Abstract
Post-translational control is a crucial mechanism for circadian timekeeping. Evolutionarily conserved kinases and phosphatases have been implicated in circadian phosphorylation and the degradation of clock-relevant proteins, which sustain high-amplitude rhythms with 24-hr periodicity in animal behaviors and physiology. Here, we report a novel clock function of the heterodimeric Ca2+/calmodulin-dependent phosphatase calcineurin and its regulator sarah (sra) in Drosophila. Genomic deletion of the sra locus dampened circadian locomotor activity rhythms in free-running constant dark after entrainment in light–dark cycles. Poor rhythms in sra mutant behaviors were accompanied by lower expression of two oscillating clock proteins, PERIOD (PER) and TIMELESS (TIM), at the post-transcriptional level. RNA interference-mediated sra depletion in circadian pacemaker neurons was sufficient to phenocopy loss-of-function mutation in sra. On the other hand, a constitutively active form of the catalytic calcineurin subunit, Pp2B-14DACT, shortened circadian periodicity in locomotor behaviors and phase-advanced PER and TIM rhythms when overexpressed in clock neurons. Heterozygous sra deletion induced behavioral arrhythmicity in Pp2B-14DACT flies, whereas sra overexpression rescued short periods in these animals. Finally, pharmacological inhibition of calcineurin in either wild-type flies or clock-less S2 cells decreased the levels of PER and TIM, likely by facilitating their proteasomal degradation. Taken together, these data suggest that sra negatively regulates calcineurin by cell-autonomously titrating calcineurin-dependent stabilization of PER and TIM proteins, thereby sustaining high-amplitude behavioral rhythms in Drosophila.
Keywords: Drosophila, circadian rhythms, calcineurin, sarah, post-translational regulation
CIRCADIAN rhythms have evolved as endogenous clock mechanisms to adaptively align behavioral and physiological processes to daily environmental changes. Circadian clocks involve three genetic components: (1) central clocks that cell-autonomously generate 24-hr rhythmicity in molecular and/or neural forms, such as daily oscillations in relative expression of a dedicated set of clock-relevant genes; (2) an input pathway that receives timing cues from the environment and entrains the endogenous clocks; and (3) an output pathway that transmits timing information in the brain to peripheral tissues to synchronize their clock phase and execute circadian physiology (Allada and Chung 2010; Bass and Takahashi 2010; Dubowy and Sehgal 2017).
The core molecular clock consists of daily rhythms in the transcriptional activation of gene promoters containing E-box sequences (CACGTG) (Hardin 2011; Zheng and Sehgal 2012). In Drosophila, a heterodimeric CLOCK/CYCLE (CLK/CYC) activator binds the E-box and induces the transcriptional expression of its target genes, such as period (per) and timeless (tim), in the early evening. per and tim transcription reaches its highest levels at midnight. Meanwhile, the cytoplasmic PER–TIM protein complex accumulates and translocates into the nucleus late at night, suppressing its own transcription by inhibiting CLK/CYC-dependent transcriptional activation. Two additional feedback loops involving the circadian transcription factors vrille, PAR-domain protein 1, and clockwork orange further support high-amplitude circadian transcription with precise 24-hr periodicity (Cyran et al. 2003; Kadener et al. 2007; Lim et al. 2007a; Matsumoto et al. 2007; Richier et al. 2008). This molecular framework of transcriptional–translational feedback loops is highly conserved among Drosophila and mammalian clocks (Hardin and Panda 2013; Lim and Allada 2013; Takahashi 2017).
On top of transcriptional control, post-translational modifications of clock proteins play crucial roles in circadian timekeeping (Tataroglu and Emery 2015; Hirano et al. 2016). In the early morning, degradation of PER and TIM derepresses CLK/CYC-dependent transcription in Drosophila, initiating another cycle of daily rhythmic expression. Several kinases and phosphatases modulate the post-translational stability, nuclear entry, and transcriptional activity of PER proteins. The DOUBLETIME (DBT)/casein kinase I ε (CKIε) and NEMO kinases phosphorylate PER and control its degradation (Kloss et al. 1998; Price et al. 1998; Ko et al. 2002; Chiu et al. 2011). DBT-dependent phosphorylation of PER also potentiates its transcriptional repression (Nawathean and Rosbash 2004; Kivimäe et al. 2008), whereas PER phosphorylation by kinases such as casein kinase II and GSK3β/SHAGGY regulates its nuclear entry (Martinek et al. 2001; Lin et al. 2002; Akten et al. 2003; Ko et al. 2010). On the other hand, protein phosphatase 1 and protein phosphatase 2A stabilize PER via TIM-dependent and TIM-independent dephosphorylation of PER, respectively (Sathyanarayanan et al. 2004; Fang et al. 2007).
In a previous study (Lim et al. 2011), we performed a reverse genetic screen of ∼4000 transgenic fly lines to discover new clock genes that regulate circadian locomotor behavior. We identified sarah (sra), a Drosophila homolog of Down syndrome critical region gene 1 (DSCR1)/regulator of calcineurin 1 (RCAN1), as a candidate gene implicated in clock function. sra was previously identified as a regulator of the Ca2+/Calmodulin-dependent serine/threonine phosphatase calcineurin (see below). Heterodimeric calcineurin consists of catalytic and regulatory subunits, which are evolutionarily conserved (Rusnak and Mertz 2000). In Drosophila, sra- and calcineurin-dependent regulation is involved in mitochondrial function (Chang and Min 2005); axonal transport (Shaw and Chang 2013); female reproductive activities such as ovulation, egg activation, and meiosis (Ejima et al. 2004; Horner et al. 2006; Takeo et al. 2010); olfactory associative learning and long-term memory (Chang et al. 2003); and courtship behaviors (Ejima et al. 2004; Sakai and Aigaki 2010). Moreover, both neuroprotective and neurotoxic effects of sra have been reported in Drosophila models of Alzheimer’s disease (Shaw and Chang 2013; Lee et al. 2016).
To date, genetic studies on Drosophila sleep have revealed the strongest association between the calcineurin–sra pathway and circadian rhythms (Nakai et al. 2011; Tomita et al. 2011). Genomic deletion of sra or a specific subunit of calcineurin (e.g., CanA-14F or CanB) causes hyperactivity and sleep loss. In addition, pan-neuronal expression of either constitutively active calcineurin subunits or transgenic calcineurin RNA interference (RNAi) modulates sleep. However, the mechanism underlying sleep regulation and its possible link to circadian clock function remains elusive.
In this study, we demonstrate that Drosophila clocks are sensitive to calcineurin activity in circadian pacemaker neurons, and that the balance of activity between calcineurin and sra drives high-amplitude behavioral rhythms. Furthermore, we provide molecular evidence that calcineurin-mediated suppression of the proteasomal degradation of PER and TIM proteins is involved in clock regulation.
Materials and Methods
Fly stocks
Flies were raised on cornmeal–yeast–agar medium at 25°C and 60% humidity in a 12 hr light/12 hr dark (LD) cycle. CanA1KO, CanA-14FKO, Pp2B-14DKO, sraKO (Takeo et al. 2006), UAS-sra (Ejima et al. 2004), and UAS-Pp2B-14DACT flies were generously provided by Toshiro Aigaki (Tokyo Metropolitan University, Japan). UAS-sraFLAG, UAS-sraWT, UAS-Pp2B-14DACT, UAS-Pp2B-14DWT, and UAS-Pp2B-14DH217Q flies were obtained from the Kyoto Stock Center (Drosophila Genomics and Genetics Resources). UAS-sraRNAi [transformant identifier (ID): 107373] and DH44-Gal4 (transformant ID:207474) were obtained from the Vienna Drosophila RNAi Center. c305-Gal4, MB247-Gal4, 30y-Gal4, c309-Gal4, 201y-Gal4, OK107-Gal4, Dilp2-Gal4, UAS-mCD8:GFP, and Df(3R)SBD45 flies were obtained from the Bloomington Drosophila Stock Center (BDSC). pdf-Gal4, tim-Gal4, cry-Gal4-16, mz520-Gal4, c929-Gal4, mai179-Gal4, and pdf-Gal4GeneSwitch lines were described previously (Kaneko and Hall 2000; Zhao et al. 2003; Grima et al. 2004; Depetris-Chauvin et al. 2011). To exclude possible genetic background issues in circadian behaviors, all mutants were isogenized by outcrossing five times to w1118 control flies (BDSC #5905). Stable mutant stocks were then reestablished by crossing to isogenic balancer lines.
Behavior analysis
Male flies (5–7 days old) were loaded individually into glass tubes (length, 6.5 cm; inner diameter, 3 mm) containing 2 cm of behavior food (5% sucrose and 2% agar) at one end, and a cotton stopper was placed on the other end. Locomotor activity was measured using a Drosophila activity monitor (Trikinetics), which counts the infrared beam crossings of individual flies in each tube every 30 min. Flies were entrained for 3 days in the LD cycle (under 400 lx of light) at 25°C, and then transferred to constant dark (DD) for 7 days at 25°C. To induce transgenic expression by pdf-Gal4GeneSwitch, two groups of transgenic flies were directly loaded onto behavior food containing either 500 μM RU486 or 1% ethanol (vehicle control) at the beginning of the first LD cycle in our behavioral runs, and their free-running behaviors were monitored on each type of food. For oral administration of a calcineurin inhibitor, wild-type flies were loaded onto behavior food containing either 1 μM cyclosporin A (CsA; Sigma [Sigma Chemical], St. Louis, MO) or 1 mM DMSO (vehicle control), and their circadian behaviors were measured as described above. Behavioral data were continuously recorded throughout behavioral runs, and then analyzed using the ClockLab software in MATLAB (Actimetrics). Circadian periods and the power of rhythmicity (power – significance) were calculated in individual flies using the χ2 periodogram, with the significance level set to an α value of 0.05). Flies with a power of rhythmicity value < 10 were defined as arrhythmic. Locomotor activity profiles were analyzed using Microsoft Excel. Morning anticipation index was calculated in individual flies by the ratio of total activity count for the last 3 hr prior to lights-on (i.e., from ZT21 to ZT0) to total activity count for the last 6 hr prior to lights-on (i.e., from ZT18 to ZT0) in the LD cycle (Seluzicki et al. 2014). Morning anticipation index in the second DD cycle was similarly calculated based on the subjective lights-on time (i.e., CT0). Behavioral data were computed as averages from three or more independent behavioral tests. Dead flies were manually scored at the end of behavioral testing, and their raw data were excluded from further analyses.
