Abstract
Background
The treatment of acute lymphoblastic leukemia (ALL) and osteosarcoma (OSC) is very effective: the vast majority of patients recover and survive for decades. However, they still need to face serious adverse effects of chemotherapy. One of these is cardiotoxicity which may lead to progressive heart failure in the long term. Cardiotoxicity is contributed mainly to the use of anthracyclines and might have genetic risk factors. Our goal was to test the association between left ventricular function and genetic variations of candidate genes.
Methods
Echocardiography data from medical records of 622 pediatric ALL and 39 OSC patients were collected from the period 1989–2015. Fractional shortening (FS) and ejection fraction (EF) were determined, 70 single nucleotide polymorphisms (SNPs) in 26 genes were genotyped. Multivariate logistic regression and multi-adjusted general linear model were performed to investigate the influence of genetic polymorphisms on the left ventricular parameters. Bayesian network based Bayesian multilevel analysis of relevance (BN-BMLA) method was applied to test for the potential interaction of the studied cofactors and SNPs.
Results
Our results indicate that variations in ABCC2, CYP3A5, NQO1, SLC22A6 and SLC28A3 genes might influence the left ventricular parameters. CYP3A5 rs4646450 TT was 17% among ALL cases with FS lower than 28, and 3% in ALL patients without pathological FS (p = 5.60E-03; OR = 6.94 (1.76–27.39)). SLC28A3 rs7853758 AA was 12% in ALL cases population, while only 1% among controls (p = 6.50E-03; OR = 11.56 (1.98–67.45)). Patients with ABCC2 rs3740066 GG genotype had lower FS during the acute phase of therapy and 5–10 years after treatment (p = 7.38E-03, p = 7.11E-04, respectively). NQO1 rs1043470 rare T allele was associated with lower left ventricular function in the acute phase and 5–10 years after the diagnosis (p = 4.28E-03 and 5.82E-03, respectively), and SLC22A6 gene rs6591722 AA genotype was associated with lower mean FS (p = 1.71E-03), 5–10 years after the diagnosis.
Conclusions
Genetic variants in transporters and metabolic enzymes might modulate the individual risk to cardiac toxicity after chemotherapy.
Electronic supplementary material
The online version of this article (10.1186/s12885-018-4629-6) contains supplementary material, which is available to authorized users.
Keywords: Anthracycline, Cardiotoxicity, Cancer, Genetic polymorphisms, Childhood cancer
Background
Acute lymphoblastic leukemia (ALL) and osteosarcoma (OSC) occur predominantly in pediatric patients. ALL is the most common childhood hematological malignancy; about 25–30% of childhood cancers are acute leukemia, 80% of which are ALL [1]. Osteosarcoma is a rare bone disease that affects 3–4 people per million and represents 3% of pediatric tumors [2]. Nowadays, the treatment of pediatric ALL is very effective: the majority of patients are cured for the long term. The 5-year event-free survival rate (EFS) is around 80% for ALL and 60% for osteosarcoma patients [2–6].
Unfortunately, despite the use of the indeed efficacious chemotherapeutic drugs, patients have to face serious side effects. Therefore, the primary goal of the scientific research is now not only to increase the survival rate, but to identify and reduce the acute and late toxic side effects of chemotherapy and to improve the quality of life in adulthood [4, 7–10]. The risk for developing health problems is increased 8-fold in pediatric cancer survivors within 30–40 years after diagnosis compared to their siblings; 50% of the sibs experience severe, disabling or life-threatening events, including death by the age of 50. One of the late toxic side effects of chemotherapy in childhood ALL is cardiotoxicity [5, 7, 11, 12]. A 30-year-old survivor might face treatment-related cardiac damage usually characteristic for much older patients [13–15]. There is a need for preventing cardiac damage especially in children, because they can live for decades after treatment [16]. Several treatment regimens introduce dose reduction in some cases to decrease late side effects, but childhood cancer survivors still require long-term follow-up for their prevention and treatment. The constant monitoring of patients is important in order to identify subclinical anomalies before the clinical symptoms occur [17–20].
Anthracyclines are among the most essential and highly effective chemotherapeutic agents in the treatment of both hematological malignancies and solid tumors (e.g. leukemia, lymphoma, breast cancer, and sarcoma) [21–25], and belong to the backbone of childhood ALL and osteosarcoma treatment protocols all around the globe [4]. However, anthracyclines damage cardiomyocytes which can manifest during the therapy, years or even decades after the exposure to chemotherapeutic agents [18, 26–30]. The pathophysiology of the toxicity is not completely understood, but it is likely that both the drug and its metabolites are cardiotoxic [31, 32]. Despite the fact that anthracycline-induced cardiotoxicity (ACT) is well known, the toxicity is unpredictable [33–35]. Rates of cardiotoxicity increase the higher the cumulative dose, with doses above 500 mg/m2 resulting in unacceptable rates of cardiac toxicity; therefore, most of the treatment protocols limit the use of these drugs below this value [18, 36]. However, there are patients with cardiac problems who received very low doses of anthracyclines while others were administered with high doses and escaped the side effect. The variable development of anthracycline cardiotoxicity suggests that the genetic background of the patients is important in this side effect [37–45].
The comparability of pharmacogenomics research results is hampered by the heterogeneity of the populations under study, applied treatment protocols and investigated parameters. Half of the studies were executed on former pediatric patient populations and there is an urgent pressure to translate these research evidences into clinical practice [46–50]. For this purpose one of the newest publications of the topic contains evidence-based clinical practice recommendations for pharmacogenomic testing. They emphasize RARG (Retinoic Acid Receptor Gamma) rs2229774, SLC28A3 (Solute Carrier Family 28 Member 3) rs7853758 and UGT1A6 (UDP Glucuronosyltransferase Family 1 Member A6) rs17863783 as genetic variants which have the strongest association with ACT [49]. SNPs in the genes of anthracycline transporters ABCB1 (ATP Binding Cassette Subfamily B Member 1) and ABCC1 (ATP Binding Cassette Subfamily C Member 1) also associated with ACT in anthracycline-treated children [37]. SLC22A7 (Solute Carrier Family 22 Member 7) and SLC22A17 (Solute Carrier Family 22 Member 17) were also in connection with cardiotoxicity among patients with osteosarcoma [2]. Studying adult patients with non-Hodgkin lymphoma Wojnowski et al. found associations between cardiotoxicity and SNPs in the NAD(P)H oxidase complex, and ABCC1 and ABCC2 (ATP Binding Cassette Subfamily C Member 2) transporters [38]. The function of cytochrome P450 enzymes is crucial in the metabolism of several drugs, among their coding genes e.g.: CYP3A5 (Cytochrome P450 Family 3 Subfamily A Member 5) had importance in this context. In a genome wide association study RARG has been identified as a promising gene [48].
These results support the hypothesis that the genetic features of the patients may influence chemotherapy-related cardiotoxicity. However, further independent studies are needed to confirm these findings. From the scientific literature and databases we selected 70 SNPs in 24 candidate genes coding for xenobiotic transporters and metabolizing enzymes, and searched for associations between these genetic variations, and acute or late left ventricular damage in pediatric acute lymphoblastic leukemia and osteosarcoma patients.
Methods
Patients
In this study, patients with pediatric acute lymphoblastic leukemia (ALL) or osteosarcoma (OSC), aged 0–18 years at diagnosis, were enrolled retrospectively (n = 680). Children with ALL had undergone chemotherapy between 1989 and 2015 in 6 Hungarian pediatric oncology centers, patients with OSC were treated between 1989 and 2015 at the Second Department of Pediatrics, Semmelweis University. Patients were excluded from the analysis because of Down syndrome (n = 7), previous cardiac problems or any concomitant disease with potential cardiac complications (adrenoleukodystrophy, agenesia renis, cardiac arrhythmia, congenital hypothyroidism, cystic fibrosis, ventricular septal defect, VACTERL) (n = 12). Detailed description of the ALL (n = 622) and OSC (n = 39) patients included in the statistical analysis is shown in Table 1.
Table 1.
Characteristics of the studied populations
| Patients with ALL | Patients with OSC | Total | |
|---|---|---|---|
| Number of patients | 622 | 39 | 661 |
| Gender n (%) | |||
| Male | 372 (60) | 27 (69) | 399 (60) |
| Female | 250 (40) | 12 (31) | 262 (40) |
| Age at diagnosis (%) | |||
| < 1 yr. n | 7 (1) | 0 | 7 (1) |
| 1–10 yr. n | 505 (81) | 9 (23) | 515 (78) |
| > 10 yr. n | 109 (18) | 30 (77) | 138 (21) |
| Mean ± SD yr | 6.39 (±4.3) | 13.1 (±3.5) | 6.6 (±4.3) |
| Median (range) yr | 5.2 (0–18) | 13.2 (5–18) | 5.3 (0–18) |
| Risk group n (%) | |||
| SR | 165 (27) | 3 (7) | 168 (25) |
| IR | 355 (57) | 24 (62) | 379 (58) |
| HR | 100 (16) | 12 (31) | 112 (17) |
| Chemotherapy protocol n (%) | |||
| Protocols before 20001 | 325 (52) | − | 325 (52) |
| Protocols after 20002 | 297 (48) | − | 297 (48) |
| OSC protocols | − | 39 | 39 |
| Anthracycline dose3 (range, mg/m2) | 60–840 | 180–360 | 60–840 |
| Anthracycline dose n (%) | |||
| ≤ 240 mg/m2 | 457 (74) | 6 (15) | 463 (70) |
| > 240 mg/m2 | 163 (26) | 33 (85) | 196 (30) |
| Patients with pathological FS4 n | 18 | 2 | 20 |
Data are reported as numbers with percentages, unless mentioned otherwise. Abbreviations: ALL, acute lymphoid leukemia; OSC, osteosarcoma; SD, standard deviation; SR, standard-risk; IR, intermediate-risk; HR, high-risk; FS, left ventricular fractional shortening. 1ALL patients treated with ALL BFM 88, ALL BFM 90, ALL BFM 95, Interfant 98, NHL BFM 90 or NHL BFM 95 protocol. 2ALL patients treated with ALL IC BFM 2002, ALL IC BFM 2009 or Interfant 2006 protocol. 3Cumulative anthracycline dose in doxorubicin or daunorubicin equivalent doses during the treatment according to protocol. 4FS below 28%
Patients with ALL were treated according to one of the following study protocols: ALL BFM (Berlin–Frankfurt–Münster) 88, ALL BFM 90, ALL BFM 95, ALL IC-BFM (ALL Intercontinental) 2002 or ALL IC-BFM 2009; Interfant 98 or Interfant 2006. The chemotherapy regimen was described in detail in our previous article [51]. Patients diagnosed with ALL and OSC were treated with anthracyclines in the first year of chemotherapy. The chemotherapy protocols differed slightly in the number or dosage of anthracycline-administrations. In the low-risk and medium-risk groups of patients with ALL the cumulative anthracycline (i.e. doxorubicin equivalent) doses were between 180 and 240 mg/m2; in the high-risk group and in the therapy of relapsed patients it were between 240 and 380 mg/m2. The anthracycline treatment of patients with osteosarcoma was based on the COSS (German-Austrian-Swiss osteosarcoma study group) -86 and COSS-96 protocols. Treatment of patients with OSC included cumulative doxorubicin doses 360 mg/m2 for standard-risk group patients or 180 mg/m2 for the high-risk group. For detailed description of the used COSS based protocols see Hegyi et al., 2016 [52]. In our cohort 29% of the patients received 12 Gy cranial radiotherapy according to the schedule of the ongoing BFM protocol (ALL BFM 90 or ALL BFM 95 protocols in 22% of the patients).
