Summary
Background
Haemostatic balance represented by low protein C (PC) and elevated factor VIII (FVIII) has been inconsistently associated with stroke and coronary heart disease (CHD) risk.
Objectives
To assess whether an elevated FVIII and a low PC would increase cardiovascular risk more than either individually.
Patients/Methods
REGARDS recruited 30,239 black and white US participants aged ≥45 between 2003–07. FVIII and PC were measured in a case-cohort sample of 646 stroke, 654 CHD, and a 1,104-person random sample with follow-up for ~4.5 years. Hazard ratios (HR) were estimated using Cox models adjusted for demographic and cardiovascular risk factors.
Results
Elevated FVIII (per SD increase) was associated with increased risk of both stroke (HR 1.26; 95% CI 1.08, 1.46) and CHD (HR 1.52; 95% CI 1.29, 1.79) while there was no association of PC per SD lower. For PC, there was a trend towards increased cardiovascular disease risk in the lowest values (bottom 5%). For stroke, there was no interaction between FVIII and low PC (pinteraction = 0.55). For CHD, the adjusted HR of FVIII per SD increase was significantly greater with PC in the bottom 5% (HR 3.59; 95% CI 1.39, 8.29) than PC in the upper 95% (HR 1.45; 95% CI 1.23, 1.71; pinteraction = 0.07).
Conclusions
Higher FVIII was associated with both CHD and stroke risk and the risk potentiated by low PC for CHD. Findings demonstrate that risks for cardiovascular diseases conferred by adverse levels of haemostasis biomarkers may be augmented by levels of other biomarkers.
Keywords: Coronary Disease, Epidemiology, Factor VIII, Protein C, Stroke
Introduction
The final common pathophysiology for most stroke and coronary heart disease (CHD) events is thrombotic vessel occlusion resulting in tissue ischemia and ultimately necrosis(1). Haemostasis in general and coagulation biomarkers specifically have been studied as potential cardiovascular disease (CVD) risk factors, with more studies on CHD than stroke and in Caucasian-origin populations than other populations(2–7). While underlying processes such as atherosclerosis(1) and atrial fibrillation(8) contribute substantially to the risk of stroke and CHD, drugs which inhibit coagulation such as vitamin K antagonists(9) and antiplatelet agents(10) reduce CVD risk, providing a rationale for assessing biomarkers reflective of haemostasis as risk factors for CVD.
We selected two coagulation proteins, factor VIII (FVIII) and protein C (PC) to assess the singular and joint effects on stroke and CHD risk based on a pre-specified hypothesis of a synergistic relationship between higher FVIII and lower PC. FVIII (a procoagulant protein) and PC (an anticoagulant protein) have been associated with venous thrombosis risk and arterial risk in many studies(3, 4, 11–13). FVIII is a critical coagulation protein which serves as a cofactor for factor IX eventually activating thrombin which cleaves fibrinogen to form a fibrin clot. PC is activated by thrombin and cleaves activated factors V and FVIII serving as a check on coagulation(14). Activated PC is especially concentrated near the endothelial surface, the site of atherosclerotic plaques(15). In essence, low PC concentrations should potentiate the pro-coagulant effects of high FVIII concentrations by reducing the inactivation of active FVIII. Thus mechanistically, an elevated FVIII and a low PC should interact synergistically to increase thrombotic potential. To our knowledge, the combined impact of a low PC and elevated FVIII has not been studied in relation to CVD risk.
To address this gap in knowledge, we assessed the association of PC and FVIII individually and together with risk of future stroke and CHD risk in the diverse bi-racial population of the REasons for Geographic and Racial Differences in Stroke (REGARDS) cohort(16, 17). We hypothesized that elevated concentrations of FVIII and low concentrations of PC would be associated with an increased risk of CVD and that a low PC would potentiate the adverse effect of an elevated FVIII.
Methods
Cohort
From 2003–2007, REGARDS recruited 30.239 individuals from the contiguous United States (U.S.). The goal for the cohort was to be 50% female, 50% African American (black), and 50% from the southeast stroke belt of the U.S. (North Carolina, South Carolina, Georgia, Alabama, Mississippi, Tennessee, Arkansas, and Louisiana), including 20% from the coastal plains of Virginia, North Carolina, South Carolina, and Georgia (the ‘buckle’ of the stroke belt). The final cohort was 55% female, 41% black, and 56% lived in the southeast. Exclusion criteria were medical conditions preventing long-term participation, active cancer or treatment for cancer in the past year, resident in or on the waiting list for a nursing home, inability to communicate in English, or self-identified race other than ‘black’ or ‘white’. Potential participants were first contacted by telephone and after verbal informed consent took part in a computer assisted telephone interview which gathered basic demographic and risk factor data. Participants then underwent an in-home visit where anthropomorphic data, medication inventory, fasting phlebotomy, and written informed consent were performed (Examination Management Systems Incorporated, Irving, Texas) with detailed methods previously published(16). The study was approved by the institutional review boards of all participating institutions.
The association of FVIII and PC with stroke and CHD were assessed in a stratified case-cohort study using methods proposed by Prentice(18, 19) with a detailed description of the study design in REGARDS previously published(20). PC and FVIII were measured in stroke and CHD cases and a 1,104-person stratified cohort random sample (CRS).
Phlebotomy
Blood samples were obtained in the fasting state the morning of the in-home visit(21). Samples were centrifuged locally and shipped overnight on gel-packs to the study laboratory where they were recentrifuged and stored at −80°C. C-reactive protein (CRP) was measured using a high-sensitivity particle enhanced immunonephelometric assay on the BNII nephelometer, (High Sensitivity CRP, Dade Behring Incorporated, Deerfield, IL). Total cholesterol, high-density lipoprotein (HDL) cholesterol and triglycerides were measured by colorimetric reflectance spectrophotometry using the Ortho Vitros Clinical Chemistry System 950IRC instrument (Johnson & Johnson Clinical Diagnostics, Rochester, NY), with low density lipoprotein (LDL) cholesterol calculated by the Friedewald equation for subjects with triglycerides <400 mg/dl. FVIII antigen (Enzyme Research Laboratories, Sound Bend, Indiana, U.S.) and PC antigen (Diagnostica Stago, Parsippany NJ, U.S.) were measured using an enzyme linked immunosorbent assay and reported as the percent (%) of normal pooled plasma. Interassay coefficients of variation varied from 4% – 8%.
Definitions
Prevalent stroke and peripheral arterial disease was based on participant self-report and prevalent CHD was based on participant self-report or electrocardiogram evidence of prior myocardial infarction. Prevalent CVD was defined as a stroke, peripheral arterial disease, or CHD at enrolment. Atrial fibrillation was defined by electrocardiogram evidence or participant self-report and left ventricular hypertrophy was defined from the baseline study electrocardiogram. Diabetes mellitus was defined as a fasting glucose ≥126mg/dL (or a nonfasting glucose ≥200mg/dL among those failing to fast) or self-reported diabetes with use of medications to control diabetes mellitus. Systolic blood pressure was the average of two seated measures after a 5-minute rest and use of antihypertensive and cholesterol-lowering medications were defined by participant self-report.