Western blotting
Male flies were entrained in LD cycles, and then transferred to DD. Groups of flies were harvested at the indicated time points during the LD cycle or the first DD cycle, and then stored at −70° prior to protein analyses. Thirty fly heads were homogenized in 120 μl of lysis buffer containing 25 mM Tris-Cl (pH 7.5), 300 mM NaCl, 10% glycerol, 1 mM EDTA, 1 µM dithiothreitol (DTT), 0.5% Nonidet P-40, 1 mM phenylmethylsulfonyl fluoride (PMSF), and Halt Protease and Phosphatase Inhibitor Single-Use Cocktail (ThermoFisher Scientific) on ice. Homogenization was performed for 1 min for each fly head sample using a motorized pellet pestle (Kimble Kontes). After centrifugation, the supernatant was mixed with 1/6 vol of 6× SDS sample buffer and then incubated at 95°C for 10 min. Protein samples were resolved by SDS-PAGE analysis, and then transferred to nitrocellulose membranes (Amersham, Piscataway, NJ). After blocking with 3% nonfat dry milk in Tris-buffered saline (TBS)-Tween 20 for 1 hr at room temperature, protein blots were incubated overnight with primary rabbit anti-PER (serum or purified, 1:3000), guinea pig anti-TIM (1:3000), mouse anti-FLAG (1:5000; Sigma), or rabbit anti-ACTIN antibody (1:5000; Sigma). Secondary antibodies used were HRP-linked anti-guinea pig IgG, anti-mouse IgG, or anti-rabbit IgG (Cell Signaling Technology). Chemiluminescence signals from immunoblots were generated using Pierce ECL Plus reagent (ThermoFisher Scientific) and visualized on a LAS3000 or LAS4000 mini (FUJI).
Quantitative transcript analysis
Flies were entrained in LD cycles and harvested as described above. Total RNA was purified from 40 fly heads using TRIzol (Invitrogen, Carlsbad, CA). After the removal of contaminating genomic DNAs by RQ I DNase digestion, 2 μg of total RNA was reverse-transcribed using M-MuLV reverse transcriptase with random hexamers (Promega, Madison, WI). Quantitative real-time PCR was performed using SYBR reaction mix (Enzynomics, Daejeon, Korea) and the following sets of gene-specific primers: sra, forward: 5′-TAC TGG TGC AGC TTG ATT CG-3′ and reverse: 5′-CCT ACC CAC CTC AAT CAT CG-3′; per, forward: 5′-CTC AAC AGG TAA CTT CAC CTG C-3′ and reverse: 5′-ACG GGA AGA AAA AGC GCG AGA A-3′; tim, forward: 5′-TGC TCC TCC TGG GGG CAG CAA-3′ and reverse: 5′-AGA GAG CTA GCG AAA GTA TTT AC-3′; glyceraldehyde 3-phosphate dehydrogenase (gapdh) (as a control), forward: 5′-CTA CCT GTT CAA GTT CGA TTC GAC-3′ and reverse: 5′-AGT GGA CTC CAC GAT GTA TTC G-3′.
Immunofluorescence assay
Adult flies were fixed in phosphate-buffered saline (PBS) containing 4% formaldehyde for 2.5 hr, and subsequently whole brains were dissected out in PBS. Alternatively, adult brains were first dissected out in PBS, transferred to PBS containing 4% formaldehyde, and incubated for 30 min at room temperature. After permeabilization in PBS containing 0.3% Triton X-100 (PBS-T), brain samples were blocked in PBS-T containing 5% normal goat serum (The Jackson Laboratory) for 30 min at room temperature. After washing in PBS-T, brain samples were incubated with primary antibodies diluted in blocking solution at 4°C for 2 days. After repeated washes, samples were incubated for 1 day with secondary antibodies diluted in PBS-T, washed intensively, and then mounted using VECTASHIELD Mounting Medium (Vector Laboratories, Burlingame, CA). Immunofluorescence images of brain were acquired on a BX51 fluorescence microscope (Olympus) or a confocal laser scanning microscope (LSM780; Zeiss [Carl Zeiss], Thornwood, NY). Quantification of PER and TIM levels in individual clock neurons was performed as described previously (Lee et al. 2017). The primary antibodies used were mouse anti-PIGMENT-DISPERSING FACTOR (PDF) (Development Studies Hybridoma Bank), rabbit anti-PER, and guinea pig anti-TIM (Choi et al. 2009). The secondary antibodies used were Alexa Fluor 594-conjugated AffiniPure F(ab’)2 fragment goat anti-guinea pig, anti-rabbit, and anti-mouse, and fluorescein isothiocyanate (FITC)-conjugated AffiniPure F(ab’)2 fragment goat anti-rabbit, anti-guinea pig, and anti-mouse (Jackson ImmunoResearch Laboratories).
S2 cell culture and transient transfection
Drosophila S2 cells were maintained in Shields and Sang M3 insect medium (Sigma) supplemented with 10% fetal bovine serum (GIBCO [Grand Island Biological], Grand Island, NY) and 1% penicillin–streptomycin (Invitrogen). Expression vectors for V5-tagged PER, TIM, or CLK proteins (pAc-PER-V5, pAc-TIM-V5, and pAc-CLK-V5, respectively) (Lim et al. 2007b) were transiently transfected into S2 cells using Effectene transfection reagent (QIAGEN, Valencia, CA). Where indicated, 20 μM CsA (Sigma), 20 μM tacrolimus (Sigma), and/or 50 μM MG132 (Sigma) were added to the transfected cells and incubated for 2 hr before protein analyses.
Data availability
The authors state that all data necessary for confirming the conclusions of the article are represented fully within the article. Supplemental material available at Figshare: https://doi.org/10.25386/genetics.6216251.
Results
sra mutants exhibit arrhythmic circadian behaviors
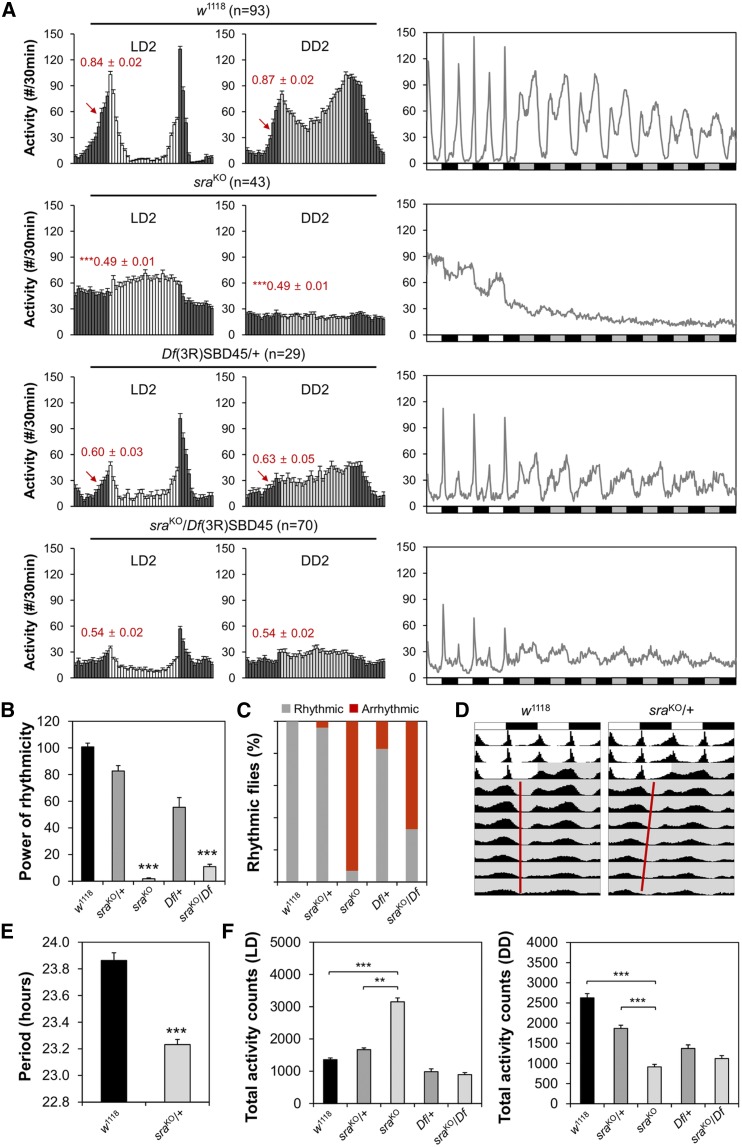
To investigate the possible role of sra in sustaining circadian rhythms, we examined circadian locomotor behaviors in individual sraKO mutant flies. Control flies exhibited robust locomotor rhythms with bimodal activity peaks in LD cycles (i.e., morning and evening peaks), and their rhythmic behaviors persisted in free-running DD cycles (Figure 1A). On the other hand, locomotor activity peaks in sraKO homozygous mutants were less evident, and their locomotor rhythms dampened rapidly in DD (Figure 1A). Quantification of free-running circadian behaviors confirmed poor rhythmicity of sraKO mutants in comparison with wild-type and heterozygous controls (Figure 1, B and C and Supplemental Material, Table S1), indicating the absence of a functional clock in sraKO mutants. In addition, the circadian periods in sraKO/+ heterozygous flies were modestly shortened (by ∼0.6 hr) (Figure 1, D and E and Table S1).
