driving cue potentiated feeding, it is possible that they share a common sensitivity to uncertainty. That is to say, uncertainty may also enhance the degree to which a CS is imbued with consummatory drive in a manner that is similar to its effects on incentive motivation. When considering the role of uncertainty in consummatory behaviors, such as foraging uncertainty has been consistently shown to potentiate feeding behaviors (Forkman, 1993; Anselme et al., 2017). Thus, uncertainty inherent in the 50% Positive Contingency training conditions may have amplified the cue potentiated feeding effect by sensitizing the consummatory drive of the CS.
6.3. Effects of JF consumption and CS-US contingency on conditioned approach
In addition to examining cue potentiated feeding, we also determined how JF and the CS-US contingency affected conditioned approach to the food cup during this same test session (food cup entries during the CS vs. the ITI). In two separate sets of experiments we found that JF consumption enhanced conditioned approach. Specifically, when JF was given continuously for 14 days the magnitude of conditioned approach was significantly increased relative to Chow groups (Fig 1F). In addition, this effect of JF scaled according to previously established CS-US contingencies such that CSs with stronger predicative validity elicited a greater magnitude of conditioned approach in JF groups (Fig 1B–E). Thus, JF did not simply enhance general responding to CSs; rather JF consumption selectively increased incentive motivation for CSs tightly linked to US delivery. That is, the relative predictive value of a given CS interacted with JF consumption to impact the magnitude of subsequent cue-triggered motivational responses. This suggests that JF consumption may result in amplification of the incentive value of CSs that are more consistently paired with palatable food. Of course, whether the enhancing effect of JF is limited to food CSs that predict palatable foods or may extend to all food CSs generally is an outstanding question that will need further investigation.
6.4. Dissociable effects of JF on conditioned approach vs. cue potentiated feeding
One intriguing aspect of the current results is that although JF amplified conditioned approach in all groups trained on a positive contingency (Exp 1 and 2), CS+ presentation did not enhance cue potentiated feeding in JF vs. Chow treated rats. In the testing procedure, the food pellets are present inside the food cup throughout testing. Thus, although rats in the JF groups were entering the food cup more frequently in response to the CS+ than rats in the Chow groups, this failed to translate into enhanced consumption. While direct comparisons between conditioned approach and cue potentiated feeding have not been conducted, lesion studies have demonstrated that cue potentiated feeding requires an intact central nucleus of the amygdala, whereas conditioned approach relies on the function of the basolateral amygdala (Parkinson et al., 2000c; Holland et al., 2002; Holland & Gallagher, 2003). Thus, the dissociable effects of JF on conditioned approach vs. cue potentiated feeding may suggest differential effects of JF on the function of these sub-regions.
Another interesting feature of the data presented here is that rates of instrumental responding and break points for the US itself were reduced in JF vs. Chow groups (Fig 4). Thus, in the same rats the food pellet US itself was less desired while motivational responses to the CS+ were enhanced by JF. It is tempting to speculate that this dissociation of JF effects on appetitive and consummatory behavior may be similar to established dissociations between “wanting” and “liking” following psychostimulant-induced sensitization (Castro et al., 2015; Robinson et al., 2016). However, it is important to note that we did not explicitly measure hedonic responses to food consumption here, and changes in the willingness to work for the US do not necessarily reflect changes in “liking” (Pecina et al., 2003). However, using the same junk-food diet used here, we previously found that that prolonged “junk-food” consumption (30 days) diminished positive orofacial hedonic responses to liquid sucrose regardless of obesity, but enhanced incentive motivation (Robinson et al., 2015). Thus, the reduced break points alongside enhanced conditioned approach following junk-food consumption found here are consistent with this previous result. Though speculative, this is also consistent with results in obese humans, where hedonic and neural responses to food itself are blunted while motivational and neural responses to food cues are enhanced (see Small, 2009 for review).