Outcomes
Detailed methods for stroke(22) and CHD(23) event ascertainment have been previously published. Participants or proxies were contacted every 6 months by telephone to ascertain stroke or stroke symptoms and potential CHD hospitalizations. Medical records, including neuroimaging and other diagnostic reports were retrieved and centrally reviewed. For stroke, adjudication was based on the World Health Organization’s definition of stroke and/or imaging consistent with stroke. Follow-up for stroke for this analysis was through September 1, 2011. For CHD, adjudication followed published guidelines for major epidemiology studies for nonfatal myocardial infarction (MI) and acute CHD death(24). MI diagnoses were based on a rising and/or falling pattern of cardiac biomarkers to a level at least twice the upper limit of normal for the biomarker; clinical signs or symptoms consistent with ischemia; and electrocardiogram findings. Acute CHD death was ascertained through interviews with proxies or next of kin about circumstances immediately prior to death, autopsy reports, hospital records, death certificates and medical history obtained at baseline. Follow-up for CHD for this analysis was complete through December 31, 2009. Individuals were censored at death, date of last known follow-up, or the last follow-up for stroke (September 1, 2011) or CHD (December 31, 2009).
Statistical Methods
Differences between cardiovascular disease cases and the CRS were assessed using the Rao-Scott χ2 statistic and means of continuous variables using weighted analysis of variance accounting for sample weighting. Correlates of PC and FVIII were assessed using weighted linear regression adjusting for age, sex (male, female), race (black, white), and region (southeast stroke belt, buckle, and the rest of the U.S.).
Cox proportional hazard models were used to calculate the hazard ratios (HR) with robust sandwich estimators to calculate the 95% confidence intervals (CI) accounting for sample weighting. The outcome was either CHD or stroke. Models for stroke excluded participants with baseline stroke, and models for CHD excluded participants with baseline CHD. As warfarin affects PC levels and CVD risk, individuals on warfarin at baseline were excluded from analysis (36 individuals in the CRS, 47 incident stroke cases, and 31 incident CHD cases). PC and FVIII were modelled in several ways to assess the association with CVD. In continuous analyses, biomarkers were modelled per standard deviation (SD) increment. We also assessed the extremes of the distribution versus the rest of the distribution (<5%, <10%, >90%, >95%) and modelled the biomarkers in quintiles. Based on these results, associations were assessed for interactions between FVIII and PC using cross-product terms.
Two models were fitted for stroke and CHD. Model 1 adjusted for demographics and model 2 additionally included traditional CVD risk factors. For stroke, model 1 adjusted for age (continuous), sex (male, female), race (black, white), region (stroke belt, stroke buckle, and rest of U.S.), and a previously identified age by race interaction term(22). Model 2 for stroke additionally adjusted for systolic blood pressure (continuous), diabetes (yes, no), current smoking (yes, no), baseline cardiovascular disease (yes, no), baseline atrial fibrillation (yes, no), baseline left ventricular hypertrophy (yes, no), and baseline use of antihypertensive medications (yes, no). For CHD, model 1 adjusted for age (continuous), sex (male, female), race (black, white), and region (stroke belt, stroke buckle, rest of U.S.). Model 2 for CHD additionally adjusted for systolic blood pressure (continuous), taking blood pressure medications (yes, no), current smoking (yes, no) total cholesterol (continuous), high density lipoprotein cholesterol (continuous), and taking cholesterol lowering medications (yes, no). Individuals with missing data were dropped from analysis. All analyses were done using SAS 9.3 (Cary, North Carolina, U.S.).
Results
Over a median of 4.5 years (interquartile range 3.1 – 5.3 years), there were 654 incident CHD events and over a median 5.8 years (interquartile range 4.1 – 7.0 years) there were 646 incident stroke events. Table 1 presents the baseline characteristics of the CRS, CHD cases, and stroke cases. Table 2 presents percentiles and the standard deviations of the distribution of FVIII and PC in the CRS. In general, individuals with stroke and CHD events were older, male, and had a higher baseline prevalence of CVD risk factors than those who did not develop CHD or stroke. Mean FVIII was higher in stroke and CHD cases than the CRS (Table 1, p <0.001) with mean PC levels borderline lower in stroke and CHD cases than the CRS (Table 1, p ~0.05). Adjusting for age, sex, race, and region; FVIII was higher with increasing age, in blacks, with current smoking, with baseline CVD, and with baseline atrial fibrillation and lower in men than women (Table 3). Adjusting for age, sex, race, and region; PC was lower with increasing age, black race, with baseline CVD and was higher in men, with increasing systolic blood pressure, and with increasing cholesterol measures (Table 3).
Table 1.
Cardiovascular Risk Factors and Biomarkers in the Cohort Random Sample and Cardiovascular Diseases Cases
| CRS* N = 1,104 |
Incident Stroke N = 646 |
Pstroke vs CRS | Incident CHD N = 654 |
PCHD vs. CRS | |
|---|---|---|---|---|---|
| Age (mean, SD) | 65 (7) | 70 (9) | <0.001 | 69 (9) | <0.001 |
| Male (N, %) | 530 (45%) | 308 (51%) | 0.008 | 387 (62%) | <0.001 |
| Black (N, %) | 541 (42%) | 261 (44%) | 0.36 | 281 (45%) | 0.43 |
| Region (N, %) | - | - | - | - | - |
| Belt | 369 (35%) | 210 (35%) | - | 250 (40%) | - |
| Buckle | 189 (18%) | 119 (20%) | 0.72 | 102 (16%) | 0.14 |
| Nonbelt | 510 (47%) | 270 (45%) | - | 271 (44%) | - |
| Current Smoking (N, %) | 155 (14%) | 108 (18%) | 0.03 | 131 (21%) | <0.001 |
| Diabetes (N, %) | 226 (22%) | 163 (28%) | 0.007 | 192 (32%) | <0.001 |
| Baseline Cardiovascular Disease (N, %) | 201 (17%) | 167 (28%) | <0.001 | 28 (4%) | <0.001 |
| Baseline CHD (N, %) | 188 (16%) | 162 (27%) | <0.001 | - | - |
| Baseline Stroke (N, %) | 82 (7%) | - | - | 66 (11%) | <0.001 |
| Atrial Fibrillation (N, %) | 68 (8%) | 60 (15%) | 0.06 | 63 (10%) | 0.008 |
| Left Ventricular Hypertrophy (N, %) | 105 (9%) | 88 (15%) | <0.001 | 72 (12%) | 0.02 |
| Body Mass Index kg/m2 (Mean, SD) | 29 (6) | 29 (6) | 0.02 | 29 (6) | 0.36 |
| Systolic Blood Pressure mmHG (Mean, SD) | 127 (15) | 134 (18) | <0.001 | 135 (20) | <0.001 |
| Blood Pressure Medications (N, %) | 551 (52%) | 286 (66%) | <0.001 | 360 (60%) | <0.001 |
| Total Cholesterol mg/dL (Mean, SD) | 190 (37) | 191 (43) | 0.79 | 192 (42) | 0.73 |
| HDL Cholesterol mg/dL (mean, SD) | 52 (16) | 50 (17) | 0.005 | 47 (14) | <0.001 |
| Taking cholesterol medications (n, %) | 339 (35%) | 203 (34%) | 0.90 | 168 (27%) | 0.41 |
| Factor VIII % (mean, SD) | 121 (44) | 132 (49) | <0.001 | 136 (57) | <0.001 |
| Protein C % (mean, SD) | 122 (22) | 120 (23) | 0.05 | 120 (28) | 0.054 |
Weighted percent
Table 2.