Informed consent was requested from legal guardians of the patients or from the participants above the age of 16 (6% of patients). The study was approved by the Ethics Committee of the Hungarian Medical Research Council and conducted according to the principles of the Declaration of Helsinki.
The patients were followed-up by echocardiography (ECHO) routinely in the clinical practice to monitor their left ventricular function. All ECHOs were performed by the pediatric cardiologists in the Hungarian pediatric oncology centers. Left ventricular end-diastolic-diameter (LVEDD) and left ventricular end-systolic diameter (LVESD) data were collected from the patients’ medical records. Left ventricular ejection fraction (EF) and left ventricular fractional shortening (FS) were determined: EF = (LVEDD3-LVESD3)/LVEDD3; FS = (LVEDD-LVESD)/LVEDD. Measurements were performed before the initiation of therapy, several times during the treatment and annually after finishing treatment. FS and EF data were analyzed in follow-up categories, which are: 1) at the diagnosis (used as a control); 2) in acute phase: during the intensive chemotherapy phase; 3) during oral maintenance chemotherapy; 4) at the end of the treatment, which is after the oral maintenance chemotherapy period completed 2 or 3 years after the diagnosis; 5) from the end of the treatment until 5 years after the diagnosis; 6) 5–10 years after the diagnosis; 7) 10–15 years after the diagnosis; 8) more than 15 years after the diagnosis. For detailed description of the follow-up categories see Table 2. Not all of the ECHO records were available, because of the retrospective data collection. Only the latest ECHO of each patient was used in each follow-up category.
Table 2.
Follow-up categories with echocardiography parameters
| Follow-up category | Patients with ALL | Patients with OSC | Total population | ||
|---|---|---|---|---|---|
| N (mean FS ± SD) |
N (mean FS ± SD) |
N (mean FS ± SD) |
Decreased not decreased FS, N1 | OR (95% CI) 2 |
|
| At the diagnosis | 358 | 29 | 387 | ||
| 41.5 ± 6.1 | 39.6 ± 4.4 | 41.4 ± 6.0 | |||
| < 1 yr. from diagnosis1 | 275 | 5 | 280 | 104 | 83 | 1.0 |
| 40.4 ± 6.1 | 40.2 ± 5.3 | 40.4 ± 6.1 | |||
| 1–2 yr. from diagnosis | 46 | 3 | 49 | 19 | 10 | 1.5 (0.6–3.4) |
| 41.4 ± 6.0 | 39.9 ± 2.3 | 41.3 ± 5.9 | |||
| End of the treatment | 287 | 28 | 315 | 105 | 98 | 0.9 (0.6–1.3) |
| 40.0 ± 5.6 | 38.4 ± 6.3 | 39.9 ± 5.7 | |||
| 2–5 yr. from diagnosis | 229 | 35 | 264 | 77 | 73 | 0.8 (0.5–1.3) |
| 40.4 ± 5.7 | 38.1 ± 5.2 | 40.1 ± 5.7 | |||
| 5–10 yr. from diagnosis | 265 | 36 | 301 | 70 | 76 | 0.7 (0.5–1.1) |
| 40.1 ± 5.5 | 40.3 ± 5.6 | 40.1 ± 5.5 | |||
| 10–15 yr. from diagnosis | 133 | 19 | 152 | 24 | 36 | 0.5 (0.3–1.0) |
| 40.4 ± 5.4 | 39.9 ± 5.2 | 40.3 ± 5.3 | |||
| > 15 yr. from diagnosis | 24 | 8 | 32 | 5 | 3 | 1.3 (0.3–5.7) |
| 37.6 ± 7.5 | 40.7 ± 6.1 | 38.4 ± 7.2 | |||
The decrease of FS was calculated patient by patient in every category compared to the individual value at diagnosis if these data were available. 1 Number of patients with a decreased FS per number of patients with a not decreased FS. 2 Compared to the second category. Abbreviations: CI, confidence interval; FS, left ventricular fractional shortening; OR, odds ratio; N, number; SD, standard deviation
The worst heart function of each patient was used to define patients for the case-control type study. Cases were those who had echocardiograms with FS ≤ 28% at any time point during the follow-up (n = 20); patients, who received the same chemotherapy but never had FS ≤ 28% were regarded as controls (n = 641).
The alteration of FS was computed and analyzed as dichotomous variable, which was defined as the difference between the FS value at diagnosis and at the end of the treatment. In this study, patients with decreased FS (n = 105) were compared to those with increased FS (n = 94). Difference between the FS value at diagnosis and at the last follow-up time point was also computed, 170 patients with decreased FS were compared to those with increased FS (n = 152).
Laboratory methods
Peripheral blood samples were taken from children with ALL in remission. DNA was isolated from blood using Qiagen isolation kits according to the manufacturer’s instructions (QIAmp DNA Blood Midi or Maxi Kit, Qiagen, Hilden, Germany).
Based on the scientific literature, 70 single nucleotide polymorphisms (SNPs) in 26 genes were selected and genotyped. These genes encode transporters involved in drug import or elimination as well as enzymes in the metabolism of the chemotherapeutic agents. Candidate genes were chosen from previous candidate gene studies in this field if the gene or gene family were found to be associated with cardiotoxicity in more than two studies (ABCB1, ABCC1, ABCC2, GSTP1, SLC22A17, SLC22A6, SLC22A7, SLC22A8, SLC28A3) [48] or were found in relation to cardiotoxicity in large-scale or genome-wide association studies (BCL2, HAS3, RARG) [53–55]. Previously not validated potential candidate genes were also selected if those were important in the transport or metabolism of cardiotoxic drugs used in chemotherapy (AKR1A1, AKR1C3, ABCG2, CEP72, CYP3A4, CYP3A5, NQO1, NQO2, MTHFR) [46]. SNPs were selected prioritized on the basis of their estimated functionality in this order: non-synonymous SNPs, SNPs in the promoter and the 3’-UTR (3′-untranslated region) region, synonymous SNPs and intronic SNPs. During the selection the minor allele frequency data of the SNPs were validated using HapMap database release No. 27 and the CEU population (CEPH: Utah residents with ancestry from northern and western Europe) [56]. Information on the selected SNPs is shown in an additional table file in more detail [see Additional file 1]. Genotyping 63 of the SNPs was conducted using TaqMan® OpenArray™ Genotyping System (Thermo Fisher Scientific, Waltham, MA, USA) following the manufacturer’s instructions at the Department of Medical Chemistry, Molecular Biology and Pathobiochemistry, Semmelweis University (Budapest, Hungary). Detailed description of this procedure can be found in the article of Banlaki et al. [57]. Other 7 SNPs were genotyped using KASPar (KBioscience Competitive Allele-Specific Polymerase chain reaction)-on-Demand prevalidated assays (LGC Genomics, Berlin, Germany) on 7900HT Fast Real-Time PCR System (Thermo Fisher Scientific Waltham, MA, USA). The genotyping was unsuccessful in the case of three SNPs; call rate for the other SNPs was higher than 87.5%.
Statistical analysis
Allele frequencies were tested by allele counting, HWE (Hardy-Weinberg equilibrium) was studied using an on-line software [58], significant violation of HWE was considered where p ≤ 8.90E-03. In our case-control and follow-up studies, univariate and multivariate logistic regression and multi-adjusted general linear model were performed to investigate the influence of genetic polymorphisms on the left ventricular parameters. The analyses were adjusted for potential confounders, which were age at the time of diagnosis (years), gender (male-female), chemotherapy protocols (before 2000, after 2000 and OSC protocols; also reflects radiotherapy), risk groups (standard, intermediate, high-risk) and cumulative dose of anthracycline (≤ or > 240 mg/m2). The analyses were performed studying the genotypes separately (11 vs. 12 vs. 22), using recessive (11/12 vs. 22) or dominant (11 vs. 12/22) models, with the common homozygotes signed as 11. EF and FS is indicated in the text with the standard error (SE) of the estimate of the mean. In order to deal with multiple comparisons the Benjamini-Hochberg false discovery rate (FDR) method with type I error rate of 10% (p ≤ 8.90E-03) was applied as correction (with 201 analyses performed for 67 SNPs and each phenotype) [59, 60]. Analyses and preparation of the figures were performed using IBM SPSS Statistics 23.0 (IBM Corporation, Armonk, NY, USA) and RStudio Version 1.0.136 (RStudio, Boston, MA, USA) programs. Estimated haplotype frequency in cases and controls and the haplotype-specific odds ratio (OR) were calculated by the Haploview 4.1 software [61]. The power of the analyses was calculated at a significance level of 0.05 using SPSS Statistics 23.0 program. Bayesian network based Bayesian multilevel analysis of relevance (BN-BMLA) method was applied to test for potential interaction of the studied cofactors and SNPs. The BN-BMLA was described in our previous article [62].