Following conditioned approach and cue potentiated feeding measures, the Zero Contingency and 50% Positive Contingency groups were tested for reinstatement following extinction training. Rats were given 4 days of extinction training followed by one CS-US pairing. Approach to the food cup during CS presentation and the ITI was then determined in the absence of food (i.e., reinstatement testing). Although neither group showed a true reinstatement effect, rats in the JF group continued to exhibit robust conditioned approach during testing, whereas conditioned approach was absent in the Chow group (Fig 3D). Taken with data above, this provides further support for the idea that JF consumption amplifies the incentive motivational properties of CSs, even when the CS-US association has been undermined by extinction training (Delamater et al., 2017).
6.5. Effects of JF vs. JF-Deprivation
In Experiment 2, we included a group that was given 30 days of continuous access to JF and then underwent 14 days of JF deprivation before testing (i.e., JF replaced with ad lib chow). The purpose of including this group was to determine how long-lasting the impacts of JF consumption were. Conditioned approach to in the 100% Positive Contingency groups was greater in the JF-Dep group compared to the Chow group (Fig 7A). Furthermore, the magnitude of approach was similar between JF-Dep and JF groups. Thus, prolonged JF consumption had long-lasting effects on incentive motivation, even withstanding a 14 day deprivation period. This is an important observation because it shows that once the incentive motivational properties of a CS have been amplified by JF consumption, voluntary reductions in caloric intake (Fig 6C) and elimination of palatable food consumption are insufficient to reverse this process. This finding may have important clinical relevance, as an individual’s diet history may be an important factor to consider when establishing treatment plans for weight loss. Of course, it is also possible that longer JF deprivation may be able to reverse this effect; this will be determined in future studies.
6.6. Relationship between junk-food effects and obesity
In the current study, rats given JF did not differ significantly in weight from Chow groups. This is not surprising given that the diet used is relatively low in fat content (~20%), and that diet exposure was relatively limited (45 days at maximum), compared to regimens usually used to induce obesity or metabolic dysfunction (typically 40–60% fat for 6–8 weeks). In addition, the distribution of weight was similar between Chow and JF groups (Fig 2G and 6A). Although we did not measure adiposity directly, these data suggest that JF induced increases in incentive motivation may not require profound weight gain or metabolic dysregulation. Indeed, this is consistent with the development of obesity, as over-consumption necessarily precedes weight gain. Of course, these effects may be further potentiated in the obese state, and/or in individuals that are susceptible to weight gain (see Ferrario, 2017 for discussion). Consistent with a role of individual susceptibility, we recently found that obesity-prone rats show stronger Pavlovian-to-instrumental transfer (PIT) than obesity-resistant rats prior to obesity (Derman & Ferrario, 2018); that is, they are more sensitive to the incentive motivational impact of a food CS. Thus, it will be worthwhile in future to determine how effects of JF described here may interact with individual difference in susceptibility to obesity.
6.7. Additional considerations and conclusions
The JF diet used here contains several different kinds of fats and sugars, as well as salt. Thus, we do not know which component or combination of components may be more important in the observed behavioral effects. However, given the current food environment and wide range of dietary fats, sugars, etc. consumed by people, we think this diet manipulation should provide results that are generalizable across species. Of course, understanding how, for example, one particular type of fat affects similar behaviors would also be informative in future.