Distribution of Biomarkers in the Cohort Random Sample
| Standard Deviation | Percentile | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| 2.5th | 5th | 10th | 20th | 40th | 60th | 80th | 90th | 95th | 97.5th | ||
| Factor VIII (%) | 44 | 61 | 67 | 77 | 86 | 104 | 122 | 152 | 177 | 202 | 158 |
| Protein C (%) | 22 | 82 | 89 | 96 | 105 | 116 | 126 | 138 | 148 | 158 | 223 |
Table 3.
Difference in Mean Factor VIII and Protein C Levels by Cardiovascular Risk Factors in the Cohort Random Sample
| Cardiovascular Risk Factor* | Factor VIII (%) | p | Protein C (%) | p |
|---|---|---|---|---|
| Age (per 10 years) | 9 | <0.001 | −2 | <0.001 |
| Male vs. female | −7 | 0.003 | 13 | <0.001 |
| Black vs. white | 11 | <0.001 | −6 | <0.001 |
| Region | - | - | - | - |
| Belt vs Nonbelt | 0.3 | 0.92 | −4 | 0.01 |
| Buckle vs. Nonbelt | 3 | 0.42 | −2 | 0.32 |
| Current Smoking (yes vs. no) | 7 | 0.05 | 0.2 | 0.90 |
| Diabetes (yes vs. no) | 5 | 0.14 | 0.5 | 0.75 |
| Baseline Cardiovascular Disease (yes vs. no) | 18 | <0.001 | −5 | 0.04 |
| Baseline CHD (yes vs. no) | 19 | <0.001 | −5 | 0.04 |
| Baseline Stroke (yes vs. no) | 20 | 0.04 | −4 | 0.21 |
| Atrial Fibrillation (yes vs. no) | 10 | 0.03 | −3 | 0.11 |
| Left Ventricular Hypertrophy (yes vs. no) | 6 | 0.23 | 3 | 0.20 |
| Body Mass Index (per 5kg/m2) | 2 | 0.06 | 0.6 | 0.16 |
| Systolic Blood Pressure (per 10mmHg) | −0.3 | 0.67 | 1 | 0.008 |
| Blood Pressure Medications (yes vs. no | 9 | 0.002 | 2 | 0.05 |
| Total Cholesterol (20 mg/dL increase) | −0.5 | 0.46 | 4 | <0.001 |
| HDL Cholesterol (per 10 mg/dL increase) | −2 | 0.06 | 2 | <0.001 |
| Taking cholesterol medications (yes vs. no) | 0.5 | 0.85 | 5 | <0.001 |
| Loge CRP (per SD (1.21 unit) increase) | 6 | <0.001 | 2 | 0.001 |
| Factor VIII % (per SD increase) | - | - | −1 | 0.36 |
| Protein C % (per SD increase) | −2 | 0.37 | - | - |
Adjusted for age, sex, race, and region.
Tables 4 and 5 present the associations of FVIII and PC with stroke and CHD. As demographic and risk-factor adjusted models demonstrated similar associations and interpretations, the risk factor-adjusted models are discussed here. FVIII was associated with both stroke and CHD risk (Table 4). Per SD higher, FVIII was associated with a HR of 1.26 (95% CI 1.08, 1.46) for stroke and a HR of 1.51 (95% CI 1.29, 1.76) for CHD with no evidence of a race interaction for stroke or CHD (pinteractions >0.1). When evaluated by the extremes of the distribution (bottom 5%, bottom 10%, top 10%, and top 5% versus the rest of the distribution), there was no association between low levels of factor VIII and stroke risk overall. However, when stratified by race, FVIII <5% versus ≥5% had a lower HR for stroke in blacks (HR 0.32; 95% CI 0.10, 1.02) than whites (HR 2.40, 95% CI 0.98, 5.26), pinteraction = 0.007, but the confidence intervals were wide. FVIII >95% were associated with increased stroke risk in both blacks and whites (HR 1.89; 95% CI 1.04, 3.41) with no significant difference by race (pinteraction = 0.99). In the quintile analysis (Q1 being the reference), stroke risk was elevated for Q2–5 of FVIII, but the point estimates were similar for Q2–Q5 (1.51, 1.29, 1.72, 1.97). For CHD, higher FVIII levels were consistently associated with increased CHD risk, with associations similar between blacks and whites. Individuals in the top 5% of FVIII levels versus the rest had a HR of 3.06 (95% CI 1.71, 5.47) for CHD. In the quintile analysis (with Q1 as the reference), risk increased monotonically with increasing quintiles (Table 4) with the HR for the 5th quintile being 2.86 (95% CI 1.65, 4.94). Results were similar for blacks and whites (pinteraction=0.77).
Table 4.