Figure 1.
Genomic deletion of sra locus impairs circadian locomotor behaviors. (A) Male flies homozygous or trans-heterozygous for sra mutant alleles show weaker anticipatory activity to lights-on (red arrows) and poor rhythmicity in free-running locomotor behaviors in DD. Activity profiles on the second day of LD cycles (LD2, left), on the second day of DD cycles (DD2, middle), or throughout the behavioral analyses (right) were averaged from individual flies. Averaged morning anticipation index values ± SEM are shown in red. *** P < 0.001 vs. wild-type as determined by Student’s t-test. n, the number of flies analyzed; white/black bars, LD cycles; and gray/black bars, DD cycles. (B) Rhythmicity in free-running locomotor behaviors was determined by measuring power (P) − significance (S) values from the χ2 periodograms of individual flies, and averaging over each genotype. *** P < 0.001 vs. wild-type or heterozygous control, as determined by one-way ANOVA with Tukey’s post hoc test. Error bars indicate SEM (n = 29–98). (C) Percentage of flies with detectable rhythmicity (P − S > 10) was calculated per each genotype. (D) Averaged actograms of wild-type or sraKO heterozygous flies were double-plotted (n = 93–98). Gray shade indicates constant dark. (E) Circadian periods in DD were averaged from rhythmic flies per each genotype. *** P < 0.001 vs. wild-type, as determined by Student’s t-test. Error bars indicate SEM (n = 93–94). (F) Daily total activity counts were averaged from individual flies per each genotype in LD (left) or DD (right) cycles. ** P < 0.01 and *** P < 0.001 as determined by one-way ANOVA with Tukey’s post hoc test. Error bars indicate SEM (n = 29–98). DD, constant dark; LD, 12 hr light/12 hr dark.
Because sraKO mutants have short baseline sleep (Nakai et al. 2011), hyperactivity might explain their loss of behavioral rhythmicity. However, previous studies have shown that short-sleep mutants can have intact circadian clocks (Liu et al. 2014; Shi et al. 2014), indicating that hyperactivity in such mutants is, in principle, separable from their circadian phenotypes. In fact, sraKO mutants exhibited lower activity in DD, when their behavioral arrhythmicity was most evident (Figure 1F). Moreover, trans-heterozygosity of the sraKO allele over a larger genomic deletion of the sra locus (i.e., a deficiency chromosome) did not significantly affect locomotor activity in LD, whereas the trans-heterozygous mutants still exhibited arrhythmic behaviors in DD (Figure 1, A–C and Table S1). Nonetheless, the weaker circadian phenotypes in sra trans-heterozygotes suggest that the effects of sra on circadian behaviors may be modulated by genetic modifiers present in the sraKO mutants or deficiency line.
Two groups of circadian pacemaker neurons, the small and large lateral ventral neurons (s-LNVs and l-LNVs), sustain behavioral rhythmicity by expressing a neuropeptide called PDF (Renn et al. 1999). Immunostaining of whole-mount fly brains with anti-PDF antibody revealed that the cell bodies and axonal projections in PDF-expressing clock neurons were similar between control and sraKO mutants (Figure S1). Accordingly, abnormal development of PDF neurons is not likely to be responsible for the arrhythmic behaviors of sraKO mutants. Together, these data suggest that sra is necessary for sustaining high-amplitude rhythms in free-running circadian behaviors.
sra mutation post-transcriptionally dampens circadian expression of PER and TIM
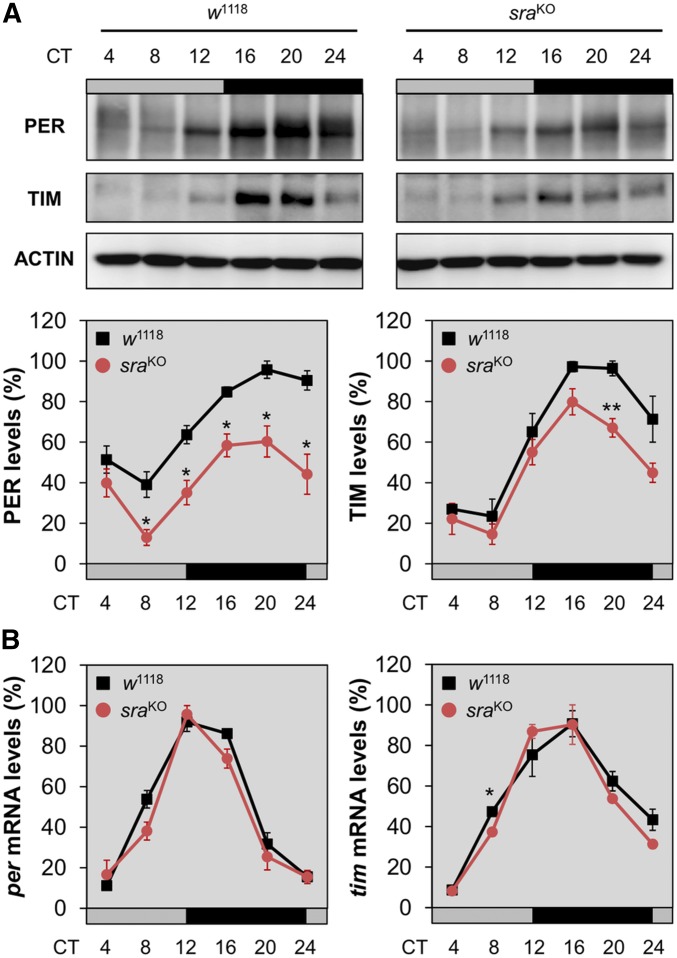
Given that sra acts as a regulator of Ca2+-dependent protein phosphatase calcineurin, we hypothesized that sra might control the post-transcriptional expression of circadian clock genes to maintain rhythmic behaviors. Accordingly, we collected control and sraKO flies every 4 hr during the first DD cycle after LD entrainment, and then quantified the relative expression of PER and TIM proteins in head extracts. This analysis revealed that circadian expression levels of both clock proteins were reduced in sraKO flies compared to the wild type (Figure 2A). Relative quantification of per and tim mRNA levels in head extracts revealed no significant difference between control and sraKO flies (Figure 2B), implying that the effects of sra on the rhythmic expression of clock proteins are likely to be post-transcriptional.
Figure 2.
sraKO mutation post-transcriptionally dampens circadian expression of PER and TIM proteins in adult fly heads. (A) Adult male flies were harvested at the indicated time points during the first DD cycle following the LD entrainment. Head extracts were immunoblotted with anti-PER, anti-TIM, and anti-actin (loading control) antibodies. Representative blot images from three independent experiments are shown. Protein band intensities in each lane were quantified using ImageJ software and normalized against the corresponding level of actin protein. The y-axis indicates % relative expression levels of PER and TIM, calculated by normalizing to the peak value in w1118 control flies (set as 100). Data represent average ± SEM (n = 3). * P < 0.05 and ** P < 0.01 vs. controls at the same time point, as determined by Student’s t-test. Gray/black bars, DD cycles; black lines, w1118 control flies; and red lines, sraKO flies. (B) Flies were harvested during the first DD cycle following the LD entrainment. Total RNAs were purified from head extracts, and relative levels of per, tim, and gapdh (normalizing control) mRNAs were quantitatively analyzed by real-time PCR. The y-axis indicates % relative expression levels of per and tim mRNAs at each time point, calculated by normalizing to the peak value in w1118 control flies (set as 100). Data represent average ± SEM (n = 3). * P < 0.05 vs. control at the same time point, as determined by Student’s t-test. CT, circadian time; DD, constant dark; LD, 12 hr light/12 hr dark.
Circadian expression of clock genes in head extracts reflects the pace of the molecular clock in peripheral tissues, but it is not necessarily coupled to rhythmic behaviors in DD. In fact, modest effects of sra on the protein oscillations in head extracts do not convincingly explain stronger behavioral phenotypes in sra mutants. Therefore, we assessed circadian expression of PER and TIM proteins in behaviorally relevant PDF neurons by immunostaining adult fly brains. PDF-expressing s-LNVs play pivotal roles in multiple aspects of circadian behaviors, including morning anticipation and free-running rhythms in DD (Grima et al. 2004; Stoleru et al. 2004). Consistent with this, PER and TIM levels in PDF neurons were significantly lower in sra mutant flies than in controls at the peak time point in LD. The effects of sra on PER and TIM levels were less evident in dorsal lateral neurons (LNds), another group of circadian pacemaker neurons that express PER and TIM, but not PDF (Figure S2), although it is possible that lack of counterstaining of all LNds in our experimental conditions might have underestimated sra effects in these clock neurons. We reason that our protein analyses using polyclonal antibodies could not have revealed more subtle, qualitative defects in the molecular oscillations that are actually responsible for sra mutant behaviors. Nonetheless, these data support the idea that sra-dependent post-transcriptional control may contribute to the robust expression of circadian clock proteins, and likely, high-amplitude rhythms in locomotor behaviors.