Results
In this study, altogether 70 SNPs were genotyped. The minor allele frequencies of the SNPs are presented in an additional table file in more detail [see Additional file 1]. Genotype distributions were in Hardy-Weinberg equilibrium except for one SNP (AKR1A1 (Aldo-Keto Reductase Family 1 Member A1) rs2934859) which was excluded from the analysis. Genotyping was unsuccessful in the case of three SNPs. Thus, the genotyping results of 66 SNPs were used for the evaluations. Minor allele frequencies in our population were found to be more than 7% for all of the SNPs. The analyses performed on the population had adequate power (≥75%) for all of the results.
Case-control analysis
The potential roles of genetic variations of candidate genes in the changes of the left ventricular function of children with ALL or OSC after anthracycline therapy were investigated. Ejection fraction (EF) and fractional shortening (FS) were used to monitor the left ventricular function. Patients who had FS ≤ 28% any time during the follow-up were regarded as cases, those who received the same chemotherapy, but never had FS ≤ 28% were regarded as controls. To assess the possible association of the genotypes with cardiotoxicity, the genotype and allele frequencies in the two groups were compared.
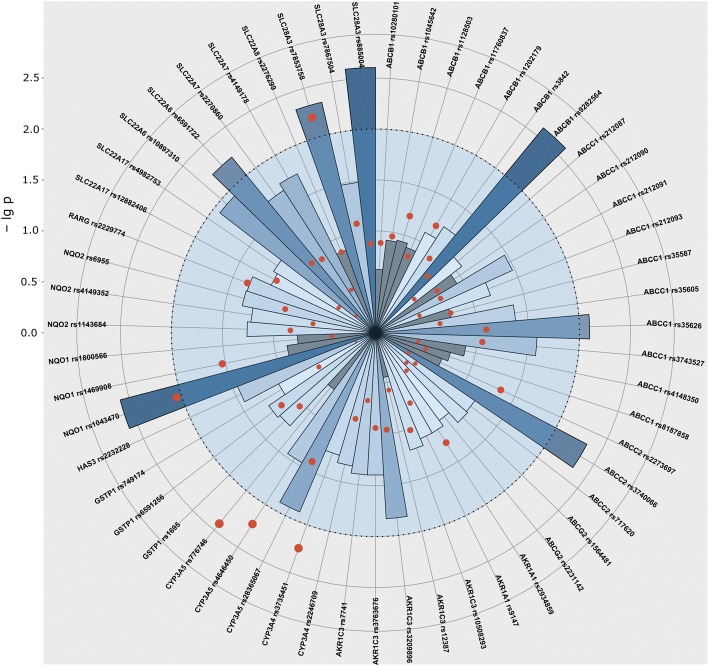
Multi-adjusted logistic regression analyses were used on the full cohort, while univariate logistic regression analyses were implemented on various subpopulations. Case-control analysis was performed for ALL patients in case of all SNPs. Among these, the ones with at least 2 cases in one group are shown in Fig. 1. Risk of pathological FS was significantly influenced by SNPs in CYP3A5 and SLC28A3 genes. CYP3A5 rs4646450 TT was 17% among ALL cases and 3% in ALL patients without pathological FS (p = 5.60E-03; OR = 6.94 (1.76–27.39)). SLC28A3 rs7853758 AA was 12% in ALL cases, while only 1% among controls (p = 6.50E-03; OR = 11.56 (1.98–67.45)). These two SNPs were analyzed in the whole population including both ALL and OSC patients.
Fig. 1.
The p values of the follow-up and case-control studies of the ALL population. Results of the analysis including data of ALL patients are presented in this Figure. The p values are illustrated in a polar coordinate system, where the circular grids represent the negative logarithm of p values (axis on the left can be projected to grids). Intermittent line indicates border at p = 0.01 (− lg p = 2). Columns show the results of the follow-up analysis of FS, darker shades of blue mean stronger significance. The lowest p values were chosen from every follow-up category and from every model, if the number of cases was above 5. (The time of diagnosis was excluded.) Results of the case-control study are shown with red dots, sizes proportional with stronger significance. Most significant results of this plot were studied further on the total cohort including osteosarcoma patients as well
The genotype distribution of the CYP3A5 rs4646450 differed significantly between cases and controls in the combined cohort (ALL and OSC patients) (p = 4.81E-03; OR = 7.25 (1.83–28.78)). Among cases (n = 20) 15% had TT genotype while this value was 2.8% in controls. The genotype distribution of the SLC28A3 rs7853758 SNP was not different between cases (10.5%) and controls (1.3%) in the combined cohort (p = 1.00E-02; OR = 9.837 (1.73–56.02) if considering the corrected p value.
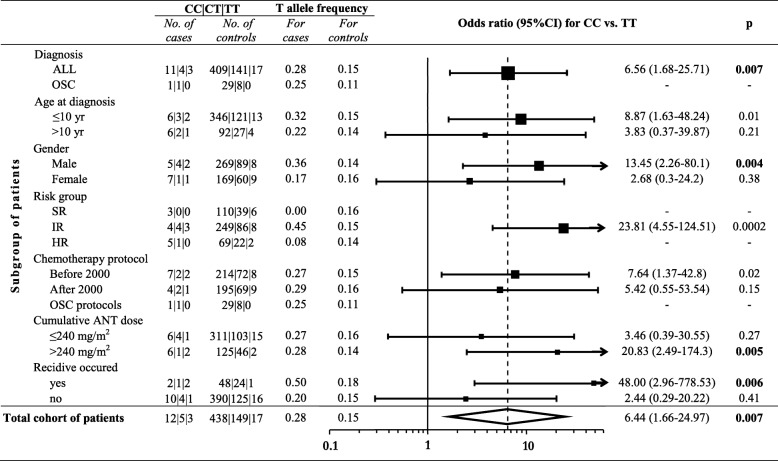
Subsequently, it was investigated whether CYP3A5 rs4646450 was associated with cardiotoxicity in various subpopulations determined by clinical characteristics of the patients (Fig. 2). The CYP3A5 rs4646450 TT genotype was associated with cardiotoxicity in patients with ALL (p = 7.00E-03; OR = 6.56 (1.68–25.71)). Similar association was found when analyzing only male patients (p = 4.00E-03; OR = 13.45 (2.26–80.1)) or intermediate-risk patients (p = 2.00E-04; OR = 23.34 (4.46–122.07)).
Fig. 2.
Odds ratios for cardiotoxicity associated with the CYP3A5 rs4646450 genotype among subgroups of patients and also in the whole cohort. Results of the univariate logistic regression analysis performed on subpopulations of patients and also on the total cohort of patients. Subpopulations are determined based on the following factors: diagnosis, age at diagnosis, gender, risk group, chemotherapy protocol, cumulative ANT dose, recidive occurred. Black boxes represent OR, the number of cases is proportional with the width of the boxes. The lengths of the horizontal lines depict the 95% confidence intervals. Analysis of OR was not accomplished if the number of cases was 0. Abbreviations: ALL, acute lymphoblastic leukemia; OSC, osteosarcoma; no, number; yr., year; SR, standard-risk; IR, intermediate-risk; HR, high-risk
Usage of radiation therapy did not associate with FS reduction below 28% in our cohort. Haplotype analyses were carried out to study the association of haplotype blocks of genes in cardiotoxicity, but no significant results were found.
Follow-up analysis
All of the SNPs were analyzed in relation with EF an FS in the acute lymphoid leukemia population in every follow-up category with all of the three models using multi-adjusted general linear model. Summary of the results from the analyses is shown in Fig. 1. Results with the lowest p value are depicted, except those with less than 5 patients in one group. Significant results of this analysis are shown in Table 3. SNPs with p values < 0.01 were analyzed in the whole population including both ALL and OSC patients.
Table 3.
Significant results of the follow-up analysis in the acute lymphoid leukemia population
| Gene | SNP | Genotype group 1 / group 2 | Mean FS % ± SE genotype group 1 (N) |
Mean FS % ± SE genotype group 2 (N) |
P value | Follow-up category |
|---|---|---|---|---|---|---|
| ABCB1 | rs9282564 | AA / AG + GG |
41.5 ± 0.7 (100) | 37.9 ± 1.1 (29) | 2 .50E-03 | 10–15 years after Dx |
| ABCC1 | rs35626 | GG / GT + TT |
41.0 ± 0.6 (92) | 39.0 ± 0.6 (127) | 7 .90E-03 | 2–5 years after Dx |
| ABCC2 | rs3740066 | GG / GA / AA | 39.5 ± 0.5 (112) | 40.8 ± 0.5 (112) / 42.9 ± 0.9 (33) | 4 .50E-03 | 5–10 years after Dx |
| NQO1 | rs1043470 | CC / CT + TT |
40.9 ± 0.5 (198) | 38.1 ± 0.9 (53) | 2 .60E-03 | acute phase |
| SLC22A6 | rs6591722 | TT + TA / AA |
40.7 ± 0.4 (227) | 37.8 ± 1.0 (28) | 5 .90E-03 | 5–10 years after Dx |
| SLC28A3 | rs7853758 | GG / GA + AA |
41.3 ± 0.7 (96) | 38.4 ± 1.1 (36) | 4 .80E-03 | 10–15 years after Dx |
| SLC28A3 | rs885004 | GG / GA + AA |
41.3 ± 0.7 (95) | 38.0 ± 1.1 (33) | 2 .50E-03 | 10–15 years after Dx |
Results are from multivariate general linear model performed on the ALL cohort adjusted for potential confounders. Abbreviations: Dx, diagnosis; FS, fractional shortening; N, number; SE, standard error
ABCC2 rs3740066 common GG genotype was associated with the poorest left ventricular function during the intensive chemotherapy phase (acute phase) in the whole population (Fig. 3). Patients with GG genotype had lower mean FS (39.5% ± 1.06) value, compared to patients with AA genotype (mean FS = 42.9% ± 1.4; p = 7.38E-03). The ABCC2 rs3740066 GG genotype was also associated with significantly lower mean FS (39.1% ± 0.5; p = 7.11E-04) at 5–10 years after the diagnosis, whereas higher mean FS rates were related to the other genotypes (40.6% ± 0.5, 42.4% ± 0.8; AG, AA, respectively).