Association of Factor VIII with Risk of Stroke and Coronary Heart Disease*
| Factor VIII Levels | All | Blacks | Whites | Pinteraction |
|---|---|---|---|---|
| Stroke: Demographic-Adjusted Model | ||||
| Per SD higher (44%) | 1.29 (1.13, 1.47) | 1.29 (1.08, 1.54) | 1.29 (1.06, 1.56) | 0.99 |
| <5% versus higher | 1.00 (0.52, 1.90) | 0.44 (0.15, 1.31) | 1.73 (0.74, 4.08) | 0.05 |
| <10% versus higher | 1.20 (0.77, 1.89) | 0.59 (0.28, 1.22) | 1.02 (0.57, 1.83) | 0.25 |
| >90% versus lower | 1.30 (0.89, 1.92) | 1.32 (0.79, 2.20) | 1.28 (0.72, 2.30) | 0.95 |
| >95% versus lower | 1.81 (1.05, 3.11) | 1.82 (0.91, 3.65) | 1.80 (0.77, 4.17) | 0.98 |
| Quintiles | - | - | - | - |
| 1 | Reference | Reference | Reference | - |
| 2 | 1.14 (0.75, 1.75) | 2.38 (1.24, 4.56) | 0.73 (0.42, 1.26) | - |
| 3 | 1.08 (0.71, 1.65) | 1.01 (0.51, 2.02) | 1.07 (0.62, 1.85) | 0.03 |
| 4 | 1.44 (0.96, 2.15) | 1.92 (1.04, 3.54) | 1.18 (0.68, 2.05) | - |
| 5 | 1.89 (1.26, 2.84) | 2.43 (1.34, 4.41) | 1.62 (0.92, 2.85) | - |
| Stroke: Risk Factor-Adjusted Model | - | |||
| Per SD higher (44%) | 1.26 (1.08, 1.46) | 1.26 (1.02, 1.55) | 1.26 (1.02, 1.56) | 0.99 |
| <5% versus higher | 0.99 (0.50, 1.93) | 0.32 (0.10, 1.02) | 2.40 (0.98, 5.26) | 0.007 |
| <10% versus higher | 0.62 (0.33, 1.17) | 0.43 (0.19, 0.99) | 0.77 (0.31, 1.90) | 0.35 |
| >90% versus lower | 1.26 (0.82, 1.93) | 1.30 (0.73, 2.33) | 1.21 (0.65, 2.26) | 0.87 |
| >95% versus lower | 1.89(1.04, 3.41) | 1.89 (0.78, 4.62) | 1.90 (0.78, 4.62) | 0.99 |
| Quintiles | - | - | - | - |
| 1 | Reference | Reference | Reference | - |
| 2 | 1.51 (0.91, 2.49) | 2.74 (1.34, 5.62) | 1.03 (0.53, 2.02) | - |
| 3 | 1.29 (0.78, 2.56) | 1.15 (0.55, 2.43) | 1.32 (0.66, 2.62) | 0.15 |
| 4 | 1.72 (1.05, 2.83) | 2.21 (1.11, 4.43) | 1.43 (0.73, 2.80) | - |
| 5 | 1.97 (1.20, 3.22) | 2.47 (1.28, 4.78) | 1.68 (0.84, 3.39) | - |
| CHD: Demographic-Adjusted Model | - | |||
| Per SD higher (44%) | 1.51 (1.29, 1.76) | 1.70 (1.40, 2.07) | 1.41 (1.16, 1.72) | 0.18 |
| <5% versus higher | 0.83 (0.44, 1.59) | 1.22 (0.50, 2.80) | 0.52 (0.20, 1.36) | 0.20 |
| <10% versus higher | 0.82 (0.53, 1.28) | 0.84 (0.44, 1.61) | 0.80 (0.44, 1.48) | 0.92 |
| >90% versus lower | 2.11 (1.40, 3.17) | 2.23 (1.37, 3.64) | 1.95 (1.00, 3.83) | 0.75 |
| >95% versus lower | 2.63 (1.52, 4.56) | 3.05 (1.56, 5.95) | 2.03 (0.78, 5.25) | 0.49 |
| Quintiles | - | - | - | - |
| 1 | Reference | Reference | Reference | - |
| 2 | 1.10 (0.70, 1.72) | 1.62 (0.83, 3.15) | 0.90 (0.50, 1.61) | - |
| 3 | 1.40 (0.89 2.19) | 1.87 (0.98, 2.54) | 1.16 (0.63, 2.15) | 0.51 |
| 4 | 2.06 (1.35, 3.16) | 2.08 (1.15, 3.78) | 2.08 (1.15, 3.80) | - |
| 5 | 2.79 (1.80, 4.34) | 2.99 (1.67, 5.37) | 2.76 (1.44, 5.31) | - |
| CHD: Risk Factor-Adjusted Model | - | |||
| Per SD higher (44%) | 1.52 (1.29, 1.79) | 1.71 (1.41, 2.09) | 1.41 (1.14, 1.74) | 0.18 |
| <5% versus higher | 0.93 (0.47, 1.84) | 1.11 (0.42, 2.90) | 0.73 (0.27, 1.93) | 0.55 |
| <10% versus higher | 0.62 (0.33, 1.17) | 0.74 (0.34, 1.59) | 0.54 (0.20, 1.42) | 0.62 |
| >90% versus lower | 1.98 (1.23, 3.19) | 2.30 (1.33, 3.98) | 1.64 (0.75, 3.62) | 0.49 |
| >95% versus lower | 3.06 (1.71, 5.47) | 3.31 (1.59, 6.90) | 2.64 (1.03, 6.77) | 0.71 |
| Quintiles | - | - | - | - |
| 1 | Reference | Reference | Reference | - |
| 2 | 1.37 (0.81, 2.32) | 1.63 (0.77, 3.44) | 1.24 (0.61, 2.53) | - |
| 3 | 1.59 (0.92, 2.77) | 2.00 (0.96, 4.15) | 1.39 (0.64, 3.01) | 0.77 |
| 4 | 2.53 (1.50, 4.26) | 2.26 (1.14, 4.49) | 2.76 (1.33, 5.74) | - |
| 5 | 2.86 (1.65, 4.94) | 3.03 (1.56, 5.89) | 2.74 (1.20, 6.24) | - |
Stroke Demographic Model adjusted for Age, gender, race, region, and an age*race interaction term.
Stroke Risk Factor Model adjusted for variables in Demographic Model and systolic blood pressure, diabetes, current smoking, cardiovascular disease, atrial fibrillation, left ventricular hypertrophy, hypertension medications.
CHD Demographic Model adjusted for age, gender, race, and region.
CHD Risk Factor Model adjusted for variables in Demographic Model and systolic blood pressure, taking blood pressure medications, diabetes, current smoking, total cholesterol, high density lipoprotein cholesterol, and taking cholesterol medications.
Percentiles and quintiles as outlined in Table 2.
Table 5.