sra depletion in circadian pacemaker neurons phenocopies sra mutation
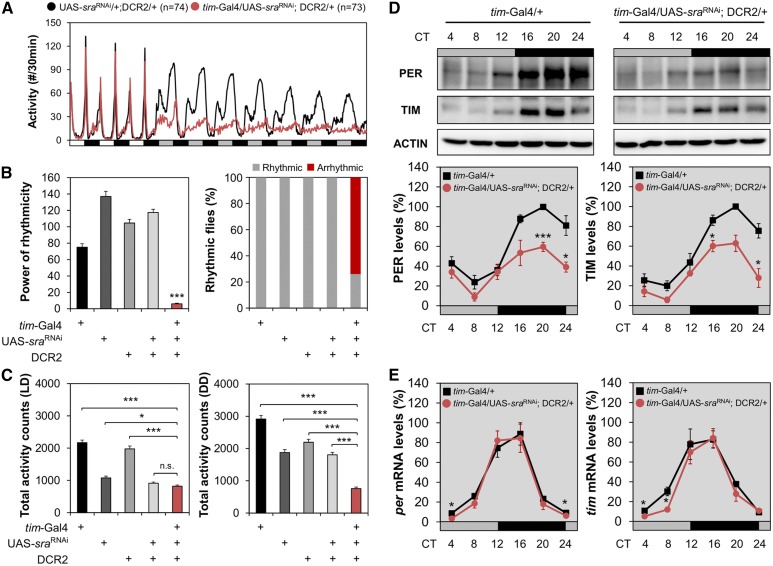
A transgenic reporter harboring the sra promoter (sra-Gal4) (Lee et al. 2016) is broadly expressed in the adult fly brain (Figure S3), suggesting that sra functions in multiple brain regions including circadian clock neurons, the mushroom body, and the pars intercerebralis. To determine whether a specific neural locus is important for sra function in circadian behaviors, we constitutively overexpressed an RNAi transgene against sra (sraRNAi) along with the RNAi-enhancing Dicer2 (DCR2) transgene in different sets of brain neurons and examined their behavioral effects. Expression of sraRNAi in tim-expressing clock cells under the control of the tim-Gal4 driver (tim-Gal4/UAS-sraRNAi; DCR2/+) was sufficient to mimic most of the circadian phenotypes of sraKO flies (Figure 3 and Table S2). sra-depleted flies lost free-running behavioral rhythms in DD (Figure 3, A and B and Table S2). Nonetheless, sraRNAi flies did not exhibit hyperactivity but their locomotor activity was rather reduced in DD (Figure 3C). In addition, sra depletion post-transcriptionally impaired PER and TIM rhythms in fly heads (Figure 3, D and E), to a similar extent as the sraKO allele.
Figure 3.
sra depletion in tim-expressing circadian clock cells is sufficient to phenocopy sraKO mutation. (A) Activity profiles were averaged from individual flies in LD cycles followed by DD. (B and C) Rhythmicity in free-running locomotor behaviors [power (P) − significance (S)], percentage of flies with detectable rhythmicity (P − S > 10), and daily total activity counts were analyzed similarly as in Figure 1. * P < 0.05 and *** P < 0.001 vs. other transgenic controls, as determined by one-way ANOVA with Tukey’s post hoc test. Error bars indicate SEM (n = 31–73). (D and E) Transgenic flies were harvested at the indicated time points during the first DD cycle following the LD entrainment. Relative expression levels (%)of PER and TIM proteins (D), or those of per and tim mRNAs (E) were quantified in head extracts similarly as in Figure 2. Data represent average ± SEM (n = 3). * P < 0.05 and ** P < 0.01 vs. controls at the same time point, as determined by Student’s t-test. CT, circadian time; DD, constant dark; LD, 12 hr light/12 hr dark; n.s., not significant; UAS: upstream activating sequence.
Because the tim-Gal4 transgene is expressed in all clock-relevant cells, including glia (Suh and Jackson 2007), we tested additional Gal4 drivers to narrow down the neural substrate of sra-dependent clocks. Although the majority of Gal4 drivers expressed in circadian pacemaker neurons affected free-running circadian behaviors, sra depletion in PDF-expressing LNVs was sufficient to dampen free-running behavioral rhythms (Figure S4 and Table S2). However, the behavioral phenotypes by the LNV-specific pdf-Gal4 or mz520-Gal4 driver were relatively modest. One possible explanation for this observation is that sra depletion in PDF neurons by these LN-specific Gal4 drivers might not be as efficient as depletion by tim-Gal4. In addition, we cannot exclude the possibility that non-PDF clock neurons positive for the tim-Gal4 or cryptochrome-Gal4 (cry-Gal4) transgene could make additional contributions to sra-dependent behavioral rhythms.
To determine whether sra effects on circadian behaviors are specific to the adult stage, we conditionally depleted sra expression in PDF neurons of adult fly brains using a gene-switch transgene (Depetris-Chauvin et al. 2011). Although baseline expression of the sraRNAi transgene by the leaky pdf-Gal4GeneSwitch decreased behavioral rhythmicity in control flies fed vehicle (ethanol)-containing food, we observed that the RU486-induced depletion of sra in PDF neurons eventually led to arrhythmic behaviors in subsequent DD cycles (Figure S5 and Table S3). Taken together, these data support the idea that sra functions in adult PDF neurons to sustain locomotor rhythms, excluding the possibility that sra affects circadian behaviors by influencing the development of PDF neurons.
sra overexpression in circadian pacemaker neurons disrupts circadian rhythms
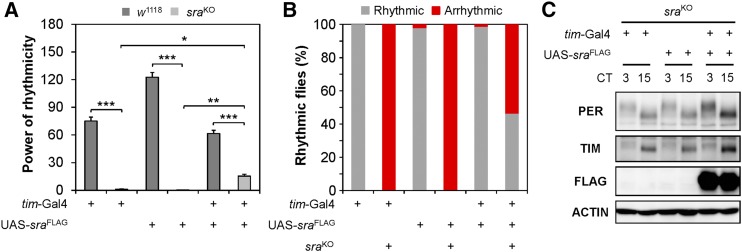
Circadian rhythms could be sensitized to a specific dosage of a clock-relevant gene. To explore this idea, we investigated whether sra overexpression could modulate circadian behaviors in wild-type flies. For this purpose, we employed three sra transgenes, encoding either wild-type or epitope-tagged sra cDNAs, independently established in previous studies (Ejima et al. 2004; Takeo et al. 2012). Each sra transgene was genetically combined with either tim-Gal4 or LN-specific Gal4 drivers to induce sra overexpression in all clock cells or PDF neurons, respectively. The strongest effects of sra overexpression were observed with the UAS-sra transgene: its expression in PDF neurons was sufficient to cause longer periodicity, with lower-amplitude rhythms in free-running behaviors, than in heterozygous controls (Table S4). However, modest dampening of behavioral rhythms with longer periods was consistently observed when sra overexpression in tim-expressing cells was genetically induced using the other sra transgenes (UAS-sraFLAG and UAS-sraWT). Different levels of sra overexpression in PDF neurons under the control of tim-Gal4 or LN-specific Gal4 drivers might explain the phenotypic differences among sra transgenic lines. An alternative (but not mutually exclusive) explanation is that overexpression or ectopic expression of sra in non-PDF neurons under the control of tim-Gal4 might have contributed to the deficient rhythms in these transgenic flies. Nonetheless, sra overexpression driven by tim-Gal4 partially rescued the disrupted behavioral rhythms in sraKO flies, and also elevated the levels of PER and TIM proteins in head extracts of sra mutants (Figure 4 and Table S4). Taken together, these results suggest that the robustness of 24-hr behavioral rhythms requires a specific dosage window of sra expression in circadian pacemaker neurons.
Figure 4.
sra overexpression in tim-expressing clock cells partially rescues circadian phenotypes in sraKO mutation. (A and B) Overexpression of a transgene encoding the FLAG-tagged sra cDNA was driven by a tim-Gal4 driver in a w1118 control or sraKO mutant background. Rhythmicity in free-running locomotor behaviors [power (P) − significance (S)] and percentage of transgenic flies with detectable rhythmicity (P − S > 10) were analyzed similarly as in Figure 1. Data represent average ± SEM (n = 47–75). Two-way ANOVA detected significant interaction between sra overexpression and sraKO mutation on power of rhythmicity [F (2, 369) = 71.67, P < 0.0001], validating the transgenic rescue. * P < 0.05, ** P < 0.01, and *** P < 0.001 as determined by Tukey’s post hoc test. (C) Flies harboring the indicated transgenes in sraKO mutant background were harvested at CT3 and CT15 during the first DD cycle following the LD entrainment. Head extracts were immunoblotted with anti-PER, anti-TIM, anti-FLAG, and anti-actin (loading control) antibodies. CT, circadian time; DD, constant dark; LD, 12 hr light/12 hr dark; UAS: upstream activating sequence.
Genetic manipulation of calcineurin expression or activity affects circadian rhythms
The Drosophila calcineurin contains one of three catalytic subunits: CanA1, CanA-14F, or Pp2B-14D (Takeo et al. 2006). Nonetheless, increased locomotor activity and short-sleep phenotypes have been reported most evidently in CanA-14FKO flies (Nakai et al. 2011). To determine whether sra collaborates with a specific calcineurin to sustain circadian rhythms, we assessed circadian behaviors in individual calcineurin mutant flies. Genomic deletion of either the CanA-14F or Pp2B-14D locus modestly dampened rhythm amplitude in free-running DD behaviors (Figure S6 and Table S5). However, the percentage of rhythmic flies was much higher than in sra mutants. This observation supports the idea that circadian phenotypes and hyperactivity are, at least in part, dissociable in calcineurin mutants. We also reasoned that functional redundancy among the three catalytic calcineurin subunits might explain their relatively weak individual phenotypes in comparison with sra mutants.