Fig. 3.
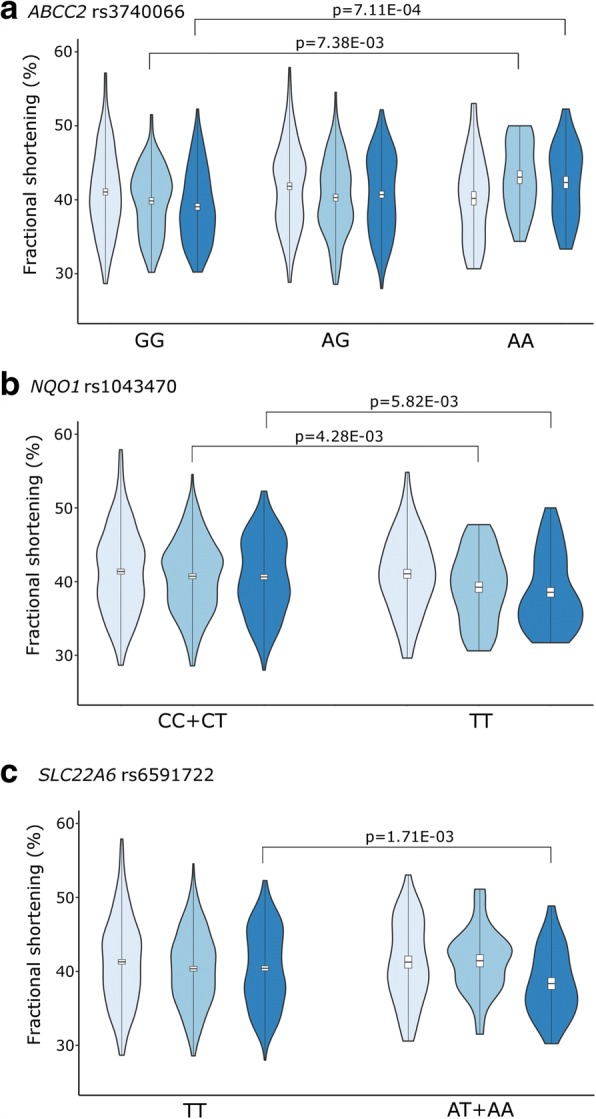
Violin plot of fractional shortening in the total population. FS (%) by genotypes is shown in different follow-up categories. Light blue is the time of diagnosis, medium blue is the time of the anthracycline administration (acute phase), dark blue is the follow-up 5–10 years after therapy. FS is indicated in box plots, box is mean ± S.D., whiskers are means ±3 S.D. Violin plot describes the distribution of FS data, records out of mean ± 3SD are not shown. A: ABCC2 rs3740066; B: NQO1 rs1043470; C: SLC22A6 rs6591722
Patients with NQO1 (NAD(P)H Quinone Dehydrogenase 1) rs1043470 rare T allele had significantly lower mean left ventricular function rates during both phases: in the intensive chemotherapy phase (acute phase) and 5–10 years after the diagnosis (Fig. 3). In the acute phase the T allele was associated with lower mean FS (38.1% ± 1.2; p = 4.28E-03), while patients with at least one C allele had FS = 40.7% ± 0.9. Between 5 and 10 years after the therapy NQO1 rs1043470 rare T allele was associated with lower mean FS (38.5% ± 0.7, p = 5.82E-03), while the values represented with the C allele were higher (FS = 40.6% ± 0.4).
SLC22A6 (Solute Carrier Family 22 Member 6) gene rs6591722 rare AA genotype was associated with lower mean FS (37.5% ± 0.9, p = 1.71E-03) 5–10 years after the diagnosis compared to values of TT and TA genotypes (FS: 40.6% ± 0.4) (Fig. 3).
The other SNPs were not associated with heart function parameters. Results regarding ejection fraction were consistent in the direction of those described above with fractional shortening.
Analysis of fractional shortening alteration
When computing alteration of fractional shortening from diagnosis until the end of the treatment or the last echo, difference of FS was more than 3% or less than 3% in 67–71% of the patients in all groups. The alteration of fractional shortening from diagnosis until the end of the treatment or the last echo ever measured was also analyzed for patients with ALL for all SNPs. Among these the ones with at least 2 cases in one group are shown in Fig. 1, if the p value was lower than the p value of case-control analysis. SNPs with p values < 0.01 are shown in Table 4. These were analyzed in the whole population including both ALL and OSC patients and were not significant.
Table 4.
Significant results of the analysis of fractional shortening alteration in the acute lymphoid leukemia population
| Gene | SNP | Genotype group 1 / group 2 | Patients with decreased FS in genotype groups N (%) | Patients with increased FS in genotype groups N (%) | P value | OR (CI 95%) |
|---|---|---|---|---|---|---|
| Alteration of FS: diagnosis vs. end of therapy | ||||||
| CYP3A4 | rs3735451 | AA / AG + GG |
74 (82) / 16 (18) |
52 (63) / 31 (37) |
5.70E-03 | 0.36 (0.18–0.74) |
| CYP3A5 | rs776746 | GG / GA + AA |
81 (91) / 8 (9) |
60 (73) / 22 (27) |
3.80E-03 | 0.26 (0.11–0.65) |
| Alteration of FS: diagnosis vs. last echocardiography | ||||||
| NQO1 | rs1043470 | CC / CT + TT |
111 (85) / 41 (15) |
112 (73) / 20 (27) |
8.90E-03 | 0.44 (0.24–0.81) |
Results are from logistic regression performed on the ALL cohort adjusted for potential confounders. Abbreviations: CI, confidence interval; FS, fractional shortening; N, number; OR, odds ratio
Bayesian network based Bayesian multilevel analysis of relevance
Bayesian network based Bayesian multilevel analysis of relevance (BN-BMLA) method was performed for SNPs in the ABCB1, ABCC1, ABCC2, ABCG2, AKR1A1, AKR1C3, CYP3A4, CYP3A5, GSTP1, HAS3, NQO1, NQO2, RARG, SLC22A17, SLC22A6, SLC22A7, SLC22A8 and SLC28A3 genes along with cofactors. This method aims to find the most probably strongly relevant variables with respect to the case-control status of the patients. The strongly relevant variables have a direct influence on the target. Values for posterior probability of strong relevance (P) range from 0 to 1, where P = 1 means that the probability of the given variable is 100% relevant with respect to the case-control status. Our analyses revealed potentially strongly relevant effects of a SNP in gene CYP3A5 (rs776746, P = 0.42), two SNPs in gene NQO1 (rs1043470 and rs1469908, P = 0.42 and 0.34, respectively), two SNPs in gene SLC28A3 (rs7853758 and rs885004, P = 0.55 and 0.36, respectively), and several cofactors (age at the time of diagnosis, P = 0.72; gender, P = 0.44; risk group, P = 0.73; diagnosis (ALL vs. OSC), P = 0.8 and cumulative dose of anthracycline, P = 0.64). Besides, several interaction effects were found between the variables. Among these, the two SNPs (rs7853758 and rs885004) in gene SLC28A3 showed the strongest interaction. However, as the number of cases was low, these interaction effects could not be confirmed with logistic regression models using interaction terms.
Discussion
In this study, we evaluated the association of 66 single nucleotide polymorphisms and anthracycline- induced cardiotoxicity (ACT) developed during or after the treatment in acute lymphoblastic leukemia and osteosarcoma patients. SNPs in four investigated genes (ABCC2, NQO1, SLC22A6 and SLC28A3) were associated with decreased FS and EF. Regarding the aforementioned genes, the acute phase and the period of 5–10 years after the diagnosis were especially important. CYP3A5 SNP appeared to be a predictor for ACT; the association was more prominent in boys, in ALL patients and in the intermediate risk group.
It must be noted that there are some potential biases of this study. Because of the retrospective data collection not all of the ECHO records were available. Therefore, the analysis of ECHO was not possible for every year; categories of follow-up were generated. Only the data of the latest ECHO of each patient were used in each follow-up category, the redundant echocardiography measurements were excluded. Nevertheless, the large patient population and long follow-up make our study notable. Also, it has to be mentioned that patients who died before the period of sample collection are underrepresented in our cohort. In our opinion, this is not a relevant bias, as late effects only manifested and have relevance in survivors. Furthermore, according to the data of the Hungarian Pediatric Cancer Registry, only three patients in our cohort did die of cardiac-related events (endocarditis, ventricular insufficiency and one patient died of cardiomyopathia). Controls have 1–7 echocardiogram assessments in our cohort (21% of the patients had only one echo and 60% of patients had 3 or more echos). Still we think that our results are real in our cohort, as statistical analyses performed using smaller cohort of controls show the same direction.