Association of Protein C with Stroke and Coronary Heart Disease*
| Protein C Levels | All | Blacks | Whites | Pinteraction |
|---|---|---|---|---|
| Stroke: Demographic Model | - | |||
| Per SD lower (22%) | 0.96 (0.84, 1.10) | 1.02 (0.81, 1.28) | 0.93 (0.79, 1.10) | 0.53 |
| <5% versus higher | 1.43 (0.83, 2.46) | 1.76 (0.86, 3.63) | 1.14 (0.51, 2.56) | 0.43 |
| <10% versus higher | 1.08 (0.73, 1.61) | 1.37 (0.80, 2.33) | 0.87 (0.49, 1.54) | 0.25 |
| >90% versus lower | 1.25 (0.81, 1.92) | 1.19 (0.60, 2.34) | 1.29 (0.74, 2.25) | 0.85 |
| >95% versus lower | 1.19 (0.65, 2.17) | 1.57 (0.52, 4.72) | 1.07 (0.53, 2.18) | 0.57 |
| Quintiles | - | - | - | - |
| 1 | 0.88 (0.59, 1.30) | 1.01 (0.57, 1.77) | 0.77 (0.45, 1.33) | - |
| 2 | 0.93 (0.63, 1.37) | 1.10 (0.61, 1.97) | 0.83 (0.50, 1.38) | 0.51 |
| 3 | 0.93 (0.63, 1.36) | 0.78 (0.44, 1.39) | 1.08 (0.64, 1.83) | - |
| 4 | 0.88 (0.59, 1.31) | 0.95 (0.51, 1.76) | 0.83 (0.49, 1.42) | - |
| 5 | Reference | Reference | Reference | - |
| Stroke: Risk Factor Model | - | |||
| Per SD lower (22%) | 1.04 (0.88, 1.23) | 0.99 (0.77, 1.26) | 1.09 (0.87, 1.35) | 0.57 |
| <5% versus higher | 1.20 (0.61, 2.35) | 1.49 (0.63, 3.52) | 0.88 (0.30, 2.60) | 0.46 |
| <10% versus higher | 1.01 (0.62, 1.65) | 1.47 (0.78, 2.80) | 0.62 (0.29, 1.33) | 0.09 |
| >90% versus lower | 0.88 (0.48, 1.60) | 1.22 (0.58, 2.59) | 0.73 (0.32, 1.67) | 0.36 |
| >95% versus lower | 0.47 (0.18, 1.25) | 1.08 (0.32, 3.58) | 0.36 (0.11, 1.19) | 0.21 |
| Quintiles | - | - | - | - |
| 1 | 0.97 (0.58, 1.63) | 1.00 (0.51, 1.95) | 0.87 (0.41, 1.85) | - |
| 2 | 1.35 (0.81, 2.24) | 1.27 (0.64, 2.54) | 1.38 (0.67 (2.78) | 0.24 |
| 3 | 1.28 (0.77, 2.13) | 0.85 (0.43, 1.70) | 1.97 (0.96, 4.01) | - |
| 4 | 0.98 (0.59, 1.62) | 1.07 (0.52, 2.19) | 0.90 (0.45, 1.81) | - |
| 5 | Reference | Reference | Reference | - |
| CHD: Demographic Model | - | |||
| Per SD lower (22%) | 0.91 (0.77, 1.07) | 1.05 (0.84, 1.30) | 0.83 (0.67, 1.03) | 0.13 |
| <5% versus higher | 1.53 (0.87, 2.67) | 1.61 (0.87, 3.01) | 1.42 (0.53, 3.81) | 0.83 |
| <10% versus higher | 1.21 (0.81, 1.82) | 1.62 (1.01, 2.63) | 0.86 (0.45, 1.65) | 0.12 |
| >90% versus lower | 1.38 (0.87, 2.19) | 1.32 (0.67, 2.58) | 1.43 (0.77, 2.64) | 0.86 |
| >95% versus lower | 1.78 (0.97, 3.28) | 2.11 (0.74, 6.02) | 1.65 (0.79, 3.46) | 0.71 |
| Quintiles | - | - | - | - |
| 1 | 0.81 (0.53, 1.23) | 1.16 (0.66, 2.05) | 0.56 (0.31, 1.02) | - |
| 2 | 0.80 (0.52, 1.22) | 0.93 (0.50, 1.73) | 0.70 (0.40, 1.25) | 0.02 |
| 3 | 0.95 (0.63, 1.44) | 0.74 (0.41, 1.35) | 1.25 (0.70, 2.25) | - |
| 4 | 0.79 (0.52, 1.21) | 1.16 (0.62, 2.16) | 0.63 (0.36, 1.10) | - |
| 5 | Reference | Reference | Reference | - |
| CHD: Risk Factor Model | - | |||
| Per SD lower (22%) | 0.99 (0.81, 1.22) | 1.06 (0.84, 1.34) | 0.95 (0.72, 1.25) | 0.52 |
| <5% versus higher | 1.74 (0.94, 3.20) | 1.65 (0.78, 3.46) | 1.87 (0.69, 5.01) | 0.84 |
| <10% versus higher | 1.41 (0.89, 2.23) | 1.78 (1.02, 3.10) | 1.08 (0.52, 2.21) | 0.27 |
| >90% versus lower | 0.95 (0.49, 1.84) | 1.12 (0.52, 2.40) | 0.87 (0.34, 2.18) | 0.67 |
| >95% versus lower | 0.96 (0.38, 2.45) | 1.55 (0.42, 5.67) | 0.82 (0.26, 2.56) | 0.45 |
| Quintiles | - | - | - | - |
| 1 | 0.93 (0.55, 1.57) | 1.07 (0.56, 2.03) | 0.74 (0.35, 1.57) | - |
| 2 | 0.99 (0.60, 1.64) | 1.02 (0.51, 2.04) | 0.94 (0.47, 1.88) | - |
| 3 | 1.04 (0.63, 1.73) | 0.62 (0.31, 1.24) | 1.71 (0.85, 3.43) | 0.03 |
| 4 | 0.83 (0.49, 1.41) | 1.02 (0.52, 2.00) | 0.72 (0.34, 1.52) | - |
| 5 | Reference | Reference | Reference | - |
Stroke Demographic Model adjusted for Age, gender, race, region, and an age*race interaction term.
Stroke Risk Factor Model adjusted for variables in Demographic Model and systolic blood pressure, diabetes, current smoking, cardiovascular disease, atrial fibrillation, left ventricular hypertrophy, hypertension medications.
CHD Demographic Model adjusted for age, gender, race, and region.
CHD Risk Factor Model adjusted for variables in Demographic Model and systolic blood pressure, taking blood pressure medications, diabetes, current smoking, total cholesterol, high density lipoprotein cholesterol, and taking cholesterol medications.
Percentiles and quintiles as outlined in Table 2.
For PC, there were no strong associations with stroke or CHD, either in the entire cohort, or stratifying by race in either the demographic model or the CVD risk factor adjusted model. For the risk-factor adjusted model, each SD lower PC was associated with a HR of 1.04 (95% CI 0.88, 1.23) for stroke and a HR of 0.91 (95% CI 0.77, 1.07) for CHD with no race interactions (pinteractions>0.10). For CHD, PC levels in the bottom 5% were associated with a non-significantly increased hazard of CHD (HR 1.74; 95% CI 0.94, 3.20) with no race interaction (pinteraction=0.84). In the quintile analysis, there was a significant race interaction for the association of PC with CHD (pinteraction=0.03), however on reviewing the point estimates for each quintile there was no apparent pattern to the differential association (Table 5).