In regard to underlying neural mechanisms, there is a limited understanding of the neural substrates of conditioned approach behavior. However, this behavior relies on activity within mesolimbic systems, as well as on the integrity of the central nucleus of the amygdala (Parkinson et al., 2000a; Parkinson et al., 2000b; Parkinson et al., 2000c; Cardinal et al., 2002). Furthermore, activity within these circuits can be profoundly altered by consumption of sugary, fatty, diets. For example, consumption of the same JF diet used here enhances acute amphetamine-induced locomotor activity compared to chow fed controls, regardless of degree of weight gain (Robinson et al., 2015). Psychostimulant induced locomotion is a well-established, though general, indicator of mesolimbic reactivity (Robinson et al., 1985; Vezina, 2004), and locomotor sensitization induced by repeated psychostimulant exposure enhances incentive motivational response to food CS (Taylor & Horger, 1999; Wyvell & Berridge, 2000; 2001). Thus, increases in amphetamine-induced locomotor activity following JF consumption are consistent with enhanced responsivity of mesolimbic circuits. This is also consistent with effects of both sugar, and high fat on responsivity to direct acting dopamine receptor agonists (e.g., McGuire et al., 2011), increases in dopamine uptake (Fordahl & Jones, 2017), and enhanced striatal excitatory transmission (Tukey et al., 2013; Oginsky et al., 2016; Dingess et al., 2017).
In sum, the data presented above add to our understanding of the psychological and behavioral alterations induced by consumption of sugary, fatty diets, and expand on previous studies examining the processes underlying cue potentiated feeding. The persistent and selective enhancement of incentive motivation in the face of reduced motivation for food itself by JF suggest that interventions to prevent weight gain must include targeting neurobehavioral responses to food CSs, as well as food itself.
Supplementary Material
Supplemental Figure 1: Latency to Approach Food Cup Across Pavlovian Conditioning Experiment 1. Average latency (±SEM) to enter the food cup following CS onset during Pavlovian conditioning. The latency to enter the food cup following CS onset was significantly longer in the 100% Negative Contingency group compared to all other groups.
Supplemental Figure 2: Counterbalancing for Diet Group Assignments Experiment. 1. Average increases (±SEM) in food cup entries during CS presentation (CS-ITI) in groups subsequently assigned to Chow or JF groups. A) 100% Negative Contingency groups. B) Zero Contingency groups. C) 50% Positive Contingency groups. D) 100% Positive Contingency groups. E) Average calories consumed per cage per day across post-training ad lib access to chow or junk-food diet. Food intake started off high and dropped across time. This is likely due to prior food restriction. Overall, caloric intake was significantly greater in the JF vs. Chow group. * = main effect of diet, p<0.05
Supplemental Figure 3: Individual Consumption Prior to and During Testing for Cue Potentiation Experiment 1. Average number of pellets consumed (±SEM) prior to and during testing for cue potentiation of feeding (Exp. 1). A) Average pellet consumption across junk-food and CS-US contingency groups during the 1 hour selective satiation period. No differences were found between any groups. B) Average pellet consumption across junk-food and CS-US contingency groups during the 5 minute baseline (BL) period. No differences were found between any groups. C) Summary of cue potentiated feeding (CS period/ [CS period + BL]) across junk-food and CS-US contingency groups. No statistical differences were found between JF and Chow groups.
Supplemental Figure 4: By Trial Data During Reinstatement Testing. A) Average rate of food cup entries (±SEM) elicited by the CS0 for the Zero Contingency Groups (CS-ITI). CS presentation did not affect the rate of food cup entries across trials. B) Average rate of food cup entries (±SEM) elicited by the CS+ for the 50% Positive Contingency groups (CS-ITI). Conditioned approach was generally greater in the JF vs. Chow group, and this effect was most apparent on the second and third trials.
Supplemental Figure 5: Counterbalancing for Diet Group Assignments Experiment 2. Average increases (±SEM) in food cup entries during CS presentation (CS-ITI) in groups subsequently assigned to Chow, JF or JF-Dep groups (Exp 2). A) 100% Negative Contingency groups. B) 100% Positive Contingency groups.
Highlights.
Post-training junk-food consumption enhances conditioned food cup approach.
This effect is limited to rats that were trained with positive CS-US contingencies.
Junk-food consumption does not alter cue potentiated feeding.
Training with a partially vs. fully predictive CS produces stronger cue potentiated feeding.
Acknowledgments
Financial Disclosure: Dr. Ferrario received funding for this research from the NIDDK, grant number R01-DK106188. Ms. Derman received funding for this research from the NIDA, grant number T32-DA007281 and from the NIDDK, grant number 1F31-DK111194-01.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Both authors of this paper declare having no financial conflicts of interest.