ABCC2
In our study, during the treatment and after 5–10 years of the therapy ABCC2 rs3740066 common GG genotype was associated with decreased FS and EF values. ABCC2 (a.k.a. MRP2; 10q24.2) is a member of the ATP binding cassette subfamily. ABCC2 is responsible for organic anion transmembrane transport and its substrates also include anticancer drugs, antibiotics and statins. The efflux activity of ABCC2 is involved in multidrug resistance. Expression of ABCC2 is at critical sites of uptake and elimination, including the hepatobiliary tract, intestine, kidney and blood-tissue barriers [63]. ABCC2 is a frequently investigated gene for instance in drug-related toxicities, in therapy-response, resistance against various drugs, in carcinogenesis and in the outcomes of osteosarcoma and leukemia [64–70]. There are also several findings in the field of cardiotoxicity regarding ABCC2. Wojnowski et al. studied acute and chronic ACT in adult patients with Non-Hodgkin lymphoma (NHL). Acute ACT was associated with one haplotype of the ABCC2 gene (rs8187694-rs8187710) [38]. Association of ABCC2 rs3740066 with cardiac parameters was previously not published in the literature. We found the same gene but different ABCC2 SNP to be associated with acute and chronic ACT. A possible explanation for this divergence might be the different phenotyping method, different target SNPs and the different population: age groups, tumor types, and chemotherapies. Armenian et al. revealed that the rare allele of ABCC2 rs8187710 was over-represented in survivors of hematopoietic cell transplantation patients who developed anthracycline-related congestive heart failure [71]. A meta-analysis of twenty-eight studies found increased risk of ACT in a strong association within ABCC2 gene, with the above mentioned rs8187710 SNP, which is near to rs3740066 [50]. There have also been several studies investigating ABCC2 rs3740066. A research of Lopez-Lopez et al. studied the methotrexate (MTX) plasma levels and SNPs in pediatric ALL patients, focusing on adverse events. They suggest rs3740066 as a predictor to prevent MTX toxicity [9]. Hegyi et al. investigated the pharmacokinetics of MTX among osteosarcoma pediatric patients. In their analysis AUC0–48 (area under the concentration–time curve) was significantly lower in patients with homozygous variant genotype of rs3740066 [52]. The potential function of rs3740066 SNP is not fully understood yet. It may modify the mRNA stability or act together with rs572344237 SNP at the transcriptional level [72].
NQO1
In our study, rs1043470 was connected with reduced cardiac function rates during the treatment and between the fifth and tenth years after the therapy. Rs1043470 is located in the 3’UTR region of both NQO1 (nicotinamide adenine dinucleotide phosphate: quinone oxidoreductase 1) and NFAT5 (Nuclear Factor Of Activated T-Cells 5) genes, as NQO1 is transcribed from the complementary strand. Nuclear factor-activated T- cell 5 (NFAT5) plays a role against hyperosmotic stress, it is also expressed in the heart. NQO1 is a cytoplasmic 2-electron reductase, it reduces quinone to hydroquinone. NQO1 prevents oxidative stress and defends against pro-oxidant drugs like anthracyclines [73]. The SNP which seemed to be relevant in our study has not been studied in the literature yet, although there are several SNPs in the NQO1 gene which were reported to be important from a clinical point of view. In a study of childhood ALL patients the outcome was worse in carriers of an NQO1 variant [74]. Dunna et al. studied the effect of rs1800566, which is only approximately 7000 base pair distance away from the rs1043470 investigated in our study. Rs1800566 (NQO1*2) was associated with poorer outcome in patients treated with anthracycline for breast cancer [75]. Szkandera et al. in a breast cancer population failed to demonstrate the effect of rs1800566 on the therapy-response of anthracyclines [76]. In a cardiomyocyte cell culture investigation of NFAT5 showed lower protein levels, but not on the mRNA level after doxorubicin treatment. Effects of doxorubicin were the degradation of NFAT5 protein and limitation of the viability of cardiomyocytes. Ito et al. proposed NFAT5 as a new positive marker of cardiomyocyte survival [77]. Lagoa et al. studied rats treated with doxorubicin. They experienced the down-regulation of Nqo1 and increasing ROS production during the therapy. They suggested using this molecule as an early biomarker in the doxorubicin cardiotoxicity [78].
Rs1043470, studied by us, is located in a 3’UTR region of both NQO1 and NFAT5. The localization of the SNPs might provide new binding sites for miRs (microRNAs) or affect the binding ability of them and these may result changes in the translation. According to the PolymiRTS Database 3.0 database, one miR binds our investigated SNP, it is called hsa-mir-6863 [79]. However, presently it is not known whether NQO1, NFAT5 or both have a role in this respect.
SLC22A6 and SLC28A3
According to our results five to ten years after the diagnosis rs6591722 of SLC22A6 gene was in correlation with lower cardiac function. This is the first time that SLC22A6 gene polymorphism is found to be associated with cardiac function. Solute Carrier Family 22 Member 6 is involved in renal excretion of organic anions, toxic ones are also included. Renal Slc22a6 was down-regulated after MTX treatment in rats [80]. In an in vitro study, indoxyl sulfate correlated adverse cardiac effects were inhibited by SL22A6, it blocked entering the toxin into cardiac cells [81].
The genotype distribution of the SLC28A3 rs7853758 was also significantly different between cases and controls. In many studies SLC28A3 rs7853758 is proved to be a very important protective genetic marker against ACT, its minor allele (A) found more often in controls than in patient cases [37, 82]. This SNP was recommended for clinical use in pharmacogenetic testing before using doxorubicin or daunorubicin in pediatric cancer patients’ treatment. [49]. This correlation for chronic cardiotoxicity was not significant in another cohort [83], nor RICOVER-60 trial found association with adverse cardiac reactions [84]. In contrast to these analyses, in our cohort the SLC28A3 rs7853758 AA genotype was more frequent among cases. However, not only in the case- control study but also in the BN-BMLA we could confirm the importance of this variant which needs further validation in larger cohorts.
CYP3A5
The CYP3A5 rs4646450 TT genotype associated with fractional shortening lower than 28% in our joined cohort. The BN-BMLA showed that the CYP3A5 rs776746 was potentially relevant in our case-control analysis. Previous studies in the literature did not find association of CYP3A5 rs4646450 with cardiac parameters [48]. CYP3A5 rs776746 AG/AA seemed to increase the risk of grade 2–4 cardiac toxicity in diffuse large B-cell lymphoma patients [85]. Our BN-BMLA analysis revealed potential strongly relevant effect of rs776746 SNP in gene CYP3A5 with respect to the case-control status of the patients. CYP3A5 gene (7q21.1) is a member of cytochrome P450 proteins involved in drug metabolism, synthesis of steroids and lipids. CYP3A5 is expressed in the liver and also in extrahepatic tissues e.g. in intestines. Genetic variability of CYP3A5 is high; it is not expressed in 20% of African and 80% of Caucasian population. SNPs in CYP3A5 (rs776746 and rs10264272) may modify its alternative splicing and protein truncation, which can result in a less active CYP3A5 [86]. Huang et al. studied the CYP3A5 enzyme activity with the presence of its different gene polymorphisms in pediatric ALL patients. They revealed that patients with rs776746 had lower enzyme activity. Rs776746 was in association with the mRNA expression, daunorubicin plasma concentration and adverse drug reactions. In this investigation the AUC of daunorubicin was higher in children with cardiotoxicity [87]. In our population the intronic CYP3A5 rs4646450 SNP, was associated with low FS (< 28%, cases) with a significantly higher OR value in males indicating gender related differences. Both male and female gender has been already shown as risk factors for developing cardiotoxicity [88–90]. Difference in the adverse drug reactions between man and woman might be explained with their genetic background and there might be associations only in one of the genders [91]. Presently, the function of this SNP is not known, however, in a study rs4646450 was in correlation with reduced protein-expression and activity of CYP3A4 in human liver [92]. However, these results require further validation.
Conclusions
In this study we confirmed that genetic variations in genes coding transporters and metabolizing enzymes might influence the anthracycline-induced cardiotoxicity. For the utilization of these findings, further validations and functional analysis of the implicated genetic variations are needed. International cooperation is required to be able to gather patient population with appropriate statistical power. The confirmed variants should be tested in clinical trials with long-term follow-up, because of late cardiotoxicity. Today we are only at the beginning of this process, but regarding the huge development of medicine in the last few decades and with the help of a user-friendly decision–support system, we can be sure that the number of usable pharmacogenomic tests will be expanded in the future, contributing to more effective personal therapies.
Additional file
Information about the studied SNPs. Information on the selected SNPs is shown in an additional table file in more detail (gene, SNP ID, chromosome, position, function, alleles and minor allele frequencies). (XLSX 14 kb)
Acknowledgements
We are thankful to all the patients and control subjects, nurses, physicians who took part in this study. We also thank Mónika Sándorné Vángor for the technical assistance.
Funding
This study was supported by National Research, Development and Innovation Office (NKFIH) Grants No. PD109200 (ÁF Semsei) and K115861 (DJ Erdélyi). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Availability of data and materials
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ABCB1
ATP Binding Cassette Subfamily B Member 1
- ABCC1
ATP Binding Cassette Subfamily C Member 1
- ABCC2
ATP Binding Cassette Subfamily C Member 2
- ACT
anthracycline-induced cardiotoxicity
- ALL BFM
Berlin–Frankfurt–Münster
- ALL
acute lymphoblastic leukemia
- AUC
area under the concentration–time curve
- BN-BMLA
Bayesian network based Bayesian multilevel analysis of relevance
- COSS
German-Austrian-Swiss osteosarcoma study group
- CYP3A4
Cytochrome P450 Family 3 Subfamily A Member 4
- CYP3A5
Cytochrome P450 Family 3 Subfamily A Member 5
- ECHO
echocardiography
- EF
ejection fraction
- FS
fractional shortening
- GWA
genome-wide association
- LVEDD
Left ventricular end-diastolic-diameter
- LVESD
Left ventricular end-systolic-diameter
- miR
microRNA
- MTX
methotrexate
- NFAT5
Nuclear Factor Of Activated T-Cells 5
- NQO1
NAD(P)H Quinone Dehydrogenase 1
- OR
odds ratio
- OSC
osteosarcoma
- RARG
Retinoic Acid Receptor Gamma
- SLC22A6
Solute Carrier Family 22 Member 6
- SLC28A3
Solute Carrier Family 28 Member 3
- SNP
single nucleotide polymorphism
- VACTERL
vertebral defects, anal atresia, cardiac defects, tracheo-esophageal fistula, renal anomalies, and limb abnormalities
Authors’ contributions
JCS performed the laboratory work, data analysis and wrote the manuscript, BE performed bioinformatics and statistical analysis. AK and NK performed genotyping. MH and OG collected clinical data and contributed to interpretation of data. AG performed statistical analysis. MAH performed genotyping. AR and LEF performed the laboratory work and collected clinical data. GTK supervised the research, contributed to experimental discussion and provided patient samples. DJE designed and supervised the study, critically reviewed the manuscript. CS supervised the research, contributed to experimental discussion and reviewed the manuscript. AFS designed the study, analyzed the data and wrote the paper. All authors read and approved the final manuscript.