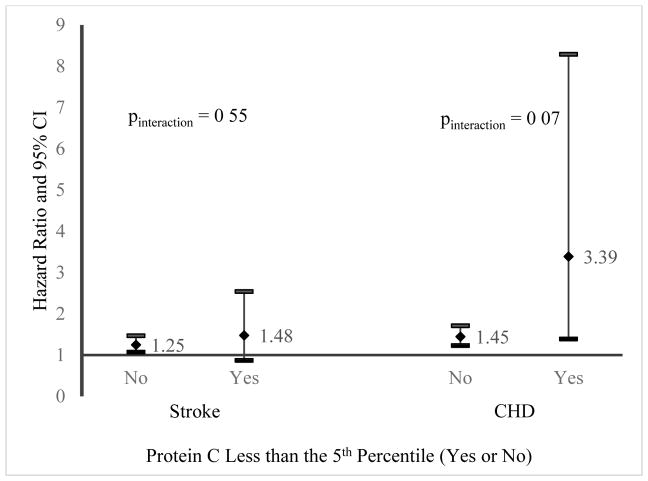
As there was a trend towards increased CHD and stroke risk with PC below the 5th percentile, we assessed the impact of PC levels above and below the 5th percentile on the association of FVIII with stroke and CHD by stratifying by PC level (figure 1). For stroke, there was no difference between the association of FVIII (per SD increment) by PC in the bottom 5th percentile (HR 1.49; 95% CI 0.87, 2.54) versus those in the top 95th percentile (HR 1.25; 95% CI 1.7, 1.47), pinteraction=0.55. However, the association of FVIII (per SD higher) with CHD was stronger in those in the bottom 5th percentile of PC (HR 3.59; 95% CI 1.39, 8.29) than those in the upper 95th percentile (HR 1.45; 95% CI 1.23, 1.71), pinteraction=0.07.
Figure 1. Cardiovascular Risk Factor Adjusted Association of Factor VIII (per Standard Deviation Increment) with Stroke and Coronary Heart Disease by Protein C Levels.
Stroke Risk Factor Model adjusted for age, sex, race, age by race interaction term, systolic blood pressure, diabetes, current smoking, cardiovascular disease, atrial fibrillation, left ventricular hypertrophy, and hypertension medications.
CHD Risk Factor Model adjusted for age, sex, race, systolic blood pressure, taking blood pressure medications, diabetes, current smoking, total cholesterol, high density lipoprotein cholesterol, and taking cholesterol medications.
We performed a secondary analysis adding C-reactive protein to the CVD risk factor models for stroke and CHD for FVIII and PC. C-reactive protein did not meaningfully change any of the associations of FVIII with stroke or CHD, nor did C-reactive protein meaningfully alter any of the associations of PC with stroke. In the CVD risk factor adjusted model, adding C-reactive protein marginally increased the association of PC below versus above the 5th percentile with CHD; HR 1.91 (95% CI 1.01, 3.60), with no signification race interaction (pinteraction=0.65). When C-reactive protein was added to the joint-association of FVIII and PC models, the results were essentially the same. For CHD, the pinteraction for FVIII (per SD higher) for PC in the bottom 5th percentile (HR 4.11; 95% CI 1.77, 9.57) versus the upper 95th percentile (HR 1.41; 95% CI 1.20, 1.67) was 0.02. For stroke, the pinteraction for VIII (per SD higher) for PC in the bottom 5th percentile (HR 1.23; 95 % CI 1.05, 1.44) versus the upper 95th percentile (HR 1.46; 95% CI 0.84, 2.55) was 0.56. Adding C-reactive protein to any other way of modelling PC did not change interpretation of the results.
Discussion
The major finding of this study was that there is a synergy in risk for CHD between higher FVIII and low PC a relationship not seen with stroke in a large bi-racial population in the United States. Elevated FVIII was associated with increased CHD risk, and to a lesser extent stroke risk, independent of traditional CVD risk factors and C-reactive protein in both blacks and whites. In contrast, low PC was only marginally associated with CHD but not stroke risk in both blacks and whites. Further, neither a low FVIII nor a high PC were protective against stroke or CHD risk.
The role of haemostasis in general, and FVIII and PC in specific in CVD risk assessment is controversial(3, 25). While adverse levels of many coagulation factors are associated with increased risk of CVD, the clinical implication is modest as haemostasis markers do not improve CVD risk assessment when added to traditional CVD risk models(3). Fundamentally, thrombosis is the final common pathway for most CVD events and iatrogenic manipulation of haemostasis with anticoagulant and antiplatelet agents reduces the risk of CVD in various populations(9, 10). This is however an over simplification of stroke and CHD which are two related, but different diseases with unique risk factors(26). Recent data suggest that the pathophysiology of CHD has a stronger link to haemostasis biomarkers than stroke(27, 28). The reason for these findings is not known for certain. We hypothesize that CHD is generally a more homogenous disease than stroke. The majority of CHD, especially in an older population, is usually the rupture of an atherosclerotic plaque causing thrombotic vessel obstruction(29). Stroke, on the other hand is more a heterogeneous disease consisting of large and small vessel occlusion and embolic occlusion(30). As stroke is likely a family of diseases, lumping stroke together as one disease may limit our ability to assess risk factors.
FVIII has long been studied as a vascular disease risk marker. Genetic and acquired low levels of factor VIII are bleeding disorders (haemophilia A and acquired haemophilia A)(31, 32) and elevated FVIII is a risk factor for stroke(4), CHD(4), and venous thrombosis(33). Our findings confirm the associations seen in prior studies in a contemporary population and extend these findings to blacks as well as demonstrate that the association is independent of inflammation as measured by CRP. Further, our findings demonstrate low FVIII concentrations and high PC concentrations are not protective against CVD when compared to the middle of the distribution. Individuals with haemophilia A (congenital factor VIII deficiency) should have lower CVD risk than the general population if low levels of FVIII are protective against CVD risk, however individual analyses and meta-analyses have failed to demonstrate a consistent lower CVD risk either in affected males or female carriers(34). Low PC is a risk factor for venous thrombosis, but the association with arterial disease is less established(2, 12, 15). Our results did not reveal an independent role for PC for stroke or CHD risk, but had only modest power to assess very low levels of protein C and CVD risk. This is congruent with prior studies; the Atherosclerosis Risk in Community Study demonstrated no association between PC levels and CHD(12). However, in contrast to the results here, the investigators did find a modest association between PC and stroke risk in the bottom quintile versus the top quintile (HR 1.38; 95% CI 1.07, 1.77) adjusting for other risk factors(12).
The joint association of PC and FVIII with CVD has not been assessed in prior research to our knowledge. The impact of PC on thrombosis risk is better known with the factor V Leiden mutation whereby factor V is resistant to inactivation by activated PC due to a point mutation. Less appreciated is that activated PC also inactivates FVIII. As activated PC inactivates FVIII we hypothesized that low PC combined with a high FVIII may potentiate CVD risk(15). Many CVD risk factors, haemostasis and inflammation biomarkers correlate with each other and prior literature assessing these biomarkers in isolation may miss important associations(3, 35). Some data supports that global measures of haemostasis such as thrombin generation(36), D-dimer(28), or the activated partial thromboplastin time(37) may be risk factors for thrombosis, but our ability to measure haemostasis in vivo is limited. For the first time, we demonstrate a potential synergy whereby low levels of one coagulation factor (PC) potentiate high levels of another (FVIII) in terms of risk for CHD, consistent with their function in vivo.