References
- Anselme P, Otto T, Gunturkun O. How unpredictable access to food increases the body fat of small passerines: A mechanistic approach. Behav Processes. 2017;144:33–45. doi: 10.1016/j.beproc.2017.08.013. [DOI] [PubMed] [Google Scholar]
- Anselme P, Robinson MJ, Berridge KC. Reward uncertainty enhances incentive salience attribution as sign-tracking. Behav Brain Res. 2013;238:53–61. doi: 10.1016/j.bbr.2012.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baladi MG, Horton RE, Owens WA, Daws LC, France CP. Eating high fat chow decreases dopamine clearance in adolescent and adult male rats but selectively enhances the locomotor stimulating effects of cocaine in adolescents. Int J Neuropsychopharmacol. 2015;18 doi: 10.1093/ijnp/pyv024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Ho CY, Richard JM, DiFeliceantonio AG. The tempted brain eats: pleasure and desire circuits in obesity and eating disorders. Brain Res. 2010;2:43–64. doi: 10.1016/j.brainres.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch LL, McPhee L, Sullivan S, Johnson S. Conditioned meal initiation in young children. Appetite. 1989;13:105–113. doi: 10.1016/0195-6663(89)90108-6. [DOI] [PubMed] [Google Scholar]
- Blaiss CA, Janak PH. The nucleus accumbens core and shell are critical for the expression, but not the consolidation, of Pavlovian conditioned approach. Behav Brain Res. 2009;200:22–32. doi: 10.1016/j.bbr.2008.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blechert J, Testa G, Georgii C, Klimesch W, Wilhelm FH. The Pavlovian craver: Neural and experiential correlates of single trial naturalistic food conditioning in humans. Physiol Behav. 2016;158:18–25. doi: 10.1016/j.physbeh.2016.02.028. [DOI] [PubMed] [Google Scholar]
- Boakes RA. Performance on learning to associate a stimulus with positive reinforcement. In: Davis H, Hurwitz HMB, editors. Operant–Pavlovian interactions. Lawrence Erlbaum Associates; New Jersey: 1977. pp. 67–97. [Google Scholar]
- Brown RM, Kupchik YM, Spencer S, Garcia-Keller C, Spanswick DC, Lawrence AJ, Simonds SE, Schwartz DJ, Jordan KA, Jhou TC, Kalivas PW. Addiction-like Synaptic Impairments in Diet-Induced Obesity. Biol Psychiatry. 2017;81:797–806. doi: 10.1016/j.biopsych.2015.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce AS, Holsen LM, Chambers RJ, Martin LE, Brooks WM, Zarcone JR, Butler MG, Savage CR. Obese children show hyperactivation to food pictures in brain networks linked to motivation, reward and cognitive control. Int J Obes. 2010;34:1494–1500. doi: 10.1038/ijo.2010.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinal RN, Parkinson JA, Hall J, Everitt BJ. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci Biobehav Rev. 2002;26:321–352. doi: 10.1016/s0149-7634(02)00007-6. [DOI] [PubMed] [Google Scholar]
- Castro DC, Cole SL, Berridge KC. Lateral hypothalamus, nucleus accumbens, and ventral pallidum roles in eating and hunger: interactions between homeostatic and reward circuitry. Front Syst Neurosci. 2015;9 doi: 10.3389/fnsys.2015.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan RS, Woo J. Prevention of overweight and obesity: how effective is the current public health approach. Int J Environ Res Public Health. 2010;7:765–783. doi: 10.3390/ijerph7030765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole S, Mayer HS, Petrovich GD. Orexin/Hypocretin-1 Receptor Antagonism Selectively Reduces Cue-Induced Feeding in Sated Rats and Recruits Medial Prefrontal Cortex and Thalamus. Sci Rep. 2015;5 doi: 10.1038/srep16143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Counotte DS, Schiefer C, Shaham Y, O'Donnell P. Time-dependent decreases in nucleus accumbens AMPA/NMDA ratio and incubation of sucrose craving in adolescent and adult rats. Psychopharmacology. 2014;231:1675–1684. doi: 10.1007/s00213-013-3294-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagher A. The neurobiology of appetite: hunger as addiction. Int J Obes (Lond) 2009 Jun;33(Suppl 2):S30–3. doi: 10.1038/ijo.2009.69. 2009. [DOI] [PubMed] [Google Scholar]