Ethics approval and consent to participate
All patients or legal guardians of the patients provided written informed consent in accordance with the Helsinki Declaration. The written informed consent was obtained from the participants or the legal guardians of participants under the age of 16 before they entered the study. The present study was approved by the local ethics committee (Ethics Committee of the Hungarian Medical Research Council). Approval file number 23310–1/2011/EKU, Date: 19th January 2012.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Judit C. Sági and Bálint Egyed contributed equally to this work.
Judit C. Sági and Bálint Egyed are co-first authors.
Electronic supplementary material
The online version of this article (10.1186/s12885-018-4629-6) contains supplementary material, which is available to authorized users.
Contributor Information
Judit C. Sági, Email: sagi.judit@med.semmelweis-univ.hu
Bálint Egyed, Email: egyed.b4lint@gmail.com.
Andrea Kelemen, Email: kelemen.andrea93@gmail.com.
Nóra Kutszegi, Email: norakutszegi@hotmail.com.
Márta Hegyi, Email: marta.hegyi@gmail.com.
András Gézsi, Email: gezsi.andras@gmail.com.
Martina Ayaka Herlitschke, Email: martina_herlit@yahoo.co.jp.
Andrea Rzepiel, Email: rzepiel.andrea@med.semmelweis-univ.hu.
Lili E. Fodor, Email: fodorlilierika@gmail.com
Gábor Ottóffy, Email: ottoffy.gabor@pte.hu.
Gábor T. Kovács, Email: kovacs.gabor1@med.semmelweis-univ.hu
Dániel J. Erdélyi, Email: erdelyi.daniel@med.semmelweis-univ.hu
Csaba Szalai, Email: szalaics@gmail.com.
Ágnes F. Semsei, Phone: +36-1-2102930, Email: semsei.agnes@med.semmelweis-univ.hu
References
- 1.Kaatsch P. Epidemiology of childhood cancer. Cancer Treat Rev. 2010;36:277–85. [DOI] [PubMed]
- 2.Vos HI, Coenen MJ, Guchelaar HJ, Te Loo DM. The role of pharmacogenetics in the treatment of osteosarcoma. Drug Discov Today. 2016;21:1775–1786. doi: 10.1016/j.drudis.2016.06.022. [DOI] [PubMed] [Google Scholar]
- 3.Garami M, Schuler D, Jakab Z. Az Országos Gyermektumor Regiszter jelentősége a gyermekonkológiai ellátásban Importance of the National Childhood Cancer Registry in the field of paediatric oncology care. Orv Hetil. 2014;155:732–739. doi: 10.1556/OH.2014.29918. [DOI] [PubMed] [Google Scholar]
- 4.Pui C, Evans W. Treatment of acute lymphoblastic leukemia. N Engl J Med. 2006;354:166–178. doi: 10.1056/NEJMra052603. [DOI] [PubMed] [Google Scholar]
- 5.Carroll WL, Raetz EA. Clinical and laboratory biology of childhood acute lymphoblastic leukemia. J Pediatr. 2012;160:10–18. doi: 10.1016/j.jpeds.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 6.Gaynon PS, Angiolillo AL, Carroll WL, Nachman JB, Trigg ME, Sather HN, et al. Long Term Results of the Children’s Cancer Group Studies for Childhood Acute Lymphoblastic Leukemia 1983–2002: a Children’s Oncology Group Report NIH Public Access. Leukemia. 2010;24:285–297. doi: 10.1038/leu.2009.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fulbright JM, Raman S, McClellan WS, August KJ. Late effects of childhood leukemia therapy. Curr Hematol Malig Rep. 2011;6:195–205. doi: 10.1007/s11899-011-0094-x. [DOI] [PubMed] [Google Scholar]
- 8.Ansari M, Sauty G, Labuda M, Gagné V, Rousseau J, Moghrabi A, et al. Polymorphism in multidrug resistance-associated protein gene 3 is associated with outcomes in childhood acute lymphoblastic leukemia. Pharmacogenomics J. 2012;1217:386–394. doi: 10.1038/tpj.2011.17. [DOI] [PubMed] [Google Scholar]
- 9.Lopez-Lopez E, Ballesteros J, Piñan MA, Sanchez de Toledo J, Garcia de Andoin N, Garcia-Miguel P, et al. Polymorphisms in the methotrexate transport pathway: a new tool for MTX plasma level prediction in pediatric acute lymphoblastic leukemia. Pharmacogenet Genomics. 2013;23:53–61. doi: 10.1097/FPC.0b013e32835c3b24. [DOI] [PubMed] [Google Scholar]
- 10.Robison LL. Late effec ts of acute lymphoblastic leukemia therapy in patients diagnosed at 0-20 years of age. Hematol Am Soc Hematol Educ Program. 2011;2011:238–42 [DOI] [PubMed]
- 11.Sundberg KK, Doukkali E, Lampic C, Eriksson LE, Arvidson J, Wettergren L. Long-term survivors of childhood Cancer report quality of life and health status in parity with a comparison group. Pediatr Blood Cancer. 2010;55:337–343. doi: 10.1002/pbc.22492. [DOI] [PubMed] [Google Scholar]
- 12.Kremer LC, van der Pal HJ, Offringa M, van Dalen EC, Voûte PA. Frequency and risk factors of subclinical cardiotoxicity after anthracycline therapy in children: a systematic review. Ann Oncol. 2002;13:819–829. doi: 10.1093/annonc/mdf167. [DOI] [PubMed] [Google Scholar]
- 13.Shelmerdine SC, Chavhan GB, Babyn PS, Nathan PC, Kaste SC. Imaging of acute and subacute toxicities of cancer therapy in children. Pediatr Radiol. 2017;47:254–266. doi: 10.1007/s00247-016-3708-6. [DOI] [PubMed] [Google Scholar]
- 14.Billett A. How should pediatric Cancer be included in the Cancer moonshot? Oncol Times. 2016;38:16–17. doi: 10.1097/01.COT.0000502636.99578.21. [DOI] [Google Scholar]
- 15.Armstrong GT, Kawashima T, Leisenring W, Stratton K, Stovall M, Hudson MM, et al. Aging and risk of severe, disabling, life-threatening, and fatal events in the childhood cancer survivor study. J Clin Oncol. 2014;32:1218–1227. doi: 10.1200/JCO.2013.51.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marder J. Childhood’s Cures Haunted By Adulthood’s ‘Late Effects’. Science. 2010;328:1474–5. [DOI] [PubMed]
- 17.Armstrong GT, Liu Q, Yasui Y, Neglia JP, Leisenring W, Robison LL, et al. Late mortality among 5-year survivors of childhood cancer: a summary from the childhood cancer survivor study. J Clin Oncol. 2009;27:2328–2338. doi: 10.1200/JCO.2008.21.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lipshultz SE, Alvarez JA, Scully RE. Anthracycline associated cardiotoxicity in survivors of childhood cancer. Heart. 2008;94:525–533. doi: 10.1136/hrt.2007.136093. [DOI] [PubMed] [Google Scholar]
- 19.Vandecruys E, Mondelaers V, De Wolf D, Benoit Y, Suys B. Late cardiotoxicity after low dose of anthracycline therapy for acute lymphoblastic leukemia in childhood. J Cancer Surviv. 2012;6:95–101. doi: 10.1007/s11764-011-0186-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruggiero A, De Rosa G, Rizzo D, Leo A, Maurizi P, De Nisco A, et al. Myocardial performance index and biochemical markers for early detection of doxorubicin-induced cardiotoxicity in children with acute lymphoblastic leukaemia. Int J Clin Oncol. 2013;18:927–933. doi: 10.1007/s10147-012-0458-9. [DOI] [PubMed] [Google Scholar]
- 21.Bayraktar S, Glück S. Systemic therapy options in BRCA mutation-associated breast cancer. Breast Cancer Res Treat. 2012;135:355–366. doi: 10.1007/s10549-012-2158-6. [DOI] [PubMed] [Google Scholar]
- 22.Mikhail M, Mekhail Y, Mekhail T. Thymic neoplasms: a clinical update. Curr Oncol Rep. 2012;14:350–358. doi: 10.1007/s11912-012-0246-8. [DOI] [PubMed] [Google Scholar]
- 23.Sokol L, Naghashpour M, Frank Glass L. Primary cutaneous B-cell lymphomas: recent advances in diagnosis and management. Cancer Control. 2012;19:236–244. doi: 10.1177/107327481201900308. [DOI] [PubMed] [Google Scholar]
- 24.dos Santos LV, Lima J, Lima CS, Sasse EC, Sasse AD. Is there a role for consolidative radiotherapy in the treatment of aggressive and localized non-Hodgkin lymphoma? A systematic review with meta-analysis. BMC Cancer. 2012;12:288. doi: 10.1186/1471-2407-12-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ray-Coquard I, Le Cesne A. A role for maintenance therapy in managing sarcoma. Cancer Treat Rev. 2012;38:368–378. doi: 10.1016/j.ctrv.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 26.Fulbright JM. Review of cardiotoxicity in pediatric Cancer patients: during and after therapy. Cardiol Res Pract. 2011;2011:1–9. doi: 10.4061/2011/942090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mulrooney DA, Yeazel MW, Kawashima T, Mertens AC, Mitby P, Stovall M, et al. Cardiac outcomes in a cohort of adult survivors of childhood and adolescent cancer: retrospective analysis of the childhood Cancer survivor study cohort. BMJ. 2009;339:b4606. doi: 10.1136/bmj.b4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yeh ET, Bickford CL. Cardiovascular complications of Cancer therapy. J Am Coll Cardiol. 2009;53:2231–2247. doi: 10.1016/j.jacc.2009.02.050. [DOI] [PubMed] [Google Scholar]