The limitations and strengths of REGARDS are those of prospective cohort studies; risk factors were measured at one time point prior to the cardiovascular events and individuals were followed for events over time. Our results may be affected by unidentified confounders and may not represent causal associations. The geographic diversity of REGARDS is the greatest strength as well as weakness of this analysis. Haemostasis biomarkers vary by race as seen in the current analysis, but most studies lack sufficient power to assess associations by race. As REGARDS is an observational cohort, the study is not suited to determine if different cardiovascular risk profiles between blacks and whites might translate into different primary prevention strategies. While we have a geographically, economically, and racially diverse population, we were limited in our ability to fully characterize risk factors and outcomes in participants. For instance, clinical evaluation of stroke events varies dramatically across the United States and despite our best efforts, a plurality of strokes cannot be subtyped. In line with the REGARDS study design, we could not assess biomarkers that are sensitive to preanalytical factors related to sample processing and shipping, such as von Willebrand factor, as individuals were recruited in their homes to allow for geographic diversity(21). As warfarin affects protein C levels and CVD risk, we excluded individuals on warfarin from analyses; as this amounted to less than 4% of the CRS, the inability to assess protein C levels in this population likely did not impact our results.
In conclusion, higher concentrations of FVIII were associated with increased CHD and to a lesser extent stroke risk, and only very low levels of PC had any potential association with CVD risk. The interaction increasing CHD risk for those with low PC and high FVIII is novel and highlights the complexity haemostasis plays in CVD risk. Future studies cannot study haemostasis in isolation and must acknowledge the complex interplay between haemostasis biomarkers, inflammation, and traditional CVD risk factors.
Extra Table.
| What is Known on This Topic |
|
| What this Paper Adds |
|
Acknowledgments
Financial Support: Funding solely from the National Institutes of Health, Department of Health and Human Service, Bethesda, MD, USA. Grant numbers: U01 NS041588, K08 HL096841, and R01 HL080477.
This research project is supported by a cooperative agreement U01 NS041588 from the National Institute of Neurological Disorders and Stroke, National Institutes of Health, Department of Health and Human Service, Bethesda, MD, USA. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke or the National Institutes of Health. Representatives of the funding agency have been involved in the review of the manuscript but not directly involved in the collection, management, analysis or interpretation of the data. The authors thank the other investigators, the staff, and the participants of the REGARDS study for their valuable contributions. A full list of participating REGARDS investigators and institutions can be found at http://www.regardsstudy.org
Additional funding was provided by an investigator-initiated grant-in-aid K08HL096841 and R01HL080477 from the National Heart, Lung, and Blood Institute National Institutes of Health, Department of Health and Human Service, Bethesda, MD, USA. Representatives from the National Heart, Lung, and Blood Institute did not have any role in the design and conduct of the study, the collection, management, analysis, and interpretation of the data, or the preparation of the manuscript.
Footnotes
Authorship Details
Obtained Funding: N.A. Zakai M.M. Safford, G. Howard. Research Design and Methods: All Authors. Statistical Analyses: N.A. Zakai and S.E. Judd. Initial Manuscript Draft: N.A. Zakai. Critical Revision and Scientific Review of the Manuscript: All Authors
References
- 1.Dzau VJ, Antman EM, Black HR, et al. The cardiovascular disease continuum validated: clinical evidence of improved patient outcomes: part I: Pathophysiology and clinical trial evidence (risk factors through stable coronary artery disease) Circulation. 2006;114(25):2850–70. doi: 10.1161/CIRCULATIONAHA.106.655688. [DOI] [PubMed] [Google Scholar]
- 2.Smith A, Patterson C, Yarnell J, et al. Which hemostatic markers add to the predictive value of conventional risk factors for coronary heart disease and ischemic stroke? The Caerphilly Study. Circulation. 2005;112(20):3080–7. doi: 10.1161/CIRCULATIONAHA.105.557132. [DOI] [PubMed] [Google Scholar]
- 3.Tzoulaki I, Murray GD, Lee AJ, et al. Relative value of inflammatory, hemostatic, and rheological factors for incident myocardial infarction and stroke: the Edinburgh Artery Study. Circulation. 2007;115(16):2119–27. doi: 10.1161/CIRCULATIONAHA.106.635029. [DOI] [PubMed] [Google Scholar]
- 4.Zakai NA, Katz R, Jenny NS, et al. Inflammation and hemostasis biomarkers and cardiovascular risk in the elderly: the Cardiovascular Health Study. Journal of thrombosis and haemostasis: JTH. 2007;5(6):1128–35. doi: 10.1111/j.1538-7836.2007.02528.x. [DOI] [PubMed] [Google Scholar]
- 5.Smith NL, Bis JC, Biagiotti S, et al. Variation in 24 hemostatic genes and associations with non-fatal myocardial infarction and ischemic stroke. Journal of thrombosis and haemostasis: JTH. 2008;6(1):45–53. doi: 10.1111/j.1538-7836.2007.02795.x. [DOI] [PubMed] [Google Scholar]
- 6.Kucharska-Newton AM, Couper DJ, Pankow JS, et al. Hemostasis, inflammation, and fatal and nonfatal coronary heart disease: long-term follow-up of the atherosclerosis risk in communities (ARIC) cohort. Arterioscler Thromb Vasc Biol. 2009;29(12):2182–90. doi: 10.1161/ATVBAHA.109.192740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhatia M, Rothwell PM. A systematic comparison of the quality and volume of published data available on novel risk factors for stroke versus coronary heart disease. Cerebrovascular diseases. 2005;20(3):180–6. doi: 10.1159/000087202. [DOI] [PubMed] [Google Scholar]
- 8.Soliman EZ, Safford MM, Muntner P, et al. Atrial fibrillation and the risk of myocardial infarction. JAMA Intern Med. 2014;174(1):107–14. doi: 10.1001/jamainternmed.2013.11912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kumar G, Goyal MK. Warfarin versus aspirin for prevention of stroke in heart failure: a meta-analysis of randomized controlled clinical trials. Journal of stroke and cerebrovascular diseases: the official journal of National Stroke Association. 2013;22(8):1279–87. doi: 10.1016/j.jstrokecerebrovasdis.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 10.Force USPST. Aspirin for the prevention of cardiovascular disease: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;150(6):396–404. doi: 10.7326/0003-4819-150-6-200903170-00008. [DOI] [PubMed] [Google Scholar]