- Delamater AR. Associative mediational processes in the acquired equivalence and distinctiveness of cues. Journal of Experimental Psychology: Animal Behavior Processes. 1998;24:467–482. [PubMed] [Google Scholar]
- Delamater AR. On the nature of CS and US representations in Pavlovian learning. Learn Behav. 2012;40:1–23. doi: 10.3758/s13420-011-0036-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delamater AR, Schneider K, Derman RC. Extinction of specific stimulus-outcome (S-O) associations in Pavlovian learning with an extended CS procedure. Journal of experimental psychology. Animal learning and cognition. 2017;43:243–261. doi: 10.1037/xan0000138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demos KE, Heatherton TF, Kelley WM. Individual differences in nucleus accumbens activity to food and sexual images predict weight gain and sexual behavior. J Neurosci. 2012;32:5549–5552. doi: 10.1523/JNEUROSCI.5958-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derman RC, Ferrario CR. Enhanced incentive motivation in obesity-prone rats is mediated by NAc core CP-AMPARs. Neuropharmacology. 2018;131:326–336. doi: 10.1016/j.neuropharm.2017.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingess PM, Darling RA, Derman RC, Wulff SS, Hunter ML, Ferrario CR, Brown TE. Structural and Functional Plasticity within the Nucleus Accumbens and Prefrontal Cortex Associated with Time-Dependent Increases in Food Cue-Seeking Behavior. Neuropsychopharmacology. 2017;12:57. doi: 10.1038/npp.2017.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrario CR, Labouebe G, Liu S, Nieh EH, Routh VH, Xu S, O'Connor EC. Homeostasis Meets Motivation in the Battle to Control Food Intake. J Neurosci. 2016;36:11469–11481. doi: 10.1523/JNEUROSCI.2338-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fordahl SC, Jones SR. High-Fat-Diet-Induced Deficits in Dopamine Terminal Function Are Reversed by Restoring Insulin Signaling. ACS Chem Neurosci. 2017;8:290–299. doi: 10.1021/acschemneuro.6b00308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forkman BA. The Effect of Uncertainty on the Food Intake of the Mongolian Gerbil. Behaviour. 1993;124:197–206. [Google Scholar]
- Galarce EM, Crombag HS, Holland PC. Reinforcer-specificity of appetitive and consummatory behavior of rats after Pavlovian conditioning with food reinforcers. Physiol Behav. 2007;91:95–105. doi: 10.1016/j.physbeh.2007.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland PC, Gallagher M. Double dissociation of the effects of lesions of basolateral and central amygdala on conditioned stimulus-potentiated feeding and Pavlovian-instrumental transfer. Eur J Neurosci. 2003;17:1680–1694. doi: 10.1046/j.1460-9568.2003.02585.x. [DOI] [PubMed] [Google Scholar]
- Holland PC, Petrovich GD, Gallagher M. The effects of amygdala lesions on conditioned stimulus-potentiated eating in rats. Physiology & Behavior. 2002;76:117–129. doi: 10.1016/s0031-9384(02)00688-1. [DOI] [PubMed] [Google Scholar]
- Keefer SE, Petrovich GD. Distinct recruitment of basolateral amygdala-medial prefrontal cortex pathways across Pavlovian appetitive conditioning. Neurobiol Learn Mem. 2017;141:27–32. doi: 10.1016/j.nlm.2017.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Globa AK, Mills F, Naef L, Qiao M, Bamji SX, Borgland SL. Consumption of palatable food primes food approach behavior by rapidly increasing synaptic density in the VTA. Proc Natl Acad Sci U S A. 2016;113:2520–2525. doi: 10.1073/pnas.1515724113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Grimm JW, Hope BT, Shaham Y. Incubation of cocaine craving after withdrawal: a review of preclinical data. Neuropharmacology. 2004;1:214–226. doi: 10.1016/j.neuropharm.2004.06.027. [DOI] [PubMed] [Google Scholar]