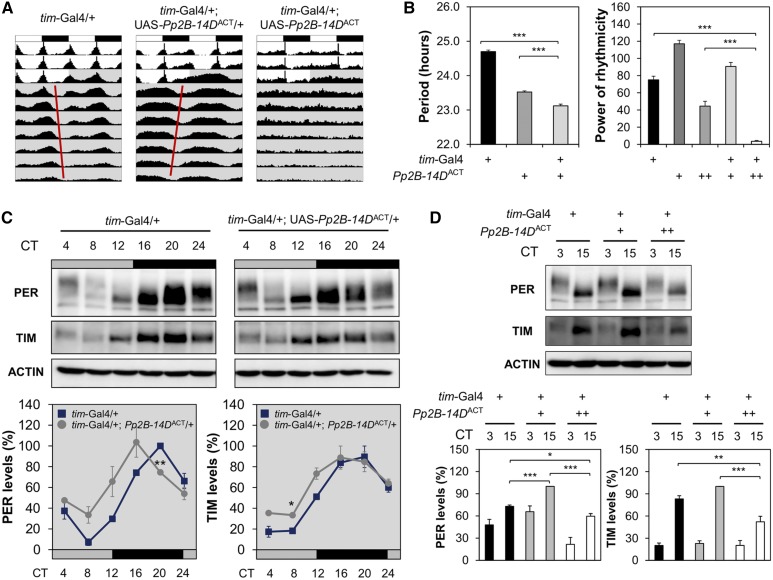
On the other hand, we observed that transgenic overexpression of constitutively active Pp2B-14D (Pp2B-14DACT), but neither a wild-type (Pp2B-14DWT) nor dominant-negative mutant (Pp2B-14DH217Q), in tim-expressing clock cells shortened circadian periods in free-running locomotor behaviors (Figure 5, A and B and Table S6). Similar to the effects of sra, Pp2B-14DACT overexpression in PDF-expressing clock neurons was sufficient to cause short-period phenotypes in DD rhythms. Circadian expression of PER and TIM proteins (as reflected by protein levels in head extracts) was accordingly phase-advanced by Pp2B-14DACT (Figure 5C). Moreover, transgenic flies overexpressing Pp2B-14DACT from two copies of the transgene were strongly arrhythmic in DD (Figure 5, A and B and Table S6), and displayed lower levels of PER and TIM proteins in head extracts (Figure 5D). Taken together, these data further support the idea that circadian clocks are sensitive to overall calcineurin activity in circadian pacemaker neurons, consistent with the effects of sra on circadian behaviors.
Figure 5.
Constitutively active form of Pp2B-14D disrupts circadian behaviors in a dose-dependent manner when overexpressed in tim-expressing clock cells. (A) Averaged actograms of flies overexpressing the constitutively active Pp2B-14DACT from a single or two copies of the transgene were double-plotted (n = 32–136). (B) Circadian periods in rhythmic flies [power (P) − significance (S) > 10] and rhythmicity in free-running locomotor behaviors (P − S) were analyzed similarly as in Figure 1. *** P < 0.001 as determined by one-way ANOVA with Tukey’s post hoc test. Error bars indicate SEM (n = 31–136). (C and D) Transgenic flies were harvested at the indicated time points during the first DD cycle following the LD entrainment. Relative expression levels (%) of PER and TIM proteins were quantified in head extracts similarly as in Figure 2. Data represent average ± SEM (n = 3). * P < 0.05 and ** P < 0.01 vs. Gal4 heterozygous controls at the same time point, as determined by Student’s t-test (C); * P < 0.05, ** P < 0.01, and *** P < 0.001 as determined by one-way ANOVA with Tukey’s post hoc test (D). CT, circadian time; DD, constant dark; LD, 12 hr light/12 hr dark; UAS: upstream activating sequence.
sra genetically titrates calcineurin activity to sustain circadian rhythms
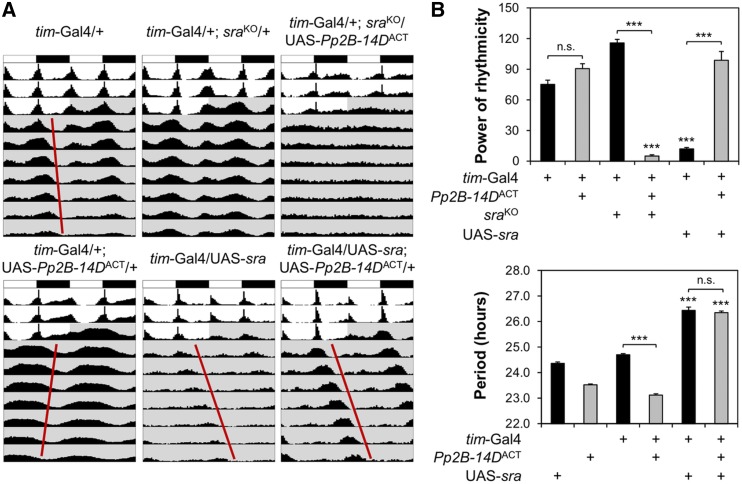
sra and Pp2B-14D interact genetically and biochemically in Drosophila models (Takeo et al. 2006; Nakai et al. 2011). To determine whether both genes function in the same genetic pathway related to circadian clocks, we tested the effects of constitutively active Pp2B-14DACT on free-running locomotor behaviors in genetic backgrounds with different dosages of sra expression. Heterozygous sraKO negligibly affected circadian behaviors in control genetic backgrounds (Figure 6 and Table S7), but substantially dampened behavioral rhythmicity in transgenic flies overexpressing Pp2B-14DACT in tim-expressing clock cells. Given that higher overexpression from two copies of the Pp2B-14DACT transgene caused arrhythmic behaviors, the genetic interaction between sraKO and Pp2B-14DACT supports the model that sra normally suppresses Pp2B-14DACT. Furthermore, we found that overexpression of wild-type sra masked short-period rhythms in Pp2B-14DACT-overexpressing flies (Figure 6 and Table S7). Moreover, Pp2B-14DACT overexpression rescued the poor rhythmicity caused by sra overexpression. Taken together, these data provide compelling genetic evidence that sra acts as a negative regulator of calcineurin to titrate its overall activity, and thereby sustains 24-hr rhythms in free-running locomotor behaviors.
Figure 6.
sra genetically suppresses Pp2B-14DACT to sustain circadian locomotor rhythms. (A) Averaged actograms of transgenic flies overexpressing the constitutively active Pp2B-14DACT in wild-type, sraKO heterozygous, or sra-overexpressing background were double-plotted (n = 29–136). (B) Rhythmicity in free-running locomotor behaviors (top) and circadian periods in rhythmic flies (bottom) were analyzed similarly as in Figure 1. Data represent average ± SEM (n = 29–136). Two-way ANOVA detected significant interaction of Pp2B-14DACT with sraKO [F (1, 344) = 185.3, P < 0.0001 for power of rhythmicity] and sra overexpression [F (1, 297) = 36.24, P < 0.0001 for power of rhythmicity; F (1, 249) = 38.52, P < 0.0001 for circadian periods], validating the genetic interaction between sra and Pp2B-14DACT on free-running locomotor behaviors in DD. *** P < 0.001 vs. the same combination of tim-Gal4 or Pp2B-14DACT transgene in wild-type, sra background as determined by Tukey’s post hoc test. CT, circadian time; DD, constant dark; n.s., not significant UAS: upstream activating sequence.
Pharmacological inhibition of calcineurin promotes proteasomal degradation of PER and TIM proteins
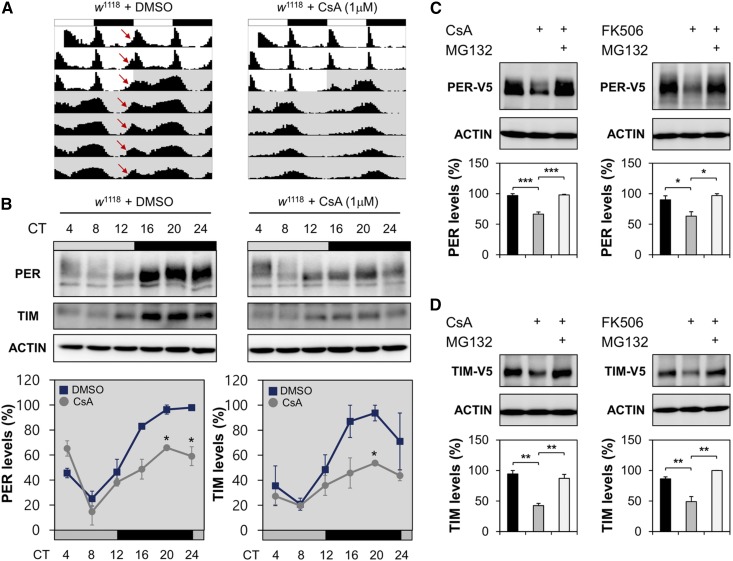
We next took a pharmacological approach to examine the behavioral and molecular consequences of calcineurin inhibition in circadian rhythms. Oral administration of a calcineurin inhibitor, CsA, to wild-type flies did not affect their viability during our tests of circadian behaviors (i.e., 3 days in LD cycle followed by 7 days in DD). However, CsA-fed flies exhibited weaker activity peaks during the subjective day in DD, as well as longer circadian periods in free-running behaviors than vehicle (DMSO)-fed flies (Figure 7A and Table S8). Circadian expression of PER and TIM proteins (as reflected by protein levels in head extracts) was also compromised in CsA-fed flies (Figure 7B).
Figure 7.
Pharmacological inhibition of calcineurin dampens circadian expression of PER and TIM proteins in wild-type fly heads and enhances their proteasomal degradation in cell culture. (A) Wild-type flies were fed on behavior food (5% sucrose and 2% agar) containing either 1 mM DMSO (vehicle control) or 1 μM CsA throughout the behavioral test, and their averaged actograms were double-plotted (n = 45–57). Locomotor activity peaks anticipatory to lights-on were indicated by red arrows. (B) Wild-type flies were entrained by three LD cycles on the behavior food containing either 1 mM DMSO or 1 μM CsA, transferred to DD, and then harvested at the indicated time points during the first DD cycle. Relative expression levels (%) of PER and TIM proteins were quantified in head extracts similarly as in Figure 2. Data represent average ± SEM (n = 2). * P < 0.05 vs. DMSO controls at the same time point as determined by Student’s t-test. (C and D) Drosophila S2 cells were transfected with the expression vector for V5-tagged PER or TIM proteins. Where indicated, 20 μM CsA, 20 μM tacrolimus (FK506), or 50 μM MG132 were incubated with transfected cells for 2 hr before harvest. Relative expression levels (%) of V5-tagged PER or TIM proteins were quantified in transfected cell extracts similarly as in Figure 2. Data represent average ± SEM (n = 3). * P < 0.05, ** P < 0.01 and *** P < 0.001 as determined by one-way ANOVA with Tukey’s post hoc test. CsA, cyclosporin A; CT, circadian time; DD, constant dark; LD, 12 hr light/12 hr dark.