- 11.Soare AM, Popa C. Deficiencies of proteins C, S and antithrombin and activated protein C resistance--their involvement in the occurrence of Arterial thromboses. J. 2010;3(4):412–5. [PMC free article] [PubMed] [Google Scholar]
- 12.Folsom AR, Ohira T, Yamagishi K, et al. Low protein C and incidence of ischemic stroke and coronary heart disease: the Atherosclerosis Risk in Communities (ARIC) Study. Journal of thrombosis and haemostasis: JTH. 2009;7(11):1774–8. doi: 10.1111/j.1538-7836.2009.03577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Folsom AR, Delaney JA, Lutsey PL, et al. Associations of factor VIIIc, D-dimer, and plasmin-antiplasmin with incident cardiovascular disease and all-cause mortality. Am J Hematol. 2009;84(6):349–53. doi: 10.1002/ajh.21429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rasche H. Haemostasis and thrombosis: an overview. Eur Heart J Suppl. 2001;3(Q):Q3–Q7. [Google Scholar]
- 15.Esmon CT. The protein C pathway. Chest. 2003;124(3 Suppl):26S–32S. doi: 10.1378/chest.124.3_suppl.26s. [DOI] [PubMed] [Google Scholar]
- 16.Howard VJ, Cushman M, Pulley L, et al. The reasons for geographic and racial differences in stroke study: objectives and design. Neuroepidemiology. 2005;25(3):135–43. doi: 10.1159/000086678. [DOI] [PubMed] [Google Scholar]
- 17.Howard VJ, Kleindorfer DO, Judd SE, et al. Disparities in stroke incidence contributing to disparities in stroke mortality. Ann Neurol. 2011;69(4):619–27. doi: 10.1002/ana.22385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barlow WE, Ichikawa L, Rosner D, et al. Analysis of case-cohort designs. Journal of clinical epidemiology. 1999;52(12):1165–72. doi: 10.1016/s0895-4356(99)00102-x. [DOI] [PubMed] [Google Scholar]
- 19.Kulathinal S, Karvanen J, Saarela O, et al. Case-cohort design in practice - experiences from the MORGAM Project. Epidemiol Perspect Innov. 2007;4:15. doi: 10.1186/1742-5573-4-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zakai NA, Judd SE, Alexander K, et al. ABO blood type and stroke risk: the REasons for Geographic And Racial Differences in Stroke Study. J Thromb Haemost. 2014;12(4):564–70. doi: 10.1111/jth.12507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gillett SR, Boyle RH, Zakai NA, et al. Validating laboratory results in a national observational cohort study without field centers: the Reasons for Geographic and Racial Differences in Stroke cohort. Clin Biochem. 2014;47(16–17):243–6. doi: 10.1016/j.clinbiochem.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Howard G, Cushman M, Kissela BM, et al. Traditional risk factors as the underlying cause of racial disparities in stroke: lessons from the half-full (empty?) glass. Stroke. 2011;42(12):3369–75. doi: 10.1161/STROKEAHA.111.625277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Safford MM, Brown TM, Muntner PM, et al. Association of race and sex with risk of incident acute coronary heart disease events. JAMA: the journal of the American Medical Association. 2012;308(17):1768–74. doi: 10.1001/jama.2012.14306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luepker RV, Apple FS, Christenson RH, et al. Case definitions for acute coronary heart disease in epidemiology and clinical research studies: a statement from the AHA Council on Epidemiology and Prevention; AHA Statistics Committee; World Heart Federation Council on Epidemiology and Prevention; the European Society of Cardiology Working Group on Epidemiology and Prevention; Centers for Disease Control and Prevention; and the National Heart, Lung, and Blood Institute. Circulation. 2003;108(20):2543–9. doi: 10.1161/01.CIR.0000100560.46946.EA. [DOI] [PubMed] [Google Scholar]
- 25.Boekholdt SM, Kramer MH. Arterial thrombosis and the role of thrombophilia. Semin Thromb Hemost. 2007;33(6):588–96. doi: 10.1055/s-2007-985755. [DOI] [PubMed] [Google Scholar]
- 26.Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation. 2017;135(10):e146–e603. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Olson NC, Cushman M, Judd SE, et al. Associations of coagulation factors IX and XI levels with incident coronary heart disease and ischemic stroke: the REGARDS study. J Thromb Haemost. 2017;15(6):1086–94. doi: 10.1111/jth.13698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zakai NA, McClure LA, Judd SE, et al. D-dimer and the Risk of Stroke and Coronary Heart Disease. The REasons for Geographic and Racial Differences in Stroke (REGARDS) Study. Thromb Haemost. 2017;117(3):618–24. doi: 10.1160/TH16-07-0519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Libby P, Theroux P. Pathophysiology of Coronary Artery Disease. Circulation. 2005;111(25):3481–8. doi: 10.1161/CIRCULATIONAHA.105.537878. [DOI] [PubMed] [Google Scholar]
- 30.Petty GW, Brown RD, Whisnant JP, et al. Ischemic Stroke Subtypes. A Population-Based Study of Incidence and Risk Factors. 1999;30(12):2513–6. doi: 10.1161/01.str.30.12.2513. [DOI] [PubMed] [Google Scholar]
- 31.Peyvandi F, Palla R, Menegatti M, et al. Coagulation factor activity and clinical bleeding severity in rare bleeding disorders: results from the European Network of Rare Bleeding Disorders. Journal of thrombosis and haemostasis: JTH. 2012;10(4):615–21. doi: 10.1111/j.1538-7836.2012.04653.x. [DOI] [PubMed] [Google Scholar]
- 32.Srivastava A, Brewer AK, Mauser-Bunschoten EP, et al. Guidelines for the management of hemophilia. Haemophilia. 2013;19(1):e1–47. doi: 10.1111/j.1365-2516.2012.02909.x. [DOI] [PubMed] [Google Scholar]
- 33.Tsai AW, Cushman M, Rosamond WD, et al. Coagulation factors, inflammation markers, and venous thromboembolism: the longitudinal investigation of thromboembolism etiology (LITE) Am J Med. 2002;113(8):636–42. doi: 10.1016/s0002-9343(02)01345-1. [DOI] [PubMed] [Google Scholar]
- 34.Kamphuisen PW, ten Cate H. Cardiovascular risk in patients with hemophilia. Blood. 2014;123(9):1297–301. doi: 10.1182/blood-2013-11-453159. [DOI] [PubMed] [Google Scholar]
- 35.Zakai NA, Olson NC, Judd SE, et al. Haemostasis biomarkers and risk of intracerebral haemorrhage in the REasons for Geographic and Racial Differences in Stroke Study. Thromb Haemost. 2017;117(9):1808–15. doi: 10.1160/TH17-03-0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ten Cate H, Hemker HC. Thrombin Generation and Atherothrombosis: What Does the Evidence Indicate? J Am Heart Assoc. 2016;5(8) doi: 10.1161/JAHA.116.003553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zakai NA, Ohira T, White R, et al. Activated partial thromboplastin time and risk of future venous thromboembolism. Am J Med. 2008;121(3):231–8. doi: 10.1016/j.amjmed.2007.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]