- McDannald MA, Setlow B, Holland PC. Effects of ventral striatal lesions on first- and second-order appetitive conditioning. The European journal of neuroscience. 2013;38:2589–2599. doi: 10.1111/ejn.12255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire BA, Baladi MG, France CP. Eating high-fat chow enhances sensitization to the effects of methamphetamine on locomotion in rats. Eur J Pharmacol. 2011;658:156–159. doi: 10.1016/j.ejphar.2011.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdaugh DL, Cox JE, Cook EW, 3rd, Weller RE. fMRI reactivity to high-calorie food pictures predicts short- and long-term outcome in a weight-loss program. Neuroimage. 2012;59:2709–2721. doi: 10.1016/j.neuroimage.2011.10.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nederkoorn C, Smulders FT, Jansen A. Cephalic phase responses, craving and food intake in normal subjects. Appetite. 2000;35:45–55. doi: 10.1006/appe.2000.0328. [DOI] [PubMed] [Google Scholar]
- Oginsky MF, Goforth PB, Nobile CW, Lopez-Santiago LF, Ferrario CR. Eating 'Junk-Food' Produces Rapid and Long-Lasting Increases in NAc CP-AMPA Receptors: Implications for Enhanced Cue-Induced Motivation and Food Addiction. Neuropsychopharmacology. 2016;41:2977–2986. doi: 10.1038/npp.2016.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandit R, Mercer JG, Overduin J, la Fleur SE, Adan RA. Dietary factors affect food reward and motivation to eat. Obes Facts. 2012;5:221–242. doi: 10.1159/000338073. [DOI] [PubMed] [Google Scholar]
- Parkinson JA, Cardinal RN, Everitt BJ. Progress in Brain Research. Elsevier; 2000a. Limbic cortical-ventral striatal systems underlying appetitive conditioning; pp. 263–285. [DOI] [PubMed] [Google Scholar]
- Parkinson JA, Cardinal RN, Everitt BJ. Limbic cortical-ventral striatal systems underlying appetitive conditioning. Prog Brain Res. 2000b;126:263–285. doi: 10.1016/S0079-6123(00)26019-6. [DOI] [PubMed] [Google Scholar]
- Parkinson JA, Robbins TW, Everitt BJ. Dissociable roles of the central and basolateral amygdala in appetitive emotional learning. Eur J Neurosci. 2000c;12:405–413. doi: 10.1046/j.1460-9568.2000.00960.x. [DOI] [PubMed] [Google Scholar]
- Pavlov PI. Conditioned reflexes: An investigation of the physiological activity of the cerebral cortex. Oxford, England: Oxford Univ. Press; 1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng XX, Lister A, Rabinowitsch A, Kolaric R, Cabeza de Vaca S, Ziff EB, Carr KD. Episodic sucrose intake during food restriction increases synaptic abundance of AMPA receptors in nucleus accumbens and augments intake of sucrose following restoration of ad libitum feeding. Neuroscience. 2015;295:58–71. doi: 10.1016/j.neuroscience.2015.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovich GD, Ross CA, Holland PC, Gallagher M. Medial prefrontal cortex is necessary for an appetitive contextual conditioned stimulus to promote eating in sated rats. J Neurosci. 2007;27:6436–6441. doi: 10.1523/JNEUROSCI.5001-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reppucci CJ, Petrovich GD. Learned food-cue stimulates persistent feeding in sated rats. Appetite. 2012;59:437–447. doi: 10.1016/j.appet.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rescorla RA. Probability of shock in the presence and absence of cs in fear conditioning. Journal of Comparative and Physiological Psychology. 1968;66:1–5. doi: 10.1037/h0025984. [DOI] [PubMed] [Google Scholar]