To determine whether the observed CsA effects on PER and TIM rhythms were driven either by behavioral dampening or by cell-autonomous effects of calcineurin inhibition, we investigated the effects of CsA on PER and TIM expression in clock-less Drosophila S2 cells. Because S2 cells barely express the endogenous clock proteins, we transiently transfected S2 cells with expression vectors encoding epitope-tagged PER or TIM, and then monitored changes in their steady-state levels in response to pharmacological treatments. CsA treatment decreased PER and TIM levels in a dose-dependent manner (Figure S7A). The proteasome inhibitor MG132 suppressed the effects of CsA on PER and TIM levels (Figure 7, C and D), indicating that CsA treatment promoted proteasomal degradation of the two proteins. Similar results were obtained using another calcineurin inhibitor tacrolimus (FK506). By contrast, neither CsA nor FK506 treatment affected steady-state levels of CLK proteins in transfected S2 cells (Figure S7B). Together, these data suggest that calcineurin specifically protects PER and TIM proteins from proteasomal degradation in a cell-autonomous manner. In addition, we reason that calcineurin-dependent stabilization of PER or TIM proteins is unlikely to be mediated by the formation of a PER–TIM complex.
Discussion
The phosphorylation status of specific residues in clock proteins changes temporally in a well-coordinated manner. Consequently, the subcellular localization, transcriptional activity, and stability of Drosophila PER and TIM proteins are collaboratively regulated by multiple protein kinases and phosphatases, thereby sustaining overt rhythms in circadian gene expression and behaviors. Previous studies in mammals showed that chemical inhibitors of the Ca2+-dependent protein phosphatase calcineurin impose their effects on a wide range of circadian physiology, including daily changes in blood pressure, phase shift by light entrainment, and locomotor rhythms (van den Dorpel et al. 1996; Katz et al. 2008). Here, we provide compelling genetic evidence that balancing the activity of calcineurin and its negative regulator sra in Drosophila circadian pacemaker neurons is crucial for high-amplitude circadian behaviors. The effects of calcineurin on Drosophila clocks are likely mediated by the proteasomal degradation of the clock proteins PER and TIM, but not by their circadian transcription. Thus, our data support the idea that Drosophila circadian rhythms are post-translationally controlled by the calcineurin–sra pathway.
The intimate interaction between circadian clocks and sleep suggests that a common pathway is responsible for the behavioral phenotypes observed in Drosophila models lacking sra and individual calcineurin genes. However, arguing against this idea, we observed clear differences between their circadian and sleep phenotypes. First, mutant flies homozygous for genomic deletion of the sra locus exhibited hyperactivity (i.e., short sleep) in LD cycles (Nakai et al. 2011), but exhibited lower locomotor activity than wild-type or heterozygous controls in subsequent DD cycles, during which their free-running behaviors actually became arrhythmic. Second, sra trans-heterozygous mutants exhibited poor rhythmicity in DD locomotor behaviors, whereas their total activity counts were comparable to those of heterozygous controls. Accordingly, circadian phenotypes in this sra mutant background were dissociable from hyperactivity or short-sleep phenotypes. Third, genomic deletion of either sra or CanA-14F shortened baseline sleep to comparable extents (Nakai et al. 2011). By contrast, we observed much severer rhythmicity phenotypes (i.e., lower percentages of rhythmic flies) in sra mutants. Finally, overexpression of a constitutively active calcineurin catalytic subunit (Pp2B-14DACT) caused shorter circadian periods or arrhythmic clocks, but had no detectable effects on baseline sleep under particular experimental conditions (Tomita et al. 2011). Together, these observations suggest sleep-independent clock regulation by the calcineurin–sra pathway.
One puzzling observation regarding Drosophila mutants in the calcineurin–sra pathway is that single deletions of either sra or the CanA-14F locus led to behavioral defects (i.e., hyperactivity, short sleep, and arrhythmic clocks) in the same direction. These data argue against the idea that sra negatively regulates calcineurin, and instead favor a model in which these factors act cooperatively or at least independently. In fact, sra and calcineurin mutants exhibit similar phenotypes in other genetic models of Drosophila and mammals, defining sra as a calcineurin activator (Vega et al. 2003; Takeo et al. 2010). Another complication comes from the observations that either loss-of-function or overexpression of sra can impair learning in olfactory conditioning (Chang et al. 2003) or behavioral rhythmicity in DD, indicating that an optimal sra dosage is required to control these behaviors.
Nonetheless, the sleep-promoting effects of sra require CanA-14F or Pp2B-14D (Nakai et al. 2011), and these nonadditive effects of sra and calcineurin mutations on the total amount of sleep support the idea that these factors participate in a common genetic pathway involved in sleep regulation. Further, we showed that strong overexpression of constitutively active Pp2B-14DACT dampened PER and TIM rhythms, and caused arrhythmic circadian behaviors, phenotypically mimicking loss of sra function. Moreover, abnormal circadian behaviors in Pp2B-14DACT-overexpressing flies were exaggerated or suppressed by genomic deletion or overexpression of sra, respectively. These pieces of genetic evidence convincingly demonstrate that sra is a negative regulator of calcineurin in the genetic pathway that regulates circadian rhythms, as exemplified in other Drosophila models (Takeo et al. 2006; Shaw and Chang 2013; Lee et al. 2016). However, it remains possible, that sra might have a stronger preference for a specific calcineurin subtype (e.g., calcineurin harboring a catalytic subunit Pp2B-14D) in regard to its clock-regulatory function. Thus, the molecular phenotypes in sraKO mutants may largely reflect the consequences of derepression of a specific calcineurin activity. On the other hand, in wild-type flies and Drosophila S2 cells, the proteasomal degradation of PER and TIM proteins could have been promoted by the overall suppression of both sra-dependent and sra-independent calcineurin activity. Another possibility is that the PER- and TIM-stabilizing effects of calcineurin might be gated by circadian clocks due to the time-dependent shuttling of these clock proteins between the nucleus and cytoplasm, or by the rhythmic changes in their phosphorylation status.
Calcineurin dephosphorylates nuclear factor of activated T-cells (NFAT) and promotes its nuclear entry to support its transcriptional regulatory activity (Hogan et al. 2003). In mammals, NFAT undergoes daily rhythmic nuclear translocation in skeletal muscle and heart, and mediates activity-dependent circadian gene expression (Sachan et al. 2011; Dyar et al. 2015). In fact, DSCR1/RCAN1 (a mammalian homolog of sra) is a transcriptional target of NFAT (Yang et al. 2000). Daily circadian oscillation in DSCR1/RCAN1 occurs in the liver and heart (Storch et al. 2002; Bray et al. 2008; Sachan et al. 2011). Because DSCR1 associates with the catalytic subunit of calcineurin and inhibits its enzymatic activity (Fuentes et al. 2000; Rothermel et al. 2000; Chan et al. 2005), the DSCR1/RCAN1–calcineurin–NFAT pathway possesses an intrinsic auto-inhibitory mechanism (Yang et al. 2000; Minami 2014). This additional layer of transcriptional feedback might supplement circadian gene expression in a Ca2+- and/or activity-dependent manner.
Conservation of circadian clock function among DSCR1/RCAN1–calcineurin homologs, and their functional interactions with other clock-regulatory factors such as cAMP response element-binding protein (Kingsbury et al. 2007; Kim and Seo 2011), CKIε/DBT (Liu et al. 2002), GSK3β/SHAGGY (Kim et al. 2009; Takeo et al. 2012), and FMRP (Wang et al. 2012), indicate that DSCR1/RCAN1–calcineurin-dependent signaling is integral to the core clock mechanism and suggest that it is derived from ancestral clocks. Future studies should seek to elucidate how the transcriptional feedback loop of the DSCR1/RCAN1–calcineurin–NFAT pathway shapes circadian transcription, as well as how post-translational regulation by DSCR1/RCAN1–calcineurin signaling elaborately contributes to high-amplitude 24-hr rhythms.
Acknowledgments
We thank Toshiro Aigaki, the Bloomington Drosophila Stock Center, the Vienna Drosophila Resource Center, and the Kyoto Stock Center (Drosophila Genomics and Genetics Resources) for reagents. This work was supported by a grant from the Korea Health Technology Research and Development Project through the Korean Health Industry Development Institute funded by the Ministry of Health and Welfare, Republic of Korea (HI16C1747) (C.L.); a grant from the National Research Foundation (NRF) funded by the Ministry of Science, ICT and Future Planning (MSIP), Republic of Korea (NRF-2017R1E1A2A02066965) (C.L.); a grant from the NRF funded by the Ministry of Education (NRF-2016R1A6A3A11932215) (J.L.); and a grant from the NRF funded by the MSIP, Republic of Korea (NRF-2016R1A2B4011111) (J.C.).
Footnotes
Supplemental material available at Figshare: https://doi.org/10.25386/genetics.6216251.