- 29.Viale PH, Yamamoto DS. Cardiovascular toxicity associated with cancer treatment. Clin J Oncol Nurs. 2008;12:627–638. doi: 10.1188/08.CJON.627-638. [DOI] [PubMed] [Google Scholar]
- 30.Iarussi D, Indolfi P, Casale F, Martino V, Di Tullio MT, Calabrò R. Anthracycline-induced cardiotoxicity in children with cancer: strategies for prevention and management. Pediatr Drugs. 2005;7:67–76. [DOI] [PubMed]
- 31.Mordente A, Meucci E, Silvestrini A, Martorana GE, Giardina B. New developments in anthracycline-induced cardiotoxicity. Curr Med Chem. 2009;16:1656–1672. doi: 10.2174/092986709788186228. [DOI] [PubMed] [Google Scholar]
- 32.Menna P, Recalcati S, Cairo G, Minotti G. An introduction to the metabolic determinants of anthracycline cardiotoxicity. Cardiovasc Toxicol. 2007;7:80–85. doi: 10.1007/s12012-007-0011-7. [DOI] [PubMed] [Google Scholar]
- 33.Lipshultz SE, Adams MJ. Cardiotoxicity after childhood cancer: beginning with the end in mind. J Clin Oncol. 2010;28:1276–1281. doi: 10.1200/JCO.2009.26.5751. [DOI] [PubMed] [Google Scholar]
- 34.Hershman DL, Neugut AI. Anthracycline cardiotoxicity: one size does not fit all! J Natl Cancer Inst. 2008;100:1046–1047. doi: 10.1093/jnci/djn241. [DOI] [PubMed] [Google Scholar]
- 35.Mody R, Li S, Dover DC, Sallan S, Leisenring W, Oeffinger KC, et al. Twenty-five-year follow-up among survivors of childhood acute lymphoblastic leukemia: a report from the childhood Cancer survivor study. Blood. 2008;111:5515–5523. doi: 10.1182/blood-2007-10-117150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boyett J, Cheng C, Pei D, Pui C, Childhood Acute Lymphoblastic Leukaemia Collaborative Group Beneficial and harmful effects of anthracyclines in the treatment of childhood acute lymphoblastic leukaemia: a systematic review and meta-analysis. Br J Haematol. 2009;145:376–388. doi: 10.1111/j.1365-2141.2009.07624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Visscher H, Ross CJ, Rassekh SR, Barhdadi A, Dubé MP, Al-Saloos H, et al. Pharmacogenomic prediction of anthracycline-induced cardiotoxicity in children. J Clin Oncol. 2012;30:1422–1428. doi: 10.1200/JCO.2010.34.3467. [DOI] [PubMed] [Google Scholar]
- 38.Wojnowski L, Kulle B, Schirmer M, Schlüter G, Schmidt A, Rosenberger A, et al. NAD(P)H oxidase and multidrug resistance protein genetic polymorphisms are associated with doxorubicin-induced cardiotoxicity. Circulation. 2005;112:3754–3762. doi: 10.1161/CIRCULATIONAHA.105.576850. [DOI] [PubMed] [Google Scholar]
- 39.Visscher H, Amstutz U, Sistonen J, Ross CJ, Hayden MR, Carleton BC. Pharmacogenomics of cardiovascular drugs and adverse effects in pediatrics. J Cardiovasc Pharmacol. 2011;58:228–239. doi: 10.1097/FJC.0b013e3182163b82. [DOI] [PubMed] [Google Scholar]
- 40.Bains OS, Takahashi RH, Pfeifer TA, Grigliatti TA, Reid RE, Riggs KW. Two allelic variants of aldo-keto reductase 1A1 exhibit reduced in vitro metabolism of daunorubicin. Drug Metab Dispos. 2008;36:904–910. doi: 10.1124/dmd.107.018895. [DOI] [PubMed] [Google Scholar]
- 41.Bains OS, Karkling MJ, Grigliatti TA, Reid RE, Riggs KW. Two nonsynonymous single nucleotide polymorphisms of human carbonyl reductase 1 demonstrate reduced in vitro metabolism of Daunorubicin and doxorubicin. Drug Metab Dispos. 2009;37:1107–1114. doi: 10.1124/dmd.108.024711. [DOI] [PubMed] [Google Scholar]
- 42.Lal S, Sandanaraj E, Wong ZW, Ang PC, Wong NS, Lee EJ, et al. CBR1 and CBR3 pharmacogenetics and their influence on doxorubicin disposition in Asian breast cancer patients. Cancer Sci. 2008;99:2045–2054. doi: 10.1111/j.1349-7006.2008.00744.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lal S, Wong ZW, Sandanaraj E, Xiang X, Ang PC, Lee EJ, et al. Influence of ABCB1 and ABCG2 polymorphisms on doxorubicin disposition in Asian breast cancer patients. Cancer Sci. 2008;99:816–823. doi: 10.1111/j.1349-7006.2008.00744.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bhatia S. Role of genetic susceptibility in development of treatment-related adverse outcomes in cancer survivors. Cancer Epidemiol Biomark Prev. 2011;20:2048–2067. doi: 10.1158/1055-9965.EPI-11-0659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lal S, Mahajan A, Chen WN, Chowbay B. Pharmacogenetics of target genes across doxorubicin disposition pathway: a review. Curr Drug Metab. 2010;11:115–128. doi: 10.2174/138920010791110890. [DOI] [PubMed] [Google Scholar]
- 46.Thorn C, Oshiro C, Marsh S, Hernandez-Boussard T, McLeod H, Klein T, et al. Doxorubicin pathways:pharmacodynamics and adverse effects. Pharmacogenet Genomics. 2011;21:440–446. doi: 10.1097/FPC.0b013e32833ffb56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rajić V, Aplenc R, Debeljak M, Prestor VV, Karas-Kuzelicki N, Mlinaric-Rascan I, et al. Influence of the polymorphism in candidate genes on late cardiac damage in patients treated due to acute leukemia in childhood. Leuk Lymphoma. 2009;50:1693–1698. doi: 10.1080/10428190903177212. [DOI] [PubMed] [Google Scholar]
- 48.Sági JC, Kutszegi N, Kelemen A, Fodor LE, Gézsi A, Kovács GT, et al. Pharmacogenetics of anthracyclines. Pharmacogenomics. 2016;17:1075–87. [DOI] [PubMed]
- 49.Aminkeng F, Ross CJ, Rassekh SR, Hwang S, Rieder MJ, Bhavsar AP, et al. Recommendations for genetic testing to reduce the incidence of anthracycline-induced cardiotoxicity. Br J Clin Pharmacol. 2016;82:683–95. [DOI] [PMC free article] [PubMed]
- 50.Leong SL, Chaiyakunapruk N, Lee SW. Candidate Gene Association studies of anthracycline-induced cardiotoxicity: a systematic review and meta-analysis. Sci Rep. 2017;7:39. doi: 10.1038/s41598-017-00075-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Erdilyi DJ, Kámory E, Csókay B, Andrikovics H, Tordai A, Kiss C, et al. Synergistic interaction of ABCB1 and ABCG2 polymorphisms predicts the prevalence of toxic encephalopathy during anticancer chemotherapy. Pharmacogenomics J. 2008;8:321–327. doi: 10.1038/sj.tpj.6500480. [DOI] [PubMed] [Google Scholar]
- 52.Hegyi M, Csordas K, Eipel O, Csagoly E, Erdelyi DJ, Semsei AF, et al. Pharmacogenetic analysis of high-dose methotrexate treatment in paediatric osteosarcoma. Oncotarget. 2017;8:9388–9398. doi: 10.18632/oncotarget.11543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aminkeng F, Bhavsar AP, Visscher H, Rassekh SR, Li Y, Lee JW, et al. A coding variant in RARG confers susceptibility to anthracycline-induced cardiotoxicity in childhood cancer. Nat Genet. 2015;47:1079–1084. doi: 10.1038/ng.3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang X, Liu W, Sun CL, Armenian SH, Hakonarson H, Hageman L, et al. Hyaluronan synthase 3 variant and anthracycline-related cardiomyopathy: a report from the Children’s oncology group. J Clin Oncol. 2014;32:647–653. doi: 10.1200/JCO.2013.50.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wheeler HE, Gamazon ER, Stark AL, Donnell PHO, Lidija K, Huang RS, et al. Genome-wide meta-analysis identifies variants associated with platinating agent susceptibility across populations. Pharmacogenomics J. 2013;13:35–43. doi: 10.1038/tpj.2011.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.HapMap database. p. http://hapmap.ncbi.nlm.nih.gov/ Accessed 14/11/16.
- 57.Banlaki Z, Elek Z, Nanasi T, Szekely A, Nemoda Z, Sasvari-Szekely M, et al. Polymorphism in the serotonin receptor 2a (HTR2A) gene as possible predisposal factor for aggressive traits. PLoS One. 2015;10:e0117792. doi: 10.1371/journal.pone.0117792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tests for deviation from Hardy-Weinberg equilibrium p. https://ihg.gsf.de/cgi-bin/hw/hwa1.pl.
- 59.B enjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc. 1995;57:289–300.
- 60.Storey JD. A direct approach to false discovery rates. J R Stat Soc. 2002;64:479–498. doi: 10.1111/1467-9868.00346. [DOI] [Google Scholar]
- 61.Haploview 4.1 software. p. http://www.broad.mit.edu/mpg/haploview/.