- Richardson NR, Roberts DC. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods. 1995;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- Robinson MJ, Burghardt PR, Patterson CM, Nobile CW, Akil H, Watson SJ, Berridge KC, Ferrario CR. Individual Differences in Cue-Induced Motivation and Striatal Systems in Rats Susceptible to Diet-Induced Obesity. Neuropsychopharmacology. 2015;40:2113–2123. doi: 10.1038/npp.2015.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MJ, Fischer AM, Ahuja A, Lesser EN, Maniates H. Roles of "Wanting" and "Liking" in Motivating Behavior: Gambling, Food, and Drug Addictions. Curr Top Behav Neurosci. 2016;27:105–136. doi: 10.1007/7854_2015_387. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Becker JB, Moore CJ, Castaneda E, Mittleman G. Enduring enhancement in frontal cortex dopamine utilization in an animal model of amphetamine psychosis. Brain Res. 1985;343:374–377. doi: 10.1016/0006-8993(85)90760-7. [DOI] [PubMed] [Google Scholar]
- Rogers PJ, Hill AJ. Breakdown of dietary restraint following mere exposure to food stimuli: interrelationships between restraint, hunger, salivation, and food intake. Addict Behav. 1989;14:387–397. doi: 10.1016/0306-4603(89)90026-9. [DOI] [PubMed] [Google Scholar]
- Rothemund Y, Preuschhof C, Bohner G, Bauknecht HC, Klingebiel R, Flor H, Klapp BF. Differential activation of the dorsal striatum by high-calorie visual food stimuli in obese individuals. Neuroimage. 2007;37:410–421. doi: 10.1016/j.neuroimage.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Small DM. Individual differences in the neurophysiology of reward and the obesity epidemic. International journal of obesity (2005) 2009;33(Suppl 2):S44–48. doi: 10.1038/ijo.2009.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeckel LE, Weller RE, Cook EW, 3rd, Twieg DB, Knowlton RC, Cox JE. Widespread reward-system activation in obese women in response to pictures of high-calorie foods. Neuroimage. 2008;41:636–647. doi: 10.1016/j.neuroimage.2008.02.031. [DOI] [PubMed] [Google Scholar]
- Taylor JR, Horger BA. Enhanced responding for conditioned reward produced by intra-accumbens amphetamine is potentiated after cocaine sensitization. Psychopharmacology. 1999;142:31–40. doi: 10.1007/s002130050859. [DOI] [PubMed] [Google Scholar]
- Tukey DS, Ferreira JM, Antoine SO, D'Amour JA, Ninan I, Cabeza de Vaca S, Incontro S, Wincott C, Horwitz JK, Hartner DT, Guarini CB, Khatri L, Goffer Y, Xu D, Titcombe RF, Khatri M, Marzan DS, Mahajan SS, Wang J, Froemke RC, Carr KD, Aoki C, Ziff EB. Sucrose ingestion induces rapid AMPA receptor trafficking. J Neurosci. 2013;33:6123–6132. doi: 10.1523/JNEUROSCI.4806-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udo T, Weinberger AH, Grilo CM, Brownell KD, DiLeone RJ, Lampert R, Matlin SL, Yanagisawa K, McKee SA. Heightened vagal activity during high-calorie food presentation in obese compared with non-obese individuals--results of a pilot study. Obes Res Clin Pract. 2014;8:006. doi: 10.1016/j.orcp.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezina P. Sensitization of midbrain dopamine neuron reactivity and the self-administration of psychomotor stimulant drugs. Neurosci Biobehav Rev. 2004;27:827–839. doi: 10.1016/j.neubiorev.2003.11.001. [DOI] [PubMed] [Google Scholar]
- W.H.O. Organization, W.H., editor. Obesity and overweight factsheet. 2017
- Weingarten HP. Conditioned cues elicit feeding in sated rats: a role for learning in meal initiation. Science. 1983;220:431–433. doi: 10.1126/science.6836286. [DOI] [PubMed] [Google Scholar]