Communicating editor: G. Bosco
Literature Cited
- Akten B., Jauch E., Genova G. K., Kim E. Y., Edery I., et al. , 2003. A role for CK2 in the Drosophila circadian oscillator. Nat. Neurosci. 6: 251–257. 10.1038/nn1007 [DOI] [PubMed] [Google Scholar]
- Allada R., Chung B. Y., 2010. Circadian organization of behavior and physiology in Drosophila. Annu. Rev. Physiol. 72: 605–624. 10.1146/annurev-physiol-021909-135815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass J., Takahashi J. S., 2010. Circadian integration of metabolism and energetics. Science 330: 1349–1354. 10.1126/science.1195027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray M. S., Shaw C. A., Moore M. W., Garcia R. A., Zanquetta M. M., et al. , 2008. Disruption of the circadian clock within the cardiomyocyte influences myocardial contractile function, metabolism, and gene expression. Am. J. Physiol. Heart Circ. Physiol. 294: H1036–H1047. 10.1152/ajpheart.01291.2007 [DOI] [PubMed] [Google Scholar]
- Chan B., Greenan G., McKeon F., Ellenberger T., 2005. Identification of a peptide fragment of DSCR1 that competitively inhibits calcineurin activity in vitro and in vivo. Proc. Natl. Acad. Sci. USA 102: 13075–13080. 10.1073/pnas.0503846102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang K. T., Min K. T., 2005. Drosophila melanogaster homolog of Down syndrome critical region 1 is critical for mitochondrial function. Nat. Neurosci. 8: 1577–1585. 10.1038/nn1564 [DOI] [PubMed] [Google Scholar]
- Chang K. T., Shi Y. J., Min K. T., 2003. The Drosophila homolog of Down’s syndrome critical region 1 gene regulates learning: implications for mental retardation. Proc. Natl. Acad. Sci. USA 100: 15794–15799. 10.1073/pnas.2536696100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu J. C., Ko H. W., Edery I., 2011. NEMO/NLK phosphorylates PERIOD to initiate a time-delay phosphorylation circuit that sets circadian clock speed. Cell 145: 357–370. 10.1016/j.cell.2011.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi C., Lee J., Lim C., Jang D., Choe J., 2009. The DOUBLETIME protein kinase regulates phosphorylation of the Drosophila PDP1epsilon. J. Neurochem. 111: 264–273. 10.1111/j.1471-4159.2009.06327.x [DOI] [PubMed] [Google Scholar]
- Cyran S. A., Buchsbaum A. M., Reddy K. L., Lin M. C., Glossop N. R., et al. , 2003. vrille, Pdp1, and dClock form a second feedback loop in the Drosophila circadian clock. Cell 112: 329–341. 10.1016/S0092-8674(03)00074-6 [DOI] [PubMed] [Google Scholar]
- Depetris-Chauvin A., Berni J., Aranovich E. J., Muraro N. I., Beckwith E. J., et al. , 2011. Adult-specific electrical silencing of pacemaker neurons uncouples molecular clock from circadian outputs. Curr. Biol. 21: 1783–1793. 10.1016/j.cub.2011.09.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubowy C., Sehgal A., 2017. Circadian rhythms and sleep in Drosophila melanogaster. Genetics 205: 1373–1397. 10.1534/genetics.115.185157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyar K. A., Ciciliot S., Tagliazucchi G. M., Pallafacchina G., Tothova J., et al. , 2015. The calcineurin-NFAT pathway controls activity-dependent circadian gene expression in slow skeletal muscle. Mol. Metab. 4: 823–833. 10.1016/j.molmet.2015.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ejima A., Tsuda M., Takeo S., Ishii K., Matsuo T., et al. , 2004. Expression level of sarah, a homolog of DSCR1, is critical for ovulation and female courtship behavior in Drosophila melanogaster. Genetics 168: 2077–2087. 10.1534/genetics.104.029934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Y., Sathyanarayanan S., Sehgal A., 2007. Post-translational regulation of the Drosophila circadian clock requires protein phosphatase 1 (PP1). Genes Dev. 21: 1506–1518. 10.1101/gad.1541607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentes J. J., Genesca L., Kingsbury T. J., Cunningham K. W., Perez-Riba M., et al. , 2000. DSCR1, overexpressed in Down syndrome, is an inhibitor of calcineurin-mediated signaling pathways. Hum. Mol. Genet. 9: 1681–1690. 10.1093/hmg/9.11.1681 [DOI] [PubMed] [Google Scholar]
- Grima B., Chelot E., Xia R., Rouyer F., 2004. Morning and evening peaks of activity rely on different clock neurons of the Drosophila brain. Nature 431: 869–873. 10.1038/nature02935 [DOI] [PubMed] [Google Scholar]
- Hardin P. E., 2011. Molecular genetic analysis of circadian timekeeping in Drosophila. Adv. Genet. 74: 141–173. 10.1016/B978-0-12-387690-4.00005-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardin P. E., Panda S., 2013. Circadian timekeeping and output mechanisms in animals. Curr. Opin. Neurobiol. 23: 724–731. 10.1016/j.conb.2013.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano A., Fu Y. H., Ptacek L. J., 2016. The intricate dance of post-translational modifications in the rhythm of life. Nat. Struct. Mol. Biol. 23: 1053–1060. 10.1038/nsmb.3326 [DOI] [PubMed] [Google Scholar]
- Hogan P. G., Chen L., Nardone J., Rao A., 2003. Transcriptional regulation by calcium, calcineurin, and NFAT. Genes Dev. 17: 2205–2232. 10.1101/gad.1102703 [DOI] [PubMed] [Google Scholar]
- Horner V. L., Czank A., Jang J. K., Singh N., Williams B. C., et al. , 2006. The Drosophila calcipressin sarah is required for several aspects of egg activation. Curr. Biol. 16: 1441–1446. 10.1016/j.cub.2006.06.024 [DOI] [PubMed] [Google Scholar]
- Kadener S., Stoleru D., McDonald M., Nawathean P., Rosbash M., 2007. Clockwork Orange is a transcriptional repressor and a new Drosophila circadian pacemaker component. Genes Dev. 21: 1675–1686. 10.1101/gad.1552607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko M., Hall J. C., 2000. Neuroanatomy of cells expressing clock genes in Drosophila: transgenic manipulation of the period and timeless genes to mark the perikarya of circadian pacemaker neurons and their projections. J. Comp. Neurol. 422: 66–94. [DOI] [PubMed] [Google Scholar]
- Katz M. E., Simonetta S. H., Ralph M. R., Golombek D. A., 2008. Immunosuppressant calcineurin inhibitors phase shift circadian rhythms and inhibit circadian responses to light. Pharmacol. Biochem. Behav. 90: 763–768. 10.1016/j.pbb.2008.05.017 [DOI] [PubMed] [Google Scholar]
- Kim S. S., Seo S. R., 2011. The regulator of calcineurin 1 (RCAN1/DSCR1) activates the cAMP response element-binding protein (CREB) pathway. J. Biol. Chem. 286: 37841–37848. 10.1074/jbc.M111.232165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y., Lee Y. I., Seo M., Kim S. Y., Lee J. E., et al. , 2009. Calcineurin dephosphorylates glycogen synthase kinase-3 beta at serine-9 in neuroblast-derived cells. J. Neurochem. 111: 344–354. 10.1111/j.1471-4159.2009.06318.x [DOI] [PubMed] [Google Scholar]
- Kingsbury T. J., Bambrick L. L., Roby C. D., Krueger B. K., 2007. Calcineurin activity is required for depolarization-induced, CREB-dependent gene transcription in cortical neurons. J. Neurochem. 103: 761–770. 10.1111/j.1471-4159.2007.04801.x [DOI] [PubMed] [Google Scholar]
- Kivimäe S., Saez L., Young M. W., 2008. Activating PER repressor through a DBT-directed phosphorylation switch. PLoS Biol. 6: e183 10.1371/journal.pbio.0060183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloss B., Price J. L., Saez L., Blau J., Rothenfluh A., et al. , 1998. The Drosophila clock gene double-time encodes a protein closely related to human casein kinase Iepsilon. Cell 94: 97–107. 10.1016/S0092-8674(00)81225-8 [DOI] [PubMed] [Google Scholar]
- Ko H. W., Jiang J., Edery I., 2002. Role for Slimb in the degradation of Drosophila Period protein phosphorylated by Doubletime. Nature 420: 673–678. 10.1038/nature01272 [DOI] [PubMed] [Google Scholar]
- Ko H. W., Kim E. Y., Chiu J., Vanselow J. T., Kramer A., et al. , 2010. A hierarchical phosphorylation cascade that regulates the timing of PERIOD nuclear entry reveals novel roles for proline-directed kinases and GSK-3beta/SGG in circadian clocks. J. Neurosci. 30: 12664–12675. 10.1523/JNEUROSCI.1586-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J., Yoo E., Lee H., Park K., Hur J. H., et al. , 2017. LSM12 and ME31B/DDX6 define distinct modes of posttranscriptional regulation by ATAXIN-2 protein complex in Drosophila circadian pacemaker neurons. Mol. Cell 66: 129–140.e7. 10.1016/j.molcel.2017.03.004 [DOI] [PubMed] [Google Scholar]
- Lee S., Bang S. M., Hong Y. K., Lee J. H., Jeong H., et al. , 2016. The calcineurin inhibitor Sarah (Nebula) exacerbates Abeta42 phenotypes in a Drosophila model of Alzheimer’s disease. Dis. Model. Mech. 9: 295–306. 10.1242/dmm.018069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim C., Allada R., 2013. Emerging roles for post-transcriptional regulation in circadian clocks. Nat. Neurosci. 16: 1544–1550. 10.1038/nn.3543 [DOI] [PubMed] [Google Scholar]