- 62.Lautner-Csorba O, Gézsi A, Erdélyi DJ, Hullám G, Antal P, Semsei ÁF, et al. Roles of genetic polymorphisms in the folate pathway in childhood acute lymphoblastic leukemia evaluated by Bayesian relevance and effect size analysis. PLoS One. 2013;8:e69843. [DOI] [PMC free article] [PubMed]
- 63.Sissung TM, Baum CE, Kirkland CT, Gao R, Gardner ER, Figg WD. Pharmacogenetics of membrane transporters: an update on current approaches. Mol Biotechnol. 2010;44:152–167. doi: 10.1007/s12033-009-9220-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vulsteke C, Pfeil AM, Maggen C, Schwenkglenks M, Pettengell R, Szucs TD, et al. Clinical and genetic risk factors for epirubicin-induced cardiac toxicity in early breast cancer patients. Breast Cancer Res Treat. 2015;152:67–76. doi: 10.1007/s10549-015-3437-9. [DOI] [PubMed] [Google Scholar]
- 65.Marsh S, Hoskins JM. Irinotecan pharmacogenomics. Pharmacogenomics. 2011;11:1003–1010. doi: 10.2217/pgs.10.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Qu J, Zhou BT, Yin JY, Xu XJ, Zhao YC, Lei GH, et al. ABCC2 polymorphisms and haplotype are associated with drug resistance in chinese epileptic patients. CNS Neurosci Ther. 2012;18:647–651. doi: 10.1111/j.1755-5949.2012.00336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sun N, Sun X, Chen B, Cheng H, Feng J, Cheng L, et al. MRP2 and GSTP1 polymorphisms and chemotherapy response in advanced non-small cell lung cancer. Cancer Chemother Pharmacol. 2010;65:437–446. doi: 10.1007/s00280-009-1046-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Andersen V, Vogel LK, Kopp TI, Sæbø M, Nonboe AW, Hamfjord J, et al. High ABCC2 and low ABCG2 gene expression are early events in the colorectal adenoma-carcinoma sequence. PLoS One. 2015;10:e0119255. doi: 10.1371/journal.pone.0119255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hattinger CM, Biason P, Iacoboni E, Gagno S, Fanelli M, Tavanti E, et al. Candidate germline polymorphisms of genes belonging to the pathways of four drugs used in osteosarcoma standard chemotherapy associated with risk, survival and toxicity in non-metastatic high-grade osteosarcoma. Oncotarget. 2016;7:61970–61987. doi: 10.18632/oncotarget.11486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhai X, Wang H, Zhu X, Miao H, Qian X, Li J, et al. Gene polymorphisms of ABC transporters are associated with clinical outcomes in children with acute lymphoblastic leukemia. Arch Med Sci. 2012;8:659–671. doi: 10.5114/aoms.2012.30290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Armenian SH, Ding Y, Mills G, Sun C, Venkataraman K, Wong FL, et al. Genetic susceptibility to anthracycline-related congestive heart failure in survivors of haematopoietic cell transplantation. Br J Haematol. 2013;163:205–213. doi: 10.1111/bjh.12516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Niemi M, Arnold KA, Backman JT, Pasanen MK, Gödtel-Armbrust U, Wojnowski L, et al. Association of genetic polymorphism in ABCC2 with hepatic multidrug resistance-associated protein 2 expression and pravastatin pharmacokinetics. Pharmacogenet Genomics. 2006;16:801–808. doi: 10.1097/01.fpc.0000230422.50962.91. [DOI] [PubMed] [Google Scholar]
- 73.Blanco JG, Leisenring WM, Gonzalez-Covarrubias VM, Kawashima TI, Davies SM, Relling MV, et al. Genetic polymorphisms in the carbonyl reductase 3 gene CBR3 and the NAD(P)H:quinone oxidoreductase 1 gene NQ01 in patients who developed anthracycline-related congestive heart failure after childhood cancer. Cancer. 2008;112:2789–2795. doi: 10.1002/cncr.23534. [DOI] [PubMed] [Google Scholar]
- 74.Krajinovic M, Labuda D, Sinnett D. Childhood acute lymphoblastic leukemia: genetic determinants of susceptibility and disease outcome. Rev Env Heal. 2001;16:263–279. doi: 10.1515/reveh.2001.16.4.263. [DOI] [PubMed] [Google Scholar]
- 75.Siegel D, Yan C, Ross D. NAD(P)H:quinone oxidoreductase 1 (NQO1) in the sensitivity and resistance to antitumor quinones. Biochem Pharmacol. 2012;83:1033–1040. doi: 10.1016/j.bcp.2011.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Szkandera J, Absenger G, Dandachi N, Regitnig P, Lax S, Stotz M, et al. Analysis of functional germline polymorphisms for prediction of response to anthracycline-based neoadjuvant chemotherapy in breast cancer. Mol Gen Genomics. 2012;287:755–764. doi: 10.1007/s00438-012-0715-7. [DOI] [PubMed] [Google Scholar]
- 77.Ito T, Fujio Y, Takahashi K, Azuma J. Degradation of NFAT5, a transcriptional regulator of osmotic stress-related genes, is a critical event for doxorubicin-induced cytotoxicity in cardiac myocytes. J Biol Chem. 2007;282:1152–1160. doi: 10.1074/jbc.M609547200. [DOI] [PubMed] [Google Scholar]
- 78.Lagoa R, Gañán C, López-Sánchez C, García-Martínez V, Gutierrez-Merino C. The decrease of NAD(P)H:quinone oxidoreductase 1 activity and increase of ROS production by NADPH oxidases are early biomarkers in doxorubicin cardiotoxicity. Biomarkers. 2014;19:142–153. doi: 10.3109/1354750X.2014.885084. [DOI] [PubMed] [Google Scholar]
- 79.PolymiRTS Database 3.0. p. http://compbio.uthsc.edu/miRSNP/ Accessed 08/03/17.
- 80.Shibayama Y, Ushinohama K, Ikeda R, Yoshikawa Y, Motoya T, Takeda Y, et al. Effect of methotrexate treatment on expression levels of multidrug resistance protein 2, breast cancer resistance protein and organic anion transporters Oat1, Oat2 and Oat3 in rats. Cancer Sci. 2006;97:1260–1266. doi: 10.1111/j.1349-7006.2006.00304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liu S, Wang BH, Kompa AR, Lekawanvijit S, Krum H. Antagonists of organic anion transporters 1 and 3 ameliorate adverse cardiac remodelling induced by uremic toxin indoxyl sulfate. Int J Cardiol. 2012;158:457–458. doi: 10.1016/j.ijcard.2012.05.022. [DOI] [PubMed] [Google Scholar]
- 82.Visscher H, Ross CJD, Rassekh SR, Sandor GSS, Caron HN, van Dalen EC, et al. Validation of variants in SLC28A3 and UGT1A6 as genetic markers predictive of anthracycline-induced cardiotoxicity in children. Pediatr Blood Cancer. 2013;60:1375–81. [DOI] [PubMed]
- 83.Hertz DL, Caram MV, Kidwell KM, Thibert JN, Gersch C, Seewald NJ, et al. Evidence for association of SNPs in ABCB1 and CBR3, but not RAC2, NCF4, SLC28A3 or TOP2B, with chronic cardiotoxicity in a cohort of breast cancer patients treated with anthracyclines. Pharmacogenomics. 2016;17:231–240. doi: 10.2217/pgs.15.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Reichwagen A, Ziepert M, Kreuz M, Gödtel-Armbrust U, Rixecker T, Poeschel V, et al. Association of NADPH oxidase polymorphisms with anthracycline-induced cardiotoxicity in the RICOVER-60 trial of patients with aggressive CD20(+) B-cell lymphoma. Pharmacogenomics. 2015;16:361–372. doi: 10.2217/pgs.14.179. [DOI] [PubMed] [Google Scholar]
- 85.Rossi D, Rasi S, Franceschetti S, Capello D, Castelli A, De Paoli L, et al. Analysis of the host pharmacogenetic background for prediction of outcome and toxicity in diffuse large B-cell lymphoma treated with R-CHOP21. Leukemia. 2009;23:1118–1126. doi: 10.1038/leu.2008.398. [DOI] [PubMed] [Google Scholar]
- 86.Ross C, Visscher H, Rassekh S, Castro-Pastrana L, Shereck E, Carleton B, et al. Pharmacogenomics of serious adverse drug reactions in pediatric oncology. J Popul Ther Clin Pharmacol. 2011;18:51. [PubMed] [Google Scholar]
- 87.Huang Z, Wang J, Qian J, Li Y, Xu Z, Chen M, et al. Effects of cytochrome P450 family 3 subfamily a member 5 gene polymorphisms on daunorubicin metabolism and adverse reactions in patients with acute leukemia. Mol Med Rep. 2017;15:3493–3498. doi: 10.3892/mmr.2017.6470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lipshultz SE, Lipsitz SR, Mone SM, Goorin AM, Sallan SE, Sanders SP, et al. Female sex and higher drug dose as risk factors for late cardiotoxic effects of doxorubicin therapy for childhood cancer. N Engl J Med. 1995;332:1738–1743. doi: 10.1056/NEJM199506293322602. [DOI] [PubMed] [Google Scholar]
- 89.Hequet O, Le QH, Moullet I, Pauli E, Salles G, Espinouse D, et al. Subclinical late cardiomyopathy after doxorubicin therapy for lymphoma in adults. J Clin Oncol. 2004;22:1864–1871. doi: 10.1200/JCO.2004.06.033. [DOI] [PubMed] [Google Scholar]
- 90.Mulrooney DA, Armstrong GT, Huang S, Ness KK, Ehrhardt MJ, Joshi VM, et al. Cardiac outcomes in adult survivors of childhood cancer exposed to cardiotoxic therapy: a cross-sectional study from the St. Jude Lifetime Cohort Ann Intern Med. 2016;164:93–101. doi: 10.7326/M15-0424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ventura-Clapier R, Moulin M, Piquereau J, Zurlo G, Garnier A. Sex differences in anthracycline cardiotoxicity. Ital J Gender-Specific Med. 2016;2:47–54. [Google Scholar]
- 92.Klein K, Thomas M, Winter S, Nussler AK, Niemi M, Schwab M, et al. PPARA: A Novel Genetic Determinant of CYP3A4 In Vitro and In Vivo. Clin Pharmacol Ther. 2012;91:1044–1052. doi: 10.1038/clpt.2011.336. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Information about the studied SNPs. Information on the selected SNPs is shown in an additional table file in more detail (gene, SNP ID, chromosome, position, function, alleles and minor allele frequencies). (XLSX 14 kb)
Data Availability Statement
The datasets analyzed during the current study are available from the corresponding author on reasonable request.