- Wolz I, Sauvaget A, Granero R, Mestre-Bach G, Bano M, Martin-Romera V, Veciana de Las Heras M, Jimenez-Murcia S, Jansen A, Roefs A, Fernandez-Aranda F. Subjective craving and event-related brain response to olfactory and visual chocolate cues in binge-eating and healthy individuals. Sci Rep. 2017;7 doi: 10.1038/srep41736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyvell CL, Berridge KC. Intra-accumbens amphetamine increases the conditioned incentive salience of sucrose reward: enhancement of reward "wanting" without enhanced "liking" or response reinforcement. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2000;20:8122–8130. doi: 10.1523/JNEUROSCI.20-21-08122.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyvell CL, Berridge KC. Incentive sensitization by previous amphetamine exposure: increased cue-triggered "wanting" for sucrose reward. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2001;21:7831–7840. doi: 10.1523/JNEUROSCI.21-19-07831.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokum S, Gearhardt AN, Harris JL, Brownell KD, Stice E. Individual differences in striatum activity to food commercials predict weight gain in adolescents. Obesity. 2014;22:2544–2551. doi: 10.1002/oby.20882. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Latency to Approach Food Cup Across Pavlovian Conditioning Experiment 1. Average latency (±SEM) to enter the food cup following CS onset during Pavlovian conditioning. The latency to enter the food cup following CS onset was significantly longer in the 100% Negative Contingency group compared to all other groups.
Supplemental Figure 2: Counterbalancing for Diet Group Assignments Experiment. 1. Average increases (±SEM) in food cup entries during CS presentation (CS-ITI) in groups subsequently assigned to Chow or JF groups. A) 100% Negative Contingency groups. B) Zero Contingency groups. C) 50% Positive Contingency groups. D) 100% Positive Contingency groups. E) Average calories consumed per cage per day across post-training ad lib access to chow or junk-food diet. Food intake started off high and dropped across time. This is likely due to prior food restriction. Overall, caloric intake was significantly greater in the JF vs. Chow group. * = main effect of diet, p<0.05
Supplemental Figure 3: Individual Consumption Prior to and During Testing for Cue Potentiation Experiment 1. Average number of pellets consumed (±SEM) prior to and during testing for cue potentiation of feeding (Exp. 1). A) Average pellet consumption across junk-food and CS-US contingency groups during the 1 hour selective satiation period. No differences were found between any groups. B) Average pellet consumption across junk-food and CS-US contingency groups during the 5 minute baseline (BL) period. No differences were found between any groups. C) Summary of cue potentiated feeding (CS period/ [CS period + BL]) across junk-food and CS-US contingency groups. No statistical differences were found between JF and Chow groups.
Supplemental Figure 4: By Trial Data During Reinstatement Testing. A) Average rate of food cup entries (±SEM) elicited by the CS0 for the Zero Contingency Groups (CS-ITI). CS presentation did not affect the rate of food cup entries across trials. B) Average rate of food cup entries (±SEM) elicited by the CS+ for the 50% Positive Contingency groups (CS-ITI). Conditioned approach was generally greater in the JF vs. Chow group, and this effect was most apparent on the second and third trials.
Supplemental Figure 5: Counterbalancing for Diet Group Assignments Experiment 2. Average increases (±SEM) in food cup entries during CS presentation (CS-ITI) in groups subsequently assigned to Chow, JF or JF-Dep groups (Exp 2). A) 100% Negative Contingency groups. B) 100% Positive Contingency groups.