- Lim C., Chung B. Y., Pitman J. L., McGill J. J., Pradhan S., et al. , 2007a Clockwork orange encodes a transcriptional repressor important for circadian-clock amplitude in Drosophila. Curr. Biol. 17: 1082–1089. 10.1016/j.cub.2007.05.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim C., Lee J., Choi C., Kim J., Doh E., et al. , 2007b Functional role of CREB-binding protein in the circadian clock system of Drosophila melanogaster. Mol. Cell. Biol. 27: 4876–4890. 10.1128/MCB.02155-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim C., Lee J., Choi C., Kilman V. L., Kim J., et al. , 2011. The novel gene twenty-four defines a critical translational step in the Drosophila clock. Nature 470: 399–403. 10.1038/nature09728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J. M., Kilman V. L., Keegan K., Paddock B., Emery-Le M., et al. , 2002. A role for casein kinase 2alpha in the Drosophila circadian clock. Nature 420: 816–820. 10.1038/nature01235 [DOI] [PubMed] [Google Scholar]
- Liu F., Virshup D. M., Nairn A. C., Greengard P., 2002. Mechanism of regulation of casein kinase I activity by group I metabotropic glutamate receptors. J. Biol. Chem. 277: 45393–45399. 10.1074/jbc.M204499200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S., Lamaze A., Liu Q. L., Tabuchi M., Yang Y., et al. , 2014. WIDE AWAKE mediates the circadian timing of sleep onset. Neuron 82: 151–166. 10.1016/j.neuron.2014.01.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinek S., Inonog S., Manoukian A. S., Young M. W., 2001. A role for the segment polarity gene shaggy/GSK-3 in the Drosophila circadian clock. Cell 105: 769–779. 10.1016/S0092-8674(01)00383-X [DOI] [PubMed] [Google Scholar]
- Matsumoto A., Ukai-Tadenuma M., Yamada R. G., Houl J., Uno K. D., et al. , 2007. A functional genomics strategy reveals clockwork orange as a transcriptional regulator in the Drosophila circadian clock. Genes Dev. 21: 1687–1700. 10.1101/gad.1552207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minami T., 2014. Calcineurin-NFAT activation and DSCR-1 auto-inhibitory loop: how is homoeostasis regulated? J. Biochem. 155: 217–226. 10.1093/jb/mvu006 [DOI] [PubMed] [Google Scholar]
- Nakai Y., Horiuchi J., Tsuda M., Takeo S., Akahori S., et al. , 2011. Calcineurin and its regulator sra/DSCR1 are essential for sleep in Drosophila. J. Neurosci. 31: 12759–12766. 10.1523/JNEUROSCI.1337-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawathean P., Rosbash M., 2004. The doubletime and CKII kinases collaborate to potentiate Drosophila PER transcriptional repressor activity. Mol. Cell 13: 213–223. 10.1016/S1097-2765(03)00503-3 [DOI] [PubMed] [Google Scholar]
- Price J. L., Blau J., Rothenfluh A., Abodeely M., Kloss B., et al. , 1998. double-time is a novel Drosophila clock gene that regulates PERIOD protein accumulation. Cell 94: 83–95. 10.1016/S0092-8674(00)81224-6 [DOI] [PubMed] [Google Scholar]
- Renn S. C. P., Park J. H., Rosbash M., Hall J. C., Taghert P. H., 1999. A pdf neuropeptide gene mutation and ablation of PDF neurons each cause severe abnormalities of behavioral circadian rhythms in Drosophila. Cell 99: 791–802. 10.1016/S0092-8674(00)81676-1 [DOI] [PubMed] [Google Scholar]
- Richier B., Michard-Vanhee C., Lamouroux A., Papin C., Rouyer F., 2008. The clockwork orange Drosophila protein functions as both an activator and a repressor of clock gene expression. J. Biol. Rhythms 23: 103–116. 10.1177/0748730407313817 [DOI] [PubMed] [Google Scholar]
- Rothermel B., Vega R. B., Yang J., Wu H., Bassel-Duby R., et al. , 2000. A protein encoded within the Down syndrome critical region is enriched in striated muscles and inhibits calcineurin signaling. J. Biol. Chem. 275: 8719–8725. 10.1074/jbc.275.12.8719 [DOI] [PubMed] [Google Scholar]
- Rusnak F., Mertz P., 2000. Calcineurin: form and function. Physiol. Rev. 80: 1483–1521. 10.1152/physrev.2000.80.4.1483 [DOI] [PubMed] [Google Scholar]
- Sachan N., Dey A., Rotter D., Grinsfelder D. B., Battiprolu P. K., et al. , 2011. Sustained hemodynamic stress disrupts normal circadian rhythms in calcineurin-dependent signaling and protein phosphorylation in the heart. Circ. Res. 108: 437–445. 10.1161/CIRCRESAHA.110.235309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai T., Aigaki T., 2010. The Drosophila calcineurin regulator, Sarah, is involved in male courtship. Neuroreport 21: 985–988. 10.1097/WNR.0b013e32833eaade [DOI] [PubMed] [Google Scholar]
- Sathyanarayanan S., Zheng X., Xiao R., Sehgal A., 2004. Posttranslational regulation of Drosophila PERIOD protein by protein phosphatase 2A. Cell 116: 603–615. 10.1016/S0092-8674(04)00128-X [DOI] [PubMed] [Google Scholar]
- Seluzicki A., Flourakis M., Kula-Eversole E., Zhang L., Kilman V., et al. , 2014. Dual PDF signaling pathways reset clocks via TIMELESS and acutely excite target neurons to control circadian behavior. PLoS Biol. 12: e1001810 10.1371/journal.pbio.1001810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw J. L., Chang K. T., 2013. Nebula/DSCR1 upregulation delays neurodegeneration and protects against APP-induced axonal transport defects by restoring calcineurin and GSK-3beta signaling. PLoS Genet. 9: e1003792 10.1371/journal.pgen.1003792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi M., Yue Z. F., Kuryatov A., Lindstrom J. M., Sehgal A., 2014. Identification of Redeye, a new sleep-regulating protein whose expression is modulated by sleep amount. Elife 3: e01473 10.7554/eLife.01473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoleru D., Peng Y., Agosto J., Rosbash M., 2004. Coupled oscillators control morning and evening locomotor behaviour of Drosophila. Nature 431: 862–868. 10.1038/nature02926 [DOI] [PubMed] [Google Scholar]
- Storch K. F., Lipan O., Leykin I., Viswanathan N., Davis F. C., et al. , 2002. Extensive and divergent circadian gene expression in liver and heart. Nature 417: 78–83. 10.1038/nature744 [DOI] [PubMed] [Google Scholar]
- Suh J., Jackson F. R., 2007. Drosophila Ebony activity is required in glia for the circadian regulation of locomotor activity. Neuron 55: 435–447. 10.1016/j.neuron.2007.06.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi J. S., 2017. Transcriptional architecture of the mammalian circadian clock. Nat. Rev. Genet. 18: 164–179. 10.1038/nrg.2016.150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeo S., Tsuda M., Akahori S., Matsuo T., Aigaki T., 2006. The calcineurin regulator sra plays an essential role in female meiosis in Drosophila. Curr. Biol. 16: 1435–1440. 10.1016/j.cub.2006.05.058 [DOI] [PubMed] [Google Scholar]
- Takeo S., Hawley R. S., Aigaki T., 2010. Calcineurin and its regulation by Sra/RCAN is required for completion of meiosis in Drosophila. Dev. Biol. 344: 957–967. 10.1016/j.ydbio.2010.06.011 [DOI] [PubMed] [Google Scholar]
- Takeo S., Swanson S. K., Nandanan K., Nakai Y., Aigaki T., et al. , 2012. Shaggy/glycogen synthase kinase 3beta and phosphorylation of Sarah/regulator of calcineurin are essential for completion of Drosophila female meiosis. Proc. Natl. Acad. Sci. USA 109: 6382–6389. 10.1073/pnas.1120367109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tataroglu O., Emery P., 2015. The molecular ticks of the Drosophila circadian clock. Curr. Opin. Insect Sci. 7: 51–57. 10.1016/j.cois.2015.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita J., Mitsuyoshi M., Ueno T., Aso Y., Tanimoto H., et al. , 2011. Pan-neuronal knockdown of calcineurin reduces sleep in the fruit fly, Drosophila melanogaster. J. Neurosci. 31: 13137–13146. 10.1523/JNEUROSCI.5860-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Dorpel M. A., van den Meiracker A. H., Lameris T. W., Boomsma F., Levi M., et al. , 1996. Cyclosporin A impairs the nocturnal blood pressure fall in renal transplant recipients. Hypertension 28: 304–307. 10.1161/01.HYP.28.2.304 [DOI] [PubMed] [Google Scholar]
- Vega R. B., Rothermel B. A., Weinheimer C. J., Kovacs A., Naseem R. H., et al. , 2003. Dual roles of modulatory calcineurin-interacting protein 1 in cardiac hypertrophy. Proc. Natl. Acad. Sci. USA 100: 669–674. 10.1073/pnas.0237225100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Zhu J. Z., Chang K. T., Min K. T., 2012. DSCR1 interacts with FMRP and is required for spine morphogenesis and local protein synthesis. EMBO J. 31: 3655–3666. 10.1038/emboj.2012.190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Rothermel B., Vega R. B., Frey N., McKinsey T. A., et al. , 2000. Independent signals control expression of the calcineurin inhibitory proteins MCIP1 and MCIP2 in striated muscles. Circ. Res. 87: E61–E68. 10.1161/01.RES.87.12.e61 [DOI] [PubMed] [Google Scholar]
- Zhao J., Kilman V. L., Keegan K. P., Peng Y., Emery P., et al. , 2003. Drosophila clock can generate ectopic circadian clocks. Cell 113: 755–766. 10.1016/S0092-8674(03)00400-8 [DOI] [PubMed] [Google Scholar]
- Zheng X., Sehgal A., 2012. Speed control: cogs and gears that drive the circadian clock. Trends Neurosci. 35: 574–585. 10.1016/j.tins.2012.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors state that all data necessary for confirming the conclusions of the article are represented fully within the article. Supplemental material available at Figshare: https://doi.org/10.25386/genetics.6216251.